-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
Eukaryotes alternate between haploid (1n) and diploid (2n) stages during their life cycles, and often seen are remarkable differences in morphology and physiology between them. Land plants are multicellular in both generations, in contrast to their presumed ancestral green algae that develop multicellularity only in the haploid stage. TALE class homeodomain transcriptional factors play a key role in the activation of diploid development in diverse lineages of eukaryotes. A gene duplication event within this family in an ancestor of land plants had profound implications for land plant evolution. We show that the two subclasses resulting from the gene duplication event act to pattern, in a complementary manner, most above ground organs of the diploid stage of the flowering plant Arabidopsis. Their opposing activities sculpt the shape of leaves from entire to pinnate and control the architecture of the plant body, and thus providing plasticity for evolutionary tinkering. These results form a foundation for understanding how these genes have been co-opted from an ancestral role of regulating diploid gene expression in a zygote to directing sporophyte land plant body architecture and provide insight into the evolution of various forms of life cycles.
Published in the journal: . PLoS Genet 11(2): e32767. doi:10.1371/journal.pgen.1004980
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004980Summary
Eukaryotes alternate between haploid (1n) and diploid (2n) stages during their life cycles, and often seen are remarkable differences in morphology and physiology between them. Land plants are multicellular in both generations, in contrast to their presumed ancestral green algae that develop multicellularity only in the haploid stage. TALE class homeodomain transcriptional factors play a key role in the activation of diploid development in diverse lineages of eukaryotes. A gene duplication event within this family in an ancestor of land plants had profound implications for land plant evolution. We show that the two subclasses resulting from the gene duplication event act to pattern, in a complementary manner, most above ground organs of the diploid stage of the flowering plant Arabidopsis. Their opposing activities sculpt the shape of leaves from entire to pinnate and control the architecture of the plant body, and thus providing plasticity for evolutionary tinkering. These results form a foundation for understanding how these genes have been co-opted from an ancestral role of regulating diploid gene expression in a zygote to directing sporophyte land plant body architecture and provide insight into the evolution of various forms of life cycles.
Introduction
Gene duplication is thought to be one of the key drivers in generating evolutionary novelty. Following gene duplication, paralogs can undergo a process of neofunctionalization, supplying a genetic basis for morphological novelty [1,2,3]. Transcription factors can undergo neofunctionalization via either a change in expression pattern or an alteration in functionality, e.g. the derivation of a repressor or inhibitor from an ancestral activator, or vice versa (e.g. [4]). Three amino acid loop extension (TALE) homeodomain transcriptional factors, characterized by having a homeodomain that has three extra amino acids between helices 1 and 2, are found in all eukaryotic lineages [5,6,7]. Plant TALE homeobox genes are classified into two subfamilies, KNOTTED-like homeobox (KNOX) and BELL-like (BELL) [8]. Whilst Chlorophyte algal KNOX genes are of a single class, a gene duplication in a common ancestor of land plants, produced two classes of KNOX genes, class I (KNOX1) and class II (KNOX2) [8,9] (Fig. 1A). KNOX genes of flowering plants have been studied for over two decades, however, the functional consequences of the KNOX gene duplication have been largely unexplored.
Fig. 1. Phylogeny and expression patterns of KNOX and BELL genes. 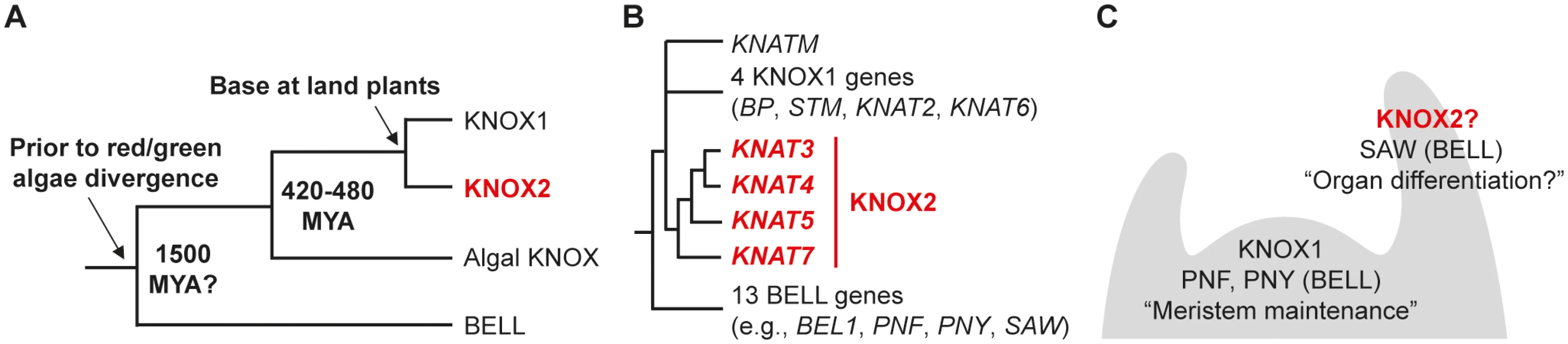
(A) Phylogenetic relationships of KNOX and BELL gene families in the plant lineage. Available sequence information suggests the gene duplication producing the KNOX and BELL genes occurred before the divergence of red and green algae. A gene duplication in the lineage leading to land plants created KNOX1 and KNOX2 genes from an ancestral algal KNOX gene. Estimated dates for some nodes are listed in millions of years before present (Mya). (B) In Arabidopsis, KNOX1, KNOX2, and BELL proteins are encoded by 4, 4, and 13 genes, respectively. In addition, KNATM encodes for a KNOX-related protein lacking a homeodomain. Detailed phylogenetic analyses of KNOX genes are presented in S1 and S2 Figs. (C) Schematic depiction of expression patterns for Arabidopsis KNOX1, KNOX2, and BELL genes based on previous literature [75] and publicly available transcriptome data (for details, see S3 and S4 Figs.). KNOX1 and some BELL genes, e.g., PNF and PNY, are primarily expressed in meristematic tissues while KNOX2 and other BELL genes such as SAW1 and SAW2 are expressed in differentiating organs. KNOX2 genes are highlighted in red. The first identified plant homeobox gene was Knotted1, a KNOX1 gene of maize [10]. Since, KNOX1 genes have been characterized in numerous flowering plants with a conspicuous loss-of-function phenotype being a failure in shoot apical meristem (SAM) maintenance. KNOX1 activity is also involved in maintenance of meristematic activity during leaf development, with prolonged activity in leaf margins observed in species with complex leaves and gain-of-function alleles result in more complex leaves. Thus, KNOX1 genes play a critical role in maintaining meristematic properties of cells in flowering plant sporophytes, the diploid generation of the land plant life cycle (reviewed in [11,12,13]). The KNOX1 genes of the moss Physcomitrella patens are only expressed in the sporophyte and mutants have decreased sporophyte growth, suggesting that KNOX1 genes have a conserved role in tissue proliferation during sporophyte development throughout land plants [12,13,14]. There is no evidence indicative of KNOX1 function in the gametophyte (haploid) generation in any characterized species, including the indeterminate meristems of the moss gametophyte, suggesting the role of KNOX1 is restricted to the diploid, sporophyte generation [14].
A functional distinction between KNOX1 and KNOX2 genes has been postulated from studies based on gene expression patterns in flowering plants. Northern blot analyses in maize demonstrated that KNOX1 gene expression is confined to less differentiated tissues whereas KNOX2 genes are broadly expressed in differentiating tissues and mature organs [9]. Similar broad expression profiles of KNOX2 genes have been reported in Arabidopsis [15,16] and tomato [17]. Characterization of spatial expression patterns in Arabidopsis revealed that KNOX2 genes have both overlapping and distinct expression patterns and that they are expressed in most tissues except for meristematic regions [15,18,19,20,21]. Despite several reports of expression patterns, comparatively little is known about KNOX2 gene function in flowering plants. One of the four Arabidopsis KNOX2 paralogs, KNAT7, is involved in secondary cell wall biosynthesis [18,19,21], and another, KNAT3, is reported to modulate ABA responses [22]. While these findings are consistent with the reported expression patterns, there exists a gap between broad expression patterns and known KNOX2 functions. For instance, unlike KNOX1 genes, which are important regulators of growth and development, it is not clear whether or not KNOX2 genes are involved in morphogenesis in flowering plants. These questions have gone unanswered owing to the paucity of functional studies on KNOX2 genes due to extensive genetic redundancy as noted by Truernit et al. [20].
From a wider perspective, a possible ancestral function of TALE homeodomain proteins is the regulation of diploid gene expression upon fusion of gametes, as is observed in the Chlorophyte alga Chlamydomonas reinhardtii and several fungi [23,24,25]. In C. reinhardtii the plus gamete expresses a BELL protein while the minus gamete expresses a KNOX protein; upon gamete fusion the KNOX and BELL proteins heterodimerize and regulate zygotic gene expression [25]. In the moss P. patens, KNOX2 genes are expressed in the egg cells and the sporophyte. Eliminating KNOX2 activity results in apospory, the development of a haploid body plan during the diploid generation, suggesting KNOX2 genes regulate the gametophyte-to-sporophyte morphological transition, a reflection of the hypothesized ancestral TALE homeodomain gene function [26]. Thus, both KNOX1 and KNOX2 mutant phenotypes in land plants are consistent with the hypothesis that ancestral function of KNOX genes was to regulate diploid gene expression. However, the seemingly different roles of KNOX1 and KNOX2 genes indicate functional diversification among land plant KNOX genes.
