-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
During nervous system development, neuronal cell bodies and their axodendritic projections are precisely positioned through transiently expressed patterning cues. We show here that two neuronally expressed, secreted immunoglobulin (Ig) domain-containing proteins, ZIG-5 and ZIG-8, have no detectable role during embryonic nervous system development of the nematode Caenorhabditis elegans but are jointly required for neuronal soma and ventral cord axons to maintain their correct position throughout postembryonic life of the animal. The maintenance defects observed upon removal of zig-5 and zig-8 are similar to those observed upon complete loss of the SAX-7 protein, the C. elegans ortholog of the L1CAM family of adhesion proteins, which have been implicated in several neurological diseases. SAX-7 exists in two isoforms: a canonical, long isoform (SAX-7L) and a more adhesive shorter isoform lacking the first two Ig domains (SAX-7S). Unexpectedly, the normally essential function of ZIG-5 and ZIG-8 in maintaining neuronal soma and axon position is completely suppressed by genetic removal of the long SAX-7L isoform. Overexpression of the short isoform SAX-7S also abrogates the need for ZIG-5 and ZIG-8. Conversely, overexpression of the long isoform disrupts adhesion, irrespective of the presence of the ZIG proteins. These findings suggest an unexpected interdependency of distinct Ig domain proteins, with one isoform of SAX-7, SAX-7L, inhibiting the function of the most adhesive isoform, SAX-7S, and this inhibition being relieved by ZIG-5 and ZIG-8. Apart from extending our understanding of dedicated neuronal maintenance mechanisms, these findings provide novel insights into adhesive and anti-adhesive functions of IgCAM proteins.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002819
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002819Summary
During nervous system development, neuronal cell bodies and their axodendritic projections are precisely positioned through transiently expressed patterning cues. We show here that two neuronally expressed, secreted immunoglobulin (Ig) domain-containing proteins, ZIG-5 and ZIG-8, have no detectable role during embryonic nervous system development of the nematode Caenorhabditis elegans but are jointly required for neuronal soma and ventral cord axons to maintain their correct position throughout postembryonic life of the animal. The maintenance defects observed upon removal of zig-5 and zig-8 are similar to those observed upon complete loss of the SAX-7 protein, the C. elegans ortholog of the L1CAM family of adhesion proteins, which have been implicated in several neurological diseases. SAX-7 exists in two isoforms: a canonical, long isoform (SAX-7L) and a more adhesive shorter isoform lacking the first two Ig domains (SAX-7S). Unexpectedly, the normally essential function of ZIG-5 and ZIG-8 in maintaining neuronal soma and axon position is completely suppressed by genetic removal of the long SAX-7L isoform. Overexpression of the short isoform SAX-7S also abrogates the need for ZIG-5 and ZIG-8. Conversely, overexpression of the long isoform disrupts adhesion, irrespective of the presence of the ZIG proteins. These findings suggest an unexpected interdependency of distinct Ig domain proteins, with one isoform of SAX-7, SAX-7L, inhibiting the function of the most adhesive isoform, SAX-7S, and this inhibition being relieved by ZIG-5 and ZIG-8. Apart from extending our understanding of dedicated neuronal maintenance mechanisms, these findings provide novel insights into adhesive and anti-adhesive functions of IgCAM proteins.
Introduction
The structural organization of an adult nervous system depends on two genetically separable processes. First, during development - the wiring phase - the soma and axonal/dendritic extensions of neurons need to be accurately positioned. This process depends on the precisely orchestrated activity of a multitude of well-characterized and dynamically acting guidance and signaling systems [1], [2], [3]. Second, during postembryonic life, dedicated maintenance factors ensure that neuronal soma, axon and dendrites maintain their precise position in neuronal ganglia and fascicles [4]. These maintenance factors counteract the various forms of mechanical and physical stress exerted onto a nervous system [4].
The need for such maintenance mechanisms, and the specific maintenance factors involved, were first identified in the nematode C. elegans. The removal of a number of distinct molecules was found to result in no apparent effect on the initial positioning of neurons and fibers during embryonic development; yet the absence of these molecules affected the maintenance of the positioning of neuronal soma and fibers. These molecules include the L1CAM-type adhesion molecule SAX-7 [5], [6], [7], the extracellular matrix protein DIG-1 [8], a specific splice form the FGF receptor EGL-15, called EGL-15A [9] and ZIG-3 and ZIG-4, members of a family of small, secreted two-Ig domain proteins [10], [11](Figure 1A). While SAX-7, DIG-1, EGL-15A and the ZIG proteins appear to be solely dedicated to a maintenance role, other proteins, such as the basement membrane protein SPON-1/Spondin or UNC-70/β–Spectrin function both during the embryonic neuronal wiring phase and postembryonically in maintenance [12], [13].
Fig. 1. Neuronal maintenance factors and the defects caused by their removal. 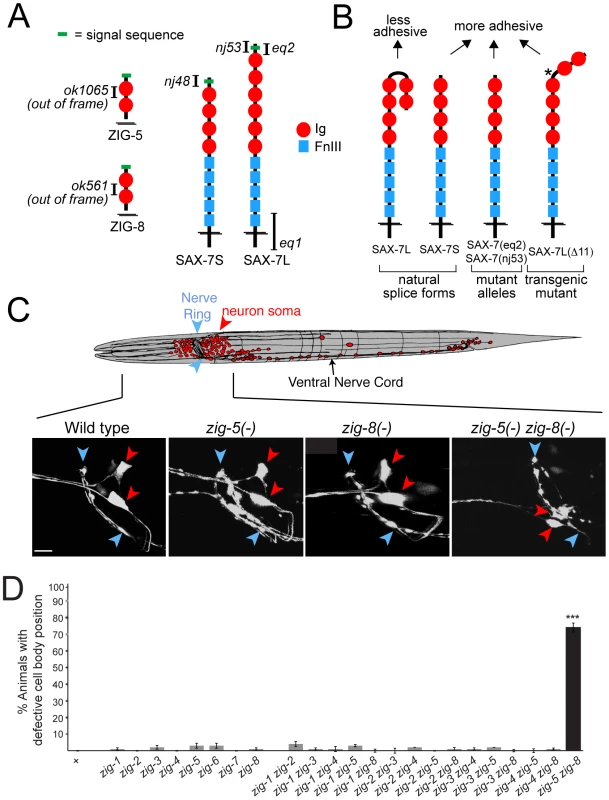
(A) Schematic protein structures and alleles used in this study. (B) Summary of previous in vitro and in vivo adhesion studies [6], [7]. Star indicates a shortened hinge region which prevents formation of the horseshoe configuration [7]. (C) ASI and ASH neuronal displacements observed in zig-5(ok1065) and zig-8(ok561) single and double mutant adult animals with the oyIs14 reporter transgene. Blue arrowheads indicate position of the nerve ring and red arrowheads position of neuronal soma, which is scored relative to position of the nerve ring (wild type: behind nerve ring; mutant: on top of to nerve ring). Anterior to left, dorsal on top. Scale bar is 5 µm. (D) Quantification of ASI and ASH neuronal displacement in single and double mutants of the zig gene family. Alleles are described in [11]. Error bars indicate s.e.p.. Proportions of different animal populations were compared using the z-test. “*” indicates p<0.001. How these maintenance factors functionally interact with one another has been unclear. In this paper, we describe the function of two previously uncharacterized ZIG proteins, ZIG-5 and ZIG-8, in maintaining neuron soma position. We tie their function specifically to the function of SAX-7, the C. elegans ortholog of the L1CAM family of vertebrate adhesion molecules. In C. elegans, SAX-7 exists in two splice forms, a short splice form (SAX-7S) and a long splice form (SAX-7L)(Figure 1B). Intriguingly, several studies have shown that the short isoform, SAX-7S, is more adhesive than the long isoform SAX-7L [6], [7], [14]. We show here that the two ZIG proteins ZIG-5 and ZIG-8 serve to prevent the SAX-7L isoform from interfering with cellular adhesion.
