-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Regulation by NLA and MicroRNA827 for Maintaining
Nitrate-Dependent Phosphate Homeostasis in
Plants need abundant nitrogen and phosphorus for higher yield. Improving plant
genetics for higher nitrogen and phosphorus use efficiency would save
potentially billions of dollars annually on fertilizers and reduce global
environmental pollution. This will require knowledge of molecular regulators for
maintaining homeostasis of these nutrients in plants. Previously, we reported
that the NITROGEN LIMITATION ADAPTATION (NLA)
gene is involved in adaptive responses to low-nitrogen conditions in
Arabidopsis, where nla mutant plants
display abrupt early senescence. To understand the molecular mechanisms
underlying NLA function, two suppressors of the
nla mutation were isolated that recover the
nla mutant phenotype to wild type. Map-based cloning
identified these suppressors as the phosphate (Pi) transport-related genes
PHF1 and PHT1.1. In addition,
NLA expression is shown to be regulated by the low-Pi
induced microRNA miR827. Pi analysis revealed that the early senescence in
nla mutant plants was due to Pi toxicity. These plants
accumulated over five times the normal Pi content in shoots specifically under
low nitrate and high Pi but not under high nitrate conditions. Also the Pi
overaccumulator pho2 mutant shows Pi toxicity in a
nitrate-dependent manner similar to the nla mutant. Further,
the nitrate and Pi levels are shown to have an antagonistic crosstalk as
displayed by their differential effects on flowering time. The results
demonstrate that NLA and miR827 have pivotal roles in
regulating Pi homeostasis in plants in a nitrate-dependent fashion.
Published in the journal: . PLoS Genet 7(3): e32767. doi:10.1371/journal.pgen.1002021
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002021Summary
Plants need abundant nitrogen and phosphorus for higher yield. Improving plant
genetics for higher nitrogen and phosphorus use efficiency would save
potentially billions of dollars annually on fertilizers and reduce global
environmental pollution. This will require knowledge of molecular regulators for
maintaining homeostasis of these nutrients in plants. Previously, we reported
that the NITROGEN LIMITATION ADAPTATION (NLA)
gene is involved in adaptive responses to low-nitrogen conditions in
Arabidopsis, where nla mutant plants
display abrupt early senescence. To understand the molecular mechanisms
underlying NLA function, two suppressors of the
nla mutation were isolated that recover the
nla mutant phenotype to wild type. Map-based cloning
identified these suppressors as the phosphate (Pi) transport-related genes
PHF1 and PHT1.1. In addition,
NLA expression is shown to be regulated by the low-Pi
induced microRNA miR827. Pi analysis revealed that the early senescence in
nla mutant plants was due to Pi toxicity. These plants
accumulated over five times the normal Pi content in shoots specifically under
low nitrate and high Pi but not under high nitrate conditions. Also the Pi
overaccumulator pho2 mutant shows Pi toxicity in a
nitrate-dependent manner similar to the nla mutant. Further,
the nitrate and Pi levels are shown to have an antagonistic crosstalk as
displayed by their differential effects on flowering time. The results
demonstrate that NLA and miR827 have pivotal roles in
regulating Pi homeostasis in plants in a nitrate-dependent fashion.Introduction
High yielding crops require the application of large amounts of nitrogen (N) and phosphorus (P) fertilizers. However, most of the crop plants are able to take up less than 40% of the applied N and P fertilizers and the rest of it is lost to the environment. This leads to an increase in crop production cost and significant global environmental damage by eutrophication of marine and fresh water ecosystems and gaseous loss to the atmosphere [1]–[3]. For instance, a 1% increase in N use efficiency worldwide would save ∼$1.1 billion annually. In addition, a ∼2.5-fold increase in N - and P-driven eutrophication of water bodies is expected by the year 2050 given current trends [1]. Therefore, developing crop varieties with higher nutrient use efficiency to restrict the excessive use of N and P fertilizer is required. For this, a comprehensive knowledge of molecular mechanisms regulating N and P homeostasis in plants is a prerequisite.
P is an essential structural component of nucleic acids and phospholipids and is a key constituent of high energy phosphate compounds such as ATP and ADP [2]. Despite the integral role of P for normal plant growth, development and yield, P availability in soil is usually the lowest of the macronutrients [4]. Even though the total P content in soil is high, its availability for plant uptake is largely restricted due to its adsorption in soil, precipitation by other cations and conversion into organic forms by microbes [2], [5]. To maintain internal P homeostasis, plants have evolved a series of adaptive responses that include induction of inorganic phosphate (Pi) transporters, change of root architecture, secretion of phosphatase and symbiosis with mycorrhizal fungi [1], [5], [6]. Genetic and molecular approaches have revealed several genes involved in Pi transport, homeostasis and adaptive responses in plants [6]. Among the regulatory genes for Pi homeostasis, PHOSPHATE STARVATION RESPONSE1 (PHR1) is a MYB transcription factor which acts in the Pi starvation signaling pathway by regulating a group of Pi starvation induced genes [7]. PHR1 has sumoylation sites and is a target of the SUMO E3 ligase SIZ1 which is a controller of Pi starvation dependent responses in Arabidopsis [8]. A SEC12-related PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 (PHF1) gene facilitates the trafficking of a key high affinity Pi transporter, PHOSPHATE TRANSPORTER1.1 (PHT1.1), which is involved in Pi acquisition [9], [10]. The pht1.1 mutant shows reduced Pi uptake in Arabidopsis [10] and its overexpression increased the Pi uptake in tobacco cells [11]. Another Pi transporter PHT1.4 might have a role in Pi uptake under high Pi conditions and a pht1.1 x pht1.4 double mutant shows a significant reduction in Pi uptake and shoot Pi content [10]. The low affinity Pi transporter PHT2.1 facilitates Pi allocation within the plant between roots and shoots and is required for Pi remobilization in old and young leaves [12]. Twenty genes with SPX domains (SYG1, Pho81 and XPR1) respond to Pi levels and SPX1 and SPX3 are proposed to regulate the expression of Pi starvation response genes [13]. Another SPX-domain containing gene PHO1 has a role in Pi loading into the xylem and possibly also in Pi signaling [14], [15]. The pho1 mutant has significantly lower levels of shoot Pi, but has normal root Pi content [14], [16]. A mutation in a ubiquitin conjugase gene PHO2 results in overaccumulation of Pi that causes Pi toxicity in Arabidopsis [17], [18]. The PHO2 gene is a target of the microRNA, miR399 [18], [19]. Like other nutrient elements, uptake of Pi also depends upon external pH and ion competition. At low pH, Pi in the H2PO4− form is present at a high proportion whereas at high pH HPO42− dominates [2]. Competition between Pi and arsenate is well known and both are taken up by the same transport system [2], [9].
N components are not only required for plant growth, but also serve as regulators of various metabolic and developmental pathways. Plants take up N mainly as nitrate (NO3−) and ammonium (NH4+), with NO3− being the predominant form in most agricultural soils [20]. NO3− and Pi are the two most important anions required for plant growth and development. However, the interaction and balance between NO3− and Pi in plants is not well studied. Here, we demonstrate the crosstalk between NO3− and Pi and the role of genes encoding an E3 ubiquitin ligase, NITROGEN LIMITATION ADAPTATION (NLA) and an E2 ubiquitin conjugase, PHO2, and a microRNA - miR827, in maintaining Pi homeostasis in Arabidopsis thaliana in a NO3− dependent manner.