To gain insight into developmental roles for KNOX2 genes in flowering plants and the genetic relationship between KNOX1 and KNOX2 classes, we undertook a genetic study of KNOX2 genes in Arabidopsis thaliana, a species in which KNOX1 gene function is well characterized. We discuss the implication of our findings on the impact of the gene duplication producing KNOX1 and KNOX2 paralogs in the course of land plant evolution.
Results
Four Arabidopsis genes encode KNOX2 proteins. As phylogenetic analyses place KNAT7 in a clade sister to the remaining Arabidopsis KNOX2 genes [9], we focused on KNAT3 (AT5G25220), KNAT4 (AT5G11060), and KNAT5 (AT4G32040) genes in this study (Fig. 1B, S1 and S2 Figs.). In contrast to KNOX1 genes, which are expressed primarily in meristematic tissues, KNOX2 gene expression occurs in differentiating organs suggesting distinct and perhaps complementary functions [15,16,20] (Fig. 1C, S3 and S4 Figs.).
KNOX2 mutant phenotypes and expression patterns
KNOX2 mutant phenotypes were characterized using null alleles (S5 Fig.). As reported previously [20], single mutants lack conspicuous aberrant phenotypes. Amongst double mutants, knat3 knat5 seedlings are distinguishable from wild type by a longer petiole and narrower lamina of cotyledons, and more deeply serrated leaf margins (Fig. 2A-B). Venation pattern is also affected in knat3 knat5 cotyledons (S6 Fig.). knat3 knat4 plants also have serrated leaves (Fig. 2I) and are sporophytically female sterile with abnormal integument development. While knat3 knat4/+ knat5 plants are also female sterile, knat3/+ knat4 knat5 plants are phenotypically wild type and produce viable seeds, facilitating characterization of segregating triple mutant plants.
Fig. 2. KNOX2 mutant phenotypes and KNAT5 expression patterns. 
(A-C) 10-day-old seedlings of wild-type (WT, A), knat3 knat5 (designated as knat35, B), and knat345 (C). (D-E) Wild-type (D) and knat345 (E) plants after bolting; 5 weeks-old plants are shown. (F-G) Representative wild-type (F) and knat345 (G) rosette leaves. (H-L) First cauline (stem) leaves of wild-type (H), knat3 knat4 (designated as knat34, I), knat3 knat4 knat5/+ (designated as knat34, 5/+, J), knat3 knat5 (K), and knat345 (L) plants; progressive loss of the KNOX2 activity results in increasingly serrated leaves. (M-N) Wild-type (M) and knat345 (N) flowers. (O-R) Mature wild-type (O, Q) and knat345 (P, R) ovules and embryo sacs. An arrow marks ectopic formation of tracheary elements. (S-V) proKNAT5:KNAT5-GUS expression in developing embryos (S-T), vegetative shoot apex (U), leaf (V), and ovules (W). proKNAT5:KNAT5-GUS is not detected in the shoot apical meristem (marked by an arrowhead) and the youngest leaf primordium (marked with an arrow). An asterisk indicates a stipule. (X) proKNAT4:GUS expression in vegetative shoot apex. For additional expression data, see S10 Fig. Plants are all in the Columbia (Col) background. Scale bars, 1 cm. Selfed knat3/+ knat4 knat5 plants segregated small, dark-green plants with deeply lobed leaves (Fig. 2C-G and S14F Fig.), a phenotype reminiscent of gain-of-function KNOX1 alleles [12,13]. PCR-based genotyping indicated these plants were triple-mutant homozygotes (knat345). Since only a single mutant allele was available for each gene, we designed an artificial miRNA (amiRNA, [27]) targeting only KNAT4, amiR159-KNAT4 (S7A Fig.), and generated knat3 knat5 plants constitutively expressing amiR159-KNAT4 under the control of the Cauliflower Mosaic Virus 35S promoter (pro35S). pro35S:amiR159-KNAT4 knat3 knat5 lines closely resembled the identified knat345 plants (S8C-D Fig.). Another amiRNA, amiR159-KNAT345–1, was designed to target KNAT3, KNAT4, and KNAT5 (S7B Fig.). pro35S:amiR159-KNAT345–1 plants also show a deeply serrated leaf phenotype (S8E and S14H Figs.). We thus conclude that this is the triple mutant phenotype. Consistent with functional redundancy among these genes, dosage-dependent enhancement of the leaf serration phenotype was observed (Fig. 2H-L). Likewise, venation pattern in cotyledons is more severely affected in knat345 plants (S6 Fig.). Floral organs homologous with leaves are also affected. Sepals and petals are narrower and partially dissected in the knat345 mutant, and integument development is defective as seen in knat3 knat4 plants (Fig. 2M-N and S9 Fig.). Ectopic formation of tracheary elements is observed in knat345 embryo sacs (Fig. 2R). Although these genes are expressed in roots [15,16,20], the morphology of primary roots in knat345 plants appeared normal (S10 Fig.).
A proKNAT5:KNAT5-GUS translational fusion line was generated to monitor expression patterns (Fig. 2S-W and S11 Fig.). In line with the mutant phenotypes, GUS activity was observed in developing leaves but excluded from the shoot apical meristem (SAM) (Fig. 2S-V and S11C Fig.). During early stages of leaf development, GUS activity was not detected in youngest leaf primordia but was observed in older leaf primordia (Fig. 2U). Reduced signal levels were observed in older leaves (S11B Fig.). Prolonged incubation detected GUS signal along cotyledon and leaf veins and in ovules (Fig. 2T and S11H-I Fig.). proKNAT5:KNAT5-GUS expression is nuclear in trichomes, supporting a role for KNAT5 in transcriptional regulation (S11 Fig.). A transcriptional fusion line, proKNAT4:GUS, was generated to examine KNAT4 expression patterns. Ten independent T1 plants were examined, all of which exhibited KNAT4 promoter activity in leaves but not in the SAM (Fig. 2X). A similar expression pattern has been described for KNAT3 using either a GUS reporter line or RNA in situ hybridization [15]. Exclusion of KNOX2 expression from the SAM is also supported by cell-type specific expression analyses of the inflorescence SAM (S3 Fig.).
Genetic evidence for KNOX2/BELL heterodimarization
KNOX and BELL heterodimerization plays a pivotal role in regulating their activities as transcription factors [13]. We speculated that the lack of BELL partners may explain why no conspicuous phenotype has been described to date upon ectopic expression of KNOX2 genes [28] (S12 Fig.). The founding BELL gene, BELL1 (BEL1), and closely related paralogs, SAWTOOTH1 (SAW1) and SAW2, represent candidates for KNOX2 partners since loss-of-function phenotypes in ovules and leaf margins resemble those of KNOX2 mutants [29,30,31]. Physical interactions have been previously proposed between these BELL and KNOX2 proteins [22,29,32]. SAW1 and SAW2 are expressed in leaves but not in meristems [29] (S3 and S4 Figs.). Thus, we co-expressed SAW2 and KNAT3 throughout the SAM by trans-activating SAW2 under the control of SHOOT MERISTEMLESS (STM) regulatory sequences (proSTM>>SAW2; >> denotes the use of transactivation system hereafter) in pro35S:KNAT3 plants [33]. pro35S:KNAT3 proSTM>>SAW2 plants lack an embryonic SAM and resemble loss-of-function stm or stm knat6 mutant plants [34,35,36] (Fig. 3A-C, 3E). Combined expression of KNAT5 and SAW2 in the proSTM region resulted in a similar phenotype (Fig. 3D), confirming that the presence of both SAW2 and KNOX2 proteins simultaneously accounts for the phenotype. Collectively, these data indicate that concurrent expression, and by proxy, heterodimerization with BELL proteins, is important for KNOX2 function and that KNOX2 activity may thus be constrained by limited access to corresponding BELL partners.
Fig. 3. Genetic interactions between BELL and KNOX2 genes. 
(A-E) 10 days-old seedlings of pro35S:KNAT3 (A), proSTM>>SAW2 (B), pro35S:KNAT3 proSTM>>SAW2 (C), proSTM>>KNAT5 proSTM>>SAW2 (D), and stm-11 (E), showing resemblance between stm (E) and plants expressing both KNOX2 and SAW2 proteins in the meristem (C, D). Defective meristems are marked with arrows. In (B-D), expression of SAW2 or KNAT5 alone, or both genes together is transactivated by the STM regulatory sequence. (F) Introducing the pro35S:amiR159-KNAT345–1 construct did not alter the seedling lethal phenotype of stm-11 plants. Shown is a 25 days-old plant. (G-J) Gynoecia from wild-type (G, emasculated and unpollinated), bel1–154 (H), knat345 (I), and bel1–154 blh1–114 (J) flowers. (I-J) Unfertilized gynoecia turn yellow. See S9 Fig. for more data. This phenotype was not observed in emasculated wild-type (G), bel1 (H), and blh1–114 plants. Plants in (B, D, E, F) are in the Landsberg erecta (Ler) background. A plant in (C) is in the Col/Ler mixed background. All other plants are in the Col background. Scale bars, 0.5 mm. A mutation in KNAT3 suppresses the gain-of-function phenotype caused by ectopic expression of another BELL gene, BLH1, suggesting that BLH1 is likely a functional partner for KNOX2 proteins [37]. This prompted us to examine genetic interactions between BLH1 and BEL1-related BELL genes, and we found that bel1 blh1 double mutants show color changes in unfertilized gynoecia as seen in knat3 knat4 and knat345 plants (Fig. 3G-J and S9 Fig.). Thus, BEL1 and BLH1 play a redundant role in gynoecium development and perhaps act in association with KNOX2 genes. More comprehensive genetic analyses as well as expression analyses are required to assign specific roles to functionally redundant BELL genes.
