-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
Embryo development involves formation of various cell types through the regulation of gene transcription, resulting in expression of cell type specific RNAs and proteins. A key regulatory step in transcription of animal genes involves the transition of RNA polymerase II (Pol II) into active elongation. At many genes, Pol II is transiently paused approximately 50 basepairs downstream of the transcription start site. Release from this promoter-proximal pausing involves the kinase P-TEFb, which phosphorylates negative elongation factors, allowing Pol II to enter into productive elongation. In this work, we have depleted a considerable amount of P-TEFb from early Drosophila embryos. We find that several genes with paused Pol II can be expressed relatively normally in P-TEFb depleted embryos, whereas expression of some non-paused genes is substantially reduced. This result suggests that also non-paused genes transit through a P-TEFb-dependent checkpoint before entering active elongation. Unexpectedly, we find less Pol II associated with these non-paused genes in P-TEFb embryos. We demonstrate that a protein complex involved in recruitment of Pol II to promoters, the Mediator complex, show the same morphological and gene expression phenotypes as P-TEFb. We propose that Mediator and P-TEFb function together in recruiting Pol II to a subset of developmental genes.
Published in the journal: . PLoS Genet 11(2): e32767. doi:10.1371/journal.pgen.1004971
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004971Summary
Embryo development involves formation of various cell types through the regulation of gene transcription, resulting in expression of cell type specific RNAs and proteins. A key regulatory step in transcription of animal genes involves the transition of RNA polymerase II (Pol II) into active elongation. At many genes, Pol II is transiently paused approximately 50 basepairs downstream of the transcription start site. Release from this promoter-proximal pausing involves the kinase P-TEFb, which phosphorylates negative elongation factors, allowing Pol II to enter into productive elongation. In this work, we have depleted a considerable amount of P-TEFb from early Drosophila embryos. We find that several genes with paused Pol II can be expressed relatively normally in P-TEFb depleted embryos, whereas expression of some non-paused genes is substantially reduced. This result suggests that also non-paused genes transit through a P-TEFb-dependent checkpoint before entering active elongation. Unexpectedly, we find less Pol II associated with these non-paused genes in P-TEFb embryos. We demonstrate that a protein complex involved in recruitment of Pol II to promoters, the Mediator complex, show the same morphological and gene expression phenotypes as P-TEFb. We propose that Mediator and P-TEFb function together in recruiting Pol II to a subset of developmental genes.
Introduction
RNA Polymerase II (Pol II) is responsible for the transcription of protein-coding genes in eukaryotes, and differs from all other RNA Polymerases in that its largest subunit contains a C-terminal domain (CTD) consisting of a heptapeptide repeat with the consensus YSPTSPS [reviewed in 1]. Phosphorylation of the CTD is linked to various steps in the transcription cycle. Hypophosphorylated Pol II is recruited to the transcription start site (TSS) and forms a pre-initiation complex. Phosphorylation of Ser5 in the heptapeptide repeat is coupled to initiation of transcription. The transition into transcription elongation involves CTD Ser2 phosphorylation. CTD phosphorylation also links transcription with RNA processing, since the CTD forms a platform for the assembly and action of enzymes involved in capping (Ser5 phosphorylated CTD), splicing and polyadenylation (Ser2 phosphorylated CTD) [reviewed in 2].
Genome-wide mapping of Pol II has shown that it pauses around 50 bp downstream of the TSS at a majority of genes in mammalian and Drosophila cells [3,4,5]. Thus, release from promoter-proximal pausing may be a rate-limiting step in the expression of thousands of genes [reviewed in 6]. Indeed, a majority of the genes involved in Drosophila embryo patterning are regulated at the level of release from pausing [7]. Paused Polymerase is Ser5 phosphorylated, and release from pausing is associated with Ser2 phosphorylation as well as phosphorylation of the elongation factors DRB sensitivity-inducing factor (DSIF) and negative elongation factor (NELF) [reviewed in 8,9].
Positive transcription elongation factor b (P-TEFb) was purified based on its activity in Drosophila nuclear extracts treated with the ATP analog 5,6-dichloro-1-ß-D-ribofuranosylbenzimidazole (DRB) [reviewed in 10]. P-TEFb consists of Cyclin-dependent kinase 9 (Cdk9) and its cyclin partner, Cyclin T (CycT). By phosphorylating the negative elongation factors DSIF and NELF, P-TEFb allows Pol II to enter into productive elongation. The P-TEFb inhibitor flavopiridol affects expression of virtually all genes in Drosophila and mouse cells [11,12], showing that release into elongation is a key regulatory event in metazoan gene expression. Active, non-paused genes are most sensitive to P-TEFb inhibition, indicating that they require P-TEFb continuously for rapid release into elongation [11].
A large fraction of P-TEFb is bound to the 7SK snRNP that sequesters the kinase in an inactive state [13]. Another fraction of P-TEFb is found in a protein complex known as the Super Elongation Complex (SEC) [reviewed in 14]. In Drosophila, the SEC contains the elongation factor ELL (eleven-nineteen lysine-rich leukemia), the ELL-associated factor Eaf, the ENL/AF9-like protein Ear, the AFF (AF4/FMR2 family)-like protein Lilliputian (Lilli), and P-TEFb [15]. The SEC appears to be particularly important for rapid induction of transcription [16]. The mechanism by which P-TEFb and the SEC are recruited to gene promoters is not understood, but recently the Mediator subunit MED26 has been suggested to be involved [17].
Embryo development in most insects involves cellularization, whereby the nuclei in a syncytium become enclosed by plasma membrane. In Drosophila melanogaster, thirteen mitotic divisions occur without cytokinesis [reviewed in 18]. Cellularization is controlled by the maternal to zygotic transition (MZT), where maternal transcripts are destabilized, and zygotic genome activation necessary for the event to initiate [reviewed in 19]. Four zygotically transcribed genes are critical, Serendipity-α (Sry-α) and bottleneck (bnk) are required for stability and organization of microfilaments [20], whereas nullo controls formation of endocytic vesicles needed for membrane trafficking [21], and slow-as-molasses (slam) localizes RhoGEF2 to the cleavage furrows that enclose the nuclei into cells [22]. Transcription of these genes requires the maternally provided Zinc-finger transcription factor Zelda [23], whereas the SEC component Lilli is necessary for Sry-α expression, but not for expression of bnk and nullo [24].
Recently, it was shown that cellularization genes and other genes transcribed in pre-cellular embryos do not show Pol II pausing, and are associated with distinct core promoter elements compared to genes activated at the major wave of the MZT [25]. Pre-cellular genes are generally short and rapidly transcribed, since the nuclear cycles are only between 8 and 13 min long at this stage and progression through mitosis causes abortion of nascent transcripts [26,27]. Consistent with their fast transcription, pre-cellular genes are shorter and more often intronless than genes activated at the MZT [25].
Previous work showed that P-TEFb depletion in Drosophila larvae results in decreased chromatin bound Ser2 phosphorylated Pol II [28], and in wing patterning defects [29]. Similarly, larval knockdown of the P-TEFb regulator dHEXIM (a component of the 7SK snRNP) results in severe leg and wing phenotypes [13]. However, the role of P-TEFb in embryogenesis is poorly understood. Here, we provide evidence for a fundamental role for P-TEFb during early development. Reduced P-TEFb levels do not grossly affect expression of most genes involved in embryo patterning, despite the presence of paused Pol II at many of these genes. Instead, expression of a subset of non-paused, rapidly transcribed genes in pre-cellular embryos is diminished by P-TEFb depletion. The same genes are affected by depleting the SEC and Mediator complexes, providing in vivo evidence that P-TEFb, the SEC and Mediator collaborate in the regulation of rapidly transcribed genes. Our results show that some non-paused genes are more reliant on P-TEFb than many paused genes in the early embryo.
Results
P-TEFb is required for early Drosophila embryo development
In order to study the role of P-TEFb in early Drosophila embryo development, we depleted its maternal contribution using short hairpin micro RNAs (shmiRNAs) expressed in the female germline [30]. These shmiRNAs are designed to effectively deplete maternal protein in ovaries [31]. We used α-Tubulin-Gal4-VP16 to drive UAS-shmiRNAs in the female germline, and collected embryos from mothers targeting P-TEFb (Cdk9 or CycT, hereafter referred to as Cdk9, CycT, or P-TEFb embryos) and embryos from control mothers expressing the α-Tubulin-Gal4-VP16 transgene alone. Cuticle preparations showed that P-TEFb embryos failed to hatch and produced variable patterning defects (Fig. 1A). We examined the extent of knock-down in 2–4 hour old embryos by RT-qPCR and by Western blotting (Fig. 1B-D). Cdk9 mRNA expression was reduced to 7% of controls in Cdk9 embryos, but was not affected in CycT embryos, whereas CycT mRNA levels were reduced to 56% of controls in CycT embryos, and increased in Cdk9 embryos (Fig. 1B). In wild-type embryos, multiple bands were detected with a CycT antibody, which may represent isoforms generated by alternative splicing as well as breakdown products (Fig. 1C). In CycT embryos, the 118 kDa CycT isoform was reduced to 27% of wild-type and the 94 kDa isoform to 65%. Interestingly, maternal Cdk9 knock-down also resulted in diminished CycT levels (24% and 71% of wild-type, Fig. 1C). This suggests that an interaction between Cdk9 and CycT stabilizes the CycT protein. Since we did not have access to a Cdk9 antibody, we generated a FLAG-tagged Cdk9 protein expressed from its endogenous promoter (Fig. 1F). We crossed this transgene into females expressing the Cdk9 shmiRNA in the germline, and into females expressing only the α-Tubulin-Gal4-VP16 driver as a control. A Western blot showed a strong reduction of FLAG-tagged Cdk9 protein in embryos from mothers with Cdk9 knock-down compared to the control (3% of control, Fig. 1D), consistent with the effect on Cdk9 mRNA levels (Fig. 1B).
