-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
A variety of extracellular factors regulate morphogenesis during development. However, coordination between extracellular signaling and dynamic morphogenesis is largely unexplored. We address the fundamental question by studying posterior crossvein (PCV) development in Drosophila as a model, in which long-range BMP transport from the longitudinal veins plays a critical role during the pupal stages. Here, we show that RhoGAP Crossveinless-C (Cv-C) is induced at the PCV primordial cells by BMP signaling and mediates PCV morphogenesis cell-autonomously by inactivating members of the Rho-type small GTPases. Intriguingly, we find that Cv-C is also required non-cell-autonomously for BMP transport into the PCV region, while a long-range BMP transport is guided toward ectopic wing vein regions by loss of the Rho-type small GTPases. We present evidence that low level of ß-integrin accumulation at the basal side of PCV epithelial cells regulated by Cv-C provides an optimal extracellular environment for guiding BMP transport. These data suggest that BMP transport and PCV morphogenesis are tightly coupled. Our study reveals a feed-forward mechanism that coordinates the spatial distribution of extracellular instructive cues and morphogenesis. The coupling mechanism may be widely utilized to achieve precise morphogenesis during development and homeostasis.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003403
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003403Summary
A variety of extracellular factors regulate morphogenesis during development. However, coordination between extracellular signaling and dynamic morphogenesis is largely unexplored. We address the fundamental question by studying posterior crossvein (PCV) development in Drosophila as a model, in which long-range BMP transport from the longitudinal veins plays a critical role during the pupal stages. Here, we show that RhoGAP Crossveinless-C (Cv-C) is induced at the PCV primordial cells by BMP signaling and mediates PCV morphogenesis cell-autonomously by inactivating members of the Rho-type small GTPases. Intriguingly, we find that Cv-C is also required non-cell-autonomously for BMP transport into the PCV region, while a long-range BMP transport is guided toward ectopic wing vein regions by loss of the Rho-type small GTPases. We present evidence that low level of ß-integrin accumulation at the basal side of PCV epithelial cells regulated by Cv-C provides an optimal extracellular environment for guiding BMP transport. These data suggest that BMP transport and PCV morphogenesis are tightly coupled. Our study reveals a feed-forward mechanism that coordinates the spatial distribution of extracellular instructive cues and morphogenesis. The coupling mechanism may be widely utilized to achieve precise morphogenesis during development and homeostasis.
Introduction
A key question in developmental biology is to address how tissue morphogenesis is regulated by a variety of extracellular signals. This includes identification of such extracellular signaling molecules and intracellular mechanisms that trigger morphogenesis. Since arrival of extracellular factors coincides with dynamic morphogenesis, there must be mechanisms to coordinate signaling and morphogenesis. The coordination can be achieved by an instructive role of morphogenesis in determining the regions where extracellular signals arrive or are activated. However, this is largely unknown, due to the complexity of morphogenesis in vivo.
The bone morphogenetic proteins (BMPs) are extracellular factors that regulate morphogenesis as well as growth and patterning [1], [2]. In Drosophila, Decapentaplegic (Dpp), a homologue of BMP2/4, is secreted either as a homodimer or a heterodimer with another BMP-type ligand (Glass bottom boat (Gbb) or Screw (Scw)). The ligands bind to the type I receptor Thickveins (Tkv) and type II receptor Punt and phosphorylate the transcription factor Mad. Then phosphorylated Mad (pMad), together with Medea, translocates into the nucleus for transcriptional regulation of various genes [3]. The nuclear accumulation of pMad can be visualized by immunostaining and used as a readout of the BMP signal.
The Rho-type small GTPases, including Rho, Rac, and Cdc42, play critical roles in actin cytoskeleton organization, cell-extracellular matrix (ECM) adhesion, cell polarity, cell cycle progression, and cell migration [4], [5]. The activities of the Rho-type small GTPases are tightly regulated by the guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). The GEFs activate the GTPases by replacing GDP with GTP, while the GAPs inactivate the GTPases by enhancing their GTP-hydrolyzing activity [6], [7]. Recent studies have shown that BMP signaling regulates epithelial morphogenesis through the Rho-type small GTPases. However, transcriptional downstream factors that link the BMP signal with the activities of the Rho-type small GTPases are largely unknown [8], [9].
Posterior crossvein (PCV) development mediated by BMP signaling during the pupal stages provides an excellent system for understanding how the long-range BMP signal regulates morphogenesis (Figure 1A, 1B). dpp is initially transcribed at the prospective longitudinal veins (LVs) (Figure 1B), then later also in the PCV region about 28 hr after pupariation (AP) [10]. In contrast, the BMP signal is detected at all the vein primordia from about 17—18 hr AP (Figure 1B′). It has been thus proposed that Dpp diffuses from the adjacent LVs (L4 and L5) toward the PCV region during 18—28 hr AP for PCV development [10]–[12]. By visualizing Dpp distribution in the pupal wing, we recently demonstrated that the Dpp-Gbb heterodimer is directionally transported from the LVs into the PCV region through two BMP-binding proteins, Short gastrulation (Sog) and Crossveinless (Cv) (Figure 1C) [13]. Cleavage of Sog by the protease Tolloid-related (Tlr) then releases the ligands to activate the receptors (Figure 1C) [12]. Interestingly, the direction of BMP transport or PCV position is prefigured independently of BMP signaling by lack of sog transcription at the PCV region about 20 hr AP [10], [13], which is thought to help generate the Sog gradient that guides BMP towards the PCV region [13]. To date, the BMP signal mediated by the BMP transport is the earliest instructive signal for PCV formation. A similar BMP transport mechanism also operates in the patterning of the early embryo [14]–[16].
Fig. 1. BMP signaling is required for PCV morphogenesis. 