KNOX2 exhibits selectivity for BELL
To further dissect BELL-KNOX interactions, plants expressing BELL and/or KNOX genes in the proSTM region were characterized. Among BELL proteins, PENNYWISE (PNY) and POUND-FOOLISH (PNF) are expressed in the SAM and act in conjunction with KNOX1 proteins to promote SAM activity [12,13]. As expected, plants expressing KNOX1 genes (STM or KNAT2) or PNY in the STM domain appeared wild type (S13B-D Fig.). In contrast, proSTM>>SAW2 plants displayed abnormal floral morphologies, such as fused sepals, reduced petals, and misshapen fruits (S13E, H Fig.), phenotypes often associated with reduced KNOX1 activity, e.g. weak stm mutants [38]. Flower development was not impacted in proSTM>>KNAT5 plants, but fused sepals are also observed in strong pro35S:KNAT3 lines (S12F Fig.). Concomitant expression of KNOX2 with PNY or KNOX1 with SAW2 did not enhance the KNOX2 or SAW2 overexpression phenotypes. We therefore conclude KNOX2 shows selectivity for BELL proteins in vivo.
KNOX2 mutant phenotype is independent of KNOX1 activity
Loss-of-function and gain-of-function KNOX2 phenotypes are reminiscent of gain-of-function and loss-of-function KNOX1 phenotypes, respectively [12,13]. To characterize the relationship between the two gene classes, loss-of-function alleles for KNOX1 and KNOX2 were combined. Plants constitutively expressing an amiRNA targeting KNAT3, KNAT4, and KNAT5, pro35S:amiR159-KNAT345–1, in KNOX1 loss-of-function (stm or bp knat2 knat6) backgrounds were examined. Neither the meristem failure of stm mutants nor the KNOX2 loss-of-function mutant leaf phenotype was suppressed in these plants (Fig. 3F and Fig. 4A-B). Similarly, neither knat2 knat3 knat5 knat6 nor bp knat345 showed significant suppression of the KNOX2 loss-of-function mutant leaf phenotype and the bp inflorescence phenotype (Fig. 4F-J). Thus, loss-of-function phenotypes of KNOX1 and KNOX2 mutants are not due to ectopic activation of KNOX2 and KNOX1, respectively. Furthermore, BP, STM, and KNAT2 expression was not altered in knat3 knat5 plants (Fig. 4K-P), arguing against mutual repression between KNOX1 and KNOX2 genes.
Fig. 4. Genetic interactions between KNOX1 and KNOX2 genes. 
(A-B) Morphology of pro35S:amiR159-KNAT345–1 plants in wild-type (A) and bp-9 knat2–5 knat6–1 mutant (B) backgrounds. 6 weeks-old plants are shown. (C-E) F1 plants expressing both proBLS:STM and pro35S:amiR159-KNAT345–1 constructs (D) show a stronger serration phenotype than either parental line (C, E). All plants shown are hemizygous for the transgene(s) and 2 weeks old. (F-G) Morphology of knat3 knat5 (F) and knat2–5 knat3 knat5 knat6–1 (G) plants grown for 5 weeks. (H) A representative bp knat345 rosette leaf with deeply lobed margins, as seen in knat345. (I-J) The bp inflorescence phenotype (I) is observed in bp knat345 infloresences (J), indicating additive effects of mutations in these genes. (H-J) Plants were grown for 2 months. (K-P) KNOX1 reporter expression in wild-type (K-M) and knat3 knat5 (N-P) plants. Shown are proBP:GUS expression in 17 days-old plants (K, N), proKNAT2:GUS expression in 20 days-old plants (L, O), and proSTM:GUS expression in 21 days-old plants (M, P). No ectopic expression of proBP:GUS was detected during stages of leaf development when lobes are forming in knat3 knat5 plants (K, N), but ectopic expression was observed at leaf serration tips after their development. proKNAT2:GUS and proSTM:GUS expression patterns in knat3 knat5 plants are similar to those in wild-type plants (L-M, O-P). Occasionally, longer incubation detected proSTM:GUS activity in the sinus of wild-type and mutant leaves (M). proKNAT2:GUS and proSTM:GUS were analyzed in the mixed genetic background (refer to S1 Table for details), and other plants are in the Col background. Scale bars in I, J, 3 mm, K, L, N, 100 μm and in M, O, P, 200 μm. Deeply lobed leaves, a phenotype characteristic of gain-of-function KNOX1 alleles, occur in Arabidopsis plants where STM is driven by the leaf specific promoter, proBLS, proBLS:STM ([39]; S14B, C Fig.). These were crossed with loss-of-function KNOX2 plants (pro35S:amiR159-KNAT345–1) to generate plants with ectopic KNOX1 and reduced KNOX2 activities in the leaves. Compared to the parental lines, F1 plants harboring both transgenes displayed more extreme leaf margin elaboration (Fig. 4C-E). The additive effects, rather than epistatic interactions, suggest it is unlikely that the two subclasses negatively regulate one another.
Antagonistic relationship between KNOX1 and KNOX2
An attractive hypothesis for the antagonism between KNOX1 and KNOX2 is that they regulate shared downstream events in an opposite manner. The complex leaf of gain-of-function KNOX1 alelles is suppressed by reduction in CUP SHAPED COTYLEDON (CUC) transcription factor activity [40] (S14C Fig.). Two CUC genes are targeted by the miR164 family of miRNAs, and expression of miR164b in young leaves using regulatory sequences of the FILAMENTOUS FLOWER (FIL) gene (designated as proFIL), proFIL:miR164b, flattens the leaf margin in wild-type plants (S14D-E Fig.). Thus, the miRNA-mediated CUC regulation plays a key role in leaf margin elaboration [41]. Introduction of proFIL:miR164b also suppressed the leaf dissection phenotype in a knat345 mutant background (S14F-G Fig.). Among miR164 targets, CUC2 plays a major role in leaf serration development [41]. We find leaf serration is largely suppressed in the cuc2 knat345 and pro35S:amiR159-KNAT345–1 cuc2 backgrounds (Fig. 5A-C and S14H-I Fig.). In addition, constitutive expression of KNOX2 (pro35S:KNAT3) can partially suppress the proBLS:STM leaf phenotype (Fig. 5D-F). Thus, a common developmental program mediates both gain-of-function KNOX1 and loss-of-function KNOX2 leaf phenotypes.
