-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
Aspergillus fumigatus is an opportunistic pathogenic fungus that primarily causes infections in the immunocompromised host. It is known that Aspergillus employs various strategies to evade immune recognition by the host's immune system. Recently, galactosaminogalactan (GAG), a new component of the Aspergillus cell wall, was discovered to have potent anti-inflammatory effects in mice making them more susceptible to Aspergillosis. In the current study we found that this anti-inflammatory property of GAG was due to its capacity to induce the potent anti-inflammatory cytokine interleukin-1 Receptor antagonist. This cytokine interferes with IL-1 signalling and thereby can reduce IL-1–induced immune responses such as T-cell responses. We also found that the induction of this anti-inflammatory cytokine by GAG correlates with increased fungal burden, and mice deficient for this cytokine were protected against aspergillosis. Additionally, we show that the capacity of GAG to induce the natural regulator of IL-1 signalling could be used in the treatment of IL-1–mediated disease such as allergy and colitis. Our study provides new insights on the immunoregulatory activity of GAG and opens up possibilities to exploit the anti-inflammatory potential of GAG as a therapy for inflammatory diseases.
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003936
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003936Summary
Aspergillus fumigatus is an opportunistic pathogenic fungus that primarily causes infections in the immunocompromised host. It is known that Aspergillus employs various strategies to evade immune recognition by the host's immune system. Recently, galactosaminogalactan (GAG), a new component of the Aspergillus cell wall, was discovered to have potent anti-inflammatory effects in mice making them more susceptible to Aspergillosis. In the current study we found that this anti-inflammatory property of GAG was due to its capacity to induce the potent anti-inflammatory cytokine interleukin-1 Receptor antagonist. This cytokine interferes with IL-1 signalling and thereby can reduce IL-1–induced immune responses such as T-cell responses. We also found that the induction of this anti-inflammatory cytokine by GAG correlates with increased fungal burden, and mice deficient for this cytokine were protected against aspergillosis. Additionally, we show that the capacity of GAG to induce the natural regulator of IL-1 signalling could be used in the treatment of IL-1–mediated disease such as allergy and colitis. Our study provides new insights on the immunoregulatory activity of GAG and opens up possibilities to exploit the anti-inflammatory potential of GAG as a therapy for inflammatory diseases.
Introduction
Aspergillus fumigatus is an opportunistic fungus that causes infections under specific conditions, of which secondary immunodeficiency is by far the largest risk factor for the development of invasive infections [1]. In order to initiate an effective host response against Aspergillus, recognition of conserved pathogen associated molecular patterns (PAMPs) by specific pattern recognition receptors (PRRs) is required.
A. fumigatus has a complex cell wall consisting of polysaccharides that play essential biological functions in fungal cell biology and host-pathogen interactions. Some of these polysaccharides are recognized by various PRRs expressed on human immune cells [2]. However, A. fumigatus employs various strategies to evade immune recognition. Aspergillus expresses surface molecules that shield PAMPs or can modulate TLR responses [3]. Several surface molecules and PAMPs of A. fumigatus have been characterized as being capable of modulating or suppressing the immune response. Rodlets and melanin, that are present on the conidial surface, shield PAMPs that elicit pro-inflammatory host responses [4], [5]. In addition, β-glucan, α-glucan and galactomannan (GM) have been shown to modulate the host immune response [6].
Another cell wall component of A. fumigatus that is capable of modulating the immune response is galactosaminogalactan (GAG) [7]. GAG is not expressed on Aspergillus conidia, but is exposed when conidia start to germinate and was found to be present in the extracellular matrix that surrounds Aspergillus hyphae in aspergilloma isolated from patients and in experimental murine invasive aspergillosis [8]. Furthermore, GAG has been shown to serve as an adhesin of Aspergillus [9], [10] and to shield β-glucan moieties on the cell wall [9]. This polysaccharide that is shed into the host environment during Aspergillus vegetative growth induces immunosuppressive effects that results in diminished neutrophil recruitment, which predisposes mice to A. fumigatus infection [7]. However, the mechanism through which GAG induces immunosuppressive effects as well as its capacity to induce similar immunosuppressive effects on the human immune response were unknown. Therefore, we investigated whether GAG can be immunosuppressive in the human host response against A. fumigatus, and we have systematically addressed the possible mechanisms responsible for the anti-inflammatory property of GAG.
Results
Galactosaminogalactan modulates human T-helper cytokine responses
To investigate whether GAG can exert immunomodulatory effects in humans, we tested whether GAG induces the production of pro - and/or anti-inflammatory cytokines in human PBMCs. GAG did not induce the proinflammatory cytokines TNFα, IL-6, IL-8, IFN-γ, IL-17, IL-5 and IL-9 (Figure 1A), neither did it induce the anti-inflammatory cytokine IL-10 (Figure 1A). To determine whether GAG modulates Aspergillus-induced innate monocyte-derived cytokines, PBMCs were stimulated for 24 hours with Aspergillus conidia (these morphotypes of A. fumigatus were selected because they do not contain GAG that would interfere with the study of the immunological function of GAG) in the presence or absence of GAG. The presence of GAG did not have a significant effect on the production of the innate cytokines TNFα and IL-6, or the anti-inflammatory cytokine IL-10 (Figure 1B). However, when the production of the characteristic T-helper cytokines IL-17, IL-22 and IFN-γ induced by A. fumigatus was investigated, the IL-17 and IL-22 responses were significantly reduced in the presence of GAG (Figure 1C). To determine whether the effects of GAG are specific for Aspergillus-driven T-helper (Th) responses, or whether GAG has a general ability to modulate human Th responses, the effects of GAG on cytokine-driven Th responses were studied. GAG significantly decreased the proinflammatory Th cytokine production induced by the cytokine combinations IL-1β/IL-23 and IL-12/IL-18 that induce IL-17/IL-22 and IFN-γ, respectively (Figure 1E). Thus, GAG can inhibit human proinflammatory Th cytokine production induced by Aspergillus and cytokine cocktails.
