-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
The Alternative Lengthening of Telomeres (ALT) pathway is a telomerase-independent pathway for telomere maintenance that is active in a significant subset of human cancers and in vitro immortalized cell lines. ALT is thought to involve templated extension of telomeres through homologous recombination, but the genetic or epigenetic changes that unleash ALT are not known. Recently, mutations in the ATRX/DAXX chromatin remodeling complex and histone H3.3 were found to correlate with features of ALT in pancreatic neuroendocrine cancers, pediatric glioblastomas, and other tumors of the central nervous system, suggesting that these mutations might contribute to the activation of the ALT pathway in these cancers. We have taken a comprehensive approach to deciphering ALT by applying genomic, molecular biological, and cell biological approaches to a panel of 22 ALT cell lines, including cell lines derived in vitro. Here we show that loss of ATRX protein and mutations in the ATRX gene are hallmarks of ALT–immortalized cell lines. In addition, ALT is associated with extensive genome rearrangements, marked micronucleation, defects in the G2/M checkpoint, and altered double-strand break (DSB) repair. These attributes will facilitate the diagnosis and treatment of ALT positive human cancers.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002772
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002772Summary
The Alternative Lengthening of Telomeres (ALT) pathway is a telomerase-independent pathway for telomere maintenance that is active in a significant subset of human cancers and in vitro immortalized cell lines. ALT is thought to involve templated extension of telomeres through homologous recombination, but the genetic or epigenetic changes that unleash ALT are not known. Recently, mutations in the ATRX/DAXX chromatin remodeling complex and histone H3.3 were found to correlate with features of ALT in pancreatic neuroendocrine cancers, pediatric glioblastomas, and other tumors of the central nervous system, suggesting that these mutations might contribute to the activation of the ALT pathway in these cancers. We have taken a comprehensive approach to deciphering ALT by applying genomic, molecular biological, and cell biological approaches to a panel of 22 ALT cell lines, including cell lines derived in vitro. Here we show that loss of ATRX protein and mutations in the ATRX gene are hallmarks of ALT–immortalized cell lines. In addition, ALT is associated with extensive genome rearrangements, marked micronucleation, defects in the G2/M checkpoint, and altered double-strand break (DSB) repair. These attributes will facilitate the diagnosis and treatment of ALT positive human cancers.
Introduction
In the absence of telomerase activity, telomeres shorten with cell division, ultimately leading to senescence. Hence, the development of human cancer is associated with an active telomere maintenance system that provides an infinite source of telomeric DNA to potentiate immortality. Although telomerase reactivation is the most common mechanism of telomeric repeat addition in cancers, a significant subset of human tumors employs a telomerase-independent telomere maintenance pathway, referred to as ALT [1].
The emerging view is that ALT maintains telomeres through homology-directed recombination (HDR) [2]. Supporting this view, ALT cells show an elevated frequency of sequence exchanges between telomeres [2]–[4], contain extrachromosomal linear and circular telomeric DNA [5]–[8], and often exhibit heterogeneously-sized telomeres [1], features consistent with hyperactive HDR. The extrachromosomal telomeric DNA has been proposed to serve as a template for the extension of telomeres by a recombination mechanism akin to Break Induced Replication (BIR) [9], [10]. In addition, ALT cells often carry altered PML bodies (ALT-associated PML bodies, or APBs) that contain telomeric DNA as well as numerous recombination factors [11]. Several proteins involved in recombination are known to be required for ALT, including the Mre11 complex, Mus81, and the SMC5/6 sumoylation pathway [6], [12]–[15].
ALT was discovered in a subset of immortalized cell lines that emerge at low frequency from human cell cultures in telomere crisis, but this pathway has increasingly been identified in human cancer. Approximately 10–15% of human cancers are suspected to use the ALT pathway based on their lack of telomerase activity in combination with certain hallmarks of ALT, such as the presence of extrachromosomal telomeric circles, APBs, and/or long and heterogeneous telomeres. Based on these criteria, ALT is relatively common in many sarcomas (osteosarcoma and some types of soft tissue sarcomas), certain endocrine tumors (pancreatic neuroendocrine tumors, paraganglioma), a subset of nervous system tumors (e.g. glioblastoma, medulloblastoma, oligodendroglioma, astrocytoma, ganglioneuroblastoma), bladder small cell carcinoma, and nonseminomatous germ cell tumors [16]–[18].
The molecular basis for the activation of the ALT pathway is not known. In vitro, the frequency of conversion to ALT is low, typically requiring many months of culturing of virally-transformed (p53/Rb deficient) human cells that have entered telomere crisis [11]. The low frequency of conversion to ALT suggests that mutations and/or infrequent epigenetic alterations are required to unleash this pathway. Indeed, in telomerase-positive mammalian cells with fully functional telomeres, telomeric recombination is stringently repressed by the TRF2, Rap1, and POT1 components of shelterin as well as by the Ku70/80 heterodimer [6], [19]–[23]. Mutations in shelterin and Ku might therefore be anticipated in ALT, a notion that is tested here.
Recently, a subset of pancreatic neuroendocrine tumors (PanNETs) were found to have ultrabright telomeric FISH signals, suggesting the presence of ALT-like long telomeres [24], [25]. These tumors also exhibited alterations in ATRX or its binding partner DAXX. Twenty-five PanNETs with ultrabright telomeric foci had ATRX/DAXX mutations or lacked nuclear ATRX/DAXX protein. In contrast, 16 PanNETs with apparently normal ATRX/DAXX lacked the aberrant telomeric staining patterns. The same correlation between inactivation of the ATRX pathway and the ALT-like phenotype was observed in pediatric glioblastoma and several other cancers [16], [26].
ATRX and DAXX act together in a replication-independent chromatin assembly pathway that deposits the histone variant H3.3 at telomeres and perhaps at other G-rich repetitive elements [27]–[31]. The function of ATRX/DAXX and histone H3.3 at telomeres is not yet clear, but diminished ATRX function has been documented to increase TTAGGG repeat containing telomeric transcripts (referred to as TERRA [32]), reduce telomeric loading of HP1α, and cause modest levels of telomere dysfunction in mouse ES (but not NIH3T3) cells as gleaned from the localization of γ-H2AX at chromosome ends [29], . ATRX deficiency also leads to a defect in sister chromatid cohesion and aberrant mitoses, culminating in the formation of micronuclei, lobulated nuclei, and chromatin bridges [33]–[35].
Because functional assessment of ALT is not possible in tumor specimens, further tests for the association of ALT with ATRX/DAXX deficiency requires analysis of in vitro immortalized cell lines. Here we describe the results of a comprehensive effort to characterize the genetic alterations and associated phenotypes of 22 human ALT cell lines. We found loss of ATRX in 90% of in vitro immortalized ALT lines, suggesting that inactivation of ATRX is a major step in generating the ALT phenotype. We also document that ALT cell lines are characterized by extensive genomic instability, formation of micronuclei, an aberrant G2/M checkpoint, and abnormal DSB repair kinetics.
Results
Most human ALT cell lines lack ATRX
We assembled a panel of 22 human ALT cell lines (Table 1), nineteen of which originated from in vitro immortalization experiments and four of which were derived from human tumors (three osteosarcomas and one lung adenocarcinoma). Many of the in vitro immortalized lines expressed SV40 - or HPV-derived oncoproteins. Seven cell lines originated from a single individual with cystic fibrosis (JFCF-6 series); an additional five cell lines were established from a Li-Fraumeni patient (IIICF series). We confirmed the absence of telomerase activity by TRAP assay (data not shown) and the presence of extra-chromosomal telomeric C-circles, supporting the interpretation that these were ALT cells [8], [17] (Table 1; Figure S1).
