-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
We demonstrated previously that 75% of infertile men with round, acrosomeless spermatozoa (globozoospermia) had a homozygous 200-Kb deletion removing the totality of DPY19L2. We showed that this deletion occurred by Non-Allelic Homologous Recombination (NAHR) between two homologous 28-Kb Low Copy Repeats (LCRs) located on each side of the gene. The accepted NAHR model predicts that inter-chromatid and inter-chromosome NAHR create a deleted and a duplicated recombined allele, while intra-chromatid events only generate deletions. Therefore more deletions are expected to be produced de novo. Surprisingly, array CGH data show that, in the general population, DPY19L2 duplicated alleles are approximately three times as frequent as deleted alleles. In order to shed light on this paradox, we developed a sperm-based assay to measure the de novo rates of deletions and duplications at this locus. As predicted by the NAHR model, we identified an excess of de novo deletions over duplications. We calculated that the excess of de novo deletion was compensated by evolutionary loss, whereas duplications, not subjected to selection, increased gradually. Purifying selection against sterile, homozygous deleted men may be sufficient for this compensation, but heterozygously deleted men might also suffer a small fitness penalty. The recombined alleles were sequenced to pinpoint the localisation of the breakpoints. We analysed a total of 15 homozygous deleted patients and 17 heterozygous individuals carrying either a deletion (n = 4) or a duplication (n = 13). All but two alleles fell within a 1.2-Kb region central to the 28-Kb LCR, indicating that >90% of the NAHR took place in that region. We showed that a PRDM9 13-mer recognition sequence is located right in the centre of that region. Our results therefore strengthen the link between this consensus sequence and the occurrence of NAHR.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003363
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003363Summary
We demonstrated previously that 75% of infertile men with round, acrosomeless spermatozoa (globozoospermia) had a homozygous 200-Kb deletion removing the totality of DPY19L2. We showed that this deletion occurred by Non-Allelic Homologous Recombination (NAHR) between two homologous 28-Kb Low Copy Repeats (LCRs) located on each side of the gene. The accepted NAHR model predicts that inter-chromatid and inter-chromosome NAHR create a deleted and a duplicated recombined allele, while intra-chromatid events only generate deletions. Therefore more deletions are expected to be produced de novo. Surprisingly, array CGH data show that, in the general population, DPY19L2 duplicated alleles are approximately three times as frequent as deleted alleles. In order to shed light on this paradox, we developed a sperm-based assay to measure the de novo rates of deletions and duplications at this locus. As predicted by the NAHR model, we identified an excess of de novo deletions over duplications. We calculated that the excess of de novo deletion was compensated by evolutionary loss, whereas duplications, not subjected to selection, increased gradually. Purifying selection against sterile, homozygous deleted men may be sufficient for this compensation, but heterozygously deleted men might also suffer a small fitness penalty. The recombined alleles were sequenced to pinpoint the localisation of the breakpoints. We analysed a total of 15 homozygous deleted patients and 17 heterozygous individuals carrying either a deletion (n = 4) or a duplication (n = 13). All but two alleles fell within a 1.2-Kb region central to the 28-Kb LCR, indicating that >90% of the NAHR took place in that region. We showed that a PRDM9 13-mer recognition sequence is located right in the centre of that region. Our results therefore strengthen the link between this consensus sequence and the occurrence of NAHR.
Introduction
Several mechanisms have been proposed to cause genomic rearrangements, notably: Non Allelic Homologous Recombination (NAHR), Non Homologous End Joining (NHEJ), Fork Stalling and Template Switching (FoSTeS) and Break-Induced Replication (BIR) [1], [2]. NAHR takes place between duplicated sequences with a high sequence identity (usually >95%) located in different genomic regions of the same chromosome [3]. These paralogous sequences or Low Copy Repeats (LCR) tend to generate polymorphic regions with deleted and duplicated alleles called Copy Number Variants (CNVs). The consensual NAHR model predicts that recombinations between LCRs located on the same chromatid result in the production of a deleted allele and a small circular molecule that will be lost by the end of the cell cycle. Recombinations between LCRs located on two distinct chromatids (whether sister-chromatids or chromatids from homologous chromosomes) result in the production of a deleted allele and a complementary duplicated allele (Figure 1A). In consequence NAHR is expected to produce an excess of deletions over duplications. This has been verified for several NAHR hotspots using sperm typing assays: on average twice as many deletions as duplications were generated de novo [4]. One study however describes similar deletion and duplication frequencies at the 7q11.23, 15q11-q13 and 22q11.2 loci, suggesting a predominant inter-chromatid NAHR [5]. This study was carried out by fluorescent in situ hybridization (FISH) which allows the detection of all numerical anomalies occurring at these loci and not only the NAHR-mediated events. This could explain at least part of the discrepancy observed between the two studies, given that a recent study of the RAI1 locus suggested that complex genetic events generate an excess of duplications [6].
Fig. 1. Strategy and validation of the detection of DPY19L2 recombined alleles by PCR. 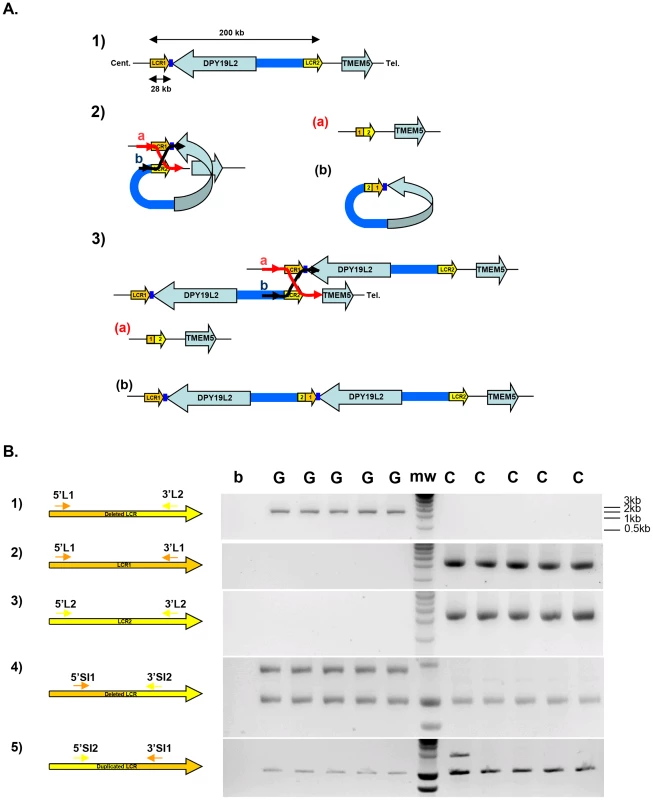
(A) Schematic representation of NAHR at the DPY19L2 locus. 1) LCR1 and LCR2 correspond to the centromeric and telomeric LCRs respectively. The two LCRs are separated by approximately 200 Kb and each measures 28 Kb. 2) NAHR can occur following the mis-alignment of Low Copy Repeats 1 and 2 located either on 1) the same chromatid and results in the production of a) a deleted allele with a recombined 1-2 LCR, and b) a small circular molecule with a recombined 2-1 LCR and the DPY19L2 gene. This small molecule will not survive through the cell cycle. 3) NAHR can occur following the mis-alignment from two distinct chromatids (whether sister-chromatids or chromatids from homologous chromosomes). This results in the production of a) a deleted allele with a 1-2 recombined LCR, and b) a complementary duplicated allele with a 2-1 recombined LCR. (B) Illustration of the specificity of the LCR-specific amplification when amplifying DNA from DPY19L2 homozygously deleted globozoospermic patients (G) and control individuals (C). 1) Primers specific to the deleted 1-2 LCR yield a 2088 nt fragment in globozoospermic patients only. 2,3) Specific amplification of LCR 1 and 2 is only obtained from non-deleted controls. 4) Co-amplification of a control locus (bottom band) with a deleted 1-2 LCR-specific sequence. 5) Co-amplification of a control locus (bottom band) with a duplicated 2-1 LCR-specific sequence. A duplicated allele is identified in one control individual (first lane after the molecular weight markers (mw)). As NAHRs events occur at fixed LCRs they tend to be recurrent, and the recombined alleles normally share a common size defined by the distance separating the two LCRs. It is well-established that meiotic recombination events, whether resulting in crossing over or producing unbalanced alleles through NAHR, are not uniformly distributed along the human genome but occur preferentially at specific hot spots [7]–[9]. Myers et al. (2008) have characterized a degenerate 13 bp sequence motif (CCNCCNTNNCCNC) that is present in approximately 40% of the identified human crossover hotspots. A three nucleotide periodicity was observed within and beyond the 13-mer core, suggesting a direct interaction with a motif binding protein [10]. Subsequent work strengthened this hypothesis as it has been proposed that PRDM9, a multi-unit zinc finger binding protein expressed mainly during early meiosis in germ cells [11], specifies hotspot usage by binding specifically to this 13 bp consensus motif [12]–[14]. PRDM9 was then shown to be highly polymorphic, different alleles seemingly providing preferred targeted recombination hotspots [14]. Berg et al. (2010) measured the recombination rate at ten crossover hotspots, five with a PRDM9 recognition motif, five without a clear motif. Men with the rarer N allele showed a heavy reduction (>30-fold) at all hotspots, even at those which did not contain an obvious PRDM9 motif [12]. Further work revealed that specific PRDM9 alleles activated different hotspots [15]. The direct correlation between PRDM9 recognition sequence and PRDM9 genotype however remains elusive, indicating that the rules governing the interaction between PRDM9 and its targeted sequences must be subtle and complex [12], [15].
