-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
African trypanosomes are parasites that cause sleeping sickness in humans. In mice models, trypanosomiasis causes loss of the spleen memory B-cell precursors, of the host memory response and of protection against certain pathogens, built up by vaccination. The phenomenon has never been studied in human sleeping sickness, but if occurring, revaccination after treatment would be required. We show that gambiense human sleeping sickness is associated with a relevant increase in memory T - and B - cells in peripheral blood, in particular T-independent memory B-cells. As measles vaccination is included in standard vaccination programs, we measured measles antibody concentrations, which, although slightly lower in sleeping sickness patients than in controls, exceeded in 95% of patients the minimum level considered protective. Anti-red blood cell IgM titres, measured to assess the T-cell independent antibody response, were one titre lower in patients than in controls, but normalized after treatment. Overall, our results in gambiense HAT patients do not suggest trypanosomiasis associated massive memory cell destruction, or loss of antibody levels, although the antibody's protective capacity remains to be confirmed.
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003947
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003947Summary
African trypanosomes are parasites that cause sleeping sickness in humans. In mice models, trypanosomiasis causes loss of the spleen memory B-cell precursors, of the host memory response and of protection against certain pathogens, built up by vaccination. The phenomenon has never been studied in human sleeping sickness, but if occurring, revaccination after treatment would be required. We show that gambiense human sleeping sickness is associated with a relevant increase in memory T - and B - cells in peripheral blood, in particular T-independent memory B-cells. As measles vaccination is included in standard vaccination programs, we measured measles antibody concentrations, which, although slightly lower in sleeping sickness patients than in controls, exceeded in 95% of patients the minimum level considered protective. Anti-red blood cell IgM titres, measured to assess the T-cell independent antibody response, were one titre lower in patients than in controls, but normalized after treatment. Overall, our results in gambiense HAT patients do not suggest trypanosomiasis associated massive memory cell destruction, or loss of antibody levels, although the antibody's protective capacity remains to be confirmed.
Introduction
Human African Trypanosomiasis (HAT) or sleeping sickness, is a vector-borne parasitic disease occurring in sub-Saharan Africa. About 70 million persons are at risk for infection and 30 000 persons are estimated to be infected [1]. The parasites concerned belong to the Trypanosoma genus and are transmitted through the bites of tsetse flies (Glossina genus). Two subspecies of Trypanosoma brucei (T.b.), T.b. gambiense and T.b. rhodesiense, are responsible for human infection, which is usually fatal if left untreated. Infection with T.b. gambiense is responsible for chronic HAT in West - and Central-Africa, and characterized by low parasite numbers. In East-Africa, infection with T.b. rhodesiense leads to acute disease with relatively high parasite loads. Control of HAT relies on a combination of accurate diagnosis of cases, treatment of detected cases, and on control of the tsetse fly vector. No vaccine is available yet.
The immunopathology of HAT remains poorly understood and most of our understanding comes from experimental T.b. brucei infections in mice, which also serve as a model for vaccine development. In T.b. brucei infected mice, host control over disease mainly relies on the T-cell independent IgM antibody response [2]–[4]. However, mice T.b. brucei infection results in decreased B-cell development in the bone marrow [5]. Lymphopoiesis, which is taken over by the spleen, is in turn abrogated by apoptosis of transitional B-cells, permanent loss of splenic marginal zone B-cells (which are important for the early antibody response against T-cell independent antigens) and depletion of follicular B-cells (which normally develop into antibody producing plasma cells and memory B-cells). As a result of B-cell dysfunction, mice become susceptible to repetitive infections by previously encountered T.b. brucei variant antigenic types [6]. Furthermore, T.b. brucei infection equally affects the protective immune response towards unrelated pathogens, as observed in two experiments. First, in mice immunized against Trichinella spiralis, it was observed that upon subsequent infection with T.b. brucei and Trichinella spiralis, the effect of vaccination was lost in T.b. brucei infected mice only [7]. Similarly, in mice vaccinated against diphtheria, tetanus and Bordetella pertussis, the vaccine mediated protective effect was abrogated in mice that were infected with T.b. brucei prior to a Bordetella pertussis challenge, while vaccinated mice that had not been infected with T.b. brucei, remained protected upon challenge with Bordetella [6]. In vivo and in vitro correlates of cell-mediated immunity were observed to be depressed as well in rabbits infected with the African trypanosome T. congolense [8].
These results indicate that T.b. brucei infections can give rise to general memory B-cell destruction in animals, and point to the possibility that T. brucei infection may destruct memory B-cell and abrogate vaccine induced protection in humans as well. If confirmed, this would imply the need of revaccination of HAT patients after anti-trypanosomal therapy and development of a vaccine against the disease might be hard to achieve [9]. However, the relevance of the experimental models for humans remained unknown. Data about leukocyte phenotypes in HAT have remained limited to one study showing increased percentages of CD19+ B-cells and activated B-cells in blood of gambiense HAT patients, as well as a relative decrease in memory and effector CD8 T-cells [10]. Evidence for an increased occurrence of vaccine preventable diseases in cured HAT patients is missing, although such relationships may be easily overlooked due to weak surveillance systems in HAT endemic countries. The vaccine-induced memory response in HAT is difficult to assess. Firstly, one is limited to vaccines that provide life-long protection and have been administered to the majority of the population and prior to trypanosomiasis infection. Secondly, loss of protection cannot be tested by challenge with the pathogen. Moreover, HAT mainly occurs in remote rural settings where no standard laboratory infrastructure or electricity is available. Although in T.b. brucei animal models, immune depression may occur despite intact antibody levels [7], we selected antibody quantification as an initial, though suboptimal, approach to assess immunological memory, taking into account that so far, nothing is known for the human situation. We opted for iso-agglutinins, which are innate antibodies against A and B carbohydrate antigens on red blood cells [11], as well as for measles vaccine antibodies, as this vaccine is part of the standard vaccination programs [12].
We addressed the following questions: (i) does gambiense HAT eliminate peripheral blood memory B-cells; (ii) are peripheral blood memory T-cells affected in gambiense HAT (iii) does gambiense HAT influence iso-agglutinin levels and antibody levels against measles, and; (iv) are these effects reversible upon cure from gambiense HAT?
Materials and Methods
Ethics statement
Before enrolment into the study, written informed consent was obtained from adult participants. In the case of minors, an assent was asked for and parents/guardians provided written informed consent. Ethical clearance for the study was obtained from the institutional review board of ITM and the ethical committees of the University Hospital in Antwerp, Belgium (study registration number B30020108363) and of the Ministry of Health of the Democratic Republic of the Congo (DR Congo).
Study population and specimen collection
Trypanosma brucei gambiense infected HAT patients and non-HAT endemic controls were prospectively enrolled (T = 0 months) in the study in DR Congo, Bandundu Province between July and December 2010. Participants were identified during HAT screening activities of the dedicated HAT mobile team of Masi-Manimba, or included at the HAT treatment centres of Masi-Manimba and Bonga-Yasa. Inclusion criteria for HAT patients were the presence of trypanosomes in blood, lymph and/or cerebrospinal fluid (irrespective of disease stage), and being 12 years or older. Exclusion criteria were pregnancy, being previously treated for HAT and being moribund. For each HAT patient, a control was included, fulfilling the following criteria: same gender and age and being and being resident in the same or a neighbouring village. Inclusion criteria for controls were absence of clinical evidence for HAT (no swollen lymph nodes or neurological symptoms), absence of trypanosome specific antibodies in whole blood detected by card agglutination test for trypanosomiasis (CATT) [13]; no trypanosomes in blood detected by the mini anion exchange centrifugation technique [14] and being 12 years or older. Exclusion criteria were identical as for HAT patients.
At enrolment, a crude assessment of the general condition (normal, good, bad, pre-moribund or moribund) was made, based on the participant's ability to eat, walk and take care of himself independently. Participants were questioned for their vaccination history (measles, diphtheria-tetanus-whooping cough, polio, Bacillus Calmette-Guérin (BCG)) and presence of a BCG scar was verified.
Whole blood was collected by venipuncture and collected in 5 ml Cyto-Chex BCT blood collection tubes (Streck, Omaha, NE, USA) and shipped within one week to the Institute of Tropical Medicine (ITM) for phenotyping. From blood sampled on heparin, plasma was prepared that was snap frozen in liquid nitrogen and shipped to ITM where specimens were stored at −70°C until use. Blood taken on EDTA was preserved in an equal volume of GE buffer (6 M guanidine hydrochloride, 0.2 M EDTA, pH 8.0) at ambient temperature until DNA extraction. Thick and thin blood films were prepared and Giemsa stained for malaria diagnosis.
The participants ABO blood group was determined using Eldoncard 2511 (Eldon Biologicals, Gentofte, Denmark). The HIV status was determined using HIV 1/2 STAT-PAK Assay (Chembio, Medford, NY, USA) which, if positive, was followed by Uni-Gold HIV (Trinity Biotech, Wicklow, Ireland), and if positive, by HIV 1/2 Oraquick ADVANCE (Orasure Technologies, Bethlehem, PA, USA) [15]. In participants positive for all 3 serological tests, HIV infection was confirmed a posteriori using PCR, following a nested method in an algorithm of three different primer sets in pol, env and LTR region [16]. CATT was performed on whole blood taken on heparin, and if positive, the plasma end-titre was determined.
HAT was treated following the guidelines of the National HAT Control Program in DR Congo.
HAT patients were revisited six months after treatment, controls at the corresponding time point (T = 7 months). The participant's general condition was re-assessed. Blood taken on heparin and on Cyto-Chex BCT blood collection tubes was processed as described above. All participants were examined for absence of trypanosomes using the mini anion exchange centrifugation technique, and in controls, CATT was repeated.
Flow cytometry
Whole blood, collected in Cyto-Chex BCT blood collection tubes (Streck, Omaha, NE, USA), was used to study T and B cell subsets by flow cytometry.
B-cells subsets were analysed using mouse anti-human monoclonal antibodies anti-CD45 PerCP (leucocytes), anti-CD20-FITC (B cells), anti-human CD27-APC (IgG1) and anti-human IgM-PE (IgG1) and appropriate IgG1 isotype controls (BD Biosciences, Erembodegem, Belgium). These combinations were used to identify B-cells (CD20), naïve B-cells (CD20+CD27−), memory B-cells (CD20+CD27+), T independent B-cells (CD20+ IgM+) and T dependent B-cells (CD20+IgM−) [17].
T-cells subsets were stained with mouse anti-human monoclonal antibodies anti-CD45 PeCP, anti-CD3 (IgG1)-PE, anti-CD45RO-FITC (IgG2a), anti-CD27-APC (IgG1) and appropriate IgG1 isotype controls (BD Biosciences, Erembodegem, Belgium). These combinations were used to identify T-cells (CD3), naïve T-cells (CD3+CD45RO−CD27+), early effector/memory T-cells (CD3+CD45RO+CD27+) and late effector/memory T-cells (CD3+CD45RO+CD27−) [18]. For the staining of B-cells, 50 µl of fixed blood was pipetted in two test tubes. Blood in both tubes was washed twice with 2 ml of phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA) to remove serum. Subsequently, a cocktail of anti-CD20/anti-CD27/anti-IgM was added to one tube and anti-CD20/isotype-control cocktail to the other. After 30 minutes of incubation, red blood cell lysing solution was added for 10 minutes, cells were washed and analysed on the flow cytometer (FACSCalibur, BD Biosciences). For the staining of the T-cells the procedure was the same with exception of the washing step with PBS-BSA which was omitted. The cells subsets were analysed using FlowJo software (Tree Star, US).
Prior to the study, the antibody cocktails were tested using whole blood from 3 normal controls. Blood collected in Cyto-Chex BCT blood collection tubes was compared to fresh blood collected in EDTA tubes. Using the above described antibody cocktails, T - and B - cell subsets could be measured in blood collected in Cyto-Chex BCT blood collection stored for at least 14 days.
Determination of IgM and IgG antibody titres against red blood cells (iso-agglutinin end-titers)
Screening for irregular anti - erythrocyte antibodies (antibodies causing agglutination but that are not A and B red blood cell carbohydrate antigen specific) was performed with ID-Diacell I–II–III (Bio-Rad, Cressier, Switzerland) using undiluted plasma. For IgG, 25 µl of plasma and 50 µl of ID-Diacell I, ID-Diacell II or ID-Diacell III cell suspension were incubated for 15 minutes at 37°C on Coombs anti-IgG ID-cards (Bio-Rad, Cressier, Switzerland). For IgM, 25 µl of plasma and 50 µl of each cell suspension were incubated for 15 minutes at 20°C on ID-cards NaCl, enzymetest and cold agglutinins (Bio-Rad, Cressier, Switzerland). After incubation, gel cards were centrifuged (ID-centrifuge, Bio-Rad, Cressier, Switzerland) for 10 minutes and the agglutination reaction was scored. Samples positive for irregular anti-erythrocyte antibodies, implying a risk for false positive iso-agglutinin reactions, were excluded from further analysis.
For assessment of antibody titres against A and B red blood cell carbohydrate antigens, plasma samples of patients with blood group O were tested with A1 and B cells (ID-Diacell ABO, Bio-Rad, Cressier, Switzerland), those from blood group A or B were tested with respectively B or A1 cells only, those from blood group AB were not tested. Two-fold serial dilution series of plasma were prepared in phosphate buffered saline (Yvsolab, Turnhout, Belgium). For IgG iso-agglutinin, 25 µl of diluted plasma and 50 µl of ID-Diacell A1 and/or B cell suspension were incubated for 15 minutes at 37°C on Coombs anti-IgG ID-cards (Bio-Rad, Cressier, Switzerland). For IgM iso-agglutinin, 50 µl of diluted plasma and 50 µl of ID-Diacell A1 and/or B cell suspension were incubated for 15 minutes at 20°C on ID-cards NaCl, enzymetest and cold agglutinins (Bio-Rad, Cressier, Switzerland). After incubation, gel cards were centrifuged (ID-centrifuge, Bio-Rad, Cressier, Switzerland) for 10 minutes and the agglutination reaction was scored. The end-titre was the highest plasma dilution still causing an agglutination reaction.
Measurement of measles antibodies
Quantitative and qualitative determination of specific IgG antibodies to measles virus was performed using Enzygnost anti-measles Virus/IgG ELISA (Siemens, Marburg, Germany), following the manufacturer instructions for the BEP III system (Siemens, Marburg, Germany). Plasma of HAT patients at inclusion and 6 months post-treatment, and corresponding plasma from the respective control were analysed in the same ELISA plate. Based on the reference included in the kit, results were expressed as mIU/ml. Samples with OD<0.1 were negative, samples with OD>0.2 were positive, samples in the grey zone with 0.1<OD<0.2 were retested. A measles antibody level of ≥200 mIU/ml is assumed to provide protection against infection in a healthy population [12], [19].
