-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
Penetration of a male copulatory organ into a suitable mate is a conserved and necessary behavioral step for most terrestrial matings; however, the detailed molecular and cellular mechanisms for this distinct social interaction have not been elucidated in any animal. During mating, the Caenorhabditis elegans male cloaca is maintained over the hermaphrodite's vulva as he attempts to insert his copulatory spicules. Rhythmic spicule thrusts cease when insertion is sensed. Circuit components consisting of sensory/motor neurons and sex muscles for these steps have been previously identified, but it was unclear how their outputs are integrated to generate a coordinated behavior pattern. Here, we show that cholinergic signaling between the cloacal sensory/motor neurons and the posterior sex muscles sustains genital contact between the sexes. Simultaneously, via gap junctions, signaling from these muscles is transmitted to the spicule muscles, thus coupling repeated spicule thrusts with vulval contact. To transit from rhythmic to sustained muscle contraction during penetration, the SPC sensory-motor neurons integrate the signal of spicule's position in the vulva with inputs from the hook and cloacal sensilla. The UNC-103 K+ channel maintains a high excitability threshold in the circuit, so that sustained spicule muscle contraction is not stimulated by fewer inputs. We demonstrate that coordination of sensory inputs and motor outputs used to initiate, maintain, self-monitor, and complete an innate behavior is accomplished via the coupling of a few circuit components.
Published in the journal: . PLoS Genet 7(3): e32767. doi:10.1371/journal.pgen.1001326
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001326Summary
Penetration of a male copulatory organ into a suitable mate is a conserved and necessary behavioral step for most terrestrial matings; however, the detailed molecular and cellular mechanisms for this distinct social interaction have not been elucidated in any animal. During mating, the Caenorhabditis elegans male cloaca is maintained over the hermaphrodite's vulva as he attempts to insert his copulatory spicules. Rhythmic spicule thrusts cease when insertion is sensed. Circuit components consisting of sensory/motor neurons and sex muscles for these steps have been previously identified, but it was unclear how their outputs are integrated to generate a coordinated behavior pattern. Here, we show that cholinergic signaling between the cloacal sensory/motor neurons and the posterior sex muscles sustains genital contact between the sexes. Simultaneously, via gap junctions, signaling from these muscles is transmitted to the spicule muscles, thus coupling repeated spicule thrusts with vulval contact. To transit from rhythmic to sustained muscle contraction during penetration, the SPC sensory-motor neurons integrate the signal of spicule's position in the vulva with inputs from the hook and cloacal sensilla. The UNC-103 K+ channel maintains a high excitability threshold in the circuit, so that sustained spicule muscle contraction is not stimulated by fewer inputs. We demonstrate that coordination of sensory inputs and motor outputs used to initiate, maintain, self-monitor, and complete an innate behavior is accomplished via the coupling of a few circuit components.
Introduction
Male copulation is the most complex behavior for C. elegans [1]. The nervous system must integrate sensory cues from the hermaphrodite to generate instructions for each step of mating. In addition, the sex-specific mating circuits monitor the progress, and then determine whether to retain the current motor pattern or to adopt a new action [2]. This is done simultaneously as they convey information to the general locomotion circuit to coordinate the male's movements with the hermaphrodite's [3]. The function of the male's nervous system is also modulated by gender determining factors, nutritional state and the presence of food odors [4]–[7].
The 302 neurons in Caenorhabditis elegans hermaphrodites and the 383 neurons in males encode a variety of general behaviors like feeding, locomotion, defecation and sensory modalities in both sexes. The circuits controlling these behaviors are comprised of a few neurons and muscles that are multifunctional in sensing, processing and commanding signals [8]–[17]. In contrast to many C. elegans hermaphrodite-studied sensory and motor actions, the male-executed copulation is a multi-component task-orientated behavior. Under an idealized mating situation, the male presses his sensory laden tail firmly on a receptive hermaphrodite, and adjusts his posture so that he laterally scans the dorsal and ventral cuticle of his mate, searching for the vulva. He would then position his tail over the vulva and extend a pair of copulatory spicules from his cloacal opening to pry apart the vulva lips. Finally, he would transfer sperm into the uterus of his mate [2], [18]–[21]. However in reality, young virgin hermaphrodites attempt to crawl away from the male during mating, and depending on the age of the hermaphrodites, their vulval slits can be too tight for males to breach immediately. Thus, the male adopts an alternative behavior, which is to produce high frequency (7–11 Hz) repetitive spicule thrusts at the vulva while maintaining contact with it, until partial spicule insertion induces the full penetration [22]–[24].
Essential cellular components for spicule insertion were identified in previous work using aged paralyzed hermaphrodites as mates. The movements of the spicules are caused by contractions of the spicule “protractor” muscles, which are attached to the base of the spicules. Rhythmic contractions of the protractors, initiated by the postcloacal sensilla neurons PCA, PCB and PCC, cause the spicules to thrust repeatedly against the vulval slit. Tonic contraction of the protractors causes the spicules to extend fully from the male tail and to penetrate the vulva. This step requires the activity of the cholinergic SPC neurons, which innervate the protractors [2], [22], [25]. Although copulation is initiated by the postcloacal and SPC sensory-motor neurons, it remains elusive how these cells generate and coordinate each step of the behavior.
Understanding the functional cellular/molecular architecture that drives circuit output is a necessity before one can appreciate why certain genes are or are not the evolutionary targets that underlie the diversity of sexual behavior and reproduction. In this study, we addressed how the C. elegans male spicule protraction circuit generates and coordinates different motor outputs to penetrate the hermaphrodite's vulva. We determined that stimulation of distinct acetylcholine receptors in posterior sex muscles is used to maintain the male's position over the vulva. This information is simultaneously relayed to the spicule protractors through electrical junctions, thus providing a mechanism for coupling spicule insertion and vulva location behavior. In addition, we identified multiple sensory inputs that control when the protractors should transit from rhythmic to sustained contraction.
Results
Males require cholinergic signaling to maintain contact with the hermaphrodite's vulva during mating
During their attempts to inseminate the hermaphrodite, C. elegans males must maintain precise contact between their cloacal opening and the vulva. In our previous study, we observed that males lacking the muscarinic acetylcholine receptor (mAChR) GAR-3 do not sustain their position over the vulva during prolonged attempts to insert their spicules. This mAChR is expressed in the cholinergic spicule-related neurons, PCB and SPC, to enhance ACh secretion from these neurons [26]. ACh secreted from the SPC neurons activates ionotropic ACh receptors on the spicule protractor muscles to induce muscle contraction, which results in tonic spicule protraction [22]. We speculated that the ionotropic ACh receptors in the cells of the spicule circuit can also be used to sustain precise vulval contact behavior; therefore in the gar-3 deletion mutant, reduced cholinergic synaptic transmission between the spicule-related neurons and the sex muscles resulted in defective vulval contact.
To address whether ionotropic ACh receptors are utilized to regulate precise vulval contact, we first asked how effectively males that lack the functional levamisole (LEV)-sensitive receptor (L-AChR) mated. The L-AChR had been the only ionotropic ACh receptor described to be involved in ACh agonist-induced spicule protraction. This receptor contains three α-subunits: UNC-38, UNC-63 and LEV-8, and two non-α subunits: UNC-29 and LEV-1 [22], [27]–[30]. We used mutants carrying the unc-29(e193) allele, which has a missense mutation that changes a proline to serine at amino acid 258 within the hypothetic first transmembrane domain of the channel [31]–[33]. LEV induces spicule protraction via activating functional UNC-29-containing L-AChRs, and the effective concentration that induces prolonged protraction for 90% of the males is 2 µM [22]. unc-29(e193) males were resistant to LEV. We observed that none of the mutant males protracted their spicules in 2 µM LEV (n = 47), compared to 87.7% of the wild-type males (n = 57, p<0.0001). Like other unc-29 loss-of-function (lf) alleles, the e193 allele causes worms to have severe uncoordinated locomotion [29], [32], which disrupts the males' ability to move backwards along the hermaphrodite during mating. We restored locomotion to the mutant males by using the upstream region of the acr-8 gene to drive unc-29 cDNA expression (Pacr-8:unc-29cDNA::SL2::GFP) in the body wall muscles, but not in the male sex muscles [34]. Similar to the unc-29(lf) mutant males, the transgenic males were also resistant to LEV; 6% of them protracted their spicules in 2 µM LEV (n = 35, p = 0.18). We assayed the mating potency of the locomotion-restored unc-29(lf) males by pairing each male with a moving hermaphrodite for 4 hours, and observed that these males could sire progeny with 83.3% of wild-type efficiency (n = 38, Figure 1A). This indicates that even though the L-AChR is used to facilitate spicule protraction in response to LEV, there are other receptors compensating for its function during male mating.
Fig. 1. Ionotropic ACh receptors are required for sustained vulval contact. 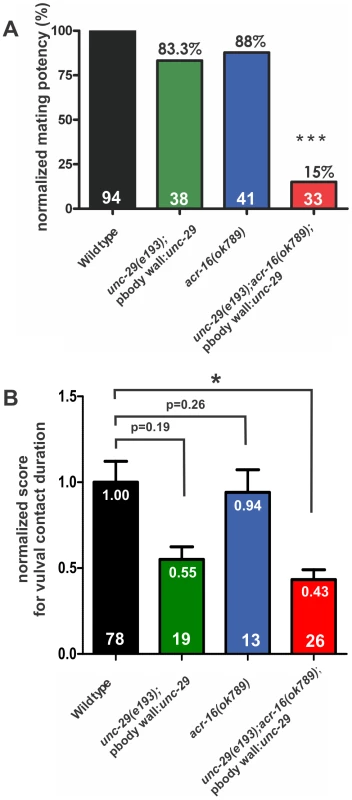
(A) Relative mating potency of mutant males normalized to wild type. The number of males assayed for each strain is listed within each bar. The number above the bar refers to the normalized mating potency value. The males were mated with free-moving hermaphrodites. Each mutant strain was compared to the wild-type males that were assayed in parallel, using the Fisher's exact test. Asterisks (***) indicate the p value <0.0001. (B) Profiles of the vulval contact duration. The males were mated into paralyzed hermaphrodites. The average duration of vulval contact for each male was normalized to the wild type mean to obtain the “normalized score for vulval contact duration”, as a measurement of the male's ability to maintain precise vulval contact (Materials and Methods). The mean of the normalized score for vulval contact duration for each strain is represented by the height of the bar, and it is also indicated by the number below the top of the bar. Error bars indicate the standard error of the mean. The number of males assayed for each strain is listed within each bar. Each mutant strain was compared to wild-type males using the Mann-Whitney non-parametric test. Asterisk (*) indicates the p value <0.005. The raw values of average duration of vulval contact for each male tested are presented in Figure S2A. Next, we asked which other ionotropic ACh receptors could be functioning redundantly with the L-AChR. One possibility was the homomeric receptor formed by the α7-like nicotinic ACh receptor (nAChR) subunit, ACR-16. Since it was previously shown to have an ancillary function with the L-AChR in the C. elegans locomotion circuit [30], [35], we asked if ACR-16 was also used during male mating behavior. After 4 hours of contact with hermaphrodites, males that contained the acr-16(ok789) deletion (acr-16(0)) achieved 88% of wild-type mating potency (n = 41, Figure 1A), suggesting that like the L-AChR, ACR-16 is not essential for male potency.
We reasoned that if the L-AChR and the ACR-16-containing nAChR were required for mating but were interchangeable, then a severe mating defect would be seen in the unc-29(lf);acr-16(0) double mutant. The unc-29(lf);acr-16(0) males are completely paralyzed. Since the acr-16(0) allele alone does not cause any gross locomotion defect [30], [35], we only needed to restore the impaired locomotion of the double mutant using the construct Pacr-8:unc-29cDNA::SL2::GFP. We quantified the velocity of backward locomotion during vulva search behavior and found that the transgenic double mutant males moved relatively well, albeit slightly slower than wild-type males; 105±33 µm/sec for wild type (n = 5 males) and 71±35 µm/sec for the double mutant (n = 5 males) (Figure S1). In our mating potency assay, we found that the transgenic unc-29(lf);acr-16(0) males could only achieve 15% of wild-type mating potency (n = 33, p<0.001, Figure 1A). This suggests that both L-AChR and the ACR-16 nAChR are used in male mating behavior, and unlike the locomotion behavior, if the function of one receptor is compromised, the other can equally compensate.
We then asked if the locomotion-restored double mutant males mated poorly because they could not insert their spicules, or if they were additionally defective in other steps of mating, such as maintaining contact with the hermaphrodite's vulva. We paired males with paralyzed unc-64(lf) hermaphrodites for them to mate, so that duration of each vulval contact could be accurately recorded [26]. During a 5 minute observation window, the majority of the males we assayed made multiple contacts with the hermaphrodites' vulva before they inserted their spicules or until the observation period ended. Then we determined the average duration of the vulval contacts for each male (Figure S2A). In order to score his ability to maintain precise vulval contact, we first normalized wild type to a score of 1 and then we compared the normalized mutant males to the wild type (Materials and Methods) (Figure 1B). We found that the unc-29(lf);acr-16(0) males sustained vulval contact for half the time of wild-type males (p<.05, Figure 1B; see Materials and Methods). In contrast to matings with moving hermaphrodites, the reduced vulval contact did not severely affect the mating potency of the mutant males when they mated with paralyzed hermaphrodites. 48.5% of the double mutant males inserted their spicules and transferred sperm into unc-64(lf) hermaphrodites during the 5 minute observation period (n = 33), not statistically different from the 69.7% of the wild type (n = 33, p = 0.13). However, failure to sustain their position over the vulva is likely to be the reason why the double mutant males cannot inseminate free-behaving hermaphrodites, as maintaining contact with a moving object is already challenging for wild-type males: only 10% of the wild-type males could inseminate free-moving hermaphrodites within 5 minutes (n = 20). We then asked if the defect in maintaining vulval contact in unc-29(lf);acr-16(0) males was caused by the loss of the L-AChR or the ACR-16 nAChR, or both. Loss of function of either receptor did not reduce the vulval contact duration with statistical significance (Figure 1B), suggesting that these genes are used redundantly to maintain vulval contact. However, although the average duration of vulval contact were not statistically significant between wild type and the mutants, the distributions of vulval contact duration were slightly different (p<0.005, F test; Figure S2A) between wild type and the unc-29(lf), as well as between wild type and the acr-16(0) mutant, suggesting that these genes on their own subtly affect the behavior.
