-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
Tight regulation of the visual response is essential for photoreceptor function and survival. Visual response dysregulation often leads to photoreceptor cell degeneration, but the causes of such cell death are not well understood. In this study, we investigated a fatty acid transport protein (fatp) null mutation that caused adult-onset and progressive photoreceptor cell death. Consistent with fatp having a role in the retina, we showed that fatp is expressed in adult photoreceptors and accessory cells and that its re-expression in photoreceptors rescued photoreceptor viability in fatp mutants. The visual response in young fatp-mutant flies was abnormal with elevated electroretinogram amplitudes associated with high levels of Rhodopsin-1 (Rh1). Reducing Rh1 levels in rh1 mutants or depriving flies of vitamin A rescued photoreceptor cell death in fatp mutant flies. Our results indicate that fatp promotes photoreceptor survival by regulating Rh1 abundance.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002833
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002833Summary
Tight regulation of the visual response is essential for photoreceptor function and survival. Visual response dysregulation often leads to photoreceptor cell degeneration, but the causes of such cell death are not well understood. In this study, we investigated a fatty acid transport protein (fatp) null mutation that caused adult-onset and progressive photoreceptor cell death. Consistent with fatp having a role in the retina, we showed that fatp is expressed in adult photoreceptors and accessory cells and that its re-expression in photoreceptors rescued photoreceptor viability in fatp mutants. The visual response in young fatp-mutant flies was abnormal with elevated electroretinogram amplitudes associated with high levels of Rhodopsin-1 (Rh1). Reducing Rh1 levels in rh1 mutants or depriving flies of vitamin A rescued photoreceptor cell death in fatp mutant flies. Our results indicate that fatp promotes photoreceptor survival by regulating Rh1 abundance.
Introduction
Retinal degeneration is a major health concern that affects one in 2000 people worldwide (http://www.sph.uth.tmc.edu/Retnet/) [1]. Although human retinopathies are heterogeneous in physiopathology and severity, they all involve loss of photoreceptor (PR) neurons, which leads to blindness. The most frequent retinal disease is retinitis pigmentosa, which is caused by mutations in one or more of at least 54 distinct genes (Retnet). Among them, the most frequently mutated gene is rhodopsin (rho in mammals) which is mutated in 30–40% of all cases of autosomal dominant retinitis pigmentosa (ADRP) (Retnet) [2]. Rhodopsin is the light-sensitive protein of PRs that activates phototransduction. Approximately 100 rho mutations have been identified, and they affect folding, trafficking and activity of the rhodopsin protein. Despite extensive study, the mechanisms of retinal degeneration remain unclear.
Retinal degeneration has been studied extensively in Drosophila [3], [4], [5]. Many mutations in Drosophila phototransduction pathway genes induce PR degeneration. The mechanism of toxicity of these mutations is related either to a defect in folding, trafficking or activity of the Drosophila Rhodopsin-1 protein (Rh1), or to an accumulation of toxic Rh1-Arrestin2 (Arr2) complexes, or to a deregulation of the Ca2+ homeostasis [4], [5]. In addition, mutations can be introduced in Drosophila genes to model human diseases. For example, the rh1P37H allele, which corresponds to rhoP23H, the most frequent mutation of rhodopsin in ADRP, has been successfully introduced into Drosophila and induces PR degeneration [6]. We recently used Drosophila to identify genes involved in PR survival [7]. Using a new method of PR visualization, called Tomato/GFP-FLP/FRT, we screened recessive lethal mutations and found fatpk10307 to be associated with PR cell death. The fatpk10307 mutation consists of the insertion of a P{lacW} element into the open reading frame of the fatty acid transport protein (fatp) gene, which has never been characterized in Drosophila. Its closest mammalian orthologs are fatp1 and fatp4 [8]. The mammalian members of the Fatp family of proteins have acyl-CoA synthetase enzymatic activity and facilitate cellular fatty acid uptake [9], [10]. Each member of the family has a specific expression pattern and function. fatp1 is expressed in muscle, heart, brain, adipose tissue and retina [11], [12], and is involved in thermogenesis and obesity. fatp4 is expressed most abundantly in the small intestine, brain, skeletal muscle, heart, skin, liver and kidney [10], [13], [14]. Loss of fatp4 in mice or in humans is associated with restrictive dermopathy related to the ichthyosis prematurity syndrome, a skin defect caused by altered lipid and fatty acid compositions [15], [16], [17].
In this work, we show that Drosophila fatp is required for PR survival. PR degeneration in a fatp null mutant is adult-onset and progressive. The onset of degeneration in the fatp mutant correlates with the time of expression of fatp in the normal adult retina. We then investigated the mechanisms of PR degeneration in the fatp mutant. We show that PRs in the fatp mutant exhibit an elevated photoresponse that is associated with high levels of Rh1. We also found that fatp mutant flies have a defect in Rh1 degradation and that reducing the level of Rh1 restored PR survival.
Results
fatp is required for PR viability in adult Drosophila
To study the role of fatp in PR viability, we first used RNA interference to reduce fatp expression. fatp-interfering RNA was specifically produced in the retina under the control of eye-specific drivers (ey-Gal4 and GMR-Gal4) (Figure 1A–1G). fatp knockdown led to a progressive loss of PRs, indicating that fatp expression is required for PR viability. To validate these findings, we used the fatpk10307 mutation, generated by the insertion of a 10.7 kb P{lacW} element into the first exon of the gene. This mutation is recessive and can be considered a null allele as it completely prevents fatp expression, abolishing the production of fatp mRNA and protein in homozygous fatpk10307 L1 larvae (Figure S1). As a consequence, fatpk10307 mutant larvae died during the second instar. To study the role of fatp in adult PR and circumvent fatpk10307 larval lethality, we used the Tomato/GFP-FLP/FRT mosaic method [7]. This new method combines mitotic recombination and cornea neutralization techniques [18], [19], [20] to allow time-course analysis of homozygous mutant clones in living flies over several days. We evaluated PR presence, identified on the basis of GFP expression, for 14 days from hatching in fatpk10307 mutant retinas (Figure 1H–1J). All fatp mutant PRs were present upon hatching but progressively disappeared starting on day four (Figure 1H, 1H′). Losses of fatp mutant PRs were statistically significant in the retinas of 8 - and 14-day-old adults (Figure 1H″, 1H′″, 1I). In contrast, little or no PR loss occurred in neighboring wild-type or heterozygous tissue, indicating that the fatpk10307 mutation was cell-autonomous (Figure 1H, 1I, 1J).
Fig. 1. Loss of fatp induces adult-onset progressive degeneration of PRs. 
