-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
Human herpesviruses can cause a wide range of serious infections. They are extremely common and individuals remain latently infected lifelong, with reactivations often causing recurrent or severe disease. T-cells are important in controlling herpesvirus infections and preventing their reactivation, so vaccines that induce T-cells are likely to improve control. Here, we examined human T-cells against VZV that might allow focused vaccine development. We identified a dominant target against which the majority of subjects had mounted a CD8 T-cell response. We found that very similar targets also exist in three other important herpesviruses, HSV-1, HSV-2 and EBV. We showed that CD8 T-cells recognizing the VZV target could also recognize the others and we hypothesized that recurrent encounter with these viruses could boost this common response. In some individuals, immunization with a VZV vaccine did cause activation of these cells, but in most it did not. This reflects the variable efficacy of the currently available VZV vaccine. Our findings suggest that T-cell targets may be shared between herpesvirus species and may therefore contribute to a novel “pan-herpesvirus” vaccine. However, current VZV vaccines cannot reliably stimulate these T-cells and new strategies will be necessary to achieve this goal.
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1004008
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004008Summary
Human herpesviruses can cause a wide range of serious infections. They are extremely common and individuals remain latently infected lifelong, with reactivations often causing recurrent or severe disease. T-cells are important in controlling herpesvirus infections and preventing their reactivation, so vaccines that induce T-cells are likely to improve control. Here, we examined human T-cells against VZV that might allow focused vaccine development. We identified a dominant target against which the majority of subjects had mounted a CD8 T-cell response. We found that very similar targets also exist in three other important herpesviruses, HSV-1, HSV-2 and EBV. We showed that CD8 T-cells recognizing the VZV target could also recognize the others and we hypothesized that recurrent encounter with these viruses could boost this common response. In some individuals, immunization with a VZV vaccine did cause activation of these cells, but in most it did not. This reflects the variable efficacy of the currently available VZV vaccine. Our findings suggest that T-cell targets may be shared between herpesvirus species and may therefore contribute to a novel “pan-herpesvirus” vaccine. However, current VZV vaccines cannot reliably stimulate these T-cells and new strategies will be necessary to achieve this goal.
Introduction
The family Herpesviridae encompasses several highly prevalent human pathogens that cause a spectrum of diseases ranging from mildly symptomatic to severe life-threatening illness [1]. All herpesvirus subfamilies (α, β, and γ) share one important characteristic: the ability to evade the immune response while persisting as latent infections in a state of minimal gene transcription. In many individuals, latent herpesviruses cause no further disease. However, reactivations do occur that lead to considerable morbidity and mortality as well as promoting onward transmission. These events are most frequent in individuals with immunosuppression or immunosenescence [2]. However, asymptomatic reactivation can also occur in immunocompetent individuals, leading to recurrent stimulation of host immunity by herpesvirus antigens [3], [4]. T cells are essential both for recovery from primary herpesvirus infections and prevention of symptomatic reactivation [5]. VZV-specific T cells that secrete Th1 cytokines and exhibit cytolytic activity are detectable following chicken pox [6]. While virus exposure also induces antibodies, the absence of antibodies in children with agammaglobulinemia does not lead to more severe disease [7]. Conversely, the waning T cell immunity that occurs with older age is associated with greater frequency and severity of reactivations [8].
The only herpesvirus vaccines currently available are against VZV. This live attenuated vaccine prevents primary infection in children (i.e. chicken pox) and, when given at high dose, reduces the frequency and/or severity of shingles in elderly adults [9]. The vaccine induces both humoral and cell-mediated immunity [10]–[12], but vaccine-induced immunity can fail and effectiveness in the elderly is relatively poor [13]. The factors underlying this are poorly understood. Rational design of herpesvirus vaccines that elicit optimal protective T cell responses therefore remains an important goal.
However, in order to achieve this, further understanding of the role of human virus-specific T cells during herpesvirus infections is required. In this study, we aimed to comprehensively analyze the breadth of the CD8 T cell response to VZV in the context of the common HLA-A*0201 allele. The VZV genome is large, containing 69 unique open reading frames. This hampers the systematic identification of T cell epitopes and the generation of tools to study them. From VZV, only 7 class I-restricted epitopes from 3 proteins (gI, gB and IE62) have been reported thus far [14]. To address this, we used in silico prediction across the entire VZV proteome for epitope mapping. Screening of these candidate peptides in VZV seropositive individuals identified an immunodominant HLA-A*0201-restricted epitope that was conserved with three other herpesviruses. In this study, we characterized the phenotype and function of CD8 T cells that recognized this conserved epitope and also examined the responsiveness of these CD8 T cells to VZV vaccination in humans.
Results
Identification of an immunodominant HLA A*0201-restricted VZV T cell epitope
We recruited 21 HLA-A*0201 positive volunteers with a history of primary VZV infection, detectable VZV IgG but no previous VZV vaccination or clinical evidence of recent reactivation (Table 1). The median age was 63 years (range 25–77 years). Epitope predictions in the context of HLA-A*0201 were made using the Immune Epitope Database consensus prediction tool with the complete published VZV sequences (Table S1). The top 0.5% of 9 - and 10-mers (367 peptides) were synthesized and peptide pools screened by IFN-γ ELISpot using PBMCs from each subject. In 15/21 subjects, the same peptide pool induced positive responses (Figure 1a). Deconvolution of this pool showed that two candidate peptides (ILIEGIFFV and MILIEGIFFV) from ribonucleotide reductase subunit 2 (RNR2) of VZV strongly induced IFN-γ production (Figure 1b). The predicted MHC-binding of the 9-mer (henceforth called ILI) had 11-fold higher affinity than the 10-mer (Table 2), so this was used to generate the A2-ILI tetramer used to label epitope-specific cells (Figure 1c). Tetramer+ cells were detected in 12/21 subjects with frequencies ranging from 0.01% to 1.8% of CD8 T cells (Figure 1d). ILI was thus identified as an immunodominant HLA-A*0201-restricted class I epitope.
