-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
The innate immune system represents a key barrier that fungal pathogens such as Candida albicans must overcome in order to disseminate through the host. C. albicans cells phagocytosed by macrophages initiate a complex program that involves a large-scale reprogramming of metabolism and transcription and results in the switch to a hyphal form that can penetrate and kill the macrophage. Though a number of signals are known to induce this morphological transition in vitro, what does so following phagocytosis has been unclear. We previously showed that C. albicans rapidly neutralizes acidic, nutrient-poor media that resembles the phagolysosome and that this is deficient in mutants impaired in amino acid import due to a mutation in STP2. In this paper, we investigate whether this happens within the macrophage as well. We show here that, in contrast to wild-type cells, stp2Δ mutants occupy an acidic phagosome and are unable to initiate hyphal differentiation. Because of this, they are more sensitive to killing and do less damage to the macrophages than cells that can neutralize the phagolysosome. We conclude that alteration of phagosomal pH is an important virulence adaptation in this species.
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003995
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003995Summary
The innate immune system represents a key barrier that fungal pathogens such as Candida albicans must overcome in order to disseminate through the host. C. albicans cells phagocytosed by macrophages initiate a complex program that involves a large-scale reprogramming of metabolism and transcription and results in the switch to a hyphal form that can penetrate and kill the macrophage. Though a number of signals are known to induce this morphological transition in vitro, what does so following phagocytosis has been unclear. We previously showed that C. albicans rapidly neutralizes acidic, nutrient-poor media that resembles the phagolysosome and that this is deficient in mutants impaired in amino acid import due to a mutation in STP2. In this paper, we investigate whether this happens within the macrophage as well. We show here that, in contrast to wild-type cells, stp2Δ mutants occupy an acidic phagosome and are unable to initiate hyphal differentiation. Because of this, they are more sensitive to killing and do less damage to the macrophages than cells that can neutralize the phagolysosome. We conclude that alteration of phagosomal pH is an important virulence adaptation in this species.
Introduction
Normally a benign commensal, Candida albicans is also the most prevalent fungal pathogen in humans. Common mucosal manifestations of candidiasis are oropharyngeal thrush and vaginitis, but C. albicans can infect virtually any body site [1], [2]. The most serious infection – disseminated hematogenous candidiasis – is the fourth most common acquired hospital infection with a mortality rate of about 40% [3], [4]. In healthy individuals the innate immune system maintains C. albicans as a commensal and, with the exception of vaginitis, C. albicans infections are associated with defects in innate immunity. A variety of factors such as neutropenia, chemotherapy, implanted medical devices, and several genetic disorders have been linked with increased risk for disseminated candidiasis, emphasizing the important role of the immune system, including phagocytes such as macrophages and neutrophils [5].
Phagocytosis is an important step in the process by which macrophages destroy foreign cells. Many pathogens have evolved strategies to avoid or subvert phagocytosis at various stages of this process. For instance, bacterial pathogens such as Pseudomonas aeruginosa and Yersinia enterolitica inhibit phagocytosis through direct inhibition or by altering cell surface structure [6], [7]. Other pathogens, such as Anaplasma phagocytophila, interfere with the endocytic process or with the activity of macrophage-derived antimicrobial factors [8], [9]. Mycobacterium tuberculosis, Salmonella typhimurium, Listeria monocytogenes and other pathogens have developed strategies to either withstand or modulate the acidic pH of the phagolysosome, and/or alter fusion of the phagosome with the lysosome to prevent killing [10]–[12]. Similarly, C. albicans has developed strategies to escape phagocytosis and killing by the macrophages. Inside the macrophage C. albicans differentiates into the filamentous hyphal form, which ruptures the macrophage allowing it to escape and resume proliferation. This morphogenetic switch is required for virulence and has therefore been well studied [13], [14]. A variety of factors can trigger morphogenesis in vitro, including neutral pH, serum, elevated CO2 concentration, physiological temperature, and N-acetyl glucosamine [15]. While several of these inducing factors (37°C, elevated CO2) act on phagocytosed cells, an acidic phagosome should inhibit germination and it has been unclear what stimulates this morphological transition. Understanding this phenomenon is further complicated by evidence suggesting that C. albicans might modulate the phagosomal milieu or alter endocytic trafficking [16]. In fact, the exact nature of the intracellular compartment(s) containing C. albicans is not clear; here we use the generic term phagosome for simplicity.
The morphogenetic change is only part of the response to phagocytosis. Genomic and proteomic profiling indicates that C. albicans responds to phagocytosis by a significant reorganization of metabolic processes [17]–[20]. The response of C. albicans within macrophages is broadly similar to that seen after nutrient starvation, including repression of translation and glycolysis and activation of metabolic pathways required to use less favored carbon sources, including the glyoxylate cycle, β-oxidation, and gluconeogenesis [17], [18], [21]. Some of these metabolic pathways have been shown to be required for full virulence or are induced in vivo in animal models of candidiasis indicating that cells experience carbon starvation in the context of the intact host [19], [22]–[24]. Growth in alternative carbon sources also alters recognition by phagocytes, drug resistance and other relevant phenotypes [25], [26].
In a recent study we demonstrated that C. albicans cells grown in acidic medium with a nutrient composition similar to that predicted to exist inside phagocytic cells rapidly alkalinize the environment [27]. This phenomenon requires amino acid catabolism and results in extrusion of as ammonia presumably derived from the amino acid. As the cells neutralize the media, they auto-induce a shift to the hyphal form [27]. We identified mutations in several genes that impaired or abolished alkalinization, with the most severe phenotype conferred by mutation of STP2, which encodes for a transcription factor that regulates expression of amino acid permeases. We previously postulated that C. albicans might modulate the pH of the phagosome [27], a suggestion also made by others [16]. Thus, the stp2Δ mutant is an excellent tool with which to probe the importance of extracellular alkalinization during contact with host phagocytes.
Here we test the ability of C. albicans cells to alkalinize the phagosomal space and the importance of neutral pH in escape from phagocytosis. We show that phagocytosed stp2Δ mutant cells do not underdo hyphal morphogenesis within macrophages, suggesting that amino acid uptake is necessary for this process. Cells lacking STP2 are defective in killing macrophages and show reduced survival upon phagocytosis. Finally, we show that wild type C. albicans cells can modulate the intraphagosomal pH, since these cells fail to co-localize with acidophilic dyes. In contrast, stp2Δ mutant cells inhabit acidic phagosomes, suggesting that the alteration of phagosomal pH by C. albicans requires amino acid catabolism, as does alkalinization in vitro. Pharmacological neutralization of the phagosome restored hyphal morphogenesis in the stp2Δ mutant cells, indicating that neutral pH is a critical inducing factor in this environment. Consistent with this, stp2Δ mutants were attenuated in virulence in a mouse model of disseminated candidiasis. Altogether our results suggest that during phagocytosis C. albicans utilizes amino acids to promote neutralization of the phagosome, stimulation of hyphal morphogenesis and escape from the macrophages and that this process contributes to fitness within the host.
Results
Construction and verification of new stp2Δ mutants
C. albicans cells grown in conditions that mimic the phagolysosome efficiently alkalinize the environment, resulting in induction of hyphal morphogenesis. We hypothesized that this phenomenon may occur within the phagosome to promote fungal survival and escape. To test this, we took advantage of cells carrying mutation in the STP2 gene, a regulator of amino acid permease expression [28], since cells lacking STP2 are defective in environmental alkalinization [27]. Because the existing stp2Δ mutant strain is apparently aneuploid [28] and uses URA3 as a disruption marker, which can be problematic when used for in vivo and ex vivo studies [29], [30], we generated two independent stp2Δ mutant strains (SVC17, SVC18) using the SAT-flipper method [31] as described in the Materials and Methods.
In order to confirm the phenotype in the newly generated strains we verified that they shared previously published phenotypes in amino acid assimilation, alkalinization and ammonia release [27], [28]. Our newly generated strains behaved identically to the previously published stp2Δ mutant strain PMRCA57 [28] when grown on multiple amino acids as the sole nitrogen source and phenotypes were fully complemented in the restored strain (Fig. S1). Next, we tested if the newly developed strains were able to alkalinize an acidic environment via production of ammonia. The stp2Δ mutant strains grew slightly more slowly than the wild type cells in alkalinization-inducing medium (YNB+1% CAA, pH 4.5), but the difference was not statistically significant (Fig. 1A). In contrast, the stp2Δ mutant cells showed a dramatic defect in alkalinization (Fig. 1B). In cultures with the wild type and complemented strains, the media pH rose from a starting pH of 4.5 to about 7.5 within seven hours, whereas cultures with the stp2Δ mutant reached only pH 5.4 at 24 hours (Fig. 1B). A similar environmental alkalinization defect was observed in the stp2Δ mutant when cells were grown in medium M199 (data not shown) and on the solid medium GM-BCP, pH 4.5 (Fig. S2). Alkalinization is associated with extrusion of volatile ammonia [27] and non-alkalinizing stp2Δ mutant colonies did not release significant quantities of ammonia when grown on GM-BCP medium (Fig. 1C). Overall, these results confirm that the newly generated stp2Δ mutant strain shows the expected phenotypes: impaired utilization of amino acids, a severe defect in the ability to modulate the environmental pH, and abrogated release of volatile ammonia during this process [27], [28].
