-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Oral Bacteria and Cancer
article has not abstract
Published in the journal: . PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003933
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003933Summary
article has not abstract
Epidemiological Associations
Over a number of years, epidemiological studies established several well-defined risk factors for cancer, including age, heredity, diet, tobacco use, chronic viral infections, and inflammation. Paradoxically, the success of these studies left little room for incorporation of any new factors or causative agents, and, consequently, the idea that a bacterial infection could contribute to cancer was generally disregarded. However, landmark studies in the early 1990s established Helicobacter pylori as a causative agent of gastric cancers, resulting in a paradigm shift regarding the relationship between microbial agents and cancers [1]. Indeed, in 1994, H. pylori became the first bacterial species to be officially recognized by the World Health Organization as a definite cause of cancer in humans. Since then, there has been a growing body of evidence supporting an association between specific microorganisms, including those in the oral cavity, and various types of cancers.
The oral cavity is inhabited by complex multispecies communities that usually exist in a balanced immunoinflammatory state with the host [2]. Certain species, such as Porphyromonas gingivalis, can disrupt this equilibrium, resulting in a dysbiotic host–microbiota interaction. Subsequently, other community constituents, such as Fusobacterium nucleatum, can become opportunistically pathogenic, and the combined effect of a dysbiotic microbial community along with a dysregulated immune response ultimately causes periodontal disease [2]. These well-studied periodontal organisms have now emerged as the focal point for the developing association between oral bacteria and cancer.
Perhaps the most likely carcinogenic link with oral bacteria is with oral squamous cell carcinoma (OSCC), one of the most common cancers worldwide. OSCC surfaces have been reported to harbor significantly higher levels of Porphyromonas and Fusobacterium compared with contiguous healthy mucosa [3]. Moreover, immunohistochemistry with P. gingivalis antibodies revealed higher levels of detection and intensity of staining in gingival carcinomas compared with healthy gingival tissue, although only a small number of cases were examined [4]. A striking association has also been demonstrated between P. gingivalis infection and pancreatic cancer. In a prospective cohort study of over 400 cases and controls, a >2-fold increase in risk of pancreatic cancer was observed among those with high levels of antibodies to P. gingivalis, after adjusting for known risk factors [5]. Similarly, in the extensive National Health and Nutrition Examination Survey III, orodigestive cancer mortality was found to be related to the levels of P. gingivalis antibodies, independent of periodontal disease [6]. Several recent studies have shown a strong association between F. nucleatum and colorectal cancer (CRC) [7]–[10]. F. nucleatum was found to be one of the more abundant species within and around CRC neoplasms, and levels of F. nucleatum correlated with the presence of lymph node metastases.
Mechanistic Basis Supporting a Role for Oral Bacteria in Cancer
Epidemiological studies associate oral bacteria temporally and spatially with certain cancers and render involvement in the initiation or progression of the disease plausible. However, it is equally plausible that early undetected cancer, or precancerous lesions, facilitate the colonization and growth of oral bacteria. If these organisms are active participants in the disease process, then a mechanistic basis that would support an etiological role should exist.
Chronic or dysregulated inflammation has long been appreciated as contributing to tumor development, in part through modulation of the tumor microenvironment [11]. Both P. gingivalis and F. nucleatum establish chronic infections that involve intracellular persistence within epithelial cells, can spread systemically and cause extra-oral infections, and have well-characterized immune disruptive properties [12]. F. nucleatum is strongly proinflammatory, and McCoy et al. [7] demonstrated a positive correlation between mRNA levels for several local cytokines and Fusobacterium species in CRC cases. Furthermore, in the ApcMin/+ mouse model of intestinal tumorigenesis, F. nucleatum recruits tumor-infiltrating immune cells, thus generating a proinflammatory microenvironment that is conducive for CRC progression [13]. The inflammatory properties of P. gingivalis are more nuanced, and the organism can exhibit both pro - and anti-inflammatory properties, depending on the context [14], [15]. In either event, P. gingivalis has a major disruptive effect on local immune responses in the periodontal area [2]; however, the possible link with tumor development has yet to be investigated in molecular detail. In addition to broadly based immune-disruptive properties, both P. gingivalis and F. nucleatum impinge upon several aspects of epithelial cell signaling that have relevance to cancer progression.