(A) Wild-type yw adult wing. (B, B′) lacZ (B) and pMad (B′) staining of dppshv-lacZ at 24 hr AP. The PCV position is indicated by arrows. (C) A current model of Sog-Cv-mediated directional BMP transport from the LVs into the PCV. (D—I) Wild-type yw pupal wing. pMad (D, E, F, G, H, I), F-actin (E′, G′, I′), and ß-integrin (E″, G″, I″) staining at 18 hr AP (D, E), 20 hr AP (F, G), and 24 hr AP (H, I). (J—O) cv70 pupal wing. pMad (J, K, L, M, N, O), F-actin (K′, M′, O′), and ß-integrin (K″, M″, O″) staining at 18 hr AP (J, K), 20 hr AP (L, M), and 24 hr AP (N, O). (D, F, H, J, L, N) Dorsal view of the PCV region. (E, G, I, K, M, O) Optical cross-sections of the PCV region. Prospective PCV positions are indicated by double-headed arrows at 18 hr AP (E–E″, K–K″). In contrast with the extracellular regulation of BMP transport, little is known about how the BMP signal regulates wing vein morphogenesis recognized by apposition at the basal side of two wing epithelial layers [17]. Furthermore, directional BMP transport toward the PCV region undergoing morphogenesis raises a question of how BMP transport and wing vein morphogenesis are coordinated [13].
A candidate for mediating PCV formation is Crossveinless-C (Cv-C), whose viable mutant allele displays a PCV-less phenotype [18]. Recently, cv-c was identified as RhoGAP88C required for a variety of embryo morphogenesis [19]. However, it remains unclear how Cv-C regulates PCV formation. Here, we show that cv-c is induced at the PCV region by the BMP signal and mediates PCV morphogenesis by inactivating various members of the Rho-type small GTPases. Intriguingly, we found that loss of cv-c inhibited Sog-Cv dependent BMP transport into the PCV region, while an ectopic Sog-Cv-dependent BMP signal was induced toward ectopic wing veins by loss of the Rho-type small GTPases. Taken together, our data suggest that Cv-C mediates a feed-forward loop coupling BMP transport and PCV morphogenesis. We also provide evidence that the initial PCV morphogenesis precedes BMP signaling and sog transcription, highlighting an instructive role of morphogenesis in guiding BMP transport.
Results
BMP signaling is required for PCV morphogenesis
To investigate how the PCV region undergoes morphogenesis, we analyzed optical cross-sections in the prospective PCV region, marked by pMad accumulation. The tissue architecture and the cell-extracellular matrix (ECM) adhesion were visualized by phalloidin staining of F-actin and immunostaining for ß-integrin [20]. At 18 hr AP, two wing epithelial layers were separated with the similar tissue architecture between the PCV region and intervein regions (Figure 1E). Around 20—21 hr AP, the wing vein lumen was formed through apposition of the basal side of the intervein regions. The apical-basal cell length in the PCV region became shorter than that in the intervein regions (Figure 1G). At 24 hr AP, the apposition continued except the PCV region (Figure 1I) and apical-basal polarity is maintained between the PCV region and intervein regions (Figure S6A–S6A‴). During 18—24 hr AP, F-actin and ß-integrin preferentially accumulated at the basal side of the intervein epithelial cells, but less at the basal side of the PCV region (Figure 1E′, 1E″, 1G′, 1G″, 1I′, 1I″). F-actin also accumulated at the apical side of the wing epithelial cells, which became more evident at the apical side of the PCV region at 24 hr AP (Figure 1I′). Ubiquitous expression of ß-integrin in the pupal wing suggests that the ß-integrin distribution is regulated posttranscriptionally (Figure S1). The lack of apposition of the two wing epithelial layers and the distinct tissue architecture in the PCV region recognized by less distribution of the ß-integrin and F-actin at the basal side are hereinafter referred to as PCV morphogenesis. We note that similar ß-integrin and F-actin distributions were observed in the LV formation [20].
Since PCV morphogenesis overlapped with the pMad accumulation (Figure 1D–1I), we investigated whether BMP signal is required for PCV morphogenesis. To test this, PCV morphogenesis was analyzed in cv70, a null allele of cv, in which pMad accumulation was absent due to lack of BMP transport (Figure 1J, 1K, 1L, 1M, 1N, 1O) [11], [13]. We found that, despite the smaller lumen size, the initial PCV morphogenesis occurred during 18—20 hr AP and was subsequently disrupted around 24 hr AP (Figure 1K, 1M, 1O). PCV morphogenesis was also analyzed in a dppshv mutant (dpps4/dpps11) [21]. In dpps4/dpps11, BMP signal was severely affected both in the LVs and CVs during pupal stages (20–26 hr AP), and consequently, distal parts of L4, L5 and PCV were not formed in the adult wing (Figure S2A–S2C, data not shown) [13]. We found that PCV morphogenesis occurred during 22–24 hr AP and was disrupted until 26 hr AP in dpps4/dpps11 (Figure S2D–S2G). These observations suggest that BMP signaling is not required for the initiation but for the maintenance of PCV morphogenesis through regulation of ß-integrin and F-actin localizations.
The initial PCV morphogenesis is independent of sog transcriptional prepattern
It has been shown that sog transcription also prepatterns the PCV position (Figure 2A) [10], [13]. To test whether the initial PCV morphogenesis is dependent on sog transcription, the initial PCV morphogenesis was analyzed at 20 hr AP in sogP129D, where sog transcriptional prepattern information and pMad signal were severely absent at the PCV region (Figure 2B–2D). We found that the initial PCV morphogenesis still occurred in sogP129D (Figure 2E–2E″). Thus, the initial PCV morphogenesis is independent of sog transcription. The initial PCV morphogenesis as well as sog transcription may be involved in guiding the BMP transport.
