-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Anti-tumor necrosis factor alpha (anti-TNF) biologic therapy is a widely used treatment for rheumatoid arthritis (RA). It is unknown why some RA patients fail to respond adequately to anti-TNF therapy, which limits the development of clinical biomarkers to predict response or new drugs to target refractory cases. To understand the biological basis of response to anti-TNF therapy, we conducted a genome-wide association study (GWAS) meta-analysis of more than 2 million common variants in 2,706 RA patients from 13 different collections. Patients were treated with one of three anti-TNF medications: etanercept (n = 733), infliximab (n = 894), or adalimumab (n = 1,071). We identified a SNP (rs6427528) at the 1q23 locus that was associated with change in disease activity score (ΔDAS) in the etanercept subset of patients (P = 8×10−8), but not in the infliximab or adalimumab subsets (P>0.05). The SNP is predicted to disrupt transcription factor binding site motifs in the 3′ UTR of an immune-related gene, CD84, and the allele associated with better response to etanercept was associated with higher CD84 gene expression in peripheral blood mononuclear cells (P = 1×10−11 in 228 non-RA patients and P = 0.004 in 132 RA patients). Consistent with the genetic findings, higher CD84 gene expression correlated with lower cross-sectional DAS (P = 0.02, n = 210) and showed a non-significant trend for better ΔDAS in a subset of RA patients with gene expression data (n = 31, etanercept-treated). A small, multi-ethnic replication showed a non-significant trend towards an association among etanercept-treated RA patients of Portuguese ancestry (n = 139, P = 0.4), but no association among patients of Japanese ancestry (n = 151, P = 0.8). Our study demonstrates that an allele associated with response to etanercept therapy is also associated with CD84 gene expression, and further that CD84 expression correlates with disease activity. These findings support a model in which CD84 genotypes and/or expression may serve as a useful biomarker for response to etanercept treatment in RA patients of European ancestry.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003394
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003394Summary
Anti-tumor necrosis factor alpha (anti-TNF) biologic therapy is a widely used treatment for rheumatoid arthritis (RA). It is unknown why some RA patients fail to respond adequately to anti-TNF therapy, which limits the development of clinical biomarkers to predict response or new drugs to target refractory cases. To understand the biological basis of response to anti-TNF therapy, we conducted a genome-wide association study (GWAS) meta-analysis of more than 2 million common variants in 2,706 RA patients from 13 different collections. Patients were treated with one of three anti-TNF medications: etanercept (n = 733), infliximab (n = 894), or adalimumab (n = 1,071). We identified a SNP (rs6427528) at the 1q23 locus that was associated with change in disease activity score (ΔDAS) in the etanercept subset of patients (P = 8×10−8), but not in the infliximab or adalimumab subsets (P>0.05). The SNP is predicted to disrupt transcription factor binding site motifs in the 3′ UTR of an immune-related gene, CD84, and the allele associated with better response to etanercept was associated with higher CD84 gene expression in peripheral blood mononuclear cells (P = 1×10−11 in 228 non-RA patients and P = 0.004 in 132 RA patients). Consistent with the genetic findings, higher CD84 gene expression correlated with lower cross-sectional DAS (P = 0.02, n = 210) and showed a non-significant trend for better ΔDAS in a subset of RA patients with gene expression data (n = 31, etanercept-treated). A small, multi-ethnic replication showed a non-significant trend towards an association among etanercept-treated RA patients of Portuguese ancestry (n = 139, P = 0.4), but no association among patients of Japanese ancestry (n = 151, P = 0.8). Our study demonstrates that an allele associated with response to etanercept therapy is also associated with CD84 gene expression, and further that CD84 expression correlates with disease activity. These findings support a model in which CD84 genotypes and/or expression may serve as a useful biomarker for response to etanercept treatment in RA patients of European ancestry.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of the synovial lining of the joint [1]. If left untreated, outcome varies from self-limited disease in a small proportion of RA patients to severe disease resulting in profound structural damage, excess morbidity and disability, and early mortality [2]. In the last twenty years, disease activity has been controlled in many patients by treatment with disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate, and the more recently developed biologic DMARDs that block inflammatory cytokines such as tumor necrosis factor-alpha (TNFa) [3]. Unfortunately, these medications are not effective in all RA patients, with up to one-third of patients failing to respond to any single DMARD [1]–[3]. Moreover, the biological mechanisms underlying treatment failure are unknown, which limits the development of clinical biomarkers to guide DMARD therapy or the development of new drugs to target refractory cases.
There are two classes of anti-TNF therapy: the TNF receptor fusion protein (etanercept), which acts as a soluble receptor to bind circulating cytokine and prevent TNF from binding to its cell surface receptor, and monoclonal antibodies that bind TNF (adalimumab, infliximab, certolizumab, and golimumab). There are undoubtedly shared mechanisms between the two drug classes (e.g., downstream signaling factors), as illustrated by similar effects on the change in inflammatory cytokines, complement activation, lymphocyte trafficking, and apoptosis [4], [5], [6]. Similarly, there are likely to be different biological factors that influence response: infliximab and adalimumab are approved for treatment of Crohn's disease; infliximab and adalimumab bind to transmembrane TNF on the surface of activated immune cells, whereas etanercept only binds soluble TNF [7]; and etanercept also binds a related molecule, lymphotoxin alpha (LTA), whereas infliximab/adalimumab do not [8].
Pharmacogenetics of response to anti-TNF therapy in RA remains in its early stages, with no single variant reaching an unambiguous level of statistical significance. Candidate gene studies suggest associations of TNFa or TNF receptor alleles, RA risk alleles or other SNPs with response to anti-TNF therapy [9], [10], [11]. Two GWAS in small sample sets (largest was 566 patients) have been performed, which identified loci with suggestive evidence for association [12], [13]. Therefore, GWAS of large sample sizes may yet uncover genetic factors associated with response to anti-TNF therapy in RA, and larger cohorts enable separate analyses of the different types of anti-TNF drugs.
Here we report a GWAS of 2,706 samples with anti-TNF treatment response data collected from an international collaboration, including previously published GWAS data [12], [13]. Our primary outcome measure was the change in disease activity score based on a joint count in 28 joints (DAS28) from baseline to 3–12 months after initiating anti-TNF therapy. Our secondary outcome measure was European League Against Rheumatism (EULAR) responder status [14], [15], where patients are classified as EULAR good responders, moderate responders or non-responders based on follow up DAS28 after treatment and overall change in DAS28. We found a highly significant association for a variant that we also show is also a strong expression quantitative trait locus (eQTL) for the CD84 gene. Our findings suggest that CD84 genotype and/or expression may prove to be a biomarker for etanercept response in RA patients.