(A–G) Consequences of fatp knockdown by RNA interference on PR viability. (A, B, C) Visualization of PRs using a cornea-neutralization method for 1-, 15- and 21-day-old PRs expressing lacZ as a control. (D, E, F) PRs expressing the fatp-interfering RNA and (G) PR quantification. Whereas all PRs were present in control retina and fatp-knocked-down retina at day one (A, D), there were significant PR losses at days 15 and 21 in fatp-knocked-down retina (B vs E, C vs F, t-test, n≥6). (H) Time-course analysis of fatpk10307 mutant mosaic retina using the Tomato/GFP-FLP/FRT method and (I) quantification. All outer PRs (R1–R6) expressed GFP (green). FLP-mediated mitotic mutant clones were visualized by the absence of rh1-tdTomato (red). Clones of the same eye were visualized in the same fly at day one, four, eight and fourteen after hatching. (H) All PRs were present at day one (scale bar = 10 µm). (H′) From day four, mutant PRs (in green) started to disappear. (H″ and H′″) Losses of mutant PRs were significant at day eight and day fourteen (paired t-test, n = 3). (I) Quantified results are expressed as mean ± SD. (J) Analysis of a control 15-day-old mosaic retina using the Tomato/GFP-FLP/FRT method. All PRs are present. (K–N) Rescue of fatpk10307-mutant PRs by re-expression of wild-type fatp. (K) Tomato/GFP-FLP/FRT visualization of 10- to 14-day-old control, (L) fatpk10307/k10307 and (M) fatpk10307/k10307+rh1>fatp mosaic retinas and (N) quantification (t-test, n> = 5). In the control (K), all PRs were present. In fatp mutant mosaic retina (L), 70% of the mutant PRs were lost. In these retinas, mutant PRs were rescued by re-expression of fatp under the control of the rh1 promoter (M). The cell-autonomous nature of the PR loss in the fatp mutant (Figure 1H–1I) suggested that fatp expression is required for PR viability. To confirm this requirement, we attempted to rescue PR viability in the fatpk10307 mutant with tissue-specific expression of wild-type fatp using the UAS/GAL4 system [21]. fatp re-expression from the rh1 promoter in the R1–R6 PRs fully rescued the fatpk10307 mutant PR phenotype (Figure 1K–1N). These results demonstrate that fatp expression in PRs is required for their survival.
Next, we performed structural and ultra-structural analyses of fatp mutant PR in resin-embedded retina sections. We used classical brightfield microscopy to examine PR integrity in whole-eye fatp mutant clones generated by the EGUF/Hid method [22] (Figure 2A–2E). PR loss started after approximately 15 days and increased progressively with age (Figure 2A–2E). Using this approach, PR loss was detected later than with the Tomato/GFP-FLP/FRT method. This may be because the Tomato/GFP-FLP/FRT method is based on the expression and targeting of GFP in the rhabdomere and thus detects early deficits whereas classical histological methods assess only the physical presence or absence of PRs. We also detected progressive and adult-onset PR loss by recording the number of nuclei between the apical and basal layers of fatp mutant retinas in horizontal cryosections (Figure S2). We then studied fatp mosaic retinas by electron microscopy (EM) to characterize further PR degeneration in fatp mutant cells (Figure 2F–2M). On EM images, we could distinguish homozygous fatp mutant PRs from the absence of pigment vesicles at the base of the rhabdomeres and in inter-ommatidial cells (IOCs) (Figure 2F). We observed several levels of PR degeneration among fatp mutant cells ranging from normal PRs to PRs that were fully degenerated and engulfed by neighboring IOCs. Most normal PRs displayed no obvious sign that would predict future degeneration (Figure 2G). Some PRs were electron-dense and their cytoplasm was contracted suggesting that they had initiated the degeneration process (Figure 2H). In these PRs, some swelling mitochondria could be observed but the rhabdomeres were still intact. In more advanced stages of degeneration, the rhabdomeres were clearly affected with disorganized microvilli structures (Figure 2I, 2J). In addition, the neighboring IOCs seemed to be activated with a typical spotted pattern in the cytoplasm. At the end of the degenerative process, PRs were phagocytosed by the neighboring IOCs and apparently digested (Figure 2K–2M). Thus, the absence of fatp causes progressive adult-onset PR degeneration.
Fig. 2. Histological analysis of fatp−/− PR degeneration. 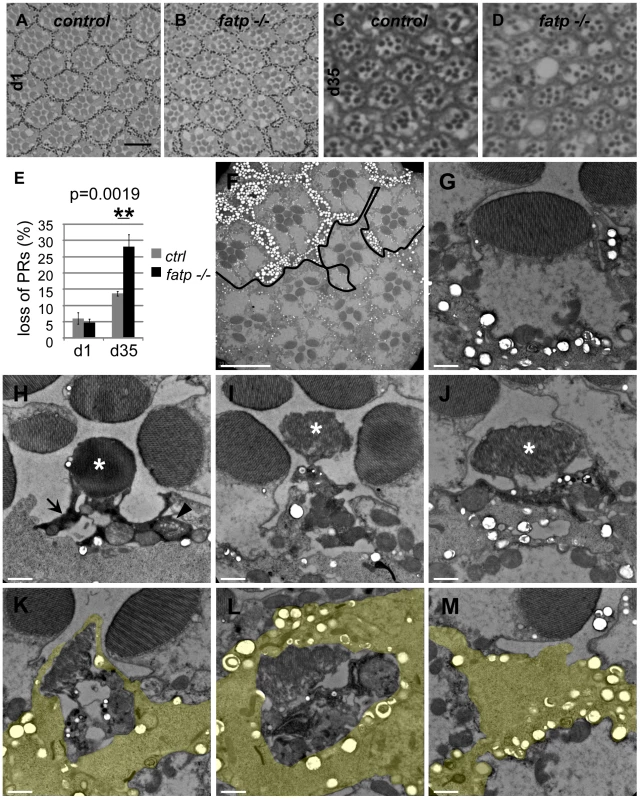
(A–E) Analysis of fatpk10307 mutant PR survival using resin-embedded tangential sections. (A, C) Control retina of 1- and 35-day-old flies (scale bar = 10 µm). (B, D) Homozygous fatpk10307 retina of 1-day-old and 35-day-old flies. (E) PR losses in fatp mutant retinas were significant at 35 days compared to those in control retinas (t-test, n = 6). (F–M) Electron microscopy analysis of fatp−/− PR degeneration. fatpk10307 mosaic retina of 15-day-old flies were analyzed. (F) Wild-type and heterozygous clones were marked with numerous large pigment granules in the IOCs and at the basis of PR rhabdomeres (white spots, above the black line) whereas homozygous mutant part exhibited rare small pigment granules (under the black line, scale bar = 10 µm). Several PRs were missing in the homozygous mutant part. (G–M) Different stages of fatpk10307/k10307 PR degeneration (scale bar = 1 µm). (G) fatpk10307/k10307 PR exhibiting no sign of degeneration. (H) The cytoplasm of fatpk10307/k10307 PRs shrank and became electron-dense (arrow). Some mitochondria were swelling (arrowhead). The rhabdomere was not much affected (*). (I–J) PRs then disintegrated. This was clearly visible at the level of the rhabdomere (*). (K, L, M) Degenerating PRs were finally phagocytosed and digested by the neighboring interommatidial cell (yellow). fatp is expressed in the adult retina
We determined the specificity of fatp expression in developing and adult Drosophila eyes. To do this, we generated an anti-Fatp antibody (C11-7) that was sensitive enough to detect ectopic Fatp and endogenous Fatp on western blots and in immunofluorescence experiments (Figure 3A–3E). No fatp expression was observed in third instar eye imaginal discs (Figure 3B), but we detected Fatp protein in PR cytoplasm juxtaposed to PR rhabdomeres and in IOCs of the adult retina (Figure 3D, 3E). To capture the fatp expression profile, we exploited the fatpk10307 enhancer trap line, which carries the β-galactosidase reporter (Figure S3). Using a β-galactosidase antibody, we detected β-galactosidase in the nuclei cell layer that includes the outer PRs and accessory cells in adult head cryosections (Figure S3A–S3D). Immunolocalization experiments revealed that small amounts of Fatp consistently co-localized with the neuronal marker ELAV in the outer PRs and that Fatp was also present in apical IOCs (Figure S3A–S3D). Thus, the fatpk10307 enhancer trap line faithfully reproduces the distribution of Fatp and can be used to follow fatp expression. Testing for β-galactosidase activity in this line revealed that fatp was also expressed in the adult optic lobe around the medulla and lamina, in the midgut and in the salivary glands but not in the eye imaginal disc of third instar larvae (Figure S3E–S3L). In conclusion, the absence of fatp expression in the developing eye disc and its presence in differentiated PRs are consistent with fatp being required for the viability of PRs in adult flies.