Fig. 1. Human CD8 T cells recognize a dominant epitope in the VZV ribonucleotide reductase subunit 2. 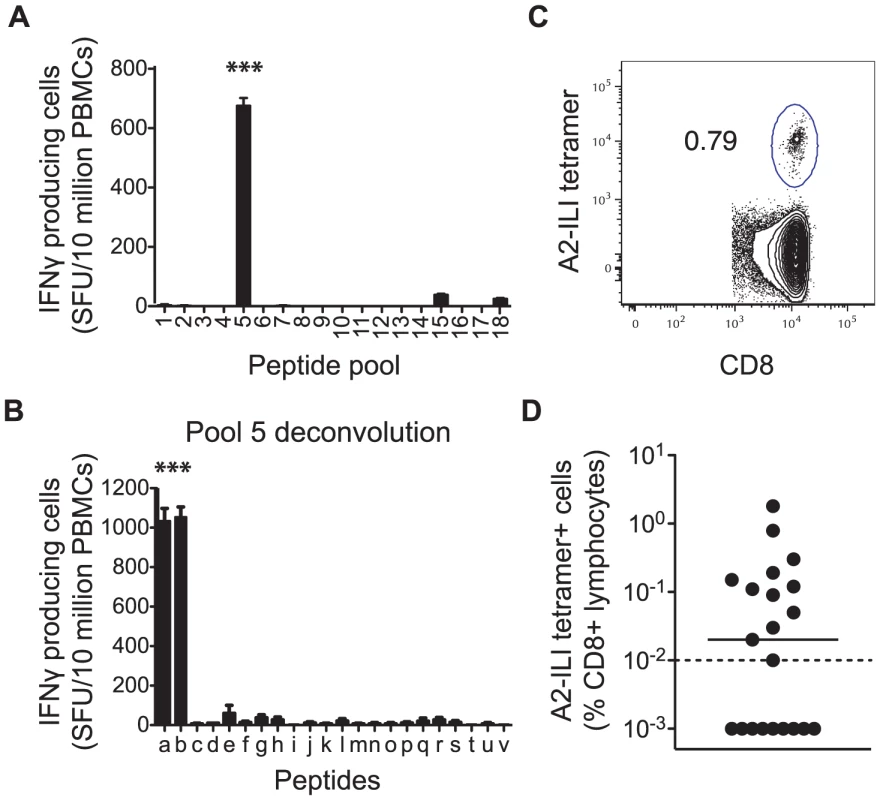
HLA-A*0201 binding predictions were used to identify candidate VZV-derived epitopes. PBMCs from 21 healthy adult HLA-A*0201+ volunteers were used in IFN-γ ELISpot assays to screen (a) peptide pools and subsequently (b) individual peptides for their ability to stimulate IFN-γ. Asterisks represent p-values<0.001 using Student's t-test. The A2-ILI tetramer was constructed and used to label epitope-specific CD8 T cells. (c) One representative donor is shown. The plot is gated on CD3+CD8+ lymphocytes. The number represents frequency of A2-ILI+ events as a percentage of CD8+ lymphocytes. (d) The frequency of A2-ILI+ CD8 T cells is shown along with the median. The dotted line represents the limit of detectability. Tab. 1. Summary of demographic data of HLA-A*0201+ subjects. 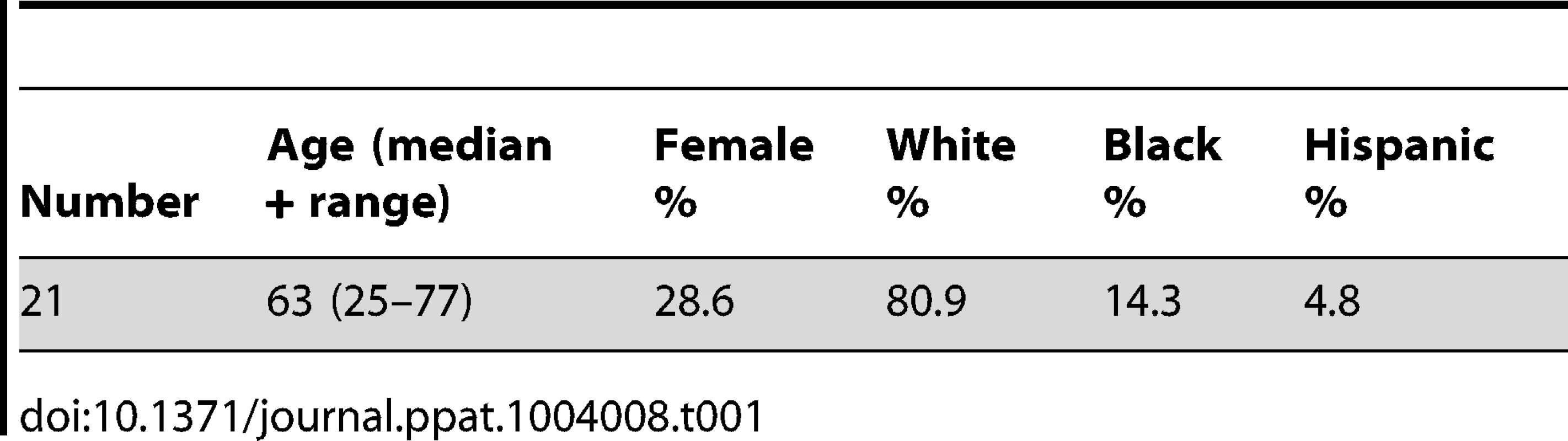
Tab. 2. RNR2 epitope homologues in human herpesviruses. 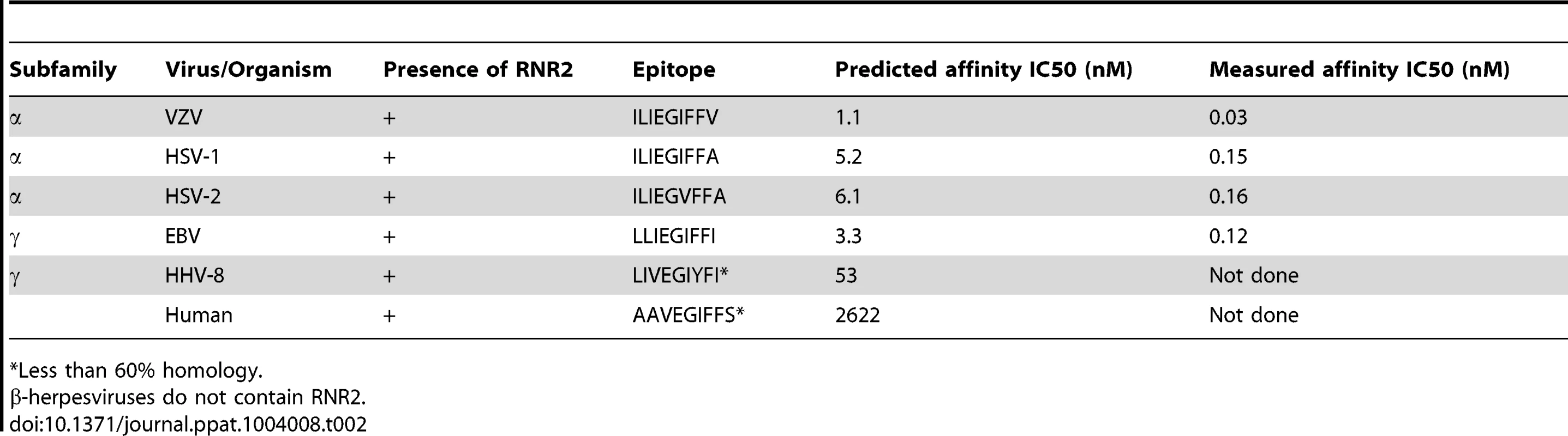
*Less than 60% homology. Phenotypic and functional heterogeneity of ILI-specific CD8 T cells between different individuals
To determine the phenotype of these ILI-specific CD8 T cells, we co-stained for memory subset markers; co-stimulatory molecules; and effector molecules. The majority of A2-ILI+ cells were CD45RA-/CCR7 - indicative of an effector memory T cell phenotype (Figure 2a & 2b). However, between individuals, these cells displayed marked heterogeneity, which allowed further categorization into one of three phenotypic groups. Phenotype 1 (6/12 subjects) was the least effector-like, expressing high levels of the co-stimulatory receptors CD27 and CD28 with no expression of the cytotoxic molecules perforin or granzyme B; most A2-ILI+ cells of phenotype 2 (5/12 subjects) still expressed CD27 and CD28 and were still negative for perforin, but now expressed granzyme B; finally, phenotype 3 (1/12 subject) described the most effector-like cells with no CD27 and CD28 expression but high perforin and granzyme B (Figure 2a & 2b). We also investigated the expression of granzyme K, a serine protease that marks less differentiated CD8 T cells [15]. This also differed between groups and was inversely associated with granzyme B expression (in keeping with previous reports). In a subset of donors, we went on to examine the differentiation markers KLRG-1 and CD127 (Figure S1a). In all subjects tested, at least half the ILI-specific cells expressed KLRG-1 (a marker commonly expressed on terminally differentiated short-lived effectors), with a trend towards progressively higher expression in phenotypes 2 and 3. A variable proportion expressed CD127 (predominantly expressed on long-lived memory cells) but there was a trend towards more CD127+ cells in phenotype 1 and fewer in phenotype 3. These data were therefore consistent with those used to classify phenotypes 1, 2 and 3, suggesting that ILI-specific cells of phenotypes 2 and 3 were more likely to be terminally differentiated.