CNVs and other unbalanced micro recombination events are involved in the aetiology of many human pathologies such as Alpha Thalassemia, Potocki-Lupski Syndrome, Charcot-Marie Tooth, Williams-Beuren syndrome, Prader Willi/Angelman syndrome, and infertility through the production of Y-chromosome microdeletions [16]. Here we focus on the DPY19L2 locus (12q14.2) which has recently been shown to be linked with Globozoospermia [17], a rare syndrome of male infertility [18] characterized by the presence of 100% round, acrosomeless spermatozoa in the patient's ejaculate (MIM #102530). Reports of familial cases pointed to a genetic component to this pathology [19]–[21], and this assumption was confirmed as a homozygous mutation of SPATA16 was identified in three siblings [22] and a homozygous missense mutation of PICK1 was identified in a Chinese patient [23]. We demonstrated recently that DPY19L2 was in fact the main locus associated with globozoospermia as 15 out of 20 analysed patients presented a 200 Kb homozygous deletion removing the totality of the gene [17]. DPY19L2 was described to have arisen, along with three other genes (DPY19L1, L3 and L4), through the expansion and evolution of the DPY19L gene family from a single ortholog found in invertebrate animals [24]. We then identified DPY19L2 point mutations and heterozygous deletions and demonstrated that 84% of the 31 globozoospermia patients analysed had a molecular alteration of DPY19L2 [25]. Others find a slightly lower incidence of DPY19L2 deletions in globozoospermia patients [26], [27]. Comparison of the spermiogenesis between wild type and Dpy19l2 knock out (KO) mice allowed us to demonstrate that Dpy19l2 is expressed in the inner nuclear membrane only in the section facing the acrosome, and that it is necessary to anchor the acrosome to the nucleus. This indicates that DPY19 proteins (DPY19L1-4 in mammals) might constitute a new family of structural transmembrane proteins of the nuclear envelope that likely participate in a function that was so far known to be only carried out by SUN proteins: constituting a bridge between the nucleoskeleton and cytoplasmic organelles and/or the cytoskeleton [28]. In our previous work we had demonstrated that DPY19L2 was homozygously deleted in a majority of patients with globozoospermia and that this deletion occurred by NAHR between two highly homologous 28 Kb LCRs located on each side of the gene [17]. Strengthening the case for the occurrence of NAHR at the DPY19L2 locus, heterozygous deletions and duplications have been identified in several large array CGH studies and this locus is classified as a CNV [29]–[33]. Surprisingly, considering that NAHR is known to generate an excess of deletions, these databases contain a large excess of duplications.
We developed a PCR assay to specifically amplify the recombined LCRs corresponding to deleted and duplicated alleles allowing the precise localisation of the breakpoints (BP). We observed that all identified BPs clustered in the center of the LCR. We analysed this region and identified a 13-mer PRDM9 pro-recombination sequences in the middle of the hotspot. We also developed a digital PCR assay that enabled us to estimate the rates of de novo deletion and duplication at this locus. Contrary to the allelic frequency observed in the general population we measured an approximate 2 fold excess of deletions over duplications. We show that the negative selection against the deleted alleles could explain this apparent paradox.
Results
Estimation of the DPY19L2 deleted and duplicated alleles' frequencies in the general population and assessment of the PCR assay's sensitivity
The DPY19L2 CNV was analysed using array CGH data available from web servers [29]–[33] for a total of 6575 control individuals, mainly from the Database of Genomic Variants (http://projects.tcag.ca/variation/). A total of 83 gains and 26 heterozygous losses are reported for the DPY19L2 CNV in this pool, indicating a threefold excess of duplications over deletions.
We wanted to confirm this result and exclude a potential technical bias towards duplications that could be caused by the presence on chromosome 7 of DPY19L2P1, a pseudogene highly homologous to DPY19L2 [24]. To this end we re-analysed the array CGH data produced for the diagnosis of syndromic mental retardation in Grenoble and Lyon hospitals, and searched for DPY19L2 deleted and duplicated alleles in this dataset. A total of 1699 array CGH profiles were re-analysed (see Figure S1 for illustration). We identified a total of 15 duplications and 3 heterozygous deletions. The recombined alleles were secondarily amplified with the long PCR primers to confirm the validity of the array CGH results. Presence of the deletion could be confirmed by our deletion-specific PCR in the three individuals putatively carrying a heterozygous deletion. DNA from 3 individuals expected to carry a duplicated allele could not be obtained. Ten out of the 12 remaining individuals putatively carrying a DPY19L2 duplication were amplified by our duplication-specific PCR. For the two individuals that could not be amplified, the duplication was nevertheless confirmed by Multiplex Ligation-dependent Probe Amplification (MLPA). Theses results show that our reanalysis of the array CGH data did not yield any false positives. Overall reanalysis of these 15 individuals showed that 2 out of 15 recombinant alleles could not be detected by our PCR assay, indicating that the breakpoints of 2/15 recombined alleles fell outside of our amplified region.
We also wanted to obtain an estimation of the frequency of the deleted and duplicated alleles in the general population using our recombination-specific PCR assay. For that we designed primers that amplified a smaller sequence which could be co-amplified with an additional pair of primers (RYR2 primers) used as a positive amplification control (Figure 1B and Table S1). This duplex PCR setup controls for poor DNA quality or technical variations. We analysed 150 control individuals originating from North Africa and 150 individuals of European origin with these two duplex PCRs (for the detection of deleted and duplicated LCRs, respectively). We identified only one heterozygous deletion in an individual of North African origin and two duplications in one European and in one North African individuals.
Overall a total of 8574 individuals have been analysed, including 6575 individuals from array CGH public databases, 1699 individuals from Grenoble-Lyon array CGH data and 300 individuals analysed by recombination-specific PCR. From these cohorts we identified 30 deletions (frequency of approximately 1/290) and 100 duplications (approximate frequency 1/85) (Table S2). These values indicate that the allele frequencies of the recombined deleted and duplicated alleles are 1.7×10−3 (95% CI: 1.2×10−3; 2.5×10−3) and 5.8×10−3 (95% CI: 4.7×10−3; 7.1×10−3), respectively. Confidence intervals (CI) were calculated assuming a binomial model, with binom.test in R.
We note that our PCR-based assay only allows the identification of breakpoints occurring between the selected primers (1392 bp). The location of the breakpoints of each CNV detected by array CGH (an unbiased approach) located in the DPY19L2 locus was scrutinised to establish if they were located within the LCR and hence were caused by NAHR (Table S3). This analysis shows that 87% of the deletions and 76% of the duplication fell within the LCR limits.
Overall, we believe that our PCR assay permits to identify the majority of recombinations occurring at the DPY19L2 locus, since: 1) amplification was obtained for all 15/15 globozoospermia patients analysed, and 2) amplification was obtained for 13/15 (87%) recombined array CGH patients.
Determination of DPY19L2 de novo recombination rates by digital PCR
As the previous results consistently showed an excess of duplications over deletions in the general population, we wanted to measure the rates of de novo duplications and deletions to verify if the observed skew was due to the selection of duplications over deletions or if more duplications were produced de novo. The rate of genetic events occurring de novo can be measured on sperm DNA since each spermatozoon is the product of meiosis and corresponds to a new haploid genome. We first tried to develop a semi-quantitative PCR assay to directly measure the frequencies of deletions and duplications using sperm from control donors (with two copies of DPY19L2). The shortest fragment that could provide a reliable specific amplification and amplify the whole breakpoint area was 1392 nt long. Reliable quantitative PCR for fragments longer than 500 nt is difficult with current techniques. We therefore resorted to performing a digital PCR. First, the DNA was serially diluted and distributed in 96-well plates so that approximately 25% of the wells produced an amplicon. The appropriate quantity of sperm DNA was determined by trial experiments for each of the two PCR assays: 50 ng of sperm DNA per well (corresponding to approximately 17,000 copies of chromosome 12, assuming one haploid genome represents 3 pg of DNA) were used for the PCR specific of the DPY19L2 deletion, and 100 ng per well (∼33,000 copies under the same assumption) were used for the duplication-specific PCR.