Results
zig-5 and zig-8 redundantly affect neuron soma and axon position
Loss of the C. elegans L1CAM ortholog sax-7 affects the maintenance of neuron soma position in the main head ganglia of C. elegans, as well as the positioning of axons in the ventral nerve cord (VNC) [5], [6], [7], [14]. Loss of two members of the zig gene family (zig-3 and zig-4) also affects the maintenance of axon positioning in the ventral nerve cord, but do not affect neuron soma position [10], [11]. To test whether zig genes may phenocopy the sax-7 effect on the maintenance of soma position in head ganglia, we analyzed deletion alleles of all presently known, eight zig gene family members. Visualizing head neuron position either with gfp reporters or by dye labeling showed no defects in any zig single mutant strain (Figure 1C, 1D). Since zig genes may act redundantly, we generated double mutant combinations of all six neuronally expressed zig genes (that is all zig genes except muscle-expressed zig-6 and zig-7; Aurelio and Hobert, 2002). This double mutant analysis led us to discover striking neuronal soma displacement defects in head ganglia of zig-5(ok1065) zig-8(ok561) double null mutant animals (Figure 1C, 1D). This defect can be observed both with cell-type specific gfp reporters (Figure 1C) as well as with dye filling of sensory neurons (47% animals affected; n = 150).
zig-5 zig-8 double mutants also display postembryonic axon position defects in the VNC (Figure 2). The C. elegans VNC is composed of unilaterally positioned motoneuron axons, located on the right side of the VNC and of axons of bilaterally symmetric neurons that extend along the left and right side of the VNC [15]. The left and right side of the VNC are separated by a midline structure, initially made of neuronal cell bodies, later of a hypodermal ridge [16]. In zig-5 zig-8 double mutants, the axons of bilaterally symmetric neurons become aberrantly positioned across the midline during larval life (Figure 2A). Similar VNC axon defects can also be observed in zig-3 and zig-4 single mutant animals [10], [11]. Yet the cellular specificity of the VNC axon flip-over defects is broader in zig-5 zig-8 double than in zig-3 and zig-4 single or double mutants, since HSN axons are affected only in zig-5 zig-8 double mutants (Figure 2B). Other than these neuronal morphology defects, zig-5 zig-8 double null mutant animals appear healthy, fertile, locomote normally and do not display other obvious morphological abnormalities. Also, the organization of muscle and epidermal tissues is normal in these mutants (assessed using specific reporters; data not shown), indicating that zig-5 and zig-8 function to maintain the integrity specifically of the nervous system.
Fig. 2. zig-5 and zig-8 affect axon positioning in the ventral nerve cord. 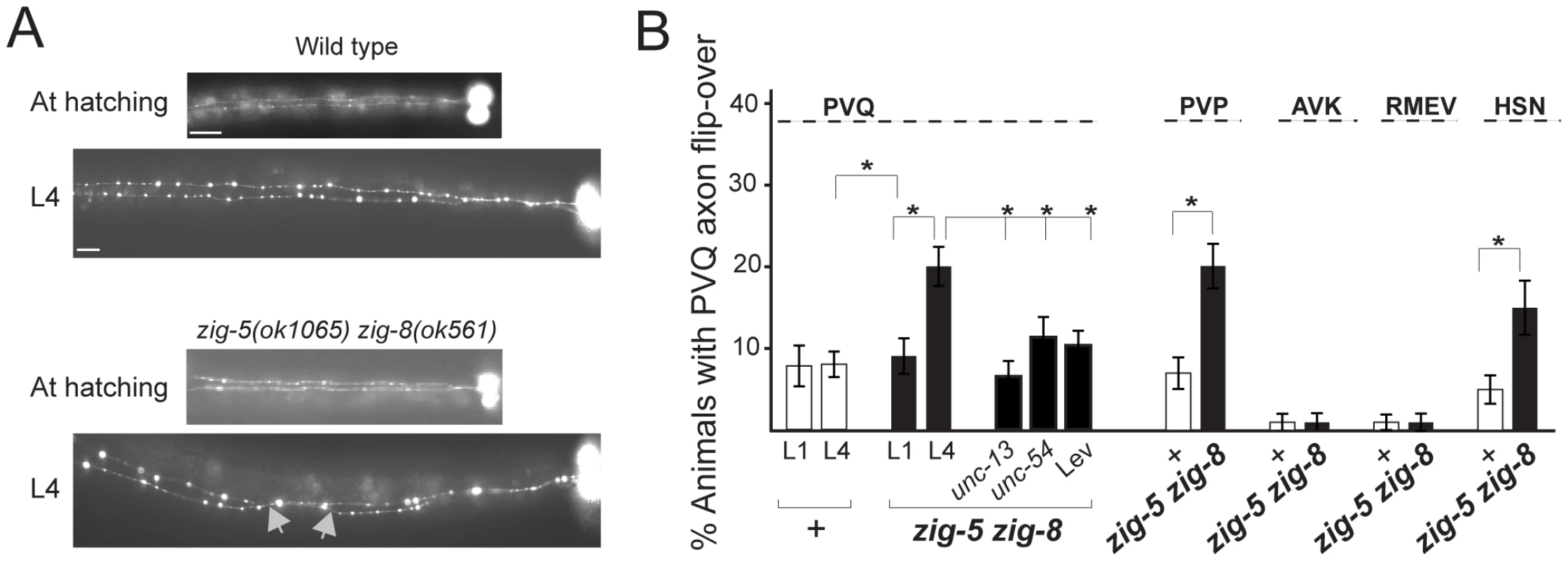
