-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
In insects, products of the male reproductive tract are essential for initiating and maintaining the female post-mating response (PMR). The PMR includes changes in egg laying, receptivity to courting males, and sperm storage. In Drosophila, previous studies have determined that the main cells of the male accessory gland produce some of the products required for these processes. However, nothing was known about the contribution of the gland's other secretory cell type, the secondary cells. In the course of investigating the late functions of the homeotic gene, Abdominal-B (Abd-B), we discovered that Abd-B is specifically expressed in the secondary cells of the Drosophila male accessory gland. Using an Abd-B BAC reporter coupled with a collection of genetic deletions, we discovered an enhancer from the iab-6 regulatory domain that is responsible for Abd-B expression in these cells and that apparently works independently from the segmentally regulated chromatin domains of the bithorax complex. Removal of this enhancer results in visible morphological defects in the secondary cells. We determined that mates of iab-6 mutant males show defects in long-term egg laying and suppression of receptivity, and that products of the secondary cells are influential during sperm competition. Many of these phenotypes seem to be caused by a defect in the storage and gradual release of sex peptide in female mates of iab-6 mutant males. We also found that Abd-B expression in the secondary cells contributes to glycosylation of at least three accessory gland proteins: ovulin (Acp26Aa), CG1656, and CG1652. Our results demonstrate that long-term post-mating changes observed in mated females are not solely induced by main cell secretions, as previously believed, but that secondary cells also play an important role in male fertility by extending the female PMR. Overall, these discoveries provide new insights into how these two cell types cooperate to produce and maintain a robust female PMR.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003395
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003395Summary
In insects, products of the male reproductive tract are essential for initiating and maintaining the female post-mating response (PMR). The PMR includes changes in egg laying, receptivity to courting males, and sperm storage. In Drosophila, previous studies have determined that the main cells of the male accessory gland produce some of the products required for these processes. However, nothing was known about the contribution of the gland's other secretory cell type, the secondary cells. In the course of investigating the late functions of the homeotic gene, Abdominal-B (Abd-B), we discovered that Abd-B is specifically expressed in the secondary cells of the Drosophila male accessory gland. Using an Abd-B BAC reporter coupled with a collection of genetic deletions, we discovered an enhancer from the iab-6 regulatory domain that is responsible for Abd-B expression in these cells and that apparently works independently from the segmentally regulated chromatin domains of the bithorax complex. Removal of this enhancer results in visible morphological defects in the secondary cells. We determined that mates of iab-6 mutant males show defects in long-term egg laying and suppression of receptivity, and that products of the secondary cells are influential during sperm competition. Many of these phenotypes seem to be caused by a defect in the storage and gradual release of sex peptide in female mates of iab-6 mutant males. We also found that Abd-B expression in the secondary cells contributes to glycosylation of at least three accessory gland proteins: ovulin (Acp26Aa), CG1656, and CG1652. Our results demonstrate that long-term post-mating changes observed in mated females are not solely induced by main cell secretions, as previously believed, but that secondary cells also play an important role in male fertility by extending the female PMR. Overall, these discoveries provide new insights into how these two cell types cooperate to produce and maintain a robust female PMR.
Introduction
The homeotic transcription factor Abdominal-B (Abd-B) specifies the identity of the four most-posterior abdominal segments of the fly (the 5th through 8th abdominal segments), as well as the genital and anal structures [1]–[3]. Each of these segments is specified by a particular pattern and level of Abd-B protein expression in the early embryo. Four segment-specific cis-regulatory domains (iab-5 through iab-8) spanning >90 kb of DNA have been shown to control the expression pattern of Abd-B, where each domain is predominantly responsible for controlling the Abd-B expression pattern in one particular segment [4]–[6] (for a review see [7]).
Extensive study has been devoted to exploring how the segment-specific expression pattern of Abd-B is achieved. Due to the striking cuticular transformations elicited by Abd-B mutations, genetic and transgenic analyses have been able to discover numerous enhancers, silencers and insulators that direct Abd-B expression in the ectoderm [8]–[16]. However, much less is known about the role of Abd-B in non-ectodermally derived tissues during later stages of development. Here, we use a 111 kb BAC-based reporter construct to identify new locations of Abd-B expression in the adult fly. We find that Abd-B is strongly expressed in the accessory gland (AG), a secretory tissue of the adult male reproductive tract that has important reproductive functions.
The AG synthesizes seminal proteins that are essential for male fertility. These >180 accessory gland proteins (“Acps”) are transferred to females during mating and cause post-mating changes in the females known collectively as the post-mating response (PMR). The PMR includes increased rates of egg-laying and ovulation, sperm storage, decreased receptivity to courting males, as well as changes in longevity, feeding, and sleep patterns (reviewed in [17], [18]). The PMR is divided into two phases. The short term response (STR) refers to changes in the above behaviors during the first ∼24 hours post-mating. It requires Acps, but not the receipt of sperm. Persistence of the PMR after 24 hr (and for up to ∼10 days) is known as the long-term response (LTR). The LTR requires Acps and stored sperm [19]–[22]. Many of the roles of Acps were initially discovered by experiments in which whole AG extracts or purified Acps were injected into unmated females [23]–[25], or by whole-tissue ablation in males [26].
Each lobe of the AG is composed of a monolayer of approximately 1000 secretory cells comprised of two morphologically distinct cell types. Roughly 96% of these cells are flat, polygonally shaped “main cells”. The remaining 4% of the cells are large, spherical, vacuole filled “secondary cells ”; these are dispersed among the main cells at the distal tip of the gland. Enhancer trapping and other studies have shown that, in addition to their morphological differences, these two secretory cell types are biochemically distinct [27]–[29]. Ablation of the main cells only [19] showed that products of these cells are essential for the PMR. These products include ovulin (Acp26Aa), an Acp that acts in the STR to stimulate ovulation [30], [31], and the sex peptide (SP, Acp70A), which is the ultimate regulator of most other PMR effects [22], [32]–[35]. SP binds to sperm within the mated female, and its active portion is gradually released from the sperm [22]. This binding and release allows SP to affect the female for as long as she contains stored sperm. A network of five other Acps is necessary for SP to bind to sperm and enter storage. The predicted protease CG10586 (Seminase) [36] appears to be necessary for both STR and LTR related events, while the predicted protease CG9997, the predicted cysteine-rich secretory protein (CRISP) CG17575, and the predicted lectins CG1656/1652 appear to be LTR specific [37]–[40]. The cellular source of each of these proteins is currently unknown.
In spite of the detailed characterization of the main cells and several specific Acps, the role of the secondary cells has remained mysterious. No PMR-associated Acps were known to be expressed exclusively in the secondary cells, and no tools have been available to specifically target those cells. Here, we identified the secondary cells of the male AG as a novel location of Abd-B expression in the adult fly. By screening an extensive collection of cis-regulatory deletions [6], [41], [42], we discovered a 2.8 kb enhancer from the iab-6 cis-regulatory domain, whose removal completely abolishes Abd-B expression in the secondary cells. Loss of Abd-B expression in the secondary cells causes those cells to develop aberrantly. Moreover, these mutant males provide their mates with substances that initiate the PMR, but are insufficient to maintain it. Our results indicate that Abd-B expression in the secondary cells is essential for their proper development and for the production of proteins important for long-term changes in female post-mating responses.
Results
Creation of Gal4 reporter BAC for Abdominal B
In order to discover new tissues in which the Abd-B gene functions, we undertook the creation of a transgenic reporter that accurately reproduces the Abd-B expression pattern throughout development. Previous studies indicated that the Abd-B gene is expressed as two isoforms, the Abd-B m and r forms, and that the expression of these two isoforms requires separate elements located within a large cis-regulatory region spanning >90 kb of DNA [43]. As the Abd-Br isoform is thought to be primarily involved in the formation of the external genitalia [44], we decided to concentrate our study on the Abd-Bm isoform, which is involved in determining segment identity. BACR24L18 is a BAC of ∼172 kb that contains the Abd-B, and much of the abd-A region of the Bithorax complex (BX-C). By recombineering, we reduced BAC24L18 to contain mostly the iab-5 to iab-8 domains required for Abd-Bm expression (removing many of the Abd-Br alternative promoters and its regulatory elements) and the Abd-Bm coding sequence (Figure 1B). A ΦC31 AttB integration sequence and a white integration marker were also added during the reduction step (Figure 1B and 1C).
Fig. 1. Extend of DNA contains in the Abd-B BAC. 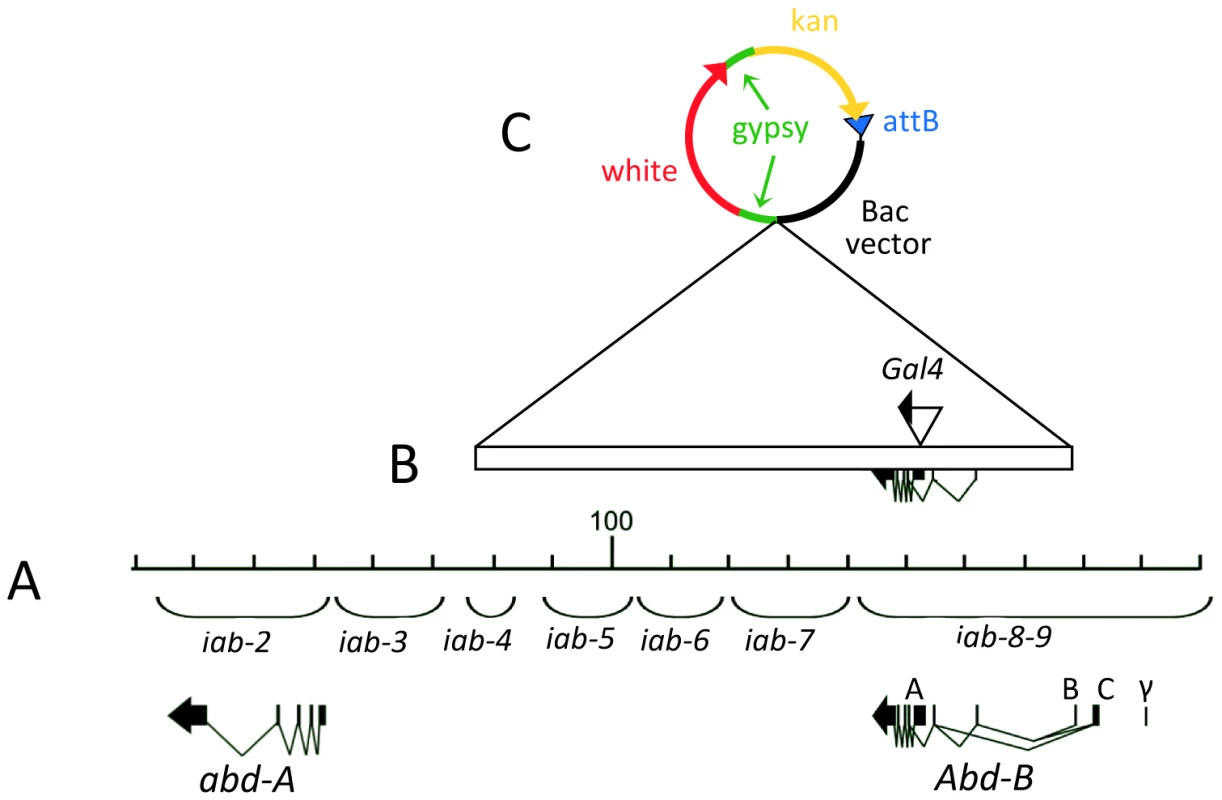
A) Molecular map of the abdominal region of the Bithorax complex numbered in kb according to [88] (Genbank U31961). The abd-A and Abd-B transcription units are drawn below the DNA line along with the extent of the segment-specific iab cis-regulatory domains iab-2 through iab-9. B) The rectangle depicts the extent of the BAC used in this study. Note that it lacks the B,C and γ promoters specific for the Abd-Br form. The Gal-4 coding sequence was inserted within the 5′UTR of the Abd-Bm form. C) The structure of the vector sequences used to propagate the BAC and to select the integration within the Drosophila genome. Note the presence of two gypsy insulator sequences flanking the mini-white sequences to prevent possible position effect on white expression (see Materials and Methods for further details). We first tested if expression derived from the sequences on this BAC were sufficient to rescue Abd-B mutant phenotypes. We integrated the Abd-B BAC into the 51C landing platform [45] and tested for complementation of two large deletions affecting Abd-B activity. We found that the presence of a copy of the BAC on the second chromosome rescues the mutant phenotypes of iab-6,7IH and iab-5,6J82 [6] (Figure S1; See Text S1). Because the sequences preserved on the BAC seemed to drive appropriate Abd-Bm expression, we proceeded to modify the BAC by recombineering to replace the first codon of the first exon of Abd-B with the sequence encoding the Gal4 transcription factor. As this sequence also adds a stop codon, the expression of Abd-B from the BAC should be eliminated, but any sequences that might be used in Abd-B gene regulation will be preserved to drive reporter gene expression. The final BAC used in the experiments was 111 kb (Figure 1). It was integrated into the 51C landing platform.