The unc-29 and acr-16 genes are expressed in the male-specific muscles
We hypothesized that in the unc-29(lf);acr-16(0) mutant, signaling from circuit components that maintain vulval contact is impaired, resulting in males that cannot sustain their position at the vulva. To address which neurons and muscles were used in the male mating circuit in this step of behavior, we first determined where unc-29 and acr-16 were expressed in the male tail. In adult males, a YFP expression construct that contains a 5.3 kb sequence upstream of the acr-16 start codon (Pacr-16:YFP) was observed to be expressed in the body wall muscles, the sexually dimorphic anal depressor muscle, and some male-specific muscles, such as the spicule protractor muscles, the gubernacular erector and retractor muscles and the anterior oblique muscles. However, it was not seen in any neuron in the spicule protraction circuit (Figure 2A-2D). Similarly, a construct that has the 1.3 kb sequence upstream of the first exon of unc-29 and the whole unc-29 genomic sequence fused to the YFP gene was expressed in the same set of muscles as well as in some ventral cord neurons and some head neurons. This non-α L-AChR subunit was not expressed on any spicule neuron either (Figure 2E–2H; Figure S3A-S3F).
Fig. 2. Male tail expression of acr-16 and unc-29. 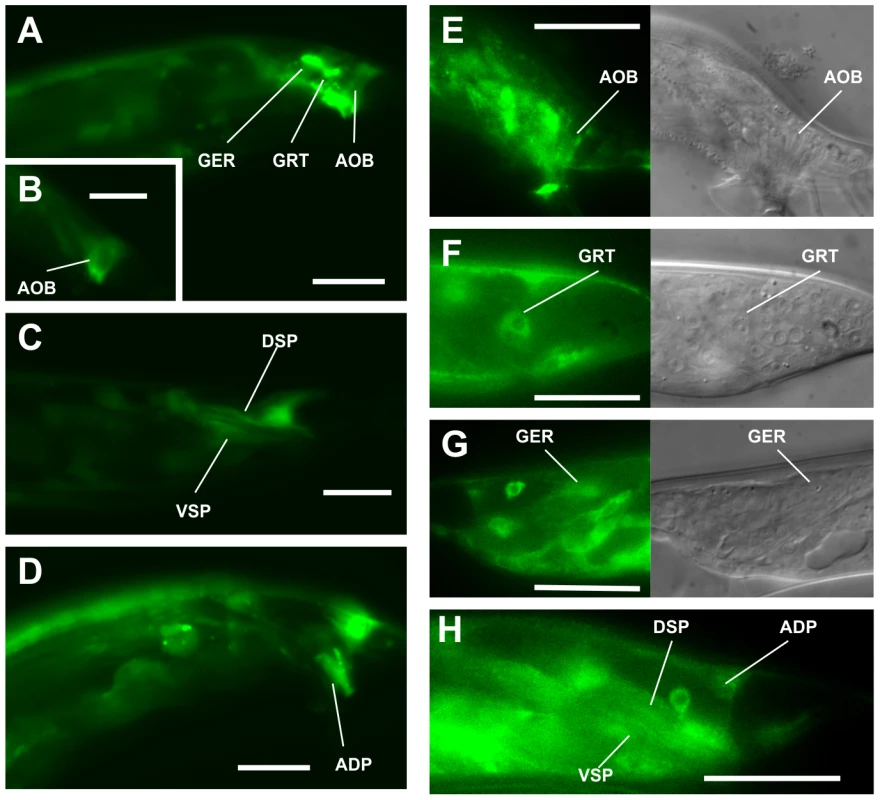
Gubernacular erector (GER), gubernacular retractor (GRT), anterior oblique (AOB), dorsal spicule protractor (DSP), ventral spicule protractor (VSP) and anal depressor (ADP) muscle. (A–D) Fluorescence images of the tail region of adult males expressing the Pacr-16:YFP construct. Scale bar, 20 µm. (E–H) Fluorescence images of the tail region of adult males expressing the Punc-29:unc-29::YFP construct. Scale bar, 20 µm. UNC-63 and UNC-38 are α-subunits required for a functional L-AChR. We found both genes were expressed in the same set of male tail muscles as unc-29, but also expressed in the SPC spicule neurons (Figure S3G–S3N). This indicates that UNC-63 and UNC-38 probably form an ionotropic ACh receptor on the SPC neurons independent of UNC-29. UNC-63 and UNC-38 have been found to form a LEV-insensitive ionotropic ACh receptor with the α-subunit ACR-12 and also with the non-α-subunits ACR-2 and ACR-3 on the ventral cord cholinergic motor neurons [36]. This is consistent with the fact that the acr-12 promoter-genomic DNA fusion construct was also expressed in the SPC neurons (Figure S3O, S3P). Thus, by using the unc-29(e193) allele in the unc-29(e193);acr-16(ok789) double mutant, we only impaired the function of L-AChR on the male sex muscles. However, it was not initially obvious how reducing UNC-29 and ACR-16 in the sex muscles affected the male's ability to maintain its position over the vulva during spicule insertion attempts.
The postcloacal sensilla neurons maintain the male tail position at the vulva
Electron micrograph reconstruction of the male has reveal extensive electrical and chemical communication between the bilateral sets of muscles and neurons in the tail region (Figure 3A) (S.W. Emmons, personal communication; S.W. Emmons, D.H. Hall, M. Xu, Y. Wang and T. Jarrell, Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm). We studied the connections of the male nervous system to determine candidate neurons that make synapses to the acr-16 and unc-29 expressing male sex muscles. Among the muscles that express both acr-16 and unc-29, the spicule protractors are innervated by the SPC cholinergic neurons; the anterior oblique muscles (left/right), and the gubernacular erector (L/R) and retractor (L/R) muscles are innervated by the postcloacal sensilla (p.c.s.) neurons (Figure 3B).
Fig. 3. Circuit diagram of cells that control spicule movement and model of prolonged vulval contact coupling to rhythmic protractor contractions. 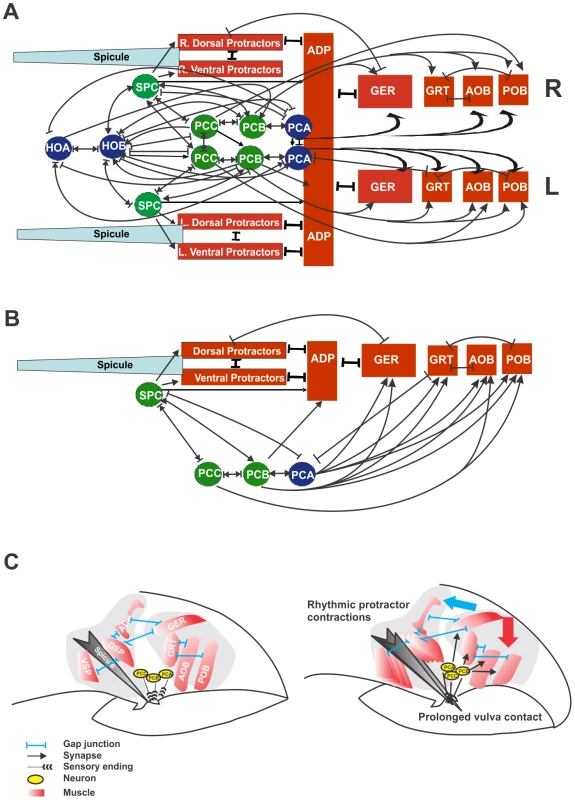
Gubernacular erector (GER), gubernacular retractor (GRT), anterior oblique (AOB), posterior oblique (POB), dorsal spicule protractor (DSP), ventral spicule protractor (VSP) and anal depressor (ADP). Arrows and bars indicate chemical synapses and gap junctions, respectively. Bi-directional arrows refer to cells that make reciprocal chemical synapses. Bi-directional arrows embedded in bars refer to cells that make gap junctions in addition to reciprocal chemical synapses. The green circles refer to cholinergic neurons. (A) Bilateral electrical and chemical connectivity diagram of male neurons and muscles that control the movements of the spicules [adapted from [25], and the Male Wiring Project]. (B) Abbreviated cartoon of limited connections between neurons and muscles discussed in this work. Refer to (Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm) for a more complete list of connections to other cells in the male. (C) Cartoon depicting changes in male tail muscles upon vulval contact. Left panel depicts relative locations and electrical junctions between the male sex muscles. The right panel depicts the shortening of the AOB and POB muscles causing a curvature in the posterior male tail (red arrow) upon chemical stimulation from the PCA, PCB and PCC neurons. Stimulated GER muscles relay their signals to the spicule protractor muscles causing rhythmic contractions (blue arrow). Previous work suggested that the precise location of the vulva is determined by three pairs of p.c.s. neurons, PCA, PCB and PCC. Operated males that contain any two of the three pairs of p.c.s. neurons can sense the precise position of the vulva [2]. However, males with zero or one pair of the p.c.s. neurons can sense the general area of the vulva via the hook sensillum neurons, but will pass over the precise location of the vulva without stopping [2], [18]. Although these neurons had been shown to sense the vulva, it had not been determined if they also function in prolonging contact with the hermaphrodite's genitalia.
To assess these neurons' role in maintaining vulval contact, we laser-ablated each individual pair of p.c.s. neurons and tested the male vulval contact efficiency. Similarly to published reports, we found that ablations of any neuronal pair do not obviously affect the males' ability to locate the precise position of the vulva, since the other two pairs can compensate (data not shown). However, like the unc-29(lf);acr-16(0) males, the operations significantly reduced the duration of vulval contact (Figure 4A; Figure S2B), suggesting that each pair of p.c.s. neurons is used to sustain contact between the cloacal opening and the hermaphrodite's vulva. Among these neurons, the PCB and PCC neurons are cholinergic [22]. The non-cholinergic PCA neurons might exercise this function using other neurotransmitters.
Fig. 4. The postcloacal sensilla (p.c.s.) neurons and the oblique muscles facilitate prolonged vulval contact. 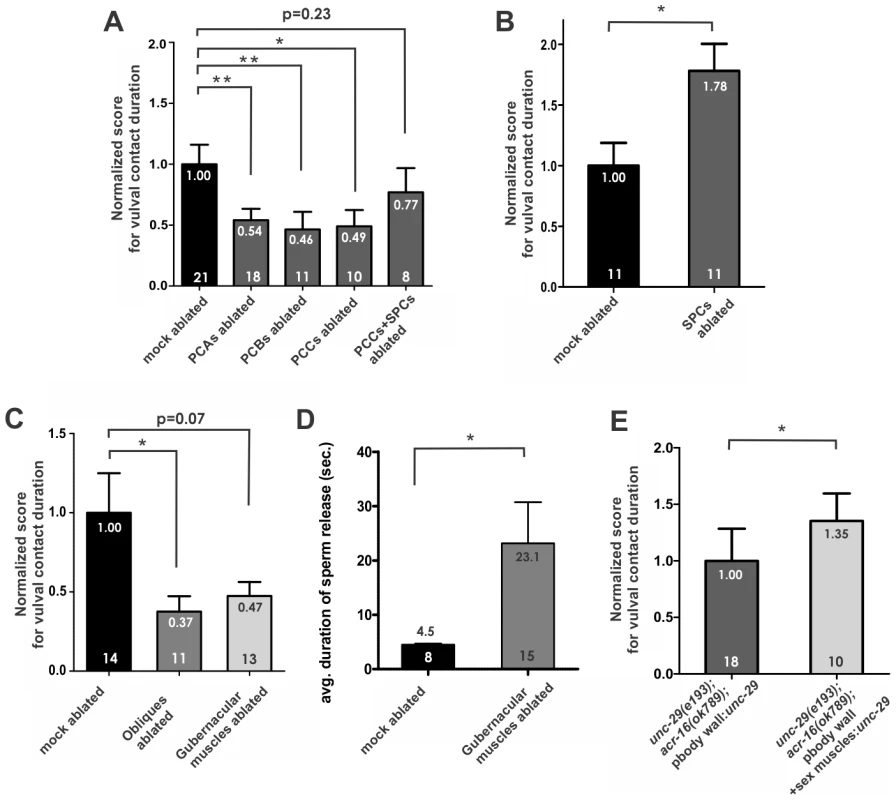
(A–C) The average duration of vulval contact for each male was normalized to the mock-ablated mean to obtain the “normalized score for vulval contact duration” (Materials and Methods). The mean of normalized score for each strain is represented by the height of the bar, and it is also indicated by the number below the top of the bar. Error bars indicate the standard error of the mean. The number of males assayed for each operation is listed within each bar. Ablated males are compared to mock-ablated males that mated with the same group of hermaphrodites. Data that are shown in the same panel were obtained in parallel, but cannot be compared directly to data presented in different panels. (D) Ablation of the gubernacular muscles increases the duration of sperm release. The average duration of sperm release is represented by the height of the bar, and it is also indicated by the number below the top of the bar. The number of males assayed for each operation is listed within each bar. Error bars indicate the standard error of the mean. (E) Restoring unc-29 in male muscles reverses the vulval contact defect of the locomotion-restored unc-29(lf);acr-16(0) males. The average duration of vulval contact for each male was normalized to the mean of the body wall muscle unc-29 rescued males, to obtain the “normalized score for vulval contact duration”. The mean of normalized score for each strain is represented by the height of the bar, and it is also indicated by the number below the top of the bar. The number of males assayed for each operation is listed within each bar. Error bars indicate the standard error of the mean. Asterisks (**) indicate the p value <0.005, (*) indicates the p value <0.05, calculated using the Mann-Whitney non-parametric test. The raw values of average duration of vulval contact or duration of sperm release for each male tested are presented in Figure S2B-S2F. The cholinergic SPC neurons innervate the spicule protractor muscles and are required for sustained protractor contraction, which results in keeping the spicules inserted into the hermaphrodite's vulva during sperm transfer [22], [25]. We observed the effect of ablating the SPC neurons on vulval contact duration, and found that unlike the p.c.s. operations, the SPC-ablated males could stay at the vulva of paralyzed hermaphrodites significantly longer than the intact control males (Figure 4B; Figure S2C). This increase in duration is not a simple result of the failure to insert the spicules, since hermaphrodites used for this assay were purposely chosen to be difficult for intact males to penetrate. Additionally, intact control males that were observed to penetrate the hermaphrodite, inserted their spicules after multiple vulval contacts, and multiple contacts were recorded for the SPC operated males as well (see Material and methods). In addition, ablating the SPC neurons could reverse the vulval contact deficiency caused by ablation of an individual pair of p.c.s. neurons (PCCs) (Figure 4A; Figure S2B). These indicate that, unlike the p.c.s. neurons, the SPC neurons are not required to sustain contact with the vulva, but might be involved in delimiting the duration of this step in mating behavior.