Fig. 3. fatp is expressed in the adult retina. 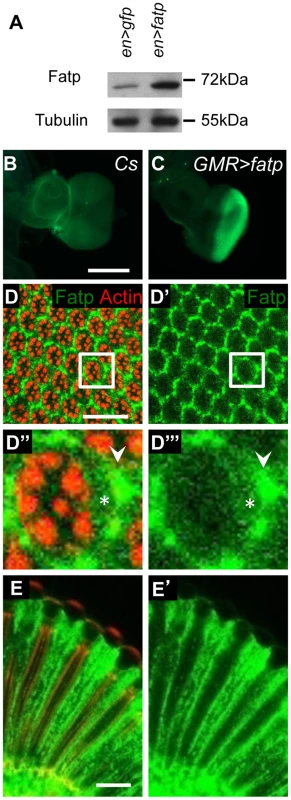
(A) Western blot analysis of en-GAL4 UAS-GFP (en>GFP) and en-GAL4 UAS-fatp (en>fatp) adult eye extracts using anti-Fatp C11-7 and anti-tubulin antibodies. Anti-Fatp C11-7 detects a single band at 72 kDa, which corresponds to the predicted molecular weight of Fatp. Endogenous and ectopically expressed Fatp were detected. Tubulin was used as a loading control. (B, C) Immunofluorescent detection of Fatp in wild-type (CS) and GMR-GAL4 UAS-fatp (GMR>fatp) third instar larva eye imaginal disc using the anti-Fatp C11-7 antibody (scale bar = 200 µm). (C) Fatp was detected in the posterior part of the disc, which corresponds to the expression profile of the GMR promoter. (D–E) Immunostaining of endogenous Fatp and Actin in whole-mount cnbw adult retina using the anti-Fatp C11-7 antibody and phalloidin. In the tangential (D) and longitudinal plans (E), Fatp immunostaining is detected in the cytoplasm of PRs (star) and accessory cells (arrowhead). Rhabdomeres are stained with phalloidin (scale bar = 20 µm). fatp regulates the visual response
We investigated whether the requirement for fatp expression for PR viability is related to the visual response. First, we tested whether PR degeneration in the fatp mutant is light-dependent. fatp mutant flies reared in normal light conditions exhibited significantly greater PR losses than fatp mutant flies reared in complete darkness (Figure 4A–4G). This indicates that light contributes to PRs loss in the fatp mutant. Then, to examine directly the visual response in the fatp mutant, we performed electoretinogram (ERG) recordings on white-eyed flies (Figure 4H, 4I): we used 8-day-old flies, an age at which the integrity of fatp mutant PRs is still intact as observed in resin-embedded retinal sections (data not shown). We obtained ERG recordings that measure the summed responses of all retinal cells. Wild-type flies exhibited a corneal negative receptor potential in response to orange light, which returned to baseline when the light was switched off (Figure 4H). The amplitude of the ERG was higher in fatpk10307 flies than controls, with no apparent difference in the kinetics of the visual response (Figure 4H, 4I). These results suggest that Fatp is a negative regulator of the visual response.
Fig. 4. The visual response is altered in fatp mutant PRs. 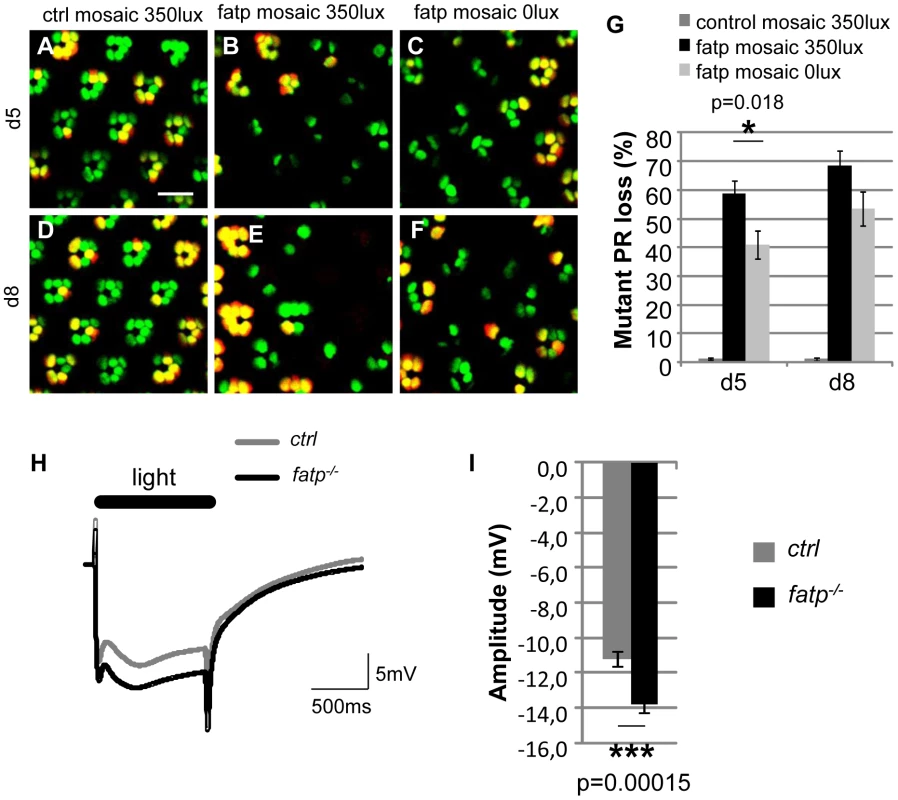
(A–F) Tomato/GFP-FLP/FRT visualization of fatpk10307 mutant mosaic retinas from 5- and 8-day-old flies reared from the pupal stage in normal room light (350 lux) or in darkness (0 lux) (scale bar = 10 µm). (G) Quantification of fatpk10307 mutant PR loss. (A, D) No PR losses occurred in control mosaic retinas from 5-day-old and 8-day-old flies reared in room light conditions. (B, E, G) In fatp mutant retinas from light-reared flies, 58.8±4.5% and 68.5±4.9% of the mutant PRs were lost in 5-day-old and 8-day-old flies, respectively. (C, F, G) PR losses were significantly lower in flies reared in the dark (t-test, n = 8). (H) ERG recordings from control and fatpk10307/k10307 8-day-old retinas and (I) quantification. Retinas were exposed to a one second flash of orange light. (I) The amplitude of the plateau is significantly higher in the fatp mutant retina than in the control retina (t-test, n = 24). The elevated levels of Rh1 cause PR loss in the fatp mutant
We wondered whether PR degeneration in the fatp mutant could be rescued by rh1 mutant alleles as previously described for light-dependent retinal degeneration mutants such as rdgC and norpA [23]. We therefore tested whether PR viability in the fatp mutant could be rescued by rh1G69D and rh1I17 (ninaEG69D and ninaEI17) alleles (Figure 5 and Figure S5). rh1G69D is a dominant negative allele whereas rh1I17 is a null allele. Although the rh1G69D allele causes retinal degeneration in old flies under constant light exposure, it could be used in our study because these mutants raised under a 12 h day/light cycle did not exhibit retinal degeneration (Figure 5C, 5H, 5M; Figure S4; and [23], [24]). Using the Tomato/GFP-FLP/FRT method, we compared PR loss in retinas in the single fatpk10307/k10307 mutant to that in fatpk10307/k10307rh1G69D/+ double mutant. PR loss was very much lower in fatpk10307/k10307rh1G69D/+ mutant retinas than in fatpk10307/k10307 mutant retinas (Figure 5A–5E). In resin-embedded eye sections, PR viability was found to be fully rescued in the fatpk10307/k10307rh1G69D/+ double mutant (Figure 5F–5J). EM showed that despite the smaller size of the rhabdomeres, PR ultra-structure was fully rescued in fatpk10307/k10307rh1G69D/+ double mutants (Figure 5K–5N). Similarly, a rescue of PR degeneration was observed in fatpk10307/k10307rh1I17/+ double mutant (Figure S5). Thus, rh1 mutant alleles rescued PR degeneration in the fatp mutant.
Fig. 5. Rh1G69D rescues PR viability in the fatp mutant. 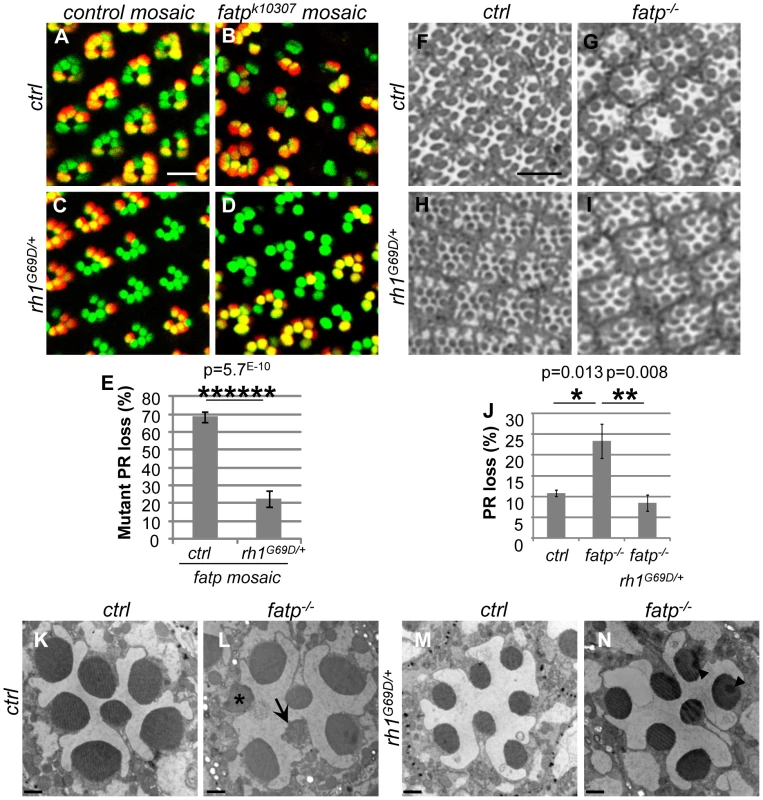
(A–E) Analysis of the survival of control, fatpk10307, rh1G69D/+ and fatpk10307rh1G69D/+ double mutant PRs using the Tomato/GFP-FLP/FRT method in 15-day-old flies (scale bar = 10 µm). (E) Quantification of mutant PR losses (t-test, n≥12). PR loss in the fatpk10307 rh1G69D/+ double mutant was dramatically lower than in the fatp single mutant. (F–J) Analysis of the survival of control, fatpk10307, rh1G69D/+ and fatpk10307 rh1G69D/+ double mutant PRs using resin-embedded tangential sections in 28-day-old flies (scale bar = 10 µm). (J) Quantification of PR loss. Significantly more PRs died in the fatp mutant than in control retina (t-test, n = 6). In the double mutant, PR losses were reduced to control levels. (K–N) Electron microscopy analysis of whole-eye control (K), fatpk10307 (L), rh1G69D/+ (M) and fatpk10307 rh1G69D/+ (N) mutants in 28-day-old flies (scale bar = 1 µm). Whereas PRs degenerated in fatpk10307 ommatidia (arrow) and were phagocytosed by IOCs (*), PRs survived in fatpk10307 rh1G69D/+ double mutant flies. Rhabdomeres of rh1G69D/+ and fatpk10307 rh1G69D/+ outer PRs were reduced in size. Arrowheads show artifactual shadows on the sample. We tested whether rh1 is dysregulated in fatp mutant PRs. We assayed Rh1 protein in fatp mutant and control retinas by western blotting (Figure 6A, 6B). The Rh1 content of fatpk10307/k10307 mutant retinas was double that of control retinas. We confirmed that Rh1 was less abundant in fatpk10307/k10307rh1G69D/+ double mutant than fatpk10307/k10307 single mutant retinas (Figure 6A, 6B). These results indicate that Rh1 metabolism is altered in the fatp mutant and that reducing Rh1 levels in fatp mutants protects the PRs. To confirm this conclusion, we examined whether rearing flies on a vitamin A-deficient medium could reduce PR loss in fatp mutant retinas (Figure 6C–6F). Vitamin A is the precursor of the Rh1 chromophore and is required for Rh1 synthesis. A vitamin A-deficient diet rescues retinal degeneration in several mutants, including rdgB, crumbs, and arrestin mutants [25], [26], [27]. In retinas of flies reared on a vitamin A-deficient diet, occasional PR degeneration was visible but most rhabdomeres were present (Figure S6). fatp mutant flies deprived of vitamin A produced no detectable Rh1 protein and the PR loss in these flies was very much lower than that in flies receiving a standard diet (Figure 6C–6F). Thus, reducing the Rh1 level rescued PR degeneration in the fatp mutant. Therefore, PR degeneration in the fatp mutant appears to be due to an over abundance of Rh1.
Fig. 6. Elevated Rh1 levels are responsible for PR loss in the fatp mutant. 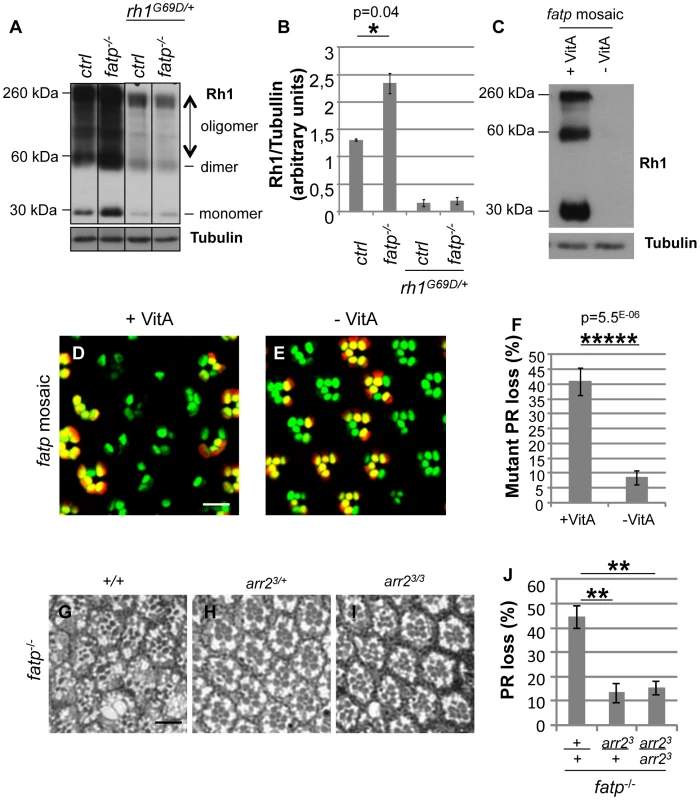
(A) Western blot analysis of Rh1 in boiled head extracts from control, fatpk10307/k10307, rh1G69D/+ and fatpk10307/k10307/rh1G69D/+ 1 to 11-day-old flies. Tubulin was used as a loading control. (B) Quantification of protein levels. Dimer and oligomer forms of Rh1 were due to the boiling of the extracts. Rh1 levels were twofold higher in the fatp mutant than in the control. The level of Rh1 was substantially lower in the rh1G69D/+ single mutant and in the fatpk10307/k10307/rh1G69D/+ double mutant. (C) Western blot analysis of Rh1 and tubulin in fatp mutant mosaic retina extracts from 5-day-old flies reared from the embryonic stage on control (+VitA) and vitamin A-deficient (-VitA) media. Tubulin was used as a loading control. Rh1 was not detectable in flies reared on vitamin A-deficient medium. (D, E) Visualization of fatp mutant mosaic retina of 5-day-old flies reared from the embryonic stage on control (D) and vitamin A-deficient (E) media using the Tomato/GFP-FLP/FRT method (scale bar = 10 µm). (F) Quantification of mutant PR loss (t-test, n≥12). fatp mutant PR viability was dramatically restored in flies reared on vitamin A-deficient medium. (G–J) Analysis of the loss of fatpk10307, fatpk10307 arr23/+ and fatpk10307 arr23/3 double mutant PRs using resin-embedded tangential sections in 34-day-old flies (scale bar = 10 µm). (H) Quantification of PR loss. In the double mutants retinas, PRs were significantly rescued (p = 0.0021 and p = 0.0016, t-test, n = 6). To test whether high levels of Rh1 are toxic to the PR, we overexpressed rh1 in developing and adult PRs (Figure S7). Ectopic expression of rh1 with the GMR driver led to the rough eye phenotype, indicating that rh1 overexpression is toxic in the developping eye (Figure S7A, S7B). We also overexpressed rh1 specifically in differentiated R1-6 PRs using the rh1 promoter. rh1 overexpression led to rhabdomere degeneration (Figure S7C, S7D). Thus, we conclude that elevated Rh1 levels in fatp mutant PRs can lead to PR pathogenesis.
Next we examined the possible involvement of Arrestin2 (Arr2) in Rh1 toxicity in fatp mutants. Stable Rh1-Arr2 complexes are toxic in adult PRs and are responsible for retinal degeneration in several mutants including norpA, rdgC, rdgB [26], [28], [29], [30]. To examine this possibility, we tested for genetic interaction between fatpk10307 and arr23 mutations (Figure 6G–6J). We found that PRs were rescued in fatp and arr2 double mutant retina, indicating that arr2 is required for the toxicity in fatp mutant PRs. Together with the excess of Rh1 detected in fatp mutant, these observations suggest that toxic Rh1-Arr2 complexes induce PR degeneration in the fatp mutant.