Results
Genome-wide association study
Clinical and GWAS data were compiled for 2,706 individuals of European ancestry from 13 collections as part of an international collaboration. Table 1 shows sample sizes, phenotypes and clinical variables for the four collections that were the units of analysis (additional details are shown in Table S1). Disease activity score based on a 28-joint count (DAS28) were collected at baseline and at one time point after anti-TNF therapy administration (mean 3.7 months, range 3–12 months). We defined our primary phenotype as a change in DAS28 (ΔDAS) from baseline (so that greater ΔDAS corresponded with better response to therapy; overall mean and standard deviation of 2.1±1.3), adjusted for baseline DAS. A secondary phenotype was used based on European League Against Rheumatism (EULAR) response criteria. EULAR ‘good response’ was defined as ending DAS<3.2 and ΔDAS>1.2; ‘non-response’ was defined as ΔDAS <0.6 or ΔDAS≤1.2, and ending DAS >5.1; and ‘moderate response’ is in between [15]. We limited our secondary analysis to a dichotomous outcome, EULAR good responders (n = 998 for all patients treated with anti-TNF therapy) versus EULAR non-responders (n = 655), excluding the moderate category based on the hypothesis that a more extreme phenotype of response would yield improved discrimination.
Tab. 1. Samples and clinical data. 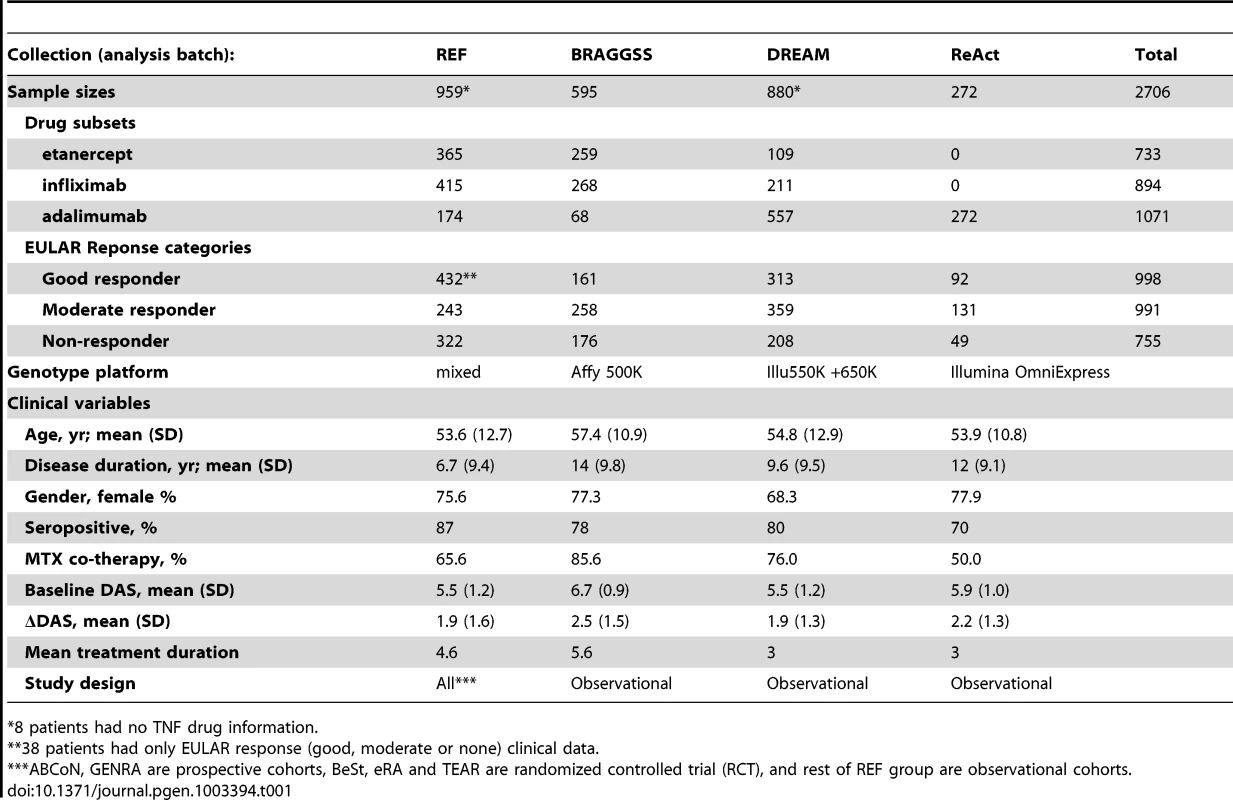
8 patients had no TNF drug information. Clinical variables were examined for association with phenotype, and therefore possible confounding in genetic association tests. In multivariate models (Table S2), only baseline DAS was strongly associated with the ΔDAS phenotype. As previously shown [11], age and gender showed univariate associations that were attenuated in the multivariate analysis. Accordingly, we used only baseline DAS as a clinical covariate, as this allowed us to maximize sample size given clinical variable missing data in some cohorts.
We performed quality control (QC) filtering and data processing of GWAS data for each of eleven genotyping batches. Genotyping array platforms are described in the Methods. HapMap2 imputation allowed us to test for association at >2 M SNPs with imputation quality scores >0.5. Genotype data were merged across several genotype batches to create four collections for genome-wide association testing. We performed linear regression association tests using baseline DAS and three principal components as covariates, and performed inverse-variance weighted meta-analysis to combine results across the four collections. Quantile-quantile plots with genomic control λGC values are shown in Figure S1. We found no evidence of systematic inflation of association test results, and no evidence of deflation for imputed versus genotyped SNPs. As a final filter, we excluded SNPs that showed strong evidence of heterogeneity across collections (Cochran's Q P<0.001).
We first analyzed all samples together (n = 2,706), regardless of drug type. We found no clear evidence of association with treatment response measured by ΔDAS (Figure 1A). Similar results were obtained using the binary phenotype of EULAR responder versus EULAR non-responder status ( Figures S1 and S2).