The oblique muscles are required for prolonging vulval contact
To test the hypothesis that the male p.c.s. neurons maintain vulval contact by innervating the acr-16 and unc-29 expressing sex muscles, we ablated the oblique muscles and the gubernacular muscles, to see which operation causes defects similarly to the p.c.s. ablations. We ablated both anterior and posterior oblique muscle nuclei in L4 males. The anterior and posterior oblique muscles lie dorsal-ventrally at the male tail near the cloacal opening, having both the dorsal and ventral ends attached to the body wall [25], [37]. Similarly to intact males, the operated males initiated backward scanning of the hermaphrodites, and they paused at the vulva and started to prod the vulva with their spicules. However, they lose vulval contact and resumed backward scanning more frequently than non-ablated males (Figure 4C, Figure S2D). The vulval contact that remained was largely due to the hermaphrodites being paralyzed. When paired with free-moving hermaphrodites, the males could not maintain the contact (average duration = 2 seconds, n = 3). The contraction of the oblique muscles might change the tail posture during mating, based on their position and shape in the male tail. We propose that contractions of these muscles sustain vulval contact through the application of force between the male cloaca and the hermaphrodite's vulva.
We then laser ablated the gubernacular erector and retractor muscles on both sides of the males at the early L4 larval stage, and observed their mating behavior when they became adults. The gubernaculum is a cuticle structure that lies posterior to the spicules, and it is thought to help guide the extension of the spicules through the male cloaca. The gubernacular erectors and retractors are muscles that attach the gubernaculum to the body wall [25], [37]. We found that the vulval contact duration for these males was slightly, but not significantly shorter than the wild type (Figure 4C; Figure S2D); suggesting that unlike the oblique muscles, the gubernacular muscles are not essential to sustain vulval contact.
Strikingly, the gubernacular muscle-ablated males showed difficulty in sperm transfer (Figure 4D; Figure S2E). In intact males, after sperm leaves the seminal vesicle, the gametes are briefly held at the distal end of the vas deferens for two to four seconds, until they are “released” out of the cloaca [2], [20]. In gubernacular muscle-ablated animals, sperm accumulated at the distal end of the vas deferens and did not efficiently drain out of the male. In contrast, from casual observation, ablating oblique muscles or other spicule-associated neurons described in this work did not seem to affect this step of behavior. Sperm transfer requires signals from ventral cord neurons, the gonad and the SPV spicule sensory neurons [2], [20], [38]. We speculate that the gubernacular muscle contractions move the gubernaculum, which lifts the adjacent tissues so that the lumen of the distal vas deferens is accessible to the cloacal opening.
The LEV-sensitive ACh receptor functions in the male sex muscles to facilitate vulval contact
Previous data suggest that the p.c.s. neurons facilitate the males' contact with the vulva through stimulating ionotropic ACh receptors on the postsynaptic oblique muscles. However, given the expression pattern of unc-29, it is also possible that the behavior is regulated by the L-AChRs on the ventral cord or head neurons. Therefore, we asked if restoring the L-AChR in the male sex muscles could reverse the vulval contact defect in the unc-29(lf);acr-16(0) males. We restored unc-29 cDNA in the body wall muscles and the male sex muscles using the 3.5 kb promoter region of the tropomyosin gene lev-11 [6] (Plev-11:unc-29cDNA::SL2::GFP). Compared to unc-29(lf);acr-16(0) males that had unc-29 expressed only in the body wall muscles, males that expressed unc-29 in both body wall and sex muscles could sustain vulval contact significantly longer (Figure 4E; Figure S2F), suggesting that the L-AChR functions in the male sex muscles to facilitate vulval contact.
The gubernacular-oblique muscle group activity directly induces repetitive protractor muscle contractions
At the vulva, the male coordinates movements of multiple muscles in his tail to effectively penetrate the vulval slit. While he maintains his precise position at the vulva via the oblique muscles, his spicules are rapidly prodding the vulval slit as a result of repetitive protractor muscle contractions. The postcloacal sensilla initiate the spicule prodding behavior [22]; however, the postcloacal sensilla neurons do not make synapses to the protractor muscles (Figure 3B; Male Wiring Project).
The gubernacular erectors and retractors, as well as the anterior and posterior oblique muscles, are innervated by two of the three pairs of p.c.s. neurons or all three of them (Figure 3B), and it is likely that these muscles are stimulated simultaneously when the p.c.s. neurons become activated. In addition, the gubernacular retractors make gap junctions to both the anterior and the posterior oblique muscles (Male Wiring Project; Figure 3B). We therefore refer to these muscles as the “gubernacular-oblique muscle group”. Interestingly, the spicule protractor muscles and the anal depressor muscle are also connected to the gubernacular erectors through gap junctions (Male Wiring Project; Figure 3B). Since gap junctions are low-resistance channels that bridge two cells and allow ions to pass directly, the cytoplasm of the cells are connected, and cell activity can be synchronized by exchanging electric transients [39], [40]. We then hypothesized that ions or other signal molecules could pass from the gubernacular-oblique muscle group through the gap junctions to induce Ca2+ transients in the protractor and the anal depressor muscles. In this way, by innervating the gubernacular-oblique muscle group, the p.c.s. neurons could initiate repetitive contractions of the protractors.
To test our hypothesis, we asked if localized activation of the gubernacular-oblique muscle group could immediately induce fast repetitive protractor muscle contractions and Ca2+ transients in these muscles. We employed the YFP-fused channelrhodopsin-2 light-gated cation channel (ChR2::YFP) to depolarize the gubernacular-oblique muscle group [41]. The 6.2 kb region upstream of the acr-18 gene was used as the promoter to drive transcription of ChR2::YFP in multiple cells, including the gubernacular erector and retractor muscles and the posterior oblique muscles, but not the spicule protractors (Figure S3Q and S3R). When ChR2 was expressed and activated by growing males in the presence of all-trans retinal, the activating blue light caused the spicule protractors to contract repetitively. When multiple sequential brief pulses of blue light were applied, the repetitive spicule movements were induced correspondingly, and coupled to the light stimulation (Figure 5A; Figure S4; Figure S5A-S5C; Video S1; see Materials and Methods). In contrast, males that grew in the absence of all-trans retinal did not respond to light stimulation (Figure 5A; Figure S5D-S5E; Video S2), since the blue light-induced transients require the presence of all-trans retinal [42].
Fig. 5. Stimulation of the gubernacular-oblique muscle group induces repetitive spicule thrusts and Ca2+ transients in the spicule protractor-anal depressor muscles. 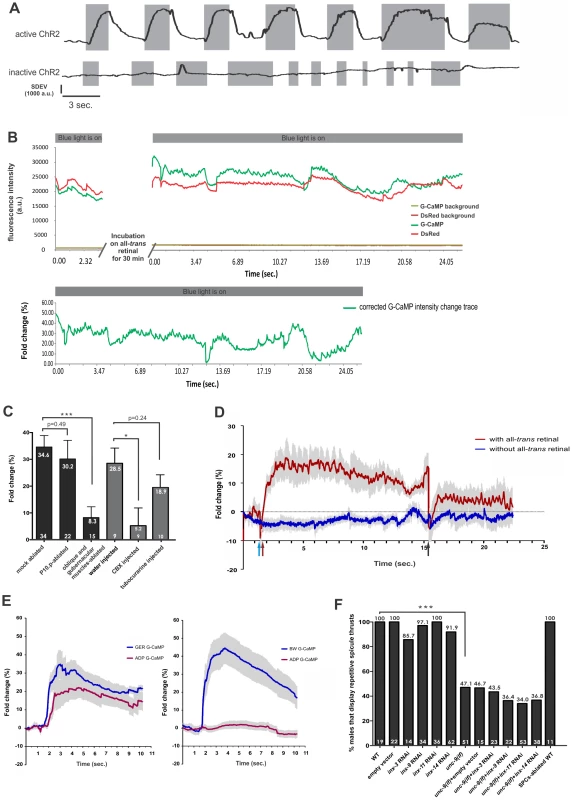
(A) Displacement of the spicule during and between brief blue light pulses. The grey regions indicate the time periods of blue light pulses. An image sequence of the male tail was captured for ∼30 seconds when light pulses were applied repetitively. A region of interest (ROI) was placed at the base of a spicule, and the standard deviation of the pixel intensity within the ROI was obtained to indicate spicule displacement (see Materials and Methods). A representative trace for males that expressed active ChR2 (in the presence of all-trans retinal) is shown in the upper panel; a representative trace for males that expressed inactive ChR2 (in the absence of all-trans retinal) is shown in the lower panel. a.u. arbitrary units. (B) Raw fluorescence and corrected intensity traces for a representative male. Upper panel: raw fluorescence intensity traces in the protractor without gubernacular-oblique muscle stimulation for the baseline recording (with inactive ChR2), and during stimulation of active ChR2 on the gubernacular-oblique muscle group. Fluorescence intensity for both G-CaMP and DsRed channels are plotted, as well as the background fluorescence intensity for each channel. The periods that the light stimuli were applied are indicated by grey bars; the numbers on the X-axis indicate the time points since the onset of the most recent light stimulus; the 30 minute incubation is indicated on the X-axis. a.u. arbitrary units. Lower panel: corrected G-CaMP fold change trace during light stimulation with active ChR2. (C) Maximal G-CaMP intensity changes in the protractors during light stimulation. The number of males assayed for each operation is listed at the bottom of each bar. The numbers below the top of the bars indicate the mean of maximal G-CaMP intensity changes of the population. Error bars indicate the standard error of the mean. Asterisk (***) indicate the p value <0.0005, (*) indicates the p value <0.05, calculated using the Mann-Whitney non-parametric test. (D) Average G-CaMP intensity changes before, during and after a pulse of blue light stimulation. The blue arrow indicates the beginning of light stimulation, the red arrow indicates the onset of G-CaMP intensity increase, and the black arrow indicates the end of light stimulation. The trace in red represents males with active ChR2. Males without active ChR2 are represented by the blue trace. The grey region around each curve represents the standard error of the mean. 4 males were measured for each trace. (E) Averaged G-CaMP intensity change traces in the anal depressor muscle (ADP), the gubernacular erector (GER) and a posterior body wall muscle (BW), upon laser microbeam stimulation of the gubernacular erector muscle or the posterior body wall muscle in the male tail. Traces represent averaged G-CaMP intensity changes in specific muscles. The grey region around each trace represents the standard error of the mean. Left panel, the gubernacular erector on one side of the males was stimulated by a laser microbeam. The energy of the laser was adjusted to the lowest level that could elicit Ca2+ transients in the muscles. N = 10 males. Right panel, a body wall muscle that has no gap junction to the protractor-anal depressor muscle group was stimulated by a laser microbeam. Ca2+ transients in the gubernacular erector and the anal depressor muscles were detected by using G-CaMP. Stimulation of the posterior body wall muscle failed to induce Ca2+ transients in the anal depressor muscle, indicating that in the left panel, Ca2+ transients in the anal depressor muscle were not caused by non-specific laser damage in the male tail. N = 11 males. (F) Effects of innexin mutant, RNAi of innexins and SPC ablation on ChR2-induced spicule thrusts. The unc-9(lf) males were compared to the wild type. The innexin RNAi strains with a wild-type genetic background were compared to the wild type that was fed with empty vector. The innexin RNAi strains with an unc-9(lf) genetic background were compared to the unc-9(lf) that was fed with empty vector-containing bacteria. The SPC-ablated wild type was compared to un-operated wild type. The numbers above the bars show the percentage of males that displayed rapid spicule thrusts upon light stimulation. The number of males assayed for each strain is listed within the bar. Asterisks (***) indicate the p value <0.0001, using the Fisher's exact test. To verify that the repetitive spicule movements were directly due to protractor muscle contractions and not to non-specific displacement of neighboring tissue, we measured Ca2+ dynamics in the spicule muscles. To detect Ca2+ level changes in the protractor muscles and the anal depressor muscle, we expressed a fluorescent Ca2+ indicator protein, G-CaMP, using one of the unc-103 promoter regions (Punc-103E) [5], [43], [44], and expressed a red fluorescent protein (DsRed) using the same promoter as an internal control (Pacr-18:ChR2::YFP+Punc-103E:G-CaMP+Punc-103E:DsRed) [5], [45], [46]. Ca2+ transients were determined by changes in the G-CaMP/DsRed intensity ratio in the same tissue. The excitation spectrum of G-CaMP overlaps with ChR2 [43], [47]. To determine the baseline of the G-CaMP/DsRed intensity ratio in the protractor-anal depressor muscles (in the absence of ChR2-mediated gubernacular-oblique muscle activation), males were first imaged under the blue excitation light prior to exposure to all-trans retinal. Afterwards, they were incubated on plates that contained all-trans retinal for 30 minutes and then reimaged again under the blue light. 30 minutes of incubation was sufficient to activate ChR2 for light stimulation (Figure S6). In the presence of all-trans retinal, we observed Ca2+transients in correlation with light-induced contractions that caused muscle length changes (Figure 5B, 5C; Video S3; see Materials and Methods). In contrast, ChR2-expressing males that grew in the absence of all-trans retinal, did not display spicule thrusts or had Ca2+ transients in the protractor muscles (data not shown, n = 30; Video S4).
Pacr-18:ChR2::YFP was also expressed in the HOA hook sensillum neuron (Figure S3T-S3V), which theoretically could induce the spicule prodding behavior independent of the p.c.s. neurons [22]. To rule out the possibility that Ca2+ transients we observed in the protractor muscles were the result of HOA neuron activity, we laser ablated the hook sensillum precursor cell P10.p in the late L2 larval stage (see Materials and Methods). When we activated ChR2 in the gubernacular-oblique muscle group of ablated adult males, we found that Ca2+ transients in the protractor muscles were still induced with a magnitude that was slightly, but not significantly lower than mock-ablated males (p>0.05, Figure 5C). This result suggests that under these conditions, HOA is not the major contributor for Ca2+ transients observed in the spicule protractor muscles.
To verify that Ca2+ transients observed in the protractor muscles were caused by excitation of the gubernacular-oblique muscle group, we ablated the gubernacular erector and retractor muscles and the anterior and posterior oblique muscles. We found that the repetitive contractions of the protractors were impaired, and Ca2+ transients observed in the ablated animals were greatly reduced (p<0.0005, Figure 5C). We reasoned that ablating nuclei at the mid-L4 larval stage severely impaired the physiology of these muscle cells and reduced the amount of ChR2 proteins on the remaining cell membranes. Thus, the cell corpses that remained after ablation could not be activated sufficiently to pass ions or signal molecules to the protractors to induce detectable Ca2+ transients. The remaining activity we saw in the protractors could be due to activity in the sphincter muscle and the spicule retractor muscles, since the upstream region of acr-18 also drove transcription in these muscles (Figure S3R, S3S). These muscles are also connected to the spicule protractors through gap junctions (Male Wiring Project). However in an intact male, due to the lack of innervation from male-specific neurons (Male Wiring Project), the sphincter and spicule retractor muscles are not likely to be the major facilitators of rhythmic spicule prodding behavior. These observations suggest that direct depolarization of the gubernacular-oblique muscle group in the absence of neuronal input can elicit Ca2+ transients in the protractor-anal depressor muscles to induce muscle contraction.
To gain a better idea of the temporal correlation between gubernacular-oblique muscle group depolarization and protractor-anal depressor muscle activities, we monitored Ca2+ levels continuously in the protractor muscles before, during and after a brief pulse of blue light stimulation of the gubernacular-oblique muscles. Using a Mosaic Imaging System (Andor™ Technology), we excited multiple regions simultaneously or sequentially without optically stimulating the whole field of view. We found that Ca2+ levels elevated gradually in the protractor muscles upon light-activation of the gubernacular-oblique muscles, and declined rapidly after the stimulation (Figure 5D; Video S5, S6; see Materials and Methods). We also stimulated the gubernacular erector muscles using a laser-induced muscle contraction method [48]. In males that did not express ChR2, abrupt release of internal Ca2+ stores in the gubernacular erector muscle, via irradiating the muscle with a low-energy laser microbeam (440 nm), also elicited Ca2+ transients in the protractor-anal depressor muscles, causing rapid muscle contraction (Figure 5E; Video S7, S8; see Materials and Methods). Interestingly, in both the optical and the laser stimulation of the gubernacular muscles, Ca2+ transients in the protractor and anal depressor muscles are induced with a delay of up to 0.3 seconds (Figure 5D; Figure 5E). The similar latency was also observed for light-induced spicule thrusts (Figure 5A; Figure S5). We speculate that this period is required for Ca2+ to travel between the muscles through gap junctions. Similar phenomenon was also observed in the C. elegans intestinal cells. Adjacent cells that are connected by gap junctions showed Ca2+ spikes that were not complete synchronous [49]. We therefore suggest that activities of the gubernacular-oblique muscle group, initiated by the p.c.s. neurons, can be transmitted to the spicule protractor muscles directly, probably through gap junctions.
Gap junctions are required for information transmission from the gubernacular-oblique muscle group to the protractor muscles
To determine if gap junctions mediate the activity transmission between the gubernacular-oblique muscles and the protractor-anal depressor muscles, we asked if the application of a gap junction inhibitor could affect Ca2+ transients in the protractor-anal depressor muscles during gubernacular-oblique muscles stimulation. Carbenoxolone (CBX) has been shown to inhibit innexin function in invertebrates [50]-[52]. We found that application of this drug reduced the light-induced Ca2+ transients in the protractor-anal depressor muscles (Figure 5C; Figure S7). To the contrary, application of tubocurarine, which blocks both nicotine-sensitive and LEV-sensitive AChRs, [27], [29], [30], [53], [54] and cause the males to be paralyzed, did not affect either repetitive protractor contractions or Ca2+ transients in response to light stimulation (Figure 5C). Although it is possible that ChR2-stimulation of the gubernacular-oblique muscles could indirectly cause some non-specific neuron to secrete neurotransmitters other than ACh to induce Ca2+ transients in the spicule protractor-anal depressor muscles, the tubocurarine result in conjunction with the CBX experiment indicates that functional gap junctions, instead of cholinergic synaptic transmission, are required for activities of the gubernacular-oblique muscles to be relayed to the spicule protractor muscles.
The C. elegans genome encodes 25 innexin proteins. To identify the innexin(s) that is important to the coupling of male sex muscles, we first determined innexin genes that are expressed in these muscles. A high-resolution expression map of all innexins has been reported in the C. elegans hermaphrodite, and five innexins are expressed in the hermaphrodite sex muscles: inx-3, inx-8, inx-9 inx-14 and unc-9 [55]. In addition, inx-11 was reported to be expressed in these muscles as well in another study [56]. The hermaphrodite sex muscles are derived from the M-lineage, same as the male sex muscles [57]; hence, it is possible that these genes are also expressed in the male sex muscles. We inspected these genes' expression patterns in males by checking GFP expression under the control of their promoter regions [55]. We found that unc-9 and inx-14 are expressed in multiple male-specific sex muscles and the sexually dimorphic anal depressor muscle. In addition, unc-9 is also expressed in the SPC and the PCB neurons in the male tail (Figure S8).
To test if these innexins are used to transmit signals among the male sex muscles, we asked if the light-induced rapid thrusts of the spicules are affected when these genes are mutated or knocked-down by RNA interference (RNAi). Males that carry the unc-9(e101) loss-of-function allele have uncoordinated locomotion [58], probably due to inhibited electrical coupling among body wall muscles, as well as neuronal UNC-9 deficiency [59]. We found that when ChR2 was expressed in the gubernacular-oblique muscles in these males, in the presence of all-trans retinal, only 47.1% of the males responded to the blue light with repetitive spicule thrusts (n = 51, p<0.0001), compared to 100% of the wild type (n = 19; Figure 5F). To the contrary, light-induced spicule thrusts were not affected by RNAi of inx-3, inx-9, inx-11 or inx-14. In addition, RNAi of these genes in the unc-9(e101) genetic background did not result in further inhibition of the behavior (Figure 5F). In theory, the reduced spicule thrusts can be resulted from a deficiency of UNC-9 in the SPC and PCB neurons. We addressed this possibility by first asking if these neurons are required for blue light-induced spicule thrusts. We reasoned that the PCB neurons do not make synapse or gap junction to the protractors; as a result, for the PCBs to mediate the “muscle-induced muscle contraction” phenomenon, they have to send signals through the SPC neurons, which innervate the protractors. Therefore, we can test whether the PCB and SPC neurons are required for the “muscle-induced muscle contraction” phenomenon by asking if light-stimulated gubernacular-oblique muscle activity can still induce spicule thrusts when the SPC neurons are ablated. We found that ablation of the SPC neurons did not have an effect (Figure 5F), suggesting that neither pair of these neurons is required and therefore a deficiency of UNC-9 in these neurons is not likely to cause reduced light-induced spicule thrusts. Taken together, these data suggest that the UNC-9 innexin is used in some of the gap junctions that mediate the direct signal transmission from the gubernacular-oblique muscles to the protractor-anal depressor muscles. Identification of additional gap junction subunits awaits further study.
The SPC neurons are necessary, but not sufficient to trigger sustained protractor contraction
Repetitive protractor contractions eventually transform into a tonic contraction, which causes the spicules to penetrate the vulva. Premature tonic protractor contraction, prior to or during the various sub-steps of mating, rarely occurs in wild-type males [60], suggesting that the triggering of tonic protractor contraction is under strict regulation. We favored the hypothesis that multiple signals are integrated by the spicule circuit to induce sustained protractor contraction at the proper moment of mating.
We used ChR2 to activate subsets of spicule circuit components, in males that were not engaged in mating [41], [61]-[63], and then asked if these cells could induce sustained spicule protraction (Figure 6A). To our surprise, when we expressed ChR2 in all muscles, using the tropomyosin Plev-11 promoter (Plev-11:ChR2::YFP), we observed that blue light induced strong body wall muscle contraction in all males, but only 5% of them displayed tonic contraction of the protractors. The remaining males responded to blue light with shallow repetitive protractor contractions with the tips of their spicules slightly protruding from the cloacal opening (n = 20, Figure 6B, Video S9). Consistent with this result, expressing ChR2 in sex muscles (the spicule protractors, the gubernacular erector and retractor muscles, the anterior and posterior oblique muscles, the sphincter and the diagonal muscles) and the sexually dimorphic anal depressor muscle, using the Punc-103E promoter (Punc-103E:ChR2::YFP), also induced repetitive shallow protractor contractions. Approximately 18% of males fully protracted their spicules (n = 17, Figure 6B, Video S10). Since light-activated ChR2 channels should depolarize the muscle cells, we speculated that some negative regulatory molecules on the spicule muscles might be keeping them from contracting tonically.
Fig. 6. Stimulation of ChR2 in different spicule circuit components. 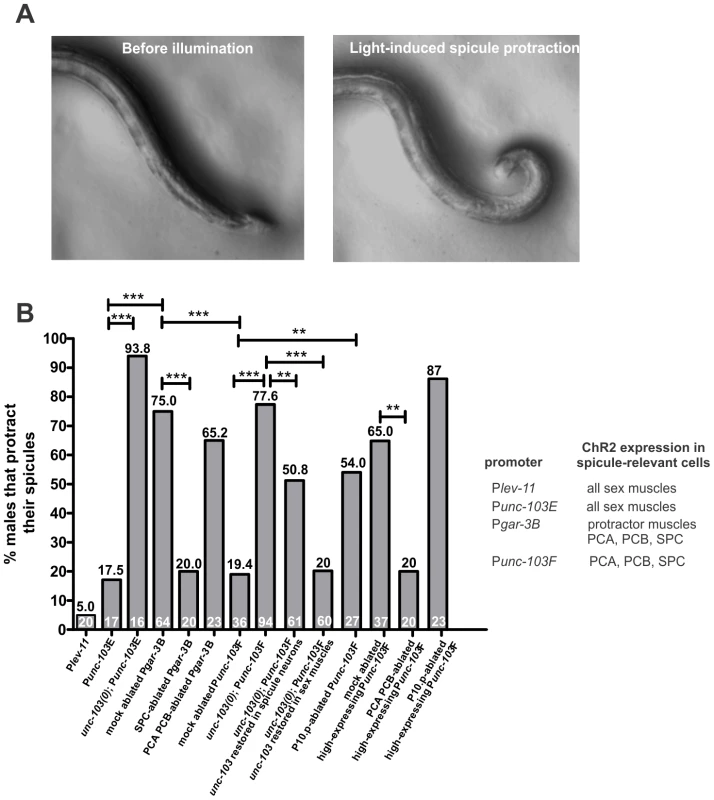
(A) Left panel, a male that expresses Pgar-3:ChR2::YFP holds his spicules inside of his tail prior to illumination. Right panel, the blue light induces sustained spicule protraction of this male. (B) Sustained spicule protraction induced by stimulation of ChR2 in different circuit components. The numbers on the vertical axis represent the percentage of males that protracted their spicules when different circuit components were activated by light-stimulated ChR2. The numbers above the bars show the percentage of males that protracted their spicules. The number of males assayed for each strain is listed within the bar. Asterisks (***) indicate the p value <0.0001, (**) indicate the p value <0.001, using the Fisher's exact test. To address if spicule activity is controlled by negative regulator molecules, we introduced a null allele of the unc-103 gene (n1213) into the Punc-103E:ChR2::YFP-expressing animals. The unc-103 gene encodes an ERG-like K+ channel. The unc-103 deletion males (unc-103(0)) occasionally protract their spicules in the absence of mating stimulation. Previous studies suggested that the K+ channel suppresses premature spicule protraction by reducing the excitability of the spicule muscles [44], [60]. In unc-103(0) males that expressed ChR2 on their sex muscles, blue light induced tonic spicule protraction in 93.8% of the population (n = 16, Figure 6B; see Materials and Methods). In contrast, the transgenic male population that grew in the absence of all-trans retinal did not protract their spicules under the blue light (n = 20). This suggests that in wild-type males, the UNC-103 K+ channel functions on the spicule muscles to counteract the ChR2-mediated depolarization.
The transition between protractor contractile states is presumed to be facilitated by the cholinergic SPC neurons. These neurons have proprioceptive sensory endings that are attached to the protractor muscles and are suggested to sense the spicule position in the vulval channel. Removal of these cells results in operated males that rhythmically prod the vulva with their spicules, but never completely insert them during mating [2], [22]. Since these neurons make synapses to the muscles, their secretions might be sufficient to evoke tonic muscle contraction. Alternatively, the ACh signals from the SPC neurons might need to act in conjunction with the rhythmically contracting protractors to induce the state change in the muscles.
To test these hypotheses, we used the promoter region of the gar-3 gene (Pgar-3B:ChR2::YFP) to express ChR2 in the SPC neurons and two of the p.c.s. neurons (PCA and PCB), in addition to the spicule protractors and the anal depressor [26]. 75% of the males responded to the fluorescent light with sustained spicule protraction (n = 64, Figure 6A and 6B; Video S11). To verify that this was due to SPC neuron activity, we laser ablated these cells, and found that blue light only caused sustained protraction in 20% of the males (n = 20, Figure 6B). In addition, ablation of the PCA and PCB neurons did not affect the light-induced behavior (n = 23, Figure 6B), presumably since the spicule muscles were already stimulated via Pgar-3B-expressing ChR2 and did not require further p.c.s. signaling.
If the p.c.s. neurons induce rhythmic spicule protractor contractions, and activation of the SPC neurons switches rhythmic contractions to tonic contraction, then artificially activating these neurons independent of the spicule muscles should cause sustained spicule protraction. To test this, we used one of the unc-103 gene promoter regions (Punc-103F) to express ChR2 in the PCA, PCB and SPC neurons [44]. Only 19.4% of males, the ones that highly expressed Punc-103F:ChR2::YFP, displayed prolonged protractor contraction in response to blue light (n = 36, Figure 6B). When we preselected males that displayed very high expression of Punc-103F:ChR2::YFP, 65% of these biased males displayed the behavior after light stimulation (n = 37, Figure 6B). Laser ablation of the PCA and PCB sensory neurons reduced the percentage of light-stimulated protraction in preselected biased males to 20% (n = 20, p = 0.002), indicating that stimulation of the SPC neurons in the absence of PCA and PCB activity does not result in efficient tonic muscle contraction.
The low efficiency of Punc-103F:ChR2::YFP-induced spicule protraction in unbiased males suggested that the spicule-prodding/protraction neurons have intrinsic properties, which to overcome, require high levels of ChR2 stimulation. We reasoned that, similar to the spicule muscles, the UNC-103 K+ channel might be offsetting ChR2 activity in the spicule neurons. When the Punc-103F:ChR2::YFP construct was introduced into the unc-103(0) males, 77.6% of the population fully protracted their spicules in response to the blue light (n = 94, Figure 6B). The unc-103 gene is expressed in both spicule-associated neurons and the male sex muscles [6], [44]. We found that restoring functional UNC-103 in the sex muscles could reestablish these males' resistance to light-stimulation; however, expressing unc-103 in the spicule-associated neurons can also restore some resistance to light stimulation (Figure 6B). Thus in wild type, the UNC-103 channel likely regulates the excitability threshold of neurons, in addition to muscles.
Why do the p.c.s. and SPC neurons promote spicule protraction at the vulva, but not efficiently under artificial non-mating conditions? We hypothesized that in addition to UNC-103, the activities of the p.c.s. and SPC neurons might be attenuated by other neurons. In intact males, spicule prodding and full protraction only occur when the males are at the vulva, which is sensed by both the p.c.s. and the hook sensillum. When the hook sensillum precursor cell, P10.p is laser-ablated, the operated males no longer sense the general area of the vulva but instead randomly prod the cuticle of the hermaphrodite with their spicules [2]. This suggests that in intact males, the cells derived from P10.p might attenuate spicule protractor activity at areas outside the vulva. The P10.p precursor cell gives rise to the hook structural cells, HOA and HOB sensory neurons and the PVZ motor neuron. The PVZ does not make synapses to the spicule-relevant neurons, but the HOA and HOB hook sensillum neurons do make direct chemical and electrical connections to the p.c.s. and SPC neurons (Figure 3A; Male Wiring Project). Thus, it is possible that these connections might negatively modulate spicule activity until the vulva is sensed.
We ablated the precursor cell P10.p in Punc-103F:ChR2::YFP-expressing males, and found that in 54% of unbiased operated males (n = 27, Figure 6B) and 87% of high-expressing operated males (n = 23, Figure 6B), the light induced prolonged protraction. In both cases, the incidence of ChR2-induced spicule protraction was higher in operated animals compared to mock-ablated. However, for unbiased males, the difference was statistically significant (p = 0.004), whereas for biased Punc-103F:ChR2::YFP high-expressing males, the increase was not statistically different over the already high protracting non-ablated males (p = 0.08). We conclude that the lineage descendants of P10.p, most likely the hook neurons, can reduce the output of ChR2-activated p.c.s. and SPC neurons, but this reduction can be partly circumvented by expressing more ChR2.
Discussion
C. elegans male mating behavior is a multi-step goal-directed behavior that utilizes several active sensing processes, as the male worm actively acquires sensory inputs using different types of “motor sampling routines” [64]. For example, sensation of the hermaphrodite during male locomotion initiates the backward vulval searching behavior; recognition of the vulva promotes the repetitive spicule thrusts as an attempt to penetrate the vulval slit; and as a result, proprioceptive signals can detect partial penetration and subsequently trigger prolonged spicule extension. The transition from one behavioral step to another is directed by one or more sensory feedback signals, and the consequential motor output is objectively purposeful and intended to obtain further sensory inputs in order to achieve the behavioral goal – vulval penetration. During this process, maintaining a precise contact with the hermaphrodite's vulva while she is moving is exceptionally challenging, as the hermaphrodite can constantly shift her direction of locomotion and can crawl on average at speeds up to ∼200 µm/second [65]. Although feedback signals must be involved in these motor behaviors, sensory feedback can be delayed and can result in motor output errors. A heuristic model called the forward internal model, which potentially involves heavy computation, is proposed as a solution to this problem in motor control. A neural “controller” region generates a command to the motor unit, while simultaneously creating a copy of the command to a hypothetical region called the “forward model”. The “forward model” then predicts the sensory consequences of the motor commands. The predicted sensory consequences are then compared with the actual sensory feedback in the “comparator” region, and the integrated information is sent back to the controller region to modify subsequent motor commands [66]. In neural systems of higher invertebrates and vertebrates, their complex brains can theoretically incorporate the computational components predicted in the forward internal model. However, for a simple organism like C. elegans that has a highly compact nervous system, we suggest that coordinating and modifying motor outputs to facilitate goal-oriented tasks can be solved by simpler, yet sufficient mechanisms.
For wild-type males, contact with the vulva and contractions of the protractor muscles take place simultaneously and rarely occur independently. This suggests that their mechanisms of execution and regulation are integrated. Efficient sensing of the vulva requires the combined functions of the bilateral set of postcloacal sensilla (p.c.s.) PCA, PCB and PCC sensory-motor neurons and the hook sensillum sensory neurons HOA and HOB. The sensory endings of the p.c.s. neurons are encased in the cuticle, suggesting that they might be mechanosensory; whereas the sensory endings of HOA and HOB neurons are opened to the environment, indicating that these neurons might sense chemical as well as mechano-signals [25]. Laser ablations of the hook sensillum cells or mutations that decrease the hook neurons' sensory functions severely reduce the ability of the male to recognize the vulva; however at low efficiency, the males can recognize the precise location of the vulva with the p.c.s. and insert their spicules [2], [18], [67]-[70]. In contrast, removal of all p.c.s. neurons results in males that can sense the general area of the vulva, but are incapable of positioning their tails over the vulval slit to insert their spicules [2], [22].
Males can utilize any two of the three bilateral pairs of the p.c.s. neurons to position the cloacal opening over the vulva. But if a male lacking any single pair of the p.c.s. neurons does not quickly insert his spicules, he will move off sooner than if he had the complete sensilla. This indicates that after the initial sensory contact, the p.c.s. neurons are continually required to maintain the male's position over the vulval slit. The maintenance of the male's position over the vulva is partly regulated by direct signaling between the p.c.s. neurons and the male anterior and posterior oblique muscles (Figure 3B, 3C; Male Wiring Project, Albert Einstein College of Medicine, http://worms.aecom.yu.edu/pages/male_wiring_project.htm). The PCB and PCC neurons are cholinergic [22]. The neurotransmitter assignment for PCA has not yet been determined, but the cell can express a reporter construct for the eat-4 vesicular glutamate transporter, suggesting it might secrete glutamate (Garcia, unpublished observation).
Ablation of the oblique muscles or reduction in ACR-16 and UNC-29-mediated muscular cholinergic signaling causes males to behave similarly to those that have only two pairs of p.c.s. neurons. The oblique muscles are located slightly posterior to the cloacal opening, and their single sarcomeres, oriented dorsal-ventrally, are attached to the lateral body wall [25]. When they contract, the posterior tail region curls ventrally. This modification in tail curvature might redirect the force, which the male applies laterally to the hermaphrodite cuticle during backing behavior, to a downward force immediately upon vulval contact (Figure 3C). We suggest that this would stabilize the male at the vulva during his repetitive attempts to insert his spicules.
The same cells and molecules that stabilize the male at the vulva also promote the insertion of the copulatory spicules. High frequency and sustained muscle contractions of the spicule-associated muscles can be stimulated by acetylcholine (ACh) secretions from the postcloacal sensilla PCB, PCC neurons and the SPC neurons, respectively. It was hypothesized in earlier studies that the cholinergic PCB, PCC and SPC neurons differentially regulate the two types of protractor contractions via secreting different amounts or rates of ACh to activate ionotropic and muscarinic ACh receptors on the protractors [22]. The SPC neurons were known to innervate the protractors [25]. However, electron micrograph reconstruction of the male tail (Male Wiring Project) indicates that the p.c.s. neurons do not form chemical synapses to the spicule muscles. Instead, the p.c.s. neurons make multiple chemical connections to each other, to the SPC neurons and to the oblique-gubernacular muscle group (Figure 3B).
Communication between the p.c.s. neurons and the protractors are relayed through electrical junctions between the gubernacular muscles and the protractor muscles, mainly through an intermediate accessory spicule muscle called the anal depressor (Figure 3B; Male Wiring Project). In adult C. elegans, gap junctions are distributed extensively throughout the nervous system. Based on serial transmission electronic microscopy images and innexin expression patterns, the gap junctions are not limited between neurons or between muscles, but also connect neuron and muscle cells, where the processes penetrate the basement membrane (Male Wiring Project) [25], [55], [71]. These electrical connections probably play important roles in regulating animal behaviors. The worm gap junctions have been reported to mediate Ca2+ transient propagation throughout the intestinal cells and body wall muscles. A few studies also indicate their role in synchronizing neuron activities [49], [59], [72], [73]. Artificially stimulating the oblique and gubernacular muscles can instantaneously induce Ca2+ transients and subsequent contractions in the protractor muscles. Thus, by utilizing gap junctions in the circuit, the p.c.s. neurons can coordinate both vulval contact and spicule penetration attempts simultaneously.
Although connected by gap junctions, different motor outputs are generated by the gubernacular-oblique muscle group and the protractor muscles. While males rhythmically prod their spicules at the vulva, they maintain a stable tail curvature. This suggests that the oblique muscles do not undergo high frequency contractions like the protractors. Direct p.c.s. stimulation of the oblique-gubernacular muscle group causes tonic contraction in these muscles. But signals transmitted from the oblique-gubernacular muscle group to the spicule muscles through gap junctions facilitate rhythmic contractions of the protractors (Figure 3B, 3C). This provides a scenario that unpatterned signal input elicits patterned motor output, and this process requires intracellular regenerative Ca2+ transients through the UNC-68 ryanodine receptor channel [22]. The pattern generating mechanism could either be present in the protractor muscles themselves, by utilizing intracellular oscillating Ca2+ transients; or it can be a result of the interplay between contractions of the protractor and the retractor muscles, since the spicules are attached by both types of muscles that cause opposite directions of spicule movements.
The core spicule circuit (the p.c.s. neurons, the gubernacular-oblique muscle group, the SPC neurons, the anal depressor muscle and the spicule protractor muscles) is connected to other parts of the nervous system via extensive synapses and gap junctions (Male Wiring Project). Thus, it is likely that activity in other behavioral circuits can cause non-specific activity (noise) in the spicule circuit. Therefore, the circuit must contain contingency mechanisms to reduce the males from fully protracting their spicules into non-vulval orifices or to random environmental stimuli. The light-gated channelrhodopsin (ChR2) was used to activate different components of the core spicule circuit. Exciting cells via ChR2 is not equivalent to natural activation caused by ligand stimulation, synaptic transmission or electrical junctions in behaving animals, but these experiments provide an idea on how activated circuit components promote differential behavioral outputs.
At least three inputs must be integrated by components of the core spicule circuit for the protractors to contract completely with the highest probability via ChR2 stimulation: removal of the negative input from cells derived from the progenitor cell P10.p, possibly from the hook sensillum neurons HOA and HOB; stimulation of rhythmic contractions in the protractors via the p.c.s. sensory-motor neurons; and the addition of positive input from the SPC proprioceptive sensory-motor neurons. During mating, contact with vulval tissue would simultaneously stimulate the p.c.s. neurons and release negative regulation from the P10.p descendants. Finally, partial spicule penetration would trigger the SPC sensory-motor neurons, through their proprioceptive sensory endings, to secrete ACh onto the rhythmically contracting protractor muscles. The ACh signals from the SPC neurons could act additively with the intracellular regenerative Ca2+ transients to transform the oscillating contractions into a sustained one. The final motor output is the result of integrating the activities of different components in the behavioral circuit. A similar observation has been reported in other reproductive behavior systems, such as in the leech, where the oscillation frequencies of ganglia M5 and M6 are integrated to determine the rhythmic twisting behavior performed during mating [74].
We propose that the circuit uses the ERG-like K+ channel UNC-103 to set up a high threshold for stimulating the circuit, so it responds maximally when all inputs are detected “coincidently”. The “coincidence detector” mechanism collapses when UNC-103 is removed, which causes the circuit to respond to stimuli that are not normally sufficient to induce spicule protraction in normal animals. This explains why unc-103 null mutant males spontaneously display a fictive mating behavior in the absence of hermaphrodites and prematurely protract their spicules at the hermaphrodite's vulva before they partially breach the vulval slit [60]. Similar mechanisms are used in nervous systems of other organisms. For example in vertebrates, the low excitability of the striatal neurons maintained by K+ channels has been implied to play an important role in preventing unintended locomotion conditions [75]. The central pattern generators in the spinal cord generate the locomotion. These networks remain inhibited, until activation of the striatum relieves this inhibition [76]. The striatum has a low excitability due to the inward rectifier K+ current [77]–[80]. Therefore, the striatum only becomes activated when it receive strong stimulation from the thalamus or the cortex, where relevant sensory inputs are integrated. Using this mechanism, the animal adapts its locomotion properly in response to environmental conditions [75], [81].
Materials and Methods
Strains
All strains were grown at 20°C on nematode growth media (NGM) plates seeded with E. Coli OP50 [58]. Pharmacological and behavioral assays were conducted between 21-23°C. All males contained him-5(e1490) on linkage group V(LGV) [82]. Additional alleles used were: unc-29(e193) [31], [33] on LGI; unc-103(n1213) [83], pha-1(e2123) [84], unc-64(e246) [58] on LGIII; acr-16(ok789) on LGV was generated by the C. elegans Gene Knockout Consortium; and unc-9(e101) [58], lite-1(ce314) on LGX [85].
Behavior assays
Mating potency assay
Males were separated from hermaphrodites at the L4 stage and put onto a fresh OP50-containing NGM plate. The next day, each male was put onto an individual plate containing a “mating lawn” of OP50 with one free-moving pha-1(e2123) hermaphrodite. 25 µl of a saturated OP50 culture was seeded on each plate the previous night. Males and hermaphrodites were incubated together for 4 hours at 20°C, after which the males were picked off. Hermaphrodites were then incubated at 25°C, and their progeny was scored three days later. Homozygotes of pha-1(e2123) cannot survive beyond the L1 stage at 25°C [84]. Plates with L2 or older progeny indicated that cross fertilization occurred. The percentage of males that can sire progeny within 4 hours was recorded for each strain. To present the data, the wild-type potency was arbitrarily set to 100%, and mating potency of mutants was calculated proportional to the wild type.
Vulval contact assay
Males and hermaphrodites were separated from each other at the L4 stage, and put onto separate OP50-containing plates. The next day, 20–30 paralyzed hermaphrodites were put on a mating lawn. One male was then put onto the mating lawn and observed with a Zeiss Stemi SV 11 microscope. The male behavior was video recorded once he started scanning the hermaphrodite with his tail. Recordings were stopped 5 minutes later or when the male inserted his spicules (whichever happened first). From these videos, we quantified how long a male spent performing each step of mating behavior using a time recording Visual Basic Macro described elsewhere [26].
To determine how well a male maintains contact with the hermaphrodite's vulva, we determined the average (not cumulative) duration of vulval contact of each male. 1-day-old paralyzed unc-64(e246) hermaphrodites were used in this assay. The vulvas of hermaphrodites at this age were not dilated due to extended egg-laying, thus, the vast majority of the males tested in this work could not penetrate the vulva for the duration of the first vulval contact (77.3% of wild type, n = 66; 78.9% of unc-29(lf) locomotion-restored males, n = 19; 92.3% of acr-16(0) males, n = 13; 87.5% of unc-29(lf);acr-16(0) locomotion-restored males, n = 32; 100% of the SPC-ablated males, n = 11), and multiple vulval contacts were recorded for these males. Therefore, the average duration of vulval contact of an individual male was averaged from multiple vulval contacts, and the efficiency of spicule insertion was not likely to affect our measurements of vulval contact duration.
To present the data in figures, the average duration of vulval contact for each male was normalized to the mean of wild type or the mock-ablated males to obtain the “normalized score for vulval contact duration”, as a measurement of male's ability to maintain precise vulval contact. We calculated the “normalized score for contact duration” of an individual male using: (nri is the number of males in the reference group, xri is the average duration of contract for an individual reference male (wild type or mock-ablated males), xi is the average duration of contact for an individual male). The height of the bar represents the mean of normalized score for each strain: (ni is the number of males tested for a specific strain or treatment). Error bars indicate the standard error of the mean.
Multiple males were analyzed for each strain or treatment. Wild-type males/un-operated males were always observed in parallel with mutant males/operated males as control. Statistic comparisons were only conducted between male samples that were observed in parallel, since the wild-type behavior can vary on a day-to-day basis. Comparison between two samples was done by using the Mann-Whitney non-parametric test.
Sperm release
The behavior of males was filmed and the duration of sperm “release” was recorded as described earlier. As defined by a previous study, sperm “release” refers to the process when sperm leaves the seminal vesicle and is release from the vas deferens [20].
Measuring velocity of backward locomotion during vulva search behavior
Twenty 48 hrs adult pha-1(e2123); him-5(e1490); lite-1(ce314);rgEx431[Phsp-16:egl-2(n693gf); Punc-103E:mDsRed; pha-1(+)] hermaphrodites were heat shocked for 30 minutes at 30°C, and then incubated at 20°C. After 3 hrs, the heat shocked-expressed EGL-2(gf) K+ channels induced complete paralysis. The hermaphrodites were placed on a 5 mm diameter lawn of E. coli OP50. Their postures were straightened out using an eyelash pick. 18–24 hrs adult males were then placed with the hermaphrodites and monitored at 60× total magnification on a stereomicroscope mounted with a Hamamatsu ImagEM CCD camera. Digital recordings (containing timestamps) were made at 30 frames per second. To facilitate quantification of the distances that the males moved during the vulval search behavior, images of a stage micrometer were acquired prior to recording the copulations. Velocity was determined as the time required for the male tail (focusing on the spicule tip) to travel 300 to 800 µm along the hermaphrodite's cuticle. The straight posture of the paralyzed hermaphrodites allowed easy determination of the males' position along the hermaphrodite's cuticle.
Pharmacology
Levamisole (LEV) (ICN Biomedicals, Aurora, OH) was prepared in distilled water and stored at −20°C. 400 µl of LEV solution at various concentrations was added to a Pyrex Spot Plate (nine 1 ml volume depressions), and ∼5–10 virgin males were put into the drug. We observed these males for 3 minutes under a Leica MZ 7.5 stereomicroscope. A male was considered sensitive to the drug if he fully protracted his spicules for >5 seconds. Fresh drug was used after ∼30 virgin males were assayed. Comparisons were made by using the Fisher's exact test.
Plasmids
Expression pattern of unc-29, acr-16, unc-38, unc-63, and acr-18
Primer sequences are provided in Table S1. A 5.3 kb region upstream of the acr-16 ATG was PCR amplified with the following primers: ATTB1acr-16 and ATTB2acr-16. A 2.4 kb region upstream of the unc-63 ATG, plus the first three codons, was PCR amplified with the following primers: attb1unc-63 and unc-63attb2. A 4.3 kb genomic region that contained 1.2 kb upstream of the unc-38 ATG and all unc-38 introns and exons up to the stop codon was PCR amplified with the following primers: attb1unc-38fus and Attb2unc-38fusnew. A 2.2 kb genomic region that contained a 1.3 kb region upstream of the unc-29 ATG and part of the unc-29 genomic sequence up to the end of the second exon was PCR amplified with the following primers: ATTB1Punc-29 and ATTB2Punc-29. A 2.3 kb genomic region that contained 1.2 kb upstream of the unc-29 ATG and all unc-29 introns and exons up to the stop codon was PCR amplified with the following primers: ATTB1Punc-29 and ATTB2U29lstex. A 6.2 kb region upstream of the acr-18 ATG was PCR amplified with the following primers: ATTB1acr-18 and ATTB2acr-18noatg. All these PCR primers contained Gateway ATTB sites, which allowed the acr-16, unc-63, unc-38, unc-29 and acr-18 PCR products to be recombined, using BP clonase (Invitrogen), into the low copy number Gateway entry vector pDG15, to generate pLR98, pLR149, pLR125, pYL21 (includes the partial unc-29 gene), pLR198 (includes the full-length unc-29 gene) and pLR162, respectively. In order to place the acetylcholine receptor sequences in front of YFP, pLR98, pLR149, pLR125, pYL21 and pLR198 were then individually recombined with the YFP destination vector pGW322YFP using LR clonase (Invitrogen) to make the plasmids pLR106, pLR152, pLR127, pYL22 and pLR199, respectively. pGW322YFP and pDG15 have been described in previous studies [6], [44].
Cell-specific expression of the unc-29 cDNA
We inserted a SL2-accepting trans-splice site between the unc-29 cDNA (including the stop codon) and the GFP gene to use GFP flourescence as a proxy for unc-29 expression. The intergenic region between gpd-2 and gpd-3, containing a SL2-accepting trans-splice site, was PCR amplified with the following primers: igrgpd3 and gpd2igr. This region was then cloned into the SmaI site of pPD95.69 (Addgene plasmid 1491) to generate pJP1. pJP1 was cut with HindIII and ApaI and then cloned into the HindIII/AvaI sites of pBR322 to generate pDG4, which contains the SL2 site in front of the GFP gene. The unc-29 cDNA was then PCR amplified from a cDNA library using the primers Func-29 and unc-29R. The unc-29 cDNA was cloned into the BamHI site of pDG4 to generate pDG5. pDG5 was then cut with XbaI, blunt-ended and then ligated with the Gateway Vector Conversion Reading frame Cassette C.1 (Invitrogen) to generate pYL16. The acr-8 and the lev-11 promoter regions were recombined into the entry vector pDG15 to generate pLR92 and pLR22, respectively [6], [34]. Then we recombined the promoter sequences in pLR92 and pLR22 into pYL16 using LR clonase (Invitrogen) to generate pYL18 and pYL20, respectively.
Cell-specific expression of ChR2
To put a Gateway destination cassette in front of ChR2, a pmyo-3::ChR2(gf)::YFP plasmid (a gift from Dr. Gottschalk, Goethe University Frankfurt) [41] was cut with HindIII and BamHI, blunt-ended and then ligated with the Gateway Vector Conversion Reading frame Cassette C.1 (Invitrogen) to generate pZL5. A TGA stop codon 50 nucleotides downstream of the XbaI site was changed to GGA to generate pLR167. To express ChR2 from the promoter region of acr-18, unc-103 (promoter E and F), lev-11 and gar-3 (promoter B), pLR162, pLR21, pLR28, pLR22 and pLR57 were subsequently recombined with pLR167 to make pLR165, pLR176, pYL41, pLR178 and pLR183, respectively. Construction of pLR21, pLR22, pLR28 and pLR57 have been described previously [5], [26], [34], [44].
Transgenics
Plasmids were co-injected with pBX1 (50 ng/µl) into strains that contained the pha-1(e2123) allele. Animals that could survive beyond the L2 stage were then kept as transgenic lines. For strains that did not have the pha-1(e2123) allele, GFP or YFP encoded on one of the injected plasmids was used to identify transgenic animals. For all injections, pUC-18 was used to make the final concentration of DNA to 200 ng/µl. The expression constructs pLR106, pLR152, pLR127, pLR199 and pYL22 were injected at 50 ng/µl into the pha-1(e2123); him-5(e1490); lite-1(ce314) hermaphrodites. Multiple male-specific muscles are located within a small region of the male tail. Sometimes it was difficult to differentiate these muscles when the majority of them were expressing YFP. The expression of pLR165 (Pacr-18:ChR2::YFP) was located on the plasma membrane and the ER, making it easier to visualize muscle expression in the male tail (Figure S3Q-S3S).
To rescue the locomotion defects of unc-29(lf) males, pYL18 (70 ng/µl) and pYL20 (20 ng/µl) were injected into unc-29(e193); him-5(e1490) or unc-29(e193); acr-16(ok789) him-5(e1490) hermaphrodites. To express G-CaMP and DsRed simultaneously in the male-specific muscles and express ChR2 in the oblique-gubernacular muscle group, pLR135 (20 ng/µl), pLR136 (50 ng/µl) and pLR165 (100ng/µl), were injected into the pha-1(e2123); him-5(e1490); lite-1(ce314) hermaphrodites. pLR35(Punc103E::G-CaMP) and pLR36(Punc-103E::DsRed) are described elsewhere [5]. To express ChR2 in specific tissues, pLR176 (50 ng/µl), pLR178 (50 ng/µl), pLR183 (100 ng/µl) and pYL41 (150 ng/µl) were injected into the pha-1(e2123); him-5(e1490); lite-1(ce314) hermaphrodites.
To restore functional unc-103 to specific tissues of the unc-103(0);Punc-103F:ChR2::YFP worms, the Punc-103F:unc-103F and Punc-103E:unc-103E constructs [44] were injected with pYL41 (Punc-103F:ChR2::YFP) (150 ng/µl)into the unc-103(0) hermaphrodites at 20 ng/µl, respectively.
Laser ablation
Cell ablations were done using the standard protocol [86]. The operation was conducted using a Spectra-Physics VSL-337ND-S Nitrogen Laser (Mountain View, Ca) attached to an Olympus BX51 microscope. L2 worms were operated on 5% agar pads containing 0.5 mM NaN3, and L4 males were operated on 5% agar pads containing 2 mM NaN3. For each operated animal, a control animal was placed on the same agar pad for the same amount of time to rule out the possibility that behavioral changes are due to the anesthetic pads.
Optical stimulation and detection of repetitive spicule thrusts
lite-1(ce314) males that expressed Pacr-18:ChR2::YFP were used. At L4 stage, males were transferred onto NGM plates with OP50 supplemented with all-trans retinal. Males with the same transgenes were also placed onto NGM plates with regular OP50 as control. All-trans retinal-containing plates were prepared the day before by spreading OP50 culture that contained 50 µM all-trans retinal (A.G. Scientific).
The next day, the males were immobilized on 10% agarose (in H2O) pad containing 0.5 µl of 0.1 µm diameter polystyrene microspheres, and covered with a coverglass [87]. Sequences of DIC images of the male tails were recorded under an Olympus BX51 microscope. A Dual View Simultaneous Imaging Systems by Photometrics (Surrey, BC) was used and adjusted to split the image signal, so that DIC image could be recorded in one field of view (field 1), and simultaneously, fluorescent signals could be recorded in the other field with a dimmer DIC image (field 2) (Figure S4A, S4B). Multiple blue light pulses were applied manually to each male tested. Once the blue light was turned on, the YFP fluorescent signal was detected in field 2 and this was used to indicate the timing of light stimulation in our recording (Figure S4C). An ROI was placed in the DIC image in field 1 at the base of one spicule (Figure S4A, S4B). During spicule thrusts, light refractivity of the region defined by the ROI changed, resulting in changes in the standard deviation of the pixel intensity within this ROI (SDEV) (Figure S4C). Therefore, the value of SDEV throughout the image sequence was used to indicate the displacement of spicule.
Optical stimulation and Ca2+ imaging
Strains used to image Ca2+ transient contained lite-1(ce314). Muscular Ca2+ transients were measured by detecting changes in fluorescence intensity of G-CaMP. The red fluorescent protein DsRed was expressed in the same set of cells as G-CaMP. Since the fluorescence intensity of DsRed does not change in response to light stimulation, it was used as an internal control. ChR2 is expressed in the gubernacular-oblique muscle group. DsRed and G-CaMP signals were recorded separately, but simultaneously via the Dual View Simultaneous Imaging Systems with an OI-11-EM filter by Photometrics (Surrey, BC). To record G-CaMP and DsRed, the transgenetic males were placed on agar pads without NaN3, and were observed under an Olympus BX51 microscope using a 40× objective. The images were recorded using a Hamamatsu ImagEM Electron multiplier (EM) CCD camera. Series of pictures were taken at the speed of ∼25 frames per second for 1 minute after the blue light was turned on. To record Ca2+ changes, each transgenic male was separated from hermaphrodites at the mid-L4 larval stage, and grown on OP50 lawns overnight. The adult male was recorded for the first time with non-functional ChR2, to obtain the baseline ratio of G-CaMP/DsRed intensity (R0). This ratio does not change within a short period, since G-CaMP and DsRed expression were under control of the same promoter. Afterwards, they were incubated on OP50 lawns that contain all-trans retinal for 30 minutes and then reimaged under blue light.
Laser stimulation and Ca2+ imaging
Laser stimulation of muscles was slightly modified from a previous study [48]. 18–24 hr adult males containing G-CaMP and DsRed in their muscles were put on a 10% agarose (in H2O) pad containing 0.5 µl of 0.1 µm diameter polystyrene microspheres, and covered with a coverslip [87]. The minimum output of a Spectra-Physics VSL-337ND-S Nitrogen Laser was adjusted to induce muscle contraction. The laser was aimed at the gubernaculum erector or a posterior body wall muscle, and one to ten pulses were applied to induce the muscles to contract. G-CaMP and DsRed fluorescence changes were then measured in the gubernaculum and body wall muscles, as well as the anal depressor and protractor muscles.
Analysis of Ca2+ imaging data
The Hamamatsu SimplePCI (version 6.6.0.0.) software was used to analyze the movies. The region-of-interest (ROI) that covers the spicule protractor and anal depressor muscles was defined manually for both G-CaMP and DsRed, and an ROI was picked far away from the male as the background region for both channels. All ROIs had the same shape and area (by using the “ROI clone” tool), and the “mean grey” of the ROIs was calculated for each frame as the fluorescence intensity. The intensity of the background region ROI was subtracted from the sample region ROI to exclude background fluorescence from other sources.
The intensity of DsRed does not change in response to Ca2+ transients. The average intensity of the DsRed was calculated through the image sequence (DsRedaverage). The intensity of DsRed for each frame (DsRedn) deviates from the “DsRedaverage” only because of photo bleaching and/or changes in muscle shape, which should contribute to G-CaMP signal change as well. To cancel out these Ca2+-unrelated changes in G-CaMP signals, the intensity of G-CaMP in each frame (G-CaMPn) was then normalized by using the equation of “G-CaMPnormalized n = G-CaMPn × DsRedaverage/DsRedn”.
For the baseline recording before all-trans retinal incubation, a ratio of “G-CaMPnormalized n/DsRedaverage” for each frame was calculated (R0 n), and the average of this ratio within the first 2-4 seconds of recording (R0) was used as the baseline signal.
For the second recording after all-trans retinal incubation, the ratio of “G-CaMPnormalized n/DsRedaverage” for each frame was also calculated (Rn). Finally, the Ca2+ level change (ΔR/R0) for this male was then calculated by “(Rn - R0)/R0×100%”. This ratio is comparable to the ΔF/F0 ratio used in other literature, except for that a G-CaMP/DsRed ratio is used instead of merely the G-CaMP intensity. The ratio ΔR/R0 was plotted over time as “corrected G-CaMP fold change trace for stimulated recording” (Figure 5B).
The maximum fold change (max. ΔR/R0) throughout the frame sequence was then calculated. Finally, the “max. ΔR/R0” was used as the maximal Ca2+ level change for each male, and was plotted and compared between treatments groups using the Mann-Whitney non-parametric test (Figure 5C).
Optical stimulation and continuous Ca2+ imaging
A demonstration unit of the Mosaic Imaging System (Andor Technology) was used to image G-CaMP and DsRed in the protractor muscles before, during and after light-stimulation of the gubernacular-oblique muscle group. The image sequences were taken at a rate of ∼72 frames/second. Males that expressed Pacr-18:ChR2::YFP, Punc-103E:G-CaMP and Punc-103E:DsRed were used in this assay. Males were immobilized by using the 10% agarose pad and polystyrene microspheres described earlier. Only the region of protractors was illuminated and monitored in the first 100 frames of images (∼1.4 seconds). Then, a region containing the gubernacular-oblique muscles was subsequently stimulated with blue light for 1000 frames of recording (∼14 seconds). The protractors were continuously monitored during this period, and were recorded for another 500 frames (∼6.9 seconds) after the end of gubernacular-oblique stimulation. The Ca2+ level change (ΔR/R0) was then calculated by “(Rn - R0)/R0×100%”. Rn and R0 were determined using methods described earlier. For some males, periodic spicule movements were seen before stimulation of the gubernacular muscles, due to pressure applied on the male tail by the coverglass.
RNA interference
RNAi was induced by feeding worms bacteria producing double stranded RNA to the target genes. Bacteria containing the target genes were obtained from the C. elegans ORF-RNAi library [88]. Bacteria with the L4440 double-T7 vector but with no target gene was used as the negative control. Bacteria were grown and induced by IPTG using a standard protocol [89]. L4 males were transferred to plates spotted with the bacteria that express dsRNA and were incubated for ∼20 hours. The adult males then were assayed for their response to light stimulation. The target gene sequences in the bacteria were verified by PCR amplification and sequencing using the universal primers [88].
Injection of carbenoxolone and tubocurarine
Males that expressed Pacr-18:ChR2::YFP, Punc-103E:G-CaMP and Punc-103E:DsRed were injected with the drugs. Carbenoxolone disodium salt (CBX) and (+)-Tubocurarine were purchased from Sigma-Aldrich (St. Louis, MO). Solution of CBX or tubocurarine was injected into the males using the same procedure as DNA microinjection. Males were imaged to obtain the baseline G-CaMP/DsRed ratio, and then were incubated on OP50 supplemented with all-trans retinal for 30 minutes. Drug or water was injected right after the incubation. These males were allowed to recover on OP50 supplemented with all-trans retinal for 1 hour, before they were imaged again to assess light-induced changes in the G-CaMP/DsRed ratio. For the injection needle not to stimulate any muscles or neurons in the male tail, one injection pulse was done at 1/3 of body length away from the posterior end of the male, the other was done in the anterior half of the male. A series of different concentrations of solution were injected for each drug to determine the proper concentration for our measurements. 7.5 mM or higher of CBX was found completely to suppress the light-induced repetitive spicule thrusts as well as the locomotion (Figure S7). Upon light stimulation, the G-CaMP signal then was measured in males that were injected with 7.5 mM CBX. The effects of different concentrations of tubocurarine were evaluated, and 7.5 mM or higher of tubocurarine was found to completely suppress the worm's locomotion. 10 mM of tubocurarine then was injected for the recordings.
Assay for blue light–induced behaviors
All strains used in this assay contained the lite-1(ce314) allele. At L4, males expressing the respective transgenes were transferred onto NGM plates with OP50 supplemented with all-trans retinal, which was prepared in the same way as described. Males with the same transgenes were also placed onto NGM plates with regular OP50 as control.
In the assay for light-induced sustained spicule protractor contraction, the SPC, PCA and PCB neurons were laser ablated in males at the mid-L4 larval stage. To remove hook associated cells, P10.p and P9.p were ablated at the L2 stage. The Plev-11:ChR2::YFP expressing males were assayed for light-evoked behavior on 5% agar pads without NaN3. All other strains were assayed for light-evoked behavior on fresh all-trans retinal-supplemented OP50 plates, and their control males were assayed on standard OP50 plates. The behavior was observed using an Olympus SZX16 microscope and recorded using a Hamamatsu ImagEM Electron multiplier (EM) CCD camera. Males were illuminated with blue light from the EXFO X-Cite 120PC Q Fluorescence Illumination System, filtered with the SZX2-FGFPA GFP filter (Ex460-495/Em510-550). Males were filmed in the absence of blue light for the first few seconds and then blue light was manually turned on for ∼10 seconds. Males that protracted their spicules in response to illumination for more than 5 seconds were counted as sustained spicule protraction positive.
The unc-103(0) males occasionally protrude their spicules in the absence of mating stimulation. Eventually, ∼30% of the virgin adult males that are separated from hermaphrodites will protract their spicules permanently. We used males whose spicules were not permanently protracted for the blue light stimulation assay. To rule out the possibility that spicule protraction scored was a result of spontaneous activity of the spicule circuit instead of light-stimulated activity, only males that retracted their spicules once the light was turned off were counted as positive for light-induced spicule protraction.
To determine the percentage of males that display rapid spicule thrusts upon blue light stimulation for the unc-9(lf) mutant and innexin RNAi strains, males were placed on 5% agar pads without NaN3, and observed under the Olympus BX51 microscope. The blue light was manually turned on for ∼5 seconds and the males that displayed repetitive spicule thrusts during this period were counted as positive.
