-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
Tomato Yellow Leaf Curl Virus Disease incited by Tomato yellow leaf curl virus (TYLCV) causes huge losses in tomato production worldwide and is caused by different related begomovirus species. Breeding for TYLCV resistance has been based on the introgression of multiple resistance genes originating from several wild tomato species. In this study we have fine-mapped the widely used Solanum chilense–derived Ty-1 and Ty-3 genes by screening nearly 12,000 plants for recombination events and generating recombinant inbred lines. Multiple molecular markers were developed and used in combination with disease tests to fine-map the genes to a small genomic region (approximately 70 kb). Using a Tobacco Rattle Virus–Virus Induced Gene Silencing approach, the resistance gene was identified. It is shown that Ty-1 and Ty-3 are allelic and that they code for a RNA–dependent RNA polymerase (RDR) belonging to the RDRγ type, which has an atypical DFDGD motif in the catalytic domain. In contrast to the RDRα type, characterized by a catalytic DLDGD motif, no clear function has yet been described for the RDRγ type, and thus the Ty-1/Ty-3 gene unveils a completely new class of resistance gene. Although speculative, the resistance mechanism of Ty-1/Ty-3 and its specificity towards TYLCV are discussed in light of the function of the related RDRα class in the amplification of the RNAi response in plants and transcriptional silencing of geminiviruses in plants.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003399
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003399Summary
Tomato Yellow Leaf Curl Virus Disease incited by Tomato yellow leaf curl virus (TYLCV) causes huge losses in tomato production worldwide and is caused by different related begomovirus species. Breeding for TYLCV resistance has been based on the introgression of multiple resistance genes originating from several wild tomato species. In this study we have fine-mapped the widely used Solanum chilense–derived Ty-1 and Ty-3 genes by screening nearly 12,000 plants for recombination events and generating recombinant inbred lines. Multiple molecular markers were developed and used in combination with disease tests to fine-map the genes to a small genomic region (approximately 70 kb). Using a Tobacco Rattle Virus–Virus Induced Gene Silencing approach, the resistance gene was identified. It is shown that Ty-1 and Ty-3 are allelic and that they code for a RNA–dependent RNA polymerase (RDR) belonging to the RDRγ type, which has an atypical DFDGD motif in the catalytic domain. In contrast to the RDRα type, characterized by a catalytic DLDGD motif, no clear function has yet been described for the RDRγ type, and thus the Ty-1/Ty-3 gene unveils a completely new class of resistance gene. Although speculative, the resistance mechanism of Ty-1/Ty-3 and its specificity towards TYLCV are discussed in light of the function of the related RDRα class in the amplification of the RNAi response in plants and transcriptional silencing of geminiviruses in plants.
Introduction
Plant pathogens are a major limiting factor for agricultural productivity worldwide. Viruses are among these and cause large yield losses in a variety of economically important crops. Although most viruses have small genomes and code for a very limited amount of proteins, they can cause a variety of disease symptoms, and the mechanisms underlying these are still mostly unknown. Plants utilize several lines of defense mechanisms to protect themselves from pathogen invasion. The mechanism that has been studied the most is resistance (R) gene-mediated resistance, which relies on the ability of a plant to recognize a pathogen and consequently trigger the hypersensitive cell death response (HR) [1]. Meanwhile, a large number of R genes have been identified, including ones responsible for the (in)direct recognition of viruses, such as Sw-5 for tospoviruses in tomato [2], Rx2 for Potato virus X [3] and the I locus for Bean common mosaic virus [4]. In addition to these dominant R genes, a second type of resistance gene is inherited recessively, which is more common in resistances to viruses compared with resistance to fungi or bacteria [5]–[6]. Most of these genes are linked to the eukaryotic translation initiation complex and negatively affect the viral RNA replication cycle [7].
RNA silencing (also called RNA interference, RNAi), is a conserved eukaryotic gene regulation mechanism that involves the biogenesis of small (s)RNA molecules of ∼21–26 nucleotides in size from perfect or imperfect long double stranded (ds)RNA molecules by an enzyme designated Dicer (mammals, insects), or Dicer-like protein (DCL) (plants) [8]. One strand of these sRNA molecules is incorporated into an RNA-induced silencing complex (RISC) and enables the latter to sense and target RNA molecules with sequence complementarity to the uploaded RNA strand for degradation or translational arrest by means of the core Argonaute (AGO) protein. In recent years, RNA silencing has become known as an antiviral defense mechanism in plants and insects in which viral double-stranded RNA replicative intermediates or secondary RNA folding structures are cleaved into primary, small-interfering (si)RNA molecules. In plants, the viral primary siRNA molecules also act as primers for the host RNA-dependent RNA polymerases (RDR) to convert (aberrant) RNA target sequences into new long dsRNAs. These in turn become processed into secondary siRNAs. This not only leads to an amplification of the siRNA signal, but also results in a distributional spread of siRNA molecules from the entire RNA target sequence, referred to as transitive silencing [9]. The amplification of siRNAs is required to mount a strong antiviral RNAi response. Arabidopsis RDR1, 2 and 6, and orthologs of these genes, have been demonstrated to be involved in this amplification and plants from which these genes have been knocked-out exhibit higher susceptibility to various plant viruses [10]–[14].
The whitefly transmitted tomato yellow leaf curl disease (TYLCD) is one of the most devastating diseases of tomato (Solanum lycopersicum) and is caused by several species of the Begomovirus genus (Geminiviridae) [15]. Tomato yellow leaf curl viruses (TYLCV) are the most widespread and currently rank 3rd among the economically and scientifically most important plant viruses worldwide [16]. They have a single-stranded circular bi-directionally organized DNA genome with six partially-overlapping open reading frames [17]. Because of their limited coding capacity they rely, like most viruses, not only on their own proteins but also on the host cell machinery for their infection cycle [18]. Since the whitefly insect vector is hard to control, breeding TYLCV resistant tomato cultivars provides an attractive strategy to manage TYLCV. All domesticated tomatoes are susceptible to TYLCV, but high levels of resistance were found in several related wild tomato species. Genetic studies have led to the mapping of five TYLCV resistance/tolerance genes which are being exploited for resistance breeding. These genes have different origins: Ty-2 was introgressed from S. habrochaites, Ty-5 (ty-5) was introgressed from S. peruvianum while Ty-1, Ty-3 and Ty-4 all originated from different S. chilense accessions [19]–[24]. So far, none of these genes have been cloned and the underlying resistance mechanisms are still unknown. In contrast with classical R-genes none of the resistances to TYLCV described so far are associated with a HR. Moreover, in almost all TYLCV resistant materials, viral replication occurs [25]–[28]. This also holds true for Ty-1/Ty-3, where in the donors (S. chilense LA1969/LA1932) as well as in a commercial line with a Ty-1 introgression (3761, A.B. Seeds, Ness Ziona, Israel) TYLCV is replicating and detectable [29]–[31], although the level does not exceed more than 10% of that in susceptible tomato cultivars.
Though many loci (i.e. Ty-1 to Ty-5) for TYLCV resistance have been described, the genes conferring resistance have not been identified. Recently, several papers have reported on host genes in a gene network contributing to the resistance originating from S. habrochaites [32]–[34]. By differential cDNA library comparisons of susceptible and resistant tomato lines before and after TYLCV inoculation, approximately 70 genes were found to be preferentially expressed in a tomato line with a resistance introgressed from S. habrochaites. For three of those, a lipocalin-like protein (SlVRSLip), a Permease I-like protein and a hexose transporter LeHT1, it was shown that their silencing (partly) compromised resistance.
In our previous study we found that Ty-1 and Ty-3 map closer than previously reported and that they might be allelic [35]. In the present study Ty-1 and Ty-3 are fine mapped, and using a Tobacco Rattle Virus (TRV) induced silencing approach, the genes have been identified and found to be allelic. They code for an RNA-dependent RNA polymerase (RDR) of the γ class, a class of RDRs for which no function is yet described. The role of this new class of resistance genes will be discussed in light of the TYLCV infection cycle.
Results
Fine-mapping of Ty-1 and Ty-3
Previously, we mapped Ty-1 in the interval between MSc05732-4 and MSc05732-14 [35]. To fine-map Ty-1, markers T0774 and SL_2.40ch06_30.891, which flank this interval, were used to screen an F2 population derived from a cross between the susceptible Fla. 7776 and a recombinant inbred line (RIL) carrying the S. chilense Ty-1 introgression. Approximately 2,000 F2 plants were screened, 13 recombinants were identified, and RILs were developed for each of these (designated R1 to R13). Four RILs (R1, 4, 12 and 5) containing the S. chilense introgression between markers Hba0161K22 and WU_M31 were resistant, while eight RILs that lacked this interval were susceptible (Figure 1A). R7, which resulted from a recombination event between markers WU-M27 and UF_TY3-P19, showed an intermediate response. These results were confirmed for the three most informative recombinants (R7, R8 and R11) (Table S1) using agroinoculation and show that Ty-1 is located between HBa0161K22 and WU_M31, an interval of approximately 70 kb.