Fig. 1. GWAS results for the ΔDAS phenotype. 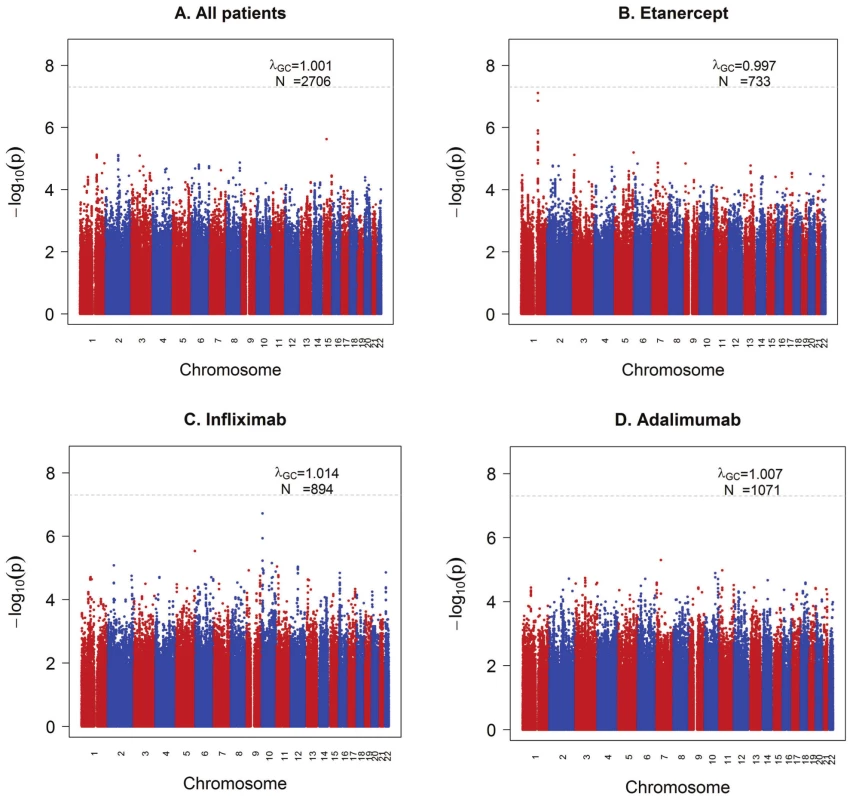
Shown are strengths of association (−Log10 P-value) for each SNP versus position along chromosomes 1 to 22. A) All samples (n = 2,706). B) Etanercept-treated patients (n = 733). C) Infliximab-treated patients (n = 894). D) Adalimumab-treated patients (n = 1,071). We next separately analyzed patients treated with either etanercept (n = 733), infliximab (n = 894) or adalimumab (n = 1,071) (Figure 1B–1D), under the hypothesis that different genetic loci affect response to the different drugs based on their mechanism of action or other biochemical properties. GWAS results are publicly available for all SNPs tested at the Plenge laboratory and RICOPILI Web sites (see URLs). GWAS results for all SNPs achieving P<10−6 from any analysis are detailed in the Table S3.
For etanercept-treated RA patients, a locus on chromosome 1q23 achieved near-genome-wide significance (rs6427528, PMETA = 8×10−8) (Figure 1B, Figure 2A, and Figure 3), but not in the infliximab or adalimumab subsets (P>0.05) (Figure S3). SNPs in linkage disequilibrium (LD) showed consistent association results (rs1503860, P = 1×10−7, r2 = 1 with rs6427528 in HapMap; three perfect-LD clusters of SNPs exemplified by rs3737792, rs10908787 and rs11265432 respectively; P<5×10−6; r2 = 0.83, 0.63 and 0.59 with rs6427528, respectively). No single collection was responsible for the signal of association, as the effect size was consistent across all collections (Figure S4). The top SNP rs6427528 was genotyped in the ReAct dataset (Illumina Omni Express genotyping chip), and was well imputed across all other datasets (imputation quality score INFO ≥0.94, which is an estimate of genotype accuracy; the range of INFO scores is 0–1, where 1 indicates high confidence). All of these SNPs had minor allele frequencies ranging from 7–10%. The SNP explains 2.6% variance in response to etanercept treatment.
Fig. 2. Association results and SNP annotations in the 1q23 CD84 locus. 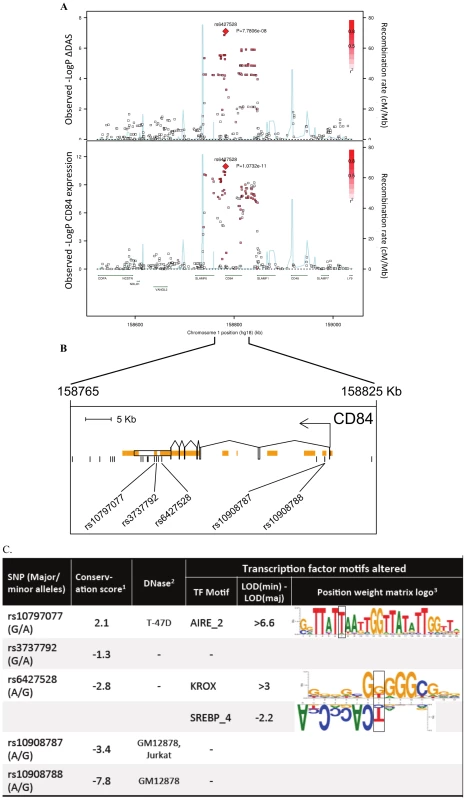
A) Regional association plots with ΔDAS (top panel) and with CD84 expression (bottom panel), showing strengths of association (−Log10 P-value) versus position (Kb) along chromosome 1. B) Schematic of CD84 gene structure (RefSeq gene model, box exons connected by diagonal lines, arrow indicates direction of transcription) with strong enhancer chromatin states (orange rectangles) and SNPs in high LD (r2>0.8) with rs6427528 (vertical ticks). SNPs in enhancers are labeled below. C) Annotations of strong-enhancer rs6427528 proxy SNPs; listed are SNP rs-ID (major and minor alleles), conservation score, cell line with DNAse footprint if present, and transcription factor binding sites altered. 1- Genomic evolutionary rate profiling (GERP) conservation score, where a score >2 indicates conservation across mammals. 2- DNase footprint data are compiled from publicly available experiments by HaploReg. 3- Position weight matrix logos show transcription factor consensus binding sites with nucleotide bases proportional to binding importance. SNP position is boxed. Note that the rs10797077 AIRE_2 and the rs6427528 SREBP_4 motifs are on the minus strand (base complements correspond to SNP alleles), with the SREBP motif shown upside down to align with the rs6427528 KROX motif on the positive strand. Data are from HaploReg. Fig. 3. 1q23/CD84 genotype association plots for ΔDAS and CD84 gene expression. 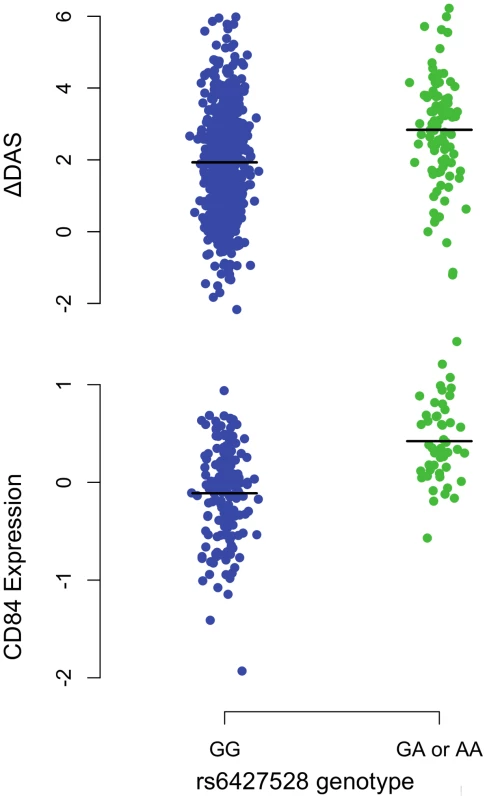
Shown are ΔDAS in our GWAS in etanercept-treated patients (top panel, n = 733; n = 634 with the GG genotype and n = 99 with the GA or AA genotype) and CD84 expression in our eQTL results (bottom panel, n = 228 non-RA patients; n = 178 with the GG genotype and n = 50 with the GA or AA genotype). The rare-allele homozygous genotype AA was observed four times in our ΔDAS GWAS and was pooled with the heterozygous GA genotype for this figure; AA homozygotes were not observed in the CD84 eQTL data. Association analyses reported in the text regressed phenotype (ΔDAS, P = 8×10−8; CD84 expression, P = 1×10−11) on minor-allele dosage (range 0–2). For patients treated with infliximab, we observed a suggestive result on chromosome 10p14 (rs12570744, P = 2×10−7). No highly significant or suggestive results were observed for the ΔDAS phenotype in patients treated with adalimumab (PMETA>10−5).
Qualitatively similar results were attained in the analysis of our secondary phenotype, EULAR good responder vs non-responder status (Figures S1 and S2). For SNPs at the 1q23 locus, the pattern of association with responder/non-responder status (etanercept-treated patients) was consistent with the results for ΔDAS (P = 6×10−3 for rs6427528 and rs1503860). We also identified potential novel associations, with suggestive results for infliximab (rs4336372, chromosome 5q35, P = 8×10−7) and adalimumab (rs940928, chromosome 2q12, P = 2×10−6).
eQTL and sequence analysis of the CD84 gene
For each SNP with P<10−6 identified by our GWAS (n = 6 independent SNPs), we searched for biological evidence to support a true positive association. We used genome-wide sequence data from the 1000 Genomes Project to search for putative functional variants in LD with the index SNP (defined as SNPs predicted to change protein-sequence or mRNA splicing). We also used genome-wide expression data to search for an expression quantitative trait locus (eQTL) in public databases and in peripheral blood mononuclear cells (PBMCs) in 228 non-RA patients and in 132 RA patients.
While we did not identify any variants disrupting protein-coding sequences or mRNA splicing, we did find that the 1q23 SNP associated with response to etanercept therapy was a strong eQTL in PBMCs (Figure 2A and Figure 3). In an analysis of 679 SNPs for cis-regulated expression of five genes in the region of LD (SLAMF6, CD84, SLAMF1, CD48, and SLAMF7), we found that rs6427528-CD84 (and SNPs in LD with it) was the top eQTL of all results (n = 228 subjects; Figure 2A). This SNP was specifically associated with CD84 expression, and was not an eQTL for other genes in the region (P>0.36 for the other genes).
We replicated our eQTL finding in 132 RA patients with both GWAS data and genome-wide expression data. PBMC expression data were available from RA patients in the Brigham RA Sequential Study (BRASS) and Autoimmune Biomarkers Collaborative Network (ABCoN) collections. We observed a significant association between rs6427528 genotype and CD84 expression (linear regression adjusted for cohort P = 0.004, rank correlation P = 0.018). The direction of effect was the same as in the PBMC samples from 228 non-RA patients. A combined analysis of RA patients and the non-RA patient eQTL data (described above) yielded rank correlation P = 3×10−10 (n = 360 total individuals).
We searched sequence data to determine if rs6427528, or any of the SNPs in LD with it, were located within conserved, non-coding motifs that might explain the eQTL data. We used HaploReg [16] to examine the chromatin context of rs6427528 and 26 SNPs in LD with it (at r2>0.50). We found that 5 SNPs occur in strong enhancers inferred from chromatin marks (Figure 2B) [17]. Two of these 5 SNPs, rs10797077 and rs6427528 (r2 = 0.74 to each other), are predicted to disrupt transcription factor binding sites, and rs10797077 occurs at a site that shows conservation across mammalian genomes [18]. Figure 2C shows the DNA sequence position weight matrices of the transcription factor binding sites changed by rs10797077 (the minor allele creates a stronger binding site for the AIRE transcription factor) and rs6427528 (the minor allele creates a binding site for KROX and SREBP).
Expression of CD84 as a biomarker of disease activity and treatment response
Because the genetic data demonstrates that the allele associated with better response is associated with higher CD84 expression, this suggests that CD84 expression itself may serve as a useful biomarker of disease activity or treatment response. We tested both hypotheses using PBMC expression data from the BRASS and ABCoN collections. First, we tested if CD84 expression is associated with cross-sectional DAS, adjusting for age, gender and cohort (Figure 4). We observed a significant inverse association between CD84 expression and cross-sectional DAS in 210 RA patients (beta = −0.3, P = 0.02, r2 = 0.02). That is, higher CD84 expression was associated with lower DAS, regardless of treatment.
