-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evidence of Inbreeding Depression on Human Height
Stature is a classical and highly heritable complex trait, with 80%–90% of variation explained by genetic factors. In recent years, genome-wide association studies (GWAS) have successfully identified many common additive variants influencing human height; however, little attention has been given to the potential role of recessive genetic effects. Here, we investigated genome-wide recessive effects by an analysis of inbreeding depression on adult height in over 35,000 people from 21 different population samples. We found a highly significant inverse association between height and genome-wide homozygosity, equivalent to a height reduction of up to 3 cm in the offspring of first cousins compared with the offspring of unrelated individuals, an effect which remained after controlling for the effects of socio-economic status, an important confounder (χ2 = 83.89, df = 1; p = 5.2×10−20). There was, however, a high degree of heterogeneity among populations: whereas the direction of the effect was consistent across most population samples, the effect size differed significantly among populations. It is likely that this reflects true biological heterogeneity: whether or not an effect can be observed will depend on both the variance in homozygosity in the population and the chance inheritance of individual recessive genotypes. These results predict that multiple, rare, recessive variants influence human height. Although this exploratory work focuses on height alone, the methodology developed is generally applicable to heritable quantitative traits (QT), paving the way for an investigation into inbreeding effects, and therefore genetic architecture, on a range of QT of biomedical importance.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002655
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002655Summary
Stature is a classical and highly heritable complex trait, with 80%–90% of variation explained by genetic factors. In recent years, genome-wide association studies (GWAS) have successfully identified many common additive variants influencing human height; however, little attention has been given to the potential role of recessive genetic effects. Here, we investigated genome-wide recessive effects by an analysis of inbreeding depression on adult height in over 35,000 people from 21 different population samples. We found a highly significant inverse association between height and genome-wide homozygosity, equivalent to a height reduction of up to 3 cm in the offspring of first cousins compared with the offspring of unrelated individuals, an effect which remained after controlling for the effects of socio-economic status, an important confounder (χ2 = 83.89, df = 1; p = 5.2×10−20). There was, however, a high degree of heterogeneity among populations: whereas the direction of the effect was consistent across most population samples, the effect size differed significantly among populations. It is likely that this reflects true biological heterogeneity: whether or not an effect can be observed will depend on both the variance in homozygosity in the population and the chance inheritance of individual recessive genotypes. These results predict that multiple, rare, recessive variants influence human height. Although this exploratory work focuses on height alone, the methodology developed is generally applicable to heritable quantitative traits (QT), paving the way for an investigation into inbreeding effects, and therefore genetic architecture, on a range of QT of biomedical importance.
Introduction
Height is a classic complex trait, which is influenced by both genetic and non-genetic factors. Observed increases in height in developed countries over the last few generations suggest that environmental factors such as nutrition and childhood healthcare play an important role in determining adult height [1], [2]. Within any one population at one point in time, 80–90% of the variation in height is explained by genetic factors [3], [4], [5], [6], [7], [8]. These characteristics, plus the fact that height is cheaply and accurately measurable and has been assessed in many thousands of study subjects, make it an attractive model for investigating the genetic architecture of quantitative traits generally [9], . Height is not merely of interest as a model quantitative trait (QT): a better understanding of the genetic mechanisms influencing height offers insights into genetic variants influencing growth and development [11]. Because height is associated with a range of complex diseases, including cancer, [12], [13], [14], [15] and because pleiotropic effects have been observed between disease-associated and height-associated genetic variants [16], [17], [18], a better understanding of the genetic mechanisms influencing height may also provide biological insights into disease mechanisms.
In a seminal work published almost a century ago, Fisher first proposed that the heritability of height results from the combined effects of many genetic variants of individually small effect size [19]. In recent years, the advent of genome-wide association studies (GWAS) has enabled this theory to be tested empirically. A GWAS of over 180,000 individuals conducted by the GIANT (Genome-wide Investigation of Anthropometric Measures) consortium found common genetic variants at more than 180 loci influencing human height [20]. Despite the undoubted success of GWAS, even this very large study discovered variants explaining in total only around 10% of phenotypic variation [20]. This “missing heritability” [21] has become an important subject of debate in genetic epidemiology because of the implications it has for future gene discovery strategies and indirectly on attempts to predict phenotype from genotype. Yang and colleagues proposed a different approach to identifying this missing heritability [22]. Instead of using GWAS to identify individual genome-wide significant SNPs associated with stature, they considered all SNPs simultaneously, allowing the entire GWAS data to be used as predictors. Using this approach, they explained up to 40% of the variance in height. This still leaves ∼40% of variance unexplained by common genetic variants. The authors of the large GIANT study cited above predict that increased GWAS sample sizes will identify more common variants of moderate-to-small effect size and will increase the proportion of heritable variation explained merely to around 20% [20], [22]). Therefore, alternative strategies are required in order to detect rarer variants, structural variants, variants of very small effect size, and interactions, including dominance and epistasis [21].
This study explores whether there is evidence for genome-wide recessive genetic effects, or inbreeding depression, on height. Inbreeding depression implies directional dominance: i.e. that dominance is on average in the same direction across loci. An association between height and genome-wide homozygosity would imply that height was influenced by the combined effects of many recessive variants of individually small effect size, scattered across the genome. On the face of it, this endeavour looks unpromising. Most pedigree and GWAS studies investigating the genetic architecture of height to date have found no strong evidence of deviation from an additive genetic model [23]. Three heritability studies have found little evidence for dominance variance [24], [25], [26]. Absence of evidence for dominance variance need not, however, be inconsistent with evidence of inbreeding depression: it can be shown that, assuming a large number of contributing loci, it is theoretically possible to have inbreeding depression in the absence of detectable dominance variance [27]. Dominance variance may be difficult to estimate in study designs where genome-wide additive and dominance coefficients are highly correlated [26]. Independently of GWAS, epidemiologists have long observed associations between parental relatedness and reduced height [28], [29], [30], [31], although not all studies have found such an association [32], [33]. A recent small study of the isolated Norfolk Island population found an association between reduced height and both parental relatedness (estimated from genealogical data) and genome-wide homozygosity (estimated from microsatellite markers) [34]. Finally, whilst many twin studies have concluded that height is purely additive, an extended twin family design using large numbers (n = 29,691) revealed a non-additive genetic component of 9.4% which was balanced by extra additive variance due to assortative mating (confounded with shared environment in twin studies). As assortative mating increases the correlation in dizygotic twins above half that in monozygotic twins, whereas dominance does the opposite, they appear to cancel each other out, so height looks perfectly additive from twins alone [35].
The aim of this study was to explore the association between genome-wide homozygosity and adult height, controlling for the effects of potential confounding factors. The study involved over 35,000 subjects, drawn from 21 population samples. We invited studies to participate in the consortium which we knew were conducted in isolated populations, where both the mean and variance in genome-wide homozygosity are higher. In this way, we optimised our chances of being able to detect an effect, should one exist. We found highly significant evidence of an inverse association between genome-wide homozygosity and height, with significant heterogeneity among sample sets.
Results
We explored the association between genome-wide homozygosity and height in 21 European or European-heritage populations (Table 1). All samples were genotyped using the Illumina platform (see Materials and Methods and Supporting Information). Because different Illumina platforms were used by different studies, we extracted the SNPs present in the Illumina HumanHap 300 panel (common to all the Illumina platforms used). The number of SNPs remaining after quality control procedures had been run on a population-by-population basis are given in Table 1, as are details of the mean age and height of the samples and the proportion of women in each sample.
Tab. 1. Sample details. 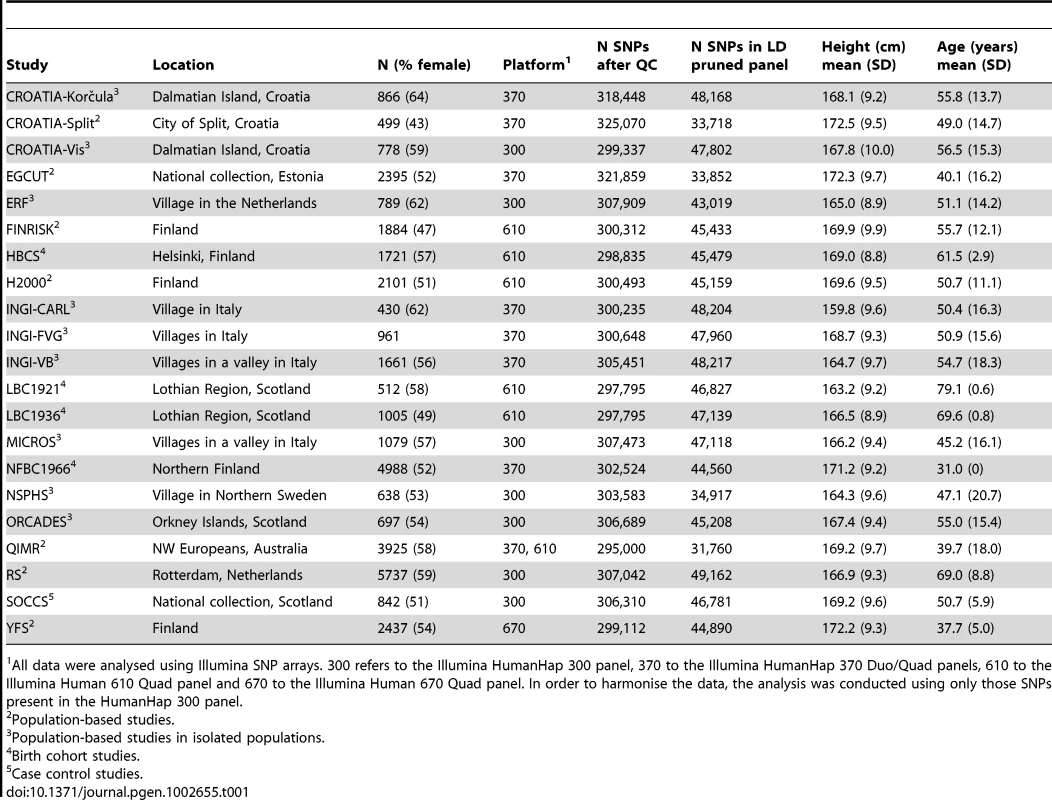
All data were analysed using Illumina SNP arrays. 300 refers to the Illumina HumanHap 300 panel, 370 to the Illumina HumanHap 370 Duo/Quad panels, 610 to the Illumina Human 610 Quad panel and 670 to the Illumina Human 670 Quad panel. In order to harmonise the data, the analysis was conducted using only those SNPs present in the HumanHap 300 panel. We used three different measures of genome-wide homozygosity. FROH is defined as the percentage of the typed autosomal genome in runs of homozygosity (ROH) greater than or equal to 1.5 Mb in length. FROH is strongly correlated with the degree of relatedness between an individual's parents [36]. FROHLD is a modification of FROH, derived using a panel of independent SNPs, where all SNPs in strong linkage disequilibrium (LD) have been removed. This is a more stringent estimate of parental relatedness: removing SNPs that are in strong LD with other SNPs means that all ROH detected are likely to be the result of recent parental relatedness and not ancient patterns of shared ancestry. The third measure we used was observed homozygosity (Fhom). This is defined as the number of observed homozygous genotypes per individual, expressed as a percentage of the number of non-missing genotypes for that individual. This is a much less precise estimate of parental relatedness, as Fhom is a single-point measure which captures all genotyped homozygous loci, not just those located in long ROH. Thus it reflects not only recent parental relatedness but also more ancient aspects of population history, such as population isolation and bottlenecks.
Figure 1 shows the sample means, with 95% confidence intervals, of these three measures of genome-wide homozygosity. Whereas in general the three measures were strongly correlated, differences were observed, particularly between FROHLD and Fhom. For example, the Estonian sample (Estonian Genome Centre of University of Tartu [EGCUT]) had the second highest mean value for Fhom, but it had one of the lowest mean values for FROHLD. For all three measures of genome-wide homozygosity there is a continuum of values. The isolate populations are generally located at the more homozygous end of the spectrum, but with considerable variation amongst the different sample sets. For example, there is almost a three-fold difference in mean FROHLD between the Northern Sweden Population Health Study (NSPHS) and ORCADES. The Finnish sample sets and some others (for example, CROATIA-Split and EGCUT) have intermediate levels of homozygosity, whilst the urban and national collections from Scotland, the Netherlands and Australia are the least homozygous. There was more than an order of magnitude difference in mean FROHLD between the most and the least homozygous population samples.
Fig. 1. Three alternative measures of mean homozygosity, with 95% confidence intervals, by population sample. 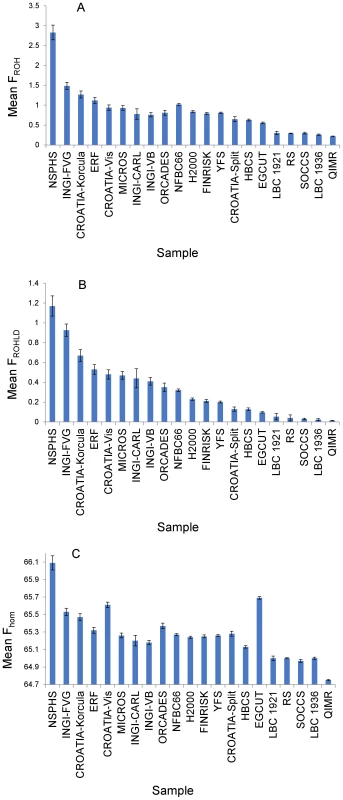
(A) shows mean FROH by population sample. FROH is defined as the percentage of the genotyped autosomal genome in ROH measuring at least 1.5 Mb. Mean values of FROH per population (with 95% confidence intervals) are: CROATIA-Korčula = 1.27 (1.18, 1.36); CROATIA-Split = 0.65 (0.59, 0.71); CROATIA-Vis = 0.94 (0.87,1.01); EGCUT = 0.56 (0.54, 0.58); ERF = 1.12 (1.04, 1.20); FINRISK = 0.79 (0.77, 0.82); HBCS = 0.63 (0.60, 0.65); H2000 = 0.84 (0.82, 0.86); INGI-CARL = 0.78 (0.65, 0.91); INGI-FVG = 1.49 (1.40, 1.58); INGI-VB = 0.76 (0.71, 0.81); LBC1921 = 0.30 (0.25, 0.35); LBC1936 = 0.26 (0.24, 0.28); MICROS = 0.93 (0.87, 0.99); NFBC1966 = 1.02 (1.00, 1.04); NSPHS = 2.83 (2.64, 3.02); ORCADES = 0.81 (0.75, 0.87); QIMR = 0.22 (0.21, 0.23); RS = 0.29 (0.28, 0.30); SOCCS = 0.30 (0.28, 0.32); YFS = 0.81 (0.79, 0.83). (B) shows mean FROHLD by population sample. FROHLD is defined as the percentage of the genotyped autosomal genome in ROH measuring at least 1.0 Mb, derived from a panel of independent SNPs. Mean values of FROHLD per population (with 95% confidence intervals) are: CROATIA-Korčula = 0.67 (0.61, 0.73); CROATIA-Split = 0.13 (0.11, 0.15); CROATIA-Vis = 0.48 (0.43, 0.53); EGCUT = 0.10 (0.09, 0.10); ERF = 0.53 (0.48, 0.58); FINRISK = 0.21 (0.20, 0.23); HBCS = 0.13 (0.11, 0.14); H2000 = 0.23 (0.22, 0.24); INGI-CARL = 0.44 (0.34, 0.54); INGI-FVG = 0.93 (0.86, 0.99); INGI-VB = 0.41 (037, 0.45); LBC1921 = 0.05 (0.02, 0.09); LBC1936 = 0.02 (0.01, 0.03); MICROS = 0.47 (0.43, 0.51); NFBC1966 = 0.32 (0.31, 0.33); NSPHS = 1.17 (1.07, 1.27); ORCADES = 0.35 (0.31, 0.39); QIMR = 0.013 (0.011, 0.015); RS = 0.04 (0.01, 0.07); SOCCS = 0.03 (0.02, 0.04); YFS = 0.20 (0.19, 0.21). (C) shows mean Fhom by population sample. Fhom is defined as the percentage of genotyped autosomal SNPs that are homozygous. Mean values of Fhom per population (with 95% confidence intervals) are: CROATIA-Korčula = 65.47 (65.43, 65.51); CROATIA-Split = 65.28 (65.25, 65.31); CROATIA-Vis = 65.61 (65.58, 65.64); EGCUT = 65.69 (65.68, 65.70); ERF = 65.32 (65.29, 65.35); FINRISK = 65.25 (65.23, 65.27); HBCS = 65.13 (65.12, 65.14); H2000 = 65.24 (65.23, 65.25); INGI-CARL = 65.20 (65.14, 65.26); INGI-FVG = 65.53 (65.49, 65.57); INGI-VB = 65.18 (65.16, 65.20); LBC1921 = 65.00 (64.97, 65.03); LBC1936 = 65.00 (64.99, 65.01); MICROS = 65.26 (65.23, 65.29); NFBC1966 = 65.27 (65.26, 65.28); NSPHS = 66.09 (66.01, 66.17); ORCADES = 65.37 (65.34, 65.40); QIMR = 64.75 (64.74, 64.76); RS = 65.00 (64.99, 65.01); SOCCS = 64.97 (64.95, 64.99); YFS = 65.26 (65.25, 65.27). The purpose of the first part of the analysis was to explore the association between height and homozygosity, as measured in different ways. First, we estimated the association between height and FROH, adjusting for age, sex and (in sample sets including related individuals) genomic kinship (Table 2, Figure S1). We found evidence for a small but strongly significant (p = 1.23×10−11) inverse association between FROH and height. This association was significant in nine of the twenty-one sample sets in the study. In nine further sample sets, confidence intervals overlapped with zero but the direction of the effect was consistent with an inverse association between FROH and height. In none of the sample sets was there a significant positive association between FROH and height. An increase of 1% in FROH was associated with a decrease of 0.012 (SE = 0.0018) in the z-score for height (approximately 0.09 cm). Using pedigree and FROH data from three separate population samples, we estimated that this is equivalent to a reduction in height of 0.7 cm in the offspring of first cousins, compared with the offspring of unrelated individuals (based on FROH differences of 6.6, 7.4 and 7.4 in the offspring of first cousins compared with the offspring of unrelated individuals in the Micro-Isolates in South Tyrol (MICROS), ORCADES and Irish data sets respectively – see Materials and Methods).
Tab. 2. Meta-analysis of the association between height and genome-wide homozygosity, adjusted for age and sex only. 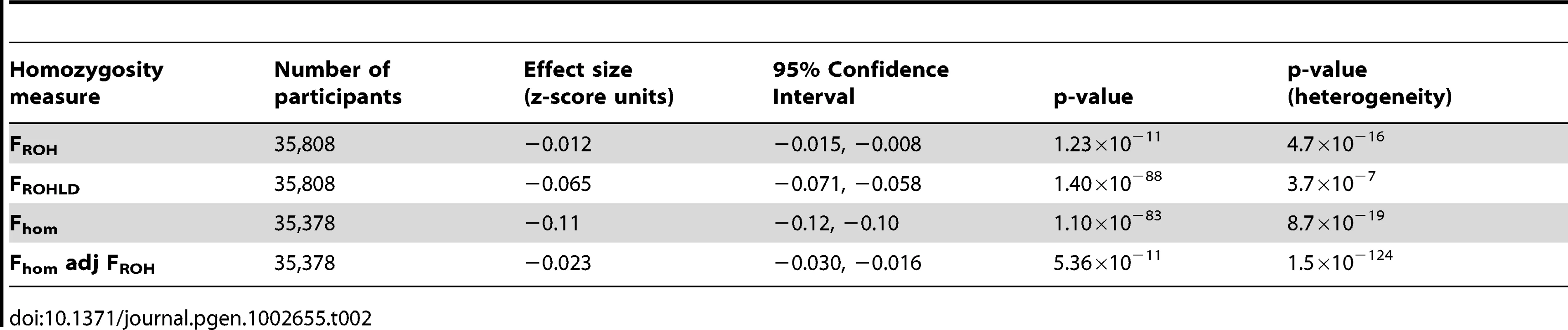
The second analysis estimated the association between height and FROHLD, adjusted for age, sex and genomic kinship. Again, there was evidence of a very strongly significant inverse association (p = 1.40×10−88) between FROHLD and height (Table 2, Figure 2). This association was significant in seven of the twenty-one sample sets in this study. In eleven further sample sets, confidence intervals overlapped with zero but the direction of the effect was consistent with an inverse association between FROHLD and height. In none of the sample sets was there a significant effect in the other direction. A 1% increase in FROHLD was associated with a decrease of 0.065 (SE = 0.0032) in the z-score for height (approximately 0.6 cm). Again using pedigree and FROHLD data from three separate population samples, this gave a much higher estimate of a reduction in height of between 2.8 and 3.3 cm in the offspring of first cousins compared with the offspring of unrelated parents (based on FROHLD differences of 2.8, 3.3 and 2.9 in the offspring of first cousins compared with the offspring of unrelated individuals in the MICROS, ORCADES and Irish data sets respectively).
Fig. 2. Forest plot of the effect of FROHLD on height. 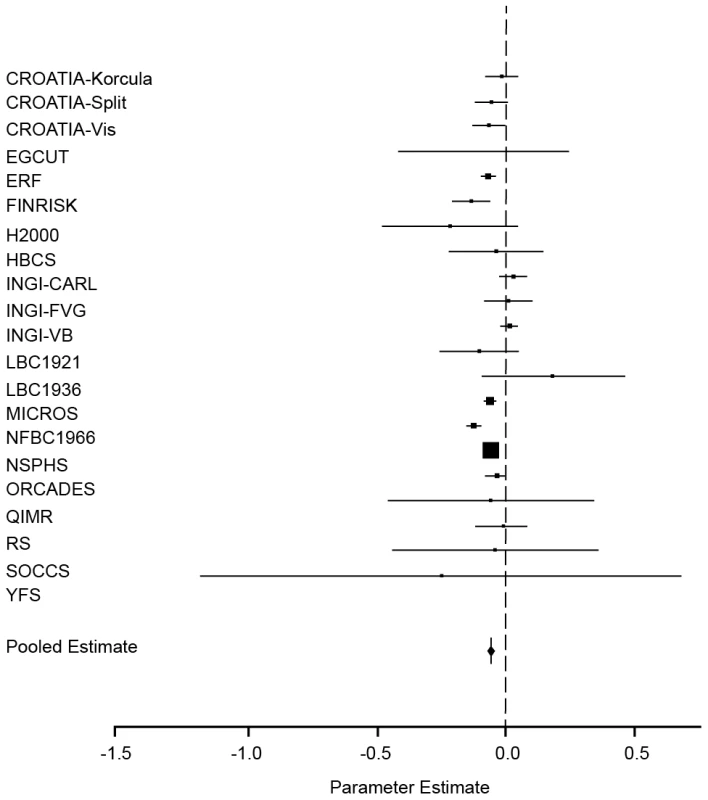
Results of a meta-analysis of the association between FROHLD and height are shown for twenty-one population samples. The model was adjusted for age and sex in all samples. Additionally, it was adjusted for genomic kinship in samples with pairs of related individuals (CROATIA-Korčula, CROATIA-Split, CROATIA-Vis, ERF, FINRISK, HBCS, H2000, INGI-CARL, INGI-FVG, INGI-VB, MICROS, NFBC1966, NSPHS, ORCADES and YFS). The plot shows estimated effect sizes (solid squares) for each population, with 95% confidence intervals (horizontal lines). Each sample estimate is weighted by the inverse of the squared standard error of the regression coefficient, so that the smaller the standard error of the study, the greater the contribution it makes to the pooled regression coefficient. The area of the solid squares is proportional to the weighting given to each study in the meta-analysis. Effect sizes in z-score units (with 95% confidence intervals) are: CROATIA-Korčula = −0.02 (−0.09, 0.04); CROATIA-Split = −0.06 (−0.1, −0.002); CROATIA-Vis = −0.07 (−0.1, −0.01); EGCUT = −0.09 (−0.04, 0.2); ERF = −0.08 (−0.1, −0.05); FINRISK = −0.1 (−0.2, −0.07); HBCS = −0.04 (−0.2, 0.1); H2000 = −0.2 (−0.5, 0.04); INGI-CARL = 0.02 (−0.03, 0.07); INGI-FVG = −0.0001 (−0.08, 0.08); INGI-VB = 0.005 (−0.03, 0.04); LBC1921 = −0.1 (−0.3, 0.04); LBC1936 = 0.2 (−0.1, 0.4); MICROS = −0.06 (−0.08, −0.05); NFBC1966 = −0.1 (−0.2, −0.1); NSPHS = −0.07 (−0.07, −0.06); ORCADES = −0.04 (−0.08, 0.001); QIMR = −0.07 (−0.5, 0.3); RS = −0.02 (−0.1, 0.08); SOCCS = −0.05 (−0.4, 0.3); YFS = −0.3 (−1.2, 0.7). The third analysis estimated the association between height and Fhom, adjusting for age and sex (Figure S2). Again, there was evidence of a very strongly significant inverse association between Fhom and height (p = 1.10×10−83). The direction of effect was consistent for fourteen sample sets, significantly so for seven of these, and not significantly different from zero but of opposite sign in the final seven studies. A 1% increase in Fhom was associated with a decrease of 0.11 (SE = 0.0057) in the z-score for height (approximately 1 cm). Again using pedigree and Fhom data from three separate population samples, this gave an estimate of a reduction in height of between 2.7 and 3.3 cm in the offspring of first cousins compared with the offspring of unrelated people, identical to the estimate obtained using FROHLD (based on Fhom differences of 2.7, 3.3 and 2.7 in the offspring of first cousins compared with the offspring of unrelated individuals in the MICROS, ORCADES and Irish data sets respectively).
We explored whether the signal observed in the Fhom analysis was driven by homozygous genotypes located in long ROH, or from the more common, homozygous genotypes resulting from the chance inheritance of identical shorter haplotypes from both parents. This analysis estimated the association between height and Fhom, adjusted for age, sex and FROH. Again, a significant association was observed, but both the magnitude and the significance of the effect were reduced compared to the previous analysis (Table 2), suggesting that most, but not all, of the signal was coming from long ROH.
Although these results were highly significant, there was also a high degree of heterogeneity across population samples. Some further analyses were performed to explore the source of this heterogeneity. Three of the twenty-one study samples (Carlantino [INGI-CARL], Lothian Birth Cohort 1936 [LBC1936] and Val Borbera [INGI-VB]) consistently showed a (non-significant) positive association between genome-wide homozygosity and height. In the LBC1936 and INGI-VB cohorts, the parameter estimate was positive for all three measures. In INGI-CARL, the parameter estimate was positive for FROH and FROHLD; however, the maximum likelihood method used to find the parameter estimate failed to converge for the Fhom analysis. Excluding these three cohorts from the FROHLD meta-analysis reduced heterogeneity considerably, whilst not eliminating it completely (p-value for heterogeneity = 0.01).
Removing these cohorts only slightly reduced heterogeneity in the Fhom (p-value for heterogeneity = 6.6×10−16) and FROH meta-analyses (p-value for heterogeneity = 1.3×10−16). For both these measures, other outliers also contributed to the heterogeneity. In the case of FROH the Rotterdam Study (RS) showed a non-significant positive association with height. Four additional cohorts showed a non-significant positive association between Fhom and height (EGCUT, CROATIA-Korčula, Queensland Institute of Medical Research [QIMR] and RS).
To summarise, these results provide evidence of a highly significant inverse association between genome-wide homozygosity and height, regardless of which homozygosity estimate was used. The weakest result was for FROH. The effect estimate for this analysis was lower than those for the other 2 homozygosity measures. The most heterogeneous result was for Fhom. The Fhom analysis was similar to FROHLD in terms of effect size and significance; however, when FROH was included in the Fhom model, although the association remained significant, the effect size fell, the p-value increased and heterogeneity increased. This suggests that the effect was being driven mainly by longer ROH which are more effectively captured by FROHLD. It is important not to overstate this, however: even after controlling for FROH, there is a significant, although highly heterogeneous inverse association between Fhom and height, which suggests that a signal is also coming from homozygous genotypes that are not found in the long ROH characteristic of parental relatedness (Table 2). Furthermore, no correlation was observed between sample mean FROHLD and effect size (r = 0.03). Correlation between these two measures would be expected if the observed effect was entirely attributable to parental relatedness of recent origin. Nevertheless, the most significant and least heterogeneous result was seen with FROHLD. Furthermore, a moderate negative correlation was observed between average FROHLD and the standard error of the effect estimate (r = −0.4), suggesting that the higher the level of parental relatedness present in the sample, the greater the precision of the effect estimate. This is because mean FROHLD is related to its standard deviation (higher mean, higher variance) and it is the variance in FROHLD that determines the standard error of the estimate of the regression coefficient (i.e. higher variance, lower standard error). For these reasons, it was decided to use FROHLD in further analyses to explore possible confounding factors.
All analyses were adjusted for age but, because the mean age of most of the population samples in this study was over 50 years at the time of genotyping, it was important to undertake additional checks to ensure that the observed effect was not confounded by the effects of osteoporotic, age-related shrinking. We used the Northern Finland Birth Cohort 1966 (NFBC1966), where all subjects were under 40 at the time of measurement. In this cohort, there was a significant inverse association (p = 0.002) between FROHLD and height, with a 1% increase in FROHLD associated with a decrease of 0.13 in the z-score for height (95% confidence interval −0.16, −0.10). This is equivalent to a reduction in height of 5.3 cm (95% confidence interval −4.1, −6.6) in the offspring of first cousins compared with the offspring of unrelated parents, a stronger effect than observed in the meta-analysis of the full sample. We also repeated the FROHLD analysis for a subset of individuals aged under 40 years of age (15 cohorts, n = 9909) and the relationship remained significant, although the effect size was much smaller (1% increase in FROHLD associated with a decrease of 0.009 in the z-score for height (95% confidence interval −0.013, −0.0049; p = 2.15×10−5). This is equivalent to a reduction in height of 0.4 cm (95% confidence interval −0.2, −0.5) in the offspring of first cousins compared with the offspring of unrelated parents.
The final stage in this analysis was to investigate possible confounding by socio-economic status (SES) of the observed association between genome-wide homozygosity and reduced height. Four of the 21 cohorts (Erasmus Rucphen Family Study [ERF], MICROS, NSPHS and QIMR) did not collect data on SES and so were excluded from further analyses. SOCCS estimated SES using a composite measure of deprivation based on residential address; however, because this was an area - rather than an individual-level estimate and because only one other cohort (ORCADES) used this measure, SOCCS was also excluded from analyses of SES. Eleven cohorts recorded an ordinal measure of educational attainment (CROATIA-Korčula, CROATIA-Split, CROATIA-Vis, EGCUT, the National FINRISK Study [FINRISK], the Health2000 Survey [H2000], FVG-Genetic Park [INGI-FVG], INGI-VB, NFBC1966, ORCADES and RS). Seven cohorts provided an ordinal measure of occupational status (EGCUT, Helsinki Birth Cohort Study [HBCS], INGI-CARL, INGI-FVG, INGI-VB, the Lothian Birth Cohort 1921 [LBC1921], LBC1936 and the Young Finns Study [YFS]); however, the maximum likelihood method used to find the parameter estimate failed to converge for INGI-FVG so this cohort was excluded from the occupational status analysis. We conducted four meta-analyses to investigate whether educational attainment or occupational status confounded the association between genome-wide homozygosity (as measured by FROHLD) and height. First, we analysed the eleven cohorts with educational attainment data available. Two meta-analyses were performed, one adjusting for age, sex, genomic kinship and FROHLD only and one adjusting for age, sex, genomic kinship, FROHLD and educational attainment. Results were then compared to assess possible confounding by educational attainment. This process was then repeated for the seven cohorts with data available on occupational status. Results are summarised in Table 3. A forest plot illustrating the results of the educational attainment meta-analyses is shown in Figure 3.
Fig. 3. Forest plot of the effect of FROHLD on height, adjusted for educational attainment. 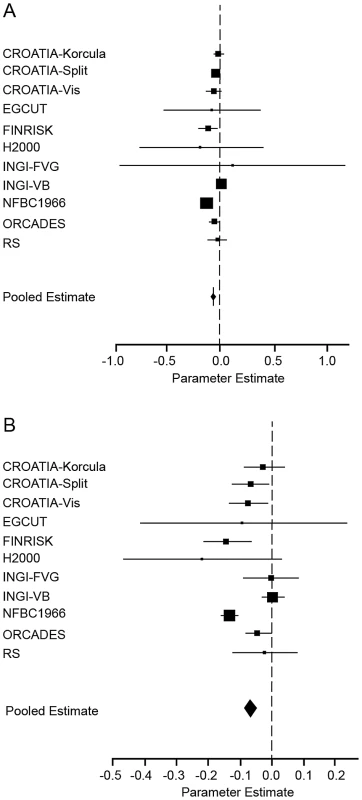
Results of a meta-analysis of the association between FROHLD and height are shown for the eleven population samples which collected data on educational attainment. (A) shows the model adjusted for age, sex and educational attainment in all samples and additionally for genomic kinship in samples with pairs of related individuals (CROATIA-Korčula, CROATIA-Split, CROATIA-Vis, FINRISK, H2000, INGI-FVG, INGI-VB NFBC1966 and ORCADES). Effect sizes in z-score units (with 95% confidence intervals) are: CROATIA-Korčula = −0.02 (−0.07, 0.04); CROATIA-Split = −0.05 (−0.08, −0.01); CROATIA-Vis = −0.06 (−0.1, 0.02); EGCUT = −0.08 (−0.5, 0.4); FINRISK = −0.1 (−0.2, −0.03); H2000 = −0.2 (−0.8, 0.4); INGI-FVG = 0.1 (−1.0, 1.2); INGI-VB = 0.009 (−0.02, 0.04); NFBC1966 = −0.1 (−0.2, −0.1); ORCADES = −0.06 (−0.1, −0.007); RS = −0.02 (−0.1, 0.08). (B) shows the model adjusted for age and sex in all samples and additionally for genomic kinship in samples with pairs of related individuals (CROATIA-Korčula, CROATIA-Split, CROATIA-Vis, FINRISK, H2000, INGI-FVG, INGI-VB, NFBC1966 and ORCADES). Effect sizes and 95% confidence intervals are as in Figure 2. The plots show estimated effect sizes (solid squares) for each population, with 95% confidence intervals (horizontal lines). Each sample estimate is weighted by the inverse of the squared standard error of the regression coefficient, so that the smaller the standard error of the study, the greater the contribution it makes to the pooled regression coefficient. The area of the solid squares is proportional to the weighting given to each study in the meta-analysis. Tab. 3. Meta-analysis assessing potential confounding of SES variables on the association between FROHLD and height. 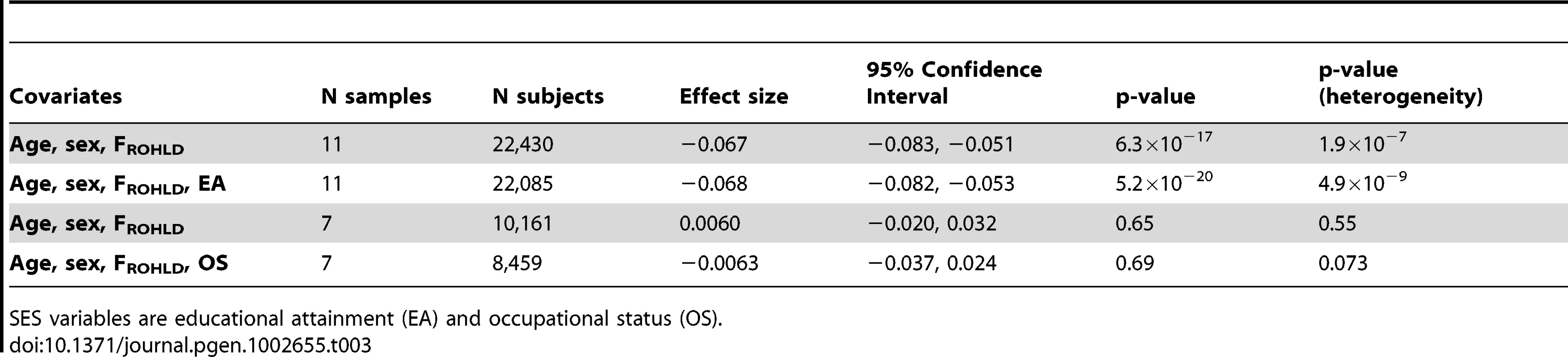
SES variables are educational attainment (EA) and occupational status (OS). Inclusion of educational attainment in the model made very little difference to the size, direction and significance of the effect. If anything, inclusion of educational attainment strengthened the association between reduced height and FROHLD, although heterogeneity was also increased. Inclusion of occupational status in the model also made very little difference: in the meta-analysis of the seven cohorts with data on occupational status, no significant association between reduced height and FROHLD was observed, either with or without the inclusion of occupational status in the model.
Discussion
This study found evidence for a strongly significant inverse association between genome-wide homozygosity and height (i.e. inbreeding depression) using three alternative estimates of genomic homozygosity, with each method capturing a somewhat different aspect of this phenomenon. Whereas all three measures are strongly correlated, there are also important differences, particularly between Fhom and both FROH measures. For example, whereas the Estonian sample (EGCUT) had the second highest mean value for Fhom, it had one of the lowest mean values for FROHLD. There are several possible explanations for this. Firstly, it may be suggestive of a small, isolated population deep in the past but with a larger population size and low levels of parental relatedness in recent generations. Secondly, ascertainment bias in the selection of SNPs may also influence these patterns, as markers present in the HumanHap300 panel are more likely to be heterozygous in NW Europeans [37]. Thirdly, it may be that the level of parental relatedness in the sample is lower than that in the population.
The strongest association between genome-wide homozygosity and reduced height was observed using FROHLD, a measure which estimates homozygosity attributable to recent parental relatedness. There is, however, an important caveat: a significant association was also observed between reduced height and Fhom, controlled for FROHLD, suggesting that homozygous genotypes not located in the long ROH characteristic of recent parental relatedness are also important. We estimated that the increased genome-wide homozygosity that is characteristic of consanguinity results in a reduction of up to 3 cm in the height of the offspring of first cousins compared with the offspring of unrelated parents. Using FROHLD, we then expanded the model to explore possible confounding factors. Firstly, we investigated the possible confounding effects of age-related shrinking. Adult height is the combined effect of growth during childhood and adolescence and loss of height during ageing [11]. There is a powerful age-cohort effect on homozygosity [38] (McQuillan and Wilson unpublished): the rapid pace of urbanisation and population mobility that we have witnessed over the past century has resulted in an observable decrease in homozygosity in younger, compared with older age cohorts. Reduced height is also associated with age, both as a cohort effect reflecting improvements in nutrition and living standards, and because as part of the natural process of ageing, adults lose height as they age due to osteoporotic changes. This process, which is particularly marked in women, may start as young as age 40 [39], with the effects accelerating with age [40]. All analyses were adjusted for age, but as an additional test, we restricted the samples to individuals aged <40. The NFBC1966 sample set provided a further check, as all subjects were aged 31 years at the time of measurement. The inverse association between FROHLD and height remained in both these analyses, suggesting that confounding as a result of the osteoporotic effects of ageing was not a major factor in these samples. The NFBC1966 analysis also suggests that the relationship between genome-wide homozygosity and height is not confounded by the simultaneous improvements in nutrition and living standards over the last century.
Secondly, we assessed possible confounding by socio-economic status. The association between low childhood SES and reduced adult stature is well established, with the likely mechanism being poor nutrition during childhood [6], although shared genetic factors cannot be excluded. There is no direct evidence on the association between genome-wide homozygosity and SES; however there is a substantial literature on the association between consanguinity, or kin marriage, and SES, albeit not in European populations, where kin marriage is rare. In South and West Asian Muslim populations, where kin marriage is customary, many studies have reported an inverse association between consanguinity and women's educational status [41], although the picture is less clear-cut in men [42]. In a large post-World War Two study of the children of consanguineous parents living in the Japanese cities of Hiroshima and Nagasaki, which used a multi-dimensional SES score, Schull and Neel found a small negative correlation between consanguinity and SES [43]. A later Japanese study also found evidence of confounding by SES, although the direction of the effect was opposite depending on the urban or rural background of the subjects [33]. SES can be estimated in a variety of different ways: the measures available to us here were educational attainment and occupational status. We grouped all the cohorts with ordinal measures of educational attainment together and performed two meta-analyses: one adjusting for age, sex and genomic kinship only and the other adjusting for age, sex, genomic kinship and educational attainment. We compared the two meta-analyses to assess the effect of educational attainment as a possible confounder. We repeated this process for the cohorts with ordinal measures of occupational status. The inclusion of either SES measure in the model made very little difference to the results. We therefore found no strong evidence for confounding by SES, although the limited data available on SES mean that confounding by SES cannot be ruled out entirely.
While we did not have access to raw intensity data with which to call hemizygous deletions, which can masquerade as ROH, two different studies give us confidence that such copy number variation will only have a very minor effect on our results. First, in the ORCADES population, removing ROH which overlapped with deletions resulted in only a 0.3% reduction in the sum length of ROH across the cohort [36]. Second, the median length of these deletions was ∼10 kb in a dataset of >7,000 European-heritage subjects, whereas the median length of ROH in the same studies was ∼2000 kb, showing that the vast majority of deletions will be smaller than the ROH under study here [44]. However, we note that an increased burden of deletions has recently been associated with short stature [45].
Our results are consistent with those of Macgregor and colleagues, who found a significant inverse association between height and both the inbreeding coefficient derived from genealogical data (Fped) (p = 0.03; n = 60) and genome-wide homozygosity (p = 0.02; n = 593) in the extreme isolate population of Norfolk Island [34]. The probable reason that they were able to see an effect with such small samples is that they observed much higher levels of parental relatedness than are present in most of the samples used in the present study, therefore the study had greater power to detect an effect. Over one quarter (26%) of their total sample had Fped>0, with mean Fped = 0.044. This contrasts with, for example, only 10% of the ORCADES sample having Fped>0, with mean Fped = 0.01 using pedigrees of a similar depth (unpublished data). Although comparable pedigree data are not available for all samples, it is probable that, with the possible exception of NSPHS, all the samples in the present study have lower levels of Fped and genome-wide homozygosity and thus lower power to detect an association with height than is the case in the Norfolk Island sample of descendants of the Bounty mutineers. Cultural attitudes to consanguinity are at best ambivalent in Europe, so marriage between first cousins is rare, even in the nine isolated population samples in our consortium, where inflated levels of parental relatedness are predicted simply as a function of population size and endogamy.
The present study's analyses provide strong evidence for an association between genomic homozygosity and reduced height; however, there is also strong evidence of heterogeneity. Although we did not find a significant positive association between FROHLD and height in any sample, there was a small number of non-significant positive associations and overall there was considerable variation in the magnitude of the observed effects among population samples. One possible explanation for this is that the observed effects are found only in individuals whose parents are closely related (e.g. as first cousins). If this were the case, however, the strongest effects would be observed in the samples with the highest levels of parental relatedness. In fact, we found no correlation between mean sample FROHLD and effect size. We also found evidence of an association after controlling for parental relatedness, suggesting that homozygous genotypes not resulting from recent parental relatedness also contribute to the observed association. The data do not, then, support the hypothesis that the more inbreeding there is in the sample, the stronger the observed effect. We did, however, find a moderate negative correlation between the mean sample FROHLD and the SE of the FROHLD effect estimate, which suggests that the more inbreeding there is in the sample the greater the power to detect an effect and therefore the more precise the estimate of the effect.
One puzzling result of this study was the discrepancy in the results of the meta-analyses of FROH and FROHLD. The difference in ROH length threshold may contribute to this discrepancy. The 1.5 Mb threshold for FROH was chosen on the basis of an empirical analysis of several European-heritage populations [36]. All individuals in all samples observed in this study, which also used the Illumina Hap300 SNP array, had ROH<1.5 Mb. ROH longer than this were more common in the offspring of related parents, although still present in most offspring of unrelated parents. With the benefit of hindsight, a longer and thus more stringent ROH length threshold may have been preferable, in terms of differentiating ROH resulting from close parental relatedness originating in recent generations from what might be termed population homogeneity resulting from population isolation deeper in the past. In contrast, the FROHLD measure does not detect ROH arising from common ancient haplotypes in the population because SNPs in LD are removed before the analysis. Any ROH detected using FROHLD are the result of parental relatedness of recent origin. For FROHLD the aim is to maximise the ROH that can be detected by setting a minimum length threshold which is as low as possible. ROH are identified by observing a string of contiguous homozygous genotypes. The greater the number of contiguous homozygous genotypes, the stronger the probability that what is observed is a true ROH (i.e. a segment where the entire stretch of unobserved intervening DNA is also homozygous), rather than just a chance observation. Because of the reduced number of SNPs, and thus reduced SNP density, in the LD-pruned SNP panels used for the FROHLD analysis, detection of ROH shorter than 1 Mb becomes unreliable: hence 1 Mb was used as the threshold.
The purpose of carrying out this analysis was to investigate possible genome-wide recessive effects on height. These results are important because by showing an association with genome-wide homozygosity rather than specific individual SNPs, we provide evidence that there is a polygenic recessive component to the genetic architecture of height: i.e. that the observed reductions in height associated with genome-wide homozygosity result from the combined effects of many recessive alleles of individually small effect size, located across the genome. The proportion of the phenotypic variance explained by FROHLD was very variable across cohorts, but the average was 0.4%. Secondly, by demonstrating that the strongest signal comes from the long ROH characteristic of parental relatedness, we provide evidence that the observed effect is primarily the result of rare, rather than common, recessive alleles. Short ROH (measuring up to 2 Mb) are a common feature of all our genomes [36] and their locations are remarkably consistent across different populations, at least within Europe [46]. In contrast, the longer ROH characteristic of parental relatedness are randomly distributed across the genome [36], can be composed of common or rare haplotypes, and as such are predicted to be enriched for rare recessive variants. Our suggestion that it is rare, rather than common, recessive variants that are driving the observed effect is consistent both with theoretical expectations [47] and with empirical data. Two recent studies found evidence that functional regions of the genome (i.e. protein coding regions or regions governing gene expression) are enriched for rare genetic variants. Zhu et al. (2011) conclude that rare, at least moderately harmful, variants constitute the majority of human functional variation [48]. Li et al. (2010) found that non-synonymous coding SNPs were much rarer than synonymous coding SNPs, suggesting that these SNPs have been subject to purifying selection, which in turn suggests that they are deleterious. They found that this pattern was stronger in the X-chromosome than in the autosomes, suggesting that most rare deleterious SNPs are recessive [49].
These findings are also important because, if there is a polygenic, rare, recessive component to the genetic architecture of height, this might also be the case for disease-associated QT of biomedical importance, such as blood pressure and lipid levels. Indeed this is more likely, if these traits are associated with fitness. A high dominance variance has been reported in systolic blood pressure (SBP) and LDL cholesterol in the Hutterites [50]. For this reason, there is a theoretical expectation that these QT will be influenced by genome-wide homozygosity. There have been many empirical studies over the years which have explored this recessive component to the genetic architecture of blood pressure and LDL cholesterol; however until genome-wide scan data became routinely affordable, this could only be investigated indirectly using inbreeding coefficients derived from genealogical data (Fped). Such measures are highly error-prone and cannot account for stochastic variation in the inheritance process. Nevertheless, various studies have found evidence of a significant positive association between blood pressure and Fped [51], [52], [53], [54], [55] although other similar studies found no such evidence [56], [57]. One small study by Campbell and colleagues replicated these findings using a genomic measure of homozygosity derived from microsatellite data [32]. Blood pressure in this Croatian island isolate population was significantly (p<0.05) higher in the offspring of consanguineous parents compared with the offspring of unrelated parents. Similarly, there is some evidence of a positive association between total cholesterol and Fped [58] and between low density lipoprotein cholesterol (LDL) and Fped [59] and of a negative association between high density lipoprotein (HDL) and Fped [60], although other studies have come up with more ambiguous results [28], [55]. The study by Campbell and colleagues found significant positive associations between both total cholesterol and LDL cholesterol and homozygosity, using a panel of microsatellite markers. All these, however, were very small studies. The ROHgen consortium is well placed to investigate these questions thoroughly: we have access to large numbers of subjects; we can replicate investigations in a diverse range of European-heritage populations and we have developed a robust methodology applicable to any number of different QT.
Materials and Methods
Ethics Statement
Each study had ethical approval for genetic research into the basis of complex traits, approved by the appropriate committees in each country. All participants provided written informed consent. As analyses were performed locally by cohort analysts, no data were shared across national boundaries.
Study Participants
This meta-analysis combined data from 21 European or European-heritage population samples: The Estonian Genome Centre University of Tartu (EGCUT), the Erasmus Rucphen Family Study (ERF), the National FINRISK Study (FINRISK) (genotyped samples from 1997, 2002 and 2007 study years), the Health 2000 Survey (H2000), the Helsinki Birth Cohort (HBCS), the Lothian Birth Cohort 1921 (LBC1921), the Lothian Birth Cohort 1936 (LBC1936) the Carlantino Project (INGI-CARL), Friuli-Venezia-Giulia-Genetic Park (INGI-FVG), Korčula (CROATIA-Korčula), Micro-Isolates in South Tyrol (MICROS), the Northern Finland 1966 Birth Cohort (NFBC1966), the Northern Sweden Population Health Study (NSPHS), the Orkney Complex Disease Study (ORCADES), Queensland Institute of Medical Research (QIMR), the Rotterdam Study (RS), the Study of Colorectal Cancer in Scotland (SOCCS), Split (CROATIA-Split), Val Borbera (INGI-VB), Vis (CROATIA-Vis) and the Young Finns Study (YFS). Most (n = 16) were population-based samples, 4 were birth cohorts and 1 was a case-control sample. Five study populations were Finnish, 4 were Scottish, 4 were Italian, 3 were Croatian, 2 were Dutch, 1 was Estonian, 1 was Swedish and 1 was Australian of NW European heritage. Most of the samples were drawn from genetically isolated populations or populations with increased homozygosity, such as the Finns. The total number of participants was 35,808. All studies were carried out after the appropriate local ethical approval had been obtained. All participants provided written informed consent. Full sample details are given in Table S1.
Measurement of Height
In all studies apart from SOCCS, height was measured by trained personnel using a stadiometer. SOCCS participants provided self-reported measurements of height. This was validated by measuring height in a subset of the sample by trained personnel using a stadiometer. There was a high concordance between the two measures.
Genotyping
All genotyping was performed on the Illumina platform but using four different SNP panels. Seven samples were genotyped using the Illumina HumanHap 300 panel, six using the Illumina HumanHap 370 Duo/Quad panels, five using the Illumina Human 610 Quad panel, one using the Illumina Human 670 Quad panel and one using both the 370 and 610 panels. In order to harmonise the data across samples, SNPs present in the HumanHap 300 panel were extracted and the analysis was conducted using these SNPs only. Quality control procedures were performed on each sample separately, with the minimum requirements as follows. Individuals with more than 5% missing genotypes were excluded. SNPs missing in more than 10% of samples were excluded, as were SNPs failing the Hardy-Weinberg equilibrium test at p<0.0001 and SNPs with minor allele frequency (MAF)<0.01.
Measures of Genome-Wide Homozygosity
These were detected using the Runs of homozygosity routine in plink [61], [62]. This slides a moving window of 5000 kb (minimum 50 SNPs) across the genome to detect long contiguous runs of homozygous genotypes. An occasional genotyping error occurring in an otherwise unbroken homozygous segment could result in the underestimation of ROH lengths. To address this, the routine allows one heterozygous and five missing calls per window.
FROH
ROH were defined as runs of at least 25 consecutive homozygous SNPs spanning at least 1500 kb, with less than a 100 kb gap between adjacent SNPs and a density of SNP coverage within the ROH of no more than 20 kb/SNP. For each individual, an F statistic termed FROH [36] was derived by summing the lengths of all ROH longer than 1500 kb and expressing this as a percentage of the typed autosomal genome (i.e. the sum of the length of all the autosomes from the first to the last SNP, excluding the centromeres). 1500 kb was chosen as the minimum length of ROH because observational studies in European populations have shown that whereas all individuals have ROH shorter than 1500 kb, ROH longer than this are more likely to be the result of parental relatedness [36]. We have shown previously that this measure is strongly correlated (r = 0.86) with pedigree-derived inbreeding coefficients [36].
FROHLD
An alternative approach to deriving an inbreeding coefficient from ROH is to start by pruning the SNP panel of SNPs in strong linkage disequilibrium (LD), in order to remove ROH that are very common due to the high frequency of ancestral haplotypes. SNP panels were pruned using the pairwise option in plink [61]. At each point, it calculates LD between each pair of SNPs in a window of 50 SNPs and removes one of each pair if LD exceeds the user-defined limit (set here at r2 = 0.1). ROH parameters were adjusted to reflect the reduced number of SNPs. The minimum number of consecutive homozygous SNPs constituting a ROH was set at 12 (probability of occurring by chance p<0.005 in all samples). The minimum length of ROH was set at 1000 kb, with no more than 250 kb gap between adjacent SNPs and a density of SNP coverage within the ROH of no more than 100 kb/SNP. Individual FROHLD statistics were then calculated as described above. This approach yields a more stringent estimate of parental relatedness, as it removes all ROH that are there simply because of parental sharing of long haplotypes that are common in the population. ROH consisting of independent SNPs will be of recent origin and will thus be enriched for rarer haplotypes. Again, this is highly correlated with the pedigree-derived inbreeding coefficient (r = 0.82 in a subset of 241 subjects from the ORCADES sample with complete pedigree information available to five ancestral generations).
Observed homozygosity (Fhom)
This is defined as the number of observed homozygous genotypes per individual, expressed as a percentage of the number of non-missing genotypes for that individual. This measure is less strongly correlated with pedigree inbreeding coefficients than the above (r = 0.76 [36]), as it counts all homozygous genotypes and not simply those found in long ROH arising from recent pedigree loops.
Statistical Analysis
All tests were two sided and a p-value threshold of 0.05 was used. In order to account for differences in mean height among population samples, all height measures are expressed as z-scores. Because genetically isolated populations are characterised by high levels of relatedness between individuals, measures of height are not independent and therefore conventional regression techniques are not appropriate. The CROATIA-Korčula, CROATIA-Split, CROATIA-Vis, ERF, FINRISK, HBCS, H2000, INGI-CARL, INGI-FVG, INGI-VB MICROS, NFBC1966, NSPHS, ORCADES and YFS samples were therefore analysed using a linear mixed polygenic model in GenABEL. This programme maximises the likelihood of the data under the polygenic model with specified covariates. It reports twice the negative maximum likelihood estimates and the inverse of the variance-covariance matrix at the point of maximum likelihood [63], [64], [65]. The z-score for height was analysed with age, sex, genome-wide homozygosity measure and either educational attainment or occupational status fitted as fixed effects. This model also fits a genomic kinship matrix, which estimates pairwise relatedness, derived on the basis of identical by state (IBS) sharing, weighted by allele frequency, so that a pair of individuals sharing a rare allele is estimated to be more closely related than a pair sharing a common allele. All other samples consist of unrelated individuals, so data were analysed in SPSS using simple linear regression, with age, sex, genome-wide homozygosity measure and either educational attainment or occupational status as covariates. Before embarking on analysis of the SOCCS data, the sample was analysed using binary logistic regression to check that height is not associated with colorectal cancer status. There was no association between height and colorectal cancer, so cases and controls were analysed as a single sample.
Meta-Analysis
Results were combined in a meta-analysis using the inverse variance method to combine effect size estimates from each sample [63]. This weights each sample estimate by the inverse of the squared standard error of the regression coefficient, so that the smaller the standard error of the study, the greater the contribution it makes to the pooled regression coefficient.
Estimation of the Reduction in Height Resulting from Increased Homozygosity in the Offspring of First Cousins Compared with the Offspring of Unrelated Individuals
In order to standardise across the different population samples in this study, we converted height measurements into z-scores. The results of each meta-analysis report a pooled estimate of the change in this z-score associated with a 1% increase in genomic homozygosity. In order to make this easier to interpret, we express this in the text as the difference in height between the offspring of first cousins and the offspring of unrelated parents. The first step in this analysis was to estimate the difference in observed genomic homozygosity between the offspring of first cousins and the offspring of unrelated parents (a more realistic approach than using the theoretical predictions of Fped = 0.0625 and 0). For each measure of genomic homozygosity we estimated this difference separately in 3 different populations where genealogical and genomic data were available for the reliable identification of the offspring of first cousins. In each population group and for each measure of genomic homozygosity, we estimated the mean difference between the offspring of first cousins and the offspring of unrelated parents. We multiplied this by the effect size estimate from the regression meta-analysis to give a z-score estimate for the reduction in height in the offspring of first cousins compared with the offspring of unrelated individuals. To convert each of these z-scores into cm, we then multiplied them by an estimate of the SD for height across the whole sample, derived by taking the SD for each sample in turn and weighting it by sample size. Two of the three populations used for this analysis were part of the main study (ORCADES and MICROS). The third was a small Irish sample, consisting of members of both settled and traveller communities in Ireland (unpublished data, JF Wilson and GL Cavalleri). We repeated this analysis separately in these three populations, partly because of the very small number of first cousin offspring in any single sample in our study and partly to ensure that the observed difference in homozygosity was not simply an artefact of one particular population sample.
Supporting Information
Zdroje
1. ColeTJ 2003 The secular trend in human physical growth: a biological view. Econ Hum Biol 1 161 168
2. OgdenCLFryarCDCarrollMDFlegalKM 2004 Mean body weight, height, and body mass index, United States 1960–2002. Adv Data 1 17
3. MacgregorSCornesBKMartinNGVisscherPM 2006 Bias, precision and heritability of self-reported and clinically measured height in Australian twins. Hum Genet 120 571 580
4. PreeceMA 1996 The genetic contribution to stature. Horm Res 45 56 58
5. SilventoinenKKaprioJLahelmaEKoskenvuoM 2000 Relative effect of genetic and environmental factors on body height: differences across birth cohorts among Finnish men and women. Am J Public Health 90 627 630
6. SilventoinenKSammalistoSPerolaMBoomsmaDICornesBK 2003 Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res 6 399 408
7. PerolaMSammalistoSHiekkalinnaTMartinNGVisscherPM 2007 Combined genome scans for body stature in 6,602 European twins: evidence for common Caucasian loci. PLoS Genet 3 e97 doi:10.1371/journal.pgen.0030097
8. VisscherPMMedlandSEFerreiraMAMorleyKIZhuG 2006 Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet 2 e41 doi:10.1371/journal.pgen.0020041
9. WeedonMNFraylingTM 2008 Reaching new heights: insights into the genetics of human stature. Trends Genet 24 595 603
10. GudbjartssonDFWaltersGBThorleifssonGStefanssonHHalldorssonBV 2008 Many sequence variants affecting diversity of adult human height. Nat Genet 40 609 615
11. WeedonMNLangoHLindgrenCMWallaceCEvansDM 2008 Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet 40 575 583
12. Davey SmithGHartCUptonMHoleDGillisC 2000 Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health 54 97 103
13. LawlorDAEbrahimSDavey SmithG 2002 The association between components of adult height and Type II diabetes and insulin resistance: British Women's Heart and Health Study. Diabetologia 45 1097 1106
14. LawlorDATaylorMDavey SmithGGunnellDEbrahimS 2004 Associations of components of adult height with coronary heart disease in postmenopausal women: the British women's heart and health study. Heart 90 745 749
15. GunnellDOkashaMSmithGDOliverSESandhuJ 2001 Height, leg length, and cancer risk: a systematic review. Epidemiol Rev 23 313 342
16. RaychaudhuriSRemmersEFLeeATHackettRGuiducciC 2008 Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet 40 1216 1223
17. SouthamLRodriguez-LopezJWilkinsJMPombo-SuarezMSnellingS 2007 An SNP in the 5′-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum Mol Genet 16 2226 2232
18. MiyamotoYMabuchiAShiDKuboTTakatoriY 2007 A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet 39 529 533
19. FisherRA 1918 The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh 52 399 433
20. Lango AllenHEstradaKLettreGBerndtSIWeedonMN 2010 Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 832 838
21. ManolioTACollinsFSCoxNJGoldsteinDBHindorffLA 2009 Finding the missing heritability of complex diseases. Nature 461 747 753
22. YangJBenyaminBMcEvoyBPGordonSHendersAK 2010 Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 565 569
23. VisscherPM 2008 Sizing up human height variation. Nat Genet 40 489 490
24. OberCAbneyMMcPeekMS 2001 The genetic dissection of complex traits in a founder population. Am J Hum Genet 69 1068 1079
25. WeissLAPanLAbneyMOberC 2006 The sex-specific genetic architecture of quantitative traits in humans. Nat Genet 38 218 222
26. VisscherPMMacgregorSBenyaminBZhuGGordonS 2007 Genome partitioning of genetic variation for height from 11,214 sibling pairs. Am J Hum Genet 81 1104 1110
27. RobertsonAHillWG 1983 Population and quantitative genetics of many linked loci in finite populations. Prc R Soc Lond B 219 253 264
28. MartinAOKurczynskiTWSteinbergAG 1973 Familial studies of medical and anthropometric variables in a human isolate. Am J Hum Genet 25 581 593
29. KrishanG 1986 Effect of parental consanguinity on anthropometric measurements among the Sheikh Sunni Muslim boys of Delhi. Am J Phys Anthropol 70 69 73
30. ZottarelliLKSunilTSRajaramS 2007 Influence of parental and socioeconomic factors on stunting in children under 5 years in Egypt. East Mediterr Health J 13 1330 1342
31. Freire-MaiaN 1983 Inbreeding studies in Brasilian schoolchildren. Am J Med Genet 16 331 355
32. CampbellHCarothersADRudanIHaywardCBiloglavZ 2007 Effects of genome-wide heterozygosity on a range of biomedically relevant human quantitative traits. Hum Mol Genet 16 233 241
33. NeelJVSchullWJYamamotoMUchidaSYanaseT 1970 The effects of parental consanguinity and inbreeding in Hirado, Japan. II. Physical development, tapping rate, blood pressure, intelligence quotient, and school performance. Am J Hum Genet 22 263 286
34. MacgregorSBellisCLeaRACoxHDyerT 2010 Legacy of mutiny on the Bounty: founder effect and admixture on Norfolk Island. Eur J Hum Genet 18 67 72
35. EavesLJHeathACMartinNG 1999 Chapter 11: Biological and Cultural Inheritance of Stature and Attitudes. CloningerCR Personality and Psychopathology Washington DC and London American Psychiatric Press Inc editor
36. McQuillanRLeuteneggerALAbdel-RahmanRFranklinCSPericicM 2008 Runs of homozygosity in European populations. Am J Hum Genet 83 359 372
37. KirinMMcQuillanRFranklinCSCampbellHMcKeiguePM 2010 Genomic runs of homozygosity record population history and consanguinity. PLoS ONE 5 e13996 doi:10.1371/journal.pone.0013996
38. NallsMASimon-SanchezJGibbsJRPaisan-RuizCBrasJT 2009 Measures of autozygosity in decline: globalization, urbanization, and its implications for medical genetics. PLoS Genet 5 e1000415 doi:10.1371/journal.pgen.1000415
39. ForsdahlAWaalerHT 1976 [Body height changes in relation to age]. Tidsskr Nor Laegeforen 96 211 215
40. NoppaHAnderssonMBengtssonCBruceAIsakssonB 1980 Longitudinal studies of anthropometric data and body composition. The population study of women in Gotenberg, Sweden. Am J Clin Nutr 33 155 162
41. HussainRBittlesAH 2000 Sociodemographic correlates of consanguineous marriage in the Muslim population of India. J Biosoc Sci 32 433 442
42. KhourySAMassadD 1992 Consanguineous marriage in Jordan. Am J Med Genet 43 769 775
43. SchullWJNeelJV 1965 The effects of inbreeding on Japanese children New York Harper and Row
44. LevinsonDFDuanJOhSWangKSandersAR 2011 Copy number variants in schizophrenia:confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry 168 302 316
45. DauberAYuYTurchinMCChiangCWMengYA 2011 Genome-wide association of copy-number variation reveals an association between short stature and the presence of low-frequency genomic deletions. Am J Hum Genet 89 751 759
46. NothnagelMLuTTKayserMKrawczakM 2010 Genomic and geographic distribution of SNP-defined runs of homozygosity in Europeans. Hum Mol Genet 19 2927 2935
47. WrightACharlesworthBRudanICarothersACampbellH 2003 A polygenic basis for late-onset disease. Trends Genet 19 97 106
48. ZhuQGeDMaiaJMZhuMPetrovskiS 2011 A genome-wide comparison of the functional properties of rare and common genetic variants in humans. Am J Hum Genet 88 458 468
49. LiYVinckenboschNTianGHuerta-SanchezEJiangT 2010 Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding variants. Nat Genet 42 969 972
50. AbneyMMcPeekMSOberC 2001 Broad and narrow heritabilities of quantitative traits in a founder population. Am J Hum Genet 68 1302 1307
51. KriegerH 1969 Inbreeding effects on metrical traits in Northeastern Brazil. Am J Hum Genet 21 537 546
52. SalehEAMahfouzAATayelKYNaguibMKBin-al-ShaikhNM 2000 Hypertension and its determinants among primary-school children in Kuwait: an epidemiological study. East Mediterranean Health Journal 6 333 337
53. RudanISmolej-NarancicNCampbellHCarothersAWrightA 2003 Inbreeding and the genetic complexity of human hypertension. Genetics 163 1011 1021
54. Badaruddoza 2004 Inbreeding effects on metrical phenotypes among north Indian children. Collegium Antropologicum 28 311 319
55. RudanIBiloglavZVorko-JovicAKujundzic-TiljakMStevanovicR 2006 Effects of inbreeding, endogamy, genetic admixture, and outbreeding on human health: a (1001 Dalmatians) study. Croat Med J 47 601 610
56. SoyannwoMAKurashiNYGadallahMHamsJel-EssawiO 1998 Blood pressure pattern in Saudi population of Gassim. Afr J Med Med Sci 27 107 116
57. BenerAHussainRAhmadST 2006 Consanguineous marriages and their effects on common adult diseases: studies from an endogamous population. Medical Principles and Practice 16 262 267
58. IsaacsASayed-TabatabaeiFAAulchenkoYSZillikensMCSijbrandsEJ 2007 Heritabilities, apolipoprotein E, and effects of inbreeding on plasma lipids in a genetically isolated population: the Erasmus Rucphen Family Study. Eur J Epidemiol 22 99 105
59. AulchenkoYSRipattiSLindqvistIBoomsmaDHeidIM 2009 Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet 41 47 55
60. EldonBJAxelssonJSigurdssonSBArnasonE 2001 Cardiovascular risk factors and relatedness in an Icelandic subpopulation. Int J Circumpolar Health 60 499 502
61. PurcellS 2007 PLINK v1.0 whole genome association analysis toolset. 1.0 ed
62. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
63. AulchenkoYS 2008 GenABEL Tutorial. Available: http://mgabionetnscru/yurii/courses/ge05-2008/GenABEL-tutorialpdf. Accessed 2011
64. AulchenkoYSRipkeSIsaacsAvan DuijnCM 2007 GenABEL: an R library for genome-wide association analysis. Bioinformatics 23 1294 1296
65. AulchenkoY 2012 GenABEL.org Manuals. Available: http://wwwgenabelorg/manuals/GenABEL/polygenichtml. Accessed 2012
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