For example, for donor A the deletion-specific PCR produced 26 positive wells. The deletion recombination frequency λ and its 95% confidence interval were then calculated as described (see Methods), resulting in a rate of de novo DPY19L2 deletion for donor A estimated at 1.9×10−5 (95% CI: 1.3×10−5; 2.7×10−5). Similarly, the duplication-specific PCR for donor A produced 23 positive wells, but because there was twice as much starting DNA this results in a rate of de novo DPY19L2 duplication estimated at 8.1×10−6 (95% CI: 5.3×10−6;1.2×10−5) for this donor (Table 1 and Figure 2).
Fig. 2. Rate of de novo deletion and duplication events occurring at the DPY19L2 NAHR hotspot determined by digital PCR on sperm from 3 control donors. 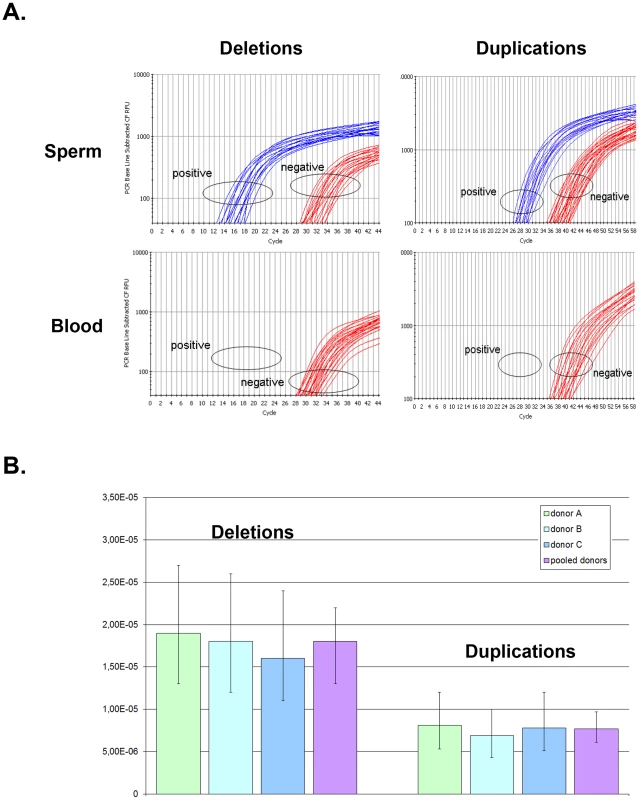
(A) Illustration of PCR results obtained by real time PCR. The left plots show amplification profiles obtained with primers specific to the recombined deleted LCR, the right plots show profiles obtained with the duplication-specific primers. No amplification was observed with either pairs of primers from 200 ng of somatic (blood) DNA, indicating that the NAHR did not occur during mitosis. Sperm DNA was diluted in order to obtain a positive amplification in approximately 25% of the wells. (B) The number of positive wells allowed estimating the frequency of de novo deletion and duplication events in three control sperms. Error bars represent 95% CIs. Tab. 1. Frequency of deleted and duplicated alleles in sperm from three control donors. 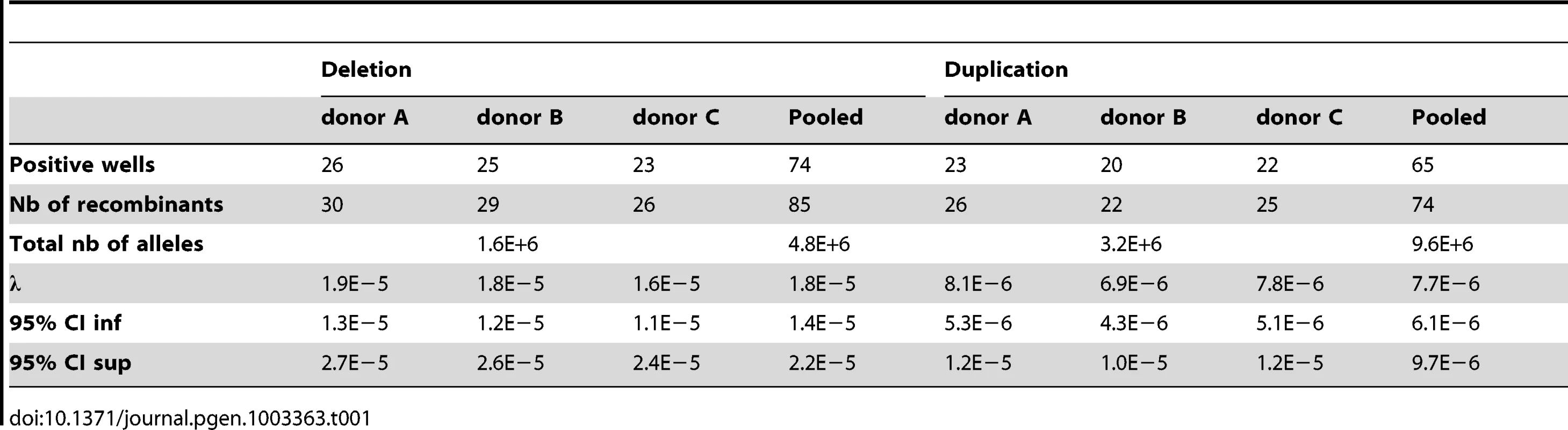
When pooling the results from the three sperm donors, more robust estimates are obtained: the de novo DPY19L2 deletion rate is estimated at 1.8×10−5 (95% CI: 1.4×10−5; 2.2×10−5), while the de novo duplication rate is estimated at 7.7×10−6 (95% CI: 6.1×10−6; 9.7×10−6) (Table 1). There is a significant approximately two-fold enrichment of deletions over duplications at the DPY19L2 NAHR hotspot.
We investigated whether differential amplification efficiency between the deletion and duplication assays could explain the observed difference between deletion and duplication de novo rates. To this end, we performed a control experiment as described (see Methods). No significant difference in amplification efficiency was observed: the deletion-specific control PCR amplified 37 wells, and the duplication-specific PCR amplified 40 wells.
Precise localisation of the recombined allele's breakpoints
Amplification of the LCRs in the deleted alleles had not been achieved in our previous study and the breakpoint minimal region had only been narrowed down to a 15 Kb region within the LCRs (8). Here we designed and validated PCR primers that amplify a 2 Kb product in deleted individuals only (Figure 1B). We quickly realised that mapping the breakpoints was complicated by the fact that many of the nucleotides that differed between LCR1 and LCR2 in the reference sequence were in fact not specific to one or the other LCR. Since mapping the breakpoints requires markers specific to each LCR, we decided to amplify and sequence the 2 Kb breakpoint region for each LCR in 20 control individuals. To achieve the specific amplification of LCR 1 and 2 we had to rely on the reference human genome sequence to design the primers. We had no way of confirming that the targeted LCRs were specifically amplified in control individuals, but no amplification was obtained when assaying twenty homozygous deleted patients, vouching for the specificity of the primers. We then amplified and sequenced LCR1 and 2 from a total of 20 control individuals: 10 of North African origin and 10 of European origin. Thirty-four nucleotides were indicated as specific to either LCR 1 or 2 in hg19 reference sequence but 14 of these were in fact arbitrarily found in the two LCRs (Table S4): we consider that these are non-LCR-specific single nucleotide polymorphisms (SNPs). The remaining 20 nucleotides were indeed LCR-specific: these 20 fixed markers were used to map the recombination breakpoints, and we used the 14 SNPs to establish a haplotype map of the patients' deleted alleles (Table S4).
Allele-specific amplification of the deleted LCR was carried out on 15 homozygously deleted globozoospermia patients. Each amplification yielded a single 2088 bp product, while the PCR was negative for all the healthy controls tested (n = 20). We sequenced all the amplicons in order to better characterize the breakpoint region. Fourteen out of the 15 patients analysed were homozygous for all markers tested. Three different breakpoints (BPs) were identified based on the presence of the 20 invariant markers. The three recombination events (BP1–3) were included in a 1153 bp maximal region (Table S4 and Figure 3). The breakpoints could not be mapped more accurately for lack of nucleotides specific to each LCR. One patient was heterozygous for markers 13 and 14, indicating that this patient was heterozygous and carried two different deleted alleles (BPs 2 and 3). If we consider that the other 14 patients carried two recombined deleted alleles each, we have a total of 14 alleles with BP1 (between markers 17 and 18), 13 alleles with BP2 (between markers 18 and 24) and 3 with BP3 (between markers 25 and 28) (Figure 3B). The 14 identified SNPs were then used to map the different haplotypes in patients presenting the same breakpoint (Table S4). This shows the presence of a total of 7 distinct haplotypes, indicating that at least 7 recombination events are at the origin of our patients' pathology (15 patients). We also observe that 5 patients with BP2 have the same haplotype and that two groups of 3 patients with BP1 have the same haplotype, suggesting the presence of several founding deletions in our patients' population. This is not surprising as all our patients came from the same region (Tunis area) and a majority had related parents (often first cousins).