Results
Identification of the nla Mutant and nla-Suppressors, phf1 and pht1.1
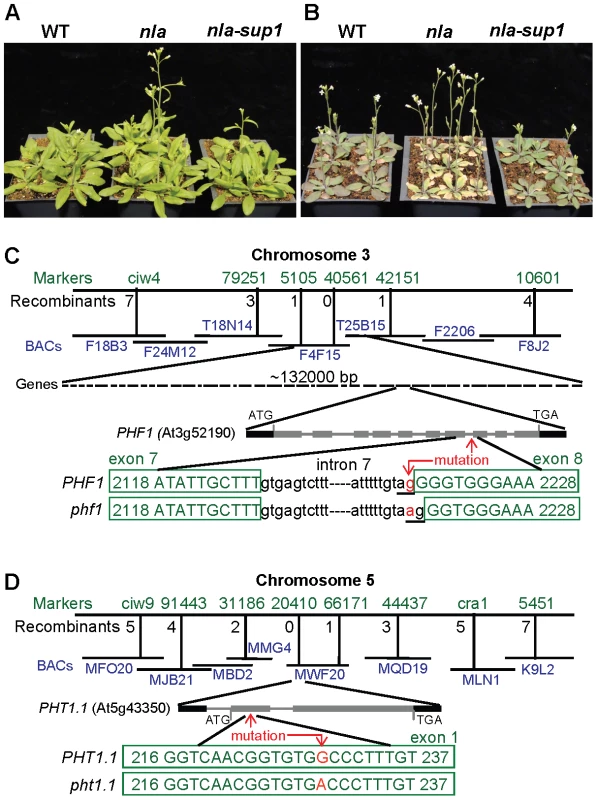
The nla mutant was identified based on its altered growth response to N limitation. nla mutant plants failed to show several adaptive responses to low-N conditions, such as the inability to accumulate anthocyanin and abrupt early senescence compared to wild type (WT) plants [21]. The nla phenotype was specific to the low-N growth condition, since at optimum-N and under other abiotic stresses, both nla and WT plants were similar in phenotype [21]. Later, it was revealed that in nla mutant plants under low-N conditions, the substrate in phenylpropanoid pathway was channeled towards lignin biosynthesis instead of anthocyanin synthesis, resulting in low anthocyanin accumulation [22]. Anthocyanin protects plants from photoinhibition damage under various stress conditions [22], [23]. A lack of anthocyanin accumulation in the nla mutant under low-N conditions means an absence of the photo-protective screen, which might result in the early senescence phenotype in nla mutant plants.
This evidence suggests that the NLA gene regulates the Arabidopsis adaptive responses under low-N. The knowledge of N regulatory genes is limited and the NLA gene might be a key component for the regulation of plant adaptation to low-N. Therefore, we initiated further studies to understand in more detail the physiological and molecular role of NLA. One approach was to generate and identify suppressor mutations in the nla mutant, which would restore the nla mutant phenotype to WT. For this nla mutant seeds were chemically mutagenised and screened at low-N supply. Two suppressor plants (nla-sup1 and nla-sup2) were identified which were phenotypically similar to WT in that the nla-suppressor plants did not show early senescence under low-N conditions (Figure 1A, 1B and Figure S1A, S1B). These suppressor genes were identified separately using map-based cloning approaches. The nla-sup1 locus was mapped to the lower arm of chromosome 3, in a ∼132 kb region with 40 annotated genes (Figure 1C). Sequencing this genomic region for each gene revealed that in PHF1 (At3g52190) a transition of a single G/C to A/T occurred at the 3′ end of intron 7 (position 2218). This transition destroyed the conserved dinucleotide, ‘AG’, which is required for proper splice recognition at the 3′ end of an intron. The resulting altered splice site led to the inclusion of the adjacent exonic ‘G’ as part of the intron (Figure 1C) leading to a 1 bp deletion in the processed mRNA. The cDNA sequence also revealed that in the nla-sup1 one ‘G’ nucleotide was missing compared to WT which causes a frameshift and generation of a premature stop codon resulting in a truncated PHF1 protein sequence (Figure 1C). The second suppressor, nla-sup2, was mapped to a location on chromosome 5. Sequencing of the genomic regions confirmed a mutation in a single G/C to A/T (position 229) in the first exon of PHT1.1 (At5g43350) gene. This transition results in a switch from alanine to threonine (position 73) in the amino acid sequence of the PHT1.1 protein (Figure 1D). PHT1.1 is a high affinity Pi uptake transporter [10] and PHF1 facilitates the trafficking of PHT1.1 [9]. To confirm that these altered genes corresponded to the suppressor mutations, their respective T-DNA insertion mutants were used to make nla x phf1 and nla x pht1.1 double mutants. Like the suppressor mutations, these double mutants also recovered the nla mutant phenotype to WT.
MicroRNA827 Suppresses the NLA Transcript Level
MicroRNAs (miRNAs) are noncoding small RNAs and suppress the expression of genes that have nearly complementary sequences by mRNA cleavage [24], [25]. The NLA gene is a putative target of a miRNA, miR827 (At3g59884) based on complementary sequences (Figure 2A and 2B). By identifying phf1 and pht1.1 as nla-suppressors, we have shown that NLA directly or indirectly targets PHF1 and PHT1.1. To know how NLA expression is regulated and whether the NLA transcript is a target of miR827, we analyzed the expression pattern of NLA and miR827 under different N and Pi regimes. Also the expression of NLA transcript was analyzed in the miR827 overexpresser (OX) and T-DNA mutant lines. The expression of miR827 is up-regulated by low-Pi conditions (Figure 2C) [26]. In contrast, the transcript level of NLA was down-regulated under low-Pi, the exact opposite response to the expression of miR827 (Figure 2D). Figure 2E shows that miR827 was overexpressed by 35-times in the OX-miR827 line and there was very low expression in the miR827-mutant plants as compared to WT. The NLA transcript level was over 3-fold down-regulated in the OX-miR827 and over 2-fold up-regulated in miR827 mutant plants compared to WT (Figure 2F). These results show that NLA is a target of miR827. Similarly, the PHO2 gene has been reported to be a target of miR399 and their expression level is also Pi dependent [18], [19].