Fig. 1. GAG inhibits Aspergillus-induced human T-helper cell cytokine production. 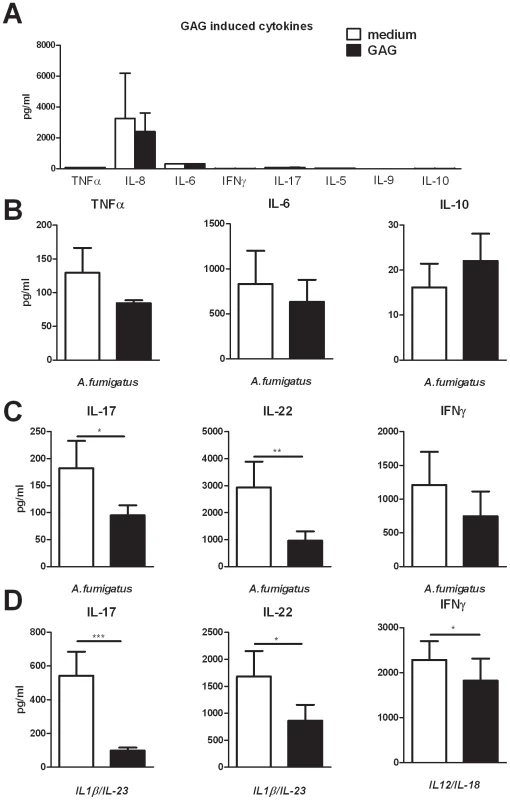
(A) TNFα, IL-6, IL-8 and IL-10 concentrations in culture supernatants of human PBMCs stimulated for 24 hours with 10 µg/ml GAG and IFN-γ, IL-17, IL-5 and IL-9 concentrations after 7 days of stimulation. (B) TNFα, IL-6 and IL-10 concentrations in culture supernatants of human PBMCs (n = 6 donors) stimulated for 24 hours with heat inactivated A. fumigatus conidia (1×107/ml) in the presence or absence of 10 µg/ml GAG. (C,D) IL-17, IL-22 and IFN-γ concentrations in culture supernatants of human PBMCs stimulated for 7 days with heat inactivated A. fumigatus conidia (1×107/ml) (n = 10 donors for IL-17 and IL-22, n = 6 donors for IFN-γ) (c), IL-1β/IL-23 (50/100 ng/ml) (n = 14 donors) or IL-12/IL-18 (50/100 ng/ml) (n = 10 donors) in the presence or absence of GAG (10 µg/ml). Data are represented as mean +/− SEM. Galactosaminogalactan induces IL-1 receptor antagonist
Human Th cytokine responses such as IL-17 and IL-22 production are highly dependent on the IL-1 receptor pathway [11], [12]. To investigate whether the observed modulation of Th cytokines by GAG was due to an interaction of GAG with the IL-1 pathway, we determined the capacity of GAG conditioned medium (culture supernatants of PBMCs that were exposed to 10 µg/ml GAG for 24 hours) to reduce IL-1β bioactivity. Indeed it was shown that GAG significantly reduced the bioactivity of IL-1β while culture supernatants of unstimulated PBMCs did not (Figure 2A). Bioactivity of the IL-1 signalling pathway is dependent on IL-1 receptor agonists (IL-1α and IL-1β) and IL-1 receptor antagonists [13]. One of the natural inhibitors of the IL-1 signalling is the interleukin-1 receptor antagonist (IL-1Ra); therefore the ability of GAG to induce IL-1Ra was tested. IL-1Ra concentrations in the supernatant of the cells stimulated with GAG were significantly increased, whereas GAG induced none of the IL-1 receptor agonists, IL-1α or IL-1β (Figure 2B), showing that GAG has the capacity to modulate immune responses by blocking the IL-1 receptor pathway.
Fig. 2. GAG induces interleukin 1 receptor antagonist. 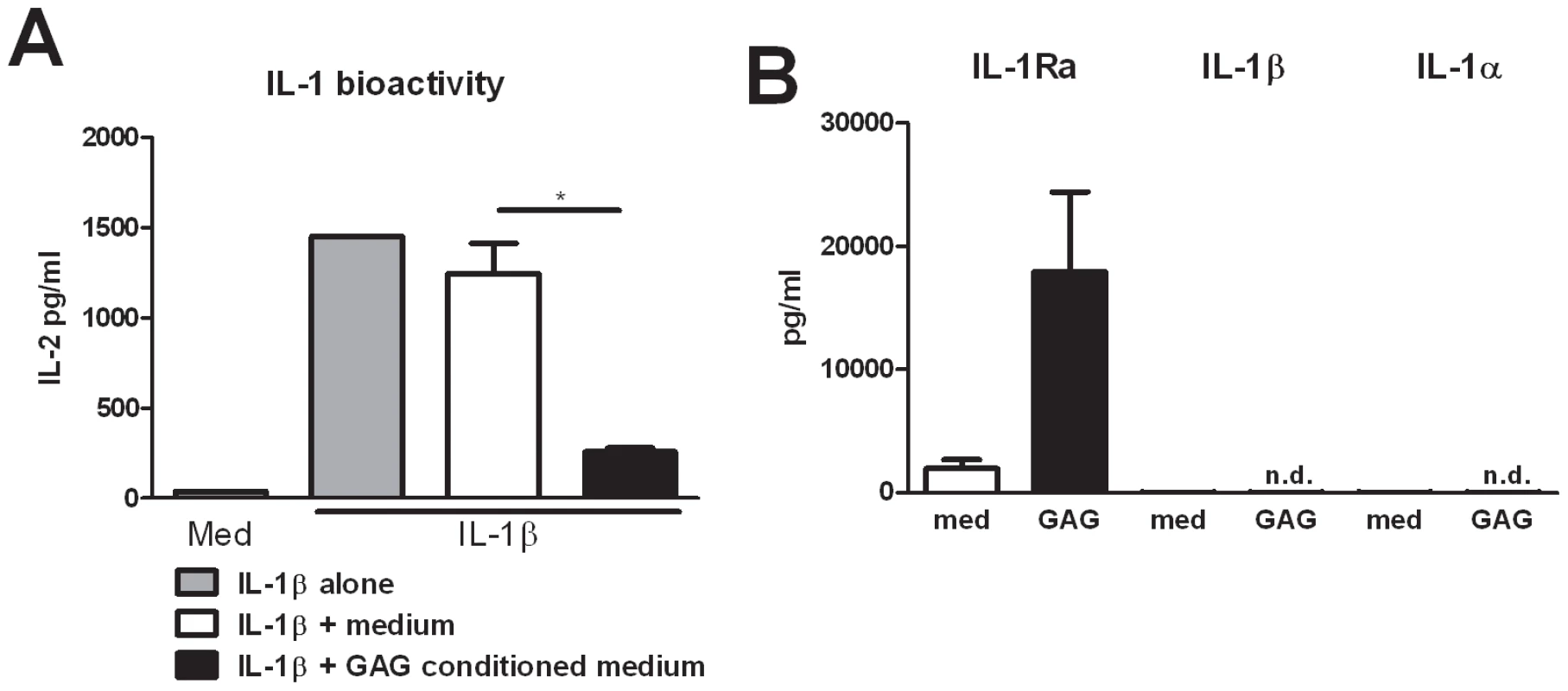
(A) IL-1 bioactivity measured as IL-2 production by NOB-1 cells stimulated with 50 ng/ml IL-1β in the presence of culture supernatant of unstimulated PBMCs (medium) or culture supernatants of PBMCs that were exposed to 10 µg/ml GAG for 24 hours (GAG conditioned medium) (n = 6 donors). (B) IL-1Ra, IL-1β and IL-1α concentrations in culture supernatants of human PBMCs stimulated with for 24 hours with 10 µg/ml GAG. Data are represented as mean +/− SEM. Suppression of IL-17 and IL-22 by galactosaminogalactan is dependent on IL-1Ra