Fig. 1. Physical maps showing control lines and introgressed fragments in the RILs used to map Ty-1 and Ty-3. 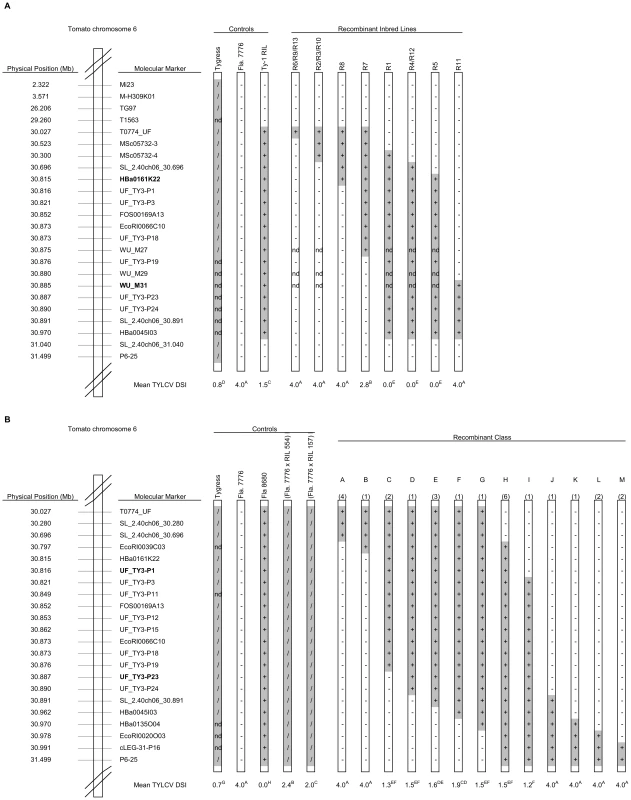
Introgressed segments of the S. chilense genome are shaded grey; genotype for each line at each marker is indicated (+ = homozygous S. chilense; / = heterozygous; − = homozygous S. lycopersicum; nd = not determined). Approximate physical positions are based on the tomato genome assembly SL_2.40, available through the Sol Genomics Network (SGN; http://solgenomics.net/). DSI = mean disease severity index as described in the Materials and Methods; within either population, different superscript letters represent statistically significant differences at P<0.05 based on Duncan's multiple range test. A: Control lines and RILs used for mapping of Ty-1. Flanking markers of the Ty-1 region, HBa0161K22 and WU_M31, are depicted in bold. B: Control lines and RILs used for mapping of Ty-3. The number of recombinants recovered in each class is given in parentheses above each recombinant chromosome. Flanking markers, UF_TY3-P1 and UF_TY3-P23 of the Ty-3 region are depicted in bold. The Ty-3 gene was previously mapped between T0774 and T1079 [21]. By screening an F2 population (n = 717) from a cross between the susceptible line Fla. 7781 with the resistant line Fla. 8680 (carrying the Ty-3 introgression from S. chilense LA2779), 30 recombinants were identified. RILs of these recombinants were generated and tested with TYLCV. Results mapped Ty-3 to the interval between T0774 and P6-25 (Table S2). To further narrow down the Ty-3 interval, RILs of two key recombinants were used to generate three F2 sub-populations, A, B and C. Screening more than 10,500 individuals of these sub-populations with markers Mi23 and P6-25 (sub-population A and B) and markers T0774 and T0834 (sub-population C) identified 309 recombinants (Table S3). Cuttings of these recombinants were evaluated for TYLCV disease severity (Table S3; control experiments, Table S4) and interval QTL mapping confirmed the location of Ty-3 between markers T0774 and P6-25, with a LOD of over 50 in an interval between markers SL_2.40ch06_30.696 and cLEG-31-P16 (Figure S1). Recombinants in this interval were further analysed by testing their RILs with TYLCV and by saturating this region with additional molecular markers (Figure 1B, Table S5). RILs carrying the S. chilense LA2779 introgression between markers UF_TY3_P1 and UF_TY3_P23 were resistant (recombinant class C to I, Figure 1B), while RILs with introgressions that did not span this region were susceptible; these results map Ty-3 to a region of approximately 71 kb that overlaps the region containing Ty-1 (Figure 2).
Fig. 2. Schematic representation of the region of interest of chromosome 6. 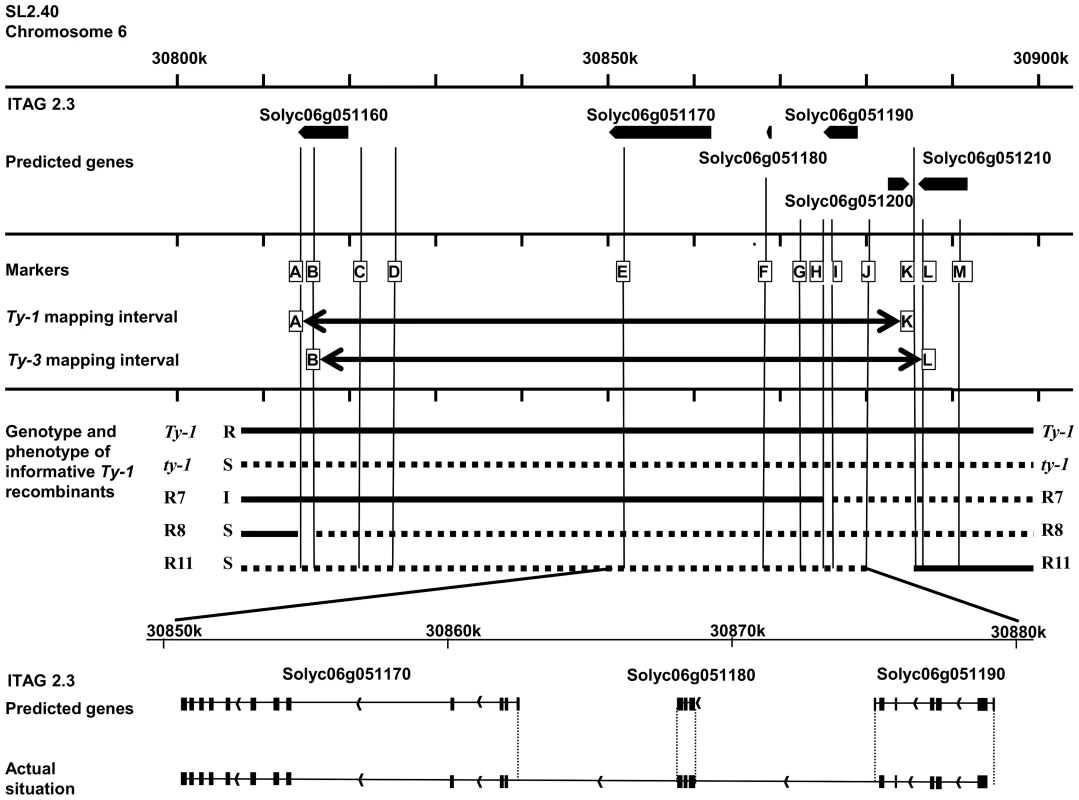
Depicted is the region 30,800,000 to 30,900,000 of chromosome 6 with the genomic annotations of the ITAG2.3 release [36]. In the first frame the six predicted genes are represented by arrows. In the next frame the markers used to genotype the recombinants in this study are shown (A = HBa0161K22, B = UF_TY3-P1, C = UF_TY3-P3, D = WU_M17, E = FOS00169A13, F = WUR_M25, G = UF_TY3-P18, H = WU_M27, I = UF_TY3-P19, J = WU_M29, K = WU_M31, L = UF_TY3-P23, M = UF_TY3-P24). In this frame also the Ty-1 and Ty-3 intervals with their flanking markers are depicted. The third frame shows the genotype of the informative recombinants used to fine map Ty-1, R7, R8 and R11, note that only for R7 the precise recombination point is known (Figure S2). Also their phenotype upon TYLCV challenge inoculation is shown (Resistant (R), Susceptible (S) and Intermediate (I)). The last frame shows the predicted splicing of gene Solyc06g051170, Solyc06g051180 and Solyc06g051190 compared with the actual situation; differences are indicated with dotted lines. Candidate genes for Ty-1 and Ty-3
According to the ITAG2.3 release of the tomato genome, the region to which Ty-1/Ty-3 mapped was predicted to contain five genes; Solyc06g051160 (408 bp), Solyc06g051170 (1728 bp), Solyc06g051180 (438 bp), Solyc06g051190 (957 bp) and Solyc06g051200 (843 bp) [36] (Figure 2). While gene Solyc06g051160 has an unknown function and Solyc06g051200 encodes a predicted ribosomal protein, the other three genes are each predicted to encode (parts of) an RNA-dependent RNA polymerase (RDR). Arabidopsis thaliana RDRs in general are approximately 3 kb in size, but these three predicted genes are all much shorter. Since the genes only share low sequence similarity they likely are not paralogous. Interestingly, the crossing-over event in the intermediate resistant R7 occurred within the candidate gene Solyc06g051190. After amplification and sequence analysis of this gene from R7 and subsequent alignment to the corresponding regions of a Ty-1 line and a ty-1 line, the recombination site in R7 could be pinpointed between two SNPs. This region covered less than 100 base pairs in which the recombination point mapped to the last part of predicted exon number 4 (Figures S2 and S3). Plants of R7 thus contained a chimeric predicted gene Solyc06g051190.