Fig. 5. KNOX1 and KNOX2 converge on CUC activity. 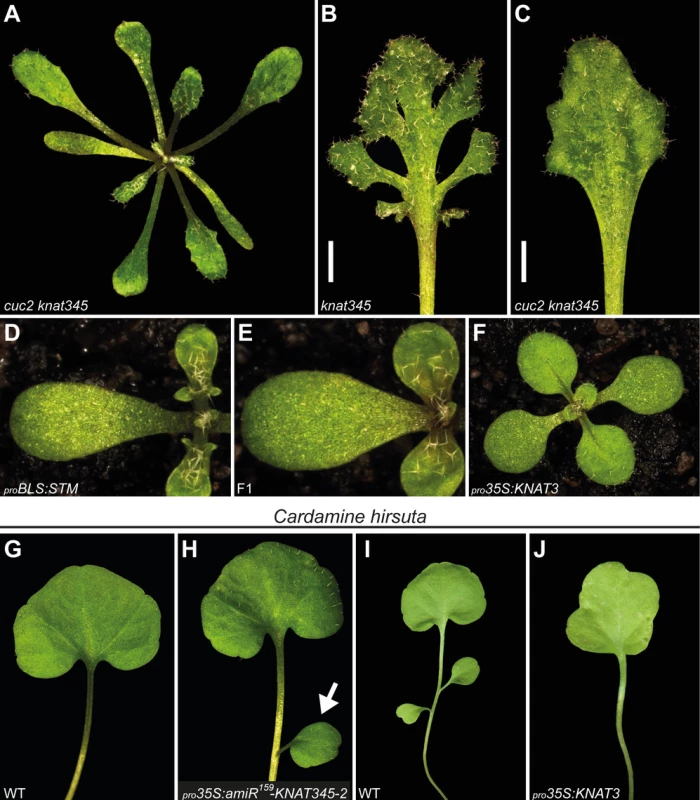
(A) One-month old cuc2 knat345 quadruple mutant. A mutation in the CUC2 gene largely suppresses the leaf serration phenotype of knat345 plants. Compare with a knat345 plant in Fig. 2E. See S13 Fig. for additional data. (B-C) Representative knat345 (B) and cuc2 knat345 (C) rosette leaves, demonstrating marginal leaf lobing is suppressed by a cuc2 mutation. Shown are the 10th leaves from 2 month-old plants of each genotype. Note that other mutant phenotypes, including leaf size and female sterility, are not suppressed by the cuc2 mutation. (D-F) pro35S:KNAT3 partially suppresses the leaf lobing phenotype of proBLS:STM plants. 12 days-old plants are shown. (G-H) Second leaves of wild-type (G) and pro35S:amiR159-KNAT345–2 (H) Cardamine hirsuta plants grown for four weeks. (G) In wild-type plants, the first and second leaves always consist of a single, undivided, lamina. (H) Reducing KNOX2 activity by consitutively expressing an amiRNA that targets Cardamine hirsuta orthologues of KNAT3, KNAT4, and KNAT5 genes (pro35S:amiR159-KNAT345–2) results in plants with an extra lateral leaflet (marked with an arrow) on the second leaf. (I-J) Third leaves removed from one month-old wild-type (I) and pro35S:KNAT3 (J) Cardamine hirsuta plants. (I) In wild-type plants, the third leaf typically consists of three leaflets. (J) Introduction of gain-of-function KNOX2 alleles (constitutive expression of the KNAT3 gene from Arabidopsis; pro35S:KNAT3) results in an undivided third leaf in strong lines. Plants in (A-F) are all in the Col background. As observed in proBLS:STM plants, elevated levels of KNOX1 activity are often associated with increased leaf complexity (reviewed in [11]). In Cardamine hirsuta, a close relative of Arabidopsis, dissected leaf development requires KNOX1 expression in leaves, and additional KNOX1 expression leads to ectopic leaflet initiation [42]. We investigated the outcome of reduction in the level of KNOX2 activity in this species. In Cardamine, leaf shape exhibits heteroblasty with leaflet number increasing in later produced leaves. Although leaflet number can vary for a particular leaf position, the first and second leaves always consist of a single, undivided, lamina, and the third leaf typically consisting of three leaflets (S15A Fig.). An amiRNA, amiR159-KNAT345–2, was designed to target three Cardamine genes homologous to Arabidopsis KNAT3, KNAT4, and KNAT5. Constitutive amiR159-KNAT345–2 expression (pro35S:amiR159-KNAT345–2; S7C Fig.) results in plants with an extra lateral leaflet on the second leaf, observed in approximately 15% of individuals (27 of 188 plants derived from 6 independent lines), indicating KNOX2 activity influences complexity of dissected leaves in Cardamine (Fig. 5G-H). Furthermore, gain-of-function KNOX2 alleles (pro35S:KNAT3) simplify leaf shape, a phenotype particularly obvious in third leaves, which are undivided in strong lines (Fig. 5I-J and S15B Fig.). Thus, reduction or increase in KNOX2 activity leads to increase or decrease in leaf complexity, respectively, in Cardamine (Fig. 5 and S15 Fig.). This observation and the deduced KNOX1/KNOX2 antagonism are in consistent with the results in Arabidopsis.
Discussion
Arabidopsis KNOX2 genes act redundantly to promote differentiation of all aerial organs in a manner broadly antagonistic to the action of KNOX1 genes. Loss-of-function KNOX2 alleles exhibit phenotypes with attributes of those of gain-of-function KNOX1 alleles, and vice versa, both in the maintenance of the shoot apical meristem and in the development of leaf complexity. In both contexts, KNOX2 functions to suppress meristematic capability, while KNOX1 promotes or maintains it. Our observations suggest that following the gene duplication giving rise to the KNOX1 and KNOX2 paralogs in an ancestor of land plants, neofunctionalization led to evolution of antagonistic biochemical activity thereby facilitating morphological evolution. Given the highly conserved nature of KNOX1 and KNOX2 genes in land plants, the antagonistic relationship may be a general phenomenon of diverse species.
Three Arabidopsis KNOX2 genes, KNAT3, KNAT4, and KNAT5, act redundantly in regulating plant development. Distinct phenotypes of double mutant combinations, however, indicate various degrees of contributions among three genes. For instance, knat3 knat4 and knat3 knat5 plants have more deeply serrated leaves, whereas knat4 knat5 plants appear phenotypically wild type. Distinctive expression patterns may explain different phenotypic consequences in mutants [20], or alternatively, the potency of three KNOX2 proteins may vary owing to structural differences, and the different relative contributions of the three genes to leaf development can be seen as a process of subfunctionalization. Although Arabidopsis KNOX2 genes are expressed in the root, no overt phenotype was recognized in triple mutant roots, perhaps due to genetic redundancy with the fourth KNOX2 gene, KNAT7. In addition to their developmental roles, KNOX2 genes may play an undetected physiological role as they are expressed in senescing leaves (S4 Fig.) and have been reported to have a role involved in seed germination and early seedling development through modulating ABA responses [22]. Characterization of the quadruple mutants and physiological experiments may illuminate additional cryptic mutant phenotypes of KNOX2 genes.
Evolution of leaf complexity
Expression of KNOX1 genes in leaves is correlated with increased leaf complexity and has been hypothesized to be influential in the evolution of leaf shape [42,43,44]. Given that seed plants leaves evolved from ancestral shoot systems, the ancestral seed plant leaf was likely complex, but fossil evidence and phylognetic analyses suggest that the ancestral angiosperm leaf may have been simple [45]. Regardless of the ancestral state, transitions from simple to more complex and vice versa have occurred repeatedly during angiosperm evolution [43,44,46]. In angiosperms, increase in leaf complexity is associated with increased KNOX1 activity while loss of KNOX1 activity in leaves results in decreasing complexity. While KNOX1 activity has been shown to play a pivotal role, other loci, such as REDUCED COMPLEXITY (RCO) in Cardamine and LEAFY (LFY) orthologues in legumes either contribute directly to modifying leaf shape or influence sensitivity to KNOX1 activity [11,47,48]. The lability of angiosperm leaf architecture may reflect that addition or loss of enhancer modules directing KNOX1 activity in leaves does not affect general plant viability.
The present study demonstrates that KNOX2 activities can also influence leaf shape—leaf dissection increases with decreasing KNOX2 activity (Fig. 2) in a dose dependent manner—raising the possibility of whether changes in KNOX2 activity could also have contributed to the evolution of leaf morphology. Just as KNOX1 gain-of-function alleles result in increases in leaf complexity, novel gain-of-function KNOX2 alleles that alter temporal or spatial expression patterns within developing leaves could contribute to the evolution from complex towards simple leaf morphology, as suggested by our experimental results in Cardamine, via acquisition of leaf specific enhancers. Alleles resulting in loss of KNOX2 activity could also contribute to increases in leaf complexity as suggested by the dose dependent changes to leaf shape in Arabidopsis, however, this may be less likely due to pleiotropic effects of loss-of-function KNOX2 alleles.
Intriguingly, in monilophytes KNOX1 gene expression is broadly similar to that of seed plants, with expression limited to less differentiated tissues including the shoot apical meristem, developing leaves, and procambial tissues [43,49,50]. KNOX2 gene expression has not been studied in detail, but similar to the situation in angiosperms, is reported to be throughout the sporophyte body [50]. In parallel with seed plants, simple leaves have evolved from more complex ancestral leaves within monilophytes [51]. Whether changes in KNOX1 or KNOX2 gene expression may be related to evolution of leaf form in monilophytes is presently unknown.
Nature of the KNOX1/KNOX2 antagonistic relationship
One plausible explanation for the opposing action of KNOX1 and KNOX2 genes is an epistatic relationship between the gene classes. While non-overlapping expression patterns have been observed between KNOX1 and KNOX2 genes, we found no evidence for mutual repression. Alternatively, KNOX1 and KNOX2 proteins may interfere one another’s activity. Such a mode of action was proposed for KNATM in Arabidopsis and PETROSELINUM (PTS)/TKD1 in tomato, both of which are KNOX-related proteins that lack a DNA-binding homeodomain [52,53]. It is suggested that these mini KNOX proteins act as passive repressors and interfere with formation of a functional complex composed of canonical KNOX and BELL proteins. That KNOX2 function depends on the availability of appropriate BELL partners to be active, argues against a similar mechanism for the KNOX1/KNOX2 antagonism. Instead, our data favor a model whereby the antagonistic roles of KNOX1 and KNOX2 are at the level of opposing modes of transcriptional regulation.
Since addition of a repressor domain causes a dominant negative phenotype, KNOX1 proteins can act as activators [39,54]. Conversely a KNOX2 protein, KNAT7, can repress transcription in a transient protoplast system [18,19], and a motif similar to known repression domains is found in the ELK domain of all KNOX2 proteins [55] (S16 Fig.). Comparison of KNOX1 and KNOX2 homeodomains reveals that the third helices, an important determinant of DNA binding specificity, are highly conserved, indicating similar DNA binding properties, at least in vitro (S16 Fig.). Concurrently expressed KNOX1 and KNOX2 proteins could thus conceivably compete with each other at some target genes. Indeed, a putative KNOX2-SAW2 complex can overcome endogenous KNOX1 activities in the meristem, as does a dominant-negative form of KNOX1 (e.g., TKN2-SRDX [39] and en298-STM [54]). However, as KNOX1 proteins have also been reported to act to repress gene expression, the activity of KNOX proteins may be modified by either BELL partners, or third parties, such as OVATE proteins that interact with KNOX/BELL heterodimers and influence both their cellular localization and transcriptional activity [32,37,56]. In a related scenario, KNOX1 and KNOX2 could act on different sets of paralogs of downstream targets. These hypotheses are not mutually exclusive, and depending on the cellular contexts, different modes of action could operate, as is the case for the yeast TALE protein, Matα2, which has different partners in different cell types (reviewed in [23]).