To study the Abd-B expression pattern, a line was established containing the Abd-B-Gal4 BAC and a UAS-GFP reporter. Initial examination of the embryonic expression pattern in these lines confirms that the Abd-B-Gal4 BAC appears to recapitulate most of the wild-type expression pattern of Abd-Bm in early embryos (Figure 2A; Figure S2; Figure S3). Later, we do observe some evidence of ectopic expression from the BAC, particularly in the ventral nerve cord (Figure S2; Figure S3). Even with the slight level of ectopic expression, the Abd-B-Gal4 BAC seems to be a useful tool, as it recapitulates known patterns of Abd-B expression even in adult and larval tissues (Figure S4).
Fig. 2. Expression patterns driven by the Abd-B-Gal4 BAC. 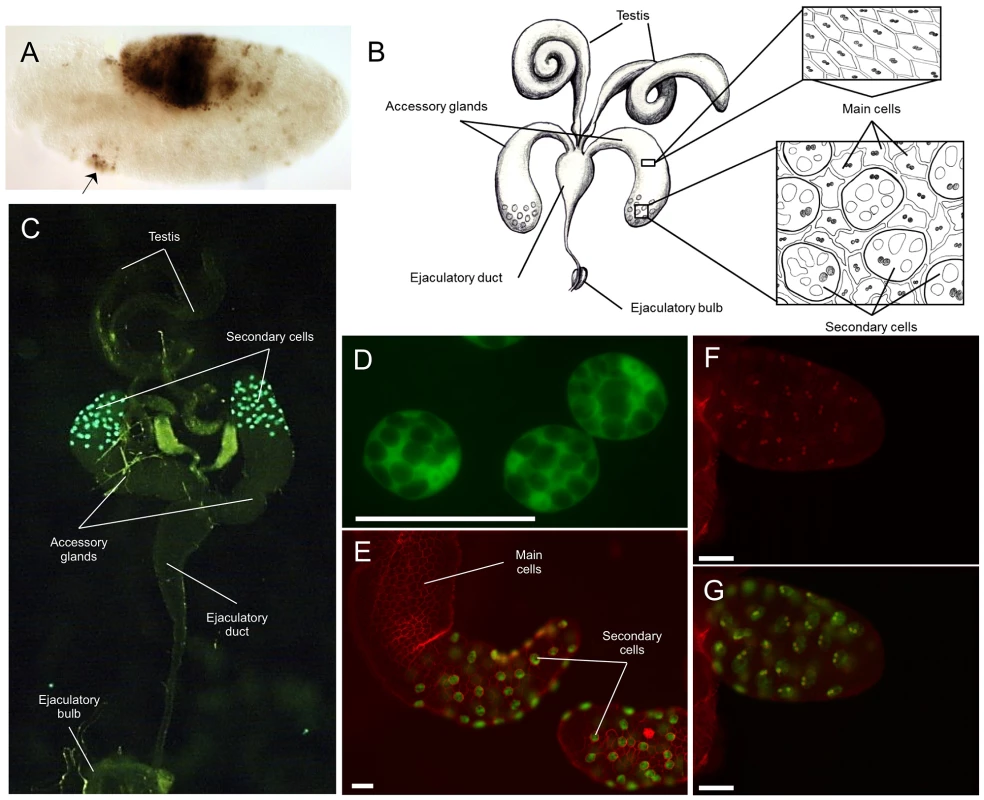
A) Embryo expressing the Abd-B-Gal4 BAC crossed to a UAS-LacZ reporter stained with an antibody directed against ß-galactosidase. Out of a slight ectopic expression anteriorly (indicated by the arrow), the expression pattern is stickingly simiar to the WT Abd-B expression pattern as documented in Figure S2. B) Cartoon depicting the male reproductive apparatus with testis, the paired accessory glands, the ejaculatory duct and ejaculatory bulb. Each accessory gland contains two secretory cell types, the main cells which make up the majority of the gland (top insert) and the secondary cells which are located at the distal tip of the gland interspersed among the main cells (bottom insert) Drawing by J. L. Sitnik; C) Picture of the male reproductive system from flies carrying the Abd-B-Gal4 BAC crossed to a UAS-GFP reporter with the secondary cells of the accessory glands showing GFP expression. The different organs composing the system are marked. D) Magnification of three secondary cells from flies carrying the Abd-B-Gal4 BAC crossed to a cytoplasmic UAS-GFP reporter. The multiple, large vacuoles, characteristic of secondary cells, can be visualized through their exclusion of the GFP protein. The two nuclei of the cells can also be seen as slightly more intense GFP signals; E) The tip of the accessory gland with GFP expressed specifically in the secondary cells driven by the Abd-B-Gal4 BAC (green), co-stained with the membrane staining dye, FM4–64, in red. The two cell types can be clearly distinguished with examples indicated with white lines.; F) Abd-B antibody staining of the tip of an accessory gland on Abd-B-Gal4 BAC, UAS-GFP flies (red). Only secondary cell nuclei are stained. G) GFP expression (green) in the same gland overlaid onto the Abd-B antibody staining (red) shown in Figure 2F. Each cell with Abd-B protein expression also express GFP. The white scale bar on figures D, F, E, and G represents 50 µm. Previously unknown location of Abdominal B expression in adult flies
Using this new reporter, we identified the adult male accessory gland (see Figure 2B) as a location of Abd-B expression (Figure 2C). More specifically, based on the expression of our Abd-B-Gal4 BAC, Abd-B appears to be specifically expressed in the secondary cells (Figure 2C, 2D, and 2E). To confirm this finding, we stained accessory glands in the presence of the Abd-B-Gal4 UAS-GFP reporter with an antibody directed against Abd-B (Figure 2F, 2G). Like the reporter, accessory gland immunostaining against the Abd-B protein shows specific staining in the secondary cells (Figure 2F, 2G). Interestingly, we also see Abd-B staining in the ejaculatory duct (Figure S4C) that is not observed with our reporter (Figure 2C). This is perhaps not surprising, as the ejaculatory duct is a structure derived from the male genital disc, a tissue that primarily expresses the Abd-Br isoform [44] (which is also recognized by our antibody).
Secondary cell enhancer
In order to examine the role of Abd-B in the development of the accessory glands, we sought a method to remove Abd-B expression exclusively in the secondary cells. Rather than use the traditional FLP-FRT system for making clones, we reasoned that in our collection of Abd-B cis-regulatory mutations [6], we may already have a deletion that specifically removes secondary cell enhancers. Given our hypothesis that Abd-B might act as a cell fate determinant in the secondary cells, we screened a set of large, overlapping deficiencies covering the Abd-B cis-regulatory region for defects in secondary cell formation (Figure 3A). To make this analysis easier, homozygous mutant flies were screened in lines that also contain a copy of our BAC reporter to mark the cells that would normally become secondary cells. Two of the lines examined, iab-6,7IH & iab-5,6J82(Figure 3C and 3D), showed a distinct morphological abnormality in the secondary cells. This abnormality can be easily seen using the cytoplasmic GFP marker. In wild-type cells, the GFP marker outlines the presence of large vacuolar structures in the secondary cells (Figure 2D, Figure 3B). In both the iab-6,7IH & iab-5,6J82 mutants, these structures appear to be absent, and consequently the GFP marker is almost uniformly distributed across the cytoplasm.
Fig. 3. Mutants affecting Abd-B expression in the accessory gland. 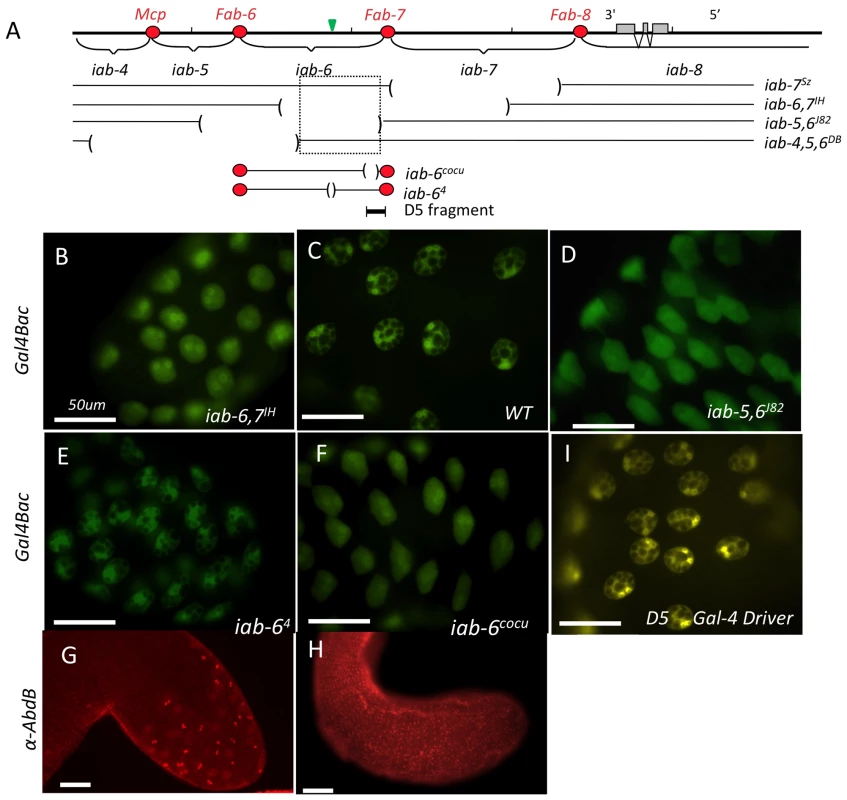
A) The Molecular map of the Abd-B gene region is shown with its extensive 3′ cis-regulatory domains iab-5 through iab-8 (the iab-4 domain regulates abd-A). The extents of the various deficiencies that were used to map the enhancer responsible for Abd-B expression in the secondary cells are shown below the molecular map. The location of DNA sequence used to make the 2.8 kb-long D5rsG4rs driver (thereby refereed as D5 Gal4 driver).is shown under the map. The red circles on the map represent the boundaries separating the parasegment-specific cis-regulatory domains of Abd-B. The green triangle above the iab-6 domain marks the iab-6 initiator. B) UAS-GFP expression driven by Abd-B-Gal4 in a WT for the BX-C. C) same as B, but in an iab-6, 7IH homozygous male or in an iab-5,6J82 homozygous male(D). Note that in the iab-6, 7IH and iab-5,6J82 background, the numerous vacuoles, characteristic of the secondary cells (visible by black holes in the GFP background), are lost. However, the vacuoles are not affected in iab-4,5,6DB. Thus, the critical region required for proper secondary cell specification based on these 3 deficiencies is indicated by the dotted-line box in panel A. E) UAS-GFP expression driven by Abd-B-Gal4 in secondary cells of iab-64 (initiator deletion) and of iab-6cocu males (F). Note the normal aspect of GFP staining in iab-64 (E) relative to the WT shown in B). In iab-6cocu however (F), the vacuoles are lost, giving rise to staining comparable to panels C and D. Panels G) and H) show iab-64 (G) and iab-6cocu (H) accessory glands stained with an Abd-B antibody. While Abd-B expression appears normal in iab-64 (G), the signal is absent in iab-6cocu (H). I) shows the tip of an accessory gland from a fly carrying the D5-Gal4 driver driving GFP expression in the secondary cells (the staining is shown in yellow to distinguish it from panels B–F depicting GFP driven by the Gal4 Bac. The white horizontal scale bars in each of the panels represents 50 µm. Although these secondary cells are not normal, we do not detect any expression of main cell-specific markers in these cells, suggesting that they are not transformed towards a main cell fate (they still express the Acp95EF lacZ reporter gene [28] and fail to express the SP lacZ reporter gene (data not shown) [29]). To test if Abd-B is capable of transforming main cells into secondary cells, we expressed Abd-B across the whole accessory gland using a paired-Gal4 driver [46]. The most common result of this ectopic expression is cell death in the main cells (data not shown). These results suggest that Abd-B expression in the secondary cells is required for morphological differentiation but may not be necessary for the initial differentiation between the two cell types.
Based on the sequences uncovered by both the iab-6,7IH & iab-5,6J82 mutations, we concluded that the iab-6 domain, responsible for Abd-B expression in segment 6, is also responsible for Abd-B expression in the secondary cells. Thus, we screened our collection of smaller iab-6 deficiencies [41] for the secondary cell phenotype. From this analysis, we were able to narrow down the location containing the secondary cell enhancer to a 2.8 kb region in iab-6 (Figure 3A, 3F and 3H). Flies lacking this 2.8 kb region (iab-6Δ5) specifically lack Abd-B protein expression in the secondary cells (Figure 3H), and show distinct secondary cell morphological defects (Figure 3F). Like the larger deficiencies above, iab-6Δ5 homozygous males lack the large vacuoles characteristic of secondary cells.
