-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
The chromosomes of eukaryotes are organized into structurally and functionally discrete domains. This implies the presence of insulator elements that separate adjacent domains, allowing them to maintain different chromatin structures. We show that the Fun30 chromatin remodeler, Fft3, is essential for maintaining a proper chromatin structure at centromeres and subtelomeres. Fft3 is localized to insulator elements and inhibits euchromatin assembly in silent chromatin domains. In its absence, euchromatic histone modifications and histone variants invade centromeres and subtelomeres, causing a mis-regulation of gene expression and severe chromosome segregation defects. Our data strongly suggest that Fft3 controls the identity of chromatin domains by protecting these regions from euchromatin assembly.
Published in the journal: . PLoS Genet 7(3): e32767. doi:10.1371/journal.pgen.1001334
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001334Summary
The chromosomes of eukaryotes are organized into structurally and functionally discrete domains. This implies the presence of insulator elements that separate adjacent domains, allowing them to maintain different chromatin structures. We show that the Fun30 chromatin remodeler, Fft3, is essential for maintaining a proper chromatin structure at centromeres and subtelomeres. Fft3 is localized to insulator elements and inhibits euchromatin assembly in silent chromatin domains. In its absence, euchromatic histone modifications and histone variants invade centromeres and subtelomeres, causing a mis-regulation of gene expression and severe chromosome segregation defects. Our data strongly suggest that Fft3 controls the identity of chromatin domains by protecting these regions from euchromatin assembly.
Introduction
The eukaryotic genome is organized into chromosomal domains of distinct structure and function. One type of chromatin structure, termed euchromatin, is decondensed and accessible most of the time and condenses only during mitosis. In contrast, the compacted heterochromatin remains condensed throughout the cell cycle except during S phase. In general, euchromatin contains transcriptionally active genes, whereas heterochromatin contains repetitive sequences and relatively few silenced genes.
Post-translational histone modifications have a crucial role in organizing chromosomes into different domains. Euchromatin is enriched in methylation of histone H3 at lysine 4 (H3K4me) and contains hyperacetylated histones [1]–[4]. This generates an open chromatin fiber, enabling better access of the transcription machinery. In contrast, heterochromatin lacks H3K4me, but is methylated at lysine 9 of histone H3 (H3K9me) and has low levels of histone acetylation [1], [5]. H3K9me recruits HP1 chromo-domain proteins which packages the chromatin into an inaccessible configuration that silences genes transcriptionally [6].
Active and silent chromatin domains are often juxtaposed along the chromosomes and this organization demands a mechanism to separate them. The borders between these functionally antagonistic chromatin domains are often defined by specialized DNA sequences, known as insulators. Insulators are generally divided into two subclasses; enhancer-blocking insulators that prevent an enhancer from communicating with a promoter when positioned between the two, and barrier insulators that limit the spread of heterochromatin into the adjacent domain [7]. How certain DNA sequences can insulate chromatin domains is uncertain. It has been suggested that the insulators recruits chromatin-modifying factors, which would counteract the propagation of adjacent chromatin structures [8], [9]. Alternatively, another model suggests that the insulators could form loops and tether chromatin to fixed nuclear substrates, resulting in the formation of topologically distinct active and inactive domains [10], [11].
The fission yeast, Schizosaccharomyces pombe, is an excellent model system to study chromatin domains. Its three chromosomes are structurally similar to those of more complex eukaryotes, and many factors involved in heterochromatin assembly are conserved [12]. S. pombe chromosomes contain relatively large blocks of heterochromatin at centromeres, subtelomeres, tandem rDNA arrays and at the silent mating-type region [1].
Three structurally and genomically distinct types of insulators have been described in S. pombe. The most studied boundary is the cluster of tRNA genes located between centromeric chromatin and the flanking pericentric heterochromatin domains. Removal of these genes causes spreading of heterochromatin and silencing of reporter genes [13]. The insulator function seems to be a general property of tRNA genes, since replacement of centromeric tRNAs with noncentromeric tRNA isotypes does not affect the function of the centromeric boundary [14]. The mechanisms of tRNA boundary function is not completely understood, but it has been shown that the transcription factor TFIIIC and RNA polymerase III are required [14]. The Pol III initiation complex is thought to exclude histones from the barrier element, thereby generating a nucleosome-free region that restricts the spreading of silent chromatin [15]. Moreover, two histone demethylases, Lsd1 and Lsd2, associate with centromeric tRNA genes, and the deletion of Lsd1 results in the propagation of pericentric heterochromatin beyond the tRNA boundaries [16].
A second type of insulator is present outside the centromeric heterochromatin domains. Two inverted repeats, IRC, flank the left and right border of centromeres 1 and 3 [1], [11]. The mode of action of the centromeric IRC barrier is unknown. However, it has been shown that it is independent of TFIIIC and Pol III [11].
Another type of inverted repeats, IR elements, flank the silent mating type cassettes [2], [17]. They contain several Pol III B Box motifs and their boundary activity requires the association of TFIIIC, but not Pol III [11].
In this study, we have identified a SNF2 family ATP-dependent chromatin-remodeling factor, Fission yeast fun thirty (Fft3), required for proper chromatin structure at centromeres and subtelomeres. Fft3 is localized to known insulator elements and in its absence, euchromatin invades centromeres and subtelomeres, causing a change in histone modifications, histone variant localization, mis-regulation of gene expression and chromosome segregation defects. Based on this phenotype of fft3Δ, we suggest that Fft3 controls the identity of these chromatin domains by protecting them from euchromatin formation.
Results
Fft3 is required for the assembly of functional centromeric chromatin
The Fun30 chromatin remodeler was identified in a screen for genes that affect chromosome stability in budding yeast [18]. This finding prompted us to investigate whether the S. pombe Fun30 homolog, Fft3, also has a role in chromosome stability.
To test this we constructed wild type and fft3Δ mutant strains bearing the Ch16 mini-chromosome. When this mini-chromosome is lost the cells form red rather than white colonies on limited adenine plates. By scoring the frequency of half-sectored colonies, the rate of chromosome loss per division can be calculated. The fft3Δ mutant shows a very high frequency of mini-chromosome loss (11%, Figure 1A) compared to wild type (0.13%). This result was confirmed by DAPI staining of mitotic chromosomes. As expected, fft3Δ cells show a higher rate of unequal chromosome segregation compared to wild type. Five percent of fft3Δ cells show a segregation defect in which none of the chromosomes separate. Instead, all DAPI-stained material stays on one side of the septum (Figure 1B). This phenotype is typical for proteins important for kinetochore formation [19], [20].