Supporting Information
Zdroje
1. EmmonsSW
LiptonJ
2003 Genetic basis of male sexual behavior. J Neurobiol 54 93 110
2. LiuKS
SternbergPW
1995 Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14 79 89
3. de BonoM
MaricqAV
2005 Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28 451 501
4. LiptonJ
KleemannG
GhoshR
LintsR
EmmonsSW
2004 Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci 24 7427 7434
5. GruningerTR
GualbertoDG
GarciaLR
2008 Sensory perception of food and insulin-like signals influence seizure susceptibility. PLoS Genet 4 e1000117 doi:10.1371/journal.pgen.1000117
6. GruningerTR
GualbertoDG
LeBoeufB
GarciaLR
2006 Integration of male mating and feeding behaviors in Caenorhabditis elegans. J Neurosci 26 169 179
7. LeeK
PortmanDS
2007 Neural sex modifies the function of a C. elegans sensory circuit. Curr Biol 17 1858 1863
8. SenguptaP
SamuelAD
2009 Caenorhabditis elegans: a model system for systems neuroscience. Curr Opin Neurobiol 19 637 643
9. SokolowskiMB
2010 Social interactions in “simple” model systems. Neuron 65 780 794
10. BarrMM
GarciaLR
2006 Male mating behavior WormBook, ed The C elegans Research Community, WormBook, doi/101895/wormbook1781 http://wwwwormbookorg
11. SchaferWF
2006 Genetics of egg-laying in worms. Annu Rev Genet 40 487 509
12. BranickyR
HekimiS
2006 What keeps C. elegans regular: the genetics of defecation. Trends Genet 22 571 579
13. RankinCH
2006 Nematode behavior: the taste of success, the smell of danger! Curr Biol 16 R89 91
14. BargmannCI
2006 Chemosensation in C. elegans WormBook, ed The C elegans Research Community, WormBook, doi/101895/wormbook11231, http://wwwwormbookorg
15. GoodmanMB
2006 Mechanosensation WormBook, ed The C elegans Research Community, WormBook, http://wwwwormbookorg January 06
16. MoriI
SasakuraH
KuharaA
2007 Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol 17 712 719
17. ZhangM
ChungSH
Fang-YenC
CraigC
KerrRA
2008 A self-regulating feed-forward circuit controlling C. elegans egg-laying behavior. Curr Biol 18 1445 1455
18. BarrMM
SternbergPW
1999 A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401 386 389
19. LiuT
KimK
LiC
BarrM
2007 FMRFamide-like neuropeptides and mechanosensory touch receptor neurons regulate male sexual turning behavior in Caenorhabditis elegans. J Neurosci 27 7174 7182
20. SchindelmanG
WhittakerAJ
ThumJY
GharibS
SternbergPW
2006 Initiation of male sperm-transfer behavior in Caenorhabditis elegans requires input from the ventral nerve cord. BMC Biol 4 26
21. WhittakerA
SternbergP
2009 Coordination of opposing sex-specific and core muscle groups regulates male tail posture during Caenorhabditis elegans male mating behavior. BMC Biol 7 33
22. GarciaLR
MehtaP
SternbergPW
2001 Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell 107 777 788
23. KleemannGA
BasoloAL
2007 Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Animal Behaviour 74 1337 1347
24. GarciaLR
LeBoeufB
KooP
2007 Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics 175 1761 1771
25. SulstonJE
AlbertsonDG
ThomsonJN
1980 The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol 78 542 576
26. LiuY
LeBoeufB
GarciaLR
2007 G alpha(q)-coupled muscarinic acetylcholine receptors enhance nicotinic acetylcholine receptor signaling in Caenorhabditis elegans mating behavior. J Neurosci 27 1411 1421
27. FlemingJT
SquireMD
BarnesTM
TornoeC
MatsudaK
1997 Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci 17 5843 5857
28. RayesD
FlaminiM
HernandoG
BouzatC
2007 Activation of single nicotinic receptor channels from Caenorhabditis elegans muscle. Mol Pharmacol 71 1407 1415
29. RichmondJE
JorgensenEM
1999 One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2 791 797
30. BallivetM
AlliodC
BertrandS
BertrandD
1996 Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J Mol Biol 258 261 269
31. LewisJA
WuCH
BergH
LevineJH
1980 The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95 905 928
32. KimJ
PooleDS
WaggonerLE
KempfA
RamirezDS
2001 Genes affecting the activity of nicotinic receptors involved in Caenorhabditis elegans egg-laying behavior. Genetics 157 1599 1610
33. LewisJA
WuCH
LevineJH
BergH
1980 Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience 5 967 989
34. LeBoeufB
GruningerTR
GarciaLR
2007 Food deprivation attenuates seizures through CaMKII and EAG K+ channels. PLoS Genet 3 e156 doi:10.1371/journal.pgen.0030156
35. TouroutineD
FoxRM
Von StetinaSE
BurdinaA
MillerDM3rd
2005 acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem 280 27013 27021
36. JospinM
QiYB
StawickiTM
BoulinT
SchuskeKR
2009 A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol 7 e1000265 doi:10.1371/journal.pbio.1000265
37. LintsR
HallDH
2009 Male muscle system, male-specific muscles. In WormAtlas doi:103908/wormatlas25
38. GowerNJ
WalkerDS
BaylisHA
2005 Inositol 1,4,5-trisphosphate signaling regulates mating behavior in Caenorhabditis elegans males. Mol Biol Cell 16 3978 3986
39. BennettMV
ZukinRS
2004 Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron 41 495 511
40. BennettMV
BarrioLC
BargielloTA
SprayDC
HertzbergE
1991 Gap junctions: new tools, new answers, new questions. Neuron 6 305 320
41. NagelG
BraunerM
LiewaldJF
AdeishviliN
BambergE
2005 Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15 2279 2284
42. NagelG
SzellasT
HuhnW
KateriyaS
AdeishviliN
2003 Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A 100 13940 13945
43. NakaiJ
OhkuraM
ImotoK
2001 A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol 19 137 141
44. ReinerDJ
WeinshenkerD
TianH
ThomasJH
NishiwakiK
2006 Behavioral genetics of caenorhabditis elegans unc-103-encoded erg-like K(+) channel. J Neurogenet 20 41 66
45. MatzMV
FradkovAF
LabasYA
SavitskyAP
ZaraiskyAG
1999 Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol 17 969 973
46. BairdGS
ZachariasDA
TsienRY
2000 Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci U S A 97 11984 11989
47. ZhangF
PriggeM
BeyriereF
TsunodaSP
MattisJ
2008 Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci 11 631 633
48. ReinerDJ
WeinshenkerD
ThomasJH
1995 Analysis of dominant mutations affecting muscle excitation in Caenorhabditis elegans. Genetics 141 961 976
49. PetersMA
TeramotoT
WhiteJQ
IwasakiK
JorgensenEM
2007 A calcium wave mediated by gap junctions coordinates a rhythmic behavior in C. elegans. Curr Biol 17 1601 1608
50. DavidsonJS
BaumgartenIM
1988 Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther 246 1104 1107
51. SchneiderNL
StenglM
2006 Gap junctions between accessory medulla neurons appear to synchronize circadian clock cells of the cockroach Leucophaea maderae. J Neurophysiol 95 1996 2002
52. BaoL
SamuelsS
LocoveiS
MacagnoER
MullerKJ
2007 Innexins form two types of channels. FEBS Lett 581 5703 5708
53. RaizenDM
LeeRY
AveryL
1995 Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141 1365 1382
54. SattelleDB
CulettoE
GrausoM
RaymondV
FranksCJ
2002 Functional genomics of ionotropic acetylcholine receptors in Caenorhabditis elegans and Drosophila melanogaster. Novartis Found Symp 245 240 257; discussion 257-260, 261-244
55. AltunZF
ChenB
WangZW
HallDH
2009 High resolution map of Caenorhabditis elegans gap junction proteins. Dev Dyn 238 1936 1950
56. StarichT
SheehanM
JadrichJ
ShawJ
2001 Innexins in C. elegans. Cell Commun Adhes 8 311 314
57. SulstonJE
HorvitzHR
1977 Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 110 156
58. BrennerS
1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
59. LiuQ
ChenB
GaierE
JoshiJ
WangZW
2006 Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. J Biol Chem 281 7881 7889
60. GarciaLR
SternbergPW
2003 Caenorhabditis elegans UNC-103 ERG-like potassium channel regulates contractile behaviors of sex muscles in males before and during mating. J Neurosci 23 2696 2705
61. FranksCJ
MurrayC
OgdenD
O'ConnorV
Holden-DyeL
2009 A comparison of electrically evoked and channel rhodopsin-evoked postsynaptic potentials in the pharyngeal system of Caenorhabditis elegans. Invert Neurosci 9 43 56
62. BaierH
ScottEK
2009 Genetic and optical targeting of neural circuits and behavior—zebrafish in the spotlight. Curr Opin Neurobiol 19 553 560
63. GuoZV
HartAC
RamanathanS
2009 Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods 6 891 896
64. SchroederCE
WilsonDA
RadmanT
ScharfmanH
LakatosP
2010 Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol 20 172 176
65. CroninCJ
MendelJE
MukhtarS
KimYM
StirblRC
2005 An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet 6 5
66. ShadmehrR
SmithMA
KrakauerJW
2010 Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33 89 108
67. YuH
PretotR
BurglinT
SternbergP
2003 Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development 130 5217 5227
68. PedenE
BarrM
2005 The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol 15 394 404
69. JaureguiA
BarrM
2005 Functional characterization of the C. elegans nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp Cell Res 305 333 342
70. BaeY-K
Lyman-GingerichJ
BarrM
KnobelK
2008 Identification of genes involved in the ciliary trafficking of C. elegans PKD-2. Developmental Dynamics 237 2021 2029
71. WhiteJG
SouthgateE
ThomsonJN
BrennerS
1986 The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Phil Trans Royal Soc London Series B, Biol Scien 314 1 340
72. MacoskoEZ
PokalaN
FeinbergEH
ChalasaniSH
ButcherRA
2009 A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458 1171 1175
73. ChenB
LiuQ
GeQ
XieJ
WangZW
2007 UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol 17 1334 1339
74. WagenaarDA
HamiltonMS
HuangT
KristanWB
FrenchKA
2010 A hormone-activated central pattern generator for courtship. Curr Biol 20 487 495
75. GrillnerS
WallenP
SaitohK
KozlovA
RobertsonB
2008 Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res Rev 57 2 12
76. GrillnerS
JessellTM
2009 Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol 19 572 586
77. NisenbaumES
WilsonCJ
1995 Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15 4449 4463
78. KreitzerAC
MalenkaRC
2008 Striatal plasticity and basal ganglia circuit function. Neuron 60 543 554
79. MermelsteinPG
SongWJ
TkatchT
YanZ
SurmeierDJ
1998 Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J Neurosci 18 6650 6661
80. WilsonCJ
KawaguchiY
1996 The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16 2397 2410
81. GrillnerS
HellgrenJ
MenardA
SaitohK
WikstromMA
2005 Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28 364 370
82. HodgkinJA
HorvitzHR
BrennerS
1979 Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91 67 94
83. ParkEC
HorvitzHR
1986 Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics 113 821 852
84. SchnabelH
SchnabelR
1990 An Organ-Specific Differentiation Gene, pha-1, from Caenorhabditis elegans. Science 250 686 688
85. EdwardsS
CharlieN
MilfortM
BrownB
GravlinC
2008 A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol 6 e198 doi:10.1371/journal.pbio.0060198
86. BargmannCI
AveryL
1995 Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol 48 225 250
87. Fang-YenC
WassermanS
SenguptaP
SamuelAD
2009 Agarose immobilization of C. elegans. Worm Breeder's Gazette 18 32
88. RualJF
CeronJ
KorethJ
HaoT
NicotAS
2004 Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14 2162 2168
89. KamathRS
Martinez-CamposM
ZipperlenP
FraserAG
AhringerJ
2001 Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2 RESEARCH0002
Štítky
Genetika Reprodukční medicína
Článek Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis inČlánek c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and PhosphodiesterasesČlánek Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- Testing for an Unusual Distribution of Rare Variants
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities!
- Initial Mutations Direct Alternative Pathways of Protein Evolution
- Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in
- Sensing of Replication Stress and Mec1 Activation Act through Two Independent Pathways Involving the 9-1-1 Complex and DNA Polymerase ε
- Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in
- Identification of a Novel Type of Spacer Element Required for Imprinting in Fission Yeast
- Chiasmata Promote Monopolar Attachment of Sister Chromatids and Their Co-Segregation toward the Proper Pole during Meiosis I
- Global Analysis of the Relationship between JIL-1 Kinase and Transcription
- H3K9me2/3 Binding of the MBT Domain Protein LIN-61 Is Essential for Vulva Development
- REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock
- A Novel Unstable Duplication Upstream of Predisposes to a Breed-Defining Skin Phenotype and a Periodic Fever Syndrome in Chinese Shar-Pei Dogs
- Polycomb Repressive Complex 2 Controls the Embryo-to-Seedling Phase Transition
- A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Germ Line
- Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits
- A Genome-Wide Association Study of Upper Aerodigestive Tract Cancers Conducted within the INHANCE Consortium
- Ancestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
- Ultra-Deep Sequencing of Mouse Mitochondrial DNA: Mutational Patterns and Their Origins
- Phenotype Restricted Genome-Wide Association Study Using a Gene-Centric Approach Identifies Three Low-Risk Neuroblastoma Susceptibility Loci
- The Toll-Like Receptor Gene Family Is Integrated into Human DNA Damage and p53 Networks
- Polycomb Targets Seek Closest Neighbours
- Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis
- c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and Phosphodiesterases
- Estimating Divergence Time and Ancestral Effective Population Size of Bornean and Sumatran Orangutan Subspecies Using a Coalescent Hidden Markov Model
- Rif1 Supports the Function of the CST Complex in Yeast Telomere Capping
- A Tradeoff Drives the Evolution of Reduced Metal Resistance in Natural Populations of Yeast
- Quantifying the Underestimation of Relative Risks from Genome-Wide Association Studies
- Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in
- Triplet Repeat–Derived siRNAs Enhance RNA–Mediated Toxicity in a Drosophila Model for Myotonic Dystrophy
- The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
- Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
- Environmental Sex Determination in the Branchiopod Crustacean : Deep Conservation of a Gene in the Sex-Determining Pathway
- Systematic Detection of Polygenic Regulatory Evolution
- The SUMO Isopeptidase Ulp2p Is Required to Prevent Recombination-Induced Chromosome Segregation Lethality following DNA Replication Stress
- Uncoupling Antisense-Mediated Silencing and DNA Methylation in the Imprinted Cluster
- Role of the Drosophila Non-Visual ß-Arrestin Kurtz in Hedgehog Signalling
- Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti–dsDNA Autoantibody Production
- COMPASS-Like Complexes Mediate Histone H3 Lysine-4 Trimethylation to Control Floral Transition and Plant Development
- H3 Lysine 4 Is Acetylated at Active Gene Promoters and Is Regulated by H3 Lysine 4 Methylation
- Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches
- A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in
- Roles of () in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- A Molecular Phylogeny of Living Primates
- Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice
- A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