As further confirmation of the importance of Abd-B and the 2.8 kb iab-6 enhancer in secondary cell development, we performed a number of control experiments. First, we crossed in a BAC transgene containing the wild-type Abd-B region and tested for rescue of the cellular phenotype. As expected, the secondary cells of males, homozygous for the iab-6Δ5 mutation but carrying one copy of the Abd-B BAC are substantially rescued (containing a number of large vacuoles) (Figure S5C). Although this rescue is quite evident, it is not complete, a fact that probably reflects a weaker level of expression from the BAC relative to the native Abd-B locus. Indeed, Abd-B staining experiments using this BAC indicate that this is the case (data not shown). Next, we created a transgene carrying the 2.8 kb region of iab-6 (called D5-Gal4) and showed that it drives expression of Gal4 in the male reproductive tract specifically in the secondary cells (Figure 3E). Using this D5-Gal4 driver, we were then able to drive expression of an Abd-B RNAi construct in the secondary cells. Knocking down Abd-B in the secondary cells was able to partially phenocopy the iab-6Δ5 mutation (Figure S6C and S6F). The strength of this phenotype could be enhanced by the inclusion of a Dicer 2 overexpression transgene in the background.
iab-6Δ5 was originally isolated in Iampietro et al [41], where they did not observe any visible external phenotype. With the discovery of the secondary cell phenotype and the strong reproductive phenotype described below, we have renamed this allele iab-6cocu (“cocu” means “cuckold” in French, reflecting that the mates of these males fail to reject other suitors).
Abd-B expression in secondary cells is independent of the initiator
Interestingly, although the secondary cell enhancer was found in the iab-6 domain, it does not seem to be regulated like other BX-C enhancers. Previous work has demonstrated that most enhancers in the BX-C function coordinately through their integration into segment-specifically activated chromatin domains [6], [47], [48]. A special domain control element, called an initiator, is thought to dictate the activity state of a domain along the A-P axis [41], [49], [50]. Thus, deletion of the iab-6 initiator is predicted to inactivate Abd-B expression in the secondary cells, because the secondary cell enhancer should be coordinately regulated with the other enhancers in the iab-6 domain. In contrast to this prediction, we observed that deletions of the iab-6 initiator, which seem to show complete transformations of A6 to A5, display wild-type accessory glands (Figure 3E and 3G). From these experiments, we conclude that Abd-B expression in the secondary cells is set up by a different mechanism than that of tissues arising early in development.
Impact of iab-6cocu on the production of main cell Acps
The iab-6cocu mutation offers the opportunity to investigate the role of the secondary cells in the PMR. First, we tested whether the main cells of these males are functional, since loss of main cell derived Acps may mask any secondary cell related phenotypes present in our mutant. We performed Western blots to examine the presence of known main cell Acps in the accessory glands of iab-6cocu males relative to two types of control males [males heterozygous for the iab-6cocu mutation (henceforth referred to as control males) and wild type males (Canton S)]. As a negative control, we included the accessory glands of DTA-E males, which lack protein production in the main cells [19] but have theoretically normal secondary cells. We used antibodies to four Acps expressed in the main cells: SP [29], Acp62F, Acp36DE [51], and ovulin; the latter Acp is also present in the secondary cells but is known to be absent in DTA-E males [52]. We detected all four Acps in the extracts from iab-6cocu males (Figure 4A). This result suggests that the main cells in iab-6cocu males are functional.
Fig. 4. Seminal fluid proteins in iab-6cocu and control males, and their mates. 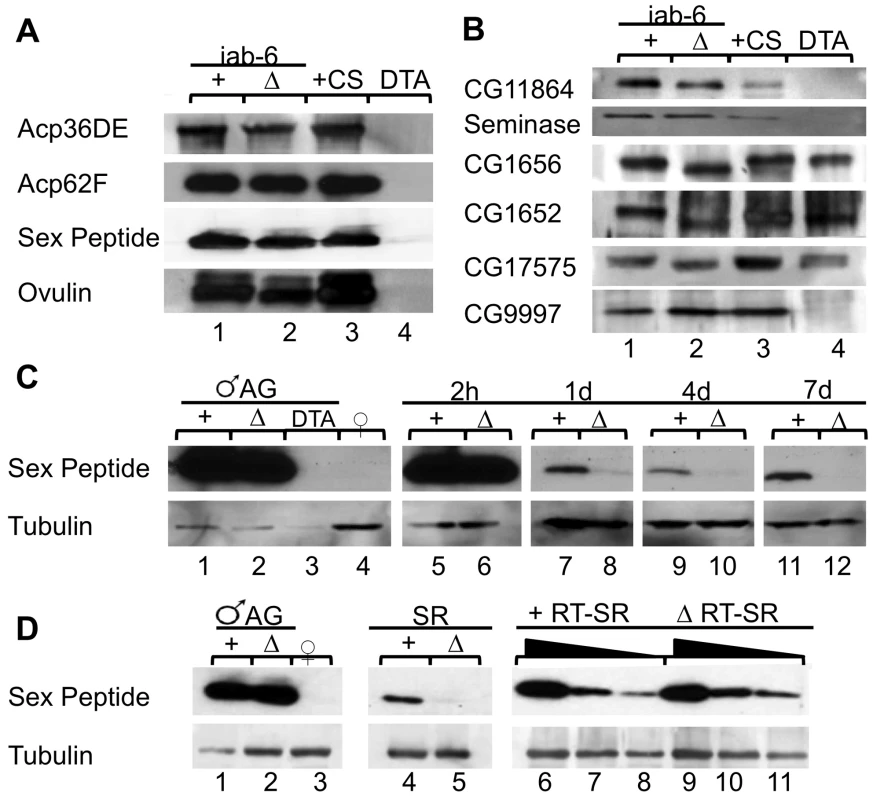
A) Western blots of accessory gland extracts from two control males (lane 1), two iab-6cocu males (lane 2), two wild type males (lane 3), and two DTA-E males (lane 4). All Acps known to be produced by the primary cell (Acp36DE, Acp62F, sex peptide, and ovulin) are present in the accessory glands of iab-6cocu males, but not DTA-E males. B) Other Acps necessary for various aspects of the PMR (CG11864, Seminase, CG1656, CG1652, CG17575, and CG9997) are present in the accessory glands of iab-6cocu males. CG1656, CG1652, and CG17575 are always detectable in DTA-E males, however their abundance is highly variable compared to controls (The western blots depicted were selectived to most clearly demonstrate the presence of these proteins in DTA-E males). C) Mates of iab-6cocu males have less SP, as detected by antibodies to SP, in the reproductive tract at 1 d ASM and all subsequent time points. Tubulin was used as a loading control for the female reproductive tracts. Accessory gland extracts from a single control male (lane 1) and iab-6cocu male (lane 2) were used as a positive control and accessory gland extracts from 2 DTA-E males (lane 3) and reproductive tract extracts from 8 virgin females (lane 4) were used as a negative control. Reproductive tract extracts from females mated to either control (+) or iab-6cocu (Δ) males at 2 h (lane 5–6, 2 RTs per), 1 d (lane 7–8, 20 RTs per), 4 d (lane 9–10, 18 RTs per), and 7 d ASM (lane 11–12, 21 RTs per). D) Mates of iab-6cocu males have dramatically less SP in the seminal receptacle (SR) at 2 h ASM. Tubulin was used as a loading control for the female reproductive tracts. Accessory gland extracts from a single control male (lane 1) and iab-6cocu male (lane 2) were used as positive controls and reproductive tract extracts from 8 virgin females (lane 3) were used as a negative control. Extracts from SRs dissected from females mated to either control (+) or iab-6cocu (Δ) males at 2 h ASM (20 SRs each, lanes 4–5). The amount of SP present in the reproductive tract (minus the SR) of mates of control and iab-6cocu males was determined in a dilution series (1∶1 (lanes 6 and 9), 1∶2 (lanes 7 and 10), and 1∶4 (lanes 8 and 11) and are equivalent to 5 RTs, 2.5 RTs, and 1.25 RTs). There is no appreciable difference in the amount of transferred SP. Egg laying in mates of iab-6cocu males
To test if the iab-6cocu mutation impacts the ability of males to induce egg-laying in their mates, we crossed iab-6cocu males, control males, and DTA-E males to virgin females. During the first 24 hours after the start of mating (ASM), the number of eggs laid by females that had mated to iab-6cocu males is comparable to that of mates of control males and is significantly higher than the number laid by females mated to DTA-E males (Figure 5A). This indicates that the iab-6cocu mutation does not impact the STR and supports the Western blot results that suggest that the main cells are normal. However, egg laying in mates of iab-6cocu males decreased dramatically at 48 hours, and the total number of eggs produced over the entire 10 day period was significantly lower than the number laid by mates of control males (Figure 5A) (note that Canton-S males behave similarly to our control males (Figure S7)). This drop in egg laying is consistent with that observed when females do not receive or fail to store/release SP. This suggests that products from the secondary cells may be necessary for maintenance of the LTR. The proportion of progeny that eclosed from eggs laid by females mated to either iab-6cocu or control males was comparable, suggesting that there is no effect of secondary cell products on hatchability (Figure 5B). Together these results suggest that the secondary cells perform a function that is essential for the maintenance of the post-mating egg-laying increase, but does not impact hatchability, and that this function is perturbed in iab-6cocu males.
Fig. 5. Egg-laying and receptivity in mates of iab-6cocu or control males. 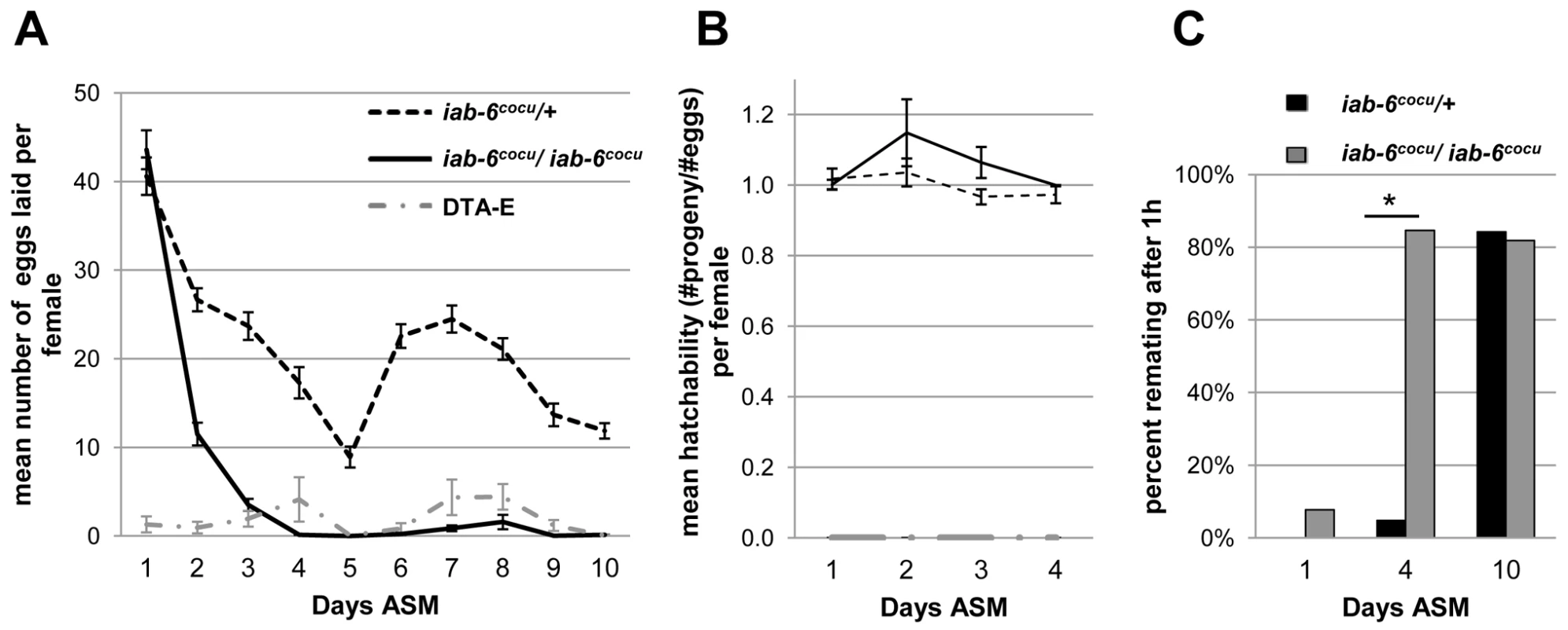
A) The mean number of eggs laid per female mated to either control males (dashed line), iab-6cocu males (solid line), or DTA-E males (grey dot dashed line) over a 10 day period. Mates of iab-6cocu males lay normal numbers of eggs during the first day after mating (WRST p = 0.300) but lay significantly fewer eggs over 10 days when compared to mates of control males (rmANOVA p = <0.0001*, Control N = 51, iab-6cocu N = 45, DTA-E N = 17). The drop in egg laying for controls on day 5 is atypical and was likely a response to food quality. B) The mean hatchability (#progeny/#eggs) per female for mates of control, iab-6cocu, and DTA-E males for days 1–4 of the egg laying results reported in (A). Days 5–10 were omitted because iab-6cocu mated females do not lay enough eggs on these days for analysis. Mates of iab-6cocu males have comparable hatching totals for the eggs that they do lay when compared to mates of control males (WRST p = 0.37). Because DTA-E males do not produce sperm, their mates are expected to show zero hatchability. (Values greater than 1 represent instances where the number of progeny produced exceeded the number of eggs counted; this under-counting can result when females lay eggs under bubbles in the medium or directly on top of previously laid eggs. Hatchability values were not normalized to 1 so as to accurately report counter error.) C) The percentage of mated females willing to mate within 1 hour of exposure to a wild type male at 1, 4, and 10 days after an initial mating. Both groups of females initially mated to iab-6cocu or control males are unreceptive (WRST p = 0.21, control n = 20, iab-6cocu n = 26). At 4 d ASM females initially mated to iab-6cocu males are significantly more receptive to courting males compared to mates of control males (WRST p = <0.0001*, control n = 21, iab-6cocu n = 26). By 10 d ASM there is no difference between mates of control or iab-6cocu males (WRST p = 0.84, control n = 19, iab-6cocu n = 22). Receptivity in mates of iab-6cocu males
Under normal conditions, mated females are less receptive to subsequent mating for more than four days after the initial mating occurred [53]. This reduction in receptivity requires the receipt of Acps [19], [23], [26]. To test whether the iab-6cocu mutation alters female receptivity to remating, we mated virgin females to either iab-6cocu or control males and then allowed these females access to a single WT male at 1 d, 4 d, or 10 d ASM. At 24 hours after the initial mating, neither group of females remated, further suggesting that the STR is intact in mates of iab-6cocu males. However, when mated females were introduced to a WT male at 4 days ASM, females which had initially mated to iab-6cocu males were significantly more receptive than mates of control males. At 10 days ASM, both groups were fully receptive (Figure 5C). Our results show that sexual receptivity is initially repressed in mates of iab-6cocu males, but that this effect is not maintained. This finding demonstrates a defect similar to those observed in known LTR-related proteins and further corroborates the LTR phenotype observed in our egg-laying experiments.