Silencing of Solyc06g051180 and Solyc06g051190 compromises resistance
To identify the Ty-1 gene from the five candidate genes predicted in the Ty-1 interval, a Tobacco Rattle Virus (TRV) based Virus Induced Gene Silencing (VIGS) approach was applied. For three out of five genes a VIGS construct could be made; TRV2-160 for Solyc06g051160; TRV2-180 for Solyc06g051180 and TRV2-190 for Solyc06g051190. The two VIGS vectors, TRV2-180 and TR2-190, are specific and both are assumed to target an individual RDR, due to low sequence similarity between Solyc06g051180 and Solyc06g051190. Several attempts to make a VIGS construct for Solyc06g051170 and Solyc06g051200 failed so experiments were done with the available constructs. When plants containing Ty-1 were agroinfiltrated with empty vector control (EV, TRV2 without an insert) or TRV2-160, and two weeks later superimposed with a TYLCV challenge, the plants maintained resistance to TYLCV. However, when either TRV2-180 or TRV2-190 was used, the resistance was compromised as observed by the appearance of TYLCV disease symptoms (Figure 3). Repeated analysis confirmed these results, which, together with the fact that both Solyc06g051180 and Solyc06g051190 are predicted RDRs located in close proximity to one another within the Ty-1/Ty-3 region, suggest that Solyc06g051180 and Solyc06g051190 might belong to one and the same gene.
Fig. 3. Silencing with constructs TRV2-180 and TRV2-190 compromises TYLCV resistance in Ty-1 and Ty-3 lines. 
Depicted are leaves of plants 6 weeks after inoculation of the TRV silencing constructs and 4 weeks after TYLCV challenge inoculation. EV, empty vector control; 160, TRV2-160; 180, TRV2-180; 190, TRV2-190. All Moneymaker (MM) plants are susceptible, constructs TRV2-180 and TRV2-190 compromise resistance in Ty-1 and Ty-3 carrying lines but not in a line with a Ty-2 introgression. Ty-1 and Ty-3 are allelic
Our initial mapping studies indicated that Ty-1 and Ty-3 could be alleles of the same gene [35], and the fine mapping of both genes to a similar marker interval strengthened this hypothesis. To test this, the Ty-1 VIGS approach was again applied to compromise TYLCV resistance in plants carrying the Ty-3; as a control, plants with resistance based on Ty-2 were included. As in the Ty-1 plants, resistance in the Ty-3 lines was compromised by TRV2-180 and TRV2-190, but not by TRV-160 (Figure 3). On the other hand, plants containing Ty-2 remained fully resistant against TYLCV after silencing with all three constructs. Altogether these data indicate that Ty-1 and Ty-3 indeed are allelic, while Ty-2 belongs to another class of resistance genes.
Solyc06g051170, Solyc06g051180, and Solyc06g051190 together code for Ty-1 and Ty-3
To test the hypothesis that Solyc06g051170, Solyc06g051180 and Solyc06g051190 were part of the same gene, and to clone the entire Ty-1 gene, several primer pairs were designed to enable RT-PCR amplification of the exons from the three predicted genes, and tested on cDNA of Ty-1 lines and TYLCV susceptible cv. Moneymaker. Primers designed on the start and stop codons of the three predicted genes did not amplify any products. However, when primers were used that were located a bit downstream of the start codon or upstream of the stop codon products were amplified, indicating that the predicted start and stop codons were wrong. To test whether the initially predicted genes were all part of one RDR-encoding ORF other primer pairs were tested. When primers targeting Solyc06g051170 were combined with Solyc06g051190 (Figure S4, F6-R4) surprisingly a product of approximately 700 bp was amplified indicating that all three predicted genes were indeed not paralogous but part of one and the same RDR gene. This was confirmed by sequence analysis of all overlapping PCR fragments obtained (Figure S4). Using a GeneRacer (Invitrogen) approach the genuine start and stop codons of the RDR gene were identified. Based on these sequences new primers (Table S6, Ty-F7-CACC and Ty-R5) were designed that supported the amplification of a product of approximately 3.1 kb from cDNA of a Ty-1 line, a Ty-3 line and from cv. Moneymaker.
Ty-1 and Ty-3 are RDR3/4/5 homologues
Sequence analysis of the amplified Ty-1/Ty-3 gene products revealed that the gene contained 19 exons. Compared with the three predicted genes the first predicted exon of Solyc06g051190 was not expressed, nor was the last exon containing the stop codon (Figure 2). For Solyc06g051180 the first exon started earlier than predicted, the last exon was shorter than predicted, again losing the stop codon. Finally for Solyc06g051170 the first predicted exon was not expressed. Alignment of the amino acid (aa) sequences of Ty-1, Ty-3 and ty-1 (the susceptible allele from tomato cv. Moneymaker) revealed high sequence identity between all alleles, with only small differences. The most significant difference was a four aa deletion in the N-terminal domain of the susceptible allele. In addition, 20 aa changes were observed, with only small differences between Ty-1 and Ty-3.
Multiple sequence alignment with the six RDRs identified in A. thaliana (Figure S5 and S6) showed a high sequence similarity to RDR3, RDR4, and RDR5 and the presence of the atypical DFDGD catalytic motif of these genes in both Ty-1 and Ty-3 alleles (Figure 4A). The homology inferred from the sequence similarity was supported by a phylogenetic analysis using an unrooted neighbor joining tree, in which Ty-1 and Ty-3 grouped in the clade containing RDR3, 4 and 5 (Figure 5). Interestingly, although the ty-1 allele (Moneymaker) appeared in the same clade, it showed less similarity to RDR3/4/5 then the Ty-1/Ty-3 allele (Figure 4B).
