-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
Rac1 is a founding member of the Rho-GTPase family and a key regulator of membrane remodeling. In the context of apoptotic cell corpse engulfment, CED-10/Rac1 acts with its bipartite guanine nucleotide exchange factor, CED-5/Dock180-CED-12/ELMO, in an evolutionarily conserved pathway to promote phagocytosis. Here we show that in the context of the Caenorhabditis elegans intestinal epithelium CED-10/Rac1, CED-5/Dock180, and CED-12/ELMO promote basolateral recycling. Furthermore, we show that CED-10 binds to the RAB-5 GTPase activating protein TBC-2, that CED-10 contributes to recruitment of TBC-2 to endosomes, and that recycling cargo is trapped in recycling endosomes in ced-12, ced-10, and tbc-2 mutants. Expression of GTPase defective RAB-5(Q78L) also traps recycling cargo. Our results indicate that down-regulation of early endosome regulator RAB-5/Rab5 by a CED-5, CED-12, CED-10, TBC-2 cascade is an important step in the transport of cargo through the basolateral recycling endosome for delivery to the plasma membrane.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002785
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002785Summary
Rac1 is a founding member of the Rho-GTPase family and a key regulator of membrane remodeling. In the context of apoptotic cell corpse engulfment, CED-10/Rac1 acts with its bipartite guanine nucleotide exchange factor, CED-5/Dock180-CED-12/ELMO, in an evolutionarily conserved pathway to promote phagocytosis. Here we show that in the context of the Caenorhabditis elegans intestinal epithelium CED-10/Rac1, CED-5/Dock180, and CED-12/ELMO promote basolateral recycling. Furthermore, we show that CED-10 binds to the RAB-5 GTPase activating protein TBC-2, that CED-10 contributes to recruitment of TBC-2 to endosomes, and that recycling cargo is trapped in recycling endosomes in ced-12, ced-10, and tbc-2 mutants. Expression of GTPase defective RAB-5(Q78L) also traps recycling cargo. Our results indicate that down-regulation of early endosome regulator RAB-5/Rab5 by a CED-5, CED-12, CED-10, TBC-2 cascade is an important step in the transport of cargo through the basolateral recycling endosome for delivery to the plasma membrane.
Introduction
The C. elegans intestine has proven to be a powerful model system for the study of epithelial cell membrane trafficking mechanisms. The worm intestine is a simple epithelial tube consisting of 20 enterocyte cells that form nine “donut-like” intestinal rings [1]. Each of these 20 cells is terminally differentiated, and each intestinal cell is maintained for the life of the animal without replacement [1].
Like mammalian intestinal epithelial cells, C. elegans enterocytes display apicobasal polarity with defined apical junctions separating the apical and basolateral domains [1]. The apical enterocyte membranes, which form the intestinal lumen, display a prominent microvillar brush border, with an overlying glycocalyx and underlying subapical terminal web rich in actin and intermediate filaments [1]. The basolateral membrane is in contact with the pseudocoelom (body cavity) and is responsible for the exchange of molecules between the intestine and other tissues of the body.
In previous studies we established three model transmembrane cargo markers for the analysis of basolateral endocytic trafficking in the C. elegans intestine: hTAC-GFP, hTfR-GFP, and MIG-14-GFP [2]–[6]. hTAC (human IL-2 receptor alpha-chain) enters cells via clathrin-independent endocytosis (CIE), while hTfR (human transferrin receptor) and MIG-14 (Wntless) enter cells via clathrin-dependent endocytosis (CDE) [7]–[9]. However, while hTAC and hTfR recycle via the recycling endosome in an RME-1/EHD-dependent manner, MIG-14 recycles via retrograde recycling to the Golgi in a retromer-dependent manner [2], [5], [8]–[12]. Thus comparison of the effects of any endocytic transport mutant on these three cargo proteins can give insight into which steps in receptor traffic are affected.
Here we focus on the function of the C. elegans Rac1 homolog CED-10 in regulation of epithelial cell endocytic trafficking. During engulfment of dead apoptotic cells, CED-10 functions in a pathway with associated proteins CED-12/ELMO, CED-5/DOCK180, and CED-2/CrkII, promoting cytoskeletal reorganization that is thought to be important for pseudopod formation/function [13]. CED-12 and CED-5 form a bipartite guanine-nucleotide exchange factor for CED-10, and are thus thought to promote conversion of inactive CED-10(GDP) to active CED-10(GTP) [14]. CED-2 physically associates with CED-5 and is thought to function as an adapter, potentially linking the protein complex to certain apoptotic corpse receptors such as MOM-5/Frizzled and/or Integrins, but not the CED-1 corpse receptor [15], [16].
Mammalian Rac1 has been reported to become GTP-loaded on endosomes, and to require Arf6-dependent recycling for membrane ruffling and its localization to the leading edge of migrating cells [17]–[20]. Despite the known association of activated Rac1 with early and recycling endosomes, little is known of the potential role of Rac1 in regulating endosome function. In this study we define an important requirement for CED-10/Rac1 in basolateral recycling in the intestinal epithelia. This function of CED-10/Rac requires CED-5 and CED-12, but not CED-2. Furthermore we connect this recycling function of CED-10 to Rab-GAP TBC-2, indicating a mechanism for the down-regulation of the RAB-5 GTPase as endocytic cargo reaches the recycling endosome.
Results
Loss of CED-10/Rac1, CED-12/Elmo, or CED-5/Dock180 leads to intracellular accumulation of recycling cargo
In order to determine if CED-10/Rac1 is required for endocytic transport, we assayed the effect of a strong Rac1 loss-of-function mutant, ced-10(n3246), on the subcellular distribution of three endocytic cargos, using confocal microscopy in the adult intestine [15]. The C. elegans intestine is known to express CED-10/Rac1 at high levels, but CED-10/Rac1 function has not been previously investigated in this tissue [21].
Interestingly, we observed strong intracellular accumulation of recycling receptor hTfR-GFP in the ced-10 mutant background, similar to that we had previously observed in known endocytic recycling mutants such as rme-1 and amph-1 (Figure 1A and 1B, quantified in 1D) [2], [4]. The abnormal intracellular accumulation of hTfR-GFP in the ced-10(n3246) mutant was completely rescued by transgenic expression of CED-10 using an intestine-specific promoter, indicating that the effect of CED-10 on recycling is cell autonomous and is not mediated by indirect effects via other tissues (Figure 1C and 1D). We also observed abnormal accumulation of another recycling cargo protein, hTAC-GFP, in ced-10 mutants, and found that accumulated intracellular hTAC-GFP colocalized with recycling endosome marker EHBP-1 (Figure 1I, 1J, 1L, and 1N–1N″) [6]. TAC and TfR are thought to be internalized independently, meet in the endosomal system, and then recycle from the recycling endosome to plasma membrane in separate carriers [3], [22]–[24]. Taken together our results indicated trapping of multiple types of cargo in the recycling arm of the endocytic pathway in ced-10/Rac1 mutants.