Fig. 2. The initial PCV morphogenesis is independent of sog transcriptional prepattern. 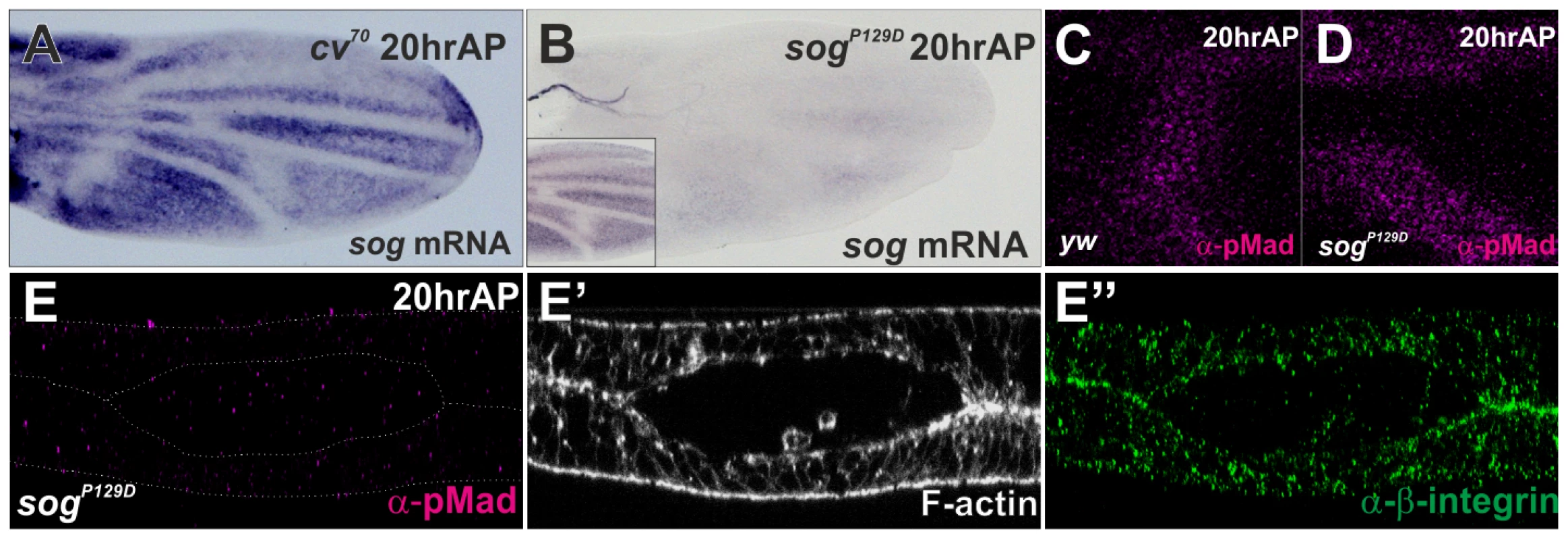
(A) in situ hybridization of sog at 20 hr AP in cv70 pupal wing. (B) in situ hybridization of sog at 20 hr AP in sogP129D and in wild-type yw pupal wing (inset). (C–D) pMad staining at PCV region at 20 hr AP in yw (C) and in sogp129D (D). (E–E″) Optical cross-sections of the PCV region. pMad (E), F-actin (E′), and ß-integrin (E″) staining at 20 hr AP in sogP129D. Cv-C mediates PCV morphogenesis by inactivating various Rho-type small GTPases
We then asked what mediates PCV morphogenesis downstream of BMP signaling. A candidate for mediating PCV morphogenesis is RhoGAP Cv-C (Figure 3A), which regulates a variety of embryo morphogenesis through regulation of the cytoskeleton [19], [22]. Indeed, optical cross-sections showed defects in PCV morphogenesis at 24 hr AP in cv-c1, a viable allele of cv-c (Figure 3B, 3C). During 20—24 hr AP, cv-c is strongly expressed in the CVs and weakly in the LVs and intervein regions along the LVs (Figure 3D, 3E). Since cv-c expression is absent from the PCV region during 20—24 hr AP in cv70 (Figure 3F, 3G) and ectopically induced by the constitutively active form of type I receptor Tkv (caTkv) at 24 hr AP (Figure 3H), cv-c transcription in the PCV region is tightly regulated by BMP signaling.