Fig. 1. Maternal knockdown of P-TEFb disrupts embryo development. 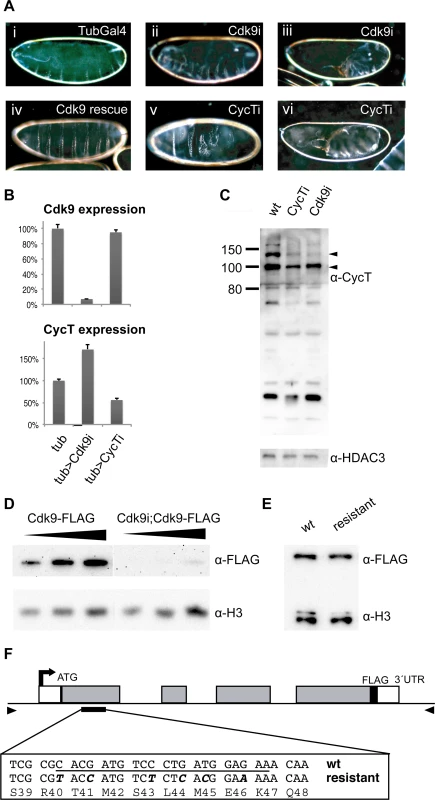
(A) Embryos were collected from females containing the maternal α-Tubulin-Gal4-VP16 (TubGal4) driver alone (i), TubGal4 and a shmiRNA targeting Cdk9 (ii, iii) or CycT (v, vi), or TubGal4, Cdk9 shmiRNA and an miRNA-resistant Cdk9 transgene (iv). Dark-field micrographs of embryo cuticle preparations show patterning defects. (B) Quantitative RT-PCR of Cdk9 and CycT expression relative four reference genes in 2–4 hour old maternally depleted embryos derived from females with TubGal4 (tub) crossed to wild-type (w1118, control), or to shmiRNAs targeting Cdk9 or CycT. Expression in control embryos was set to 100%. Error bars denote S.E.M. n = 6. (C) Western blot showing CycT levels in wild-type 2–4 h embryos and in embryos depleted of maternal CycT or Cdk9. HDAC3 was used as a loading control. Molecular weight markers are indicated to the left, and arrowheads point to the predicted 118 and 94 kDa CycT isoforms. (D) Western blot of FLAG epitope-tagged Cdk9 expressed from its endogenous promoter demonstrates reduced Cdk9 protein in Cdk9 miRNA depleted 2–4 h embryos. Histone H3 was used as a loading control. (E) Western blot of FLAG-tagged Cdk9 transgenes in adult flies with either the native DNA sequence or an miRNA resistant sequence shows comparable levels of protein. Histone H3 was used as a loading control. (F) A schematic drawing of the Cdk9 locus. The sequence targeted by the miRNA is underlined, and the nucleotide changes in the miRNA resistant transgene highlighted in bold and italic. The arrowheads indicate the positions of the primers used to amplify the genomic region used in the transgenes. To examine if the P-TEFb phenotype was due to off-target effects, we mutated the transgenic Cdk9 construct in the miRNA binding site to make a miRNA-resistant gene using synonymous codons already existing in other parts of the gene (Fig. 1F). A FLAG-tagged version of the miRNA-resistant transgene was integrated in the same genomic position as the FLAG-tagged wild-type transgene, and the expression levels compared in adult flies by Western blot (Fig. 1E). No difference in expression was seen, the wild-type transgene and the miRNA-resistant transgene produced a similar amount of protein. This shows that the synonymous codons do not impair translation of Cdk9 protein. However, the FLAG-tagged transgene failed to rescue the Cdk9 mutant phenotype. We therefore produced a non-tagged miRNA-resistant Cdk9 transgenic fly and crossed it into females expressing the Cdk9 shmiRNA in the germline, which rescued the embryonic phenotype and lethality (Fig. 1A and S1 Table). This shows that the embryonic phenotype was due to depletion of P-TEFb, and was not caused by off-target effects. It also demonstrates that the FLAG-tag interferes with Cdk9 function. Taken together, our results suggest that miRNAs targeting P-TEFb efficiently and specifically reduce P-TEFb protein levels and disrupt embryo development.
Maternal P-TEFb depletion results in loss of cells from the embryo posterior and in cellularization defects
Maternal knock-down of P-TEFb resulted in a posterior-specific loss of cells in embryos prior to gastrulation (Fig. 2). Differential interference contrast (DIC) microscopy showed embryos with normal morphology in the syncytial blastoderm stage (Fig. 2Aii and iii), with the phenotype appearing in cellularized embryos, where a substantial part of the embryo posterior was missing cells (Fig. 2Av and vi). Gastrulation occurred but was delayed, as the cephalic and ventral furrows were formed later than in wild-type. Depletion of Cdk9 or CycT resulted in undistinguishable phenotypes, strongly indicating that the two subunits of P-TEFb function together in the embryo.
Fig. 2. P-TEFb embryos show cellularization defects and lose cells in the embryo posterior. 
(A) Differential interference contrast (DIC) microscopy reveals a posterior loss of cells that appear prior to gastrulation in P-TEFb depleted embryos. Cellularizing P-TEFb embryos look normal (ii and iii), whereas cellularized embryos are missing cells in the posterior (v and vi). Compare to wild-type (wt) embryos in (i) and (iv). (B, C) Confocal images of wild-type (Bi, Ci, iii, v) and P-TEFb embryos immunostained with anti-phosphotyrosine (pTYR) antibody that marks the plasma membrane (green) and anti-Histone 4 marking the nuclei (red). Cells are lost from the posterior in P-TEFb embryos (Bii and iii). In addition, cell shape changes as well as multi-nucleated cells are evident in Cdk9 depleted embryos (Cii), the nuclei do not elongate as in wild-type (compare Ciii with iv), and some nuclei are present outside the basal side of cells in Cdk9 embryos (yellow arrowheads in C iv and vi). In cellularized P-TEFb embryos, mesodermal cells are not properly arranged (Bii, iii, and C vi). Embryos were generated as in Fig. 1, and are oriented with anterior to the left and dorsal side up in A and B. In order to follow when nuclei disappear from the posterior, we used live imaging with RFP-tagged histone H2Av. Time-lapse movies of Cdk9 depleted embryos showed that the nuclei migrated to the cortex in the entire embryo during division cycle 10 (S2 Movie), as in wild-type embryos (S1 Movie). The posterior phenotype originated from an abnormal loss of posterior nuclei from the cortex prior to germ-band extension. Even though no cells remained in the posterior after this migration, Cdk9-depleted embryos still underwent gastrulation. However, germ band extension did not proceed normally. Sporadic folds appeared and twisting of the embryo was seen in many cases (S2 Movie).
Wild-type and P-TEFb embryos were stained with a phosphotyrosine antibody, which labels the cell membrane, and with a histone H4 antibody to label the nuclei (Fig. 2B and C). In addition to missing cells in the posterior (Fig. 2B), defects in cell shape as well as multinucleated cells were found in P-TEFb embryos (Fig. 2C). Another feature of P-TEFb embryos was that nuclei and cells were not aligned laterally in the mesoderm, but were occasionally located on top of other cells. Nuclei could also be found outside of the basal cell membranes after cellularization (Fig 2C). This shows that cellullarization is perturbed in P-TEFb embryos.
Serendipity-α gene expression is controlled by P-TEFb and the Super Elongation Complex
To further explore the cellularization phenotype, we examined expression of genes involved in this process by RNA in situ hybridization. Knock-down of either Cdk9 or CycT resulted in a reduction in Serendipity-α (Sry-α) mRNA abundance (Fig. 3A). Since a component of the super elongation complex (SEC), Lilliputian (Lilli), has previously been shown to activate Sry-αexpression [24], depletion of the SEC shows similar gene expression changes as P-TEFb during early development. Therefore, we investigated if the posterior phenotype could also be seen in lilli mutant embryos. We generated embryos lacking maternal Lilli by inducing germ-line clones with the strong lilliXS575 allele [24]. Although this phenotype was not previously reported, we observed loss of posterior cells in lilli mutant embryos (Fig. 3B). The cell-depleted area was somewhat smaller in lilli than in P-TEFb depleted embryos (compare Fig. 3B with Fig. 2A). We next examined if another component of the SEC complex showed the same phenotype. We used a shmiRNA targeting the elongation factor dELL [32] to knock-down maternal dELL protein. Offspring derived from these mothers also showed loss of cells from the embryo posterior (Fig. 3B).