Fig. 1. The Fun30 ATP-dependent chromatin remodeler Fft3 is required for accurate chromosome segregation. 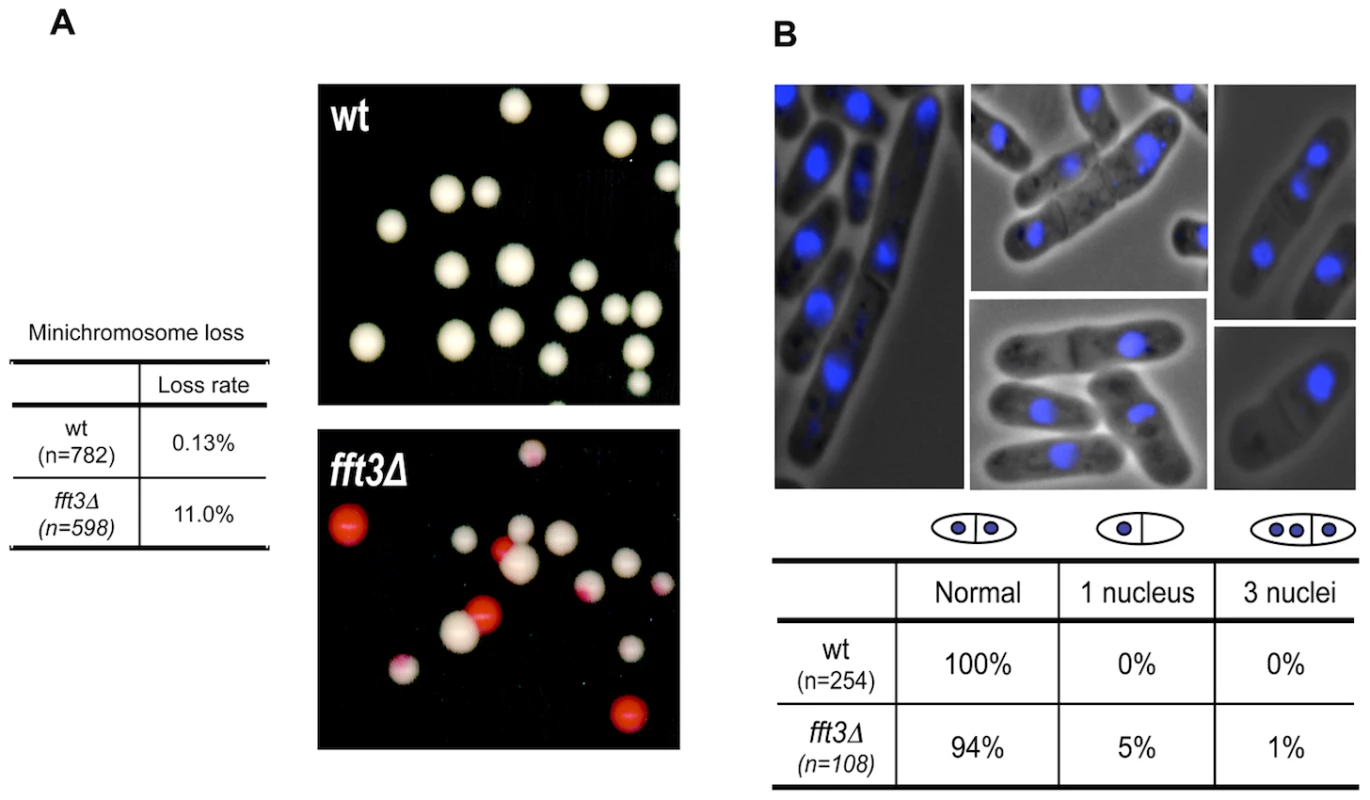
A) Left: A table showing data from the Ch16 mini-chromosome loss colony color-sectoring assay. Wild type (Hu199) and fft3Δ (Hu1666) colonies containing a mini-chromosome were picked from plates selecting for the mini-chromosome (Ade-), and plated onto YE+ low adenine plates. The colonies retaining the mini-chromosome are white, and loss events result in red sectors. The mini-chromosome loss frequency was quantified by determining the amount of half-sectored colonies. Right: A photograph showing the red-sectoring phenotype of fft3Δ cells. B) Top: Microscope images showing fft3Δ (Hu1867) cells stained with DAPI. Bottom: A table showing the frequencies of aberrant mitosis in wt (Hu29) and fft3Δ (Hu1867) cells. The S. pombe centromeres consist of a central core domain (cnt), where the kinetochore is formed, surrounded by inverted repeats. Closest to the cnt are the inner repeats (imr) flanked by the outer repeats (otr), which are composed of dg and dh elements [21], [22] (Figure S1). The otr regions are assembled into heterochromatin [23], [24]. The central core domain possesses a different chromatin structure from the outer repeats. It is not heterochromatic, though there is transcriptional silencing. Placement of a ura4+ gene within this centromeric region results in its transcriptional silencing [25]. We found that cells carrying a deletion for fft3 show an increased sensitivity to FOA as compared to wt cells when the ura4+ gene is inserted to the central core domain (Figure S1A), indicating that the ura4+ genes is upregulated. A slight upregulation of the ura4+ genes was also supported by RT-PCR analysis (Figure S1B).
Taken together our findings suggest that Fft3 contributes to the unique chromatin structure underlying the kinetochore.
Fft3 is localized at tRNA and IRC insulator elements
To investigate whether Fft3 has a direct role at centromeres, we analyzed the localization of Fft3-myc fusion protein using a genome-wide ChIP-chip assay. The experiment clearly revealed that Fft3 is located at the central core domains of all three centromeres (Figure 2 and Figure S2). In this domain histone H3 is replaced by the variant histone Cnp1 (S. pombe homolog of CENP-A). The Cnp1 chromatin is surrounded by heterochromatin methylated at lysine 9 of histone H3 [1], [23]. At the transition between the central core and the otr regions we observed a sharp decrease in Fft3 localization. Fft3 continues to be depleted from the entire heterochromatin domains in both directions until the border between otr and the surrounding euchromatin, where we also detected prominent peaks of Fft3. A similar localization pattern was observed for chromosomes 1 and 2 (Figure S2).
Fig. 2. Fft3 associates with known centromeric insulators and the central core domain. 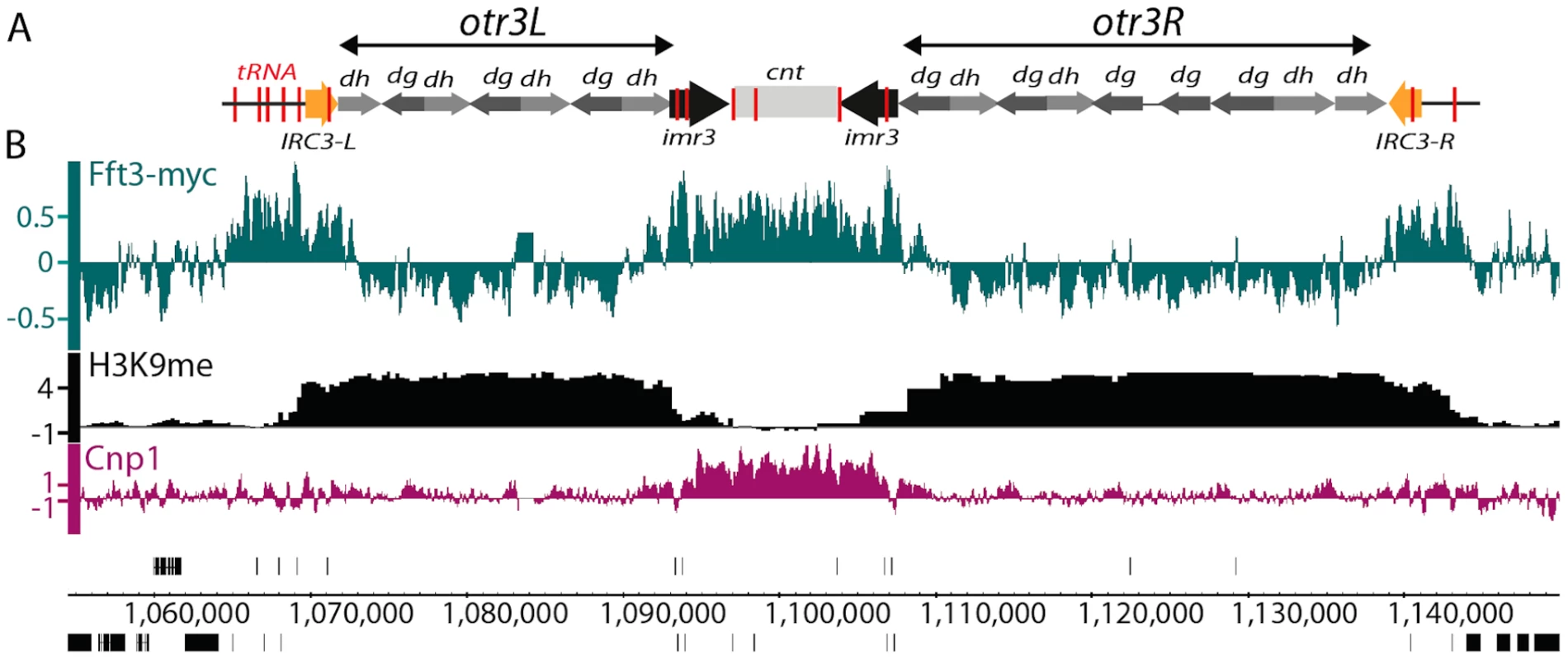
A) A schematic presentation of centromere 3. tRNA genes are marked in red and the IRC elements in yellow. B) A genome browser view of cen3 showing the ChIP-chip occupancy profile for Fft3-myc (green), H3K9me2 (black) and Cnp1 (purple). Data on the Y-axis are presented in log2 scale and the X-axis shows genome positions in base pairs. H3K9me2 data are from [1]. Clusters of tRNA genes demark the transition between the imr/cnt and otr at all centromeres in S. pombe. These genes have been shown to act as insulators and prevent heterochromatin from spreading into the Cnp1-containing chromatin [13]. Notably, our ChIP-chip data shows that Fft3 is enriched over these tRNA genes. tRNA clusters are also present at five of the six centromere extremities. Fft3 is enriched over these tRNA clusters as well. Fft3 localization is not restricted to centromeric tRNA genes. We also found that Fft3 was enriched (2-fold) over 156 out of the 175 (89%) tRNA genes throughout the genome (data not shown).