Fig. 4. CD84 expression level and clinical features. 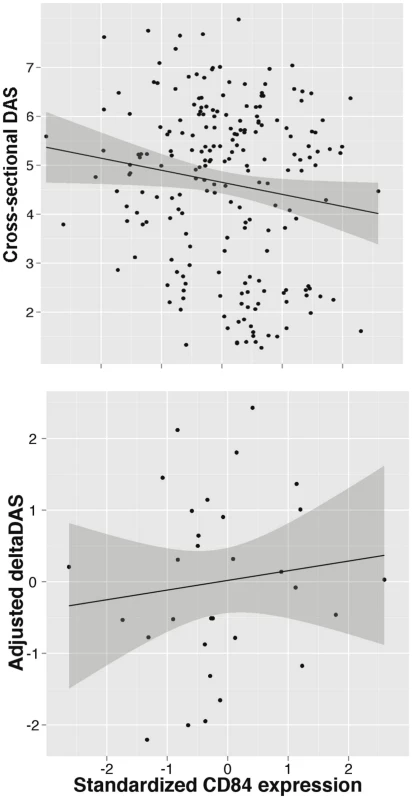
Analyses are shown in RA patients from the BRASS and ABCoN registries, for baseline DAS (top panel, n = 210; R2 = 0.02, p = 0.02) and ΔDAS (bottom panel, n = 31; R2 = 0.001, p = 0.46). Best-fit linear regression lines are shown in black, with shaded regions showing linear regression model (slope and intercept) 95% confidence intervals. CD84 expression levels were quantile normalized, and ΔDAS values were adjusted for age, gender and baseline DAS. Second, we tested CD84 for association with our primary treatment response phenotype, ΔDAS. The sample size for this analysis was smaller than for the cross-sectional analysis, as we required that patients be on anti-TNF therapy and have pre - and post-treatment DAS. We found that CD84 expression levels showed a non-significant trend towards an association with ΔDAS in 31 etanercept-treated patients (beta = 0.2, r2 = 0.002, P = 0.46) and in all 78 anti-TNF-treated patients (beta = 0.14, r2 = 0.004, P = 0.4). The effect is in the same direction one would predict based on the genetic association at rs6427528: the allele associated with better response is also associated with higher CD84 expression (Figure 3), and in 31 RA patients, higher CD84 expression (regardless of genotype) is associated with a larger ΔDAS (i.e., better response; Figure 4).
Replication of genetic data in a small, multi-ethnic cohort
Since most of the samples available to us as part of our international collaboration were included in our GWAS, few additional samples were available for replication. In addition, the remaining samples available to us were from different ethnic backgrounds. Nonetheless, we sought to replicate the associations of rs6427528 with ΔDAS in these additional samples. We genotyped 139 etanercept-treated patients from a rheumatoid arthritis registry in Portugal (Reuma.pt) and 151 etanercept-treated patients from two Japanese collections (IORRA, n = 88 patients on etanercept and Kyoto University, n = 63 on etanercept). Replication sample sizes, clinical data and results for these two SNPs are shown in Table S4. Based on the observed effect size in the GWAS and observed allele frequency in the replication samples, we had 32% power to replicate this finding in the Portuguese samples and 17% power to replicate this finding in the Asian samples at P<0.05. The same association analysis as for GWAS was carried out: linear regression assuming an additive genetic model and using ΔDAS as phenotype, adjusted for baseline DAS. Replication results are shown in Figure 5.
Fig. 5. Replication and overall results for the CD84 SNP rs6427528. 
Forest plot shows each cohort, sample size and linear regression beta coefficient estimates with symbol size proportional to cohort sample size and thin horizontal lines showing beta 95% CIs. Inverse variance weighted meta-analysis results are shown in bold for GWAS, GWAS+European (Portuguese) replication samples, and for GWAS+European+Asian (Japanese) replication samples, with diamond widths indicating beta 95% CIs. While the SNPs fail to replicate in these patient collections at P<0.05, the direction of effect is the same in the Portuguese and Kyoto replication samples as in our GWAS. In a combined analysis limited to subjects of European-ancestry (GWAS data and Portuguese replication samples), rs6427528 remained highly suggestive (P = 2×10−6). Including the Japanese subjects, the overall GWAS+replication combined meta-analysis P-value remained suggestive (P = 5×10−4).
Discussion
Here we present the largest GWAS to date on anti-TNF therapy response in 2,706 RA patients. We find a significant association at the 1q23/CD84 locus in 733 etanercept treated patients (P = 8×10−8), but not in RA patients treated with drugs that act as a monoclonal antibody to neutralize TNF (infliximab or adalimumab). The allele associated with a larger ΔDAS (i.e., better response) was associated with higher CD84 expression in PBMCs from non-RA patients (P = 1×10−11) and in RA patients (P = 0.004).
We first conducted a GWAS of both categories of anti-TNF drugs (the soluble receptor drug, etanercept, and two monoclonal antibody drugs, infliximab and adalimumab). However, this analysis revealed no strongly associated SNPs. When we subset our GWAS by each of the three individual drugs, several SNPs in the 1q23 locus were highly significant in etanercept-treated patients, and SNPs in three other loci (10p15, 5q35 and 2q12) were associated in infliximab or adalimumab subset analyses. Furthermore, the top SNPs for each analysis (Table S3) showed little correlation across the three anti-TNF drugs. This simple observation suggests that genetic control of treatment response may be different for different drugs. This finding is consistent with the clinical observation that RA patients who fail one anti-TNF drug may still respond to a different anti-TNF drug, albeit at lower rates of response [19]. If confirmed in larger samples and more comprehensive analyses, then this could have major implications for how physicians prescribe these drugs.
The most significant finding from our GWAS was a set of equivalent SNPs in LD with each other from the 1q23 locus in etanercept-treated RA patients (Figure 1 and Figure 2A). While the top SNP did not reach genome-wide significance in predicting treatment response, it did reach genome-wide significance as an eQTL in PBMCs (P = 1×10−11; Figure 2A). This finding indicates that the SNP (or another variant in LD with it) is indeed biologically functional in a human tissue that is important in the immune response. Two SNPs, rs10797077 and rs6427528, disrupt transcription factor binding sites, and represent excellent candidates for the causative allele to explain the effect on CD84 expression (Figure 2C).
Our findings suggest that CD84 genotype and/or expression could be a biomarker for etanercept treatment response among individuals of European ancestry. The genetic and expression data predict that CD84 expression should be positively associated with treatment response (i.e., higher expression is associated with better response; Figure 3). While we did not observe a significant association between CD84 levels and ΔDAS, we did observe a trend consistent with this prediction (Figure 4). Importantly, we note that power was extremely limited with the small sample sizes for which we had CD84 expression as well as drug response data (n = 31 RA patients treated with etanercept).
