-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
During development, tissue-specific transcription factors regulate both protein-coding and non-coding genes to control differentiation. Recent studies have established a dual role for the transcription factor Pax6 as both an activator and repressor of gene expression in the eye, central nervous system, and pancreas. However, the molecular mechanism underlying the inhibitory activity of Pax6 is not fully understood. Here, we reveal that Trpm3 and the intronic microRNA gene miR-204 are co-regulated by Pax6 during eye development. miR-204 is probably the best known microRNA to function as a negative modulator of gene expression during eye development in vertebrates. Analysis of genes altered in mouse Pax6 mutants during lens development revealed significant over-representation of miR-204 targets among the genes up-regulated in the Pax6 mutant lens. A number of new targets of miR-204 were revealed, among them Sox11, a member of the SoxC family of pro-neuronal transcription factors, and an important regulator of eye development. Expression of Trpm/miR-204 and a few of its targets are also Pax6-dependent in medaka fish eyes. Collectively, this study identifies a novel evolutionarily conserved mechanism by which Pax6 controls the down-regulation of multiple genes through direct up-regulation of miR-204.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003357
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003357Summary
During development, tissue-specific transcription factors regulate both protein-coding and non-coding genes to control differentiation. Recent studies have established a dual role for the transcription factor Pax6 as both an activator and repressor of gene expression in the eye, central nervous system, and pancreas. However, the molecular mechanism underlying the inhibitory activity of Pax6 is not fully understood. Here, we reveal that Trpm3 and the intronic microRNA gene miR-204 are co-regulated by Pax6 during eye development. miR-204 is probably the best known microRNA to function as a negative modulator of gene expression during eye development in vertebrates. Analysis of genes altered in mouse Pax6 mutants during lens development revealed significant over-representation of miR-204 targets among the genes up-regulated in the Pax6 mutant lens. A number of new targets of miR-204 were revealed, among them Sox11, a member of the SoxC family of pro-neuronal transcription factors, and an important regulator of eye development. Expression of Trpm/miR-204 and a few of its targets are also Pax6-dependent in medaka fish eyes. Collectively, this study identifies a novel evolutionarily conserved mechanism by which Pax6 controls the down-regulation of multiple genes through direct up-regulation of miR-204.
Introduction
Lineage-specific transcription factors (TFs) such as Pax6 direct the development of multiple tissues through the regulation of gene networks that execute discrete developmental programs. Pax6 is essential for normal development of the central nervous system (CNS), pancreas, olfactory system and eye (reviewed in [1], [2]). Pax6 is considered a “master regulator” of eye development as it specifies the multiple cell lineages that comprise the eye in vertebrate and invertebrate species [3].
During embryonic development, Pax6 protein is known to activate several target genes using two DNA-binding domains and a proline-serine-threonine transcription activating domain [4]–[6]. Pax6 may also enhance gene expression by recruiting chromatin-remodeling enzymes and alleviating heterochromatin repression [4], [7], [8]. In contrast, Pax6 has been found to function as a repressor of the lens crystallin genes Cryabb1 and Crygf, and of the photoreceptor TF Crx [4], [9]–[11]. Since repression of crystallin genes was shown to be independent of the transactivation domain of Pax6, it was proposed that inhibition is mediated by competition for promoter occupancy with other TFs [4], [9], [10]. However, additional mechanisms of Pax6-dependent gene repression remain to be identified.
MicroRNAs (miRNAs) direct post-transcriptional repression of a wide array of genes by adhering to miRNA-specific sequences in the 3′ untranslated region (UTR) of mRNAs [12]. In this way, a single type of miRNA can essentially bind dozens of different mRNA transcripts [13]. Therefore, regulation of a miRNA gene by a tissue-specific TF can facilitate the down-regulation of batteries of genes.
To ascertain the roles of miRNAs in eye development, somatic mutations of the miRNA-maturation enzyme gene Dicer1 were examined in the mouse lens and retinal progenitors cells (RPCs, [14]–[16]). When Dicer1 was knocked out at the lens placode (LP) stage, lens development proceeded to primary lens fiber cell differentiation; however, secondary lens fiber cell differentiation was aborted and lens epithelium (LE) cells ceased to divide, undergoing apoptosis. Therefore, it is evident that miRNAs play an important role in the late stages of lens development. Somatic mutation of Dicer1 in RPCs revealed multiple activities of miRNAs in their specification, differentiation and survival [15], [16].
To date, there is limited information on the function of specific miRNAs in the eye. Probably the most extensively studied example is miR-204. In medaka fish (Oryzias latipes; ol), ol-miR-204 was shown to affect lenticular and retinal development via repression of Meis2 and its transcriptional target Pax6 [17]. In addition, miR-204 was found to contribute to the epithelial physiology of human retinal pigmented epithelium (RPE) [18], [19]. However, the activity and regulation of miR-204 in the mammalian lens and retina remain unknown. The coding region for the mouse miR-204 resides in intron 6 of the transient receptor potential cation channel M3 gene (Trpm3 [20]). miR-204 appears to be concomitantly expressed with Trpm3 in the eye and CNS [18], [21], [22]. In the post-natal mouse eye, its pattern resembles that of Pax6 (Figure 1;[21], [23]).
Fig. 1. Trpm3/miR-204 expression is dependent on Pax6 activity during eye development. 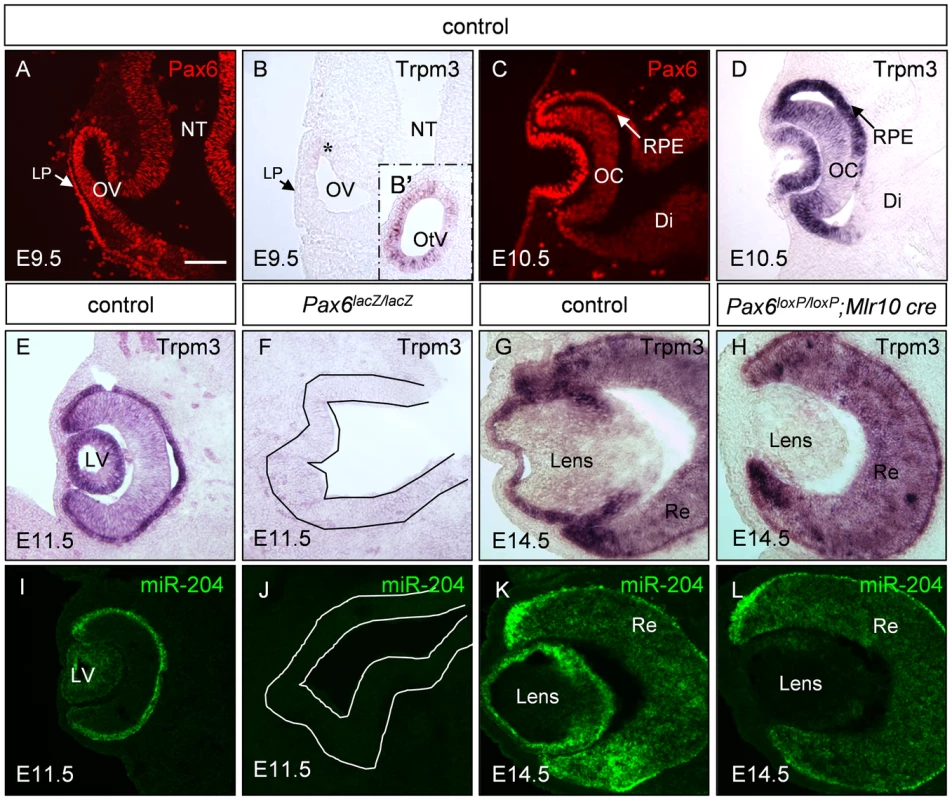
Paraffin sections of control (A–E,G,I,K), Pax6lacZ/lacZ (F,J) and Pax6loxP/loxP;Mlr10-cre (H,L) stained for Trpm3 mRNA (B,D,E–H), Pax6 protein (A,C, red) and miR-204 (I–L, green). Trpm3 expression begins after E9.5 in the developing eye (B,D), although it is already active in the otic vesicle on E9.5 (B′ inset). Trpm3 and miR-204 are lost from the optic rudiment of Pax6lacZ/lacZ embryos (F,J; optic cup rudiment is traced with a line) and in the lens of Pax6loxP/loxP;Mlr10-cre mutants (H,L). Di, diencephalon; LP, lens placode; LV, lens vesicle; OV, optic vesicle; NT, neural tube; OC, optic cup; OtV, otic vesicle; RPE, retinal pigmented epithelium; Re, developing retina. Scale bar = 100 µM. The present study was aimed at elucidating the molecular mechanism of Pax6-dependent transcriptional repression through unbiased analysis of up-regulated genes in Pax6-mutant lenses. We show that inhibition of gene expression by Pax6 is at least partly mediated through direct activation of miR-204. In addition, we identify Sox11 as a novel target for miR-204 in lens and retinal development. Finally, both regulation of Trpm/miR-204 by Pax6 and inhibition of Sox11 are shown to be conserved in vertebrates. This study is the first to reveal that miRNAs are part of the Pax6 genetic network in different vertebrate species, adding to the known repertoire of Pax6 activities in the course of organ development.
Results
Large-scale changes in the lens transcriptome as a result of Pax6 deletion
To identify new Pax6 genetic targets in the developing lens, an expression microarray was performed on embryonic day 14.5 (E14.5) lenses from controls and somatic mutants of Pax6 (Pax6loxP/loxP;Mlr10-cre, [24]). Out of 28,853 mouse genes, 315 were altered at a cutoff of 2.0-fold and a P-value below 0.05. Of these, the expression levels of 235 genes were increased upon Pax6 deletion, while only 83 genes were reduced (P-values and fold change are shown Figure S1 and the genes are listed in Table S1). A bias toward up-regulated genes - 2.8∶1 in the lens, has also been reported in the embryonic neocortex (1.8∶1, 600 up-regulated vs. 339 down-regulated genes; [25]). This raised the possibility of Pax6's role in directly or indirectly repressing the expression of a large number of genes.
To identify common targets of Pax6, we compared the list of altered genes in the lens and embryonic neocortex [25]. Interestingly, only 22 genes were found to be regulated similarly in both systems, with 18 up-regulated and 4 down-regulated in both the lens and neocortex (Table 1). The four genes that were down-regulated in both lens and neuronal lineages are potential direct transcriptional targets of Pax6. One of these - Trpm3 - contains both a coding region for Trpm3 and a non-coding miR-204 sequence. Trpm3 is a melastatin-like cation channel which is sensitive to steroids, active in insulin-producing beta cells and a chemo - and thermosensor in the somatosensory system [20], [26], [27], while miR-204 has been documented to play a role in ocular lineages in fish and mammals [17], [18] and thus may mediate Pax6's inhibitory activity in the eye.