Fig. 3. P-TEFb and the Super Elongation Complex (SEC) display similar phenotypes. 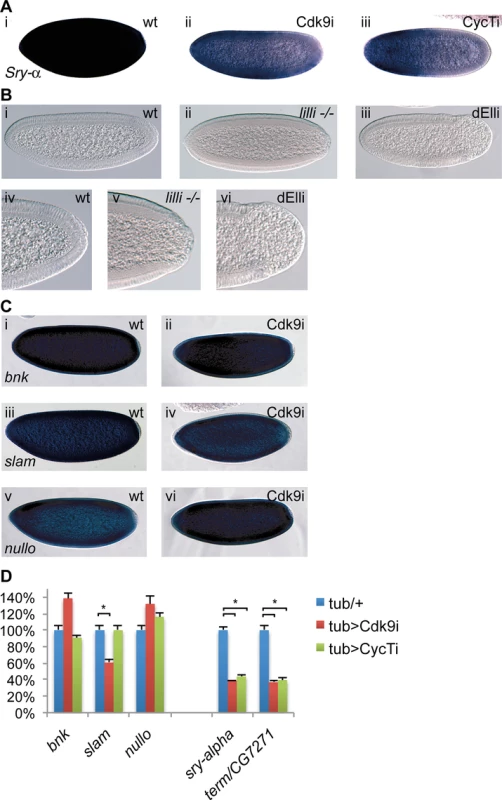
(A) RNA in situ hybridization using a digoxigenin-labeled Serendipity-α (Sry-α) probe demonstrates decreased levels of Sry-αmRNA in embryos depleted of maternal Cdk9 (ii) or Cyclin T (iii) compared to wild-type (i). (B) DIC micrographs of wild-type (wt, i), or embryos derived from females with germline clones of the SEC component dAFF4/Lilliputian (lilli -/-, ii) or females expressing TubGal4 and a shmiRNA targeting the SEC subunit dEll (dELLi, iii) show the same posterior phenotype as in P-TEFb embryos. A close up of the same embryos is shown below (iv-vi). (C) Expression of the cellularization genes bottleneck (bnk, i, ii), slow-as-molasses (slam, iii, iv), and nullo (v, vi) is comparable between wild-type and embryos depleted of maternal Cdk9. (D) Quantification of cellularization gene expression by RT-qPCR. Columns show average values in 2–4h embryos with S.E.M. (n = 5–6) and control values were set to 100%. Relative expression was normalized to a mean of four reference genes (beta-tubulin, GAPDH, RpL32, and 28SrRNA). *indicates P<0.05, two-tailed paired Student’s t-test. To find more genes that are sensitive to P-TEFb or SEC depletion, we examined a subset of genes that have a very rapid induction during the maternal to zygotic transition (MZT), since the SEC is known to be involved in rapid induction of genes [16]. We manually selected a set of genes that peak in zygotic expression during 2–4 h of development, but had low or no transcript levels in 0–2 hour old embryos (none or little maternal transcript, Flybase data [33]). Several genes involved in cellularization were found, including Sry-α, bnk, slam and nullo. We investigated the expression of these genes in Cdk9 depleted embryos by in situ hybridization, but did not observe reduced mRNA levels of bnk, slam, or nullo, suggesting that they are activated by a mechanism not sensitive to reduced P-TEFb levels (Fig. 3C). Similarly, embryos derived from lilli germline clones were previously demonstrated to downregulate Sry-α, but showed no change in the protein levels of Nullo and Bnk [24]. We also used quantitative RT-PCR to measure the mRNA levels of these cellularization genes in Cdk9 and CycT embryos, which largely confirmed the results obtained by in situ hybridization (Fig. 3D). We observed small changes in bnk, slam and nullo expression in Cdk9 and CycT embryos, although slam expression was reduced in Cdk9 but not in CycT embryos (Fig. 3D). By contrast, Sry-α expression was reduced by both Cdk9 and CycT knock-down (Fig. 3D), as was also observed by in situ hybridization (Fig. 3A). This shows that reducing the levels of P-TEFb and the SEC is of little consequence for the expression of several genes in the early embryo and selectively affects gene expression.
Terminus is a P-TEFb-sensitive gene that is essential for embryo development
Among other genes having a rapid zygotic expression, we focused on the gene terminus. Its mRNA is not maternally provided, but the gene is activated at high levels throughout the embryo during the MZT, with the highest expression in the most posterior part of the embryo (Fig. 4A and [34]). Expression then rapidly decreases in most cells until the mRNA is only localized to the posterior pole, just prior to germ band extension (Fig. 4A and [34]). A BLAST search showed that the coding DNA sequence of term is 99.1% homologous to the gene CG7271. Since CG7271 is located next to the term locus in a head to head orientation, it is likely the result of a recent gene duplication. Given the high sequence similarity between CG7271 and term, we did not expect the term probe to distinguish between the two gene products. We therefore refer to the pattern detected by this probe as term/CG7271 (Fig. 4A). In Cdk9 knock-down embryos, we observed a strong reduction in term/CG7271 expression (Fig. 4B). This phenotype could be rescued with the miRNA-resistant Cdk9 transgene (Fig. 4B). A similar effect on term/CG7271 expression was observed in CycT and ELL embryos, as well as in lilli germline clone embryos (Fig. 4B). We also measured term/CG7271 expression in P-TEFb embryos by RT-qPCR, and detected reduced term/CG7271 levels in both Cdk9 and CycT knock-down embryos (Fig. 3D).
Fig. 4. The P-TEFb and SEC-regulated gene terminus (term) is essential in early embryos. 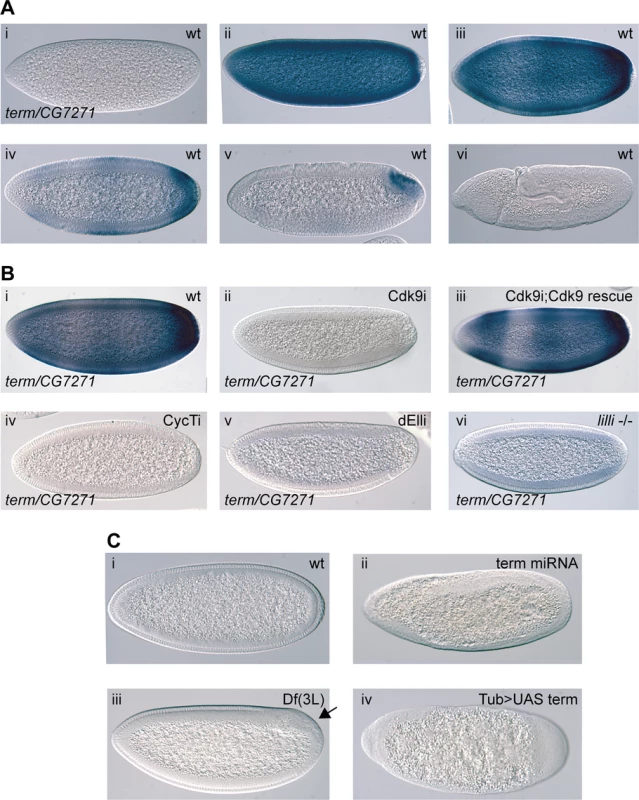
(A) In situ hybridization showing term/CG7271 expression in wild-type during different embryo stages (i-vi). The transcripts become concentrated to the posterior in cellularized embryos (iv and v) (B) Expression of term/CG7271 is severely reduced in embryos depleted of maternal Cdk9 (ii) compared to wild-type (i), and rescued by the miRNA-resistant transgene in Cdk9 embryos (iii). Greatly diminished term/CG7271 levels are also observed in embryos depleted of maternal CycT (iv) or dEll (v), and in embryos from lilli germline clones (vi). (C) Knockdown of zygotic term by crossing TubGal4 females with Term shmiRNA males (ii), eliminating Term and CG7271 by deletion in embryos derived from the deficiency Df(3L)BSC416 (iii), or over-expressing Term by crossing TubGal4 females with UAS-term males (iv) results in severe morphological defects in early embryos, including a failure to form a cellular blastoderm. A wild-type cellularizing embryo (i) is shown for comparison. To test if Term is required for embryo development, we crossed males containing a shmiRNA transgene targeting term with females providing maternal Gal4-VP16 to the early embryo, thereby knocking down zygotic Term expression. This shmiRNA targets a part of the 5’ UTR that is unique to term. Cuticle preparations from Term depleted embryos showed many embryos without any cuticle, suggesting an early arrest in development. Fixed embryos were observed with DIC microscopy, which revealed a variety of defects, including a failure to form a cellular blastoderm (Fig. 4C). In embryos homozygous for a deficiency that removes both term and CG7271 as well as several other genes, we observed morphological defects to a variable extent that include a failure to form cells in part of the embryo (arrow in Fig. 4C iii). These phenotypes are not as severe as Term knock-down, indicating both on-target and off-target effects of the shmiRNA. However, the phenotypes of this deficiency are consistent with a role for term/CG7271 in embryo development. To further analyze the function of Term, we overexpressed HA and FLAG-tagged Term ubiquitously in early embryos with maternally provided Gal4-VP16. This resulted in severe morphological defects already at the blastoderm stage (Fig. 4C iv). The term loss of function and gain of function morphological phenotypes appear at an earlier stage than those of P-TEFb and SEC, but support a function for Term in embryo development. Although these results do not fully explain the P-TEFb phenotypes, they indicate that changes to expression of Term in combination with other gene expression differences may mediate some of the P-TEFb and SEC morphological defects.
Patterning genes are expressed in P-TEFb knock-down embryos
Many genes involved in embryo patterning are regulated by release from Pol II promoter-proximal pausing [7]. Interestingly, posterior group genes as well as genes required for development of pole cells and the embryonic termini were expressed relatively normally, despite the loss of cells from the posterior in Cdk9 embryos. As shown in Fig. 5A-D, nanos (nos) and polar granule component (pgc) mRNA is present at the posterior of pre-cellular Cdk9 knock-down embryos, indicating that the subsequent loss of cells from this part of the embryo is not due to lack of pole plasm. Similarly, tailless (tll) mRNA was present in the embryo posterior in cellularizing Cdk9 depleted embryos, although cells were beginning to disappear at this stage (Fig. 5E and F).
Fig. 5. The expression of many patterning genes is relatively normal in P-TEFb embryos. 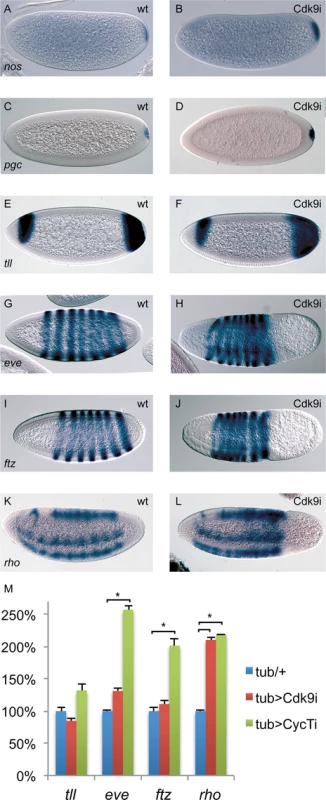
Wild-type embryos (A, C, E, G, I, K) and embryos depleted of maternal Cdk9 (B, D, F, H, J, L) were hybridized with probes detecting nanos (nos), polar granule component (pgc), tailless (tll), even-skipped (eve), fushi-tarazu (ftz), and rhomboid (rho) RNA. Relatively normal expression patterns were observed in Cdk9 embryos. (M) Expression of tll, eve, ftz, and rho in 2–4h P-TEFb embryos quantified by RT-qPCR (n = 4–6). Relative expression was normalized to a mean of four reference genes as described in Fig. 3. *indicates P<0.05, two-tailed paired Student’s t-test. We also examined some well-studied zygotically expressed developmental genes involved in anterior-posterior and dorsal-ventral patterning. Expression of even-skipped (eve) and fushi-tarazu (ftz) occur in a 7-striped pattern in alternate parasegments in wild-type embryos (Fig. 5G and I). Although compressed by the absence of cells in the posterior, 7 stripes of eve and ftz mRNA were present in Cdk9 embryos (Fig. 5H and J). We further examined expression of rhomboid (rho), which is present in two lateral stripes where it is activated by the Dorsal protein morphogen, as well as in dorsal parts of the embryo where it is expressed in response to Dpp signaling (Fig. 5K). Both the Dorsal-dependent and Dpp-dependent parts of the rho expression pattern were observed in Cdk9 embryos (Fig. 5L). Using RT-qPCR, we confirmed that expression of the patterning genes tll, eve, ftz, and rho were not diminished by Cdk9 or CycT knockdown (Fig. 5M). Instead, expression of eve, ftz, and rho were up-regulated in CycT embryos, whereas only rho expression was elevated in Cdk9 embryos (Fig. 5M). Up-regulation of eve and ftz in CycT, but not in Cdk9 embryos, indicates that CycT knock-down affects these genes independently of P-TEFb. Increased rho expression could be an indirect effect due to altered expression of rho regulators, or could be due to a negative function for P-TEFb at this gene. Since pausing promotes a nucleosome-free promoter region [35], one possibility is that P-TEFb depletion causes stronger pausing at rho that leads to a more open chromatin environment and elevated transcription. Still, the in situ hybridization and RT-qPCR results together suggest that expression of most genes involved in early embryo patterning is not grossly altered by reduced P-TEFb amounts.