Fig. 2. A2-ILI tetramer+ CD8 T cells display distinct phenotypic patterns between individuals. 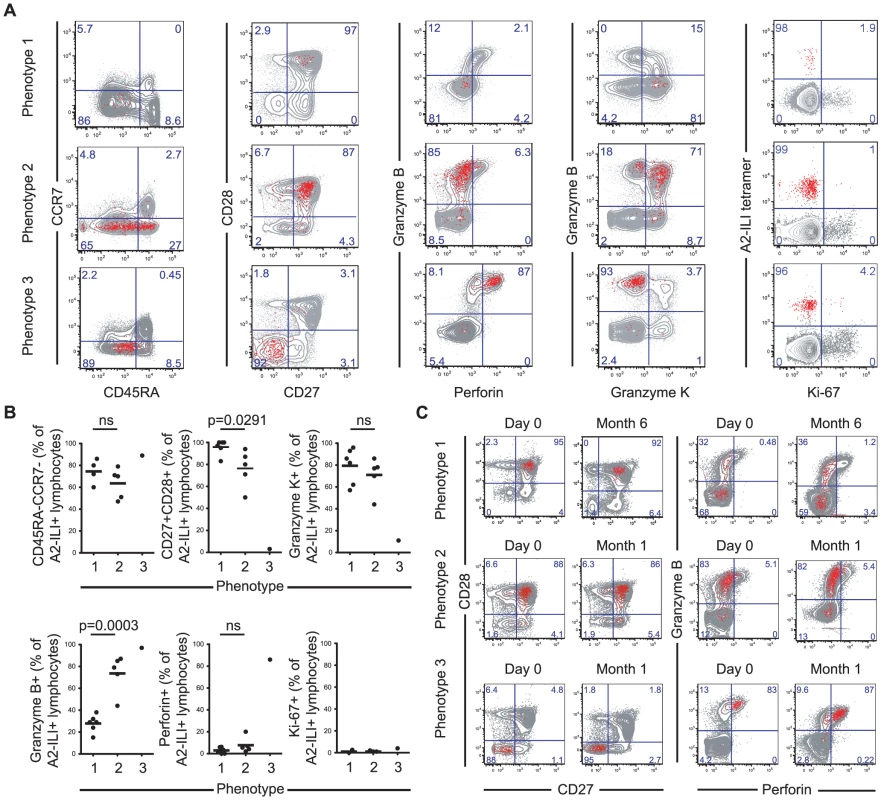
A2-ILI tetramer+ CD8 T cells were co-stained with anti-CD45RA, CCR7, CD27, CD28, granzyme B, granzyme K, perforin, and Ki-67. Phenotypic markers were analysed by flow cytometry. (a) Subjects were categorized according to the combination of phenotypic markers expressed. One representative donor of each phenotype is shown. Numbers represent percentage of CD3+CD8+ lymphocytes. (b) The frequency of A2-ILI+ CD8 T cells in every subject expressing each marker is summarized. The medians are shown and p-values derived from Student's t-test. (c) PBMCs were collected at baseline (day 0) and one month or six months later. A2-ILI tetramer+ CD8 T cells were co-stained with anti-CD27, CD28, granzyme B, and perforin. Phenotypic markers were analysed by flow cytometry. Representative plots from one subject of each phenotype are shown. Primary VZV infection results in a multi-system disease that includes skin and neurotropic phases and antigen-specific cells may therefore need to localise to a variety of tissues. In most individuals, a major proportion of ILI-specific cells expressed the integrin CD62L, which allows homing to lymphoid organs (Figure S1b). In addition, a variable proportion expressed CCR5, indicating potential for homing to inflamed tissues. With neither marker was there a significant difference in frequency of expression between the 3 phenotypes. A2-ILI+ cells displayed no expression of cutaneous lymphocyte antigen (CLA) but a variable proportion did express the integrin α4β7. Again, there was no correlation with advancing phenotypic group.
One possible explanation for the heterogeneity of phenotype might have been recent or on-going activation, since the combination of markers characteristic of phenotype 3 would also be expected to occur in short-lived effector T cells. We therefore also analysed the expression of Ki-67 to determine whether any of these cells had undergone recent proliferation (Figure 2a & 2b). In a few subjects a minority of A2-ILI+ cells did express Ki-67. However, these never made up more than 5% of the population and there was no evidence that ILI-specific cells of phenotype 2 or 3 were more likely to have had recent proliferative activity. This was supported by analysis of the activation markers CD38 and HLA-DR, neither of which was up-regulated on ILI-specific CD8 T cells (Figure S1c).
In addition, the combinations of markers that segregated the phenotypic groupings did not change over time. Subjects were sampled at intervals between 1 and 6 months from baseline and the frequencies of ILI-specific cells expressing these combinations of markers remained stable (Figure 2c). These data therefore suggested that the ILI-specific memory T cell populations had been observed in a quiescent state and that, while they might express one of several stable phenotypes in any single subject, they were heterogeneous between individuals.
Functional capacity of ILI-specific CD8 T cells is associated with their phenotype and expression of inhibitory receptors
In view of their phenotypic differences, we proceeded to examine the functional capacity of ILI-specific CD8 T cells by measuring their ability to produce cytokines and undergo proliferation (Figure 3). Comparing the proportions of A2-ILI+ CD8 T cells capable of producing IFN-γ and IL-2, ILI-specific cells with phenotype 1 had the greatest cytokine producing capacity with a mean of 76% (range 37–100%) expressing IFN-γ (Figure 3b). As the phenotype changed from 1 to 2 and 3, the capacity of ILI-specific cells to produce IFN-γ fell. Furthermore, phenotype 1 ILI-specific cells were also the most polyfunctional, with a mean of 29% (range 22–51%) also staining for IL-2 (Figure 3c). Again, as the phenotype advanced, fewer ILI-specific cells produced this cytokine. In subjects in whom ILI-specific cells were at sufficiently high frequency for the assay, we then analyzed their in vitro proliferative capacity. This indicated that ILI-specific CD8 T cells from the individual displaying phenotype 3 were markedly impaired in their proliferation compared with those with phenotype 1 (Figure 3d). Thus the phenotypic and functional patterns displayed by the ILI-specific populations implied that they had been driven, to varying extents between individuals, towards more terminal differentiation with characteristics reminiscent of functional exhaustion.
