-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
Consumption of sugar and lipid (fat) enriched food increases the risk of developing metabolic diseases and cancers. However, lipids are essential molecules for life, as they are the major components of cell membranes. Metabolism refers to biochemical reactions that transform nutrients into molecules required by an organism, although toxic by-products can also formed. Sugars or their derivatives are likely to induce toxic effects by forming stable conjugates with proteins. To neutralize their toxic potential, sugars are metabolized and stored as fat. Here, we have used the fruitfly model to investigate the consequences of lipogenesis deficiency upon ingestion of sugar-enriched diets. We show that lipogenesis deficient animals are dramatically sensitive to dietary sugar. Further, we have identified the sugar by-product responsible for intracellular toxicity, in the context of lipogenesis inhibition. Our study reveals that inhibiting lipogenesis does not disrupt cellular growth if extracellular lipids are available. In contrast lipogenesis inhibition may have deleterious consequences due to accumulation of toxic by-products. The efficacy of lipogenic inhibitors in fighting cancers and metabolic diseases is currently under investigation. Therefore, to evaluate the clinical benefit of these inhibitors, accumulation of the toxic molecules should be monitored in both sick and healthy cells.
Published in the journal: . PLoS Genet 11(2): e32767. doi:10.1371/journal.pgen.1004995
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004995Summary
Consumption of sugar and lipid (fat) enriched food increases the risk of developing metabolic diseases and cancers. However, lipids are essential molecules for life, as they are the major components of cell membranes. Metabolism refers to biochemical reactions that transform nutrients into molecules required by an organism, although toxic by-products can also formed. Sugars or their derivatives are likely to induce toxic effects by forming stable conjugates with proteins. To neutralize their toxic potential, sugars are metabolized and stored as fat. Here, we have used the fruitfly model to investigate the consequences of lipogenesis deficiency upon ingestion of sugar-enriched diets. We show that lipogenesis deficient animals are dramatically sensitive to dietary sugar. Further, we have identified the sugar by-product responsible for intracellular toxicity, in the context of lipogenesis inhibition. Our study reveals that inhibiting lipogenesis does not disrupt cellular growth if extracellular lipids are available. In contrast lipogenesis inhibition may have deleterious consequences due to accumulation of toxic by-products. The efficacy of lipogenic inhibitors in fighting cancers and metabolic diseases is currently under investigation. Therefore, to evaluate the clinical benefit of these inhibitors, accumulation of the toxic molecules should be monitored in both sick and healthy cells.
Introduction
Deregulation of metabolism occurs in several pandemic human diseases whose incidence has dramatically increased due to changes in lifestyle and extended lifespan. These disorders include metabolic syndrome and type 2 diabetes (T2D) that are typified by insulin resistance and elevated levels of glucose and triacylglycerols (TAGs) in the plasma [1,2]. However, insulin resistance does not directly depend on an increase in TAG levels, but is rather a consequence of diacylglycerol and/or ceramides accumulation [1,3,4], whose levels increase as adipose tissue reaches a saturating point [5,6]. Cancer cells also exhibit metabolic perturbations characterized in part by a dramatic increase in glycolysis and fatty acid (FA) synthesis [7,8]. These changes emphasize direct links between sugar catabolism and FA synthesis.
Recent studies support the notion that glycation of proteins, DNA and/or phospholipids is likely to be responsible for the toxic effects induced by excess sugar [9,10]. The resulting compounds, advanced-glycation-end-products (AGEs), maybe responsible for vascular complication, nephropathy and retinal degeneration in T2D patients [11,12]. Glycation is a spontaneous reaction that occurs between an amine group and a carbonyl group of sugars or α-oxoaldehydes [13]. The latter include methylglyoxal (MG) that largely derives from spontaneous oxidation of the glycolytic intermediates dihydroxyacetone-phosphate (DHAP) and glyceraldhehyde-3-phosphate (G3P) [14]. The glyoxalase system [15], an enzymatic system composed of glyoxalase 1 (Glo1) and glyoxalase 2, maintains tolerable levels of MG.
In healthy organisms, circulating glucose is taken up by cells and is used to produce energy through glycolysis and the citric acid cycle. In postprandial condition, dietary glucose is used to synthesize glycogen in the liver and muscles. Excess glucose is also used for FA synthesis in hepatocytes and adipocytes. Synthesis of FA first requires carboxylation of acetyl-CoA to malonyl-CoA by the enzyme ACC (Acetyl-CoA carboxylase) [16]. Next, the Fatty acid synthase (FASN according to the current mammalian nomenclature) sequentially incorporates several malonyl-CoA molecules onto an acetyl-CoA primer to form a long chain FA (LCFA) [17].
Drosophila genetics has proven a powerful model system to investigate metabolic regulation at the level of the organism [18,19,20]. We previously demonstrated that in larvae, ACC is cell-autonomously required for the synthesis and storage of TAGs in the fat body (FB) [21], an insect organ with hepatic and adipose functions. We also provided evidence that within the oenocytes—abdominal cells with a hepatic-like function [22]—ACC is required to maintain the watertightness of the tracheal system [21].
Here, we have focused on the Drosophila FASN orthologs, of which only one (FASNCG3523) is ubiquitously expressed. By directing inducible RNA-interfering (RNAi) to FASNCG3523 and glycogen synthase (GlyS), we observed that the larval FB synthesizes both TAGs and glycogen. Next, we observed that expression of FASNCG3523 is induced by dietary sugar and that FASNCG3523 deficient animals are extremely sensitive to moderate increases in dietary sugar. Furthermore, we provide evidence that the activity of FASN and Glo1 cooperate both at the organismal and cellular level to protect against sugar toxicity.
Results
FASN genes in Drosophila
To investigate the physiological consequences of FA synthesis defect in Drosophila, we focused on the ortholog of the anabolic enzyme FASN, encoded by three distinct genes (FASNCG3523, FASNCG3524, FASNCG17374) [21]. Previous reports show that in larval tissues, FASNCG3523 is ubiquitously expressed, while FASNCG3524 and FASNCG17374 are mostly expressed in the carcass, which is comprised of epidermal cells, oenocytes and skeletal muscles [23]. To corroborate these findings, transcript levels of the three FASN genes were monitored using quantitative-PCR (RT-Q-PCR) in third stage larvae (L3) separated in two fractions, the internal organs, which can be easily removed and the leftover carcass. Consistently, FASNCG3523 transcripts were detected at high levels in both fractions, whereas FASNCG3524 and FASNCG17374 transcripts were detected at high levels in the carcass, but minimally in the internal organs (S1A Fig.).
To determine whether these enzymes are essential, we made use of the binary Gal4/UAS system to direct specific RNAi to each FASN gene and to the ACC orthologue [21]. Ubiquitous knockdown of these genes caused lethality at late embryogenesis for ACC, at L1 stage for FASNCG3523, at the L2 stage for FASNCG17374, while no phenotype was observed for FASNCG3524 (Table 1). The lethality at the L2 stage observed in FASNCG17374-RNAi knockdown resembled the phenotype previously observed when inducing this RNAi using an oenocyte specific driver [21], typified by a defect in the watertightness of the tracheal system (S1B–S1C Fig.). Therefore, we have used a svp-gal80 transgene to inhibit Gal4 in the oenocytes [22]. Driving FASNCG17374-RNAi in the entire animal, except in the oenocytes, resulted in a total rescue of the lethal phenotype (Table 1 and S1D Fig.), indicating that FASNCG17374 does not serve an essential function in other tissues. To get further insights into the organ-specific function of these enzymes, we used the Cg-gal4 and Mef2-gal4 drivers, which are specific to the FB and the muscles, respectively. When induced in either tissue, knockdown to any of either gene did not affect viability (Table 1). Nonetheless, muscle knockdown of ACC or FASNCG3523, but not of FASNCG3524 or FASNCG17374, led to a motility defect in adult flies (Table 1). Taken together, these findings indicate that the synthesis of LCFA is not essential in either the FB or the muscles. However, consistent with previous studies reporting that muscle-specific knockdown of ACC affects body homeostasis and motility of adult flies [24,25], our findings indicate that FA synthesis plays an important role in muscle development and/or activity.
Tab. 1. Genotypic analysis of ACC and of the 3 FASN genes using ubiquitous and tissues-targeted knockdown. 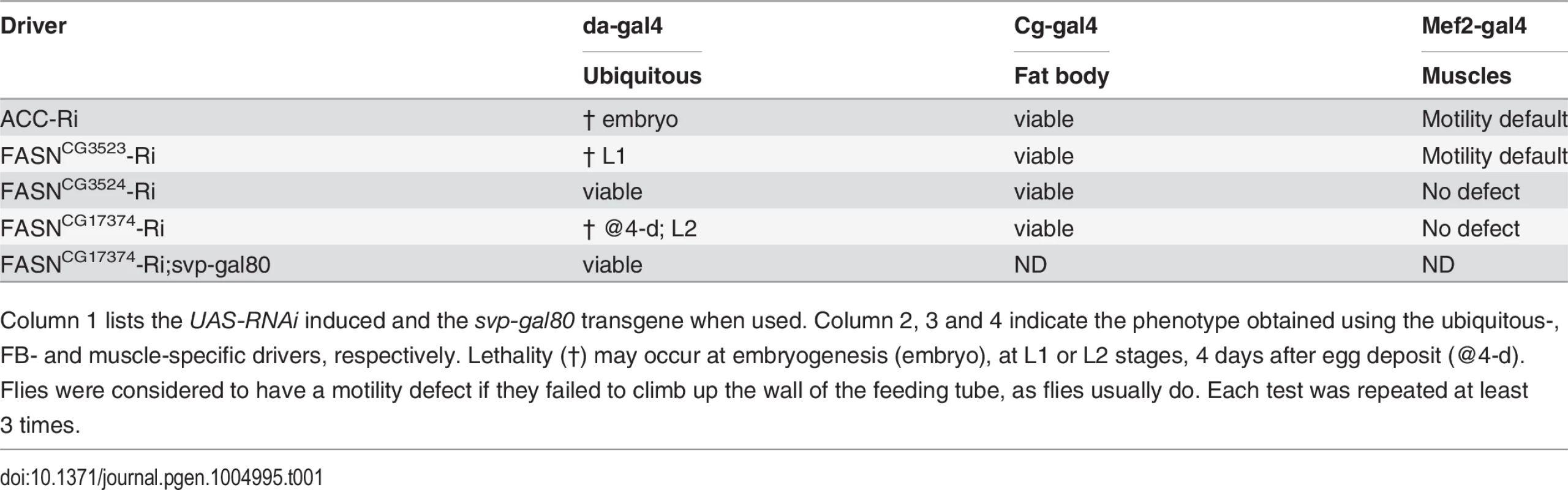
Column 1 lists the UAS-RNAi induced and the svp-gal80 transgene when used. Column 2, 3 and 4 indicate the phenotype obtained using the ubiquitous-, FB- and muscle-specific drivers, respectively. Lethality (†) may occur at embryogenesis (embryo), at L1 or L2 stages, 4 days after egg deposit (@4-d). Flies were considered to have a motility defect if they failed to climb up the wall of the feeding tube, as flies usually do. Each test was repeated at least 3 times. FASNCG3523 knockdown affects TAG and Glycogen levels
We previously reported that FB-knockdown of ACC results in a decrease in total TAG levels [21]. To determine which of the three FASN members is necessary for LCFA synthesis in the FB, RNAi to each FASN gene was induced using the FB-specific driver. Consistent with the finding that FASNCG3523 is the only FASN gene expressed in internal organs (S1A Fig.), total TAG levels were dramatically reduced in FASNCG3523 but not in FASNCG17374 and FASNCG3524 knockdowns (S2A Fig.).