Fig. 2. Regulation of <i>NLA</i> expression by miR827. 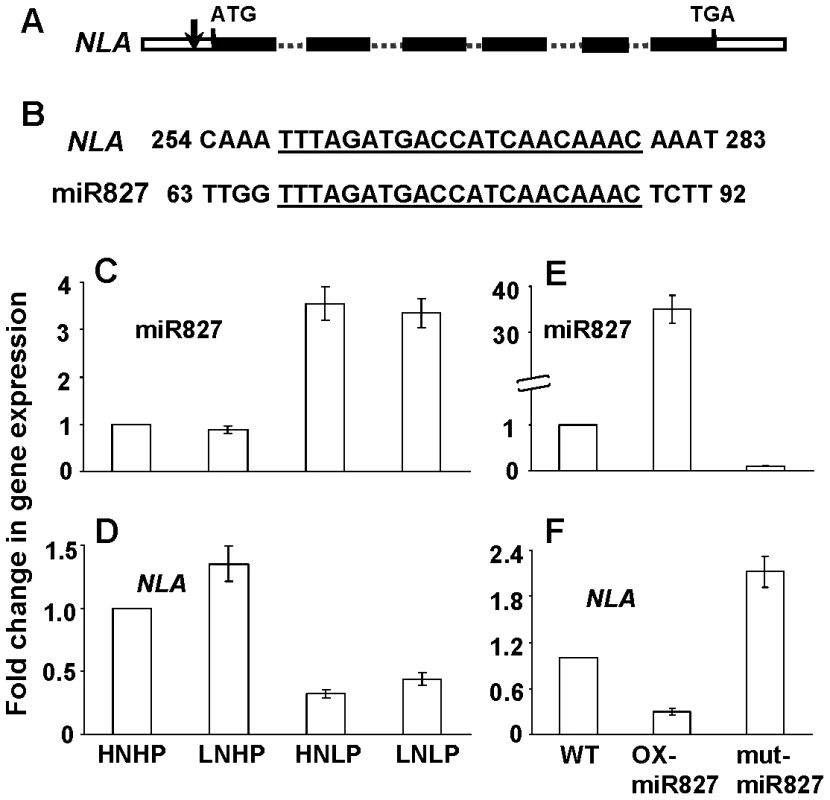
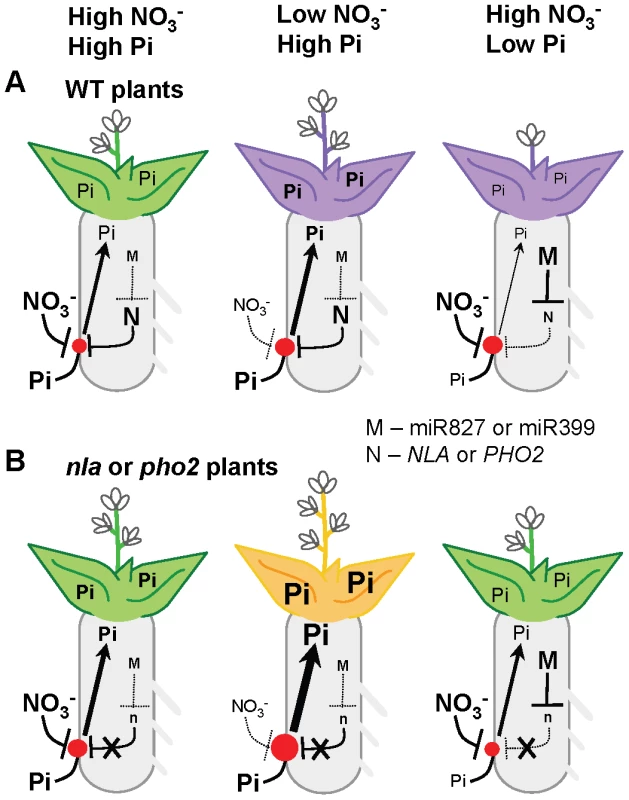
The nla Mutant Overaccumulates Pi in Shoots Specifically at Low NO3− Supply
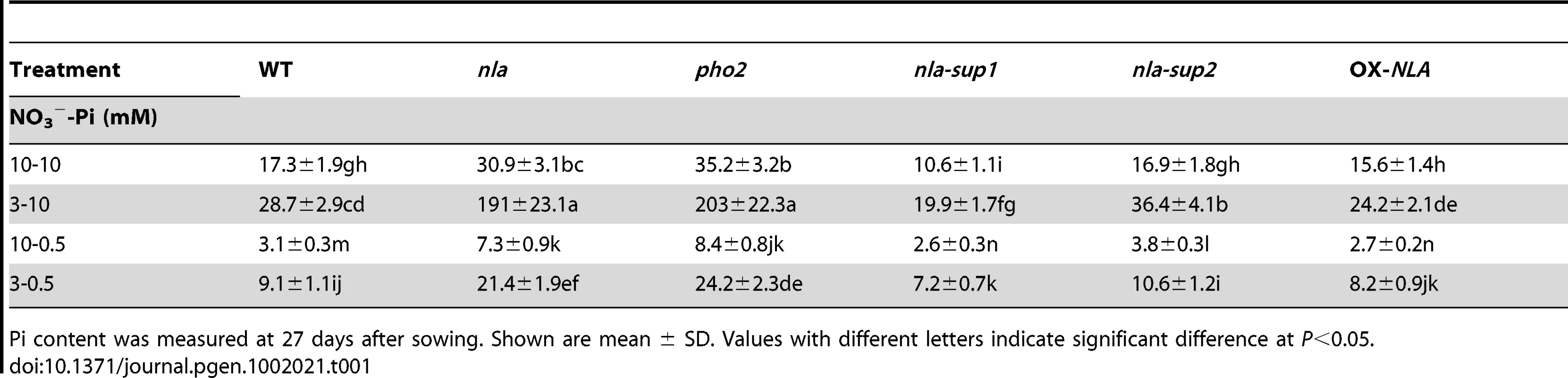
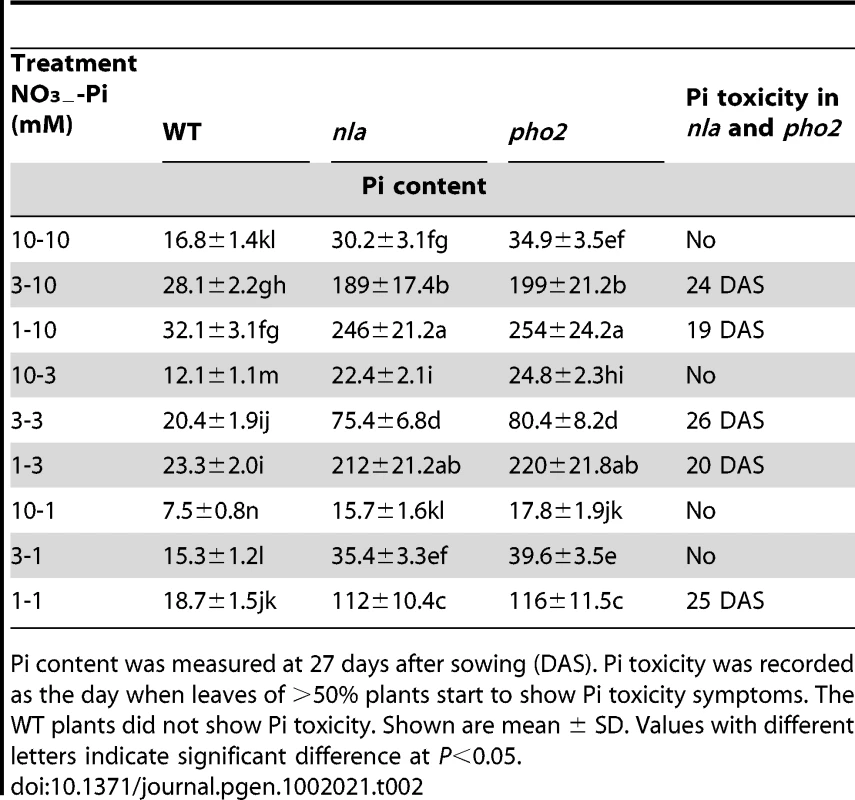
It has been shown previously that phf1 and pht1.1 mutants accumulate less Pi than WT [9], [10]. Therefore, the identification of the nla-suppressors as phf1 and pht1.1 mutations (Figure 1) and the transcript change of NLA with Pi levels (Figure 2C) indicate that Pi accumulation in the nla mutant might be impaired. Pi analysis reveals that under sufficient N-P conditions of 10 mM NO3−-10 mM Pi, where the nla mutant is phenotypically similar to WT (Figure 1A), Pi content in the nla mutant shoots was ∼1.8-fold higher than WT (Table 1). Interestingly, under the relatively low NO3− (3 mM NO3−-10 mM Pi) regime where the nla mutant shows early senescence (Figure 1B), Pi content in the nla mutant shoots increased ∼6.6-fold compared to WT (Table 1). P usually makes up ∼0.2% of plant dry matter and visual symptoms of Pi toxicity appear when P constitutes >1% of dry matter [2], [27]. Pi content in the nla mutant shoots at 3 mM NO3−-10 mM Pi regime makes up ∼2% of dry matter and its senescence phenotype likely is due to Pi toxicity, which leads to chlorosis or necrosis starting from the leaf margins (Figure 1B). Whereas, the nla mutant grown at low-Pi regimes accumulated Pi below P toxicity limits (Table 1). Also the nla suppressors, nla-sup1 and nla-sup2 as well as OX-NLA had a visual phenotype and Pi content similar to WT (Figure 1, Figure S1 and Table 1). Interestingly, the previously described Pi overaccumulator, pho2 mutant [17], [28], shows a phenotype and Pi content similar to the nla mutant (Table 1, Figure S1C and S1D). Pi toxicity in the nla and pho2 mutants was evident only when NO3− supply was relatively low with plants grown on 3 mM NO3−-10 mM Pi, while at the 10 mM NO3−-10 mM Pi regime the plants were phenotypically similar to WT (Figure S1C and S1D). This led to the obvious assumption that NO3− is also playing a role in Pi accumulation. This crosstalk is evident when Pi supply was constant and NO3− supplies were variable, where Pi content in WT shoots increased with decreasing NO3− applications (Table 1 and Table 2). In the nla and pho2 mutants, Pi accumulation accelerated and the appearance of Pi toxicity symptoms occurred earlier in accordance with decreasing NO3− supply at a given Pi level (Table 2). Pi toxicity in these mutants occurred only under low NO3− availability. This can be ascribed to the additive effects of lack of negative regulation by NLA or PHO2 and minimal suppression of NO3− on Pi accumulation.
