-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Polycomb Targets Seek Closest Neighbours
article has not abstract
Published in the journal: . PLoS Genet 7(3): e32767. doi:10.1371/journal.pgen.1002031
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002031Summary
article has not abstract
Eukaryotic chromosomes occupy discrete territories with preferred positions within the cell nucleus, and establish extensive intra - and inter-chromosomal interactions. The mechanisms underlying chromatin interactions and their roles in gene activity and cellular function remain unclear. Nor is it clear to what extent individual loci are free to explore the entire nuclear space, or are constrained by their genomic context. At the local level, interactions between distant enhancer and promoter sequences, detected by 3C (chromosome conformation capture) technologies, have suggested a multi-step mechanism of gene regulation, involving protein binding to enhancer sequences followed by long-range chromatin contacts and activation of the target gene [1], [2]. Long-range interactions have also been described amongst distant, actively transcribed genes, which co-localise at transcription factories. However, long-range interactions are not only limited to events associated with gene activation, but also to those associated with gene repression, including for target genes of Polycomb group (PcG) proteins.
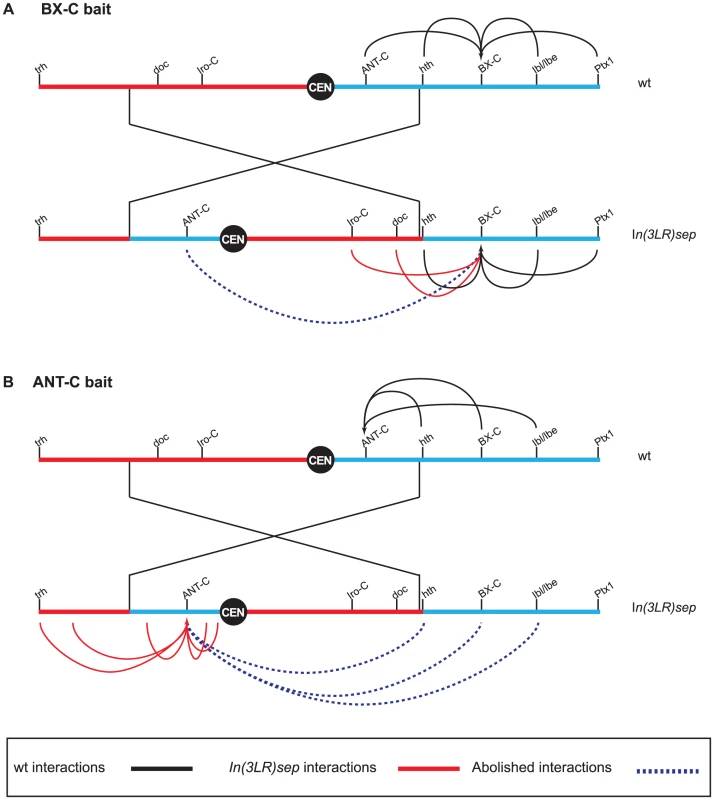
PcG proteins are involved in the stable repression of many key developmental genes in eukaryotes. In Drosophila melanogaster, they are concentrated in the nuclear space as discrete foci known as PcG bodies, which colocalise with stably repressed Homeotic genes [3]. Homeotic genes in D. melanogaster are organised into two gene complexes, separated by approximately 10 Mb on the same chromosome arm (Figure 1): the Antennapedia (ANT-C) complex specifies regions of the head and the anterior thorax, while the Bithorax (BX-C) complex is involved in the formation of the posterior thorax and the abdomen. Gene silencing of the BX-C in the anterior thorax requires long-range chromosomal interactions mediated by the two major Polycomb repressive complexes (PRC1 and PRC2), which bind to cis-regulatory elements known as Polycomb response elements (PREs) and modify histones [4]. Some PREs interact over large distances with their target promoters, establishing higher-order three-dimensional chromatin structures in the nucleus [5].
Despite the suggestion that PcG proteins are involved in long-range chromatin interactions, no systematic approach to address whether interactions among Polycomb domains represent a general phenomenon has been conducted. In this issue, Tolhuis et al. [6] describe an adapted Chromosome Conformation Capture on Chip (4C) assay to map genome-wide interactions of four established Polycomb domains in larval brain tissue. Due to the limitation of available cellular material, the authors introduced a linear amplification of 4C PCR products using a T7 RNA amplification procedure prior to hybridisation to a specialised microarray, covering 92% of the non-repetitive fly genome. The authors also developed a novel computational analysis of 4C data, which evaluates the statistical significance of interacting regions and identifies the exact boundaries of regions known as discrete interacting domains (DIDs). To eliminate chromatin interactions caused by linear rather than by spatial proximity, the data is fitted with a monotonously declining smoothing line, which reduces the number of interactions close to the bait without abolishing long-range interactions.