We previously observed that in Cg>ACC-RNAi (Cg-gal4 directing ACC-RNAi) animals the drop in whole larvae TAG levels was accompanied by an increase in glycogen storage [21]. Thus, to investigate the physiological relationship between TAG and glycogen storage, RNAi transgenes to either ACC or FASNCG3523 was combined with an RNAi transgene to the gene encoding the unique Drosophila GlyS. Single or dual knockdowns were induced in either the FB, the muscles or in both tissues. Total amounts of TAG, glycogen, trehalose, glucose and protein were measured in 0–5h prepupae, as this is a convenient phase to stage the animals after the feeding period. Prepupal weighing revealed that animals expressing GlyS-RNAi in combination with either ACC-RNAi or FASNCG3523-RNAi in both the muscles and the FB exhibited the most prominent reduction in body weight (S2B Fig. and S1 Table). Total TAG levels decreased dramatically when either ACC-RNAi or FASNCG3523-RNAi were induced in the FB but not in the muscles (S2C Fig.); this decrease was roughly similar when either RNAi were induced in both tissues (S2C Fig.). Furthermore, TAG levels measured in control, ACC-RNAi- or FASNCG3523-RNAi-expressing animals were not markedly modified by the expression of the GlyS-RNAi (S2C Fig.). Total glycogen levels decreased when GlyS-RNAi was expressed in either the FB or the muscles, and decreased further when GlyS-RNAi was expressed in both tissues (S2D Fig.), indicating that in prepupae both organs contribute to glycogen storage. This finding contrasts with a previous study reporting that in larvae, glycogen can be detected in skeletal muscles only [26]. Therefore, since glycogen is unlikely to be transported between organs, it is conceivable that FB glycogen synthesis mostly occurs at late larval stage. Unexpectedly, driving either ACC-RNAi or FASNCG3523-RNAi in the muscles provoked a moderate decrease in glycogen levels (S2D Fig.). This may be a consequence of muscle dysfunction linked to the above mentioned motility defect (Table 1). Importantly, FB-knockdown to ACC or FASNCG3523 provoked a very strong increase in total glycogen levels that is not observed when co-expressing GlyS-RNAi (S2D Fig.), indicating that this extra-glycogen is synthesized inside the FB. Furthermore, we observed that trehalose levels (S2E Fig.) in part mirrored the variations observed with in glycogen (S2D Fig.), as shown by a strong correlation (S2F Fig.). Since energy stores are mobilized during metamorphosis, the decrease in trehalose levels might be a direct consequence of reduced glycogen breakdown. However, when ACC-RNAi or FASNCG3523-RNAi was expressed in the FB, glycogen but not trehalose levels increased dramatically (S2D–S2F Fig.), suggesting that trehalose levels cannot be increased in the prepupae. Finally, neither glucose (S2G Fig.) nor protein (S2H Fig.) levels exhibited severe perturbation in any of the tested genotypes. Taken together these results indicate that at the end of larval life, glycogen accumulates in both the muscles and the FB, whereas TAGs accumulate mainly in the FB. Further, a reduction in TAG storage can, in part, be compensated for by an increase in glycogen storage.
Direct links between FA synthesis and sugar metabolism
Considering that the synthesis of glycogen and TAG constitutes a metabolic mechanism to safely store high quantities of glucose, we hypothesized that the anabolic enzymes FASN, ACC and GlyS, are induced by dietary sugar. Therefore larvae were fed a low carbohydrate diet (LCD) or a sucrose-supplemented diet (SSD). Using RT-Q-PCR, the expression of ACC, GlyS and the three FASN genes was monitored in larvae fed on 0% (LCD), 5%-, 10% - and 20%-SSDs (S2 Table). FASNCG17374 expression was insensitive to increases in dietary sugar, while the expression of all the other genes was enhanced by sucrose (Fig. 1A). This response was observed following a 5%-SSD but was not further enhanced by 10% - and 20%-SSD, indicating that a moderate increase in dietary sugar elicits an adaptive metabolic response.
Fig. 1. GlyS, ACC and FASNCG3523 expression is induced by and protects from dietary sugar. 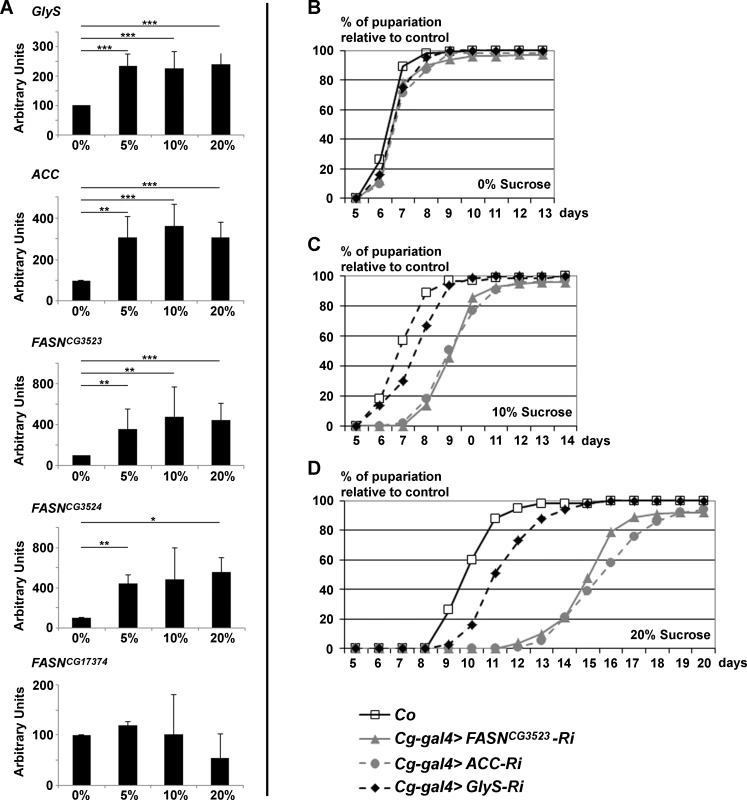
(A) RT-Q-PCR (means calculated from 3 samples of 10 feeding L3 larvae) to GlyS, ACC, FASNCG3523, FASNCG3524 and FASNCG17374 in response to increasing concentration of dietary sucrose (0%, 5%, 10%, 20%). (B-D) Developmental delay was measured at puparium formation of larvae fed a LCD (B), a 10%-SSD (C) or a 20%-SSD (D). The Cg-gal4 driver was used to direct RNAi to GlyS, ACC or FASNCG3523 within the FB. The Cg-gal4 was combined with a UAS-Dcr2 transgene to strengthen the RNAi effect. Controls (Co) were progeny resulting from the cross between Cg-gal4 females and w- balanced males. In (B-D), each curve represents at least 300 animals; experiment repeated twice. Next, we wondered whether the synthesis of FA may protect against excess dietary sugar. Considering that the FB is the main storage organ, RNAi to ACC, FASNCG3523, or GlyS were induced with the Cg-gal4 driver and the duration of larval development was monitored by following the onset of metamorphosis. When fed LCD, no developmental delay was observed in control, Cg>ACC-RNAi, Cg>FASNCG3523-RNAi or Cg>GlyS-RNAi larvae (Fig. 1B). In contrast, when fed a 10%-SSD, the onset of metamorphosis was delayed by roughly two days in Cg>ACC-RNAi and Cg>FASNCG3523-RNAi larvae, while Cg>GlyS-RNAi larvae were only slightly delayed (Fig. 1C and lines 1–3, S3 Table). The effect was enhanced for larvae fed a 20%-SSD. Control larvae exhibited a 3-day delay, Cg>GlyS-RNAi larvae exhibited a 4-day delay, whereas Cg>ACC-RNAi and Cg>FASNCG3523-RNAi larvae exhibited approximately an 8-day developmental delay (Fig. 1D and lines 4–6, S3 Table). Together, these findings indicate that FA synthesis is a crucial metabolic pathway, which buffers the developmental defects induced by excess dietary sugar.
Generation of FASN mutants
To gain further insights into the physiological requirements of LCFA synthesis we generated FASN mutants. As shown above, FASNCG3523 is an essential gene ubiquitously expressed, whereas FASNCG17374 sustains the synthesis of an essential FA only within the oenocytes (Table 1 and S1C–S1D Fig.). FASNCG3524 is not essential (Table 1) and may be redundant with FASNCG3523, as these two genes are in tandem on the second chromosome (Fig. 2A) and both are induced by dietary sugar (Fig. 1A). Therefore, we took advantage of two FRT-containing P-elements, located within the FASNCG3524 and the FASNCG3523 genes (Fig. 2A). Flipase recombination between the FRT sequences of these two P-elements resulted in a complete deletion of FASNCG3524, hereafter referred to as FASNΔ24. The resulting chimeric P-element links the 5’ region of FASNCG3524 to most of the FASNCG3523 genomic sequences (Fig. 2A). To generate a null FASNCG3523 mutant, we performed a remobilization of the chimeric P-element and looked for imprecise excisions that remove part of the FASNCG3523 gene. 22 excisions were recovered, one of them (hereafter referred to as FASNΔ24-23) removed 1200-bp of the FASNCG3523 gene (Fig. 2A), including the first methionine codon and the sequence coding half of the β-ketoacyl synthase (KS) domain [17].
Fig. 2. Characterization of FASN mutants. 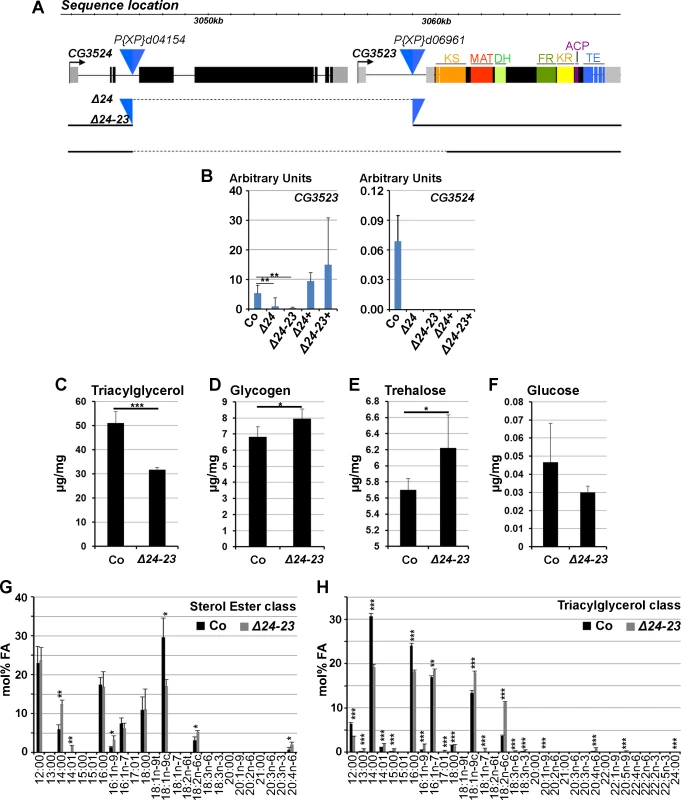
(A) Locus of the FASNCG3523, FASNCG3524 genes. The blue triangles show the two P-element insertions used to generate the FASNΔ24 deficiency. The dotted lines indicate the genomic sequences removed in FASNΔ24 and FASNΔ24-23 mutants. The ketoacyl synthase (KS), malonyl acyl transferase (MAT), dehydratase (DH), enoyl reductase (ER) ketoreductase (KR), acyl carrier protein (ACP) and thioesterase (TE) domains of FASNCG3523 are indicated. (B) RT-Q-PCR (means calculated from 3 samples of 10 feeding L3 larvae) to FASNCG3523 (left) and FASNCG3524 (right) in w- control (Co), FASNΔ24 (Δ24) and FASNΔ24-23 (Δ24-23) mutants rescued by dietary lipids or the UAS-FASNCG3523 transgene (Δ24+ and Δ24-23+). (C-F) Concentration of TAGs (C), glycogen (D), trehalose (E) and glucose (F) in w- control (Co) or FASNΔ24-23 (Δ24-23) mutant prepupae raised on the rescuing lipid media. (G-H) FA profiles of the sterol esters (G) and TAGs (H) classes from either w- control (Co) or FASNΔ24-23 (Δ24-23) mutant prepupae raised on a beySD. Fatty acid species [71] are indicated at the bottom of each panel. TAGs values are means calculated from 5 samples of 150 mg 0–5h prepupae; glucose, trehalose and glycogen values are means calculated from 4 samples of 500 mg 0–5h prepupae. Fatty acid profiles represent means calculated from 3 samples of 100 mg 0–5h prepupae. Both FASNΔ24 and FASNΔ24-23 are lethal at the L1 larval stage. RT-Q-PCR analysis showed that FASNCG3524 expression could not be detected in either mutants fed a lipid-supplemented diet (Fig. 2B and see below). In addition, FASNCG3523 transcript levels were severely reduced in homozygous FASNΔ24 larvae and barely detectable in homozygous FASNΔ24-23 larvae (Fig. 2B). Therefore, both mutations delete FASNCG3524, however, FASNΔ24 appears to be a hypomorphic mutant and FASNΔ24-23 a null mutant for FASNCG3523.
To ascertain that the L1 lethality observed in both mutants was solely due to FASN deficiency, rescue experiments were performed, using UAS lines expressing either FASNCG3524 or FASNCG3523 cDNA. Ubiquitous overexpression revealed that FASNCG3524 cDNA could partially rescue the lethality of FASNΔ24-23 mutants to the pupal stage, although none emerged as adults (S4 Table). In contrast, ubiquitous overexpression of FASNCG3523 cDNA did not rescue the lethal phenotype in either FASN mutants and induced embryonic lethality when driven with any of the ubiquitous gal4-lines tested (S4 Table). However, one of the UAS-FASNCG3523 lines was able to partially rescue the lethal phenotype to pupal or adult stages in both mutants in the absence of gal4 drivers (S4 Table). Consistently, RT-Q-PCR analysis revealed that FASNCG3523 but not FASNCG3524 transcripts were detected at high levels in both FASN mutant rescued animals (Fig. 2B), indicating that an endogenous promoter could drive the expression of this UAS-FASNCG3523 transgene. These findings show that both FASNΔ24 and FASNΔ24-23 are bona fide mutants and suggest that FASNCG3523 protein levels should be maintained within a precise window of expression.
Rescue of FASN mutants with dietary lipids
To determine whether the lethal phenotype could be rescued by dietary lipids, a LCD was supplemented with lipids (S2 and S5 Tables). Interestingly, supplementing a LCD with soy lipids could in part rescue the lethality of the hypomorph FASNΔ24 mutant to pupal or adult stages (S5 and see below) but not the lethality of the null FASNΔ24-23 mutant (S5 Table). We therefore, supplemented a LCD with various dietary lipids, including oils, margarine, butter and egg yolk alone or in combination. In isolation, none of the dietary lipids could rescue lethality of FASNΔ24-23 mutants, although a few larvae grew and developed to the L2 or L3 stages (S5 Table). In contrast, a LCD supplemented with butter and egg yolk (beySD) (S2 Table) could partially rescue lethality of both FASNΔ24 and FASNΔ24-23 mutants (S5 Table). To evaluate the metabolic consequences of the FASN deletion, TAG, glycogen, trehalose and glucose levels were measured in the FASNΔ24-23 mutant and control prepupae fed a beySD. FASNΔ24-23 prepupae exhibited a net decrease in TAG levels (Fig. 2C) associated with a moderate increase in glycogen and trehalose levels (Fig. 2D-E), whereas glucose levels were not significantly modified (Fig. 2F). Then, we performed a detailed analysis of FA composition of the TAGs, the sterol esters, and the various phospholipid classes. This analysis revealed that the relative FA content of the various phospholipids was not significantly modified (S3A–S3F Fig.). In each phospholipid class, palmitic acid (16 : 00) was always the most abundant FA component, although palmitoleic (16 : 01), stearic (18 : 00) oleic (18 : 01) and linoleic (18 : 02) acids were also highly represented. In contrast, the relative FA content in the sterol ester and TAG classes significantly varied in FASNΔ24-23 mutants versus controls (Fig. 2G-H). For the sterol ester class, oleic acid was less abundant in the mutants than in the control; however, this deficit was compensated for with higher levels of myristic (14 : 00), myristoleic (14 : 01), palmitoleic, linoleic (18 : 02) and arachidonic (20 : 04) acids (Fig. 2G). For the TAG class, control prepupae contained a higher proportion of saturated lauric (12 : 00), myrictic and palmitic acids, whereas mutants contained a higher proportion of unsaturated myristoleic, palmitoleic, oleic, linoleic and arachidonic acids (Fig. 2H). Together, these findings suggest that dietary lipids provide phospholipid precursors in sufficient amounts to compensate for the loss of FASN. Further, the difference in the FA composition of the TAG class in mutant versus control animals suggests that the structure of the TAGs is not critical.
Moderate sucrose supplementation is dramatic for FASN deficient animals
Next, we investigated sucrose sensitivity in FASN mutant. Importantly, about 40% of the FASNΔ24 mutants fed a soy-lipid supplemented diet and of the FASNΔ24-23 mutant fed a beySD underwent metamorphosis onset (S4A Fig. and Fig. 3A). As shown by standard deviation values the percentages of rescue was highly variable. Nonetheless, addition of 10% sucrose to either lipid supplemented diet, resulted in a total lethality at L1 stage for both FASN mutants (S4A Fig. and Fig. 3A). These findings indicate that individuals that are unable to synthesized FAs are extremely sensitive to moderate increases in dietary sucrose. Moreover, less than half of the control larvae were able to pupariate when fed a lipid supplemented diet (S4A Fig. and Fig. 3A). The lipotoxicity was markedly suppressed when beySD was supplemented with 10% sucrose (Fig. 3A), possibly due to a reduction in the feeding rate (see below).