P. gingivalis
Cancer cells, by definition, are defective in functional cell death pathways, and tumorigenesis is initiated when cells are freed from growth restraints. Epithelial cell responses to P. gingivalis infection include both changes to apoptosis and cell division (Figure 1). In primary cultures of gingival epithelial cells, P. gingivalis is strongly antiapoptotic and, indeed, can suppress chemically induced apoptosis [16]. P. gingivalis activates Jak1/Akt/Stat3 signaling that controls intrinsic mitochondrial apoptosis pathways [16], [17]. At the mitochondrial membrane, the activity of proapoptotic Bad is inhibited, and the Bcl2 (antiapoptotic):Bax (proapoptotic) ratio is increased, consequently curtailing the release of the apoptosis effector cytochrome c [18]. Further downstream, activation of both caspase-9 and the executioner caspase-3 is blocked. Remarkably, P. gingivalis possesses multiple mechanisms for inhibition of apoptosis in epithelial cells. Expression of microRNAs (miRs) is modulated, and up-regulation of miR-203 leads to inhibition of the negative regulator SOCS3 and subsequent suppression of apoptosis [19]. P. gingivalis secretes a nucleoside diphosphate kinase (NDK), which can function as an ATPase and prevent ATP-dependent apoptosis mediated through the purinergic receptor P2X7 [20]. Another potential role for NDK is diminishing ATP activation of P2X7 receptors on dendritic cells, which will impede activation of the NLRP3/ASC/caspase-1 inflammasome. This, in turn, will reduce secretion of IL-1β, which is important for the priming of IFNγ-producing tumor–antigen-specific CD8+ T cells [21]. In concert with suppression of apoptosis, P. gingivalis can accelerate progression through the S-phase of the cell cycle by manipulation of cyclin/CDK (cyclin-dependent kinase) activity and reducing the level of the p53 tumor suppressor [22]. A fimbrial-deficient mutant of P. gingivalis does not display this activity, suggestive of a role for the FimA adhesin in elevating epithelial cell proliferation. A role for LPS (lipopolysaccharide) in the dysregulation of p53 has been established [23], and the extent to which P. gingivalis LPS can target p53 requires further investigation.
Fig. 1. Interactions between P. gingivalis and epithelial cells that could produce an oncogenic phenotype. 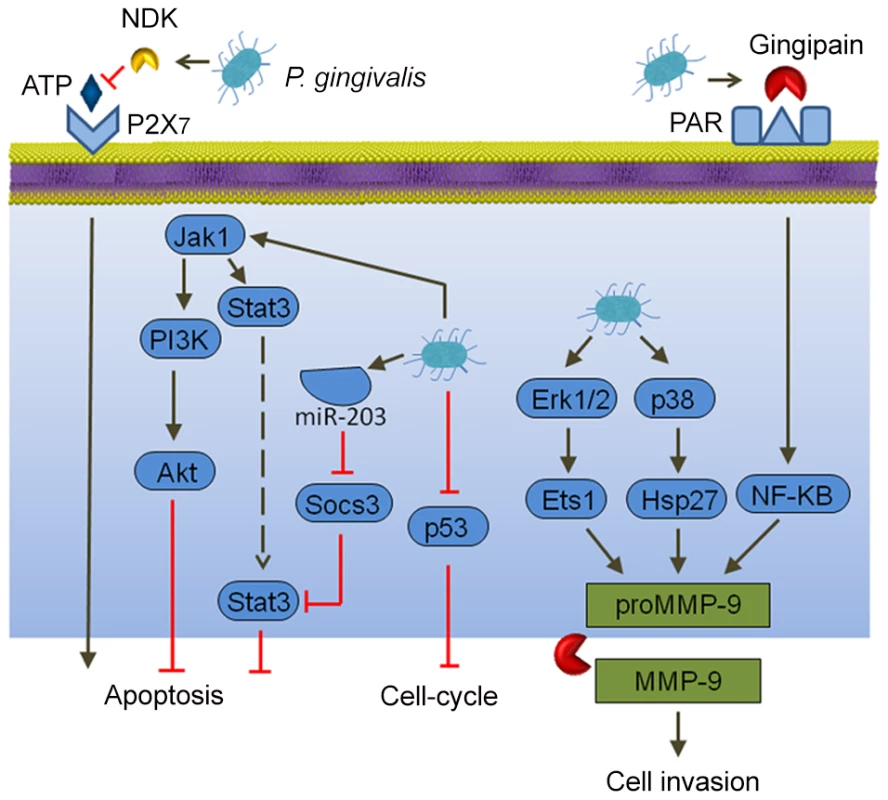
Extracellular P. gingivalis secrete gingipains, which activate Protease Activated Receptor (PAR) leading to promatrix metalloprotease (MMP)-9 production, and they also convert proMMP-9 to mature MMP-9, along with nucleoside diphosphate kinase (NDK), which cleaves ATP and prevents activation of the proapoptotic P2X7 receptor. Intracellular P. gingivalis activate antiapoptotic Jak-Stat signaling and inhibit expression of the p53 tumor suppressor. Additionally, Erk 1/2 and p38 are activated, which also elevates proMMP-9 expression. In both primary gingival epithelial cells and OSCC cells, P. gingivalis can induce the expression of the B7-H1 and B7-DC receptors [24]. These receptors are up-regulated in cells originating from a variety of cancers and contribute to chronic inflammation. Furthermore, B7-H1 expression promotes the development of regulatory T cells (Treg), which suppress effector T cells, and thus could contribute to immune evasion by oral cancers. Another impact of P. gingivalis on OSCC cells is in promoting cellular invasion. P. gingivalis infection activates the ERK1/2-Ets1, p38/HSP27, and PAR2/NF-ΚB pathways to induce promatrix metalloproteinase (MMP)-9 expression [25]. Gingipains, cysteine proteinases produced by P. gingivalis, play a dual role in this process. They both engage the PAR2 receptor and cleave the MMP-9 proenzyme into the mature active form. MMP-9 degrades basement membrane and extracellular matrix, which promotes carcinoma cell migration and invasion, thus allowing carcinoma cells to enter the lymphatic system and blood vessels for dissemination and metastatic growth at remote sites. In this manner, P. gingivalis may contribute to OSCC metastasis.