Fig. 1. C. albicans stp2Δ mutant cells fail to change the environmental pH. 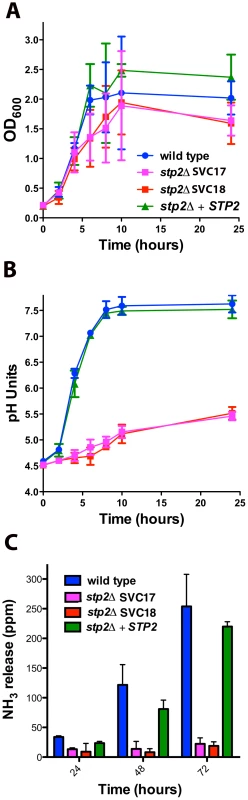
A. C. albicans strains of the indicated genotypes were grown in YNB+1% CAA, pH 4.5 at 37°C and growth was assessed by measuring OD600 at the indicated time points. B. The pH of the same cultures was measured. C. C. albicans strains were grown on GM-BCP agar to promote alkalinization and ammonia release was quantified as noted in Materials and Methods. Each experiment was performed in triplicate. By generating a neutral pH, alkalinization induces C. albicans to switch from yeast to hyphal form, which is not observed when alkalinization is repressed by glucose or buffering [27]. Therefore, we assessed the morphology of the stp2Δ mutant cells grown under alkalinizing conditions (Fig. 2A). As expected, the majority of the wild type cells (∼85%) and of the STP2 complemented cells (∼75%) switched to the hyphal morphology as the media neutralized. In contrast, fewer than 5% of the stp2Δ mutant cells had switched to the hyphal form within seven hours, at which point the pH of the medium was 4.8. Representative images of each strain prior to and after alkalinization of the medium are presented in Fig. 2B. To verify that the observed defect in morphological switch is due to the inability of the stp2Δ mutants to raise the environmental pH under these conditions and not due to an inherent defect in responding to neutral pH, we assessed hypha formation in the wild type and stp2Δ mutant cells in standard YPD medium at pH 4.5 or 7.3 (average pH achieved by wild-type cells). We did not observe any difference in the morphologies of the wild type and the stp2Δ mutant cells (Fig. 2C). Thus, the inability of the stp2Δ mutant cells to switch to the hyphal form in alkalinizing conditions is not due to a general morphological defect in these cells.
Fig. 2. Cells lacking STP2 do not induce hyphal morphogenesis under alkalinization conditions. 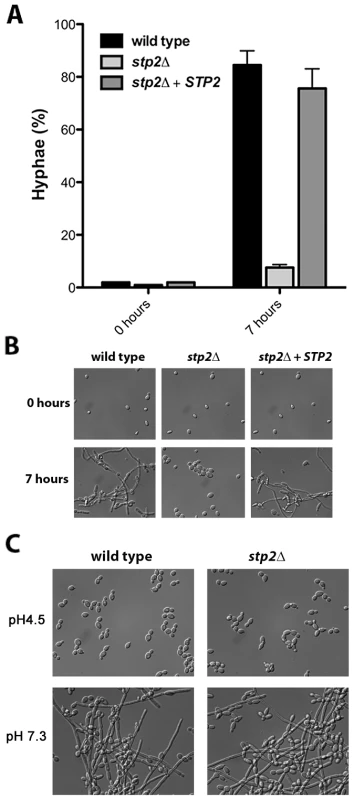
A. Strains of the indicated genotype were grown overnight in YPD and diluted to OD600 ∼0.2 in YNB + 1% CAA, pH 4.5. Cellular morphology was assessed in photomicrographs of samples after seven hours by counting at least 100 cells per condition. B. Representative images from the experiment in (A) are shown. C. C. albicans wild type or stp2Δ strains were grown overnight in YPD, then transferred to YPD medium at pH 4.5 or 7.3. Cells were incubated at 37°C and after 24 hours samples were collected and photographed. The data in panels B and C are for a single experiment, but are representative of multiple replicates. The scale bars in panels B and C are 20 µm. stp2Δ mutant cells fail to escape phagocytosis
The ability to change the environmental pH from acidic to neutral could have important effects on the host-pathogen interaction, since many niches in the host, such as oral cavity, vagina and the skin, have acidic pH. Another environment where pH is critical is the phagolysosome, where acidic pH activates lysosomal proteases and hydrolases, potent defensive enzymes, to destroy the pathogen [32]. Therefore, we asked whether the stp2Δ mutant cells have a morphogenetic defect upon internalization by macrophages. First, we monitored the interaction of stp2Δ mutant cells with the macrophages using live cell video microscopy. As expected, the majority of wild type C. albicans cells initiated hyphal morphogenesis within one hour of phagocytosis (Fig. 3A; Movies S1 and S2 are the wild-type and stp2Δ mutant, respectively). Hypha formation continued and eventually allowed C. albicans to escape phagocytosis by rupturing the macrophages. In contrast, the phagocytosed stp2Δ mutant cells showed a severely impaired ability to form hyphae. Importantly, stp2Δ cells that were not phagocytosed switched to the hyphal forms, indicating that stp2Δ mutant cells have no inherent defect in responding to hyphal-inducing conditions in this medium. To calculate what percentage of the cells underwent hyphal morphogenesis during phagocytosis, we carefully observed the time-lapse images of phagocytosed wild type or stp2Δ mutant cells and counted both total phagocytosis events and morphogenesis. At one hour of co-culture about 14% of the phagocytosed wild type cells and 8% of the stp2Δ mutant cells had initiated hyphal morphologensis (Table 1). However, significant differences in hyphal morphogenesis between the two strains were observed at later time points: at three hours of co-culture about 75% of the wild type cells were found as germ tubes or hyphae, whereas only 31% of the stp2Δ mutant cells have switched to the hyphal morphology. We noted that stp2Δ mutant cells that had begun to germinate prior to phagocytosis continued to form hyphae within the phagosomes, but those that were ingested as yeast rarely switched morphology (data not shown). Thus, stp2Δ mutant cells have a substantial reduction in hyphal morphogenesis following phagocytosis.
Fig. 3. Cells lacking STP2 fail to form hyphae and escape from phagocytosis. 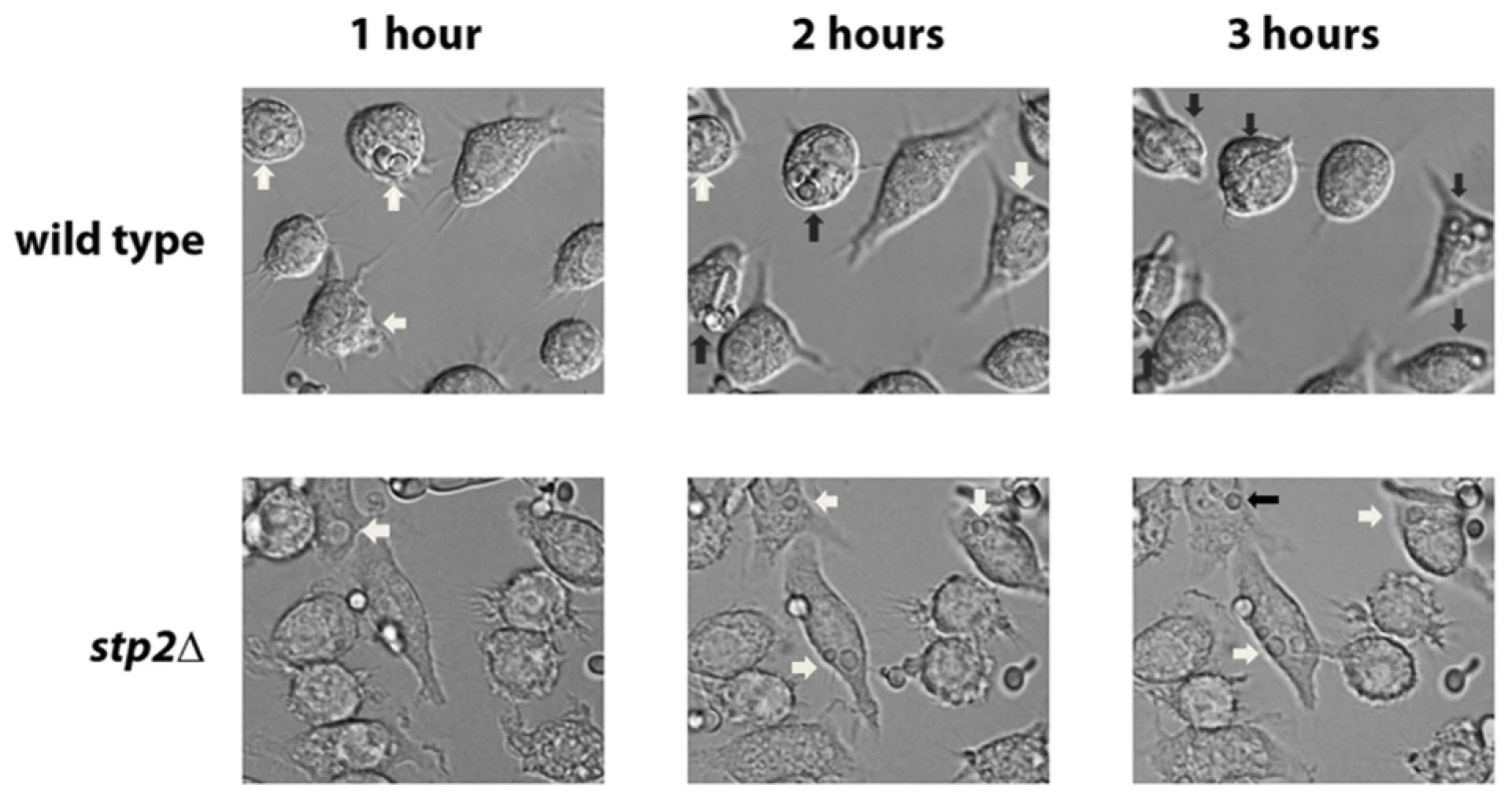