The specificity of the 4C assay was confirmed by the identification of previously reported interactions between the Homeotic gene clusters. Interestingly, the majority of DIDs coincide with Polycomb domains (defined from Polycomb and H3K27me3 maps) showing that Homeotic genes preferentially interact with other Polycomb domains despite being separated by mega-bases of intervening sequences. Moreover, preference for Polycomb domains is not limited to the Homeotic gene clusters, as complementary experiments with non-Homeotic PcG target genes revealed comparable findings suggesting that the majority of PcG target genes have a preference for Polycomb domains. A small subset of interactions did not coincide with Polycomb domains, raising the possibility that interactions may represent inactive regions of the genome coming together to form an inactive nuclear compartment. Comparisons with gene expression data suggest that interactions between Polycomb domains cannot simply be attributed to general interactions between transcriptionally inactive loci. However, as DIDs have low genomic resolution, with average sizes of 170 Kb and containing many genes, it is possible that any correlation with gene expression might be diluted if expression levels within each DID are confounded by active genes next to the PREs driving the interactions. Higher resolution analyses will help clarify this aspect of the interactions between Polycomb-regulated genes in Drosophila.
Most (95%) of the long-range chromatin interactions detected were confined to the chromosome arm containing the bait for the assay (intra-chromosomal interactions), although a few inter-chromosomal interactions were also observed. To decipher the mechanisms limiting interactions to a single chromosome arm, 4C experiments were repeated in a fly strain carrying a pericentric inversion for chromosome 3 (In(3LR)sep) that now places ANT-C on the opposite chromosome arm from BX-C (Figure 1). These studies provide a means to distinguish between two models for long-range interactions. First, long-range interactions may be driven by high affinity for specific DNA elements irrespective of their genomic distance. Second, they result mostly from topological constrains in the nuclear space, with local interactions amongst similarly regulated genes being favoured. The absence of interactions across the inversion breakpoints and the formation of new interactions between Polycomb domains located in the same chromosome arm suggest that the interactions are mostly constrained by overall chromosome architecture (Figure 1). The authors do not observe any correlative change in PcG gene regulation in mutant flies, which may be due to the redundancy of Polycomb domains.
Taken together, the work by Tolhuis and colleagues suggests that the nature of the interacting Polycomb domains is not important, but rather that the complement of all interactions may contribute to PcG-mediated gene silencing across the cell population. These conclusions are partly in disagreement with recent findings from Bantignies et al., who report that the disruption of long-range interactions between ANT-C and BX-C result in specific phenotypic perturbations [3]. However, the phenotypic changes were only observed in sensitized genetic backgrounds, suggesting that interactions with other Polycomb domains may functionally complement the loss of long-range interactions. Despite the suggestion that a compensatory network may exist, the observed phenotypic changes by Bantignies and colleagues demonstrate that the resulting spatial network does still not fully reflect the appropriate regulatory environment required for correct PcG-mediated silencing.
The findings from Tolhuis and colleagues are consistent with a substantial degree of genome flexibility and dynamics, which are constrained by overall chromosome topology. Together with the identification of infrequent inter-chromosomal interactions between repressed PcG targets, this work highlights a pressing question in the field regarding the functional significance of such low-frequency chromatin interactions. Are they simply a reflection of the variability of chromatin interactions across a cell population, due to the stochastic behaviour of gene expression and chromatin organisation? Alternatively, do these interactions echo epigenetic differences in cells, which are diluted in 3C-based technologies that study populations of cells? Analyses of interaction profiles within single cells would help assess the variability of chromatin conformations across the cell population, but are currently limited due to the relatively small number of sequence partners that can be investigated by fluorescence in situ hybridization. This would be important to fully understand the mechanisms that establish chromatin interactions, their dynamic behaviour and their roles in gene regulation.
Zdroje
1. HakimOSungMHHagerGL
2010
3D shortcuts to gene regulation.
Curr Opin Cell Biol
22
305
313
2. SextonTBantigniesFCavalliG
2009
Genomic interactions: chromatin loops and gene meeting points in
transcriptional regulation.
Semin Cell Dev Biol
20
849
855
3. BantigniesFRoureVCometILeblancBSchuettengruberB
2011
Polycomb-dependent regulatory contacts between distant Hox loci
in Drosophila.
Cell
144
214
226
4. SimonJAKingstonRE
2009
Mechanisms of polycomb gene silencing: knowns and
unknowns.
Nat Rev Mol Cell Biol
10
697
708
6. LanzuoloCRoureVDekkerJBantigniesFOrlandoV
2007
Polycomb response elements mediate the formation of chromosome
higher-order structures in the bithorax complex.
Nat Cell Biol
9
1167
1174
6. TolhuisBBlomMKerkhovenRPagieLTeunissenH
2011
Interactions among polycomb domains are guided by chromosome
architecture.
PLoS Genet
7
e1001343
doi:10.1371/journal.pgen.1001343
Štítky
Genetika Reprodukční medicína
Článek Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis inČlánek c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and PhosphodiesterasesČlánek Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- Testing for an Unusual Distribution of Rare Variants
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities!
