-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
Animals acquire predictive values of sensory stimuli through reinforcement. In the brain of Drosophila melanogaster, activation of two types of dopamine neurons in the PAM and PPL1 clusters has been shown to induce aversive odor memory. Here, we identified the third cell type and characterized aversive memories induced by these dopamine neurons. These three dopamine pathways all project to the mushroom body but terminate in the spatially segregated subdomains. To understand the functional difference of these dopamine pathways in electric shock reinforcement, we blocked each one of them during memory acquisition. We found that all three pathways partially contribute to electric shock memory. Notably, the memories mediated by these neurons differed in temporal stability. Furthermore, combinatorial activation of two of these pathways revealed significant interaction of individual memory components rather than their simple summation. These results cast light on a cellular mechanism by which a noxious event induces different dopamine signals to a single brain structure to synthesize an aversive memory.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002768
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002768Summary
Animals acquire predictive values of sensory stimuli through reinforcement. In the brain of Drosophila melanogaster, activation of two types of dopamine neurons in the PAM and PPL1 clusters has been shown to induce aversive odor memory. Here, we identified the third cell type and characterized aversive memories induced by these dopamine neurons. These three dopamine pathways all project to the mushroom body but terminate in the spatially segregated subdomains. To understand the functional difference of these dopamine pathways in electric shock reinforcement, we blocked each one of them during memory acquisition. We found that all three pathways partially contribute to electric shock memory. Notably, the memories mediated by these neurons differed in temporal stability. Furthermore, combinatorial activation of two of these pathways revealed significant interaction of individual memory components rather than their simple summation. These results cast light on a cellular mechanism by which a noxious event induces different dopamine signals to a single brain structure to synthesize an aversive memory.
Introduction
Mechanisms underlying memory can be as simple as a modulation of monosynaptic connection in the gill withdraw reflex of Aplysia [1]. Alternatively memory formation and storage may require dynamic interaction of distinct neuropiles in a brain [2]. In Drosophila melanogaster, a neuronal circuit centered on the mushroom body (MB) is important for the formation and storage of odor memory [3], [4], [5], [6]. Signals of odor and shock are integrated in the MB for memory formation [7], [8], [9]. Identity of the odor is represented by a small subset of Kenyon cells [10], [11], [12], which are major intrinsic neurons of the MB and categorized into the α/β, α'/β' and γ neurons (Figure 1A) [13]. According to their projection patterns in the lobes, these neurons can be further classified into 8 subtypes: α/βp, α/βs and α/βc (also known as the pioneer α/β, the early α/β, or the late α/β respectively [14]) for the posterior, surface or core layers of the α/β lobes; α'/β'a, α'/β'm and α'/β'p for the anterior, middle and posterior layers of α'/β' lobes; γd and γmain for the dorsal and main layer of the γ lobe [15], [16]. The Kenyon cells receive a dopamine signal that mediates aversive reinforcement for odor memory formation [17], [18]. Expression of DopR, a D1-like dopamine receptor also known as dDA1, in the γ neurons is fully sufficient to rescue the mutant defect in aversive odor memory [18]. Activation of many types of dopamine neurons using TH-GAL4 can substitute for the aversive stimulus that induces odor memory [19], [20]. In flies, dopamine neurons from protocerebral anterior median (PAM), protocerebral posterior lateral 1 and 2ab (PPL1 and PPL2ab) clusters terminate in the entire MB (Figure 1B–1C) [21], [22], [23]. Individual neurons in these clusters terminate distinct subdomains along the longitudinal axis of the MB lobes. The application of noxious stimuli, such as electric shock, activates only a subset of dopamine neurons [21], [24], indicating that the response property greatly varies among individual cells within a cluster. Consistent with this observation, activation of specific subsets of these clusters, such as MB-M3 and MB-MP1 neurons, can induce aversive odor memory [25]. This dopamine input presumably modulates the pre-synaptic output of odor-representing Kenyon cells and drives memory formation [8], [9].
Fig. 1. GAL4 drivers for dopaminergic neurons that project to the mushroom body. 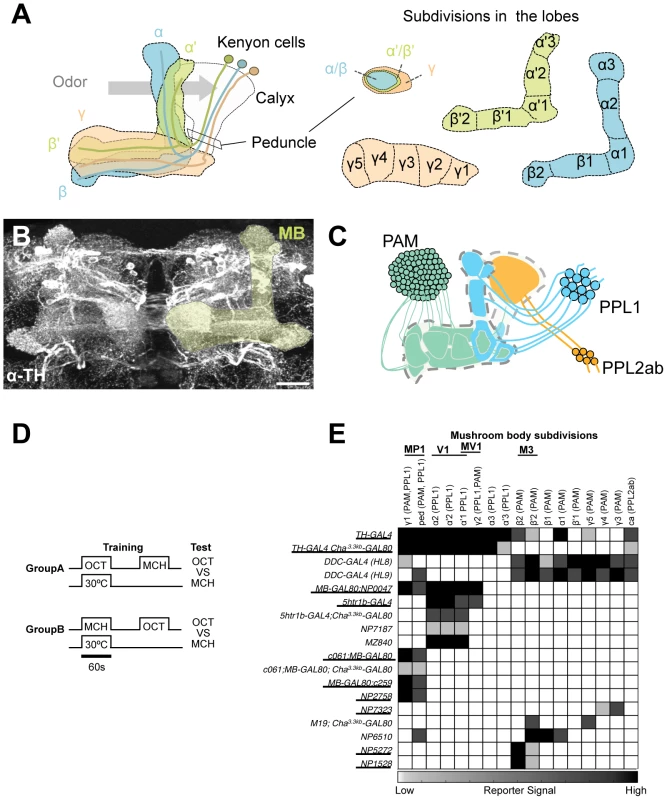
(A) Schematic diagrams of the mushroom body (left) and subdivisions in the lobe system of the MB (right). Kenyon cells are the major intrinsic neurons of the MB. They have dendritic terminals forming the structure called calyx, where odor signals are conveyed from the antennal lobes. From the calyx, Kenyon cell axons project anteriorly through the peduncle to the lobes. α, α', β, β', and γ indicate the lobes of the MB. In the peduncle, the parallel axon fibers of Kenyon cell subtypes are organized in concentric layers. The spur is contributed exclusively by the neurons projecting to the γ lobe (γ1). The terminals of the extrinsic neurons define the subdivision in the lobes along the longitudinal axis of Kenyon cell axon bundles [15]. (B) Confocal projection of the MB region (rectangle in A; light green overlay) labeled with the antibody against tyrosine hydroxylase (TH; frontal view; dorsal up). Many types of dopaminergic neurons project to the entire MB. Scale bar represents 20 µm. (C) The MB is supplied by three distinct clusters of TH-immunoreactive cells (PAM, PPL1, and PPL2ab) [21], [22]. (D) The conditioning protocol for dTRPA1-induced memory (see Material and Methods for detail). (E) Terminal distribution of dopamine neurons in the MB in GAL4 drivers used in this study. The gray scale represents subjectively determined intensity of terminals in the MB. Drivers that induced significant aversive memory are underlined. See Figure S2 for the fraction of GAL4 expressing cells per cluster. See [21], [22] for the description of the clusters. Besides aversive reinforcement, dopamine is responsible for a wide range of physiological functions including appetitive memory, some of which are mapped in the MB [17], [21], [24], [26], [27], [28], [29]. This implies the functional differentiation of the MB subdivisions by different sets of dopamine neurons. While both MB-M3 and MB-MP1 neurons can induce aversive odor memory, these neurons terminate in spatially segregated subdomains of the MB. MB-M3 neurons primarily synapses in the medial tip of the β lobe, whereas MB-MP1 neurons terminates in the spur of the γ lobe (γ1) and the peduncle of the α/β neurons [15]. Blocking the output of MB-M3 neurons during memory acquisition preferentially affects labile 2-hour memory [25], indicating the partial contribution of MB-M3 neurons to aversive reinforcement of electric shock. This raises the question of how the other reinforcement pathways to the MB are coordinated to synthesize a complete aversive odor memory. Considering the selective phenotype of MB-M3 neurons and differential functions of the lobe systems [25], [30], [31], [32], [33], it is likely that electric shock induces qualitatively different memory traces in parallel. In this study, we challenge this hypothesis by activating and inactivating individual dopamine pathways separately. By conducting further behavioral screening of GAL4 driver lines, we identify a third type of dopamine neurons that are capable to induce aversive odor memory. Using these tools to manipulate different dopamine pathways to the MB, we characterize each memory component in isolation and show how their interaction shapes the temporal stability of aversive memory.
Results
Screening dopamine neurons for formation of aversive memory
To systematically identify reinforcement pathways to the MB, we elaborated our previous dTRPA1-based behavioral screening by testing 10 additional GAL4 lines for different subsets of dopamine neurons. Dopamine synthesis in MB-projecting neurons of these lines was confirmed with immunoreactive signals to Tyrosine Hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis. The pattern of TH signals coincides well with that of dopamine itself in the brain (Figure S1). This collection of GAL4 drivers covers most, if not all, dopamine neurons in the fly brain including those projecting to the MB (Figure S2) [25]. For activation of target neurons, we expressed the thermo-sensitive cation channel dTRPA1 [34]. We elevated temperature to 30°C for 60 sec only during the presentation of an odor, as in the standard conditioning protocol (Figure 1D). This temperature shift by itself had a slight aversive effect as seen in the tendency of conditioned odor avoidance of control groups [25]. Contingent activation with the odor presentation should induce significant conditioned avoidance of the paired odor, if a given GAL4 line drives dTrpA1 within neurons that signal aversive reinforcement. We found functional heterogeneity of dopamine neurons in inducing immediate aversive memory (Figure 1E). Activation of particular cell types in the PAM and PPL1 clusters induced aversive memory, whereas drivers labeling other types in the same clusters did not (Figure 1E; see below for the detailed description).