Fig. 3. Sugar sensitivity of FASN null mutants. 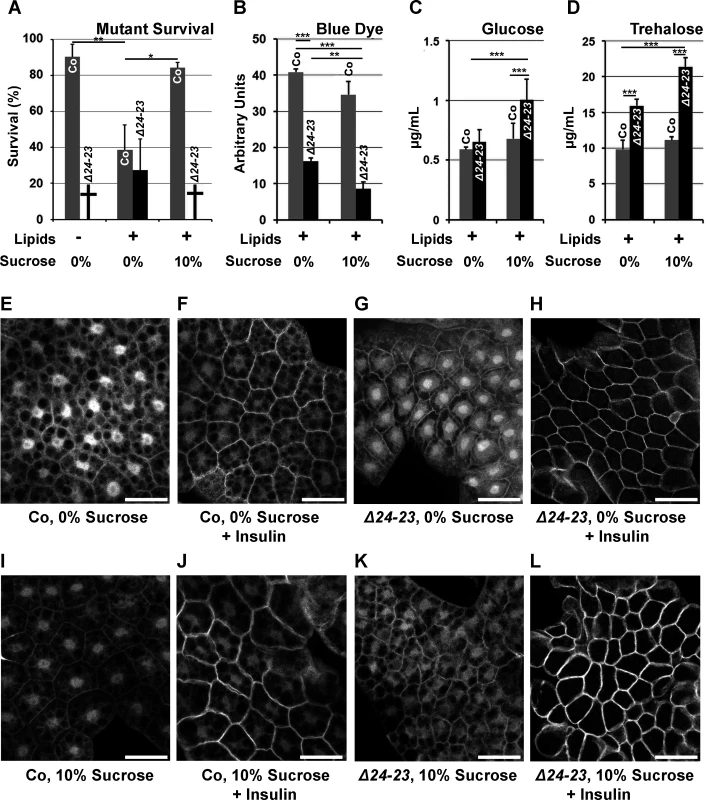
(A) Survival until metamorphosis or early larval lethality (†) of w- control (Co) and FASNΔ24-23 mutant (Δ24-23) animals fed either a LCD (Lipids -) or a beySD (Lipids +) with (10%) or without (0%) additional sugar. For each condition, the mean of survival rate was calculated by assessing the number of newly hatched larvae reaching metamorphosis (groups of 100 L1 larvae placed in 5 separate tubes). (B-D) Metabolic measurements from w- control (Co) or FASNΔ24-23 mutant (Δ24-23) larvae that were fed a beySD until L2/L3 transition and then transferred onto fresh media (0%) or media containing additional sucrose (10%) for 24h. (B) Blue dye accumulation in 24h old L3 larvae after feeding on a tinted media for 1h (means calculated from 3 samples of 10 feeding L3 larvae); experiments repeated 3 times. (C-D) Circulating glucose (C) and trehalose (D) levels from bled larvae (means calculated from 4 samples of 20 to 30 bled L3 larvae); experiment repeated 3 times. (E-L) Membrane localization of tGPH in FB explants incubated with (F,H,J,L) or without (E,G,I,K) insulin (0,5 μM) (for quantification of tGPH intensity see S4B Fig.). FB explants were dissected from w- control (E,F,I,J) or FASNΔ24-23 (G,H,K,L) L3 larvae that were fed a beySD (E-H) or the same media supplemented with 10% sucrose (I-L) for 24h. For each genotype, at least 10 larvae were dissected; experiment repeated twice. Scale bars: 20μm. Since metabolic analysis is easier to perform on late rather than early larvae—which are very small—, a diet-shift protocol was established. FASNΔ24-23 mutant and control larvae were fed a beySD until the L2/L3 transition, transferred onto the same feeding media with or without 10% sucrose supplementation and left to develop 24h or 40h. First, to evaluate the feeding rate, larvae were transferred onto fresh media stained with brilliant blue FCF dye, and absorption of stained food was evaluated from whole larval extracts after one hour. Colorimetric measurement revealed that FASNΔ24-23 mutants contained much less food in their gut than control animals (Fig. 3B), and that sucrose supplementation also reduced the stained food content in both FASNΔ24-23 and control larvae (Fig. 3B). The lower gut content suggests that food uptake was reduced, although we could not exclude an increase in stool elimination. Next, levels of circulating sugars in larval hemolymph were measured. Interestingly, neither glucose nor trehalose levels increased in control larvae fed a 10%-sucrose supplemented beySD (Fig. 3C-D), suggesting that this feeding protocol does not induce a diabetic-like phenotype. Nonetheless, FASNΔ24-23 mutants fed a beySD exhibited a moderate increase in trehalose levels (Fig. 3D), while glucose levels remained unchanged (Fig. 3C). In contrast, after 24h of feeding on a 10%-sucrose supplemented beySD, both glucose and trehalose levels were strongly increased (Fig. 3C-D). Considering that increases in levels of circulating sugar is a hallmark of diabetes [27], the insulin response was evaluated in the FB of larvae expressing a tGPH reporter [28]. FBs were dissected from larvae fed a beySD with or without a 10%-sucrose supplement, and membrane translocation of tGPH was analyzed after 20 mn incubation with or without insulin. When grown on either feeding media, both control and mutant FBs were highly responsive to insulin stimulation (Fig. 3E-L and S4B Fig.) indicating that neither the FASNΔ24-23 mutant nor control larvae exhibit a T2D-like phenotype when fed a 10%-sucrose supplemented beySD. Importantly, the membrane-GFP fluorescence induced by insulin stimulation was much higher in FASNΔ24-23 mutant than in control larvae (Fig. 3F,H,J,L and S4B Fig.), suggesting that the former were hypersensitive to insulin. Together, our findings indicate that FASNΔ24-23 mutant animals are highly sensitive to dietary sugar but do not exhibit a T2D-like phenotype.
FASN and Glo1 cooperation
Since an increase in AGEs is linked to high levels of circulating sugar in T2D patients [12], we compared the amounts of AGEs in whole control or FASN mutant larvae. In L3 larvae transferred onto fresh beySD for 24h, the amounts of AGEs were higher in FASNΔ24-23 mutants than in controls. This was the case regardless or whether the beySD was supplemented with 10% sucrose (Fig. 4A). In older L3 larvae transferred on fresh beySD for 40h, the amounts of AGEs were strongly increased in FASNΔ24-23 mutants compare to controls (Fig. 4B). In addition, exposure to 10%-sucrose supplemented beySD further increased AGE levels in FASNΔ24-23 mutants (Fig. 4B), suggesting that FA synthesis constitutes a metabolic pathway to restrict AGE accumulation.
Fig. 4. AGE metabolism and FASN deficiency. 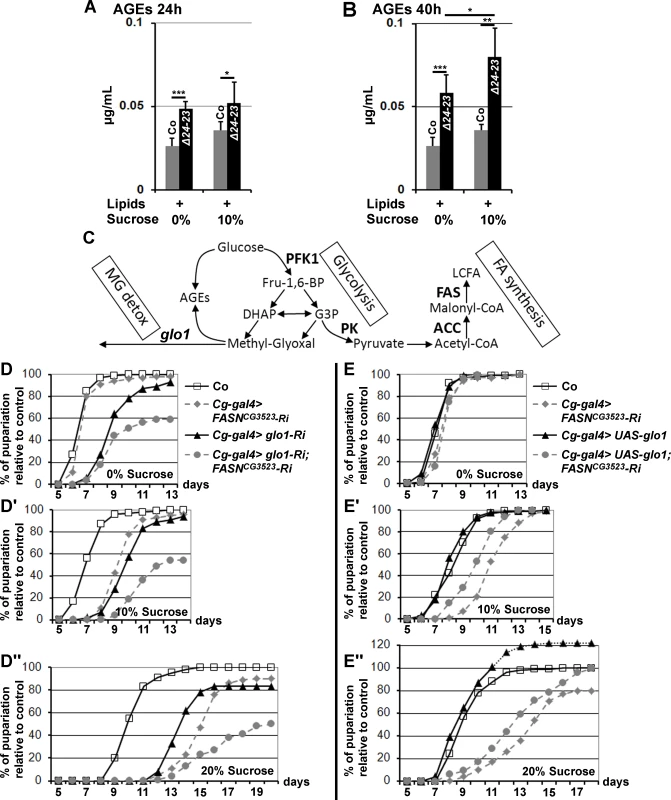
(A-B) AGE levels in w- control (Co) and FASNΔ24-23 mutant (Δ24-23) L3 larvae raised for 24h (A) or 40h (B) (calculated from 5 samples of 10 L3 larvae); lipid and sucrose complements are indicated as in Fig. 3A-D; experiment repeated twice. (C) Metabolic links between glucose catabolism, FA synthesis, AGE formation and MG detoxification. Enzymes are indicated in bold characters. (D-D”,E-E”) Developmental delay measured at puparium formation of larvae fed a LCD (D,E), a 10%-SSD (D’,E’) or a 20%-SSD (D”,E”). (D-D”) The Cg-gal4 driver was used to direct RNAi to glo1, FASNCG3523 or both together within the FB. In (D-D”), each curve represents at least 300 animas; experiment repeated twice. (E-E”) The Cg-gal4 driver was used to direct FASNCG3523-RNAi, UAS-glo1, or both together. Controls (Co) are progeny resulting from the cross between Cg-gal4 females and w- balanced males. In (E-E”), each curve represents at least 700 animals. To further investigate the effects of dietary sucrose, we performed RNAi knockdown to two glycolytic enzymes encoded by single genes, Phosphofructokinase 1 (PFK1) and Pyruvate kinase (PK) that catalyze an early and the last glycolytic steps, respectively (Fig. 4C). FB-targeted knockdown to either PFK1 or PK did not result in a phenotypic defect in larvae fed a LCD, as developmental times did not differ markedly from controls (S5A Fig.). However these larvae were very sensitive to sucrose. When fed a 10%-SSD, both RNAi-knockdown larvae exhibited a significant developmental delay (S5B Fig. and lines 7–8, S3 Table). Moreover, when fed a 20%-SSD, the developmental delay was further increased for Cg>PFK1-RNAi larvae, whereas most of the Cg>PK-RNAi animals died during larval life (S5C Fig. and lines 9–10, S3 Table). The difference in sucrose sensitivity suggests either that PK-RNAi induces a more efficient knockdown than PFK1-RNAi, or that some glycolytic intermediates produced downstream of the enzymatic step catalyzed by PFK1 are extremely toxic.
Following the glycolytic step catalyzed by PFK1, an Aldolase cleaves fructose 1,6 bisphophate (Fru-1,6-BP) in the trioses phosphate, DHAP or G3P. Either metabolite leads to pyruvate, or to the highly reactive glycating α-oxoaldehyde MG via a non enzymatic reaction (Fig. 4C). We therefore used UAS-RNAi to the single glo1 ortholog that encodes an MG neutralizing enzyme. FASNCG3523-RNAi and glo1-RNAi were induced independently or together in the FB and the duration of larval development was monitored. When fed a LCD, glo1-RNAi larvae exhibited a moderate developmental delay (Fig. 4D and line 11, S3 Table). This developmental delay was slightly prolonged when fed a SSD, although not to the same extent as FASNCG3523-RNAi larvae, which were much more sensitive to dietary sucrose (Fig. 4D-D” and lines 11,14,17, S3 Table). Furthermore, animals dually expressing FASNCG3523-RNAi and glo1-RNAi in their FB exhibited a high rate of larval lethality and a developmental delay that dramatically increased concurrently with sucrose concentration (Fig. 4D-D” and lines 12–13,15–16,18–19, S3 Table). Conversely, FB-overexpression of Glo1 was able to partially compensate for the developmental delay induced by an increase dietary sugar (Fig. 4E-E” and lines 20,22, S3 Table). FB-overexpression of Glo1 was also able to partially suppress the strong developmental delay of FASNCG3523-RNAi larvae grown on SSD (Fig. 4E-E” and lines 21,23, S3 Table). In each assay, the percentage of pupae is relative to the number of their SM5-TM6B siblings (see material and methods). Intriguingly, when fed a 20%-SSD, the ratio of UAS-glo1 larvae relative to the number of their SM5-TM6B siblings was higher than the control ratio, reaching a maximum at roughly 120% (Fig. 4E”). Furthermore, we also observed that when testing homozygous w- control flies, the rate of larval lethality was significantly higher in 20%-SSD than in LCD or in 10%-SSD (S5D Fig.). This observation suggests that in the Glo1-overexpressing assay, a significant number of the SM5-TM6B siblings underwent lethality when fed a 20%-SSD and that Glo1 overexpression suppresses this lethality. In contrast in the control assay all the larvae underwent the same rate of lethality irrespective of the SM5-TM6B balancers. Together, these findings indicate that sucrose toxicity can be alleviated by overexpression of Glo1 and conversely, the deleterious effects are exacerbated when both FA synthesis and Glyoxalase activity are simultaneously dampened.
Cell-autonomous sucrose toxicity
To determine whether a lack of FA synthesis induces cell-autonomous defects, we generated flip-out recombination during embryogenesis and analyzed the resulting clones in the FB of feeding larvae at the end of the L3 stage. Interestingly, the size of FASNCG3523-RNAi cells was almost normal in larvae fed a LCD, but drastically reduced in larvae fed a 20%-SSD (S6A–S6B,S6M Fig.). A similar phenotype was observed for PK-RNAi flip-out cells (S6C–S6D,S6M Fig.), although the size reduction observed in larvae fed a 20%-SSD, varied a lot depending on the experiment, possibly because of variability in RNAi efficiency. In contrast, PFK1-RNAi flip-out cells were insensitive to dietary sucrose since cell size remained unchanged irrespective of sucrose supplementation (S6E–S6F,S6M Fig.). To perform genetic interactions at the cellular level, we generated MARCM clones either mutant (FASNΔ24-23) or wild-type (FASN+). Firstly, the sucrose sensitivity of FASNΔ24-23 cells was evaluated in the FB of larvae raised on media containing increasing quantities of sucrose. For larvae fed a LCD, the size of FASNΔ24-23 cells was slightly reduced compare to neighboring control cells (Fig. 5A,M). However, as the sucrose content in the diet increased, a concomitant reduction in the size of FASNΔ24-23 cells was observed (Fig. 5B-D,M). This cell size reduction was not correlated with lipid content, as Nile red staining revealed that FASNΔ24-23 cells were severely depleted in LDs, irrespective of sugar supplementation (S6G–S6I Fig.). Next, we generated FASNΔ24-23 MARCM clones expressing PFK1-RNAi or PK-RNAi. Under these conditions, the size of FASNΔ24-23 cells, expressing either RNAi was hardly reduced in larvae fed a LCD (Fig. 5E,G,N). However, in larvae fed a 20%-SSD, the size of FASNΔ24-23 cells remained unaffected when expressing PFK1-RNAi (Fig. 5F,N), but were dramatically reduced when expressing PK-RNAi (Fig. 5H,N). The phenotypic suppression produced by PFK1-RNAi, suggests that an intermediate metabolite, downstream of PFK1 (Fig. 4C), is responsible for the size reduction of FASNΔ24-23 cells observed in SSD-fed larvae. Therefore, MARCM clones, expressing glo1-RNAi were analyzed. Interestingly, MARCM FASN+ clones expressing only the glo1-RNAi were insensitive to sucrose supplementation (Fig. 5I-J,O). Nonetheless, FASNΔ24-23 cells expressing glo1-RNAi exhibited an extreme size reduction in larvae fed LCD (Fig. 5K,O) accompanied by a severe decrease in nucleus size (see below). Furthermore, these clonal cells could not be observed when larvae were fed 20%-SSD, suggesting that these cells were eliminated during development. Conversely, FASNΔ24-23 MARCM cells overexpressing glo1 were of normal size in larvae fed a 20%-SSD (Fig. 5L,P). However, neither FASNΔ24-23 MARCM cells in LCD-fed larvae, nor FASN+ MARCM cells were affected in size by Glo1 overexpression (S6J–S6L,S6M Fig.). Together, these findings indicate that Glo1 can compensate for cell size reduction due to a sugar-dependent FA-synthesis defect, but is unlikely to promote cellular growth.
Fig. 5. Cell-autonomous defect in FASN mutant FB cells. 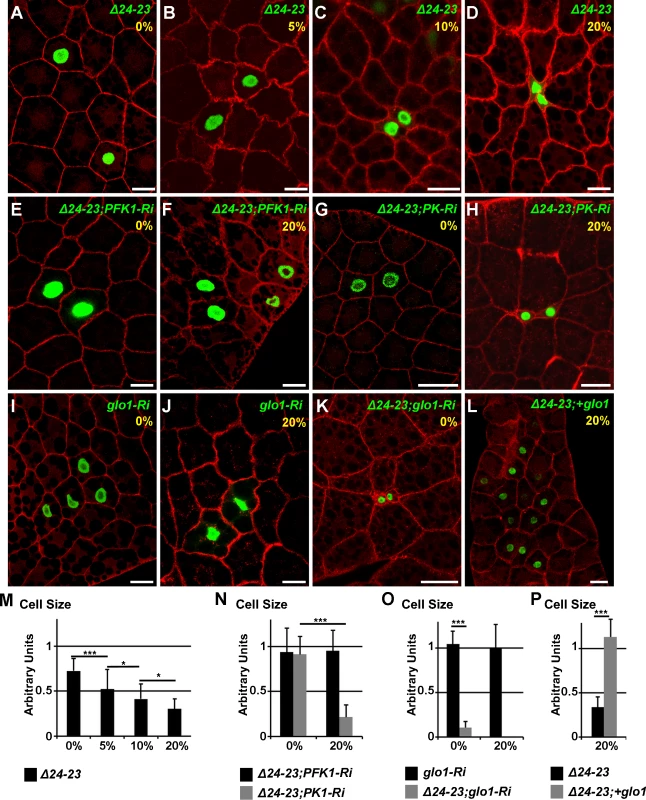
(A-L) Phalloidin staining of FB from feeding L3 larvae containing MARCM clones labeled with GFP. At the top right corner of each image, the genotype of the clonal cells and the percentage of sucrose supplementation are shown in green and yellow, respectively. FASNΔ24-23 (Δ24-23), PFK1-RNAi (PFK1-Ri), PK-RNAi (PK-Ri), glo1-RNAi (glo1-Ri) and UAS-glo1 (+glo1). Scale bars: 20μm. (M-P) Size ratio between at least ten clonal cells and the neighbouring control cells, as shown in A, B, C, D (M), E, F, G, H (N), I, J, K (O) and L (P). For each condition, at least 10 larvae were dissected; whilst searching for FASNΔ24-23 clones expressing glo1-RNAi in larvae fed a 20%-SSD at least 40 animals were dissected. Finally, an antibody to MG-derived AGEs (MG-AGEs) was used for immunostaining. In FASNΔ24-23 clonal cells the amounts of MG-AGEs were barely detectable in larvae fed a LCD (Fig. 6A-C) but were dramatically increased in larvae fed a 20%-SSD (Fig. 6D-F). Importantly, increased MG-AGE levels induced by 20%-SSD were abolished in FASNΔ24-23 MARCM clones expressing either PFK1-RNAi (Fig. 6G-I) or UAS-glo1 (Fig. 6J-L). Furthermore, in larvae fed a LCD, FASNΔ24-23 clones expressing glo1-RNAi exhibited a strong accumulation of MG-AGEs (Fig. 6M-O). Nucleus size in these clones, was also dramatically reduced (Fig. 6O,O’). Taken together, these findings indicate that FA synthesis and Glyoxalase activity cooperate in a cell-autonomous manner to neutralize the toxicity of dietary sugar, which may result in cellular growth defects or putative cell elimination.
Fig. 6. Cell-autonomous accumulation of MG-AGEs in FASN mutant FB cells. 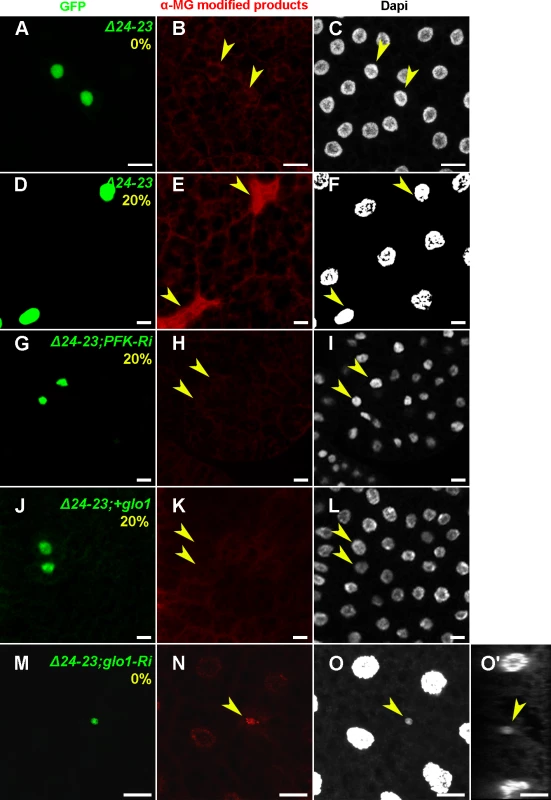
Immunodetection of MG-AGEs (B,E,H,K,N) and DAPI staining (C,F,I,L,O,O’) in FB from feeding L3 larvae containing MARCM clones labeled with GFP (A,D,G,J,M). (A-F) Accumulation of MG-AGEs in FASNΔ24-23 MARCN clones of L3 larvae fed either a LCD (A-C) or a 20%-SSD (D-F). (G-L) Suppression of MG-AGE accumulation in FASNΔ24-23 MARCN clones of L3 larvae fed a 20%-SSD by either PFK1-RNAi (G-I) or UAS-glo1 (J-L). (M-O) Accumulation of MG-AGEs and extreme nuclear size reduction in FASNΔ24-23 MARCN clones expressing glo1-RNAi of L3 larvae fed a LCD. (O’) is an orthogonal section of (O). Scale bars: 20μm. For each condition, at least 10 larvae were dissected. Discussion
In this study, we investigated the role of FA synthesis in regulating homeostasis in response to dietary sugar. To maintain tolerable levels of circulating sugars, organisms synthesize and store macromolecules in appropriate organs. In contrast to previous studies in insects, which report that the majority of TAGs stored in the FB are of dietary origin [29,30], we observed that in Drosophila, the larval FB is a lipogenic organ. However, in FASNΔ24-23 mutant fed a beySD, TAG levels were decreased but not abolished. This indicates that as in mammalian hepatocytes and adipocytes [2,31,32], TAGs stored in the Drosophila larval FB originate from either food assimilation or de novo synthesis. Together, our findings confirm that metabolic pathways act within an integrative network to maintain homeostasis and support the notion that in term of post-feeding macromolecules storage (TAGs and glycogen), the Drosophila larval FB constitutes an alternative model for mammalian liver and adipose tissue (Fig. 7A).
Fig. 7. Glucose metabolic fate in the larval FB. 
(A) Under normal conditions, glucose that enters into FB cells is mainly used through glycolysis and FA synthesis for TAG storage. Glucose is also stored as glycogen. Small amounts of MG are formed; thus Glo1 activity is not critical. (B) In FASN mutant FB cells, FA synthesis is abolished and glycogen synthesis is increased. Excess of sugar provokes a dramatic increase in MG levels and elicits Glo1 activity. Our FASN mutants are lethal at L1 stage, and this lethality can be recued by a beySD. Rescue of FASNΔ24 but not of FASNΔ24-23 mutants by soy lipid extracts likely reflects the strength of the mutation since FASNCG3523 is still weakly expressed in the hypomorphic mutant. Consistently a SREBP mutant that down-regulates but does not abolished the expression of several FA anabolic enzymes including FASNCG3523, could also be rescued by soy lipid extracts [33]. Rescue of the lethal phenotype by dietary lipids, as well as the minor phenotype observed in FASNΔ24-23 clonal cells, suggests that neighboring cells or organs can provide FAs to those that are deficient. This may be achieved through lipophorin activity [34]. Intriguingly, we found that in contrast to other lipid-supplemented media, a mix of butter and egg yolk could rescue the FASNΔ24-23 lethal phenotype. TAGs cannot be directly assimilated by enterocytes; first they require digestive lipases to cleave TAGs to di-acyl-glycerol (DAG), mono-acyl-2-glycerol (MAG) and free FAs (FFAs) [35,36]. In several mammalian species, lipids interact with bile acids to form micelles prior to enzyme cleavage and enterocyte absorption. However, it has been reported that in rats and human infants, FFAs may interact with calcium or magnesium ions to form soaps that are hardly assimilated [37,38,39]. In insects, lipid emulsifiers are poorly characterized although glycolipid or amino acid complexes are likely to be involved in lipid assimilation [40,41]. As egg yolk lipoproteins are highly efficient emulsifiers [42], they may help solubilize lipids, thereby favoring their absorption. The composition in FAs and their positions on the glycerol backbone vary depending on the origin of the TAGs. Regarding FA synthesis, FASNΔ24-23 mutants are expected to lack palmitic acid. Analysis of various oils and fats, revealed that TAGs found in butter contain high quantities of palmitic acid in position sn-2 of the glycerol [36]. Thus, assimilation of MAGs resulting from butter digestion, are high in palmitic acid. Hence, it is possible that MAGs are better assimilated than FFAs in our FASNΔ24-23 mutants. In order to fully understand the process of lipid absorption in FASNΔ24-23 mutants, extensive analysis, including the precise measurement of ingested and excreted FAs, will be required.
Rescue of lethality of FASN mutant by a lipid-supplemented diet indicates that FA synthesis deficiency can be compensated for by an appropriate lipid diet. Previous studies in Drosophila have reported that the FA composition of the various lipid classes varies depending on the diet [43,44]. Here, we show that the relative FA composition of phospholipids is not significantly different in FASNΔ24-23 rescued mutants and control animals fed a beySD. These findings not only confirm that diet contributes to phospholipid composition, but reveal that in the presence of an exogenous lipid supply, the essential FASN enzyme becomes dispensable for phospholipid synthesis. In contrast, sterol esters and TAGs exhibit variation in their FA composition. Compared to controls, TAGs from mutants contain less saturated FAs and more long chain unsaturated FA, suggesting that expression of desaturases and elongases [45] may be increased in FASN mutants. The high variability in TAG composition suggests that TAG structure is not a crucial parameter, which strengthens the notion that TAG synthesis constitutes a metabolic strategy to neutralize the potential toxicity of nutrients. Previous studies suggested that the fat tissue fulfills a protective role against excess sugar. In agreement with this, it has been shown that fat transplantation in lipoatrophic mice reverses T2D [46,47] and that in genetically induced obese mice, a decrease in adipose FASN expression is linked to T2D progression [48]. In addition, mice and flies with defects in ChREBP—a transcriptional activator of lipogenic enzyme expression—do not survive increases in dietary sugar levels [44,49,50]. However, it was unknown that FASN activity also protects against sugar toxicity. This finding is in contrast to a previous report which showed that in flies, lethality induced by ubiquitous expression of FASNCG3523-RNAi can be partially rescued by dietary sugar [50]. Here, we demonstrate that FA synthesis protects against dietary sugar at both a systemic and cell-autonomous level. The media used in our study contained low concentrations of digestible sugar, 64, 164 and 264 mg/ml for the LCD, 10%-SSD and 20%-SSD, respectively. In other studies, which used Drosophila larvae as a model for sugar tolerance, the concentration of digestible sugar was 86, 140 or 80 mg/ml for the low carbohydrate media and 377, 380 or 230 mg/ml for the sugar enriched media [27,50,51]. Importantly, while circulating sugar levels increase in FASNΔ24-23 animals, these mutants do not exhibit a T2D-like phenotype and become insulin hypersensitive. Therefore, as previously suggested [44,49,50], disrupting FA synthesis provides a convenient model to investigate the effect of glucotoxicity independent of lipotoxicity.
Here, we provide evidence to propose that FASN and Glo1 cooperate both in a systemic and in a cell-autonomous manner to protect against the deleterious effect of dietary sucrose. Our findings indicate that when FA synthesis is very active, as in the FB of Drosophila larvae, Glo1 activity is dispensable in term of neutralizing the few toxic metabolites produced through sugar catabolism (Fig. 7A). Conversely, the detoxifying activity of Glo1 becomes critical when FASN activity is disrupted in the larval FB (Fig. 7B). Thus, the observed decrease in lipogenic enzyme expression in the adipose tissue of a diabetic mouse model [48], may require an increase in Glo1 activity. If lipogenic enzyme expression is also decreased in T2D patients, the increase in glycating agents [52] may result not only from an increase in circulating sugar but also from a decrease in FA synthesis. For a few decades, pathological damage induced by excess sugar was thought to be a consequence of AGE formation [53], a paradigm substantiated by recent studies on experimental diabetic nephropathy [9,10,54]. Consistent with a study in Caenorhabditis elegans, reporting that Glo1 overexpression protects against glucose toxicity [55], we show that manipulating Glo1 levels in the larval FB modulate a sugar-induced developmental delay. Studies in diabetic models and patients mostly focused on AGE levels in body fluids [56,57,58,59], although alterations to intracellular products have also been reported [60,61,62]. At the cellular level, glo1 knockdown in FB cells induces a cell-autonomous phenotype, only when clones are also FASN deficient. This phenotype results in either an extreme reduction in cell size or elimination of cells, when larvae are fed LCD or SSD, respectively. The number of FB cells is determined during a proliferative phase at embryogenesis. During larval life, FB cells do not divide, but undergo a rapid cell growth phase [63,64]. The lack of a visible phenotype in glo1-deficient cells indicates that even when larvae are fed SSD, Glo1 does not affect the growth process of FB cells. In contrast, increasing quantities of sucrose in the food, even to moderate levels, induces a size reduction of FASN mutant cells. This phenotype is unlikely to depend directly on sugar since addition of moderate amounts of sucrose to food media does not markedly increase circulating sugar levels. In contrast, it is likely to directly depend on an increase of intracellular MG, since the cell size reduction is suppressed if FASN mutant cells are either deficient in PFK1 or overexpressing glo1 cDNA. In summary, our findings suggest that FASN activity is dispensable in sustaining cell growth but plays a key role in protecting against the potentially toxicity of MG produced through glycolysis.
In conclusion, we have demonstrated that FA synthesis constitutes a metabolic strategy to restrict the production of intermediate toxic molecules, suggesting that obesity is not a harmful process, as long as storage capacity is not overwhelmed. Furthermore, our study highlights the need for caution when using FA synthesis inhibitors to treat cancers and metabolic diseases, as they might provoke negative side effects.
Materials and Methods
Fly stocks and genetics
Fly strains: P[tGPH] [28], daughterless(da)-gal4, Mef2-gal4, actin5C>CD2>gal4,UAS-GFP, P[w[+mC] = tubP-GAL80]LL10,P[ry[+t7.2] = neoFRT]40A, UAS-Dcr-2 (Bloomington Stock Center); Inducible RNA-interfering (UAS-RNAi) lines to ACC (VDRC 108631), FASNCG3523 (VDRC 29349), FASNCG3524 (VDRC 4290), GlyS (VDRC 35136), glo1 (remobilized on chromosome III from VDRC 26832), PFK1 (VDRC 3017), PK (remobilized on chromosome III from VDRC 49533); FASCG17374-RNAi, svp-gal80, Cg-gal4 [21]. The P-element insertions (Exelixis collection) P[XP]v(2)k05816d04154 and P[XP]CG3523d06961 were used to generate deficiency as described [65]. All the fly lines were isogenized from single males in a white1118 mutant (w-) background. For clonal analysis FASNΔ24-23 was over SM5-TM6B,Tb- balancers [21]; For survival and metabolic analyses, FASN mutants were balanced by a CyO GFP-labelled chromosome.
The results presented for ubiquitous or tissue-targeted UAS-RNAi lines—including the corresponding controls—were obtained with a UAS-Dcr-2 that strengthens the RNAi effect. Developmental delays were evaluated from overnight egg collection and the number of prepupae formed was counted every morning. For each assay, several tubes were collected, overcrowded tubes were discarded and the numbers of prepupae were pooled. As some of the transgenes used in the genetic combinations were homozygous lethal, all the lines (driver, RNAi, w- control) were balanced with co-segregating SM5-TM6B,Tb- balancers that lead to non-mendelian offspring distribution. Therefore, for each assay, the number of RNAi-expressing Tb+ larvae was divided by the final number of Tb- larvae and all assays were normalized to the control ratio. For controls, a similar calculation was done from the offspring of driver females crossed with w-;SM5-TM6B males and this control ratio was adjusted to reach 100%.
Molecular biology
To generate the overexpressing lines, the locus of FASCG3523, and FASCG3524 were recovered by gap repair and the endogenous promoter replaced by a UAST [66]. glo1 cDNA was amplified from GH24818 (DGRC) and cloned into the pUAST vector. Plasmid constructs were injected by BestGene.
RT-Q-PCR were performed as previously described [21] using the following primers:
FASNCG3523 (5’-F CTTCTTCATTTCCCCGA-3’ and 5’-CGAAGGAGTATCCGGC-3’)
FASNCG3524 (5’-CTTTGACAATATGCTCTAC-3’ and 5’-AAGTCCGGAGTGTCCAG-3’)
FASNCG17374 (5’-F ATCAGCTCCAACCTCTAC-3’ and 5’-GGGCTACATGCAAGTCT-3’)
ACC (5’-TTGGGAAACTCATTCGTG-3’ and 5’-CCAGGACCTTGGCATTA-3’)
GlyS (5’-CCCCTCATACTACGAGC-3’ and 5’-CGATATAGCGGCGATCC-3’)
Immunocytochemistry
Flip-out clones were performed as described [21]. MARCM clones [67] were heat-shock induced at 4–6h embryogenesis and the larvae were allowed to grow on various sucrose-supplemented media until mid/late L3 stage. FB were dissected as described [21] but fixed with 3.7% formaldehyde in PBT (PBS 0.1% Tween20). FB were stained with Phalloidin-Rhodamine B (sigma) at 625 ηg/ml for 2h at RT, extensively washed and mounted in DABCO (sigma). Relative cell size was expressed as a ratio, clonal:neighboring control cells, which was estimated using the image-j software. The insulin responsive assay was performed as described [27]. tGPH quantification was measured in squares (10X10 pixels) positioned either at the membrane or at the nuclei. Measurements for each assay were recorded from 8 cells taken from 2 different FBs. For each cell, the maximum fluorescence intensity at the membrane was divided by the maximum fluorescence intensity at the nucleus and the mean ratio was plotted (S4B Fig.). Nile Red staining was performed as previously described [21]. For MG-AGE Immunostaining, dissected FB were fixed as described above, but blocked for 20 mn in PBS containing 0.1% Triton X100 and 2% bovine serum albumin. Samples were incubated overnight at 4°C with diluted (1 : 400) MG-AGE antibody (Cell Biolabs), extensively washed and incubated for 2h at room temperature with secondary antibody and DAPI in the blocking solution. Samples were finally washed in PBT and mounted in DABCO. Image acquisitions were obtained using a Nikon TE2000-U or a Leica SP8 confocal laser-scanning microscopes.
Metabolic measurements
TAGs, protein, glucose, trehalose and glycogen measurements were performed as previously described [21]. To measure circulating sugar, 6μl samples of hemolymph were collected from 20 to 30 bled L3 larvae. For AGE measurement, 5 samples of 10 L3 larvae were washed in PBS and crushed at 4°C in a Precellys 24; extracts were cleared 10 mn at 4° C in a microfuge at maximum speed. Extracts were diluted 100X in PBS and 100 μL of this diluted extract were treated with an ELISA kit (Cell Biolabs STA-317). AGEs estimation evaluated from spectrophotometric dosage at 450nm, was normalized to the protein concentration of each sample. To measure feeding rates, food media was tinted with 0,1% brilliant blue FCF. 3 samples of 10 L3 larvae were collected, frozen, extracted in 200μl water and centrifuged for 7min at maximum speed. The final volume was adjusted to 800μl and measured at 629ηm. Lipidomics were performed in triplicates of 100 mg 0–5h prepupae. Lipids were extracted and analyzed by GC-MS as described [68].
Statistical analysis
Statistical analyses were performed with R version 3.0.2 [69]. Error bars in figures stand for empirical standard deviations measured independently from the replicates in each category. Significance for the statistical tests was coded in the following way based on the p-values: ***: 0 < p < 0.001; **: 0.001 < p < 0.01; *: 0.01 < p < 0.05. In all the graphs, the error bars represent the standard deviations.
For S3 Table (corresponding to Fig. 1C, 1D, 4D, 4E, and S5A–S5C Fig.), the effect of the genotype was tested with one-way ANOVAs on developmental rates. Developmental rates (in units of days-1) were computed as the inverse of developmental duration to pupation. Lethality (evaluated for each developmental curve and corrected with the lethality rates for control measured in S5D Fig.) was accounted for by including a corresponding number of (unobserved) lethal events (individuals with a developmental rate of 0). Since all nine ANOVAs detected a significant effect of the genotype, pairwise comparisons between genotypes were tested with a post-hoc Tukey "Honest significant difference" test [70] for each sub-figure, and the biologically-relevant comparisons are reported.
Supporting Information
Zdroje
1. Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148 : 852–871. doi: 10.1016/j.cell.2012.02.017 22385956
2. Patel P, Abate N (2013) Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance. J Obes 2013 : 489187. doi: 10.1155/2013/489187 23691287
3. Chavez JA, Summers SA (2012) A ceramide-centric view of insulin resistance. Cell Metab 15 : 585–594. doi: 10.1016/j.cmet.2012.04.002 22560211
4. Farese RV Jr., Zechner R, Newgard CB, Walther TC (2012) The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab 15 : 570–573. doi: 10.1016/j.cmet.2012.03.004 22560209
5. Taubes G (2009) Insulin resistance. Prosperity's plague. Science 325 : 256–260. doi: 10.1126/science.325_256 19608888
6. Brookheart RT, Michel CI, Schaffer JE (2009) As a matter of fat. Cell Metab 10 : 9–12. doi: 10.1016/j.cmet.2009.03.011 19583949
7. Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7 : 763–777. 17882277
8. Menendez JA, Vazquez-Martin A, Ortega FJ, Fernandez-Real JM (2009) Fatty acid synthase: association with insulin resistance, type 2 diabetes, and cancer. Clin Chem 55 : 425–438. doi: 10.1373/clinchem.2008.115352 19181734
9. Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, et al. (2011) Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem 286 : 1374–1380. doi: 10.1074/jbc.M110.144097 21056979
10. Giacco F, Du X, D'Agati VD, Milne R, Sui G, et al. (2014) Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63 : 291–299. doi: 10.2337/db13-0316 24062246
11. Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114 : 597–605. 16894049
12. Rabbani N, Thornalley PJ (2013) Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 22 : 309–317.
13. Monnier VM, Cerami A (1981) Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science 211 : 491–493. 6779377
14. Kalapos MP (2008) The tandem of free radicals and methylglyoxal. Chem Biol Interact 171 : 251–271. doi: 10.1016/j.cbi.2007.11.009 18164697
15. Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14 : 287–371. 8277832
16. Barber MC, Price NT, Travers MT (2005) Structure and regulation of acetyl-CoA carboxylase genes of metazoa. Biochim Biophys Acta 1733 : 1–28. 15749055
17. Maier T, Leibundgut M, Ban N (2008) The crystal structure of a mammalian fatty acid synthase. Science 321 : 1315–1322. doi: 10.1126/science.1161269 18772430
18. Baker KD, Thummel CS (2007) Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6 : 257–266. 17908555
19. Kuhnlein RP (2012) Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. Lipid droplet-based storage fat metabolism in Drosophila. J Lipid Res 53 : 1430–1436. doi: 10.1194/jlr.R024299 22566574
20. Rajan A, Perrimon N (2013) Of flies and men: insights on organismal metabolism from fruit flies. BMC Biol 11 : 38. doi: 10.1186/1741-7007-11-38 23587196
21. Parvy JP, Napal L, Rubin T, Poidevin M, Perrin L, et al. (2012) Drosophila melanogaster Acetyl-CoA-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet 8: e1002925. doi: 10.1371/journal.pgen.1002925 22956916
22. Gutierrez E, Wiggins D, Fielding B, Gould AP (2007) Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445 : 275–280. 17136098
23. Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 : 715–720. 17534367
24. Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, et al. (2012) Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab 16 : 97–103. doi: 10.1016/j.cmet.2012.06.005 22768842
25. Schnorrer F, Schonbauer C, Langer CC, Dietzl G, Novatchkova M, et al. (2010) Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 464 : 287–291. doi: 10.1038/nature08799 20220848
26. Ruaud AF, Lam G, Thummel CS (2011) The Drosophila NR4A nuclear receptor DHR38 regulates carbohydrate metabolism and glycogen storage. Mol Endocrinol 25 : 83–91. doi: 10.1210/me.2010-0337 21084378
27. Pasco MY, Leopold P (2012) High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS One 7: e36583. doi: 10.1371/journal.pone.0036583 22567167
28. Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA (2002) Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell 2 : 239–249. 11832249
29. Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21 : 23–46. 11375428
30. Van Hoof D, Rodenburg KW, van der Horst DJ (2003) Lipophorin receptor-mediated lipoprotein endocytosis in insect fat body cells. J Lipid Res 44 : 1431–1440. 12754276
31. Jensen-Urstad AP, Semenkovich CF (2012) Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta 1821 : 747–753. doi: 10.1016/j.bbalip.2011.09.017 22009142
32. Nakamura MT, Yudell BE, Loor JJ (2014) Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53 : 124–144. doi: 10.1016/j.plipres.2013.12.001 24362249
33. Kunte AS, Matthews KA, Rawson RB (2006) Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab 3 : 439–448. 16753579
34. Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, et al. (2012) Lipoproteins in Drosophila melanogaster—assembly, function, and influence on tissue lipid composition. PLoS Genet 8: e1002828. doi: 10.1371/journal.pgen.1002828 22844248
35. Berry SE (2009) Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: an overview and implications for cardiovascular disease. Nutr Res Rev 22 : 3–17. doi: 10.1017/S0954422409369267 19442321
36. Michalski MC, Genot C, Gayet C, Lopez C, Fine F, et al. (2013) Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog Lipid Res 52 : 354–373. doi: 10.1016/j.plipres.2013.04.004 23624223
37. Tomarelli RM, Meyer BJ, Weaber JR, Bernhart FW (1968) Effect of positional distribution on the absorption of the fatty acids of human milk and infant formulas. J Nutr 95 : 583–590. 5665659
38. Innis SM, Dyer R, Nelson CM (1994) Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids 29 : 541–545. 7990660
39. Carnielli VP, Luijendijk IH, van Beek RH, Boerma GJ, Degenhart HJ, et al. (1995) Effect of dietary triacylglycerol fatty acid positional distribution on plasma lipid classes and their fatty acid composition in preterm infants. Am J Clin Nutr 62 : 776–781. 7572708
40. Turunen S, Crailsheim K (1996) Lipid and sugar absorption. In: Lehane MJ, Billingsley PF, editors. Biology of Insect Midgut. London: Chapman & Hall. pp. 293–320.
41. Lemaitre B, Miguel-Aliaga I (2013) The digestive tract of Drosophila melanogaster. Annu Rev Genet 47 : 377–404. doi: 10.1146/annurev-genet-111212-133343 24016187
42. Anton M, Martinet V, Dalgalarrondo M, Beaumal V, David-Briand E, et al. (2003) Chemical and structural characterisation of low-density lipoproteins purified from hen egg yolk. Food Chemistry 83 : 175–183.
43. Carvalho M, Sampaio JL, Palm W, Brankatschk M, Eaton S, et al. (2012) Effects of diet and development on the Drosophila lipidome. Mol Syst Biol 8 : 600. doi: 10.1038/msb.2012.29 22864382
44. Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, et al. (2013) Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J Biol Chem 288 : 8028–8042. doi: 10.1074/jbc.M112.371047 23355467
45. Guillou H, Zadravec D, Martin PG, Jacobsson A (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res 49 : 186–199. doi: 10.1016/j.plipres.2009.12.002 20018209
46. Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI (2000) Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 275 : 8456–8460. 10722680
47. Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, et al. (2000) Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105 : 271–278. 10675352
48. Lan H, Rabaglia ME, Stoehr JP, Nadler ST, Schueler KL, et al. (2003) Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 52 : 688–700. 12606510
49. Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K (2004) Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A 101 : 7281–7286. 15118080
50. Havula E, Teesalu M, Hyotylainen T, Seppala H, Hasygar K, et al. (2013) Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet 9: e1003438. doi: 10.1371/journal.pgen.1003438 23593032
51. Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, et al. (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4 : 842–849. doi: 10.1242/dmm.007948 21719444
52. Furusyo N, Hayashi J (2013) Glycated albumin and diabetes mellitus. Biochim Biophys Acta 1830 : 5509–5514. doi: 10.1016/j.bbagen.2013.05.010 23673238
53. Thornalley PJ (1988) Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 254 : 751–755. 3196289
54. Brouwers O, Niessen PM, Miyata T, Ostergaard JA, Flyvbjerg A, et al. (2013) Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 57 : 224–235. doi: 10.1007/s00125-013-3088-5 24162587
55. Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, et al. (2009) C. elegans as model for the study of high glucose - mediated life span reduction. Diabetes 58 : 2450–2456. doi: 10.2337/db09-0567 19675139
56. Phillips SA, Mirrlees D, Thornalley PJ (1993) Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor Statil. Biochem Pharmacol 46 : 805–811. 8373434
57. Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS (1999) Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 48 : 198–202. 9892243
58. Karachalias N, Babaei-Jadidi R, Rabbani N, Thornalley PJ (2010) Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia 53 : 1506–1516. doi: 10.1007/s00125-010-1722-z 20369223
59. Kesavan SK, Bhat S, Golegaonkar SB, Jagadeeshaprasad MG, Deshmukh AB, et al. (2013) Proteome wide reduction in AGE modification in streptozotocin induced diabetic mice by hydralazine mediated transglycation. Sci Rep 3 : 2941. doi: 10.1038/srep02941 24126953
60. Cantero AV, Portero-Otin M, Ayala V, Auge N, Sanson M, et al. (2007) Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-beta: implications for diabetic atherosclerosis. Faseb J 21 : 3096–3106. 17504976
61. Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, et al. (2010) Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia 53 : 989–1000. doi: 10.1007/s00125-010-1677-0 20186387
62. Morgan PE, Sheahan PJ, Pattison DI, Davies MJ (2013) Methylglyoxal-induced modification of arginine residues decreases the activity of NADPH-generating enzymes. Free Radic Biol Med 61C: 229–242.
63. Britton JS, Edgar BA (1998) Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125 : 2149–2158. 9570778
64. Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E (2000) Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev 14 : 2689–2694. 11069885
65. Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, et al. (2004) Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36 : 288–292. 14981519
66. Venken KJ, He Y, Hoskins RA, Bellen HJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314 : 1747–1751. 17138868
67. Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24 : 251–254. 11311363
68. Portier K, Guichardant M, Debouzy JC, Crouzier D, Geraud I, et al. (2007) In vitro effects of oxygen on physico-chemical properties of horse erythrocyte membrane. Environ Toxicol Pharmacol 23 : 340–346. doi: 10.1016/j.etap.2006.12.002 21783778
69. R_Core_Team (2013) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. doi: 10.1007/s12070-013-0687-x 25621273
70. Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5 : 99–114. 18151955
71. Gunstone FD, Harwood JL, Dijkstra AJ (2007) The Lipid Handbook (3rd Edition): CRC Press, Boca Raton</References> doi: 10.1093/jxb/erm028 25506957
Štítky
Genetika Reprodukční medicína
Článek 2014 Reviewer Thank YouČlánek Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic TranslationČlánek Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate CellsČlánek K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in ArabidopsisČlánek A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis inČlánek Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in MiceČlánek The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- Systematic Cell-Based Phenotyping of Missense Alleles Empowers Rare Variant Association Studies: A Case for and Myocardial Infarction
- African Glucose-6-Phosphate Dehydrogenase Alleles Associated with Protection from Severe Malaria in Heterozygous Females in Tanzania
- Genomics of Divergence along a Continuum of Parapatric Population Differentiation
- microRNAs Regulate Cell-to-Cell Variability of Endogenous Target Gene Expression in Developing Mouse Thymocytes
- A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in
- Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
- Partially Redundant Enhancers Cooperatively Maintain Mammalian Expression Above a Critical Functional Threshold
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Mutations in Result in Ocular Coloboma, Microcornea and Cataracts
- A Genome-Wide Hybrid Incompatibility Landscape between and
- Recurrent Evolution of Melanism in South American Felids
- Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate Cells
- Tissue Expression Pattern of PMK-2 p38 MAPK Is Established by the miR-58 Family in
- Essential Role for Endogenous siRNAs during Meiosis in Mouse Oocytes
- Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- RR-1 Cuticular Protein TcCPR4 Is Required for Formation of Pore Canals in Rigid Cuticle
- GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
- K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in Arabidopsis
- A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis in
- HYPER RECOMBINATION1 of the THO/TREX Complex Plays a Role in Controlling Transcription of the Gene in Arabidopsis
- Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan
- Structured Observations Reveal Slow HIV-1 CTL Escape
- An Integrative Multi-scale Analysis of the Dynamic DNA Methylation Landscape in Aging
- Combining Natural Sequence Variation with High Throughput Mutational Data to Reveal Protein Interaction Sites
- Transhydrogenase Promotes the Robustness and Evolvability of Deficient in NADPH Production
- Regulators of Autophagosome Formation in Muscles
- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Eye Selector Logic for a Coordinated Cell Cycle Exit
- Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations
- The DNA Polymerase δ Has a Role in the Deposition of Transcriptionally Active Epigenetic Marks, Development and Flowering
- Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice
- Membrane Recognition and Dynamics of the RNA Degradosome
- P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
- is a Long Non-coding RNA in JNK Signaling in Epithelial Shape Changes during Drosophila Dorsal Closure
- A Pleiotropy-Informed Bayesian False Discovery Rate Adapted to a Shared Control Design Finds New Disease Associations From GWAS Summary Statistics
- Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers
- and Hyperdrive Mechanisms (in Mouse Meiosis)
- Elevated In Vivo Levels of a Single Transcription Factor Directly Convert Satellite Glia into Oligodendrocyte-like Cells
- Systemic Delivery of MicroRNA-101 Potently Inhibits Hepatocellular Carcinoma by Repressing Multiple Targets
- Pooled Sequencing of 531 Genes in Inflammatory Bowel Disease Identifies an Associated Rare Variant in and Implicates Other Immune Related Genes
- Abscission Is Regulated by the ESCRT-III Protein Shrub in Germline Stem Cells
- Temperature Stress Mediates Decanalization and Dominance of Gene Expression in
- Transcriptome Wide Annotation of Eukaryotic RNase III Reactivity and Degradation Signals
- The Exosome Component Rrp6 Is Required for RNA Polymerase II Termination at Specific Targets of the Nrd1-Nab3 Pathway
- Sex-specific -regulatory Variation on the X Chromosome
- Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex
- Modeling of the Human Alveolar Rhabdomyosarcoma Chromosome Translocation in Mouse Myoblasts Using CRISPR-Cas9 Nuclease
- Asymmetry of the Budding Yeast Tem1 GTPase at Spindle Poles Is Required for Spindle Positioning But Not for Mitotic Exit
- TIM Binds Importin α1, and Acts as an Adapter to Transport PER to the Nucleus
- Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
- The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
- Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
- Region-Specific Activation of mRNA Translation by Inhibition of Bruno-Mediated Repression
- An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish ()
- Interaction between the tRNA-Binding and C-Terminal Domains of Yeast Gcn2 Regulates Kinase Activity In Vivo
- Hyper-Acetylation of Histone H3K56 Limits Break-Induced Replication by Inhibiting Extensive Repair Synthesis
- Prodomain Removal Enables Neto to Stabilize Glutamate Receptors at the Neuromuscular Junction
- Recent Selective Sweeps in North American Show Signatures of Soft Sweeps
- Identification and Functional Analysis of Healing Regulators in
- A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2
- Drosophila Casein Kinase I Alpha Regulates Homolog Pairing and Genome Organization by Modulating Condensin II Subunit Cap-H2 Levels
- The Hippo Pathway Regulates Homeostatic Growth of Stem Cell Niche Precursors in the Ovary
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