To demonstrate that IL-17, IL-22, and IFN-γ production by human PBMCs is indeed dependent on the IL-1 receptor pathway and that IL-1Ra can inhibit the production of these Th cytokines, Th1 and Th17 inducing stimuli were studied in the presence or absence of IL-1Ra. Addition of IL-1Ra reduced IL-17, IL-22, and IFN-γ induction by both Aspergillus conidia and by IL-1/IL-23 and IL-12/IL-18 cytokine combinations (Figure 3A). To determine whether the immunosuppressive effect of GAG was mediated through the induction of IL-1Ra, PBMCs were stimulated with IL-1β/IL-23 and GAG in the presence or absence of neutralizing anti-IL-1Ra antibodies. GAG reduced IL-17 and IL-22 levels significantly, which was not observed in the presence of neutralizing anti-IL-1Ra antibodies, demonstrating that the inhibitory effects of GAG on Th cytokine production are dependent on IL-1Ra (Figure 3B).
Fig. 3. Suppression of IL-17 and IL-22 by GAG is dependent on IL-1Ra. 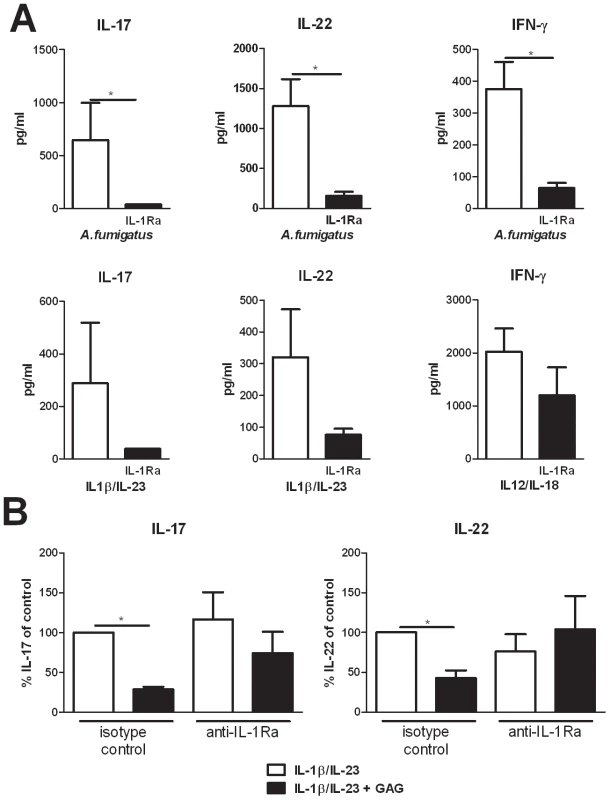
(A) IL-17, IL-22 and IFN-γ concentrations in culture supernatants of PBMCs stimulated for 7 days with heat inactivated A. fumigatus conidia 1×107/ml, IL-1β/IL-23 (50/100 ng/ml) or IL-12/IL-18 (50/100 ng/ml) in the presence or absence of recombinant human IL-1Ra (10 ng/ml). Data are represented as mean +/− SEM. (B) Inhibition of IL-1β/IL-23 (50/100 ng/ml) induced IL-17 and IL-22 by GAG (10 µg/ml) in human PBMCs in the presence of isotype control (10 µg/ml) or anti-IL-1Ra (10 µg/ml). The IL-17 and IL-22 production by IL-1β/IL-23 in absence of GAG was set at 100% and mean percentage changes relative to the control are represented +/− SEM. Galactosaminogalactan induces IL-1Ra in vivo and IL-1Ra increases susceptibility to aspergillosis
The in vitro stimulations described above suggest that the immunomodulatory effects of GAG are due to inhibition of IL-1 bioactivity by inducing IL-1Ra. To assess whether this has relevant consequences in vivo, we measured IL-1Ra transcription in the lungs of mice infected with Aspergillus with or without the administration of GAG. Induction of Il1ra was increased in the presence of GAG (Figure 4A). To determine which cells are responsible for the induction of Il1ra, we isolated macrophages, neutrophils and epithelial cells from the lungs of naïve mice. Macrophages and neutrophils, but not epithelial cells, expressed Il1ra after stimulation with Aspergillus in the presence of GAG (Figure 4B). Interestingly, not all microbiological stimuli can prime for increased GAG-induced Il1ra, since pre-stimulation with LPS did not increase Il1ra induction by GAG (Figure 4B).