Fig. 4. Catalytic domain and polymorphism of Ty-1 and Ty-3. 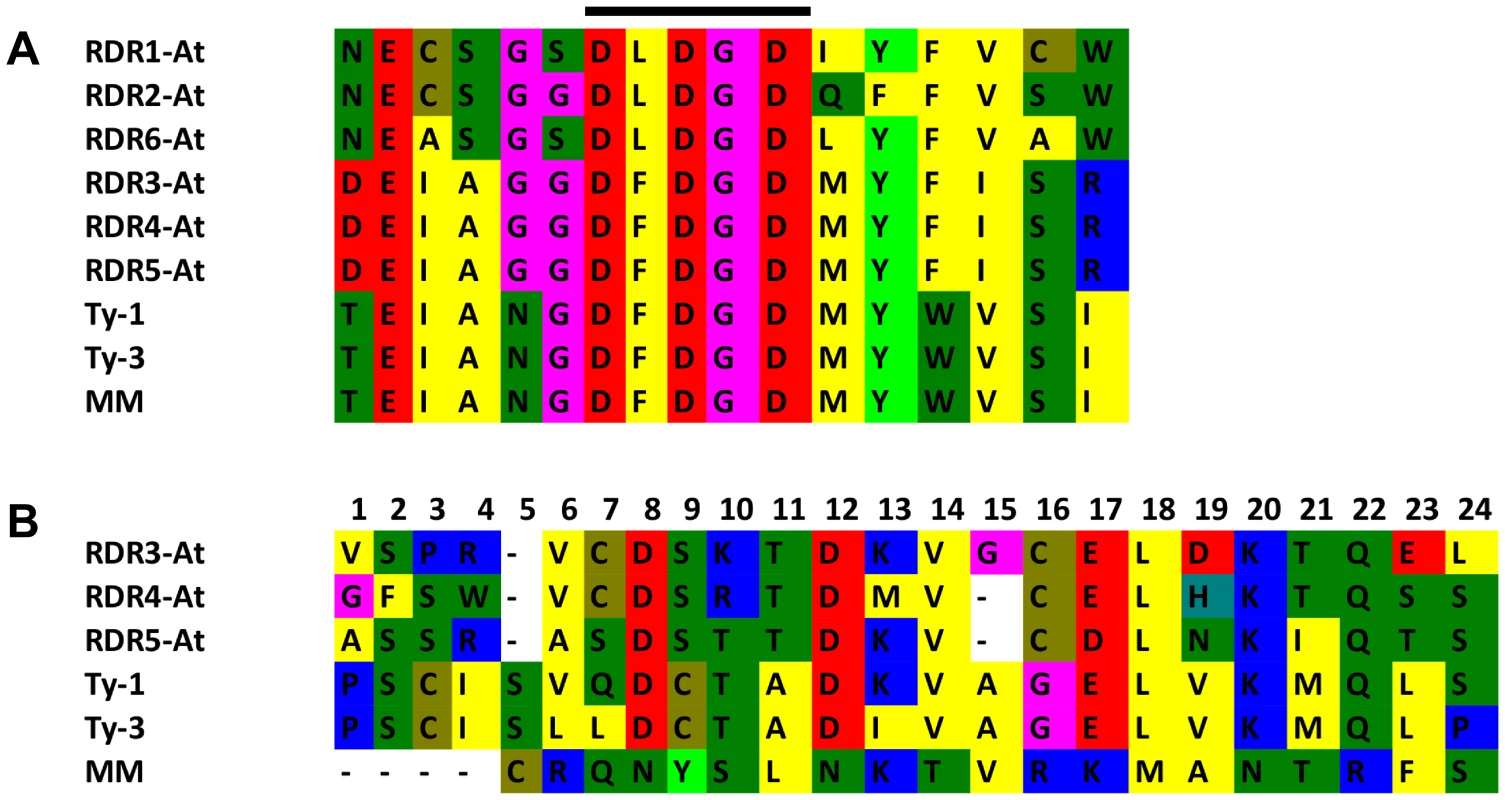
A: Alignment of the catalytic domain and 6 amino acids up- and downstream of the A. thaliana RDR1-6, Ty-1, Ty-3 and Moneymaker (MM) alleles. The catalytic domain is indicated with a black bar above the alignment. B: All 24 polymorphisms between Moneymaker and S. chilense are shown. The RDR3-5 A. thaliana amino acids depicted are taken from a Clustel W alignment (Figure S5 and S6). Fig. 5. Neighbour joining tree of protein sequences of <i>A. thaliana</i> RDR1-6, <i>Ty-1</i>, <i>Ty-3</i>, and the susceptible Moneymaker allele (MM). 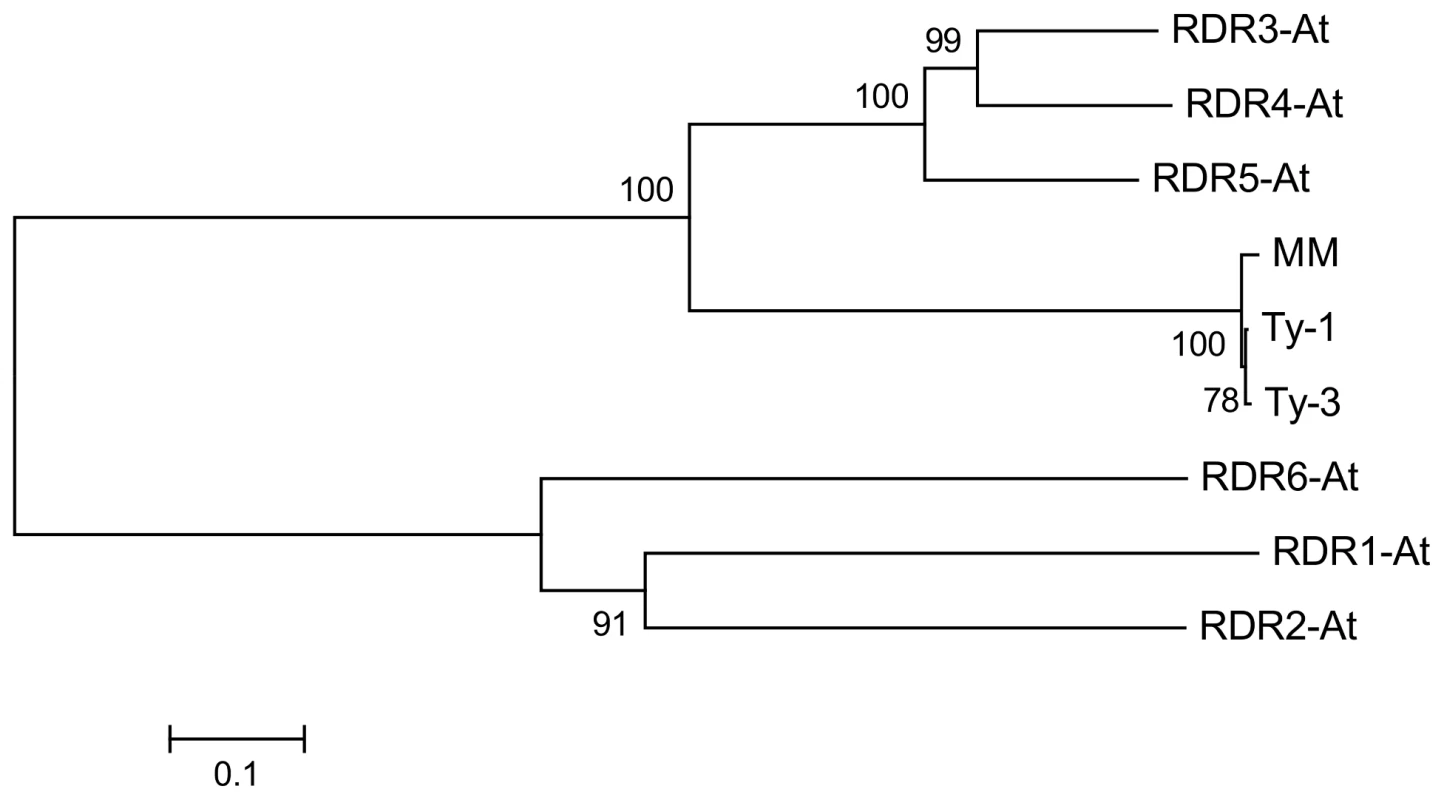
Ty-1 is relatively high expressed
Considering a potential role of the Ty-1 encoded RDR in mounting a strong antiviral RNAi response, its transcriptional expression level was analyzed. To this end, a time-series experiment was performed during which expression of the resistant Ty-1 and the susceptible ty-1 allele was quantified upon TYLCV-challenge via agroinoculation in both tomato lines. The expression level of the specific allele was measured by qPCR at several time points (Figure 6). The results showed that at all time points the basic transcription level of the Ty-1 allele was significantly higher compared to the ty-1 allele. In the resistance line, no significant difference was observed for the Ty-1 expression between mock and TYLCV inoculated plants at all time points. However, in the susceptible Moneymaker line, the expression of the ty-1 allele was induced upon TYLCV inoculation at 12 and 19 days. Compared with day 0 of resistant and susceptible lines, the respective expression of Ty-1 and ty-1 was decreased at day 5 and increased at day 19.