Tab. 1. Description of ALT lines used in this study and summary of results. 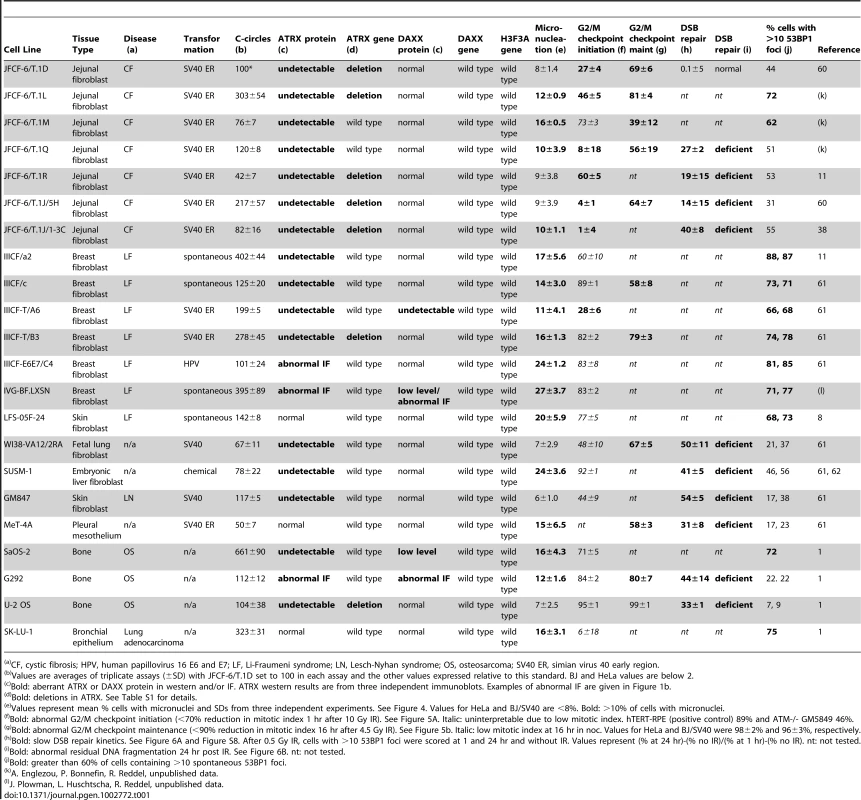
CF, cystic fibrosis; HPV, human papillovirus 16 E6 and E7; LF, Li-Fraumeni syndrome; LN, Lesch-Nyhan syndrome; OS, osteosarcoma; SV40 ER, simian virus 40 early region. The ALT cell lines and non-ALT controls (hTERT-immortalized SV40-transformed BJ fibroblasts and the telomerase-positive HPV-E6/E7 expressing HeLa cervical tumor cell line) were tested for the expression of ATRX and DAXX (Figure 1A–1C and data not shown). Repeated immunoblots on independently harvested whole cell extracts indicated that among the 22 ALT cell lines, only six (G292, IIICF-E6E7/C4, IVG-BF.LXN, LFS-05F-24, SK-LU-1, and MeT-4A), contained detectable full length ATRX protein and one of these (IVG-BF.LXN) appeared to lack DAXX. Of the six cell lines expressing ATRX, three (G292, IIICF-E6E7/C4, and IVG-BF.LXN) showed very low immunofluorescence signals for ATRX and/or DAXX (Figure 1B), whereas the other three lines displayed the punctate nuclear staining pattern for ATRX and DAXX expected for this PML body component [36], [37]. Thus, 19 out of 22 ALT lines had an alteration in the expression of ATRX and/or DAXX. As each of the seven JFCF-6-derived ALT lines lacked detectable ATRX, we considered the possibility that the parental JFCF-6 culture might have been deficient in ATRX expression. However, a non-ALT JFCF-6 line contained ATRX (Figure 1C), indicating that the lack of ATRX in the other JFCF-6 lines was potentially correlated with the ALT phenotype.
Fig. 1. Deficiency in ATRX/DAXX correlates with ALT. 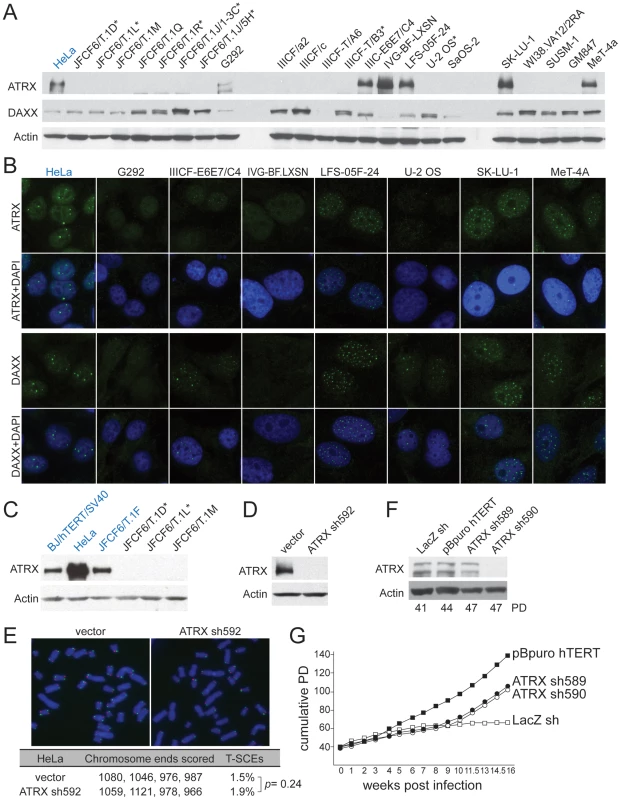
A, Immunoblot for ATRX and DAXX in the indicated ALT lines. ATRX was detected with A301-045 (Bethyl Labs). DAXX was detected with A301-353A (Bethyl Labs). Asterisk: cell lines with deletions in the ATRX gene (see Table 1 and Table S2). HeLa cells (blue) were used as a positive control. B, IF for ATRX and DAXX in the indicated cell lines. HeLa cells (blue) are used as a positive control. C, Immunoblot for ATRX in additional non-ALT, positive control cells (blue) relative to the indicated ALT lines. D, Immunoblot showing >90% reduction of ATRX protein in HeLa cells expressing ATRX shRNA592. E, T-SCEs, a measure for telomeric recombination, assessed by Chromosome Orientation (CO)-FISH on metaphases harvested from HeLa cells expressing vector or ATRX shRNA592. The average percentage of T-SCEs from four independent experiments is shown, with a p value derived from a two-tailed, unpaired t test. F, Immunoblot showing >90% reduction of ATRX protein in cells expressing ATRX shRNA590 but not with shRNA589. G, Growth curves showing immortalization of SV40-transformed BJ fibroblasts infected with an hTERT expressing retrovirus but no immortalization after infection with the effective (sh590) and ineffective (sh589) shRNAs to ATRX. All ALT cell lines were tested for genetic changes in ATRX, DAXX, and H3.3 (H3F3A) using primers designed to PCR-amplify all exons from these genes for Sanger sequencing (Table 1; Table S1). Failure to amplify multiple consecutive exons was interpreted as a deletion event only when flanking exons did amplify. By that criterion, 6 cell lines (JFCF-6/T.1D, JFCF-6/T.1L, JFCF-6/T.1R, JFCF-6/T.1J/5H, JFCF-6/T.1J/1-3C, and IIICF-T/B3) harbored large deletions in the ATRX gene that ranged from 4 to 26 exons in size (Table 1; Table S1).
Sequencing of genes that did not harbor detectable deletions revealed no potentially inactivating genetic changes. Nine of those cell lines lacked detectable ATRX protein suggesting that genetic alterations not detectable by Sanger sequencing, such as translocations, promoter or splicing mutations, or epigenetic changes may underlie their lack of expression. No genetic alterations were detected in DAXX or the H3F3A gene encoding H3.3 (Table 1).
These data provide strong evidence that loss of ATRX expression is involved in either the initiation or maintenance of the ALT pathway in human cells. To test whether deficiency in ATRX was sufficient to induce the telomere-telomere recombination typical of ALT cells, we used an shRNA to deplete ATRX from HeLa cells and used Chromosome-Orientation FISH (CO-FISH) to measure sequence exchanges between sister telomeres (Telomere Sister Chromatid Exchanges or T-SCEs). Despite repression of ATRX by more than 95%, the frequency of T-SCEs was not significantly altered (Figure 1D and 1E). In addition, repression of ATRX or DAXX in SV40-transformed BJ fibroblasts failed to induce escape from crisis (Figure 1F and 1G; Figure S2), whereas expression of hTERT readily immortalized the cells (Figure 1G; Figure S2). After a prolonged period in crisis (6 weeks), immortal clones emerged from the culture in which ATRX expression was suppressed with ATRX sh590. However, a parallel culture in which ATRX was not suppressed efficiently (ATRX sh589) also yielded an immortal subpopulation (Figure 1F and 1G) and in both cases, the immortal cells expressed telomerase and lacked telomeric C-circles (Figure S2A and S2B), indicating that they had not activated ALT. These observations argue that this level of ATRX/DAXX suppression is not sufficient to activate the ALT pathway.
No overt changes in shelterin or 299 potentially ALT relevant genes
The lack of induction of ALT by shRNA-mediated suppression of ATRX and DAXX prompted us to inspect the ALT cell line panel for changes in the status of genes, pathways, and proteins with possible relevance to the activation of ALT. Because HDR is normally repressed at telomeres by shelterin, we examined the expression of all six shelterin components in the ALT lines by repeated immunoblotting (Figure S3). Using this approach on all 22 ALT lines failed to reveal a consistent change in the abundance or appearance of the six shelterin proteins. In addition, exome sequencing (see below) failed to reveal mutations in the genes encoding TRF1, TRF2, Rap1, POT1, TIN2, and TPP1.
A second entity associated with telomeres is the telomeric RNA, TERRA [32]. As mouse ATRX deficient cells were reported to have elevated TERRA levels and selected ALT cells were previously found to have higher levels of TERRA [31], [38], we examined the expression of this RNA in the ALT lines. Quantitative Northern analysis confirmed that the ALT lines generally have higher TERRA levels than telomerase-positive cells, but some ALT lines (e.g. JFCF-6/T.1D and IIICF-E6E7/C4) approximated the levels of TERRA found in BJ/hTERT/SV40 and HeLa cells (Figure S4). There was no clear correlation between increased TERRA and ATRX status since the cell lines with apparently normal ATRX/DAXX (LFS-05F-24, SK-LU-1, and MeT-4A) had TERRA levels comparable to, or higher than, cell lines lacking ATRX (e.g. JFCF-6/T.1D, JFCF-6/T.1R, and JFCF-6/T.1L) (Figure S4).
In addition to shelterin and TERRA, we analyzed 299 genes with potential relevance to telomeres and the ALT pathway. This gene set included genes encoding proteins involved in telomere maintenance, DNA damage repair and signaling, chromosome duplication and segregation, and cell cycle regulation (Table S2). The coding regions of these genes were analyzed by massively parallel 454 sequencing on genomic DNA derived from 14 of the 22 ALT lines. We focused on ALT lines generated in vitro as opposed to tumor-derived ALT lines to exclude genetic changes selected for during tumorigenesis. The JFCF-6 and IIICF ALT lines are derived from two individuals, allowing identification of SNPs specific to the individual as well as potential ALT-correlated changes. After excluding known SNPs, the remaining sequence data were filtered to exclude apparent SNPs present in less than 25% of the reads. The rare SNPs that eluded the threshold filter affected 20 genes (Table S3). Within the JFCF-6 and IIICF cell line sets, a nucleotide change in multiple members of either set was interpreted as being present prior to the acquisition of the ALT phenotype, and thus not relevant. The variable occurrence of these SNPs in the multiple ALT lines derived from one individual (e.g. only two of the five IIICF derived lines have the BLM K323R) is likely due to chromosome losses occurring during or after telomere crisis. The florid chromosome instability exhibited by these cell lines (see below) makes this an appealing and parsimonious explanation. Some of these SNPs were also found in other cell lines (e.g. MDC1 C1559G in LSF-05F-24 and IVG-BF.LXSN) and deemed irrelevant based on their presence in the IIICF and/or JFCF-6 lines prior to the induction of ALT. Based on these criteria, no ALT specific change was observed in the five IIICF-derived ALT lines or in IVG-BF.LXN. Three of the JFCF-6 derived ALT lines had a specific change that could not be attributed to a pre-existing SNP (A436S in Suv420H2, S553F/S470F in PRMT5, and D2579V in Ki67; Table S3). Since these genes were not affected in the other 13 ALT lines analyzed, they are unlikely to be critical to ALT. Similarly, LSF-05F-24 contained changes in five genes that are not affected in the other 13 cell lines. Since none of these alterations were found in a substantive fraction of the ALT cell lines, it is unlikely that these genes represent clear contributors to the ALT phenotype.
Extensive genome rearrangements in ALT
As ALT-relevant genetic alterations might be identified from recurrent regions of gene copy number changes, we analyzed the ALT cell line panel using Affymetrix 6.0 single nucleotide polymorphism (SNP) arrays to identify regions of recurrent copy number alteration. When we examined either individual cells (Figure 2A and 2B) or the entire set as a group (GISTIC analysis, Figure S5), we found that the number and complexity of these gains and losses were at least as extensive as the alterations in human cancers with genome instability [39]. When we considered all of the ALT cell lines as a group, we identified 18 regions of significant focal copy number gain, and many more regions of copy number loss (Figure S5).
Fig. 2. Copy number analysis showing extensive genome rearrangements in ALT lines. 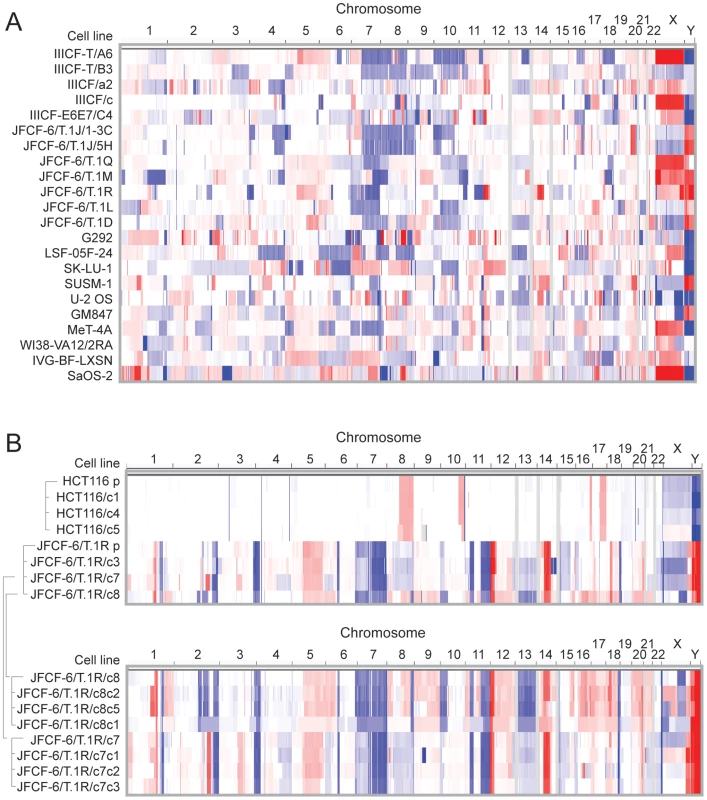
SNP array copy number results are shown for (A) the ALT cell lines and (B) the 1st and 2nd generation subclones derived from JFCF-6/T.1R (indicated by the brackets) and subclones derived from the telomerase-positive HCT116 control. Segmented copy number data is shown for each chromosome, by genomic position in columns and by cell line in rows. The color scale ranges from red (amplification; log2 copy number ratio of 1.5) through white (neutral; 2 copies in diploid lines, log2 ratio of 0) to blue (deletion; log2 ratio of −1.5). The genome rearrangements in the ALT lines are a likely consequence of the telomere dysfunction these cells experienced prior to their immortalization. Consistent with this idea, SKY analysis of five ALT lines (JFCF-6/T.1R, JFCF-6/T.1Q, IVG-BF.LXN, IIICF/c, and LFS-05F-24) showed frequent non-reciprocal translocations, deletions, complex rearrangements, and hyper-triploid chromosome numbers (Figure 3), all of which can be caused by telomere dysfunction [40], [41]. Previous cytogenetic analysis of two ALT cell lines (Saos-2 and ZK-58) also indicated frequent translocations, deletions, and complex rearrangements [42].
Fig. 3. Abnormal karyotypes in ALT lines. 
Representative metaphases from 5 cell lines showing subtetraploid karyotype with high number of rearranged chromosomes (up to 60%). Structural rearrangements are labeled as following: del(Z)- deletion of chromosome Z; der(Z) - multiple aberrations within single chromosome Z; chromosome, denoted with two or more numbers indicate rearrangement involving two or more partners. In cells that have been immortalized by telomerase activation, the telomere-driven genome instability is largely dampened, resulting in a rearranged but now relatively stable karyotype [43], [44]. We asked whether ALT similarly stabilizes the genome by examining two generations of clonal descendants of the JFCF-6/T.1R ALT line. Although the clones exhibited features in common with the parental cell line, each of the clones showed new regions of copy number gain or loss (Figure 2B). For instance, a new gain of a segment on chromosome 2q was observed in one single cell clone of the parental line (and all the clones derived from it), and in another clone (and its derivatives) an overlapping segment showed a new deletion. Many other changes, including examples on chromosomes 6, 12, 14, 15, 16 and 21, showed obvious new gains or losses as compared to the parental clone, with independent clones showing different alterations. In contrast, we found only rare clonal alterations in single cell clones derived from the telomerase-expressing HCT116 cell line. These observations suggest that the JFCF-6/T.1R and perhaps other ALT lines have ongoing genome instability as well as highly rearranged genomes.
We considered that ongoing genome instability in the ALT lines could be due to defects in mitosis and/or dicentric chromosomes resulting from telomere dysfunction and ATRX deficiency. Defects in mitosis and dicentric chromosomes can give rise to lagging chromosomes that can form micronuclei, which were recently shown to engender massive genomic alterations [45]. We therefore examined the ALT lines for the spontaneous occurrence of micronuclei. Fifteen of the 22 ALT lines showed a high frequency (10–30%) of cells with micronuclei (Table 1; Figure 4A and 4B). As expected, SV40-transformed hTERT-expressing BJ fibroblasts and HeLa cells showed the low level of spontaneous micronucleation (<8%) previously noted in other transformed human cells [46]. Micronucleation frequencies of up to 15% were previously only observed in cells experiencing high levels of DNA damage ensuing from γ-irradiation, in genetic contexts associated with high levels of spontaneous DNA damage, and in genomically unstable tumor cell lines (e.g. [47], [48]; Neill Ganem and David Pellman, pers. comm.). Thus, many ALT cell lines show an unusually high frequency of micronucleation, indicative of ongoing genome instability. The micronucleation phenotype of ALT lines is likely to be in part due to the absence of ATRX since depletion of ATRX with two shRNAs induced the formation of micronuclei in BJ/hTERT/SV40 and HeLa cells (Figure 4C and 4D), consistent with a previous report on the induction of lobulated nuclei after ATRX depletion [35].
Fig. 4. Frequent micronucleation in ALT cells may be attributable to loss of ATRX. 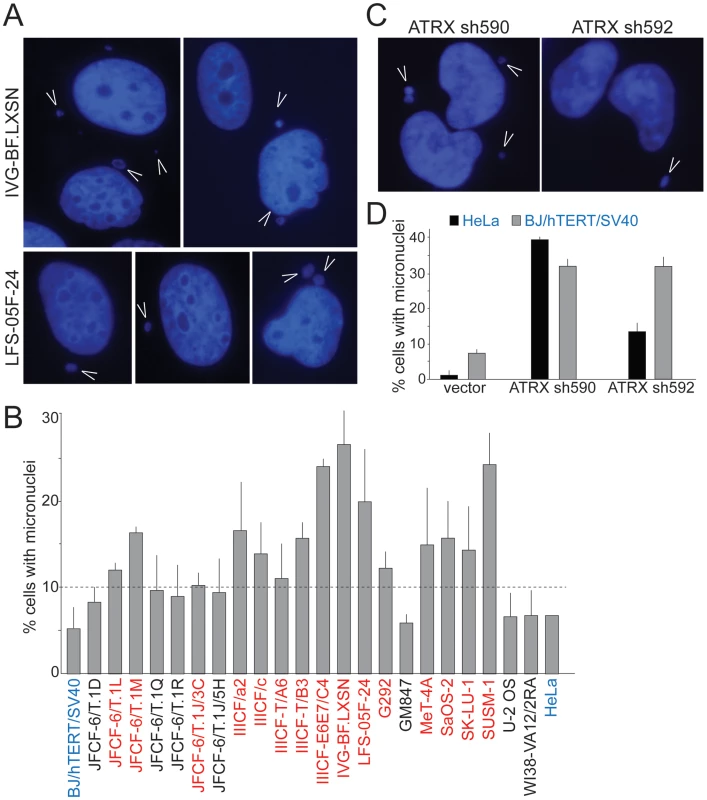
A, Examples of micronuclei (arrowheads) in two ALT cell lines. Blue: DAPI stain for DNA. B, Graph showing micronucleation frequencies in the ALT cell lines and two telomerase positive controls (blue). Values are means ±SD from three experiments (>100 nuclei each). Red: cell lines with >10% micronucleation frequency (dashed line). HeLa cells were analyzed once. C, Examples of micronuclei (arrowheads) in HeLa cells expressing two independent ATRX shRNAs (see Figure 1 for immunoblots). D, Graph showing micronucleation frequencies in two telomerase positive cell lines infected with vector or the indicated ATRX shRNAs. Values are means ±SEM from two experiments (200 nuclei each). G2/M checkpoint function and DSB repair kinetics in ALT lines
The signs of ongoing genome instability in ALT cells led us to examine the functionality of DNA damage checkpoints and DSB repair. As the ALT lines were expected to have an impaired G1/S DNA damage checkpoint due to their p53 deficiency, we focused our efforts on the G2/M checkpoint. Entry into mitosis is blocked upon activation of the ATM and/or ATR signaling pathways. ATM signaling is primarily responsible for the initiation of the checkpoint, whereas ATR signaling ensures the maintenance of the arrest [49]. To determine the efficiency of initiation and maintenance of the G2/M checkpoint, subconfluent cells were subjected to ionizing radiation, and the mitotic index was determined by flow cytometric quantitation of histone H3 (S10) phosphorylation. One hour after treatment with 10 Gy ionizing radiation (IR), the mitotic index of control hTERT-immortalized Retinal Pigment Epithelial cells (RPE/hTERT) was reduced approximately ten-fold (from 2.7% to 0.3%), whereas reduction in the mitotic index of ATM-deficient GM5849 cells was less than two-fold (from 4.1% to 2.3%) (Table 1; Figure 5A). Of fourteen ALT lines that had a sufficiently high mitotic index to yield interpretable results, half showed a severe defect in G2/M checkpoint initiation (Figure 5A; Table 1). In six ALT lines, the checkpoint defect was more severe than that of ATM-deficient cells. Qualitatively similar results were observed in response to low dose (1 Gy) IR treatment, with the same cell lines exhibiting defects as at the high dose (data not shown). Similarly, 10 of 11 ALT lines tested displayed a deficiency in the maintenance of the G2/M checkpoint, monitored at 16 hr after γ-irradiation with 4.5 Gy (Table 1; Figure 5B). Some of the ALT lines were proficient in the initiation of the checkpoint but failed to maintain it (e.g., IIICF/c). Collectively, we observed impaired G2/M checkpoint function in 11 out of 17 ALT lines tested. This defect in the G2/M checkpoint is not seen in established non-ALT human cell lines. For example, normal G2/M arrest following γ-irradiation is observed in HT1080, HCT116, IMR90 (JHJP, unpubl. data), 293T [50], MCF7, and genetically complemented HCC1937 cells [51]. The decrement in the G2/M checkpoint did not reflect a global impairment of DNA damage response (DDR) signaling in the ALT cells, since phosphorylation of CHK2, an outcome of ATM signaling, was readily apparent upon IR treatment in all cell lines, but undetectable in GM5849 (ATM-deficient) cells (Figure S6). Indeed, CHK2 phosphorylation was evident in several of the ALT cell lines prior to irradiation, consistent with the finding that the ALT phenotype is associated with chronic genotoxic stress (Figure S6).
Fig. 5. Many ALT lines have a defect in the G2/M checkpoint initiation and/or maintenance. 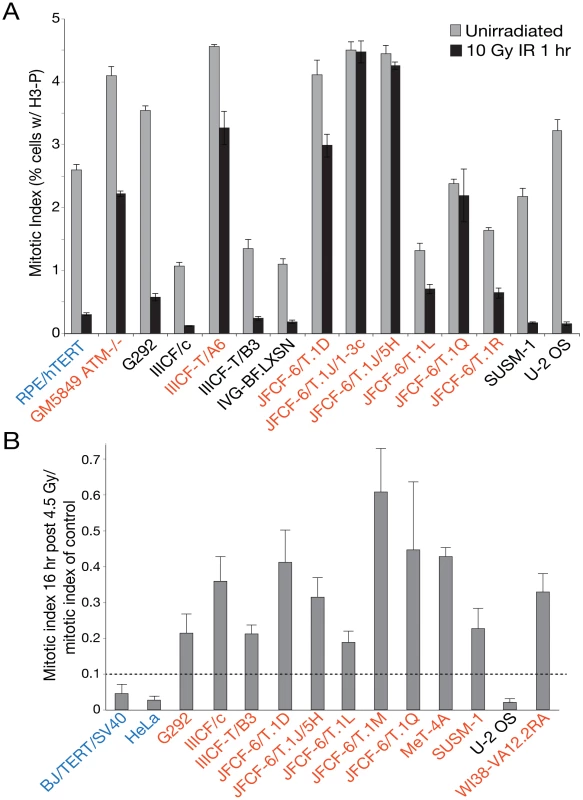
A, Bar graph depicting the results of an assay for the initiation of the G2/M checkpoint at 1 hr after irradiation of the indicated cell lines. RPE/hTERT is a positive control (in blue). Mean ± SD for triplicate experiments are shown. B, Bar graph depicting the results of an assay for the maintenance of the G2/M checkpoint at 16 hr after IR. BJ/hTERT/SV40 is a positive control (in blue). Mean ± SD for triplicate experiments are shown. See Table 1 for summary. Cell lines in red have a defect in the G2/M checkpoint. In addition to an abnormal G2/M checkpoint, many of the ALT cell lines showed unusually slow DSB repair kinetics. To assay this aspect of the DDR, cells were γ-irradiated with 0.5 Gy and the fraction of cells with 10 or more 53BP1 foci was scored after 1 and 24 hr as well as in non-irradiated controls (Figure 6A; Figure S7; Table 1). As expected, non-ALT cell lines (HeLa and hTERT immortalized SV40-transformed BJ) showed a nearly complete disappearance of the IR-induced DNA damage foci at 24 hr. In contrast, 11 out of 12 ALT cell lines retained a significant fraction of the induced 53BP1 DNA damage foci after a day (Table 1; Figure 6A). This result was corroborated with a pulsed field gel electrophoresis (PFGE)-based gel assay for the disappearance of IR-induced DNA fragmentation after 24 hr (Figure 6B; Table 1). We also noted that approximately half of the ALT cell lines had a high basal level of cells containing 10 or more 53BP1 foci, further confirming that ALT cells experience ongoing genome damage (Table 1). These spontaneous 53BP1 foci could be the result of both genome-wide DNA breaks and the presence of dysfunctional telomeres [52]. Their deficiency in the G2/M checkpoint, combined with the absence of a functional p53 pathway likely explains why these cells proliferate despite severe genome damage.
Fig. 6. Deficiency in DSB repair in ALT lines. 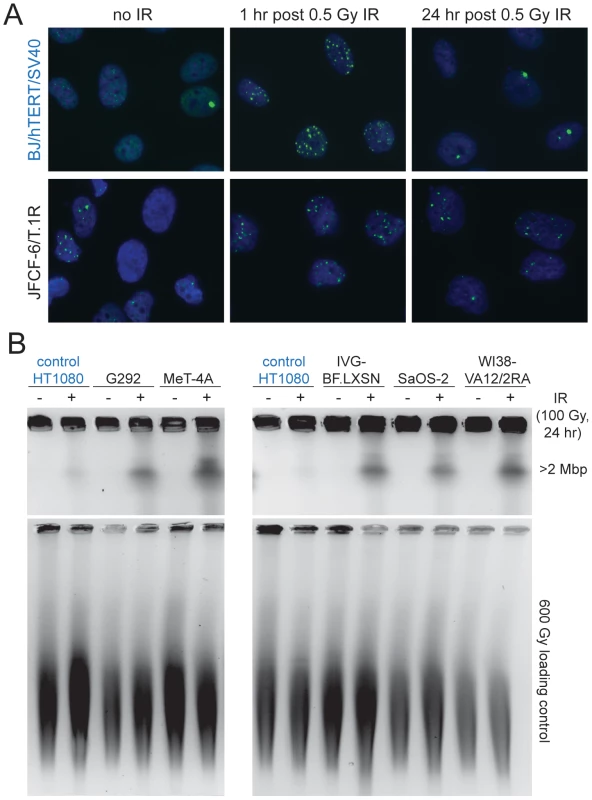
A, Example of assay for 53BP1 foci in the positive control (BJ/hTERT/SV40, blue) (top) and an ALT line (bottom). Cells were treated as indicated above the panels and stained for DNA with DAPI (blue) in conjunction with IF for 53BP1 (green). Note the higher level of 53BP1 foci in non-irradiated ALT cells and incomplete DSB repair at 24 hr after IR. B, Example of PFGE-assay for DSB repair in the indicated cell lines. Cells were treated with 100 Gy IR 24 hr before harvesting and residual DSBs were evaluated based on the DNA fragments released from agarose embedded cells into a PFGE gel. Plugs were treated with 600 Gy to fragment the DNA and run in parallel to serve as a control for loading. DNA was stained with ethidium bromide after electrophoresis. HT1080 (fibrosarcoma cell line) was used as a positive control (in blue). Discussion
Here we report that ATRX is either undetectable or severely depleted from PML bodies in ∼90% of human ALT cell lines tested, including ALT lines derived in vitro. This result establishes a strong correlation between the initiation or maintenance of ALT and deficiency in the ATRX/DAXX pathway. In a subset of cases, the loss of ATRX is due to large deletions within the ATRX gene, further underscoring the relevance of ATRX status to ALT. We expect that alterations in ATRX/DAXX in cells and tumors will be a useful indicator of the presence of the ALT pathway. However, manipulating ATRX/DAXX expression failed to unleash ALT, suggesting that deficiency of these proteins is not sufficient for the ALT phenotype and pointing to the need to identify the cooperating (epi-) genetic changes. In addition, a key issue for further investigation is the mechanism by which the loss of ATRX/DAXX and concomitant lack of deposition of H3.3 into telomeric chromatin might allow or promote telomere recombination. We note that it remains possible that the ATRX/DAXX pathway has an indirect effect, for instance by repressing genes that promote telomere recombination.
Our data have uncovered a hitherto unappreciated level of genome instability in ALT cells. All ALT lines, including cell lines that arose from in vitro immortalization experiments, harbor scrambled genomes and have signs of ongoing genome instability. The ALT lines have frequent micronuclei, a high basal level of DNA damage foci, and elevated checkpoint signaling in absence of exogenous damage. Furthermore, in one ALT cell line, repeated subcloning established frequent copy number alterations. These features of ALT raise the possibility that tumors employing this pathway will have unique vulnerabilities that could offer clinical utility, such as has been shown for cells lacking BRCA1 that are sensitive to PARP inhibition [53]. This possibility underscores the importance of establishing a signature for the ALT phenotype.
In this context, it is notable that G2/M checkpoint deficiency emerged as a prominent attribute of many ALT cells in this study. It is likely that the diminished G2/M checkpoint response allows these cells to proliferate, despite a considerable burden of DNA damage both at telomeres and elsewhere in the genome. G2/M checkpoint deficiency is an example of a potential vulnerability unique to ALT cells. Indeed, G2/M checkpoint inhibitors to enhance efficacy of clastogenic therapies are currently being developed and evaluated in clinical trials [54].
The altered G2/M response could not be ascribed to mutations in known components of the ATM or ATR signaling pathways. Furthermore, DNA damage signaling was demonstrably intact since CHK2 phosphorylation could be induced by exogenous DNA damage. The implication of ATRX and DAXX in the ALT phenotype may have some relevance to the checkpoint defects observed, as the involvement of chromatin remodelers in the DDR becomes increasingly clear [55]. Nevertheless, identification of the mechanisms underlying G2/M checkpoint dysfunction in ALT cells is warranted given the data presented here.
Our results would appear to exclude the possibility that the ALT cells arise from a single dominant mutation in addition to p53/Rb loss. Loss of ATRX/DAXX function appears to be required for ALT but it seems likely that a defect in the G2/M checkpoint is also needed in order for the cells to proliferate, given their high level of spontaneous DNA damage. The view that multiple steps are required to allow ALT-mediated immortalization is consistent with the low frequency by which it arises, even in SV40 - or HPV-transformed cells.
Materials and Methods
Cell lines
ATL cell lines are described in Table 1. CellBank Australia provided cell line quality control. All cell lines were verified by 16-locus STR profiling and confirmed to be free of Mycoplasma species, or were analyzed within 10 population doublings of being obtained from ATCC. Non-ALT cell lines were obtained from the ATCC. The HeLa cell line (subclone HeLa1.3) was described previously [56].
Sequence analysis of ATRX, DAXX, and H3.3 genes
Primers were synthesized by Invitrogen (San Diego, CA) to cover all exons of ATRX, DAXX, and H3F3A1 (H3.3). PCR was performed in 5 µl reactions containing 1× PCR Buffer (67 mM Tris-HCl, pH 8.8, 6.7 mM MgCl2, 16.6 mM NH4SO4, 10 mM 2-mercaptoethanol), 1 mM dNTPs (Invitrogen, San Diego, CA), 1 µM forward and 1 µM reverse primers, 6% DMSO, 2 mM ATP,0.25 U Platinum Taq (Invitrogen, San Diego, CA) and 3 ng DNA. Reactions were carried out in 384-well ABI 9700 thermocyclers (Applied Biosystems, Foster City, CA) using a touchdown PCR protocol: 1 cycle of 96°C for 2 min; 3 cycles of 96°C for 10 sec, 64°C for 10 sec, 70°C for 30 sec; 3 cycles of 96°C for 10 sec, 61°C for 10 sec, 70°C for 30 sec; 3 cycles of 96°C for 10 sec, 58°C for 10 sec, 70°C for 30 sec; 41 cycles of 96°C for 10 sec, 57°C for 10 sec, 70°C for 30 sec; 1cycle of 70°C for 5 min. The PCR products were evaluated for presence and size by electrophoresis on 2% agarose gels and sequenced as described (refs. 25 and 57 [25], [57]). All failed PCRs were repeated at least two more times.
Targeted sequencing of candidate ALT genes
Coding regions of 299 genes were amplified from genomic DNA of 15 samples. Following PCR amplification, products were normalized and pooled prior to emulsion PCR amplification of single stranded DNA and 454 pyrosequencing. Sequencing was performed using a FLX Titanium machine at Agencourt Bioscience Corp. (Beverly MA), to a depth of 20× coverage per sample per amplicon (attempted). Assembly and mapping of reads was performed using AVA software.
Immunoblotting
Immunoblotting was performed using standard procedures on whole cell extracts. ATRX was detected primarily with A301-045 (Bethyl Labs) or with HPA001906 (Sigma Aldrich). DAXX was detected with A301-353A (Bethyl Labs). Immunoblotting for CHK2-P was done with CHK2-T69 (Cell Signaling) and total CHK2 was detected with clone 7 Ab (Millipore). Shelterin components were analyzed as described previously [56].
Immunofluorescence
Cells grown on glass coverslips were fixed with 3% paraformaldehyde for 10 min at room temperature, washed three times with PBS, and permeabilized with 0.5% Triton X-100 in PBS for 10 min on ice. Cells were then washed four times with PBS and blocked for 30 min at room temperature with PBG (0.2% cold water fish gelatin (Sigma), 0.5% BSA, in PBS). Primary antibodies recognizing ATRX and DAXX (Bethyl Laboratories, Inc., see above) were diluted in PBG and incubated on cells for 1.5 h at 37°C. Following three washes with PBG, cells were incubated with FITC-conjugated donkey anti-rabbit secondary antibody (Life Technologies), diluted in PBG, for 30 min at 37°C. Cells were washed three times with PBS and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI).
Quantitative RT–PCR
RNA was extracted from cell lines using the RNeasy kit (Qiagen). 0.5 µg of RNA was reverse transcribed using RT-Advantage kit (Clontech) in a volume of 20 µl according to the manufactures instructions. Following reverse transcription 180 µl of water was added and 5 µl was used for qPCR with specific primers and SYBR green mix (Applied Biosystems). Primers: DAXX 5′: AGACGGTTTCTGAGCATCATC; DAXX 3′: AGAGGAGCTAGGGGCTTCTG; TERT 5′: GCCTTCAAGAGCCACGTC; TERT 3′: CCACGAACTGTCGCATGT.
Analysis of copy number alterations
Affymetrix 6.0 SNP data were generated at the Broad Institute for 22 ALT cell lines and 6 single cell ALT (JFCF-6/T.1R) and non-ALT (HCT116) cell line clones. Single cell clones were generated by fluorescence activated cell sorting (FACS) sorting into 96 well plates. Normalized copy number estimates (log2 ratios) were made and segmented by the Circular Binary Segmentation algorithm as previously described [58]. The GISTIC 2.0 algorithm was performed as previously described on the resulting segmented copy number data from the 22 ALT lines [39], [59]. The boundaries of the peak amplified and deleted regions identified by GISTIC 2.0 were set with at least 95% confidence to include the target gene(s).
Functional analysis of DAXX and ATRX
SV40-transformed BJ fibroblasts at PD 38 were infected with either ATRX - or DAXX-specific shRNAs (Sigma) with the following target sequences: ATRXsh589: GCAGATTGATATGAGAGGAAT; ATRXsh590: CGACAGAAACTAACCCTGTAA; ATRXsh592: CCGGTGGTGAACATAAGAAAT; DAXXsh800 : GAAGGGATGGACTAAGCTAAT; DAXXsh801: TCACCATCGTTACTGTCAGAA; DAXXsh802: GCCACACAATGCGATCCAGAA. LacZ-specific shRNA was used as negative control, while infection with hTERT was used as a positive control. Cells were selected with puromycin (0.5 µg/ml) for 2 days, and at least 106 cells of each sub-line were maintained in culture for 110 days. Cultures were split at <80% confluence, and growth medium was replaced every 3 days. Cells were counted at each passage and cumulative PDs were recorded. The suppression of ATRX in HeLa1.3 cells was achieved with ATRXsh592.
T-SCE assay for Telomeric Recombination
Cells were grown in 10 µM BrdU∶BrdC (3∶1) for 16 hr with the addition of 0.1 µg/ml colcemid (Roche) for the final 2 h. Slides were treated with 0.5 mg/ml RNAse A for 10 min at 37°C, stained with 0.5 µg/ml Hoechst 33258 in 2× SSC for 15 min at room temperature, and exposed to 365-nm UV light (Stratalinker 1800 UV irradiator, 5400 J/m2). Following digestion with Exonuclease III (Promega, 10 U/µl, 2×10 min) at room temperature, slides were dehydrated through an ethanol series (70%, 90%, 100%) and incubated sequentially with TAMRA-TelG 5′-[TTAGGG]3-3′ and FITC-TelC 5′-[CCCTAA]3-3′ probes at room temperature. The percentage of chromosome ends displaying telomeric sister chromatid exchanges (T-SCEs) was calculated from four independent experiments.
SKY
Cells were treated with colcemid for 1 hr, harvested, pelleted, re-suspended in a hypotonic solution of 0.075 M KCl for 18 min, fixed in Carnoy's fixative (3∶1 methanol∶glacial acetic acid), and washed four times with Carnoy's fixative. All fixed samples were spread on slides for staining or hybridization. Chromosomes were stained with Giemsa or Hoechst to visualize chromosomal abnormalities. Spectral karyotyping (SKY) was performed on mitotic samples according to the SkyPaint DNA kit H-5 for human chromosomes procedure (Applied Spectral Imaging, SKY000029) and imaged on a Nikon Eclipse E6000 microscope equipped with the SD300 Spectracube and Spectral Imaging acquisition software.
G2/M checkpoint analysis
To assess G2/M checkpoint initiation, subconfluent cells were exposed to γ-irradiation and harvested at 1 hr with non-irradiated cells for mitotic index measurement by flow cytometry. Briefly, cell pellets were fixed in 70% ethanol, washed twice with cold PBS, and permeabilized with 0.25% Triton X-100 in PBS for 15 min on ice. Cells were then rinsed with PBS containing 1% BSA and stained with anti-phospho-histone H3 S10 antibody (Cell Signaling) for 1.5 hr at room temperature. Following two washes with PBS containing 1% BSA, cells were stained with a FITC-conjugated donkey anti-mouse secondary antibody (Life Technologies) for 30 min at room temperature. Cells were washed twice with PBS and then stained with propidium iodide (25 µg/ml) in the presence of 0.1 mg/ml RNase. Flow cytometry was performed on a BD Biosciences FACSCalibur, and the percentage of mitotic cells was determined as those that were FITC-positive and contained 4N DNA content. To assess maintenance of the G2/M checkpoint, nocodazole (1 µg/ml) was added 1 hr after irradiation, and cells were incubated at 37°C for 16 hr. Cells were then harvested and processed as described above. To account for differences in the rate of cell cycle progression between cell lines, the mitotic index of irradiated cells was normalized to that of non-irradiated cells treated with nocodazole for 16 hr.
Micronucleation
Asynchronously growing cells were fixed and examined for the presence of micronuclei after DAPI staining for DNA. Data were obtained from three independent experiments with >100 nuclei examined in each.
C-circle assay
For the C-circle assay, DNA from cell lines was isolated using the DNeasy Blood and Tissue Qiagen kit, digested with HinfI and RsaI, and quantified using Hoechst fluorimetric analysis. DNAs were diluted to approximately 3 ng/µl, quantified again, and the dilutions were adjusted to exactly 3 ng/µl. The assay was performed using 30 ng of quantified DNA as described by Henson et al. [8] using dot-blotting with an end-labeled [AACCCT]4 telomeric oligonucleotide probe. C-circle values in Table 1 were derived from three independent DNA preparations for each cell line.
DSB repair assays
Cells grown on glass coverslips were irradiated with 0.5 Gy IR and incubated at 37°C for 1 hr or 24 hr. Irradiated and non-irradiated cells were fixed with 3% paraformaldehyde for 10 min at room temperature, washed three times with PBS, and permeabilized with 0.5% Triton X-100 for 10 min on ice. Cells were then washed four times with PBS and blocked for 30 min at room temperature with PBG (0.2% cold water fish gelatin (Sigma), 0.5% BSA, in PBS). Primary 53BP1 (Novus) antibody was diluted in PBG and incubated on cells for 1 hr at 37°C. Following three washes with PBG, cells were incubated with FITC-conjugated donkey anti-rabbit secondary antibodies (Life Technologies), diluted in PBG for 30 min at 37°C. Cells were washed three times with PBS and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). The percentage of cells containing >10 53BP1 foci per nucleus was calculated from three independent experiments.
For the PFGE assay, subconfluent cells were irradiated with 100 Gy IR and incubated at 37°C for 24 hr. Irradiated and non-irradiated cells were washed once with PBS, trypsinized, and counted. One million cells per sample (in duplicate) were centrifuged and washed a second time with PBS. Cell pellets were resuspended in 50 µl 10 mM Tris pH 7.2, 20 mM NaCl, 50 mM EDTA and pre-warmed at 55°C for 10 min. The cell suspension was then mixed with 50 µl of 2% agarose/PBS at 55°C and solidified in plastic molds (BioRad). Agarose plugs were incubated with 1 mg/ml Proteinase K, 0.2% sodium deoxycholate, 1% N-lauroylsarcosine-sodium salt, 100 mM EDTA, pH 8.0 for 20 hr at 50°C and then washed four times for 1 hr with 20 mM Tris/HCl, 50 mM EDTA, pH 8.0. Duplicate plugs were irradiated with 600 Gy IR, and all samples were equilibrated in 0.5× TBE for 30 min. Agarose plugs were inserted into the wells of a 1% agarose gel and sealed with 1% agarose. PFGE was carried out using a CHEF-II apparatus in 0.5× TBE buffer at 6 V/cm with pulse times of 70 sec (15 hr) and 120 sec (11 hr). Following electrophoresis, the gel was stained with 0.5 µg/ml ethidium bromide in TBE for 30 min.
Supporting Information
Zdroje
1. BryanTMEnglezouADalla-PozzaLDunhamMAReddelRR 1997 Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med 3 1271 1274
2. DunhamMANeumannAAFaschingCLReddelRR 2000 Telomere maintenance by recombination in human cells. Nat Genet 26 447 450
3. BechterOEShayJWWrightWE 2004 The Frequency of Homologous Recombination in Human ALT Cells. Cell Cycle 3 457 549
4. Londono-VallejoJADer-SarkissianHCazesLBacchettiSReddelRR 2004 Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res 64 2324 2327
5. CesareAJGriffithJD 2004 Telomeric DNA in ALT cells is characterized by free telomeric circles and heterogeneous t-loops. Mol Cell Biol 24 9948 9957
6. WangRCSmogorzewskaAde LangeT 2004 Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119 355 368
7. TokutakeYMatsumotoTWatanabeTMaedaSTaharaH 1998 Extra-chromosomal telomere repeat DNA in telomerase-negative immortalized cell lines. Biochem Biophys Res Commun 247 765 772
8. HensonJDCaoYHuschtschaLIChangACAuAY 2009 DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol 27 1181 1185
9. McEachernMJHaberJE 2005 Telomerase-independent telomere maintenance in yeast. de LangeTLundbladVBlackburnE Telomeres Cold Spring Harbor, New York Cold Spring Harbor Laboratory Press 199 224
10. HensonJDNeumannAAYeagerTRReddelRR 2002 Alternative lengthening of telomeres in mammalian cells. Oncogene 21 598 610
11. YeagerTRNeumannAAEnglezouAHuschtschaLINobleJR 1999 Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res 59 4175 4179
12. JiangWQZhongZHHensonJDNeumannAAChangAC 2005 Suppression of alternative lengthening of telomeres by Sp100-mediated sequestration of the MRE11/RAD50/NBS1 complex. Mol Cell Biol 25 2708 2721
13. ComptonSAChoiJHCesareAJOzgurSGriffithJD 2007 Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Cancer Res 67 1513 1519
14. PottsPRYuH 2007 The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol 14 581 590
15. ZengSXiangTPanditaTKGonzalez-SuarezIGonzaloS 2009 Telomere recombination requires the MUS81 endonuclease. Nat Cell Biol 11 616 623
16. HeaphyCMSubhawongAPHongSMGogginsMGMontgomeryEA 2011 Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol 179 1608 1615
17. HensonJDReddelRR 2010 Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett 584 3800 3811
18. HensonJDHannayJAMcCarthySWRoydsJAYeagerTR 2005 A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer Res 11 217 225
19. SfeirAKabirSvan OverbeekMCelliGBde LangeT 2010 Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science 327 1657 1661
20. KibeTOsawaGAKeeganCEde LangeT 2010 Telomere protection by TPP1 Is mediated by POT1a and POT1b. Mol Cell Biol 30 1059 1066
21. RaiRZhengHHeHLuoYMultaniA 2010 The function of classical and alternative non-homologous end-joining pathways in the fusion of dysfunctional telomeres. EMBO J 29 2598 2610
22. WangYGhoshGHendricksonEA 2009 Ku86 represses lethal telomere deletion events in human somatic cells. Proc Natl Acad Sci U S A 106 12430 12435
23. CelliGBLazzerini DenchiEde LangeT 2006 Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat Cell Biol 8 885 890
24. HeaphyCMde WildeRFJiaoYKleinAPEdilBH 2011 Altered telomeres in tumors with ATRX and DAXX mutations. Science 333 425
25. JiaoYShiCEdilBHde WildeRFKlimstraDS 2011 DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331 1199 1203
26. SchwartzentruberJKorshunovALiuXYJonesDTPfaffE 2012 Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482 226 231
27. DranePOuararhniKDepauxAShuaibMHamicheA 2010 The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev 24 1253 1265
28. LawMJLowerKMVoonHPHughesJRGarrickD 2010 ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell 143 367 378
29. WongLHMcGhieJDSimMAndersonMAAhnS 2010 ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res 20 351 360
30. LewisPWElsaesserSJNohKMStadlerSCAllisCD 2010 Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A 107 14075 14080
31. GoldbergADBanaszynskiLANohKMLewisPWElsaesserSJ 2010 Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140 678 691
32. AzzalinCMReichenbachPKhoriauliLGiulottoELingnerJ 2007 Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318 798 801
33. BaumannCViveirosMMDe La FuenteR 2010 Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet 6 e1001137 doi:10.1371/journal.pgen.1001137
34. De La FuenteRViveirosMMWigglesworthKEppigJJ 2004 ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev Biol 272 1 14
35. RitchieKSeahCMoulinJIsaacCDickF 2008 Loss of ATRX leads to chromosome cohesion and congression defects. J Cell Biol 180 315 324
36. IshovAMVladimirovaOVMaulGG 2004 Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J Cell Sci 117 3807 3820
37. XueYGibbonsRYanZYangDMcDowellTL 2003 The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci U S A 100 10635 10640
38. NgLJCropleyJEPickettHAReddelRRSuterCM 2009 Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res 37 1152 1159
39. BeroukhimRMermelCHPorterDWeiGRaychaudhuriS 2010 The landscape of somatic copy-number alteration across human cancers. Nature 463 899 905
40. DavoliTDenchiELde LangeT 2010 Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 141 81 93
41. ArtandiSEChangSLeeSLAlsonSGottliebGJ 2000 Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406 641 645
42. ScheelCSchaeferKLJauchAKellerMWaiD 2001 Alternative lengthening of telomeres is associated with chromosomal instability in osteosarcomas. Oncogene 20 3835 3844
43. CounterCMAvilionAALeFeuvreCEStewartNGGreiderCW 1992 Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. Embo J 11 1921 1929
44. ChinKDe SolorzanoCOKnowlesDJonesAChouW 2004 In situ analyses of genome instability in breast cancer. Nat Genet 36 984 988
45. CrastaKGanemNJDagherRLantermannABIvanovaEV 2012 DNA breaks and chromosome pulverization from errors in mitosis. Nature 482 53 58
46. BakouKStephanouGAndrianopoulosCDemopoulosNA 2002 Spontaneous and spindle poison-induced micronuclei and chromosome non-disjunction in cytokinesis-blocked lymphocytes from two age groups of women. Mutagenesis 17 233 239
47. StrackerTHWilliamsBRDerianoLTheunissenJWAdelmanCA 2009 Artemis and nonhomologous end joining-independent influence of DNA-dependent protein kinase catalytic subunit on chromosome stability. Mol Cell Biol 29 503 514
48. Lu-HesselmannJAbendMvan BeuningenD 2004 Comparison of endogenous TP53 genomic status with clonogenicity and different modes of cell death after X irradiation. Radiat Res 161 39 47
49. BrownEJBaltimoreD 2003 Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev 17 615 628
50. KimSTXuBKastanMB 2002 Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev 16 560 570
51. AbreuEAritonovskaEReichenbachPCristofariGCulpB 2010 TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol 30 2971 2982
52. CesareAJKaulZCohenSBNapierCEPickettHA 2009 Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol 16 1244 1251
53. TurnerNTuttAAshworthA 2005 Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol 5 388 393
54. BucherNBrittenCD 2008 G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 98 523 528
55. JacksonSPBartekJ 2009 The DNA-damage response in human biology and disease. Nature 461 1071 1078
56. TakaiKKHooperSBlackwoodSGandhiRde LangeT 2010 In vivo stoichiometry of shelterin components. J Biol Chem 285 1457 1467
57. SjoblomTJonesSWoodLDParsonsDWLinJ 2006 The consensus coding sequences of human breast and colorectal cancers. Science 314 268 274
58. Cancer Genome Atlas Research Network 2008 Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455 1061 1068
59. MermelCHSchumacherSEHillBMeyersonMLBeroukhimR 2011 GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol 12 R41
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