To verify that both the egg-laying and receptivity phenotypes are caused specifically by the loss of Abd-B expression in the secondary cells, we also performed these experiments using BAC rescued iab-6cocu flies (Figure S5) and D5-Gal4::Abd-B RNAi flies (Figure S6). In both cases, these experiments confirm a role for Abd-B in producing the PMR phenotypes. Again, as with the cellular phenotypes mentioned above, both the rescue and phenocopying was incomplete, though clearly significant. Given the caveats involved in these experiments regarding the level and timing of protein expression, this was perhaps not unexpected and demonstrate a strong relationship between the celluar and the behavioral phenotypes. Nonetheless, these data clearly point to a major role for Abd-B expression in the secondary cells in modulating the PMR.
The production of LTR-associated Acps in iab-6cocu males
Our results suggest that the secondary cells are necessary for the processes required for long-term PMR maintenance. Therefore, the iab-6cocu mutation likely impacts proteins required for the LTR. While the Acps that are produced and transferred to females have been extensively described, [51], [54]–[57] little is known about their cellular origins. Thus, we investigated the possibility that some of the known PMR-related Acps, and more specifically those involved in the LTR, could be produced in the secondary cells or in both cell types, and thus, may be absent in iab-6cocu males.
We performed Western blots to examine the presence of known LTR Acps in the accessory glands of iab-6cocu males relative to control males. As a negative control for main cell expressed Acps, we included DTA-E males. Any secondary cell-expressed Acp should still be produced in these males, but main cell expressed Acps should not. We used antibodies to six Acps that regulate the PMR; one STR associated Acp (CG11864) and five LTR associated Acps (CG10586 (Seminase), CG9997, CG17575, CG1656, and CG1652 [36], [38]–[40]). All of these Acps were present in iab-6cocu males (Figure 4B). Surprisingly, three of the Acps associated with the SP pathway (CG17575, CG1656, and CG1652) were also present in AG extracts from DTA-E males suggesting that they may be secondary cell expressed. Supporting this hypothesis, RNAi of these proteins in the secondary cells knocksdown their expression, while leaving a main cell-expressed control protein, Acp62F, unchanged (Figure S8). The remainder of the Acps, CG9997, CG11864, and Seminase, are likely all expressed primarily or exclusively in the main cells. Further, these results support our previous conclusion that the secondary cells in iab-6cocu males maintain a distinct expression profile from main cells and still produce some secondary cell proteins.
A role for secondary cells in SP storage
The LTR defects seen in mates of iab-6cocu males are consistent with those associated with failure to store or release SP [51], [55]–[57]. However, iab-6cocu males produce SP and all known LTR Acps. Still, it is possible that the iab-6cocu mutation interferes with the ability of SP to enter storage and thus maintain the LTR. We tested for this by performing Western blots using SP antibodies. Both control and iab-6cocu males transfer SP to their mates, and there are comparable amounts of SP in the female reproductive tract by 2 h ASM. However, by 24 hours ASM and continuing to 6 days thereafter, mates of iab-6cocu males have significantly less stored SP (Figure 4C; Figure S9). To distinguish between premature loss of SP from the seminal receptacle (SR) versus failure of SP to be stored in the SR initially, we performed Western blots of SRs dissected from females mated to either iab-6cocu or control males. Significantly less SP is present in the SR of mates of iab-6cocu males at 2 h ASM compared to mates of control males, while the amount of SP present in the remainder of the reproductive tract is comparable, though slightly higher in mates of iab-6cocu males (Figure 4D; Figure S9). These results suggest that iab-6cocu males transfer normal amounts of SP but that this SP fails to enter the SR. The reduction/absence of stored SP at later time points (1–7 days ASM) is likely responsible for the reduction in egg laying and the increase in receptivity seen in mates of iab-6cocu males.
Sperm competition in mates of iab-6cocu males
SP also plays a role in sperm competition [58], which occurs when ejaculates from two males are present within the same female reproductive tract [59]. For example, in circumstances where SP null males are the first male to mate with a given female, they sire a higher percentage of the total progeny (P1, #progeny from first male/total progeny) than control males [33]. To test whether the iab-6cocu mutation also affects P1, we mated iab-6cocu and control males to cn bw females and, after 3 days, allowed them to mate with cn bw males. The iab-6cocu males had significantly higher P1 than control males consistent with a problem in SP presence or storage (Figure 6A).
Fig. 6. Sperm storage and use by mates of iab-6cocu or control males. 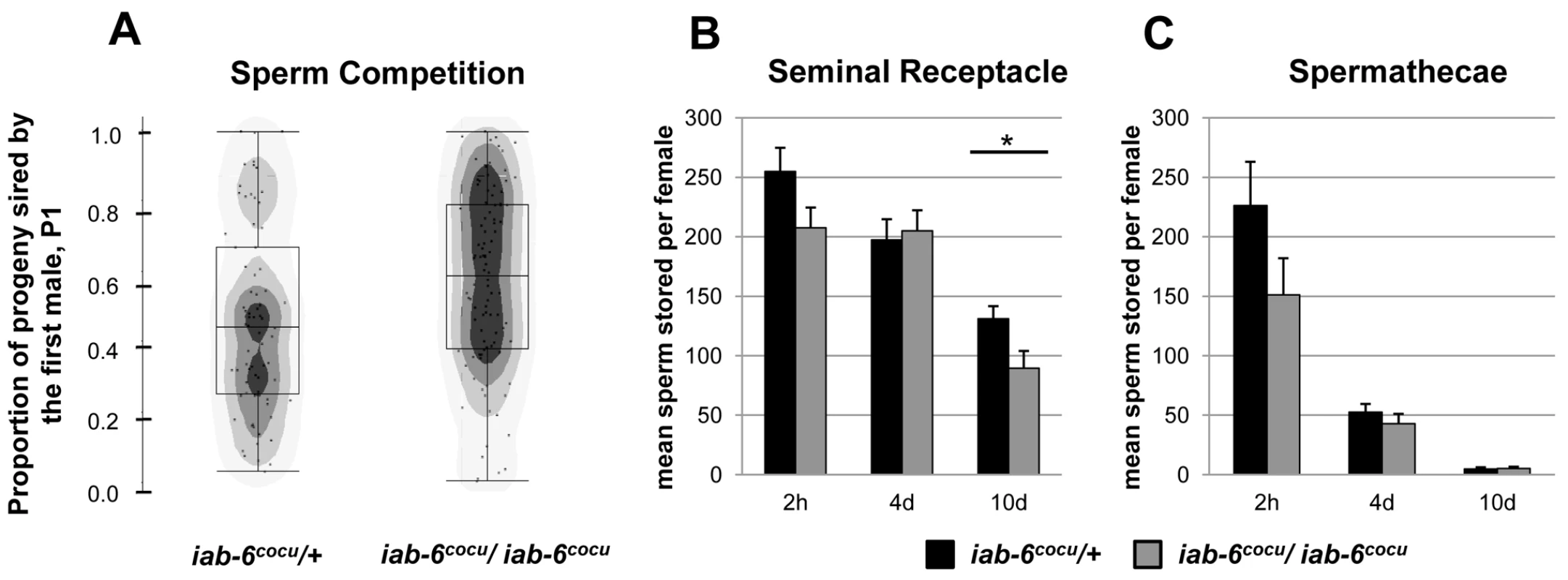
A) For sperm competition assays cn bw females were first mated to either control (left) or iab-6cocu (right) as the first male and allowed to mate a second time with a cn bw male. The proportion of progeny sired by iab-6cocu males when acting as the first male (P1, # progeny from first male/total progeny) was significantly higher when compared to females who first mated with control males (WRST p = 0.038*, control N = 74, iab-6cocu N = 98). B&C) Counts of sperm stored in mates of control (black) and iab-6cocu (grey) males at 2 h, 4 d, and 10 d ASM. B) Mates of iab-6cocu males have wild type numbers of sperm present in the seminal receptacle at 2 h (WRST p = 0.10, control N = 8, iab-6cocu N = 11) and 4 d ASM (WRST p = 0.96, control N = 10, iab-6cocu N = 8) but fewer at 10 d ASM (WRST p = 0.017*, control N = 19, iab-6cocu N = 12) when compared to mates of control males. C) Mates of iab-6cocu males show wild type numbers of sperm stored in the spermathecae at all time points. 2 h (WRST p = 0.13, control N = 7, iab-6cocu N = 10); 4 d (WRST p = 0.38, control N = 10, iab-6cocu N = 7); 10 d (WRST p = 0.77, control N = 17, iab-6cocu N = 16). Sperm storage in mates of iab-6cocu males
One possible explanation for the reduction in stored SP in mates of iab-6cocu males is a defect in sperm entry into storage or an increase in the rate at which sperm are released from storage. To test this, we counted sperm present in both female sperm storage organs at 2 hours, 4 days, and 10 days ASM. Females mated to either control or iab-6cocu males store sperm at comparable levels at 2 hours ASM and appear to retain normal numbers through 4 days ASM (Figure 6B and 6C). These results suggest that initial sperm storage and release is normal in mates of iab-6cocu males and that the reduced level of SP in the seminal receptacle at 2 hours ASM is not due to a failure to adequately store sperm. Mates of iab-6cocu males do not show the stereotypical sperm over-retention phenotype seen with knockdown of other LTR related proteins [33] and instead show a slight but significant decrease in stored sperm within the SR at 10 days ASM when compared to controls (Figure 6B). This is not wholly surprising, as the iab-6cocu mutation does not result in the absence of a single gene product, but likely several that contribute to different aspects of the PMR. It is possible that the secondary cells produce, modify, or transfer some product necessary for sperm to be retained within the female sperm storage organs. When SP is absent, this product may be regulated improperly resulting in the typical sperm over-retention phenotype. A loss of, or reduction in this product, combined with the SP retention defect, may explain these results.
Several Acps display defects in glycosylation, stability, or protein abundance within the reproductive tract of females mated to iab-6cocu males
Our Western blots and RNAi data showed that three of the known LTR specific proteins (CG1656, CG1652, and CG17575) are produced in the secondary cells and that one (CG9997) is likely produced by main cells (Figure 4B and Figure S7). We considered the possibility that failure to transfer one or all of these Acps to the female during mating could contribute to the SP storage defect seen in mates of iab-6cocu males. To test this, we performed Western blots on the reproductive tracts of females mated to either iab-6cocu or control males at 15 m, 30 m, and 1 h ASM using antibodies to CG9997, CG1656, CG1652, and CG17575. Although all four Acps are transferred to females and are present in the female reproductive tract at all time points tested, their abundance, gel mobility, or processing appear to be abnormal in mates of iab-6cocu males (Figure 7).