Fig. 3. Details of the DPY19L2 LCR1 and 2 and of the NAHR hotspot. 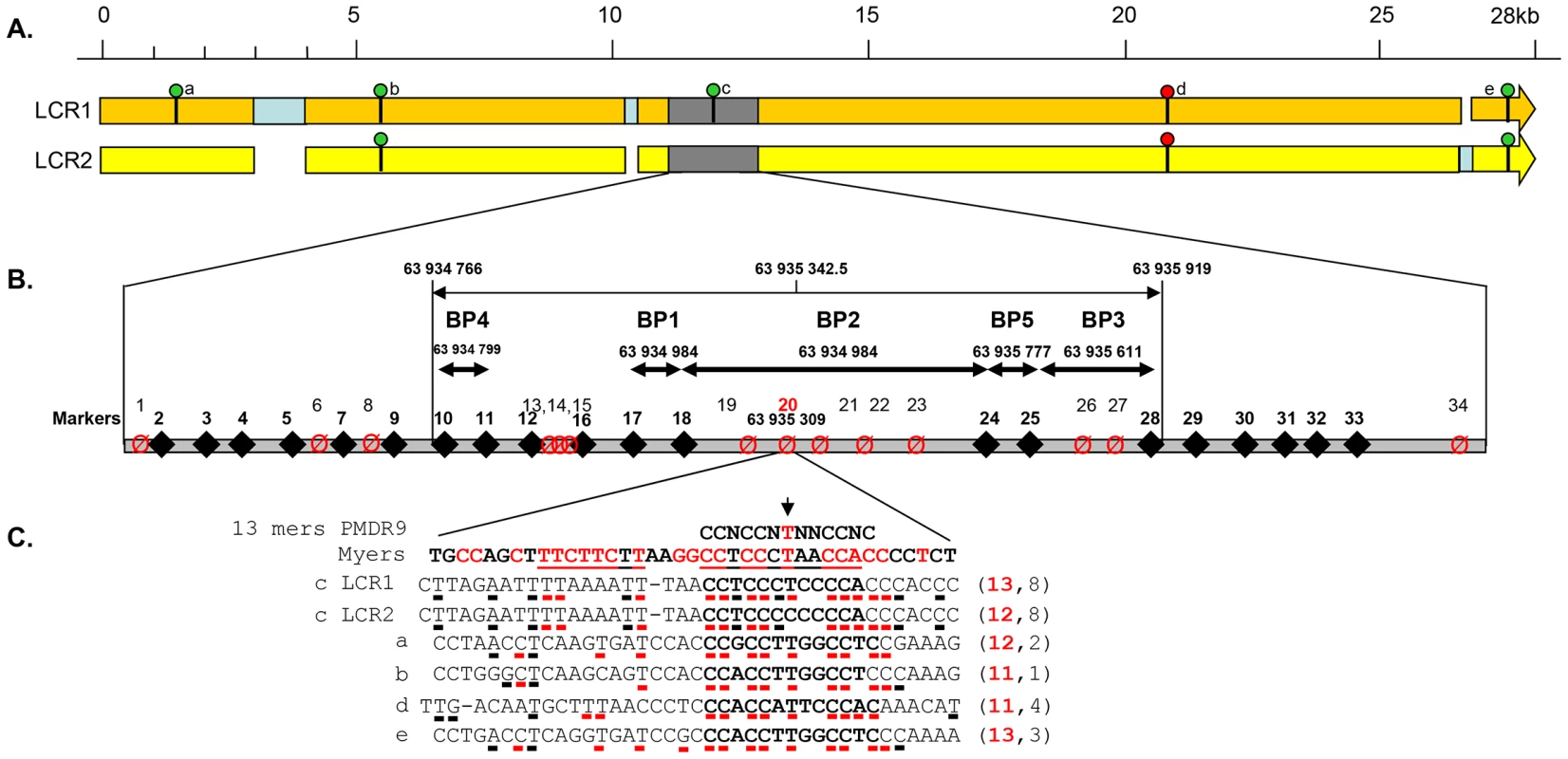
(A) Detailed scaled representation of the 28.2 Kb LCR 1 (orange) and 27 Kb LCR2 (yellow). Pale blue rectangles correspond to sequences specific to one of the LCRs facing a gap in the other LCR. The presence of a 13 bp consensus PRDM9 recognition site (CCNCCNTNNCCNC) on LCR1 or LCR2 is indicated by a green circle when identified on the forward DNA strand and by a red circle when identified on the reverse strand (GTGGNNAGGGTGG). The LCR arrows point toward the chromosome 12 telomere. (B) The analysed recombination region is represented in grey. The positions of LCR-specific markers (diamonds and bold numbering) and variable nucleotides (crossed circles) are represented. Details of the markers' sequences and localisations are indicated in Table S2. The five identified breakpoints (BP1–BP5) are shown as double arrows. One PRDM9 consensus sequence is localised in the centre of BP2, the central and most frequent breakpoint. (C) The central nucleotide from the consensus sequence corresponds to one of the identified SNPs (snp 20). A perfect match for the consensus sequence is present on LCR1, while the central thimine is replaced by a cytidine in LCR2. The 39 nt surrounding the 5 matches to the PRDM9 consensus sequence identified in LCR1 and 2 (sites a–e) are compared with the consensus sequence described in Myers et al [8], [11]. Highly conserved nucleotides are red. For each locus the number of nucleotides identical to the consensus sequence is indicated on the right. One and three deletions were identified respectively in the 300 individuals analysed by PCR and in the 1699 Grenoble-Lyon array CGH patients group. There were 3 occurrences of BP2 and 1 of BP3. Overall, including the globozoospermia patients, a total of 34 somatic deleted alleles were examined, resulting in the detection of three different recombination breakpoints. Fourteen alleles (41.2%) had a deletion between markers 17 and 18 (BP1), 16 alleles (47.0%) between markers 18 and 24 (BP2), and 4 alleles (11.8%) were recombined between markers 25 and 28 (BP3) (Figure 4 top left).
Fig. 4. Distribution of deleted and duplicated breakpoints observed from somatic DNA (left two panels) and sperm DNA (right two panels). 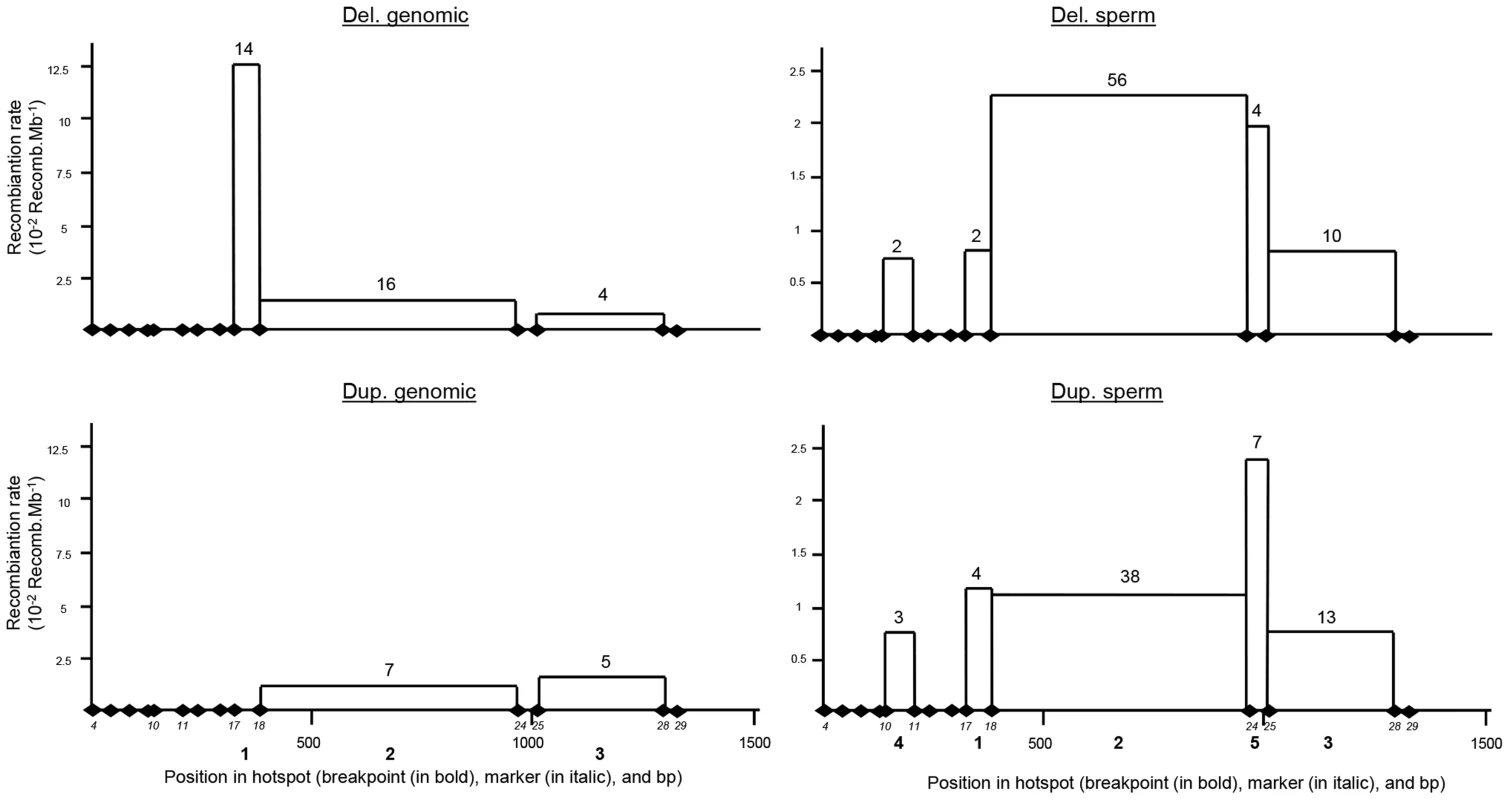
Somatic deletions were identified from sequence analysis of 15 homozygous deleted patients and two heterozygous deleted control individuals. Somatic duplications were identified from 12 positive control individuals. Data from sperm were pooled from three control donors. Two and fifteen genomic duplicated alleles were detected respectively in the 300 control individuals analysed by PCR and in the Grenoble-Lyon array CGH patients. Only 12 duplicated alleles could be sequenced (for lack of DNA from 3 control subjects and because two of the subjects had breakpoints falling outside the range of the duplication-specific PCR). Seven alleles (58.3%) corresponded to the reciprocal alleles of deletion 2 (BP2) with a recombination between markers 18 and 24, and 5 alleles (41.70%) corresponded to the reciprocal alleles of deletion 3 (BP3) with a recombination between markers 25 and 28 (Figure 3B).