An additional centromeric insulator element has been identified in S. pombe. At centromeres 1 and 3 the boundaries between pericentromeric heterochromatin and surrounding euchromatin coincide with the location of IRC elements [1]. Deletion of IRC1-L causes spreading of H3K9me from the centromere into the surrounding euchromatin, suggesting that these elements function as heterochromatin barriers [2]. Our Fft3 occupancy map clearly shows that Fft3 is also enriched over these elements (Figure 2 and Figure S2).
Taken together, our Chip-chip analysis demonstrates Fft3 occupancy over all known centromeric insulator elements in S. pombe, suggesting that the remodeling factor could have a function at these boundaries.
The chromatin structure of centromeric domains and boundaries is altered in fft3Δ cells
Most tRNA genes reside in nucleosome free regions and it has been suggested that this nucleosome-free gap is sufficient to provide a boundary function for the tRNA genes [26]. In budding yeast, the RNA Pol III complex and a chromatin remodeler, RSC, are thought to be involved in generating such a histone-depleted region [9]. To test whether the Fun30 remodeler could have a function in disassembling nucleosomes over centromeric tRNA genes we analyzed the distribution of histone H3 by ChIP-chip experiments.
Our data shows that H3 is enriched over the entire pericentromeric heterochromatin regions (Figure 3A (blue), and Figure S3). As expected, we observed a sharp decrease in H3 density over the tRNA genes surrounding the central domain, and at the tRNA and IRC elements flanking the right and left borders of the pericentric region. The H3 levels are low over the central domain, where instead the histone H3 variant, Cnp1 is enriched (Figure 3C (red)). ChIP-chip analysis of fft3Δ mutant cells revealed that H3 remained depleted over the tRNA genes and IRC boundary element, indicating that Fft3 is not involved in evicting histones from the insulators. However, our analysis showed that in fft3Δ cells, H3 appears in the imr regions (Figure 3A; blue). This increase of H3 was validated by quantitative PCR of ChIP samples (Figure 3D). We found a 3.5 fold increase of H3 levels at imr3 in fft3Δ vs wt (P value 0.0002; T-test). Consistent with this, the Cnp1 levels were 2.7 fold reduced (P value 0.001; T-test) at imr3 in fft3Δ cells (Figure 3C (red), and 3D). Outside the imr and central core domain the Cnp1 signals are very low (near background) in wild type and sometimes even lower in fft3Δ cells. Therefore, since the Cnp1 protein is basically undetectable in these regions we do not think the ratio changes observed outside the imr and central core regions are biologically meaningful.
Fig. 3. Centromeric boundary function is impaired in fft3Δ cells. 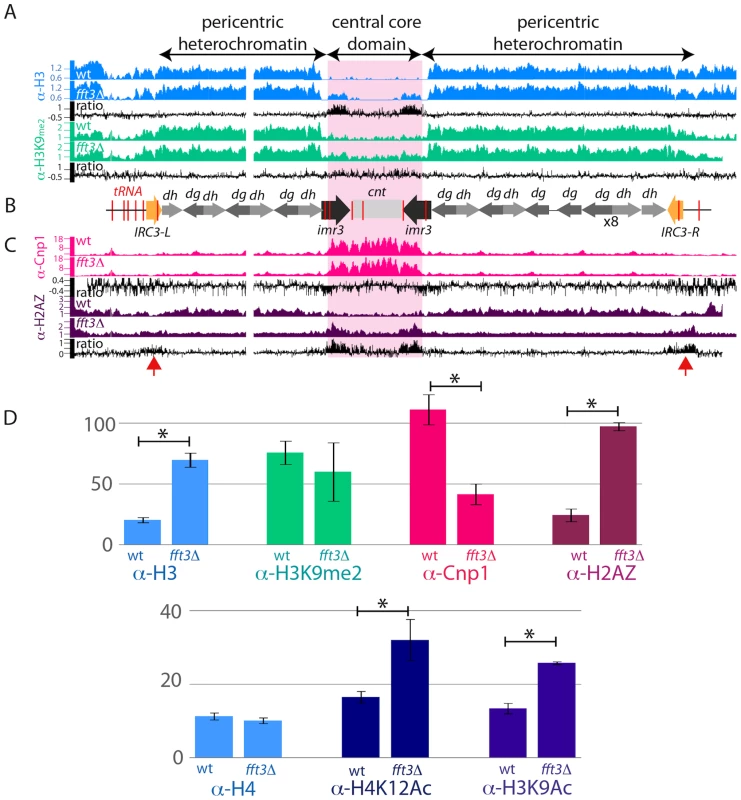
A) The ChIP-chip distributions of histone H3 (blue) and H3K9me2 (green) at centromere 3 in wt and fft3Δ cells are shown in Genome Browser images. Y-axis: Linear scale. Mutant/wt ratios are indicated in black in log2 scale. B) A schematic diagram of centromere 3. tRNA genes are marked in red and the IRC elements in yellow. C) Genome browser images showing ChIP-chip data for Cnp1 (red) and H2A.ZPht1 (purple). Y-axis: Linear scale. Mutant/wt ratios are shown in black in log2 scale. D) Bar diagrams showing the results from real-time quantitative PCR analysis of ChIP signals. The enrichments at imr3 are relative to the act1+ euchromatic control locus except for the Cnp1 enrichment which is relative to cen1. The ChIP signals were normalized to input samples from the same chromatin extract. The error bars represent S.D. values from triplicate samples. *indicates a significant difference between wt and mutant (P<0.01; T-test, two-tailed, unpaired). Several insulators have been shown to stop the propagation of heterochromatin [11], [13], [16]. Therefore, we wanted to investigate whether the H3 that appears in the central core region in fft3Δ cells is methylated at lysine 9. Our ChIP-chip and ChIP/quantitative PCR experiments revealed that the H3 was not methylated at K9 (Figure 3A (green) and 3D). Surprisingly, we found that the H3 that appears in the imr region in fft3Δ instead was acetylated at K9. We observed a 2-fold increase of H3K9ac in fft3Δ compared to wt (P value, 0.006; T-test; Figure 3D). We also found that the degree of acetylation of histone H4 at K12 was 2-fold increased at imr3 in fft3Δ (P value 0.01; T-test; Figure 3D, bottom panel), but the histone H4 levels remained unchanged. Both of these acetylation marks, and especially H3K9ac, are characteristic of active euchromatin in S. pombe [27].