Log phase wild type cells or stp2Δ mutant cells were co-cultured with RAW264.7 macrophages in RPMI medium. The interaction was observed using time-lapse microscopy as noted in Materials and Methods. Representative images are shown. White arrows indicate cells in yeast morphology, whereas black arrows point to germinated cells. Tab. 1. Morphology changes in phagocytosed <i>C. albicans</i> cells. 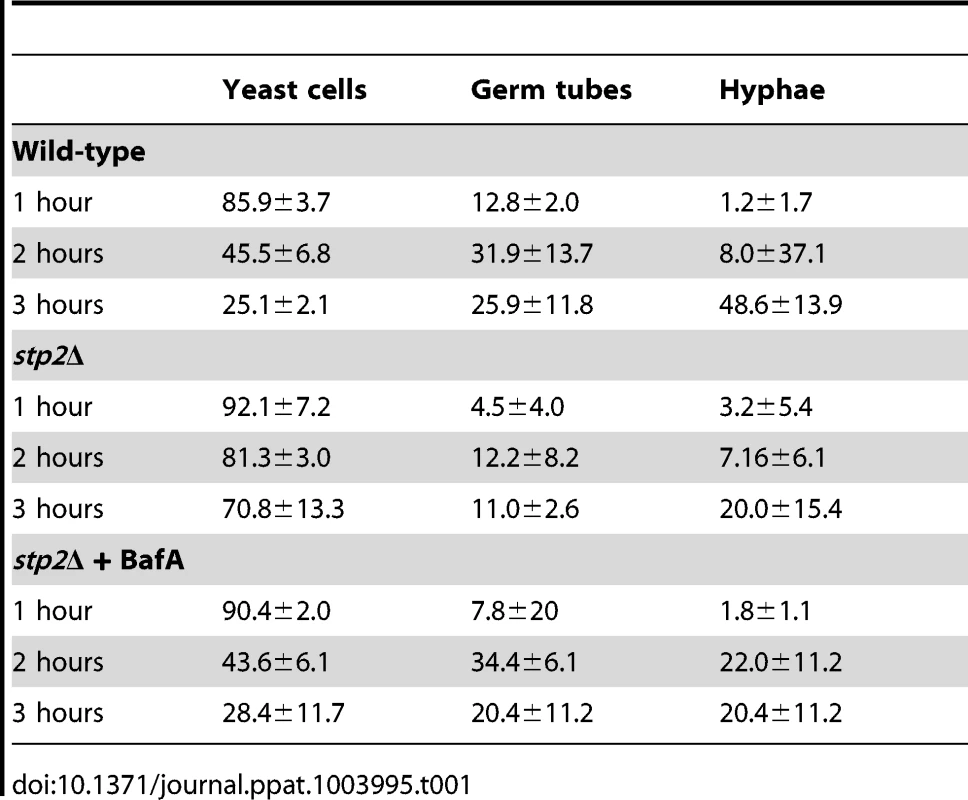
We sought to understand whether the impaired hyphal growth might be caused by altered recognition by macrophages, as has been observed in some mutations that affect the cell wall proteome [33], [34]. We monitored the uptake of C. albicans cells by macrophages by applying the membrane-impermeant chitin binding dye Calcofluor White (CW) to distinguish between external versus internalized cells. We did not observe any significant differences in uptake of live C. albicans cells tested, indicating that recognition of stp2Δ mutant cells by the macrophages is not impaired (Fig. 4). At 30 min about 30% of the wild type cells, stp2Δ mutant cells and STP2 complemented cells were internalized and this increased to about 60% at one hour. In contrast, phagocytosis of the heat killed cells was far more effective, with the majority of the cells internalized by 30 minutes (Fig. 4), which may be explained by alterations in the cell wall caused by the heat killing [35]. Thus, the observed morphogenesis defect in the stp2Δ mutants was not due to differential recognition by the RAW264.7 macrophages.
Fig. 4. The phagocytosis rate of stp2 mutant cells by RAW264.7 macrophages does not differ from the wild-type cells. 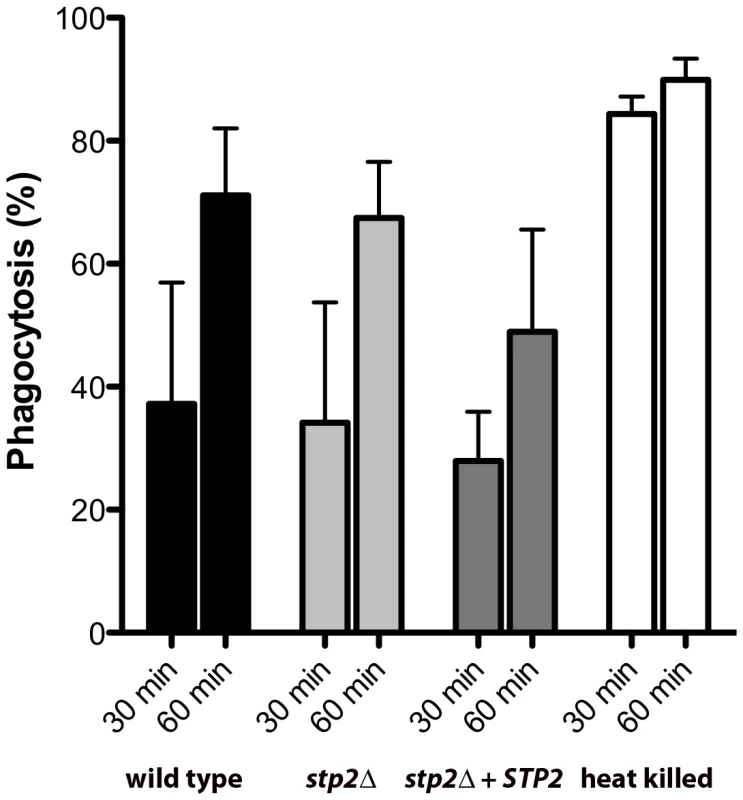
Cells of the indicated genotypes were co-cultured with RAW264.7 macrophages for 30–60 minutes, stained with Calcofluor White, and fixed with paraformaldehyde. The percentage of phagocytosis was determined as the number of cells unstained by Calcofluor White relative to the total number of C. albicans cells. At least 100 cells per condition were counted. Hyphal growth permits C. albicans cells to escape phagocytosis by lysing the macrophage. We hypothesized that the hyphal growth defect of the stp2Δ mutant strain would result in less damage to the phagocyte. For this purpose we measured loss of macrophage membrane integrity based on lactate dehydrogenase (LDH) release by the cells after five hours of co-culture. At this point about 32% of the RAW264.7 macrophages were killed (Fig. 5A); in contrast, damage caused by the stp2Δ mutant strain was significantly reduced (16% killed). The complemented STP2 strain damaged macrophages to about the same extent as the wild type (Fig. 5A). This data clearly indicates that STP2 is required for successful escape from phagocytosis.
Fig. 5. Cells lacking STP2 show reduced survival during phagocytosis and reduced capacity to kill RAW264.7 macrophages. 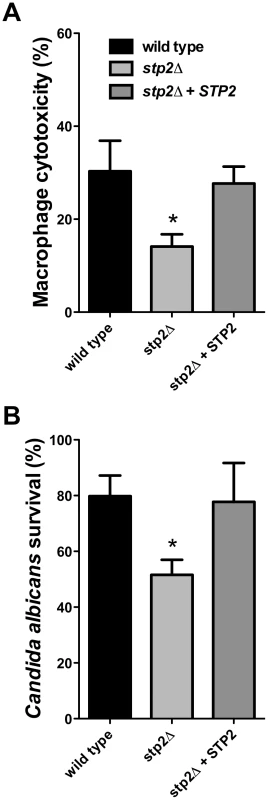
A. C. albicans cells of the indicated genotype were co-cultured with RAW264.7 macrophages for five hours. Cytotoxicity was determined by release of LDH, calculated as described in Materials and Methods. B. Killing of C. albicans by the immune cells was determined through an end point dilution assay in which serially dilutions of C. albicans cells were co-cultured with the RAW264.7 macrophages and colony formation was observed 24–48 hours after initiation of the experiment. Percent survival was calculated as described in Materials and Methods. Statistical analysis on the data was performed using a one way anova, followed by Tukey's multiple comparison; asterisks indicate p<0.05 compared to the other conditions. Results are the average of three replicate experiments. Since we observed that the stp2Δ mutant has a reduced ability to form hyphae and escape phagocytosis, we asked whether it was more efficiently killed by the immune cells. To test C. albicans survival following phagocytosis we used an end-point dilution survival assay as previously reported [36]. Only 54% of the stp2Δ mutant strains survived the macrophage attack while about 80% of the wild type cells and 78% of the STP2 complemented cells survived (Fig. 5B). Thus, the reduced ability to escape macrophages leads to more effective killing.
Growth of stp2Δ mutant cells is not impaired by different stress conditions mimicking the macrophage phagosome
The impaired interaction with macrophages could results from enhanced sensitivity to host-derived stresses, which we tested using in vitro growth assays. Phagocytosed C. albicans cells appear to utilize non-fermentable carbon sources for nutrition rather than glucose [18]. Therefore, we tested growth in minimal medium supplemented with ethanol, acetate or glucose as the sole carbon source and found no meaningful differences (Fig. S3). Further, the stp2Δ mutant strain was not more sensitive to conditions that mimic other aspects of the phagolysosomal environment, including oxidative stress, nitrosative stress, or iron depleting conditions (Fig. S4). Thus, the stp2Δ mutants are not more sensitive to conditions found in the macrophage phagosome.
Phagosomal neutralization promotes C. albicans escape from the macrophages
Given the Stp2p-mediated alkalinization in vitro, we hypothesized that the defect in hyphal growth in phagocytosed cells reflected a difference in the pH of phagosomes containing wild type versus stp2Δ mutant cells, i.e. neutral versus acidic. To test our hypothesis we pretreated RAW264.7 macrophages with the acidophilic dye Lysotracker Red DN99 (LR) to allow accumulation of the dye in acidic organelles (lysosomes). FITC-labeled C. albicans cells were allowed to interact with the LR-loaded macrophages for up to two hours. As expected, heat killed cells were found in acidic phagosomes one hour after co-culture (Fig. 6). In contrast, C. albicans wild type cells rarely localized to acidic phagosomes as noted by the diffuse LR staining, suggesting that the pH of the C. albicans-containing phagosome is over 5.5 and that these cells alter the nature of this compartment relative to heat-killed cells. In contract, cells lacking STP2 localized to acidic phagosomes, similar to the heat killed cells; complementation of STP2 restored the wild type phenotype (Fig. 6). To estimate the difference in the pH of phagosomes containing wild-type versus stp2Δ mutant cells, we measured the fluorescence intensity of LR relative to FITC surrounding phagocytosed cells, with the rationale that a higher ratio indicated a lower pH (Fig. 7). The LR:FITC ratio for heat killed cells in acidic phagosomes was about 2.0 at 60 min (Fig. 7). In contrast, the LR:FITC ratio for wild-type or STP2 complemented cells was 1.2 and 0.8 respectively, while the stp2Δ mutant cells had an LR/FITC ratio of 1.9, close to that of heat killed cells. Stp2p-dependent phagosomal neutralization by C. albicans was also observed following phagocytosis by bone marrow-derived macrophages (data not shown). Thus, there is a substantial difference in the pH of phagosomes containing wild-type versus stp2Δ mutants, suggesting that the alkalinization process we have described in vitro also occurs within the phagocyte.
Fig. 6. C. albicans alkalinizes the macrophage phagosome. 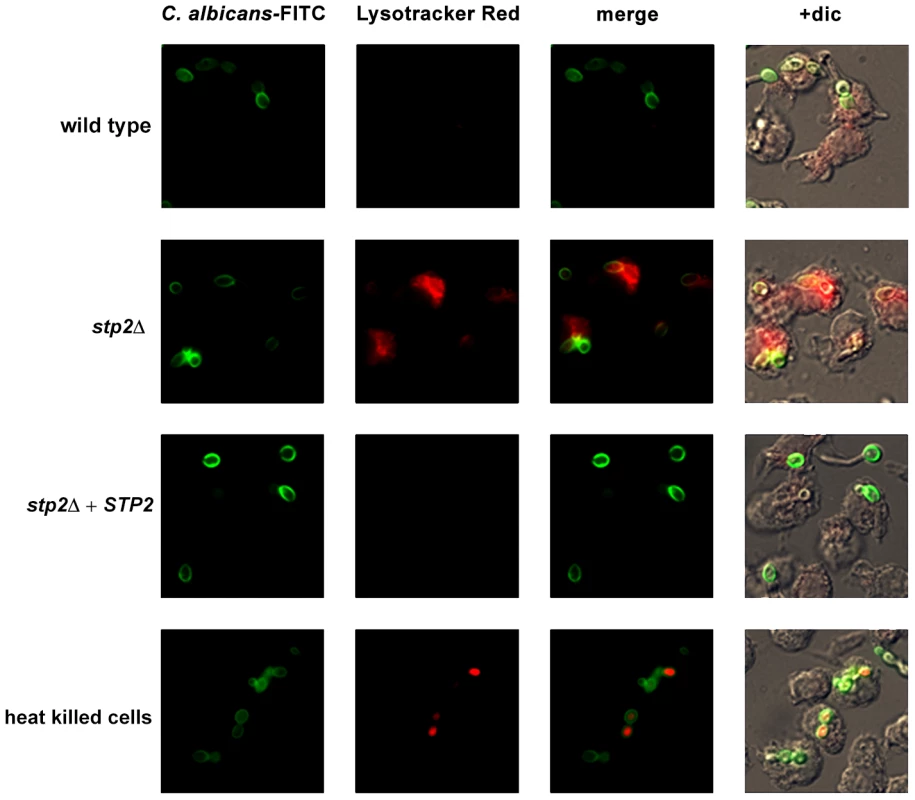
FITC-stained C. albicans wild type, stp2Δ mutant, STP2 complemented cells or heat-killed wild type cells were co-cultured with RAW264.7 macrophages preloaded with Lysotracker Red at a 1∶1 ratio and imaged after one hour of co-culture. Representative images are shown. Fig. 7. C. albicans cells alkalinize the macrophage phagosome. 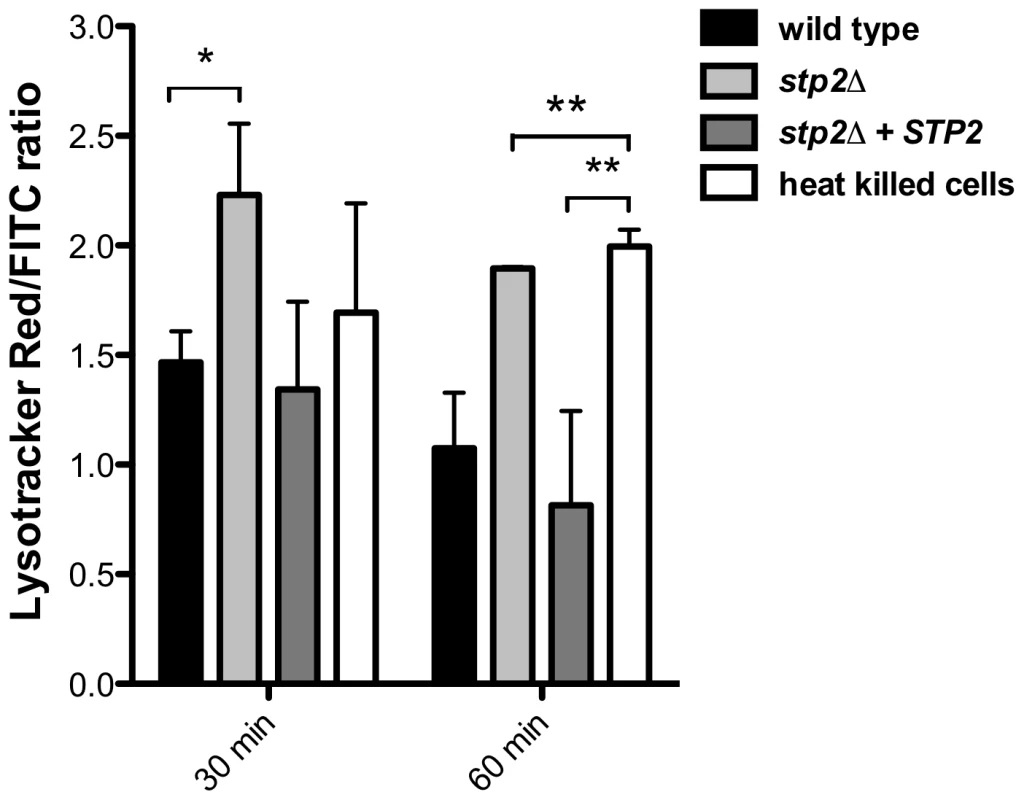
FITC-stained wild type, stp2Δ mutant, STP2 complemented or heat killed wild type cells were co-cultured with macrophages preloaded with Lysotracker Red. The cultures were fixed at the indicated times, imaged and phagosomal pH was calculated as described in Materials and Methods. Statistical analysis was performed using a two-way anova; asterisks indicate p<0.05 compared to the other conditions. Data from three independent experiments is shown. Next, we asked if failure to neutralize the phagosomal environment alone explains the hyphal defect of stp2Δ cells. To test this, we created neutral phagosomes by specifically inhibiting the vacuolar ATPase (v-ATPase) of the macrophages using bafilomycin A. We verified that the phagosomes were neutralized by obtaining the LR/FITC ratio after one hour of co-culture of C. albicans, which was about 1.0 for all the cells tested (Fig. S5). Importantly, bafilomycin A completely suppressed the hyphal morphogenesis defects of phagocytosed stp2Δ mutant cells (Fig. 8). Indeed, the proportion of stp2Δ hyphal cells in bafilomycin-treated macrophages was essentially the same as that of the wild-type control, indicating that neutralization of the phagosome is a key factor for hyphal morphogenesis in these cells (Table 1). Bafilomycin A1 did not affect C. albicans growth or morphogenesis in macrophage-free RPMI medium (data not shown). Altogether, our data suggests that the observed differences in stp2Δ morphogenesis, macrophage survival and reduced macrophage killing are due to inability to neutralize the phagosomal environment. More importantly, neutralization of the phagosome promotes hyphal morphogenesis and phagosomal escape in the fungal pathogen.
Fig. 8. Neutralization of the phagosome is sufficient for hyphal morphogenesis and escape from the macrophages. 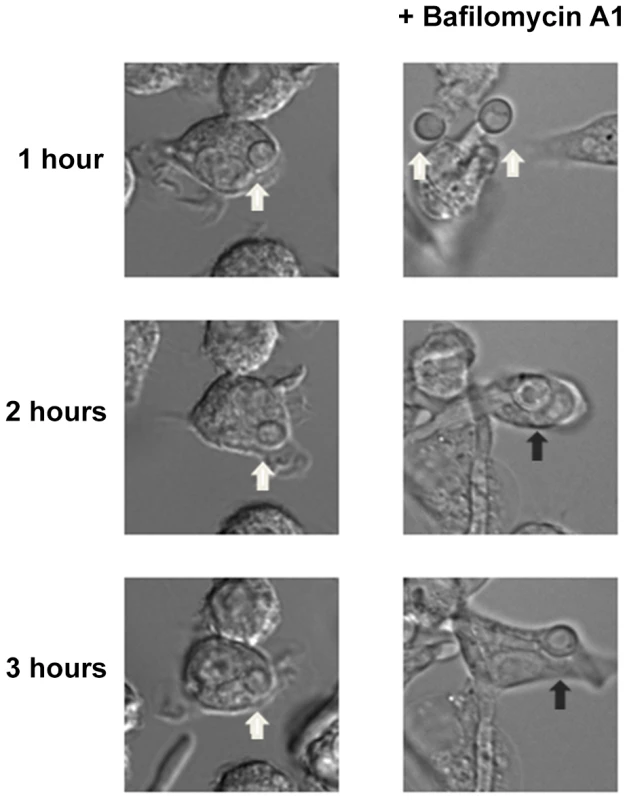
Cells lacking STP2 were co-cultured at 1∶1 ratio with RAW264.7 macrophages that were pre-treated with 50 nM Bafilomycin A1. Co-cultures were imaged using time-lapse microscopy. Representative images are shown, but the experiments were performed in triplicate and morphogenesis was quantitated in Table 1. White arrows point to C. albicans cells in yeast morphology, whereas black arrows show germinated cells. Environmental alkalinization can occur in body niches colonized by C. albicans
Since C. albicans can alkalinize the phagosome and this process contributes to hyphae formation and escape from the macrophages, we asked if alkalinization may alter the host-pathogen interaction in other body niches colonized by C. albicans. Because both the oral and vaginal cavities are acidic, we tested C. albicans alkalinization on media that mimics the composition of vaginal fluid (vaginal simulating fluid or VSF) and human saliva (artificial saliva or AS). Alkalinization in AS was remarkably rapid, rising from pH 4.5 to 7.0 within five hours and further to pH 8.4 at 24 hours of incubation (Fig. 9A). Similarly, these cells steadily raised the pH of the VSF from starting pH of 4.2 to 7.0 (Fig. 9B), though this was much slower, occurring over 72 hours. Interestingly, stp2Δ mutant cells showed a marked delay in alkalinization on AS, where the pH reached only 5.3 at five hours (Fig. 9A), but the alkalinization of VSF did not differ from that of the controls (Fig. 9B). The significant difference in the time required to fully alkalinize VS and AS, and the differential requirement of Stp2p in the alkalinization processes, suggests that multiple mechanisms for environmental alkalinization by C. albicans may exist.
Fig. 9. Environmental alkalinization by C. albicans occurs under host niche-simulating conditions. 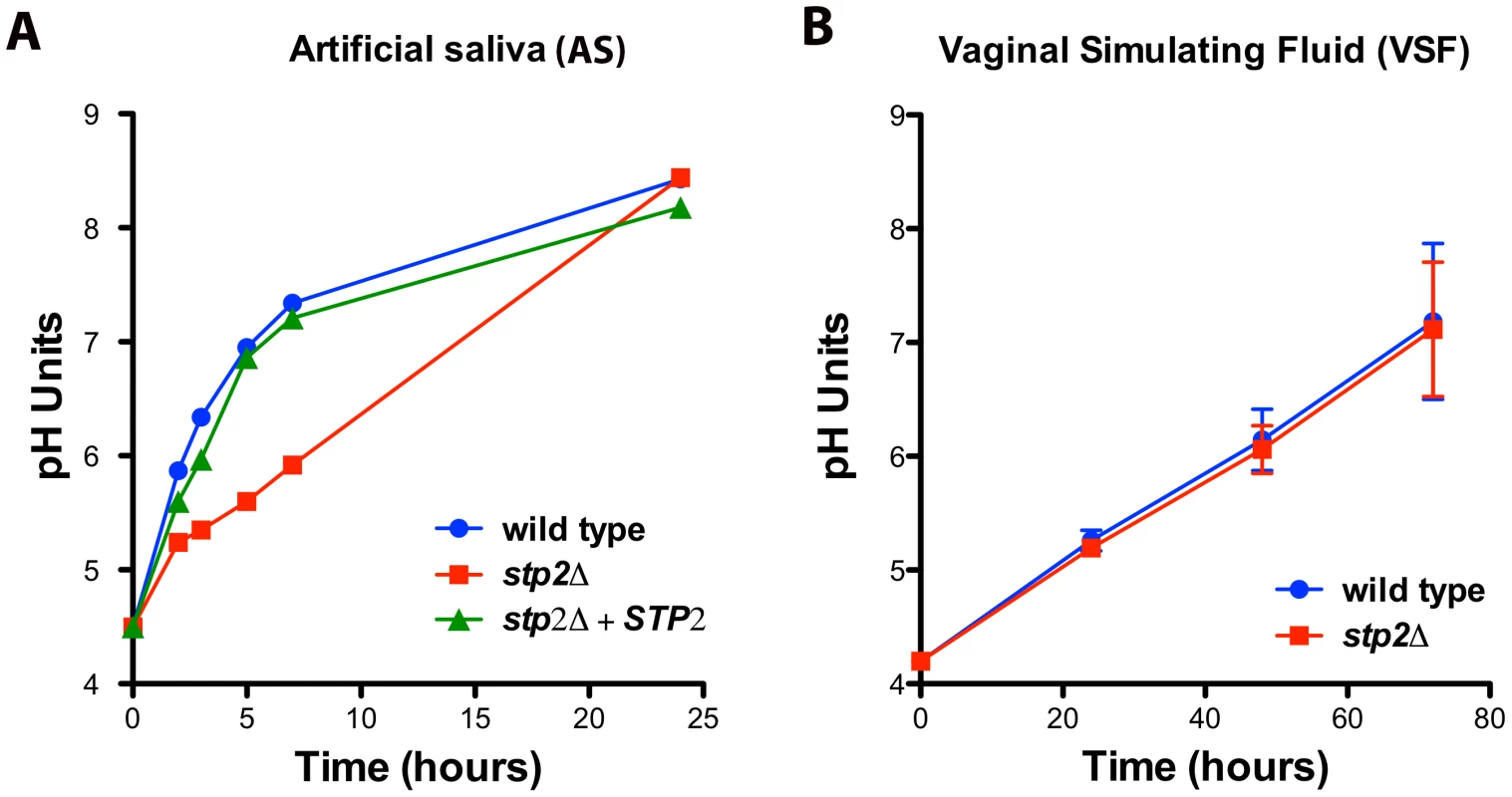
Wild-type cells, stp2Δ mutant or complemented cells were grown in A. artificial saliva, pH 4.5 or B. vaginal simulating fluid, pH 4.2. The pH change of the growing cultures was measured at the indicated time points. The experiment was performed in triplicate. Stp2p is important for full virulence in a mouse model
The decreased survival upon phagocytosis by the macrophages of stp2Δ mutants raised the question whether these strains would be impaired during disseminated infection. Therefore, we tested stp2Δ mutant strains in the standard mouse tail-vein injection model of disseminated hematogenous candidiasis. The stp2Δ mutant strain SVC23 was attenuated in virulence compared to the STP2 complemented or the wild type strain SVC21 (Fig. 10), with a mean time to death of 2.4 days for the wild-type strain compared to 5.9 days for the mutant strain, indicating that Stp2p is required for full virulence in this animal model of infection. The statistical analysis showed that the survival curve for this mutant is significantly different compared to the wild type or the complemented cells (p = 0.0011 or 0.0024, respectively). We also tested the survival of a second, independent stp2Δ mutant strain SVC22, which had a mean time to death 5.3 days (data not shown). This modest attenuation is consistent with the partial reduction of hyphal induction and macrophage toxicity in phagocytosed cells.
Fig. 10. Mutation in C. albicans STP2 causes attenuation of virulence. 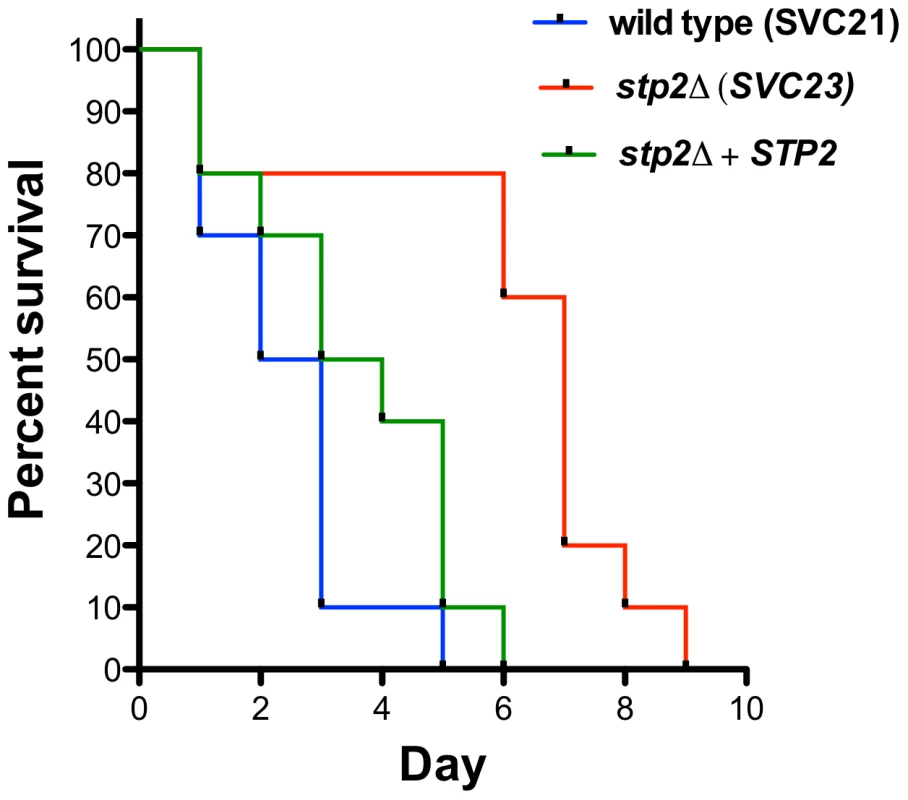
Outbred ICR mice were injected via the tail vein with 106 cells of the wild type strain SVC21, stp2Δ mutant strain SVC23, or the STP2 complemented cells resuspended in phosphate buffered saline. Ten animals per strain were used and the animals were monitored for signs of infection as described in Materials and Methods. The statistical analysis showed that the mutant survival curves are significantly different compared to the wild type or the complemented cells, with a P value of 0.0011 and 0.0024, respectively. Discussion
Here we show that C. albicans can neutralize the macrophage phagosome to induce hyphal morphogenesis and escape from the immune cell and we propose the first mechanism for this phenomenon: extrusion of ammonia as a byproduct of amino acid catabolism within the host cell. This conclusion comes from the analysis of strains lacking Stp2p, a transcriptional regulator of amino acid permease genes that is required for amino acid-driven alkalinization in vitro [27]. Cells lacking Stp2p are defective in hypha formation during phagocytosis, show reduced survival upon interaction with the immune cell and are defective in killing the macrophages. These cells grow at wild type rates on alternative carbon sources and show normal resistance to a variety of stress conditions encountered in the phagosome, indicating that the observed defects are not due to an enhanced sensitivity to macrophage-derived stresses. However, stp2Δ mutant cells switched to the hyphal form when within neutral phagosomes, indicating a key role for pH in inducing germination of phagocytosed cells. Alkalinization is also observed in artificial saliva and vaginal simulating fluid, indicating that there may be Candida-induced pH changes at body sites normally occupied by C. albicans, such as the oropharyngeal tract and vulvovaginal tract, underlining the effect of this phenomenon on microbial and human physiology. These defects are reflected in a modest attenuation of virulence in a mouse model of disseminated candidiasis.
The intracellular fate of C. albicans cells is not well understood. A few studies on the phagosome-lysosome fusion following C. albicans ingestion by macrophages exist: two studies concluded that the fusion is inhibited, two studies concluded that fusion occurs, while another got both results depending on the assay used [37]–[41]. A key distinction in these papers was whether fusion was monitored by acidification using dyes such as acridine orange or lysotracker or by colocalization with endocytic markers such as LAMP-1. Our data provides a possible explanation for these contradictory reports, since we show that C. albicans can alkalinize the phagosome and that neutralization of the phagosome promotes hyphal morphogenesis and escape from the host cell. The exact fate of C. albicans after phagocytosis remains unclear: studies report C. albicans localization in mature phagolysosmes [37], late endosomes [39], or the endoplasmic reticulum [16]. Our data does not directly address this question, which clearly requires additional study, but we demonstrate a difference in the pH of the Candida-containing compartment between the wild-type strain and stp2Δ mutants, the latter of which behave similarly to killed cells. The simplest explanation is that the wild-type cells occupy a fused phagolysosome in which acidification is outcompeted by ammonia release from the fungal cell, though we cannot exclude differences in trafficking between these two strains.
We indicate here the importance phagosomal neutralization for C. albicans escape from the macrophages, however additional factors may also contribute to this process, including nutrient availability, CO2 concentration, presence of reactive oxygen species, and modulation of the iron content. The effect of these morphogenetic stimuli is likely combined, since mutants in hyphal morphogenesis, cell wall synthesis, or nutrient assimilation fail to escape from phagocytosis [16], [33]. The exact role of each of these factors on hyphal morphogenesis during phagocytosis is yet to be determined, but here we provide for the first time evidence that neutral pH is a critical component since mutants defective in environmental alkalinization remain in acidic phagosomes and do not switch to the hyphal form.
It has been well documented that pathogens manipulate the phagosomal environment to promote survival by neutralizing the phagolysosome, preventing phagosome-lysosome fusion and/or reducing the production of reactive oxygen or nitrogen species [42]–[45]. Phagosomal neutralization is also observed with Helicobacter pylori, which uses an extracellular urease to produce ammonia to elevate phagosomal pH and retard acquisition of late endosomal markers, allowing survival [43], [46]. Other fungal pathogens, such as Candida glabrata, Histoplasma capsulatum, and Cryptococcus neoformans alter phagosomal pH, but the mechanisms to achieve the pH increase differ from the one reported here. For instance, C. glabrata inhibits phagolysosomal fusion and survives phagocytosis without damaging the host cells or eliciting a proinflammatory immune response but, surprisingly, this does not require viable cells [47]. In contrast, H. capsulatum resides in neutralized mature phagosomes [48], [49]. The mechanism for this is unknown, but may involve permeabilization of the phagosomal membrane via the saposin-like protein Cbp1, which is secreted into the phagosomal space [50]. Similarly, C. neoformans appears to destabilize the phagosomal membrane such that there is exchange of luminal contents with the cytosol [51]. Thus, our findings are consistent with other fungal pathogens, though the mechanism we propose is quite different. Pharmacological inhibition of acidification also compromises macrophage antimicrobial activity: artificial elevation of the phagosomal pH using NH4Cl, chloroquine or Bafilomycin A1 has also been found to impair killing of phagocytosed Staphylococcus aureus, Bordetella pertusis and the fungal pathogen Aspergillus fumigatus [42], [44], [45]. Further, addition of NH4Cl to normal resident mouse peritoneal macrophages reduced the rate of killing of the non-pathogenic S. cerevisiae [52]. Likewise, we observe hypha formation and escape from Bafilomycin A1-treated phagosomes of the stp2Δ mutant cells that normally are defective in these processes. In the context of C. albicans the elevated pH of the phagosome will promote hyphal morphogenesis and escape from phagocytosis.
Our data suggests that this alkalinization phenomenon is driven by metabolic changes resulting from nutrient starvation imposed by the phagocyte; metabolism of non-fermentable carbon sources appears to be important during infection as disruption of some these carbon metabolic pathways impairs virulence [19], [21]–[23]. Glucose limitation within the phagosome might be one of the mechanisms macrophages use to prevent survival of the ingested cells in a manner analogous to iron limitation within the host. For example, enzymes involved in the gluconeogenesis and the glyoxylate shunt are also required for colonization and persistence of the phagosomal pathogens Leishmania manor and M. tuberculosis [53], [54]. Similarly, 3-hydroxy-methylglutaryl coenzyme A lyase, the enzyme catalyzing the last step of leucine catabolism, is important for survival of the fungal pathogen Histoplasma capsulatum within macrophages [55]. Amino acid utilization is important for C. albicans pathogenicity too, since we show that stp2Δ mutants have reduced survival during phagocytosis and a modest reduction in virulence. Similarly, cells lacking DUR1,2 and ATO5, genes involved in detoxification and export of excess amines produced during amino acid catabolism, exhibit significant delay in hyphal morphogenesis and survival upon phagocytosis, further highlighting the importance of amino acid utilization within the host ([56]; H. Danhof and M. Lorenz, unpublished).
The source of amino acids within the macrophage is not clear, but they are a potentially plentiful nutrient source in the host. Numerous amino acid auxotrophic mutants retain full virulence in the mouse model, including those for leucine, arginine, tryptophan, and histidine [57]–[60]; this is the basis for the genetic methodology developed by Noble and Johnson [61]. This demonstrates that sufficient amino acids are accessible in the host to support growth of the pathogen, in decided contrast to the profound avirulence of nucleotide auxotrophs [29], [30], [60], [62]. We note that previous transcriptional profiling of phagocytosed cells suggested that they were starved for carbon but not for amino acids, as no amino acid biosynthetic genes were upregulated, with the exception of the arginine pathway, which we have shown is induced by oxidative stress [18], [63]. Importantly, we propose these amino acids are being used as a source of carbon and not just as the building blocks of proteins. Consistent with this, mutations of both STP2 and CSH3, an ER chaperone for amino acid permeases, block alkalinization and confer virulence defects [27], [64]. The presence of extracellular amino acids activates STP2 post-translationally to increase the relative levels of the amino acid permeases CAN1, GAP1 and GAP2, the oligopeptide transporters OPT1 and OPT3, and the secreted aspartyl protease SAP2; most of these genes are significantly induced in phagocytosed cells [18], [28]. Together, these indicate that amino acids are a key source of carbon, nitrogen or both within the host. Our preliminary results showing Stp2p-independent alkalinization during growth in vaginal simulating fluid indicates some redundancy with other processes; perhaps transport and utilization of oligopeptides generated from the protein in this media. In summary, we show here that catabolism of amino acids as a primary nutrient is a key determinant of the host-pathogen interaction with both phagocytes and in whole animal models. Further studies are necessary to define the most important catabolic pathways and host niches in which amino acid-driven pH changes have the biggest impact.
Materials and Methods
Strains and growth media
C. albicans strains were propagated under standard conditions in YPD medium (1% yeast extract, 2% peptone, 2% glucose). For growth on plates, 2% agar was added to the medium. To select for nourseothricin-resistant (NouR) transformants, 200 µg/ml of nourseothricin (Werner Bioagents, Jena, Germany) was added to the YPD agar plates [31]. Alkalinization experiments were performed in minimal yeast nitrogen base (YNB) medium (0.17% yeast nitrogen base, 0.5% ammonium sulfate) prepared without glucose and supplemented with 1% casamino acids as the sole carbon source. For alkalinization on solid medium and for assessment of NH3 release by alkalinizing colonies cells were grown on GM-BCP medium, which contained 1% yeast extract, 30 mM CaCl2, 3% glycerol, 0.01% bromocresol purple, 2% agar and adjusted pH 4.5 [65]. Other carbon sources or chemical stressors were added to YNB medium as indicated in the results and figures. In some experiments environmental alkalinization was monitored in artificial saliva, pH 4.5 (which included, per liter, 1.7 g yeast nitrogen base without amino acids and ammonium sulfate, 5.0 g casamino acids, 1.1 g KCl, 0.5 g NaCl, 14 mg choline chloride, 10 mg sodium citrate, 1.0 mg ascorbate, 2.5 g mucin), a formulation modified from [66] or in vaginal simulating fluid, pH 4.2 (per liter, 2 g glucose, 0.16 g glycerol, 2 g lactic acid, 1 g acetic acid, 0.018 g bovine serum albumin, 0.4 g urea, 1.4 g KOH, 0.222 CaCl2, 3.51 g NaCl, 0.25 g mucin), a formulation modified from [67]. Utilization of different amino acids as the sole nitrogen source was assessed as previously described [28], using succinate buffered YNB medium supplemented with 2% glucose and 50 µM histidine, pH 6.0 and supplemented with the indicated amino acids at 1 mM. RAW264.7 macrophages were maintained in RPMI with glutamine and HEPES, supplemented with Penicillin/Streptomycin and 10% inactivated Fetal Bovine Serum.
Strain construction
C. albicans strains lacking STP2 were generated using the SAT-flipper method as described previously [31]. In short, approximately 300 bp of homology immediately to the 5′ or 3′ of the STP2 ORF were amplified by PCR and cloned between the ApaI/XhoI and SacI/SacII sites of pSFS1. The resulting SAT1–FLP cassette was used to transform C. albicans SC5314 strain by electroporation with selection on YPD-Nou. Genomic DNA was isolated and cassette integration confirmed in the selected candidates via PCR. To eliminate the nourseothricin selection marker, the mutant strain was induced with 1% BSA in YNB medium for 4 days and the NouS colonies were selected. This process was repeated to generate the independently-derived homozygous disruptants SVC17 and SVC18 (stp2Δ::FRT/stp2Δ::FRT).
Complementation of the mutant strain used plasmid pSV-4, a SAT1-marked version of CIp10 expressing STP2 under its native promoter. To generate this plasmid the URA3 gene from Cip10 [68] was excised using BamHI and SacI and replaced by the SAT1 gene from the plasmid pSFS1 [31] to generate pAG6. Next, pSV-4 was generated by cloning the entire STP2 ORF into the ApaI and XhoI sites of pAG6. This plasmid was linearized with StuI and used to transform SC5413 or stp2Δ mutant cells to generate the strains SVC21 (STP2/STP2 RPS10/rps10:Clp10-SAT1) and SVC20 (stp2Δ::FRT/stp2Δ::FRT RPS10/rps10::Clp10-STP2-SAT1), respectively. The stp2Δ mutant strain was also transformed with linearized pAG6 to produce the stp2Δ strains SVC22 and SVC23 (stp2Δ::FRT/stp2Δ::FRT RPS10/rps10::Clp10). The previously generated stp2Δ mutant strain PMRCA57 (stp2Δ4::dpl200-URA3/stp2Δ2::CaNAT1/stp2Δ5::MPA) [28], was used for phenotype comparison where noted.
Alkalinization and ammonia release assays
Alkalinization experiments were performed as previously described [27], with the following modifications: The minimal medium YNB was supplemented with 1% casamino acids as the sole carbon source and pH adjusted to 4.5. C. albicans cells were grown in YPD medium overnight, washed in dH2O and diluted to OD600 = 0.2 in the alkalinization medium. Cells were incubated at 37°C with aeration and growth, pH changes, and cellular morphology were assessed at the indicated times. Cellular morphology was scored by analyzing photomicrographs of at least 150 cells per condition. Experiments were performed at least in triplicate and analyzed using Prism 5.0 (GraphPad) software.
Ammonia release by C. albicans cells during alkalinization was assessed using acid traps as previously described [27], [65]. In brief, cells were grown in YPD medium overnight, washed in dH2O and resuspended at an OD600 of 1.0 in dH2O. Cells were spotted onto solid GM-BCP medium at pH 4.5; reservoirs containing 10% citric acid were placed underneath the colonies. Cells were incubated at 37°C and samples from the acid trap collected at 24, 48 or 72 hours after initiation of the experiment. Ammonia was quantified using Nessler's reagent, as described [27]. Experiments were performed in triplicate and data plotted using GraphPad Prism software.
Growth assays
C. albicans strains were grown in YPD medium overnight, washed in dH2O and diluted in the testing medium to final OD600 of 0.2. For growth on alternative carbon sources we tested YNB medium supplemented with 0.01% or 0.1% acetate, 0.2% or 2% ethanol or 2% glucose. To test the effect of different stress conditions on growth, YNB medium was supplemented with 2% glucose and H2O2, DETA NONOate (Cayman Chemical), or bathophenanthroline disulfonate (BPS; Acros Organics), as indicated in the figures. Assays were performed in triplicate in 96 well plate format using a SynergyMx (Biotek) plate reader at 30°C with 1 minute agitation prior to the OD600 reading every 10 minutes for 16 hours.
Live interaction with the macrophages
To assess the interaction of single C. albicans cells with the macrophages we seeded 3×106 cells RAW264.7 macrophages to 35 mm glass bottom dishes (MatTek) and allowed them to adhere for four hours. C. albicans cells were grown in YPD medium overnight, diluted 1∶100 in fresh medium and grown for 3 hours at 30°C. Cells were then washed in dH2O and 3×106 cells were resuspended in CO2-independent RPMI medium (HyClone) and co-cultured with the macrophages at 37°C. Images of the Candida-macrophage interaction were taken at 3 minute intervals for up to 10 hours using an Olympus IX81 automated inverted microscope with a temperature-controlled stage. Images from at least 10 fields were analyzed using SlideBook software. Percent hyphal morhogenesis during phagocytosis was calculated by obtaining percentage of phagocytosed cells using the following formula: (germ tubes + hyphal cells/total amount of cells)×100. Experiments were performed in triplicate.
Macrophage cytotoxicity assay
C. albicans toxicity on macrophages was assessed using CytoTox96 Non-Radioactive Cytotoxicity assay (Promega) as follows: tissue culture macrophages were seeded at 2.5×105 cells per well of a 96 well plate in phenol red-free RPMI and incubated overnight at 37°C, 5% CO2. C. albicans cells were grown to log phase in YPD media, washed in PBS and co-cultured with macrophages at a 3∶1 ratio for 5 hours. Calculation of lactate dehydrogenase (LDH) release by infected macrophages was then determined according to the manufacturer's protocol relative to maximum LDH release from lysed macrophages and corrected for spontaneous release of LDH by the macrophages or C. albicans alone. The experiment was performed in triplicate.
End-point dilution assay
C. albicans survival during interaction with the RAW264.7 macrophages was assessed as previously described [36]. Briefly, macrophages were collected and resuspended in fresh RPMI medium. Cells were seeded at 2.5×104 cells/well in 96 well plates and grown overnight at 37°C, 5% CO2. Log-phase C. albicans cells were washed in dH2O and resuspended in fresh RPMI medium. 1×104 cells/well were added to wells with or without macrophages, followed by six serial 1∶5 dilutions. After 48 hours at 37°C in 5% CO2, microcolonies of C. albicans were counted using an inverted microscope in wells in which individual colonies could be distinguished. Results were presented as the ratio of (number of colonies in the presence of macrophages/number of colonies without macrophages)×100. The experiment was performed in triplicate.
Lysotracker Red assay
For co-cultures with RAW264.7 cells, 1×106 cells/ml were seeded onto glass coverslips in 12-well plates and allowed to adhere for 2 hours. Next, 1 mM Lysotracker Red DM99 (Molecular Probes) was added to the cells and incubated for 2 hours to ensure concentration of the dye in the lysosomes. In some experiments 50 nM Bafilocymin A1 (Alexis Biochemicals) was added to the cells and incubated for 30 minutes prior to co-culture. C. albicans cells were grown overnight in YPD, diluted 1∶100 in fresh YPD, and grown for 3 hours at 30°C. Then cells were washed in dH2O, stained with 1 µM FITC for 15 minutes and excess dye was removed by washing three times in PBS. Control cells were heat killed by incubation for 60 minutes at 65°C. Cells were diluted to 1×106 cells/ml in phenol red-free RPMI medium and co-cultured with macrophages for the indicated time points. Cultures were washed once in PBS, stained with Calcofluor white (35 µg/ml for 1 minute) to label non-phagocytosed cells, and fixed in 2.7% paraformaldehyde for 20 minutes. To estimate the relative phagosomal pH, regions that included a phagocytosed C. albicans cell and including approximately 0.5 µm of the adjoining area were selected for at least 100 cells per condition. Lysotracker Red (LR) and FITC intensities were determined and corrected by subtracting background intensities in an area without phagocytosed C. albicans cells before calculating the LR/FITC ratio. Percentage of phagocytosed cells was calculated as (Calcofluor White stained cells/total cells)×100. Image analysis was performed using SlideBook 5.0 Image Software. All experiments were performed at least in triplicate.
In vivo virulence assays
Disseminated infections with C. albicans were performed as previously described [23]. Briefly, C. albicans strains were grown to mid-log phase in YPD overnight. Next, cells were collected via centrifugation, washed, and resuspended in phosphate-buffered saline. Ten female 21–25 g ICR mice per strain were infected via tail vein injection with 1×106 C. albicans cells in 100 µl PBS. The mice were monitored twice daily for signs of infection and euthanized when moribund. Survival data were plotted using Prism 5 (GraphPad Software) and analyzed using the log-rank test.
Ethics statement
All animal work was performed under protocols approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston (protocol HSC-AWC-12-099). Procedures adhered to the U.S National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition.
Supporting Information
Zdroje
1. Kwon-Chung KJ, Bennett JE (1992) Medical Mycology. Philadelphia: Lea & Febiger.
2. Odds FC (1988) Candida and candidosis. Philadelphia: Bailliere Tindall.
3. WisplinghoffH, BischoffT, TallentSM, SeifertH, WenzelRP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39 : 309–317.
4. DiekemaD, ArbefevilleS, BoykenL, KroegerJ, PfallerM (2012) The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis 73 : 45–48.
5. HajjehRA, SofairAN, HarrisonLH, LyonGM, Arthington-SkaggsBA, et al. (2004) Incidence of bloodstream infections due to Candida species and n vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42 : 1519–1527.
6. Garrity-RyanL, KazmierczakB, KowalR, ComolliJ, HauserA, et al. (2000) The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun 68 : 7100–7113.
7. GrosdentN, Maridonneau-PariniI, SoryMP, CornelisGR (2002) Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect Immun 70 : 4165–4176.
8. HuangB, HubberA, McDonoughJA, RoyCR, ScidmoreMA, et al. (2010) The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes. Cell Microbiol 12 : 1292–1307.