Tab. 1. Pax6 targets that are reduced in developing lens (E14.5) and forebrain (E12.5). 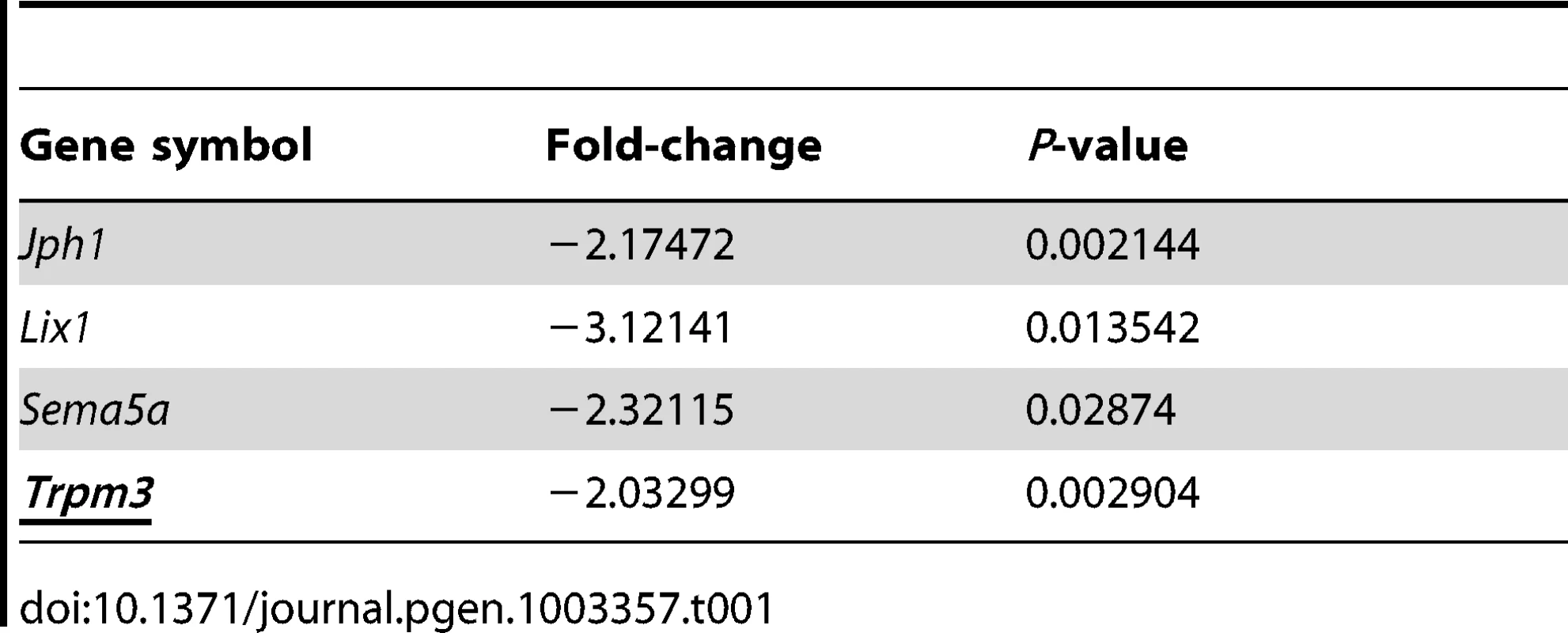
Pax6 is required for Trpm3/miR-204 expression during lens development
Trpm3 transcript distribution in the post-natal eye shows some overlap with Pax6 expression [18], [21], [22]. Considering the possibility that Pax6 regulates Trpm3 expression directly during eye development, we compared the spatio-temporal expression patterns of Pax6 protein and Trpm3 mRNA during eye morphogenesis and differentiation (Figure 1). Pax6 expression initiates in the neural tube on around E8.5 [28]. On E9.5, Pax6 is detected in the ocular progenitors of the optic vesicle (OV) and LP (Figure 1A). On E9.5, Trpm3 is not yet detected in most ocular progenitors cells, and is barely observed in a small population of cells in the dorsal OV (Figure 1B, asterisk). Expression of Trpm3 on E9.5 was, however, evident in the otic vesicle, the primordium of the inner ear (Figure 1B′ inset). In contrast, from E10.5 onwards, Trpm3 mRNA was co-expressed with Pax6 protein in the lens and optic cup (OC) lineages. Both were strongly expressed in the invaginating lens vesicle and in the inner and outer layers of the OC (Figure 1C, 1D). Trpm3 expression seemed to be restricted to the developing eye, inner ear and choroid plexus and did not extend to Pax6-positive cells of the developing diencephalon (Figure 1D, Figure S2A). The expression of Trpm3 continued to follow that of Pax6 during later stages of eye development and was detected in the inner nuclear layer and ciliary body after birth (Figure 1E, 1G; Figure S2B). Therefore, Trpm3 expression in the developing eye began only after the establishment of ocular progenitor domains. From that stage onwards, Trpm3 expression recapitulated the complex ocular pattern of Pax6 expression, suggesting that Pax6 regulates Trpm3 expression in the developing lens and OC derivatives.
To determine whether Pax6 simultaneously regulates Trpm3 and its hosted non-coding miR-204, Pax6 systemic knockout (Pax6lacZ/lacZ, E11.5) and Pax6 lens-specific conditional mutants (Pax6loxP/loxP;Mlr10-cre, E14.5) were examined (Figure 1). On E11.5, both Trpm3 and miR-204 were highly expressed in the lens vesicle and in the outer layer of the OC that contains the progenitors of the RPE, whereas lower levels were detected in the inner OC, where the RPCs reside (Figure 1E, 1I). Upon Pax6 ablation in Pax6lacZ/lacZ knock-out mice, Trpm3/miR-204 expression on E11.5 was lost from the optic rudiment (Figure 1F, 1J). On E14.5, Trpm3 and miR-204 were both detected in the LE and RPE. In the inner layer of the OC, expression was more prominent in the distal regions containing the ciliary body and iris primordia (Figure 1G, 1K). When Pax6 was removed specifically in the lens lineage on E14.5 (Pax6loxP/loxP;Mlr10-cre, [24]), both Trpm3 and miR-204 were lost from the mutant lens (Figure 1H, 1L). A reduction in Trpm3 expression was also detected in Pax6-deficient OC (Pax6loxP/loxP;a-cre [29], see below) and in progenitors of the iris and ciliary body (Pax6loxP/loxP;Dct-Cre mutants, Figure S3). These findings strongly support genetic regulation of Trpm3/miR-204 by Pax6 in several ocular tissue types.
Pax6 directly binds Trpm3 regulatory sequences in vitro and in vivo
To determine whether Pax6 regulation of the Trpm3/miR-204 gene is direct, we utilized chromatin immunoprecipitation (ChIP) assay to identify the binding region, in conjunction with in-silico TF binding site searches, followed by electrophoretic mobility shift assay (EMSA) to precisely locate Pax6 binding sites. The mammalian Trpm3 gene contains three transcription start sites based on the RefSeq data presented in the UCSC genome browser. A preliminary ChIP-on-chip experiment performed using chromatin prepared from E13.5 whole eyes indicated Pax6 binding within the region shown in Figure 2B. To examine whether Pax6 indeed binds to these Trpm3 regulatory sequences in vivo, quantitative ChIP PCR was performed on wild-type P0 lenses using anti-Pax6 antibodies. Two amplicons: A1 (chr19 : 22,524,578–697) and A2 (chr19 : 22,525,042–126) within the candidate regulatory region were enriched 16.3-fold (P = 0.0043) and 25.9-fold (P = 0.0098), respectively, compared to an upstream non-specific region (NSR-chr19:NSR-chr19 : 22,523,967–4079).
Fig. 2. Pax6 directly binds a Trpm3 enhancer sequence in vitro and in vivo. 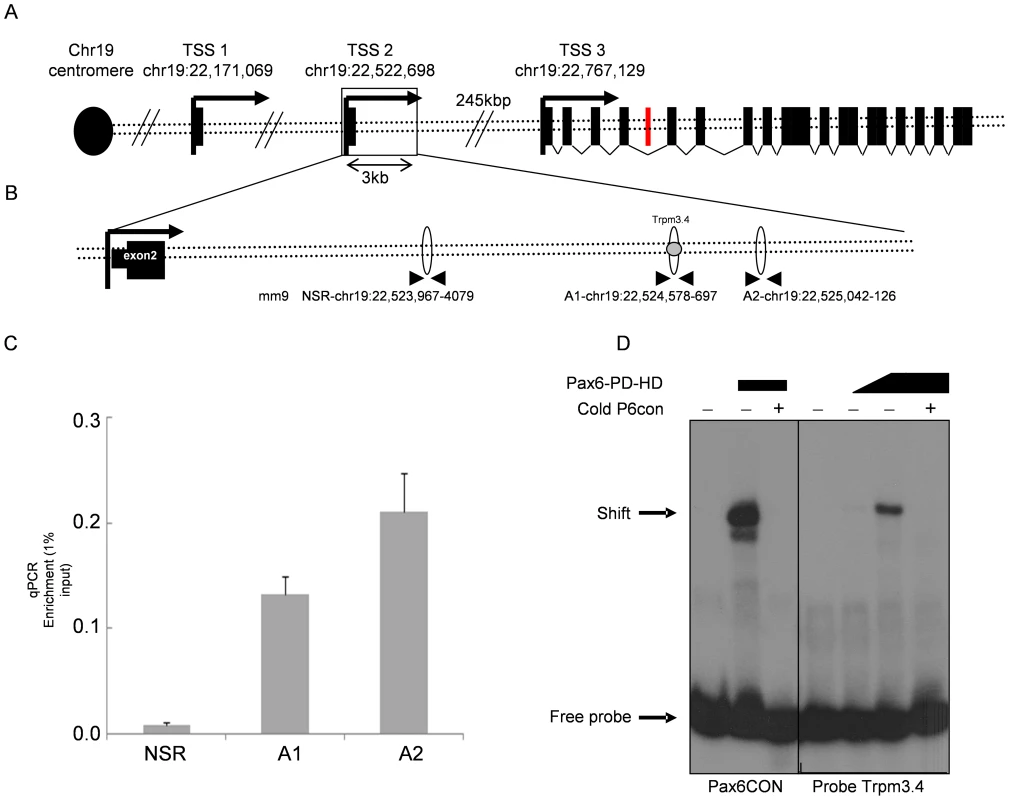
(A) Schematic diagram of the murine Trpm3 locus. Three transcription start sites (TSS, black arrows) are distributed across 600 kb. The black rectangles represent Trpm3 exons. The red rectangle represents the miR-204-encoding sequence in intron 6. (B) Area downstream of TSS2. Ellipses represent real-time qRT-PCR amplicons. Gray circle represents the location of EMSA probe Trpm3.4. (C) Results of real-time qRT-PCR on DNA from newborn lens ChIP experiment. Y-axis represents relative quantity of template DNA divided by the amount of template of the same reaction in 1% of input DNA. P-values for A1 = 0.0043, A2 = 0.0098, error bars indicate SD. (D) Acrylamide gel of radioactive EMSA probe Trpm3.4. Pax6CON is an oligonucleotide with the consensus binding site of Pax6.. For the Trpm3.4 probe, the first lane is Trpm3.4 probe only, lane 2 is probe+1∶10 flag-Pax6, lane 3 is probe+Pax6-flag, lane 4 is probe with Pax6-flag and competition by cold probe. We tested four sequences (Trpm3.1–4) containing putative Pax6 binding sites using EMSA (prediction of target sites was performed as described in [30], EMSA described in [31], and the sequence similarities of Trpm3.1–Trpm3.4 to Pax6 binding matrices are presented in Table S2, Table S3). The probe for Trpm3.4 showed strongest binding to a flag-Pax6 protein containing both the paired domain and homeodomain (Pax6-PD-HD, Figure 2D). Increased concentrations of Pax6 protein produced stronger complex formation, while the presence of cold oligonucleotide competitors inhibited the binding (P6CON, Figure 2D). Therefore, we concluded that Pax6 directly binds Trpm3 regulatory sequences located downstream of the second promoter of Trpm3.
miR-204 binds the 3′ UTR of Sox11 and down-regulates its expression
Since miR-204 is down-regulated in ocular tissues depleted in Pax6, we next examined which downstream genes might be regulated by miR-204 in the developing mouse eye. Since most miRNA target sites can be found in the 3′ UTR segment of the target genes, we searched for putative miR-204 binding sites in the 3′ UTRs of all mouse genes (TargetScan Mouse, www.targetscan.org). From the list of genes containing conserved putative binding sites for miR-204, 35 were found to be up-regulated in the Pax6 mutant lens with at least a 1.5-fold change (Table 2). Statistical analysis revealed significant over-representation of miR-204 target genes among the genes negatively regulated by Pax6 (hypergeometric test, P = 0.019), making them good candidates for miR-204-mediated repression in the developing lens. Of these, Sox11 was selected due to its reported roles in ocular development [32]. Interestingly, Sox11 is encoded by a single exon and a long, 5,301-bp 3′ UTR, and was the only gene from this list that had three highly conserved putative miR-204 target sequences at positions 545–551, 916–923 and 3848–3855 (TargetScan Mouse). Sox11 is a member of the SoxC group of TFs, which are required for neurogenesis in the embryo and adult [33]–[36].
Tab. 2. Putative miR-204 targets found to be upregulated in Pax6 CKO lenses. 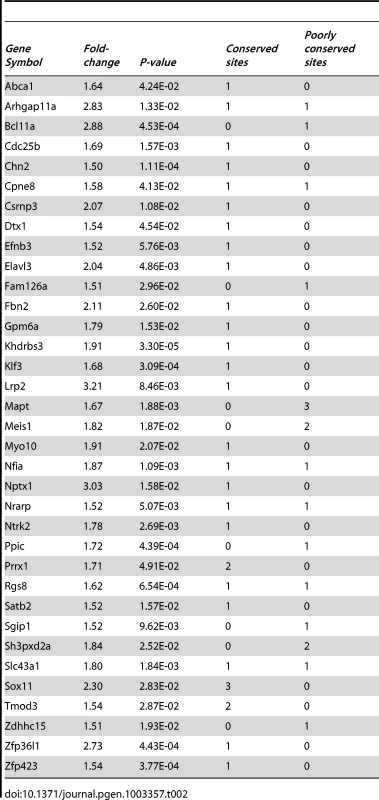
To examine whether miR-204 down-regulates Sox11 expression, we utilized mouse neuroblastoma 2a (Neu-2a) cells, which show high expression of Sox11 [37]. The cells were transfected with either a miR-204 mimic plasmid (miR-204-miRVec) or a scrambled vector (scramble-miRVec) and were examined for Sox11 immunofluorescence. On average, cells transfected with scrambled miRNA had fluorescence levels similar to those of non-transfected cells (1∶1.03, n = 163), whereas cells transfected with miR-204 had significantly lower levels (1∶0.87, n = 150, P = 0.0104, Figure 3A–3C). This was confirmed at the mRNA level in a human lens cell line (H36CE) and mouse Neu-2a transfected with a mimic miR-204 (see below and Figure S8). Therefore, miR-204 is able to down-regulate the level of Sox11 transcript and protein in culture.
Fig. 3. miR-204 down-regulates the expression of Sox11. 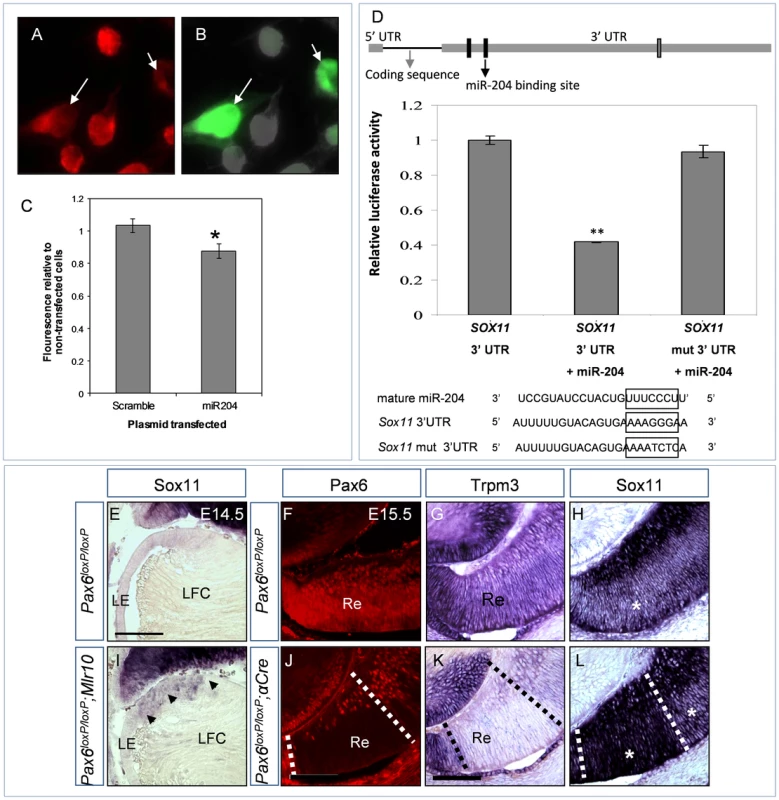
(A–B) Sox11 immunofluorescence (A, red) and EGFP (B, green) in Neu-2a cells transfected with miR-204-miRVec plasmid and pCAG-GFP. White arrows indicate GFP-positive transfected cells with visibly reduced Sox11 immunofluorescence. (C) Quantification of Sox11 immunofluorescence in cultures transfected with either scramble-miRVec plasmid or a miR-204-miRVec control plasmid. Y-axis is Sox11 fluorescence of transfected cells divided by that of non-transfected cells in the same image field. Error bars are SEM (P = 0.0104, n = 163;150 cells). (D) Illustration of Sox11 cDNA including the untranslated and coding regions drawn in relative proportion. The three predicted miR-204 binding sites in the 3′ UTR are indicated as rectangles. Black-labeled rectangles were tested in the luciferase reporter assay. The mutated site is marked with a black arrow. Graph presents the relative luciferase luminescence in cells transfected with wild-type Sox11 3′ UTR, wild-type 3′ UTR and miR-204, or mutated 3′ UTR and miR-204. Error bars represent SEM (P = 0.0011, n = 3). Below are alignments of miR-204 RNA sequence, with wild-type and mutated Sox11 3′ UTR regions used in transfections. (E–L) Cryosections of control (E–H), Pax6loxP/loxP;Mlr10-cre (I) and Pax6loxP/loxP;a-Cre distal optic cup (J–L) stained with riboprobe against Sox11 (E,I,H,L) or Trpm3 (G,K) and Pax6 immunofluorescence (F,J, red). Arrowheads in (I) indicate up-regulation in the enlarged transition zone of the Pax6loxP/loxP;Mlr10-cre lens. (F–H) Adjacent sections of the same control animal: asterisk in (H) marks intermediate level of Sox11 staining in outer retina. (J–L) Adjacent sections of the same Pax6loxP/loxP;a-Cre distal retina: dotted lines demarcate area of Pax6 deletion, asterisks mark outer retina with elevated levels of Sox11 (H). LE, lens epithelium; LFC, lens fiber cell; Re, retina. Scale bar = 100 µm. To determine whether Sox11 down-regulation is a result of direct binding, part of the wild-type 3′ UTR of Sox11, containing the first two putative miR-204 binding sites, was cloned downstream of the luciferase gene and compared to a mutated version of the 3′ UTR. Upon co-transfection of the wild-type Sox11 3′ UTR with miR-204, luciferase activity was reduced by 59% (P = 0.0012). In contrast, when we used a mutated version of the site that had the highest probability of conserved targeting (based on TargetScan version 6.2, position 914–921 on the 3′ UTR), luciferase levels were restored to control levels (96%, P = 0.20, Figure 3D arrow). Therefore, miR-204 binds the 3′ UTR of Sox11 and down-regulates its expression. Several miRNA binding sites on the gene's 3′ UTR usually exhibit additive effects ([38]). Yet, in the case of Sox11, mutating one site was sufficient to rescue most of the effect of miR-204. This reveals the importance of this site for the regulation of Sox11, although the other sites may contribute as well.
Pax6 negatively controls Sox11 expression in vivo in the lens and retina
Sox11 has been shown to be required for ocular development in mice. In Sox11−/− mutant embryos, the OC fails to close, resulting in a coloboma, a folded retina and a small lens that remains attached to the cornea [32]. This phenotype is reminiscent of heterozygous Pax6 mutations, suggesting a positive interaction between Pax6 and Sox11 in the context of early eye development [32]. However, we did not detect reduced expression of Sox11 transcript in Pax6lacZ/lacZ embryos in the OV or SE on E9.5 (Figure S4). Therefore, while both Pax6 and Sox11 are required for lens vesicle detachment and OC morphogenesis, Pax6 is not required for the expression of Sox11.
In contrast, we identified a negative regulatory interaction between Pax6 and Sox11 at later stages of eye development as observed by the 2.3-fold elevation in Sox11 revealed by the microarray results above. To validate these findings, we characterized Sox11 expression in control and Pax6loxP/loxP;Mlr10-cre eyes on E14.5. In the wild-type, Sox11 transcripts were detected in the retina but not in the LE, in agreement with previous reports (Figure 3E, 3H, [32]). In contrast to previous studies, which utilized a lacZ reporter [32],[39], we did not detect Sox11 transcript in the lens fiber cells (E14.5, Figure 3E). This up-regulation of the lacZ reporter may be due to loss of miR-204 binding sites within the 3′ UTR, which were replaced with the lacZ gene, or to persistence of β-galactosidase protein in the slow-metabolizing lens fibers. Sox11 mRNA was elevated at the equator of Pax6loxP/loxP;Mlr10-cre lenses (Figure 3I, arrowheads), similar to the area of elevation seen with Sox2 [24] and Sox9 (Figure S5) in Pax6 mutant lenses. The elevation of Sox11 transcript in the Pax6loxP/loxP;Mlr10-cre lens validates Pax6's negative regulation of Sox11 in the lens.