The Pi toxicity symptom in the nla mutant appears mainly after bolting [21] and the above results are from plants at 27 days after sowing (DAS) when plants were already flowering. Therefore, Pi contents at the initiation of bolting (22 DAS) and before bolting (17 DAS) were also analyzed (Table S1). Pi accumulation at 22 and 17 DAS followed a similar trend to that of 27 DAS under varying NO3−-Pi regimes. However, net Pi accumulation in shoots at a specific NO3−-Pi regime was much lower at 17 DAS than 27 DAS (Table S1 and Table 1) and the nla mutant did not show Pi toxicity before bolting. This indicates that the accumulation of Pi accelerates with the transition of Arabidopsis from the vegetative to the reproductive stage. The roots are the initial sites of contact with Pi, but root growth and architecture are similar between nla and WT at varying NO3−-Pi regimes [21]. In conjunction with this, at low NO3− availability the fold differences for Pi content in roots of the nla mutant vs WT was much less than that observed in shoots (Table S2 and Table S3). This suggests that most of the Pi taken up by roots was not retained by them, but was transported towards the shoots.
A radiolabelled 33Pi uptake assay was conducted to assess whether the overaccumulation of Pi in the nla and pho2 mutants was due to increased Pi uptake. Indeed, the nla and pho2 mutants showed a higher 33Pi uptake rate than WT, nla-sup1, nla-sup2 and OX-NLA plants (Figure 3). NLA is an E3 Ubiquitin Ligase and has two distinct domains, SPX and RING. The nla is a deletion mutation (Col background) with a disrupted RING domain [21]. To ascertain if a different type of nla mutation would exert similar effects, a T-DNA insertion mutant in the same gene but in the ecotype Wassilewskija (Ws) (FLAG_352A03) was obtained. The nla mutant (Ws background) showed a similar Pi toxicity phenotype and Pi content as that of the nla mutant (Col background) under low NO3−-high Pi conditions (Figure S3).
For the detailed analysis of Pi homeostasis maintenance and the complementation study, several overexpresser (Table 3A) and double mutant (Table 3, Double Mutants) lines were generated. As shown above, miR827 suppresses NLA expression (Figure 2F). Indeed, Pi content in the OX-miR827 plants was ∼6.4-fold higher than WT. Pi toxicity and Pi content were similar in OX-miR827 and nla mutant plants. Whereas, the miR827 mutant had Pi content similar to the OX-NLA plants (Table 3). The PHF1 gene facilitates the trafficking of the key Pi uptake transporter, PHT1.1, and their mutant lines had lower Pi content than WT (Table 3C). Hence, their overexpression lines would be expected to accumulate higher Pi and might show Pi toxicity, as observed in the nla and pho2 mutants. However, the increase in Pi content in OX-PHF1 and OX-PHT1.1 lines was only ∼1.5-fold compared to WT, much lower than the Pi toxicity limit (Table 3A). Further, to confirm that phf1 and pht1.1 are nla-sup1 and nla-sup2 suppressor mutations, respectively, the WT cDNAs of PHF1 and PHT1.1 driven by the cauliflower mosaic virus 35S promoter (35S) were transformed into nla-sup1 and nla-sup2 mutants, respectively. The transformed nla-sup1-OX-PHF1 and nla-sup2-OX-PHT1.1 plants (generated using the genomic sequences of PHF1 and PHT1.1, respectively) grown at low NO3− had Pi content similar to the nla mutant (Table 3). In addition, the nla mutant was crossed with T-DNA mutants of phf1 and pht1.1. The double mutants, nla x phf1 and nla x pht1.1 had Pi contents similar to nla-sup1 and nla-sup2, respectively (Table 3, Double Mutants and Control Plants). The nla and pho2 plants were transformed with 35S:PHF1 and 35S:PHT1.1. However, these OX lines had no further increase of Pi contents over nla and pho2 mutants (Table 3). The nla x pho2 double mutant had no significant additive increase of Pi content compared to nla or pho2 mutants (Table 3). This indicates overlapping roles of nla and pho2 in Pi overaccumulation and their involvement in the same functional pathway. This is further confirmed in that pho2 x phf1 and pho2 x pht1.1 also restored Pi content to WT levels, like that of nla x phf1 and nla x pht1.1, respectively (Table 3, Double Mutants). PHT1.4 and PHT2.1 are also Pi transporters, but their mutants had Pi content similar to WT under high Pi conditions (Table 3, Control Plants), suggesting they may have roles in Pi reallocation within plants [6], [10], [12]. In agreement, nla x pht1.4 and nla x pht2.1 double mutants accumulated high Pi levels (Table 3, Double Mutants). The PHO1 gene is involved in Pi loading to the xylem [14] and the pho1 mutant shoots have very low Pi content (Table 3, Control Plants) [14]. The nla x pho1 double mutant gave expected results of having very low shoot Pi content compared to WT (Table 3, Double Mutants), showing that the pho1 mutation is able to restrict Pi loading for transport from roots towards shoots in the nla mutant. PHR1 regulates expression of some Pi-responsive genes [7]. The phr1 mutant had lower shoot Pi content than WT. Also the nla x phr1 mutant had a decrease in Pi content compared to the nla mutant (Table 3, Double Mutants and Control Plants), but not enough to avoid Pi toxicity. SIZ1 gene encodes a SUMO E3 ligase, mutation of which had slightly higher Pi content than WT (Table 3, Control Plants) [8]. However, nla x siz1 had no further increase in Pi levels over the nla mutant (Table 3, Control Plants).