Phylogenetic analyses indicate land plant KNOX1 and KNOX2 genes are derived from a single, ancestral KNOX gene. We hypothesize that subsequent to the KNOX1/KNOX2 gene duplication, accumulating structural differences endowed a new mode of action to at least one paralog. Therefore a possible evolutionary scenario could have an ancestral KNOX protein acting primarily as a transcriptional activator, with the evolution of a transcriptional repressor following gene duplication and neofunctionalization. The evolution of a repressor from an ancestral activator may be a common event, with several instances documented in plant transcription factor families [52,53,57,58,59,60]. Thus, within the context of land plant KNOX genes two types of negative regulators, in which the modes of repressor action are mechanistically different, may have evolved. Mini KNOX proteins act to inhibit KNOX activity by interacting with and sequestering BELL proteins [52,53], as opposed to antagonistic action at the level of downstream gene expression as we propose for KNOX2. The latter provides more flexibility due to the potential to act independently. Accompanying divergence in protein functionality, our data provides additional evidence for nearly complementary expression patterns of KNOX1 and KNOX2 genes in Arabidopsis thaliana. In contrast, in P. patens KNOX1 and KNOX2 genes exhibit both overlapping and distinctive expression patterns [14,26]. Changes in cis-regulatory sequences must have contributed to the establishment of complementary expression patterns during land plant evolution. Flexibility in gene regulatory networks governing meristematic maintenance and differentition engendered by the combination of changes in protein functionality and expression pattern could provide plasticity enabling morphological evolution.
Diversification of KNOX/BELL modules
Heterodimerization between BELL and KNOX proteins is important for translocation of the complex into the nucleus [13]. BELL-KNOX2 heterodimerization may also be critical for providing specificity or increasing affinity of DNA binding (e.g. [61]). Although studies based on the yeast two-hybrid technique suggest physical interactions between BELL and KNOX proteins in a rather nonspecific manner [29,32], our genetic data suggest KNOX2 proteins interact in planta with a subset of BELL proteins, including those of the BEL1/SAW1/SAW2 clade. KNOX1 proteins rely on a distinct set of BELL proteins, e.g. PNY and PNF (reviewed in [12,13]). Due to an obligate heterodimerization requirement, the activity of a KNOX/BELL pair may be limited by the protein with the more restricted expression domain. In Arabidopsis KNOX2 functions appear to be regulated by restricted availability of corresponding BELL partners [29] (Fig. 3).
Similar to KNOX genes, land plant BELL genes evolved from a single gene in the algal ancestor [9]. However, the diversification of paralogs followed a different trajectory in the two families since BELL genes do not fall into discrete functional clades (S17 Fig.). For instance, KNOX1-interacting BELL genes (PNY and PNF) form a sister clade with KNOX2-interacting BELL genes (BEL1 and SAW1/2). Moreover, genetic interactions implicate BLH1, from a phylogenetically distinct clade, as a KNOX2 partner since knat3 alleles suppress the phenotype induced by ectopic BLH1 embryo sac expression [37]. These phylogenetic relationships might be expected if the genome of the land plant common ancestor encoded a single BELL protein that interacted with both KNOX1 and KNOX2 proteins. As the BELL gene family diversified, subfunctionalization would have restricted interactions of BELL paralogs to specific KNOX1 or KNOX2 partners.
KNOX1/KNOX2 gene duplication and land plant evolution
The defining feature of land plants is the formation of an embryo—a multicellular diploid generation. One prominent feature within land plant evolution is the transition from a gametophyte-dominant life cycle to a sporophyte-dominant life cycle [62,63]. This process is regarded as progressive sterilization and elaboration of vegetative organs [62], and in flowering plants, the gametophyte is reduced to a ephemeral structure of only a few cells that is dependent on a sporophyte body that can live up to thousands of years. If the ancestral KNOX-BELL genetic program regulated gene expression in a single celled zygote [25], it follows that during the course of land plant evolution, the KNOX/BELL module has been recruited to control numerous aspects of sporophyte development, with KNOX1/BELL modules promoting meristematic maintenance and continued growth and KNOX2/BELL modules promoting differentiation. In some cases, there is resemblance to a presumed ancestral function, such as in P. patens where KNOX2 genes regulate the gametophyte-to-sporophyte morphological transition [14,26]. In other cases, however, KNOX/BELL modules direct the development of novel structures, such as sporophyte shoot meristems and leaves (Fig. 6), that evolved later in land plant evolution, suggesting the duplication and diversification of the KNOX/BELL genetic module is linked with the evolution of morphological diversity in the land plant sporophyte. Neofunctionalization, exemplified by opposing activities between KNOX1 and KNOX2 genes in Arabidopsis, may underlie the molecular mechanism of key innovations and modification of body plans in the land plant history, through elaboration of transcriptional networks.
Fig. 6. Proposed KNOX functions during land plant evolution. 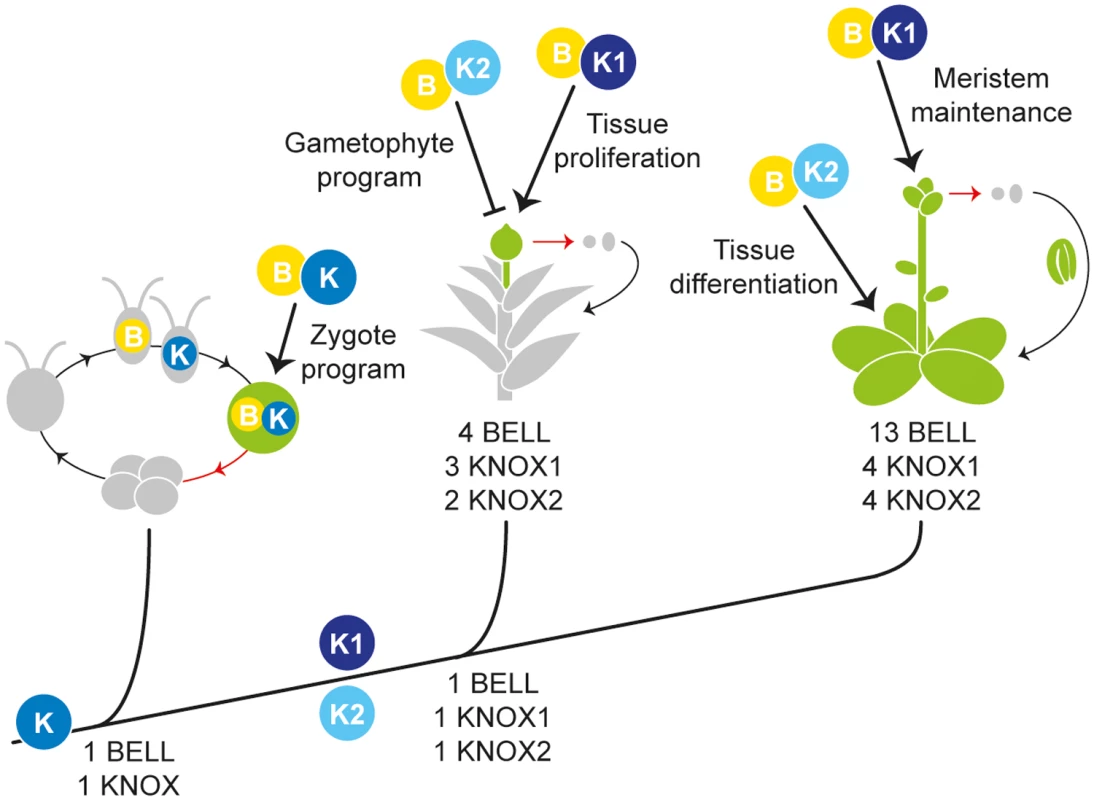
Along the phylogeny of plants, the primary functions for BELL (depicted as B) and KNOX (K) proteins, as well as the gene copy number, are presented for Chlamydomonas (a unicellular Chlorophyte alga), Physcomitrella (a moss), and Arabidopsis (a flowering plant). The ancestral conditions at branches were deduced from our phylogenetic analyses (S1 and S17 Figs.). In each life cycle, a red arrow indicates meiosis, and haploid (grey) and diploid (green) stages are color-coded. In Chlamydomonas the plus gamete expresses a BELL (depicted as B) protein while the minus gamete expresses a KNOX (K) protein; upon gamete fusion the KNOX and BELL proteins heterodimerize and regulate zygotic gene expression. Prior to the origin of land plants, a gene duplication in an ancestral KNOX gene generated two subclasses, KNOX1 (K1) and KNOX2 (K2) genes. In Physcomitrella, KNOX1 activity maintains tissue proliferation during sporophyte (diploid) development while KNOX2 represses the haploid genetic program during the diploid generation. In Arabidopsis, KNOX1 activity promotes meristem maintenance, and our study demonstrates that KNOX2 activity promotes tissue differentiation, perhaps via repression of meristematic functions, in the diploid generation. We propose that (1) the gene duplication producing KNOX1 and KNOX2 paralogs and ensuing neofunctionalization was instrumental in the evolution of a complex multicellular diploid generations in land plants and (2) the diversification of KNOX/BELL modules during land plant evolution facilitated the evolution of ever more complex diploid sporophyte body plans. The role of TALE genes in fungi and Chlamydomonas can be viewed as promotion of cellular specialization in the diploid zygote and progression towards a meiotic state. The life cycle of land plants arose by an interpolation of mitotic divisions between fertilization and meiosis. Thus there is cell proliferation and a delay in meiosis in the diploid generation. KNOX1 genes prevent differentiation and maintain an undifferentiated state of the cells, enabling the cells to proliferate and develop a multicellular body in the sporophyte generation. In organisms with two heteromorphic multicellular generations, such as land plants, the developmental programs for each must be tightly controlled—a role suggested for KNOX2 genes in preventing the haploid gametophyte genetic program to be active during the diploid sporophyte generation in Physcomitrella. We hypothesize the duplication and diversification of the KNOX/BELL genetic module was instrumental in the evolution of a diploid embryo such that multicellular bodies develop in both haploid gametophyte and diploid sporophyte generations known as alternations of generations [25,26]. Alternations of generations have evolved independently in phylogenetically diverse eukaryotic lineages [64,65], prompting the question of whether similar TALE class genetic diversification may be found in these lineages.