The position of the meiotic recombination events (deletion and duplication) obtained from three sperm donors were also characterized by DNA sequencing. A total of 74 de novo deleted alleles and 65 de novo duplicated alleles were sequenced. All recombination events (from both duplications and deletions) clustered into five breakpoints (Figure 4). Two of them are new (BP4 and BP5) i.e. not previously identified in globozoospermic patients or in the CGH control cohort. The number and percentages of deleted and duplicated breakpoints respectively are: BP1 : 2 (2.7%) and 4 (6.1%); BP2 : 56 (75.7%) and 38 (58.5%); BP3 : 10 (13.5%) and 13 (20%); BP4 : 2 (2.7%) and 3 (4.6%) and BP5 : 4 (5.4%) and 7 (10.8%) (Figure 4). BP2 is by far the most frequent BP, followed by BP3, explained by the fact that these two breakpoints correspond to the largest regions. Interestingly in sperm, the distributions of the deleted and duplicated breakpoints are quite similar. This is logical as the duplicated alleles are expected to be the reciprocal alleles of some of the deleted alleles. In genomic DNA the correlation is not as good, and we note that the frequency of the deleted BP1 is particularly high. Most of the deleted alleles come from globozoospermia patients (and a few detected in CGHarray patients) most of whom were recruited in Tunis. As suggested by the shared haplotypes observed between some deleted patients (Table S4) a founder's effect is likely to account for some of the most frequent deletions, in particular BP1.
PRDM9 genotyping of the sperm donors
Sequencing of the PRDM9 ZF array was performed in the 3 sperm donors. All three donors were homozygous for the A allele which represents over 90% of the European alleles. It comprises 13 copies of the 84-bp ZF repeat that binds the 13-bp Myers recombination motif [12], [14]. This result is concordant with the ethnicity of the donors.
Detailed analysis of LCR1 and 2
A comparison of the two LCRs is presented in Figure 3A. The illustration was produced from the results of a megablast search (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM). All identified recombined alleles (n = 185) cluster between markers 10 and 28 within a 1153 bp region. This recombination hotspot is roughly located in the middle of the 28 Kb LCR (Figure 3A). Five 13 bp PRDM9 consensus recognition sites (CCNCCNTNNCCNC) are present along the LCR (Figure 3A). One of these sites is located in the centre of the 1153 bp hotspot (less than 35 nt away from the hotspot median position) (Figure 3B). We note that the most central BP (BP2) which encompasses the 13 bp site, represents 117 out of 185 recombined alleles or 63% of the detected recombined alleles (Figure 4). Given that five PRDM9 consensus recognition sites are found within the 28 Kb LCR1 sequence, the probability that a site would occur by chance less than 35 bp away from the centre of the hotspot is 1−(1−5/28000)70 = 0.012.
The Thymin at the centre of the consensus recognition site (CCNCCNTNNCCNC) was present only in the reference sequence of LCR1. Sequence analysis of our control individuals showed that this nucleotide was in fact a SNP (Marker 20 in Figure 3 and Table S4) with a T allele frequently found in both LCR1 and LCR2 (Table S4). In our globozoospermia patients we observed that all patients with the BP1 (with marker 20 located after the breakpoint thus on LCR2 sequence) have the T allele, indicating the presence of a T allele on LCR2 of the original unrecombined allele (Table S4). Conversely patients with BP3 (with marker 20 located before the breakpoint thus on LCR1 sequence) have the C allele indicating the presence of a C allele on LCR1 of the original unrecombined allele. All patients with BP2 have the C allele. As marker 20 is located within the breakpoint maximal sequence we can only conclude that at least a C allele was present on either LCR1 or LCR2 of the original unrecombined allele.
We sequenced LCR1 and LCR2 of our three sperm donors and realised that all where homozygous for the C allele at both LCR1 and LCR2, suggesting that the presence of the thymine in the CCNCCNTNNCCNC consensus sequence is not necessary to initiate recombination in the DPY19L2 LCR central region. Myers et al. [8], [11] indicated that although the core 13-mer recognition sequence was associated with recombination hotspots, the recognition motif extended beyond the core sequence with preferentially associated nucleotides identified within a 39 bp sequence encompassing the PRDM9 core sequence. We therefore aligned this extended motif with the sequence of the 5 PRDM9 motifs identified within the LCR (Figure 3C). We observe a good correlation within all 5 sequences, especially for the nucleotides that had been shown to be significantly associated with hotspots (indicated in red in Figure 3C). We also observe that the sequence central to our recombination hotspot (motif c) presents the highest homology (53%) with Myers' extended recognition sequence (Figure 3C).
Discussion
It appears paradoxical that de novo deletions are produced twice more frequently than de novo duplications during meiosis, while duplicated alleles are three times more frequent than deleted alleles in the general population. We investigated whether this could be explained parsimoniously through the combined effects of selection and mutation. Men carrying a homozygous deletion of DPY19L2 are 100% infertile, but currently there is no evidence that a heterozygous deletion of DPY19L2 causes a phenotype or that homozygous women are affected. Additionally, the deleted allele is rare. Under these assumptions, according to the General Selection Model (GSM), natural selection results in a decrease in the frequency of the deleted allele of approximately q2/2 per generation, where q is the frequency of the deleted allele (see Methods). Given that the deleted allele has a frequency of 1.7×10−3 (95% CI: 1.2×10−3; 2.5×10−3) in the general population according to our combined control data, the GSM predicts that this frequency decreases by 1.5×10−6 (95% CI: 7×10−7;3.1×10−6) per generation. Conversely, deleted alleles are produced de novo by NAHR at an estimated rate of 1.8×10−5 (95% CI: 1.4×10−5; 2.2×10−5) according to our digital PCR data. Assuming the allele frequency is at an equilibrium, these two rates should balance out. In fact they are somewhat similar but the 95% confidence intervals do not overlap. However the CIs only represent the uncertainty induced by the sampling procedure, i.e. the fact that the allele frequency and recombination rate are estimated from a sample of the whole population: they do not take into account experimental biases or imperfections that may exist at various steps. In addition, the GSM is a theoretical model that assumes an infinite population size and panmixia, whereas in practice stochastic effects and population structure (including for example any potential consanguinity or local founder effects) come into play. These could result in a significantly increased impact of purifying selection on the deleted allele, so that the frequency decrease resulting from selection and the de novo production of deleted alleles through NAHR may in fact cancel out.