Fig. 3. Cv-C mediates PCV morphogenesis downstream of BMP signaling by inactivating various Rho-type small GTPases. 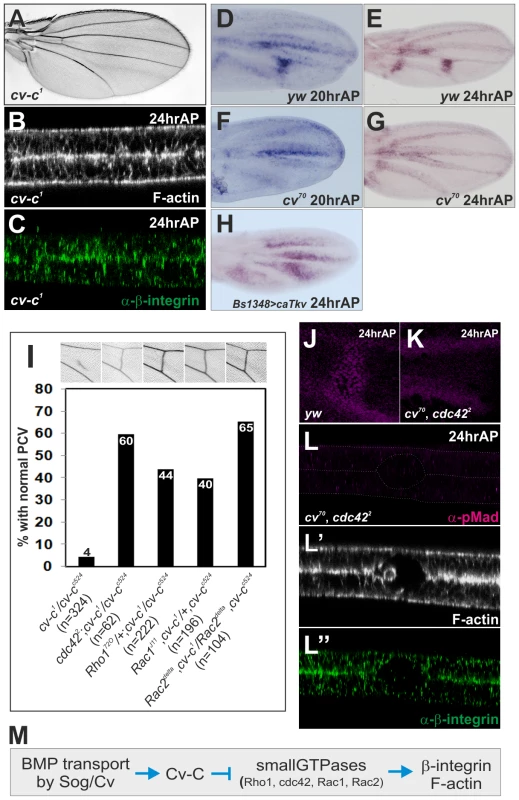
(A) Adult wing of cv-c1. (B, C) Optical cross-sections of the PCV region. F-actin (B) and ß-integrin (C) staining at 24 hr AP in cv-c1. (D—H) in situ hybridization of cv-c at 20 hr AP (D) and at 24 hr AP (E) in wild-type yw, and at 20 hr AP (F) and at 24 hr AP (G) in cv70, and at 24 hr AP in BS1348>caTkv (H). BS1348-Gal4 is intervein-specific Gal4 driver. (I) Genetic interactions of cv-c with various small GTPases. Representative adult wings around the PCV region of each genotype are shown above. (J, K) pMad staining at 24 hr AP in wild-type yw (J) and in cv70, cdc422 double mutant (K). (L—L″) Optical cross-sections of the PCV region. pMad (L), F-actin (L′), and ß-integrin (L″) staining at 24 hr AP in cv70, cdc422 double mutant. (M) A schematic pathway regulating PCV morphogenesis downstream of BMP signaling. We then asked if Cv-C regulates PCV morphogenesis by inactivating the Rho-type small GTPases. In this case, adult PCV defects in cv-c may be rescued by reducing the activities of Rho-type small GTPases. Indeed, we found that severe adult PCV defects in cv-c1/cv-cc524 were efficiently restored by mutant alleles of Cdc42, Rho1, Rac1, or Rac2 (Figure 3I). Consistently, when Rho1 activity was visualized by a GFP-based sensor that binds to the active form of Rho1 in the dorsal wing layer [23], Rho1 activity was low in the PCV region and high at the basal side of the intervein region at 24 hr AP (Figure S3A). In contrast, GFP alone was uniformly distributed in the dorsal wing layer at 24 hr AP (Figure S3B). The Rho1 protein also less accumulated at the basal side of the PCV region at 24 hr AP (Figure S3D). The similar distribution of Rho1 activity was also observed in the dorsal wing layer at 20 hr AP in cv70, even though cv-c was not expressed in the PCV region (Figure 3F, Figure S3C). This suggests that Rho1 activity in the initial PCV morphogenesis is regulated independently of BMP signaling and Cv-C.
We then asked if inactivation of the Rho-type small GTPases is critical for PCV morphogenesis downstream of BMP signaling. In this case, defects in PCV morphogenesis due to lack of BMP signaling may be rescued by reducing activities of Rho-type small GTPases. Indeed, by using the viable cdc42 allele cdc422 [24], we found that defects in PCV morphogenesis in cv70 (Figure 1N, 1O) were efficiently rescued at 24 hr AP in cv70, cdc422 double mutant independently of pMad signal (Figure 3J–3L). Taken together, these data suggest that Cv-C is induced in the PCV region by BMP signaling and regulates ß-integrin and F-actin distribution by inactivating various Rho-type small GTPases (Figure 3M).
Cv-C is non-cell-autonomously required for BMP signaling
Unexpectedly, we found that loss of cv-c affects BMP signaling in the PCV region. In cv-c1, pMad signal in the PCV region was almost absent during 20–24 hr AP (Figure 4A–4D). To investigate how Cv-C is involved in BMP signaling, mutant clones of cv-cc524, a null allele of cv-c, were generated using the MARCM system [25]. Since BMP ligands are produced in the LVs of both wing layers and diffuse into the PCV region to activate BMP signal in both layers (Figure 1) [13], we analyzed the effects of cv-c mutant clones on BMP signal in both layers. We found that pMad accumulation appeared normal within small mutant clones (Figure 4E, 4F), suggesting that cv-c mutant cells can activate BMP signal. In such cv-c mutant clones, the apical-basal cell length became longer with F-actin accumulation at the basal side (Figure S4). In contrast, when mutant clones covered about half of the PCV region in one wing layer, pMad accumulation was normal in a few mutant cells adjacent to wild-type cells but attenuated in the middle of the clones (Figure 4G). When mutant clones straddled almost all the PCV region in one wing layer, pMad signal was severely affected within clones (Figure 4I). Interestingly, in these cases, pMad signal was also attenuated in the PCV region in the other wing layer (Figure 4H, 4J). When mutant clones in both layers were overlapped at the PCV region, pMad signal was more effectively inhibited within overlapping double-sided clones (Figure 4K–4N) than single-sided mutant clones (dashed arrows Figure 4G, 4L), and was sometimes lost even in the wild-type cells adjacent to mutant clones (arrows in Figure 4K, 4K″, 4M, 4M″). These results indicate that Cv-C is non-cell autonomously required for BMP signaling in the PCV region. The non-autonomous effects of cv-c mutant clones on BMP signal across the wing layer (Figure 4H, 4J, 4K, 4M) indicate that BMP ligands transported into one wing layer activate BMP signal in both wing layers. BMP ligands derived from the wild-type wing layer probably cross the lumen to activate BMP signal within cv-c mutant cells or wild-type cells between single-sided clones (Figure 4G, 4L).