Fig. 1. ced-10 and ced-12 mutants display abnormal trafficking of recycling cargo in the C. elegans intestine. 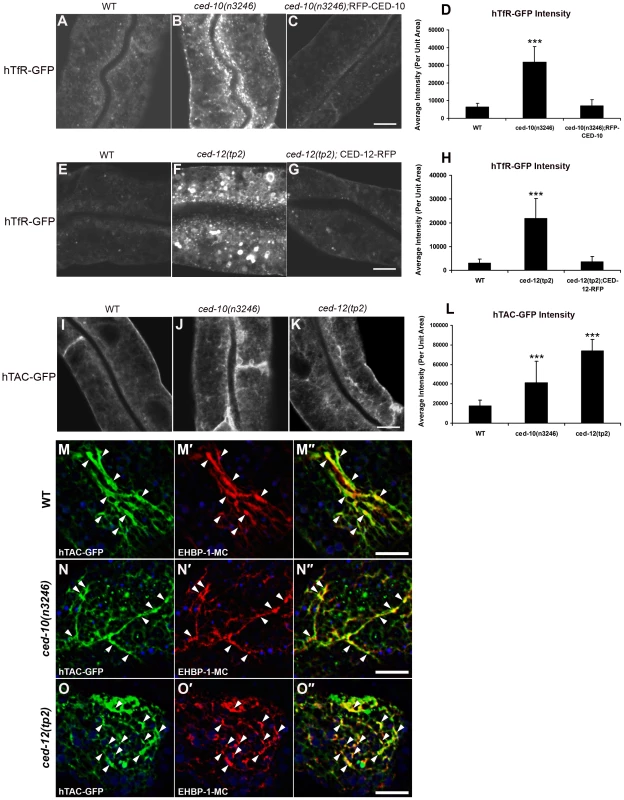
(A–C) Confocal images of the worm intestine in live intact animals expressing a GFP-tagged CDE cargo protein that recycles via the recycling endosome, the human transferrin receptor (hTfR-GFP). The ced-10 mutant phenotype is rescued by intestine-specific expression of RFP-CED-10. (D) Quantification of hTfR-GFP puncta intensity. (E–G) Intracellular hTfR-GFP accumulates in ced-12(tp2) mutants. The CED-12 mutant phenotype is rescued by intestine-specific expression of CED-12-RFP. (H) Quantification of hTfR-GFP puncta intensity. (I–K) Representative confocal images of the worm intestine in living intact young adult animals expressing a GFP-tagged CIE cargo protein that recycles via the recycling endosome, the IL-2 receptor alpha chain (hTAC-GFP). Wild type (N2), ced-10(n3246), and ced-12(tp2) mutant animals are shown. (L) Quantification of hTAC-GFP intensity. (M–O″) Confocal images of the worm intestine in live intact animals expressing GFP-tagged IL-2 receptor alpha chain (hTAC-GFP) and mCherry-tagged C. elegans EHBP-1 (EHBP-1-MC), a recycling endosome marker. Wild-type animals (M–M″), ced-10(n3246) mutant animals (N–N″), and ced-12(tp2) mutant animals (O–O″) are shown. Error bars represent standard deviations from the mean (n = 18 each, 6 animals of each genotype sampled in three different regions of each intestine). Asterisks indicate a significant difference in the one-tailed Student's T-test (***p<0.0001). Scale bar, 10 µm. Furthermore, we found that ced-12(tp2) and ced-5(n1812) mutants displayed the same aberrant intracellular accumulation of hTfR-GFP and hTAC-GFP found in ced-10 mutants, indicating that the CED-12/CED-5 Rac exchange factor complex is also required for the recycling process (Figure 1E, 1F, 1H, 1K and 1L; Figure S2A–S2D and S2I). The abnormal intracellular accumulation of hTfR-GFP in the ced-12 mutant was completely rescued by transgenic expression of CED-12 using an intestine-specific promoter, again indicating an intrinsic requirement for CED-12 in the intestinal cells (Figure 1G and 1H).
Importantly, ced-10 and ced-12 mutants had no effect on the subcellular localization of MIG-14-GFP, indicating that CED-10/Rac1 and CED-12/ELMO are somewhat cargo specific in their effects, and are not required for retrograde recycling from endosomes to the Golgi (Figure S1A–S1G). Since MIG-14 shares its uptake route with hTfR, but is not thought to enter the recycling endosome, these results suggest that CED-10/Rac1 is required for specific membrane trafficking events associated with recycling endosome dependent cargo.
No defect in hTAC-GFP or hTfR-GFP localization was found in ced-2(e1752) mutants, indicating that CED-2 is not required for the endocytic transport of these cargos (Figure S3A–S3D, S3I). Since CED-2 is required for phagocytic dead cell engulfment, this result indicates that the observed defects in endocytic traffic are not indirect effects of failed phagocytosis. This is also consistent with previous studies of the C. elegans intestinal cells, indicating that after embryogenesis the intestinal cells are not involved in the clearance of apoptotic cell corpses, do not perform phagocytosis, and do not migrate [1], [25]. Our results showing that CED-2 is not required in the intestine for hTfR and hTAC trafficking also indicates that not all CED-10 associated factors are shared between phagocytic and endocytic regulation.
Loss of CED-10, CED-12, or CED-5 disrupts endosome morphology
In order to help determine which step in trafficking is affected by loss of CED-10/Rac1 and its exchange factor CED-5/CED-12, we performed morphometric analysis of a wide-variety of marker proteins associated with endocytic organelles in the intestine of ced-10 and ced-12 mutant animals. This set of markers has been used successfully in previous studies to gain insight into the specific defects associated with endocytic transport mutants [2]–[6]. We noted over-accumulation of GFP-tagged early endosome regulator RAB-5 in ced-10 and ced-12 mutants (Figure 2A–2C, quantified in 2M and Figure S4). The average GFP-RAB-5 puncta intensity increased by about 8-fold and 12-fold, respectively, in ced-10 and ced-12 mutants (Figure 2M). We also noted in both mutants abnormal morphology of basolateral recycling endosomes labeled by GFP-RAB-10, ARF-6-GFP, GFP-RME-1, and SDPN-1-GFP (Figure 2D–2L, quantified in 2M, 2N, Figure S4, and Figure S5A–S5C, quantified in S5J). By contrast, markers for late endosomes (GFP-RAB-7), and apical recycling endosomes (GFP-RAB-11) were unperturbed (Figure S5D–S5I and S5K). We found that ced-5 mutants, but not ced-2 mutants, also displayed defective recycling endosome morphology (Figures S2E–S2H, S3E–S3H, S2J and S3J). Taken together with the cargo accumulation results, these specific changes in endosomal morphology indicate that a particular branch of the endocytic pathway, including the early endosomes and basolateral recycling endosomes, but not late endosomes or apical recycling endosomes, require CED-10/Rac1 activity for their normal function.