Alternatively, it is possible that heterozygously deleted men suffer a fitness penalty. This can be taken into account within the GSM, and one can calculate the relative fitness of heterozygous individuals such that the GSM-predicted decrease of the deleted allele's frequency compensates the measured NAHR-induced production of new deleted alleles. In fact, assuming women are not affected, a 98% relative fitness of heterozygous men is sufficient (see Methods). Such a small effect could have easily remained undetected, and this scenario cannot be ruled out. This potential selection could be caused by meiotic segregation distortion as was observed for the T/t mouse locus [34]. Finally we only studied the recombination rate in male germ cells and we cannot exclude the possibility that the frequency and ratio of deletion and duplication might be different in female gametes.
All in all we believe the rates are reconcilable: whether the discrepancy observed when assuming heterozygous individuals have no phenotype is due to imperfections in the data and/or to population structure which disrupts the theoretical GSM model, or whether heterozygously deleted men suffer a small fitness penalty, we propose that the frequency decrease due to purifying selection and the de novo production of deleted alleles through NAHR cancel out, and that the frequency of the deleted DPY19L2 allele is today at a selection-recombination equilibrium in the population. On the other hand, to the best of our knowledge there is no evidence that the duplicated DPY19L2 allele is either deleterious or advantageous. We therefore assume that the duplicated DPY19L2 allele is not under selection, so its frequency can increase in the population by recurrent NAHR. This resolves the paradox.
Liu and colleagues (2011) proposed that the frequency of NAHR occurring between two paralogous LCRs was proportional to the LCR length and sequence homology but inversely proportional to the distance between the LCRs [6]. The authors logically proposed that the probability of ectopic chromosome synapsis increases with LCR length, and that ectopic synapsis is a necessary precursor to ectopic crossing-over. Here we measured that the average rate of de novo recombination (deletion plus duplication) by NAHR at the DPY19L2 recombination hotspot was 2.6×10−5. This rate is higher than what was measured at other loci such as the Williams-Beurren syndrome (WBS) locus or the LCR17p locus [4]. In our case the relatively small LCR size (28 Kb) is compensated by the proximity of the repeats (200 Kb) compared with much greater distances separating the paralogous LCRs for WBS and LCR17p. DPY19L2 LCR1 and 2 also present a very high sequence identity (98%) which could also reinforce their synapsis and recombination. Our results are in agreement with previous work suggesting that the distance separating the two LCRs, as well as their sequence homology and length are parameters likely influencing recombination frequency.
We observed that >90% of DPY19L2 NAHR events occurred within a 1.2 Kb region located in the centre of the 28 Kb LCR, suggesting the presence of a pro-recombination sequence within this hotspot. Myers et al. (2008) have characterized a degenerate 13 bp sequence motif that is present in approximately 40% of the identified human hotspots and which constitutes a PRDM9 recognition signal [12]–[14]. PRDM9 codes for a zinc finger array which catalyses the trimethylation of the lysine 4 of histone H3 (H3K4me3) [11]. This PRDM9-mediated post-translational histone modification likely initiates the recruitment of the recombination initiation complex, creating a favourable chromatin environment and allowing access of SPO11 to the DNA. SPO11 then initiates the formation of double-strand breaks (DSBs) which will be repaired by homologous recombination [35]. Here we identified a hotspot of NAHR located in the centre of a 28 Kb LCR. We showed that a PRDM9 13-mer recognition sequence is present at the epicentre of all the identified breakpoints. We however realised that the thymine, central to the 13-mer motif (CCNCCNTNNCCNC), was a T/C SNP, each nucleotide being found arbitrarily within LCR1 or LCR2. Following this observation one can wonder if recombination events at the DPY19L2 hotspot occur preferentially in the presence of fully matching PRDM9 13-mer alleles. We measured the frequency of de novo recombination in sperm from three donors. As it happens, sequencing revealed that all three were homozygous for the C allele on both LCR1 and LCR2. This indicates that, at this locus, the presence of the 13-mer exact match is not necessary to initiate recombination. This observation is concordant with what was described previously at different loci and confirms that PRDM9 tropism for the 13-mer recognition site might not be very strong and/or that other mechanisms also intervene in the choosing of double strand break localization [12], [15]. One explanation can come from the extended sequence surrounding the 13-mer motif. Myers and colleagues (2008) [10] described a 39 bp pro-recombination sequence encompassing the 13-mer motif. We observe a greater than 50% sequence identity for the complete 39-mer sequence, indicating that a good match to the extended motif might be at least as important as a perfect match of the core 13-mer motif.
We identified a total of 5 distinct breakpoints (BP), all localized within a 1.2 Kb region located in the centre of the 28 Kb LCR. Others have described the localization of the deletions of globozoospermia patients [22]. They described a total of 9 separate BPs in the DPY19L2 LCR. Looking at the precise localizations of the described BPs, we noticed that the nucleotides used to delimit BPs 1–6 in that study are in fact nucleotides that we identified as SNPs (markers 19–23 and 26), which strongly questions the validity of the BP localization in that study. Reanalyzing the presented data and using LCR-specific markers only, we conclude that Elinati et al. (2012) BPs 1, 2, 4, 5, 6 fall within the boundaries of “our” BP2 and that “their” BP3 corresponds to “our” BP3. This illustrates the difficulty in precisely identifying the localization of BPs and demonstrates that this can only be achieved with a high level of confidence after confirmation that the markers used to define the BP positions are indeed locus-specific. From our reanalysis, Elinati et al. (2012) identified deletions in 27 globozoospermia patients, 23 had our BP2, one had BP3 and one had a BP that fell just outside of our studied region. These results thus confirm the importance of the recombination hotspot described here. Two additional BPs (BP8 and 9) were also identified in Elinati's study which fell well outside of our recombination hotspot. This might constitute a second, less frequent recombination hotspot within the LCRs. We noticed that these two BPs are located 1200 bp telomeric from the 13-mer PRDM9 site d (as indicated in Figure 3A). Thus this second putative hotspot is further away from a consensus 13-mer motif than our hotspot (the greatest distance of the BPs we identified from the 13-mer is 600 bp), but we can question again the accuracy of the positioning of these two breakpoints. Here, while analyzing the array CGH recombined patients we identified two recombined alleles which did not fall within our studied BP area. It is possible that these recombination events are also located within this second putative hotspot.
With the DPY19L2 locus we believe that we have a good model to study the effect of the PRDM9 recognition site on NAHR. We plan to accurately position the yet uncharacterized BPs in relation to other PRDM9 sites. We are also currently screening an anonymized sperm bank to identify donors that are homozygous for the central 13-mer PRDM9 recognition T allele and/or who present rarer PRDM9 alleles to investigate how the recombination rate is affected by both the PRDM9 genotype and the extended PRDM9 recognition motif. We believe that although much work remains to be done, our study illustrates and consolidates the hotspot models described previously. In a moving environment we can imagine that the central region of the LCR will have the most opportunities to synapse with its paralogous sequence. The presence of an extended PRDM9 recognition motif in the centre of the LCR then very likely contributes to DSB and NAHR. The combination of these parameters therefore probably explains why approximately 90% of the breakpoints occurred within a few hundred nucleotides from the most centrally located PRDM9 recognition site.
Materials and Methods
Ethics statement
All patients, family members and anonymous DNA and sperm donors gave their written informed consent, and all national laws and regulations were respected. Ethical approval was obtained from Grenoble CHU review board.
Information on patients and control individuals
We previously reported that 15 out of 20 patients with globozoospermia had a homozygous deletion of the DPY19L2 region [17]. These patients are included in this study. All patients are unrelated apart from two who are brothers. All patients originated from North Africa (Tunisia, n = 12; Morocco, n = 2 and Algeria, n = 1).
Array CGH data from a total of 1699 control anonymous individuals were re-analysed. These analyses had been carried out as a diagnosis for syndromic mental retardation either at Grenoble or Lyon's hospital. As our aim was to identify DPY19L2 centred CNVs in this cohort of patients and since there is no known link between DPY19L2 and mental retardation, we believe that this cohort can serve as a control in this study. All individuals agreed to the anonymous use of their DNA in genetic studies and signed an informed consent. The fertility and ethnic origin of these individuals was not documented. All were French citizens. We estimate that in excess of 90% of these individuals are of European origin and that the vast majority of the others are of North African origin.
There was no gender selection but this cohort contained approximately 2/3rd of males.
Array CGH results from these patients were scrutinized for the DPY19L2 region.
Three hundred control individuals were analysed independently with recombinant DPY19L2-specific PCR (deleted and duplicated) to identify deleted and duplicated alleles. One hundred and fifty individuals originated from North Africa (Algeria, Morocco, and Tunisia) and 150 originated from Europe. All individuals gave their informed consent to constitute an anonymous DNA bank. Non-recombined LCR1 and 2 of twenty of these individuals were amplified and sequenced to identify LCR-specific SNPs. There was no gender selection and this cohort contained a similar number of males and females.