Fig. 4. Cv-C is non-cell-autonomously required for BMP signaling. 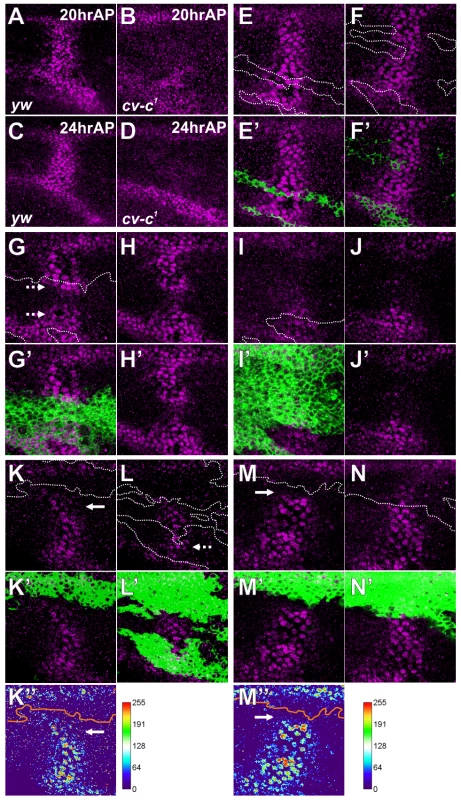
(A—D) pMad staining at 20 hr AP (A, B) and at 24 hr AP (C, D) in wild-type yw (A, C) and cv-c1 (B, D). (E—N) Effect of homozygous cv-cc524 clones (green, E′—N′) on pMad (purple, E—N) at 24 hr AP. (E–J, M, N) A single confocal image from different wing layers of the same wing (E—F, G—H, I—J, and M—N). (K, L) Maximum intensity projections from a single wing layer of the same wing. Clone boundaries were marked by white line in E—N and by orange line in K″, M″. Mutant cells accumulating pMad signal in the PCV region are marked by dashed arrows (G, L). Wild-type cells lacking pMad accumulation in the PCV region are marked by arrows (K, M). Cv-C is required for Sog-Cv-dependent BMP transport into the PCV region
The non-cell-autonomous function of Cv-C on BMP signaling can be mediated through BMP transporters or BMP-binding protein Crossveinless-2 (Cv-2), which facilitates ligand-receptor binding in a short-range manner [13], [26]. We found partial PCV defects in wings transheterozygous for cv-c and sog, or for cv-c and cv, but not for cv-c and cv-2 (Figure 5A–5D). The genetic interaction between Cv-C and BMP transporters suggests that Cv-C is involved in BMP transport toward the PCV region. To test this, we visualized Dpp distribution in the PCV region by expressing functional GFP-dpp in the LVs [13], [27]. In the wild-type background, GFP-Dpp dots accumulated at the PCV region at 24 hr AP, where pMad signal is positive (62.5±10.1 dots, n = 13 wings) (Figure 5E, 5H). In contrast, GFP-Dpp dots and pMad accumulation were significantly reduced at the PCV region at 24 hr AP (3.6±1.2 dots, n = 14 wings), and consequently the adult PCV defect was not rescued in cv-c1 mutant background (Figure 5F, 5H). When GFP was expressed in the LVs, GFP dots did not accumulate at the PCV region (0.1±0.1 dots, n = 8 wings) (Figure 5G, 5H). Taken together, these data indicate that Cv-C is required for Sog-Cv-dependent BMP transport into the PCV region.
Fig. 5. Cv-C is required for Sog-Cv-dependent BMP transport into the PCV region. 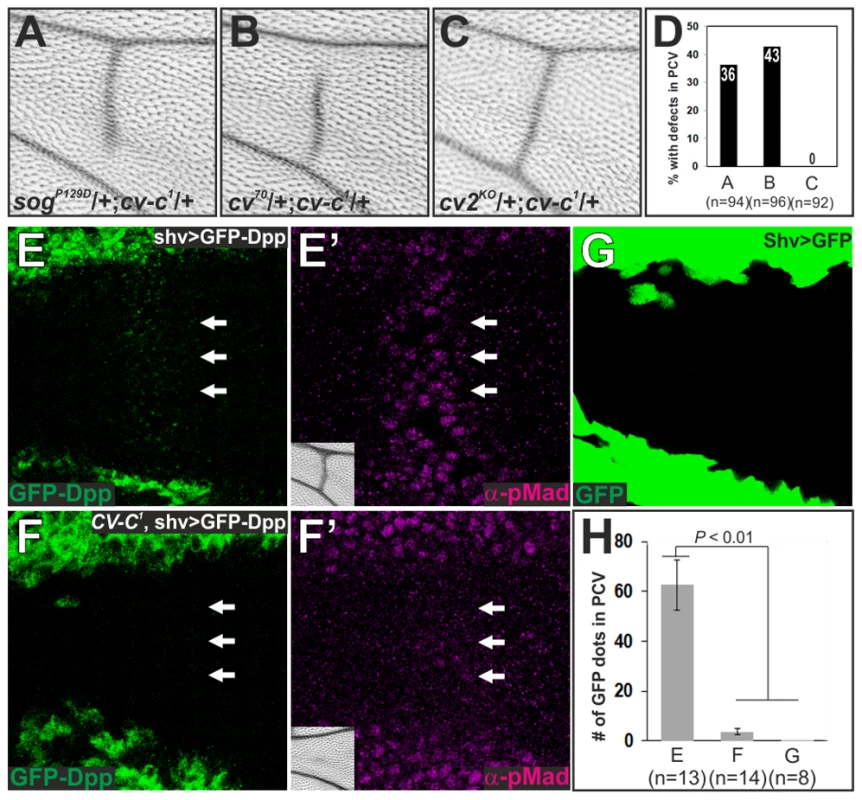
(A–C) Genetic interaction of cv-c with sog (A), cv (B), or cv-2 (C). Representative adult wings around the PCV region of each genotype are shown. (D) Quantification of adult wing phenotypes in A–C. (E, F) GFP-Dpp distribution (E, F) and pMad staining (E′, F′) at 24 hr AP and adult PCV region (inset) in wild-type yw (shv>GFP-Dpp) (E, E′) and in cv-c (cv-c1, shv>GFP-Dpp) (F, F′). The PCV position is indicated by arrows. (G) GFP distribution at 24 hr AP in shv>GFP. Despite strong GFP signal in LVs, no dots were observed in the PCV region. (H) Quantification of the number of GFP dots in E, F and G. Statistical significance was determined using Mann-Whitney U test. Sog-Cv-dependent BMP signaling is induced at the ectopic wing veins by loss of the Rho-type small GTPases
How does Cv-C regulate BMP transport? Since cv-c is required for PCV morphogenesis, we hypothesized that BMP transport is coupled with wing vein morphogenesis. To test this, we analyzed cdc422, in which ectopic CVs were frequently observed in the adult wings (Figure 6A, 6G) [28], [29]. We found that ectopic pMad signal was induced in the future ectopic CVs at 24 hr AP in cdc422 without changing dpp transcription (Figure 6B, 6B′). The ectopic pMad signal and CVs formation in cdc422 were completely cancelled in sogP129D, cdc422 or cv70, cdc422 double mutant (Figure 6C–6G). Optical cross-sections revealed that ectopic pMad signal was detected at the ectopic wing vein regions (arrow in Figure 6B) at 24 hr AP in cdc422 (Figure 6H, 6I). We found that the ectopic wing vein morphogenesis occurred independently of pMad signal in the corresponding region (arrow in Figure 6C) in sogP129D, cdc422 double mutant (Figure 6J, 6K). These data indicate that Sog-Cv-dependent BMP transport was guided toward ectopic wing veins by loss of cdc42. Sog-Cv-dependent ectopic pMad accumulation and ectopic adult wing veins were also induced by loss of Rho1 (Figure S5). We note that BMP signaling independent wing vein morphogenesis at 24 hr AP by loss of cdc42 (Figure 3L, Figure 6J and 6K) never induced adult wing veins (Figure 6D, 6F, 6G), suggesting that additional factors are required to form adult wing veins downstream of BMP signal after 24 hr AP.