However, most GAL4 drivers used in these experiments have expression outside the target dopamine neurons. To scrutinize whether the induced memory is due to the target cells, we suppressed dTRPA1 expression selectively in dopamine neurons using TH-GAL80 [35] and examined whether TH-GAL80 suppresses memory formation induced by thermo-activation. We found that aversive memory is indeed attributable to dopamine neurons in most cases. TH-GAL80 did not fully silence dTRPA1-dependent memory of MB-GAL80;c259 and NP2758, which could be due to either an incomplete suppression of GAL4 in dopamine neurons or a potential contribution of non-dopaminergic cells in these drivers (see below for MB-GAL80;c259 and [25] for NP2758). To further narrow down the expression pattern to a single dopamine cell type, we combined Cha3.3kb-GAL80 with some drivers. The Cha3.3kb fragment drives strong expression not only in choline acetyltransferase-positive neurons but also non-cholinergic cells presumably due to ectopic expression [36].
Dopamine biosynthesis requires the enzymes TH and Dopa Decarboxylase (DDC) and these enzymes are expected to be in all dopamine neurons. TH-GAL4 and two versions of DDC-GAL4 (HL8 and HL9) label the majority, but not all, dopamine neurons. TH-GAL4 labels all cells in the PPL1 and PPL2ab clusters and a small subset of the PAM cluster including MB-M3 neurons (Figure 2A, 2E, 2I, 2M, 2Q). Activation of these neurons induced robust aversive memories even in the dTrpA1 mutant background (Figure 2U, Figure S4), suggesting that activation of dopamine neurons alone can substitute an aversive unconditioned stimulus. Cha3.3kb-GAL80 suppressed transgene expression in some of the PAM cluster cells and three cells in the PPL1 cluster of TH-GAL4 (Figure 2B, 2F, 2J, 2N, 2R). The silenced cells include MB-M3 neurons and at least one MB-MP1 neuron [25]. TH-GAL4 Cha3.3kb-GAL80/UAS-dTrpA1 flies however showed a similar level of aversive memory as TH-GAL4/UAS-dTrpA1 flies (Figure 2U). This result implies functional redundancy of Cha-GAL80-positive MB-M3 neurons and MB-MP1 neuron in immediate memory.
Fig. 2. Memories induced by thermo-activation with drivers that label many types of dopamine neurons. 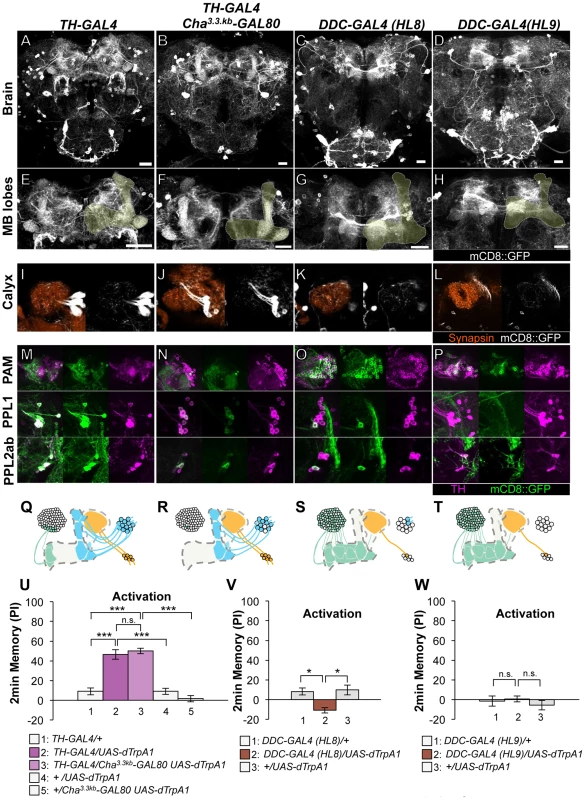
(A–D) Expression of mCD8::GFP in the central brain in drivers described above (frontal view; dorsal up). (E–H) Magnification of the anterior brain region including the MB-lobes (shaded). Scale bars represent 20 µm. (I–L) Magnification of the posterior regions centered on the MB calyx. (M–P) TH-immunoreactivity in three clusters of dopamine neurons that project to the MB. (Q–T) Diagrams illustrate the dopamine neurons projecting to the MB labeled in each driver. (U–W) Immediate memories induced by transient thermo-activation of dTRPA1-expressing cells. Memory of experimental group GAL4/UAS-dTrpA1 is compared with that of control groups (i.e. GAL4/+, +/UAS-dTrpA1). For experiments with GAL80, the performance of GAL4/GAL80 UAS-dTrpA1 is compared with those of GAL4/UAS-dTrpA1, GAL4/+ and +/GAL80 UAS-dTrpA1. n = 9–20. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; *** P<0.001; n.s. not significant. HL8 and HL9 label the majority of PAM cluster cells, one or no PPL1 cells and several PPL2ab cells (Figure 2C–2D, 2G–2H, 2K–2L, 2O–2P, 2S–2T). Although these drivers also label serotonergic neurons, some of which project to the MB [37], [38], terminals in the calyx are TH immunoreactive (Figure S3), suggesting that they belong to the PPL2ab cluster. With HL8 and HL9, dTRPA1 activation did not induce significant aversive memory (Figure 2V–2W), consistent with a previous report using light-dependent activation [19]. Because these drivers label many cell types, the roles of individual dopamine neurons might be obscured in final memory scores by antagonizing functions each other.
As to the PPL1 cluster neurons, two drivers labeling MB-MP1 neuron (i.e. MB-GAL80;NP0047 and MB-GAL80;c259) induced very robust aversive memories (Figure 3), which is fully consistent with the previous result using other drivers c061;MB-GAL80 and NP2758 [25]. TH-GAL80 silenced GAL4 activity selectively in the dopamine neurons in these drivers (Figure 3B, 3F, 3J, 3D, 3H, 3L). Memory induced with these drivers was also significantly suppressed by TH-GAL80, although it was not complete with MB-GAL80;c259 (Figure 3R). Therefore, we used c061;MB-GAL80, but not MB-GAL80;c259, for the following experiment to manipulate MB-MP1 neuron.
Fig. 3. Memories induced by thermo-activation with MB-GAL80;NP0047 and MB-GAL80;c259. 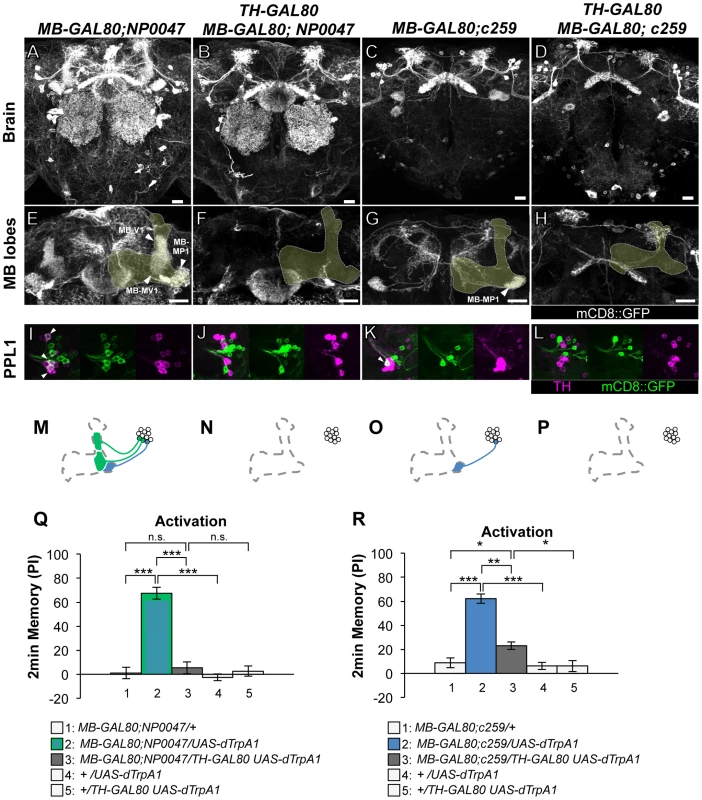
(A–D) Expression of mCD8::GFP in the central brain in drivers described above (frontal view; dorsal up). (E–H) Magnification of the anterior brain region including the MB-lobes (shaded). Scale bars represent 20 µm. (I–L) TH-immunoreactivity in the PPL1 cluster of dopamine neurons. Arrowheads indicate colocalization of TH and GFP. (M–P) Diagrams illustrate the dopamine neurons projecting to the MB labeled in each driver. (Q–R) Immediate memories induced by transient thermo-activation of dTrpA1 expressing cells. (Q) n = 12–16. (R) n = 16–17. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; *** P<0.001; n.s. not significant. The GAL4 driver line 5htr1b-GAL4 labels one MB-V1 neuron and one MB-MV1 neuron in the PPL1 cluster (Figure 4A, 4E, 4I, 4L). 5htr1b-GAL4 induced a slight but significant aversive memory (Figure 4P). TH-GAL80 suppressed most of the GAL4-positive cells of 5htr1b-GAL4 (Figure 4B, 4F, 4M) and accordingly dTRPA1-induced memory (Figure 4P). We combined Cha3.3kb-GAL80 with 5htr1b-GAL4 that preferentially silenced MB-MV1 neuron but did not abolish expression in MB-V1 neuron and other GAL4-positive cells (Figure 4C, 4G, 4J, 4N). 5htr1b-GAL4 Cha3.3kb-GAL80 did not induce memory (Figure 4Q), suggesting the critical role of MB-MV1. Consistently, in NP7187 and MZ840, which label one MB-V1 neuron, activation did not induce significant memory (Figure 4D, 4H, 4K, 4O, 4R) [25]. Thus, memory induced with 5htr1b-GAL4 is likely to be an effect of MB-MV1 neuron activation. In accordance with this interpretation, calcium imaging revealed that MB-MV1 neuron robustly responds to electric shock, whereas MB-V1 neuron preferentially responds to odors [21]. Considering the importance of the vertical lobes in olfactory learning [8], [9], [39], [40], [41], [42], MB-MV1 neuron might act together with MB-V1 neuron during memory acquisition.