Fig. 6. Ty-1 expression is elevated in resistant lines. 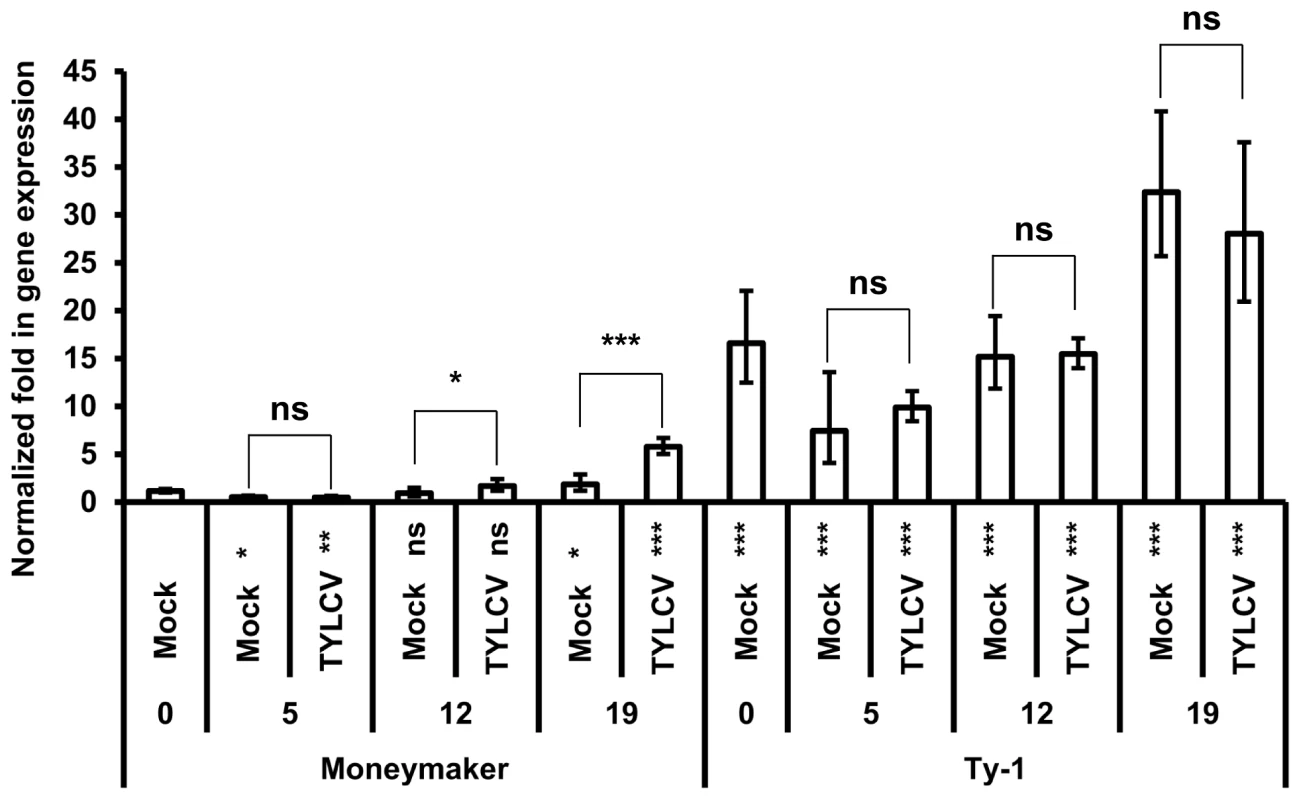
Normalized fold in gene expression of the ty-1 (susceptible) and Ty-1 (resistant) allele. Numbers below x-axis indicate days after inoculation. Values are normalized against the Moneymaker day 0 sample, bars represent means and standard deviation of five biological replicas. Asterisks under the x-axis represent significant differences to the Moneymaker day 0 sample, asterisks above the bars represent significant differences between Mock or TYLCV treatment. (* = P<0.05, ** = P<0.01, *** = P<0.001, ns = not significant). Discussion
Nowadays many dominant and recessive virus resistance genes are well characterized and used in breeding of various crops. Most of these genes either do not allow/prevent viral replication or limit this to the first cells of entry in the host. The TYLCV resistance genes Ty-1 and Ty-3 are different from these because they lead to a level of virus tolerance (rather than immunity). Plants carrying these genes and challenged by the virus still show low levels of viral replication and systemic spread but with moderate (as with Ty-3) or no (as with Ty-1) visual symptoms. Recently we observed that the S. chilense LA1969 derived Ty-1 and the S. chilense LA2779 derived Ty-3 map close to each other and that they might be allelic [35]. Here we show by fine mapping and functional analysis that Ty-1 and Ty-3 are alleles of the same gene and code for RNA-dependent RNA polymerases from a class of functionally unknown RDR genes.
Sequence data shows that most of the SNPs that are present in Ty-1 are also present in Ty-3, which is logical since both alleles originate from S. chilense accessions. The most striking difference between Ty-1/Ty-3 and the ty-1 allele is a deletion of 4 amino acids in the first amino-terminal part of the protein. However, it is not likely that this deletion solely causes a functional loss, since recombinant R7 contains a chimeric RDR – with the N-terminal part of ty-1, and still confers partial resistance to TYLCV.
Recently, three genes have been reported which are involved in different networks related to TYLCV resistance introgressed from S. habrochaites [32]–[34]. Of the three identified genes, SlVRSLip functions downstream LeHT1 within the same network, while Permease I-like protein functions in a different network [32]–[34]. In another study, 18 host genes with a potential role in Tomato Yellow Leaf Curl Sardinia Virus (TYLCSV) infection were identified. Interestingly, almost half of these genes had a role in posttranslational modifications [37]. Whether RDRs encoded by Ty-1 and Ty-3 play a role in one any of these networks remains to be analysed.
RDRs are defined by a conserved catalytic domain and are found in RNA viruses and multicellular organisms (plants, fungi and invertebrate animals), but so far are not described in vertebrates and insects. For RNA viruses, the RDR is required to enable replication of its RNA genome to render viral progeny [38]. In multicellular organisms, three major classes of eukaryotic RDRs have been described and some of their functions have been unraveled. The first class is presented by RDRα and members of these are found in plants, animals and fungi. The class of RDRβ genes has been found only in animals and fungi while RDRγ members are only found in plants and fungi [39]. In the model plant A. thaliana a total of six RDRs have been identified [40]. Three of them belong to the RDRα type, i.e. RDR1, RDR2 and RDR6, and are characterized by a catalytic DLDGD motif. The other three belong to the RDRγ class of genes and are denoted RDR3, RDR4 and RDR5 (also referred to as RDR3a, RDR3b and RDR3c, respectively). Members of this class have an atypical DFDGD motif in the catalytic domain [40].
The RDRα genes are all known to be involved in RNA silencing, specifically in the amplification of the siRNA signal and resulting in transitive silencing. RNA silencing is generally accepted as a defense system against viral invasion, and is induced by viral dsRNA replicative intermediates or folding structures [41]. Geminiviruses are also targeted by RNAi, as observed by the synthesis of geminivirus-specific siRNAs, (small-RNA directed) viral DNA methylation and post-transcriptional gene silencing of the protein-coding genes [42]–[45]. Although geminiviruses contain a single stranded DNA genome, siRNAs have been observed to originate from the entire virus genome although their distribution was not always equal. The siRNAs are postulated to originate in two ways; 1) as a result of DCL processing from dsRNA molecules that are generated by RDR from bidirectional geminivirus transcripts with overlapping 3′ ends, and 2) mRNA folding structures [42]–[43], [45]–[46].
It is proposed that plants employ silencing of DNA by RNA-directed methylation as a strategy to repress geminivirus replication/transcription [47]. This is supported by two major observations; methylation of geminivirus DNA greatly reduces its ability to replicate in protoplasts [48], and the identification of geminivirus RNA silencing suppressor proteins (RSS) C2, C4 and V2 that exert their activity by interference in the process of DNA methylation and transcriptional gene silencing [49]–[56]. Antiviral RNAi defense against geminiviruses thus seems to mostly rely on a methylation-based defence, a process that involves the action of siRNA-directed methylation pathway component Ago4. Although several studies have pointed towards the involvement of RDR1 and RDR6 in the biogenesis of geminivirus-specific siRNAs, the involvement of other antiviral RDRs in this cannot yet be excluded [10], [57].
Besides their role in RNAi, several studies have described other (endogenous) functions of the RDRα (1, 2 and 6) genes [58], e.g. being involved in herbivore resistance (RDR1) [59], female gamete formation (RDR2 and 6) [60] or in developmental timing (RDR6) [61]. While a knockdown of RDR from the RDR1/2/6 class renders plants highly susceptible to many different viruses [11], their transcriptional up-regulation has been observed to lead to (elevated) resistance levels against different plant viruses [62].Viruses are able to counteract RNAi by coding for viral RSS proteins, and many of these have been shown to sequester siRNAs and prevent their uploading into RISC [63]. The presence of a viral RSS, however, does not seem to enable viruses to overcome elevated levels of resistance caused by transcriptional up-regulation of the RDR1/2/6 class of genes.
For RDR3, RDR4, and RDR5 a function has not yet been described [64]. How to explain the resistance mechanism of the Ty-1/Ty-3 encoded RDRs remains speculative at present. The resistance spectrum of these alleles is not well studied; Ty-3 also provides resistance to the bipartite Tomato mottle virus (ToMoV), but studies describing disease tests with other geminiviruses on Ty-1/Ty-3 carrying lines are not available [21]. These genes act specifically on geminiviruses; what then is the identity of the (conserved?) Avr protein, and what are the characteristics of resistance breaking isolates? Considering the role of the DLDGD type of RDRs (1,2 and 6) in the generation of secondary siRNAs, irrespective of the RNA virus involved, it is tempting to propose a role of the DFDGD type of RDRs (3,4 and 5), and thus of Ty-1/Ty-3, in the formation of dsRNA too. Since Ty-1/Ty-3 lines are resistant to TYLCV, but still allow for a symptomless (Ty-1) or an almost symptomless (Ty-3) infection with low titres of TYLCV, a resistance strategy as earlier described for the RDRα (1,2 and 6) genes could be possible, where transcriptional up regulation provides (elevated) resistance levels against different plant viruses.
In light of this, transcriptional expression analysis of Ty-1 showed elevated expression levels in resistant lines compared to those in susceptible lines, even without TYLCV challenging. Whether differences in the Ty-1 vs. ty-1 protein or just those in transcriptional expression levels, or even a combination of both, are the cause of resistance remains to be investigated. However, since we did not observe hyper-susceptibility in tomato Moneymaker after silencing of the susceptible allele, as what is observed for Potato Virus X (PVX) and potato potyvirus Y (PVY) after silencing of Nicotiana benthamiana RDR6 [14], a function of ty-1 in resistance is highly unlikely. The functionality and transcriptional upregulation of Ty-1 thus seems the most plausible reason to explain the resistance. To solve this issue, transgenic tomato lines (over)expressing either the resistant allele or the susceptible allele will be made. Analysis of the expression level and protein sequence of Ty-1/ty-1 in other resistant/susceptible tomato varieties and wild species will additionally be informative and experiments for these are currently being prepared.