Fig. 6. Sog-Cv-dependent BMP signaling is induced along ectopic wing vein morphogenesis by loss of cdc42. 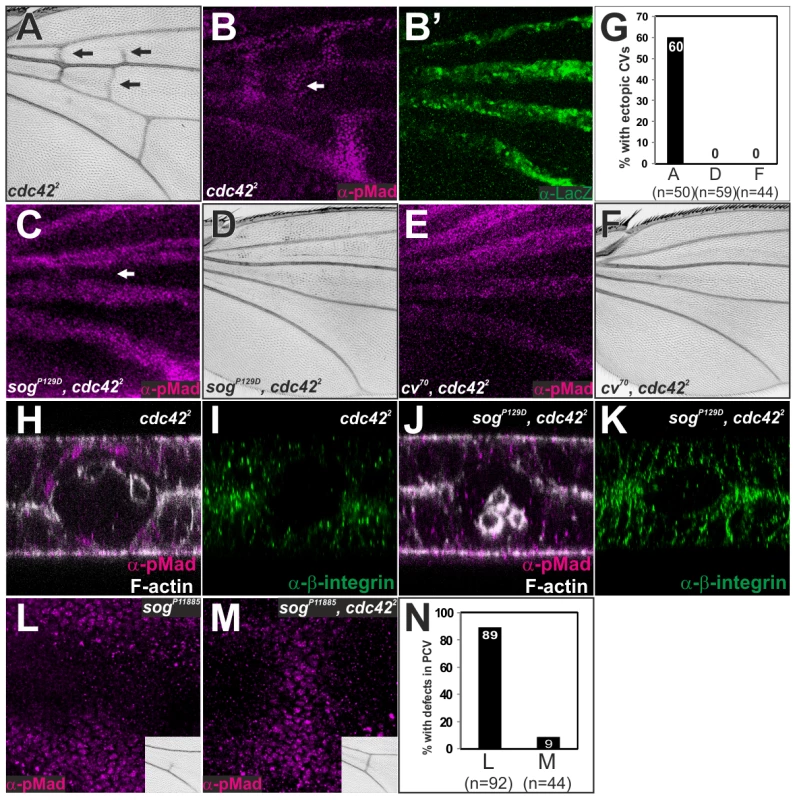
(A) Adult wing of cdc422. (B, B′) pMad (B) and lacZ staining (B′) in cdc422 ; dppshv-lacZ/+. (C—F) pMad staining (C, E) and adult wing (D, F) of sogP129D, cdc422 (C, D) and cv70, cdc422 (E, F). (G) Quantification of the phenotypes in A, D and F. (H—K) Optical cross-sections of the ectopic CV region (white arrows in B and C). pMad and F-actin (H, J) and ß-integrin (I, K) staining in cdc422 (H, I) and sogP129D, cdc422 (J, K). The relative ß-integrin accumulation at the basal side of the ectopic CV region by comparing to that of intervein regions is 32±2% (n = 7 wings) in cdc422 (I) and 37±3%, (n = 6 wings) in sog, cdc42 (K). (L, M) pMad staining and adult PCV (inset) in sogP11885 (L) and in sogP11885, cdc422 (M). (N) Quantification of the adult wing phenotypes in L and M. All pupal wings were fixed at 24 hr AP. We then addressed whether PCV morphogenesis plays an instructive role in BMP transport. In this case, despite loss of pMad signal in cv70, cdc422, or sogP129D, cdc422 double mutant due to lack of BMP transport (Figure 6C, 6E), defects in pMad signal in weak alleles of BMP transporters may be rescued by reducing the activities of the Rho-type small GTPases. Indeed, we found that defects of pMad and adult PCV in sogp11885, a weak hypomorphic allele of sog, were efficiently restored in sogp11885, cdc422 double mutant (Figure 6L–6N). These results suggest that Sog-Cv-mediated BMP transport is tightly coupled with wing vein morphogenesis by loss of the Rho-type small GTPases.