Fig. 2. Abnormal accumulation of early and recycling endosomes markers in ced-10(n3246) and ced-12(tp2) mutants. 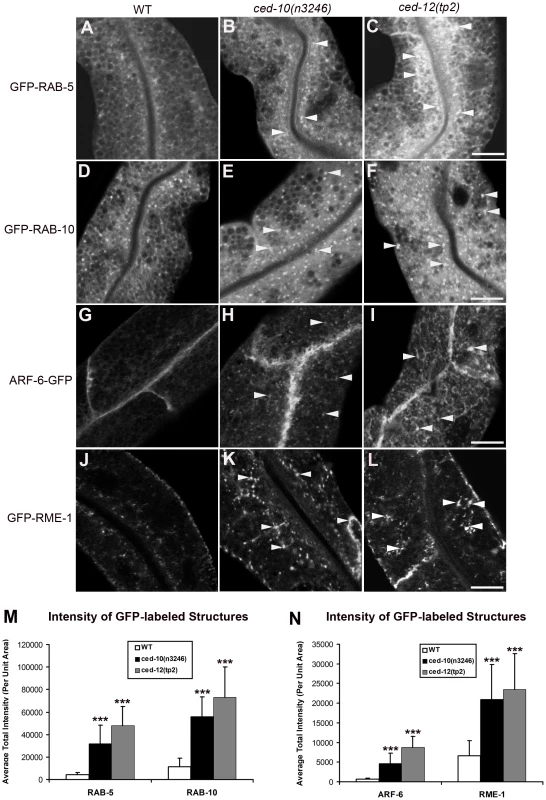
Representative confocal images are shown for GFP-RAB-5 (A–C), GFP-RAB-10 (D–F), ARF-6-GFP (G–I), and GFP-RME-1 (J–L). Quantifications of average puncta intensity are shown in (M–N). All images were collected from living intact young adult animals expressing GFP-tagged proteins specifically in the intestinal epithelial cells. Error bars represent standard deviation from the mean (n = 18 each, 6 animals of each genotype sampled in three different regions of each intestine). Asterisks indicate a significant difference in the one-tailed Student's t-test (***p<0.0001). Scale bar represents 10 µm. CED-10 and CED-12 are associated with early and recycling endosomes
If CED-10 and CED-12 function directly in endosomal regulation, then we would expect to find them associated with endosomes in wild-type cells. Thus we sought to determine the subcellular localization of CED-10 and CED-12 in the intestinal epithelial cells using functional tagged forms of the proteins. GFP-CED-10 localized strongly to the apical domain (likely to the microvilli) and to intracellular puncta in the cytoplasm (Figure 3A). CED-12-RFP colocalized well with GFP-CED-10 to the intracellular puncta (Figure 3B and 3C). However, CED-12-RFP also strongly labeled a subapical band that displayed significant overlap with the GFP-CED-10 labeled apical band (Figure 3A, 3B and 3C). It is not clear if the partial apical overlap represents the presence of both proteins on certain subapical structures, or rather represents two distinct apical localizations for CED-10 and CED-12 that are very near one-another. The simplest interpretation of these results is that the functionally important site of CED-10/CED-12 interaction for the recycling of basolateral cargo is on the intracellular puncta (endosomes – see below), although we cannot exclude important interactions on other subcellular compartments.
Fig. 3. CED-10 localizes to early and recycling endosomes, and colocalizes with CED-12, in the intestine. 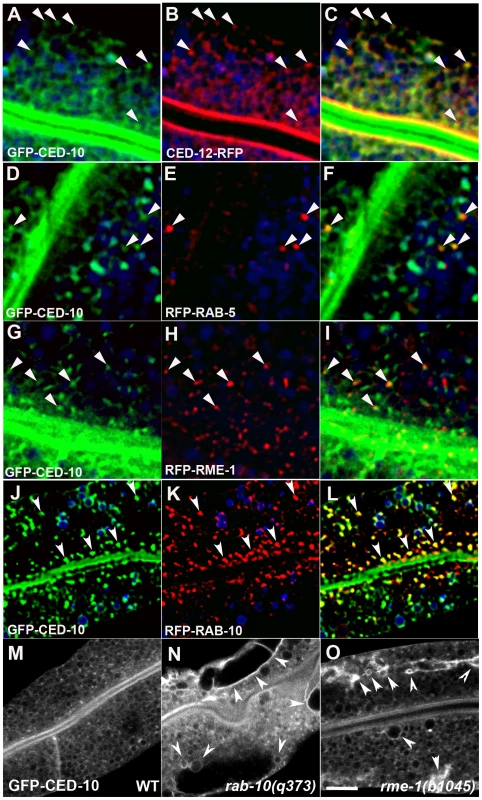
All images are from wide-field deconvolved, or confocal, 3-D image stacks acquired in intact living animals expressing GFP and RFP tagged proteins specifically in intestinal epithelial cells. (A–C) GFP-CED-10 colocalizes with CED-12-RFP on intracellular puncta. Arrowheads indicate structures labeled by both GFP-CED-10 and CED-12-RFP. (D–F) GFP-CED-10 colocalizes with RFP-RAB-5 on a subset of early endosomes. Arrowheads indicate endosomes labeled by both GFP-CED-10 and RFP-RAB-5. (G–I) GFP-CED-10 partially colocalizes with RFP-RME-1 on basolateral recycling endosomes. Arrowheads indicate endosomes labeled by both GFP-CED-10 and RFP-RME-1. (J–L) GFP-CED-10 colocalizes extensively with RFP-RAB-10 on recycling endosomes. Arrowheads indicate endosomes labeled by both GFP-CED-10 and RFP-RAB-10. (M–O) GFP-CED-10 localizes to the abnormally enlarged endosomes present in rab-10 and rme-1 mutants. Arrows mark the grossly enlarged early and recycling endosomes. In each image autofluorescent lysosome-like organelles are shown in blue in all three channels, whereas GFP appears only in the green channel and RFP only in the red channel. Signals observed in the green or red channels that do not overlap with signals in the blue channel are considered bone fide GFP or RFP signals, respectively. Scale bar, 10 µm. We identified the intracellular puncta labeled by CED-10 as endosomes by performing a series of colocalization studies with a previously established set of intestine-specific compartment markers [2]–[6]. CED-10 appeared specifically enriched on endosomes along the early and recycling pathway. We observed direct overlap of intestinal GFP-CED-10-labeled puncta and a subset of early endosomes marked by RFP-RAB-5 (Figure 3D, 3E and 3F). GFP-CED-10 showed the strongest colocalization with recycling endosome marker RFP-RAB-10 (Figure 3J, 3K and 3L), and displayed less overlap with later acting recycling endosome protein RFP-RME-1 (Figure 3G–3I). Similarly, we also observed colocalization between CED-12-GFP and markers of early endosomes and recycling endosomes (Figure S6J–S6L, and data not shown). Little overlap was observed between GFP-CED-10 and markers for late endosomes (GFP-RAB-7), the Golgi (MANS-GFP), or multi-vesicular bodies (GFP-HGRS-1/Hrs) indicating specificity in endosome-type associated with CED-10 (Figure S6A–S6I).
In order to confirm the endosomal localization of CED-10 we assayed for changes in the localization of GFP-CED-10 in rab-10 and rme-1 mutant backgrounds where the morphology of specific types of endosomes is specifically disrupted. Indeed we found that loss of either RAB-10 or RME-1 disrupted GFP-CED-10 localization (Figure 3M–3O). In rab-10 and rme-1 mutants, GFP-CED-10 labeled the grossly enlarged endosomes that were produced (Figure 3M–3O). Since our previous work showed that rme-1 mutants accumulate enlarged basolateral recycling endosomes without affecting early endosomes, and rab-10 mutants accumulate enlarged early endosomes and display reduced numbers of recycling endosomes, our results further indicate the residence of CED-10/Rac1 on both early and recycling endosome types [2], [3].
TBC-2 functions with CED-10 to mediate recycling
While most Rac1 effectors are thought to be plasma membrane localized, recent work identified the TBC-domain Rab-GAP protein Armus (TBC1D2) as an endosome associated Rac1 effector [26]. This work showed that active GTP-bound Rac1 binds to Armus, regulating the trafficking of E-cadherin, and thus cell adhesion, in MDCK cells [26]. The C. elegans homolog of Armus is TBC-2, a TBC-domain protein recently shown to function as a RAB-5 GAP important for the regulation of endocytosis and phagocytosis in vivo [27], [28]. Genetic analysis indicated that in the absence of TBC-2, RAB-5 activity is abnormally high, and early and late endosomes of the intestine are enlarged [27]. The enlargement of late endosomes in tbc-2 mutants could be phenocopied by expression of constitutively active RAB-5(Q78L), and could be suppressed by depletion of RAB-5, RAB-7, or the HOPS complex. Thus it was proposed that hyperactive RAB-5 in tbc-2 mutants leads to increased RAB-7 activity, and apparent increased lysosomal degradative activity [27], [29].
Previous studies did not determine if TBC-2/Armus is important for endocytic recycling. Since Rab GTPases generally function sequentially as cargo progresses along membrane trafficking pathways, one might expect that de-activation of the early acting GTPase RAB-5 is required to allow proper functioning of recycling endosomes associated with later acting GTPases such as RAB-10 [30]. Thus we sought to determine if TBC-2 functions with CED-10/Rac1 in C. elegans, and if TBC-2 function is required for endocytic recycling.
In agreement with the work on mammalian Armus, we observed clear and consistent interactions between GFP::TBC-2 and CED-10 in GST pull-down experiments from C. elegans lysates. We found that GFP::TBC-2 interacts with a constitutively active mutant form of CED-10, G12V, or wild-type CED-10 loaded with GTPγS, but not wild-type CED-10 loaded with GDP (Figure 4A and 4B). Thus TBC-2 interacts specifically with activated CED-10, indicating that the physical interaction between Rac1/CED-10 and Armus/TBC-2 is evolutionarily conserved.
Fig. 4. TBC-2 is recruited to endosomes by CED-10. 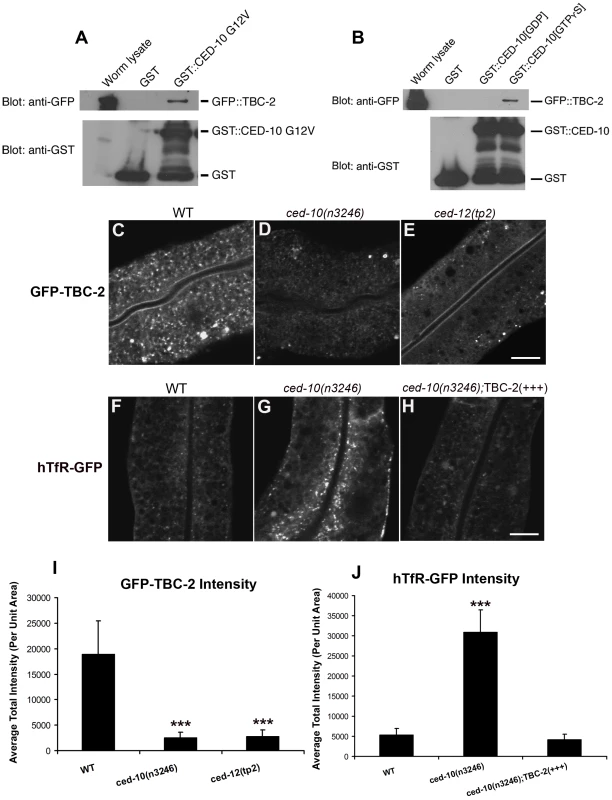
(A) Glutathione beads loaded with recombinant GST or GST-CED-10(G12V), or (B) GST-CED-10(GDP) or GST-CED-10(GTPγS) were incubated with worm lysates containing GFP::TBC-2 and then washed to remove unbound proteins. Bound proteins were eluted and analyzed by Western blot using anti-GFP (top) or anti-GST (bottom) antibodies. Worm lysate represents 1% of the input. No GFP::TBC-2 was detected in non-transgenic worm lysates (not shown). (C–G) Representative confocal micrographs for GFP-TBC-2 and hTfR-GFP in various genetic backgrounds are shown. All images were collected from living intact adult animals expressing intestine-specific transgenes. (C–E) GFP-TBC-2 localization to endosomes is strongly reduced in ced-10 and ced-12 mutants. (F–H) hTfR-GFP overaccumulation in ced-10 mutants is suppressed by over-expression of RFP-TBC-2. (I–J) Quantification of average puncta and tubule intensities for the indicated genotypes are shown. Asterisks indicate a significant difference in the one-tailed Student's T-test (***p<0.0001). For all the presented data, error bars represent standard deviations from the mean (n = 18 each, 6 animals of each genotype sampled in three different regions of each intestine). Scale bar, 10 µm. To determine if CED-10 is important for TBC-2 recruitment to endosomes, we examined the subcellular localization of GFP-TBC-2 in ced-10 and ced-12 mutants. We found that the normal punctate endosomal distribution of GFP-TBC-2 was disrupted in animals lacking CED-10 or CED-12 (Figure 4C–4E). The intensity of GFP-TBC-2 endosomal labeling was reduced by 6 to 7-fold in ced-10 and ced-12 mutants (Figure 4I). These results indicate that CED-10 and CED-12 are required in vivo for the efficient recruitment of TBC-2 to endosomal membranes, likely through direct binding of CED-10(GTP) to TBC-2.
To determine if TBC-2 is important for endocytic recycling, we analyzed the localization of recycling endosome markers and recycling cargo markers in the tbc-2(tm2241) deletion mutant. We found that basolateral recycling endosome markers GFP-RME-1 and SDPN-1-GFP were severely perturbed in tbc-2 mutants in a manner similar to that found in ced-10, ced-12, and ced-5 mutants (Figure 5E–5H and 5J). Loss of TBC-2 also resulted in intracellular accumulation of hTAC-GFP and hTfR-GFP, similar to the phenotype observed in ced-10, ced-12, and ced-5 mutants (Figure 5A–5D and 5I). hTAC-GFP in tbc-2 mutants colocalized with recycling endosome marker EHBP-1-MC, indicating cargo trapping in recycling endosomes (Figure 5K–5K″). Furthermore, we reasoned that if the role of TBC-2 in recycling was to convert RAB-5(GTP) to RAB-5(GDP), then expression of GTPase-defective RAB-5 should also interfere with cargo recycling. Consistent with this model we found strong intracellular accumulation of hTAC-GFP and hTfR-GFP in the intestinal cells of animals expressing GTPase-defective RAB-5(Q78L) (Figure 6A–6F). Taken together these results indicate that the recycling of clathrin-independent cargo hTAC and clathrin-dependent cargo hTfR require TBC-2-depedent RAB-5 down-regulation. These results indicate that TBC-2 function is critical for the basolateral endocytic recycling pathway.
Fig. 5. TBC-2 is required for recycling endosome morphology and function. 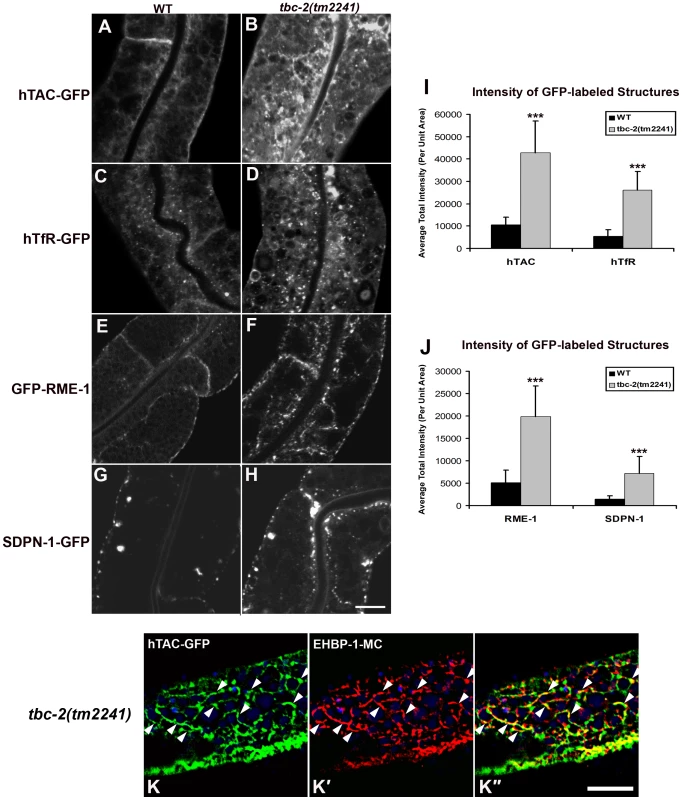
Representative confocal micrographs are shown for hTAC-GFP, hTfR-GFP, GFP-RME-1 and SDPN-1-GFP, in a tbc-2 mutant background. All images were collected from living intact adult animals expressing intestine-specific transgenes. (A–D) Recycling cargos hTAC-GFP and hTfR-GFP over-accumulate in tbc-2 mutants. (I) Quantification of hTAC-GFP and hTfR-GFP puncta and tubule intensity in the intestine of living intact wild-type and tbc-2 mutant animals. (E–H) Recycling endosome markers GFP-RME-1 and SDPN-1-GFP also over-accumulate in tbc-2 mutants. (J) Quantification of puncta and tubule intensity of GFP-RME-1 and SDPN-1-GFP per unit area. (K–K″) Confocal images of the intestinal epithelium in live intact tbc-2(tm2241) mutant animals expressing recycling cargo hTAC-GFP and recycling endosome marker EHBP-1-MC. Autofluorescent lysosomes are shown in blue. Asterisks indicate a significant difference in the one-tailed Student's t test (***p<0.0001). Error bars represent standard deviations from the mean (n = 18 each, 6 animals of each genotype sampled in three different regions of each intestine). Scale bar, 10 µm. Fig. 6. Expression of GTPase-defective RAB-5 interferes with the trafficking of recycling cargo. 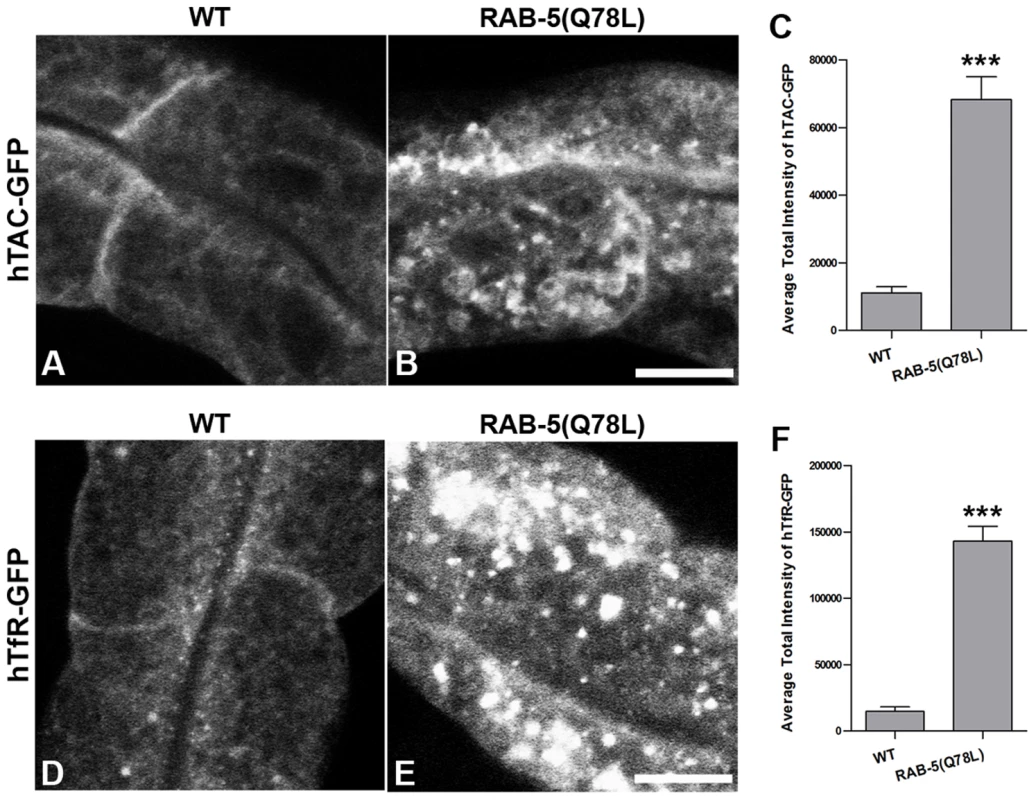
(A and B) Confocal images of recycling cargo hTAC-GFP in the intestinal epithelium. Wild-type animals (A) and animals expressing of GTPase-defective RAB-5 (tagRFP-RAB-5(Q78L)) (B) are shown. (C) Quantification of hTAC-GFP puncta and tubule intensity. (D and E) Confocal images of recycling cargo hTfR-GFP in the intestinal epithelium. Wild-type animals (D) and animals expressing of GTPase-defective RAB-5 (tagRFP-RAB-5(Q78L)) (E) are shown. (F) Quantification of hTfR-GFP puncta intensity. Error bars represent standard deviations from the mean (n = 18 each, 6 animals of each genotype sampled in three different regions of each intestine). Asterisks indicate a significant difference in the one-tailed Student's T-test (***p<0.0001). Scale bar, 10 µm. We also sought further evidence that TBC-2 functions downstream of CED-10/Rac1 in the recycling pathway. We reasoned that if the ced-10 recycling phenotype is due to poor recruitment of TBC-2 to endosomes, then overexpression of TBC-2 might ameliorate ced-10 mutant defects. Indeed, we found that the overexpression of RFP-tagged TBC-2 completely rescued the abnormal accumulation of recycling cargo hTfR-GFP in ced-10 mutant animals (Figure 4F–4H and 4J). These results strongly suggest that TBC-2 is a key CED-10/Rac1 effector required for endocytic recycling.