Materials and Methods
Plant material and growth conditions
Arabidopsis thaliana accessions Columbia and Landsberg erecta (Ler) were used as wild type in most experiments. proKNAT2:GUS was generated in the C24 background and introgressed into Ler. Cardamine hirsuta ‘Oxford strain’ is a kind gift of A. Hay and M. Tsiantis. Plants were grown under long-day (18 hours light) or short-day (10 hours light) conditions at 20°C. knat3 and knat5 alleles are gift from V. Sundaresan and G. Pagnussat. bp-9 knat2–5 knat6–1 seeds are gift from V. Pautot. T-DNA insertion alleles for BELL and KNOX genes were obtained from the Arabidopsis Biological Resource Center (ABRC) or the Nottingham Arabidopsis Stock Center (NASC). Mutant and transgenic lines have been described previously: bp-9 knat2–5 knat6–1 [66]; stm-11 [67]; proBP:GUS [68]; proKNAT2:GUS [69]; Op:KNAT2 and Op:STM [39]; and proSTM:LhG4 [70]. The mutant and transgenic lines used in this study are listed in S1 Table. Homozygous mutant lines were identified by polymerase chain reaction (PCR)-based genotyping. Sequences of genotyping primers are available in S2 Table. The details of the transactivation system was previously described [33].
Genetics
Multiple mutants combining knat3, knat4, and knat5 alleles were generated by crossing, and genotypes were confirmed by PCR-based genotyping. To generate bel1 blh1 double mutant, blh1 plants were crossed with bel1 plants, and the resulting F2 plants were examined. bel1 plants were identified based on self-sterility, and among them, plants with yellow gynoecia segregated and were confirmed to be bel1 blh1 double mutant plants by PCR-based genotyping. knat2 knat3 knat5 knat6 and bp knat345 plants were identified among F2 plants originating from a cross between bp knat2 knat6 and knat345 plants, and their genotypes were confirmed by PCR-based genotyping. cuc2 knat345 plants were identified in a F2 population derived from a cross between cuc2 and knat345 plants. To generate proFIL:miR164b lines in the knat345 mutant, self-fertile knat3 knat5 plants were transformed with the proFIL:miR164b construct, and tranformants were selected by resistance to herbicide Basta. Single insertion lines were selected and crossed with knat3/+ knat4 knat5 plants. Among F1 plants, self-fertile knat3/+ knat4/+ knat5 plants carrying the proFIL:miR164b transgene were selected, and F2 seeds were collected; proFIL:miR164b knat345 plants were identified in the resultant F2 population. To characterize the effects of the pro35S:amiR159-KNAT345–1 transgene in mutant backgrounds, the mutant plants were directly transformed with the pro35S:amiR159-KNAT345–1 construct, and transformants were selected by resistance to Basta. As stm null alleles are seedling lethal, heterozygous plants were used for transformation. More than twenty T1 plants for each background were examined, and phenotypes consistently observed among independent lines were reported.
Semi-quantitative RT-PCR
RNA was extracted, using the RNeasy Plant Mini Kit (Qiagen), from 10-day-old seedlings grown on half-strength MS medium supplemented with 0.5% sucrose. RNA samples were treated with on-column DNaseI (Qiagen) and purified. SMARTScribe reverse transcriptase was used for cDNA synthesis (Clontech), and PCR reactions were performed using Ex Taq (Takara). Oligo sequences used for PCR reactions are described in S3 Table.
Plasmid construction and plant transformation
amiRNAs were designed using the Arabidopsis pre-miR159a backbone (S7 Fig.) and synthesized (GenScript). For construction of the proKNAT5:KNAT5-GUS reporter construct, the genomic sequence spanning the KNAT5 locus (from the next upstream annotated gene [At4g32030] to the next downstream annotated gene [At4g32050]) was used, and the stop codon was replaced with the GUS coding sequence. For construction of the proKNAT4:GUS reporter construct, an approximately 6.6-kb region of the sequence directly upstream of the KNAT4 coding sequence was amplified using BAC T5K6 as PCR template and cloned into pCRII-TOPO (Invitrogen). The KNAT4 upstream sequence was subcloned into the pRITA vector, which contains the GUS coding sequence and the terminator sequence from the nopaline synthase gene. For constitutive expression, the amiRNA sequences or the KNAT3 coding sequence were cloned into the ART7 vector, which contains the Cauliflower mosaic virus pro35S sequence and the terminator sequence from the octopine synthase gene. KNAT5, SAW2, and PNY coding sequences were amplified from Ler cDNA and cloned downstream of an Lac Op array [33] to generate responder cassettes used in the transcription activation system. All constructs were subcloned into pMLBART or pART27 binary vector and were introduced into Agrobacterium tumefaciens strain GV3001 by electroporation. Transgenic lines were generated by Agrobacterium-mediated transformation, and transformants were selected on soil on the basis of resistance to the BASTA or kanamycin. Primers used to clone the various cDNAs and promoters are described in S2 Table.
Histology and microscopy
Scanning electron microscopy was performed according to Alvarez and Smyth [71]. For light microscopy, cleared samples were prepared. Leaf samples were fixed overnight in 9 : 1 (v:v) ethanol:acetic acid at room temperature. After rehydration in a graded ethanol series, samples were rinsed with water and were cleared with chloral hydrate solution [1 : 8:2 (v:w:v) glycerol:chloral hydrate:water]. For histochemical analysis of GUS activity, samples were infiltrated with GUS staining solution [0.2% (w/v) Triton X-100, 2 mM potassium ferricyanide, 2 mM potassium ferrocyanide, and 1.9 mM 5-bromo-4-chloro-3-indolyl-β-glucuronide in 50 mM sodium phosphate buffer, pH 7.0] and incubated at 37°C.
Phylogenetic analyses
Publically available KNOX and BELL coding nucleotide sequences representing taxa across land plants were manually aligned as amino acid translations using Se-Al v2.0a11 (http://tree.bio.ed.ac.uk/software/seal/). We excluded ambiguously aligned sequence to produce alignments for subsequent Bayesian analysis. Bayesian phylogenetic analysis was performed using Mr. Bayes 3.2.1 [72,73]. Three separate analyses were performed. The first included Chlorophyte algal and land plant KNOX sequences (S1 Fig.); the second included only land plant KNOX2 sequences (S2 Fig.); and the third included land plant BELL sequences (S17 Fig.). The fixed rate model option JTT + I was used based on analysis of the alignments with ProTest 2.4 [74]. Sequence alignments and command files used to run the Bayesian phylogenetic analyses are provided upon request.