Trpm3/miR-204 is expressed in the progenitors of the OC and similar to Pax6, its expression is higher in the peripheral region of the OC, which is populated by the progenitors of the iris and ciliary body (Figure 1C, 1G, 1K and Figure 3G). While Sox11 is also expressed in the OC, its expression is lowest in the iris and ciliary body progenitors (not shown), intermediate in the neuroblastic layer (NBL) and highest in the ganglion cell layer (Figure 3H). We next examined whether Pax6 may also function in the OC to modulate Sox11 using Pax6loxP/loxP;a-Cre embryos [29]. In the region depleted of Pax6 (Figure 3J, dotted lines), Trpm3 was reduced (Figure 3K, dotted lines), while in the same region, Sox11 transcript level was markedly elevated (Figure 3L, small asterisk in area demarcated by dotted lines). The area of elevation corresponded to the Pax6−;Crx− population of RPCs in mutated Pax6loxP/loxP;a-Cre retinas [11].
Therefore, Pax6 down-regulates the expression of Sox11 in progenitors of the lens and in a subclass of RPCs. Combined with the results above, these findings suggest that Pax6 modulates Sox11 via miR-204-based repression.
Pax6 regulation of Trpm/miR-204 is evolutionarily conserved in vertebrates
As miR-204 was previously shown to be expressed and to regulate eye morphogenesis in medaka fish [17], we hypothesized that Pax6 regulation of medaka miR-204 is evolutionarily conserved.
Interestingly, while ol-Trpm3 is not expressed in the medaka at stage 24 (st 24), the expression of ol-Trpm1 corresponded with the pattern of expression of murine Trpm3 and miR-204 (mm-Trpm3, Figure 1E and Figure 4A, 4C). The ol-Trpm1 was expressed in the LP, neuroretina, ciliary marginal zone, ciliary body and presumed RPE (Figure 4C, 4F), suggesting that both ol-Trpm3 and ol-Trpm1 may have undergone a process of divergent gene functionalization, a common evolutionary process in teleost species [40]. To identify additional paralogs for both ol-Trpm1 and ol-Trpm3 genes in medaka fish, we used both available ol-Trpm1 (ENSORLT00000009435) and ol-Trpm3 (ENSORLT00000013467) sequences as queries to search public databases for the orthologous genomic locus. This search did not retrieve any additional ol-Trpm1/3 genes in the medaka genome (data not shown). Furthermore, comparison of the protein sequences together with phylogenetic analysis of many known vertebrate Trpm1 and Trpm3 proteins (human, caw, mouse, rat, chicken, Xenopus, zebrafish, Table S6) using PHYLIP package tools [41] identified ol-Trpm1 as more closely related to the mammalian Trpm3 than to mammalian Trpm1 (Figure S6). In support of these data, mouse–medaka comparative genomic analysis using MultiZ and Medaka Chain/Net package at UCSC (http://genome.ucsc.edu/) highlighted the locus for medaka ol-Trpm1 as a possible syntenic region to the mouse Trpm3 locus (Figure S7).
Fig. 4. Characterization of the medaka ol-Trpm1/miR-204 regulatory region. 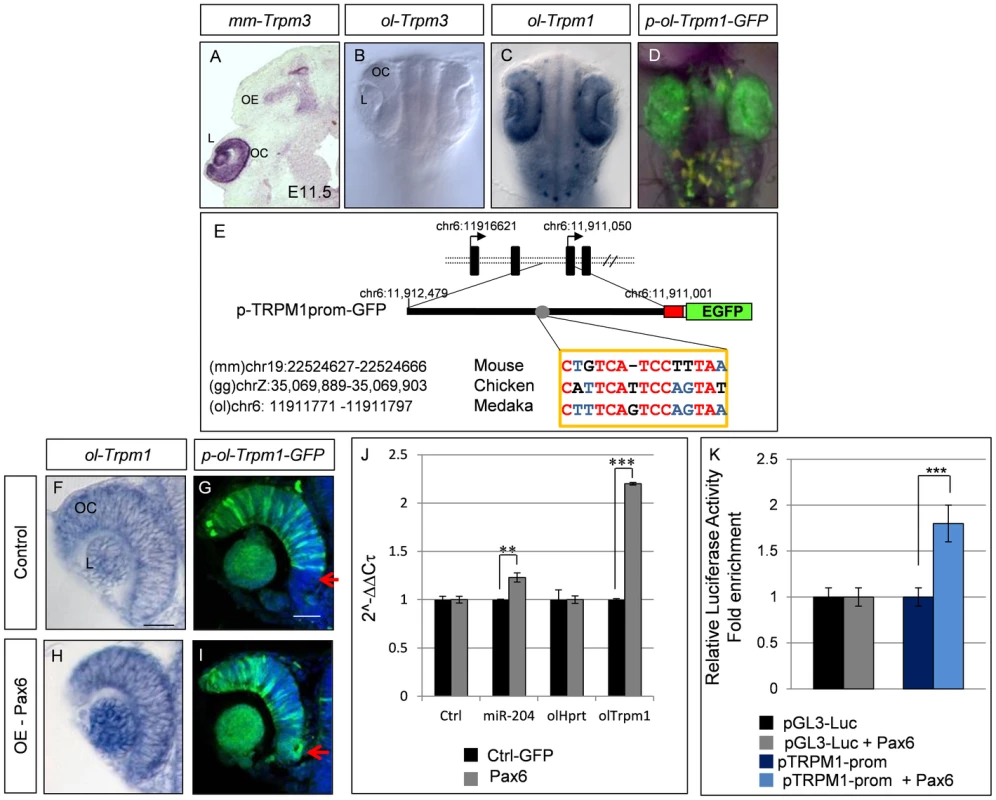
(A) RNA in-situ hybridization on frontal eye sections of E11.5 wild-type mouse embryos with the mouse mm-Trpm3 probe. (B–D) Bright-field dorsal views of embryos at stage 24 of development; whole-mount in-situ hybridization with (B) ol-Trpm3 or (C) ol-Trpm1 probes and (D) epifluorescence of cI-transgenic embryos for EGF expression from p-ol-Trpm1-GFP transgene. (E) The p-ol-Trpm1-GFP construct includes 1.5 kb upstream of the coding region of ol-Trpm1. The red box represents the minimal TK promoter. The sequence with similarty to the Trpm3.4 Pax6-binding site is indicated. The numbers indicate respective genomic locations in the three indicated genomes. Conserved nucleotides between the three species are indicated in red or blue (two out of three analyzed species), and non-conserved nucleotides are in black. (D, G) EGFP expression in the whole (D) or a section (G) of the eye of p-ol-Trpm1-GFP-transgenic embryos recapitulates endogenous ol-Trpm1 expression pattern (C,F). (H,I) Pax6 mRNA over-expression activates both ol-Trpm1 and EGFP expression in the ventral retina (red arrow) of the OC. (J) Fold-changes (expressed as 2-ΔΔCt values) in miR-204 and ol-Trpm1 quantified by qRT-PCR, from Pax6- compared to GFP-injected embryos. (K) Relative luciferase luminescence upon transfection of HeLa cells with Pax6 expression plasmid with or without ol-Trpm1 promoter sequences. **P<0.001; ***P<0.0001. Abbreviations: L, Lens; OC, optic cup; OE, olfactory epithelium; NC neural crest melanocytes. Scale bar in C: 20 µm. In medaka, ol-miR-204 is located within an ol-Trpm1 intron. Utilizing phylogenetic footprinting in sequences from related species [42], we identified putative Pax6 cis-regulatory elements within the first 1.5 kb upstream of the ol-Trpm1 coding region (Figure 4E). This region included a Pax6 binding site that resembled the Trpm3.4 region bound by Pax6 in vitro (Figure 2B, Figure 4E). To test the regulatory potential of these sequences, the medaka 1.5-kb genomic fragment was amplified and fused with a nuclear EGFP reporter to generate the p-ol-Trpm1P-GFP construct (Figure 4E). p-ol-Trpm1P-GFP was then assayed for possible enhancer activity in medaka embryos [42]. Three stable transgenic p-ol-Trpm1P-GFP lines showed comparable and robust EGFP expression in the developing fish eye in a pattern that mimicked the reported spatio-temporal distribution of the expression pattern of both ol-Trpm1 and miR-204 in medaka ([17], Figure 4D, 4G), at both embryonic (Figure 4C, 4D) and adult (not shown) stages. We thus concluded that this region contains the sequences that mediate Trpm1/miR-204 expression in the medaka fish.
To examine whether Pax6 controls ol-Trpm1/miR-204 expression in vivo, we tested whether over-expression could expand ol-Trpm1/miR-204's expression domains. Injections of murine Pax6 mRNA (75–100 ng/µl) in p-ol-Trpm1P-GFP-transgenic embryos expanded and enhanced EGFP expression in both the lens and ventral retina of the OC (stage 24; Figure 4I, red arrow). Notably, no ectopic EGFP expression was observed in regions other than the retina, supporting tissue-specific Pax6-mediated activation of ol-Trpm1/miR-204 expression. Likewise, Pax6 over-expression in wild-type embryos expanded ol-Trpm1 mRNA distribution (Figure 4H) and increased both miR-204 and ol-Trpm1 transcript levels as detected by quantitative (q) RT-PCR (Figure 4J). In agreement with these results, transient transfection of HeLa cells with murine Pax6 activated a luciferase reporter construct containing the Trpm1P fragment significantly more than the luciferase activity observed in controls (Figure 4K). Collectively, these findings revealed that Pax6 is able to regulate the Trpm1/miR-204 promoter in fish, similar to its regulation of Trpm3/miR-204 in mice.
miR-204 mediates Pax6 suppression of Sox11 and additional genes in the lens and in medaka embryos
To further evaluate the contribution of miR-204 to the phenotype observed following loss of Pax6, we tested the response of a subset of seven additional genes (Cpn8, Satb2, Hcn2, Nfia, Myo10, Fbn2, and Elavl3) that were both up-regulated in Pax6-negative lenses and contained putative miR-204 binding sites in their 3′ UTR (Table 2) to over-expression of hsa-miR-204 mimic. Most of these genes were examined in the human lens H36CE cell line, whereas Elavl3, a member of the Hu family of neuronal RNA binding proteins, was examined in mouse Neu-2a cells (Figure S8) because of its low expression levels in the lens cell line (data not shown). From the eight genes that were tested, including Sox11, six were down-regulated in response to hsa-miR-204 mimic; the five reduced in H36CE cells were also significantly elevated following transfection with hsa-miR-204 inhibitor (Cpn8, Nfia, Myo10, Fbn2, Sox11; Figure 5A). In Neu-2a cells, Elavl3, Sox11 and Myo10 were significantly reduced (Figure S8). Luciferase assay conducted on the Elavl3 3′ UTR further supported direct regulation of Elavl3 by miR-204, and antibody labeling revealed expansion of Elavl3 expression to the posterior lens cells of Pax6loxP/loxP;Mrl10-Cre mutants (Figure S8, [43]).