NO3− and Pi Have an Antagonistic Interaction
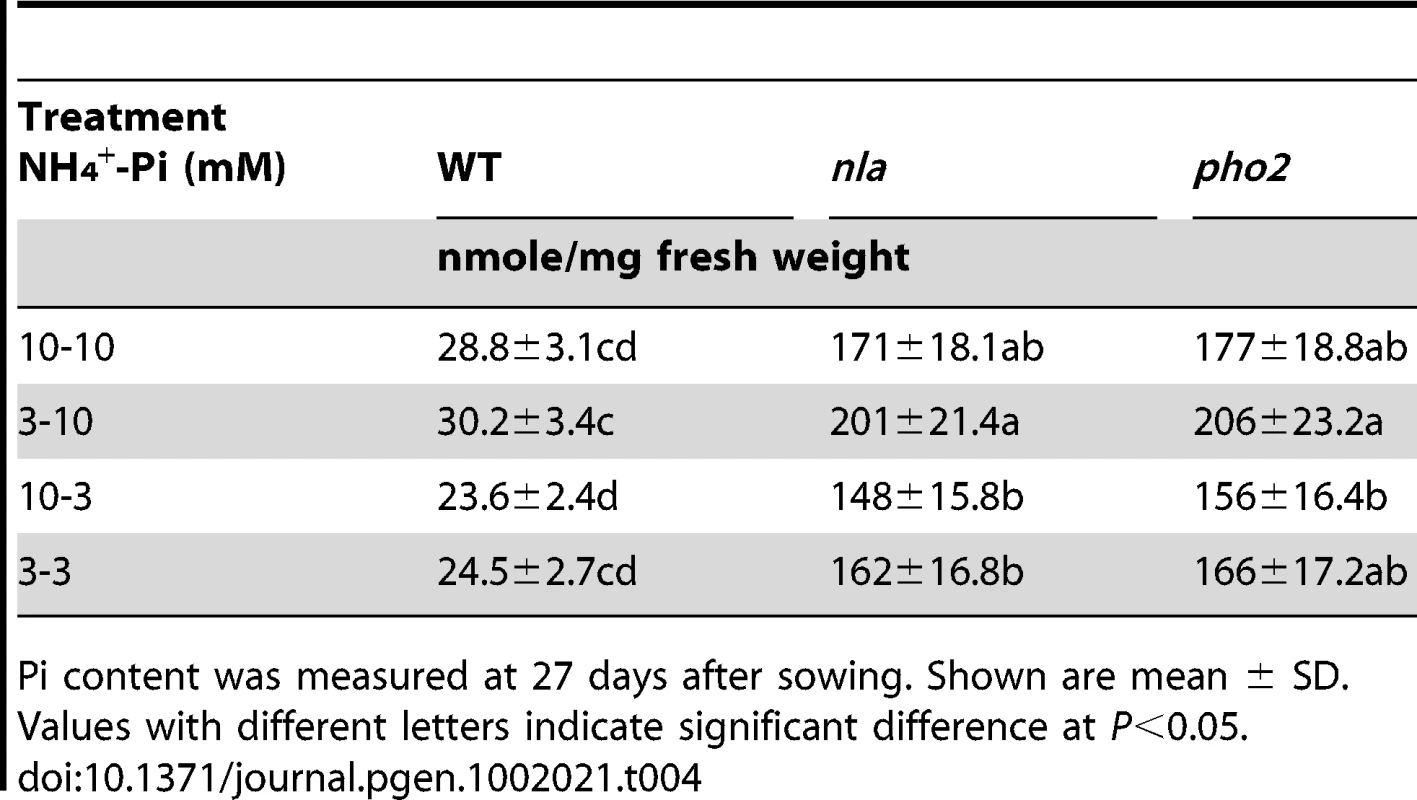
Accumulation of Pi increased with decreasing NO3− supply (Table 1). The N content was analyzed to confirm whether the changing Pi application would also exert similar suppression on N accumulation. In fact, N content increased with decreasing Pi supply (Table S4). However, the suppression by Pi on N accumulation was quite low (Table S4) compared to the suppression by NO3− on Pi accumulation (Table 1). The differential suppression by these ions could be ascribed to their contrasting mobility in soil, given that NO3− diffusion is 3–4 times faster than Pi [29]. N is available in both anionic and cationic forms, NO3− and NH4+, respectively, with NO3− being dominant in most soils and also the preferred form taken up by most plants, including Arabidopsis [20]. To analyze whether the antagonistic interaction of Pi was generalized to both NO3− and NH4+, the plants were grown in different NH4+-Pi regimes. Surprisingly, even at high NH4+ supply, Pi accumulation was much higher in nla and pho2 mutants (Table 4) and these mutant plants showed Pi toxicity (Figure S4). In contrast to this, equimolar concentration of NO3− suppressed Pi accumulation below toxicity limits in the nla and pho2 mutants (Table 1, Figure 1 and Figure S1). This suggests that Pi has an antagonistic crosstalk with NO3−, but not with NH4+.
Another important finding was the interaction between the NO3− and Pi applications for their effect on flowering time in Arabidopsis. For this, the plants were grown under varying relative supplies of NO3− and Pi as described in the materials and methods. Plants flowered earlier when grown under low NO3− compared to high NO3− conditions. In contrast, growth with low-Pi delayed flowering compared to high Pi. Further, the interaction between these two nutrients had a cumulative effect given that the plants grown at 3 mM NO3−-10 mM Pi flowered significantly earlier than plants at 10 mM NO3−-3 mM Pi (Figure 4A, Figure S2). In addition, the nla mutant, which had higher Pi accumulation, flowered significantly earlier compared to the phf1 mutant which had low Pi accumulation (Figure 4A). Expression levels of various genes which regulate flowering time in Arabidopsis were also changed in a similar fashion by NO3−-Pi levels. FLOWERING LOCUS C (FLC) is a floral repressor [30] and its expression was lowest at 3 mM NO3−-10 mM, where plants flowered earliest. Further, the expression of FLC was lower in nla mutant leaves than in phf1 (Figure 4B). FLOWERING LOCUS T (FT), LEAFY (LFY) and APETALA1 (AP1) are positive regulators of flowering time [30], and their expression was significantly higher in leaves of plants grown on the 3 mM NO3−-10 mM Pi than at 10 mM NO3−-3 mM Pi. Also the expression of these genes was higher in the nla than in the phf1 mutant (Figure 4C–4E). The effect of varying NO3− and Pi regimes on change of expression of FLC, FT, LFY and AP1 genes indicates that the autonomous and gibberellin pathways for flowering are affected. Since, Pi and gibberellin levels are positively correlated in Arabidopsis [31] and gibberellin is known to promote flowering [32], which was reflected by early flowering at high Pi content (Figure 4A).
Discussion
The identification of the nla suppressors as phf1 and pht1.1 and analysis of Pi levels in the nla mutant grown under different N and Pi regimes demonstrated that NLA has a role in regulating Pi homeostasis in plants. Further, the expression of NLA itself is regulated by a miR827 in a Pi dependent manner. Interestingly, comparing the phenotype of the nla and pho2 mutants has shown a similar phenotypic pattern under varying NO3− and Pi regimes and both mutants overaccumulate Pi under low NO3− and high Pi conditions. Figure 5 shows the proposed model for the maintenance of Pi homeostasis via the regulation by the NLA and PHO2 genes and the crosstalk between NO3− and Pi. The NLA and PHO2 genes, along with higher NO3− applications, suppress Pi uptake in Arabidopsis WT plants. Plants grow normally as long as both NO3− and Pi supplies are sufficient, whereas, if either of them is limiting, plants start to accumulate anthocyanin as a stress symptom (Figure 5A). In the nla or pho2 mutants, uptake of Pi is higher than in WT. However, when suppression by NO3− is also minimal when plants are grown under low NO3− and high Pi conditions, Pi accumulation is accelerated to toxic levels leading to necrotic senescence (Figure 5B). Thus, these mutants can grow normally even when Pi supply is sub-optimal, without showing Pi deficiency symptoms (Figure 5B) and would have optimal internal Pi content in shoots (Table 1).