Fig. 4. GAG induces IL-1Ra in vivo and IL-1Ra increases susceptibility to aspergillosis. 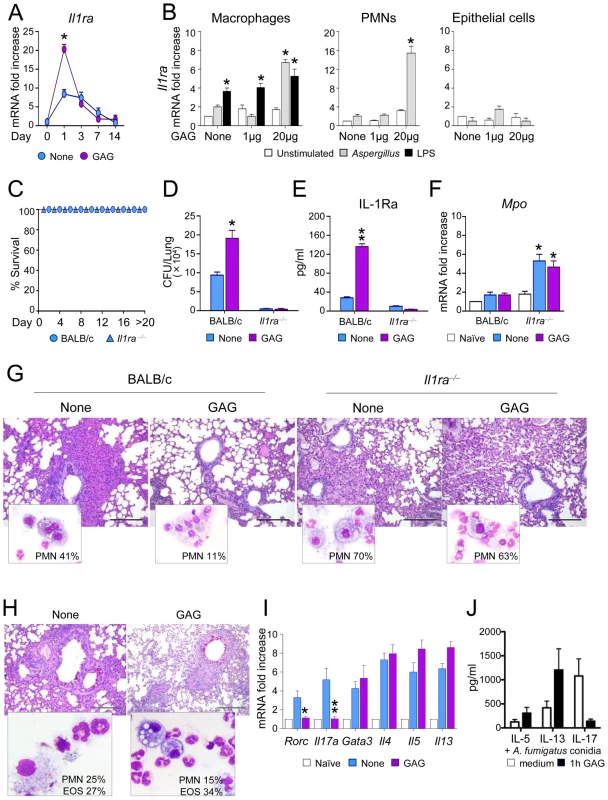
BALB/c and Il1ra−/− mice were intranasally infected with Aspergillus conidia and treated with GAG (250 μg/kg intranasally) the day of infection, and 1, 2 and 3 days post-infection. (A) Il1ra mRNA expression in lung homogenates of mice with invasive aspergillosis, (B) Il1ra mRNA expression in purified cells from lungs of naive mice pre-stimulated with Aspergillus conidia or LPS for 1 hour, and exposed to different GAG concentrations for an additional 18 hours. (C) Survival, (D) fungal growth (CFU/lung, mean +/−SEM), (E) protein levels of IL-1Ra, (F) Mpo expression in lung homogenates, and (G) BAL morphometry [% polymorphonuclear (PMNs) cells and lung histology (PAS stained sections, bars indicate 20× magnification) of Aspergillus-infected mice with or without GAG treatment. Assays were done a day after the last GAG treatment. (H) BAL morphometry [% PMNs or eosinophils (Eo)] and lung histology (PAS stained sections, bars indicate 20× magnification), and (I) expression of Th transcription factors and cytokines in total cells from the draining lymph nodes in mice with ABPA and treated with or without GAG. Naïve means uninfected mice, and none means untreated mice and/or unstimulated cells. (J) IL-5, IL-13 and IL-17 concentrations in culture supernatants of PBMCs pre-incubated 1 h either with medium or GAG (10 μg/ml). After washing, the cells were stimulated for 7 days with heat inactivated 1×107/ml A. fumigatus conidia (n = 4 donors). Data are represented as mean +/− SEM. *, p<0.05; **, p<0.01. To investigate the significance of IL-1Ra in vivo and to determine whether the effects induced by GAG are dependent on IL-1Ra, we studied the effects of GAG in wild type (WT) and Il1ra−/− mice with invasive aspergillosis. Il1ra−/− mice were highly resistant to invasive aspergillosis, as indicated by long-term survival (Figure 4C) and reduced fungal burden (Figure 4D). Administration of GAG resulted in increased protein levels of IL-1Ra in the lungs of wild-type mice during infection (Figure 4E). In line with previous observations, GAG increased the susceptibility to invasive aspergillosis in WT mice but not in Il1ra−/− mice (Figure 4D). Il1ra−/− mice had increased Mpo expression (Figure 4F) and PMN influx in their respiratory tract (Fig. 4G). As expected, administration of GAG reduced inflammatory PMN recruitment in WT but not in Il1ra−/− mice (Figure 4G). These data demonstrate that IL-1Ra has an important role in invasive aspergillosis, and support the concept that the induction of IL-1Ra by GAG may have important clinical consequences.
In ABPA, GAG administration decreased PMN recruitment, but not eosinophilic infiltration in the BAL and lung of allergic mice (Figure 4H), a finding consistent with decreased Th17 but not Th2 cell responses in the draining lymph nodes (Figure 4I). To address whether GAG would have similar effects on human Th2 responses, PBMCs isolated form healthy subjects were pre-incubated for 1 h with GAG and subsequently stimulated with Aspergillus conidia for 7 days. Similar to mice, IL-17 production decreased in the presence of GAG, but not Th2 cytokines such as IL-5 and IL-13 (Figure 4J). Thus, GAG has the potential to ameliorate Th17-dependent immunopathology in ABPA.