Diminished Pol II CTD Ser2 levels in P-TEFb knock-down embryos does not explain the gene expression phenotypes
P-TEFb phosphorylates elongation factors and the Pol II CTD on Ser2 in order to release the polymerase into active elongation. We immunostained embryos to examine the localization of Ser2 phosphorylated Pol II. During the first nuclear division stages, a Pol II Ser2 signal was detected in the cytoplasm, presumably coming from polymerases not bound to chromatin (Fig. 6A). Interestingly, this signal showed an increase in Cdk9 embryos compared to wild-type. In later stage, cellularizing embryos, nuclear Pol II Ser2 was detected in all somatic cells, but not in the transcriptionally silent pole cells (Fig. 6A). Compared to wild-type embryos, reduced levels of Ser2 phosphorylated Pol II was detected in Cdk9 embryos at this stage (Fig. 6A). Quantification of the embryo stainings demonstrated that 59 +/ - 1% of Ser2 phosphorylation remained in Cdk9 embryos. We further prepared extracts from embryos depleted of Cdk9 or Cyclin T and investigated global Pol II CTD Ser2 phosphorylation levels by Western blot. Embryo extracts from either Cdk9 or CycT knock-down cellularizing embryos showed lower levels of Pol II Ser2 phosphorylation (Fig. 6B), compared to the hypophosphorylated Pol IIa form of the CTD (Fig. 6B). The same results were obtained with a different Pol II Ser2 antibody (S1 Fig.). In these experiments, 39% and 43% of the phospho-Ser2 per CTD signal remained in Cdk9 and CycT embryos, respectively (Fig. 6B). Interestingly, despite different levels of Cdk9 and CycT depletion (Fig. 1), we find similar levels of Ser2 phosphorylation in Cdk9 and CycT embryos (Fig. 6B). One possibility is that the active pool of P-TEFb is depleted to the same extent in Cdk9 and CycT embryos, despite different knock-down efficiencies. Another possibility is that the activities of other Ser 2 kinases are affected by either Cdk9 or CycT depletion to the same degree. Regardless, our data show that P-TEFb knock-down during oogenesis did not reduce Pol II Ser2 phosphorylation at this stage. Instead, increased Pol II Ser 2 phosphorylation levels were found in early embryos prior to zygotic transcription. However, during cellularization, reduced amounts of Ser2 phosphorylated Pol II accumulated in the somatic nuclei of P-TEFb depleted embryos.
Fig. 6. Pol II occupancy is reduced at genes affected by P-TEFb depletion. 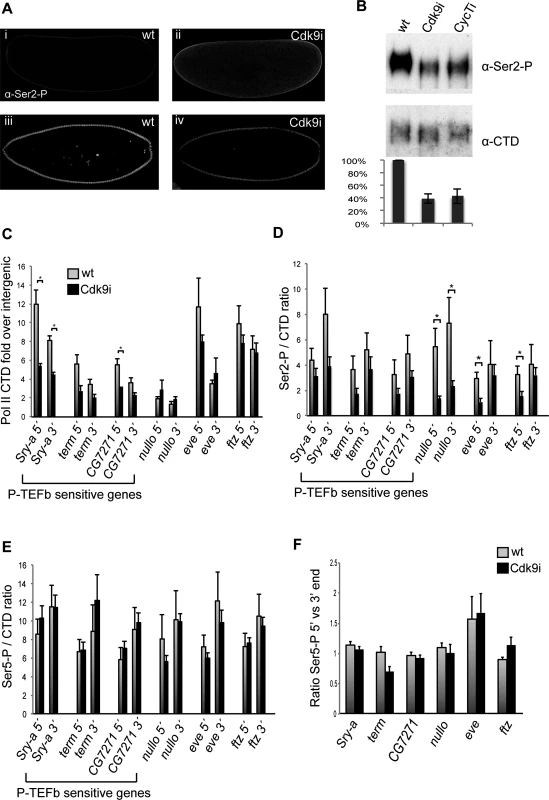
(A) Confocal images of wild-type embryos (i, iii) and embryos depleted of maternal Cdk9 (ii, iv) stained with an antibody recognizing phosphorylated Pol II CTD Ser2 (Ser2-P, Abcam ab5095). In pre-cellular embryos, an elevated Ser2-P signal was observed in the cytoplasm in Cdk9 embryos (ii) compared to wild-type (i), indicating that the maternal contribution of Ser2 phosphorylated Pol II was increased in Cdk9 embryos. In cellularizing embryos, less Ser2-P was detected in nuclei of Cdk9 embryos (iv) compared to wild-type nuclei (iii). (B) Western blot with extracts from 0–5h old embryos show a decrease in Ser2-P in embryos depleted of maternal Cdk9 or CycT. The monoclonal antibody 8WG16 recognizing the Pol II CTD was used as a loading control. The ratio of Ser2-P to CTD signal was quantified from 3 biological replicates. (C-F) Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) of 2–4h wild-type or Cdk9 embryo extracts using antibodies recognizing the Pol II CTD, Pol II Ser2 phosphorylation, and Pol II Ser5 phosphorylation. (C) Pol II occupancy plotted as CTD enrichment relative the intergenic locus IG2c. Less Pol II associates with Sry-α and CG7271 in Cdk9 embryos. (D) Less Ser2-P per CTD was observed in Cdk9 embryos compared to wild-type. (E) The Ser5-P/CTD ratio was comparable in wild-type and Cdk9 embryos. (F) The ratio of Pol II Ser-5 signal at the 5’ end versus the 3’ end. No increase at the 5’ end was detected in Cdk9 embryos. Error bars show standard error of the mean (n = 3–5). * indicates P<0.05, two-tailed unpaired Student´s t-test (calculations in S4 Table). Next, we investigated the status of RNA Pol II at P-TEFb regulated genes by chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR). We used antibodies against the Pol II CTD, as well as phosphorylated forms of the Pol II CTD, in extracts from 2–4h old embryos. We designed primers against the P-TEFb regulated genes Sry-α, term and CG7271, against nullo, eve, and ftz that are unchanged in Cdk9 embryos, as well as against an intergenic control locus (IG2c). We plotted CTD occupancy as fold enrichment over the intergenic region (IG2c), and observed less Pol II occupancy in Cdk9 embryos at Sry-α and CG7271, whereas no significant difference was detected at genes unaffected by Cdk9 depletion (Fig. 6C, percent input values are shown in S4 Table and in S2 Fig.). This indicates that recruitment or stability of Pol II at some genes is impaired in Cdk9 embryos.
We thereafter investigated the phosphorylation status of the polymerases associated with these genes, and normalized the amount of phosphorylation against total CTD signal. The level of Ser2 phosphorylated Pol II was decreased at all loci in Cdk9 embryos, although statistically significant differences were only obtained at insensitive genes (Fig. 6D). The result is consistent with the reduced global levels of Ser2 phosphorylation observed in Cdk9 embryos at this stage (Fig. 6A and B), but does not explain why expression of some genes were affected and others not. Ser5 phosphorylated Pol II occupancy was comparable between wild-type and Cdk9 embryos (Fig. 6E). Consistent with this finding, global Ser5 phosphorylation was unaffected in P-TEFb embryos (S3 Fig.). To measure pausing, we plotted the occupancy of Ser5 phosphorylated Pol II at the transcription start site (TSS) versus the 3’ end of the genes (Fig. 6F). Among the tested genes, we could only detect pausing for the eve gene, consistent with its high pausing index calculated from GRO-seq data [7]. Surprisingly, the ratio of Ser5 phosphorylated Pol II at the 5’ versus the 3’ end did not change significantly in Cdk9 embryos for any of these genes (Fig. 6F), indicating that the gene expression changes observed were not caused by increased promoter-proximal pausing. Similar results were obtained with the CTD antibody (S2 Fig.).
Taken together, our results demonstrate reduced Pol II Ser2 phosphorylation levels in cellularizing Cdk9 embryos, but this cannot explain the selective gene expression changes that occur in these embryos. Instead, Pol II occupancy is reduced at genes whose expression is diminished.
Mediator complex subunits phenocopy P-TEFb in early embryos
How P-TEFb is targeted to genes is not well understood, but recent results have suggested that the Mediator subunit MED26 could be involved [17]. We therefore used shmiRNAs targeting 26 individual Mediator subunits to deplete their maternal contribution. Although MED26 knock-down did not produce an embryonic phenotype, several other Mediator subunits did (S2 Table). Both MED20 and MED22 embryos showed loss of cells from the posterior (Fig. 7A). Further analysis of MED22 embryos showed a phenotype that is virtually identical to P-TEFb embryos, including diminished Sry-α and term/CG7271 expression, but no reduction in slam expression (Fig. 7B). These results provide in vivo evidence that P-TEFb and the Mediator complex cooperate in the regulation of gene expression.