- Initial Mutations Direct Alternative Pathways of Protein Evolution
- Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in
- Sensing of Replication Stress and Mec1 Activation Act through Two Independent Pathways Involving the 9-1-1 Complex and DNA Polymerase ε
- Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in
- Identification of a Novel Type of Spacer Element Required for Imprinting in Fission Yeast
- Chiasmata Promote Monopolar Attachment of Sister Chromatids and Their Co-Segregation toward the Proper Pole during Meiosis I
- Global Analysis of the Relationship between JIL-1 Kinase and Transcription
- H3K9me2/3 Binding of the MBT Domain Protein LIN-61 Is Essential for Vulva Development
- REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock
- A Novel Unstable Duplication Upstream of Predisposes to a Breed-Defining Skin Phenotype and a Periodic Fever Syndrome in Chinese Shar-Pei Dogs
- Polycomb Repressive Complex 2 Controls the Embryo-to-Seedling Phase Transition
- A Role for Set1/MLL-Related Components in Epigenetic Regulation of the Germ Line
- Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits
- A Genome-Wide Association Study of Upper Aerodigestive Tract Cancers Conducted within the INHANCE Consortium
- Ancestral Mutation in Telomerase Causes Defects in Repeat Addition Processivity and Manifests As Familial Pulmonary Fibrosis
- Ultra-Deep Sequencing of Mouse Mitochondrial DNA: Mutational Patterns and Their Origins
- Phenotype Restricted Genome-Wide Association Study Using a Gene-Centric Approach Identifies Three Low-Risk Neuroblastoma Susceptibility Loci
- The Toll-Like Receptor Gene Family Is Integrated into Human DNA Damage and p53 Networks
- Polycomb Targets Seek Closest Neighbours
- Widespread Hypomethylation Occurs Early and Synergizes with Gene Amplification during Esophageal Carcinogenesis
- c-di-GMP Turn-Over in Is Controlled by a Plethora of Diguanylate Cyclases and Phosphodiesterases
- Estimating Divergence Time and Ancestral Effective Population Size of Bornean and Sumatran Orangutan Subspecies Using a Coalescent Hidden Markov Model
- Rif1 Supports the Function of the CST Complex in Yeast Telomere Capping
- A Tradeoff Drives the Evolution of Reduced Metal Resistance in Natural Populations of Yeast
- Quantifying the Underestimation of Relative Risks from Genome-Wide Association Studies
- Population-Based Resequencing of Experimentally Evolved Populations Reveals the Genetic Basis of Body Size Variation in
- Triplet Repeat–Derived siRNAs Enhance RNA–Mediated Toxicity in a Drosophila Model for Myotonic Dystrophy
- The FUN30 Chromatin Remodeler, Fft3, Protects Centromeric and Subtelomeric Domains from Euchromatin Formation
- Viral Genome Segmentation Can Result from a Trade-Off between Genetic Content and Particle Stability
- Environmental Sex Determination in the Branchiopod Crustacean : Deep Conservation of a Gene in the Sex-Determining Pathway
- Systematic Detection of Polygenic Regulatory Evolution
- The SUMO Isopeptidase Ulp2p Is Required to Prevent Recombination-Induced Chromosome Segregation Lethality following DNA Replication Stress
- Uncoupling Antisense-Mediated Silencing and DNA Methylation in the Imprinted Cluster
- Role of the Drosophila Non-Visual ß-Arrestin Kurtz in Hedgehog Signalling
- Differential Genetic Associations for Systemic Lupus Erythematosus Based on Anti–dsDNA Autoantibody Production
- COMPASS-Like Complexes Mediate Histone H3 Lysine-4 Trimethylation to Control Floral Transition and Plant Development
- H3 Lysine 4 Is Acetylated at Active Gene Promoters and Is Regulated by H3 Lysine 4 Methylation
- Diverse Roles and Interactions of the SWI/SNF Chromatin Remodeling Complex Revealed Using Global Approaches
- A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in
- Roles of () in Oocyte Nuclear Architecture, Gametogenesis, Gonad Tumors, and Genome Stability in Zebrafish
- A Molecular Phylogeny of Living Primates
- Roles of the Espin Actin-Bundling Proteins in the Morphogenesis and Stabilization of Hair Cell Stereocilia Revealed in CBA/CaJ Congenic Jerker Mice
- A Cholinergic-Regulated Circuit Coordinates the Maintenance and Bi-Stable States of a Sensory-Motor Behavior during Male Copulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Whole-Exome Re-Sequencing in a Family Quartet Identifies Mutations As the Cause of a Novel Skeletal Dysplasia
- Origin-Dependent Inverted-Repeat Amplification: A Replication-Based Model for Generating Palindromic Amplicons
- FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration
- Limited dCTP Availability Accounts for Mitochondrial DNA Depletion in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání