-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
Sexual reproduction is thought to be a highly divergent process due to fast evolution and speciation. For example, sperm from one species can seldom fertilize eggs from another species, indicating that different molecular machinery for fertilization is applied in different species. In contrast to this divergent view, ovulation, the process of liberating mature eggs from the ovary, is a general phenomenon throughout the Metazoa. We provide evidence that basic mechanisms of ovulation are conserved. Like mammalian follicles, Drosophila follicles consist of single oocytes surrounded by a layer of follicle cells. Drosophila follicles degrade their posterior follicle cells to allow the oocyte to rupture into the oviduct during ovulation. The residual postovulatory follicles reside in the ovary, accumulate yellowish pigmentation, and produce the steroid hormone ecdysone, features which resemble the mammalian corpus luteum. We also showed that matrix metalloproteinase, a type of proteinase proposed to degrade the mammalian follicle wall during ovulation, is required in Drosophila for posterior follicle cell degradation and ovulation. These findings are particularly important because this simple genetic model system will speed up the identification of many conserved regulators required for regulating matrix metalloproteinase activity and ovulation in human, processes that influence ovarian cancer formation and cancer metastasis.
Published in the journal: . PLoS Genet 11(2): e32767. doi:10.1371/journal.pgen.1004989
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004989Summary
Sexual reproduction is thought to be a highly divergent process due to fast evolution and speciation. For example, sperm from one species can seldom fertilize eggs from another species, indicating that different molecular machinery for fertilization is applied in different species. In contrast to this divergent view, ovulation, the process of liberating mature eggs from the ovary, is a general phenomenon throughout the Metazoa. We provide evidence that basic mechanisms of ovulation are conserved. Like mammalian follicles, Drosophila follicles consist of single oocytes surrounded by a layer of follicle cells. Drosophila follicles degrade their posterior follicle cells to allow the oocyte to rupture into the oviduct during ovulation. The residual postovulatory follicles reside in the ovary, accumulate yellowish pigmentation, and produce the steroid hormone ecdysone, features which resemble the mammalian corpus luteum. We also showed that matrix metalloproteinase, a type of proteinase proposed to degrade the mammalian follicle wall during ovulation, is required in Drosophila for posterior follicle cell degradation and ovulation. These findings are particularly important because this simple genetic model system will speed up the identification of many conserved regulators required for regulating matrix metalloproteinase activity and ovulation in human, processes that influence ovarian cancer formation and cancer metastasis.
Introduction
Ovulation, the liberation of a mature oocyte from the ovary, is one of the critical events of metazoan reproduction. In mammals, where ovulation has been studied most thoroughly, several important steps have been identified [1–4]. First, among a cohort of mature ovarian follicles, a dominant follicle arises. Eventually, proteolytic enzymes are locally activated that digest a small part of the dominant follicle’s wall and extracellular matrix, releasing the oocyte into the oviduct [5]. Finally, residual follicular cells remodel the ruptured follicle into the yellowish corpus luteum, an endocrine body that secretes the steroid hormones progesterone, estrogen, and other factors. While much has been learned, genetically testing the roles proposed for specific genes and pathways has been difficult. For example, the importance of matrix metalloproteinases (Mmps) in ovulation has not been demonstrated using knockout mice, possibly due to redundancy [6–9]. A genetically tractable system containing fewer redundant genes such as Drosophila would greatly facilitate ovulation studies. However, ovulation in Drosophila has not been well characterized and is not known to involve the same processes as mammalian ovulation.
The Drosophila female reproductive system is anatomically similar to mammals, having two ovaries connected by lateral and common oviducts to the uterus, where fertilization occurs and one egg is retained prior to laying (Fig. 1A) [10]. Ovulation does not follow a simple cycle, however. Multiple eggs are laid when suitable food resources are available [11], and ovulation follows each oviposition to replenish the uterus. Egg laying and ovulation are extensively regulated by octopaminergic neural inputs [12–14] and can be elicited by peptides transferred in semen from the male [15–18]. Ovulation requires reproductive tract secretions controlled by the NR5a class nuclear hormone receptor Hr39 [19]. A mammalian ortholog, LRH-1, is required in mouse granulosa cells for ovulation, to maintain progesterone production in the corpus luteum and for decidual cell function in the uterus [20,21]. These similarities highlight the potential value of Drosophila as a genetically tractable model of ovulation.
Fig. 1. Drosophila follicle cells remain in the ovary following ovulation and form a corpus luteum. 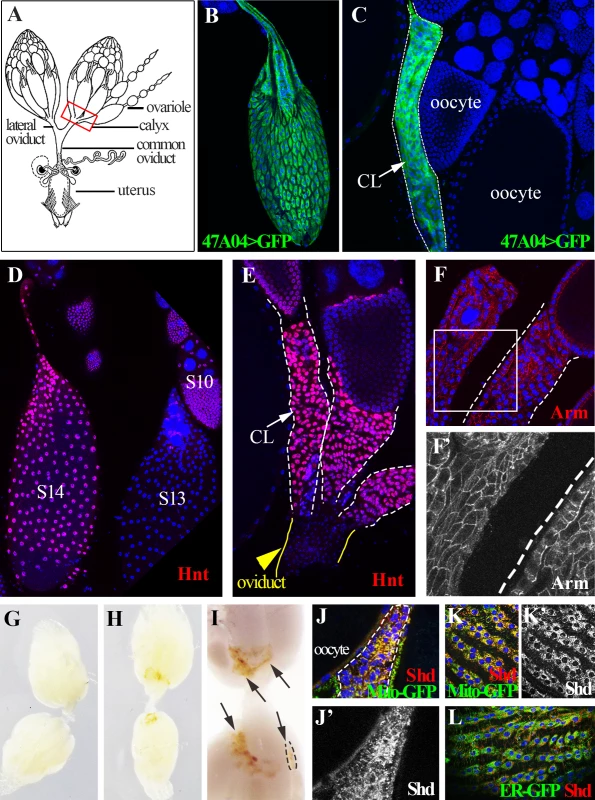
(A) A schematic diagram of female reproductive system. The red squared area is the calyx where post-ovulatory follicle cells are located. (B) R47A04-Gal4 driving UAS-GFP expression (R47A04>GFP) is specifically in follicle cells of S14 egg chambers (mature follicles). (C) Follicle cells from post-ovulatory follicles (green; outlined) remain in the ovary after the egg enters the oviduct and form a corpus luteum (CL). (D) Hnt accumulates in S14 follicle cells after decreasing in S13. (E) Hnt expression continues in CL cells. (F and F’) CL cells continue to express Arm, an adherens junction component (Red in F, white in F’) suggesting that they maintain their apical-basal polarity. G) Ovaries of virgin females before egg laying. No pigmentation is found in the ovary. (H, I) Ovaries of mated females after egg laying. Yellow pigmentation (arrows) is found in the CL. A single CL is outlined. (J-L) Shade (Shd) is expressed in CL cells (dash-line outlined in J and J’) and S14 follicle cells (K, K’, and L). Shd expression co-localized with a mitochondria marker (yellow in J and K), but not with an endoplasmic reticulum marker (L). Here, we show that a similar follicle rupturing process leads to Drosophila ovulation. Posterior follicle cells of a mature egg chamber are first degraded and the residual follicle cells are squeezed toward the anterior while the oocyte moves posteriorly into the lateral oviduct. Membrane-tethered Mmp2, but not Mmp1, functions in follicle cells to control follicle rupture and oocyte release. Residual follicle cells remain in the ovary, accumulate yellowish pigmentation, express ecdysone biosynthetic genes, and persist for an extended period before degradation, events reminiscent of the corpus luteum in mammals. Our data indicate that the cellular and molecular regulation of ovulation has been more conserved than previously thought.
Results
Follicles rupture to release the oocyte and form a corpus luteum
The fate of Drosophila follicle cells after ovulation has not been clearly described. If ovulation involves a programmed rupture of the follicular layer as in mammals, then most follicle cells would remain behind in the ovary after the egg is released into the oviduct. Alternatively, follicle cells might degenerate randomly or some might accompany the oocyte into the oviduct and the uterus. Using a mature (stage 14) follicle cell marker (Fig. 1B, Methods), we observed a cluster of GFP-labeled cells at the posterior end of each ovariole in the basal (calyx) region of ovary (Fig. 1C). In contrast, few, if any, follicle cells leave the ovary upon ovulation because GFP-labeled cells were not seen associated with eggs in the oviduct or uterus.
Post-ovulation mammalian follicles transform into the corpus luteum and similar behavior was reported previously in several other insects [22]. Consequently, we termed each cluster of residual Drosophila ovarian follicle cells as a corpus luteum (CL), and observed that CL cells continue a program of gene expression. The zinc-finger transcription factor Hindsight (Hnt), a major follicle cell regulator [23], is upregulated in stage 14 follicle cells and is expressed in CL cells (Fig. 1D-E). Expression of the adherens junction protein Arm (Fig. 1F-F’), suggests that CL cells maintain apical-basal epithelial polarity. Most dramatically, CL cells acquire a yellowish pigmentation (Fig. 1G-I) and express the ecdysone biosynthetic enzymes [24,25] Shade (Shd) in mitochondria (Fig. 1J-L) and Phantom (Phm) in the endoplasmic reticulum (ER; S1A Fig.). To examine the functional significance of steroid biosynthetic gene expression, we knocked down expression of shd and phm, as well as dib (disembodied, encoding another enzyme in the ecdysone synthesis pathway), in CL and mature follicle cells. These females all laid significantly fewer eggs than control females (S1B Fig.). This functional requirement for steroid biosynthetic enzymes supports the view that the Drosophila CL and mature follicle cells produces a steroid hormone such as ecdysone or 20-hydroxyecdysone, consistent with a recent finding that follicle cells in middle stages of oogenesis produce ecdysone to regulate border cell migration [26] and also reminiscent of steroid hormone production by the mammalian corpus luteum. We observed that only one CL is present in each ovariole, hence each CL must either degrade in situ over time following ovulation, or be extruded from the ovariole when the next egg is ovulated.
The organization of the CL reflects its origin in the follicle. All CL cells are labeled by a mature follicle cell driver, suggesting that cells from other sources are absent (Fig. 2A). Little cellular rearrangement occurs, since only anterior or middle cells of the corpus luteum were labeled by lines specifically expressed in anterior or middle stage 14 follicle cells (Fig. 2B-C). Lines specifically expressed in the posterior follicle cells did not label the CL, suggesting that these cells were degraded during ovulation (Fig. 2D).
Fig. 2. Posterior follicle cells are removed to release the oocyte and initiate corpus luteum formation. 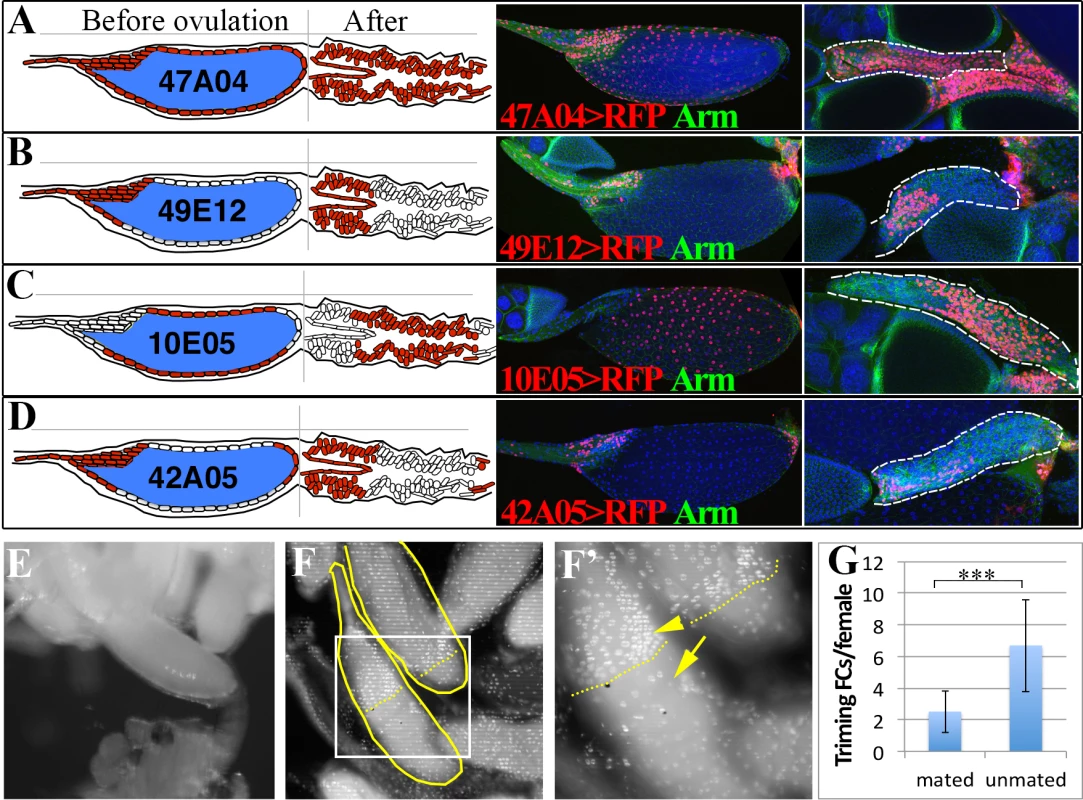
(A-D) Schematic (left panels) and real (right panels) expression patterns of Gal4 drivers in follicle cells of mature egg chambers and corpus luteum. Anterior follicle cells (B and D) reside at the anterior part of the corpus luteum, the middle follicle cells (C) reside at the middle portion of the corpus luteum, while the posterior follicle cells (D) are degraded in the corpus luteum. (E) An egg partially extruded into one lateral oviduct. (F-F’) DAPI stained calyx region showing the presence of two stage 14 trimming follicles (outlined) with no follicle cells at the posterior ends. The one with most posterior follicle cells trimmed was half-way in the lateral oviduct. The posterior end of follicle cells (brighter and larger nuclei pointed with an arrowhead) was marked by dashed lines, and the oviduct cells (faint and smaller nuclei) was marked by an arrow. F’ is the higher magnification of the squared region in F. (G) Histogram showing the number of follicles undergone trimming in mated (optimal ovulation) and unmated (no ovulation) females. Student’s T-test is used (*** P<0.001). Trimming of posterior follicle cells and protrusion precedes ovulation
Drosophila ovaries each usually contain at least 15 mature follicles, one per ovariole, oriented with their posterior ends facing the oviduct, raising the question of how one particular follicle is selected for ovulation. We examined ovary pairs from females cultured under conditions favorable for egg laying and found that at most one mature follicle protrudes significantly into a lateral oviduct (Fig. 2E). The protruding follicle always lacked posterior follicle cells covering the part of egg inside the lateral oviduct (Fig. 2F). We termed this process of losing posterior follicle cells as “trimming”. The trimmed follicle’s location indicates that trimming and protrusion represent preludes to ovulation. Frequently, another stage 14 follicle was present that had lost a smaller area of posterior follicle cells, but did not protrude (Fig. 2F-G and S2A-C Fig.), which likely represents the next follicle to ovulate. These observations show that a follicle is preselected in Drosophila well before ovulation, undergoes trimming, and awaits the next ovulation event while protruding into the lateral oviduct. In flies that were laying few eggs, for example in unmated females, up to 6 trimmed follicles could be present per female (Fig. 2G), but the follicle with the greatest level of trimming continues to protrude into the oviduct and remains in a position poised for ovulation.
Gelatinase activity is associated with trimming and egg release
The study of explanted mammalian follicles strongly implicates matrix metalloproteinases (Mmps) as important contributors to oocyte release [5]. In these follicles, Mmp activity is localized to the apex region where rupture will later occur [6]. We carried out gelatinase assays in situ to measure localized Mmp activity within Drosophila follicles before and during ovulation. High localized activity was found at the posterior end of one mature follicle in each ovary pair while a second follicle sometimes had lesser activity (Fig. 3A); the location of the activity correlated with the site of follicle cell trimming at the posterior (Fig. 3B). As eggs begin to enter the oviduct, the fraction of the follicular surface with gelatinase activity (Fig. 3C) increased from posterior to anterior, and matched where follicle cells no longer reside (Fig. 3C’). In eggs that have nearly separated from their follicle cells, gelatinase activity covered the entire surface (Fig. 3D), however, the anterior and middle follicle cells remained in a mass at the base of the ovary (Fig. 3D’). These data tightly associate Mmp activity with posterior follicle cell trimming, and suggest that more anterior Mmp activity subsequently degrades just the extracellular matrix separating the oocyte from intact middle and anterior follicle cells.
Fig. 3. Mmp2 is required for ovulation and CL formation. 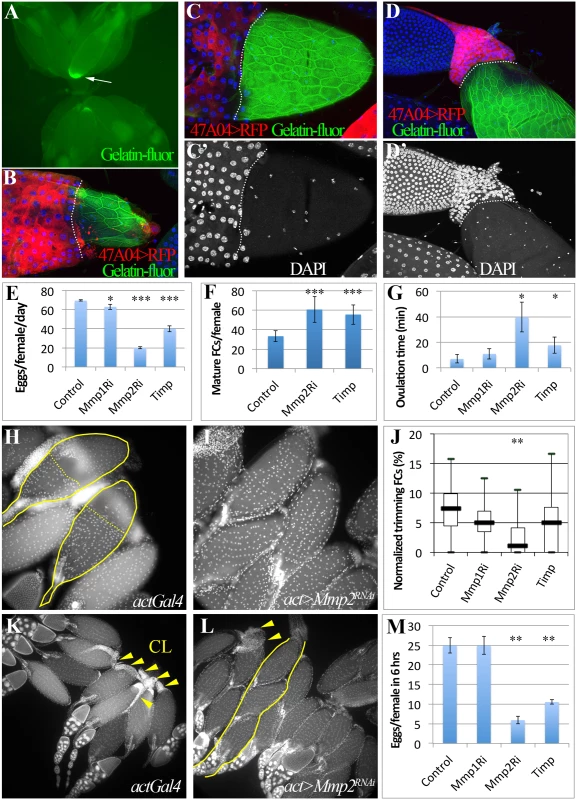
(A) In situ zymography showing one preselected follicle with high gelatinase activity at its posterior end in the entire ovary pair. (B-D) Correlation of follicle cell trimming and gelatinase activity (green in B-D). During early (B), middle (C-C’), and late (D-D’) ovulation, gelatinase activity is covering all the egg chamber area lost follicle cells. Posterior leading edge of the follicle cell layer was marked by dashed lines. Smaller nuclei (white in C’ and D’) are oviduct cells. (E-G) Egg laying (E), mature follicles in ovary (F), and the average ovulation time (G) of females of actGal4 or actGal4 driven Mmp1RNAi, Mmp2RNAi, or Timp expression in adult. (H-I) Follicle cell trimming in actGal4 control (H) and actGal4/Mmp2RNAi (I) ovaries. Mature egg chambers with posterior follicle cell trimmed were outlined with solid line and the posterior edge of the residual follicle cells were marked with dashed line. Follicle cell nuclei are elucidated by DAPI signal. (J) A quantification of trimming follicles in ovaries. (K-L) More corpora lutea (arrowheads) are found in actGal4 control ovaries (K) than those in actGal4/Mmp2RNAi ovaries (L) 6 hours after mating. One ovariole with three mature egg chambers was outlined in (K). (M) Number of eggs laid in 6 hours after mating. T-test is used (*** P<0.001, ** P<0.01, * P<0.05). Mmp2 is genetically required for ovulation and CL formation
Drosophila has two genes encoding matrix metalloproteinases, mmp1 and mmp2, and one Timp (Tissue inhibitor of matrix metalloproteinase)[27]. Genetically reducing Mmp2 but not Mmp1 expression dramatically lowered egg laying (Fig. 3E; S3 Fig.). Mature egg chambers accumulated in Mmp2 knockdown females (Fig. 3F), indicating a block in ovulation, and the average time required to ovulate (see Methods) increased fivefold (Fig. 3G and Table 1). Similarly, overexpressing Timp, a protein that inhibits both Mmp1 and Mmp2 activity, also decreased egg laying and increased egg retention and ovulation time (Fig. 3E-G, Table 1). These data show that Mmp2 enzymatic activity is required for normal ovulation in Drosophila.
Tab. 1. The effect of Mmp activity on egg laying, egg distribution in reproductive tract, and egg laying time. 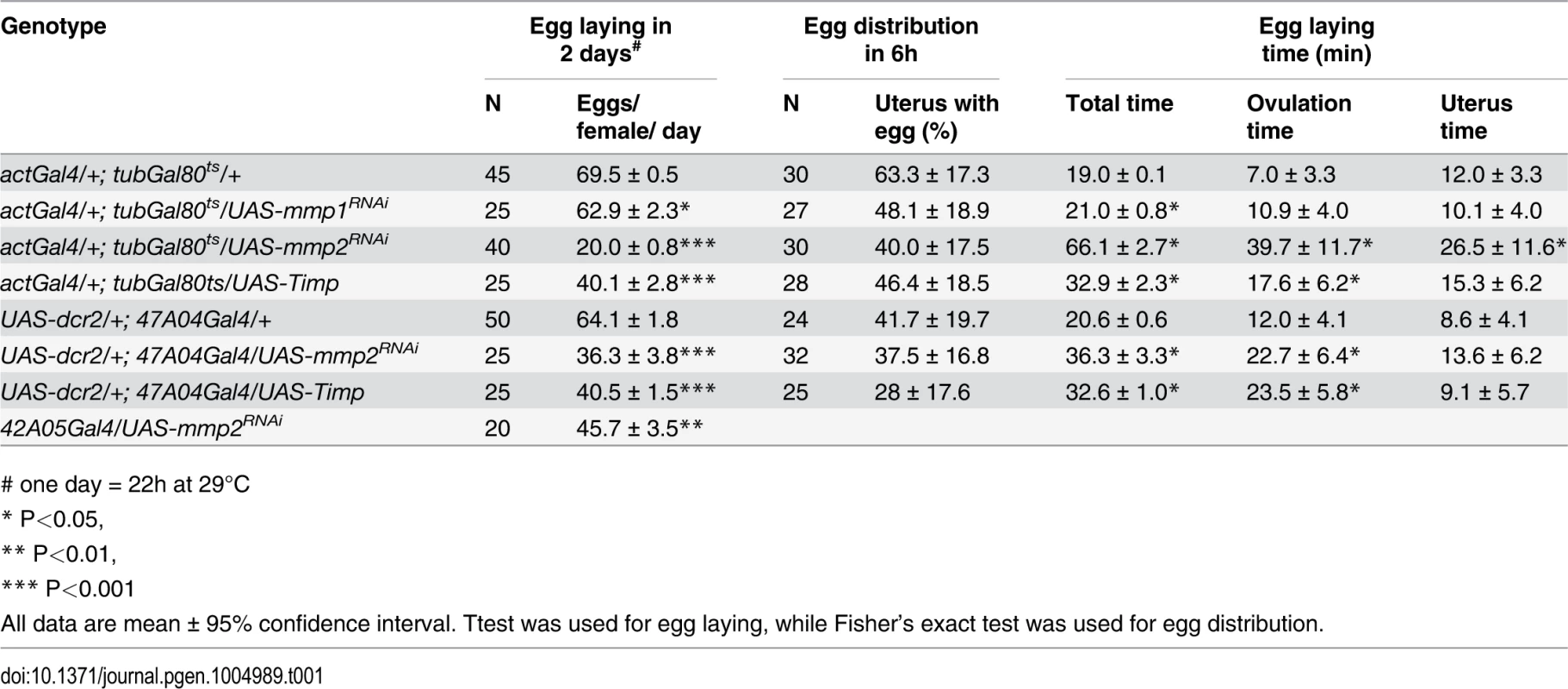
# one day = 22h at 29°C Mmp2 was also required for follicle trimming (Fig. 3H-I and Table 2). The rate of trimming was reduced at least three fold in Mmp2 knock down animals both before mating and at 6-hour post mating (Fig. 3J and Table 2). In addition, Mmp2 knock down ovaries lacked corpus luteum structures (Fig. 3K-L), and instead accumulated mature egg chambers (Fig. 3L). Both Mmp2 knock down females and females expressing Timp, displayed severe egg laying defects even within 6 hours of mating (Fig. 3M). Thus, Mmp2 activity is required in adult females for follicle cell trimming, ovulation, corpus luteum formation, and egg laying.
Tab. 2. The effect of Mmp activity for follicle cell trimming. 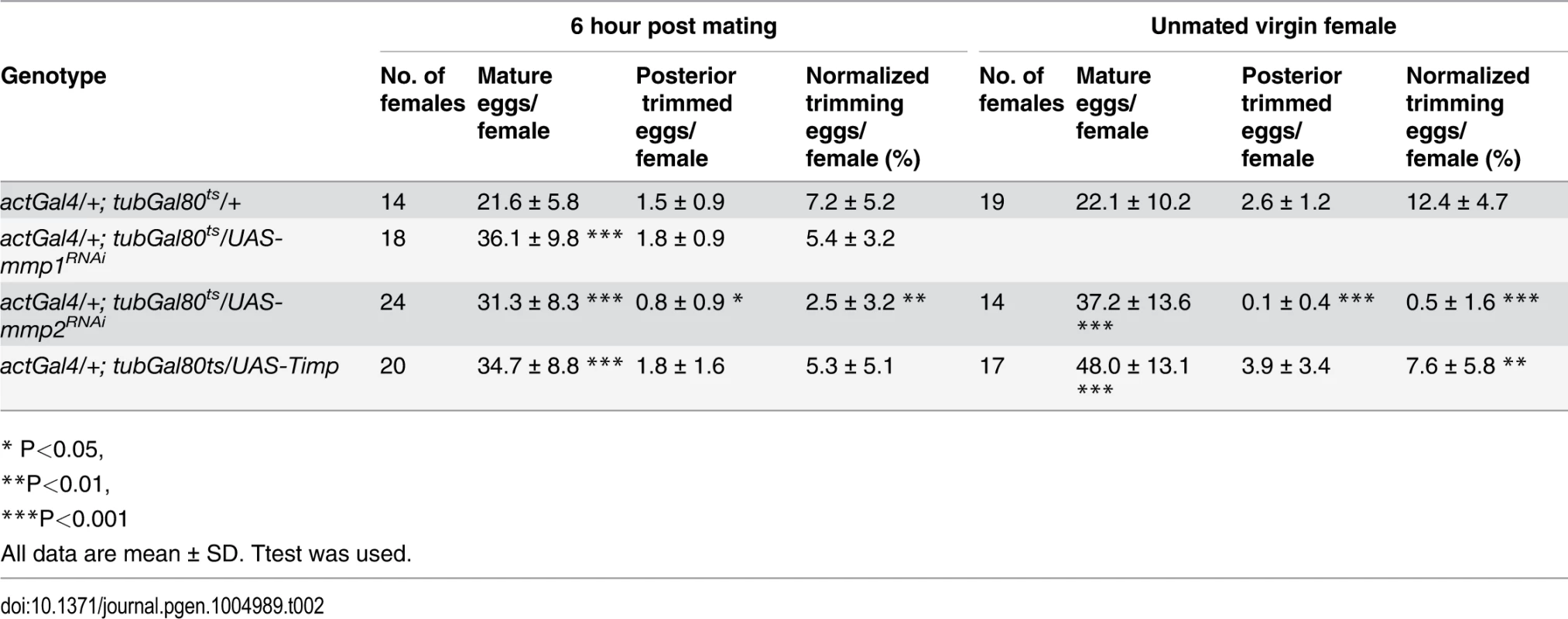
* P<0.05, Mmp2 is expressed and functions in follicle cells to control ovulation
We generated an in vivo Mmp2::GFP fusion allele at its normal genomic location by swapping an in-frame GFP exon into a MiMIC transposon inserted within an Mmp2 coding intron (S4 Fig.). We also employed a Gal4 enhancer trap line (see Methods), which mimics Mmp2 expression during pupal imaginal disc eversion, to monitor Mmp2 transcription. Mmp2 fusion protein and RNA are specifically expressed in posterior follicle cells in all mature stage 14 follicles but not in earlier follicles (Fig. 4A-C, S4C Fig. and S5A-D Fig.). Mmp2 is also expressed in some anterior follicle cells that help form dorsal eggshell structures. The reporters show expression at the posterior edge of surviving follicle cells during trimming, and in anterior and posterior corpus luteum cells (Fig. 4D-E and S5D Fig.).
Fig. 4. Mmp2 functions in follicle cells to control ovulation. 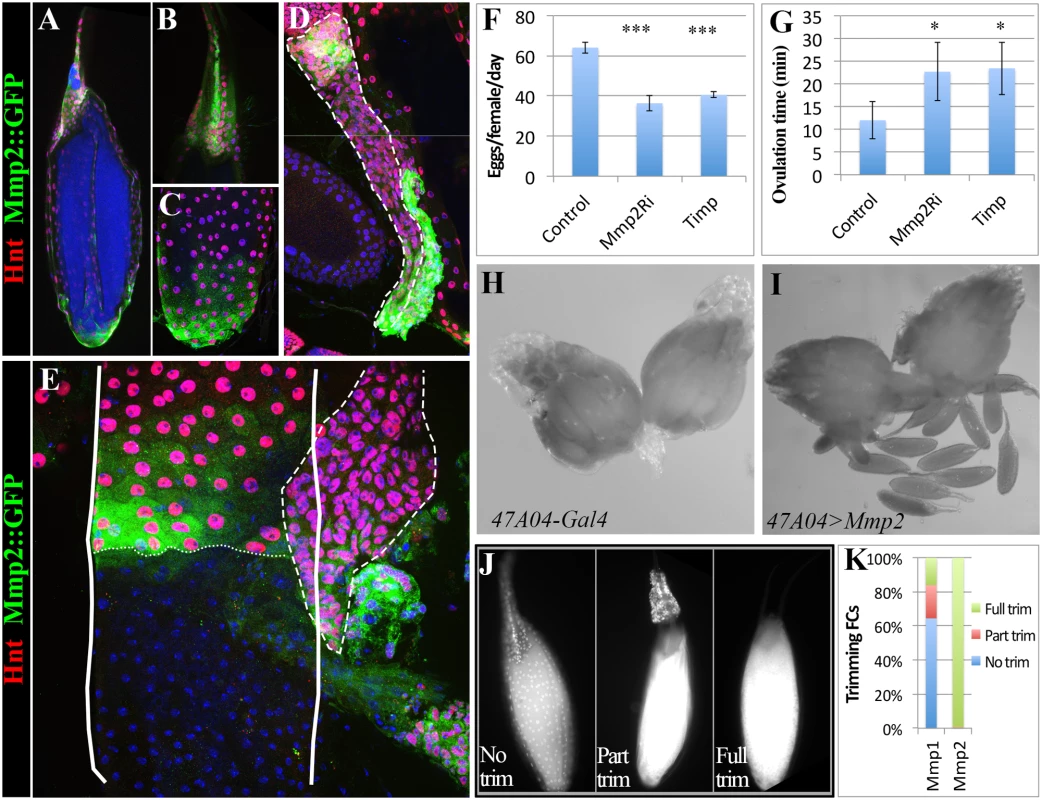
(A-C) Mmp2 expression in mature egg chambers indicated by Mmp2::GFP fusion protein. Mmp2 is highly expressed in both anterior (B) and posterior (C) follicle cells. Hnt (Red) is used to mark follicle cells. (D) Mmp2::GFP is expressed in anterior and posterior corpus luteum (outlined with dashed line). (E) Mmp2::GFP is expressed in the posterior edge of the trimming follicle cells when the egg is half way in the oviduct. The egg chamber is outlined by solid line, the posterior edge of follicle cells is marked by small dashed lines, and the corpus luteum is outlined by big dashed line. Smaller nuclei without Hnt expression are from oviduct epithelial cells. (F-G) The egg laying (F) and ovulation time (G) of females with 47A04-Gal4 or 47A04-Gal4 driven Mmp2RNAi or Timp expression in mature follicle cells. T-test is used (*** P<0.001, *P<0.05). (H-I) Ectopic Mmp2 in mature follicle cells is sufficient to cause egg release from ovary into abdominal cavity. (J) DAPI staining to indicate the follicle cell trimming of egg chambers released from ovary ectopic Mmp2 expression in (I). (K) Quantification of egg chamber trimming. Egg chambers already released from ovaries were collected from abdominal cavity of females with 47A04 driven Mmp1 or Mmp2 expression. 149 and 144 released egg chambers were collected from Mmp1 and Mmp2 flies respectively. We interfered with Mmp2 expression specifically in mature follicle cells by using a mature follicle cell driver (R47A04) to express Mmp2 RNAi or to overexpress Timp, and observed that ovulation and egg laying were defective (Fig. 4F-G and Table 1). The defect is not likely due to disruption of Mmp2 in neurons as R47A04 is not expressed in sensory neurons innervating the female reproductive tract (S5E-F Fig.). Action outside of neurons is also supported by the observation that knocking down Mmp2 with a more restricted mature follicle cell driver (R42A05: expressed in posterior and anterior mature follicle cells; Fig. 2D) showed a similar egg laying defect (Table 1), although one of lower severity. When Mmp2 was overexpressed in mature follicle cells, mature eggs ruptured and were released into the female abdominal cavity (Fig. 4H-I). Most such eggs lacked covering follicle cells (Fig. 4J-K). When Mmp1 was ectopically expressed in mature follicle cells with the same Gal4 driver, fewer follicles ruptured into the abdominal cavity and most released eggs retained some follicle cells (Fig. 4K). Consequently, Mmp2 is required in mature follicle cells to trim the follicular layer leading to ovulation, and its level of expression must be controlled or normal activity regulation may be overwhelmed.
Discussion
Despite different biological strategies of ovulation in mice and Drosophila, our studies reveal strong similarities in the underlying mechanisms. In both species, a follicle is selected before ovulation, and its oocyte is released at an appropriate time by inducing Mmp proteolytic activity, either in the apex region (mouse) or at the follicle posterior (Drosophila). Mmp2 activation is likely controlled by pro-domain processing, and may also be modulated at the level of protein secretion and/or by the presence of the endogenous inhibitor Timp. Pharmacological inhibition of Mmp activity prevents ovulation in vitro in mice [28,29], as well as in other vertebrate and primate species [30,31]. However, knockouts of individual Mmp genes have not been reported to affect ovulation, presumably due to redundancy, although individual Mmp gene knockouts frequently have specific phenotypic effects (reviewed in [9]). In contrast, our studies clearly show a genetic requirement of Mmp2 but not Mmp1 for trimming, ovulation and CL formation. However, follicles from MMP2 RNAi females still retained some gelatinase activity based on the in situ assay, and our experiments cannot rule out that MMP1 or other proteases also contribute to follicle trimming.
The value of Drosophila for studies of ovulation is further illustrated by the discovery that after ovulation, residual follicle cells form a corpus luteum. The corpus luteum (Latin for “yellow body”) was first described by Volcherus Coiter in 1573, but its relationship to ovulation rather than pregnancy was not understood until the early 19th century [32]. The existence of a pigmented structure in insect ovaries was also recognized in the 19th century, at least in a few species [22]. However, it has remained unclear whether a CL exists in Drosophila, whether it is a universal feature of insect oogenesis, whether the CL functions in reproduction, and whether any such functions have been conserved during evolution. Our studies indicate that a CL is formed in Drosophila and that Mmp2 activity is required for its production. The mammalian CL contains at least two cell types, small CL cells which are thought to be derived from thecal cells, and large CL cells that produce progesterone. Our gene expression studies suggest that at least two cell types are also likely in the Drosophila CL [33]. Some anterior CL cells may derive from stretch cells, which never secrete eggshell proteins [34].
The Drosophila CL may function at least in part by producing the steroid hormones, ecdysone or 20-hydroxyecdysone, as suggested by continuous expression of genes encoding P450 enzymes Phantom and Shade in CL cells. Mated females are known to have a higher ecdysone titer than unmated females [35], consistent with the idea that the CL contributes substantially to ecdysone production. A common role in steroid hormone production might explain the conserved pigmentation of the CL. In mammals, carotenoid accumulation is beneficial to gametogenesis and is associated with steroid hormone production. These molecules may influence free radical balance, which might otherwise interfere with steroid production [36]. Alternatively, carotenoids may simply accumulate because they are found within the circulating lipoprotein particles that must be taken up to support steroid production [37]. The easy of genetic manipulation in Drosophila may allow the biochemical nature and function of the yellow pigmentation in the CL to be further characterized.
Finally, we propose that a major function of the CL in Drosophila is to control the maturation of younger follicles in the ovariole, and to select mature follicles for the next ovulation. In their location at the posterior end of each ovariole, CL cells are well positioned to govern the orderly usage of mature follicles. If CL cell secretory activity decreases with age, the corpus luteum in each ovariole might communicate the elapsed time since an ovariole was last used, for example by local inhibition, promoting the relatively uniform usage of all ovarioles. Although the large scale organization of follicles within the mammalian ovary is less obvious, signals from its corpora lutea might likewise spatially control follicle maturation. Knowledge that some fundamental aspects of ovulation are similar in Drosophila and mammals will accelerate the study of these and many other questions.
Materials and Methods
Drosophila genetics
Flies were reared on standard cornmeal-molasses food at 25°C unless otherwise indicated. The following Gal4 lines from the Janelia Farm collection [38] were used to label follicle cells and corpus luteum cells: R47A04 (Oamb), R49E12 (5-HT2A), R10E05 (AstC-R2), and R42A05 (kay). To knockdown mmp1 or mmp2 or overexpress Timp in adult flies, actGal4/Cyo; tubGal80ts virgin females were crossed to the following lines at 18°C and shifted to 29°C immediately after adult eclosion: UAS-mmp1RNAi (Bloomington Drosophila stock center, B31489), UAS-mmp1RNAi2 [39], UAS-mmp1RNAi3 (Vienna Drosophila RNAi Center, V108894), UAS-mmp1E225A (a dominant negative form of Mmp1) [40], UAS-mmp2RNAi [39], UAS-mmp2RNAi2 (VDRC, V107888), UAS-mmp2RNAi3 (BDSC, B31371), UAS-Timp [41]. To knock down mmp1 or mmp2 or overexpress Timp in follicle cells of mature egg chambers, UAS-dcr2; R47A04 virgin females were crossed to the RNAi lines described above at 29°C. To knock down ecdysone synthesis genes, UAS-dcr2; R47A04 virgin females were crossed shdRNAi (VDRC, V17203), dibRNAi (VDRC, V101117), or phmRNAi (VDRC, V108359). To overexpress mmp1 or mmp2 in mature follicle cells, R47A04 virgin females were crossed to UAS-mmp1 or UAS-mmp2 [41] at 21°C. Control flies were derived from specific Gal4 driver crossed to wild-type Oregon-R. Mmp2::GFP fusion genes were generated through recombinase mediated cassette exchange of MiMIC insertion (MI02914) in the third coding intron of mmp2 (S4 Fig.) [42]. Mmp2-Gal4 line is from an Gal4 enhancer trap [43], and UAS-RedStinger (BDSC, B8547) and UASpGFP-act79B; UAS-mCD8-GFP were used as reporters. sqh-EYFP-Mito (BDSC, B7194) and sqh-EYFP-ER (BDSC, B7195) were used for tracking mitochondria and endoplasmic reticulum, respectively.
Egg laying and ovulation time
Egg laying and ovulation was determined essentially as described [19]. Virgin females were aged for four to five days, and fed with wet yeast one day before experiments. To measure egg laying time (the average time between successive ovipositions), five females were mated to 10 Oregon-R males in each bottle covered with a molasses plate at 29°C and five or more bottles were set up for each genotype. The molasses plates were replaced every 22 hours and the number of eggs laid was counted for 44 hours and used to determine the average time required per egg. Egg laying time (min) was then calculated as 22 hr x 60 min / eggs laid per 22hr. To determine ovulation time, about 30 single-pair matings with one virgin female and one Oregon-R male were carried out for each genotype at 29°C for 6 hours, an interval sufficient for all females to reach a steady state level of ovulation and egg laying. Females were then frozen in -80°C for four minutes, their reproductive tracts were dissected to identify eggs inside the reproductive tract, and the percentage of females with an egg in the uterus or actively ejecting out of the uterus (U%) was calculated. Free eggs were never observed in the common oviducts in control or mutant flies (N = 196), indicating that eggs spend a negligible amount of time moving through the oviducts. In addition, we never observe more than one egg in the female reproductive tract (N = 196). Therefore, the egg laying time is partitioned into the uterus time (the average time eggs reside in the uterus or actively ejecting) and the ovulation time (the average time eggs prepare to be released from the ovary), which includes the time when the follicle protrudes into the lateral oviduct, because our study indicates that these eggs are in the process of ovulation and have not yet been released from the ovary (Fig. 2E-F, 3D, and 4E). Uterus time was then calculated as egg laying time x U%, and ovulation time = egg laying time x (1—U%). The ovulation time is a proxy for the average time needed for an egg to be released from the ovary while flies are laying eggs rapidly at steady state. The ovulation process is complex and is known to be affected by many factors, such as glandular secretions, male peptides, moisture, and egg laying substrates, whose influences are aggregated using this approach. The 95% confidence intervals were calculated correspondingly.
Follicle cell trimming
Females were frozen in -80°C for four minutes before dissection. Ovaries were dissected out immediately afterwards, fixed with 4% EM Grade paraformadehyde, and stained with DAPI. Care was taken to make sure that two ovaries from single female were intact after staining and mounted in the same well on slides by carefully separating the ovarioles. The number of trimming follicles was scored according to the criteria that a quarter of the egg chamber at the posterior end has no follicle cells covering, and the number of mature follicles was scored according to their fully elongated dorsal appendage. The normalized trimming follicles were then calculated by the number of trimming follicles divided by the number of mature follicles in each female. For follicle cell trimming analysis in Fig. 2G, three to four days old w1118 mated or unmated females were used. For analyzing trimming in females with Mmp knock down, three to four days old virgin females were mated with Oregon-R males for six hours before dissection. For trimming with misexpressing mmp1 and mmp2, follicles were directly collected from female abdominal cavity.
Immunostaining, in situ zymography, and microscopy
Antibody staining followed a standard procedure [44]. Briefly, tissues were fixed in 4% EM-Grade paraformadehyde for 15 minutes and blocked in PBTG (PBS+ 0.2% Triton+ 0.5% BSA+ 2% normal goat serum). Primary antibodies were incubated overnight at 4°C and secondary antibodies were incubated for two hours at room temperature, followed by DAPI staining. The following primary antibodies were used: mouse anti-Hnt (1 : 75) and anti-Arm (1 : 40) from Developmental Study Hybridoma Bank, rabbit anti-Shd (1 : 250; a gift from Michael O’Connor) and anti-Phm [25] (1 : 250), rabbit anti-GFP (1 : 4000, Invitrogen), and rabbit anti-RFP (1 : 2000, MBL international). Secondary antibodies were Alexa 488 goat anti-rabbit and 546 goat anti-mouse and anti-rabbit (1 : 1000, Invitrogen). Images were acquired using the Leica TCS SP5 confocal microscope, and assembled using Photoshop software (Adobe, Inc.). Images for yellow pigmentation of the corpus luteum were taken with the Macropod with Canon 6D camera and Olympus SZX16 stereomicroscope with Olympus DP72 color camera.
The in situ zymography technique for gelatinase activity was performed as previously described with minor modifications [45]. Ovaries were dissected in pre-warmed Grace’s media and incubated immediately in 100 μg/ml DQ-gelatin conjugated with fluorescein (Invitrogen) for an hour. To increase substrate penetration, the peritoneal muscle sheath was broken at the ovarian anterior. Ovaries were then fixed in 4% EM-Grade Paraformadehyde for 15 minutes and mounted for visualization.
Supporting Information
Zdroje
1. Conti M, Hsieh M, Musa Zamah A, Oh JS (2012) Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 356 : 65–73. doi: 10.1016/j.mce.2011.11.002 22101318
2. Espey LL, Richards JS (2006) Ovulation. In: Neill JD, editor. Physiology of Reproduction. Amsterdam: Academic Press, Vol. 1. pp. 425–474.
3. Fan H-Y, Liu Z, Mullany LK, Richards JS (2012) Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol Cell Endocrinol 356 : 74–79. doi: 10.1016/j.mce.2011.12.005 22197887
4. Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN (1998) Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 145 : 47–54. doi: 10.1016/S0303–7207(98)00168–3 9922098
5. Ohnishi J, Ohnishi E, Shibuya H, Takahashi T (2005) Functions for proteinases in the ovulatory process. Biochim Biophys Acta BBA—Proteins Proteomics 1751 : 95–109. doi: 10.1016/j.bbapap.2005.05.002 25626174
6. Curry TE, Osteen KG (2003) The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr Rev 24 : 428–465. doi: 10.1210/er.2002–0005 12920150
7. Curry T, Smith M (2006) Impact of Extracellular Matrix Remodeling on Ovulation and the Folliculo-Luteal Transition. Semin Reprod Med 24 : 228–241. doi: 10.1055/s-2006–948552 16944420
8. McCord LA, Li F, Rosewell KL, Brännström M, Curry TE (2012) Ovarian Expression and Regulation of the Stromelysins During the Periovulatory Period in the Human and the Rat. Biol Reprod 86 : 78. doi: 10.1095/biolreprod.111.095588 22116802
9. Gill SE, Kassim SY, Birkland TP, Parks WC (2010) Mouse Models of MMP and TIMP Function. In: Clark IM, editor. Matrix Metalloproteinase Protocols. Methods in Molecular Biology. Totowa, NJ: Humana Press, Vol. 622. pp. 31–52. Available: http://link.springer.com/10.1007/978-1-60327-299-5. Accessed 2 June 2014. doi: 10.1007/978-1-60327-299-5_2 20135274
10. Spradling AC (1993) Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, Vol. I. pp. 1–70.
11. Yang CH, Belawat P, Hafen E, Jan LY, Jan YN (2008) Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319 : 1679–1683. doi: 10.1126/science.1151842 18356529
12. Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A (2003) Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol 264 : 179–190. doi: 10.1016/j.ydbio.2003.07.018 14623240
13. Monastirioti M (2003) Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol 264 : 38–49. doi: 10.1016/j.ydbio.2003.07.019 14623230
14. Lee HG, Rohila S, Han KA (2009) The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One 4: e4716. doi: 10.1371/journal.pone.0004716 19262750
15. Yapici N, Kim YJ, Ribeiro C, Dickson BJ (2008) A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451 : 33–37. doi: 10.1038/nature06483 18066048
16. Hasemeyer M, Yapici N, Heberlein U, Dickson BJ (2009) Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61 : 511–518. doi: 10.1016/j.neuron.2009.01.009 19249272
17. Rubinstein CD, Wolfner MF (2013) Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci 110 : 17420–17425. doi: 10.1073/pnas.1220018110 24101486
18. Yang C, Rumpf S, Xiang Y, Gordon MD, Song W, et al. (2009) Control of the Postmating Behavioral Switch in Drosophila Females by Internal Sensory Neurons. Neuron 61 : 519–526. doi: 10.1016/j.neuron.2008.12.021 19249273
19. Sun J, Spradling AC (2013) Ovulation in Drosophila is controlled by secretory cells of the female reproductive tract. Elife 2. Available: http://elife.elifesciences.org/content/2/e00415.short. Accessed 13 March 2014.
20. Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, et al. (2008) Liver receptor homolog 1 is essential for ovulation. Genes Dev 22 : 1871–1876. doi: 10.1101/gad.472008 18628394
21. Zhang C, Large MJ, Duggavathi R, Demayo FJ, Lydon JP, et al. (2013) Liver receptor homolog-1 is essential for pregnancy. Nat Med. doi: 10.1038/nm.3435 24466584
22. Büning J (1994) The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution. Springer. 424 p.
23. Sun J, Deng W-M (2007) Hindsight Mediates the Role of Notch in Suppressing Hedgehog Signaling and Cell Proliferation. Dev Cell 12 : 431–442. doi: 10.1016/j.devcel.2007.02.003 17336908
24. Petryk A, Warren JT, Marqués G, Jarcho MP, Gilbert LI, et al. (2003) Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci 100 : 13773–13778. doi: 10.1073/pnas.2336088100 14610274
25. Warren JT, Petryk A, Marqués G, Parvy J-P, Shinoda T, et al. (2004) Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol 34 : 991–1010. doi: 10.1016/j.ibmb.2004.06.009 15350618
26. Domanitskaya E, Anllo L, Schüpbach T (2014) Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev Biol 386 : 408–418. doi: 10.1016/j.ydbio.2013.12.013 24373956
27. Page-McCaw A (2008) Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Semin Cell Dev Biol 19 : 14–23. doi: 10.1016/j.semcdb.2007.06.004 17702617
28. Brännström M, Woessner JF Jr, Koos RD, Sear CH, LeMaire WJ (1988) Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology 122 : 1715–1721. doi: 10.1210/endo-122–5–1715 2452070
29. Reich R, Tsafriri A, Mechanic GL (1985) The involvement of collagenolysis in ovulation in the rat. Endocrinology 116 : 522–527. doi: 10.1210/endo-116–2–522 2981665
30. Chaffin CL, VandeVoort CA (2013) Follicle growth, ovulation, and luteal formation in primates and rodents: A comparative perspective. Exp Biol Med 238 : 539–548. doi: 10.1177/1535370213489437 23856905
31. Ogiwara K, Takano N, Shinohara M, Murakami M, Takahashi T (2005) Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proc Natl Acad Sci U S A 102 : 8442–8447. doi: 10.1073/pnas.0502423102 15941829
32. Watt J (1915) Contributions to Embryology. Carnegie Institution of Washington Publication no. 222. 85–86 p.
33. Tootle TL, Williams D, Hubb A, Frederick R, Spradling A (2011) Drosophila Eggshell Production: Identification of New Genes and Coordination by Pxt. PLoS ONE 6: e19943. doi: 10.1371/journal.pone.0019943 21637834
34. Parks S, Spradling AC (1987) Spatially regulated expression of chorion genes during Drosophila oogenesis. Genes Dev 1 : 497–509. doi: 10.1101/gad.1.5.497.
35. Harshman LG, Loeb AM, Johnson BA (1999) Ecdysteroid titers in mated and unmated Drosophila melanogaster females. J Insect Physiol 45 : 571–577. doi: 10.1016/S0022–1910(99)00038–4 12770342
36. Sawada M, Carlson JC (1996) Intracellular regulation of progesterone secretion by the superoxide radical in the rat corpus luteum. Endocrinology 137 : 1580–1584. doi: 10.1210/endo.137.5.8612488 8612488
37. Schweigert FJ (2003) Research note: changes in the concentration of beta-carotene, alpha-tocopherol and retinol in the bovine corpus luteum during the ovarian cycle. Arch Für Tierernähr 57 : 307–310. 4740118
38. Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, et al. (2012) A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep 2 : 991–1001. doi: 10.1016/j.celrep.2012.09.011 23063364
39. Uhlirova M, Bohmann D (2006) JNK-and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J 25 : 5294–5304. 17082773
40. Glasheen BM, Kabra AT, Page-McCaw A (2009) Distinct functions for the catalytic and hemopexin domains of a Drosophila matrix metalloproteinase. Proc Natl Acad Sci U A 106 : 2659–2664. doi: 10.1073/pnas.0804171106 19196956
41. Page-McCaw A, Serano J, Sante JM, Rubin GM (2003) Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell 4 : 95–106. doi: 10.1016/S1534-5807(02)00400-8 12530966
42. Venken KJ, Schulze KL, Haelterman NA, Pan H, He Y, et al. (2011) MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods 8 : 737–743. 21985007
43. Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T (2007) Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U A 104 : 2721–2726. doi: 10.1073/pnas.0611666104 17301221
44. Sun J, Spradling AC (2012) NR5A Nuclear Receptor Hr39 Controls Three-Cell Secretory Unit Formation in Drosophila Female Reproductive Glands. Curr Biol 22 : 862–871. doi: 10.1016/j.cub.2012.03.059 22560612
45. Vidal M, Salavaggione L, Ylagan L, Wilkins M, Watson M, et al. (2010) A role for the epithelial microenvironment at tumor boundaries: evidence from Drosophila and human squamous cell carcinomas. Am J Pathol 176 : 3007–3014. doi: 10.2353/ajpath.2010.090253 20363916
Štítky
Genetika Reprodukční medicína
Článek 2014 Reviewer Thank YouČlánek Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic TranslationČlánek Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate CellsČlánek K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in ArabidopsisČlánek A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis inČlánek Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in MiceČlánek The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- Systematic Cell-Based Phenotyping of Missense Alleles Empowers Rare Variant Association Studies: A Case for and Myocardial Infarction
- African Glucose-6-Phosphate Dehydrogenase Alleles Associated with Protection from Severe Malaria in Heterozygous Females in Tanzania
- Genomics of Divergence along a Continuum of Parapatric Population Differentiation
- microRNAs Regulate Cell-to-Cell Variability of Endogenous Target Gene Expression in Developing Mouse Thymocytes
- A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in
- Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
- Partially Redundant Enhancers Cooperatively Maintain Mammalian Expression Above a Critical Functional Threshold
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Mutations in Result in Ocular Coloboma, Microcornea and Cataracts
- A Genome-Wide Hybrid Incompatibility Landscape between and
- Recurrent Evolution of Melanism in South American Felids
- Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate Cells
- Tissue Expression Pattern of PMK-2 p38 MAPK Is Established by the miR-58 Family in
- Essential Role for Endogenous siRNAs during Meiosis in Mouse Oocytes
- Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- RR-1 Cuticular Protein TcCPR4 Is Required for Formation of Pore Canals in Rigid Cuticle
- GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
- K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in Arabidopsis
- A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis in
- HYPER RECOMBINATION1 of the THO/TREX Complex Plays a Role in Controlling Transcription of the Gene in Arabidopsis
- Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan
- Structured Observations Reveal Slow HIV-1 CTL Escape
- An Integrative Multi-scale Analysis of the Dynamic DNA Methylation Landscape in Aging
- Combining Natural Sequence Variation with High Throughput Mutational Data to Reveal Protein Interaction Sites
- Transhydrogenase Promotes the Robustness and Evolvability of Deficient in NADPH Production
- Regulators of Autophagosome Formation in Muscles
- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Eye Selector Logic for a Coordinated Cell Cycle Exit
- Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations
- The DNA Polymerase δ Has a Role in the Deposition of Transcriptionally Active Epigenetic Marks, Development and Flowering
- Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice
- Membrane Recognition and Dynamics of the RNA Degradosome
- P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
- is a Long Non-coding RNA in JNK Signaling in Epithelial Shape Changes during Drosophila Dorsal Closure
- A Pleiotropy-Informed Bayesian False Discovery Rate Adapted to a Shared Control Design Finds New Disease Associations From GWAS Summary Statistics
- Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers
- and Hyperdrive Mechanisms (in Mouse Meiosis)
- Elevated In Vivo Levels of a Single Transcription Factor Directly Convert Satellite Glia into Oligodendrocyte-like Cells
- Systemic Delivery of MicroRNA-101 Potently Inhibits Hepatocellular Carcinoma by Repressing Multiple Targets
- Pooled Sequencing of 531 Genes in Inflammatory Bowel Disease Identifies an Associated Rare Variant in and Implicates Other Immune Related Genes
- Abscission Is Regulated by the ESCRT-III Protein Shrub in Germline Stem Cells
- Temperature Stress Mediates Decanalization and Dominance of Gene Expression in
- Transcriptome Wide Annotation of Eukaryotic RNase III Reactivity and Degradation Signals
- The Exosome Component Rrp6 Is Required for RNA Polymerase II Termination at Specific Targets of the Nrd1-Nab3 Pathway
- Sex-specific -regulatory Variation on the X Chromosome
- Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex
- Modeling of the Human Alveolar Rhabdomyosarcoma Chromosome Translocation in Mouse Myoblasts Using CRISPR-Cas9 Nuclease
- Asymmetry of the Budding Yeast Tem1 GTPase at Spindle Poles Is Required for Spindle Positioning But Not for Mitotic Exit
- TIM Binds Importin α1, and Acts as an Adapter to Transport PER to the Nucleus
- Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
- The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
- Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
- Region-Specific Activation of mRNA Translation by Inhibition of Bruno-Mediated Repression
- An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish ()
- Interaction between the tRNA-Binding and C-Terminal Domains of Yeast Gcn2 Regulates Kinase Activity In Vivo
- Hyper-Acetylation of Histone H3K56 Limits Break-Induced Replication by Inhibiting Extensive Repair Synthesis
- Prodomain Removal Enables Neto to Stabilize Glutamate Receptors at the Neuromuscular Junction
- Recent Selective Sweeps in North American Show Signatures of Soft Sweeps
- Identification and Functional Analysis of Healing Regulators in
- A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2
- Drosophila Casein Kinase I Alpha Regulates Homolog Pairing and Genome Organization by Modulating Condensin II Subunit Cap-H2 Levels
- The Hippo Pathway Regulates Homeostatic Growth of Stem Cell Niche Precursors in the Ovary
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