Fig. 7. The Mediator subunits MED20 and MED22 phenocopy P-TEFb in early embryos. 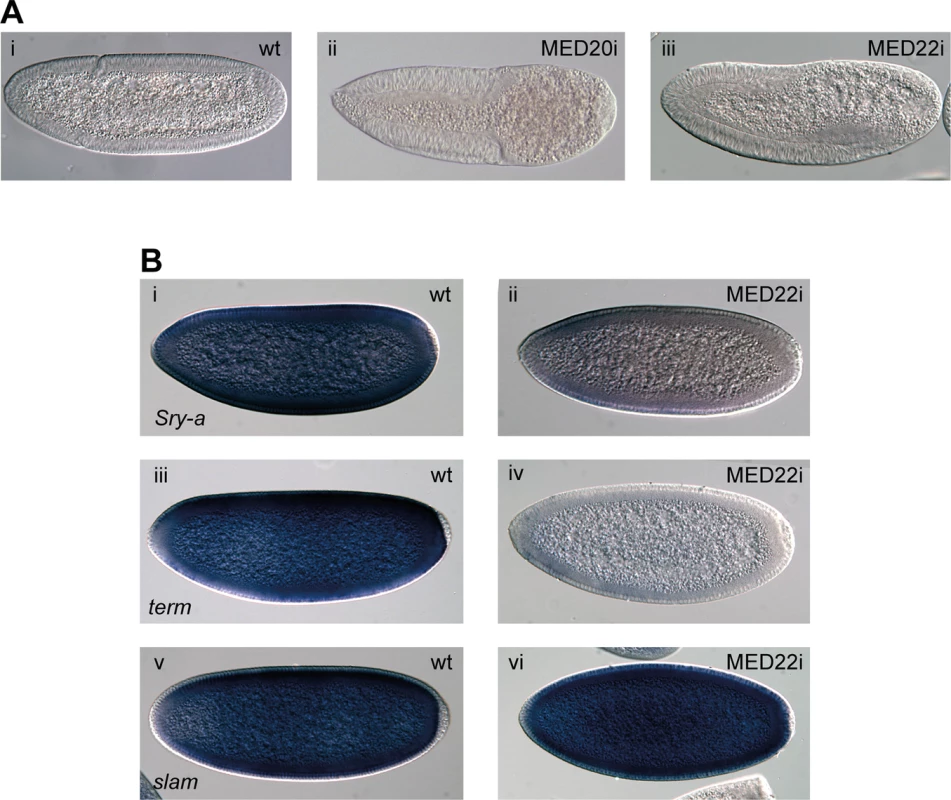
(A) DIC microscopy of cellularized wild-type (i) and maternally depleted MED20 (ii) or MED22 (iii) embryos show lack of cells in the posterior upon Mediator knock-down. (B) In situ hybridization shows that expression of Sry-α (ii) and term (iv) is compromised, whereas slam (vi) expression is unaffected in embryos depleted of maternal MED22. Wild-type embryos (i, iii, v) are shown for comparison. Discussion
A subset of non-paused, pre-cellular genes are most sensitive to P-TEFb depletion
The established function of P-TEFb is to phosphorylate the RNA Pol II CTD as well as the elongation factors DSIF and NELF, allowing Pol II to enter into productive elongation [reviewed in 10]. Here, we demonstrate that embryos from which a substantial amount of the P-TEFb maternal load has been reduced show specific gene expression and morphological phenotypes. We find that some non-paused genes are more sensitive to diminished P-TEFb levels than many paused genes, consistent with recent P-TEFb inhibitor studies [11]. This provides in vivo evidence that also non-paused genes transit through a P-TEFb-dependent checkpoint.
P-TEFb inhibition or knock-down leads to a global decrease in Ser2 phosphorylation [28,36]. In cellularizing Drosophila embryos, we see a similar reduction in Ser2 phosphorylation (Fig. 6). However, the global effect on Ser2 phosphorylation does not explain the selective gene expression changes. High transcription does not explain the sensitivity to P-TEFb depletion either, since term and CG7271 are expressed at similar levels to slam and bnk (S4 Fig. and [7]). Instead, the P-TEFb down-regulated genes that we have identified are non-paused, which would suggest that they require P-TEFb continuously for efficient release into elongation. However, the state of pausing is not the only determinant for sensitivity to P-TEFb depletion since bnk, slam and nullo are also non-paused, rapidly and highly induced in pre-cellular embryos and regulated by the transcription factor Zelda, but not affected by P-TEFb knock-down. Moreover, it is possible that among the many paused genes in the early embryo, some that are also down-regulated by P-TEFb depletion could be detected by further investigation. Moreover, although both rho and eve are highly paused genes in the embryo [7], expression of rho, but not eve, is increased upon Cdk9 knock-down. Therefore, as yet unidentified features of P-TEFb-regulated genes confer sensitivity to reduced P-TEFb levels.
Surprisingly, the ratio of Pol II at the promoter versus the 3’ end at P-TEFb down-regulated genes does not change in Cdk9 embryos (Fig. 6F). Since no difference in Pol II distribution along the tested genes could be noted, Pol II appears to be released into elongation to the same extent in P-TEFb and wild-type embryos. Rather, it appears that compared to unaffected genes, less Pol II associates with genes whose expression is reduced in P-TEFb embryos (Fig. 6C). Thus, lowered Pol II occupancy may explain diminished transcription of some genes in P-TEFb embryos. Consistent with this idea, analysis of global run-on sequencing (Gro-seq) data suggests that the Sry-α and CG7271 genes are regulated at the Pol II recruitment step, and not by release from pausing in early embryos [7]. Furthermore, Pol II ChIP-seq has shown that none of the P-TEFb down-regulated genes ever display pausing during development [25].
Why is there less Pol II associated with some genes in P-TEFb embryos? It is possible that P-TEFb regulates expression of transcription factors during oogenesis that are needed for Pol II recruitment to P-TEFb-regulated genes. One such candidate is Zelda, which is required for zygotic genome activation [23]. Since zelda embryos show phenotypes similar to P-TEFb and also regulates the P-TEFb-sensitive genes Sry-α, term, and CG7271 [23], we tested if maternal transcript levels of zelda are affected in P-TEFb embryos. In situ hybridization of P-TEFb depleted embryos showed that zelda mRNA levels are comparable to wild type embryos (S5 Fig.), suggesting that P-TEFb is not controlling maternal expression of zelda. P-TEFb might control transcription of other maternal factors that play a role in Pol II recruitment to P-TEFb-sensitive genes. Another possibility is that P-TEFb has a more direct function in recruiting Pol II to a specific set of promoters. This alternate function of P-TEFb and SEC could be evolutionarily conserved, since knocking down the SEC component ELL2 in mouse embryonic stem cells affects Pol II occupancy at the non-paused Cyp26a1 gene [16]. A recent study showed that whereas a majority of genes in mouse embryonic stem cells accumulate Pol II at the promoter after P-TEFb inhibition, around 20% showed a decrease in Pol II promoter occupancy [11]. Yet one more possibility is that cross talk between pausing and initiation explains why these genes and P-TEFb down-regulated genes in the Drosophila embryo have reduced Pol II occupancy. Inhibiting release into elongation may feed back on transcription initiation and result in decreased Pol II levels.
P-TEFb, SEC and Mediator share phenotypes in the Drosophila embryo
A fraction of P-TEFb is present in the Super Elongation Complex (SEC). The SEC components ELL and Lilliputian (dAFF4) have previously been shown to be required for embryo development and segmentation [24,37]. We demonstrate that P-TEFb and these SEC components display similar morphological and gene expression phenotypes in early embryos (Figs. 3 and 4), providing in vivo evidence that P-TEFb function is mediated at least in part as a component of the SEC. However, lilli mutant embryos are different in some respects from P-TEFb knock-down embryos. Expression of some genes, including ftz, is reduced in lilli mutant embryos [24], but not by P-TEFb depletion (Fig. 5). This could be because this lilli allele is a stronger loss of function mutation that reduces Lilli protein levels more than P-TEFb is reduced by the shmiRNAs. Alternatively, this SEC component has functions that do not require P-TEFb. Importantly, many other chromatin and transcriptional regulators that we knocked-down using shmiRNAs did not share embryonic phenotypes with P-TEFb and SEC components, demonstrating the specificity of the phenotype and allowing for the identification of factors that contribute to P-TEFb and SEC function in the embryo.
The Mediator complex was purified based on its ability to mediate activated transcription by bridging upstream transcription factors with Pol II, but additional functions for Mediator have emerged recently [reviewed in 38]. The Mediator subunit MED26 interacts with Eaf, a member of the SEC, and recruits elongation factors to promoters in mammalian cells [17]. It has also been shown that mammalian MED23 can recruit P-TEFb by interacting with Cdk9 [39], and that HIF1a can recruit the SEC via the CDK8 Mediator subunit in response to hypoxia [40]. We find that depletion of several Drosophila Mediator subunits phenocopy P-TEFb embryos and result in identical gene expression changes (Fig. 7). Our results are consistent with a Mediator-SEC interaction that is important for gene transcription in vivo, and indicate that Mediator and SEC function together not only to control elongation, but also in recruiting Pol II to some developmental genes.
P-TEFb and germ cells
In many organisms, germ cells are specified early during embryo development. In order to prevent these cells from differentiating into somatic cells, mRNA expression is transiently, but globally, repressed [reviewed in 41]. A common strategy to specifically prevent Pol II transcription has evolved that involves inhibition of Ser2 phosphorylation [41]. In Drosophila, polar granule component (pgc) is the germ plasm factor that represses Pol II transcription and Ser2 phoshorylation in the pole cells [42,43], by preventing P-TEFb from associating with chromatin [42]. We find that pgc is expressed and that pole cells are generated in P-TEFb embryos (Fig. 5), despite loss of cells from the embryo posterior at later stages (Fig. 2).
In C. elegans, the PIE-1 protein binds to CycT and prevents Ser2 phosphorylation in the germline blastomeres [44,45,46]. In contrast to somatic cells, loss of Cdk9 from mature germ cells has little effect on Ser2 phosphorylation, whereas Cdk12 loss abolishes Ser2 phosphorylation [47]. Interestingly, Cdk12 and Ser2 phosphorylation are not required for C. elegans germline development and function, whereas Cdk9 is essential [47]. Thus, P-TEFb has substrates other than the Pol II CTD that are needed for C. elegans germline function.