(A) PVQ axon flip-over (red arrowhead) in zig-5 zig-8 double mutants, observed first in later larval stages, but not earlier. Ventral view, anterior is to the left. Grey arrows indicate the location of axon flip-over. Scale bar is 5 µm. (B) Quantification of axon flip-over defects in zig-5 zig-8 double mutants, using transgenes oyIs14, hdIs29, bwIs2, oxIs12 and zdIs13, respectively. Single mutants zig-5 and zig-8 are wild type [11]. Animals were paralyzed with unc mutants or levamisole (Lev). Proportions of different animal populations were compared using the z-test. “*” indicates p<0.01. The zig-5 zig-8 double mutant phenotype can be rescued by genomic DNA clones that encompass these genes (Table 1) and can be phenocopied by RNAi (Table 2). Both zig-5 and zig-8 have previously been reported to be expressed in many neuronal and non-neuronal cell types in the head of the worm [zig-5: [10], zig-8: [17]], as well as the PVT tail neuron which extends its axons along the ventral nerve cord into the nerve ring [10]. The PVT neuron bears a critical role in conveying the function of zig gene family members in controlling maintenance of axon position in the ventral nerve cord [10], [11]. However, laser ablation of PVT does not affect head neuron position (0/57 PVT-ablated animals showed defects), and we therefore surmise that secretion of ZIG-5 and ZIG-8 from its many cellular sources in the head is required for maintaining cell body position. Even though we consider this model the most parsimonious based on the expression patterns of zig-5 and zig-8 in many head neurons, we have not been able to experimentally corroborate this notion as we observed no rescue of the mutant phenotypes by driving expression of zig-5 and/or zig-8 under control of variety of different heterologous promoters (tested promoters: neuronal unc-14, osm-6, sra-6, muscle myo-3, hypodermis dpy-7; injected at different concentrations from 0.1 to 125 ng/µL) and with different co-injection markers (pRF4, ttx-3::mCherry). Heterologous expression of zig-5 and zig-8 (under unc-14, myo-3 and dpy-7 promoter; 3 lines each) do not induce defects in a wild-type background, indicating that the lack of rescue through heterologous expression is not the result of overexpression. Since rescue with genomic clones is also just partial, it is conceivable that the correct dosage of zig-5 and zig-8 is critical for their function, yet difficult to achieve in transgenic animals. We note that we have also have problems expressing ZIG-5 and ZIG-8 in heterologous tissue culture cells.
Tab. 1. Rescue of zig-5 zig-8 mutant phenotypes. 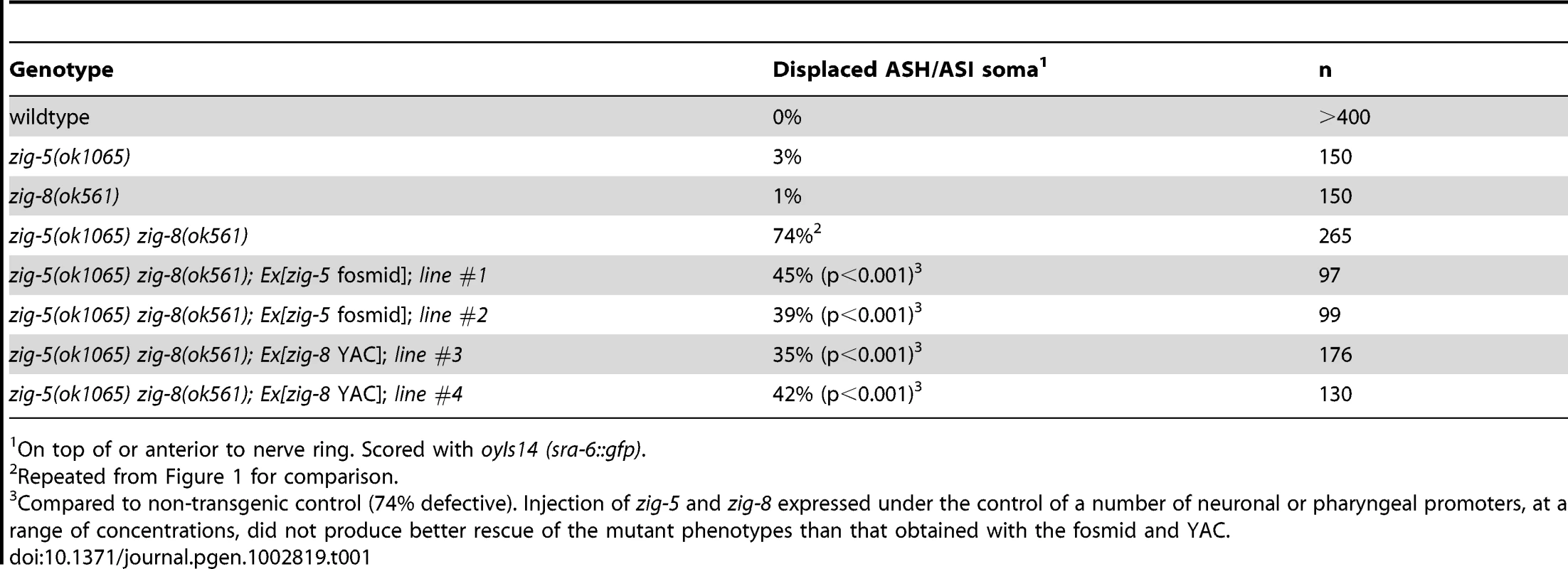
On top of or anterior to nerve ring. Scored with oyIs14 (sra-6::gfp). Tab. 2. zig-5 zig-8 neuron position defects are maintenance defects. 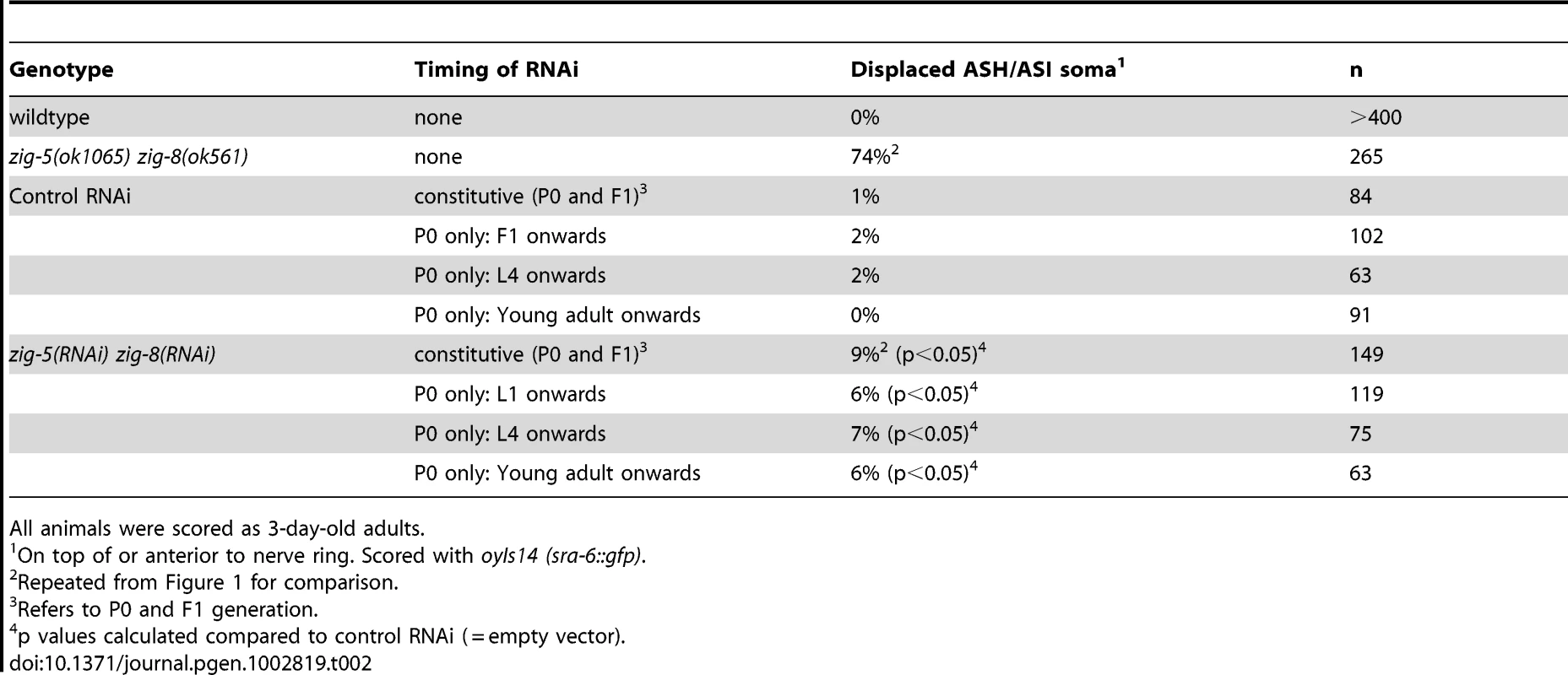
All animals were scored as 3-day-old adults. zig-5 and zig-8 maintain neuroanatomy
As in the case of sax-7 mutants [5], [6], [7], [14], the neuronal defects of zig-5 zig-8 double null mutants appear to be reflective of maintenance rather than developmental defects. First, the neuronal soma position defects of zig-5 zig-8 double mutants manifest themselves only postembryonically, long after birth and initial placement of neuronal cell bodies in the embryo (Figure 3). That is, animals in early larval stages appear completely indistinguishable from wild type and the phenotype manifests itself fully only in adult animals (Figure 3). Likewise, the axons of PVP and PVQ are initially positioned normally along the ventral midline during embryogenesis, but later become displaced during larval growth (Figure 2B). Second, the soma position defect can be evoked also in wild-type animals upon postembryonic knockdown of zig-5 and zig-8 by RNAi (Table 2). Third, the soma and axon displacement defects of the zig-5 zig-8 double null defects can be suppressed through prevention of locomotion of the animal, achieved either by genetic means or through drug treatment (Figure 2B, Figure 3B). A similar suppression of maintenance defects is a hallmark of all previously described maintenance mutants [6], [8], [10], [11] and indicates that ZIG-5 and ZIG-8, like other maintenance factors, serve to counteract the effects of physical movement, enabling neuronal soma and axonal projection to appropriately maintain their position.
Fig. 3. The zig-5 and zig-8 soma positioning defects are maintenance defects. 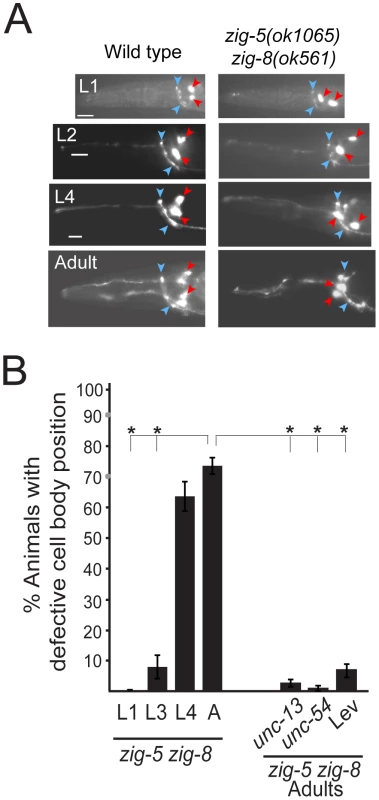
(A) Soma displacement defects in zig-5 zig-8 animals are only apparent at late larval and adult stages. See legend to Figure 1C for explanation of symbols. Scale bar is 5 µm. (B) Quantification of ASI and ASH neuronal displacement in zig-5 zig-8 double mutants at different developmental stages and when paralyzed in unc mutant backgrounds or on the drug levamisole (Lev). Proportions of different animal populations were compared using the z-test. “*” indicates p<0.001. Loss of zig-5 and zig-8 results in aberrant SAX-7L activity
The zig-5 zig-8 double null mutant phenotype, both in terms of the head soma positioning and ventral cord axon positioning defects, is similar to the null phenotype of sax-7 (Figure 4A, 4B). We set out to test whether these loci act in the same pathway by examining whether combinations of null alleles show similar phenotypes (which would argue for acting in the same pathway) or enhance each other's phenotype (which would argue for acting in separate pathways). While the null phenotype of sax-7 is completely penetrant for the soma positioning defect, the axon positioning defect is only partially penetrant, therefore allowing to do the genetic interaction test of sax-7 and the zig genes. We find that the sax-7(nj48) null mutant phenotype is not enhanced in zig-5 zig-8; sax-7 triple mutant animals, suggesting that these genes act in a similar genetic pathway (Figure 4C).
Fig. 4. Genetic interactions between zig-5, zig-8 and sax-7. 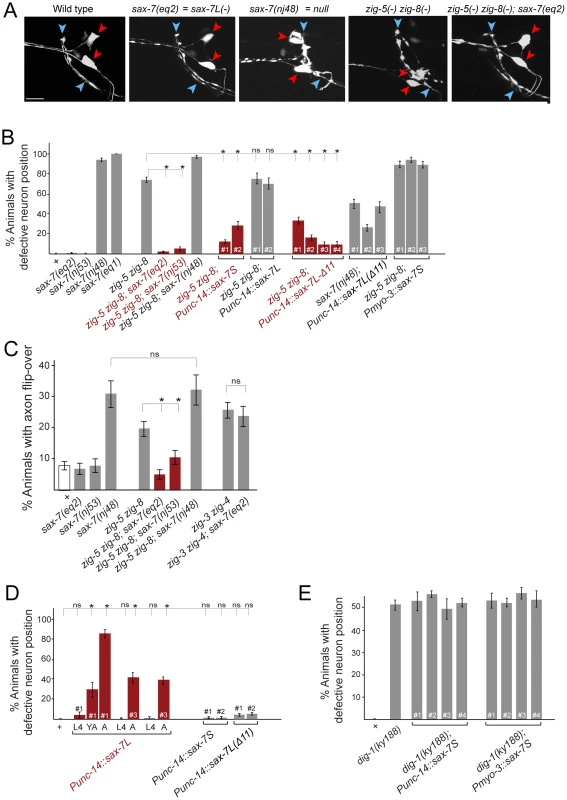
(A) ASI and ASH neuronal displacements in mutant adult animals scored with the oyIs14 reporter transgene. Wild-type and zig-5 zig-8 double mutant pictures are the same as shown in Figure 1C and shown for comparison only. See legend to Figure 1C for explanation of symbols. Scale bar is 5 µm. (B) Quantification of ASI and ASH neuronal position in genetic different backgrounds. “punc-14” is a panneuronal driver. Punc-14::sax-7L: DNA was injected at 75 ng/uL (line 1) or 50 ng/uL (lines 2 and 3). Punc-14::sax-7S and Punc-14::sax7Δ11: DNA was injected at 50 ng/uL. Proportions of different animal populations were compared using the z-test. “*” indicates p<0.001. (C) Genetic interactions of zig-5, zig-8 and sax-7 in controlling axon positioning in the ventral nerve cord. Quantification of PVQ axon flip-overs with transgene oyIs14. Error bars indicate s.e.p. Proportions of different animal populations were compared using the z-test. “*” indicates p<0.01. (D) Effect of ectopic expression of various forms of sax-7 in a wild-type background. ASI and ASH neuronal position are quantified. Proportions of different animal populations were compared using the z-test. “*” indicates p<0.001. (E) Quantification of ASI and ASH neuronal position in dig-1(ky188) mutant animals. “Punc-14” is a panneuronal driver for expression of SAX-7S. There are no statistically significant differences between dig-1 animals and any of the transgenic dig-1 animals expressing sax-7S. To further investigate the genetic interaction of zig-5, zig-8 and sax-7, we considered different isoforms of the sax-7 locus. sax-7 produces two distinct splice forms, a longer isoform, sax-7L, that displays the canonical, L1CAM-type 6 Ig/5 FnIII-domain architecture and a shorter isoform, sax-7S, which lacks the first two Ig domains [6], [14] (Figure 1A). Cell aggregation assays in tissue culture as well as transgenic rescue experiments with the two different isoforms have firmly established that the short isoform, sax-7S, is more adhesive than the long isoform, sax-7L [6], [7], [14]. Based on structural studies of various IgCAM proteins, including L1CAM family members themselves, the first 4 Ig domains of SAX-7L are expected to be configured in a horseshoe conformation in which Ig1 and Ig2 fold back onto Ig3 and Ig4 (Figure 1B) and it is this horseshoe configuration that is thought to engage in homophilic interactions [18], [19], [20], [21], [22], [23], [24]. It is therefore curious that the SAX-7S form, as well as a mutant version of SAX-7L that is unable to adopt the horseshoe configuration (through shortening of the hinge region between Ig2 and Ig3, “SAX-7L(Δ11)”; Figure 1B), is more adhesive than the presumably horseshoe-configured, wild-type SAX-7L protein [6], [7], [14]. Further, consistent with the first two Ig domains being dispensable for effective homophilic adhesion, two alleles, eq2 and nj53, that exclusively disrupt the sax-7L form, but not the sax-7S form [6], [14], neither affect soma nor axon position (Figure 4B; see Figure 1A for schematic presentation of the sax-7 isoform specific alleles).
Unexpectedly, we find that the two sax-7L-specific alleles each completely suppress the soma displacement defect of zig-5 zig-8 double mutants: While three quarters of zig-5 zig-8 double mutants display soma positioning defects, almost no triple mutant animals do (Figure 4A, 4B). Similarly, the sax-7L isoform specific alleles also suppress the VNC axon defects of zig-5 zig-8 double mutants (Figure 4C). These results indicate that zig-5 and zig-8 function is not required (i.e. their loss produces no phenotype) as long as the sax-7L isoform is not present. In other words, in wild-type animals, SAX-7L has the potential to disrupt cell adhesion, but this disruptive ability is counteracted by zig-5 and zig-8. This disruptive function is uncovered in zig-5 zig-8 mutants.