Fig. 7. Post translational modification, stability, and abundance of seminal fluid proteins in mates of iab-6cocu or control males. 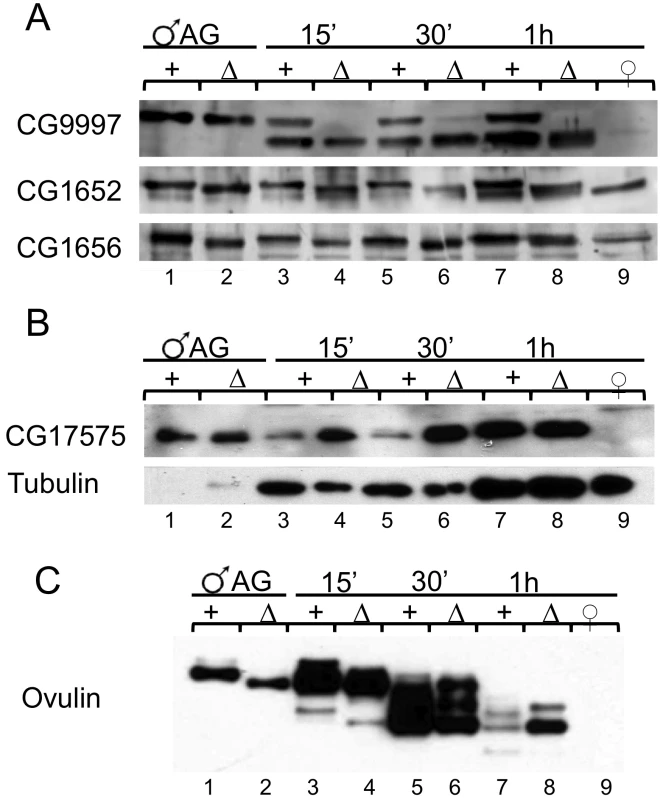
Western blots using antibodies of known LTR-associated Acps CG9997, CG1656, CG1652, and CG17575 as well as STR Acp ovulin (Acp26Aa). Accessory gland extracts from a single control (lane 1) and iab-6cocu male (lane 2) were used as positive controls and reproductive tract extracts from 4 virgin females (lane 9) were used as a negative control. Extracts from the reproductive tracts of females mated to control (+) or iab-6cocu (Δ) were collected at 15′ (lanes 3–4, 2 RTs per), 30′ (lane 5–6 s, 3 RTs per), and 1 h ASM (lanes 7–8, 6 RTs per). A) Full length CG9997 is produced by iab-6cocu males but is not present in the reproductive tracts of their mates. The smaller processed form of CG9997 is present in mates of iab-6cocu suggesting that CG9997 is transferred. Both CG1656 and CG1652 are transferred to females normally by iab-6cocu males, but both of these proteins run at a lower apparent molecular weight than in control males. B) iab-6cocu males transfer more CG17575 to their mates than control males. Tubulin was used as a loading control for the female reproductive tracts. C) Both mates of control and iab-6cocu males receive ovulin. However, the ovulin produced by iab-6cocu males also runs at a lower apparent molecular weight than in controls. iab-6cocu males carry out abnormal glycosylation of several Acps
Both CG1656 and CG1652 run at a lower apparent molecular weight in iab-6cocu males compared to control males (Figure 7A, Figure 4B). Ovulin, likewise, shows reduced apparent molecular weight in iab-6cocu males (Figure 7C, Figure 4A). The gel mobility differences for these proteins in iab-6cocu versus control males is evident both within AG extracts as well as across time points (15 m, 30 m, and 1 h ASM) within the female reproductive tract of their mates. Ovulin is normally processed inside the female reproductive tract [60]. This processing appears to occur properly in mates of iab-6cocu males, although the apparent molecular weight of some cleavage products is altered. It is unlikely that these differences in apparent molecular weight are caused by sequence differences or background effects. The controls used for these experiments are heterozygous for all of the chromosomes in the iab-6cocu mutant line. Thus, if the gel mobility differences were caused by sequence differences, we would expect to see two bands indicating the WT and altered version of each protein. Further, ovulin and CG1656/1652 are located on separate arms of chromosome 2 and are necessary for different aspects of the PMR. Together, these observations suggest that the gel mobility differences may be a result of posttranslational modification.
Ovulin is a glycoprotein [52], but little is known about the posttranslational modifications of CG1656 and CG1652. To test whether differences in glycosylation underlie the gel mobility differences observed, we treated extracts from control and iab-6cocu males with PNGaseF. This treatment, which removes N-linked glycosylation [61], [62], resulted in loss of the apparent molecular weight differences between iab-6cocu and control flies for all three proteins (Figure 8, CG1652 not shown). These results suggest that the secondary cells contribute to the regulation of posttranslational modifications, and more specifically glycosylation, of Acps. They are also the first evidence of the presence of N-linked glycosylation on CG1656 and CG1652.
Fig. 8. Glycosylation measurements of seminal fluid proteins in iab-6cocu or control males. 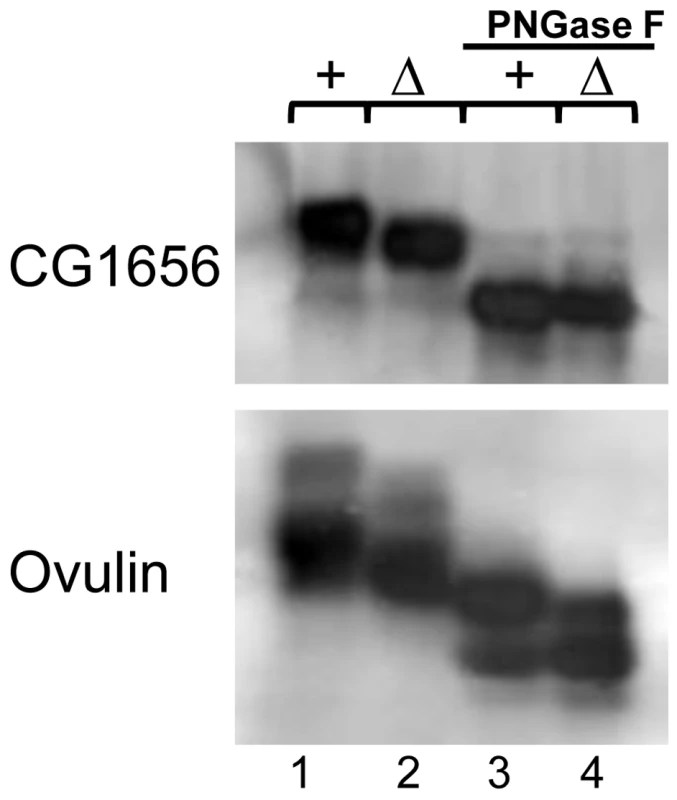
PNGase F assays were used to examine glycosylation states of CG1656 and ovulin or determine whether N-linked glycosylation differences underlie the gel differences seen between control (+) and iab-6cocu (Δ) males. Untreated (lanes 1–2) and PNGase F treated (lanes 3–4). In both cases the gel mobility differences seen between control and iab-6cocu males are absent after PNGase F treatment suggesting that in iab-6cocu males both proteins are improperly glycosylated. To verify that the glysocylation differences are caused specifically by the loss of Abd-B expression in the secondary cells, we also looked at the gel mobility of CG1656 and CG1652 in BAC rescued iab-6cocu flies (Figure S5, CG1652 not shown). The rescue BAC was able to restore proper glycosylation in the AGs of some males but not others. This is consistent with the partial rescues we have observed, especially if the glycosylation phenotype is dose dependant and the BAC males are on the threshold. Still, these results support the connection between Abd-B expression in the secondary cells and proper glycosylation of Acps and suggests that there may be a connection between these glycosylation differences and the PMR.
Mates of iab-6cocu males display abnormal stability or abundance of some Acps
In wild type males, CG9997 is transferred to females as a full length protein (45 kD) and is processed in the female reproductive tract to a smaller form (36 kD). Both products persist in the female for longer than 1 h. Males with the iab-6cocu mutation produce full length CG9997 but the full length form does not persist inside the female reproductive tract (Figure 7A). A similar increase in processing or instability of the full length product was seen in males that do not produce or transfer CG1656/CG1652 [39]; its biological relevance is currently unknown. Since iab-6cocu males produce and transfer CG1656/CG1652, our results suggest that in addition to these two proteins, the products of the secondary cells are essential for regulating the stability of CG9997 inside the female. As observed with the other LTR Acps we assayed, CG17575 is produced and transferred to females by iab-6cocu males. However, CG17575 is present in higher amounts in the reproductive tract of females mated to iab-6cocu males at all time points when compared to controls (Figure 7B; Figure S9). Whether this difference is due to increased transfer or failure to degrade CG17575 within the tract is unclear.
Discussion
Here, we report that Abd-B is expressed in the secondary cells of the Drosophila male AG and, using mutations that specifically remove Abd-B from these cells, uncover roles for this previously unstudied but important reproductive cell type. Furthermore, we show that Abd-B expression in these mesodermally-derived cells does not fit the “initiator” paradigm developed for the segment-identity function of Hox genes in ectodermal tissues. And finally, we demonstrate that the secondary cells of the male AG synthesize products necessary for maintenance of the seminal fluid's effects on the female PMR.
New insights into Abd-B gene regulation
Due to the large size and complexity of the Abd-B regulatory region, we created a BAC - reporter construct to monitor Abd-B expression in the adult fly. When combined with fluorescent markers, this method allowed us to bypass the technical issues of antibody penetration and the laborious dissections needed for in situ hybridization or immunohistochemistry to identify a novel area of Abd-B expression in the adult. Overall, the BAC reporter is able to accurately reproduce the known, complex Abd-B expression pattern; indeed, our BAC construct seems to more-faithfully reproduce Abd-Bm expression than even a previously isolated transposon insert in the Abd-B promoter (Abd-B-Gal4LDN) [63]. Furthermore, by combining our BAC reporter with pre-existing deletion mutations, we were able to discover the function of a vital gene in an adult tissue without the need to create mitotic clones.
From the standpoint of Hox gene regulation, our discovery of the secondary cell enhancer is quite interesting because, unlike other cell-type specific enhancers from the BX-C, the secondary cell enhancer does not seem to be regulated by a domain initiator [6], [41], [49]. Most cell-type specific enhancers from the BX-C are not intrinsically restricted along the A-P axis. They are restricted only to a specific cell-type and gain A-P restriction through clustering in a BX-C domain. For example, in a transgene assay, an Abd-B enhancer from the iab-7 domain (called 11X) drives expression in the tracheal placodes in all segments. However, in the BX-C, this enhancer seems to be active only in the Abd-B expression domain [6]. The clustering of enhancers to one area of the chromosome is thought to allow all of the enhancers to be coordinately regulated along the A-P axis as a domain through the changing of the local chromatin environment. The Polycomb (Pc) repression machinery is thought to be critical for this process by creating repressive chromatin over inactive domains ([6] and refs. therein). Specialized elements, called initiators, seem to read an A-P segmental address and act as domain activators, probably by preventing Pc repressive complexes from establishing on active domains [41].
The domain model predicts that deletion of an initiator element should prevent domain activation, leaving all enhancers in its domain inactive [41]. Based on this paradigm and the fact that the secondary cell enhancer was found in the iab-6 domain, we expected that the deletion of the iab-6 initiator would abolish Abd-B expression in the secondary cells. However, we found that Abd-B expression in the secondary cells of initiator mutants was normal. This finding argues against the strictest interpretation of the initiator model. We can propose several hypotheses to resolve this discrepancy. For example, the Pc repression system is known to act on many genes during development, but its main targets appear to be the homeotic genes during the establishment of segment identity. It is possible that late in development, after cells have made initial cell fate decisions (and the segment identity role of the homeotic genes might be less important), the targets of Pc silencing might change. Such loosening of Pc silencing over the Abd-B cis-regulatory regions would allow previously silenced enhancers to become available for regulating Abd-B expression so that it could perform other functions, such as in the secondary cells.
Alternatively, the difference in Abd-B gene regulation that we observe in secondary cells may reflect the cellular origin of the secondary cells. Most BX-C cis-regulatory mutations were isolated based on cuticular phenotypes and confirmed using antibody staining in the epidermis and CNS. These tissues are of ectodermal origin, unlike the mesodermally-derived secondary cells [64]. Perhaps, the rules governing the coordination of Hox expression in the ectoderm do not hold true in the other germ layers. Consistent with this, BX-C genes are expressed differently in the gut visceral mesoderm than in the ectoderm [65].
Evolutionary considerations may provide some explanation for why the fly uses different means of controlling Abd-B expression in embryonic segment identity specification vs. in later reproductive tissues. Abd-B class Hox proteins play roles in the formation of the external genitalia in both arthropods and mammals [66]–[70]. Due to the similarity in expression pattern and function, it has been proposed that Abd-B's role in the formation of the genitalia predates its role in segmental identity [67] [71]. Here, we have shown that Abd-B is also important for correct development of cells within the Drosophila male AG that produces many seminal fluid proteins required for male reproductive success. The mammalian orthologues of Abd-B, the Hox9 to 13 class of genes, are expressed in the developing seminal vesicle and prostate gland, both seminal protein secreting organs [72], [73]. The analogy in function between these organs, and their similarity in gene expression patterns suggests that the role of Abd-B class genes in the male reproductive tract might be an ancient, conserved function, potentially independent of its role in segmental identity. In this light, it would not be surprising that Abd-B regulation in the secondary cells escapes the domain regulation seen for Abd-B function in segment identity determination. The cis-regulatory domains for segment identity could have been added separately, possibly through co-opting the abd-A gene regulatory regions, as suggested by transvection studies [74], [75]. In any case, the adding of cis-regulatory sequences and the consequent constraints of the domain model on Abd-B would necessarily have to preserve its late function in the secondary cells.
The function of the secondary cells of the male accessory gland
Previous studies have shown that the male AG produces Acps required to initiate and maintain a range of PMRs in females. Further, diptheria-toxin mediated-ablation of accessory gland main cells demonstrated that products of these cells are essential for the PMR. The importance of the main cells was further strengthened by the discovery that they produce the Acp sex peptide (SP), which is essential for many aspects of the PMR and whose persistence allows maintenance of PMR effects (i.e. the LTR) [29]. Additional Acps important for other aspects of the PMR (e.g. Acp36DE, ovulin) were also found to be produced by main cells. Thus, until now, the role of the secondary cells was unknown and no methods to directly target these cells were available.
Using the iab-6cocu regulatory mutant of Abd-B, we demonstrated that the secondary cells make a unique contribution to maintenance of the female's post-mating changes in egg-laying, receptivity, sperm competition, and sperm storage (summarized in Figure 9). Our results are consistent with findings about secondary cell function obtained independently by Minami et al., from their study of dve mutants [76]. The inability of iab-6cocu males to maintain the PMR in their mates is consistent with perturbation of the function of the “LTR network”. These LTR network proteins are needed to promote the association of SP with sperm in the SR, an association that is required for SP-mediated maintenance of the PMR [22], [39]. The results of our study also show that three of these LTR network Acps, CG1656, CG1652, and CG17575 are all produced in the secondary cells while CG9997 and Seminase are primarily or exclusively main cell expressed. This is the first direct evidence that Acps from both cell types work together in a complex pathway. While all reported LTR specific network proteins (CG9997, CG1656, CG1652, and CG17575) are present in the iab-6cocu mutant, they are all abnormal, either in amount/stability inside the female reproductive tract or in glycosylation state; how these changes result in the SP storage defect is an important area for future study.
Fig. 9. Summary/model. 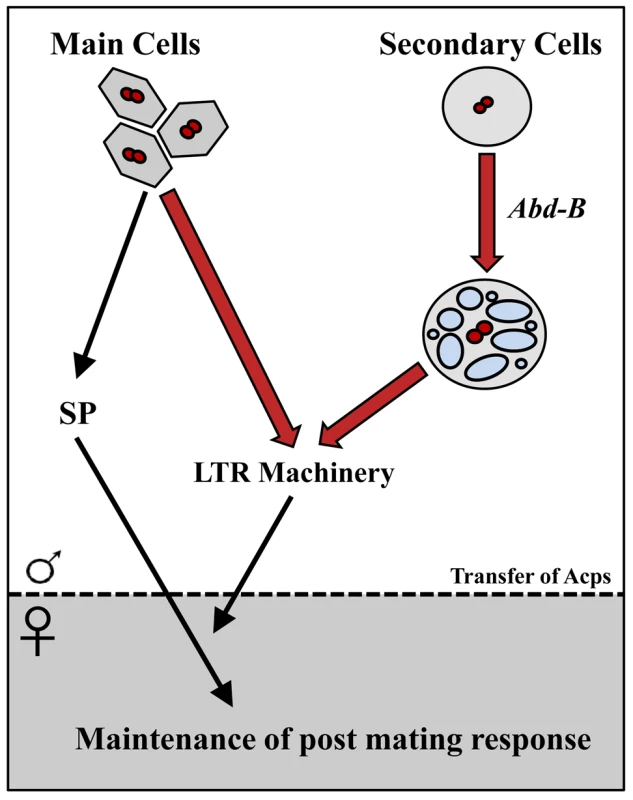
Bold arrows delineate the new findings in this paper. The Drosophila male accessory gland consists of two secretory cell types: main cells and secondary cells. Main cells produce seminal proteins essential for inducing post-mating responses (PMR) in mated females; the function (if any) of secondary cells was unknown. Sex Peptide (SP) from the main cells induces many aspects of PMR, but persistence of its effects requires other seminal proteins (the LTR machinery: CG9997, CG17575, CG1652 and CG1656), whose cellular source was unknown. Here, we showed that the Hox gene Abd-B is essential for normal development of the secondary cells, but with regulatory characteristics (not shown in this figure) that differ from those used in segment identity. By deleting the secondary-cell regulatory element of Abd-B, we obtained males with abnormal secondary cells. Mates of those males failed to maintain the PMR, indicating that secondary cells play an essential role in reproduction: their products, along with main cell products, allow persistence of SP in mated females, thus prolonging their post-mating responses. The phenotype of iab-6cocu males shows one exception to the standard LTR phenotype. Mates of SP null males (or males knocked down for any of the other 4 LTR related proteins) have high rates of sperm retention; this phenotype is lacking in mates of iab-6cocu mutants. The mechanisms behind the release of sperm from storage and this sperm retention phenotype are unknown. Loss of Abd-B expression most likely impacts a wide variety of Acps and potentially cellular functions associated with vacuoles (such as transmembrane transport). Thus, it is possible that one or a combination of the proteins affected by this mutation (some as yet unidentified) may negatively impact normal sperm storage independent of the influence of Abd-B on the storage of SP in the seminal receptacle. This could result in masking the over-retention of sperm seen in SP nulls due to the loss or abnormal function of a protein or proteins necessary for the retention phenotype to occur. Further work investigating the role of individual secondary cell associated Acps in the LTR, as well as sperm storage, may be helpful in determining how these processes function.
The iab-6cocu mutant revealed another unique role for secondary cells: regulating glycosylation of at least three seminal proteins (ovulin, CG1656, and CG1652); the impact of glycosylation on the functions of these proteins is as yet unknown. Our findings show that CG1656 and CG1652 are produced by the secondary cells. However, ovulin is produced in main cells as well as secondary cells, and is detected in the AG lumen as well as in the vacuoles of the secondary cells [52]. As such, we suggest three possibilities for how secondary cells might mediate glycosylation: 1) through the secretion of glycosylation substrates that can be taken up and used by main cells, 2) through the secretion of glycosyation regulators directly into the lumen where they could modify Acps from both cell types that are present there, or 3) that Acps like ovulin and other main cell products are taken up into these vacuoles and then modified before being secreted into the lumen as mature, glycosylated proteins. Future dissection of the glycosylation phenotypes in the iab-6cocu mutant males will help shed light on the role glycosylation plays in regulating the PMR and how this process is regulated in the tissue as a whole.
It is important to note that our Abd-B regulatory mutant still has secondary cells, or at least precursor cells poised to become secondary cells. The initial differentiation step that allows for Abd-B expression in the secondary cells is not perturbed by the iab-6cocu deletion. As such, although we have already found a new and unanticipated role for secondary cells in regulating the PMR, it is possible that these cells play additional roles. Future cell ablation experiments using tools derived from this study will allow tests of such additional roles.
In conclusion, we have shown that each of the two cell types of the Drosophila AG plays important roles in producing the female PMR: the main cells producing several Acps to initiate and maintain the PMR, and the secondary cells providing products to aid in temporally extending the response. We expect that through the action of various combinations of transcription factors, like Abd-B, the two cell-type lineages have diverged into distinct, and specialized cell-types. Although these two cell types perform vitally intertwined functions, they are maintained as separate cell types. This suggests that there may be a requirement for compartmentalization of their functions or products, or that the two cell types evolved separately for some other purpose and were functionally associated afterward. It is, therefore, of great future interest to identify the specific products of each of these cell types and to determine how they work in conjunction to mediate the full reproductive effect of seminal secretions.
Materials and Methods
Creation of Abd-B rescue BAC
The Abd-B rescue BAC was constructed from BACR24L18 (size - 171936 bp; AC095018), which contains the Abd-B region of the BX-C. We first constructed a pW25-based vector [77] to be used to add sequences to the BAC for site-specific integration into the Drosophila genome. The original white gene carried by pW25 was replaced by a Su(Hw) insulator-flanked white gene from the SUPor-P plasmid [78] by amplifying it with NotI-forward and AscI-PmeI reverse (see Table 1 for primer list). These primers carry restriction sites for insertion of the product into pw25 after appropriate enzyme digestion. A fragment containing the kanamycin resistance gene (KanR) was amplified from pIGCG21 [79] using the following primers: PmeI5′Kan, 5′attB3′KanAS. Next, an AttB sequence was PCR amplified using the primers 3′Kan5′attBS, and PmeI3′attBAS. The KanR and AttB fragments were then mixed together with the PmeI5′Kan and PmeI3′attBAS primers for a final overlap PCR reaction. The resulting PCR fragment, containing the KanR gene and an AttB sequence flanked by PmeI sites, was cloned into pGemTeasy. After sequencing, this fragment was excised with PmeI and cloned into the unique PmeI site of the modified pW25 vector (resulting in pW25/Kan-AttB).
Tab. 1. List of primers used to perform the different constructs described in Materials and Methods. 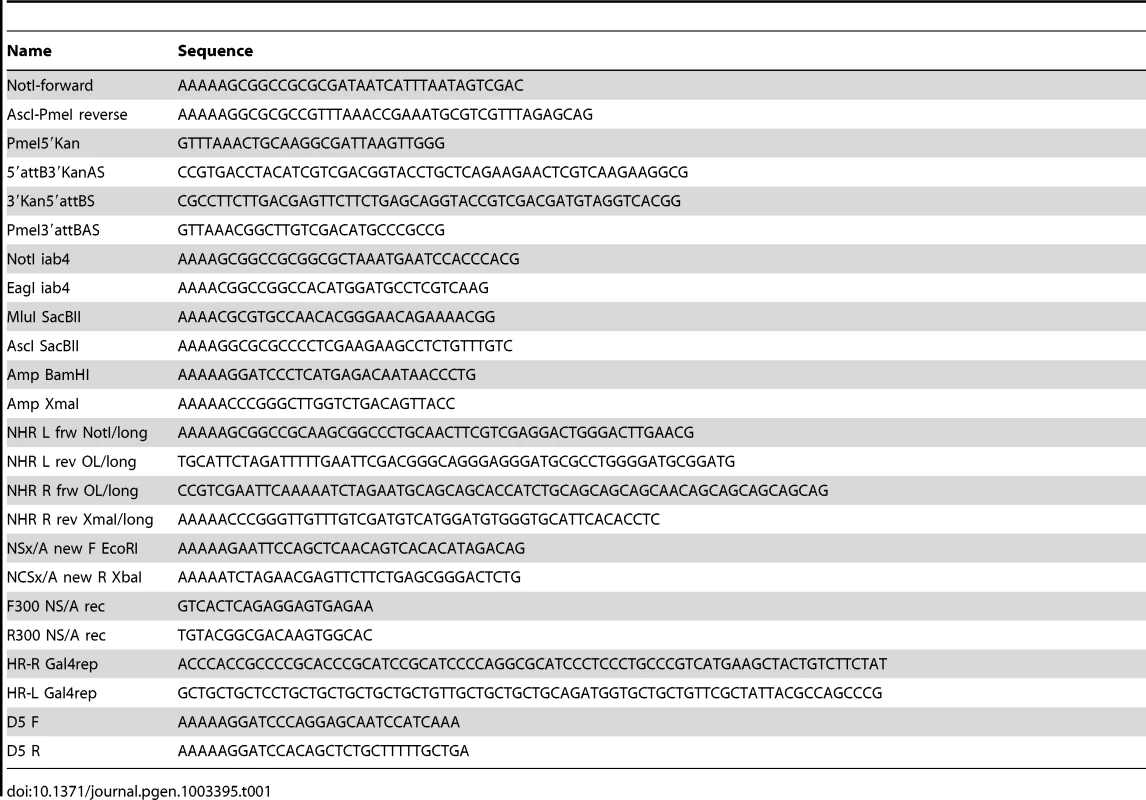
To mediate recombination in bacteria, two homology regions were then added to this vector. First, an 859 bp fragment from the iab4 region (iab4 HR: 101409–102267 coordinates in the BACR24L18) was made by PCR with the primers: NotI iab4 and EagI iab4. The resulting PCR fragment was cut with NotI and inserted into the unique NotI site of the modified pW25. A second homology fragment was designed to target the SacBII gene present on the BAC backbone. Using the PCR primers: MluI SacBII and AscI SacBII, a 525 bp fragment from SacBII was amplified (SacBII HR: 921–1445 coordinates in the BACR24L18). The resulting 525 bp fragment was digested with AscI and MluI, and inserted into the unique AscI site of pW25/Kan-AttB. Clones for both homology regions were selected in an orientation required by the homologous recombination process to function correctly. The completed construct was digested with the ISce-I endonuclease and the fragment containing the homology regions flanking the KanR gene, the white gene and the AttB site was gel purified. This fragment was then used to recombineer BAC24L18 using the protocol of Soren Warming [80]. The resulting BAC, called iab4-SacBII BACR24L18D (108528 bp), contains the region of the BX-C from about iab-4 to the Abd-B m promoter. The overall structure of this BAC was verified by restriction enzyme mapping [using three restriction digests (EcoRI, XmaI, BamHI) (data not shown)].
Abd-B Gal4 reporter BAC
Using iab4-SacBII BACR24L18D as a base we used recombineering to replace the start codon of the Abd-Bm isoform with sequence encoding the Gal4 transcription factor. First, a negative/positive selection cassette was created using the SacB gene and the Ampicillin resistance (AmpR) gene. SacBII was digested out of the plasmid pSK2-SACBK MAR using BamHI and EcoRI and cloned in pHSS7 [81]. The AmpR gene was then amplified with primers (Amp BamHI and Amp XmaI) carrying a BamHI and XmaI site. The amplified AmpR fragment was digested with BamHI and XmaI and cloned into a pHSS7-SacBII digested with the same enzymes, producing pHSS7-SacBII/Amp.
The SacBII/Amp cassette was flanked by two large homology regions using an overlap PCR strategy, as follows: The primers NHR L frw NotI/long and NHR L rev OL/long were first used to amplify the region 39201 bp to 40325 bp of iab4-SacBII BACR24L18D. At the same time, the primers NHR R frw OL/long and NHR R rev XmaI/long were used to amplify the 40326 to 41590 bp region of iab4-SacBII BACR24L18D. The two reaction products contain a 27 bp region of complementarity to mediate overlap PCR. Thus, the two fragments were mixed together with the primers NHR L frw NotI/long and NHR R rev XmaI/long in an overlap PCR reaction. The resulting fragment, containing the two homology domains fused together, was digested with NotI and XmaI and cloned into pHSS7 (pHSS7-NHR-L/NHR-R). Next, from the previously created pHSS7-SacBII/Amp, the double selection cassette was amplified with primers NSx/A new F EcoRI and NCSx/A new R XbaI. Digesting this PCR fragment with EcoRI and XbaI produced a fragment that could be inserted between the two homology domains of pHSS7-NHR-L/NHR-R creating pHSS7-NHR-L/SacBII-Amp/NHR-R.
The primers F300 NS/A rec and R300 NS/A rec were used to amplify a fragment from pHSS7-NHR-L/SacBII-Amp/NHR-R for recombineering. This fragment contained 300 bp of the homology regions on both sides of the cassette carrying the SacBII and AmpR genes. Once again recombineering was performed using the protocol of Soren Warming [80], where the target BAC was iab4-SacBII BACR24L18D. BAC DNA purified from the resulting ampicillin resistant/sucrose sensitive colonies were verified by extensive restriction enzyme digests. The new BAC was named iab4-SacBII BACR24L18D N S/A ins.
To replace the SacBII/AmpR cassette, the Gal4 gene (with a synthetic polyA tail) was PCR amplified from the plasmid pTnT Gal4 (unpublished, pTNT base vector from Promega Corp., Madison, Wisconsin, USA) using the following primers: HR-R Gal4rep and HR-L Gal4rep. These primers contain 55 bp homology regions to mediate recombineering to the BX-C sequences just flanking the SacBII/AmpR cassette. The resulting targeting fragment was then phosphorylated by T4 kinase in order to improve the recombination reactions. After standard preparation of the recombineering DY380 strain containing iab4-SacBII BACR24L18D N S/A ins, bacterial colonies were selected on LB agar plates containing 10% sucrose. Restriction digestion of the candidate colonies with BamHI was performed in order to confirm the correct replacement of the SacBII/Amp cassette with Gal4.
Injections of BACs
Using the PhiC31 system ([45]; www.flyc31.org), site 51C on the second chromosome was chosen for integration of the BACs into the fly genome. For better integration frequencies, all BACs were isolated on the day of injection using the NucleoBond PC 20 (Macherey-Nagel ref 740571) miniprep kit and resuspended in injection buffer [82]. Embryos were injected with BAC DNA (at about 50–100 ng/ul) through the chorion using the Eppendorf system (FemtoJet & TransferMan NK 2) equipped with Femtotips II glass needles. Integration efficiency was about 5%, based on the total number of fertile adults that yielded at least one integrant.
Creation of a specific secondary cell Gal4 driver based on the cocu enhancer
The 2.8 kb putative enhancer sequence removed in iab-6cocu was amplified by PCR using primers D5 F and D5 R. Both of these primers contain a BamHI site at their 5′ ends. The amplified DNA fragment (called D5) was cloned into the BamHI site of the pChs-Gal4 plasmid, which contains a minimal Hsp70 promoter upstream of the coding sequence for Gal4 and the HSP70 3′UTR (Drosophila Genomics Resource Center [83]. Although clones with the enhancer in both orientations were isolated, we proceeded using a clone where the D5 R primer containing end was closest to Gal4. The D5-Gal4 cassette was then digested out of the pChs-Gal4 vector with NotI and cloned into the NotI site of pattB [45]. An insertion with the Gal4 coding sequence next to the white gene was selected for injection. This construct was integrated by Genetic Services Inc (Cambridge, Mass) into the VK00001 (59D3) platform [84]. The resulting integrant is named D5rsG4rs and referred as to D5-Gal4 in the text.
Fly crosses and strains
All crosses were done using standard genetic techniques. iab-7Sz, iab-6,7IH, iab-5,6J82, and iab-4,5,6DB are described in [6]. The lines iab-6Δ5 and iab-64 are described in [41]. The line iab-6Δ5 was described as a deficiency without any phenotypic consequence. Following the LTR phenotype identified in this work the line was renamed iab-6cocu (reflecting that mates of these males fail to reject other suitors; “cocu” means “cuckold” in French). The BAC-AbdBGal4,w+, UAS-GFP/Cy line carrying the Abd-B Gal4 BAC reporter and a UAS-GFP marker on the second chromosome was created for this study by recombining a chromosome carrying the BAC and a UAS-GFP chromosome. The BAC reporter chromosome cannot exist as a homozygote. The 4.4E transgenic lacZ reporter line is described in [6]. The Gal4 expressing lines driven by a paired enhancer ((w−; prd-mf5.2,w+/CyO), (w−;prd-mf5.4,w+), (w−;prd-mf5.5,w+), (w−;prd-mf9.3,w+), (w−;prd-mf9.7,w+)) were obtained from Makus Noll's laboratory [46]. They were used in a cross with a UAS-AbdBm [85] flies for the experiment in which we tested for the ability of Abd-Bm to transform main cells into secondary cells.
All flies for fertility and fecundity assays, tests of receptivity and sperm competition, Western blotting, sperm counts, and PNGase F assays were raised at room temperature (23±1°C). Females were aged 3–5 days from eclosion in groups of 7–11 in glass vials on standard yeast-glucose media with added yeast. Males were aged 3–5 days from eclosion in groups of 10–20 in glass vials on standard yeast-glucose media.
Antibody, X-Gal, and FM4–64 staining
Antibody and X-Gal staining on embryos and dissected accessory glands was performed as described in [12] and [42] respectively, using a 20 min fixation. The Abd-B primary antibody, obtained from the Developmental Studies Hybridoma Bank, was diluted 1∶4. Goat-anti-mouse secondary antibody, coupled to Alexa Fluor 488/555 X (Invitrogen AG), used to reveal Abd-B localization was used at 1∶500 dilution. Goat HRP coupled anti-mouse was obtained from Biorad and used at 1∶1'500 dilution. Staining with FM4–64 dye was done by placing a drop of the dye onto a microscope slide and placing a freshly dissected gland into it. The glands were immediately covered with a cover slip and visualized using fluorescent microscope at 555 nm.
Fertility/fecundity, receptivity, sperm counts, Western blotting, and sperm competition assays
In all assays, we used 3–5 day old virgin females from a wild type strain (Canton-S for fertility/fecundity assays, receptivity assays, sperm counts, and for Western blotting experiments; and cn bw for sperm competition assays). Females were placed singly in glass vials with food and allowed access to an iab-6cocu, control male (heterozygous for the iab-6cocu mutation), DTA-E, or WT Canton-S male. Pairs were watched to confirm that mating had occurred. The male was removed upon dismounting. All statistical analysis was performed with the Jmp9 software.
In the fertility/fecundity assays, after mating, individual females were housed for 24 hours in glass vials on yeast-glucose media. After 24 hours each female was transferred to a fresh vial, and the eggs laid in the previous vial were counted. This process was repeated for a total of 10 days. Upon eclosion, all progeny from each vial were counted. Hatchability (# progeny/# eggs) was calculated per day and across the 10-day period for each female. Values greater than 1 represent instances where the number of progeny produced exceeded the number of eggs observed. This is an accurate representation of counter error, and was not normalized to 1. Small levels of counter error has a greater impact on hatchability for females that lay few eggs. Comparisons of egg and progeny production between control and experimental females were performed using a Wilcoxon non-parametric test and statistics comparing the overall 10 day trends were performed using a repeated measures ANOVA.
Receptivity assays
After mating, individual females were kept in a vial on yeast-glucose media for 1 day, 4 days, or 10 days after the start of mating (ASM). Each female was then moved to a fresh vial and provided with a single Canton S male. After addition of the single Canton S male, couples were observed at 15 min time intervals for one hour, and the proportion of successful matings was recorded. The original vials were kept to check for progeny from the first mating. Females that did not produce viable progeny were discarded from the assay. Comparisons between the remating frequency of control and experimental females were conducted using a one way ANOVA.
Sperm counts
After mating females were either frozen in liquid nitrogen at 2 h ASM or kept in glass vials on yeast-glucose media for 4 days and 10 days ASM and then frozen. The female reproductive tracts were removed and stained with orcein (as described in [33], [86], [87]. Sperm were visualized and counted using a transillumination microscope at 1000× magnification. Comparisons between the number of sperm present in control and experimental females were performed using a Wilcoxon non-parametric tests.
Sperm competition assays
After mating, individual females were housed for 3 days on yeast-glucose media. Females were then allowed access to a single cn bw male for 7 hours. Couples were observed for the first 4 hours in 15 min time intervals to determine the percent remating. After 7 hours the cn bw male was removed and the females were transferred individually to fresh vials and allowed to lay eggs for 4 days. They were then transferred individually to fresh food vials and allowed to lay eggs for an additional 4 days. Progeny were collected from each vial and assessed for the presence of red eyes (control or iab-6cocu male sire) or white eyes (cn bw sire). P1 was calculated as # progeny sired by the first male/total progeny. Comparisons between the P1 of control and experimental females were performed using a one way ANOVA and by Wilcoxon non-parametric tests.
Western blots
Females were frozen in liquid nitrogen at 15′, 30′, 1 h, and 2 h time points ASM and stored at −20°C until dissection. For later time points (1–7 days), females were kept individually on yeast-glucose media at room temperature before being frozen in liquid nitrogen and stored at −20°C until dissection. Males were also frozen in liquid nitrogen and stored at −20°C until dissection. Preparation of protein samples and Western blot analyses were performed as in [38], [39] except that gels in this study were 5–15% acrylamide gradient gels and were run at 100v for 1.5–2 hours. Due to size differences in organs across males and the lack of an optimal loading control for male accessory glands, all comparative samples contain an identical number of reproductive tracts (male or female).
PNGase F assays were performed using reagents from New England Biolabs Inc. Male accessory glands from 10 iab-6cocu males and 10 iab-6cocu heterozygous control males were dissected in 1×PBS and transferred to 10 ul 1× Glycoprotein Denaturing Buffer (GDB). Samples were ground and heated in GDB for 10 min at 100°C. Then 2 ul 10×G7 Reaction Buffer, 2 ul 10% NP40, and 4 ul ddH2O were added. Each sample was split into 9 ul aliquots. 1 ul PNGase F was added to one aliquot and 1 ul ddH2O was added to the other. All samples were then incubated for 1 hour at 37°C and frozen at −80°C overnight. 10 ul SDS sample buffer was added to each sample and then the samples were boiled for 5 min at 100°C. Western blots were performed as previously described except that 10.6% acrylamide gels were used and run at 40 v for 16 h to ensure adequate separation.
Supporting Information
Zdroje
1. Sanchez-HerreroE, VernosI, MarcoR, MorataG (1985) Genetic organization of Drosophila bithorax complex. Nature 313 : 108–113.
2. KarchF, WeiffenbachB, PeiferM, BenderW, DuncanI, et al. (1985) The abdominal region of the bithorax complex. Cell 43 : 81–96.
3. LewisEB (1978) A gene complex controlling segmentation in Drosophila. Nature 276 : 565–570.
4. Sanchez-HerreroE (1991) Control of the expression of the bithorax complex genes abdominal-A and abdominal-B by cis-regulatory regions in Drosophila embryos. Development 111 : 437–449.