Fig. 3. ILI-specific CD8 T cells display functional impairment associated with their phenotype. 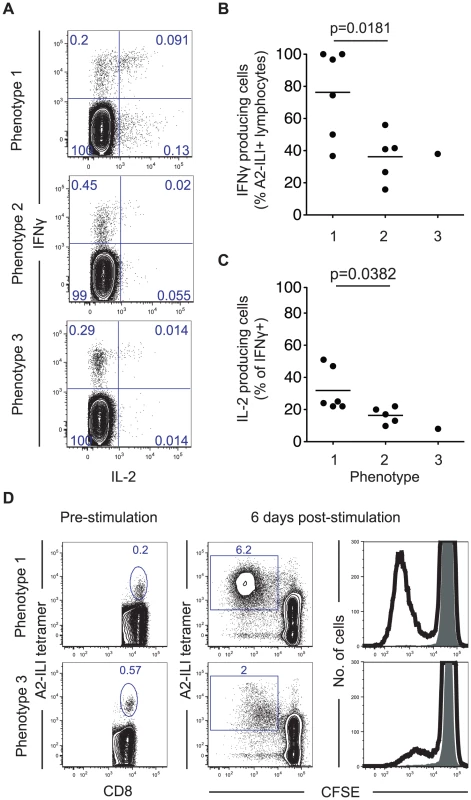
(a) PBMCs were stimulated with ILIEGIFFV peptide in vitro for 6 hours then co-stained for intracellular IFN-γ and IL-2. Numbers represent frequency as a percentage of CD8+ lymphocytes. The frequency of (b) IFN-γ+ cells as a proportion of A2-ILI tetramer+ cells and (c) IL-2 producing cells as a percentage of IFN-γ+ CD8 T cells are shown. P-values are derived from Student's t-test. (d) PBMCs from HLA-A*0201+ volunteers were stained with CFSE and stimulated with ILIEGIFFV peptide in vitro for 6 days. A2-ILI tetramer+ cells were then analyzed by flow cytometry. Numbers represent frequency as a percentage of CD8+ lymphocytes. Two representative donors are shown. To investigate the potential mechanism underlying this, we examined the association between differentiation phenotype and the expression of the inhibitory receptors PD-1 and 2B4, which are associated with exhaustion and up-regulated on virus-specific CD8 T cells during chronic antigen stimulation [16]. On ILI-specific cells, as the differentiation phenotype progressed, both PD-1 and 2B4 expression increased (Figure 4a). This was associated with a trend towards decreased capacity to produce cytokines such that as the frequency of cells expressing PD-1 and 2B4 increased, the frequency of IFN-γ producing cells fell (Figure 4b).
Fig. 4. Expression of inhibitory receptors is associated with increased ILI-specific CD8 T cell frequency but impaired functionality. 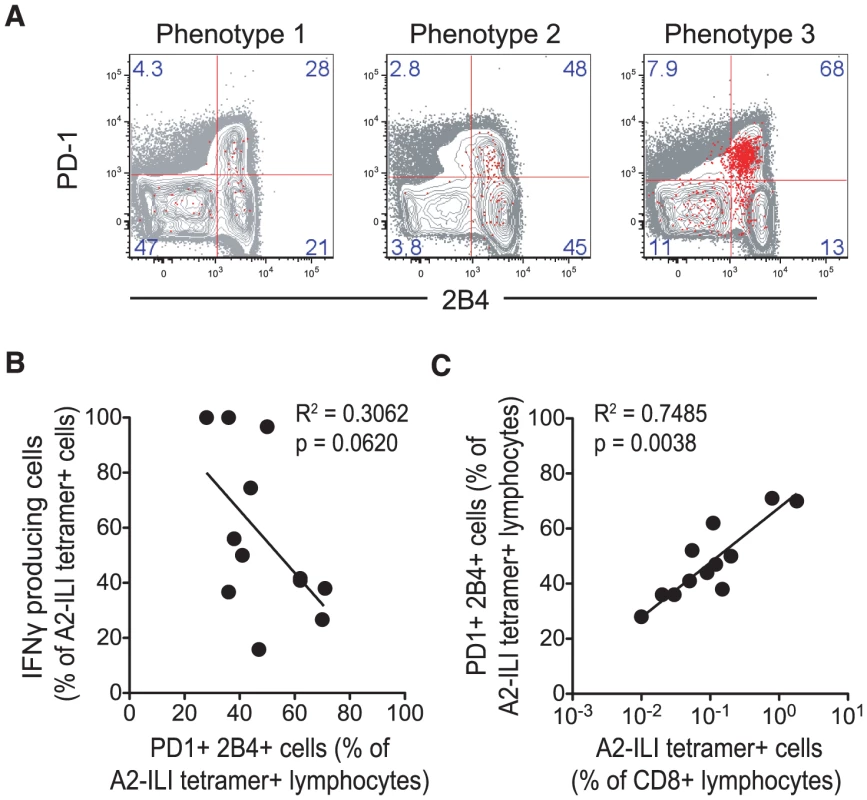
A2-ILI-tetramer+ CD8 T cells were co-stained with anti-PD-1 and 2B4, and analysed using flow cytometry. (a) Representative donors with each phenotype are shown. Numbers represent frequency of events as a percentage of CD3+CD8+ lymphocytes. (b) Non-linear regression and Spearman's rank correlation were used to show the association between the frequency of IFN-γ producing A2-ILI+ cells and their frequency of PD-1 and 2B4 co-expression. (c) Non-linear regression and Spearman's rank correlation were used to show the association between the frequency of A2-ILI tetramer+ CD8 T cells and their expression of both PD-1 and 2B4. P-values for Spearman's rank correlation are shown. In chronic viral infections such as HIV, antigen-specific CD8 T cells are abundant, driven by continuous antigenic stimulation via the T cell receptor [17]. However, this increase in the frequency is balanced by increasing expression of inhibitory markers including PD-1, leading to functional exhaustion. Thus, although the frequency of memory T cells is increased, their functionality is restrained. Although herpesviruses do not continually produce antigenic proteins during latent infection, a strong correlation between the size of the population and the frequency of ILI-specific CD8 T cells that co-expressed both inhibitory receptors was seen (Figure 4c). These data imply that recurrent antigen exposure, for example via reactivation, may have driven the proliferation of these cells, thus increasing their frequency but also inducing the expression of inhibitory markers, which in turn affects their functional capacity.
The epitope recognized by ILI-specific CD8 T cells is conserved between α - and γ-herpesviruses
RNR is one of a number of widely conserved proteins [18], [19]. We therefore hypothesized that the ILI epitope might be well conserved between the human herpesviruses. Indeed, we found that not only was the epitope present in all recorded VZV sequences but that there were also conserved homologues in the α-herpesviruses HSV-1 (ILIEGIFFA) and HSV-2 (ILIEGVFFA), and the γ-herpesvirus EBV (LLIEGIFFI)(Table 2). In contrast, poor homology was seen in the RNR2 of the γ-herpesvirus HHV-8, while the RNR2 gene is absent in the β-herpesviruses CMV, HHV-6 and HHV-7. Furthermore, the homologous epitope from the human RNR has little sequence identity with those of the herpesviruses and therefore unlikely to be responsible for any auto-reactive responses.