Interestingly, we detect elevated levels of Pol II Ser 2 phosphorylation in pre-cellular P-TEFb embryos (Fig. 6), indicating that P-TEFb is not responsible for Ser2 phosphorylation in the Drosophila female germline. We used shmiRNAs targeting Cdk12 and CycK in the germline, but these females failed to produce eggs, demonstrating that Cdk12 is required for oogenesis. Thus, there are both similarities and differences between the C. elegans and Drosophila germline, but in both organisms P-TEFb appears to function differently in germ cells and somatic cells.
Terminus is essential for embryo development
Staining of nuclei and the plasma membrane in P-TEFb embryos demonstrated cellularization defects, including multinucleated cells, and showed that cells are also lost from the posterior after cellularization (Fig. 2). Damaged nuclei or nuclei with cell cycle defects can trigger a similar phenotype, nuclear fallout, thereby preventing them from becoming somatic nuclei [48]. It is possible that P-TEFb depletion causes cell cycle perturbations that result in the observed phenotype, although no obvious chromosome segregation defects were detected in the embryo posterior. Another possibility is that gene expression is perturbed in the embryo posterior by P-TEFb depletion, causing the nuclei to detach from the cortex.
A rather small number of zygotically transcribed genes are known to control cellularization. We have identified an additional Zelda, SEC, and P-TEFb regulated gene that could be involved in this process. The gene terminus (term) was identified based on its blastoderm-specific expression [34]. Expression becomes restricted to the posterior in cellularized embryos, at the same time as we observe cell loss in the embryo posterior in P-TEFb embryos. We discovered that shmiRNA knockdown of Term or deletion of a large genomic region that includes Term is lethal to embryos and results in morphological defects, including a failure to form a cellular blastoderm (Fig. 4). Over-expression of Term similarly causes morphological deformations of early embryos (Fig. 4). These results show that Term is essential for early development, and indicate that Term may play a role in cellularization. However, the term phenotypes are different from the lack of cells observed in the posterior of cellularized P-TEFb embryos. We therefore favor the idea that the P-TEFb phenotype is caused by multiple gene expression changes.
The molecular function of Term is unexplored. It encodes a 428 amino acid (aa) protein with a single C2H2-type zinc-finger. Term is closely related to CG7271, 423 out of the 428 aa are identical. Term also shows 28% aa homology to the CG6885 gene product, in which the zinc-finger is also conserved. These three genes have very similar gene expression profiles with transcription restricted to early zygotic activation (Flybase RNA-seq data, [33]). However, none of these genes are conserved outside the Drosophila genus, suggesting that species within this clade have adopted them to perform an essential early embryonic function.
P-TEFb depleted embryos express promoter-proximal paused patterning genes
A large fraction of the genes involved in early embryo patterning, both the ones controlling anterior-posterior development and those involved in dorsal-ventral patterning, contain a promoter-proximal paused Pol II. Recent Gro-seq experiments have indicated that the majority of these are regulated by release from pausing [7]. Given the function of P-TEFb in releasing Pol II from pausing into active elongation, it could be expected that these paused genes would be susceptible to reduced P-TEFb amounts. Indeed, mitotic Cdk9 clones in imaginal discs demonstrated various patterning defects and reduced expression of Hox genes that contain paused Pol II [29]. However, inhibiting P-TEFb activity with flavopiridol showed that highly paused genes are less susceptible to P-TEFb inhibition than non-paused genes [11], indicating that they experience release from pausing less frequently than non-paused genes. We found that the majority of segmentation and dorsal-ventral genes are expressed in a relatively normal embryonic pattern, despite the morphological changes and loss of cells from P-TEFb knock-down embryos. Instead, we found that some non-paused genes are most sensitive to reduced P-TEFb levels.
Our results are consistent with a model for metazoan gene transcription where all genes require P-TEFb-mediated escape from pausing, and where non-paused genes rely most heavily on rapid release into elongation. In this model, non-paused genes will be most sensitive to diminished P-TEFb levels. Our results are also in line with studies of the SEC in mammalian cells, which showed that P-TEFb and SEC components are enriched on highly transcribed genes that are rapidly induced [16]. SEC depletion resulted in decreased Pol II occupancy of both paused and non-paused genes [16]. Although P-TEFb and the SEC may directly regulate Pol II recruitment to rapidly transcribed genes in conjunction with the Mediator complex, P-TEFb-regulated release from pausing could also feedback on transcription initiation.
Materials and Methods
Primer sequences are listed in S3 Table.
Molecular cloning, transgenic flies and crosses
The Cdk9 gene region was PCR amplified with primers containing an XbaI overhang cdk9GR2_f and cdk9GR2_r that bind 410 bp upstream of the Cdk9 TSS and 970 bp downstream of the 3´ UTR. The PCR product was cloned into the pAttB vector [49], resulting in the plasmid pAttB-Cdk9. A TY1-V5-FLAG tag was attached to the C-terminus of Cdk9 directly upstream of the stop codon by in vivo recombineering. A selection cassette having TY1-V5-FRT-KanR-RpsL-FRT-FLAG was PCR amplified from the pTAGNG vector [50], (kindly provided by Mihail Sarov) using the primers cdk92TY_f + cdk9flag_R with overhangs containing homology to the Cdk9 C-terminal region. In vivo recombineering of the tag into pAttB-Cdk9 was carried out by growing the pAttB-Cdk9 plasmid in a HME71 bacterial strain [51], kindly provided by the Donald Court lab. Cells were heat shocked to induce Red recombineering proteins followed by electroporation of the PCR amplified tagging cassette into the cells. Colonies were selected for Kanamycin-resistance, and verification of correctly recombineered pAttB-Cdk9 vector performed by DNA sequencing. The Cdk9 construct was then electroporated into 10-beta E. coli cells together with the pSC101-BAD-Flpe-tet plasmid provided by Francis Stewart. The KanR-RpsL sequence in the tagged pAttB-Cdk9 vector was flipped out by inducing FLP expression with 0.2% L-arabinose. Clones were selected for streptomycin resistance confirming the loss of the KanR-RpsL sequence. This left only the TY1-V5-FLAG-tag and an FRT scar in frame in the C-terminus of Cdk9, which was verified by sequencing.
A miRNA resistant sequence was introduced into pattB-Cdk9 by PCR amplification of the entire vector using two phosphorylated primers, cdk9GR3_f + cdk9GR3_r, both having overhangs containing one half of the miRNA resistant sequence each. The PCR reaction was Dpn I-treated and blunt-ligated to circularize the PCR product into a plasmid. Correct clones were identified by restriction digestion and DNA sequencing.
All pAttB-Cdk9 transgenic constructs were introduced into the attP40 landing site on the second chromosome, and combined with the TRIP shmiRNA lines on chromosome 3. In order to verify the existence of the Cdk9 shmiRNA on the third chromosome after combination with the miRNA-resistant transgene, we performed PCR with valium20_f + valium20_r primers.
The shmiRNA knockdown strains (created by the Harvard TRiP project) used were:
Cdk9: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS01391}attP2 (hairpin ID SH01793.N) CycT: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS00776}attP2 (hairpin ID SH00839.N) dEll: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS00277}attP2 (hairpin ID SH00582.N), term: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.GLV21046}attP2 (hairpin ID SH01121.VP)
Cdk12: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS00155}attP2 (hairpin ID SH00274.N)
CycK: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS01003}attP2 (hairpin ID SH01772.N)
MED20: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS01051}attP2 (hairpin ID SH01723.N)
MED22: y1 sc* v1; P{y[+t7.7] v[+t1.8] = TRiP.HMS01047}attP2 (hairpin ID SH01717.N)
Additional Mediator subunit hairpin IDs are listed in S2 Table.
The w*; P{w[+mC] = matalpha4-GAL-VP16}V2H strain was used as maternal Gal4 driver. pUAS-Term-HA-FLAG was a kind gift from Krishnaswamy VijayRaghavan, the DPIM project. The w*; P{w[+mC] = matalpha4-GAL-VP16}V2H driver was combined with w*; P{w[+mC] = His2Av-mRFP1}III.1, made homozygous for both transgenes and used for live imaging. Females containing lilli germ-line clones were generated as previously described [52], using the stocks lilliXS575 FRT40A/CyO, hsFLP w1118; Adv1/CyO and ovoD1 FRT40A/Dp(?;2)bwD, S1 wgSp-1 Ms(2)M1 bwD/CyO. Embryos were collected from the w1118; Df(3L)BSC416/TM6C, Sb stock, which contains a deficiency that removes term, CG7271, CG6885, and around 50 additional genes.
Collection of knock-down embryos
The w*; P{w[+mC] = matalpha4-GAL-VP16}V2H strain was used as maternal Gal4 driver. At least 50 virgin homozygous TubGal4 females were placed in food bottles together with males carrying the miRNA transgene of interest. The progeny, F1 females and males, were allowed to mate with each other in the food bottles for 2–4 days before being transferred to embryo collection cages. Embryos were collected from the cages on fruit juice agar plates supplemented with yeast.
miRNA resistant rescue assay
Overnight embryos were handpicked and placed on yeast-covered juice agar plates and put in standard fly food bottles. Eclosed live flies as well as dead flies stuck in the food were counted as rescued.
In situ hybridization
The whole-mount embryo RNA in situ hybridization protocol was modified from the protocol used in [53,54]. Probes against slam, bnk, nullo and Sry-α were generated by PCR from genomic DNA, A-overhangs added with Taq-polymerase and TA-cloned into pGEM-T Easy (Promega). Anti-sense DIG-UTP labeled RNA probes were made with SP6 or T7 RNA polymerase. For term/CG7271, pgc and zelda, an SP6 overhang was directly added to the PCR primer, and the PCR product was used as a template for in vitro transcription. Probes for eve, ftz, tll, nos, and rho were generated previously.