ß-integrin links Sog-Cv-dependent BMP signaling and PCV morphogenesis
What is the molecular mechanism that couples BMP transport and wing vein morphogenesis? We found that ectopic pMad accumulation by loss of cdc42 or Rho1 was associated with low ß-integrin at the basal side of wing epithelial cells (Figure 6H–6K, Figure S5). Integrins have been previously proposed to regulate Sog protein distribution from the intervein regions into the LVs during the pupal stages [30]. This raises the possibility that integrins may link Sog-Cv-dependent BMP transport and PCV morphogenesis. Indeed, we found that ectopic adult wing veins including ectopic or thickened CVs were induced in the ß-integrin myospheroid (mys) mutants, mys1/mysnj42or mysnj42 (Figure 7A, 7B, 7H) [30], [31], in a sog - or cv-dependent manner (Figure 7A–7C, 7G–7K). Although anterior crossvein (ACV) development requires BMP transport [11], wing vein fragments were often observed at the ACV region in mysnj42, sogP129D or cv70, mysnj42 double mutant (Figure 7I, 7J), which may reflect dpp expression in a part of ACV region during pupal stages [10]. Ectopic pMad accumulation at the PCV region was also observed in mys1/mysnj42 in a sog-dependent manner (Figure 7D–7F). In fact, quantification of GFP-Dpp distributions along apical-basal axis in wings expressing GFP-Dpp in the LVs showed accumulation of GFP-Dpp dots at 21±2% (n = 125 dots, 3 wings) from the basal surface in the PCV region (Figure S6E), where ß-integrin is less distributed (Figure 1). Furthermore, we found that pMad and adult PCV defects in cv-c1/cv-cc524 were efficiently rescued in mysnj42, cv-c1/cv-cc524 double mutants (Figure 7L–7N). This indicates that low ß-integrin activity promotes BMP transport at the basal side of the PCV region downstream of Cv-C. Taken together, these data suggest that BMP transport is coupled with PCV morphogenesis through ß-integrin.
Fig. 7. ß-integrin links Sog-Cv-dependent BMP signaling and PCV morphogenesis. 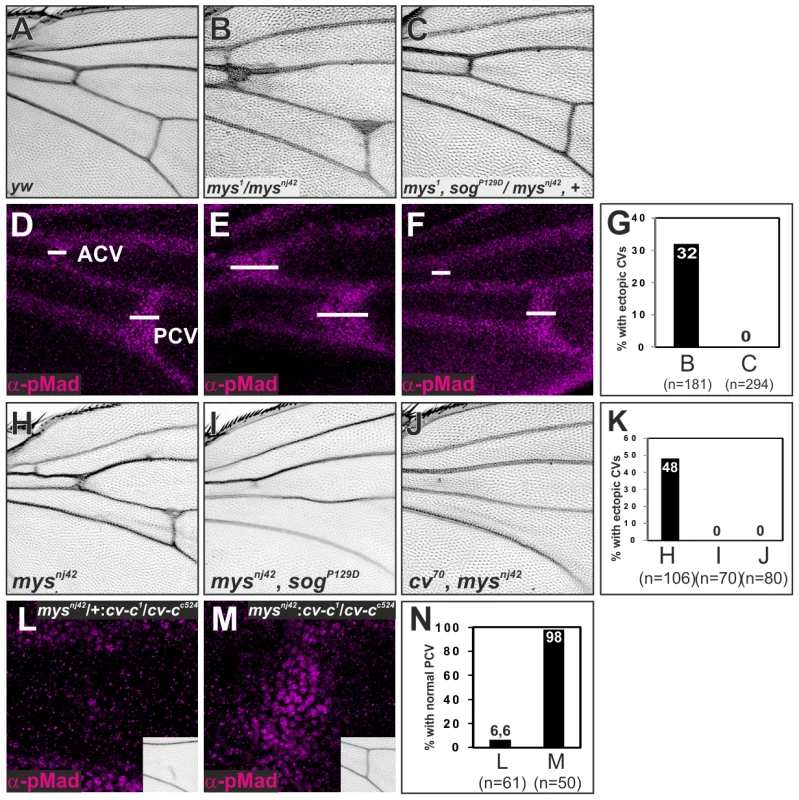
(A—F) Adult wings (A—C) and pMad staining (D—F) of wild-type yw (A, D), mys1/mysnj42 (B, E), and mys1, sogP129D/mysnj42, + (C, F). (G) Quantification of adult wing phenotypes in B and C. (H—J) Adult wings of mysnj42 (H), mysnj42, sogp129D (I), and cv70, mysnj42 (J). (K) Quantification of adult wing phenotype in H—J. (L, M) pMad staining at 24 hr AP and adult PCV (insets) in mysnj42/+; cv-c1/cv-cc524 (L) and mysnj42; cv-c1/cv-cc524 (M). (N) Quantification of adult wing phenotype in L and M. Discussion
Despite the critical roles of BMP signaling in various morphogenetic processes, little is known about its transcriptional downstream factors that mediate morphogenesis. Using Drosophila PCV morphogenesis as a model, we found that RhoGAP Cv-C is induced by BMP signaling and mediates PCV morphogenesis through inactivation of various Rho-type small GTPases. Furthermore, we found that Cv-C is required non-cell-autonomously for BMP transport into the PCV region, while BMP signaling is induced at the ectopic wing veins by loss of the Rho-type small GTPases. These results suggest that Cv-C mediates a feed-forward loop coupling BMP transport and PCV morphogenesis.
How does the activity of Rho-type small GTPases control morphogenesis in the pupal wing? Recent studies have revealed that cellular compartmentalization of the Rho-type small GTPases is critical to regulate epithelial morphogenesis [9], [23], [32]. We found a similar biased distribution of Rho1 activity in the pupal wing. Rho1 activity is high at the basal side of the intervein regions and low in the PCV region (Figure S3). Since overexpression of constitutively active form of Rho1 as well as Rho1 RNAi shortens apical-basal cell lengths in the intervein region [33], localized Rho1 activity rather than total activity is associated with the apical-basal cell length. Localized activities of Rho-type small GTPases then regulate ß-integrin and F-actin accumulation at the basal side (Figure 6H–6K) and maintain apical-basal cell length in the intervein region probably by mediating cell-ECM adhesion as in the larval wing epithelium [34]. In the PCV region, we showed that Cv-C is induced by BMP signal and inactivates various Rho-type small GTPases to induce shorter apical-basal lengths (Figure 3). Interestingly, BMP signaling has been previously linked with the localized Rho1 activity that triggers apical-basal cell elongation in the larval wing imaginal disc [9]. Thus, BMP signaling may positively or negatively regulate the compartmentalization of Rho-type small GTPases in a tissue-dependent manner.