Lastly the DPY19L2 CNV was also analysed from array CGH data available from web servers [29]–[33] for a total of 6575 control individuals, mainly from the Database of Genomic Variants (http://projects.tcag.ca/variation/). Most of these individuals originated from Europe (75%), Africa (18%) or Asia. Individual CNV could however not be linked to a particular individuals and its geographical origin. The location of the breakpoints of each CNV located in the DPY19L2 locus was scrutinised to establish if they were located within the LCR and hence were caused by NAHR (Tables S2 and S3).
DNA extraction
Genomic DNA was extracted either from peripheral blood leucocytes using a guanidium chloride extraction procedure [36] or from saliva using Oragene DNA Self-Collection Kit (DNAgenotech, Ottawa, Canada).
Sperm DNA was extracted from 2 ml of semen which were transferred to a 25 ml Falcon Tube (BD Biosciences). Ten ml of PBS was added, mixed gently and centrifuged at 3,000 rpm for 5 minutes. Supernatant was discarded and the pellet was resuspended again in 10 ml of PBS, mixed and centrifuged as before. Pellets were then resuspended in 1 ml digestion buffer (NTE buffer 0.5 mM NaCl, 10 mM Tris-HCl pH 7.5, 5 mM EDTA, pH 8 (100∶10∶1), 0.4% SDS), 25 µl of 10 mg/ml proteinase K solution (Sigma) were added and the mix was incubated overnight at 42°C with occasional mixing. Three hundred microliters of the contents of each Falcon tube were transferred into SafeLock tubes (Eppendorf). An equal volume of phenol/chloroform/isoamyl alcohol (25∶24∶1) was added and mixed gently until emulsified. The tube was centrifuged at 3,000 rpm for 5 minutes. We repeated this process a second time, adding an equal volume of chloroform/isoamyl alcohol. The upper aqueous layer was transferred into a clean Eppendorf tube. The aqueous layers from the two phenol/chloroform extractions were combined and an ethanol precipitation was performed: 25 µl 3M sodium acetate pH 5.4 and 1 ml 100% ethanol were added to the aqueous phase, mixed gently and centrifuged as before. The pellets were washed twice with 70% ethanol and finally resuspended in 300 µl TE buffer (10 mM Tris-Cl pH 8.0, 0.1 mM EDTA, pH 8.0 (10∶1)) by incubating overnight at 50°C with gentle shaking.
Information about the sperm donors
DNA was extracted from three fertile anonymous donors of European origin with normal sperm parameters and of similar age (between 30 and 35 years old). In each case a spermogram was realised according to WHO's 2010 guidelines [37]. Sperm concentration ranged between 60–120×106 spz/ml, with, in each case less than 1×106 leucocytes/ml. We therefore considered that the presence of this small percentage of leucocytes had a negligible effect on the quantification of the sperm (only) DNA and on the ensuing calculations.
A molecular analysis was carried out to determine PRDM9 ZF array genotype. PCR amplification and sequencing of the PRDM9 ZF array were performed using primers and protocols as described previously ([12]. PCR and sequencing primer sequences are listed in the Table S1.
Amplification and sequencing of the LCRs
All primers were designed to have at least their 3′ nucleotide specific to the LCR of interest (Table S1). PCR primers were designed to amplify specifically LCR 1 or LCR 2, in order to perform a sequence comparison of the two LCRs. For each recombined LCR locus (resulting from deletion or duplication), two sets of specific primers were designed (Figure 1B). The external primers (long primers) were used for sequencing analysis. They were also used as an outer primer for the digital PCR that was devised to measure the rate of de novo recombination in sperm. The short internal primers (SI) were used in duplex with RYR2 primers that were used as a positive amplification control. These two sets of primers were used to detect the presence of recombined alleles in the 300 control individuals. They were also used as inner primers for the digital PCR.
PCR amplification was carried out on an Applied Biosystems genAmp 2700 thermocycler. Due to the high sequence homology between the two LCRs, the use of a precise annealing temperature was critical. The same thermocycler had to be used throughout the study as small variations in block temperature could introduce discrepancies in the amplification. Both the long and short PCR cycles were preceded by a 7 minutes denaturation at 95°C and followed by a 10 minutes elongation at 72°C. The specific annealing temperature of each primer set is indicated in Table S1. Thirty-five cycles were carried out for the long PCRs, with 30 seconds of denaturation at 95°C, 30 seconds of annealing and 2 minutes of elongation at 72°C. Forty-five cycles were carried out for the short PCRs with 30 seconds of denaturation at 95°C, 20 seconds of annealing and 2 minutes of elongation at 72°C.
We performed the long and short PCRs in 1× Takara Ex Taq buffer (Takara), 250 µM dNTPs (Takara dNTP mixture), 300 nM each primer, 1 unit Takara Ex Taq Takara) with 200 ng of somatic DNA in a total volume of 25 µl.
All sequences (native LCR 1 and 2 and deleted and duplicated LCRs) were carried out with BigDye Terminator v3.1 (Applied Biosystems Courtaboeuf, France) on an ABI 3130XL (Applied Biosystems, Courtaboeuf, France).
Oligonucleotide array CGH was performed with the Agilent 105K or 180K Human Genome CGH Microarray (Agilent Technologies, Santa Clara, CA, USA) (Hospices Civils de Lyon array CGH Platform and CHU Grenoble array CGH Platform). Extracted DNAs were labelled according to the instructions of the supplier and incubated overnight. The samples were purified and hybridised as described previously [17].
Graphical display and analysis of the data were performed with the Agilent DNA Analytics software version 4.0.81 (statistical algorithm: ADM-2, sensitivity threshold: 2.5, window: 0.5). A value of zero represents equal fluorescence intensities between sample and reference DNA. Copy-number losses shift the value to the left (≤−1), and copy-number gains shift it to the right (≥0.58).
The design of the MLPA probes, MLPA reaction and data analysis were performed according to the recommendation of the MRC-Holland synthetic protocol (www.mlpa.com) and as described in Coutton et al. (2012) [25].
Sperm assay design and digital PCR for sperm NAHR breakpoints mapping
We designed two nested LCR-specific PCRs as described in the PCR section. In addition, we designed a TaqMan dual labeled probe (Table S1) to allow the second step of the nested PCRs to be run on Biorad iCycler IQ real time PCR detection. We tested each recombinant-specific combination of primers for specificity and sensitivity on negative and positive (DPY19L2 deleted and duplicated) control blood DNA (Figure 1B). Each of the two rearrangements was assayed on DNA extracted from three unrelated sperm donors. Each donor was confirmed to carry two copies of DPY19L2 by MLPA analysis (data not shown). We note that our assay will not distinguish triplications of the DPY19L2 locus, which are likely to occur at extremely low frequencies.
We performed the first LCR-specific PCR (long PCR) in 1× Takara Ex Taq buffer (Takara), 250 µM dNTPs (Takara dNTP mixture), 300 nM of each primer, 1 unit Takara Ex Taq (Takara), using sufficient copies of template DNA to give approximately 24 positive wells per 96-well plate (exact quantities determined empirically by successive dilutions) and 2.5 mM MgCl2, in a total volume of 50 µl. Following thermal cycling we incubated 10 µl of the long PCR products with 5 µl of Exosap-IT PCR Clean-up Kit (GE Healthcare) for 15 min at 37°C to digest the long PCR primers followed by enzyme inactivation at 80°C for a further 15 min. Two µl of 10× diluted long PCR products was used as a template in the second PCR (short PCR). In the short PCR we used the same concentrations of buffer, dNTPs, primers and enzyme as in the Long PCR, but the total volume was 25 µl and we added a dual-labeled probe (final concentration 250 nM; Eurofins MWG Operon) (Table S1). To map the locations of breakpoints we re-amplified wells that we had previously identified as positive in the long PCR plate using the short primers and sequenced these amplicons.
The quantity of input sperm DNA was experimentally determined by serial dilutions to obtain approximately 24 positive breakpoint-specific amplifications per 96-well plate. The number of positive amplifications was then counted to estimate the number of recombinants in the input sperm. Each well contains a sample drawn from the input DNA without replacement, hence the number of recombinants in a given well is appropriately modeled using a hypergeometric distribution. We note that this hypergeometric distribution has often been approximated in the literature by Poisson (6, 22) or binomial (23) distributions, but although such approximations are acceptable we find no need for them in this study, as the direct calculation is simple. Indeed, using the hypergeometric distribution the probability that a well contains no recombinants is:
where N is the total number of copies of chromosome 12 in the input DNA (i.e. 1.6×106 for the deletion assay and 3.2×106 for the duplication assay, see Results section on digital PCR), W = N/96 is the number of copies per well, and R is the total number of recombinants. The value of R such that this probability is closest to the observed ratio of negative wells (i.e. one minus the fraction of wells that produced a positive amplification) is easily found by tabulation. This leads to an estimation of the de novo recombination rate λ = R/N, and a 95% confidence interval is calculated by modeling the initial dilution to obtain the input DNA using the binomial distribution (with binom.test in the R stats package, http://www.r-project.org).In order to evaluate the amplification efficiency of our duplication/deletion assays, we used as positive controls genomic DNA from one heterozygous duplicated individual and from one heterozygous deleted individuals. We believe that this type of control is more accurate than the use of cloned recombinant deleted and duplicated alleles as this reduces dilution factors. More importantly it reproduces faithfully the possible inhibitions due to the presence of the over majoritarian non-target genomic DNA or the potential amplification of homologous sequences that are present in the actual quantifying experiments.