F. nucleatum
Epithelial cell responses to F. nucleatum infection are also consistent with carcinogenesis (Figure 2). Signaling molecules targeted by F. nucleatum include kinases involved in cell cycle control, and, as a result, F. nucleatum can elevate cell proliferation and migration [26]. F. nucleatum also activates p38, leading to the secretion of MMP-9 and MMP-13 (collagenase 3). Similar to MMP-9, MMP-13 plays an important role in tumor invasion and metastasis. Recently, a more direct relationship between F. nucleatum and CRC was demonstrated whereby the fusobacterial adhesin FadA binds to E-cadherin on colon cancer cells and activates β-catenin signaling [27]. This pathway leads to increased transcriptional activity of oncogenes, Wnt, and pro-inflammatory cytokines, as well as stimulation of CRC cell proliferation. In vivo relevance was established by the finding that fadA gene levels in colon tissue from patients with CRC were >10-fold higher compared with normal individuals.
Fig. 2. Interactions between F. nucleatum and epithelial cells that could produce an oncogenic phenotype. 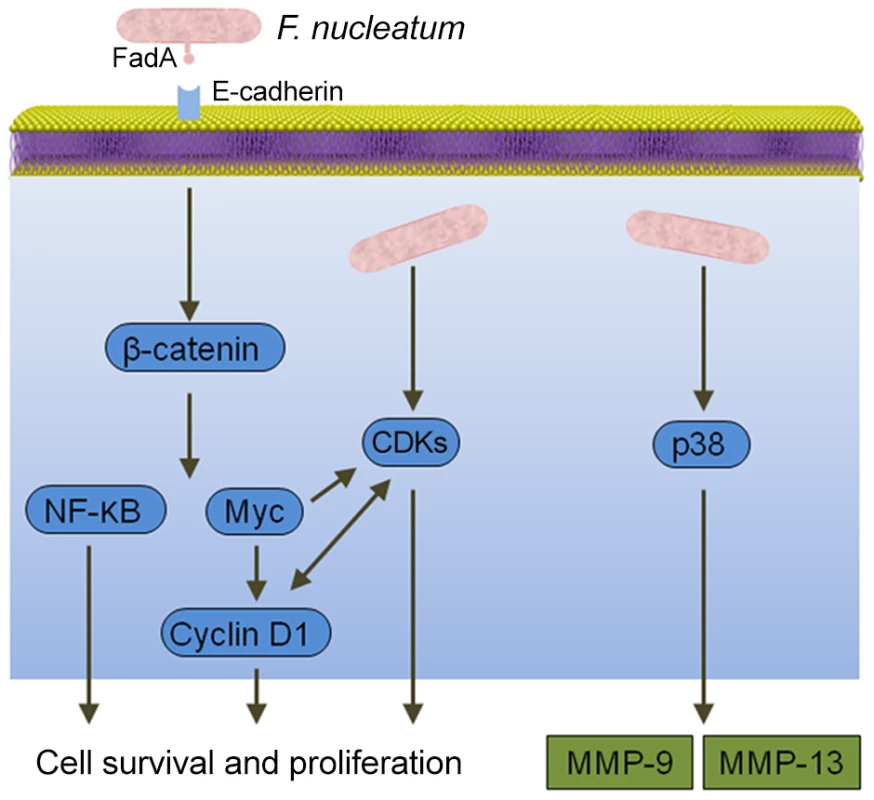
Binding of the FadA adhesin to E-cadherin activates β-catenin signaling, resulting in activation of genes that control cell survival and proliferation. F. nucleatum also activates several cyclin dependent kinases (CDKs) and p38, which controls the production of matrix metalloproteases MMP-9 and MMP-13. Conclusions
Both P. gingivalis and F. nucleatum have attributes consistent with a role in cancer development and progression. The question then arises as to why the widespread infection with these organisms leads to disease in only a limited number of individuals. Part of the answer may relate to the community nature of oral infections and the potential constraining influence of other bacteria. However, another consideration is the multifactorial etiology of cancer, and, within this framework, specific oral bacteria and their associated inflammatory insults may play a contributory, but not exclusive, role.