NO3− and Pi supply had antagonistic interactions for their accumulation in plants. However, the suppression by a higher supply of NO3− on Pi accumulation was larger than the suppression by higher supply of Pi on N content (Table 1 and Table S4). This could be due to two reasons, first that NO3− might have preferential uptake over Pi, since the requirement for N (>1% of dry matter) by most plants is usually higher than for P (<0.5% of dry matter). Second, the NO3− uptake system might not require negative regulators, since overaccumulation of N is not toxic to the plants and excess NO3− is stored in the vacuoles [33]. In contrast, NLA and PHO2 serve as negative regulators of the Pi uptake system and are in turn regulated by miRNAs, which is necessary for the plants to avoid Pi toxicity caused by its overaccumulation. Further, the antagonistic interaction between NO3− and Pi was reflected by their inverse effect on flowering time in Arabidopsis, given that the plants flowered early under low-N conditions and it took a longer time to flower under low-Pi conditions (Figure 4A and Figure 5). Delayed flowering by low-Pi level could be via gibberellin signalling, since low gibberellin levels are known to delay flowering [32] and Pi and gibberellin levels are positively correlated [31]. In contrast, the plants flowered early under low NO3− conditions which could be either through a general stress management strategy to complete the life cycle earlier or through direct N affects on the flowering pathways. In agreement with the antagonistic crosstalk between NO3− and Pi for their uptake and inverse effects on flowering time observed here, the contrasting effects of NO3− and Pi on root architecture in Arabidopsis were reported earlier [34]. In that case, the primary root length decreased with increasing NO3− supply and increased with increasing Pi availability.
NLA is an E3 ligase and PHO2 is an E2 conjugase protein. The Arabidopsis genome contains 37 E2 and ∼1300 E3 genes [35] and their proteins have potential roles for ubiquitination of target proteins destined to be degraded by the ubiquitin-26S proteasome. This pathway is employed by plants for the degradation and removal of proteins to maintain optimal growth and development. It would be interesting to study how NLA and PHO2 proteins interact to coordinate signals that specify the target proteins involved in Pi acquisition. PHF1 and PHT1.1 are likely direct or indirect targets of NLA and PHO2. This is supported by our results that mutations of phf1 or pht1.1 in the nla and pho2 mutant background successfully reduced Pi overaccumulation and restored their Pi contents similar to WT. The nla mutant had high Pi accumulation while the OX-NLA plants do not have a significantly lower Pi than does WT (Table 1). The simplest explanation is that once a threshold level of NLA is reached, then increasing NLA does not change phenotype with regards Pi accumulation. In addition, NLA is an E3 ubiquitin ligase which is part of the E1–E2–E3 ubiquitination pathway to target proteins that are degraded by the 26S proteasome pathway. Hence, the co-ordination of both pathways might be necessary for the complete degradation of PHF1 and PHT1.1 to a level which can significantly reduce the Pi content in OX-NLA plants, compared to WT plants. The role of NLA and PHO2 in Pi homeostasis is further supported in that they are regulated by the low-Pi induced miRNAs, miR827 (Figure 2) and miR399 [18], [19], respectively. The miR827 and miR399 are expressed at low levels under high-Pi conditions with the concomitant higher expression of NLA and PHO2. This is necessary to prevent the overloading of Pi into plants. Under low-Pi supply, increased expressions of miRNAs repress NLA and PHO2 transcript levels and the repression of Pi transporters is alleviated (Figure 5). Given that Pi availability in soil is usually limited, this insures that sufficient Pi accumulation occurs in plants.
Over the next 50 years, it will be essential to increase crop yields by almost double to meet the needs of the growing world population. Increasing fertilizer use for higher crop yields would lead to large economic costs and vastly add to the environmental load of crop production. Therefore, the development of crops with improved genetics for nutrient use efficiency is absolutely crucial for the sustainability of crop production. We have demonstrated that NLA and PHO2 (their regulation by miR827 and miR399) have pivotal roles in NO3− regulated control of Pi homeostasis. In fertilized agricultural soils, Pi availability is typically low when compared to NO3−, although more fixed N is required for maximal plant growth. It should be possible by modulating the expression of NLA or PHO2 to develop crop cultivars that better utilize the available P in balance with N supply.
Materials and Methods
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Columbia was used in the experiments unless otherwise stated. The seeds were stratified at 4°C for 3 d and sown in nutrient-free LB2 soil (SunGro Horticulture Canada Ltd, http://www.sungro.com/). The plants were grown in controlled growth chambers at 16 h light/8 h dark, 23°C day/18°C night, light intensity 200 µE m−1 s−1 and 65% relative humidity. The nutrient solution was applied once a week for 4 weeks and contained 2 mM MgSO4, 1 mM CaCl2, 100 µM Fe-EDTA, 50 µM H3BO3, 12 µM MnSO4, 1 µM ZnCl2, 1 µM CuSO4 and 0.2 µM Na2MoO4. N and P were supplied in the form of KNO3 and KH2PO4 (pH 6.0), respectively, with varying concentration as mentioned in the results. The nutrient levels used in the experiments are relative levels, but not absolute levels. To avoid the discrepancies of maintaining the absolute nutrient levels in soil between different experiments, we have previously developed the defined nutrient growth conditions [21]. The application of nutrient solution once every week for 4 weeks to Arabidopsis with varying N supplies showed that 10 mM NO3− is sufficient-N for optimum plant growth and yield, 3 mM NO3− is moderately low-N where plants start to develop adaptive responses required to deal with low-N conditions and 1 mM NO3− is a severe-N limiting condition [21] (Figure S5A). The varying P levels showed that 10 mM Pi is sufficient-P for optimum plant growth and yield, 3 mM Pi is moderately low to sufficient-P with a slight appearance of red color in leaves indicating the start of anthocyanin accumulation, 1 mM Pi is a moderately low-P condition with higher anthocyanin accumulation and significantly lower plant growth and yield and 0.5 mM Pi is a severe-P limiting condition (Figure S5B). Shoots and roots were harvested separately at the indicated days, frozen in liquid nitrogen and stored at −80°C until use. The T-DNA insertion mutant lines for different genes obtained from the Arabidopsis Biological Resource Center and used in the experiments are: phf1 (SALK_144943), pht1.1 (SALK_088586), pht1.4 (SALK_103881), pht2.1 (SALK_094069), siz1 (SALK_065397), phr1 (SALK_067629), and miR827 (SALK_020837).
Isolation of nla Mutant Suppressors and Positional Cloning
The homozygous nla mutant (in Col background) seeds were soaked overnight at 4°C in 100 mM phosphate buffer pH 7.5 and then treated with 0.4% ethyl methanesulfonate (EMS) for 8 h at room temperature. The seeds were washed thoroughly and sown in LB2 soil supplied with 3 mM KNO3− and 10 mM KH2PO4 and other nutrients as above. The M1 plants were grouped into 120 sets, with each set having a pool of 36–40 plants. The seeds from each set were harvested separately. About 200 M2 plants within each set were grown on 3 mM KNO3− and 10 mM KH2PO4 to screen for putative nla-suppressor plants which would recover the nla mutant phenotype similar to WT. Two putative nla-suppressors (nla-sup1 and nla-sup2) plants were identified and were backcrossed twice with nla-Col mutant plants. Simultaneously, to achieve the nla mutation in Ler genetic background, the homozygous nla-Col plant was crossed with Ler WT plant and the nla-Ler plant obtained from this cross was further backcrossed nine times with Ler WT plant to produce genetically clean nla-Ler. The nla-suppressor-Col plant was crossed with nla-Ler plant. Among the segregating F2 progeny, which was grown at 3 mM KNO3− and 10 mM KH2PO4, ∼500 plants showing the WT phenotype were selected for mapping. The first round of mapping was performed as described by Luckowitz et al. [36]. The second round of fine mapping was accomplished using the simple sequence length polymorphism (SSLP) markers prepared from the Arabidopsis genome sequence (www.arabidopsis.org). The mapping schemes are shown in Figure 1. We identified two independent putative nla-suppressors, with the mapping for each done separately. One suppressor, nla-sup1, was linked to the PHF1 (At3g52190) gene and the other, nla-sup2, was linked to the PHT1.1 (At5g43350) gene.