We tested whether the elevated Rh1 levels in fatp mutant PRs were due to deregulation of rh1 expression and/or degradation of Rh1. We first analyzed rh1 expression in fatp mutant retinas using a rh1-lacZ reporter to monitor rh1 transcription. We did not detect higher levels of lacZ in fatp mutant retinas than in control retinas (). This shows that rh1 transcription is not upregulated by the fatp mutation. Presumably, therefore, fatp regulates Rh1 levels post-transcriptionally. To test for the involvement of fatp in Rh1 degradation, we forced Rh1 degradation by illuminating the retina with blue light as previously described [31]. Blue illumination maintains Rh1 in an active conformation and induces its degradation. In white-eyed control flies blue light illumination for 6 h induced a significant loss of Rh1 for the retinas whereas the same treatment of white-eyed fatp mutants did not result in decreased Rh1 abundance (Figure S8C, S8D). This suggests that light induced-Rh1 degradation is impaired in fatp mutant PRs and this may explain why there is more Rh1 in these retinas than controls.
Discussion
Mutations resulting in inactive Rh1, impaired visual responses and PR degeneration have been studied extensively [4], [5]. In this study, we describe the phenotype of fatpk10307, the first mutation known to exhibit elevated Rh1 levels leading to loss of PRs.
We show that fatp expression is required for PR viability in adult Drosophila. In the absence of fatp, PRs degenerate progressively during adulthood. Moreover, we demonstrate that the requirement for fatp in adult PRs is cell-autonomous, which is in agreement with the presence of Fatp in adult PRs and with its absence from the developing eye imaginal disc. The age-dependent PR degeneration in the fatp mutant is reminiscent of Drosophila models of ADRP [3], [6]. We thus propose that fatp-associated degeneration is a new model of late-onset PR degeneration.
Our results indicate that PR death in fatp mutants is a consequence of elevated levels of Rh1. We demonstrate that reducing Rh1 levels, as a consequence of rh1 mutation or a vitamin A-deficient diet, efficiently restored PR viability in fatp mutants (Figure 5, Figure 6, and Figure S5). Thus, the accumulation of Rh1 is toxic for the PRs in fatp mutants. One possibility is that Rh1 associates with Arr2, forming toxic Rh1-Arr2 complexes as in norpA, rdgC, rdgB mutants [26], [28], [29], [30]. Indeed, we found that disrupting either rh1 or arr2 rescued PR loss in fatp mutants (Figure 5, Figure 6, and Figure S5). This genetic evidence is consistent with Rh1-Arr2 complexes causing PR degeneration in fatp mutant. Definitive proof of this mechanism requires the direct assessment of Rh1/Arr2 complexes in fatp mutants. Also, we cannot exclude the existence of additional toxic mechanisms. For example, the elevated visual response in fatp mutants (Figure 4) may contribute to PR death because of a defect in Ca2+ homeostasis.
We found that Rh1 protein levels were elevated in fatp mutant retinas and that this was probably due to decreased Rh1 degradation (Figure S8C, S8D). fatp may regulate sphingolipid metabolism, which controls Rh1 trafficking and degradation [32], [33]. In support of this hypothesis, the total ceramide content is higher in the skin of fatp4−/− than control mice [15]. Ceramidase facilitates the endocytic turnover of Rh1 and rescues retinal degeneration in arr2 and phospholipase C mutants [32], [34]. Similarly, Drosophila Fatp may limit ceramide levels and inhibit endocytic turnover of Rh1. It is also possible that fatp regulates Rh1 synthesis, but we show that loss of fatp does not affect rh1 gene transcription (Figure S8A, S8B). Alternatively, Fatp may regulate the synthesis or recycling of the retinal chromophore required for Rh1 synthesis. In mammals, Fatp1 inhibits two enzymes of the visual cycle in vitro, LRAT and RPE65, which respectively produce and consume retinyl-ester, a fatty acid-linked form of the chromophore [12]. A similar visual cycle was recently described in Drosophila [35], [36], [37]. Therefore, chromophore synthesis or recycling may be increased in the fatp mutants, resulting in upregulated Rh1 synthesis. In support of this hypothesis, we showed that inhibiting chromophore synthesis in vitamin A-deficient medium fully rescued PR viability in fatp mutant retinas (Figure 6D–6F). Nevertheless, the mechanisms by which fatp may regulate the visual cycle remain to be elucidated.
The visual response was higher in the fatp mutant retina than control retina but the mechanisms involved are unclear. Previous work has shown that the ERG amplitude depends on the ratio between the level of Rh1 and the Rh1 kinase activity of gprk1 [38]: decreasing gprk1 activity resulted in higher ratios and elevated ERG amplitudes whereas increasing gprk1 activity resulted in lower ratios and lower ERG amplitudes [38]. Whether there is a disequilibrium between phosphorylated/unphosphorylated forms of Rh1 in fatp mutant retinas remains to be explored. Alternatively, fatp may be required for the production of lipid metabolites that regulate the phototransduction cascade. Indeed, it has been suggested that polyunsaturated fatty acids, which are potential diacyl-glycerol metabolites, act on TRP/TRPL channels [39], [40], [41]. Thus, the elevated visual response may be the consequence of a lipid metabolite dysregulation in fatp mutant retinas.
In conclusion, fatp mutation is a new model of retinal pathology in flies in which the up-regulation of Rh1 contributes to progressive PR degeneration. Whether a similar pathological mechanism exists in human retinal diseases remains to be determined.
Materials and Methods
Drosophila stocks
For RNA interference, the UAS-dicer; ey-Gal4, GMR-Gal4/Cyo; rh1-GFP line (kind gift of C Desplan) was crossed with the UAS-RNAi line against fatp (VDRC #48719). The FRT40A fatpk10307/Cyo and rh1-Gal4, ey-FLP; FRT40A rh1-tomatoninaC/Cyo; UAS-GFPninaC lines were described previously [7]. The ninaEG69D line (named rh1G69D in the text) was previously used in [42]. The rh1I17 (ninaEI17) allele was obtained from bloomington (BL#5701). Whole-eye mutant clones were generated using the; FRT40A GMR-hid CL EGUF/Cyo; line [22]. The UAS-rh1 and arr23 lines were a kind gift of HD Ryoo and N Colley respectively. To obtain white-eyed flies carrying Pw+ transgenes, we used the pWIZ construct that expresses an iRNA against the white gene [43]. Flies were reared on standard corn medium at 25°C in a 12-h light/12-h dark environment unless noted otherwise. Vitamin A-deficient medium contained yeast (12 g), agar (1,5 g), sucrose (7,5 g), cholesterol (0,03 g), sodium methyl-4-hydroxybenzoate (1.15M, 3.75 mL) and propionic acid (0.72 mL) in distilled water (150 mL).
Generation of UAS-fatp transgenic line
fatp cDNA (SD05207, Gold cDNAs Collection) was recovered from BDGP DGRC in pOT2 vector. fatp cDNA was cloned (XhoI/EcoRI) into a pUAST-w+-attB transgenic fly vector. Best Gene, Inc (CA, USA) generated transgenic lines using PhiC31 integrase-mediated transgenesis [44]. The vector DNA was injected in embryos carrying attP docking sites (strain 9750 at 65B2). w+ embryos were selected and stable transgenic fly stocks established.