To further probe the disruptive activity of SAX-7L, we tested whether expression of SAX-7L above its usual level in an otherwise wild-type background may cause soma and axon positioning defects. Using a pan-neuronal unc-14 driver to express sax-7L, we indeed find such transgenic animals display severe soma and axon position defects that are virtually indistinguishable from a complete loss of sax-7L/S function or loss of zig-5 and zig-8 function (Figure 4D shows the soma defect; the axon defect is 58% penetrant, n = 86). The sax-7L overexpression-induced defects are maintenance defects as they are only evident in late larval and adult stages, but not earlier, after hatching (Figure 4D). Overexpression of sax-7S using the same driver does not induce any defects and neither does the sax-7L isoform when it is converted into a more adhesive form through shortening of the hinge region between Ig2 and Ig3 (sax-7L(Δ11), as shown in Figure 1B) (Figure 4D). This result can be interpreted to mean that overexpression of SAX-7L overwhelms the ability of ZIG-5/ZIG-8 to convert SAX-7L to a more adhesive form, thereby revealing the disruptive function of SAX-7L. However, the overexpression effect of SAX-7L is not alleviated in animals that carry arrays with extra copies of zig-5 and zig-8, but this experiment is not easily interpretable in light of the issues that we discuss above with transgenic zig-5 and zig-8 expression.
We considered the possibility that the disruptive function of SAX-7L, which is revealed upon removal of zig-5 and zig-8, lies in opposing the adhesive function of SAX-7S. To test this possibility, we examined whether overexpression of SAX-7S may overcome the antagonistic function of endogenous SAX-7L and therefore may abrogate the need for zig-5 and zig-8. To this end, we generated transgenic animals that overexpress sax-7S in a zig-5 zig-8 double mutant background. We find that pan-neuronal sax-7S expression completely rescues the soma positioning defects of zig-5 zig-8 double mutants (Figure 4B). Expressing sax-7S from a muscle specific promoter does not rescue the zig-5 zig-8 defects (Figure 4B). Neuronal overexpression of the long isoform sax-7L also does not rescue the zig-5 zig-8 defects (Figure 4B). However, if the sax-7L isoform is converted into a more adhesive form through shortening of the hinge region between Ig2 and Ig3 (sax-7L(Δ11), as shown in Figure 1B), sax-7L becomes able to rescue the zig-5 zig-8 double null mutant phenotype (Figure 4A, 4B). We therefore conclude that providing additional copies of non-horseshoe-configured sax-7 can compensate for the loss of zig-5 and zig-8. This compensatory effect is not simply caused by the addition of unspecific “glue”, since SAX-7S is not able to rescue the neuronal soma position defects of animals lacking the dig-1 maintenance factor (Figure 4E).
In conclusion, our results suggest that ZIG-5 and ZIG-8 exert their activity on neuronal architecture through their genetic interactions with SAX-7. All genetic interaction tests are schematically summarized in Figure 5.
Fig. 5. Genetic interactions of zig-5, zig-8 and sax-7. 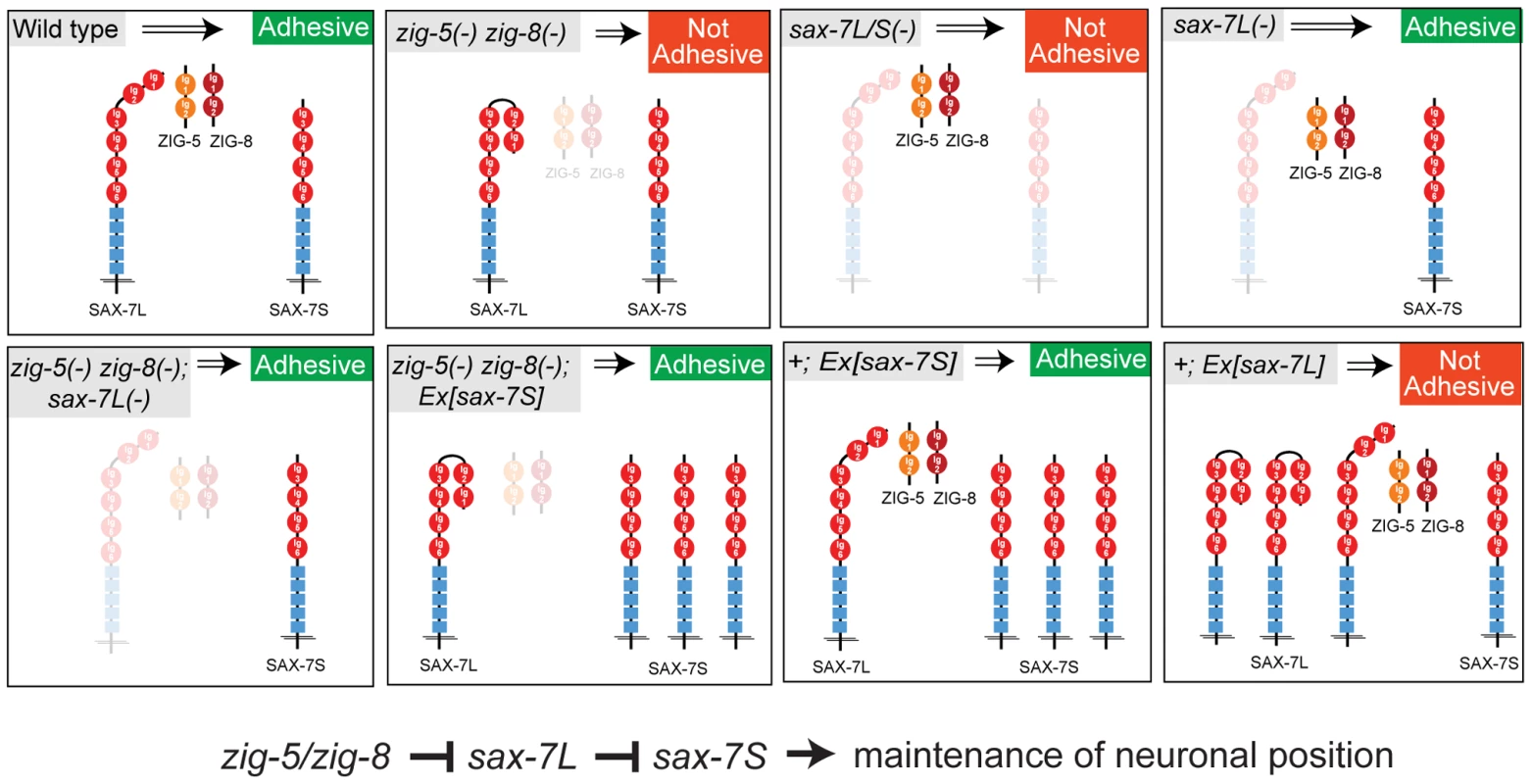
This figure summarizes the genetic interaction data described in this paper. The interaction of ZIG proteins with SAX-7 is context-dependent
The head neuron position defects described above concern neurons in the most populated ganglia of the head, the two lateral ganglia which containing about 50 neurons. In the smaller ventral head ganglion, the adhesive function of SAX-7 may be regulated in a different manner. This is because the soma positioning defects of the two ventral ganglion neuron types AIY and AVK, that are observed in sax-7 null mutant animals [7], are not phenocopied in zig-5 zig-8 double null mutant animals (0/50 animals show defects). Yet, as in neurons of the lateral ganglia, the sax-7 defect again is more efficiently rescued by sax-7S compared to sax-7L [7]. Conceivably, another combination of zig genes may act to promote sax-7 gene function in this cellular context.
We also examined the reverse and asked whether zig gene function can generally be explained zig genes affecting sax-7 gene function. To this end we turned to zig-3 zig-4 double mutants in which head soma position is unaffected, but positioning of axons in the ventral nerve cord fails to be maintained [11]. Similar axon maintenance defects are observed in animals lacking sax-7 [7], but are not observed in the sax-7L-specific allele eq2 (Figure 4C). In this case, eq2 is not able to suppress the zig-3 zig-4 double mutant phenotype (Figure 4C). However, as mentioned above, eq2 can suppress the axon positioning defects of zig-5 zig-8 double mutants (Figure 4C). In conclusion, the interaction of zig genes and sax-7 depends on the type of ZIG proteins that evoke the defects and it depends on cellular context, possibly because the adhesive substrate for specific neuronal ensembles may differ at distinct locations in the worm.
Discussion
Our analysis has revealed the function of two previously unstudied, secreted ZIG proteins, ZIG-5 and ZIG-8, thereby further broadening the concept of the requirement of specific factors for maintaining neuronal anatomy. Together with the previously characterized zig-3 and zig-4 genes, four of the eight presently known zig genes have now been specifically implicated in nervous system maintenance. The spectrum of activities of these four zig genes is partially overlapping (in the VNC), but also distinct (soma positioning in head ganglia). Given these precedents, it is conceivable that the remaining zig genes also have functions in maintaining nervous system architecture, perhaps affecting distinct subset of ganglia or even just individual neurons which have so far escaped attention.
Moreover, we have provided first hints toward the mechanism, but also diversity of ZIG protein function, by demonstrating that two ZIG family members (zig-5 and zig-8, but not zig-3 or zig-4) genetically interact with an IgCAM protein, the L1CAM protein SAX-7. These interaction results are summarized in Figure 5. Given the involvement of L1CAMs in various neurological diseases, a detailed understanding of this family of proteins is much warranted [25]. We have provided here novel and unexpected insights into mode of regulation of the adhesive activities of the L1CAM protein SAX-7.
We found that the likely horseshoe-configured SAX-7L isoform of SAX-7, previously shown to provide less homophilic adhesiveness than the shorter isoform SAX-7S [6], [7], has in fact an anti-adhesive activity in an in vivo context. This anti-adhesive activity is counteracted in wild-type animals by the two ZIG proteins ZIG-5 and ZIG-8, and thereby revealed only by either removal of ZIG-5 and ZIG-8 or by overexpression of SAX-7L. SAX-7L may engage SAX-7S in multimers in cis that are not able to engage in homophilic interactions in trans. ZIG-5 and ZIG-8 may be able to break up those complexes, for example by opening the SAX-7L horseshoe configuration, thereby converting SAX-7L into a more adhesive state and/or freeing up SAX-7S, which can then engage in homophilic trans interactions. In vitro cell aggregation assays, which we have not been able to undertake so far due to our inability to heterologously produce ZIG proteins, may address these possibilities in the future.
The in vivo studies on SAX-7 protein function have yielded results that are unexpected if one considers that several in vitro studies have provided evidence for horseshoe-configured IgCAMs engaging in homophilic interactions [21], [22], [23], [24]. The SAX-7 case argues for additional and alternative types of homophilic interactions of IgCAM molecules that are not only independent of a horseshoe configuration but also more adhesive than the horseshoe configuration. SAX-7S may be able to engage in other versions of previously described IgCAM interactions, such as the zipper mechanism proposed for the IgCAM superfamily member NCAM [26]. It is also conceivable that the adhesive mechanisms of IgCAM proteins may have diverged in the course of evolution and that the SAX-7/ZIG adhesion pathway may not be phylogenetically conserved.
Interestingly, while the activity of zig-5 and zig-8 can be explained entirely through their effect on sax-7 (as best evidenced by the complete suppression of the zig-5 zig-8 double mutant phenotype by sax-7L-specific alleles), an interaction with sax-7 is not the only way the ZIG family members operate. First, the genetic interactions of zig-5 and zig-8 with sax-7 are apparent in some ganglia and axonal tract, but not others. And second, the mutant phenotype of zig-3 and zig-4, even though similar to those of zig-5 and zig-8 in the context of the VNC, shows no genetic interaction with the sax-7L isoform. The existence of other maintenance factors, such as the extracellular matrix proteins DIG-1, F-spondin or the FGF receptor EGL-15 all point to a diversity of mechanisms involved in maintenance of nervous system integrity [4].
On a mechanistic level, our findings support the hypothesis [4] that maintenance of tissue integrity is controlled through a finely tuned and tightly regulated balance of adhesive and anti-adhesive forces (Figure 5). The function of SAX-7L may lie in modulating the strongly adhesive function of SAX-7S at stages and in tissues where high adhesiveness is not desired. This anti-adhesiveness of SAX-7L may in turn be eliminated by ZIG proteins when high degree of adhesiveness is required, such as during postembryonic life to maintain neuron position. It will be interesting to see whether modulation of the homophilic interaction of IgCAM proteins through interaction with other Ig domain proteins is a common theme in maintenance of neuronal positioning. The wealth of Ig domain proteins, many of them secreted, in vertebrate and invertebrate genomes [27], suggests that many such modulatory interactions remain to be discovered.
Materials and Methods
Strains and transgenes
The nature and structure of all zig and all sax-7 mutant alleles were previously described in detail [6], [11], [14] and some of them are schematically shown in Figure 1A. Genotyping was done by PCR. sax-7 transgenes are described in [7]. Transgenes used to rescue zig-5 zig-8 double mutant phenotype were obtained by injecting fosmid WRM063cG06 containing the zig-5 gene (injected at 20 ng/µL along with ttx-3::mCherry at 100 ng/µL) and the YAC Y39E4B, containing the zig-8 gene (injected at 10 ng/µL along with pRF4 rol-6(su1006d) at 100 ng/µL).
Scoring of neuroanatomy
The following gfp transgenes were used to score anatomy, using a Zeiss Axioplan 2 microscope: oyIs14: Is[sra-6::gfp], hdIs29: Is[sra-6::DsRed2; odr-2::gfp] oxIs12 Is[unc-47::gfp], bwIs2 Is[flp-1::gfp] (described in [11]. Cell body position was examined in three - to five-days old adults, i.e. worms that have lived for 3 to 5 days after the L4 to young adult molt. The position of the soma of ASI and ASH neurons is normally posterior to the nerve ring, and was scored defective when the cell body of at least one neuron was anterior to the nerve ring, on top of it, or contacting it.
Ventral nerve cord anatomy was examined in freshly hatched L1 larvae (<30 min post-hatch) and in L4 larvae. The axons of the pairs of bilateral neurons examined are normally separate and lie on either side of the ventral midline. An axon was scored defective when a segment of its length was located on the opposite side of the ventral nerve cord and contacted the axon on the other side.
For the paralysis on levamisole experiment, embryos were placed on plates containing 50 µM levamisole and seeded with OP50 bacteria, were allowed to reach the L4 and adult stages, and were examined as above.
All phenotypes were scored as percent animals defective and results are shown with error bars representing the standard error of proportion. Statistical significance was calculated using the z-test to compare the proportion of abnormal animals of two genotypes. When using the same control for multiple comparisons, the P value was multiplied by the total number of comparisons.
RNA interference
oyIs14 L4 hermaphrodites were placed on bacteria harboring plasmids to express dsRNA corresponding to the genes zig-5 and zig-8 (J. Ahringer library), or the empty vector (L4440). The inserts for the zig-5 and zig-8 plasmids were verified by sequencing. The two bacterial strains containing the zig-5 and the zig-8 plasmids were grown separately overnight. Bacterial cultures were concentrated by centrifugation, mixed and added to RNAi plates. A day later, these animals were transferred onto fresh plates containing the RNAi bacteria. F1 animals were scored for the morphology of the chemosensory neurons at days 3–5 of adulthood. Animals were also placed on RNAi plates at the L1, L4 and young adult stages, and their neuroanatomy was examined when they reached 3–5 days of adulthood. Similar experiments were carried out in the rrf-3;oyIs14, eri-1;lin-15b;oyIs14 genetic backgrounds, as well as with RNAi of zig-5 in zig-8 mutant background, and vice versa, but failed to elicit stronger defects.
Zdroje
1. Tessier-LavigneMGoodmanCS 1996 The molecular biology of axon guidance. Science 274 1123 1133
2. KolodkinALTessier-LavigneM 2010 Mechanisms and Molecules of Neuronal Wiring: A Primer. Cold Spring Harb Perspect Biol
3. YuTWBargmannCI 2001 Dynamic regulation of axon guidance. Nat Neurosci 4 Suppl 1169 1176
4. BenardCHobertO 2009 Looking beyond development: maintaining nervous system architecture. Curr Top Dev Biol 87 175 194
5. ZallenJAKirchSABargmannCI 1999 Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development 126 3679 3692
6. SasakuraHInadaHKuharaAFusaokaETakemotoD 2005 Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. Embo J 24 1477 1488
7. PocockRBenardCYShapiroLHobertO 2008 Functional dissection of the C. elegans cell adhesion molecule SAX-7, a homologue of human L1. Mol Cell Neurosci 37 56 68
8. BenardCYBoyanovAHallDHHobertO 2006 DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development 133 3329 3340
9. BülowHEBoulinTHobertO 2004 Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron 42 367 374
10. AurelioOHallDHHobertO 2002 Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science 295 686 690
11. BenardCTjoeNBoulinTRecioJHobertO 2009 The small, secreted immunoglobulin protein ZIG-3 maintains axon position in Caenorhabditis elegans. Genetics 183 917 927
12. WooWMBerryECHudsonMLSwaleREGoncharovA 2008 The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development 135 2747 2756
13. HammarlundMJorgensenEMBastianiMJ 2007 Axons break in animals lacking beta-spectrin. J Cell Biol 176 269 275
14. WangXKweonJLarsonSChenL 2005 A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev Biol 284 273 291
15. WhiteJGSouthgateEThomsonJNBrennerS 1986 The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B Biological Sciences 314 1 340
16. HobertOBülowH 2003 Development and maintenance of neuronal architecture at the ventral midline of C. elegans. Curr Opin Neurobiol 13 70 78
17. Hunt-NewburyRViveirosRJohnsenRMahAAnastasD 2007 High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol 5 e237
18. FreigangJProbaKLederLDiederichsKSondereggerP 2000 The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell 101 425 433
19. SchurmannGHaspelJGrumetMEricksonHP 2001 Cell adhesion molecule L1 in folded (horseshoe) and extended conformations. Mol Biol Cell 12 1765 1773
20. SuXDGastinelLNVaughnDEFayeIPoonP 1998 Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science 281 991 995
21. HeYJensenGJBjorkmanPJ 2009 Cryo-electron tomography of homophilic adhesion mediated by the neural cell adhesion molecule L1. Structure 17 460 471
22. MeijersRPuettmann-HolgadoRSkiniotisGLiuJHWalzT 2007 Structural basis of Dscam isoform specificity. Nature 449 487 491
23. SawayaMRWojtowiczWMAndreIQianBWuW 2008 A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell 134 1007 1018
24. LiuHFociaPJHeX 2011 Homophilic adhesion mechanism of neurofascin, a member of the L1 family of neural cell adhesion molecules. J Biol Chem 286 797 805
25. ChenLZhouS 2010 “CRASH”ing with the worm: insights into L1CAM functions and mechanisms. Dev Dyn 239 1490 1501
26. SorokaVKolkovaKKastrupJSDiederichsKBreedJ 2003 Structure and interactions of NCAM Ig1-2-3 suggest a novel zipper mechanism for homophilic adhesion. Structure 11 1291 1301
27. RougonGHobertO 2003 New Insights into the Diversity and Function of Neuronal Immunoglobulin Superfamily Molecules. Annu Rev Neurosci 26 207 238
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