Histone H2A.Z is a universally conserved histone variant that replaces the conventional H2A protein in a significant fraction of nucleosomes [3]. Normally H2A.Z is absent from all centromeric regions, including both the Cnp1 containing inner domain and the pericentric heterochromatin [3]. Surprisingly, we observed that in the absence of Fft3, the H2A.Z levels increase by 4-fold at imr3 (P value 0.00003; T-test; Figure 3C (purple), and 3D). Moreover, in fft3Δ cells, the H2A.Z levels clearly increased at ICR elements and at the tRNA genes flanking the pericentric regions (see red arrows in Figure 3). In euchromatin H2A.Z is localized to gene promoters and correlates well with H3K9ac and H4K12ac [3]. Taken together these results indicate that Fft3 has a novel role at insulator elements shielding the centromeric central core domain from euchromatin formation.
The above experiments show that the properties of the central core domains are altered in the fft3Δ mutant. Next, we wanted to investigate whether the pericentric heterochromatin domains are also affected. Paradoxically, heterochromatin domains in S. pombe are more sensitive to cleavage of the micrococcal nuclease (MNase) indicating a reduced nucleosome density [28], [29]. The heterochromatin showed a reduced sensitivity to MNase in the fft3Δ mutant cells, indicating an altered chromatin structure (Figure 4 and Figure S4). Thus, Fft3 is required for a proper chromatin structure of both the pericentric and the central core chromatin domains.
Fig. 4. Fft3 is affecting chromatin structure at pericentric heterochromatin. 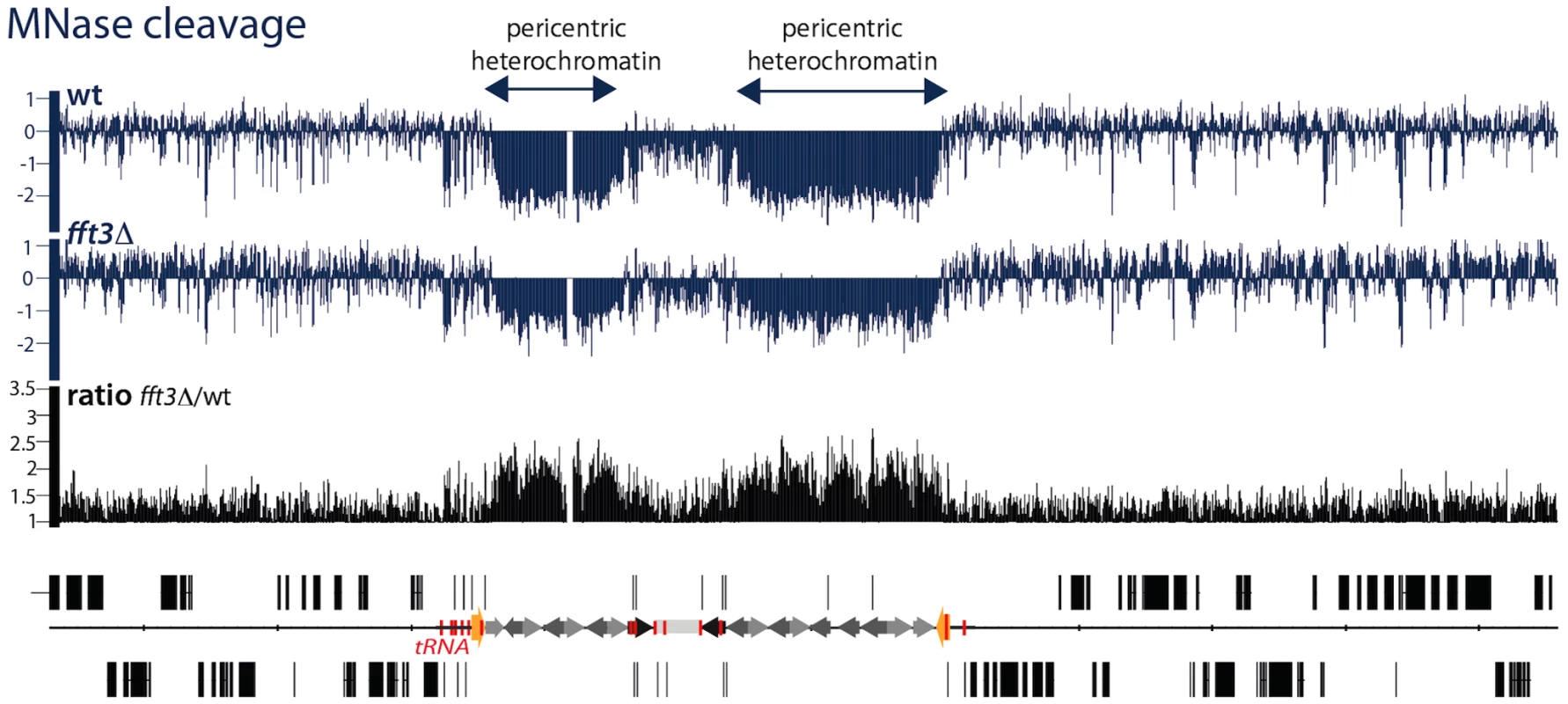
A genome browser view showing MNase cleavage maps (blue) from wt and fft3Δ cells. Y-axis: Log2 scale. Data are from [28]. The mutant/wt ratio is shown in black in linear scale. tRNA genes are shown in red and IRC elements in yellow. Fft3 represses subtelomeric genes
Our experiments indicated that Fft3 is required to maintain a proper centromeric chromatin structure. Although Fft3 is localized to centromeres, it is possible that Fft3 also affects centromeres via changes in gene expression of centromeric components. To address this concern, expression profiling of fft3Δ cells was performed. mRNA from logarithmically growing fft3Δ cells was compared to mRNA from wild type cells. Using a 2-fold cutoff for changes in gene expression, we found that 61 genes were up-regulated and 15 genes were down-regulated in fft3Δ versus wild type. Importantly, no genes known to be involved in centromere function are affected, thus arguing against any indirect effects of Fft3 on centromeric chromatin.
Interestingly, further analysis of the expression profiling data revealed that, more than half of the up-regulated genes (61%) lay within the subtelomeric regions of chromosomes 1 and 2 (Figure 5). Moreover, 74% of the upregulated genes on chromosome 2 are located within 100 kb from the chromosome ends (Figure 5B). This clustering of affected genes near tel2 is highly significant (5.39e-44, hypergeometric probability). Thus, in addition to its role in protecting centromeric chromatin domains, Fft3 is also ensuring a proper silent chromatin structure at the subtelomeres.
Fig. 5. Fft3 is required for silencing of subtelomeric genes. 
A) Chromosomal view of gene expression changes in fft3Δ versus wt. The position of upregulated genes (2-fold cutoff) is indicated in red. The subtelomeric regions of chromosome 2 are shown in more detail. B) Venn diagram showing the overlap between genes upregulated on chromosome 2 in fft3Δ cells and all subtelomeric genes 100 kb from tel2. The P value represents the hypergeometric probability. Fft3 counteracts euchromatin formation in subtelomeric regions
Fft3 could perform its telomeric gene silencing function directly or indirectly: (1) Fft3 could be located all over the telomeric silenced domain and facilitate subtelomeric chromatin-assembly; (2) It could function at the silenced genes themselves by binding and repressing their promoters; or (3) it may function as a component of an insulator that maintains the integrity of the subtelomeric domains. To distinguish between these possibilities, we analyzed the Fft3-myc ChIP-chip data. We found that the most prominent noncentromeric peaks of Fft3 were seen about 100 kb away from the telomeres (red arrows in Figure 6A), strongly suggesting that Fft3 has a function at subtelomeric boundaries.
Fig. 6. Fft3 is localized at subtelomeric boundaries and prevents euchromatin assembly in ST-chromatin. 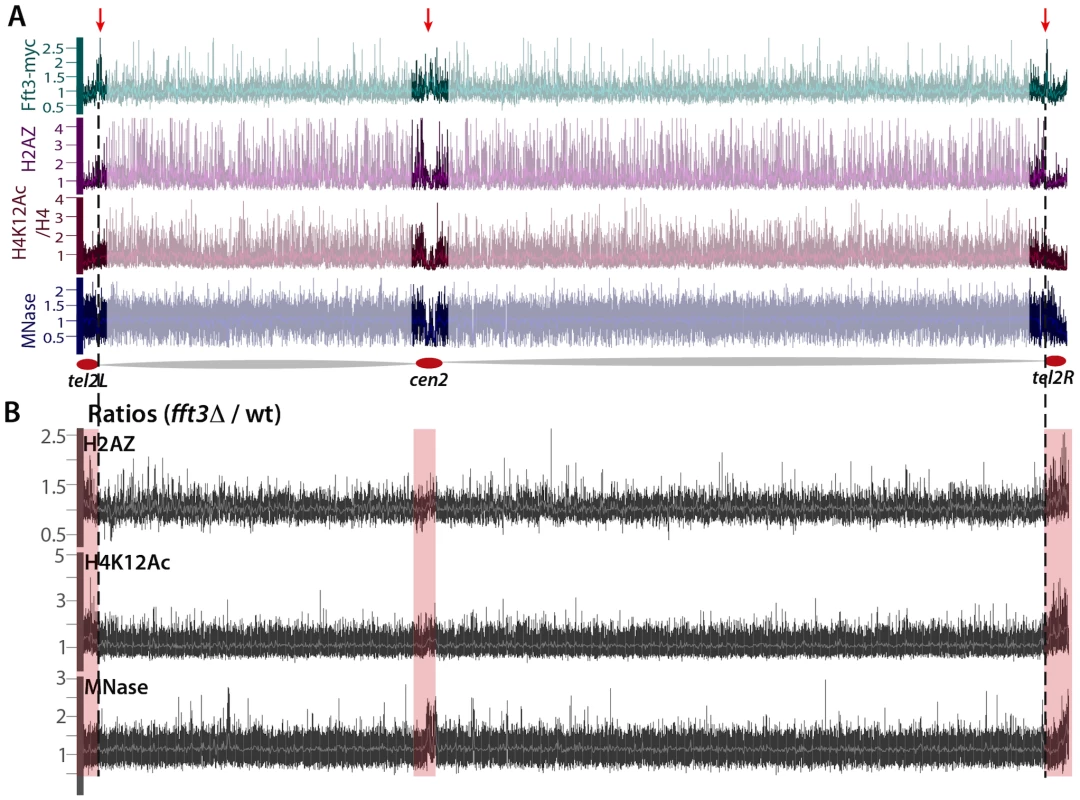
A) Genome browser images showing the chromosome 2 occupancy of Fft3-myc (green, Y-axis: Log2 scale), H2A.Z in wt (purple, Y-axis: linear scale), H4K12ac in wt corrected for H4 occupancy (dark red, Y-axis: linear scale), and Micrococcal Nuclease (MNase) digested chromatin in wt (blue, Y-axis: linear scale). The MNase data are from [28]. B) Genome browser views showing mutant/wt ratios for H2A.Z and H4K12ac ChIP-chip, and for MNase digested chromatin. Centromeric and subtelomeric regions are highlighted with light-pink rectangles. We recently showed that the subtelomeric regions represent a distinct class of chromatin, subtelomeric chromatin (ST-chromatin), with different properties than bulk eu - or heterochromatin [3]. ST-chromatin is characterized by depletion of acetylated histones (including histone H4K12 acetylation), H3K4 methylation and H2A.Z. At the very end of the chromosome arms, there is a transition from ST-chromatin to bulk subtelomeric heterochromatin methylated on H3K9 (Figure S5).
The transition between euchromatin and the ST-chromatin is sharp, with a drastic reduction of H2A.Z, H4K12ac and H3K4me (Figure 6A and [3]). Interestingly, the peaks of Fft3 occupancy appear to be situated exactly over these transitions (see the red arrows in Figure 6A), suggesting the Fft3 binding could mark the transition from euchromatin to ST-chromatin. To explore this further, we performed ChIP-chip experiments for H2A.Z and H4K12ac in wild type and fft3Δ mutant cells. Our analysis shows that, when Fft3 is removed, these two euchromatin marks expand beyond the euchromatin domain into the ST-chromatin (see ratios in Figure 6B, and Figure S6). These results strongly indicate that Fft3 protects both centromeric chromatin and ST chromatin from euchromatin assembly.
Paradoxically, in S. pombe, heterochromatin has a wider spacing between nucleosomes and a lower nucleosome occupancy as compared to euchromatin [28], [29]. To address whether Fft3 is affects the chromatin structure of heterochromatin domains, we compared the MNase digestion patterns of wild type and fft3Δ cells. Deletion of Fft3 did not detectably alter the global chromatin structure (Figure 6B, Figure S7, and [28]). However, our data revealed striking changes in the MNase digestion patterns across the centromeric and ST-chromatin. There is a tendency for a reduced MNase digestion in fft3Δ as compared to wild type cells, indicating that pericentric heterochromatin domains and ST-chromatin become more euchromatic.
From these data we conclude that Fft3 marks the boundaries between euchromatin and subtelomeres and that it prevents euchromatin formation in the ST-chromatin domains.
Altered chromatin structure at the tel2L subtelomeric transition zone
As described above, Fft3 is localized at the transition zone between euchromatin and ST-chromatin. At the left subtelomere on chromosome II, this transition zone coincides exactly with the presence of four long terminal repeats (LTRs) located just upstream of the promoters of four copies of one gene encoding a membrane transporter (Figure 7A). The four LTR elements, the four transporter genes, and the sequences between them are 100% identical at the nucleotide level, suggesting that this region arose from gene duplication events. At this transition a sharp decrease in H2A.Z, H4K12ac and H3K4me2 is seen (Figure 6 and Figure S5), indicating that this region might function as an insulator element.
Fig. 7. Fft3 is localized to the tel2L subtelomeric transition zone. 
A) Left panel: A genome browser view of subtelomere 2L. ChIP-chip data for Fft3-myc (green) and H3 (blue) are shown. A subtelomeric transition zone consisting of four copies of a membrane transporter gene and four LTR elements (red) is highlighted in blue. Right panel (top): Bar diagrams showing quantification of Fft3-myc ChIP signals by real time PCR. Enrichment at LTR elements, membrane transporter genes (using primers for SPBPB10D8.05c), and cnt1 was measured relative to centromeric dg repeats. Background normalization was performed using -antibody samples. The grey bars represent signals from the non-tagged control strain (Hu29). The error bars represent S.D. values from triplicate samples. *indicates a significant difference between Fft3-myc and the non-tagged control strain (P<0.01; T-test, two-tailed, unpaired). Right panel (bottom): Bar diagrams showing quantification of H3 ChIP in wt (blue) and fft3Δ (black) on LTR elements and membrane transporter genes relative to an euchromatin control gene (SPAC32A11.03c). The ChIP signals were normalized to input samples from the same chromatin extract. The error bars represent S.D. from triplicate samples. *indicates a significant difference between wt and fft3Δ cells (P<0.01; T-test, two-tailed, unpaired). B) A genome browser view of mononucleosomal signals in wt after MNase digestion. Data are from [28]. Insulators and barrier elements are often characterized by a special chromatin structure that manifests itself as a constitutive DNaseI hypersensitive site [30]–[32]. It has been suggested that hypersensitive sites signify a disruption of the regular nucleosome repeat along the DNA fiber and that they function as binding sites for sequence-specific factors. In agreement with this notion, we found that the LTR region was highly sensitive to MNase cutting (Figure 7B), indicating that this region has typical features of an insulator.