Live fluorescent imaging of PRs
CO2-anesthetized flies were placed in a 35 mm cell culture dish half-filled with 1% agarose, covered with water at 4°C and observed using an upright 510 Zeiss confocal fluorescent microscope as described [20]. For the time-course study of age-dependent PR death in single flies, after visualization, each living fly was detached from the agarose, dried and transferred to a vial containing fly food medium.
Resin-embedded tangential sections
Tangential sections of adult eyes were performed as described [45]. PR viability was determined by counting the number of intact rhabdomeres on retina tangential plastic sections. At least 200 ommatidia from three different animals were scored per experimental condition.
Transmission electron microscopy
Drosophila eyes were dissected and fixed overnight at 4°C in 1.5% glutaraldehyde, 1% paraformaldehyde and 0.1M PIPES buffer (pH 7.4). After washing, eyes were post-fixed at room temperature in 1% OsO4, 0.1M PIPES (pH 7.4). They were then deshydrated with successive ethanol solutions followed by anhydrous propylen oxyde. Eyes were infiltrated with increasing concentrations of epoxy resin (EMbed 812 from EMS) in propylen oxyde for 1 day at room temperature and samples were mounted in pure resin into silicone embedding molds. Polymerization was performed at 60°C for 2 days. Ultrathin sections of 60 nm were stained with lead citrate and examined with a transmission electron microscope (Philips CM120) operating at 80 kV.
Generation of anti-Fatp C11-7 antibody
Two rabbits were immunized with two peptides of Fatp, Peptide 1: YQTSKGRYELLTPQ at the C-terminus of the protein and Peptide 2: NNNSETEKNIPQAK in the middle of the protein. The serum from the two rabbits were pooled and affinity purified.
Immunostainings
Horizontal eye cryosections were performed using a cryostat microtome (Microm HM505E) and deposited on superfrost Plus slides (Thermo). Third instar larval imaginal discs were dissected in 1X PBS. For whole-mount retina, we followed the protocol described in [46]. Briefly, Drosophila heads were bisected in the middle with a scalpel. Brain tissue was removed to expose retina underneath. Cryosections, imaginal discs and whole-mount retinae were fixed in 4% PFA for 15 min. After washing in PBS+Triton X-100 (0.3%), the following antibodies were used in PBS+Triton X-100 (0.1%)+Normal Goat Serum (5%, Sigma) overnight at 4°C: anti-fatp C11-7 (1/200), anti-ELAV (1/500, DSHB), anti-β Galactosidase (1/500, MP Cappell). After washing, samples were stained with the following appropriate secondary antibodies: anti-rabbit (Alexa 488 1/500, Invitrogen), anti-rat (alexa 633 1/500, Invitrogen). For whole-mount retina, phalloidin-rhodamine (1/200, Sigma) was also used to stain rhabdomeres. Samples were mounted in DAPI mounting media (Vectashield, AbCys). Fluorescent images were obtained using Zeiss 510 and 710 confocal microscopes.
Electroretinogram
For ERG recordings, white-eyed flies were analyzed. Cold-anesthetized flies were immobilized in clay. A tungsten electrode (0.5–1 MΩ, Intracell) was inserted in the back of the head and a glass electrode filled with 3 M KCl (2–6 MΩ) was poked through the cornea. Flies were dark-adapted for 2 min before recording. An orange LED (591 nm, 2800 mcd, 40° beam, LY 5436-VBW-1, Osram, France) was placed at 1 cm from the head. The flash intensity reaching the eye was 650 µW/cm2, as measured with a PM100D power meter and S121C photodiode (Thorlabs, Maisons-Laffitte, France). Signals were filtered at 2 kHz and digitized at 10 kHz, using a MultiClamp 700A amplifier, a Digidata 1322A interface and pClamp-8 software (Molecular Devices, Sunnyvale, USA). Flash intensity and duration were controlled through pClamp and the Digidata analog output.
Histological detection of β-galactosidase activity
Horizontal adult eye sections were performed using a cryostat microtome (Microm) and deposited on superfrost Plus slides (Thermo). Third instar laval imaginal discs, midgut and salivary gland were dissected in 1X PBS. Samples were fixed 5 min in PBS 0.25% gluteraldehyde. They were stained in a solution of 7.2 mM Na2HPO4, 2.8 mM NaH2PO4, 150 mM NaCl, 1 mM MgCl2, 3 mM K3[Fe(CN)6], 3 mM K4[Fe(CN)6], containing a 1/30 dilution of X-Gal (30 mg/ml in dimethyl formamide). After washing in PBS, samples were mounted in DAPI mounting media (Vectashield, AbCys).
RT–PCR
mRNA was extracted from 20 retinas and 40 embryos using QIAshredder and RNeasy Mini kits (Qiagen). 100 ng of mRNA was used to synthesize cDNA using the Enhanced Avian RT First Strand Synthesis Kit (Sigma Aldrich) following manufacturer's instruction. Briefly, mRNA is incubated at 70°C for 10 min with dNTP (0.5 mM) and oligodT (3.5 µM) and then incubated at 50°C for 1 h with 1X buffer, reverse transcriptase enzyme (20U) and RNase inhibitor (20U). PCR was performed using GoTaq (Promega, 2U) in GreenGoTaq Buffer (Promega, 1X) with 200 µM dNTP and two pairs of primers (200 nM each, fatp forward: GGATTTTTGCTGTGCTCGTC, fatp reverse: ACCACATCGCCCTTTTTGTA, rp49 forward: CGGATCGATATGCTAAGCTGT, rp49 reverse: GCGCTTGTTCGATCCGTA). rp49 amplification was used as an internal control. cDNA was first denatured for 5 min at 95°C and amplified during 35 cycles: 95°C for 30 s, 59°C for 30 s, 72°C for 42 s; followed by an incubation at 72°C for 7 min. Amplified cDNA was segregated in 1.5% agarose gel.
Western blot
10 Drosophila adult heads were homogenized in 30 µL Laemmli buffer (10% glycerol, pH 6.8 0.5M Tris, 10% SDS, 1% bromophenol blue, 1% β-mercaptoethanol, 100 mM DTT) and centrifuged for 30 min at 12,000 g. Supernatant was boiled for 5 min and 10 µL was loaded onto a 12% acrylamide gel (Biorad) and transferred onto nitrocellulose membranes (Whatman). Anti-Fatp C11-7 (1/200), anti-Rh1 (1/1000, 4C5, DSHB) and anti-Tubulin (1/1000, Sigma) antibodies were incubated overnight at 4°C and appropriate HRP-coupled secondary anti-mouse and anti-rabbit antibodies (1∶10 000, Biorad) were then incubated for 2 h at RT. Chemiluminescent detection was carried out using a ECL kit (GE Healthcare Life Sciences). Protein band quantification was carried out using ImageJ software.