Quantitative RT-PCR
Total RNA was extracted from embryos using Trizol (Invitrogen) according to the manufacturer´s protocol. For one biological replicate 20 ul embryos were collected. A total of 1 ug from each RNA sample was subjected to DNase digestion (Sigma) and cDNA synthesis (Applied Biosystems) according to the manufacturer´s protocol. Samples were confirmed for quality and quantity using agarose gel electorphoresis and Nanodrop. Out of 200 μl (10x diluted) total cDNA, 2 μl was subsequently used for RT-qPCR in a total volume of 20 μl using CFX96 (BioRad instruments, Sweden) with EvaGreen supermix (Solis BioDyne). Resulting Ct-values were converted into raw quantities with the ΔCt method using Genorm, which were used to calculate the geometric mean of four reference genes ß-tubulin, RpL32 28SrRNA and GAPDH, serving as normalization factors for each cDNA sample. The stability measure M [55], for the mean of the reference genes was below 1.5 for all the conditions tested. Relative expression levels were calculated where expression in control embryos derived from mothers with only the TubGal4 transgene was set to 100%.
Immunoflourescence and live imaging
Embryos were collected and fixed as for RNA in situ hybridization and stained in PBT buffer using α-phosphotyrosine (1 : 150, 4G10, Millipore 05–321), α-H4 (1 : 400, Abcam ab10158) or α-Pol II Ser2 (1 : 200, Abcam ab5095). Cy3 and Alexa-488 flourescent secondary antibodies (Invitrogen) were used at a 1 : 400 dilution. Embryos were visualized in either a Zeiss LSM 780 or a Zeiss LSM 510 Meta confocal microscope, and images acquired and adjusted with Zen software (Zeiss).
For widefield live imaging, a fly strain carrying the Tubulin-Gal4-VP16 transgene and a Histone 2Av-RFP fusion transgene was created. The RFP flies were then crossed with the Cdk9 miRNA strain or used directly as a wild-type control. Embryos were collected for 30–60 min and dechorionated in bleach, then aligned on a gas-permeable membrane on top of two silicone bars and covered in halocarbon oil. Embryos were imaged every 2 min on a Zeiss AxioImagerZ1 microscope equipped with a Zeiss plan-apochromat 20X/0.75NA objective. Imaging was controlled by AxioVison software. Images were assembled with ImageJ software into time-lapse movies. Contrast and brightness settings were applied to the movies with ImageJ for better visualization.
Western blot
Protein extracts were resolved on SDS-PAGE, transferred to PVDF membrane, and incubated with primary antibodies diluted in PBS with 2% BSA: α-Histone3 (1 : 5000, Abcam ab1791), α-CyclinT (1 : 1000, gift from David Price), α-FLAG (1 : 2000, M2, Sigma F3165), α-CTD (1 : 500, 8WG16, Abcam ab817), α-Ser2 (1 : 500, Abcam ab5095), α-Ser2 (1 : 1000, clone 3E10, Millipore 04–1571), α-Ser5 (1 : 1000, Abcam ab5131), rat α-HDAC3 (1 : 1000, [56]) or α-alpha-Tubulin (1 : 2000, Abcam ab18251). HRP-labeled secondary antibodies (1 : 10 000 in PBS with 3% milk) were detected with ECL Select reagent (GE Healthcare) and the membrane exposed to a Luminescent Image Analyzer (LAS-100plus, Fujifilm). Quantification used Fuji Gauge Image software, and CycT signal was compared to HDAC3 loading control, whereas Cdk9-FLAG signal was compared to histone H3.
Chromatin immunoprecipitation
Chromatin IP was performed as described in [57], and 3–5 independent biological replicates were produced for each antibody. A dounce homogenizer was used to grind 2–4h old wild-type or Cdk9 Drosophila embryos and a Diagenode Bioruptor was used to sonicate the material before IP. The antibodies used were α-Pol II CTD (8 μl, 8WG16, Abcam ab817), α-Pol II Ser2 (7 μl, Abcam ab5095), α-Ser5 (5 μl, Abcam ab5131). Twenty-five or 30 μl Protein A/G-coated magnetic bead mix slurry and 40–50 μl of embryos were used for each IP. ChIP samples were eluted in 160 μl 0.1x TE pH 8 and duplicates with 2 μl DNA each analyzed by quantitative PCR using a Bio-Rad CFX96 machine and HOT FIREPol EvaGreen master mix (Solis BioDyne). From each IP, the percent of input signal was obtained using the average of the two duplicates, and the Ser5/CTD and Ser2/CTD ratios calculated using these values. Pol II occupancy and Pol II 5’/3’ ratios were calculated by normalizing CTD percent input signal against an intergenic locus. Student´s unpaired t-test for two-sample equal variance was used to find statistically significant differences (p<0.05) between control and Cdk9 samples. All values and calculations can be found in S4 Table.
Supporting Information
Zdroje
1. Corden JL (1990) Tails of RNA polymerase II. Trends Biochem Sci 15 : 383–387. 2251729
2. Hsin JP, Manley JL (2012) The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev 26 : 2119–2137. doi: 10.1101/gad.200303.112 23028141
3. Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA (2007) A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130 : 77–88. 17632057
4. Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, et al. (2007) RNA polymerase is poised for activation across the genome. Nature Genetics 39 : 1507–1511. 17994021
5. Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, et al. (2007) RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nature Genetics 39 : 1512–1516. 17994019
6. Adelman K, Lis JT (2012) Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nature Reviews Genetics 13 : 720–731. doi: 10.1038/nrg3293 22986266
7. Saunders A, Core LJ, Sutcliffe C, Lis JT, Ashe HL (2013) Extensive polymerase pausing during Drosophila axis patterning enables high-level and pliable transcription. Genes Dev 27 : 1146–1158. doi: 10.1101/gad.215459.113 23699410
8. Gilmour DS (2009) Promoter proximal pausing on genes in metazoans. Chromosoma 118 : 1–10. doi: 10.1007/s00412-008-0182-4 18830703
9. Peterlin BM, Price DH (2006) Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23 : 297–305. 16885020
10. Zhou Q, Li TD, Price DH (2012) RNA Polymerase II Elongation Control. Ann Rev Biochem 81 : 119–143. doi: 10.1146/annurev-biochem-052610-095910 22404626
11. Henriques T, Gilchrist DA, Nechaev S, Bern M, Muse GW, et al. (2013) Stable pausing by RNA polymerase II provides an opportunity to target and integrate regulatory signals. Mol Cell 52 : 517–528. doi: 10.1016/j.molcel.2013.10.001 24184211
12. Jonkers I, Kwak H, Lis JT (2014) Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife 3: e02407. doi: 10.7554/eLife.02407 24843027
13. Nguyen D, Krueger BJ, Sedore SC, Brogie JE, Rogers JT, et al. (2012) The Drosophila 7SK snRNP and the essential role of dHEXIM in development. Nucleic Acids Research 40 : 5283–5297. doi: 10.1093/nar/gks191 22379134
14. Luo Z, Lin C, Shilatifard A (2012) The super elongation complex (SEC) family in transcriptional control. Nat Rev Mol Cell Biol 13 : 543–547. doi: 10.1038/nrm3417 22895430
15. Smith ER, Lin C, Garrett AS, Thornton J, Mohaghegh N, et al. (2011) The little elongation complex regulates small nuclear RNA transcription. Mol Cell 44 : 954–965. doi: 10.1016/j.molcel.2011.12.008 22195968
16. Lin C, Garrett AS, De Kumar B, Smith ER, Gogol M, et al. (2011) Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC). Genes Dev 25 : 1486–1498. doi: 10.1101/gad.2059211 21764852
17. Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CAS, et al. (2011) Human Mediator Subunit MED26 Functions as a Docking Site for Transcription Elongation Factors. Cell 146 : 92–104. doi: 10.1016/j.cell.2011.06.005 21729782
18. Sullivan W, Theurkauf WE (1995) The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr Opin Cell Biol 7 : 18–22. 7755985
19. Tadros W, Lipshitz HD (2009) The maternal-to-zygotic transition: a play in two acts. Development 136 : 3033–3042. doi: 10.1242/dev.033183 19700615
20. Mazumdar A, Mazumdar M (2002) How one becomes many: blastoderm cellularization in Drosophila melanogaster. BioEssays 24 : 1012–1022. 12386932
21. Sokac AM, Wieschaus E (2008) Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization. Dev Cell 14 : 775–786. doi: 10.1016/j.devcel.2008.02.014 18477459
22. Wenzl C, Yan S, Laupsien P, Grosshans J (2010) Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by Slam. Mech Dev 127 : 371–384. doi: 10.1016/j.mod.2010.01.001 20060902
23. Liang HL, Nien CY, Liu HY, Metzstein MM, Kirov N, et al. (2008) The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 456 : 400–403. doi: 10.1038/nature07388 18931655
24. Tang AH, Neufeld TP, Rubin GM, Muller HA (2001) Transcriptional regulation of cytoskeletal functions and segmentation by a novel maternal pair-rule gene, lilliputian. Development 128 : 801–813. 11171404
25. Chen K, Johnston J, Shao W, Meier S, Staber C, et al. (2013) A global change in RNA polymerase II pausing during the Drosophila midblastula transition. Elife 2: e00861. doi: 10.7554/eLife.00861 23951546
26. Rothe M, Pehl M, Taubert H, Jackle H (1992) Loss of gene function through rapid mitotic cycles in the Drosophila embryo. Nature 359 : 156–159. 1522901
27. Shermoen AW, O'Farrell PH (1991) Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell 67 : 303–310. 1680567
28. Eissenberg JC, Shilatifard A, Dorokhov N, Michener DE (2007) Cdk9 is an essential kinase in Drosophila that is required for heat shock gene expression, histone methylation and elongation factor recruitment. Molecular Genetics and Genomics 277 : 101–114. 17001490
29. Chopra VS, Hong JW, Levine M (2009) Regulation of Hox gene activity by transcriptional elongation in Drosophila. Curr Biol 19 : 688–693. doi: 10.1016/j.cub.2009.02.055 19345103
30. Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, et al. (2011) A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8 : 405–407. doi: 10.1038/nmeth.1592 21460824
31. Staller MV, Yan D, Randklev S, Bragdon MD, Wunderlich ZB, et al. (2013) Depleting gene activities in early Drosophila embryos with the "maternal-Gal4-shRNA" system. Genetics 193 : 51–61. doi: 10.1534/genetics.112.144915 23105012
32. Gerber M, Ma J, Dean K, Eissenberg JC, Shilatifard A (2001) Drosophila ELL is associated with actively elongating RNA polymerase II on transcriptionally active sites in vivo. EMBO J 20 : 6104–6114. 11689450
33. St Pierre SE, Ponting L, Stefancsik R, McQuilton P (2014) FlyBase 102—advanced approaches to interrogating FlyBase. Nucleic Acids Research 42: D780–788. doi: 10.1093/nar/gkt1092 24234449