Fig. 4. Memories induced by thermo-activation with 5htr1b-GAL4 and NP7187. 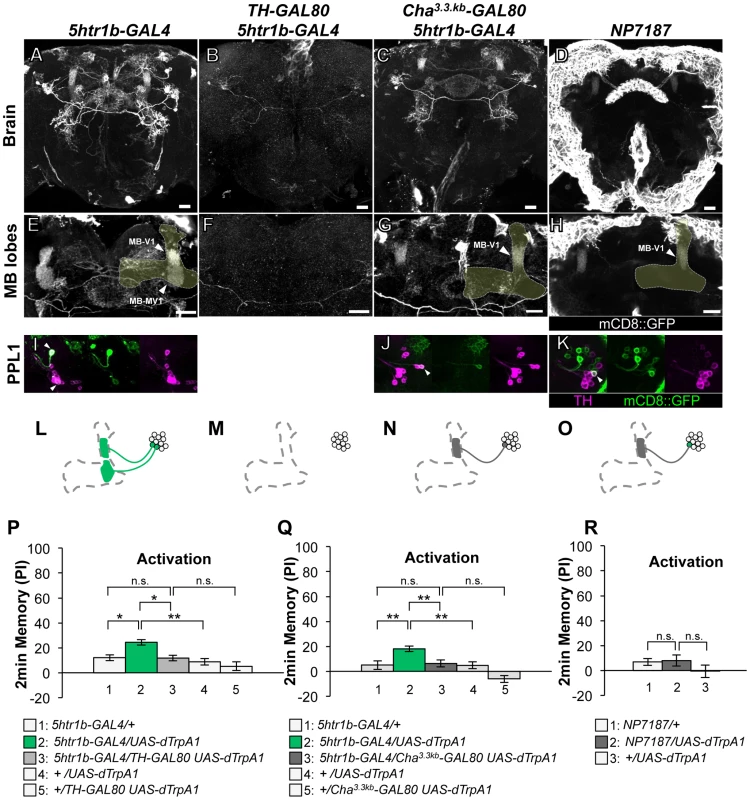
(A–D) Expression of mCD8::GFP in the central brain in drivers described above (frontal view; dorsal up). (E–H) Magnification of the anterior brain regions including the MB-lobes (shaded). Scale bars represent 20 µm. (I–K) TH-immunoreactivity in the PPL1 cluster of dopamine neurons. Arrowheads indicate colocalization of TH and GFP. (L–O) Diagrams illustrate the dopamine neurons projecting to the MB labeled in each driver. (P–R) Immediate memories induced by transient thermo-activation of dTrpA1 expressing cells. (P) n = 20–22. (Q) n = 16. (R) n = 12. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; ** P<0.01; n.s. not significant. Thermo-activation with NP7323, which labels a mixture of cell types in the PAM cluster (MB-M2), induced a slight but significant aversive memory (Figure 5A, 5C, 5E, 5G, 5I). With MZ19;Cha3.3kb-GAL80, which labels approximately ten PAM cluster cells, activation did not have significant effect (Figure 5B, 5D, 5F, 5H, 5J). These results imply functional heterogeneity in memory formation of PAM cluster neurons. However, we could not test whether the induced memory in NP7323 can be attributed to non-dopaminergic GAL4-positive neurons, because TH-GAL80 does not suppress GAL4 expression in the majority of PAM cluster cells.
Fig. 5. Memories induced by thermo-activation with NP7323 and MZ19;Cha3.3kb-GAL80. 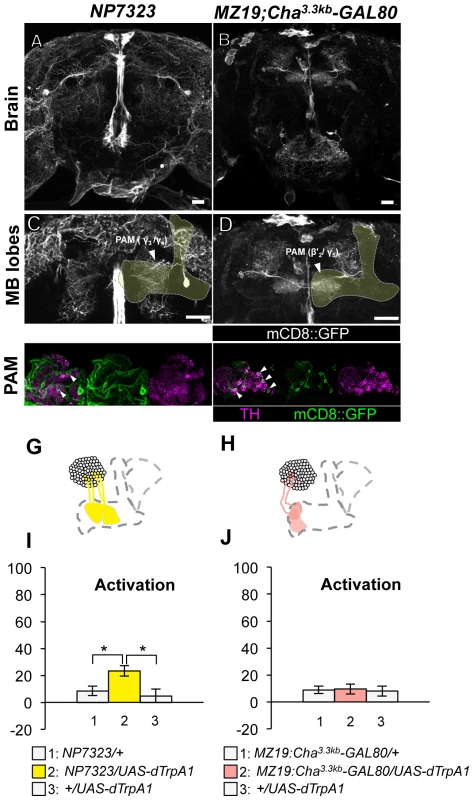
(A–B) Expression of mCD8::GFP in the central brain in drivers described above (frontal view; dorsal up). (C–D) Magnification of the anterior brain region including the MB-lobes (shaded). Scale bars represent 20 µm. (E–F) TH-immunoreactivity in the PAM cluster of dopamine neurons. Arrowheads indicate colocalization of TH and GFP. (G–H) Diagrams illustrate the dopamine neurons projecting to the MB labeled in each driver. (I–J) Immediate memories induced by transient thermo-activation of dTrpA1 expressing cells. (I) n = 14–16. (J) n = 12. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; n.s. not significant. These results, together with our previous report [25], point to three distinct dopamine pathways to the MB that induce aversive memory; MB-M3 neurons in the PAM cluster and MB-MP1 neuron and MB-MV1/MB-V1 neurons in the PPL1 cluster. The dopamine neurons of the three distinct functional groups terminate in segregated MB subdivisions along the trajectory of Kenyon cell axon bundles: MB-M3 neurons to βsp2 and β'a2, MB-MP1 neuron to γ1 and the core of the peduncle where the α/β neurons innervate, MB-MV1 neuron to γ2 and α'1, MB-V1 neuron to α2, α'2 and a part of α'1 (Figure 1E; see [15] for the description of MB subdivisions). Because blocking all of these neurons with TH-GAL4 did not abolish shock-induced aversive memory, we reserve the possibility that additional types of neurons may contribute to aversive memory formation (e.g. MB-M2 neurons in NP7323 and serotonin neurons [35]).
Anatomical characterization of the dopamine neurons
To characterize the individual dopamine pathways to the MB, we determined cellular identity by counting TH-immunoreactive cells in single and combined drivers (Figure 6A–6B). When two drivers label identical dopamine neurons, the number of cells in combined drivers should not differ from those in respective single drivers. Identical MB-V1 neuron is labeled in 5htr1b-GAL4, MZ840, NP7187, NP0047. 5htr1b-GAL4 and NP0047 label the same MB-MV1 neuron. c061;MB-GAL80, NP0047 and NP2758 label the same MB-MP1 neuron, while c061;MB-GAL80 and MB-GAL80;c259 may label another MB-MP1 neuron in addition to that in NP2758 and NP0047 [25].
Fig. 6. Identity of labeled cells in the PPL1 cluster. 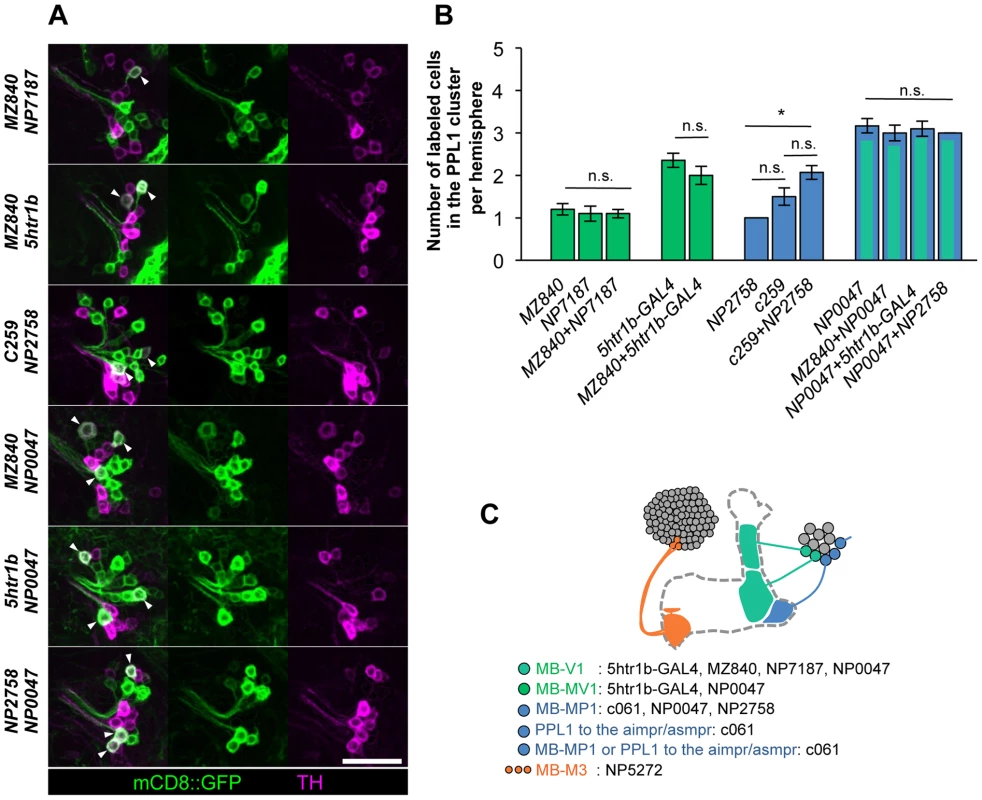
(A) TH-immunoreactivity in the PPL1 cluster of dopamine neurons in the combination of GAL4 drivers. Arrowheads indicate colocalization of TH and GFP. Scale bar represents 20 µm. (B) Counting of mCD8::GFP-labeled dopamine neurons in the PPL1 cluster. * P<0.05; n.s. not significant. (C) A diagram illustrating the identity and the number of dopamine neurons labeled in respective GAL4 drivers. See [25], [26] for the data of c061, NP2758, and MZ840. Expression of presynaptic markers in these dopamine neurons revealed that their arbors in the MB are presynaptic terminals (Figure 7A–7C) [25]. The processes of these dopamine neurons in the protocerebrum contained many fewer output sites, implying a dendritic nature (Figure 7A–7C). To compare the distribution of the dendrites, we performed a non-rigid intensity-based transformation of brains [43] and 3D image analysis of the dopamine neurons. We used TH immunolabelling as landmarks to transform brains. Computational alignment of the MB-M3 neurons, MB-MV1/V1 neurons, and MB-MP1 neuron revealed that each cell type has a unique pattern of dendrite distribution in the superior and inferior protocerebral regions, and they are partially overlapping with each other (Figure 7D, Video S1). Furthermore, we aligned the neurons that did not induce significant aversive memory (Figure 4 and Figure 5) and found that their processes outside the MB have different distribution and focus (Figure 7E, Video S2). When the memory-inducing and non-inducing neurons are separately pooled and superimposed in one brain, the dendrites of these groups are largely segregated, especially in the anterior inferior medial protocerebrum (Figure 7F, Video S3). These results imply that the dopamine neurons for aversive memory may partially share some neuronal input that is distinct from dopamine neurons that do not induce aversive memory. Cellular identification of presynaptic neurons requires more precise anatomical and functional analyses.
Fig. 7. Projection of specific dopamine neurons that induce aversive odor memory. 
Double labeling of dopamine neurons by membrane and presynaptic markers UAS-Syt::HA;UAS-mCD8::GFP driven by (A) NP2758, (B) 5htr1b-GAL4, (C) NP6510. Scale bars represent 20 µm. (D) Projection of registered brains labeling dopamine neurons that induce aversive memory. The anterior inferior medial protocerebrum (aimpr) is commonly innervated by these neurons (arrowhead). (E) Registration of control brains that do not induce aversive memory. The prominent processes project in the lateral protocerebrum (arrowhead). (F) The two functional groups of dopamine neurons are separately pooled and presented in different colors (green and magenta, respectively) for comparison. The MB lobes are shown in gray. Scale bars represent 20 µm. Differential requirement of three dopamine pathways for shock-induced memory
Based on cell counting and the behavioral screen, we selected drivers NP5272, 5htr1b-GAL4, c061;MB-GAL80 to further characterize three MB-M3 neurons, one MB-MV1 neuron/one MB-V1 neuron, and one or two MB-MP1 neurons, respectively (Figure 6C). We addressed whether these dopamine neurons function for mediating electric shock reinforcement. To test their necessity during shock conditioning, we transiently blocked corresponding neurons by expressing Shits1 [44]. Previous studies reported that blocking many dopamine neurons with TH-GAL4 (including MB-M3, MB-MP1 and MB-MV1/MB-V1 neurons) severely impaired memory irrespective of retention times [19], [25], [45], [46]. Notably, the transient Shits1 block of each dopamine pathway impaired specific temporal components of aversive memory of shock (Figure 8). The block with NP5272 preferentially impaired 2-hour memory, but not significantly affected 2-min or 9-hour memory (Figure 8A) [25].
Fig. 8. Requirement of the identified dopamine neurons for shock reinforcement. 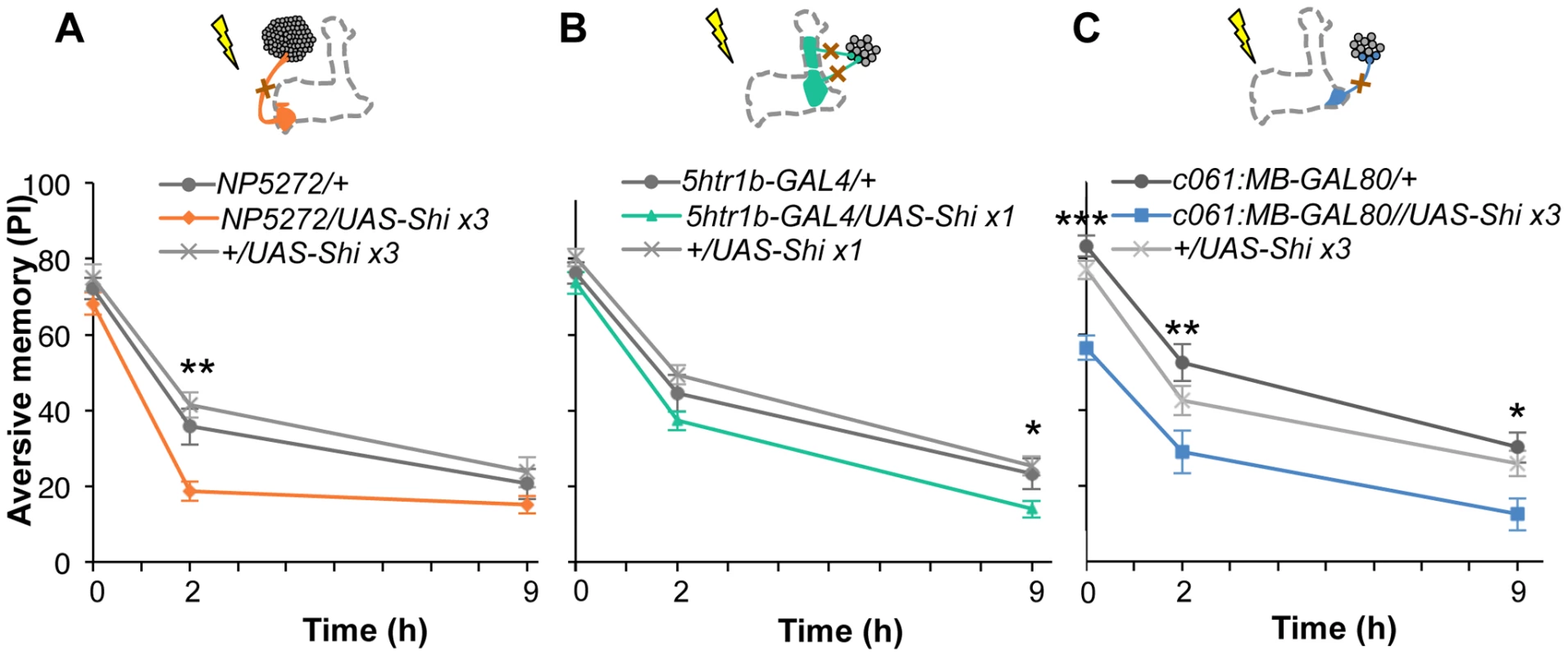
Output of the targeted neurons is blocked with Shits1 during conditioning by shifting up temperature to 33°C for 30 min prior to the training. Memory is tested immediately following the training or two or nine hours after keeping flies at permissive temperature (25°C). (A) NP5272/UAS-shits1 shows significantly impaired 2-hour memory, while the effect on immediate and 9-hour memory is marginal. n = 12–16. (B) Blocking with 5htr1b-GAL4 preferentially impaired 9-hour memory, whereas 2-min and 2-hour memory is not significantly affected. Because of memory impairment at permissive temperature with three copies of UAS-shits1, one copy of UAS-shits1 was used (see also Figure S5). n = 16–28. (C) Memories at all retention times are significantly impaired by blocking with c061;MB-GAL80. n = 16–24. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; ** P<0.01; n.s. not significant. We found that the block with 5htr1b-GAL4 with multiple copies of UAS-shits1 caused gradual memory impairment over time, leaving the immediate memory intact, although this fly had a significant phenotype at a permissive temperature (Figure S5). We reproduced the impairment of 9-hour memory with a single copy of UAS-shits1 (Figure 8B). This impairment of 5htr1b-GAL4/UAS-shits1 in consolidated memory was due to the transient block during training, since the same inhibition during consolidation or retrieval did not significantly affect the performance (Figure 9A–9D).
Fig. 9. Temporal requirement of the MB-MP1 and MB-MV1/MB-V1 during training. 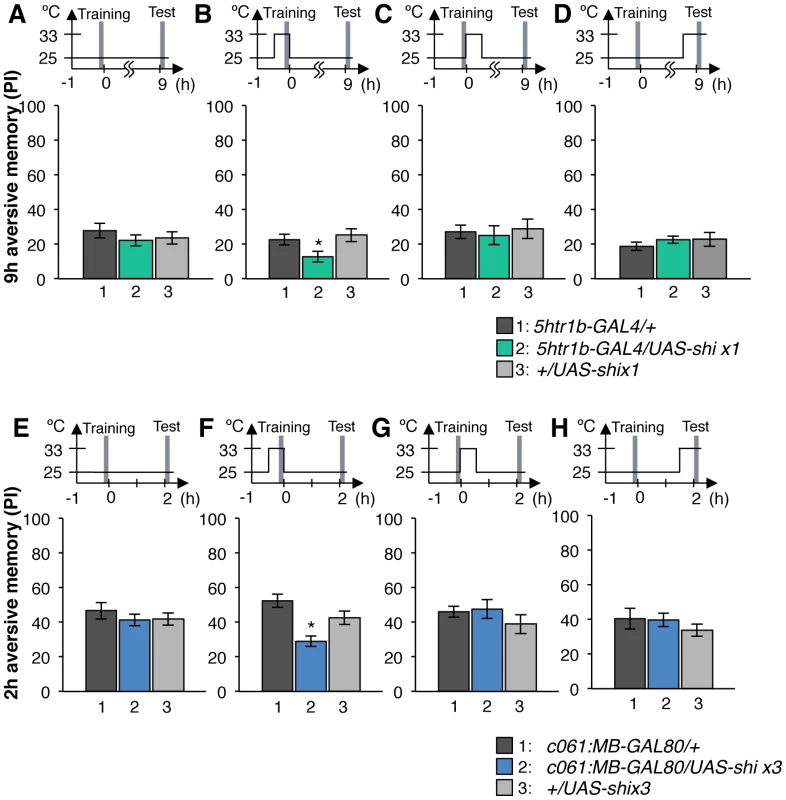
(A–D) Output of MB-MV1/V1 is selectively required for the formation of consolidated memory (9 h). At permissive temperature, 5htr1b-GAL4/UAS-shi ts1 show indistinguishable memory performance compared to the control genotypes (A). The transient block for 30 min including the training phase causes a slight but significant impairment of 9 hour memory (B), while the same blockade immediately after training (C) or during test (D) did not (n = 12–28). (E–H) c061;MB-GAL80/UAS-shi ts1 show impaired 2-h memory, only when they are trained at restrictive temperature (F). Permissive temperature (E), the same temperature shift after training (G), or during test (H) does not significantly affect the memory performance (n = 12–20). In contrast to these defects in selective memory phases, the block with c061;MB-GAL80 significantly impaired all tested memory phases (i.e. 2 min, 2 hours and 9 hours after training; Figure 8C), although a previous study found no significant impairment of 3-hour memory [26]. This is presumably due to the higher restrictive temperature in this study (33°C compared to 31°C), since the effect of Shits1 is sensitive to a small temperature difference [47]. The observed memory impairment with c061;MB-GAL80 should not be attributed to a defect in detecting odor or electric shock itself, as their reflexive avoidance was normal (Figure S6D). Furthermore, blocking after training or experiments at permissive temperature did not cause this phenotype (Figure 9E–9H). We also confirmed that blocking neurons outside our target dopamine neurons by combining GAL80 lines did not impair memory (Figure S6A–S6C and S6E–S6F). Taken together, we propose that electric shock punishment recruits multiple types of dopamine neurons MB-MP1, MB-M3 and MB-MV1/MB-V1 to form parallel memory traces with distinct temporal stability. Additional dopamine neurons might be recruited for signaling aversive reinforcement, since blocking all these dopamine pathways with TH-GAL4 leaves residual memory in contrast to complete abolishment of memory in dDA1 receptor mutants and neuron-specific TH mutants [17], [18], [25], [45], [46].
Dopamine pathways induce memories with distinct temporal dynamics
Contribution of each dopamine neuron to the synthesis of total memory depends on both the magnitude of initial memory and its stability over time. We found that the magnitude of dTRPA1-induced immediate memory depended on the activation temperature and cell type (Figure 10). This implies that the amount of dopamine input represents the strength of reinforcement.
Fig. 10. Magnitude of immediate memory depends on the cell type and dTRPA1 activation. 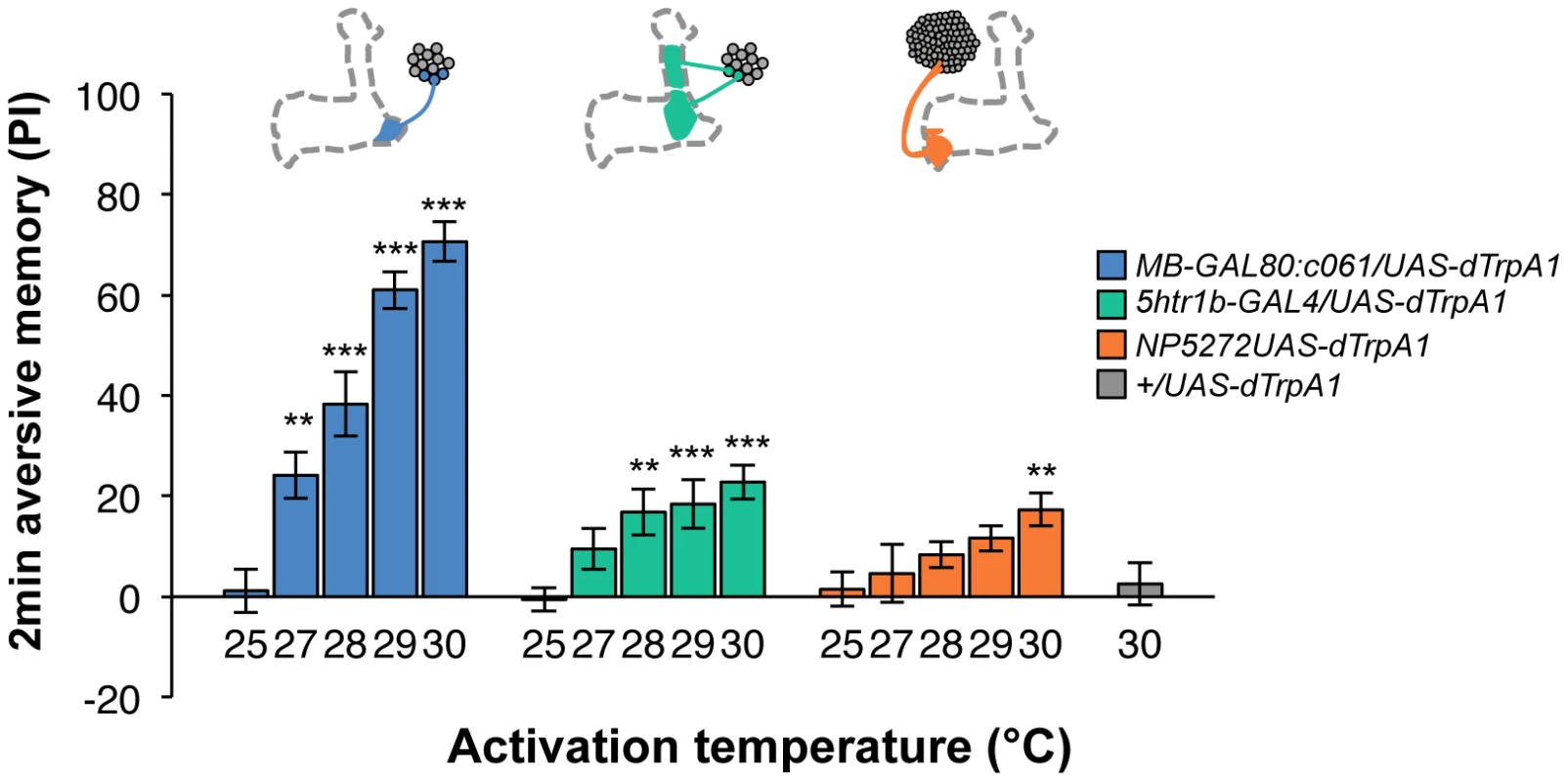
Thermo-activation of individual dopamine pathways with various temperatures from 25°C to 30°C induces different degrees of immediate memories. c061;MB-GAL80/UAS-dTrpA1 formed significant memory at 27°C compared to flies with no activation (25°C). Memory performance steeply increased with the elevation of activation temperature. In contrast, 5htr1b-GAL4 and NP5272 cause more modest immediate memory with the lowest activation temperature for inducing significant memory at 28°C and 30°C, respectively. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; ** P<0.01; *** P<0.001 by Dunnett's multiple comparison test with 25°C control following one-way ANOVA. n = 12–16. To better understand the role of the distinct reinforcement pathways, we characterized the retention of each memory component in isolation by activating the individual dopamine neurons. Memories induced by these drivers showed remarkable differences in decay dynamics (Figure 11). Initially robust memory induced with c061;MB-GAL80 decayed rapidly over 9 hours. In contrast, memory induced with 5htr1b-GAL4 was highly stable and still significantly present after 9 hours, although initial memory was moderate. This differential memory decay is not due to the magnitude of initial memory, because the equivalent initial memory with NP5272 or with milder activation using c061;MB-GAL80 disappeared completely within 3 hours. In contrast to the similar decay dynamics of memories induced with NP5272 and c061;MB-GAL80 (Figure 11), the requirement of these neurons for the retention of shock-induced memories is qualitatively different (Figure 8). The genetic background or the effect of dTRPA1 at permissive temperature is an unlikely cause of the faster/slower memory decay, because the memory retention of the dTRPA1-expressing flies was indistinguishable when trained with electric shock (Figure S7). Thus, these results suggest that each dopamine pathway to the MB establishes a memory component with unique temporal stability.
Fig. 11. Individual dopamine pathways induce memories with distinct retention dynamics. 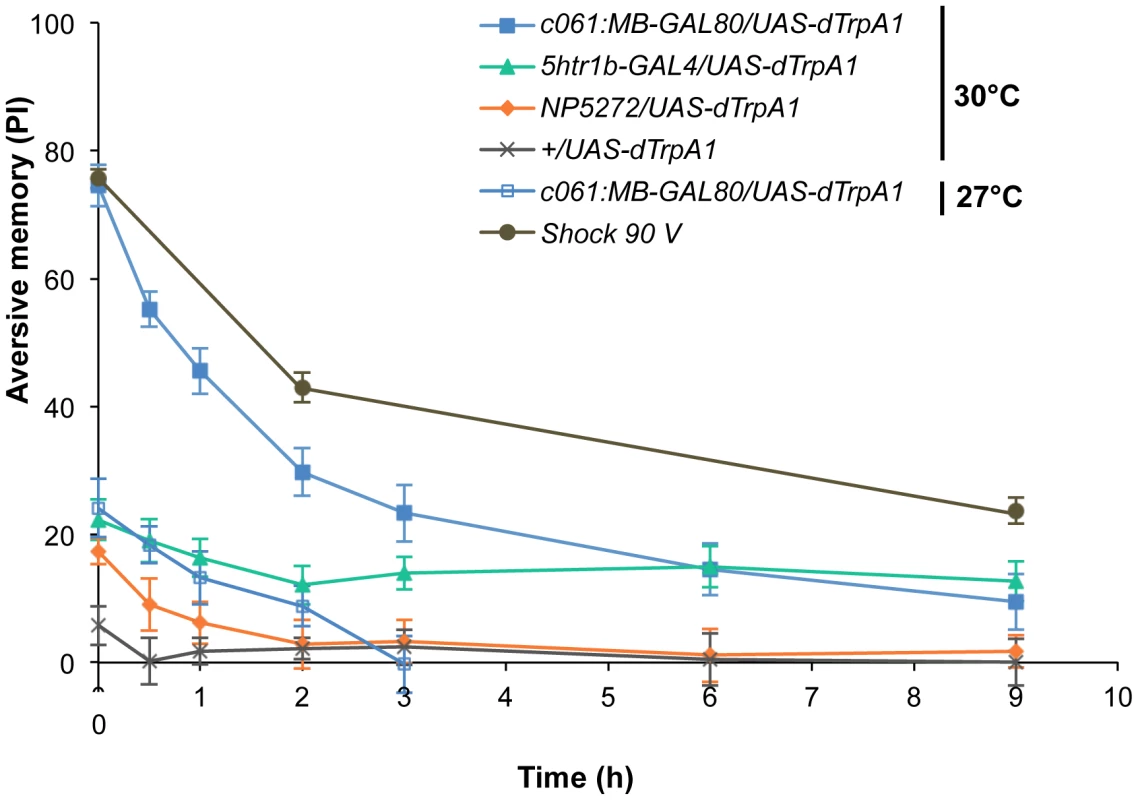
Flies expressing dTRPA1 in different dopamine neurons are trained at 30°C and tested at various retention times. c061;MB-GAL80/UAS-dTrpA1 are also trained at 27°C. For comparison, averaged performance of all these genotypes after electric shock conditioning is also plotted (gray; see Figure S7 for memory dynamics of individual genotype). Memories induced by different dopamine neurons decayed with significantly distinct temporal dynamics (P<0.05; significant interaction [genotype×retention time] in two-way ANOVA). Points and error bars represent the mean and s.e.m., respectively. n = 16–26. Functional synergy and redundancy of multiple memories
The results of activation and inactivation experiments appear to be inconsistent: A simple sum of memory performances by activation of these pathways does not explain selective memory impairments when they are blocked (Figure 8 and Figure 11). For instance, the effect of the blocking MB-M3 neurons with NP5272 was most pronounced for 2-hour memory (Figure 8A), whereas memory induced by activation of the same cells with NP5272 did not last 2 hours (Figure 11). The reason of this apparent discrepancy could be technical, such as the copy number of the effectors and temperature regimes. Alternatively it may suggest a synergistic interaction between memories induced by MB-M3 neurons and other dopamine neurons. We noticed another case of possible interaction that suggests functional redundancy; activation with NP5272 or 5htr1b-GAL4 induced immediate memory (Figure 11), whereas the block with these drivers did not show significant short-term defect (Figure 8A–8B).
To test the various forms of interaction between memory components, we first measured activation of MB-M3 neurons together with other reinforcement pathways. To activate MB-MP1, MB-MV1 and MB-V1 neurons together, we took another driver MB-GAL80;NP0047 from the activation screening (Figure 3Q), and measured the retention of dTRPA1-induced memory. In comparison to MB-GAL80;NP0047 alone, additional activation with NP5272 did not significantly improve the performance of immediate memory (Figure 12A), supporting the redundancy of MB-M3 neurons and the others for immediate memory. This is in line with no significant requirement of MB-M3 neurons for immediate memory induced by electric shock (Figure 8A). In contrast, combinatorial activation significantly improved performance in 2-hour retention (Figure 12A), suggesting the synergistic contribution of MB-M3 neurons to 2-hour memory and recapitulating the selective impairment of 2-hour shock memory upon blocking with NP5272 (Figure 8A). Also combinatorial activation of MB-M3 and MB-V1/MB-MV1 neurons with NP5272 and 5htr1b-GAL4 caused a similar pattern of interaction in immediate and 2-hour memories (Figure 12B). In contrast, activation of MB-M3 and MB-V1 neurons without MB-MV1 neuron in NP5272 and MZ840 did not induce any 2-hour memory (Figure 12C). Also NP5272 activation was not significantly additive across all tested retention times compared to c061;MB-GAL80 single activation (Figure 12D). Thus, we propose that MB-MV1, but not MB-MP1 or MB-V1 neurons, likely interacts with MB-M3 neurons for 2-hour memory. The enhancement of 2-hour memory was likely due to an increase of anesthesia-sensitive memory (ASM), because the effect disappeared by 9 hour when the memory is primarily consisted of anesthesia resistant memory (ARM). Indeed, MB-M3 neurons activation did not contribute to the increase of ARM (Figure 13A, 13C). Memories induced by thermo-activation with 5htr1b-GAL4 and c061;MB-GAL80 were at least partially ARM (Figure 13).
Fig. 12. Combinatorial activation of different dopamine neurons shapes the stability of odor memory. 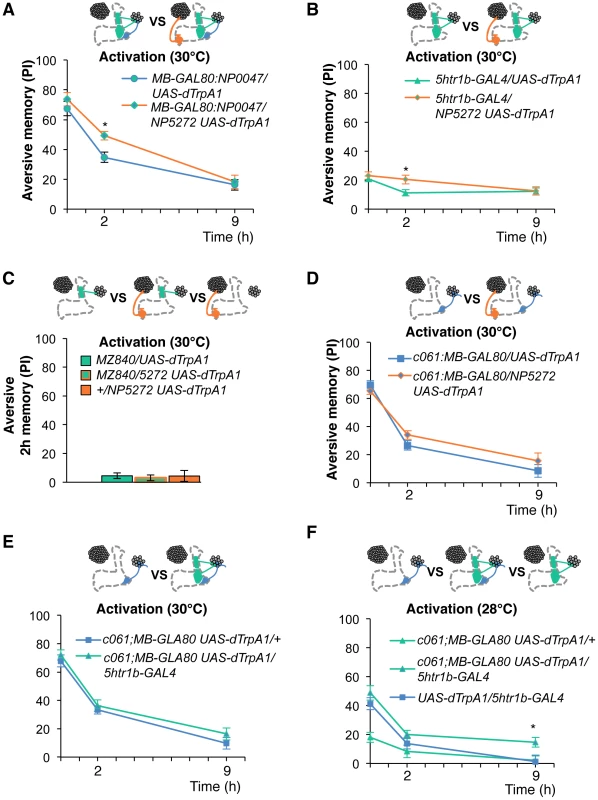
Retention of memories by activating different combinations of dopamine neurons. Diagram above each graph depicts dopamine neurons targeted by the driver combination. (A) Memory of MB-GAL80;NP0047/NP5272 UAS-dTrpA1 is significantly higher than that of MB-GAL80;NP0047/UAS-dTrpA1 at 2 hours, but not at the other tested time points. n = 16–22. (B) Memory of 5htr1b-GAL4 NP5272/UAS-dTrpA1 is significantly higher than that of 5htr1b-GAL4/UAS-dTrpA1 specifically at 2-hour retention. n = 16–26. (C) Flies do not form significant 2 h memory with MZ840 that label MB-V1, but not MB-MV1. The addition of NP5272 does not improve the memory. n = 16. (D) Memory of c061;MB-GAL80 NP5272/UAS-dTrpA1 is not significantly different from that of c061;MB-GAL80/UAS-dTrpA1 at all retention time, although it tended to be higher. n = 16–26. (E) Compared to c061;MB-GAL80/UAS-dTrpA1, combinatorial activation with c061;MB-GAL80 5htr1b-GAL4 does not significantly improve the performance at all retention times, if cells are activated at 30°C. n = 16. (F) Milder dTRPA1-activation at 28°C reveals that the performance of c061;MB-GAL80/UAS-dTrpA1 and 5htr1b-GAL4/UAS-dTrpA1 is redundant and interdependent for immediate and 9-hour memories, respectively. This resembles the increasing memory impairments upon blocking with 5htr1b-GAL4 (Figure 8B). n = 16–22. Bars and error bars represent the mean and s.e.m., respectively. * P<0.05; n.s. not significant. Fig. 13. ARM after combinatorial activation of different dopamine neurons. 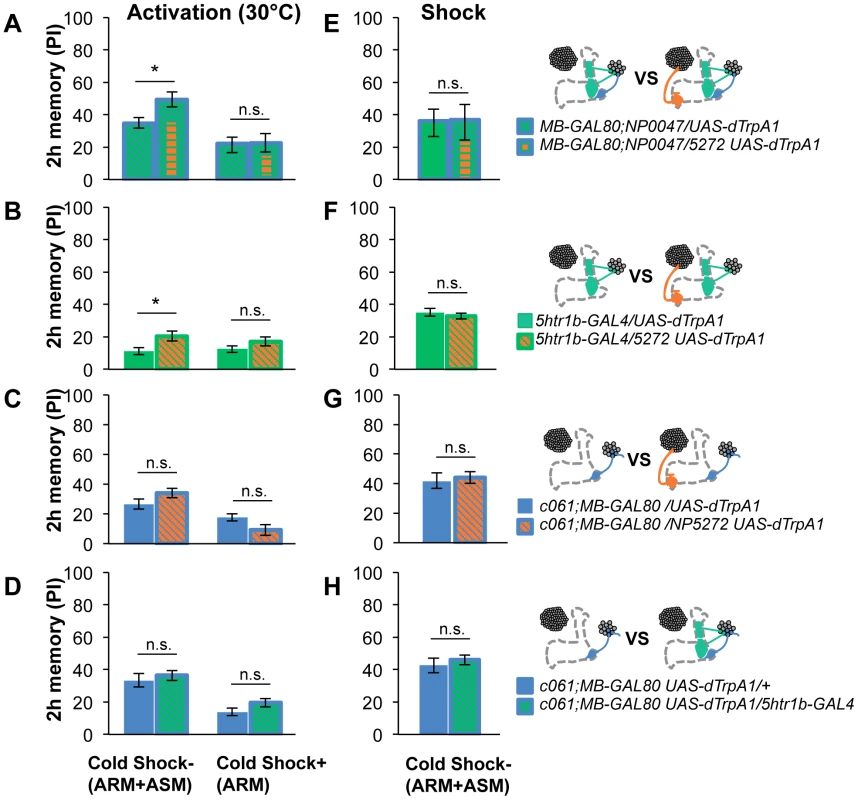
(A–D) Flies are trained by transient activation of dTrpA1 expressing cells using respective GAL4 drivers and tested 2 hours later. (A) n = 12–26. (B) n = 26. (C) n = 16–26. (D) 16–24. (E–H) At permissive temperature (25°C), 2-h memory with electric shock is not significantly different in flies expressing dTrpA1 with single and specific combinations of drivers that are used for thermo-activation. Bars and error bars represent the mean and s.e.m., respectively (n = 12–18). * P<0.05; n.s. not significant. For (E), bars and error bars represent median and interquartile range, because the data points were not normally distributed. Combinatorial thermo-activation with 5htr1b-GAL4 and c061;MB-GAL80 was not significantly different from single activation by c061;MB-GAL80 (Figure 12E). Milder activation at 28°C however revealed interaction (Figure 12F) that features gradual memory impairments upon blocking with 5htr1b-GAL4 (Figure 8B). Ectopic expression of dTRPA1 or its effect at permissive temperature alone is an unlikely cause of the interaction, since 2-hour memory of all these genotypes was normal when they were trained with electric shock (Figure 13E, 13F, 13G, 13H). Altogether, these results suggest that MB-MV1 and MB-M3 neurons are redundant for immediate memory when MB-MP1 neuron is activated, and different modulatory interactions of MB-MV1/MB-M3 neurons and MB-MV1/MB-MP1 neurons tune the stability of memory.
Discussion
Different memory traces in the MB via distinct dopamine neurons
Using freely behaving animals, we demonstrated that at least three essential dopamine pathways to the MB can together synthesize aversive memory that shares similar temporal characteristics with shock-induced memory. They arborize in the different subdomains in the MB. Functional imaging of dopamine neurons revealed that response to electric shock significantly differ between cell types [21]. These results indicate shock reinforcement recruits a specific set of the dopamine pathways to the MBs and induces multiple memory traces in Kenyon cells through different receptors [18], [30], [32]. The Drosophila MB is subdivided into domains, that are defined by specific combinations of intrinsic and extrinsic neurons (Figure 1A) [15]. Each dopamine pathway for memory induction intersects the specific axonal compartment of Kenyon cells (Figure 1E, Figure 7A–7B) [15], [21], and we found that the memory components induced by the distinct dopamine neurons interact to tune the stability of collective memory (Figure 12). Therefore, multiple memory traces formed in spatially segregated synapses in the MB would interact with each other.
One of the possible underlying mechanisms is intracellular interaction of the dopamine inputs to a single Kenyon cell. In the sensory neurons of Aplysia, simultaneous application of serotonin to different parts of a neuron - the cell body and presynaptic terminals of a sensory neuron - synergistically induces intermediate and long-term facilitation of the sensorimotor synapses [48]. Alternatively, subcellular memory traces may interact at the circuit level via interneurons, such as the DPM and APL neurons, for which previous studies showed their essential role in memory consolidation [6], [38], [49], [50]. A recent behavioral and imaging study revealed that the spontaneous activity of MB-MV1 and MB-MP1 after olfactory conditioning is coordinated to control the stability of memory [51]. Such network level interaction can also be implemented as a neuronal integration of outputs from the multiple memories. Considering parallel formation and the interaction of distinct memory components by different Kenyon cell populations [7], [30], [31], [32], [33], [52], the latter scenario might be more likely.
Recruitment of dopamine neurons by noxious stimuli
A combined neuroanatomical and computational analysis identified that the dendrites of MB-M3, MB-MV1 and MB-MP1 neurons form a cloud cluster in the protocerebrum, and they project into the segregated domains of the MB (Figure 7). This neuronal configuration implies that a punishment signal undergoes parallel processing in the MB via distinct dopamine pathways to induce different memory traces. The response profile of MB-projecting dopamine neurons is indeed distinct, suggesting that they receive input from different neurons [21]. Characterization of presynaptic neurons innervating the dendritic regions of the dopamine neurons will help identifying a cellular mechanism of parallel processing of reinforcement.
Multiple roles of dopamine neurons
Recent studies including our results highlight distinct functions of single dopamine neurons. Especially, MB-MP1 neurons are required for: 1) Suppression of the retrieval of appetitive memory when flies are not starved [26]; 2) Formation of aversive odor memory by mediating electric shock (this study); 3) Regulation of long-term memory by synchronized spontaneous activity together with MB-MV1 after spaced training [51]. Although these functions seem incompatible at the first glance, we suggest context-dependent roles of single dopamine neurons.
Functions of MB-MP1 neurons in suppression of conditioned odor approach and aversive reinforcement signaling may be reasonable, as it is not appropriate timing for an animal to follow appetitive memory when challenged by a noxious stimulus (aversive reinforcement). Implementing these two functions to the same MB-extrinsic neuron can be an elegant design by evolution, since both appetitive and aversive memories are formed and stored in the MB. Furthermore, in the light of a recent study, the results of MB-MP1 neurons suggests that dopamine may play opposing roles in the formation and the degradation of ARM (Figure 13) [51]. A key difference of the opposing actions of PPL1 neurons is that Kenyon cells are simultaneously activated by an odor. Therefore, the effect of dopamine release from MB-MP1/MB-MV1 neurons on Kenyon cell synapses might be dependent on the state of the cells. These distinct modes of dopamine action may be better characterized by physiological means, such as functional imaging or electrophysiology.
Materials and Methods
Fly strains
We generated flies for behavioral and anatomical studies using the GAL4/GAL80 lines and transgenes listed in Table S1. 5htr1b-GAL4 (II) was generated with the enhancer fragment of 5htr1b gene. The fragment (−542–+2606) was amplified using the primer pair GTC AAATTCGGTCTGGCATT and CTTGCCTATGATGGTGACG by PCR and cloned into the pCRII-TOPO® vector (Invitrogen). After verifying the sequence, the fragment was cloned into the p221-4 GAL4 vector (gift from E. Knust). UAS-UAS-Shits1 ×3 is a combination of P-element insertion on X and multiple insertions on third chromosome, which is identical to Shi2 in [44], [47]. UAS-Shits1 ×1 is isolated from multiple insertions on third chromosome by recombination, obtained from T. Préat lab. For experiments with UAS-Shits1 and UAS-dTrpA1, flies were raised at 18°C and 25°C, respectively, at 60% relative humidity. UAS-Shits1 and UAS-dTrpA1 flies were aged 8–14 and 7–12 days after eclosion, respectively, to allow sufficient accumulation of effecter proteins without age-related memory impairment. For anatomical studies, females of 5–10 days after eclosion at 25°C were analyzed.
Behavioral assays
For olfactory conditioning, we used 4-methylcyclohexanol and 3-octanol diluted in the paraffin oil (1∶10). One odor was presented for 1 min at elevated temperature or with 12 pulses of electric shocks (90 V) (Figure 1D). Subsequent to 1-min air flush, another odor was presented for 1 min. The reciprocal group of flies was trained by the protocol in which the identity of odors was altered. After a given retention time, the conditioned odor response was measured in a T-maze for two minutes. Then, a performance index (PI) was calculated by taking the mean preference of the two reciprocal groups [53]. The first odor was paired with reinforcement in a half of experiments and the second odor was paired with reinforcement in another half so that the effect of the order of reinforcement is canceled [53]. The protocol for conditioning with thermo-activation by dTRPA1 was essentially same as the standard protocol of olfactory conditioning using electric shock [45], [53], [54], except that flies were transferred to the pre-warmed T-maze in the climate box only during the presentation of one of the two odorants (60 sec) [25]. To minimize the noxious effect of heat itself, we used moderate temperature (30°C) for activation. This temperature shift by itself scarcely induced a significant memory in control genotypes [25]. For measuring the ARM, trained flies were transferred into pre-cooled tube on ice for 60 sec at 100 min after training, then transferred back to the pre-warmed tube at 25°C.
Statistics
Statistical analyses were performed using Prism (GraphPad Software). Most of the tested groups did not violate the assumption of the normal distribution and the homogeneity of variance. Therefore, mean performance indices were compared with t-test, or Dunnett's multiple comparison test or Bonferroni-post test for selected pairs following one-way or two-way ANOVA. For the groups that violated the assumption of parametric statistics, Mann-Whitney test (Figure 13E) or Dunn's multiple comparison test following Kruskal-Wallis-test was applied (Figure 9D).
Immunohistochemistry
The brain and thoracicoabdominal ganglion were prepared for immunolabeling and analyzed as previously described [16], [25], [54]. For dopamine staining (Figure S1), brains were fixed with 0.6% glutaraldehyde in PBS for 30 min; the unreacted aldehyde groups were subsequently reduced with 0.1% (w/v) sodium tetraborhydrate. Brains were embedded in 7% agarose and thick (150 um) sections were obtained with Laica Vibratome and labelled with the polyclonal antibody against conjugated dopamine (MoBiTech). Frontal optical sections of brain samples were taken with confocal microscopy, Olympus FV1000 or Leica SP2. For evaluating the effect of GAL80, brains to be compared were scanned with identical microscopy setting. Images of the confocal stacks were analyzed with the open-source software Image-J [55].
Image registration
Intensity-based affine and non-rigid registration of whole brains were performed with the toolbox elastix [43]. Confocal images of entire brains of GAL4/UAS-mCD8::GFP were acquired at 1024×512 pixel resolution. All brains were registered to a representative brain using counterstaining with TH as a reference channel. TH signals are mainly composed of sparse bright landmarks and a detectable background, allowing affine registration. The transformations computed with the TH channel were then applied to the mCD8::GFP channel. For some samples, non-rigid registration based on BSpline interpolation has been applied following affine transformation. Critical parameters, such as grid spacing, were empirically optimized. The accuracy of the registration was evaluated manually based on the matching of anti-TH signals. To compare positions of dendritic arbors of dopamine neurons, we selected samples with a small registration error (<20 µm) and signal from the other GAL4 expressing cells was manually masked (see Figure 4A, Figure 5B, and [25] for the GAL4 expression patterns), and mCD8::GFP channels of different drivers were blended as different colors using ImageJ. The final images in Figure 7D–7F and Videos S1, S2, S3 show only regions surrounding the MB. Employed GAL4 drivers: NP5272, NP2758, 5htr1b-GAL4 (Figure 7D and Video S1); MZ840, NP6510 and MZ19;Cha3.3kb-GAL4 (Figure 7E and Video S2); NP5272, NP2758, 5htr1b-GAL4, NP6510 and MZ19;Cha3.3kb-GAL4 (Figure 7F and Video S3).
Supporting Information
Zdroje
1. FrostWNCastellucciVFHawkinsRDKandelER 1985 Monosynaptic connections made by the sensory neurons of the gill - and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci U S A 82 8266 8269
2. MedinaJFRepaJCMaukMDLeDouxJE 2002 Parallels between cerebellum - and amygdala-dependent conditioning. Nat Rev Neurosci 3 122 131
3. McGuireSEDeshazerMDavisRL 2005 Thirty years of olfactory learning and memory research in Drosophila melanogaster. Progress in neurobiology Prog Neurobiol 76 328 347
4. HeisenbergM 2003 Mushroom body memoir: from maps to models. Nat Rev Neurosci 4 266 275
5. GerberBTanimotoHHeisenbergM 2004 An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol 14 737 744
6. KeeneACWaddellS 2007 Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci 8 341 354
7. ZarsTFischerMSchulzRHeisenbergM 2000 Localization of a short-term memory in Drosophila. Science 288 672 675
8. GervasiNTchenioPPréatT 2010 PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron 65 516 529
9. TomchikSMDavisRL 2009 Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron 64 510 521
10. WangYGuoHFPologrutoTAHannanFHakkerI 2004 Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci 24 6507 6514
11. TurnerGCBazhenovMLaurentG 2008 Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 99 734 746
12. HoneggerKSCampbellRATurnerGC 2011 Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci 31 11772 11785
13. CrittendenJRSkoulakisEMHanKAKalderonDDavisRL 1998 Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 5 38 51
14. LinHHLaiJSChinALChenYCChiangAS 2007 A map of olfactory representation in the Drosophila mushroom body. Cell 128 1205 1217
15. TanakaNKTanimotoHItoK 2008 Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 508 711 755
16. AsoYGrübelKBuschSFriedrichABSiwanowiczI 2009 The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet 23 156 172
17. KimYCLeeHGHanKA 2007 D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 27 7640 7647
18. QinHCressyMLiWCoravosJSIzziSA 2012 Gamma Neurons Mediate Dopaminergic Input during Aversive Olfactory Memory Formation in Drosophila. Curr Biol
19. Claridge-ChangARoordaRDVrontouESjulsonLLiH 2009 Writing memories with light-addressable reinforcement circuitry. Cell 139 405 415
20. SchrollCRiemenspergerTBucherDEhmerJVöllerT 2006 Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16 1741 1747
21. MaoZDavisRL 2009 Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3 5
22. NässelDRElekesK 1992 Aminergic neurons in the brain of blowflies and Drosophila: dopamine - and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res 267 147 167
23. WhiteKEHumphreyDMHirthF 2010 The dopaminergic system in the aging brain of Drosophila. Front Neurosci 4 205
24. RiemenspergerTVöllerTStockPBuchnerEFialaA 2005 Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 15 1953 1960
25. AsoYSiwanowiczIBräckerLItoKKitamotoT 2010 Specific Dopaminergic Neurons for the Formation of Labile Aversive Memory. Curr Biol 20 1445 1451
26. KrashesMJDasGuptaSVreedeAWhiteBArmstrongJD 2009 A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139 416 427
27. ZhangKGuoJZPengYXiWGuoA 2007 Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science 316 1901 1904
28. BangSHyunSHongSTKangJJeongK 2011 Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet 7 e1001346 doi:10.1371/journal.pgen.1001346
29. KaunKRAzanchiRMaungZHirshJHeberleinU 2011 A Drosophila model for alcohol reward. Nat Neurosci 14 612 619
30. BlumALLiWCressyMDubnauJ 2009 Short - and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol 19 1341 1350
31. AkalalDBWilsonCFZongLTanakaNKItoK 2006 Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem 13 659 668
32. IsabelGPascualAPreatT 2004 Exclusive consolidated memory phases in Drosophila. Science 304 1024 1027
33. KrashesMJKeeneACLeungBArmstrongJDWaddellS 2007 Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53 103 115
34. HamadaFNRosenzweigMKangKPulverSRGhezziA 2008 An internal thermal sensor controlling temperature preference in Drosophila. Nature 454 217 220
35. SitaramanDZarsMLaferriereHChenYCSable-SmithA 2008 Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A 105 5579 5584
36. KitamotoTIkedaKSalvaterraPM 1995 Regulation of choline acetyltransferase/lacZ fusion gene expression in putative cholinergic neurons of Drosophila melanogaster. J Neurobiol 28 70 81
37. LiHChaneySRobertsIJForteMHirshJ 2000 Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol 10 211 214
38. LeePTLinHWChangYHFuTFDubnauJ 2011 Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proc Natl Acad Sci U S A 108 13794 13799
39. YuDKeeneACSrivatsanAWaddellSDavisRL 2005 Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell 123 945 957
40. PascualAPreatT 2001 Localization of long-term memory within the Drosophila mushroom body. Science 294 1115 1117
41. YuDAkalalDBDavisRL 2006 Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron 52 845 855
42. SéjournéJPlaçaisPYAsoYSiwanowiczITrannoyS 2011 Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci 14 903 910
43. KleinSStaringMMurphyKViergeverMAPluimJP 2010 elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29 196 205
44. KitamotoT 2001 Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol 47 81 92
45. SchwaerzelMMonastiriotiMScholzHFriggi-GrelinFBirmanS 2003 Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23 10495 10502
46. RiemenspergerTIsabelGCoulomHNeuserKSeugnetL 2011 Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A 108 834 839
47. SchwaerzelMHeisenbergMZarsT 2002 Extinction antagonizes olfactory memory at the subcellular level. Neuron 35 951 960
48. SherffCMCarewTJ 1999 Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science 285 1911 1914
49. WuCLShihMFLaiJSYangHTTurnerGC 2011 Heterotypic gap junctions between two neurons in the drosophila brain are critical for memory. Curr Biol 21 848 854
50. PitmanJLHuetterothWBurkeCJKrashesMJLaiSL 2011 A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol 21 855 861
51. PlaçaisPYTrannoySIsabelGAsoYSiwanowiczI 2012 Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci 15 592 599
52. McGuireSELePTOsbornAJMatsumotoKDavisRL 2003 Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302 1765 1768
53. TullyTQuinnWG 1985 Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 157 263 277
54. ThumASJenettAItoKHeisenbergMTanimotoH 2007 Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci 27 11132 11138
55. RasbandWS 1997–2011 ImageJ, U.S.: National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