Supporting Information
Zdroje
1. Ohno S (1970) Evolution by Gene Duplication. Heidelberg, Germany: Springer-Verlag.
2. Taylor JS, Raes J (2004) Duplication and divergence: The evolution of new genes and old ideas. Annu Rev Genet 38 : 615–643. 15568988
3. Conant GC, Wolfe KH (2008) Turning a hobby into a job: How duplicated genes find new functions. Nat Rev Genet 9 : 938–950. doi: 10.1038/nrg2482 19015656
4. Davis RL, Turner DL (2001) Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 20 : 8342–8357. 11840327
5. Bertolino E, Reimund B, WildtPerinic D, Clerc RG (1995) A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J Biol Chem 270 : 31178–31188. 8537382
6. Burglin TR (1997) Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res 25 : 4173–4180. 9336443
7. Derelle R, Lopez P, Le Guyader H, Manuel M (2007) Homeodomain proteins belong to the ancestral molecular toolkit of eukaryotes. Evol Dev 9 : 212–219. 17501745
8. Mukherjee K, Brocchieri L, Burglin TR (2009) A Comprehensive Classification and Evolutionary Analysis of Plant Homeobox Genes. Mol Biol Evol 26 : 2775–2794. doi: 10.1093/molbev/msp201 19734295
9. Kerstetter R, Vollbrecht E, Lowe B, Veit B, Yamaguchi J, et al. (1994) Sequence-Analysis and Expression Patterns Divide the Maize Knotted1-Like Homeobox Genes into 2 Classes. Plant Cell 6 : 1877–1887. 7866030
10. Vollbrecht E, Veit B, Sinha N, Hake S (1991) The Developmental Gene Knotted-1 Is a Member of a Maize Homeobox Gene Family. Nature 350 : 241–243. 1672445
11. Efroni I, Eshed Y, Lifschitz E (2010) Morphogenesis of Simple and Compound Leaves: A Critical Review. Plant Cell 22 : 1019–1032. doi: 10.1105/tpc.109.073601 20435903
12. Hake S, Smith HMS, Holtan H, Magnani E, Mele G, et al. (2004) The role of knox genes in plant development. Annu Rev Cell Dev Biol 20 : 125–151. 15473837
13. Hay A, Tsiantis M (2010) KNOX genes: versatile regulators of plant development and diversity. Development 137 : 3153–3165. doi: 10.1242/dev.030049 20823061
14. Sakakibara K, Nishiyama T, Deguchi H, Hasebe M (2008) Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella patens but do function in sporophyte development. Evol Dev 10 : 555–566. doi: 10.1111/j.1525-142X.2008.00271.x 18803774
15. Serikawa KA, MartinezLaborda A, Kim HS, Zambryski PC (1997) Localization of expression of KNAT3, a class 2 knotted1-like gene. Plant J 11 : 853–861. 9161040
16. Serikawa KA, MartinezLaborda A, Zambryski P (1996) Three knotted1-like homeobox genes in Arabidopsis. Plant Mol Biol 32 : 673–683. 8980519
17. Janssen BJ, Williams A, Chen JJ, Mathern J, Hake S, et al. (1998) Isolation and characterization of two knotted-like homeobox genes from tomato. Plant Mol Biol 36 : 417–425. 9484482
18. Li EY, Bhargava A, Qiang WY, Friedmann MC, Forneris N, et al. (2012) The Class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus. New Phytol 194 : 102–115. doi: 10.1111/j.1469-8137.2011.04016.x 22236040
19. Li EY, Wang SC, Liu YY, Chen JG, Douglas CJ (2011) Ovate Family Protein4 (Ofp4) Interaction with Knat7 Regulates Secondary Cell Wall Formation in Arabidopsis Thaliana. Plant J 67 : 328–341. doi: 10.1111/j.1365-313X.2011.04595.x 21457372
20. Truernit E, Siemering KR, Hodge S, Grbic V, Haseloff J (2006) A map of KNAT gene expression in the Arabidopsis root. Plant Mol Biol 60 : 1–20. 16463096
21. Zhong RQ, Lee CH, Zhou JL, McCarthy RL, Ye ZH (2008) A Battery of Transcription Factors Involved in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell 20 : 2763–2782. doi: 10.1105/tpc.108.061325 18952777
22. Kim D, Cho YH, Ryu H, Kim Y, Kim TH, et al. (2013) BLH1 and KNAT3 modulate ABA responses during germination and early seedling development in Arabidopsis. Plant J 75 : 755–766. doi: 10.1111/tpj.12236 23663178
23. Herskowitz I (1989) A Regulatory Hierarchy for Cell Specialization in Yeast. Nature 342 : 749–757. 2513489
24. Kues U, Richardson WVJ, Tymon AM, Mutasa ES, Gottgens B, et al. (1992) The Combination of Dissimilar Alleles of the a-Alpha and a-Beta Gene Complexes, Whose Proteins Contain Homeo Domain Motifs, Determines Sexual Development in the Mushroom Coprinus-Cinereus. Genes Dev 6 : 568–577. 1348484
25. Lee J-H, Lin H, Joo S, Goodenough U (2008) Early Sexual Origins of Homeoprotein Heterodimerization and Evolution of the Plant KNOX/BELL Family. Cell 133 : 829–840. doi: 10.1016/j.cell.2008.04.028 18510927
26. Sakakibara K, Ando S, Yip HK, Tamada Y, Hiwatashi Y, et al. (2013) KNOX2 Genes Regulate the Haploid-to-Diploid Morphological Transition in Land Plants. Science 339 : 1067–1070. doi: 10.1126/science.1230082 23449590
27. Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, et al. (2006) Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18 : 1134–1151. 16603651
28. Serikawa KA, Zambryski PC (1997) Domain exchanges between KNAT3 and KNAT1 suggest specificity of the kn1-like homeodomains requires sequences outside of the third helix and N-terminal arm of the homeodomain. Plant J 11 : 863–869. 9161041
29. Kumar R, Kushalappa K, Godt D, Pidkowich MS, Pastorelli S, et al. (2007) The Arabidopsis BEL1-LIKE HOMEODOMAIN proteins SAW1 and SAW2 act redundantly to regulate KNOX expression spatially in leaf margins. Plant Cell 19 : 2719–2735. 17873098
30. Reiser L, Modrusan Z, Margossian L, Samach A, Ohad N, et al. (1995) The Bell1 Gene Encodes a Homeodomain Protein Involved in Pattern-Formation in the Arabidopsis Ovule Primordium. Cell 83 : 735–742. 8521490
31. Robinson-Beers K, Pruitt RE, Gasser CS (1992) Ovule Development in Wild-Type Arabidopsis and 2 Female-Sterile Mutants. Plant Cell 4 : 1237–1249. 12297633
32. Hackbusch J, Richter K, Muller J, Salamini F, Uhrig JF (2005) A central role of Arabidopsis thaliana ovate family proteins in networking and subcellular localization of 3-aa loop extension homeodomain proteins. Proc Natl Acad Sci U S A 102 : 4908–4912. 15781858
33. Moore I, Galweiler L, Grosskopf D, Schell J, Palme K (1998) A transcription activation system for regulated gene expression in transgenic plants. Proc Natl Acad Sci U S A 95 : 376–381. 9419383
34. Barton MK, Poethig RS (1993) Formation of the Shoot Apical Meristem in Arabidopsis-Thaliana—an Analysis of Development in the Wild-Type and in the Shoot Meristemless Mutant. Development 119 : 823–831.
35. Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, et al. (2006) KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 18 : 1900–1907. 16798887
36. Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 : 66–69. 8538741
37. Pagnussat GC, Yu HJ, Sundaresana V (2007) Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 19 : 3578–3592. 18055603
38. Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T (1996) The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J 10 : 967–979. 9011081
39. Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, et al. (2009) Stage-Specific Regulation of Solanum lycopersicum Leaf Maturation by Class 1 KNOTTED1-LIKE HOMEOBOX Proteins. Plant Cell 21 : 3078–3092. doi: 10.1105/tpc.109.068148 19820191
40. Hasson A, Plessis A, Blein T, Adroher B, Grigg S, et al. (2011) Evolution and Diverse Roles of the CUP-SHAPED COTYLEDON Genes in Arabidopsis Leaf Development. Plant Cell 23 : 54–68. doi: 10.1105/tpc.110.081448 21258003
41. Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, et al. (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18 : 2929–2945. 17098808
42. Hay A, Tsiantis M (2006) The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet 38 : 942–947. 16823378
43. Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, et al. (2002) Homologies in leaf form inferred from KNOXI gene expression during development. Science 296 : 1858–1860. 12052958
44. Piazza P, Bailey CD, Cartolano M, Krieger J, Cao J, et al. (2010) Arabidopsis thaliana Leaf Form Evolved via Loss of KNOX Expression in Leaves in Association with a Selective Sweep. Curr Biol 20 : 2223–2228. doi: 10.1016/j.cub.2010.11.037 21129970
45. Doyle JA (1998) Phylogeny of vascular plants. Annu Rev Ecol Syst 29 : 567–599.
46. Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, et al. (2011) The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol 38 : 535–552.