The CD84 gene is a compelling candidate for immune response, belonging to the CD2 subset of the immunoglobulin superfamily. It has been implicated in T-cell activation and maturation [20]. CD84 localizes to the surface of CD4+ and CD8+ T cells, and acts as a costimulatory molecule for IFN-gamma secretion [21]. CD84 is also expressed in B-cells, monocytes and platelets. CD84 has not been previously implicated in genetic studies of RA risk, disease activity, disease severity, or treatment response.
A limitation of our study is the small sample size available for replication (n = 290 etanercept-treated patients), and the lack of replication observed for the top CD84 SNP (rs6427528) among patients of Portuguese and Japanese ancestry. The simplest explanation is that our original observation in the GWAS data represents a false positive association. However, the eQTL and gene expression data argue against this possibility. Explanations for a false negative finding in our replication collections include: (1) lack of power, especially if the effect size observed in the GWAS represents an over-estimate of the true effect size (the Winner's Curse) – we estimate that we had 32% and 17% power (at P = 0.05) to detect an association in the Portuguese and Japanese sample collections, respectively; (2) clinical heterogeneity, which is always a possibility in pharmacogenetic studies, especially those conducted in different countries; and (3) ethnic differences, including different patterns of LD between the underlying causative allele (which is as yet unknown) and marker SNPs tested in our study. We did observe subtle differences in local patterns of LD between Asians and Europeans using genetic data from the 1000 Genomes Project (Figure S5). We note that the rs6427528 minor allele A has a frequency of ∼5–10% in European and East Asian populations, and ∼50% in the African YRI population (HapMap2 and 1000 Genomes); therefore, it may be of interest to test African American samples in replication.
What are the options for increasing sample size in pharmacogenetic studies, thereby providing an opportunity to replicate our CD84 genetic and expression findings? While it might seem trivial to collect more samples through traditional registries, this is extremely challenging for phenotypes pertaining to treatment efficacy. To underscore this point, we highlight our study design, where we organized samples and clinical data from 16 different collections across 7 different countries in order to obtain the samples for the current study. Going forward, non-traditional strategies to collect biospecimens linked with clinical data (e.g., online registries, electronic medical records) may be required to achieve clinical collections of sufficient size to discover pharmacogenomic predictors of efficacy.
In conclusion, we conducted the largest GWAS to date for response to anti-TNF therapy in RA patients. Our genetic and expression data suggest that CD84 genetic variants and/or expression levels could be developed as predictive biomarkers for etanercept treatment response in RA patients of European ancestry.
Methods
Samples and clinical data
All patients met 1987 ACR criteria for RA, or were diagnosed by a board-certified rheumatologist. In addition, patients were required to have at least moderate disease activity at baseline (DAS>3.2). All patients gave their informed consent and all institutional review boards approved of this study. A total of 13 collections from across 5 countries were included in GWAS [11], [12], [13], [22]: Autoimmune Biomarkers Collaborative Network (ABCoN) from the U.S. (N = 79); the Genetics Network Rheumatology Amsterdam (GENRA, N = 53); the Dutch Behandelstrategieen voor Rheumatoide Arthritis (BeSt, N = 85); the U.K. Biological in Rheumatoid arthritis Genetics and Genomics Study Syndicate (BRAGGSS, N = 140); the U.S. Brigham Rheumatoid Arthritis Sequential Study (BRASS, N = 55); the Swedish Epidemiological Investigation of Rheumatoid Arthritis (EIRA, N = 298); the Immunex Early Rheumatoid Arthritis study (eRA N = 57); the Swedish Karolinska Institutet study (KI, N = 77); the Netherlands collection from Leiden University Medical Center (LUMC, N = 43); and the U.S. Treatment of Early Aggressive RA (TEAR, N = 109). We refer to these collections as the American College of Rheumatology Research and Education Foundation (REF) collection, as funding for GWAS genotyping was provided by the “Within Our Reach” project. We included additional samples from BRAGGSS (N = 595) [12]; the Dutch Rheumatoid Arthritis Monitoring registry (DREAM) in the Netherlands, and the ApotheekZorg (AZ) database (which facilitates the Dutch distribution of adalimumab; N = 880) [23], [24], together referred to as DREAM; and the French Research in Active Rheumatoid Arthritis (ReAct, N = 272) [25].
Additional samples were collected for replication of SNPs in the 1q23 locus. These included the Rheumatic Diseases Portuguese Register (Reuma.pt, N = 378) from the Portuguese Society of Rheumatology (SPR), which captures more than 90% of patients treated with biological therapies and managed in rheumatology departments across Portugal [26]. Additional replication samples (N = 374) of East Asian ancestry were included from the IORRA and Kyoto University Hospital registries, part of the Japanese Genetics and Allied research in Rheumatic diseases Networking consortium (GARNET) [27].
Clinical data were collected in each cohort, including disease activity scores at baseline and at least one time point after treatment, gender, age, methotrexate use, as well as autoantibody status (RF or CCP). The composite disease activity scores for 28 joints (DAS28) included laboratory values for erythrocyte sedimentation rate (ESR) for most samples and C-reactive protein (CRP) for 191 samples in the REF collection (ABCoN, BRASS and eRA cohorts). DAS28 values were available at baseline and at 3–12 months after initiating anti-TNF therapy. Our primary phenotype was defined as ΔDAS = baseline DAS - end DAS, and responder status was also determined according to EULAR criteria for start and end DAS [15]. Clinical variables were assessed for association with phenotype in multivariate linear or logistic regression models for both the ΔDAS and EULAR responder-status phenotypes. Clinical variables that were significant in these analyses were retained as covariates in genetic association tests, except for methotrexate co-therapy. Including a covariate for methotrexate co-therapy reduced sample size substantially due to missing clinical data, so results were compared for our primary analysis and a secondary analysis with the covariates (and with reduced sample size) and the results were verified not to be impacted (not shown).