We tested the recognition of homologous peptides from VZV, HSV-1, HSV-2 and EBV by intracellular cytokine staining (Figure 5a & 5b). In all 11 donors tested, each peptide was capable of stimulating cytokine production. This occurred even when those individuals had no serological evidence of previous infection with the originating virus, with the response to the HSV-1 epitope equivalent to that against the one from VZV even in subjects who were HSV-1 seronegative (Figure 5b & Table 3). Conversely, the reduced response to the HSV-2 epitope occurred even in seropositive individuals. All subjects had been recruited on the basis of seropositivity to VZV and all but three volunteers also had evidence of previous EBV infection (Table 3). In contrast, only 11/21 subjects were positive for HSV-1 IgG and only 3 for HSV-2. Individuals with serological evidence of 3 or more herpesvirus infections were more likely to have detectable A2-ILI+ responses (9/12 subjects) while those with no detectable ILI-specific cells were more likely to have only EBV and/or VZV (6/9 subjects). These data therefore suggest that co-infection with more than two α - or γ-herpesviruses may increase the likelihood of generating a cross-reactive ILI-specific response.
Fig. 5. ILI-specific CD8 T cells are broadly reactive, recognizing homologous epitopes conserved between alpha- and gamma-herpesviruses. 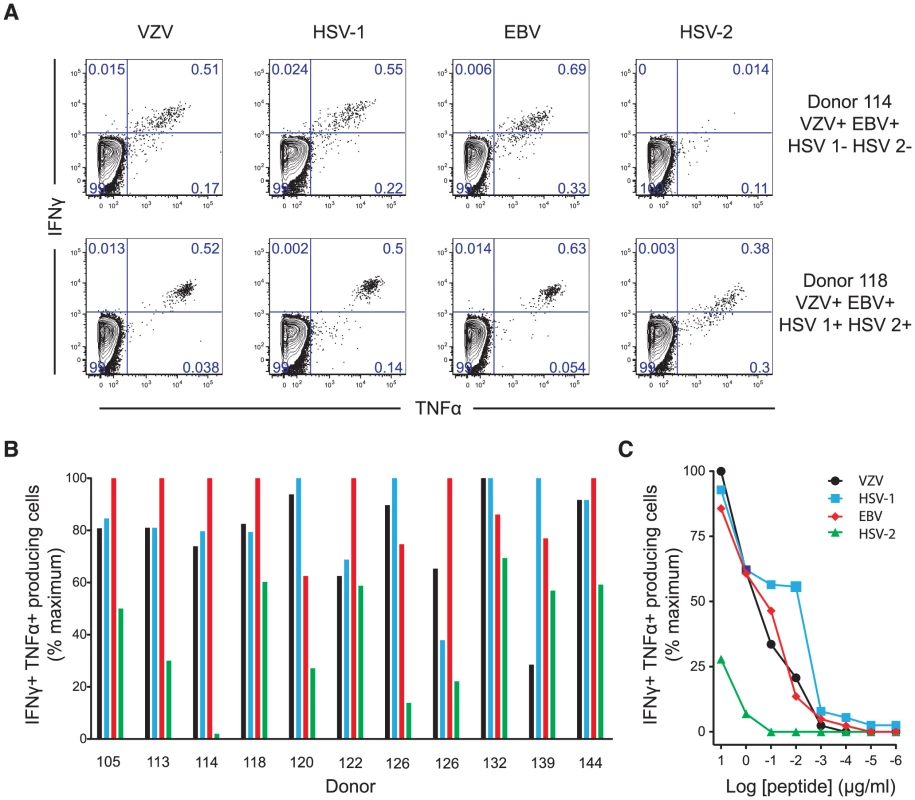
Homologous epitopes to ILIEGIFFV were identified by sequence similarity in HSV-1, HSV-2 and EBV. (a) The capacity of each epitope to stimulate cytokine production was measured by in vitro peptide stimulation. Two representative subjects are shown with their serostatus with respect to the herpesviruses tested. Numbers represent the percentage of CD3+CD8+ lymphocytes. (b) PBMCs from HLA-A*0201+ individuals with detectable ILI-specific responses underwent in vitro stimulation with each epitope at a concentration of 10 µg/ml followed by staining with anti-CD3, CD8, IFN-γ and TNF-α. The frequency of cytokine producing cells stimulated by the epitopes from VZV (black), HSV-1 (light blue), EBV (red), and HSV-2 (green) as a percentage of maximal cytokine producing cell frequency is shown. (c) The ability of each epitope to induce cytokine production was assessed by titration of stimulating peptide concentrations. One representative subject (subject 118, seropositive for all herpesviruses tested) is shown. The frequency of cytokine producing cells stimulated by the epitopes from VZV (black), HSV-1 (light blue), EBV (red), and HSV-2 (green) as a percentage of maximal cytokine producing cell frequency is shown. Tab. 3. Donor details and serology. 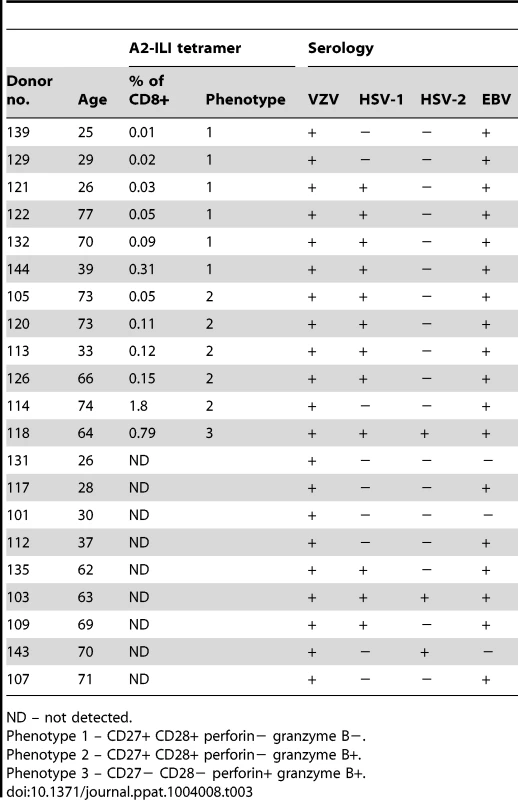
ND – not detected. In silico prediction suggested that residues at the anchor motifs of the VZV peptide (position 2 and the C-terminus) conferred optimal binding, while in vitro binding measurements showed that all four epitopes displayed extremely high affinities of <0.2 nM (Table 2). However, in vitro stimulation of CD8 T cells showed that the HSV-2 epitope was much less effective at stimulating cytokine production than those from VZV, HSV-1 and EBV (Figure 5a, 5b & 5c). Despite the calculated and measured binding affinities, peptide titrations showed that the epitopes from VZV, HSV-1 and EBV induced similar responses in any given individual while the peptide from HSV-2 only stimulated cytokines at its highest concentrations (Figure 5c). We therefore inferred that isoleucine at position 6, absent in the HSV-2 epitope, must be important for efficient TCR recognition.
These data support the hypothesis that herpesviruses are capable of inducing and boosting this epitope-specific response in a cross-reactive manner. Reactivations of one or several of these viruses may cause expansion of this population, increasing its size but also driving the cells towards an increasingly differentiated phenotype.