IL-1Ra induction by Galactosaminogalactan can be exploited as a therapy for IL-1–mediated inflammatory diseases
Since IL-1Ra treatment can be beneficial for autoinflammatory diseases such as chronic granulomatous disease CGD colitis in humans [14], we investigated whether GAG could be beneficial in experimental DSS-induced colitis in mice with chronic granulomatous disease (CGD). The administration of GAG resulted in the amelioration of clinical signs of colitis (weight loss and stool consistency) (Figure 5A-B) and of inflammatory lesions (Figure 5C) in both wild-type and CGD mice. However the protective effects of GAG were most apparent in CGD mice. GAG induced IL-1Ra and, consistently, reduced IL-1β and IL-17 (Figure 5D). Concomitantly, there was an increased production of IL-10, an anti-inflammatory cytokine that plays an important role in the protection of colitis [15], [16]. The effects of GAG on CGD colitis were similar to those of IL-1Ra administration (unpublished data).
Fig. 5. GAG protects mice from experimental DSS-induced colitis. 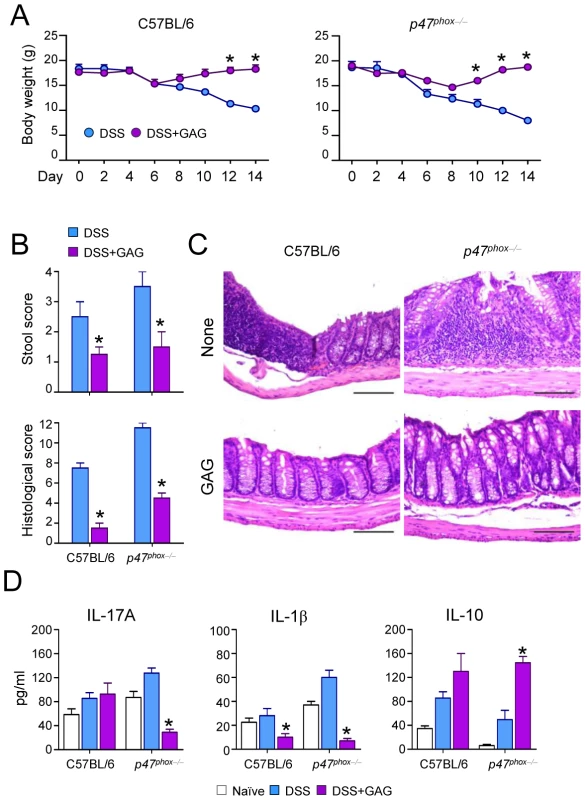
(A) Body weight losses, (B) stool and histological score, (C) histology of colonic sections and (D) cytokine concentrations present in total colonic cells a day after the 7-day of DSS rest in C57BL/6 and p47phox−/− (CGD) mice with or without GAG treatment. *P<0.05, GAG treated vs. untreated mice. Discussion
In the original report describing GAG [7], it was shown that GAG has anti-inflammatory effects in mice. However, the mechanism through which GAG elicits its immunomodulatory effects remained a question at that time. In the present study, we demonstrate that GAG induces its anti-inflammatory effects by inducing the potent anti-inflammatory cytokine IL-1 receptor antagonist.
IL-1Ra can inhibit the activation of the IL-1 pathway by binding to the IL-1R type 1 receptor and prevents recruitment of the IL-1R accessory protein that is required for signalling. It has been repeatedly shown that IL-1 is an essential proinflammatory cytokine of the innate immunity. A deficient IL-1 pathway is also detrimental for the host, since it is an important protective pathway required to fight infection [17]. Thus the IL-1 axis is a potent pro-inflammatory pathway that needs to be tightly regulated, and IL-1Ra is a crucial player in this regulation. Therefore, it is rather surprising that the role of IL-1Ra in invasive fungal infection has not been studied in detail to date. We observed that the absence of IL-1Ra completely protects mice from developing invasive pulmonary aspergillosis, underscoring the importance of the IL-1 pathway in clearance of an acute invasive Aspergillus infection. The observation that GAG induces IL-1Ra in vivo identifies GAG as a potent anti-inflammatory molecule that suppresses the IL-1 pathway, subsequently resulting in increased susceptibility to invasive aspergillosis. The relevance of the IL-1 pathway in aspergillosis is underscored by the fact that polymorphisms IL-1 gene cluster polymorphisms are associated with susceptibility to develop in invasive pulmonary aspergillosis [18], and that dectin-1 knockout mice display increased fungal burden and mortality during invasive aspergillosis, which is dependent on IL-1 [19].
One of the major risk factors that increases susceptibility to invasive aspergillosis is neutropenia [20], and neutrophils are crucial for clearing invasive germinating and hyphal forms of Aspergillus infection [21]. GAG has been shown to inhibit neutrophil recruitment to the lung, which is at least partly due to neutrophil apoptosis [7]. We observed that in the presence of GAG, IL-1Ra increased during invasive aspergillosis, which correlated with decreased PMN recruitment, and therefore increased fungal burden. In contrast, Il1ra−/− mice displayed increased neutrophil influx when exposed to Aspergillus, which could explain the resistance of Il1ra−/− mice to invasive aspergillosis, since they can rapidly and efficiently clear Aspergillus conidia due to their increased neutrophil response. In addition to the induction of IL-1Ra by GAG in vitro and in vivo, we observed that the inhibitory effects of GAG on the proinflammatory Th cytokine response in human PBMCs could be restored in the presence of a neutralizing antibody against human IL-1Ra. Furthermore, the increased susceptibility to invasive aspergillosis induced by GAG is not observed in Il1ra−/− mice. These observations strengthen the hypothesis that the anti-inflammatory properties of GAG are dependent on IL-1Ra.
The anti-inflammatory properties of GAG were present at a concentration of 10 µg/ml, which is a relevant concentration in vivo, since Aspergillus can secrete GAG in a concentration of 50 µg/ml (data not shown). The finding that antibodies against GAG are present in human serum [8] suggests that there is an adequate exposure of GAG to trigger the immune system. In the present study we were also able to demonstrate that these antibodies do not inhibit the effect of GAG, since we observed significant effects of GAG on IL-1Ra induction and inhibition of IL-17 in the presence of human serum that contained measurable concentrations of antibodies against GAG (data not shown). The relevance of GAG is highlighted by its presence in the extracellular matrix in aspergilloma resected from patients and mice with aspergillosis [8]. It is therefore expected that GAG plays a role in the immunological synapse between host immune cell and the mycelium, not only by inducing anti-inflammatory responses through IL-1Ra but also by shielding β-glucan from recognition, which has been proposed previously [9].
It must be taken into account that in the setting of chronic inflammation in which neutrophils and increased Th17 responses are detrimental for the host, IL-1Ra plays a protective role, due to its significant capability to suppress the IL-1 signaling pathway. This hypothesis is in line with the observation that Il1ra−/− mice develop spontaneous destructive arthritis that is IL-1 and Th17 dependent [22]. The importance of IL-1Ra in controlling IL-1 mediated proinflammatory responses in humans is underlined by a disease called deficiency of IL-1Ra (DIRA). This disease is characterized by the absence of IL-1Ra and severe Th17 mediated responses with neutrophil influx in the skin and bones of these patients, subsequently resulting in severe skin inflammation and osteomyelitis [23]. Therefore, the timing of IL-1Ra induction is of utmost importance to protect the host from infection and overwhelming inflammation. Chronic allergic aspergillosis is associated with excessive inflammation, with increased production of IL-1 and IL-22 [24]. We have demonstrated in the present study that administration of GAG induces IL-1Ra and is able to decrease IL-22 production. Therefore we investigated the effect of GAG in a murine model of allergic bronchopulmonary aspergillosis (ABPA). We observed that the administration of GAG reduces the amount of neutrophils, but not eosinophils in ABPA. Additionally, Th17 responses were downregulated, but not Th2 responses. It is therefore tempting to speculate that administration of GAG can be beneficial in the setting of chronic allergic inflammation that is associated with excessive neutrophil-driven inflammation by reducing Th17 dependent pathology by inhibiting the IL-1 pathway. In addition, GAG also protected CGD mice from experimental colitis. Therefore, we envisage a model in which GAG on the one hand might be detrimental for the host in the setting of an acute infection, and on the other hand could be beneficial for the host during chronic inflammation driven by IL-1. Next to the identification of GAG or IL-1Ra as a therapeutic target for invasive aspergillosis, it is the first time that a polysaccharide produced by a human pathogen has been identified as an inducer of IL-1Ra by cells of the innate immunity without inducing proinflammatory responses, and which has been demonstrated to have therapeutic capacity in IL-1 mediated disease. The search of the sensing and signal transduction cascade activated by this polysaccharide will now be the center of future research.
The data presented here brings new questions into light and opens opportunities for future research. First, one of the most interesting observations is the complete protection of IL-1Ra knockout mice to invasive pulmonary aspergillosis. This opens new treatment strategies that target IL-1Ra in the setting of an acute invasive fungal infection. Second, the significant induction of IL-1Ra by GAG makes GAG or a derivative structure of GAG a potential treatment compound for IL-1–mediated diseases, such as joint, bone and muscle diseases and even very common inflammatory diseases such as diabetes and gout [25]. Previously, we have shown that mitogenic stimulation of monocyte derived macrophages and lymphocytes by αCD3/αCD28 coated beads, or recombinant cytokine-induced IL-17 and IFN-γ production is inhibited in the presence of live A. fumigatus [26]. Although these changes in cytokine responses were attributed to changes in tryptophan and kynurenine, it is tempting to speculate that GAG secretion by live A. fumigatus could have attributed to the decreased IL-17 production.
In conclusion, our results demonstrate that GAG has potent anti-inflammatory effects in mice and humans that can be explained by the capability of GAG to induce IL-1Ra. These observations help to explain one of the immune-evasive mechanisms of A. fumigatus. Moreover, inhibition of GAG or IL-1Ra might prove beneficial in the treatment of acute invasive pulmonary aspergillosis, and GAG might be exploited for treatment of IL-1–mediated inflammatory diseases.
Materials and Methods
Ethics statement
All studies with human blood samples were conducted in the Radboud University Nijmegen Medical Centre and the use of healthy volunteers was approved by the institutional ethics review board. Peripheral venous blood samples from healthy volunteers were obtained after written informed consent was provided.
All animal studies were conducted within the University of Perugia and were performed according to the Italian Approved Animal Welfare Assurance A–3143–01. Legislative decree 245/2011-B regarding the animal license was obtained by the Italian Ministry of Health lasting for three years (2011–2014). Infections were performed under avertin anesthesia and all efforts were made to minimize suffering.
Galactosaminogalactan
Galactosaminogalactan (GAG) was isolated from A. fumigatus culture supernatant and purified from the urea-soluble fraction as previously described [7]. Lyophilized GAG was resuspended in 10 mM HCl at 2 mg/ml and used in a final concentration of 10 µg/ml. Before using GAG in stimulation experiments it was incubated with polymixin B to neutralize potential contamination of lipopolysaccharide.
Stimuli and reagents
A clinical isolate of Aspergillus fumigatus V05-27, which has been previously characterized was used for stimulations [27]. Conidia and hyphae were prepared and heat-killed as previously described [6]. A concentration of 1×107/ml was used in the experiments, unless otherwise indicated. Recombinant human IL-1β, IL-23, IL-12 and IL-18 were purchased from R&D Systems (Minneapolis, MN, USA) and were used in end concentrations of 100 ng/ml, 50 ng/ml, 10 ng/ml and 50 ng/ml respectively. Recombinant human (rh) IL-1Ra (Amgen, Inc., Thousand Oaks, CA, USA) was used to antagonize IL-1β signalling at a final concentration of 10 ng/ml. Anti-humanIL-1Ra (R&D) was used to block IL-1Ra in a final concentration of 10 µg/ml, and was compared to isotype control.
PBMC isolation and stimulation
PBMCs were isolated as described previously [28]. The cells were counted using a particle counter (Beckmann Coulter, Woerden, The Netherlands) and the cell number was adjusted to 5×106/ml. PBMCs were plated in 96-well round-bottom plates (Corning, NY, USA) at a final concentration of 2,5×106/ml and in a total volume of 200 μl. Cells were pre-stimulated for 1 hour with medium or 10 µg/ml GAG. Following prestimulation, the PBMCs were stimulated with culture medium, heat killed A. fumigatus conidia (1×107/ml), IL-1β/IL-23 (100 and 50 ng/ml respectively) or IL-12/IL-18 (10 and 50 ng/ml respectively). These experiments were also performed in the presence of 10 μg/ml anti-IL-1Ra antibody or isotype control. Plates were incubated at 37°C, 5% CO2 for 24 hours, 48 hours or 7 days. 7 day cultures were supplemented with 10% human serum. In this serum we detected anti-GAG antibodies as described previously [7]. After incubation, culture supernatants were collected and stored at -20°C until cytokine measurements were performed.
IL-1 bioassay
The murine cell line NOB-1 responds to both human or mouse IL-1 by production of IL-2, furthermore these cells are unresponsive to other cytokines like tumor necrosis factor (TNF), colony stimulating factors (CSFS), IL-3, IL-5, IL-6 and IFN-γ [29]. NOB-1 cells were plated in 96 well flat-bottom plates at a final density of 1×106 cells/ml and were stimulated for 24 hour using culture supernatants of unstimulated PBMCs or PBMCs stimulated in presence of GAG (GAG conditioned medium). After 24 hours of incubation at 37°C, 5% CO2 the culture supernatants of the NOB-1 cells were collected and IL-2 production by the NOB-1 cells was measured by ELISA (R&D systems).
Cytokine measurement
Cytokines were measured using commercially available ELISAs (R&D Systems)(Biolegend, San Diego, CA, USA) (Sanquin, Amsterdam, The Netherlands) according to the protocols supplied by the manufacturer. IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-1Ra and IL-10 were measured in culture supernatants of 24 hour cultures, and IL-5, IL-9, IL-13, IL-17, IL-22 and IFN-γ were measured in culture supernatants of 7 day cultures.
Mice
Female, 8 - to 10-weeks old, BALB/c (wild-type, WT) mice were purchased from Charles River (Calco, Italy). Breeding pairs of homozygous Il1ra−/− mice on the BALB/c background, were kept under specific-pathogen free conditions at the breeding facilities of the University of Perugia, Perugia, Italy. Experiments were performed according to the Italian Approved Animal Welfare Assurance 229-2011-B.
Fungal infection, allergy and treatment
Viable conidia from the A. fumigatus Af293 strain were obtained as described [30]. For infection mice were anesthetized in a small plastic cage, containing 3% Isofluoran (Isofluran Forene Abbot Scandinavia AB, Solna) before intranasal (i.n.) instillation of a suspension of 2×107 resting conidia/20 µl saline. Mice were treated with 250 μg/kg i.n. of GAG the day of infection and on days 1 to 3 post infection. Mice were monitored for survival, fungal growth (colony forming unit/organ, mean ± SEM), as described [31], histopathology, myeloperoxidase (Mpo) and Il1ra mRNA expression in lung cells and IL-1Ra production. For histology, sections (3–4 ìm) of paraffin-embedded tissues were stained with periodic acid-Schiff (PAS) reagent. For allergy, mice received an i.p. and s.c. injection of 100 µg of A. fumigatus culture filtrate extract (CCFA) dissolved in incomplete Freund's adjuvant (Sigma) followed by two consecutive intranasal injections (a week apart) of 20 µg CCFA. A week after the last intranasal challenge, mice received 107 Aspergillus resting conidia and evaluated a week later (16424201). GAG (250 μg/kg i.n.) or vehicle alone was administered daily, for a week, in concomitance with the Aspergillus infection.
Collection of broncho-alveolar lavage (BAL) fluid
Lungs were filled thoroughly with 1 ml aliquots of pyrogen-free saline through a 22-gauge bead-tipped feeding needle introduced into the trachea. The lavage fluid was collected in a plastic tube on ice and centrifuged at 400 g, 4°C, for 5 min. For differential BAL cell counts, cytospin preparations were made and stained with May - Grünwald Giemsa reagents (Sigma-Aldrich). At least 200 cells per cytospin preparation were counted and the absolute number of each cell type was calculated. Photographs were observed using a BX51 microscope (Olympus, Milan, Italy) and images were captured using a high-resolution DP71 camera (Olympus).
Dextran sulfate sodium–induced colitis
Mice received either regular drinking water (control) or 2.5% dextran sulfate sodium (DSS) in drinking water for 7 days and then allowed to recover by drinking water alone for an additional 7 days. GAG was given intraperitoneally (1 mg/kg) daily for a week. Weight changes were recorded daily, and the day after the 7-days of rest mice were killed and tissues were collected for histology and cytokine analysis. Colonic sections were stained with H&E [32]. To assess colitis severity, stool and histological scores were used that recently were introduced and proven sensitive to experimental therapy [33].
Cell purification and cell cultures
Purified peritoneal CD11b+ Gr-1+ polymorphonuclear neutrophils (PMNs) (>98% pure on FACS analysis) were obtained as described [34]. Lung epithelial cells were isolated as described [35] Murine macrophages were isolated from total lung cells after 2 hours plastic adherence at 37°C. PMNs, epithelial cells and macrophages were exposed to unopsonized Aspergillus conidia at the ratio of 1∶1 or LPS (10 ng/ml) at 37°C for 1 hour in the presence of different concentrations (1 or 20 µg/ml) of GAG for 18 hours before the assessment of Il1ra mRNA expression.
Statistical analysis
The differences between the various stimulations were analyzed with the Wilcoxon signed rank test (p-value of <0.05 was considered statistically significant). All experiments were performed at least twice and data represent cumulative results of all experiments performed and are presented as mean +/ − standard error of the mean (SEM) unless otherwise indicated. Data was analyzed using GraphPad Prism v5.0.
Zdroje
1. ChaiLY, HsuLY (2011) Recent advances in invasive pulmonary aspergillosis. Curr Opin Pulm Med 17 : 160–166.
2. RomaniL (2011) Immunity to fungal infections. Nat Rev Immunol 11 : 275–288.
3. ChaiLY, NeteaMG, VonkAG, KullbergBJ (2009) Fungal strategies for overcoming host innate immune response. Med Mycol 47 : 227–236.
4. AimaniandaV, BayryJ, BozzaS, KniemeyerO, PerruccioK, et al. (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460 : 1117–1121.
5. ChaiLY, NeteaMG, SuguiJ, VonkAG, van de SandeWW, et al. (2010) Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 215 : 915–920.
6. ChaiLY, KullbergBJ, VonkAG, WarrisA, CambiA, et al. (2009) Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect Immun 77 : 2184–2192.
7. FontaineT, DelangleA, SimenelC, CoddevilleB, van VlietSJ, et al. (2011) Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog 7: e1002372.
8. LoussertC, SchmittC, PrevostMC, BalloyV, FadelE, et al. (2010) In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12 : 405–410.
9. GravelatFN, BeauvaisA, LiuH, LeeMJ, SnarrBD, et al. (2013) Aspergillus Galactosaminogalactan Mediates Adherence to Host Constituents and Conceals Hyphal beta-Glucan from the Immune System. PLoS Pathog 9: e1003575.
10. SheppardDC (2011) Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr Opin Microbiol 14 : 375–379.
11. GuoL, WeiG, ZhuJ, LiaoW, LeonardWJ, et al. (2009) IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A 106 : 13463–13468.
12. Ben-SassonSZ, Hu-LiJ, QuielJ, CauchetauxS, RatnerM, et al. (2009) IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A 106 : 7119–7124.
13. DinarelloCA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117 : 3720–3732.
14. van de VeerdonkFL, NeteaMG, DinarelloCA, van der MeerJW (2011) Anakinra for the inflammatory complications of chronic granulomatous disease. Neth J Med 69 : 95.
15. GrouxH, O′GarraA, BiglerM, RouleauM, AntonenkoS, et al. (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389 : 737–742.
16. GlockerEO, KotlarzD, KleinC, ShahN, GrimbacherB (2011) IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sci 1246 : 102–107.
17. van de VeerdonkFL, NeteaMG, DinarelloCA, JoostenLA (2011) Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol 32 : 110–116.
18. SainzJ, PerezE, Gomez-LoperaS, JuradoM (2008) IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol 28 : 473–485.
19. WernerJL, MetzAE, HornD, SchoebTR, HewittMM, et al. (2009) Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182 : 4938–4946.
20. MarrKA, CarterRA, BoeckhM, MartinP, CoreyL (2002) Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100 : 4358–4366.
21. SchaffnerA, DouglasH, BraudeA (1982) Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest 69 : 617–631.
22. KoendersMI, DevesaI, MarijnissenRJ, Abdollahi-RoodsazS, BootsAM, et al. (2008) Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum 58 : 3461–3470.
23. AksentijevichI, MastersSL, FergusonPJ, DanceyP, FrenkelJ, et al. (2009) An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 360 : 2426–2437.
24. LillyLM, GessnerMA, DunawayCW, MetzAE, SchwiebertL, et al. (2012) The beta-Glucan Receptor Dectin-1 Promotes Lung Immunopathology during Fungal Allergy via IL-22. J Immunol 189(7): 3653–60.
25. DinarelloCA, SimonA, van der MeerJW (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11 : 633–652.
26. ChaiLY, van de VeerdonkF, MarijnissenRJ, ChengSC, KhooAL, et al. (2010) Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology 130 : 46–54.
27. NeteaMG, WarrisA, Van der MeerJW, FentonMJ, Verver-JanssenTJ, et al. (2003) Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J Infect Dis 188 : 320–326.
28. van de VeerdonkFL, MarijnissenRJ, KullbergBJ, KoenenHJ, ChengSC, et al. (2009) The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5 : 329–340.
29. GearingAJ, BirdCR, BristowA, PooleS, ThorpeR (1987) A simple sensitive bioassay for interleukin-1 which is unresponsive to 10(3) U/ml of interleukin-2. J Immunol Methods 99 : 7–11.
30. BonifaziP, D′AngeloC, ZagarellaS, ZelanteT, BozzaS, et al. (2010) Intranasally delivered siRNA targeting PI3K/Akt/mTOR inflammatory pathways protects from aspergillosis. Mucosal Immunol 3 : 193–205.
31. BozzaS, PerruccioK, MontagnoliC, GazianoR, BellocchioS, et al. (2003) A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102 : 3807–3814.
32. TakedatsuH, MichelsenKS, WeiB, LandersCJ, ThomasLS, et al. (2008) TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology 135 : 552–567.
33. EngelMA, LefflerA, NiedermirtlF, BabesA, ZimmermannK, et al. (2011) TRPA1 and substance P mediate colitis in mice. Gastroenterology 141 : 1346–1358.
34. BellocchioS, MorettiS, PerruccioK, FallarinoF, BozzaS, et al. (2004) TLRs govern neutrophil activity in aspergillosis. J Immunol 173 : 7406–7415.
35. YouY, RicherEJ, HuangT, BrodySL (2002) Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 283: L1315–1321.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