Genotyping and data processing
A total of eleven genotyping batches were processed separately. (1) BRASS samples were genotyped using Affymetrix 6.0 chip [28]; (2) WTCCC samples were genotyped on Affymetrix 500K chip [12]. All other cohorts were genotyped using Illumina platform arrays (see Table 1). Our American College of Rheumatology Research Education Fund (REF) collection was made up of smaller cohorts from throughout North America and Europe, including BRASS samples. Also included in REF: (3) ABCoN [13] and (4) EIRA [29] were separately genotyped on the Illumina 317K genotyping array; (5) eRA on the Illumina 550K chip; and (6) GENRA, BeSt, BRAGGSS (a subset of N = 53 samples), KI and LUMC were genotyped in one batch, and (7) BRAGGSS (N = 87) and TEAR were genotyped in a second batch, both using Illumina 660k chips, at the Broad Institute (8–10). DREAM and AZ samples were genotyped in three batches, one on 550K chip and two on 660K chips (manuscript in preparation), and (11) ReAct samples were genotyped on Illumina OmniExpress chips. Quality control (QC) filtering was done in each genotyping batch, including filtering individuals with >5% missing data, and filtering SNPs with >1% missing data, minor allele frequency (MAF) <1% and Chi-squared test of Hardy Weinberg equilibrium PHWE<10−5. We then used individual-pairwise identity-by-state estimates to remove occasional related and potentially contaminated samples. Data processing and QC were performed in PLINK [30]. Principal Components Analysis (PCA) was performed using EIGENSTRAT [31] (default settings) on the combined dataset using 20,411 SNPs genotyped across all datasets. Ethnicity outliers including all individuals of non-European decent were identified and removed, and the first three eigenvectors were used as covariates in GWAS.
Imputation was conducted on each of eleven datasets separately, using the IMPUTE v1 software [32] and haplotype-phased HapMap Phase 2 (release 22) European CEU founders as a reference panel. Imputation of BRASS and EIRA was previously reported [28], [33], and we followed the same imputation procedures for the remaining datasets. Imputation yielded posterior genotype probabilities as well as imputation quality scores at SNPs not genotyped with a minor allele frequency ≥1% in HapMap CEU. We removed imputed SNPs with imputation ‘info’ scores <0.5 or MAF <1% in any of the datasets.
Expression profile and eQTL data
Gene expression levels were quantified using mRNA derived from peripheral blood mononuclear cells (PBMCs) using Affymetrix Human Genome U133 Plus 2.0, for 255 multiple sclerosis patients in the Comprehensive Longitudinal Investigation of MS at the Brigham and Women's Hospital [34], either untreated (N = 83) or treated with interferon-beta (N = 105) or glatiramer acetate (N = 67). The raw intensity values were subject to quality control based on the recommended pipeline available in the simpleaffy and affyPLM R Bioconductor packages, and were then normalized using GCRMA (N = 228). The data are available on the Gene Expression Omnibus website (GSE16214). Expression levels for 17,390 probes mapping to 9,665 Ensembl transcripts were adjusted for confounding factors including age, gender, drug and batch using principle components and Bayesian factor analysis [35], and used in eQTL association analyses. Genotype data were collected on the Affymetrix 550K GeneChip 6.0 platform as a part of a previously published study [36]. Allelic dosages from imputed data (HapMap Phase II CEU samples; >2 million SNPs, MACH imputation quality >0.1 and MAF> = 0.05) were used for association analysis. Cis-eQTLs were identified +/−1 Mb of transcription start sites (TSS) in the 1q23 locus region. Significance was evaluated by 10,000 permutations per gene, and false discovery rates were calculated based on cis-eQTL analyses in the total of 9,665 genes [37].
Additional expression profile data were available for subsets of samples that were part of two cohorts in our GWAS. Expression data from patients enrolled in the BRASS registry have been previously published [38]. Expression data were collected on Affymetrix Gene Chip U133 Plus 2 microarrays. BRASS patients had either cross-sectional expression data (n = 132, assayed at the time the patient was enrolled in BRASS) or pre - and post-treatment expression data (n = 17 samples, 8 treated with etanercept). Of these, n = 87 patients had expression and GWAS data. For patients with pre - and post-treatment data, we used the “baseline” pre-treatment expression data for cross-sectional analysis. In ABCoN, 65 RA patients (n = 23 treated with etanercept) had both pre - and post-treatment expression data, as well as ΔDAS clinical data [39], and n = 45 patients had expression and GWAS data. As with BRASS, we use the “baseline” pre-treatment expression data for cross-sectional analysis. For ABCoN expression profile data were collected on Illumina Human WG6v3 microarrays and were quantile normalized according to Illumina recommended protocols. Within both BRASS and ABCoN, expression data were normalized to the mean and standard deviation within each collection. For prospective analyses of expression data and ΔDAS, we combined BRASS and ABCoN to include 31 etanercept-treated patients and 78 anti-TNF-treated patients.
Statistical analyses
In our primary GWAS analysis, we tested each SNP for association with ΔDAS using linear regression adjusted for baseline DAS and the first 3 PCA eigenvectors in each collection. In our secondary GWAS analysis, we modeled SNPs predicting EULAR good response versus EULAR non-response using logistic regression, again adjusting for start-DAS value and the first three eigenvectors. Association analysis was done using SNPTEST [32] assuming an additive genetic model. Genomic control λGC values [40] for genotyped SNPs only and all SNPs were calculated, and no inflation or deflation was observed in the distributions of association test results. We then conducted inverse variance-weighted meta-analysis to combine results across the four datasets, and conducted Cochran's Q tests for heterogeneity using the β coefficients [41]. We further divided samples into 3 subsets according to drug (etanercept, infliximab or adalimumab). GWAS analysis for each group followed the same analysis procedure. Meta-analysis and heterogeneity tests were conducted using SAS. Expression analyses utilized linear regression or Spearman rank correlation, also using SAS. We tested for effects of cohort, age, gender and concurrent methotrexate, and results are shown using significant covariates as indicated.
Supporting Information
Zdroje
1. KlareskogL, CatrinaAI, PagetS (2009) Rheumatoid arthritis. Lancet 373 : 659–672.
2. ScottDL, WolfeF, HuizingaTW (2010) Rheumatoid arthritis. Lancet 376 : 1094–1108.
3. McInnesIB, SchettG (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365 : 2205–2219.
4. AeberliD, SeitzM, JuniP, VilligerPM (2005) Increase of peripheral CXCR3 positive T lymphocytes upon treatment of RA patients with TNF-alpha inhibitors. Rheumatology (Oxford) 44 : 172–175.
5. AgnholtJ, DahlerupJF, KaltoftK (2003) The effect of etanercept and infliximab on the production of tumour necrosis factor alpha, interferon-gamma and GM-CSF in in vivo activated intestinal T lymphocyte cultures. Cytokine 23 : 76–85.
6. CatrinaAI, TrollmoC, af KlintE, EngstromM, LampaJ, et al. (2005) Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum 52 : 61–72.