The implications of oral bacterial involvement in cancer are many. The detection of P. gingivalis or F. nucleatum in precancerous lesions could be used as a poor prognosis indicator. Improved oral hygiene and treatment of periodontitis may be useful in limiting the development or spread of cancer. Finally, since well-characterized virulence factors of P. gingivalis and F. nucleatum, such as the FimA and FadA adhesins, may function as effector molecules in the transition of normal epithelial cells to cancerous cells, they may provide novel targets for therapeutic intervention.
Zdroje
1. KimSS, RuizVE, CarrollJD, MossSF (2011) Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett 305 : 228–238.
2. HajishengallisG, LamontRJ (2012) Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 27 : 409–419.
3. NagyKN, SonkodiI, SzokeI, NagyE, NewmanHN (1998) The microflora associated with human oral carcinomas. Oral Oncol 34 : 304–308.
4. KatzJ, OnateMD, PauleyKM, BhattacharyyaI, ChaS (2011) Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci 3 : 209–215.
5. MichaudDS (2013) Role of bacterial infections in pancreatic cancer. Carcinogenesis 34 : 2193–2197.
6. AhnJ, SegersS, HayesRB (2012) Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 33 : 1055–1058.
7. McCoyAN, Araujo-PerezF, Azcarate-PerilA, YehJJ, SandlerRS, et al. (2013) Fusobacterium is associated with colorectal adenomas. PLOS One 8: e53653.
8. CastellarinM, WarrenRL, FreemanJD, DreoliniL, KrzywinskiM, et al. (2012) Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22 : 299–306.
9. KosticAD, GeversD, PedamalluCS, MichaudM, DukeF, et al. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22 : 292–298.
10. ChenW, LiuF, LingZ, TongX, XiangC (2012) Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLOS One 7: e39743.
11. Rakoff-NahoumS (2006) Why cancer and inflammation? Yale J Biol Med 79 : 123–130.
12. HanYW, WangX (2013) Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res 92 : 485–491.
13. KosticAD, ChunE, RobertsonL, GlickmanJN, GalliniCA, et al. (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14 : 207–215.
14. TakeuchiH, HiranoT, WhitmoreSE, MorisakiI, AmanoA, et al. (2013) The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. PLOS Pathog 9: e1003326.
15. LamontRJ, JenkinsonHF (1998) Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev 62 : 1244–1263.
16. MaoS, ParkY, HasegawaY, TribbleGD, JamesCE, et al. (2007) Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol 9 : 1997–2007.
17. YilmazO, JungasT, VerbekeP, OjciusDM (2004) Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun 72 : 3743–3751.
18. YaoL, JermanusC, BarbettaB, ChoiC, VerbekeP, et al. (2010) Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol 25 : 89–101.
19. MoffattCE, LamontRJ (2011) Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun 79 : 2632–2637.
20. YilmazO, YaoL, MaedaK, RoseTM, LewisEL, et al. (2008) ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol 10 : 863–875.
21. AymericL, ApetohL, GhiringhelliF, TesniereA, MartinsI, et al. (2010) Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 70 : 855–858.
22. KuboniwaM, HasegawaY, MaoS, ShizukuishiS, AmanoA, et al. (2008) P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect 10 : 122–128.
23. TangX, AsanoM, O'ReillyA, FarquharA, YangY, et al. (2012) p53 is an important regulator of CCL2 gene expression. Curr Mol Med 12 : 929–943.
24. GroegerS, DomannE, GonzalesJR, ChakrabortyT, MeyleJ (2011) B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 216 : 1302–1310.
25. InabaH, SugitaH, KuboniwaM, IwaiS, HamadaM, et al. (2013) Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol 16 : 131–145.
26. UittoVJ, BaillieD, WuQ, GendronR, GrenierD, et al. (2005) Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect Immun 73 : 1171–1179.
27. RubinsteinMR, WangX, LiuW, HaoY, CaiG, et al. (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14 : 195–206.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