Plant Transformation
For the complementation and overexpression analysis, the coding and/or genomic sequences of NLA (At1g02860), PHF1 and PHT1.1 genes and a 300 bp region including miR827 sequences were amplified by PCR and cloned into gateway-compatible vectors pEarleyGate 100 and/or pEarleyGate 101 [37]. The plasmids were transformed into Agrobacterium tumefaciens strain EHA105 and then transformed into WT Col, nla-sup1, nla-sup2, nla and/or pho2 plants as described by Clough and Bent [38]. The transgenic plants were selected with the herbicide selection BASTA (active ingredient glufosinate ammonium). Five independent OX lines carrying a single transgene copy for all the constructs were generated. At least two independent lines for each construct were used in the experiments and representative data of one OX line is given in the results.
Biochemical Assays
Frozen shoot and root tissue was used for the following biochemical assays. Pi and total P contents were measured as described by Chiou et al. [18], which is originally adapted from the protocol mentioned by Ames [39]. The percentage of total N in the dried tissues was measured by the Micro-Dumas combustion analysis method using a Carlo Erba NA1500 C/N analyzer, (Carlo Erba Strumentazione, Milan, Italy).
Pi Uptake Assay
The plants were grown for 12 day on agar plates (1% sucrose and 0.8% agar) with 1 mM KNO3 and 0.5 mM KH2PO4 with the other nutrient concentrations the same as in the soil experiments. Ten plants were pooled and roots were immersed in 7 ml nutrient solution, except with the replacement of KH2PO4 by [33P]orthophosphate (10.2×10−2 MBq/7 ml; Perkin-Elmer). The plants were incubated for 2 and 4 h and then rinsed with nutrient solution without 33P. The plants were weighed and lysed in 500 µl of 30% H2O2 and 200 µl of perchloric acid for 1 h at 70°C and 5 ml scintillation liquid was added to each sample, incubated over night and the activity measured by scintillation counter.
Quantitative Real-Time PCR
Total RNA was isolated using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). To eliminate any residual genomic DNA, total RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI, USA). The cDNA was synthesized from total RNA by using the Reverse Transcription System kit (Promega). Primer Express 2.0 software (Applied Biosystems, Forster City, CA, USA) was used to design the primers. Primer sequences for each gene are given in Table S5. Real-time PCR was performed as described previously [40]. Relative quantification (RQ) values for each target gene were calculated by the 2−ΔΔCΤ method [41] using UBIQUITIN10 (UBQ10) and/or GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE A SUBUNIT (GAPA) as an internal reference gene. To ensure the validity of the 2−ΔΔCΤ method, twofold serial dilutions of cDNA were used to create standard curves, and the amplification efficiencies of the target and reference genes shown to be approximately equal.
Statistical Analysis
Statistical analysis was done by Fisher's protected LSD test using SAS statistical software (SAS Institute, Inc., NC). The results shown are representative of three independent experiments and within each experiment treatments were replicated three times, unless otherwise stated.
Supporting Information
Zdroje
1. PoirierYBucherM
2002
Phosphate transport and homeostasis in
Arabidopsis;
SomervilleCRMeyerowitzE
American Society of Plant Biologists
Rockville, MD
doi:10.1199/tab.0024,
http://www.aspb.org/publications/Arabidopsis.
2. MarschnerH
1995
Mineral nutrition of higher plants.
London
Academic Press
3. GoodAGShrawatAKMuenchDG
2004
Can less yield more? Is reducing nutrient input into the
environment compatible with maintaining crop production?
Trends Plant Sci
9
12
597
605
4. BarberSAWalkerJMVaseyEH
1963
Mechanisms for movement of plant nutrients from soil and
fertilizer to plant root.
J Agri Food Chem
11
3
204
207
5. RaghothamaKG
1999
Phosphate acquisition.
Annu Rev Plant Physiol Plant Mol Biol
50
665
693
6. LinWYLinSIChiouTJ
2009
Molecular regulators of phosphate homeostasis in
plants.
J Exp Bot
60
5
1427
1438
7. RubioVLinharesFSolanoRMartinACIglesiasJ
2001
A conserved MYB transcription factor involved in phosphate
starvation signaling both in vascular plants and in unicellular
algae.
Genes Dev
15
16
2122
2133
8. MiuraKRusASharkhuuAYokoiSKarthikeyanAS
2005
The Arabidopsis SUMO E3 ligase SIZ1 controls
phosphate deficiency responses.
Proc Natl Acad Sci USA
102
21
7760
7765
9. GonzalezESolanoRRubioVLeyvaAPaz-AresJ
2005
PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific
SEC12-related protein that enables the endoplasmic reticulum exit of a
high-affinity phosphate transporter in
Arabidopsis.
Plant Cell
17
12
3500
3512
10. ShinHShinHSDewbreGRHarrisonMJ
2004
Phosphate transport in Arabidopsis: Pht1;1 and
Pht1;4 play a major role in phosphate acquisition from both low - and
high-phosphate environments.
Plant J
39
4
629
642
11. MitsukawaNOkumuraSShiranoYSatoSKatoT
1997
Overexpression of an Arabidopsis thaliana high-affinity phosphate
transporter gene in tobacco cultured cells enhances cell growth under
phosphate-limited conditions.
Proceedings of the National Academy of Sciences of the United States of
America
94
13
7098
7102
12. VersawWKHarrisonMJ
2002
A chloroplast phosphate transporter, PHT2;1, influences
allocation of phosphate within the plant and phosphate-starvation
responses.
Plant Cell
14
8
1751
1766
13. DuanKYiKKDangLHuangHJWuW
2008
Characterization of a sub-family of Arabidopsis
genes with the SPX domain reveals their diverse functions in plant tolerance
to phosphorus starvation.
Plant J
54
6
965
975
14. HamburgerDRezzonicoEPetetotJMCSomervilleCPoirierY
2002
Identification and characterization of the Arabidopsis
PHO1 gene involved in phosphate loading to the
xylem.
Plant Cell
14
4
889
902
15. WangYRibotCRezzonicoEPoirierY
2004
Structure and expression profile of the Arabidopsis PHO1 gene
family indicates a broad role in inorganic phosphate
homeostasis.
Plant Physiology
135
1
400
411
16. PoirierYThomaSSomervilleC
Schiefelbein J
1991
A Mutant of Arabidopsis Deficient in Xylem Loading of
Phosphate.
Plant Physiology
97
3
1087
1093
17. AungKLinSIWuCCHuangYTSuCL
2006
pho2, a phosphate overaccumulator, is caused by a nonsense
mutation in a MicroRNA399 target gene.
Plant Physiol
141
3
1000
1011
18. ChiouTJAungKLinSIWuCCChiangSF
2006
Regulation of phosphate homeostasis by microRNA in
Arabidopsis.
Plant Cell
18
2
412
421
19. FujiiHChiouTJLinSIAungKZhuJK
2005
A miRNA involved in phosphate-starvation response in
Arabidopsis.
