-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
As sessile organisms, plants have to cope with diverse environmental constraints that may vary through time and space, eventually leading to changes in the phenotype of populations through fixation of adaptive genetic variation. To fully comprehend the mechanisms of evolution and make sense of the extensive genotypic diversity currently revealed by new sequencing technologies, we are challenged with identifying the molecular basis of such adaptive variation. Here, we have identified a new variant of a molybdenum (Mo) transporter, MOT1, which is causal for fitness changes under artificial conditions of both Mo-deficiency and Mo-toxicity and in which allelic variation among West-Asian populations is strictly correlated with the concentration of available Mo in native soils. In addition, this association is accompanied at different scales with patterns of polymorphisms that are not consistent with neutral evolution and show signs of diversifying selection. Resolving such a case of allelic heterogeneity helps explain species-wide phenotypic variation for Mo homeostasis and potentially reveals trade-off effects, a finding still rarely linked to fitness.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002814
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002814Summary
As sessile organisms, plants have to cope with diverse environmental constraints that may vary through time and space, eventually leading to changes in the phenotype of populations through fixation of adaptive genetic variation. To fully comprehend the mechanisms of evolution and make sense of the extensive genotypic diversity currently revealed by new sequencing technologies, we are challenged with identifying the molecular basis of such adaptive variation. Here, we have identified a new variant of a molybdenum (Mo) transporter, MOT1, which is causal for fitness changes under artificial conditions of both Mo-deficiency and Mo-toxicity and in which allelic variation among West-Asian populations is strictly correlated with the concentration of available Mo in native soils. In addition, this association is accompanied at different scales with patterns of polymorphisms that are not consistent with neutral evolution and show signs of diversifying selection. Resolving such a case of allelic heterogeneity helps explain species-wide phenotypic variation for Mo homeostasis and potentially reveals trade-off effects, a finding still rarely linked to fitness.
Introduction
Some of the most important constraints that plants have to adapt to are those related to soil properties [1], [2]. These are also possibly some of the least well studied constraints, because they are spatially heterogeneous, thus not prone to typical geographic clines [3], [4], and require analysis at the local/population scale [5]. In this context, quantitative genetics approaches hold great promise to reveal the genetic basis of adaptation by enabling the identification of the molecular origin of phenotypic differences between populations or even between species [6], [7]. One of the benefits of identifying the causative polymorphism(s) and/or gene(s) explaining natural phenotypic variation is that it allows direct testing for correlations between environmental factors populations may be responding to and the occurrence of the target genetic polymorphism. This contrasts with working indirectly through populations phenotype, which may reflect contradictory patterns and trade-offs, if not genetic drift [5], [8]. Moreover, this approach also enables direct testing for fitness advantages or potential cost of adaptation (i.e. antagonistic pleiotropy, an expected argument for local adaptation), that may be masked by linkage to deleterious mutations or genetic drift in reciprocal transplant experiments [9], [10]. Molecularly identified examples of potentially-adaptive variation are still largely lacking and the debate is open as to the scale and rate of adaptive evolution [11], [12].
In this work, we aimed at identifying the molecular bases of natural variation in accumulation of an essential micronutrient and understanding the ecological significance of this diversity. We describe both the fitness trade-offs of this variation and its potential adaptive advantage in the environment, revealing a system that is unlikely to have remained neutral.
Results/Discussion
Bay-0 and Shahdara, two strains (accessions) derived from wild populations of Arabidopsis thaliana, show contrasted growth behavior when grown on acidic peatmoss substrate (Figure 1). Using a segregating population derived from the cross of these two accessions, we determined the Shahdara growth defect to be segregating from a major-effect recessive locus on chromosome 2, as confirmed by a near-isogenic line derived from a residual heterozygous interval in one of the recombinant inbred lines (HIF084; Figure 1). Additional recombinant lines were phenotyped and genotyped to pinpoint the causative interval to 80 kb (Figure S1), covering 19 annotated genes. Among these, one gene appeared as a good functional candidate: MOT1 was previously linked to the transport and homeostasis of the essential micronutrient molybdenum (Mo) in the plant [13], [14], an element which availability is known to vary with soil pH [15]. Indeed, a T-DNA insertion mutant in the MOT1 gene (mot1.1; Figure S1) shows a phenotype similar to Shahdara on peatmoss and –contrary to the Bay and Col alleles – the Sha allele is not able to restore wild-type growth when combined with the mutant allele in F1 hybrids (Figure 2). Moreover, we show that defective growth is complemented by either increasing soil pH with additional CaCO3 mixed to the peatmoss (Figure S2) or increasing Mo availability (without altering soil pH) by adding Mo in the watering solution (Figure 3). Hence, genetic and chemical complementation shows that acidic soil pH is responsible for reducing Mo-bioavailability and that, combined with a defective allele at MOT1, this results in the typical Mo-deficiency syndromes of reduced leaf Mo contents, strongly altered growth and development, necrosis [16]. These observations of a significant phenotypic consequence of variation at MOT1 provide a model for the potential adaptive significance of this variation that goes beyond the simple variation in Mo content revealed previously [13], [14].
Fig. 1. Acidic peatmoss substrate induces severe growth defect linked to the Shahdara allele. 
When grown on peatmoss at a pH close to 5, Shahdara is subject to severe growth and developmental arrest, necrosis and death, contrary to Bay-0 which develops normally. In the cross between these two strains, this phenotype is entirely controlled by a single locus (Figure S1), as confirmed by near-isogenic line ‘HIF084’ segregating solely for a region of chromosome 2. Fig. 2. Mutant analysis and allelic complementation confirms MOT1 as the likely causative gene. 
Peatmoss phenotype of diverse genotypes is shown, including Bay-0 and Shahdara parental lines, mot1.1 mutant and its wild-type genetic background (Col-0), and F1 plants from complementation crosses between these genotypes. Unlike other alleles, the Shahdara allele at MOT1 is not able to rescue the mutant phenotype. Fig. 3. Chemical complementation links growth defect with Mo shortage. 
Accessions with potentially functional (Bay-0, Col-0) and defective MOT1 alleles (Ler, Shahdara, mot1.1) were grown on peatmoss substrate watered with nutrient solution containing either traces of Mo (‘Control’) as in Figure 1, or 1 mM Na2MoO4 (‘+Na2MoO4’). pH was checked to remain unchanged across treatments at ∼5. Although we find that Landsberg erecta (Ler) has a similar behaviour than Shahdara in our conditions (Figure 3), this defective allele (MOT1Ler) used initially to reveal the gene's activity [13], [14] is functionally different from the MOT1Sha defective allele. MOT1Sha doesn't bear the promoter 53 bp-deletion as in Ler (Figure S1) and in fact is not showing MOT1Ler-like transcriptional down-regulation compared to MOT1Bay or MOT1Col (Figure S3). Instead, MOT1Sha seems defined by a single amino-acid change in the protein relative to Bay-0 and Col-0 (Figure S1), strongly suggesting that MOT1Sha is hypofunctional. However, the MOT1 protein produced from the Sha allele is still able to increase Mo accumulation when heterologously expressed in yeast (Figure S4).
We then genotyped a random worldwide sample of ∼300 accessions for the Sha-like amino-acid change and the Ler-like 53-bp deletion and find that these alleles are both present at intermediate frequencies (15–20%) among the populations. Sequencing 102 of these accessions for the whole gene and promoter region revealed that the very conserved MOT1Sha haplotype is indeed clearly defined solely by the D104Y amino-acid change, while the MOT1Ler genotype is more complex and diverse (Table S1). All Sha-like and Ler-like accessions that have been phenotyped show that both MOT1Sha and MOT1Ler haplotypes are perfectly associated with defective growth under acidic soil conditions (Table S1) and complementation crosses with five additional Sha-like accessions confirm allelism to mot1.1 (Figure S5). Taking into account this allelic heterogeneity now explains most of the species variation toward low-Mo contents revealed in previous work [13] (http://www.ionomicshub.org/arabidopsis/). This form of complexity –in addition to genetic heterogeneity – is probably more frequent than previously thought in many organisms and is likely to help explain part of the missing heritability [17], [18].
Regarding MOT1 defective haplotypes, MOT1Sha is confined to ‘West-Asia’ (including Russia) with a high frequency among these populations (Figure S6) and displays a very low polymorphism level (πSha = 0,00016) in comparison to other haplotype clusters, including the worldwide-distributed MOT1Ler allele (πLer = 0,0017; Table S1). This may translate a recent and rapid expansion of the Sha allele through ‘West-Asia’, which could be due to neutral processes such as gene surfing associated with post-glaciation recolonization events from Central Asia [19]. This may also witness local positive selection events in favour of the Sha allele. Indeed, patterns of nucleotide polymorphisms at MOT1 in the sample of 102 accessions strongly deviate from the expectation under the strict neutrality model, contrarily to two control loci, PI and COI1 (Table S2). Negative values of Tajima's D reveal an excess of rare alleles at MOT1, suggesting the possible occurrence of at least one past selective sweep that has targeted this locus. Other well documented evolutionary processes such as population expansion after the last glaciation event [20] and population genetic structure [21] could also have contributed to the excess of rare alleles observed at the genome-wide level [22], as well as at the MOT1 locus in A. thaliana. Nevertheless, the HKA and McDonald-Kreitman tests, which do not rely on the frequency spectrum, support the hypothesis of diversifying selection at the species level (Tables S2 and S3). The excess of within-species polymorphisms relatively to inter-specific divergence and the excess of non-synonymous polymorphisms observed at MOT1 may result from the selection of different haplotypes at the worldwide scale. Interestingly, this trend is also clear when considering only accessions from ‘West Asia’, suggesting that the selection process could happen at different geographical scales.
Our own documented collection of wild populations from diverse regions in ‘West-Asia’ allowed us to investigate potential relationships between MOT1 alleles and environmental parameters described precisely at the population site, especially soil properties. We saw no relationship with soil pH (indeed, none of the described populations were facing acidic soil conditions), but there was an obvious trend for populations with the defective MOT1Sha allele to grow on soils with high water-extractable Mo content (Figure 4). This may indicate that the defective Sha allele is a protective response to Mo accumulation in environments with excess Mo. Indeed, under such conditions in the laboratory, we observe a strong decrease in fitness (through the total number of seeds produced per plant) in all genotypes (Figure S7), indicating that plants have to find the right balance between Mo deficiency and Mo toxicity, a trade-off that could be resolved partly through variation in function of the Mo transporter MOT1. Moreover, we show that a defective MOT1 allele (either mot1.1 or MOT1Sha) is accompanied by a slightly increased average seed mass (another component of fitness) specifically under Mo-toxic conditions (Figure S7), the outcome of which is difficult to estimate in nature [23]. It is however worth noting that previous studies in A. thaliana have shown positive effects of increased seed size for example on subsequent root and shoot growth [24] or seedling survival under limiting conditions [25].
Fig. 4. West-Asian populations highlight the correlation between MOT1Sha allele and high native soil Mo content. 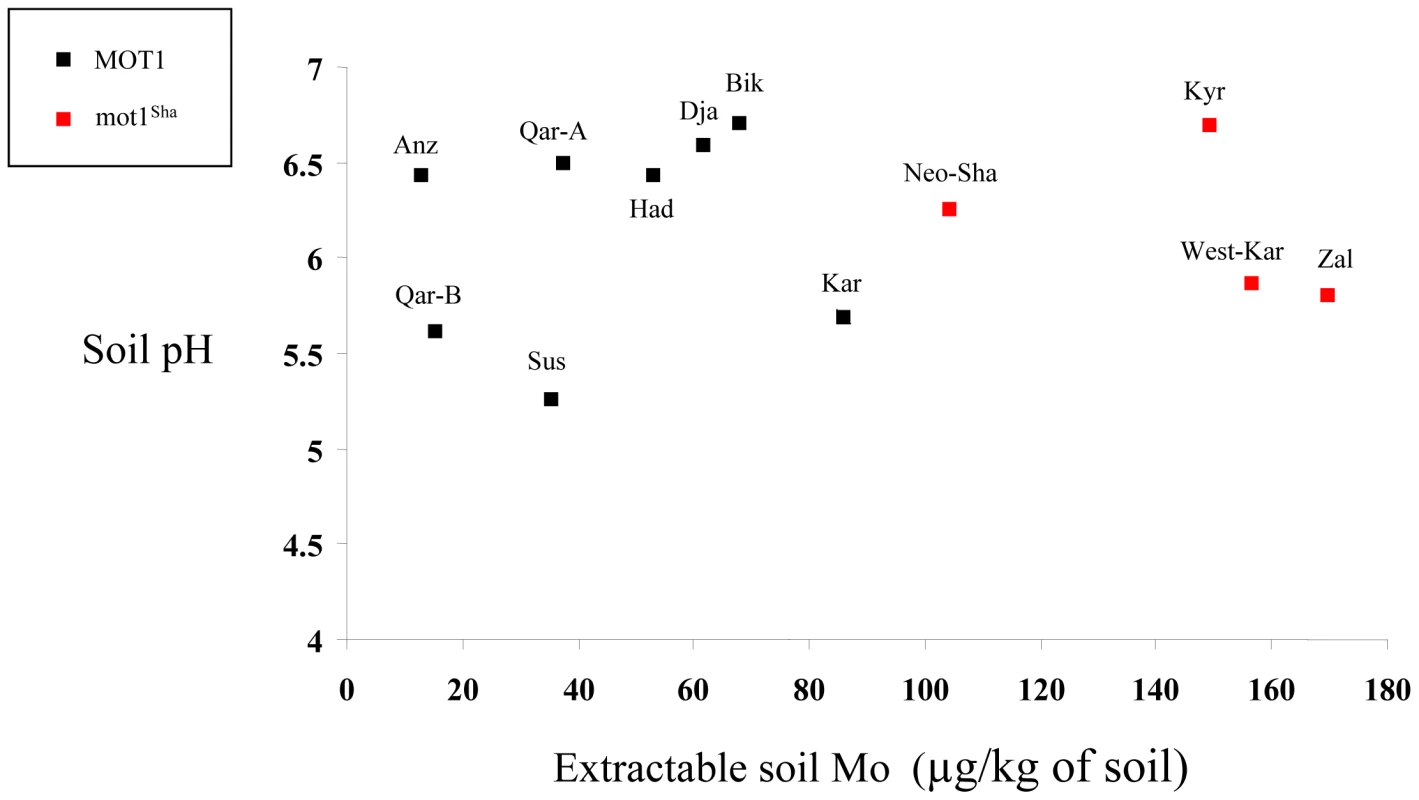
Soil parameters (pH, extractable Mo) from the precise original collection site are represented for independent populations carrying (red dots) or not (black dots) the MOT1Sha allele. In summary, we have identified a new functional variant at MOT1 that contributes to explain most of the species' diversity in Mo homeostasis, and associated phenotypes that provide likely explanations for its non neutral evolution and its correlation to native soil. It is still a rare finding to be able to relate functional genetic variants to fitness or trade-off effects [26]–[28], and even more to associate this variation to the environment [3], [7]. Our work indicates that environmental parameters of importance, such as soil properties, may be heterogeneously distributed and therefore require local description [5] and study of local adaptation [9], [11], which is greatly facilitated by the identification of the causative locus.
Materials and Methods
All accessions used and the Bay-0×Shahdara RIL set [29] were obtained from Versailles Arabidopsis Stock Centre (http://dbsgap.versailles.inra.fr/vnat/). Heterogeneous Inbred Family ‘HIF084’ was derived from RIL084 (segregating for the region of interest) as previously described [30]. New collections of A. thaliana accessions were partly described previously [31] and are shown at http://www.inra.fr/vast/collections.htm. T-DNA insertion mutant mot1.1 corresponds to line SALK_118311 as described [13], [14]. Genetic complementation tests were performed on F1 plants issued from the cross of diverse accessions to mot1.1 or its wild-type background.
Acidic soil assays were performed on ‘Floratorf’ peatmoss (Floragard, Germany) mixed with CaCO3 (4 g per liter of dry peatmoss) to maintain a soil pH∼5, watered with classical nutrient solution and grown under typical long-day conditions at 20°C. Chemical complementations were achieved in the same condition but, either with 8 g CaCO3 per liter of peatmoss to reach a pH∼6, or using a watering solution supplemented with 1 mM Na2MoO4. Mo toxicity was tested on regular fertilised soil mix (pH = 6) watered with nutrient solution supplemented with 7 mM Na2MoO4, or not (control).
MOT1 sequencing, qPCR analysis of expression (normalised against GAPDH and PP2A), functional characterisation in yeast were performed as previously described [13]. Extractable Mo was determined in soils by the method of Soltanpour and Schwab [32] using ICP-MS as the detector.
A total of 102 A. thaliana accessions (including 48 accessions known to maximize A. thaliana diversity [33] and 44 accessions from ‘West-Asia’) and 5 A. halleri accessions (I-14, I-16, F-1, PL-22 and TZC; obtained from H. Frérot at Univ. Lille [34]) were sequenced at MOT1 (including 1 kb upstream and 0.3 kb downstream for A. thaliana accessions) and at two reference loci, COI1 (At2g39940; 2,600 bp coding sequence) and PI (At5g20240; 2,150 bp coding sequence). Those genes were either used previously as reference or shown to have a neutral pattern of polymorphisms in A. thaliana [35], [36]. Sequences were aligned using Codoncode Aligner v3.7.1. and subsequent alignments were improved visually.
Intraspecific analyses i.e. nucleotide diversity estimated by π [37] and θw [38], and Tajima's D statistics [39] were calculated using DNAsp v5.10.01 on the whole region sequenced. Ten thousands coalescent simulations under the strict Wright-Fisher neutral model assuming no recombination and conditioning on S were performed to estimate statistical significance of Tajima's D.
For interspecific analyses, the orthologous of MOT1 and of the two control loci in A. halleri were used. The McDonald-Kreitman test [40] was performed by using DNAsp v5.10.01 in order to test for possible excess or deficiency in replacement substitutions at MOT1. Singletons were discarded for this analysis in order to reduce the contribution of slightly deleterious mutations (expected at very low frequencies and unlikely to become fixed). Neutral index was calculated as previously described [41]. Divergence between A. thaliana and A. halleri, defined as the average number of nucleotide differences between populations per gene, was calculated using DNAsp v5.10.01 and used to perform HKA tests with the multilocus HKA program available from J. Hey laboratory (http://genfaculty.rutgers.edu/hey/software).
Supporting Information
Zdroje
1. KarrenbergSWidmerA 2008 Ecologically relevant genetic variation from a non-Arabidopsis perspective. Curr Opin Plant Biol 11 156 162
2. NordEALynchJP 2009 Plant phenology: a critical controller of soil resource acquisition. J Exp Bot 60 1927 1937
3. BaxterIBrazeltonJNYuDHuangYSLahnerB 2010 A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genet 6 e1001193 doi:10.1371/journal.pgen.1001193
4. StinchcombeJRWeinigCUngererMOlsenKMMaysC 2004 A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc Natl Acad Sci USA 101 4712 4717
5. TrontinCTisnéSBachLLoudetO 2011 What does Arabidopsis natural variation teach us (and does not teach us) about adaptation in plants? Curr Opin Plant Biol 14 225 231
6. HanikenneMTalkeINHaydonMJLanzCNolteA 2008 Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453 391 395
7. TurnerTLBourneECVon WettbergEJHuTTNuzhdinSV 2010 Population resequencing reveals local adaptation of Arabidopsis lyrata to serpentine soils. Nat Genet 42 260 263
8. Alonso-BlancoCAartsMGBentsinkLKeurentjesJJReymondM 2009 What has natural variation taught us about plant development, physiology, and adaptation? Plant Cell 21 1877 1896
9. AndersonJTWillisJHMitchell-OldsT 2011 Evolutionary genetics of plant adaptation. Trends Genet 27 258 266
10. HerefordJ 2009 A quantitative survey of local adaptation and fitness trade-offs. Am Nat 173 579 588
11. Fournier-LevelAKorteACooperMDNordborgMSchmittJ 2011 A map of local adaptation in Arabidopsis thaliana. Science 334 86 89
12. HancockAMBrachiBFaureNHortonMWJarymowyczLB 2011 Adaptation to climate across the Arabidopsis thaliana genome. Science 334 83 86
13. BaxterIMuthukumarBParkHCBuchnerPLahnerB 2008 Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). PLoS Genet 4 e1000004 doi:10.1371/journal.pgen.1000004
14. TomatsuHTakanoJTakahashiHWatanabe-TakahashiAShibagakiN 2007 An Arabidopsis thaliana high-affinity molybdate transporter required for efficient uptake of molybdate from soil. Proc Natl Acad Sci USA 104 18807 18812
15. MengelKKirkbyEA 2001 Principles of plant nutrition; Springer, editor Berlin Springer
16. MendelRR 2011 Cell biology of molybdenum in plants. Plant Cell Reports 30 1787 1797
17. Lango AllenHEstradaKLettreGBerndtSIWeedonMN 2010 Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 832 838
18. WoodARHernandezDGNallsMAYaghootkarHGibbsJR 2011 Allelic heterogeneity and more detailed analyses of known loci explain additional phenotypic variation and reveal complex patterns of association. Hum Mol Genet 20 4082 4092
19. BeckJBSchmuthsHSchaalBA 2008 Native range genetic variation in Arabidopsis thaliana is strongly geographically structured and reflects Pleistocene glacial dynamics. Mol Ecol 17 902 915
20. FrancoisOBlumMGJakobssonMRosenbergNA 2008 Demographic history of european populations of Arabidopsis thaliana. PLoS Genet 4 e1000075 doi:10.1371/journal.pgen.1000075
21. PlattAHortonMHuangYSLiYAnastasioAE 2010 The scale of population structure in Arabidopsis thaliana. PLoS Genet 6 e1000843 doi:10.1371/journal.pgen.1000843
22. NordborgMHuTTIshinoYJhaveriJToomajianC 2005 The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3 e196 doi:10.1371/journal.pbio.0030196
23. BergelsonJRouxF 2010 Towards identifying genes underlying ecologically relevant traits in Arabidopsis thaliana. Nat Rev Genet 11 867 879
24. ElwellALGronwallDSMillerNDSpaldingEPDurham BrooksTL 2011 Separating parental environment from seed size effects on next generation growth and development in Arabidopsis. Plant Cell Environ 34 291 301
25. KrannitzPGAarssenLWDowJM 1991 The effect of genetically based differences in seed size on seedling survival in Arabidopsis thaliana (Brassicaceae). Am J Bot 78 446 450
26. KroymannJDonnerhackeSSchnabelrauchDMitchell-OldsT 2003 Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc Natl Acad Sci USA 100 14587 14592
27. TodescoMBalasubramanianSHuTTTrawMBHortonM 2010 Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature 465 632 636
28. ZhenYDhakalPUngererMC 2011 Fitness benefits and costs of cold acclimation in Arabidopsis thaliana. Am Nat 178 44 52
29. LoudetOChaillouSCamilleriCBouchezDDaniel-VedeleF 2002 Bay-0×Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor Appl Genet 104 1173 1184
30. LoudetOGaudonVTrubuilADaniel-VedeleF 2005 Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor Appl Genet 110 742 753
31. KronholmILoudetOde MeauxJ 2010 Influence of mutation rate on estimators of genetic differentiation–lessons from Arabidopsis thaliana. BMC Genet 11 33
32. SoltanpourPPSchwabAP 1977 A new soil test for simultaneous extraction of macro - and micronutrients in alkaline soils. Comm Soil Sci Plant Anal 8 195 207
33. McKhannHICamilleriCBerardABataillonTDavidJL 2004 Nested core collections maximizing genetic diversity in Arabidopsis thaliana. Plant J 38 193 202
34. GodeCDecombeixIKosteckaAWasowiczPPauwelsM 2012 Nuclear microsatellite loci for Arabidopsis halleri (Brassicaceae), a model species to study plant adaptation to heavy metals. Am J Bot
35. CaldwellKSMichelmoreRW 2009 Arabidopsis thaliana genes encoding defense signaling and recognition proteins exhibit contrasting evolutionary dynamics. Genetics 181 671 684
36. CorkJMPuruggananMD 2005 High-diversity genes in the Arabidopsis genome. Genetics 170 1897 1911
37. NeiM 1987 Molecular Evolutionary Genetics; Press CU, editor New York Columbia Univ. Press
38. WattersonGA 1975 On the number of segregating sites in genetical models without recombination. Theor Popul Biol 7 256 276
39. TajimaF 1989 Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585 595
40. McDonaldJHKreitmanM 1991 Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 652 654
41. RandDMKannLM 1996 Excess amino acid polymorphism in mitochondrial DNA: contrasts among genes from Drosophila, mice, and humans. Mol Biol Evol 13 735 748
Štítky
Genetika Reprodukční medicína
Článek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