The observed resistance specificity of Ty-1/Ty-3 against TYLCV does seem to contradict the idea that its transcriptional up regulation provides (elevated) resistance levels against other geminiviruses unless people have somehow overlooked a partial resistance to other, distinct geminiviruses. Furthermore, it is possible that the RDRγ (3, 4 and 5) class of genes may be involved in the generation of siRNAs that will mainly direct methylation of DNA and thereby support transcriptional silencing of geminivirus DNA genomes. If this hypothesis is true, this could explain why these genes will not confer (partial) resistance to most other plant viruses, of which ∼75% harbours an RNA genome and thus cannot be transcriptionally silenced by the siRNA-directed DNA methylation pathway.
The possibility of an alternate route for dsRNA formation during geminivirus infections, besides the one involving RDR1/2/6, is being supported by the observations that mutants lacking RDR1, RDR2 and RDR6 still revealed basal levels of RNA silencing and siRNA biogenesis, and plants infected with TYLCV only showed a moderate increase in susceptibility to geminiviruses in plants deficient in RDR2 and 6 [11], [47]. Whether the Ty-1/Ty-3 encoded RDR represents a player in this, and how the resistance mechanism acts, will be a challenge to investigate in the near future.
Materials and Methods
Plant material
For fine-mapping Ty-1 from S. chilense accession LA1969, a TYLCV-resistant commercial hybrid Tygress with an introgression between markers Mi23 and P6-25, reflecting the same interval as described by Verlaan et al. (2011), was used. This Ty-1 introgression was done by Jaap Hoogstraten of the Royal Sluis Seed Company, and it is different from the LA1969 Ty-1 introgression that was done in Israel [19]. This hybrid was self-pollinated to produce F2 progeny. Through two cycles of selection for recombination in this F2 population, two recombinants were identified and used to generate RILs by selfing and selection with marker genotyping for homozygous introgressions. The first recombination event resulted in a resistant RIL containing a S. chilense introgression flanked by markers Mi23 and HBa0045I03 and was used as a control (named as Ty-1 RIL, Figure 1) in all Ty-1 experiments. Another recombination event resulted in a resistant RIL containing a S. chilense introgression between markers T0774 and HBa0045I03. The susceptible Fla. 7776 was crossed to this inbred and an F2 population was generated. Approximately 2000 F2 plants were screened for recombination between the markers T0774 and SL_2.40ch06_30.891 and 13 recombinants were identified. These recombinants were selfed to develop F4 RILs as described before. RILs were evaluated, along with the controls Fla. 7776, Tygress and the Ty-1 RIL in fall 2011. Four week-old seedlings were inoculated with TYLCV for 11 days then transplanted to the field on 4 October in a non-randomized trial with two replications of 4-plant plots. TYLCV disease severity was evaluated on each plant 6 weeks after exposure to whiteflies. For the three most informative recombinants (R7, R8 and R11) results were confirmed in the greenhouse using agroinoculation as described below.
Fla. 8680, which contains Ty-3 within an approximately 27 cM introgression from the S. chilense accession LA2779, was crossed to the susceptible breeding line Fla. 7781 to produce an F2 population. F2 plants (n = 717) were individually screened in fall 2006 for recombination between the molecular markers C2_At2g39590 and T0834, located near the distal ends of the introgression. Recombinants selected from this F2 population were used to develop RILs as described above. The F4 and F5 RILs were evaluated for resistance in fall 2007 and spring 2008, respectively, in a randomized complete block design with three blocks and 12-plant plots. To further fine-map the Ty-3 locus, three F2 sub-populations were developed using two key recombinants, i.e. 554 and 157 (Table S3). Sub-population A was an F4 generated by self-pollinating F3 progeny of recombinant 554 which were heterozygous for the introgression; sub-population B was an F2 derived from a cross between the susceptible breeding line Fla. 7776 and the F5 RIL of recombinant 554 (RIL 554). Sub-population C was also an F2 developed from a cross of Fla. 7776 and the F5 RIL of recombinant 157 (RIL 157). Seeds of all three sub-populations were sown and leaf tissue was collected from each plant at approximately 5 weeks after sowing. Sub-populations A and B were screened with the markers Mi23 and P6-25, and the markers T0774 and T0834 were used to screen sub-population C. Recombinants were transplanted to the field, along with controls, in early to mid-March, 2009. Controls included the TYLCV resistant commercial hybrids Tygress and SecuriTY 28, the resistant inbreds Fla. 8680 and Fla. 8602, the susceptible inbreds Horizon and Fla. 7776, RILs 554 and 157 and their F1 hybrids with Fla. 7776. One month after transplanting to the field, 6–8 cuttings were taken from each plant, rooted in a 1∶1 perlite, fine vermiculite media under mist for 2 weeks, then inoculated with whiteflies viruliferous for TYLCV for 11 days. Inoculated cuttings were transplanted to the field on 11 May in a non-randomized design with 3 replications of 2-plant plots, with the exception that only 2 replications were planted for recombinants having cross-overs outside the T0774 to P6-25 interval. TYLCV disease severity was evaluated on each plant at 5–6 weeks after exposure to whiteflies.
Self-pollinated seed was harvested from all original recombinant plants, and progeny were grown out in summer 2009 from 26 individuals with recombination between markers SL_2.40ch06_30.696 and cLEG-31-P16. Plants homozygous for the recombined introgression were selected for producing RILs. These RILs were grown in spring 2010, along with the controls Fla. 7776, Fla. 8680, the F1 hybrids between Fla. 7776 and each of RILs 554 and 157, and the commercial hybrid Tygress. Three week-old seedlings were inoculated with TYLCV for two weeks then transplanted to the field on 23 March in a randomized complete block design with three blocks and six-plant plots. TYLCV disease severity was evaluated on each plant at seven weeks after exposure to whiteflies.
TYLCV inoculation and disease evaluation
Whitefly mediated inoculation: Plants were inoculated with whiteflies viruliferous for the TYLCV-IL strain according to the method of [65] with some modifications. Briefly, plants were exposed to viruliferous whiteflies in growth chambers for the specified period of time. After inoculation, the whiteflies were killed by treating plants with an insecticidal soap and with Admire (imidacloprid), and the plants were then transplanted to the field. Plants were rated for disease severity on a 0 to 4 disease severity index scale as described by Scott et al. (1996), where 0 = no symptoms and 4 = severe symptoms and stunting. Intermediate scores such as 1.5, 2.5, etc. were incorporated to allow for more precise disease severity ratings.
Agrobacterium mediated inoculation: An infectious TYLCV-IL clone (pTYCz40a) was used for agroinoculation using the method as described in [35]. Briefly, A. tumefaciens LBA4404 was transformed, cultured in LB, pelleted and resuspended in infiltration medium at an OD600 of 0.5. Three week old seedlings were infiltrated by pressure inoculation in the leaves with a needle-less syringe. For the VIGS experiments the agro infiltration was done two weeks after TRV inoculation.
DNA extraction, molecular marker design and testing, and statistical analysis
DNA was extracted from young leaves using the cetyltrimethyl ammonium bromide (CTAB) protocol of [66] with minor modifications as described by [67]. Molecular markers used in this study were either publicly available, or were designed using the software Primer3 [68] from Ty-3-region BAC-end sequences, FOS-end sequences, the draft tomato genome available through the Sol Genomics Network (SGN; http://solgenomics.net/) [36], or from a private database of S. lycopersicum sequences. Polymerase chain reaction (PCR) parameters, primer sequences, restriction enzymes, and detection methods were described by [69] or [35]. Additional molecular markers designed are described in Table S5 and Figure S3, and used the same PCR parameters described by [69]. Analyses of variance, se calculations, and Duncan's multiple range tests were performed in SAS (Version 9.1; SAS Institute, Cary, NC). Mapping and interval analysis of Ty-3 was performed in Windows QTL Cartographer 2.0 (2007, N.C. State University) using mean disease severity of the cuttings for each recombinant and a subset of molecular markers specific to the Ty-3 region.
Generation of TRV vectors for silencing
For gene silencing, the TRV based VIGS system as described in [70] was used. Briefly, fragments of approximately 350 base pairs of Solyc06g051160, Solyc06g051180 and Solyc06g051190 were amplified from Ty-1 cDNA using primers compatible with the Gateway system (Table S6). After cloning to pENTR the inserts were sequenced to confirm their identity. Positive clones were selected for further processing of the inserts into the TRV2 vector and subsequently transformed to Agrobacterium tumefaciens strain GV3101.