Analysis of the Fft3 occupancy map revealed that Fft3 is present at these LTR sequences, but not at the membrane transporter genes (Figure 7A). The ChIP-chip data also revealed that histone H3 is enriched over the transporter genes coding regions and depleted from the intergenic regions and the LTR elements. The occupancy of Fft3 at LTR elements was confirmed by quantitative PCR analysis. Interestingly, quantitative PCR also showed that the H3 density at the LTRs was increased about 2-fold in fft3Δ cells (P = 0.002; T-test), while the H3 density at the membrane transporter genes remained unchanged. This suggests that Fft3 is interacting with the LTR sequences where it stimulates nucleosome disassembly or eviction. Thus, Fft3 is required for a proper chromatin structure of a subtelomeric transition zone.
Discussion
Active and silent chromatin domains are often juxtaposed along the chromosome arms, but they need to be separated in order to maintain chromosome stability and correct gene expression levels. The spreading of chromatin domains beyond their natural borders is blocked by insulators. Previous studies have identified such elements surrounding the heterochromatin domains in fission yeast, but the mechanisms of boundary function are not well understood.
In S. pombe, all described insulators act as barrier elements that restrict the spread of silenced chromatin [11], [13], [16]. In this study we describe a mechanism in which euchromatin is blocked from entering certain chromatin domains. Our study shows that Fft3, an ATP-dependent chromatin-remodeling factor, is localized to known centromeric insulators and at a subtelomeric transition zone. In cells depleted for Fft3, euchromatin is assembled in the centromeric central cores and subtelomeric chromatin domains, involving a change of histone modifications, incorrect incorporation of histone variants and mis-regulation of gene expression. This strongly suggests that Fft3 controls the identity of chromatin domains by shielding them from euchromatin formation.
S. pombe utilizes at least two independent pathways to maintain silent heterochromatin at telomeres [33]. The first pathway involves Taz1, a homolog of mammalian TRF1/2, which binds directly to the telomere-associated sequence (TAS) and recruits factors essential for heterochromatin formation [34]. The other pathway requires another cis acting element consisting of cenH-like sequences that display extensive sequence homology with the dg and dh repeats found at centromeres. These repeats function as DNA templates for siRNA production. The siRNA are processed by the RNAi-RITS pathway [1], [33] and histones become methylated on histone H3 at lysine 9. H3K9me then spreads inward along the chromosome arms. Approximately 20 kb into the chromosome arms, the spreading of H3K9me terminates and instead ST-chromatin is formed [3]. The mechanism of this transition is not understood. A second transition occurs around 100 kb, where ST-chromatin is replaced by bulk euchromatin. This transition is relatively sharp with large changes of H2A.Z and H4K12ac levels. In this study, we have shown that Fft3 plays an important role in this second transition. When Fft3 is removed, the integrity of ST-chromatin is lost. Euchromatin marks, such as histone acetylation and enrichment of histone variant H2AZ appear instead. As a result of this, genes in the normally silent subtelomere become expressed. In agreement with this, we showed from MNase cleavage patterns that the telomeric chromatin structure is changed and becomes more euchromatin-like in fft3Δ mutant cells. Interestingly, two independent studies found that Fft3 co-purifies with the heterochromatic Swi6 protein[35] [36]. It is possible that this interaction is important to protect the integrity of the subtelomere chromatin structure.
Fft3 is localized to a subtelomeric transition zone containing genes and LTR elements arisen from gene duplication events. Duplicated segments at transition zones seem to be a conserved feature. In human, more than half of the transition regions between euchromatin and centromeric heterochromatin contain duplicated segments and multiple duplicones are concatenated together to form larger block of wall-to-wall duplications [37]. Interestingly, it has been demonstrated that two demethylases, Lsd1 and Lsd2, with roles in centromeric boundary function, are also localized to the S. pombe tel2L transition zone. The demethylases are present at the promoters of the membrane transporter genes where they demethylate H3K9me3 [16].
We could also show that Fft3 regulates the chromatin structure at the centromeres. In fft3Δ mutant cells H3 levels are increased and Cnp1 levels are reduced in the central core domain. Previously described centromeric insulators inhibit heterochromatin spreading [11], [13], [16]. Fft3 is located at these elements but does not participate in the heterochromatin blocking. Instead, similarly to what happens at subtelomeres, we see euchromatin marks such as histone acetylation and H2A.Z appearing in the central core region in the absence of Fft3. We propose that the insulator elements at centromeres have a novel function in addition to heterochromatin barrier function. This additional function involves Fft3 mediated protection from euchromatin formation in the central core region. It is not clear what the ‘source’ of the newly formed euchromatin is which renders the centromere dysfunctional and leads to severe chromosome segregation defects. Simple spreading models can be ruled out since the central core region is flanked by pericentric heterochromatin. The euchromatin assembly could be mediated by recruitment of trans-acting components. Alternatively it could spread from the euchromatin regions that are flanking the pericentric heterochromatin. In this scenario the pericentric regions would be skipped by looping out of the pericentric domain via clustering of the tRNAs in imr and IRC regions.
Insulator elements that protect chromosome domains from euchromatin have previously been described in Drosophila. For example, the scs and scs' (specialized chromatin structures) elements insulate the expression of the white reporter gene from euchromatin [38], and boundaries between bithorax domains protect inactive domains from the open configuration of the neighboring domain [39]. It has been suggested that insulator elements exert their function by partitioning chromosomal domains into “higher-order” loops either by interacting with each other or with fixed nuclear structure such as the nuclear matrix or nuclear pores [10], [11], [40], [41]. It has been suggested that S. pombe also uses a looping mechanism to create functional insulator elements. The Pol III transcription factor TFIIIC is recruited to insulator elements flanking the mating loci and is thought to tether these boundaries to the nuclear periphery [11]. In addition, tRNA and other Pol III transcribed genes dispersed throughout the chromosomes localize to the nuclear periphery at centromeres, and this localization is mediated by condensin [42]. Fft3 is localized to tRNA genes both in fission and budding yeast [43] and it is possible that Fft3 somehow cooperates with condensin in the tethering of tRNA to the centromere.
The Drosophila Su(Hw) (suppressor of hairy-wing) insulator protein form insulator bodies that interact with the nuclear matrix. This tethering to the matrix is required for its insulating function [7]. Moreover, the vertebrate insulator protein CTCF also interacts with the nuclear matrix [44]. Interestingly, it was recently shown that Fft3 also is tightly associated with the nuclear matrix [45].
A synthetic screen for barrier activity in S. cerevisiae identified proteins involved in nuclear transport as insulator proteins [10]. Since transport proteins localize to the nuclear pore complex, these results led to the model that tethering insulators to nuclear pores aids in the formation of loops. Recently, it was shown that Fft3 binds to the Ran nuclear transporter Spi1 [45] and fft3Δ shows synthetic lethality with mutations of several genes encoding nuclear transport proteins and nucleoporins [46]. This indicates that Fft3 maybe involved in forming chromatin loops by attaching insulator elements to the nuclear matrix or pores. This would isolate a signal generated in one chromatin domain from reaching the domain in the next loop. This could explain how Fft3 shields off centromeres and subtelomeres from euchromatin formation.
The Fun30 family of chromatin remodeling factors, represented by SMARCAD1 in humans [47], Etl1 in mouse [48], and Fun30 in budding yeast [18] is a group of conserved proteins that has received little attention and their molecular functions are relatively unknown. We have shown that the S. pombe FUN30 homolog, Fft3, protects centromeres and subtelomeres from euchromatin. This type of insulating activity could to be a conserved property of the FUN30 chromatin remodeling factors. It was recently shown that the S. cerevisiae homolog, FUN30, binds and alters the nucleosome positioning at the HMR barrier element and is required for silencing of the HMR domain [43]. Thus it is possible that FUN30 in budding yeast has a similar shielding function as Fft3 in fission yeast. Our study identifies a new biological function for the Fun30 class of chromatin remodeling factors at centromeres and in ST-chromatin, and expands our understanding of the molecular composition and machinery involved in partitioning neighboring chromatin domains. Further characterization of insulators and their role in preserving distinct chromatin configurations will help us better understand the higher order organization of chromosomes.