5. CelnikerSE, SharmaS, KeelanDJ, LewisEB (1990) The molecular genetics of the bithorax complex of Drosophila: cis - regulation in the Abdominal-B domain. Embo J 9 : 4277–4286.
6. MihalyJ, BargesS, SiposL, MaedaR, CleardF, et al. (2006) Dissecting the regulatory landscape of the Abd-B gene of the bithorax complex. Development 133 : 2983–2993.
7. MaedaRK, KarchF (2006) The ABC of the BX-C: the bithorax complex explained. Development 133 : 1413–1422.
8. KarchF, GalloniM, SiposL, GauszJ, GyurkovicsH, et al. (1994) Mcp and Fab-7: molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of Drosophila melanogaster. Nucleic Acids Res 22 : 3138–3146 Issn: 0305-1048.
9. GruzdevaN, KyrchanovaO, ParshikovA, KullyevA, GeorgievP (2005) The Mcp Element from the bithorax Complex Contains an Insulator That Is Capable of Pairwise Interactions and Can Facilitate Enhancer-Promoter Communication. Mol Cell Biol 25 : 3682–3689.
10. BusturiaA, BienzM (1993) Silencers in abdominal-B, a homeotic Drosophila gene. Embo J 12 : 1415–1425 Issn: 0261-4189.
11. HagstromK, MullerM, SchedlP (1997) A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax comple. Genetics 146 : 1365–1380.
12. HagstromK, MullerM, SchedlP (1996) Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev 10 : 3202–3215.
13. ZhouJ, AsheH, BurksC, LevineM (1999) Characterization of the transvection mediating region of the abdominal - B locus in Drosophila. Development 126 : 3057–3065.
14. ZhouJ, BaroloS, SzymanskiP, LevineM (1996) The Fab-7 element of the bithorax complex attenuates enhancer-promoter interactions in the Drosophila embryo. Genes Dev 10 : 3195–3201.
15. GyurkovicsH, GauszJ, KummerJ, KarchF (1990) A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. Embo J 9 : 2579–2585 Issn: 0261-4189.
16. BargesS, MihalyJ, GalloniM, HagstromK, MüllerM, et al. (2000) The Fab-8 boundary defines the distal limit of the bithorax complex iab-7 domain and insulates iab-7 from initiation elements and a PRE in the adjacent iab-8 domain. Development 127 : 779–790.
17. AvilaFW, SirotLK, LaFlammeBA, RubinsteinCD, WolfnerMF (2011) Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56 : 21–40.
18. SirotLK, LaFlammeBA, SitnikJL, RubinsteinCD, AvilaFW, et al. (2009) Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet 68 : 23–56.
19. KalbJM, DiBenedettoAJ, WolfnerMF (1993) Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc Natl Acad Sci U S A 90 : 8093–8097.
20. LiuH, KubliE (2003) Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A 100 : 9929–9933.
21. ChapmanT, BanghamJ, VintiG, SeifriedB, LungO, et al. (2003) The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci U S A 100 : 9923–9928.
22. PengJ, ChenS, BusserS, LiuH, HoneggerT, et al. (2005) Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol 15 : 207–213.
23. MerleJ (1968) [Ovarian function and sexual receptivity of Drosophila melanogaster after implantation of fragments of the male genital tract]. J Insect Physiol 14 : 1159–1168.
24. ChenPS, Stumm-ZollingerE, AigakiT, BalmerJ, BienzM, et al. (1988) A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell 54 : 291–298.
25. Garcia-BellidoA (1964) [the Secretion of Paragonia as a Stimulus for the Fecundity of Drosophila Melanogaster Females]. Z Naturforsch B 19 : 491–495.
26. XueL, NollM (2000) Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc Natl Acad Sci U S A 97 : 3272–3275.
27. BertramMJ, AkerkarGA, ArdRL, GonzalezC, WolfnerMF (1992) Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech Dev 38 : 33–40.
28. DiBenedettoAJ, HaradaHA, WolfnerMF (1990) Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory gland gene. Dev Biol 139 : 134–148.
29. Styger D (1992) Molekulare analyse des Drosophila melanogaster sex-peptid Gens. Zürich, Switzerland: University of Zürich.
30. HeifetzY, VandenbergLN, CohnHI, WolfnerMF (2005) Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci U S A 102 : 743–748.
31. ChapmanT, HerndonLA, HeifetzY, PartridgeL, WolfnerMF (2001) The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc Biol Sci 268 : 1647–1654.
32. IsaacRE, LiC, LeedaleAE, ShirrasAD (2010) Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci 277 : 65–70.
33. AvilaFW, Ravi RamK, Bloch QaziMC, WolfnerMF (2010) Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186 : 595–600.
34. RoginaB (2009) The effect of sex peptide and calorie intake on fecundity in female Drosophila melanogaster. Scientific World Journal 9 : 1178–1189.
35. KubliE (1992) The sex-peptide. Bioessays 14 : 779–784.
36. LaFlammeBA (2012) The Drosophila seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet 8: e1002435.
37. RamKR, WolfnerMF (2007) Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet 3: e238.
38. Ravi RamK, JiS, WolfnerMF (2005) Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol 35 : 1059–1071.
39. RamKR, WolfnerMF (2009) A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci U S A 106 : 15384–15389.
40. Ravi RamK, SirotLK, WolfnerMF (2006) Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci U S A 103 : 18674–18679.
41. IampietroC, GummallaM, MuteroA, KarchF, MaedaRK (2010) Initiator elements function to determine the activity state of BX-C enhancers. PLoS Genet 6: e1001260.
42. GalloniM, GyurkovicsH, SchedlP, KarchF (1993) The bluetail transposon: evidence for independent cis-regulatory domains and domain boundaries in the bithorax complex. Embo J 12 : 1087–1097 Issn: 0261-4189.
43. ZavortinkM, SakonjuS (1989) The morphogenetic and regulatory functions of the Drosophila Abdominal - B gene are encoded in overlapping RNAs transcribed from separate promoters. Genes Dev 3 : 1969–1981.
44. ForondaD, EstradaB, de NavasL, Sanchez-HerreroE (2006) Requirement of Abdominal-A and Abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development 133 : 117–127.
45. BischofJ, MaedaRK, HedigerM, KarchF, BaslerK (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104 : 3312–3317.
46. JiaoR, DaubeM, DuanH, ZouY, FreiE, et al. (2001) Headless flies generated by developmental pathway interference. Development 128 : 3307–3319.
47. PeiferM, KarchF, BenderW (1987) The bithorax complex: control of segmental identity. Genes Dev 1 : 891–898.
48. BenderW, HudsonA (2000) P element homing to the Drosophila bithorax complex. Development 127 : 3981–3992.
49. SimonJ, PeiferM, BenderW, O'ConnorM (1990) Regulatory elements of the bithorax complex that control expression along the anterior-posterior axis. Embo J 9 : 3945–3956 Issn: 0261-4189.
50. SinghPB, BrownD (1997) Modelling the activity of the Ultrabithorax parasegment-specific regulatory domains around their anterior boundaries. J Theor Biol 186 : 397–413.
51. WolfnerMF, HaradaHA, BertramMJ, StelickTJ, KrausKW, et al. (1997) New genes for male accessory gland proteins in Drosophila melanogaster. Insect Biochem Mol Biol 27 : 825–834.
52. MonsmaSA, HaradaHA, WolfnerMF (1990) Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev Biol 142 : 465–475.
53. ManningA (1967) The control of sexual receptivity in female Drosophila. Anim Behav 15 : 239–250.
54. YamamotoMT, TakemoriN (2010) Proteome profiling reveals tissue-specific protein expression in the male reproductive system of Drosophila melanogaster. Fly (Austin) 4 : 36–39.
55. WalkerMJ, RylettCM, KeenJN, AudsleyN, SajidM, et al. (2006) Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome Sci 4 : 9.
56. TakemoriN, YamamotoMT (2009) Proteome mapping of the Drosophila melanogaster male reproductive system. Proteomics 9 : 2484–2493.
57. FindlayGD, YiX, MaccossMJ, SwansonWJ (2008) Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol 6: e178.
58. ChowCY, WolfnerMF, ClarkAG (2010) The genetic basis for male x female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics 186 : 1355–1365.
59. ParkerGA (1970) Sperm competition and its evolutionary consequences in the insects. Bio rev 45 : 525–567.
60. ParkM, WolfnerMF (1995) Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev Biol 171 : 694–702.
61. MaleyF, TrimbleRB, TarentinoAL, PlummerTHJr (1989) Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180 : 195–204.
62. PlummerTHJr, TarentinoAL (1991) Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology 1 : 257–263.
63. de NavasL, ForondaD, SuzanneM, Sanchez-HerreroE (2006) A simple and efficient method to identify replacements of P-lacZ by P-Gal4 lines allows obtaining Gal4 insertions in the bithorax complex of Drosophila. Mech Dev 123 : 860–867.
64. AhmadSM, BakerBS (2002) Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109 : 651–661.
65. BienzM, SaariG, TremmlG, MullerJ, ZustB, et al. (1988) Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell 53 : 567–576.
66. FreelandDE, KuhnDT (1996) Expression patterns of developmental genes reveal segment and parasegment organization of D. melanogaster genital discs. Mech Dev 56 : 61–72.
67. DamenWG, TautzD (1999) Abdominal-B expression in a spider suggests a general role for Abdominal-B in specifying the genital structure. J Exp Zool 285 : 85–91.
68. DolleP, Izpisua-BelmonteJC, BrownJM, TickleC, DubouleD (1991) HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev 5 : 1767–1767.
69. WarotX, Fromental-RamainC, FraulobV, ChambonP, DolleP (1997) Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development 124 : 4781–4791.
70. van der HoevenF, SordinoP, FraudeauN, Izpisua-BelmonteJC, DubouleD (1996) Teleost HoxD and HoxA genes: comparison with tetrapods and functional evolution of the HOXD complex. Mech Dev 54 : 9–21.
71. KelshR, DawsonI, AkamM (1993) An analysis of abdominal-B expression in the locust Schistocerca gregaria. Development 117 : 293–305.
72. HuangHF, LiMT, Von HagenS, ZhangYF, IrwinRJ (1997) Androgen modulation of the messenger ribonucleic acid of retinoic acid receptors in the prostate, seminal vesicles, and kidney in the rat. Endocrinology 138 : 553–559.
73. ThomsonAA, MarkerPC (2006) Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation 74 : 382–392.
74. HendricksonJE, SakonjuS (1995) Cis and trans interactions between the iab regulatory regions and abdominal-A and abdominal-B in Drosophila melanogaster. Genetics 139 835–848Issn: 0016-6731.
75. HopmannR, DuncanD, DuncanI (1995) Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics 139 : 815–833 Issn: 0016-6731.
76. MinamiR, WakabayashiM, SugimoriS, TaniguchiK, KokuryoA, et al. (2012) The homeodomain protein defective proventriculus is essential for male accessory gland development to enhance fecundity in Drosophila. PLoS One 7: e32302.
77. GongWJ, GolicKG (2004) Genomic deletions of the Drosophila melanogaster Hsp70 genes. Genetics 168 : 1467–1476.
78. PattonJS, GomesXV, GeyerPK (1992) Position-independent germline transformation in Drosophila using a cuticle pigmentation gene as a selectable marker. Nucleic Acids Res 20 : 5859–5860.
79. LeeEC, YuD, Martinez de VelascoJ, TessarolloL, SwingDA, et al. (2001) A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73 : 56–65.
80. WarmingS, CostantinoN, CourtDL, JenkinsNA, CopelandNG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36.
81. SmithD, WohlgemuthJ, CalviBR, FranklinI, GelbartWM (1993) hobo enhancer trapping mutagenesis in Drosophila reveals an insertion specificity different from P elements. Genetics 135 : 1063–1076.
82. Drosophila protocols -Sullivan W, Ashburner M. and Scott H. (2000) Cold Spring harbor Laboratory Press: p353.
83. ApitzH, KambacheldM, HohneM, RamosRG, StraubeA, et al. (2004) Identification of regulatory modules mediating specific expression of the roughest gene in Drosophila melanogaster. Dev Genes Evol 214 : 453–459.
84. VenkenKJ, HeY, HoskinsRA, BellenHJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314 : 1747–1751.
85. Castelli-GairJ, GreigS, MicklemG, AkamM (1994) Dissecting the temporal requirements for homeotic gene function. Development 120 : 1983–1995.
86. NeubaumDM, WolfnerMF (1999) Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153 : 845–857.
87. MuellerJL, LinklaterJR, Ravi RamK, ChapmanT, WolfnerMF (2008) Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics 178 : 1605–1614.
88. MartinCH, MayedaCA, DavisCA, EricssonCL, KnafelsJD, et al. (1995) Complete sequence of the bithorax complex of Drosophila. Proc Natl Acad Sci U S A 92 : 8398–8402 Issn: 0027-8424.
89. SimonJ, ChiangA, BenderW (1992) Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development 114 : 493–505 Issn: 0950-1991.
90. DietzlG, ChenD, SchnorrerF, SuKC, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