9. MottJ, RikihisaY, TsunawakiS (2002) Effects of Anaplasma phagocytophila on NADPH oxidase components in human neutrophils and HL-60 cells. Infect Immun 70 : 1359–1366.
10. ParkYK, BearsonB, BangSH, BangIS, FosterJW (1996) Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol Microbiol 20 : 605–611.
11. ShaughnessyLM, HoppeAD, ChristensenKA, SwansonJA (2006) Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol 8 : 781–792.
12. VandalOH, PieriniLM, SchnappingerD, NathanCF, EhrtS (2008) A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14 : 849–854.
13. LoHJ, KohlerJR, DiDomenicoB, LoebenbergD, CacciapuotiA, et al. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90 : 939–949.
14. SavilleSP, LazzellAL, MonteagudoC, Lopez-RibotJL (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2 : 1053–1060.
15. Brown AJ (2002) Morphogenetic signaling pathways in Candida albicans. In: Calderone R, editor. Candida and candidiasis. Washington: ASM Press.
16. Fernandez-ArenasE, BleckCK, NombelaC, GilC, GriffithsG, et al. (2009) Candida albicans actively modulates intracellular membrane trafficking in mouse macrophage phagosomes. Cell Microbiol 11 : 560–589.
17. FradinC, De GrootP, MacCallumD, SchallerM, KlisF, et al. (2005) Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol 56 : 397–415.
18. LorenzMC, BenderJA, FinkGR (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3 : 1076–1087.
19. LorenzMC, FinkGR (2001) The glyoxylate cycle is required for fungal virulence. Nature 412 : 83–86.
20. Rubin-BejeranoI, FraserI, GrisafiP, FinkGR (2003) Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc Natl Acad Sci U S A 100 : 11007–11012.
21. Fernandez-ArenasE, CabezonV, BermejoC, ArroyoJ, NombelaC, et al. (2007) Integrated proteomics and genomics strategies bring new insight into Candida albicans response upon macrophage interaction. Mol Cell Proteomics 6 : 460–478.
22. BarelleCJ, PriestCL, MaccallumDM, GowNA, OddsFC, et al. (2006) Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol 8 : 961–971.
23. RamirezMA, LorenzMC (2007) Mutations in Alternative Carbon Utilization Pathways in Candida albicans Attenuate Virulence and Confer Pleiotropic Phenotypes. Eukaryot Cell 6 : 280–290.
24. VieiraN, CasalM, JohanssonB, MacCallumDM, BrownAJ, et al. (2010) Functional specialization and differential regulation of short-chain carboxylic acid transporters in the pathogen Candida albicans. Mol Microbiol 75 : 1337–1354.
25. EneIV, AdyaAK, WehmeierS, BrandAC, MacCallumDM, et al. (2012) Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 14 : 1319–1335.
26. EneIV, ChengSC, NeteaMG, BrownAJ (2013) Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect Immun 81 : 238–248.
27. VylkovaS, CarmanAJ, DanhofHA, ColletteJR, ZhouH, et al. (2011) The Fungal Pathogen Candida albicans Autoinduces Hyphal Morphogenesis by Raising Extracellular pH. MBio 2: e00055–11.
28. MartinezP, LjungdahlPO (2005) Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol Cell Biol 25 : 9435–9446.
29. LayJ, HenryLK, CliffordJ, KoltinY, BulawaCE, et al. (1998) Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun 66 : 5301–5306.
30. BrandA, MacCallumDM, BrownAJ, GowNA, OddsFC (2004) Ectopic Expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell 3 : 900–909.
31. ReussO, VikA, KolterR, MorschhauserJ (2004) The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341 : 119–127.
32. HuynhKK, GrinsteinS (2007) Regulation of vacuolar pH and its modulation by some microbial species. Microbiol Mol Biol Rev 71 : 452–462.
33. McKenzieCG, KoserU, LewisLE, BainJM, Mora-MontesHM, et al. (2010) Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 78 : 1650–1658.
34. SheX, ZhangL, ChenH, CalderoneR, LiD (2013) Cell surface changes in the Candida albicans mitochondrial mutant goa1Delta are associated with reduced recognition by innate immune cells. Cell Microbiol 15 : 1572–1584.
35. WheelerRT, FinkGR (2006) A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2: e35.
36. RochaCR, SchroppelK, HarcusD, MarcilA, DignardD, et al. (2001) Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 12 : 3631–3643.
37. BalestrieriB, MaekawaA, XingW, GelbMH, KatzHR, et al. (2009) Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J Immunol 182 : 4891–4898.
38. KaposztaR, MarodiL, HollinsheadM, GordonS, da SilvaRP (1999) Rapid recruitment of late endosomes and lysosomes in mouse macrophages ingesting Candida albicans. J Cell Sci 112 (Pt 19) 3237–3248.
39. MarcilA, GadouryC, AshJ, ZhangJ, NantelA, et al. (2008) Analysis of PRA1 and its relationship to Candida albicans - macrophage interactions. Infect Immun 76 : 4345–4358.
40. MorN, GorenMB (1987) Discrepancy in assessment of phagosome-lysosome fusion with two lysosomal markers in murine macrophages infected with Candida albicans. Infect Immun 55 : 1663–1667.
41. NewmanSL, HollyA (2001) Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun 69 : 6813–6822.
42. BidaniA, ReisnerBS, HaqueAK, WenJ, HelmerRE, et al. (2000) Bactericidal activity of alveolar macrophages is suppressed by V-ATPase inhibition. Lung 178 : 91–104.
43. GordonAH, HartPD, YoungMR (1980) Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 286 : 79–80.
44. Ibrahim-GranetO, PhilippeB, BoletiH, Boisvieux-UlrichE, GrenetD, et al. (2003) Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun 71 : 891–903.
45. SchneiderB, GrossR, HaasA (2000) Phagosome acidification has opposite effects on intracellular survival of Bordetella pertussis and B. bronchiseptica. Infect Immun 68 : 7039–7048.
46. SchwartzJT, AllenLA (2006) Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J Leukoc Biol 79 : 1214–1225.
47. SeiderK, BrunkeS, SchildL, JablonowskiN, WilsonD, et al. (2011) The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 187 : 3072–3086.
48. EissenbergLG, GoldmanWE, SchlesingerPH (1993) Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med 177 : 1605–1611.
49. NewmanSL, GooteeL, HiltyJ, MorrisRE (2006) Human macrophages do not require phagosome acidification to mediate fungistatic/fungicidal activity against Histoplasma capsulatum. J Immunol 176 : 1806–1813.
50. BeckMR, DekosterGT, CistolaDP, GoldmanWE (2009) NMR structure of a fungal virulence factor reveals structural homology with mammalian saposin B. Mol Microbiol 72 : 344–353.
51. JohnstonSA, MayRC (2010) The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog 6: e1001041 doi: 10.1371/journal.ppat.1001041
52. HartPD, YoungMR (1991) Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J Exp Med 174 : 881–889.
53. OpperdoesFR, SzikoraJP (2006) In silico prediction of the glycosomal enzymes of Leishmania major and trypanosomes. Mol Biochem Parasitol 147 : 193–206.
54. PelosiA, SmithD, BrammananthR, TopolskaA, Billman-JacobeH, et al. (2012) Identification of a novel gene product that promotes survival of Mycobacterium smegmatis in macrophages. PLoS One 7: e31788.
55. IsaacDT, CoadyA, Van ProoyenN, SilA (2013) The 3-hydroxy-methylglutaryl coenzyme A lyase HCL1 is required for macrophage colonization by human fungal pathogen Histoplasma capsulatum. Infect Immun 81 : 411–420.
56. GhoshS, NavarathnaDH, RobertsDD, CooperJT, AtkinAL, et al. (2009) Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect Immun 77 : 1596–1605.
57. Alonso-MongeR, Navarro-GarciaF, MoleroG, Diez-OrejasR, GustinM, et al. (1999) Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol 181 : 3058–3068.
58. JacobsenID, BrunkeS, SeiderK, SchwarzmullerT, FironA, et al. (2009) “Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy”. Infect Immun 78 : 1066–1077.
59. KingsburyJM, McCuskerJH (2009) Cytocidal amino acid starvation of Saccharomyces cerevisiae and Candida albicans acetolactate synthase (ilv2{Delta}) mutants is influenced by the carbon source and rapamycin. Microbiology 156 : 929–939.
60. KirschDR, WhitneyRR (1991) Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect Immun 59 : 3297–3300.
61. NobleSM, JohnsonAD (2005) Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell 4 : 298–309.
62. DonovanM, SchumukeJJ, FonziWA, BonarSL, Gheesling-MullisK, et al. (2001) Virulence of a phosphoribosylaminoimidazole carboxylase-deficient Candida albicans strain in an immunosuppressed murine model of systemic candidiasis. Infect Immun 69 : 2542–2548.
63. Jimenez-LopezC, ColletteJR, BrothersKM, ShepardsonKM, CramerRA, et al. (2013) Candida albicans induces arginine biosynthetic genes in response to host-derived reactive oxygen species. Eukaryot Cell 12 : 91–100.
64. MartinezP, LjungdahlPO (2004) An ER packaging chaperone determines the amino acid uptake capacity and virulence of Candida albicans. Mol Microbiol 51 : 371–384.
65. PalkovaZ, JanderovaB, GabrielJ, ZikanovaB, PospisekM, et al. (1997) Ammonia mediates communication between yeast colonies. Nature 390 : 532–536.
66. WongL, SissonsC (2001) A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol 46 : 477–486.
67. Tomás MS, Nader-Macías ME (2007) Effect of a medium simulating vaginal fluid on the growth and expression of beneficial characteristics of potentially probiotic lactobacilli. In: Méndez-Vilas A, editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Badajoz, Spain: Formatex. pp. 732–739.
68. MuradAM, LeePR, BroadbentID, BarelleCJ, BrownAJ (2000) CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16 : 325–327.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