Materials and Methods
S. pombe strains used are listed in Table S1. Standard procedures were used for growth and genetic manipulation.
Central core silencing assay
Cell suspensions of wild type (FY412) and fft3Δ (Hu1321) strains were 5-fold diluted, spotted on non-selective plates (PMG), - ura plates, and counter-selective FOA plates (1.0 g/l, US Biologicals) and incubated at 30°C for 3 days.
Ura4 expression profiling
Total RNA was extracted from Hu303 (wild-type), FY412 (CC2::ura4+), Hu1321 (fft3Δ CC2::ura4+), FY340 (TM1::ura random integrant), and Hu111 (ura4-DS/E) as described earlier [49]. Contaminating DNA was removed with TURBO DNA-free kit (ambion). RT-PCR was performed using SuperScipt II Reverse Transcriptase (invitrogen) and Oligo(dT)12–18 primers (invitrogen). The primers used for the ura4 PCR are listed in Table S2.
ChIP-chip
DNA was immunoprecipitated as described earlier [50], using 10 µl of anti-Cnp1 antiserum, 2 µl of anti-myc (9E10), 2 µl of anti-H4K12ac (ab1761, abcam), 1.5 µg of anti H3 (ab1791, abcam), 2 µl of anti-H4 (Pan 05-858, upstate), 5 µg of anti-H3K9me2 (07-441, upstate), or 2 µl of anti-H3K9Ac (07-352, Millipore) antibodies per 100 µl chromatin extract.
Real-time quantitative PCR was preformed in the presence of SYBR Green using the Applied Biosystems 7500 real-time PCR machine. The primers used are listed in Table S2.
H3K9me Chip-chip data (Figure 2, Figure S2, and Figure S5) and genome-wide MNase digestion maps are from previous studies [1], [28].
Microarray analysis
Immunoprecipitated DNA was amplified to 5 µg DNA as described in [50], with the exception that in the second PCR 5 mM dUTP was added to the reaction.
Fragmentation, labeling and hybridization to the Affymetrix GeneChip S. pombe Tiling 1.0FR was performed by Affymetrix core facility at Novum (BEA) according to Affymetrix standard protocols.
Raw data from Affymetrix (.CEL format) were analyzed with Affymetrix Tiling Analysis Software (TAS) v1.1 and visualized with Affymetrix Integrated Genome Browser (IGB). A tiling analysis group (.TAG file) for a two-sample analysis containing 2 ChIP experiments as the ‘treatment’ group and 2 input samples as the ‘control’ group was created in TAS. The data were normalized using quantile normalization plus scaling and run with a bandwidth of 20.
Microarray data have been deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/) under the accession code E-MEXP-3044.
Expression profiling
Total RNA from wild type and fft3Δ strains was purified as described earlier [49] and hybridized to Affymetrix GeneChip S. pombe Tiling 1.0FR according to protocol and recommendations from Affymetrix. Data was normalized in TAS as described above and normalized data was analyzed using GeneSpring software (Agilent). A standard cutoff value of 2-fold was used for gene expression changes.
Supporting Information
Zdroje
1. CamHP
SugiyamaT
ChenES
ChenX
FitzGeraldPC
2005
Comprehensive analysis of heterochromatin - and RNAi-mediated epigenetic control of the fission yeast genome.
Nat Genet
37
809
819
2. NomaK
AllisCD
GrewalSI
2001
Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries.
Science
293
1150
1155
3. BuchananL
Durand-DubiefM
RoguevA
SakalarC
WilhelmB
2009
The Schizosaccharomyces pombe JmjC-protein, Msc1, prevents H2A.Z localization in centromeric and subtelomeric chromatin domains.
PLoS Genet
5
e1000726
doi:10.1371/journal.pgen.1000726
4. HebbesTR
ClaytonAL
ThorneAW
Crane-RobinsonC
1994
Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain.
EMBO J
13
1823
1830
5. BraunsteinM
RoseAB
HolmesSG
AllisCD
BroachJR
1993
Transcriptional silencing in yeast is associated with reduced nucleosome acetylation.
Genes Dev
7
592
604
6. NomaK
SugiyamaT
CamH
VerdelA
ZofallM
2004
RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing.
Nat Genet
36
1174
1180
7. DormanER
BusheyAM
CorcesVG
2007
The role of insulator elements in large-scale chromatin structure in interphase.
Semin Cell Dev Biol
18
682
690
8. OkiM
ValenzuelaL
ChibaT
ItoT
KamakakaRT
2004
Barrier proteins remodel and modify chromatin to restrict silenced domains.
Mol Cell Biol
24
1956
1967
9. DhillonN
RaabJ
GuzzoJ
SzyjkaSJ
GangadharanS
2009
DNA polymerase epsilon, acetylases and remodellers cooperate to form a specialized chromatin structure at a tRNA insulator.
EMBO J
28
2583
2600
10. IshiiK
AribG
LinC
Van HouweG
LaemmliUK
2002
Chromatin boundaries in budding yeast: the nuclear pore connection.
Cell
109
551
562
11. NomaK
CamHP
MaraiaRJ
GrewalSI
2006
A role for TFIIIC transcription factor complex in genome organization.
Cell
125
859
872
12. WoodV
GwilliamR
RajandreamMA
LyneM
LyneR
2002
The genome sequence of Schizosaccharomyces pombe.
Nature
415
871
880
13. ScottKC
MerrettSL
WillardHF
2006
A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains.
Curr Biol
16
119
129
14. ScottKC
WhiteCV
WillardHF
2007
An RNA polymerase III-dependent heterochromatin barrier at fission yeast centromere 1.
PLoS ONE
2
e1099
doi:10.1371/journal.pone.0001099
15. OkiM
KamakakaRT
2005
Barrier function at HMR.
Mol Cell
19
707
716
16. LanF
ZaratieguiM
VillenJ
VaughnMW
VerdelA
2007
S. pombe LSD1 homologs regulate heterochromatin propagation and euchromatic gene transcription.
Mol Cell
26
89
101
17. ThonG
BjerlingP
BunnerCM
Verhein-HansenJ
2002
Expression-state boundaries in the mating-type region of fission yeast.
Genetics
161
611
622
18. OuspenskiII
ElledgeSJ
BrinkleyBR
1999
New yeast genes important for chromosome integrity and segregation identified by dosage effects on genome stability.
Nucleic Acids Res
27
3001
3008
19. TakahashiK
ChenES
YanagidaM
2000
Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast.
Science
288
2215
2219
20. TakahashiK
YamadaH
YanagidaM
1994
Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality.
Mol Biol Cell
5
1145
1158
21. ClarkeL
AmstutzH
FishelB
CarbonJ
1986
Analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe.
Proc Natl Acad Sci U S A
83
8253
8257
22. ChikashigeY
KinoshitaN
NakasekoY
MatsumotoT
MurakamiS
1989
Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites.
Cell
57
739
751
23. NakayamaJ
RiceJC
StrahlBD
AllisCD
GrewalSI
2001
Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly.
Science
292
110
113
24. PartridgeJF
BorgstromB
AllshireRC
2000
Distinct protein interaction domains and protein spreading in a complex centromere.
Genes Dev
14
783
791
25. AllshireRC
JaverzatJP
RedheadNJ
CranstonG
1994
Position effect variegation at fission yeast centromeres.
Cell
76
157
169
26. BiX
YuQ
SandmeierJJ
ZouY
2004
Formation of boundaries of transcriptionally silent chromatin by nucleosome-excluding structures.