47. Hofer J, Turner L, Hellens R, Ambrose M, Matthews P, et al. (1997) UNIFOLIATA regulates leaf and flower morphogenesis in pea. Curr Biol 7 : 581–587. 9259553
48. Vlad D, Kierzkowski D, Rast MI, Vuolo F, Dello Ioio R, et al. (2014) Leaf Shape Evolution Through Duplication, Regulatory Diversification, and Loss of a Homeobox Gene. Science 343 : 780–783. doi: 10.1126/science.1248384 24531971
49. Harrison CJ, Corley SB, Moylan EC, Alexander DL, Scotland RW, et al. (2005) Independent recruitment of a conserved developmental mechanism during leaf evolution. Nature 434 : 509–514. 15791256
50. Sano R, Juarez CM, Hass B, Sakakibara K, Ito M, et al. (2005) KNOX homeobox genes potentially have similar function in both diploid unicellular and multicellular meristems, but not in haploid meristems. Evol Dev 7 : 69–78. 15642091
51. Vasco A, Moran RC, Ambrose BA (2013) The evolution, morphology, and development of fern leaves. Front Plant Sci 4. doi: 10.3389/fpls.2013.00544 24575101
52. Kimura S, Koenig D, Kang J, Yoong FY, Sinha N (2008) Natural variation in leaf morphology results from mutation of a novel KNOX gene. Curr Biol 18 : 672–677. doi: 10.1016/j.cub.2008.04.008 18424140
53. Magnani E, Hake S (2008) KNOX lost the OX: The Arabidopsis KNATM gene defines a novel class of KNOX transcriptional regulators missing the homeodomain. Plant Cell 20 : 875–887. doi: 10.1105/tpc.108.058495 18398054
54. Markel H, Chandler J, Werr W (2002) Translational fusions with the engrailed repressor domain efficiently convert plant transcription factors into dominant-negative functions. Nucleic Acids Res 30 : 4709–4719. 12409462
55. Mitsuda N, Ohme-Takagi M (2009) Functional Analysis of Transcription Factors in Arabidopsis. Plant Cell Physiol 50 : 1232–1248. doi: 10.1093/pcp/pcp075 19478073
56. Wang S, Chang Y, Guo J, Chen JG (2007) Arabidopsis Ovate Family Protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J 50 : 858–872. 17461792
57. Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci U S A 102 : 7748–7753. 15894619
58. Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, et al. (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. Embo Journal 25 : 605–614. 16424903
59. Wenkel S, Emery J, Hou BH, Evans MMS, Barton MK (2007) A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell 19 : 3379–3390. 18055602
60. Liu C, Xi WY, Shen LS, Tan CP, Yu H (2009) Regulation of Floral Patterning by Flowering Time Genes. Dev Cell 16 : 711–722. doi: 10.1016/j.devcel.2009.03.011 19460347
61. Viola IL, Gonzalez DH (2009) Binding properties of the complex formed by the Arabidopsis TALE homeodomain proteins STM and BLH3 to DNA containing single and double target sites. Biochimie 91 : 974–981. doi: 10.1016/j.biochi.2009.04.021 19442701
62. Bower FO (1908) Origin of a land flora: a theory based on the facts of alternation. London: MacMillan and Co.
63. Haig D (2008) Homologous versus antithetic alternation of generations and the origin of sporophytes. Bot Rev 74 : 395–418.
64. Svedelius N (1927) Alternation of generations in relation to reduction division. Botanical Gazette 83 : 362–384.
65. Cock JM, Godfroy O, Macaisne N, Peters AF, Coelho SM (2014) Evolution and regulation of complex life cycles: a brown algal perspective. Curr Opin Plant Biol 17 : 1–6. doi: 10.1016/j.pbi.2013.09.004 24507487
66. Ragni L, Belles-Boix E, Gunl M, Pautot V (2008) Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 20 : 888–900. doi: 10.1105/tpc.108.058230 18390591
67. Long JA, Barton MK (1998) The development of apical embryonic pattern in Arabidopsis. Development 125 : 3027–3035. 9671577
68. Ori N, Eshed Y, Chuck G, Bowman JL, Hake S (2000) Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127 : 5523–5532. 11076771
69. Dockx J, Quaedvlieg N, Keultjes G, Kock P, Weisbeek P, et al. (1995) The Homeobox Gene Atk1 of Arabidopsis-Thaliana Is Expressed in the Shoot Apex of the Seedling and in Flowers and Inflorescence Stems of Mature Plants. Plant Mol Biol 28 : 723–737. 7647303
70. Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, et al. (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15 : 1566–1571. 16139212
71. Alvarez J, Smyth DR (1999) CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126 : 2377–2386. 10225997
72. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 : 754–755. 11524383
73. Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP (2001) Evolution—Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294 : 2310–2314. 11743192
74. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21 : 2104–2105. 15647292
75. Smith HMS, Hake S (2003) The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15 : 1717–1727. 12897247
76. Byrne ME, Groover AT, Fontana JR, Martienssen RA (2003) Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130 : 3941–3950. 12874117
77. Rutjens B, Bao DP, van Eck-Stouten E, Brand M, Smeekens S, et al. (2009) Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J 58 : 641–654. doi: 10.1111/j.1365-313X.2009.03809.x 19175771
78. Obayashi T, Nishida K, Kasahara K, Kinoshita K (2011) ATTED-II Updates: Condition-Specific Gene Coexpression to Extend Coexpression Analyses and Applications to a Broad Range of Flowering Plants. Plant Cell Physiol 52 : 213–219. doi: 10.1093/pcp/pcq203 21217125
79. Yadav RK, Girke T, Pasala S, Xie MT, Reddy V (2009) Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc Natl Acad Sci U S A 106 : 4941–4946. doi: 10.1073/pnas.0900843106 19258454
80. Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, et al. (2007) An “Electronic Fluorescent Pictograph” Browser for Exploring and Analyzing Large-Scale Biological Data Sets. Plos One 2. doi: 10.1371/journal.pone.0001317 18338032
81. Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31 : 3406–3415. 12824337
Štítky
Genetika Reprodukční medicína
Článek 2014 Reviewer Thank YouČlánek Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic TranslationČlánek Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate CellsČlánek K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in ArabidopsisČlánek A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis inČlánek Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in MiceČlánek The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- Systematic Cell-Based Phenotyping of Missense Alleles Empowers Rare Variant Association Studies: A Case for and Myocardial Infarction
- African Glucose-6-Phosphate Dehydrogenase Alleles Associated with Protection from Severe Malaria in Heterozygous Females in Tanzania
- Genomics of Divergence along a Continuum of Parapatric Population Differentiation
- microRNAs Regulate Cell-to-Cell Variability of Endogenous Target Gene Expression in Developing Mouse Thymocytes
- A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in
- Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
- Partially Redundant Enhancers Cooperatively Maintain Mammalian Expression Above a Critical Functional Threshold
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Mutations in Result in Ocular Coloboma, Microcornea and Cataracts
- A Genome-Wide Hybrid Incompatibility Landscape between and
- Recurrent Evolution of Melanism in South American Felids
- Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate Cells
- Tissue Expression Pattern of PMK-2 p38 MAPK Is Established by the miR-58 Family in
- Essential Role for Endogenous siRNAs during Meiosis in Mouse Oocytes
- Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- RR-1 Cuticular Protein TcCPR4 Is Required for Formation of Pore Canals in Rigid Cuticle
- GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
- K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in Arabidopsis
- A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis in
- HYPER RECOMBINATION1 of the THO/TREX Complex Plays a Role in Controlling Transcription of the Gene in Arabidopsis
- Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan
- Structured Observations Reveal Slow HIV-1 CTL Escape
- An Integrative Multi-scale Analysis of the Dynamic DNA Methylation Landscape in Aging
- Combining Natural Sequence Variation with High Throughput Mutational Data to Reveal Protein Interaction Sites
- Transhydrogenase Promotes the Robustness and Evolvability of Deficient in NADPH Production
- Regulators of Autophagosome Formation in Muscles
- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Eye Selector Logic for a Coordinated Cell Cycle Exit
- Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations
- The DNA Polymerase δ Has a Role in the Deposition of Transcriptionally Active Epigenetic Marks, Development and Flowering
- Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice
- Membrane Recognition and Dynamics of the RNA Degradosome
- P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
- is a Long Non-coding RNA in JNK Signaling in Epithelial Shape Changes during Drosophila Dorsal Closure
- A Pleiotropy-Informed Bayesian False Discovery Rate Adapted to a Shared Control Design Finds New Disease Associations From GWAS Summary Statistics
- Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers
- and Hyperdrive Mechanisms (in Mouse Meiosis)
- Elevated In Vivo Levels of a Single Transcription Factor Directly Convert Satellite Glia into Oligodendrocyte-like Cells
- Systemic Delivery of MicroRNA-101 Potently Inhibits Hepatocellular Carcinoma by Repressing Multiple Targets
- Pooled Sequencing of 531 Genes in Inflammatory Bowel Disease Identifies an Associated Rare Variant in and Implicates Other Immune Related Genes
- Abscission Is Regulated by the ESCRT-III Protein Shrub in Germline Stem Cells
- Temperature Stress Mediates Decanalization and Dominance of Gene Expression in
- Transcriptome Wide Annotation of Eukaryotic RNase III Reactivity and Degradation Signals
- The Exosome Component Rrp6 Is Required for RNA Polymerase II Termination at Specific Targets of the Nrd1-Nab3 Pathway
- Sex-specific -regulatory Variation on the X Chromosome
- Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex
- Modeling of the Human Alveolar Rhabdomyosarcoma Chromosome Translocation in Mouse Myoblasts Using CRISPR-Cas9 Nuclease
- Asymmetry of the Budding Yeast Tem1 GTPase at Spindle Poles Is Required for Spindle Positioning But Not for Mitotic Exit
- TIM Binds Importin α1, and Acts as an Adapter to Transport PER to the Nucleus
- Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
- The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
- Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
- Region-Specific Activation of mRNA Translation by Inhibition of Bruno-Mediated Repression
- An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish ()
- Interaction between the tRNA-Binding and C-Terminal Domains of Yeast Gcn2 Regulates Kinase Activity In Vivo
- Hyper-Acetylation of Histone H3K56 Limits Break-Induced Replication by Inhibiting Extensive Repair Synthesis
- Prodomain Removal Enables Neto to Stabilize Glutamate Receptors at the Neuromuscular Junction
- Recent Selective Sweeps in North American Show Signatures of Soft Sweeps
- Identification and Functional Analysis of Healing Regulators in
- A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2
- Drosophila Casein Kinase I Alpha Regulates Homolog Pairing and Genome Organization by Modulating Condensin II Subunit Cap-H2 Levels
- The Hippo Pathway Regulates Homeostatic Growth of Stem Cell Niche Precursors in the Ovary
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