34. Baldarelli RM, Mahoney PA, Salas F, Gustavson E, Boyer PD, et al. (1988) Transcripts of the Drosophila blastoderm-specific locus, terminus, are concentrated posteriorly and encode a potential DNA-binding finger. Developmental Biology 125 : 85–95. 3334721
35. Gilchrist DA, Dos Santos G, Fargo DC, Xie B, Gao Y, et al. (2010) Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell 143 : 540–551. doi: 10.1016/j.cell.2010.10.004 21074046
36. Shim EY, Walker AK, Shi Y, Blackwell TK (2002) CDK-9/cyclin T (P-TEFb) is required in two postinitiation pathways for transcription in the C-elegans embryo. Genes Dev 16 : 2135–2146. 12183367
37. Eissenberg JC, Ma J, Gerber MA, Christensen A, Kennison JA, et al. (2002) dELL is an essential RNA polymerase II elongation factor with a general role in development. Proceedings of the National Academy of Sciences of the United States of America 99 : 9894–9899. 12096188
38. Carlsten JO, Zhu X, Gustafsson CM (2013) The multitalented Mediator complex. Trends Biochem Sci 38 : 531–537. doi: 10.1016/j.tibs.2013.08.007 24074826
39. Wang W, Yao X, Huang Y, Hu X, Liu R, et al. (2013) Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb. Transcription 4 : 39–51. doi: 10.4161/trns.22874 23340209
40. Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, et al. (2013) HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 153 : 1327–1339. doi: 10.1016/j.cell.2013.04.048 23746844
41. Nakamura A, Shirae-Kurabayashi M, Hanyu-Nakamura K (2010) Repression of early zygotic transcription in the germline. Curr Opin Cell Biol 22 : 709–714. doi: 10.1016/j.ceb.2010.08.012 20817425
42. Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A (2008) Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature 451 : 730–733. doi: 10.1038/nature06498 18200011
43. Martinho RG, Kunwar PS, Casanova J, Lehmann R (2004) A noncoding RNA is required for the repression of RNApolII-dependent transcription in primordial germ cells. Curr Biol 14 : 159–165. 14738740
44. Batchelder C, Dunn MA, Choy B, Suh Y, Cassie C, et al. (1999) Transcriptional repression by the Caenorhabditis elegans germ-line protein PIE-1. Genes Dev 13 : 202–212. 9925644
45. Ghosh D, Seydoux G (2008) Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics 178 : 235–243. doi: 10.1534/genetics.107.083212 18202370
46. Seydoux G, Dunn MA (1997) Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124 : 2191–2201. 9187145
47. Bowman EA, Bowman CR, Ahn JH, Kelly WG (2013) Phosphorylation of RNA polymerase II is independent of P-TEFb in the C. elegans germline. Development 140 : 3703–3713. doi: 10.1242/dev.095778 23903194
48. Sullivan W, Daily DR, Fogarty P, Yook KJ, Pimpinelli S (1993) Delays in anaphase initiation occur in individual nuclei of the syncytial Drosophila embryo. Mol Biol Cell 4 : 885–896. 8257792
49. Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proceedings of the National Academy of Sciences of the United States of America 104 : 3312–3317. 17360644
50. Ejsmont RK, Sarov M, Winkler S, Lipinski KA, Tomancak P (2009) A toolkit for high-throughput, cross-species gene engineering in Drosophila. Nature Methods 6 : 435–U452. doi: 10.1038/nmeth.1334 19465918
51. Thomason LC, Costantino N, Shaw DV, Court DL (2007) Multicopy plasmid modification with phage lambda red recombineering. Plasmid 58 : 148–158. 17434584
52. Chou TB, Perrimon N (1996) The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144 : 1673–1679. 8978054
53. Jiang J, Hoey T, Levine M (1991) Autoregulation of a Segmentation Gene in Drosophila—Combinatorial Interaction of the Even-Skipped Homeo Box Protein with a Distal Enhancer Element. Genes Dev 5 : 265–277. 1671662
54. Tautz D, Pfeifle C (1989) A Non-Radioactive Insitu Hybridization Method for the Localization of Specific Rnas in Drosophila Embryos Reveals Translational Control of the Segmentation Gene Hunchback. Chromosoma 98 : 81–85. 2476281
55. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3: RESEARCH0034. 12184808
56. Qi D, Bergman M, Aihara H, Nibu Y, Mannervik M (2008) Drosophila Ebi mediates Snail-dependent transcriptional repression through HDAC3-induced histone deacetylation. EMBO J 27 : 898–909. doi: 10.1038/emboj.2008.26 18309295
57. Holmqvist PH, Boija A, Philip P, Crona F, Stenberg P, et al. (2012) Preferential genome targeting of the CBP co-activator by Rel and Smad proteins in early Drosophila melanogaster embryos. PLoS Genetics 8: e1002769. doi: 10.1371/journal.pgen.1002769 22737084
Štítky
Genetika Reprodukční medicína
Článek 2014 Reviewer Thank YouČlánek Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic TranslationČlánek Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate CellsČlánek K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in ArabidopsisČlánek A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis inČlánek Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in MiceČlánek The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- Systematic Cell-Based Phenotyping of Missense Alleles Empowers Rare Variant Association Studies: A Case for and Myocardial Infarction
- African Glucose-6-Phosphate Dehydrogenase Alleles Associated with Protection from Severe Malaria in Heterozygous Females in Tanzania
- Genomics of Divergence along a Continuum of Parapatric Population Differentiation
- microRNAs Regulate Cell-to-Cell Variability of Endogenous Target Gene Expression in Developing Mouse Thymocytes
- A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in
- Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
- Partially Redundant Enhancers Cooperatively Maintain Mammalian Expression Above a Critical Functional Threshold
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Mutations in Result in Ocular Coloboma, Microcornea and Cataracts
- A Genome-Wide Hybrid Incompatibility Landscape between and
- Recurrent Evolution of Melanism in South American Felids
- Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate Cells
- Tissue Expression Pattern of PMK-2 p38 MAPK Is Established by the miR-58 Family in
- Essential Role for Endogenous siRNAs during Meiosis in Mouse Oocytes
- Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- RR-1 Cuticular Protein TcCPR4 Is Required for Formation of Pore Canals in Rigid Cuticle
- GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
- K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in Arabidopsis
- A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis in
- HYPER RECOMBINATION1 of the THO/TREX Complex Plays a Role in Controlling Transcription of the Gene in Arabidopsis
- Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan
- Structured Observations Reveal Slow HIV-1 CTL Escape
- An Integrative Multi-scale Analysis of the Dynamic DNA Methylation Landscape in Aging
- Combining Natural Sequence Variation with High Throughput Mutational Data to Reveal Protein Interaction Sites
- Transhydrogenase Promotes the Robustness and Evolvability of Deficient in NADPH Production
- Regulators of Autophagosome Formation in Muscles
- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Eye Selector Logic for a Coordinated Cell Cycle Exit
- Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations
- The DNA Polymerase δ Has a Role in the Deposition of Transcriptionally Active Epigenetic Marks, Development and Flowering
- Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice
- Membrane Recognition and Dynamics of the RNA Degradosome
- P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
- is a Long Non-coding RNA in JNK Signaling in Epithelial Shape Changes during Drosophila Dorsal Closure
- A Pleiotropy-Informed Bayesian False Discovery Rate Adapted to a Shared Control Design Finds New Disease Associations From GWAS Summary Statistics
- Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers
- and Hyperdrive Mechanisms (in Mouse Meiosis)
- Elevated In Vivo Levels of a Single Transcription Factor Directly Convert Satellite Glia into Oligodendrocyte-like Cells
- Systemic Delivery of MicroRNA-101 Potently Inhibits Hepatocellular Carcinoma by Repressing Multiple Targets
- Pooled Sequencing of 531 Genes in Inflammatory Bowel Disease Identifies an Associated Rare Variant in and Implicates Other Immune Related Genes
- Abscission Is Regulated by the ESCRT-III Protein Shrub in Germline Stem Cells
- Temperature Stress Mediates Decanalization and Dominance of Gene Expression in
- Transcriptome Wide Annotation of Eukaryotic RNase III Reactivity and Degradation Signals
- The Exosome Component Rrp6 Is Required for RNA Polymerase II Termination at Specific Targets of the Nrd1-Nab3 Pathway
- Sex-specific -regulatory Variation on the X Chromosome
- Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex
- Modeling of the Human Alveolar Rhabdomyosarcoma Chromosome Translocation in Mouse Myoblasts Using CRISPR-Cas9 Nuclease
- Asymmetry of the Budding Yeast Tem1 GTPase at Spindle Poles Is Required for Spindle Positioning But Not for Mitotic Exit
- TIM Binds Importin α1, and Acts as an Adapter to Transport PER to the Nucleus
- Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
- The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
- Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
- Region-Specific Activation of mRNA Translation by Inhibition of Bruno-Mediated Repression
- An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish ()
- Interaction between the tRNA-Binding and C-Terminal Domains of Yeast Gcn2 Regulates Kinase Activity In Vivo
- Hyper-Acetylation of Histone H3K56 Limits Break-Induced Replication by Inhibiting Extensive Repair Synthesis
- Prodomain Removal Enables Neto to Stabilize Glutamate Receptors at the Neuromuscular Junction
- Recent Selective Sweeps in North American Show Signatures of Soft Sweeps
- Identification and Functional Analysis of Healing Regulators in
- A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2
- Drosophila Casein Kinase I Alpha Regulates Homolog Pairing and Genome Organization by Modulating Condensin II Subunit Cap-H2 Levels
- The Hippo Pathway Regulates Homeostatic Growth of Stem Cell Niche Precursors in the Ovary
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