Mol Cell Biol
24
2118
2131
27. WirenM
SilversteinRA
SinhaI
WalfridssonJ
LeeHM
2005
Genomewide analysis of nucleosome density histone acetylation and HDAC function in fission yeast.
EMBO J
24
2906
2918
28. LantermannAB
StraubT
StralforsA
YuanGC
EkwallK
2010
Schizosaccharomyces pombe genome-wide nucleosome mapping reveals positioning mechanisms distinct from those of Saccharomyces cerevisiae.
Nat Struct Mol Biol
17
251
257
29. GarciaJF
DumesicPA
HartleyPD
El-SamadH
MadhaniHD
2010
Combinatorial, site-specific requirement for heterochromatic silencing factors in the elimination of nucleosome-free regions.
Genes Dev
24
1758
1771
30. UdvardyA
MaineE
SchedlP
1985
The 87A7 chromomere. Identification of novel chromatin structures flanking the heat shock locus that may define the boundaries of higher order domains.
J Mol Biol
185
341
358
31. ChungJH
WhiteleyM
FelsenfeldG
1993
A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila.
Cell
74
505
514
32. NasmythKA
1982
The regulation of yeast mating-type chromatin structure by SIR: an action at a distance affecting both transcription and transposition.
Cell
30
567
578
33. KanohJ
SadaieM
UranoT
IshikawaF
2005
Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres.
Curr Biol
15
1808
1819
34. CooperJP
NimmoER
AllshireRC
CechTR
1997
Regulation of telomere length and function by a Myb-domain protein in fission yeast.
Nature
385
744
747
35. FischerT
CuiB
DhakshnamoorthyJ
ZhouM
RubinC
2009
Diverse roles of HP1 proteins in heterochromatin assembly and functions in fission yeast.
Proc Natl Acad Sci U S A
106
8998
9003
36. MotamediMR
HongEJ
LiX
GerberS
DenisonC
2008
HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms.
Mol Cell
32
778
790
37. HorvathJE
BaileyJA
LockeDP
EichlerEE
2001
Lessons from the human genome: transitions between euchromatin and heterochromatin.
Hum Mol Genet
10
2215
2223
38. KellumR
SchedlP
1991
A position-effect assay for boundaries of higher order chromosomal domains.
Cell
64
941
950
39. GyurkovicsH
GauszJ
KummerJ
KarchF
1990
A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation.
EMBO J
9
2579
2585
40. GerasimovaTI
ByrdK
CorcesVG
2000
A chromatin insulator determines the nuclear localization of DNA.
Mol Cell
6
1025
1035
41. YusufzaiTM
TagamiH
NakataniY
FelsenfeldG
2004
CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species.
Mol Cell
13
291
298
42. IwasakiO
TanakaA
TanizawaH
GrewalSI
NomaK
2010
Centromeric localization of dispersed Pol III genes in fission yeast.
Mol Biol Cell
21
254
265
43. Neves-CostaA
WillWR
VetterAT
MillerJR
Varga-WeiszP
2009
The SNF2-family member Fun30 promotes gene silencing in heterochromatic loci.
PLoS ONE
4
e8111
doi:10.1371/journal.pone.0008111
44. YusufzaiTM
FelsenfeldG
2004
The 5′-HS4 chicken beta-globin insulator is a CTCF-dependent nuclear matrix-associated element.
Proc Natl Acad Sci U S A
101
8620
8624
45. OhbaT
NishijimaH
NishitaniH
NishimotoT
2008
Schizosaccharomyces pombe Snf2SR, a novel SNF2 family protein, interacts with Ran GTPase and modulates both RanGEF and RanGAP activities.
Genes Cells
13
571
582
46. RoguevA
BandyopadhyayS
ZofallM
ZhangK
FischerT
2008
Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast.
Science
322
405
410
47. AdraCN
DonatoJL
BadovinacR
SyedF
KherajR
2000
SMARCAD1, a novel human helicase family-defining member associated with genetic instability: cloning, expression, and mapping to 4q22-q23, a band rich in breakpoints and deletion mutants involved in several human diseases.
Genomics
69
162
173
48. SoininenR
SchoorM
HenselingU
TepeC
Kisters-WoikeB
1992
The mouse Enhancer trap locus 1 (Etl-1): a novel mammalian gene related to Drosophila and yeast transcriptional regulator genes.
Mech Dev
39
111
123
49. XueY
HaasSA
BrinoL
GusnantoA
ReimersM
2004
A DNA microarray for fission yeast: minimal changes in global gene expression after temperature shift.
Yeast
21
25
39
50. Durand-DubiefM
EkwallK
2009
Chromatin immunoprecipitation using microarrays.
Methods Mol Biol
529
279
295
Štítky
Genetika Reprodukční medicína
Článek Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis inČlánek c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and PhosphodiesterasesČlánek Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- Testing for an Unusual Distribution of Rare Variants
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities!
- Initial Mutations Direct Alternative Pathways of Protein Evolution
- Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in
- Sensing of Replication Stress and Mec1 Activation Act through Two Independent Pathways Involving the 9-1-1 Complex and DNA Polymerase ε
- Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in
- Identification of a Novel Type of Spacer Element Required for Imprinting in Fission Yeast
- Chiasmata Promote Monopolar Attachment of Sister Chromatids and Their Co-Segregation toward the Proper Pole during Meiosis I
- Global Analysis of the Relationship between JIL-1 Kinase and Transcription
- H3K9me2/3 Binding of the MBT Domain Protein LIN-61 Is Essential for Vulva Development
- REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock
- A Novel Unstable Duplication Upstream of Predisposes to a Breed-Defining Skin Phenotype and a Periodic Fever Syndrome in Chinese Shar-Pei Dogs
- Polycomb Repressive Complex 2 Controls the Embryo-to-Seedling Phase Transition
- A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Germ Line
- Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits
- A Genome-Wide Association Study of Upper Aerodigestive Tract Cancers Conducted within the INHANCE Consortium
- Ancestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
- Ultra-Deep Sequencing of Mouse Mitochondrial DNA: Mutational Patterns and Their Origins
- Phenotype Restricted Genome-Wide Association Study Using a Gene-Centric Approach Identifies Three Low-Risk Neuroblastoma Susceptibility Loci
- The Toll-Like Receptor Gene Family Is Integrated into Human DNA Damage and p53 Networks
- Polycomb Targets Seek Closest Neighbours
- Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis
- c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and Phosphodiesterases
- Estimating Divergence Time and Ancestral Effective Population Size of Bornean and Sumatran Orangutan Subspecies Using a Coalescent Hidden Markov Model
- Rif1 Supports the Function of the CST Complex in Yeast Telomere Capping
- A Tradeoff Drives the Evolution of Reduced Metal Resistance in Natural Populations of Yeast
- Quantifying the Underestimation of Relative Risks from Genome-Wide Association Studies
- Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in
- Triplet Repeat–Derived siRNAs Enhance RNA–Mediated Toxicity in a Drosophila Model for Myotonic Dystrophy
- The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
- Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
- Environmental Sex Determination in the Branchiopod Crustacean : Deep Conservation of a Gene in the Sex-Determining Pathway
- Systematic Detection of Polygenic Regulatory Evolution
- The SUMO Isopeptidase Ulp2p Is Required to Prevent Recombination-Induced Chromosome Segregation Lethality following DNA Replication Stress
- Uncoupling Antisense-Mediated Silencing and DNA Methylation in the Imprinted Cluster
- Role of the Drosophila Non-Visual ß-Arrestin Kurtz in Hedgehog Signalling
- Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti–dsDNA Autoantibody Production
- COMPASS-Like Complexes Mediate Histone H3 Lysine-4 Trimethylation to Control Floral Transition and Plant Development
- H3 Lysine 4 Is Acetylated at Active Gene Promoters and Is Regulated by H3 Lysine 4 Methylation
- Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches
- A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in
- Roles of () in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- A Molecular Phylogeny of Living Primates
- Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice
- A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