For blue light-induced degradation of Rh1, white-eyed heads were exposed to 10 mW blue light for 6 h and homogenized in Tris-buffered saline (20 mM Tris (pH 7.5), 150 mM NaCl) containing 0.5% Triton X-100 and protease inhibitors. After centrifugation, the supernatant was mixed with an equal volume of 2X Laemmli buffer and loaded onto a 12% acrylamide gel as described above.
Supporting Information
Zdroje
1. SohockiMMDaigerSPBowneSJRodriquezJANorthrupH 2001 Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 17 42 51
2. LeeESFlanneryJG 2007 Transport of truncated rhodopsin and its effects on rod function and degeneration. Invest Ophthalmol Vis Sci 48 2868 2876
3. MollereauBDomingosPM 2005 Photoreceptor differentiation in Drosophila: from immature neurons to functional photoreceptors. Dev Dyn 232 585 592
4. WangTMontellC 2007 Phototransduction and retinal degeneration in Drosophila. Pflugers Arch 454 821 847
5. ShiehBH 2011 Molecular genetics of retinal degeneration: A Drosophila perspective. Fly (Austin) 5
6. GalyARouxMJSahelJALeveillardTGiangrandeA 2005 Rhodopsin maturation defects induce photoreceptor death by apoptosis: a fly model for RhodopsinPro23His human retinitis pigmentosa. Hum Mol Genet 14 2547 2557
7. GambisADourlenPStellerHMollereauB 2011 Two-color in vivo imaging of photoreceptor apoptosis and development in Drosophila. Dev Biol 351 128 134
8. WatkinsPAMaiguelDJiaZPevsnerJ 2007 Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res 48 2736 2750
9. WatkinsPA 2008 Very-long-chain acyl-CoA synthetases. J Biol Chem 283 1773 1777
10. GimenoRE 2007 Fatty acid transport proteins. Curr Opin Lipidol 18 271 276
11. SchafferJELodishHF 1994 Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79 427 436
12. GuignardTJJinMPequignotMOLiSChassigneuxY 2010 FATP1 inhibits 11-cis retinol formation via interaction with the visual cycle retinoid isomerase RPE65 and lecithin:retinol acyltransferase. J Biol Chem 285 18759 18768
13. HerrmannTBuchkremerFGoschIHallAMBernlohrDA 2001 Mouse fatty acid transport protein 4 (FATP4): characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene 270 31 40
14. StahlA 2004 A current review of fatty acid transport proteins (SLC27). Pflugers Arch 447 722 727
15. HerrmannTvan der HoevenFGroneHJStewartAFLangbeinL 2003 Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol 161 1105 1115
16. MoulsonCLMartinDRLugusJJSchafferJELindAC 2003 Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci U S A 100 5274 5279
17. KlarJSchweigerMZimmermanRZechnerRLiH 2009 Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet 85 248 253
18. GolicKG 1991 Site-specific recombination between homologous chromosomes in Drosophila. Science 252 958 961
19. MollereauBWernetMFBeaufilsPKillianDPichaudF 2000 A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech Dev 93 151 160
20. PichaudFDesplanC 2001 A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development 128 815 826
21. BrandAHPerrimonN 1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
22. StowersRSSchwarzTL 1999 A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152 1631 1639
23. KuradaPO'TousaJE 1995 Retinal degeneration caused by dominant rhodopsin mutations in Drosophila. Neuron 14 571 579
24. ColleyNJCassillJABakerEKZukerCS 1995 Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci U S A 92 3070 3074
25. HarrisWAStarkWS 1977 Hereditary retinal degeneration in Drosophila melanogaster. A mutant defect associated with the phototransduction process. J Gen Physiol 69 261 291
26. AllowayPGHowardLDolphPJ 2000 The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 28 129 138
27. JohnsonKGraweFGrzeschikNKnustE 2002 Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr Biol 12 1675 1680
28. ChinchoreYMitraADolphPJ 2009 Accumulation of rhodopsin in late endosomes triggers photoreceptor cell degeneration. PLoS Genet 5 e1000377 doi:10.1371/journal.pgen.1000377
29. SatohAKReadyDF 2005 Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol 15 1722 1733
30. KiselevASocolichMVinosJHardyRWZukerCS 2000 A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28 139 152
31. XuHLeeSJSuzukiEDuganKDStoddardA 2004 A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. Embo J 23 811 822
32. AcharyaUPatelSKoundakjianENagashimaKHanX 2003 Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science 299 1740 1743
33. YonamineIBambaTNiralaNKJesminNKosakowska-CholodyT 2011 Sphingosine kinases and their metabolites modulate endolysosomal trafficking in photoreceptors. J Cell Biol 192 557 567
34. AcharyaUMowenMBNagashimaKAcharyaJK 2004 Ceramidase expression facilitates membrane turnover and endocytosis of rhodopsin in photoreceptors. Proc Natl Acad Sci U S A 101 1922 1926
35. WangXWangTJiaoYvon LintigJMontellC 2010 Requirement for an enzymatic visual cycle in Drosophila. Curr Biol 20 93 102
36. WangXWangTNiJDvon LintigJMontellC 2012 The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J Neurosci 32 3485 3491
37. MontellC 2012 Drosophila visual transduction. Trends Neurosci
38. LeeSJXuHMontellC 2004 Rhodopsin kinase activity modulates the amplitude of the visual response in Drosophila. Proc Natl Acad Sci U S A 101 11874 11879
39. HardieRCMartinFChybSRaghuP 2003 Rescue of light responses in the Drosophila “null” phospholipase C mutant, norpAP24, by the diacylglycerol kinase mutant, rdgA, and by metabolic inhibition. J Biol Chem 278 18851 18858
40. ChybSRaghuPHardieRC 1999 Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 397 255 259
41. LeungHTTseng-CrankJKimEMahapatraCShinoS 2008 DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 58 884 896
42. MendesCSLevetCChatelainGDourlenPFouilletA 2009 ER stress protects from retinal degeneration. EMBO J 28 1296 1307
43. LeeYSCarthewRW 2003 Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30 322 329
44. FishMPGrothACCalosMPNusseR 2007 Creating transgenic Drosophila by microinjecting the site-specific phiC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protoc 2 2325 2331
45. DomingosPMMlodzikMMendesCSBrownSStellerH 2004 Spalt transcription factors are required for R3/R4 specification and establishment of planar cell polarity in the Drosophila eye. Development 131 5695 5702
46. DomingosPMBrownSBarrioRRatnakumarKFrankfortBJ 2004 Regulation of R7 and R8 differentiation by the spalt genes. Dev Biol 273 121 133
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