7. ScallonBJ, MooreMA, TrinhH, KnightDM, GhrayebJ (1995) Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine 7 : 251–259.
8. GudbrandsdottirS, LarsenR, SorensenLK, NielsenS, HansenMB, et al. (2004) TNF and LT binding capacities in the plasma of arthritis patients: effect of etanercept treatment in juvenile idiopathic arthritis. Clin Exp Rheumatol 22 : 118–124.
9. PlantD, PrajapatiR, HyrichKL, MorganAW, WilsonAG, et al. (2012) Replication of association of the PTPRC gene with response to anti-tumor necrosis factor therapy in a large UK cohort. Arthritis Rheum 64 : 665–670.
10. PrajapatiR, PlantD, BartonA (2011) Genetic and genomic predictors of anti-TNF response. Pharmacogenomics 12 : 1571–1585.
11. CuiJ, SaevarsdottirS, ThomsonB, PadyukovL, van der Helm-Van MilAH, et al. (2010) Rheumatoid arthritis risk allele PTPRC is also associated with response to anti-tumor necrosis factor alpha therapy. Arthritis Rheum 62 : 1849–1861.
12. PlantD, BowesJ, PotterC, HyrichKL, MorganAW, et al. (2011) Genome-wide association study of genetic predictors of anti-tumor necrosis factor treatment efficacy in rheumatoid arthritis identifies associations with polymorphisms at seven loci. Arthritis Rheum 63 : 645–653.
13. LiuC, BatliwallaF, LiW, LeeA, RoubenoffR, et al. (2008) Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol Med 14 : 575–581.
14. PrevooML, van 't HofMA, KuperHH, van LeeuwenMA, van de PutteLB, et al. (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38 : 44–48.
15. van GestelAM, PrevooML, van 't HofMA, van RijswijkMH, van de PutteLB, et al. (1996) Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 39 : 34–40.
16. WardLD, KellisM (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40: D930–934.
17. ErnstJ, KheradpourP, MikkelsenTS, ShoreshN, WardLD, et al. (2011) Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473 : 43–49.
18. CooperGM, StoneEA, AsimenosG, GreenED, BatzoglouS, et al. (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15 : 901–913.
19. SolimanMM, HyrichKL, LuntM, WatsonKD, SymmonsDP, et al. (2012) Rituximab or a second anti-TNF therapy for rheumatoid arthritis patients who have failed their first anti-TNF? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res (Hoboken)
20. TangyeSG, NicholsKE, HareNJ, van de WeerdtBC (2003) Functional requirements for interactions between CD84 and Src homology 2 domain-containing proteins and their contribution to human T cell activation. J Immunol 171 : 2485–2495.
21. MartinM, RomeroX, de la FuenteMA, TovarV, ZapaterN, et al. (2001) CD84 functions as a homophilic adhesion molecule and enhances IFN-gamma secretion: adhesion is mediated by Ig-like domain 1. J Immunol 167 : 3668–3676.
22. PadyukovL, LampaJ, HeimburgerM, ErnestamS, CederholmT, et al. (2003) Genetic markers for the efficacy of tumour necrosis factor blocking therapy in rheumatoid arthritis. Ann Rheum Dis 62 : 526–529.
23. CoenenMJ, EnevoldC, BarreraP, SchijvenaarsMM, ToonenEJ, et al. (2010) Genetic variants in toll-like receptors are not associated with rheumatoid arthritis susceptibility or anti-tumour necrosis factor treatment outcome. PLoS ONE 5: e14326 doi:10.1371/journal.pone.0014326.
24. ToonenEJ, CoenenMJ, KievitW, FransenJ, EijsboutsAM, et al. (2008) The tumour necrosis factor receptor superfamily member 1b 676T>G polymorphism in relation to response to infliximab and adalimumab treatment and disease severity in rheumatoid arthritis. Ann Rheum Dis 67 : 1174–1177.
25. Miceli-RichardC, CometsE, VerstuyftC, TamouzaR, LoiseauP, et al. (2008) A single tumour necrosis factor haplotype influences the response to adalimumab in rheumatoid arthritis. Ann Rheum Dis 67 : 478–484.
26. CanhaoH, FaustinoA, MartinsF, FonsecaJE (2011) Reuma.pt - the rheumatic diseases portuguese register. Acta Reumatol Port 36 : 45–56.
27. OkadaY, TeraoC, IkariK, KochiY, OhmuraK, et al. (2012) Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet
28. StahlEA, RaychaudhuriS, RemmersEF, XieG, EyreS, et al. (2010) Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42 : 508–514.
29. PlengeRM, SeielstadM, PadyukovL, LeeAT, RemmersEF, et al. (2007) TRAF1-C5 as a Risk Locus for Rheumatoid Arthritis – A Genomewide Study. N Engl J Med 357 : 1199–1209.
30. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
31. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
32. MarchiniJ, HowieB, MyersS, McVeanG, DonnellyP (2007) A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 : 906–913.
33. StahlEA, WegmannD, TrynkaG, Gutierrez-AchuryJ, DoR, et al. (2012) Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet
34. GauthierSA, GlanzBI, MandelM, WeinerHL (2006) A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev 5 : 532–536.
35. StegleO, PartsL, DurbinR, WinnJ (2010) A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS Comput Biol 6: e1000770 doi:10.1371/journal.pcbi.1000770.
36. De JagerPL, JiaX, WangJ, de BakkerPI, OttoboniL, et al. (2009) Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 41 : 776–782.
37. StrangerBE, NicaAC, ForrestMS, DimasA, BirdCP, et al. (2007) Population genomics of human gene expression. Nat Genet 39 : 1217–1224.
38. ParkerA, IzmailovaES, NarangJ, BadolaS, LeT, et al. (2007) Peripheral Blood Expression of Nuclear Factor-kappaB-Regulated Genes Is Associated with Rheumatoid Arthritis Disease Activity and Responds Differentially to Anti-Tumor Necrosis Factor-alpha versus Methotrexate. J Rheumatol 34 : 1817–1822.
39. BatliwallaFM, BaechlerEC, XiaoX, LiW, BalasubramanianS, et al. (2005) Peripheral blood gene expression profiling in rheumatoid arthritis. Genes Immun 6 : 388–397.
40. DevlinB, RoederK, WassermanL (2001) Genomic control, a new approach to genetic-based association studies. Theor Popul Biol 60 : 155–166.
41. de BakkerPI, FerreiraMA, JiaX, NealeBM, RaychaudhuriS, et al. (2008) Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet 17: R122–128.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