Statistical analysis
For analysis, only results for which the corresponding matched sample result at the same time point was available were taken into account. Comparisons of quantitative results between controls and HAT patients and between 0 and 7 months were performed with the Wilcoxon Signed Rank Test (SigmaPlot 11). Comparisons of quantitative results between first and second stage patients were performed with the Mann-Whitney Rank Sum test. Data are presented as medians with interquartile range (IQR). Differences in proportions between controls and HAT patients were assessed with McNemar Chi square (STATA 10.0). A p-value of ≤0.05 was considered as significant.
Results
Study population
In total, 117 controls and 117 gambiense HAT patients were included. Median age was 28 years, 45% of the participants were male. Respectively 9.9% of participants suffered from malaria (13 HAT patients and 9 controls positive/223 thick blood films) and 1 control had HIV. Overall vaccination coverage reported by the study population ranged between 88.4% (183/207) for polio and 100% for BCG, and 80.6% (183/227) of participants had a BCG scar. The general condition for all study participants was judged good to normal. Among the participants, 51.3% had blood group O, 30.2% A, 15.5% B and 3.0% AB. For none of the above parameters, there were significant differences in proportions between HAT patients and controls, except for polio vaccination, reported by 83.3% of controls versus 93.3% of HAT patients (p = 0.002).
Among the HAT patients, 97.4% (114/117) were positive in CATT on whole blood (median plasma titre 16, IQR 8–16), 77.4% (48/62) had trypanosomes in the lymph node fluid after successful lymph node puncture, and respectively 43.4% (36/83) and 89.3% (100/112) had trypanosomes in blood detected by the micro-haematocrit centrifugation technique or in the mini-anion exchange centrifugation technique. Cerebrospinal fluid median white blood cell counts were 5/µl (IQR 2–43) and trypanosomes were observed during the cell count in 15.7% (18/115). About half (56/116) of the included HAT patients were in the meningo-encephalitic disease stage (>5 white blood cells/µl or trypanosomes in cerebrospinal fluid).
Respectively 111/117 HAT patients and 105/117 controls were revisited after a median of 211 (IQR 197–241 days, T = 7 months) and 204 days (IQR 178–246) respectively. At revisit, all participants were in good general condition. Although one control had become CATT positive, no trypanosomes were detected in any of the study participants.
Memory B-cells during and after HAT