Live attenuated VZV vaccine (Zostavax) poorly stimulates proliferation of ILI-specific CD8 T cells
In order to examine the ability of the current VZV vaccine to boost ILI-specific responses, we immunized the study cohort with the live attenuated Zostavax vaccine and tracked the A2-ILI+ response. Following vaccination, the majority of subjects had no detectable change in the frequency of ILI-specific cells irrespective of their pre-vaccination frequency (Figure 6a). A greater than 2-fold increase in epitope-specific cells was seen in only one vaccinee (subject 105, phenotype 2). This individual had one of the lower starting frequencies at 0.05% and incremented to 0.17% at day 14 post-vaccination (Figure 6a & 6b). The increased ILI+ CD8 T cell frequency was associated with up-regulation of Ki-67 in 66% of epitope-specific cells, indicative of proliferation (Figure 6c & 6d). This occurred between 7 and 14 days post-vaccination, with Ki-67 completely down-regulated by day 28. In one additional subject (subject 126), there was evidence of minimal proliferation peaking at day 14 but no overall increase in the frequency of ILI-specific cells (Figure 6c). However, even in the best responder, the overall increase in the frequency of ILI-specific cells was modest despite the higher frequencies being maintained to day 28. Therefore live attenuated VZV vaccine only induced proliferation of ILI-specific cells in a single subject and even in the responding individual, the response was quantitatively poor.
Fig. 6. The live attenuated vaccine Zostavax cannot efficiently induce proliferation of ILI-specific CD8 T cells in most individuals. 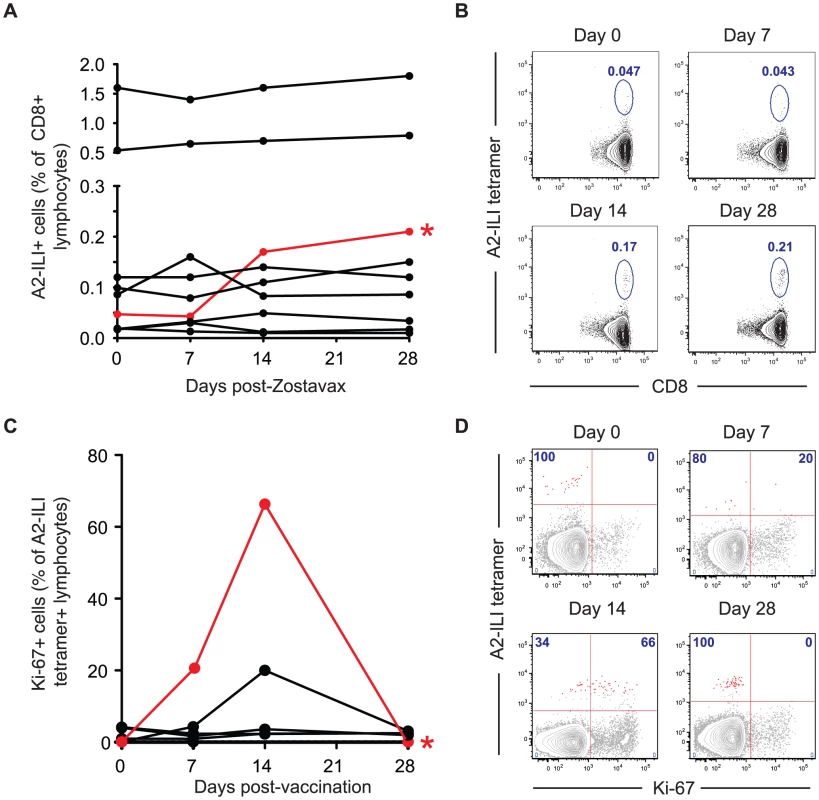
HLA-A*0201+ subjects were immunized with Zostavax. Blood was collected pre-vaccination and at 7, 14 and 28 days post-vaccination. (a) Whole blood was stained with anti-CD3, CD8 and A2-ILI tetramer. The frequency of A2-ILI+ cells post-vaccination in donors with detectable responses is shown. The only responder (subject 105) is marked in red and by an asterisk. (b) FACS plots indicating A2-ILI+ CD8 T cells from the responding donor are shown. Plots are gated on CD3+CD8+ lymphocytes and numbers represent the frequency of A2-ILI+ cells as a percentage of CD8+ lymphocytes. (c) PBMCs were co-stained with anti-Ki-67. The proportion of A2-ILI+ cells expressing Ki-67 post-vaccination in donors with detectable responses is shown. The single responder is marked in red and by an asterisk. (d) FACS plots show the expression of Ki-67 in A2-ILI+ CD8 T cells in the single responder. Plots are gated on CD3+CD8+ lymphocytes and numbers represent the percentage of A2-ILI+ cells with or without Ki-67 expression. Discussion
Several T cell epitopes have previously been described that are conserved between HSV-1 and HSV-2 [20], including the epitopes described here [21]. However, it is interesting to find that these conserved epitopes exist more widely in viruses as divergent as the α - and γ-herpesviruses. Here, we have shown that this epitope is, in fact, broadly conserved between 4 different clinically important herpesvirus species despite their sequence divergence. Furthermore, all epitopes were capable of stimulating CD8 T cells even in individuals with no evidence of previous exposure to that virus. Earlier studies have indeed noted that HSV-specific T cells are detectable in a proportion of individuals seronegative for HSV. It was then proposed that these T cells might be induced by subclinical infection or exposure without infection, but our findings suggest that cross-reactivity of T cells may be an alternative explanation [22].
The frequency of ILI-specific CD8 T cells varied widely between individuals. We hypothesize that the number of herpesvirus infections and frequency of reactivations is responsible for these differences. Since herpesviruses are highly prevalent, multiple viruses invariably co-exist within a host and new herpesvirus infections may contribute to further stimulation of cross-reactive T cell populations. Furthermore, although herpesviruses downregulate their transcriptional machinery during latency, chronic expression of some viral genes still occurs [23], and subclinical reactivation has also been widely described. During stress, VZV can be detected in blood or saliva by PCR while HSV-2 often sheds in the absence of genital ulceration [4], [24]. Chronic or periodic antigen exposure may therefore boost T cell numbers over time.
However, in many experimental systems, increasing the number of antigen-specific T cells can also lead to immunopathology and there is increasing evidence in natural infections that this can be controlled by a number of feedback mechanisms. Under conditions of continuous antigen exposure in chronic infections such as HIV, antigen-specific T cells may be driven to proliferate to high frequencies and epitope-specific CD8 T cells identified by tetramer labelling are abundant [17]. However, constitutive expression of inhibitory receptors including PD-1 and 2B4 is also induced by chronic antigen stimulation, leading to reduction in further proliferative capacity and cytokine production [25]–[27]. In tandem with a decrease in co-stimulatory signalling via the down-regulation of CD27 and CD28, immunopathology is restrained despite the higher frequency of potential effector cells. Although continuous production of antigen does not occur in the same way during herpesvirus infections, recurrent antigen stimulation during reactivations may lead to a similar process.