Curr Biol
15
22
2038
2043
20. CrawfordNMFordeBG
2002
Molecular and developmental biology of inorganic nitrogen
nutrition.;
SomervilleCRMeyerowitzE
American Society of Plant Biologists
Rockville, MD
doi:
10.1199/tab.0011, http://www.aspb.org/publications/Arabidopsis.
21. PengMHannamCGuHLBiYMRothsteinSJ
2007
A mutation in NLA, which encodes a RING-type
ubiquitin ligase, disrupts the adaptability of Arabidopsis
to nitrogen limitation.
Plant J
50
2
320
337
22. PengMHudsonDSchofieldATsaoRYangR
2008
Adaptation of Arabidopsis to nitrogen limitation
involves induction of anthocyanin synthesis which is controlled by the NLA
gene.
J Exp Bot
59
11
2933
2944
23. Chalker-ScottL
1999
Environmental significance of anthocyanins in plant stress
responses.
Photochemistry and Photobiology
70
1
1
9
24. CarringtonJCAmbrosV
2003
Role of microRNAs in plant and animal
development.
Science
301
5631
336
338
25. BartelDP
2004
MicroRNAs: Genomics, biogenesis, mechanism, and
function.
Cell
116
2
281
297
26. PantBDMusialak-LangeMNucPMayPBuhtzA
2009
Identification of nutrient-responsive
Arabidopsis and rapeseed microRNAs by comprehensive
real-time polymerase chain reaction profiling and small RNA
sequencing.
Plant Physiol
150
3
1541
1555
27. JonesJB
1998
Phosphorus toxicity in tomato plants: When and how does it
occur?
Commun Soil Sci Plant Analysis
29
11-14
1779
1784
28. DelhaizeERandallPJ
1995
Characterization of a phosphate-accumulator mutant of
Arabidopsis thaliana.
Plant Physiol
107
1
207
213
29. TinkerPBNyePH
2000
Solute movement in the rhizosphere:
Oxford
Oxford University Press
30. MouradovACremerFCouplandG
2002
Control of flowering time: Interacting pathways as a basis for
diversity.
Plant Cell
14
S111
S130
31. JiangCGaoXLiaoLHarberdNPFuX
2007
Phosphate starvation root architecture and anthocyanin
accumulation responses are modulated by the gibberellin-DELLA signaling
pathway in Arabidopsis.
Plant Physiol
145
4
1460
1470
32. BlazquezMAGreenRNilssonOSussmanMRWeigelD
1998
Gibberellins promote flowering of Arabidopsis by
activating the LEAFY promoter.
Plant Cell
10
5
791
800
33. De AngeliAMonachelloDEphritikhineGFrachisseJMThomineS
2006
The nitrate/proton antiporter AtCLCa mediates nitrate
accumulation in plant vacuoles.
Nature
442
7105
939
942
34. LinkohrBIWilliamsonLCFitterAHLeyserHMO
2002
Nitrate and phosphate availability and distribution have
different effects on root system architecture of
Arabidopsis.
Plant J
29
6
751
760
35. SmalleJVierstraRD
2004
The ubiquitin 26S proteasome proteolytic pathway.
Annu Rev Plant Biol
55
555
590
36. LukowitzWGillmorCSScheibleWR
2000
Positional cloning in Arabidopsis. Why it feels
good to have a genome initiative working for you.
Plant Physiol
123
3
795
805
37. EarleyKWHaagJRPontesOOpperKJuehneT
2006
Gateway-compatible vectors for plant functional genomics and
proteomics.
Plant J
45
4
616
629
38. CloughSJBentAF
1998
Floral dip: a simplified method for Agrobacterium-mediated
transformation of Arabidopsis thaliana.
Plant J
16
6
735
743
39. AmesBN
1966
Assay of inorganic phosphate, total phosphate and
phosphatases.
Methods Enzymol
8
115
118
40. KantSKantPRavehEBarakS
2006
Evidence that differential gene expression between the halophyte,
Thellungiella halophila, and Arabidopsis
thaliana is responsible for higher levels of the compatible
osmolyte proline and tight control of Na+ uptake in
T. halophila.
Plant Cell Environ
29
7
1220
1234
41. LivakKJSchmittgenTD
2001
Analysis of relative gene expression data using real-time
quantitative PCR and the 2−ΔΔCΤ
method.
Methods
25
4
402
408
Štítky
Genetika Reprodukční medicína
Článek c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and PhosphodiesterasesČlánek Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- Testing for an Unusual Distribution of Rare Variants
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities!
- Initial Mutations Direct Alternative Pathways of Protein Evolution
- Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in
- Sensing of Replication Stress and Mec1 Activation Act through Two Independent Pathways Involving the 9-1-1 Complex and DNA Polymerase ε
- Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in
- Identification of a Novel Type of Spacer Element Required for Imprinting in Fission Yeast
- Chiasmata Promote Monopolar Attachment of Sister Chromatids and Their Co-Segregation toward the Proper Pole during Meiosis I
- Global Analysis of the Relationship between JIL-1 Kinase and Transcription
- H3K9me2/3 Binding of the MBT Domain Protein LIN-61 Is Essential for Vulva Development
- REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock
- A Novel Unstable Duplication Upstream of Predisposes to a Breed-Defining Skin Phenotype and a Periodic Fever Syndrome in Chinese Shar-Pei Dogs
- Polycomb Repressive Complex 2 Controls the Embryo-to-Seedling Phase Transition
- A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Germ Line
- Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits
- A Genome-Wide Association Study of Upper Aerodigestive Tract Cancers Conducted within the INHANCE Consortium
- Ancestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
- Ultra-Deep Sequencing of Mouse Mitochondrial DNA: Mutational Patterns and Their Origins
- Phenotype Restricted Genome-Wide Association Study Using a Gene-Centric Approach Identifies Three Low-Risk Neuroblastoma Susceptibility Loci
- The Toll-Like Receptor Gene Family Is Integrated into Human DNA Damage and p53 Networks
- Polycomb Targets Seek Closest Neighbours
- Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis
- c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and Phosphodiesterases
- Estimating Divergence Time and Ancestral Effective Population Size of Bornean and Sumatran Orangutan Subspecies Using a Coalescent Hidden Markov Model
- Rif1 Supports the Function of the CST Complex in Yeast Telomere Capping
- A Tradeoff Drives the Evolution of Reduced Metal Resistance in Natural Populations of Yeast
- Quantifying the Underestimation of Relative Risks from Genome-Wide Association Studies
- Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in
- Triplet Repeat–Derived siRNAs Enhance RNA–Mediated Toxicity in a Drosophila Model for Myotonic Dystrophy
- The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
- Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
- Environmental Sex Determination in the Branchiopod Crustacean : Deep Conservation of a Gene in the Sex-Determining Pathway
- Systematic Detection of Polygenic Regulatory Evolution
- The SUMO Isopeptidase Ulp2p Is Required to Prevent Recombination-Induced Chromosome Segregation Lethality following DNA Replication Stress
- Uncoupling Antisense-Mediated Silencing and DNA Methylation in the Imprinted Cluster
- Role of the Drosophila Non-Visual ß-Arrestin Kurtz in Hedgehog Signalling
- Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti–dsDNA Autoantibody Production
- COMPASS-Like Complexes Mediate Histone H3 Lysine-4 Trimethylation to Control Floral Transition and Plant Development
- H3 Lysine 4 Is Acetylated at Active Gene Promoters and Is Regulated by H3 Lysine 4 Methylation
- Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches
- A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in
- Roles of () in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- A Molecular Phylogeny of Living Primates
- Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice
- A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání