-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Five Things to Know about Genetically Modified (GM) Insects for Vector Control
article has not abstract
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003909
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003909Summary
article has not abstract
Five Things to Know about Genetically Modified (GM) Insects for Vector Control
1. Why (and how to) use GM vectors for vector control?
Vector-borne diseases cause immense suffering and economic damage. Vector control remains a key element of mitigation and control strategies, particularly for pathogens such as dengue viruses for which there are no specific drugs or vaccines. Yet existing vector control tools are limited; toxic chemicals are the mainstay but difficult to deliver due to vector behaviour, emerging resistance, and/or environmental concerns. Genetically modified vectors—presently only mosquitoes—offer complementary new approaches to integrate with the best existing methods. Modified mosquitoes will actively seek out wild mosquitoes as mates, with high species specificity and minimal off-target effects.
Within this overall scheme, many different genetic modifications have been proposed, all delivered via this mating-based mechanism (“vertical transmission”). These may be classified according to the persistence of the modification: “self-sustaining” genetic systems are intended to persist or spread invasively in the wild population after an initial release period, while “self-limiting” systems will disappear relatively rapidly unless maintained by more releases. Another classification is by intended effect: “population suppression” strategies aim, like most current vector control programmes, to reduce the number of vector mosquitoes in the target area, while “population replacement” strategies aim to reduce the ability of affected mosquitoes to transmit specified pathogens, with any reduction in total number of mosquitoes being incidental. In either case, the intended result is fewer competent vectors, thereby reducing the force of infection. In computer simulations, several such strategies are capable of eliminating transmission in the programme area.
These approaches are not entirely new. Some proposals [1] are simply applications of modern genetics to improve on the classical Sterile Insect Technique (SIT) [2], in which radiation-sterilised insects are released to mate with wild counterparts and thereby reduce the reproductive potential of the target pest population, leading to suppression or even local elimination. SIT has been used successfully on large and small scales against some major agricultural pests. This close relationship to an existing method means that the rollout, use, strengths, and weaknesses of such self-limiting population suppression strategies are fairly predictable and well understood. For self-sustaining strategies, looser analogies may be drawn with classical biological control, in which an exotic predator or parasite is introduced with the intention that it should establish permanently and thereby help control the pest. This analogy highlights both key strengths of self-sustaining systems—potential long-term benefit without further human action—and weaknesses—relative lack of control post-release—relative to self-limiting ones. Simulation modelling is a vital tool to inform strain development and risk assessment and mitigation, especially of the more invasive self-sustaining systems in which release is essentially irreversible.
2. How GM mosquitoes are made
Inserting DNA into an insect's chromosome (“genetic transformation”) is currently accomplished by means of a transposon-based system (Figure 1) [3]. The DNA of interest is placed between the ends of a suitable transposon (e.g., piggyBac, Minos, mariner, or Hermes). This plasmid is micro-injected into embryos, along with “helper” transposase (as mRNA or plasmid). The helper transposase acts on the transposon ends and, at very low but nonzero frequency, causes the transposon to “jump” from the injected plasmid into the insect's chromosomes (Figure 1B). Each transposon has its characteristic insertion site (e.g., TTAA for piggyBac), but these are present in so many copies in the genome that insertion is essentially random. The inserted DNA, lacking its own transposase gene (“non-autonomous” transposon), is then stably integrated in the insect's genome.
Fig. 1. Creating a new transgenic strain. 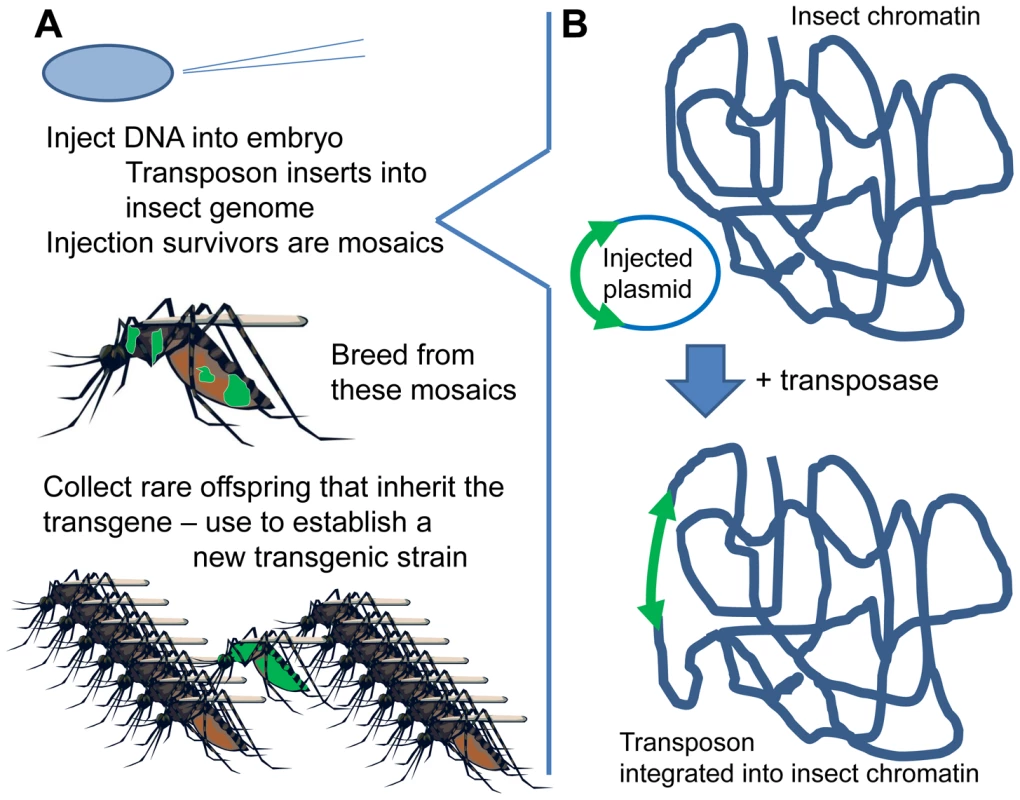
(A) DNA is injected into insect eggs; offspring of injection survivors are screened for presence of the marker gene indicating transgene presence. (B) Transposase-mediated transgene insertion. The DNA is injected into syncytial embryos, an early developmental stage before cells form, in which there are many nuclei within a shared cytoplasm. The injected DNA can therefore reach any of the nuclei. If some germline cells are transformed, then the offspring arising from them will carry the inserted DNA in all their cells—a new, transformed individual from which a transformed line can be established by simply breeding (Figure 1A). A marker gene, usually a fluorescent protein, is incorporated into the genetic construct to identify these rare, transformed insects. The transformation process is now routine in several important vector mosquitoes, such as Aedes aegypti, Anopheles stephensi, and An. gambiae.
Transformation by homologous integration is not available for mosquitoes, though long–recognition-site nucleases may facilitate this. However, site-specific integration has been developed by inserting “docking sites” on transposons and then allowing targeted integration into these engineered sites [4]. This is very valuable for some purposes: for example, comparing the effects of two different constructs. Such experiments were previously confounded by “position effect:” regulatory elements in the flanking chromatin interact with inserted DNA and affect its expression, so the same construct in different locations often gives slightly different expression and phenotype. This can be advantageous by allowing the experimenter to fine-tune expression simply by screening a panel of insertion lines, but it causes problems for other types of experiments.
Other approaches to genetic modification [5]–[9], for example, artificial infection with maternally transmitted Wolbachia pipientis bacteria or paratransgenesis (genetically engineering the vector's symbionts), are beyond the scope of this article.
3. Progress in the field
Some proposed strategies exist only as attractive-looking simulations [10], others as proof-of-principle in Drosophila or mosquitoes, but some have already entered advanced cage and field trials. This is remarkable progress given the low investment in this area by funding agencies, relative to insecticides, drugs, or vaccines, and how recently the molecular genetic tools and techniques were invented.
The first field trials of GM mosquitoes involved a self-limiting, population-suppression, sterile-male system known as RIDL (Release of Insects carrying a Dominant Lethal genetic system) [11]. Trials have shown that lab-reared, genetically engineered A. aegypti RIDL males can compete successfully for mates in the field [12], have similar field performance (e.g., longevity) to an unmodified comparator strain [13], and that sustained release of such males can suppress a target field population [14]. These data are extremely encouraging for the further development of this RIDL approach and, also, for GM mosquito methods generally.
SIT-related methods require the release of considerable numbers of modified male mosquitoes. Though the economics and timescales needed to achieve significant disease reduction look highly attractive [15], [16], in some instances even more powerful methods may be desirable. More invasive genetic systems are being developed, which models predict would require far fewer mosquitoes to be released [17]–[19]. Although the costs of post-release monitoring and stewardship of self-sustaining systems should not be underestimated, these aggressive systems are likely to be far cheaper to deploy against very widely distributed pests and species complexes, the main trade-off being lack of control post-release. Cost of development is also higher, but for all these systems development is a one-time cost that looks trivial relative to the potential benefit.
4. It's not just the genetics
Developing promising genetic strategies and modified strains that embody them is a necessary step, but far from sufficient. New technologies need to win public acceptance. The idea of dealing with a dangerous mosquito by releasing more of them is hardly intuitive! This is compounded by public concerns over the use of genetic approaches. Regulatory systems are also challenged by these new methods; for both the public and regulators, “self-sustaining” methods intending to lead to the permanent presence of novel genetic traits in wild vector populations may be especially problematic. Recombinant DNA methods may have an advantage in that frameworks already exist in many countries to regulate environmental use of genetically engineered organisms, albeit typically written with GM crops in mind. Mechanisms for appropriate oversight of other (non-recombinant) methods of genetic modification may be harder to devise.
Even from a purely technical perspective, success depends on more than good genetics. Efficient methods for rearing high-quality mosquitoes are required, particularly for those methods requiring relatively large numbers to be released. A key limitation for developing such methods is the lack of good proxy measure for field quality. Improved understanding of vector ecology would also allow more efficient use of these genetic tools. Ultimately, “success” will be defined in terms of epidemiological outcomes, and a further challenge is how best to demonstrate the efficacy of genetic vector control methods in reducing disease transmission [20].
5. Applications and limitations—What makes a good target?
Genetic methods depend on the vertical (parent-to-offspring) inheritance of one or more novel traits. Mating between modified vectors and wild conspecifics is therefore crucial to all such methods. However, mating barriers may exist between populations, or even different cryptic species with complete barriers to gene flow. In addition to natural mating barriers, selection and genetic drift may cause artificially reared laboratory strains to diverge significantly relative to wild strains, leading to poor mating. Highly invasive genetic systems may be able to cross incomplete hybridisation barriers; this spreading ability may be a significant advantage in such settings, while simultaneously a source of concern from a regulatory perspective.
More prosaically, the manipulations associated with introducing the modified trait likely require that the target species be reasonably easily reared in the laboratory. Though not a fundamental limitation, the need to develop adequate rearing methods would add to the time and cost of developing genetic control tools in species where such methods are not already established.
Genetic control methods target a single species (or species complex). From an economic perspective, this may be highly attractive when a single—or perhaps two or three—dominant vector species can be targeted, less so, elsewhere. Dengue, for which A. aegypti is the dominant vector worldwide, clearly fits this criterion. If multiple vector species are present but some are well controlled by other methods, genetic control may be useful as part of integrated vector management; this may apply in some African malaria contexts. Though development has so far focused on vectors of human diseases, many vectors of livestock and plant diseases will likely also prove amenable to genetic control methods.
More generally, genetic methods should not be seen as “magic bullets” that will single-handedly solve a problem but rather as new and powerful approaches with specific strengths and limitations. That such new approaches are needed seems beyond doubt. These should be combined with existing methods to provide programme managers with more options for effective, safe, and sustainable vector control. The prospects for genetic methods to contribute to effective control of vector-borne diseases look very promising.
Zdroje
1. AlpheyL, BenedictMQ, BelliniR, ClarkGG, DameDA, et al. (2010) Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis 10 : 295–311.
2. Dyck VA, Hendrichs J, Robinson AS,editors (2005) Sterile Insect Technique: Principles and practice in Area-Wide Integrated Pest Management. The Netherlands: Springer. 801 p.
3. FraserMJJr (2012) Insect Transgenesis: Current Applications and Future Prospects. Annu Rev Entomol 57 : 267–289.
4. NimmoD, AlpheyL, MeredithJ, EgglestonP (2006) High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol 15 : 129–136.
5. AlpheyL (2014) Genetic Control of Mosquitoes. Annu Rev Entomol 59 : 205–224.
6. AlpheyL, McKemeyA, NimmoD, OveidoMN, LacroixR, et al. (2013) Genetic control of Aedes mosquitoes. Pathog Glob Health 107 : 170–179.
7. WangS, Jacobs-LorenaM (2013) Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol 31 : 185–193.
8. Aksoy S, Weiss B, Attardo GM (2008) Paratransgenesis applied for control of tsetse transmitted sleeping sickness. In: Aksoy S, editor. Transgenesis and the Management of Vector-Borne Disease (Advances in Experimental Medicine and Biology). New York: Springer.
9. HurwitzI, FieckA, ReadA, HilleslandH, KleinN, et al. (2011) Paratransgenic Control of Vector Borne Diseases. Int J Biol Sci 7 : 1334–1344.
10. MarshallJM, HayBA (2012) General Principles Of Single-Construct Chromosomal Gene Drive. Evolution 66 : 2150–2166.
11. ThomasDD, DonnellyCA, WoodRJ, AlpheyLS (2000) Insect population control using a dominant, repressible, lethal genetic system. Science 287 : 2474–2476.
12. HarrisAF, NimmoD, McKemeyAR, KellyN, ScaifeS, et al. (2011) Field performance of engineered male mosquitoes. Nat Biotechnol 29 : 1034–1037.
13. LacroixR, McKemeyAR, RaduanN, Kwee WeeL, Hong MingW, et al. (2012) Open Field Release of Genetically Engineered Sterile Male Aedes aegypti in Malaysia. PLOS One 7: e42771 doi:10.1371/journal.pone.0042771
14. HarrisAF, McKemeyAR, NimmoD, CurtisZ, BlackI, et al. (2012) Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol 30 : 828–830.
15. AtkinsonMP, SuZ, AlpheyN, AlpheyLS, ColemanPG, et al. (2007) Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci U S A 104 : 9540–9545.
16. AlpheyN, AlpheyL, BonsallMB (2011) A Model Framework to Estimate Impact and Cost of Genetics-Based Sterile Insect Methods for Dengue Vector Control. PLOS One 6: e25384 doi:10.1371/journal.pone.0025384
17. BurtA (2003) Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci 270 : 921–928.
18. DeredecA, BurtA, GodfrayHCJ (2008) The Population Genetics of Using Homing Endonuclease Genes in Vector and Pest Management. Genetics 179 : 2013–2026.
19. ChenC-H, HuangH, WardCM, SuJT, SchaefferLV, et al. (2007) A Synthetic Maternal-Effect Selfish Genetic Element Drives Population Replacement in Drosophila. Science 316 : 597–600.
20. WolbersM, KleinschmidtI, SimmonsCP, DonnellyCA (2012) Considerations in the Design of Clinical Trials to Test Novel Entomological Approaches to Dengue Control. PLOS Negl Trop Dis 6: e1937 doi:10.1371/journal.pntd.0001937
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