Fig. 5. miR-204 affects expression of multiple genes and mediates Pax6 suppression of gene expression. 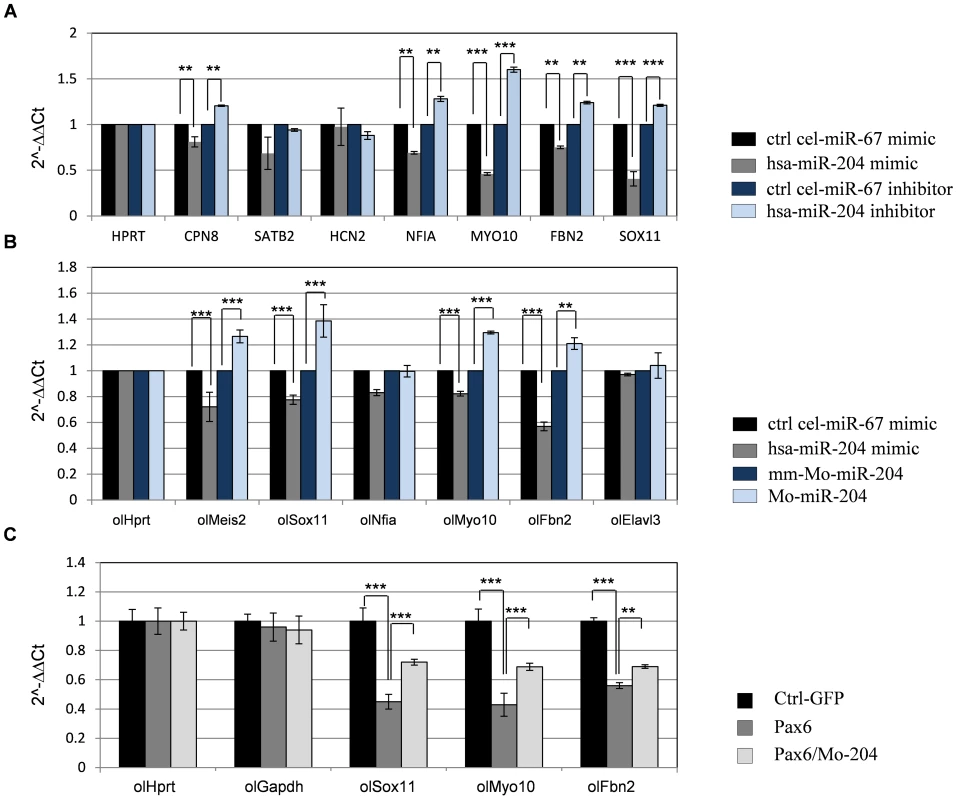
(A) H36CE cells transfected with hsa-miR-204 mimic or control miRNA (cel-miR-67 mimic) or hsa-miR-204 inhibitor or control miRNA inhibitor (cel-miR-67 inhibitor). Significant reduction following over-expression of hsa-miR-204 mimic and significant elevation following transfection with hsa-miR-204 inhibitor was detected by qPCR (expressed as 2-ΔΔCt values) for transcripts of Sox11, Cpn8, Nfia, Myo10 and Fbn2. Error bars are SD (**P<0.001 and ***P<0.0001, n = 3). (B) Fold-change (expressed as 2-ΔΔCt values) in mRNA levels of indicated medaka genes quantified by qRT-PCR, from stage 24 embryos injected with hsa-miR-204 mimic compared to control cel-miR-67mimic, morpholino against miR-204 (Mo-miR-204) and mismatched morpholino (mm-Mo-miR-204). 400 embryos were pooled for each assay, and technical triplicate experiments were independently executed at least three times. (C) Fold-change in mRNA levels of the indicated genes quantified by qRT-PCR from embryos injected with Pax6, Pax6/Mo-miR-204 and pGFP-expressing plasmid as a control. Results are shown as means ± SD, 250 embryos pooled for each assay. Technical triplicate experiments were independently executed at least three times, n = 3, **P<0.001, ***P<0.0001. As the expression pattern and regulation of miR-204 seem to be conserved between fish and mammals ([17], Figure 4), we also expected conservation of miR-204 activity. To test this, medaka embryos were injected with either hsa-miR-204 mimic for over-expression of miR-204, or with a morpholino (Mo-miR-204) for knock - down of the endogenous hsa-miR-204 [17]. Control embryos were injected with either an unrelated control Caenorhabditis elegans cel-miR-67 mimic or a six-base mismatched morpholino (MM-Mo-miR-204). The expression of ol-Sox11, ol-Nfia, ol-Fbn2, ol-Myo10, and ol-Elavl3 was monitored by qRT-PCR in embryos collected at stage 24, when the ocular phenotype of ol-miR-204 was first documented (Figure 5B, [17]). Ol-Meis2 was also monitored as it is a known target of miR-204 in medaka [17]. ol-Meis2, ol-Sox11, ol-Fbn2 and ol-Myo10 transcripts were significantly reduced following treatment with hsa-miR-204 mimic, by 28±3.7%, 23±2.8%, 43±3,4% and 21±1% relative to the control, respectively (Figure 5B); these genes were up-regulated following treatment with the Mo-miR-204 by 26.5±4.9%, 38.5±7.8%, 21±4% and 29.4±1.1%, respectively, relative to the control (Figure 5B). This finding supports the regulation of ol-Sox11 as well as ol-Myo10, and ol-Fbn2 by miR-204 in medaka, similar to the regulatory interaction observed in mammals.
We next examined Pax6 regulation of ol-Sox11, ol-Fbn2 and ol-Myo10. Injection of mouse Pax6 mRNA into medaka embryos resulted in reduced expression of these three genes compared with control embryos injected with EGFP mRNA (ol-Sox11 was reduced to 45±5.1%, ol-Myo10 was reduced to 43±6.3%, and ol-Fbn2 to 56.4±2.1% Figure 5C). This reduction was significantly alleviated when embryos were co-injected with Mo-miR-204 (ol-Sox11 was rescued by 28.5±1%, ol-Myo10 by 26±3%, and ol-Fbn2 by 18.4±1.4%) (Figure 5C), indicating that at least part of the repression is mediated through miR-204 activity.
Collectively, and similar to mammals, the expression of ol-Sox11, ol-Fbn2 and ol-Myo10 is negatively regulated by Pax6 and ol-miR-204 in medaka fish. These findings reveal several novel targets of miR-204 in the lens and demonstrate how Pax6 direct control of miR-204 can simultaneously inhibit multiple genes, thus tightly regulating normal lens fate and physiology.
Discussion
In this paper we establish Pax6 as an indirect negative regulator of gene expression through miR-204. We demonstrate that Pax6 regulation of miR-204 is conserved in vertebrates through regulation of the host gene Trpm3 in mice and ol-Trpm1 in medaka. The study identifies several targets of miR-204 repression in the lens, among them Sox11, an important factor for eye and CNS development. Additional new targets for miR-204 include neuronal factors and genes involved in cell motility. Pax6 regulation of miR-204 explains part of the complex and divergent inhibitory activity of Pax6 in ocular progenitor cells, which is required to establish and maintain the identity of ocular tissues.
miR-204 in ocular physiology and development
In mammals, miR-204 has a closely related paralog the miR-211. miR-204 and miR-211 differ by one or two nucleotides, depending on the species. However, they have the same seed-region sequence. Classified as a subfamily of miRNAs, they show the same set of predicted targets (TargetScan [12]). Interestingly, miR-211 first appears in mammals through the evolution of one of the two copies of miR-204, which is present in two identical copies in the genomes of early vertebrates and fish, including medaka fish [17]. In future studies it would be of interest to distinguish the different expression pattern of Trpm1/3 paralog genes and the functional activity of their hosted miRNAs in the different lineages.
The roles of miR-204 in the eye have been recently examined using a primary culture of human fetal RPE. In those cells, miR-204 was shown to down-regulate levels of TgfbR2 and Snai2, genes known to be involved in the epithelial-to-mesenchymal transition (EMT, [18]). The authors provided evidence of this regulation helping to maintain epithelial phenotype and prevent EMT of the RPE [18]. EMT is characteristic of TGFβ-induced anterior subcapsular cataract formation, a pathology also associated with reduced dosage of Pax6 [44]–[46]. Interestingly, secondary cataract has recently been associated with alterations in miR-204 expression [47]. With this in mind, it is possible that part of Pax6's activity in inhibiting cataract formation is mediated by the activity of miR-204 in the lens and that restoration of miR-204 levels may prevent anterior subcapsular cataract formation.
In addition, the novel miR-204 targets in lens development uncovered here include genes involved in cell motility Myo10 [48], cell matrix and regulation of the TGFβ pathway Fbn2 [49], and genes that function in neuronal or glia cells Sox11 [37], Elavl3 [43], Cpne8 [50] and Nfia [51]. The identification of multiple genes responding to miR-204, and the finding that some of these targets are conserved among vertebrates (Sox11, Fbn2, Myo10), suggest a major effect of miR-204 in regulating LE fate and cell behavior. Future studies should evaluate the contribution of each of the identified targets to maintaining lens physiology (Figure 6). Furthermore, as miR-204 is expressed in several epithelial tissues that originate from the neuroectoderm, including the ciliary body, iris and choroid plexus, it is possible that miR-204 plays a more general role in maintenance of a non-neuronal fate of various epithelia within the CNS.
Fig. 6. Model of Pax6 genetic regulation of Sox11 during ocular development. 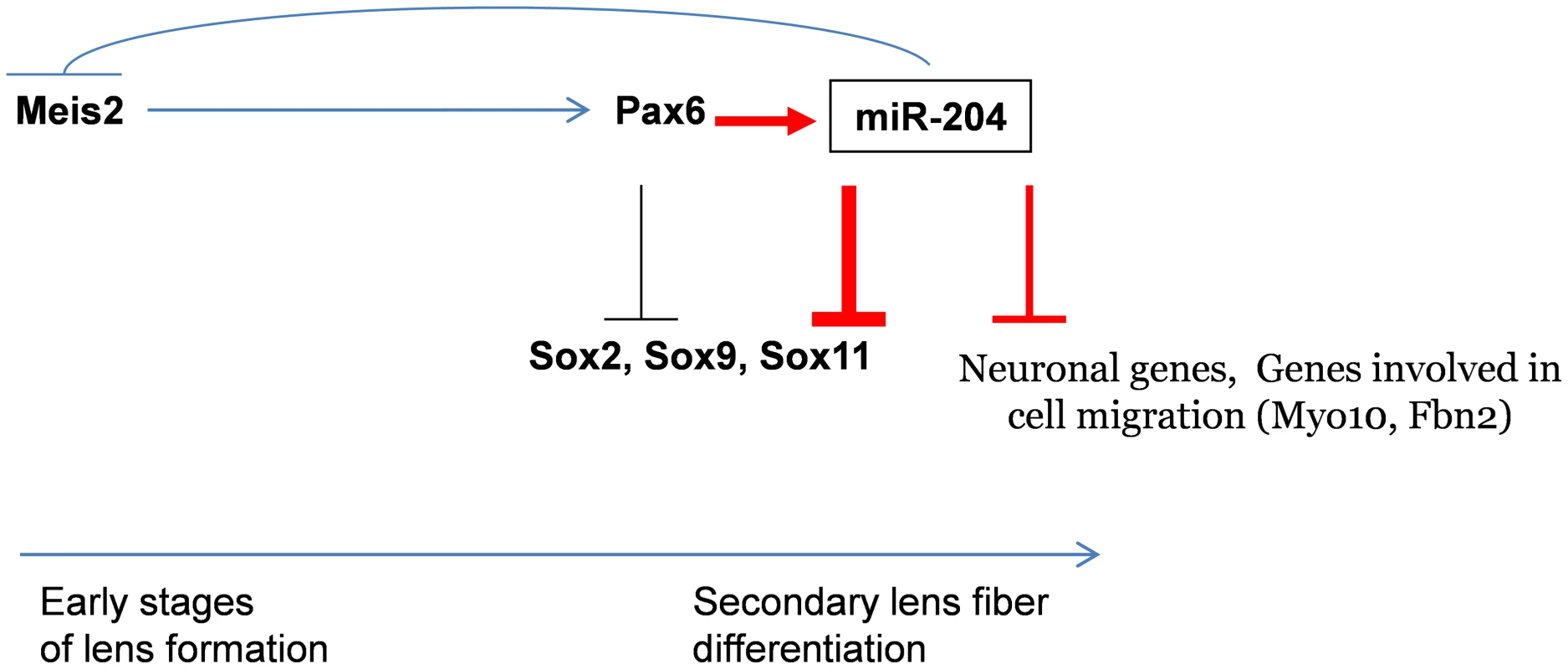
Pax6 directly regulates miR-204 by binding and activating expression of the host genes Trpm3 in mammals and ol-Trpm1 in fish. During later stages of lens development, miR-204 reinforces inhibition of Sox11. miR-204 is upstream of several genes involved in neurogenesis and cell motility. The findings in medaka suggest that during the early stages of LP formation, ol-miR-204 and Pax6 may co-regulate each other via a negative feedback loop through the established Meis2-Pax6 pathway. Red arrows indicate new data presented here. miR-204 was found to negatively regulate the expression of ol-Meis2 in the eye of medaka fish [17]. Murine Meis1 and Meis2 have been found to be upstream regulators of Pax6 during induction of the LP [52]. It was therefore proposed that miR-204 regulates ol-Pax6 in the lens through regulation of ol-Meis2 [17]. The findings presented here suggest a negative feedback loop between Meis1/2, Pax6 and miR-204 (Figure 6). This regulatory loop may only be relevant at early stages of lens development, because in the mouse lens, Meis1 and Meis2 expression is lost before E12.5 [53], whereas Pax6 and miR-204 expression is maintained throughout development (Figure 1). Further analysis is required to substantiate the relevance of the miR-204–Meis1/2–Pax6 feedback loop in maintaining correct Pax6 dosage during early stages of lens development.
Pax6 regulation of neuronal SOX genes
A previous global analysis of gene expression revealed a significant number of neuron-specific genes that are up-regulated in Pax6-heterozygous lenses [31]. This raised the hypothesis that Pax6 has a dual role: promoting lenticular genes, while simultaneously suppressing competing programs, such as neural development [31]. The altered expression profile in the Pax6-null embryonic lens reported here further revealed over-representation of neurogenic genes using several tools for seeking gene-ontology enrichment DAVID, (NIH, [54]), Expander (Tel-Aviv University, [55]), GATHER (Duke University, [56]) and Ontologizer2.0 (Computational Biology Group, [57]). Among these, four SOX members—Sox2, Sox6, Sox9 and Sox11—were up-regulated upon Pax6 deletion. All four are required for nervous system development [33], [34], [58]–[64]. Three of these genes were found to be regulated by Pax6 at the late stages of lens development in situ: Sox2 [24], Sox11 (Figure 3) and Sox9 (Figure S5). The propensity for Pax6 to repress SOX genes in the lens could be a way of silencing competing genetic programs.
In this paper, we focused on the regulatory interaction between Pax6 and Sox11 to determine the mechanism underlying Pax6 inhibitory activity during organ formation. In the eye, Sox11 is required for normal development of both lens and retina [32]. The expression of Sox11 in the lens overlaps with that of Pax6 during the early LP stage but is reduced to below detection levels by in-situ hybridization (ISH) during the lens vesicle stage (E11, not shown). In contrast, Pax6 expression is maintained in the LE through adulthood. This expression pattern supports negative regulation of Sox11 by Pax6 from the lens vesicle stage onward. The similar phenotypes of Sox11 and Pax6 mutants suggest that during early stages of LP formation, Pax6 is a positive regulator of Sox11 [32]. However, our analysis of Sox11 expression in Pax6lacZ/lacZ mutants does not support this notion (Figure S3). The current study provides evidence for inhibition of Sox11 by Pax6, mediated by direct miR-204 repression from the stage at which Trpm3/miR-204 expression begins (Figure 1). We propose that Pax6 initially functions in parallel to Sox11 for growth and morphogenesis of the LP. At later stages, Pax6 is required for the initiation of Trpm3/miR-204 expression in both retinal and lens progenitors. In cells that express miR-204, the regulation of Sox11 by Pax6 becomes inhibitory, contributing to loss of Sox11 expression from the lens fibers (Figure 3 and Figure 6). This is further supported by the observation that over-expression of either Pax6 or miR-204 in medaka, results in reduced expression of Sox11, and that Pax6 inhibition can be rescued by knock-down of miR-204 (Figure 5). Hence, this study reveals a new level of regulatory hierarchy mediated by Pax6 during the acquisition of cell fate.
Combinatorial interactions between miRNAs and TFs have been suggested based on in-silico strategies which identified a prevalent TF-miRNA feed-forward loop in which miRNA and TFs co-regulate target genes, as well as each other [65], [66]. We therefore considered the possibility that Pax6 might negatively regulate Sox11 transcription directly, in addition to repressing expression through miR-204. We did not, however, obtain substantial evidence for this possibility when examining the effects of Pax6 on Sox11 regulatory regions in cell culture (HeLa and 293T cells, D. G., and R. A-P., data not shown). Nevertheless, it is possible that binding sites, other than those tested here, play a role in the regulation of Sox11 by Pax6, and that Pax6 represses Sox11 in the presence of co-factors which are only available in the restricted developmental context of the embryonic lens in vivo.
In the retina, Sox11 exhibits a dynamic pattern of expression. It is expressed in the OV and later in the inner OC. Sox11 levels are lower in RPCs than in post-mitotic neurons in the ganglion cell layer (Figure 3), in accordance with its importance in neurogenic precursors in the CNS along with Sox4 [33], [58]. It is worth noting that while Sox11 is detected in the ganglion cell layer during embryogenesis it is subsequently reduced and is not detected in the postnatal retina (K. G, R. A-P unpublished observation). Pax6 however, is maintained in terminally differentiated ganglion and amacrine cell types. Pax6 may therefore contribute to the down-regulation of Sox11 in neuronal precursors during their terminal differentiation. Pax6 is known to play a dual role in RPCs—down-regulating the cone-rod homeobox gene Crx and maintaining proliferation in the peripheral retina, while maintaining pluripotency of the more central cells of the OC [11], [29]. Therefore, Pax6 regulation of Sox11 during retinogenesis is expected to be part of a larger regulatory network, and may be repressed by miR-204 in only a subset of RPCs.
In conclusion, this study reveals novel involvement of miRNA-based gene repression during lens and retinal development. Pax6, a regulator of multiple processes during eye development, is shown to execute part of its gene regulation through direct activation of miR-204.
Materials and Methods
Mouse lines
The mouse lines employed in this study have been previously described: Pax6loxP [67], Mlr10-cre [68], αCre [29], Pax6lacz [69]. All animal work was conducted according to national and international guidelines and approved by the Tel Aviv University review board.
Statistical analysis
All data were examined using two-tailed student's t-test unless otherwise stated.
Microarray analysis
Pax6loxP/loxP;Mlr10-cre and Pax6loxP/loxP E14.5 lenses were dissected; 20 lenses from each litter were pooled, resulting in three control (Pax6loxP/loxP) and three Pax6loxP/loxP;Mlr10-cre samples. RNA purification was performed using Qiashredder and RNeasy (Qiagen). Total RNA (300 ng) was used to generate sense-strand cDNA, which was fragmented, biotin-labeled and hybridized to Affymetrix GeneChip 1.0ST microarrays. Microarray analysis was performed using Partek Genomics Suite (Partek Inc., MO, USA; www.partek.com). Differentially expressed genes with P-values lower than 0.05 and with a fold-change cutoff of 1.5 are listed in Table S1.
MiRNA–target enrichment analysis
We calculated the significance of miR-204-target enrichment among the up-regulated genes (fold change >1.5, P-value<0.05, n = 754 genes) using TargetScan Mouse predictions (version 5.2; http://www.targetscan.org/mmu_50/) and the hypergeometric test. The background dataset for the calculation included the differentially expressed genes (fold-change cutoff >1.5 and P-value<0.05, n = 1,013 genes). P-values were corrected using the false discovery rate (FDR) method [70].
Immunofluorescence and ISH on sections
Immunofluorescence analysis was performed on 10-µM paraffin sections or 14-µM frozen sections as described previously [67], using the following primary antibodies: rabbit anti-Pax6 (1∶400, Covance, # prb-278b), rabbit anti-Sox9 (1∶200, Chemicon, ab5535), mouse anti-Elavl3 (1∶200, Invitrogen, A21272) and goat anti-CrystallinαA (1∶1,000, Santa Cruz, sc-22389). Secondary antibodies were conjugated to alexa594 donkey anti rabbit/goat (1∶1000, Invitrogen, A-21207/A-11058) or alexa488 donkey anti mouse/goat (1∶1000, Invitrogen, A-21202/A-11055).
mRNA ISH was performed as described previously [71]. Plasmids for antisense transcription were: Trpm3 [21] and Sox11—kindly donated by Kirsten Kuhlbrodt [72]. For miRNA ISH, hsa-miR-204 miRCURY LNA Detection probe (working concentration 1/150 µM/µl, Exiqon, cat number 88076-15) were hybridized to frozen sections as described previously [73].
EMSA
HEK293T cells were transfected with p3Xflag-CMV-10 conjugated to the first 270 amino acids of Pax6 containing PD and HD binding domains. Nuclear extracts were obtained as previously described [74]. Nuclear extract (1 µl) or 1∶10 diluted nuclear extract was incubated for 10 min on ice in 8.5 mM HEPES pH 7.9, 30 mM KCl, 1.5 mM MgCl2, 0.4 mM DTT and 2 µg polydI/dC (Sigma). Binding with 1 µl double-stranded 5′-γ-ATP-labeled probe (30,000 cpm) was performed at room temperature for 20 minutes and 200 ng of “cold” Pax6 consensus site was used for competition (P6CON; Wolf et al., 2009). EMSA probes are listed in Table S2.
Quantitative ChIP
Groups of 400 pooled newborn lenses were fixed in 1% formaldehyde for 10–15 minutes at room temperature. The cross-linking reaction was quenched with 0.125 M glycine. The tissues were sonicated in 4 ml lysis buffer (1 mM EDTA pH 8, 0.5 mM EGTA pH 8, 10 mM Tris-HCl pH 8) by Fisher Sonic Dismembrator Model 500 for 14 min (30 s on/30 s off, amplification = 30%). Aliquots of the sheared chromatin prepared from 40 lenses were incubated with 5 µg rabbit anti-Pax6 antibody (Millipore, Cat# AB2237, epitope C-terminus) bound to 20 µl protein G-coated magnetic beads (Invitrogen). The immunoprecipitates were washed three times and resuspended in a buffer containing 10 mM Tris–HCl pH 8.0, 100 mM NaCl, and 25 mM EDTA supplemented with 0.1 mg/ml RNaseA and 0.2 mg/ml proteinase K. After 2 h incubation at 55°C, the cross-linking was reversed by overnight incubation at 65°C. Genomic DNA was eluted into 250 µl of water using QIAquick Spin Gel Purification kit (Qiagen).
Amounts of each specific DNA fragment were determined by real-time qPCR using a standard curve generated for each primer set with 0.04, 0.2, and 1% input DNA samples. The copy number of each DNA fragment was compared to the copy number of that fragment in the input DNA. A control antibody (rabbit IgG, Millipore, Cat# NI01) was included for each set of qPCR experiments. The PCR products were between 80 and 130 bp in length. Primers used for PCR are listed in Table S2. The reactions were analyzed using a 7900 ABI PRISM PCR Instrument and 2× SYBR mix (Applied Biosystems). The parameters were: 95°C/10 min followed by 45 cycles of 94°C/10 s, 60°C/20 s and 72°C/30 s.
Immunofluorescence quantification of protein levels
For Sox11 repression by miR-204, Neuro-2a cells (ATCC) were grown on coverslips in 24-well plates for 24 h and then co-transfected with 500 ng of pCAG-GFP expression plasmid and either 500 ng of scrambled-miRVec or miR-204-miRVec expression plasmid containing the pre-miRNA of miR-204 [75]. Cells were fixed for 5 min in 4% (v/v) paraformaldehyde and then immunolabeled as described previously [37] using goat anti-Sox11 (1∶100, Santa Cruz) and rabbit anti-GFP (1∶500, Rockland Immunochemicals). Cell immunofluorescence was measured using ImageJ (NIH) as described previously [24]. Each pCAG-GFP-positive cell (green channel), co-transfected with either miR-204-miRVec or scrambled-miRVec, was measured for Sox11 fluorescence intensity (red channel). The fluorescence level of each transfected cell was divided by the average (red) fluorescence of non-transfected cells in the same field, resulting in a Sox11 index level for each cell calibrated for random image conditions. Sox11 index levels of all miR-204-miRVec-transfected cells were then compared to those of scrambled-miRVec-transfected controls. Fluorescence was measured blindly for the miR-204-miRVec - or scrambled-miRVec-transfected cells. In all measurements, the cells that had a mega-nucleus or multiple neurites were considered differentiated and were therefore not counted.
Teleost sequence analysis and plasmid construction
The available teleost Trpm1 genomic sequences were retrieved (http://genome.ucsc.edu/) and aligned for putative regulatory modules on the basis of sequence conservation [42]. A 1.5-kb region of ol-Trpm1 genomic sequence containing the start codon using the primers: ol-Trpm1 forward 5′-TGTATCATGAGCCGCTAATG-3′ and ol-Trpm1 reverse 5′-GCAGCACCAGGAGAAGGCTC-3′, was cloned in frame with an EGFP reporter gene into the pSKII-ISceI-EGFP and pGL3 basic vectors [76] to create the Trpm1P:GFP and Trpm1:Luc constructs.
Phylogenetic analysis
Sequence alignments were performed using the VISTA [77] and Multalign programs [78], which are available at the corresponding websites: [http://genome.lbl.gov/vista/index.shtml and [http://bioinfo.genopole-toulouse.prd.fr/multalin/multalin.html]. The criterion used for comparison was a minimum 75% nucleotide identity with a window size of over 100 bp. Phylogenetic analysis was performed using the PHYLIP package and the results were plotted using the Tree-view software package as previously described [41]). The syntenic genomic region was analyzed using MultiZ and Medaka Chain/Net package at UCSC (http://genome.ucsc.edu/).
Establishment of transgenic fish lines
The Cab inbred (CI) medaka strain was used throughout this study and aged with Iwamatsu staging [79]. Transgenesis and live EGFP monitoring was performed as described previously [42]. Three independent stable transgenic lines were generated for the tested construct.
mRNA injection of medaka embryos
In-vitro synthesis of mouse Pax6 mRNA (cDNA from [80]) was performed as described previously [76]. Pax6 mRNA was injected at 50–200 ng/µl to observe dose-dependent phenotypes. Selected working concentrations were 75–100 ng/µl. Control embryos were injected with 15 ng/µl of EGFP mRNA [76].
Cell transfection and luciferase assays
For miRNA-repression studies (Figure 3D), a 506-bp fragment of the Sox11 3′ UTR (spanning two miR-204 binding sites) or 484 bp of Elavl3's 3′ UTR was amplified by PCR from mouse brain cDNA and XhoI–NotI restriction sites were added with the following primers: Sox11 (F) 5′-ACACTCGAGCTGTTACTCTAGGGAGTTGA-3′ and (R) 5′-AAGGTCAAGCGGCCGCAAAGGGAAGAAGTGCCTGAA-3; Elavl3 (F) 5′-ACACTCGAGCAATGGTGCCCTACTCAGG-3′ and (R) 5′-AGGTCAAGCGGCCGCTTCCTGTGGCCATGTTTGCT-3′.
The 3′ UTRs were cloned downstream of the Renilla luciferase reporter (psiCHECK™-2, Promega). For site-directed mutation, the miR-204 binding site on the 3′ UTRs (second binding site for Sox11), was mutated by PCR with PfuUltra II Fusion HS DNA Polymerase (Genex). The mutagenesis primers were: Sox11 (F) 5′-TTTGTACAGTGAAAATCTCACAATCTTGCTGTGT-3′; Elavl3 (F) 5′-CTATTTTTGTAAAAACTCCAAAAGACCTCGTGGA-3′ and complementary reverse primers. Products were then incubated with DPN1 (New England BioLabs) for digestion of the source plasmid. Co-transfection of miRVec-miR-204 [75] and the Renilla-3′ UTR plasmid was in HEK293T cells with TransIT-LT1 Transfection Reagent (Mirus). After 48 h, firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System.
Regulation of promoter activity in cell culture (Figure 4K) was performed in HeLa cells. Cells were co-transfected with the construct (100 ng), expression vector pcDNA3/Pax6 (100 ng) and RL-TK plasmid with Renilla luciferase (10 ng) as a transfection-efficiency control. Transfection was performed using Fugene Transfection Reagent (Roche) following the manufacturer's protocol [76]. Cells were harvested 48 h after transfection. Reporter activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Each assay was performed in duplicate, with three biological repeats.
For functional studies of miR-204 in cell culture (Figure 5A, Figure S8) the human lens cell line H36CE (Porter et al. 1998) was transfected and qRT-PCR experiments were performed as described [81]. H36CE cells were transfected with either 50 nM miRIDIAN™ Dharmacon microRNA Mimics (hsa-miR-204 mimic or control cel-miR-67) or 80 nM miRIDIAN™ Dharmacon microRNA Inhibitor (hsa-miR-204 inhibitor or negative control cel-miR-67). Neu-2a cells were transfected using HiPerFect (Qiagen) with hsa-miR-204 mimic (Ambion) or miR negative control (Applied Biosystems) oligonucleotide. The isolation of RNA and qRT-PCR for detection of miR-204 were as previously described [82]. Total cDNA was generated using SuperScript III (Invitrogen) and qRT-PCR was performed with FastStart Universal SYBR Green Master (Roche). Primers used for qRT-PCR are listed in Table S4 and sequences of for functional studies of miR204 are listed in Table S5.
qRT–PCR analysis of gene expression in injected embryos
At least 250 embryos were pooled in each assay. cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System for RT-PCR using random hexamers (Invitrogen). ol-Hprt was used as the endogenous control as previously described [17]. Detection of miR-204 was performed by TaqMan MicroRNA Assays (Applied Biosystems) following the manufacturer's protocol. Each assay was performed in duplicate. Primers used for qRT-PCR are listed in Table S4.
Supporting Information
Zdroje
1. OsumiN, ShinoharaH, Numayama-TsurutaK, MaekawaM (2008) Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26 : 1663–1672.
2. ShahamO, MenuchinY, FarhyC, Ashery-PadanR (2012) Pax6: A multi-level regulator of ocular development. Prog Retin Eye Res 31 : 351–376.
3. GehringWJ (1996) The master control gene for morphogenesis and evolution of the eye. Genes Cells 1 : 11–15.
4. CveklA, YangY, ChauhanBK, CveklovaK (2004) Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int J Dev Biol 48 : 829–844.
5. CzernyT, BusslingerM (1995) DNA-binding and transactivation properties of Pax-6: three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5). Mol Cell Biol 15 : 2858–2871.
6. EpsteinJA, GlaserT, CaiJ, JepealL, WaltonDS, et al. (1994) Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev 8 : 2022–2034.
7. HeS, PirityMK, WangWL, WolfL, ChauhanBK, et al. (2010) Chromatin remodeling enzyme Brg1 is required for mouse lens fiber cell terminal differentiation and its denucleation. Epigenetics Chromatin 3 : 21.
8. YangY, StopkaT, GolestanehN, WangY, WuK, et al. (2006) Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. Embo J 25 : 2107–2118.
9. DuncanMK, HaynesJI2nd, CveklA, PiatigorskyJ (1998) Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol Cell Biol 18 : 5579–5586.
10. YangY, ChauhanBK, CveklovaK, CveklA (2004) Transcriptional regulation of mouse alphaB - and gammaF-crystallin genes in lens: opposite promoter-specific interactions between Pax6 and large Maf transcription factors. J Mol Biol 344 : 351–368.
11. Oron-KarniV, FarhyC, ElgartM, MarquardtT, RemizovaL, et al. (2008) Dual requirement for Pax6 in retinal progenitor cells. Development 135 : 4037–4047.
12. LewisBP, BurgeCB, BartelDP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120 : 15–20.
13. GrishokA, PasquinelliAE, ConteD, LiN, ParrishS, et al. (2001) Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106 : 23–34.
14. LiY, PiatigorskyJ (2009) Targeted deletion of Dicer disrupts lens morphogenesis, corneal epithelium stratification, and whole eye development. Dev Dyn 238 : 2388–2400.
15. GeorgiSA, RehTA (2010) Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J Neurosci 30 : 4048–4061.
16. DavisN, MorE, Ashery-PadanR (2011) Roles for Dicer1 in the patterning and differentiation of the optic cup neuroepithelium. Development 138 : 127–138.
17. ConteI, CarrellaS, AvellinoR, KaraliM, Marco-FerreresR, et al. (2010) miR-204 is required for lens and retinal development via Meis2 targeting. Proc Natl Acad Sci U S A 107 : 15491–15496.
18. WangFE, ZhangC, MaminishkisA, DongL, ZhiC, et al. (2010) MicroRNA-204/211 alters epithelial physiology. Faseb J 24 : 1552–1571.
19. AdijantoJ, CastorinoJJ, WangZX, MaminishkisA, GrunwaldGB, et al. (2012) Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNA-204/211 expression. J Biol Chem 287 : 20491–20503.
20. GrimmC, KraftR, SauerbruchS, SchultzG, HarteneckC (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278 : 21493–21501.
21. KaraliM, PelusoI, MarigoV, BanfiS (2007) Identification and characterization of microRNAs expressed in the mouse eye. Invest Ophthalmol Vis Sci 48 : 509–515.
22. DeoM, YuJY, ChungKH, TippensM, TurnerDL (2006) Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn 235 : 2538–2548.
23. RyanDG, Oliveira-FernandesM, LavkerRM (2006) MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis 12 : 1175–1184.
24. ShahamO, SmithAN, RobinsonML, TaketoMM, LangRA, et al. (2009) Pax6 is essential for lens fiber cell differentiation. Development 136 : 2567–2578.
25. SansomSN, GriffithsDS, FaedoA, KleinjanDJ, RuanY, et al. (2009) The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 5: e1000511 doi:10.1371/journal.pgen.1000511.
26. WagnerTF, LochS, LambertS, StraubI, MannebachS, et al. (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 10 : 1421–1430.
27. VriensJ, OwsianikG, HofmannT, PhilippSE, StabJ, et al. (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70 : 482–494.
28. GrindleyJC, DavidsonDR, HillRE (1995) The role of Pax-6 in eye and nasal development. Development 121 : 1433–1442.
29. MarquardtT, Ashery-PadanR, AndrejewskiN, ScardigliR, GuillemotF, et al. (2001) Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105 : 43–55.
30. XieQ, CveklA (2011) The orchestration of mammalian tissue morphogenesis through a series of coherent feed forward loops. J Biol Chem 286 : 43259–43271.
31. WolfLV, YangY, WangJ, XieQ, BraungerB, et al. (2009) Identification of pax6-dependent gene regulatory networks in the mouse lens. PLoS ONE 4: e4159 doi:10.1371/journal.pone.0004159.
32. WurmA, SockE, FuchshoferR, WegnerM, TammER (2008) Anterior segment dysgenesis in the eyes of mice deficient for the high-mobility-group transcription factor Sox11. Exp Eye Res 86 : 895–907.
33. BergslandM, WermeM, MalewiczM, PerlmannT, MuhrJ (2006) The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev 20 : 3475–3486.
34. HaslingerA, SchwarzTJ, CovicM, Chichung LieD (2009) Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci 29 : 2103–2114.
35. Penzo-MendezAI (2010) Critical roles for SoxC transcription factors in development and cancer. Int J Biochem Cell Biol 42 : 425–428.
36. BhattaramP, Penzo-MendezA, SockE, ColmenaresC, KanekoKJ, et al. (2010) Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat Commun 1 : 9.
37. JankowskiMP, CornuetPK, McIlwrathS, KoerberHR, AlbersKM (2006) SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 143 : 501–514.
38. NielsenCB, ShomronN, SandbergR, HornsteinE, KitzmanJ, et al. (2007) Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. Rna 13 : 1894–1910.
39. SockE, RettigSD, EnderichJ, BoslMR, TammER, et al. (2004) Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol 24 : 6635–6644.
40. RaviV, VenkateshB (2008) Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev 18 : 544–550.
41. EsteveP, Lopez-RiosJ, BovolentaP (2004) SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech Dev 121 : 687–701.
42. ConteI, BovolentaP (2007) Comprehensive characterization of the cis-regulatory code responsible for the spatio-temporal expression of olSix3.2 in the developing medaka forebrain. Genome Biol 8: R137.
43. BitelCL, Perrone-BizzozeroNI, FrederiksePH (2010) HuB/C/D, nPTB, REST4, and miR-124 regulators of neuronal cell identity are also utilized in the lens. Mol Vis 16 : 2301–2316.
44. LovicuFJ, SchulzMW, HalesAM, VincentLN, OverbeekPA, et al. (2002) TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol 86 : 220–226.
45. de IonghRU, WederellE, LovicuFJ, McAvoyJW (2005) Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs 179 : 43–55.
46. LovicuFJ, AngS, ChorazyczewskaM, McAvoyJW (2004) Deregulation of lens epithelial cell proliferation and differentiation during the development of TGFbeta-induced anterior subcapsular cataract. Dev Neurosci 26 : 446–455.
47. HoffmannA, HuangY, Suetsugu-MakiR, RingelbergCS, TomlinsonCR, et al. (2012) Implication of the miR-184 and miR-204 competitive RNA network in control of mouse secondary cataract. Mol Med 18 : 528–538.
48. SousaAD, BergJS, RobertsonBW, MeekerRB, CheneyRE (2006) Myo10 in brain: developmental regulation, identification of a headless isoform and dynamics in neurons. J Cell Sci 119 : 184–194.
49. BrinckmannJ, HunzelmannN, KahleB, RohwedelJ, KramerJ, et al. (2010) Enhanced fibrillin-2 expression is a general feature of wound healing and sclerosis: potential alteration of cell attachment and storage of TGF-beta. Lab Invest 90 : 739–752.
50. LloydSE, MaythamEG, GrizenkovaJ, HummerichH, CollingeJ (2010) A Copine family member, Cpne8, is a candidate quantitative trait gene for prion disease incubation time in mouse. Neurogenetics 11 : 185–191.
51. KangP, LeeHK, GlasgowSM, FinleyM, DontiT, et al. (2012) Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74 : 79–94.
52. ZhangX, FriedmanA, HeaneyS, PurcellP, MaasRL (2002) Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev 16 : 2097–2107.
53. HeineP, DohleE, Bumsted-O'BrienK, EngelkampD, SchulteD (2008) Evidence for an evolutionary conserved role of homothorax/Meis1/2 during vertebrate retina development. Development 135 : 805–811.
54. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
55. ShamirR, Maron-KatzA, TanayA, LinhartC, SteinfeldI, et al. (2005) EXPANDER–an integrative program suite for microarray data analysis. BMC Bioinformatics 6 : 232.
56. ChangJT, NevinsJR (2006) GATHER: a systems approach to interpreting genomic signatures. Bioinformatics 22 : 2926–2933.
57. BauerS, GrossmannS, VingronM, RobinsonPN (2008) Ontologizer 2.0–a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 24 : 1650–1651.
58. BergslandM, RamskoldD, ZaouterC, KlumS, SandbergR, et al. (2011) Sequentially acting Sox transcription factors in neural lineage development. Genes Dev 25 : 2453–2464.
59. EpiskopouV (2005) SOX2 functions in adult neural stem cells. Trends Neurosci 28 : 219–221.
60. GrahamV, KhudyakovJ, EllisP, PevnyL (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39 : 749–765.
61. Hamada-KanazawaM, IshikawaK, OgawaD, KanaiM, KawaiY, et al. (2004) Suppression of Sox6 in P19 cells leads to failure of neuronal differentiation by retinoic acid and induces retinoic acid-dependent apoptosis. FEBS Lett 577 : 60–66.
62. PocheRA, RavenMA, KwanKM, FurutaY, BehringerRR, et al. (2008) Somal positioning and dendritic growth of horizontal cells are regulated by interactions with homotypic neighbors. Eur J Neurosci 27 : 1607–1614.
63. SasaiY (2001) Roles of Sox factors in neural determination: conserved signaling in evolution? Int J Dev Biol 45 : 321–326.
64. YokoiH, YanYL, MillerMR, BreMillerRA, CatchenJM, et al. (2009) Expression profiling of zebrafish sox9 mutants reveals that Sox9 is required for retinal differentiation. Developmental biology 329 : 1–15.
65. ShalgiR, LieberD, OrenM, PilpelY (2007) Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol 3: e131 doi:10.1371/journal.pcbi.0030131.
66. ZhouQ, MeltonDA (2008) Extreme makeover: converting one cell into another. Cell Stem Cell 3 : 382–388.
67. Ashery-PadanR, MarquardtT, ZhouX, GrussP (2000) Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev 14 : 2701–2711.
68. ZhaoH, YangY, RizoCM, OverbeekPA, RobinsonML (2004) Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci 45 : 1930–1939.
69. St-OngeL, Sosa-PinedaB, ChowdhuryK, MansouriA, GrussP (1997) Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 387 : 406–409.
70. BenjaminiY, DraiD, ElmerG, KafkafiN, GolaniI (2001) Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125 : 279–284.
71. YaronO, FarhyC, MarquardtT, AppleburyM, Ashery-PadanR (2006) Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development 133 : 1367–1378.
72. KuhlbrodtK, HerbarthB, SockE, EnderichJ, Hermans-BorgmeyerI, et al. (1998) Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem 273 : 16050–16057.
73. XuS, WitmerPD, LumayagS, KovacsB, ValleD (2007) MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem 282 : 25053–25066.
74. Hay-KorenA, CaspiM, ZilberbergA, Rosin-ArbesfeldR (2011) The EDD E3 ubiquitin ligase ubiquitinates and up-regulates beta-catenin. Mol Biol Cell 22 : 399–411.
75. VoorhoevePM, le SageC, SchrierM, GillisAJ, StoopH, et al. (2006) A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124 : 1169–1181.
76. ConteI, Marco-FerreresR, BeccariL, CisnerosE, RuizJM, et al. (2010) Proper differentiation of photoreceptors and amacrine cells depends on a regulatory loop between NeuroD and Six6. Development 137 : 2307–2317.
77. FrazerKA, PachterL, PoliakovA, RubinEM, DubchakI (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Res 32: W273–279.
78. CorpetF (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16 : 10881–10890.
79. IwamatsuT (2004) Stages of normal development in the medaka Oryzias latipes. Mech Dev 121 : 605–618.
80. MuiSH, KimJW, LemkeG, BertuzziS (2005) Vax genes ventralize the embryonic eye. Genes Dev 19 : 1249–1259.
81. GennarinoVA, SardielloM, AvellinoR, MeolaN, MaselliV, et al. (2009) MicroRNA target prediction by expression analysis of host genes. Genome Res 19 : 481–490.
82. LevyC, KhaledM, IliopoulosD, JanasMM, SchubertS, et al. (2010) Intronic miR-211 Assumes the Tumor Suppressive Function of Its Host Gene in Melanoma. Mol Cell
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