In this context, our data suggest that the differentiation phenotype may act as a biomarker for frequency of reactivation. The phenotypic groups defined by stable expression of differentiation markers in the absence of recent proliferation (as evidenced by Ki-67 expression) may therefore be indicative of antigen exposure history. Furthermore, exhaustion may be one potential mechanism for the impaired T cell function that permits symptomatic reactivations in the elderly. If exhaustion could be reversed, there might be the possibility of enhancing antigen-specific immune responses in this population.
Although T cell immunity is believed to be essential for the control of herpesvirus infections, little is known about the role of T cells in the efficacy of the only currently available herpesvirus vaccines, Zostavax (which protects against shingles) and Varivax (which prevents chicken pox). Both are based on the live attenuated vOka strain of VZV, which has been passaged over 30 times in both human and animal cells. Despite the fact that Zostavax contains 14 times more virus than Varivax (which is administered to children), our data indicate that it does not efficiently stimulate a secondary ILI-specific response in seropositive adults. The explanation for this is likely to be multi-factorial. The numerous mutations that the vOka strain has acquired are likely to have contributed to poor replicative capacity and altered immunogenicity [28]. However, our data also suggest that VZV-specific CD8 T cells in many adults are intrinsically suboptimal, with a balance of co-stimulatory and inhibitory receptors that favors decreased responsiveness to antigen stimulation and impaired functionality. This may partially explain the incomplete protection provided against shingles and why VZV vaccine confers no cross-protection against other herpesviruses despite the presence of this cross-reactive CD8 T cell population in some individuals. Furthermore, VZV, HSV-1 and EBV have all been shown to evade host immunity by interfering with antigen presentation [29]–[31]. Therefore neither existing vaccines nor natural infection are therefore likely to induce cross-reactive T cell responses of sufficient magnitude to provide clinically relevant cross-protection. In addition, it is possible that conserved epitopes such as the ILI homolog are not equally processed and presented in all herpesvirus infections, even when the originating protein (e.g. RNR2) is expressed. The presentation of ILI homologs by HSV-1/2 and EBV in the absence of previous VZV infection was beyond the scope of our study and although CD8+ T cells specific for ILI homologs from HSV-1 and HSV-2 have been described, the VZV serostatus of donors in those studies was not assessed [21]. It therefore remains to be definitively shown whether the cross-reactive CD8+ T cell response demonstrated in vitro is effective in vivo.
However, in view of the evolutionary relatedness of the herpesvirus subfamilies, we hypothesize that there are still more conserved epitopes to be discovered. Vaccines that induce cross-reactive CD8 T cells might provide protection against clinical disease caused by multiple strains or even species of virus pathogens. The existence of such epitopes could open the way to the development of novel “pan-herpesvirus” vaccines if they can be made to induce responses of sufficient magnitude and functionality. Using the tools we have developed, further identification of the proteins from which similar conserved epitopes are derived may lead us to such a goal.
However, since even natural infection by virulent herpesviruses cannot adequately induce cross-protectivity, development of an effective pan-herpesvirus vaccine will require not only the identification of further cross-reactive epitopes across the major HLA supertypes but also completely new methods to specifically enhance the stimulation of cross-reactive CD8 T cells. In particular, we anticipate that novel adjuncts such as inhibitory receptor blockade will be required to tip the balance of signals that coordinate these responses in the direction of virus-specific T cell activation in order to overcome their relatively functionally impaired state. We anticipate that these strategies as well as advances in the rational design of improved immunogens will ultimately be necessary to achieve a vaccine that effectively protects against the wide array of diseases caused by herpesvirus infection.
Methods
Study subjects
All studies were approved by the Emory University Institutional Review Board (IRB #00050285). Study subjects provided written informed consent prior to participation. Clinical information is detailed in Table 1. Twenty-one healthy HLA-A*0201 +ve adults were recruited. Blood was obtained at baseline and multiple time points post-vaccination with the live attenuated Zostavax vaccine (Merck). Study subjects had a history of varicella zoster infection and serologic status against VZV was tested using the VZV IgG ELISAII (Wampole Laboratories, NJ, USA). HLA class I loci were genotyped using the sequence-base typing (SBT) method as recommended by the 13th International Histocompatibility Workshop (Tilanus et al. 2002).
Bioinformatic analyses and peptide selection
The capacity of all VZV vOka (GI: 26665420, Acc. No. AB097932) derived 9 - and 10-mer peptides to bind HLA A*02 : 01 was predicted using the command-line version of the consensus prediction tool available on the Immune Epitope Database (IEDB) web site (http://tools.immuneepitope.org/main/html/tcell_tools.html). Peptides were selected if they scored in the top 0.5% of predictions for each length. Additional peptides from non-vOka VZV strains (Table S1) were also included if they similarly scored in the top 0.5% of predictions. To assign gene names and locus tags to each peptide, genome sequences were run through an ORF finding algorithm (http://mobyle.pasteur.fr/cgi-bin/portal.py?form=getorf), and the corresponding data copied from the information available for orthologous Dumas proteins. MHC-epitope binding predictions were made with the Stabilized Matrix Method using the tool on the IEDB website. All peptides used in this study were synthesized by Mimotopes (Victoria, Australia) as crude material, and resuspended at 20 mg/ml in 100% DMSO (v/v).
MHC-peptide binding assays
Quantitative assays to measure the binding of peptides to HLA A*02 : 01 class I molecules are based on the inhibition of binding of a radiolabeled standard peptide (HBV core 18–27 analogue, FLPSDYFPSV). MHC molecules were purified by affinity chromatography from the EBV transformed homozygous cell line JY, and assays performed, as described previously. Peptides were tested at six different concentrations covering a 100,000-fold dose range in three or more independent assays, and the concentration of peptide yielding 50% inhibition of the binding of the radiolabeled probe peptide (IC50) was calculated. Under the conditions used, where [radiolabeled probe] < [MHC] and IC50 ≥ [MHC], the measured IC50 values are reasonable approximations of the true Kd values.
PBMC and plasma isolation
PBMCs were isolated using BD Vacutainer CPT tubes, washed, and resuspended in RPMI 1640 with 10% FCS (v/v) for immediate use or frozen in fetal calf serum with 10% dimethyl sulfoxide (v/v) for subsequent analysis. Plasma samples were saved at −80°C for subsequent analysis.
Ex vivo IFN-γ ELISPOT assay
Gamma interferon (IFN-γ) enzyme-linked immunospot (ELISpot) assays were performed using 2×105 PBMC stimulated with peptide pools (10 µg/ml/peptide). Peptide pools yielding positive responses were deconvoluted, by testing individual peptides at 10 µg/ml. After 20 h of incubation at 37°C, plates were developed, and responses were calculated. Positive wells contained ≥20 spot-forming units (SFU)/106 cells and a P value of ≤0.05 using a Student's t test in at least 2 experiments.