The DNA concentration was measured by Nanodrop (ThermoScientific) and DNA quality was evaluated using an agarose gel electrophoresis (0.8%). No smear or fragments were observed. Considering that a human diploid genome represents 6 pg of DNA, we performed serial dilutions of the duplication and deletion controls to obtain a concentration of 1.5 pg/µl. One microliter of each solution was aliquoted in a 96-well plate, so that approximately 25% of the wells are expected to contain a recombinant allele (as we used heterozygous controls who carry only one copy of the deleted or duplicated alleles). The number of positive wells was then counted when amplifying deleted and duplicated DNA.
Calculations with the General Selection Model
Given a locus with two alleles (e.g. wild-type DPY19L2 allele and deleted allele), and noting q the frequency of the minor (deleted) allele, the GSM predicts the change in allele frequency Δq at each generation given the relative fitness of each genotype. In our case the homozygous wild-type is used as a reference (fitness 1), and the homozygous deleted men are known to be 100% infertile while the deletion is considered to have no effect in women, hence the fitness of the homozygous deleted genotype is 0.5. Let W be the relative fitness of the heterozygous genotype, and p = 1-q the frequency of the wild-type allele. Note that q≈1.7×10−3, hence p≈998×10−3. The GSM therefore simplifies to: Δq≈−q[W(q−p)+p−q/2].
In the first scenario, heterozygous individuals are assumed to have no phenotype, hence W = 1 and the equation simplifies to: .
In the second scenario, we no longer assume W = 1 and instead wish to calculate the value of W such that the GSM-predicted Δq exactly compensates the de novo rate of production of deleted alleles through NAHR, i.e. Δq = −1.8×10−5. Turning the previous equation around, we obtain: . Substituting the values of p, q and Δq, this yields W = 0.99. Assuming that heterozygous women have no phenotype, we finally obtain a relative fitness of 98% for heterozygous males.
Supporting Information
Zdroje
1. GuW, ZhangF, LupskiJR (2008) Mechanisms for human genomic rearrangements. Pathogenetics 1 : 4.
2. SmithCE, LlorenteB, SymingtonLS (2007) Template switching during break-induced replication. Nature 447 : 102–5.
3. LupskiJR, StankiewiczP (2005) Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 1: e49 doi:10.1371/journal.pgen.0010049
4. TurnerDJ, MirettiM, RajanD, FieglerH, CarterNP, et al. (2008) Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet 40 : 90–5.
5. MolinaO, AntonE, VidalF, BlancoJ (2011) Sperm rates of 7q11.23, 15q11q13 and 22q11.2 deletions and duplications: a FISH approach. Hum Genet 129 : 35–44.
6. LiuP, LacariaM, ZhangF, WithersM, HastingsPJ, et al. (2011) Frequency of nonallelic homologous recombination is correlated with length of homology: evidence that ectopic synapsis precedes ectopic crossing-over. Am J Hum Genet 89 : 580–8.
7. ArnheimN, CalabreseP, Tiemann-BoegeI (2007) Mammalian meiotic recombination hot spots. Annu Rev Genet 41 : 369–99.
8. PaigenK, PetkovP (2010) Mammalian recombination hot spots: properties, control and evolution. Nat Rev Genet 11 : 221–33.
9. KongA, ThorleifssonG, GudbjartssonDF, MassonG, SigurdssonA, et al. (2010) Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467 : 1099–103.
10. MyersS, FreemanC, AutonA, DonnellyP, McVeanG (2008) A common sequence motif associated with recombination hot spots and genome instability in humans. Nat Genet 40 : 1124–9.
11. HayashiK, YoshidaK, MatsuiY (2005) A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438 : 374–8.
12. BergIL, NeumannR, LamKW, SarbajnaS, Odenthal-HesseL, et al. (2010) PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans. Nat Genet 42 : 859–63.
13. MyersS, BowdenR, TumianA, BontropRE, FreemanC, et al. (2010) Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 327 : 876–9.
14. BaudatF, BuardJ, GreyC, Fledel-AlonA, OberC, et al. (2010) PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327 : 836–40.
15. BergIL, NeumannR, SarbajnaS, Odenthal-HesseL, ButlerNJ, et al. (2011) Variants of the protein PRDM9 differentially regulate a set of human meiotic recombination hotspots highly active in African populations. Proc Natl Acad Sci U S A 108 : 12378–83.
16. SasakiM, LangeJ, KeeneyS (2010) Genome destabilization by homologous recombination in the germ line. Nat Rev Mol Cell Biol 11 : 182–95.
17. HarbuzR, ZouariR, PierreV, Ben KhelifaM, KharoufM, et al. (2011) A Recurrent Deletion of DPY19L2 Causes Infertility in Man by Blocking Sperm Head Elongation and Acrosome Formation. Am J Hum Genet 88 : 351–61.
18. DamAH, FeenstraI, WestphalJR, RamosL, van GoldeRJ, et al. (2007) Globozoospermia revisited. Hum Reprod Update 13 : 63–75.
19. Florke-GerloffS, Topfer-PetersenE, Muller-EsterlW, MansouriA, SchatzR, et al. (1984) Biochemical and genetic investigation of round-headed spermatozoa in infertile men including two brothers and their father. Andrologia 16 : 187–202.
20. KilaniZ, IsmailR, GhunaimS, MohamedH, HughesD, et al. (2004) Evaluation and treatment of familial globozoospermia in five brothers. Fertil Steril 82 : 1436–9.
21. NistalM, HerruzoA, Sanchez-CorralF (1978) [Absolute teratozoospermia in a family. Irregular microcephalic spermatozoa without acrosome]. Andrologia 10 : 234–40.
22. DamAH, KoscinskiI, KremerJA, MoutouC, JaegerAS, et al. (2007) Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet 81 : 813–20.
23. LiuG, ShiQW, LuGX (2010) A newly discovered mutation in PICK1 in a human with globozoospermia. Asian J Androl 12 : 556–60.
24. CarsonAR, CheungJ, SchererSW (2006) Duplication and relocation of the functional DPY19L2 gene within low copy repeats. BMC Genomics 7 : 45.
25. CouttonC, ZouariR, AbadaF, Ben KhelifaM, MerdassiG, et al. (2012) MLPA and sequence analysis of DPY19L2 reveals point mutations causing globozoospermia. Hum Reprod 27 : 2549–58.
26. ElinatiE, KuentzP, RedinC, JaberS, Vanden MeerschautF, et al. (2012) Globozoospermia is mainly due to DPY19L2 deletion via non-allelic homologous recombination involving two recombination hotspots. Hum Mol Genet 21 : 3695–702.
27. KoscinskiI, ElinatiE, FossardC, RedinC, MullerJ, et al. (2011) DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet 88 : 344–50.
28. PierreV, MartinezG, CouttonC, DelarocheJ, YassineS, et al. (2012) Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development 139 : 2955–65.
29. ShaikhTH, GaiX, PerinJC, GlessnerJT, XieH, et al. (2009) High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res 19 : 1682–90.
30. PintoD, MarshallC, FeukL, SchererSW (2007) Copy-number variation in control population cohorts. Hum Mol Genet 16 Spec No. 2: R168–73.
31. ItsaraA, CooperGM, BakerC, GirirajanS, LiJ, et al. (2009) Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet 84 : 148–61.
32. de SmithAJ, TsalenkoA, SampasN, SchefferA, YamadaNA, et al. (2007) Array CGH analysis of copy number variation identifies 1284 new genes variant in healthy white males: implications for association studies of complex diseases. Hum Mol Genet 16 : 2783–94.
33. ConradDF, PintoD, RedonR, FeukL, GokcumenO, et al. (2010) Origins and functional impact of copy number variation in the human genome. Nature 464 : 704–12.
34. SchimentiJ (2000) Segregation distortion of mouse t haplotypes the molecular basis emerges. Trends Genet 16 : 240–3.
35. GreyC, BarthesP, Chauveau-Le FriecG, LangaF, BaudatF, et al. (2011) Mouse PRDM9 DNA-binding specificity determines sites of histone H3 lysine 4 trimethylation for initiation of meiotic recombination. PLoS Biol 9: e1001176 doi:10.1371/journal.pbio.1001176
36. JeanpierreM (1987) A rapid method for the purification of DNA from blood. Nucleic Acids Res 15 : 9611.
37. World Health Organization (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Délka menstruačního cyklu jako marker ženské plodnosti
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