Discussion
Rab conversion has recently emerged as a general principle of membrane traffic, acting as a key regulator of vectorial transport of cargo along the secretory and endocytic pathways [30]. Countercurrent cascades of Rab GEFs and Rab GAPs have been proposed to mediate such Rab conversion [30]. In the simplest form of such a cascade, the early acting Rab recruits the GEF for the next Rab along the pathway, while the later acting Rab recruits the GAP for the earlier acting Rab, producing a directional exchange of Rab-GTPases as cargo progresses along the pathway.
One well-studied Rab conversion event occurs during the early to late endosome transition, and during phagosome maturation, via a RAB-5 to RAB-7 switch [31]–[33]. RAB-5(GTP) recruits the SAND-1(Mon1)/CZZ-1 heterodimer, which in turn acts to displace RABX-5 (Rabex-5), a key RAB-5 GEF [32], [33]. SAND-1/Mon1 also aids in RAB-7 recruitment, in part through interactions with the HOPS complex, a shared RAB-5/RAB-7 effector [32]. Recent evidence in yeast further indicates that the Mon1/Czz1 dimer is a Ypt7 (Rab7) GEF, acting to activate Ypt7 (Rab7) as the endosome matures [34].
TBC-2 also appears to play a role in this process as a RAB-5 GAP. tbc-2 null mutants produce grossly enlarged early and late endosomes, a phenotype very similar to that produced upon expression of constitutively active RAB-5(Q78L) [27]. These tbc-2(-) phenotypes can be suppressed by partial knockdown of RAB-5, RAB-7, or components of the HOPS complex, suggesting that TBC-2 is normally required to dampen the RAB-5 driven cascade that activates RAB-7 [27].
Importantly, our current study shows that TBC-2 also strongly influences the recycling arm of the endocytic pathway, through interaction with CED-10/Rac1. This is particularly interesting because little is known of how the transition from early endosome to recycling endosome is achieved or how Rab GEFs and GAPs might be involved. Most studies on the early endosome to recycling endosome transition have focused on the joint Rab5/Rab4 effector Rabaptin5, or the neuron-specific Rab4 effector GRASP-1 [35], [36]. Our results indicate that CED-10/Rac1 resides on early and recycling endosomes where it is likely activated by the CED-5/CED-12 bipartite GEF. This is reminiscent of the activation of Rac1 by a different Rho-GEF, Tiam1, on endosomes of migrating mammalian cells [19]. Our results are the first to clearly show that CED-10/Rac1 is required for the recycling process, and is not simply a recycling cargo. Furthermore our work provides mechanistic insight into this requirement, showing that CED-10 acts to recruit TBC-2 to endosomal membranes.
These results indicate that down-regulation of RAB-5 by TBC-2 is an important aspect of cargo recycling, and may serve as part of a program for Rab conversion along the recycling pathway. This is consistent with early work on Rab5 indicating that overexpressed Rab5(Q78L) inhibits transferrin recycling in HeLa cells [37]. It remains unclear if direct down-regulation of RAB-7 is also important to promote recycling, since the TBC-2 homolog Armus was originally described as a Rab7 GAP, and C. elegans TBC-2 displays some GAP activity toward RAB-7 in vitro, albeit at a lower level than its activity toward RAB-5 [26], [27].
In one important respect the ced-10 mutant phenotype differs from that of tbc-2 null mutants, in that RAB-7-positive late endosomes appear insensitive to CED-10 activity (Figure S5G and S5H). This suggests that while TBC-2 is generally important for regulating RAB-5, its interaction with CED-10 is mainly important for regulating RAB-5 along the recycling arm of the endocytic pathway. The specificity of the ced-10 mutant phenotype suggests that TBC-2 maintains some activity and membrane localization that is CED-10 independent, perhaps by direct lipid binding and/or interactions with additional endosomal proteins [28].
The mechanisms that give rise to recycling endosomes are not clear, but most work suggests that recycling endosomes are formed from fission products that leave the early endosome as it matures toward a late endosome [7], [38]. Fission products released from the Trans-Golgi also contribute to the recycling endosome [39]. Once endosomal and Golgi-derived fission products fuse with one another and with pre-existing recycling endosomes, they would be expected to take on new recycling endosome-specific characteristics, including changing their phosphoinositide content and Rab-GTPase activities. Given the plethora of evidence that RAB-10 regulates basolateral recycling in many polarized cells, as first shown in the C. elegans intestine, one possibility is that the CED-10/TBC-2 interaction acts to promote a RAB-5 to RAB-10 transition [2], [40], [41]. In addition, endosomes are thought to contain functionally distinct subdomains [42], [43]. The recycling endosome may maintain a RAB-5 positive fusogenic domain for incoming vesicles, and likely maintains multiple distinct tubular budding domains that accumulate outgoing cargo destined for different cellular compartments such as the basolateral plasma membrane, apical plasma membrane, and Golgi [42]. Thus another possible role for CED-10 to TBC-2 signaling is to provide negative feedback from RAB-10 to RAB-5 that maintains distinct RAB-10 and RAB-5 subdomains on the common recycling endosome. Both the Rab transition or subdomain maintenance models are consistent with the partial overlap in localization of RAB-5 and RAB-10 observed on basolateral endosomes of the C. elegans intestine, and both models predict that RAB-10 will be involved in recruiting or activating CED-10 and TBC-2 on recycling endosomes [2]. Future work will be directed at testing these models.
Interestingly, previous work in MDCK cells expressing constitutively active Rac1(V12) suggested that Rac1 could play a role in regulating the function of the common recycling endosome [44]. In that work Jou et al. identified a Rac1(V12)-induced morphological defect in recycling endosomes that contain both apical and basolateral cargos. However pulse-chase analysis in Rac1(V12) MDCK cells defined defects in apically-directed common endosome-dependent functions (basolateral-to-apical transcytosis, apical recycling, and apical secretion), but surprisingly found no defect in basolateral recycling. The physiological relevance of these results were unclear because expression of dominant negative Rac1(N17) did not affect recycling, and efficient knockdown methods were not available at that time to directly assay Rac1 loss-of-function. It would be of interest to revisit the MDCK system to investigate more fully the role of Rac1 and Armus in Rab5 down-regulation at the common recycling endosome, directly assaying for phylogenetic conservation of the mechanisms that we have defined here in the C. elegans intestine.
Our data connecting CED-10 to TBC-2 also has important implications for phagocytosis/engulfment, since CED-10/Rac1 and TBC-2 are both known to function early in the apoptotic corpse phagocytosis pathway. The potential importance of an interaction between CED-10 and TBC-2 during that process remains unexplored, but a function in promoting endocytic recycling may contribute to lamellipodia formation [28], [45]. RAB-10 has also recently been implicated in regulating phagosome maturation, and thus may be relevant to understanding the phagocytic functions of CED-10/Rac1 and TBC-2 [46].
Materials and Methods
General methods and strains
All C. elegans strains were derived originally from the wild-type Bristol strain N2. Worm cultures, genetic crosses, and other C. elegans husbandry were performed according to standard protocols [47]. Strains were maintained at 20°C. A complete list of strains used in this study can be found in Table S1—under a sub-heading “Transgenic and mutant strains used in this study”.
Plasmid and transgenic worm strain construction
The CED-10 expression plasmid was created by PCR amplification and Gateway cloning of the ced-10 cDNA, lacking a start codon, into the Gateway entry vector pDONR221 (Invitrogen, Carlsbad, CA). To create N-terminally tagged GFP or RFP/mCherry transgenes for CED-10 or RAB-5(Q78L) for expression specifically in the worm intestine, Gateway destination vectors were used that contain the promoter region of the intestine-specific gene vha-6 cloned into the C. elegans pPD117.01 vector, a Gateway cassette followed by a GFP or RFP/mCherry coding sequence and then the unc-119 gene of C. briggsae.
To construct C-terminally tagged GFP or mCherry transgenes for CED-5/-12 for expression in the worm intestine, cDNA sequences of C. elegans ced-5 and ced-12 lacking a stop codon were cloned individually into Gateway entry vector pDONR221 by PCR and BP reaction, and then transferred into intestinal expression vectors by Gateway recombination cloning LR reaction according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). All plasmids used in this study were sequenced and complete plasmid sequences are available on request.
Low copy integrated transgenic lines for all of these plasmids were obtained using the microparticle bombardment method [48].
Microscopy and image analysis
Live worms were mounted on 2% agarose pads containing 100 mM tetramisole (MP Biomedicals, OH) in M9 buffer. Most GFP versus mCherry/RFP colocalization experiments were performed on L3 and L4 larvae expressing GFP and mCherry/RFP markers. Young adult hermaphrodites expressing GFP were used for taking confocal images. Images taken in the DAPI channel were used to identify broad-spectrum intestinal autofluorescence caused by lipofuscin-positive lysosome-like organelles [25], [49].
Multi-wavelength fluorescence images were obtained using an Axiovert 200 M (Carl Zeiss MicroImaging, Oberkochen, Germany) microscope equipped with a digital CCD camera (C4742–12ER, Hamamatsu Photonics, Hamamatsu, Japan). Metamorph software version 6.3r2 (Universal Imaging, West Chester, PA) was utilized for image acquisition and Z-stacks of images were deconvolved with AutoDeblur Gold software ver 9.3 (AutoQuant Imaging, Watervliet, NY).
To obtain images of GFP fluorescence without interference from autofluorescence, the spectral fingerprinting function of a Zeiss LSM510 Meta confocal microscope system (Carl Zeiss MicroImaging) was used as described previously [2]. Quantification of confocal images was performed with MetaMorph software version 6.3r2. The same threshold values were used for all images within a given experiment. For each marker comparison, at least six animals were analyzed. Three randomly selected regions of per animal were analyzed using circular regions of defined area. Quantification of fluorescence intensities or object count was performed. The average total intensity or average puncta number was calculated. Student's t-test was used to determine the difference between the different groups.
Preparation of worm extracts, GST protein purification, and pull-downs
Worm extracts were prepared from a mixed-stage population of wild-type and tbc-2(tm2241); vhIs12[Pvha-6::GFP::tbc-2+Cb-unc-119] animals. Worms were grown on egg plates, harvested with 0.1 M NaCl, floated in 30% fresh sucrose solution, and washed three times with 0.1 M NaCl. The worm pellet was frozen in liquid nitrogen and stored at −80°C. Frozen worm pellets were thawed on ice. An equal volume of fresh ice-cold lysis buffer (50 mM HEPES pH 7.4, 1 mM EGTA, 150 mM KCl, 1 mM MgCl2, 10% glycerol, 0.2% Triton X-100, Protease inhibitors: Pepstatin, Leupectin, Aprotinin, Sodium Orthovanadate, and PMSF) was added. One mL of the suspension was subjected to 35 strokes in a 2-mL pyrex tissue homogenizer (Thermo Fisher Scientific, Waltham, MA) at 4°C. The suspension was centrifuged twice at 12,000 g for 10 minutes and once for 20 minutes at 4°C. The resulting supernatant was recovered.
The plasmid pGEX-5X-CED-10 was constructed by PCR amplification of ced-10 from a cDNA template (kindly provided by Dr. Erik Lundquist, University of Kansas) using the primers 5′-GAT CGG ATC CCC CAA GCG ATC AAA TGT GTC GT-3′ and 5′-GAT CCT CGA GTT ACT TGC TCT TTT TGG CTC TTT-3′. The resulting PCR product was cloned in plasmid pGEX-5X-2 (GE Healthcare Life Sciences, Buckinghamshire, England) using the BamHI and XhoI restriction sites.
To purify the GST proteins, overnight cultures (10 mL) of BL21 E. coli transformed with pGEX-5X-2 vectors were diluted 10-fold in 2X YT with 100 mg/mL ampicillin, grown for one hour at 37°C with shaking. Protein expression was induced with 0.5 mM IPTG and shaking at 25°C for 2 hours. The culture was centrifuged at 5,000 g for 10 minutes at 4°C and the pellet was resuspended in 10 mL ice-cold PBS, centrifuged at 3,000 g for 10 minutes at 4°C, and the pellet was resuspended in 1 mL PBS containing 1 mM PMSF and 7 mM ß-mercaptoethanol. Cells were sonicated 10 seconds on ice, freeze/thawed three times in liquid nitrogen and a 20°C water bath, and mixed with 100 µL of 10% Triton X-100. The sample was centrifuged at 12,000 g for 5 minutes at 4°C. The supernatant was gently mixed with 100 µL of 50% glutathione sepharose beads coated with 5% BSA. The sample was centrifuged 500 g for 60 seconds at room temperature and washed with 1 mL of ice-cold PBS two times, centrifuged for 10 seconds at room temperature and the pellet was resuspended in 1 mL PBS was stored at 4°C for not more than 4 weeks.
For the pull-down assays, 10 µg of the purified GST-CED-10 (or GST as the negative control) was added to 800 µL of worm lysate, 20 µL of extra glutathione sepharose beads (3X washed with PBS and pre-coated with 5% BSA), 0.2 mM GDP or GTPγS (Sigma-Aldrich, St. Louis, MO) and 10 mM MgCl2, and then incubated for 2 hours rotating at 4°C. The mixture was washed 3 times with ice-cold lysis buffer (without protease inhibitors) and proteins were eluted in 60 µL 2X Laemmli buffer for 20 minutes at 65°C. The samples were centrifuged at 500 g for one minute at room temperature and 25 µL of eluted proteins were carefully collected from the solution above the beads and analyzed by SDS-polyacrylamide-gel electrophoresis using 8% resolving gel and blotted onto nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). GFP::TBC-2 was detected using a goat anti-GFP antibody (Rockland Inc., Gilbertville, PA) and a donkey anti-goat antibody (Santa Cruz Biotechnology, CA) at a 1∶1000 and a 1∶10,000 dilution, respectively. To detect GST and GST::CED-10 proteins, the nitrocellulose membrane was reprobed with a rabbit anti-GST antibody and a goat anti-rabbit antibody (Sigma-Aldrich, St. Louis, MO) at a 1∶2000 and a 1∶10,000 dilution, respectively.
Supporting Information
Zdroje
1. McGheeJD The C. elegans Research Community, editor 2007 The C. elegans intestine. WormBook doi/10.1895/wormbook.1.133.1
2. ChenCCSchweinsbergPJVashistSMareinissDPLambieEJ 2006 RAB-10 is required for endocytic recycling in the Caenorhabditis elegans intestine. Mol Biol Cell 17 1286 1297
3. ShiAPantSBalklavaZChenCCFigueroaV 2007 A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr Biol 17 1913 1924
4. PantSSharmaMPatelKCaplanSCarrCM 2009 AMPH-1/Amphiphysin/Bin1 functions with RME-1/Ehd1 in endocytic recycling. Nat Cell Biol 11 1399 1410
5. ShiASunLBanerjeeRTobinMZhangY 2009 Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. Embo J 28 3290 3302
6. ShiAChenCCBanerjeeRGlodowskiDAudhyaA 2010 EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Mol Biol Cell. 21 2930 2943
7. GrantBDDonaldsonJG 2009 Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10 597 608
8. PanCLBaumPDGuMJorgensenEMClarkSG 2008 C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 14 132 139
9. YangPTLorenowiczMJSilhankovaMCoudreuseDYBetistMC 2008 Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 14 140 147
10. LinSXGrantBHirshDMaxfieldFR 2001 Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol 3 567 572
11. CaplanSNaslavskyNHartnellLMLodgeRPolishchukRS 2002 A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. Embo J 21 2557 2567
12. GrantBDCaplanS 2008 Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic 9 2043 2052
13. KinchenJMRavichandranKS 2007 Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci 120 2143 2149
14. BrugneraEHaneyLGrimsleyCLuMWalkSF 2002 Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 4 574 582
15. ReddienPWHorvitzHR 2000 CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol 2 131 136
16. CabelloJNeukommLJGunesdoganUBurkartKCharetteSJ 2010 The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol 8 e1000297 doi:10.1371/journal.pbio.1000297
17. RadhakrishnaHAl-AwarOKhachikianZDonaldsonJG 1999 ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci 112 Pt 6 855 866
18. KooTHEipperBADonaldsonJG 2007 Arf6 recruits the Rac GEF Kalirin to the plasma membrane facilitating Rac activation. BMC Cell Biol 8 29
19. PalamidessiAFrittoliEGarreMFarettaMMioneM 2008 Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134 135 147
20. DonaldsonJGPorat-ShliomNCohenLA 2009 Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal 21 1 6
21. LundquistEAReddienPWHartwiegEHorvitzHRBargmannCI 2001 Three C. elegans Rac proteins and several alternative Rac regulators control axon guidance, cell migration and apoptotic cell phagocytosis. Development 128 4475 4488
22. NaslavskyNWeigertRDonaldsonJG 2003 Convergence of non-clathrin - and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell 14 417 431
23. NaslavskyNWeigertRDonaldsonJG 2004 Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell 15 3542 3552
24. WeigertRYeungACLiJDonaldsonJG 2004 Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell 15 3758 3770
25. ClokeyGVJacobsonLA 1986 The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev 35 79 94
26. FrasaMAMaximianoFCSmolarczykKFrancisREBetsonME 2010 Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol 20 198 208
27. ChotardLMishraAKSylvainMATuckSLambrightDG 2010 TBC-2 regulates RAB-5/RAB-7-mediated endosomal trafficking in Caenorhabditis elegans. Mol Biol Cell 21 2285 2296
28. LiWZouWZhaoDYanJZhuZ 2009 C. elegans Rab GTPase activating protein TBC-2 promotes cell corpse degradation by regulating the small GTPase RAB-5. Development 136 2445 2455
29. ChotardLSkorobogataOSylvainMAShrivastavaSRocheleauCE 2010 TBC-2 is required for embryonic yolk protein storage and larval survival during L1 diapause in Caenorhabditis elegans. PLoS ONE 5 e15662 doi:10.1371/journal.pone.0015662
30. HutagalungAHNovickPJ 2011 Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91 119 149
31. RinkJGhigoEKalaidzidisYZerialM 2005 Rab conversion as a mechanism of progression from early to late endosomes. Cell 122 735 749
32. PoteryaevDDattaSAckemaKZerialMSpangA 2010 Identification of the switch in early-to-late endosome transition. Cell 141 497 508
33. KinchenJMRavichandranKS 2010 Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 464 778 782
34. NordmannMCabreraMPerzABrockerCOstrowiczC 2010 The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 20 1654 1659
35. DenekaMNeeftMPopaIvan OortMSprongH 2003 Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. Embo J 22 2645 2657
36. HoogenraadCCPopaIFutaiKMartinez-SanchezEWulfPS 2010 Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol 8 e1000283 doi:10.1371/journal.pbio.1000283
37. StenmarkHPartonRGSteele-MortimerOLutckeAGruenbergJ 1994 Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. Embo J 13 1287 1296
38. HsuVWPrekerisR 2010 Transport at the recycling endosome. Curr Opin Cell Biol 22 528 534
39. AngALTaguchiTFrancisSFolschHMurrellsLJ 2004 Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167 531 543
40. BabbeyCMAhktarNWangEChenCCGrantBD 2006 Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell 17 3156 3175
41. SchuckSGerlMJAngAManninenAKellerP 2007 Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic 8 47 60
42. ThompsonANesslerRWiscoDAndersonEWincklerB 2007 Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell 18 2687 2697
43. SonnichsenBDe RenzisSNielsenERietdorfJZerialM 2000 Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149 901 914
44. JouTSLeungSMFungLMRuizWGNelsonWJ 2000 Selective alterations in biosynthetic and endocytic protein traffic in Madin-Darby canine kidney epithelial cells expressing mutants of the small GTPase Rac1. Mol Biol Cell 11 287 304
45. StruckhoffECLundquistEA 2003 The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C. elegans. Development 130 693 704
46. CardosoCMJordaoLVieiraOV 2010 Rab10 regulates phagosome maturation and its overexpression rescues Mycobacterium-containing phagosomes maturation. Traffic 11 221 235
47. BrennerS 1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
48. PraitisVCaseyECollarDAustinJ 2001 Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 1217 1226
49. HermannGJSchroederLKHiebCAKershnerAMRabbittsBM 2005 Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Mol Biol Cell 16 3273 3288
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