Tetramer and antibody staining for flow cytometry
MHC class I tetramer was prepared in-house. Surface staining of T cells was achieved by addition of tetramer to whole blood, incubation for 10 minutes at room temperature, followed by addition of antibody co-stains for 20 minutes. Whole blood was preferred to thawed PBMCs for tetramer labelling due to greater consistency and signal intensity. Following lysis of erythrocytes using BD FACS Lysing solution (BD Biosciences), cells were either fixed using 2% formaldehyde (v/v) or permeabilized using the BD Cytofix/Cytoperm kit for intra-cellular staining. The following antibodies were used for surface and intra-cellular staining: CD3-PerCP, CD8-Horizon V500, CD8-APCH7, Ki-67-FITC, Bcl-2-PE, HLA-DR-Horizon V450, HLA-DR-PerCP, Perforin-FITC, Granzyme B-Horizon V450, CCR7-PE, CD27-Horizon V450, CD27-FITC, CD28-PECy7, CD28-PE (all BD Biosciences) and CD38-PECy7 (eBioscience), Granzyme B-PE (Caltag), Granzyme K-PE (Santa Cruz), CD45RA-FITC (Beckman Coulter), PD-1-PE and 2B4-PerCPCy5.5 (Biolegend). Intra-cellular cytokine staining with IFN-γ-FITC, TNF-α-APC, and IL-2-PerCpCy5.5 (all BD Biosciences) was undertaken after in vitro stimulation of PBMCs using peptide for 6 hours. Flow cytometry analysis was performed BD LSRII and BD FACSCanto flow cytometers. Flow cytometry data were analyzed using FlowJo software.
In vitro proliferation assay
PBMC were labeled for 7 minutes with 2.5 µM carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes) in PBS at room temperature. Cold FCS was then added and cells were washed extensively with RPMI 1640 plus 10% FCS. CFSE-labeled cells were incubated with or without the ILIEGIFFV peptide (10 µg/ml) for 6 days. Responding CD8 T cells were subsequently identified by tetramer staining.
Supporting Information
Zdroje
1. Fields BN, Knipe DM, Howley PM, editors (2007) Fields' Virology. London: Wolters Kluwer/Lippincott Williams & Wilkins. 2664 p.
2. WilsonA, SharpM, KoropchakCM, TingSF, ArvinAM (1992) Subclinical Varicella-Zoster Virus Viremia, Herpes Zoster, and T Lymphocyte Immunity to Varicella-Zoster Viral Antigens after Bone Marrow Transplantation. J Infect Dis 165 : 119–126.
3. MehtaSK, CohrsRJ, ForghaniB, ZerbeG, GildenDH, et al. (2004) Stress-induced subclinical reactivation of varicella zoster virus in astronauts. Journal of Medical Virology 72 : 174–179.
4. PapaevangelouV, QuinlivanM, LockwoodJ, PapaloukasO, SideriG, et al. (2013) Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect 19: E245–51.
5. WeinbergA, LevinMJ (2010) VZV T cell-mediated immunity. Curr Top Microbiol Immunol 342 : 341–357.
6. ArvinAM (1996) Immune responses to varicella-zoster virus. Infect Dis Clin North Am 10 : 529–570.
7. GoodRA, ZakSJ (1956) Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics 18 : 109–149.
8. Patterson-BartlettJ, LevinMJ, LangN, SchödelFP, VesseyR, et al. (2007) Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 25 : 7087–7093.
9. OxmanMN (2010) Zoster Vaccine: Current Status and Future Prospects. Clin Infect Dis 51 : 197–213.
10. DiazPS, SmithS, HunterE, ArvinAM (1989) T lymphocyte cytotoxicity with natural varicella-zoster virus infection and after immunization with live attenuated varicella vaccine. J Immunol 142 : 636–641.
11. LevinMJ, MurrayM, RotbartHA, ZerbeGO, WhiteCJ, et al. (1992) Immune Response of Elderly Individuals to a Live Attenuated Varicella Vaccine. J Infect Dis 166 : 253–259.
12. LevinMJ, MurrayM, ZerbeGO, WhiteCJ, HaywardAR (1994) Immune Responses Of Elderly Persons 4 Years After Receiving A Live Attenuated Varicella Vaccine. J Infect Dis 170 : 522–526.
13. OxmanMN, LevinMJ, JohnsonGR, SchmaderKE, StrausSE, et al. (2005) A Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older Adults. New England Journal of Medicine 352 : 2271–2284.
14. FreyCR, SharpMA, MinAS, SchmidDS, LoparevV, et al. (2003) Identification of CD8+ T Cell Epitopes in the Immediate Early 62 Protein (IE62) of Varicella-Zoster Virus, and Evaluation of Frequency of CD8+ T Cell Response to IE62, by Use of IE62 Peptides after Varicella Vaccination. J Infect Dis 188 : 40–52.
15. HarariA, EndersFB, CelleraiC, BartP-A, PantaleoG (2009) Distinct Profiles of Cytotoxic Granules in Memory CD8 T Cells Correlate with Function, Differentiation Stage, and Antigen Exposure. J Virol 83 : 2862–2871.
16. YoungbloodB, WherryEJ, AhmedR (2011) Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS 7 : 50–57.
17. DayCL, KaufmannDE, KiepielaP, BrownJA, MoodleyES, et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443 : 350–354.
18. JacobsonJG, LeibDA, GoldsteinDJ, BogardCL, SchafferPA, et al. (1989) A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 173 : 276–283.
19. HeinemanTC, CohenJI (1994) Deletion of the varicella-zoster virus large subunit of ribonucleotide reductase impairs growth of virus in vitro. Journal of Virology 68 : 3317–3323.
20. LaingKJ, DongL, SidneyJ, SetteA, KoelleDM (2012) Immunology in the Clinic Review Series; focus on host responses: T cell responses to herpes simplex viruses. Clinical & Experimental Immunology 167 : 47–58.
21. JingL, HaasJ, ChongTM, BrucknerJJ, DannGC, et al. (2012) Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. Journal of Clinical Investigation 122 : 654–673.
22. PosavadCM, WaldA, HoskenN, HuangML, KoelleDM, et al. (2003) T Cell Immunity to Herpes Simplex Viruses in Seronegative Subjects: Silent Infection or Acquired Immunity? J Immunol 170 : 4380–4388.
23. RessingME, HorstD, GriffinBD, TellamJ, ZuoJ, et al. (2008) Epstein-Barr virus evasion of CD8+ and CD4+ T cell immunity via concerted actions of multiple gene products. Seminars in Cancer Biology 18 : 397–408.
24. SchifferJT, CoreyL (2013) Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 19 : 280–288.
25. ZajacAJ, BlattmanJN, Murali-KrishnaK, SourdiveDJ, SureshM, et al. (1998) Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188 : 2205–2213.
26. BarberDL, WherryEJ, MasopustD, ZhuB, AllisonJP, et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439 : 682–687.
27. MasopustD, HaS-J, VezysV, AhmedR (2006) Stimulation History Dictates Memory CD8 T Cell Phenotype: Implications for Prime-Boost Vaccination. J Immunol 177 : 831–839.
28. QuinlivanM, BreuerJ, SchmidDS (2011) Molecular studies of the Oka varicella vaccine. Expert Review of Vaccines 10 : 1321–1336.
29. HillA, JugovicP, YorkI, RussG, BenninkJ, et al. (1995) Herpes simplex virus turns off the TAP to evade host immunity. Nature 375 : 411–415.
30. HislopAD, RessingME, van LeeuwenD, PudneyVA, HorstD, et al. (2007) A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med 204 : 1863–1873.
31. AbendrothA, KinchingtonPR, SlobedmanB (2010) Varicella Zoster Virus Immune Evasion Strategies. Curr Top Microbiol Immunol 342 : 155–71.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