TRV infection by agrobacterium-mediated infiltration
A 3 ml culture of A. tumefaciens strain GV3101 containing the TRV replicons was grown overnight at 28°C, 200 RPM in appropriate selective LB medium. Cultures were transferred to 20 mL LB containing proper selection pressure, 10 mM MES and 200 µM acetosyringone, and further grown overnight in a 28°C shaker. A. tumefaciens cells were pelleted, and resuspended in infiltration buffer (20 g/L sucrose, 5 g/L MS salts (no vitamins), 10 mM MES) to a final OD600 of 1. Agro infiltration was performed on cotyledons of 10 day old seedlings using pressure inoculation with a 2, 5 mL syringe without a needle.
Phylogenetic analysis
A neighbour joining tree with a bootstrap value of 1000 was generated using MEGA version 5 [71]. Arabidopsis RDR sequences were downloaded from The Arabidopsis Information Resource (www.arabidopsis.org) [72].
Quantitative RT–PCR
For gene expression analysis, 17 day old seedlings were agroinoculated as described above. For the mock treatment infiltration buffer without bacteria was used. Top leaves of plants were harvested 0, 5, 12 and 19 days after TYLCV inoculation and grinded in liquid nitrogen using mortar and pestle. Total RNA was extracted by using the RNeasy Plant Mini Kit (Qiagen) as described by the manufacturer. One µg RNA was digested using DNase I (Amp. Grade) following the manufacturers protocol (Invitrogen) and cDNA was synthesized using the iScript cDNA Synthesis Kit following the protocol (Bio-Rad). Quantitative Real-Time PCR was performed in 10 µl reactions in a Bio-Rad iCycler iQ5 using SYBR Green Supermix (Bio-Rad) according to the protocol provided by the manufacturer.
For quantitative RT-PCR of Ty-1/ty-1 the forward primer 180-F1 (5′-GGCAAAATATGCAGCCAGGCTTTCC-3′) and the reverse primer 180-R1 (5′-TCAGTATGTATACGAGGTTCGCCGT-3′) were used. As a reference the ACT gene was used as described by [73] with primers: ACT-F (5′-GAAATAGCATAAGATGGCAGACG-3′) and ACT-R (5′-ATACCCACCATCACACCAGTAT-3′). Gene expression levels were calculated using the ΔΔCt method as described by [74].
Supporting Information
Zdroje
1. JonesJDG, DanglJL (2006) The plant immune system. Nature 444 : 323–329.
2. BrommonschenkelSH, FraryA, FraryA, TanksleySD (2000) The Broad-Spectrum Tospovirus Resistance Gene Sw-5 of Tomato Is a Homolog of the Root-Knot Nematode Resistance Gene Mi. Molecular Plant-Microbe Interactions 13 : 1130–1138.
3. BendahmaneA, QuerciM, KanyukaK, BaulcombeDC (2000) Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. The Plant Journal 21 : 73–81.
4. VallejosCE, Astua-MongeG, JonesV, PlylerTR, SakiyamaNS, et al. (2006) Genetic and Molecular Characterization of the I Locus of Phaseolus vulgaris. Genetics 172 : 1229–1242.
5. KangBC, YeamI, JahnMM (2005) Genetics of plant virus resistance. Annual Review of Phytopathology 43 : 581–621.
6. Truniger V, Aranda MA, Gad L, John PC (2009) Chapter 4 - Recessive Resistance to Plant Viruses. Advances in Virus Research: Academic Press. pp. 119–231.
7. RobagliaC, CarantaC (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends in Plant Science 11 : 40–45.
8. BernsteinE, CaudyAA, HammondSM, HannonGJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409 : 363–366.
9. SijenT, FleenorJ, SimmerF, ThijssenKL, ParrishS, et al. (2001) On the Role of RNA Amplification in dsRNA-Triggered Gene Silencing. Cell 107 : 465–476.
10. WangX-B, WuQ, ItoT, CilloF, LiW-X, et al. (2010) RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proceedings of the National Academy of Sciences 107 : 484–489.
11. Garcia-RuizH, TakedaA, ChapmanEJ, SullivanCM, FahlgrenN, et al. (2010) Arabidopsis RNA-Dependent RNA Polymerases and Dicer-Like Proteins in Antiviral Defense and Small Interfering RNA Biogenesis during Turnip Mosaic Virus Infection. The Plant Cell Online 22 : 481–496.
12. Diaz-PendonJA, LiF, LiW-X, DingS-W (2007) Suppression of Antiviral Silencing by Cucumber Mosaic Virus 2b Protein in Arabidopsis Is Associated with Drastically Reduced Accumulation of Three Classes of Viral Small Interfering RNAs. The Plant Cell Online 19 : 2053–2063.
13. PandeySP, GaquerelE, GaseK, BaldwinIT (2008) RNA-Directed RNA Polymerase3 from Nicotiana attenuata Is Required for Competitive Growth in Natural Environments. Plant Physiology 147 : 1212–1224.
14. SchwachF, VaistijFE, JonesL, BaulcombeDC (2005) An RNA-Dependent RNA Polymerase Prevents Meristem Invasion by Potato Virus X and Is Required for the Activity But Not the Production of a Systemic Silencing Signal. Plant Physiology 138 : 1842–1852.
15. FauquetC, BriddonR, BrownJ, MorionesE, StanleyJ, et al. (2008) Geminivirus strain demarcation and nomenclature. Archives of Virology 153 : 783–821.
16. ScholthofKBG, AdkinsS, CzosnekH, PalukaitisP, JacquotE, et al. (2011) Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology 12 : 938–954.
17. Gronenborn B (2007) The Tomato Yellow Leaf Curl Virus Genome and Function of its Proteins. In: Czosnek H, editor. Tomato Yellow Leaf Curl Virus Disease: Springer Netherlands. pp. 67–84.
18. Hanley-BowdoinL, SettlageSB, RobertsonD (2004) Reprogramming plant gene expression: a prerequisite to geminivirus DNA replication. Molecular Plant Pathology 5 : 149–156.
19. ZamirD, Ekstein MichelsonI, ZakayY, NavotN, ZeidanM, et al. (1994) Mapping and introgression of a tomato yellow leaf curl virus tolerance gene, Ty-1. Theoretical and Applied Genetics 88 : 141–146.
20. HansonP, GreenS, KuoG (2006) Ty-2, a gene on chromosome 11 conditioning geminivirus resistance in tomato. Report of the Tomato Genetics Cooperative 56 : 17–18.
21. JiY, SchusterDJ, ScottJW (2007) Ty-3, a begomovirus resistance locus near the Tomato yellow leaf curl virus resistance locus Ty-1 on chromosome 6 of tomato. Molecular Breeding 20 : 271–284.
22. JiY, ScottJW, SchusterDJ, MaxwellDP (2009) Molecular mapping of Ty-4, a new Tomato yellow leaf curl virus resistance locus on chromosome 3 of Tomato. Journal of the American Society for Horticultural Science 134 : 281–288.
23. AnbinderI, ReuveniM, AzariR, ParanI, NahonS, et al. (2009) Molecular dissection of Tomato leaf curl virus resistance in tomato line TY172 derived from Solanum peruvianum. Theoretical and Applied Genetics 119 : 519–530.
24. HuttonSF, ScottJW, SchusterDJ (2012) Recessive Resistance to Tomato yellow leaf curl virus from the Tomato Cultivar Tyking Is Located in the Same Region as Ty-5 on Chromosome 4. HortScience 47 : 324–327.
25. Narasegowda MaruthiM, CzosnekH, VidavskiF, TarbaS-Y, MiloJ, et al. (2003) Comparison of Resistance to Tomato Leaf Curl Virus (India) and Tomato Yellow Leaf Curl Virus (Israel) among Lycopersicon Wild Species, Breeding Lines and Hybrids. 109 : 1–11.
26. Perez de CastroA, DiezMJ, NuezF (2005) Evaluation of breeding tomato lines partially resistant to Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl virus derived from Lycopersicon chilense. Canadian Journal of Plant Pathology 27 : 268–275.
27. PicoB, FerriolM, iezMJD, NuezF (1999) Developing tomato breeding lines resistant to Tomato yellow leaf curl virus. Plant Breeding 118 : 537–542.
28. PicoB, SifresA, EliaM, Jose DiezM, NuezF (2000) Searching for new resistance sources to Tomato yellow leaf curl virus within a highly variable wild Lycopersicon genetic pool. Acta Physiologiae Plantarum 22 : 344–350.
29. Pérez de Castro A, Julián O, Díez M Genetic control and mapping of Solanum chilense LA1932, LA1960 and LA1971-derived resistance to Tomato yellow leaf curl disease. Euphytica: 1–12.
30. LapidotM, FriedmannM, LachmanO, YehezkelA, NahonS, et al. (1997) Comparison of resistance level to Tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Disease 81 : 1425–1428.
31. FargetteD, LeslieM, HarrisonBD (1996) Serological studies on the accumulation and localisation of three tomato leaf curl geminiviruses in resistant and susceptible Lycopersicon species and tomato cultivars. Annals of Applied Biology 128 : 317–328.
32. SadeD, EybishtzA, GorovitsR, SobolI, CzosnekH (2012) A developmentally regulated lipocalin-like gene is overexpressed in Tomato yellow leaf curl virus-resistant tomato plants upon virus inoculation, and its silencing abolishes resistance. Plant Molecular Biology 80 : 273–287.
33. EybishtzA, PeretzY, SadeD, GorovitsR, CzosnekH (2009) Tomato yellow leaf curl virus infection of a resistant tomato line with a silenced sucrose transporter gene LeHT1 results in inhibition of growth, enhanced virus spread, and necrosis. Planta 231 : 537–548.
34. EybishtzA, PeretzY, SadeD, AkadF, CzosnekH (2009) Silencing of a single gene in tomato plants resistant to Tomato yellow leaf curl virus renders them susceptible to the virus. Plant Molecular Biology 71 : 157–171.
35. VerlaanMG, SzinayD, HuttonSF, de JongH, KormelinkR, et al. (2011) Chromosomal rearrangements between tomato and Solanum chilense hamper mapping and breeding of the TYLCV resistance gene Ty-1. The Plant Journal 68 : 1093–1103.
36. BombarelyA, MendaN, TecleIY, BuelsRM, StricklerS, et al. (2011) The Sol Genomics Network (solgenomics.net): growing tomatoes using Perl. Nucleic Acids Research 39: D1149–D1155.
37. Lozano-DuránR, Rosas-DíazT, LunaAP, BejaranoER (2011) Identification of Host Genes Involved in Geminivirus Infection Using a Reverse Genetics Approach. PLoS ONE 6: e22383 doi:10.1371/journal.pone.0022383.
38. PochO, BlumbergBM, BougueleretL, TordoN (1990) Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. Journal of General Virology 71 : 1153–1162.
39. ZongJ, YaoX, YinJ, ZhangD, MaH (2009) Evolution of the RNA-dependent RNA polymerase (RdRP) genes: Duplications and possible losses before and after the divergence of major eukaryotic groups. Gene 447 : 29–39.
40. WasseneggerM, KrczalG (2006) Nomenclature and functions of RNA-directed RNA polymerases. Trends in Plant Science 11 : 142–151.
41. DingS-W, VoinnetO (2007) Antiviral Immunity Directed by Small RNAs. Cell 130 : 413–426.
42. ChellappanP, MasonaM, VanitharaniR, TaylorN, FauquetC (2004) Broad Spectrum Resistance to ssDNA Viruses Associated with Transgene-Induced Gene Silencing in Cassava. Plant Molecular Biology 56 : 601–611.
43. RibeiroSG, LohuisH, GoldbachR, PrinsM (2007) Tomato Chlorotic Mottle Virus Is a Target of RNA Silencing but the Presence of Specific Short Interfering RNAs Does Not Guarantee Resistance in Transgenic Plants. Journal of Virology 81 : 1563–1573.
44. YadavRK, ChattopadhyayD (2011) Enhanced viral intergenic region-specific short interfering RNA accumulation and DNA methylation correlates with resistance against a geminivirus. Molecular Plant-Microbe Interactions 24 : 1189–1197.
45. YangX, XieY, RajaP, LiS, WolfJN, et al. (2011) Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection. PLoS Path 7: e1002329 doi:10.1371/journal.pgen.1002329.
46. VanitharaniR, ChellappanP, FauquetCM (2005) Geminiviruses and RNA silencing. Trends in Plant Science 10 : 144–151.
47. RajaP, SanvilleBC, BuchmannRC, BisaroDM (2008) Viral Genome Methylation as an Epigenetic Defense against Geminiviruses. Journal of Virology 82 : 8997–9007.
48. BroughCL, GardinerWE, InamdarNM, ZhangX-Y, EhrlichM, et al. (1992) DNA methylation inhibits propagation of tomato golden mosaic virus DNA in transfected protoplasts. Plant Molecular Biology 18 : 703–712.
49. VanitharaniR, ChellappanP, PitaJS, FauquetCM (2004) Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. Journal of Virology 78 : 9487–9498.
50. BuchmannRC, AsadS, WolfJN, MohannathG, BisaroDM (2009) Geminivirus AL2 and L2 Proteins Suppress Transcriptional Gene Silencing and Cause Genome-Wide Reductions in Cytosine Methylation. Journal of Virology 83 : 5005–5013.
51. VoinnetO, PintoYM, BaulcombeDC (1999) Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proceedings of the National Academy of Sciences 96 : 14147–14152.
52. WangH, BuckleyKJ, YangX, BuchmannRC, BisaroDM (2005) Adenosine Kinase Inhibition and Suppression of RNA Silencing by Geminivirus AL2 and L2 Proteins. Journal of Virology 79 : 7410–7418.
53. WangH, HaoL, ShungC-Y, SunterG, BisaroDM (2003) Adenosine Kinase Is Inactivated by Geminivirus AL2 and L2 Proteins. The Plant Cell Online 15 : 3020–3032.
54. van WezelWR, DongX, LiuH, TienP, StanleyJ, et al. (2002) Mutation of three cysteine residues in Tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Molecular Plant-Microbe Interactions 15 : 203–208.
55. ZhangZ, ChenH, HuangX, XiaR, ZhaoQ, et al. (2011) BSCTV C2 Attenuates the Degradation of SAMDC1 to Suppress DNA Methylation-Mediated Gene Silencing in Arabidopsis. The Plant Cell Online 23 : 273–288.
56. LunaAP, MorillaG, VoinnetO, BejaranoER (2012) Functional analysis of gene silencing suppressors from Tomato yellow leaf curl disease viruses. Molecular Plant-Microbe Interactions 25 : 1294–1306.
57. MuangsanN, BeclinC, VaucheretH, RobertsonD (2004) Geminivirus VIGS of endogenous genes requires SGS2/SDE1 and SGS3 and defines a new branch in the genetic pathway for silencing in plants. The Plant Journal 38 : 1004–1014.
58. VoinnetO (2008) Use, tolerance and avoidance of amplified RNA silencing by plants. Trends in Plant Science 13 : 317–328.
59. PandeySP, BaldwinIT (2007) RNA-directed RNA polymerase 1 (RdR1) mediates the resistance of Nicotiana attenuata to herbivore attack in nature. The Plant Journal 50 : 40–53.
60. Olmedo-MonfilV, Duran-FigueroaN, Arteaga-VazquezM, Demesa-ArevaloE, AutranD, et al. (2010) Control of female gamete formation by a small RNA pathway in Arabidopsis. 464 : 628–632.
61. PeragineA, YoshikawaM, WuG, AlbrechtHL, PoethigRS (2004) SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes & Development 18 : 2368–2379.
62. LeibmanD, WolfD, SaharanV, ZelcerA, AraziT, et al. (2011) A High Level of Transgenic Viral Small RNA Is Associated with Broad Potyvirus Resistance in Cucurbits. Molecular Plant-Microbe Interactions 24 : 1220–1238.
63. WuQ, WangX, DingS-W (2010) Viral Suppressors of RNA-Based Viral Immunity: Host Targets. Cell Host & Microbe 8 : 12–15.
64. WillmannMR, EndresMW, CookRT, GregoryBD (2011) The Functions of RNA-Dependent RNA Polymerases in Arabidopsis. The Arabidopsis Book e0146.
65. GriffithsP, ScottJ (2001) Inheritance and linkage of tomato mottle virus resistance genes derived from Lycopersicon chilense accession LA 1932. Journal of the American Society for Horticultural Science 126 : 462–467.
66. DoyleJJ, DoyleJL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical bulletin 19 : 11–15.
67. FultonTM, ChunwongseJ, TanksleySD (1995) Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Molecular Biology Reporter 13 : 207–209.
68. RozenS, SkaletskyH (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132 : 365–386.
69. HuttonSF, ScottJW (2011) A collection of polymorphic markers useful for fine-mapping the Ty-3 locus from Solanum chilense accession LA2779. Report of the Tomato Genetics Cooperative 61 : 12–14.
70. LiuY, SchiffM, Dinesh-KumarS (2002) Virus-induced gene silencing in tomato. The Plant Journal 31 : 777–786.
71. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28 : 2731–2739.
72. LameschP, BerardiniTZ, LiD, SwarbreckD, WilksC, et al. (2011) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research
73. LøvdalT, LilloC (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Analytical Biochemistry 387 : 238–242.
74. LivakKJ, SchmittgenTD (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25 : 402–408.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