An overview of the B-cell phenotyping results in HAT patients and controls is presented in Table 1, and an example of a dot plot of CD27 and IgM expression on B-cell subsets (CD20+), in a HAT patient and a control is shown in Figure 1. The percentage of CD20+ B-cells in HAT patients was significantly higher than in controls (median 1.5 times higher, p<0.001). Although the percentage of CD20+ cells had decreased 6 months after treatment of HAT, it still remained significantly higher than in controls (p<0.001). Within the CD20+ subset, the percentages of CD27+ memory B-cells and IgM+ B-cells were significantly higher in HAT than in controls (increases of the median of respectively 2.3 and 3.6 times, p<0.001). After HAT treatment the percentage of CD27+ cells within the B-cell (CD20+) subset still remained significantly higher than in controls (p = 0.001), while no significant difference could be observed anymore for the percentage of IgM+ B-cells (p = 0.7). The most striking change within the B-cells subset was the more than 6-fold increase of the percentage CD27+IgM+ cells (Q2 in Figure 1) in HAT patients compared to controls (p<0.001). After treatment, this subset returned to normal percentages. HAT was associated with only minor differences in the CD27+IgM − subset (Q1 in Figure 1, p = 0.004) of B cells. The relative decrease of naive (CD27−) B-cells in HAT was mainly due to a decrease of CD27−IgM − cells (Q4 in Figure 1, p<0.001) while the percentage of CD27−IgM+ cells within the B-cell subset had increased (Q3 in Figure 1, p<0.001). For none of the B-cell phenotypes studied, significant differences between stage 1 and stage 2 HAT patients were observed (0.2<p<0.9).
Fig. 1. Flow cytometry dot plot of the CD20+ B-cell population in HAT and in a control. 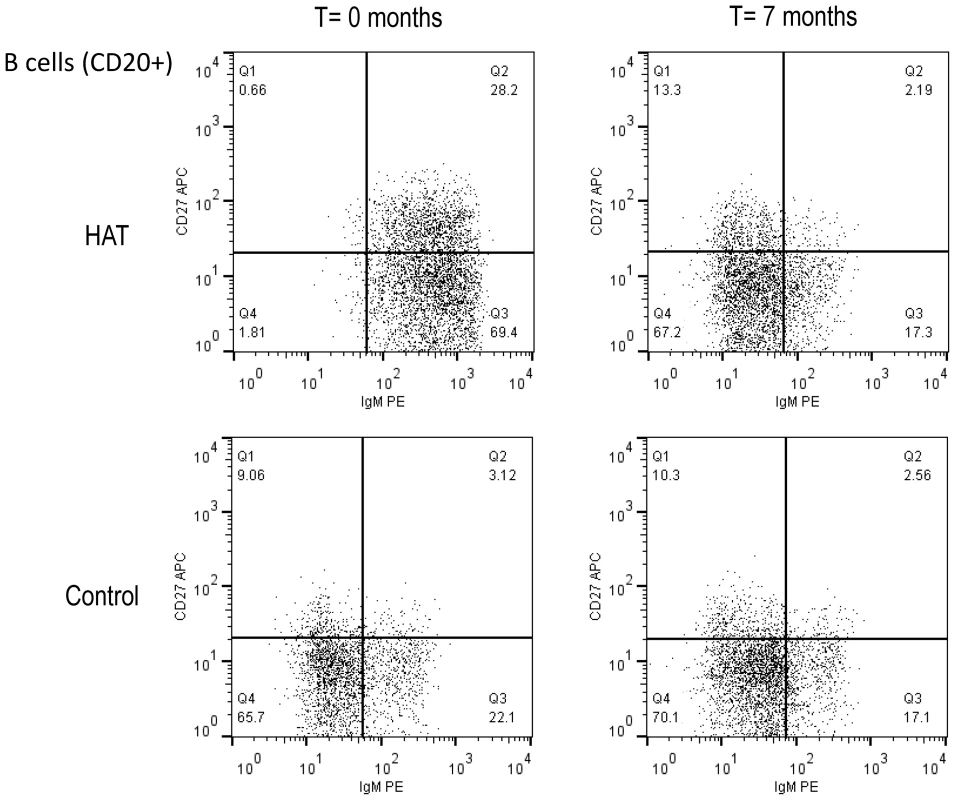
B-cell subsets were based on the CD27 and IgM cell surface markers. A HAT patient before and after treatment (T = 0 and 7 months), and a healthy control subject at the same time points are shown. Cut-offs for considering a cell surface marker positive or negative were based on isotype controls and are shown as solid lines, and subdivide the graph into 4 quadrants (Q1–Q4). B-cell subsets in each quadrant are expressed as percentages of CD20+ B-cells. Tab. 1. Peripheral blood B-cell subsets in HAT and in controls. 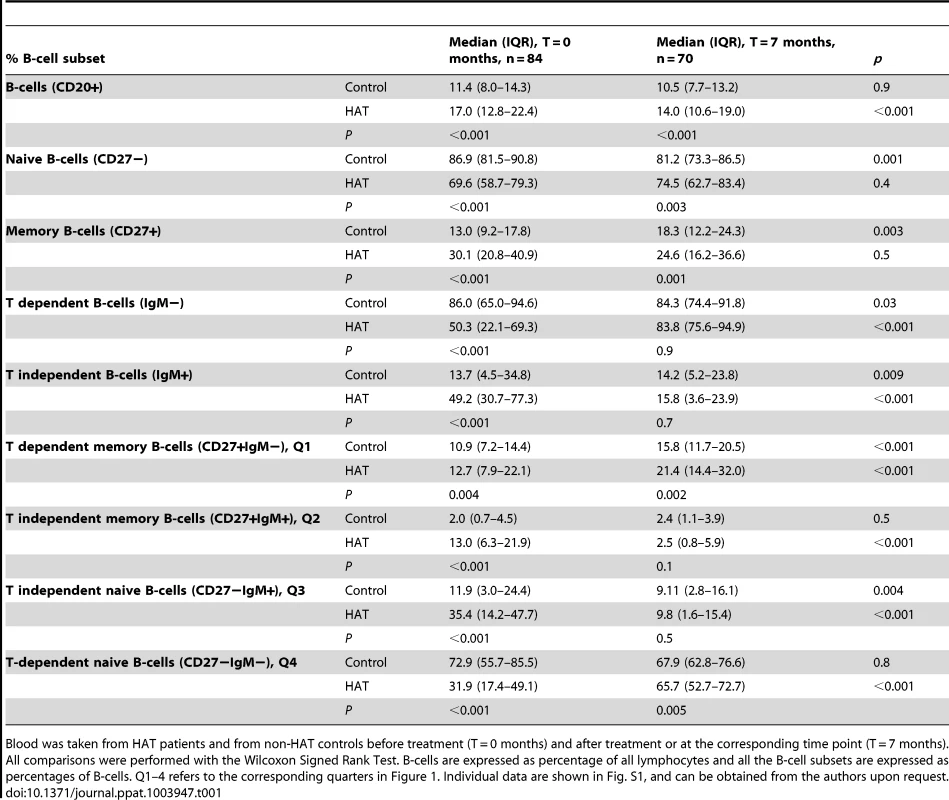
Blood was taken from HAT patients and from non-HAT controls before treatment (T = 0 months) and after treatment or at the corresponding time point (T = 7 months). All comparisons were performed with the Wilcoxon Signed Rank Test. B-cells are expressed as percentage of all lymphocytes and all the B-cell subsets are expressed as percentages of B-cells. Q1–4 refers to the corresponding quarters in Figure 1. Individual data are shown in Fig. S1, and can be obtained from the authors upon request. Memory T-cells during and after HAT
A summary of the T-cell phenotypes is presented in Table 2, and an example of a dot plot of the CD27 and CD45RO expression on T-cell subsets (CD3+), in a HAT patient and a healthy control subject is shown in Figure 2.
Fig. 2. Flow cytometry dot plot of the CD3+ T-cell population in HAT and in a control. 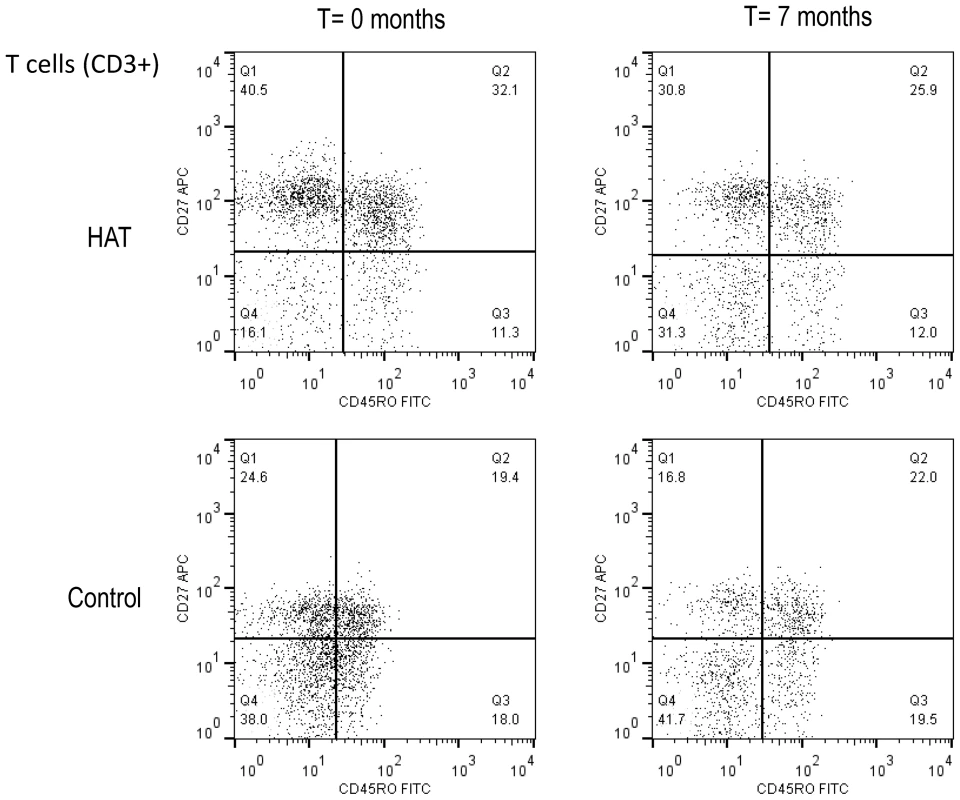
T-cell subsets were based on the CD27 and CD45RO cell surface markers. A HAT patient before and after treatment (T = 0 and 7 months), and a control at the same time points are shown. Cut-offs for considering a cell surface marker positive or negative are shown as solid lines, and subdivide the graph into 4 quadrants (Q1–Q4). T-cell subsets in each quadrant are expressed as percentages of CD3+ T cells. Tab. 2. Peripheral blood T-cell subsets in HAT and in controls. 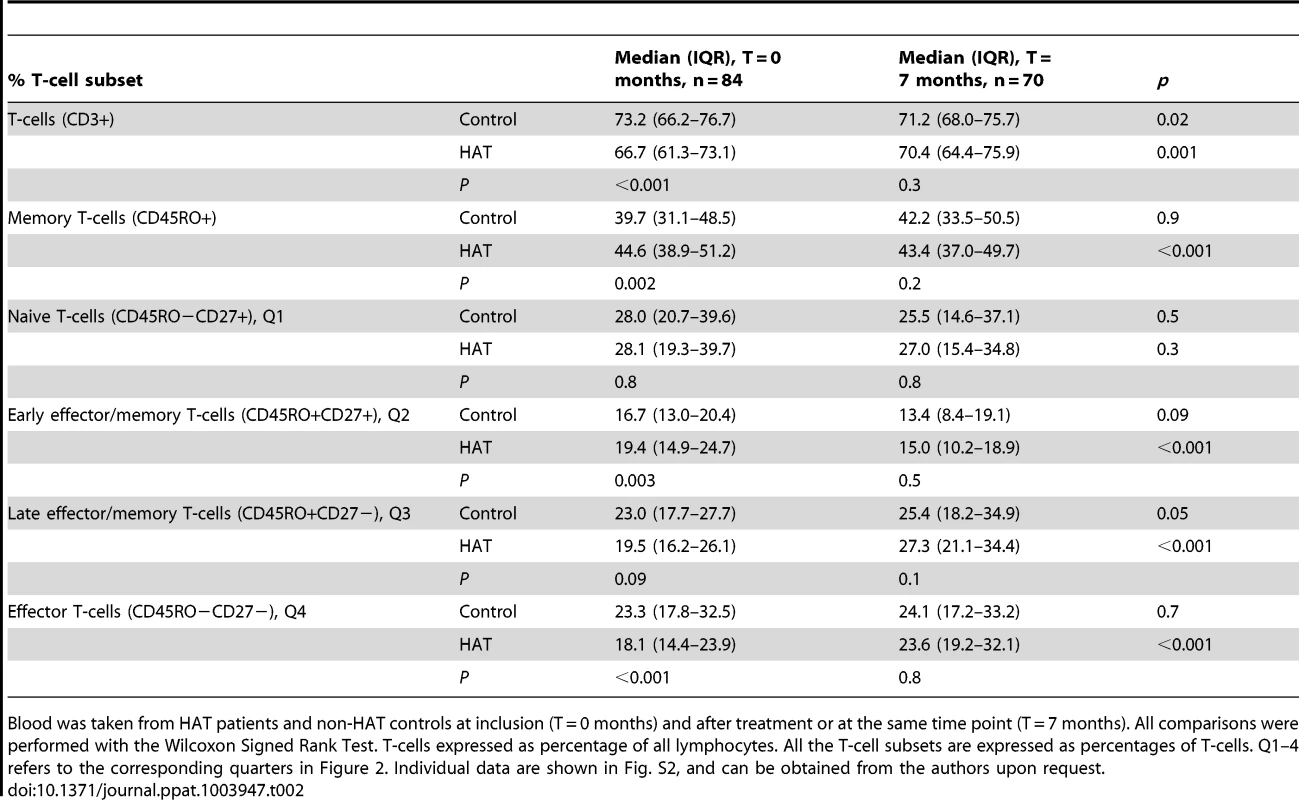
Blood was taken from HAT patients and non-HAT controls at inclusion (T = 0 months) and after treatment or at the same time point (T = 7 months). All comparisons were performed with the Wilcoxon Signed Rank Test. T-cells expressed as percentage of all lymphocytes. All the T-cell subsets are expressed as percentages of T-cells. Q1–4 refers to the corresponding quarters in Figure 2. Individual data are shown in Fig. S2, and can be obtained from the authors upon request. The percentage of CD3+ T-cells was significantly lower in HAT than in controls, and returned to normal 6 months after treatment. Within the T-cells subset, memory T-cells were significantly increased (CD45RO+, p = 0.002), which was due to a relative increase in early effector/memory (CD45RO+CD27+) T-cells in HAT (1.2 fold increase of the median, p = 0.003, Figure 2 Q2). After treatment, the observed differences in memory T-cell subsets between HAT and controls disappeared. No difference was observed in percentage of naïve (CD45RO − CD27+) T-cells between HAT patients and controls (p = 0.8) while the percentage of late effector (CD45RO−CD27−) T-cells was significantly lower in HAT than in controls (p<0.001, Figure 2 Q4), but normalized after treatment.
No differences were observed in function of the disease stage for any of the measured T-cell subsets (p>0.08).
IgG and IgM iso-agglutinin end-titres
Screening for irregular anti-erythrocyte IgG with ID-Diacell I–II–III cells revealed respectively 9/116 and 12/104 reactive controls at T = 0 months and T = 7 months (9 at both time points), and 7/116 and 5/109 reactive HAT patients (4 at both time points). At T = 0 months or T = 7 months, respectively, 7/116 and 4/104 controls (4 at both time points), and 6/116 and 5/109 HAT patients (4 at both time points) reacted for irregular anti-erythrocyte IgM. At inclusion, there was no difference in anti-A or anti-B IgG end titers between controls and HAT patients (table 3). For IgM, at time of inclusion median anti-A1 and anti-B iso-agglutinin end-titres were significantly lower in HAT patients than in controls (p<0.004). After treatment, at T = 7 months, the anti-A1 IgM iso-agglutinin end-titre had increased significantly in HAT patients (p<0.01), but remained lower for anti-B IgM.
Tab. 3. Iso-agglutinin end-titres in HAT and in controls. 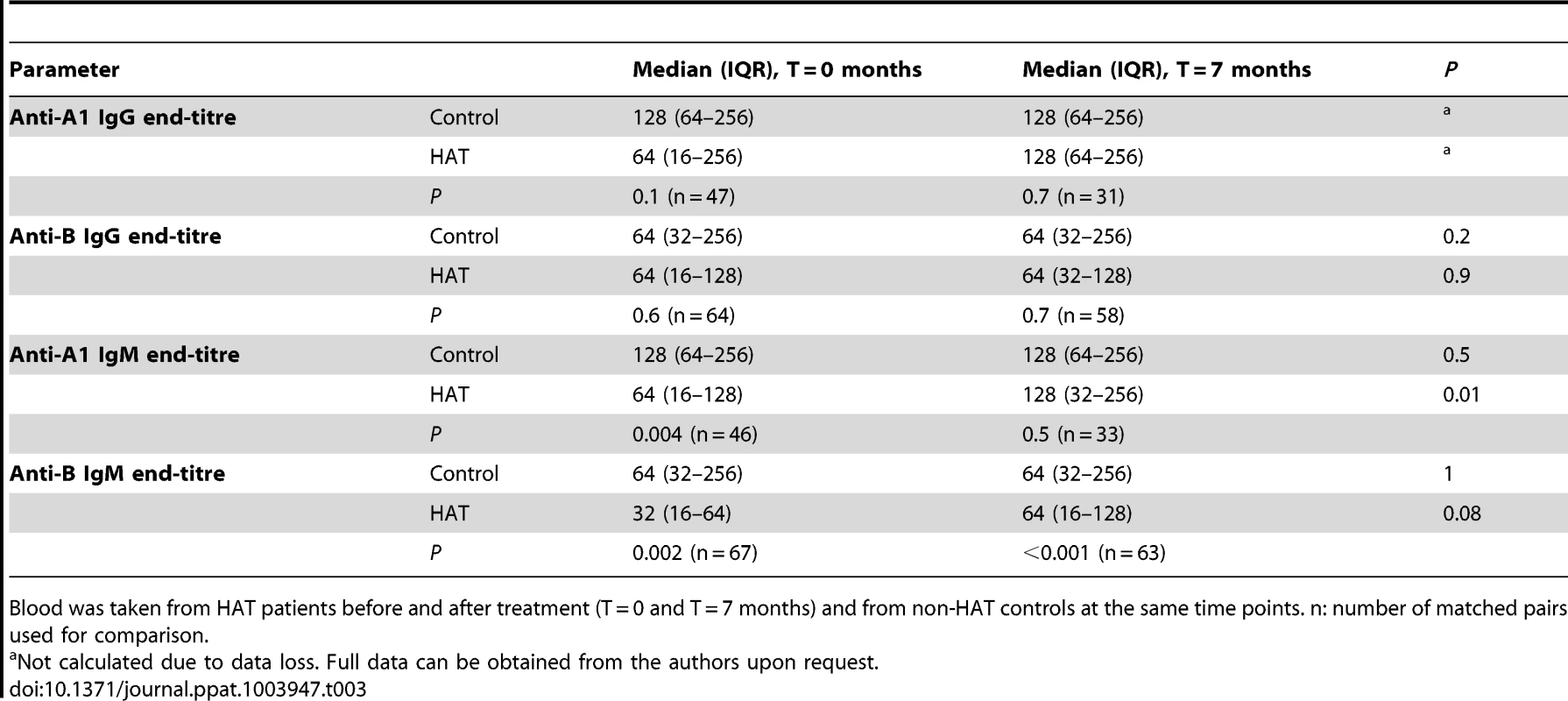
Blood was taken from HAT patients before and after treatment (T = 0 and T = 7 months) and from non-HAT controls at the same time points. n: number of matched pairs used for comparison. There was no difference in iso-agglutinin end-titres between stage 1 and stage 2 HAT patients (p values>0.1), except for anti-B IgM which was one titer lower in stage 2 (p = 0.05).
Antibody levels against measles
Measles antibody concentrations in HAT patients at inclusion and after treatment and in controls at corresponding time points are summarized in Figure 3. At inclusion, the median antibody concentration in HAT patients (1500 mIU/ml, IQR 643–3300) was significantly lower than in controls (2250 mIU/ml, IQR 940–4675). Seven months later, the antibody concentration in the treated HAT patients (1700 mIU/ml, IQR 790–4300) remained significantly lower than in controls (2600 mIU/ml, IQR 1000–5500) although in both groups, the antibody level had increased significantly compared to inclusion (p<0.001 and p = 0.006 respectively). There was no difference in measles antibody concentration between stage 1 and stage 2 HAT (p = 0.7).
Fig. 3. Box plot of measles antibody concentrations in HAT and controls. 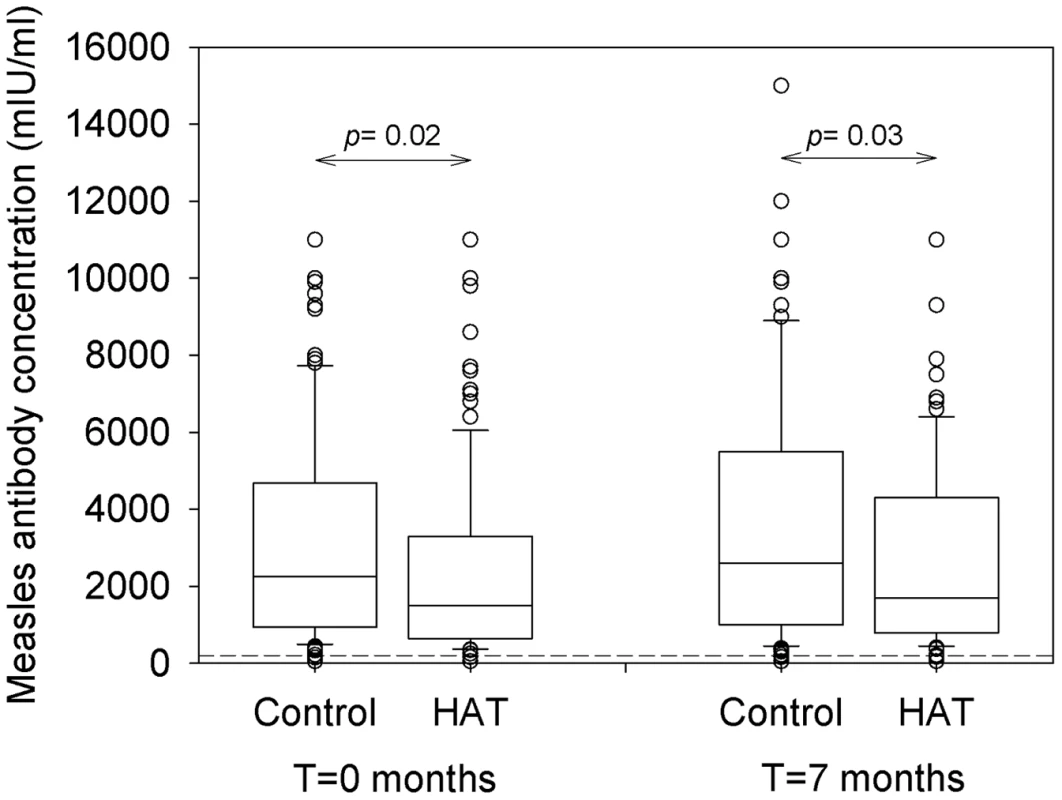
Blood was taken from HAT patients and non-HAT controls before treatment (T = 0 months, n = 116), and after treatment or at the same time point (T = 7 months, n = 99). The cut-off value for protective immunity of ≥200 mIU/ml is indicated by the dashed line. Full data can be obtained from the authors upon request. A measles antibody level superior to the cut-off assumed to provide protection against infection was present in 94.8% (110/116) of HAT patients and in 98.3% (114/116) of controls, at inclusion and 7 months later. There was no difference in proportions of HAT patients and controls exceeding this cut-off (p = 0.3).
No relationship between high measles antibody levels and self-reported vaccination against measles, polio, diphtheria-tetanus-pertussis, BCG or presence of a BCG scar could be observed (p = 0.6–1).
Discussion
Our results suggest that the issue of B-cell dysfunction that troubles mouse models for trypanosomiasis, might not be that severe in human African trypanosomiasis patients infected with T.b. gambiense. In gambiense HAT patients compared to controls, significantly higher percentages of memory B - and memory T-cells were present in peripheral blood. After treatment, the percentage of memory T-cells normalized and the percentage of memory B-cells did not yet normalize. Iso-agglutinin IgM end-titres were slightly lower in gambiense HAT, and normalized only partially after treatment. Although anti-measles antibody levels were, and remained, lower in gambiense HAT patients than in controls, no significant difference could be observed in the number of individuals with levels above the international cut-off for protection.
Memory cell populations in experimental T.b. brucei infection have exclusively been studied in bone marrow and spleen [5]. For T. vivax experimental mice infections, peripheral blood data are available as well [20]. In the human host, only peripheral blood is readily accessible. Until now, data on peripheral blood lymphocyte subsets in HAT are rare, due to the important logistic challenges related to conducting research in settings like DR Congo. The observed relative B-cell increase is consistent with previous findings [10] and in line with polyclonal B-cell activation and proliferation of cells of the B lymphoid series previously described for HAT [21]. The upregulation of Fas (CD95) expression in gambiense HAT, measured by Boda et al., led these authors to suggest a poor conversion of B-cells into memory B-cells [10]. In our study, we observed a relative increase of CD27+IgM+ B-cells which are defined as T independent memory B-cells [17] in HAT. In T. vivax experimental mice infections, the fall in number of B-cells in the lymphoid organs is similar to experimental T.b. brucei infections. In peripheral blood it is accompanied by an increase in the number of transitional IgM+IgD − B-cells and switched IgM−IgD − plasma/memory cells and by a decrease in naive B-lymphocytes [20]. Although the marker combinations used to identify B-cells in these experimental T. vivax studies were different from ours, the results for peripheral blood are similar, if we assume that the memory B-cells defined by CD27+ in HAT, are similar to the IgD − population in T. vivax infected mice [17].
We confirm the moderate relative T-cell decrease in gambiense HAT observed previously by Boda et al., associated to the relative expansion of B-cells. The present observation of a relative increase in early memory T-cells (CD45RO+CD27+) seems to corroborate earlier findings of larger numbers of CD4+CD45RA−CD62L+ cells in HAT with CD8+CD45RA−CD62L+ cells remaining constant [10]. As previously suggested [10], there were no differences in lymphocyte subsets according to the disease stage.
HIV also causes a significant increase in the memory (CD45RA−CD45RO+) phenotype CD8 subset [22] and reduces the CD27+ memory B-cell population [23]. The HIV prevalence in our study population was low and is not expected to affect the overall results. Malaria, which is associated with B - and T-cell exhaustion and an increase in an atypical CD19+CD27−CD21−CD10 − memory B-cell population [24], is not expected to account for differences between the control and HAT population since the frequency of occurrence of trophozoites in blood was similar in both groups. However, malaria, or other infections, might account for variation in some cell phenotypes in time, as was observed in the control group. This underlines the importance to sample controls at similar time points as HAT patients and to perform a matched statistical analysis, to maximally eliminate external variation.
The loss of the host's capacity to recall vaccine-induced memory responses, as has been described for laboratory animals [6], [7], can in humans, for ethical reasons, not be tested by challenge with a pathogen. Therefore, the Multitest cell-mediated immunity (Pasteur-Mérieux, Lyon, France), an intradermal skin test to measure delayed hypersensitivity as a marker for T-lymphocyte response, was considered but this test was no longer available anymore at time of the project. Neither was it feasible to set-up of facilities for cell culture or ELISPOT. We therefore had to rely on surrogate markers and opted for the quantification of iso-agglutinins and measles antibodies.
Natural IgM antibodies against A and B carbohydrate antigens are T-cell independent, while a T-cell dependent antibody response results in higher affinity IgG1 and IgG3 antibodies [11]. In the presence of an intact immune system, iso-agglutinins to the missing A or B red blood cell carbohydrate antigens are always found, even if there has been no exposure to red blood cells carrying these antigens. These antibodies were therefore used to asses T-cell independent and T-cell dependent humoral immunity. The lower IgM iso-agglutinin titers observed in HAT patients, might indeed point to a moderate effect of gambiense HAT on the T-independent antibody response [6], but seems reversible upon treatment.
Measles were selected for antibody quantification for the following reasons. A high proportion of the population is expected to have antibodies against measles since the measles vaccine is part of the standard vaccination programs [12]. Half-life of measles IgG antibodies has been estimated at around 3000 years so they should be measurable in all subjects that have been infected or were successfully immunized [25]. Moreover, in healthy individuals, the absolute level of antibodies needed to fully protect against infection is known, as well as the concentration below which no protection is obtained anymore [26], [27]. Vaccine coverage in the last 33 years in DR Congo has been estimated by WHO-UNICEF as 21–95% for BCG and 17–90% for the measles vaccine respectively [28]. Presence of a BCG scar in 80.6% of study participants indicated rather high vaccine coverage, and measles antibody levels above the cut-off were present in 96.6%. Measles antibody levels were comparable to levels observed in pregnant women in Belgium and in a Swedish volunteer group, in which concentrations were measured with the same ELISA kit [29], [30], or in an adult population in Addis-Abeba [31]. The increase in measles antibody concentrations in HAT patients and controls 7 months after inclusion, is unlikely due to a technical bias since samples taken at both time points were analyzed in the same ELISA plate. The rise might have been caused by a natural exposure to measles, as measles outbreaks regularly occur in DR Congo, also during the study period [32]. Although existing data are contradictory, presence of antibodies does not necessarily reflect presence of antibody secreting memory B-cells, as continuous antibody secretion might be due to long-lived plasma cells rather than on-going activation of memory B-cells [30]. Measles vaccine induces both humoral and cellular immune responses comparable to those following natural infection [33]. We withheld from revaccination, since live virus measles vaccination is not recommended for immune-suppressed patients [27], a condition to be expected during HAT, and since revaccination was judged not to be in the patient's best interest, even after treatment for HAT.
Overall, our results do not exclude an impairment of humoral and cellular immunity during gambiense HAT. Indeed, when given during gambiense HAT infection, a reduced response to typhoid vaccine has been observed, as well as diminished reactions to skin test antigens [34]. Similar observations have been made in domestic animals, where the antibody response to and/or efficacy of vaccination against e.g. contagious bovine pleuropneumonia [35], foot and mouth disease [36], swine fever [37], antrax spore [38] and Brucella abortus [39] were affected when given during infection with various animal trypanosomes. Moreover, in the immunized T.b. brucei - Trichinella spiralis co-infection experimental model, the anti-Trichinella IgG1 response was not affected [7] although protection was partially lost. Due to polyclonal B-cell activation, characteristic for trypanosomiasis infection, specific functional antibodies may be replaced by non-protective, low affinity, cross-reactive antibodies [40]. Although for measles, antibody concentrations remained above the cut-off, we cannot exclude that they have become unfunctional in HAT and their protective capacity may have been lost. The lack of a functionality test is an important difference with previously published experimental mice studies [6], [7] and represents the main limitation of the actual study. It might therefore be worth to further assess the protective capacity of the measles antibodies against infection, e.g. using a functional assay such as the plaque reduction neutralization test [41]. However, none of the study participants mentioned a measles episode while being questioned for their vaccination history, although as mentioned above, natural exposure might have occurred. Of interest, the agglutinating capacity of the iso-agglutinin antibodies was only moderately affected in our assay.
As discussed above, other limitations inherent to our study are mainly related to research in humans instead of in laboratory animals, and to studying a disease that typically occurs in rural Africa, far from high-tech environments. In this context, blood specimens were collected on a blood stabilizer, intended to preserve peripheral blood samples' qualitative and quantitative leukocyte subset characteristics and allowing collection and storage of blood specimens for immunophenotyping by flow cytometry. Even using this stabiliser, preliminary experiments demonstrated that some lymphocyte subset cell markers (e.g. the cell surface marker CCR7, which we had originally selected to be used in combination with CD27 to better identify T memory subsets [18] were not optimally preserved, thus antibody cocktails had to be adapted accordingly. As we did not perform absolute counting of lymphocyte numbers, the observed changes in lymphocyte sub-populations are relative. For the iso-agglutinins, the participants blood group has to be taken into account, but the blood group was not used as a matching criterion at time of collection. In the settings we were working, in practice, it would have been difficult to identify a matched control for the patients with rarer B and AB blood groups. Data for HAT patients and controls that had different blood groups and were not tested against the same red blood cell carbohydrate antigen, were therefore lost. However, similarly lower IgM end-titers in HAT patients were also observed when statistical analysis was performed without matching the results for HAT patients and their corresponding controls (data not shown).
Overall, our results in gambiense HAT patients do not suggest trypanosomiasis associated massive memory cell destruction, or loss of antibody levels, although the antibody's protective capacity remains to be confirmed. So far there have never been epidemiological signals/reports that HAT patients, before or after treatment, were at increased risk of having vaccine-preventable diseases (measles or others) compared to the rest of the population. One should however acknowledge that epidemiological surveillance is generally weak in rural Africa and that such occurrences might have been missed. If some degree of immunity loss may exist in HAT patients infected with T.b gambiense, it does not seem of clinical relevance. At least for measles, our data indicate that antibody levels remain intact. Some open questions remain. Functionality of measles antibodies should be confirmed to completely ensure that revaccination after gambiense HAT, would not be necessary. It could also be interesting to assess activity of other vaccine-induced antibodies, as the decay of measles antibody concentrations is extremely slow and since we cannot exclude that other vaccines might depend more on memory cell dependent antibody production. Differences in immune-suppression and B-cell apoptosis observed between gambiense HAT and experimental infections may be linked to the differences in parasitemia between T.b. gambiense HAT and experimental infections [5], [34]. As previously suggested [5], [34], it might therefore be worth to perform similar investigations in acute T.b. rhodesiense HAT, which is characterized by higher parasitemia, and for which no data on peripheral blood memory T - and B-cells or on acquired immunity are available.
Supporting Information
Zdroje
1. SimarroPP, CecchiG, FrancoJR, PaoneM, DiarraA, et al. (2012) Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis 6: e1859.
2. RadwanskaM, MagezS, StijlemansB, GeuskensM, PaysE (2000) Comparative analysis of antibody responses against HSP60, invariant surface glycoprotein 70, and variant surface glycoprotein reveals a complex antigen-specifioc pattern of immunoglobulin isotype switching during infection by Trypanosoma brucei. Infect Immun 68 : 848–860.
3. ReinitzDM, MansfieldJM (1990) T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect Immun 58 : 2337–2342.
4. BaralTN, De BaetselierP, BrombacherF, MagezS (2007) Control of Trypanosoma evansi infection is IgM mediated and does not require a Type I inflammatory response. J Infect Dis 195 : 1513–1520.
5. BockstalV, GuirnaldaP, CaljonG, GoenkaR, TelferJC, et al. (2011) T. brucei infection reduces B lymphopoiesis in bone marrow and truncates compensatory splenic lymphopoiesis through transitional B-cell apoptosis. PLoS Pathog 7: e1002089.
6. RadwanskaM, GuirnaldaP, De TrezC, RyffelB, BlackS, et al. (2008) Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog 4: e1000078.
7. OnahDN, WakelinD (2000) Murine model study of the practical implication of trypanosome-induced immunosuppression in vaccine-based disease control programmes. Vet Immunol Immunopathol 74 : 271–284.
8. MansfieldJM, WallaceJH (1974) Suppression of cell-mediated immunity in experimental African trypanosomiasis. Infect Immun 10 : 335–339.
9. La GrecaF, MagezS (2011) Vaccination against trypanosomiasis: can it be done or is the trypanosome truly the ultimate immune destroyer and escape artist? Hum Vaccin 1225–1233 18203 [pii];10.4161/hv.7.11.18203 [doi].
10. BodaC, CourtiouxB, RoquesP, PervieuxL, VatungaG, et al. (2009) Immunophenotypic lymphocyte profiles in human African trypanosomiasis. Public Library of Science One 4: e6184.
11. OuwehandWH, WallingtonTB (2004) Adaptive immunity and transfusion. Vox Sang 87: S35–S38.
12. World Health Organization (1995) Immunization policy. WHOGPV/GEN/95.03 Rev.1
13. MagnusE, Van MeirvenneN, VervoortT, Le RayD, WéryM (1978) Use of freeze-dried trypanosomes in the indirect fluorescent antibody test for the serodiagnosis of sleeping sickness. Ann Soc Belg Méd Trop 58 : 103–109.
14. BüscherP, Mumba NgoyiD, KaboréJ, LejonV, RobaysJ, et al. (2009) Improved models of mini anion exchange centrifugation technique (mAECT) and modified single centrifugation (MSC) for sleeping sickness diagnosis and staging. PLoS Negl Trop Dis 3: e471.
15. LejonV, Mumba NgoyiD, IlungaM, BeelaertG, MaesI, et al. (2010) Low specificities of HIV diagnostic tests caused by Trypanosoma brucei gambiense sleeping sickness. J Clin Microbiol 48 : 2836–2839.
16. VandammeAM, FransenK, DebaisieuxL, MarissensD, SprecherS, et al. (1995) Standardization of primers and an algorithm for HIV-1 diagnostic PCR evaluated in patients harbouring strains of diverse geographical origin. J Virol Methods 51 : 305–316.
17. TangyeSG, GoodMF (2007) Human IgM+ CD27+ B cells: Memory B cells or “Memory” B cells? J Immunol 179 : 13–19.
18. AppayV, van LierRA, SallustoF, RoedererM (2008) Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73 : 975–983.
19. DavidkinI, JokinenS, BromanM, LeinikkiP, PeltolaH (2008) Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 197 : 950–956.
20. Blom-PotarMC, ChamondN, CossonA, JouvionG, Droin-BergereS, et al. (2010) Trypanosoma vivax infections: pushing ahead with mouse models for the study of Nagana. II. Immunobiological dysfunctions. PLoS Negl Trop Dis 4: e0793 10.1371/journal.pntd.0000793 [doi].
21. GreenwoodBM, WhittleHC (1980) The pathogenesis of sleeping sickness. Trans R Soc Trop Med Hyg 74 : 716–725.
22. PrinceHE, JensenER (1991) Three-color cytofluorometric analysis of CD8 cell subsets in HIV-1 infection. J Acquir Immune Defic Syndr 4 : 1227–1232.
23. De MilitoA, MorchC, SonnerborgA, ChiodiF (2001) Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 15 : 957–964.
24. IllingworthJ, ButlerNS, RoetynckS, MwacharoJ, PierceSK, et al. (2013) Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol 190 : 1038–1047.
25. AmannaIJ, CarlsonNE, SlifkaMK (2007) Duration of humoral immunity to comon viral and vaccine antigens. N Engl J Med 357 : 1903–1915.
26. PlotkinSA (2008) Correlates of vaccine-induced immunity. Clin Infect Dis 47 : 401–409.
27. Strebel PM, Papania MJ, Dayan GH, Halsey NA (2008) Measles vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia: Saunders Elsevier. pp. 353–398.
28. World Health Organization (2013) WHO vaccine-preventable diseases: monitoring system. 2013 global summary. http://apps who int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=COD.
29. LeuridanE, HensN, HutseV, IevenM, AertsM, et al. (2010) Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ 340: c1626.
30. KakoulidouM, Ingelman-SundbergH, JohanssonE, CagigiA, FaroukSE, et al. (2013) Kinetics of antibody and memory B cell responses after MMR immunization in children and young adults. Vaccine 31 : 711–717 S0264-410X(12)01624-6 [pii];10.1016/j.vaccine.2012.11.031 [doi].
31. EnquselassieF, AyeleW, DejeneA, MesseleT, AbebeA, et al. (2003) Seroepidemiology of measles in Addis Ababa, Ethiopia: implications for control through vaccination. Epidemiol Infect 130 : 507–519.
32. GroutL, MinettiA, HurtadoN, FrancoisG, FermonF, et al. (2013) Measles in Democratic Republic of Congo: an outbreak description from Katanga, 2010–2011. BMC Infect Dis 13 : 232.
33. World Health Organization (2009) Measles vaccines: WHO position paper. Wkly Epidemiol Rec 84 : 349–360.
34. GreenwoodBM, WhittleHC, MolyneuxDH (1973) Immunosuppression in Gambian trypanosomiasis. Trans R Soc Trop Med Hyg 67 : 846–850.
35. IlemobadeAA, AdegboyeDS, OnoviranO, ChimaJC (1982) Immunodepressive effects of trypanosomal infection in cattle immunized against contagious bovine pleuropneumonia. Parasite Immunol 4 : 273–282.
36. SharpeRT, LangleyAM, MowatGN, MacAskillJA, HolmesPH (1982) Immunosuppression in bovine trypanosomiasis: response of cattle infected with Trypanosoma congolense to foot-and-mouth disease vaccination and subsequent live virus challenge. Res Vet Sci 32 : 289–293.
37. HollandWG, DoTT, HuongNT, DungNT, ThanhNG, et al. (2003) The effect of Trypanosoma evansi infection on pig performance and vaccination against classical swine fever. Vet Parasitol 13 : 115–123.
38. MwangiDM, MunyuaWK, NyagaPN (1990) Immunosuppression in caprine trypanosomiasis: effects of acute Trypanosoma congolense infection on antibody response to anthrax spore vaccine. Trop Anim Health Prod 22 : 95–100.
39. RurangirwaFR, MusokeAJ, NantulyaVM, TabelH (1983) Immune depression in bovine trypanosomiasis: effects of acute and chronic Trypanosoma congolense and chronic Trypanosoma vivax infections on antibody response to Brucella abortus vaccine. Parasite Immunol 5 : 267–276.
40. LambertPH, BerneyM, KazyumbaG (1981) Immune complexes in serum and in cerebrospinal fluid in African trypanosomiasis. Correlation with polyclonal B cell activation and with intracerebral immunoglobulin synthesis. J Clin Invest 67 : 77–85.
41. CohenBJ, AudetS, AndrewsN, BeelerJ (2007) Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 26 : 59–66.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