Our study revealed that RhoGAP Cv-C also regulates BMP transport through PCV morphogenesis (Figure 6, Figure 7). How then is BMP transport coupled with wing vein morphogenesis? Our data showed that GFP-Dpp is basally accumulated at the PCV region (Figure S6) [13]. Furthermore, a recent study showed that vittelogenin-like protein Crossveinless-d (Cv-d) is secreted from hemocytes to regulate BMP signaling in the PCV region [35]. These observations suggest that BMP is transported into the PCV region through the lumen (Figure 8) [13]. The lumen may provide physical space for BMP transport. However, pMad accumulates in the PCV region even before the lumen is formed (Figure 1). We found that BMP transport is rather associated with ß-integrin distribution (Figure 1, Figure 6, Figure 7). Since Sog-Cv dependent BMP signal is induced in the ß-integrin-free regions adjacent to the LVs (Figure S5), low level of ß-integrin activity guides BMP transport from the LVs probably by affecting Sog distribution [30]. Since integrins physically interact with Sog [30], we presume that the Sog protein gradient is formed along the ECM to direct Sog-Cv-BMP complex towards the PCV region (Figure 8). BMPs are then released from Sog-Cv by Tlr and activate the signal in the PCV region on the both wing layers. In this model, BMP transport is effectively blocked when cv-c mutant clones are overlapped in both layers (Figure 4K–4N). In contrast, when cv-c mutant clones are generated in one wing layer, BMP transport is attenuated in cv-c mutant cells, but BMP ligands can be supplied from the other wing layer to activate BMP signal in cv-c mutant cells (dashed arrows Figure 4G, 4L). However, since total amounts of BMP ligands are reduced in the PCV region, BMP signaling range is affected in both wing layers (Figure 4H, 4J).
Fig. 8. A model of coupling of BMP transport and PCV morphogenesis. 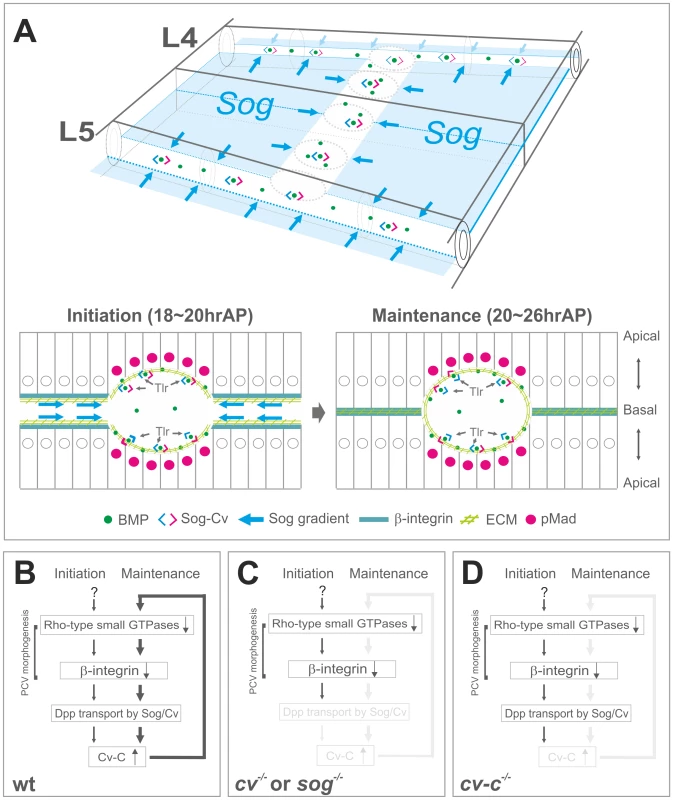
(A) Schematic overview of BMP transport and cross-section in the PCV region. BMP transport appears to occur through the ECM along PCV morphogenesis. (B) In wild-type pupal wing, the initial PCV morphogenesis is induced by inactivation of Rho-type small GTPases independently of BMP signaling. BMP transport is then guided toward the PCV region through low ß-integrin activity (∼18 hr AP). BMP signaling induces RhoGAP Cv-C to maintain PCV morphogenesis and BMP transport through a feed-forward loop (20∼26 hr AP). (C) In cv or sog pupal wing, the initial PCV morphogenesis occurs but is not maintained, due to the absence of BMP transport. (D) In cv-c pupal wing, BMP signaling and PCV morphogenesis are not maintained without a feed-forward loop. How is Sog gradient established along the ECM? ECM components may help Sog distribution. Collagen IV has been recently shown to regulate BMP signal in a variety of developmental processes [36], [37]. In contrast with Collagen IV accumulation at the basal side of the larval wing imaginal disc (Figure S6D) [38], Collagen IV accumulated as few punctate spots at the basal side and in the hemocytes in the pupal wing (Figure S6A–S6C). Since punctate Collagen IV signal is randomly distributed at the basal side, Collagen IV may not be actively involved in BMP signal at the PCV region but probably degraded by hemocytes for remodeling the pupal wing [39]. Another ECM component Laminin has been shown to genetically interact with Sog [30]. Thus Laminin may be involved in regulating Sog distribution. Recent studies also identified novel extracellular factors that regulate BMP signal via heparan sulfate proteoglycans (HSPGs) [40], [41]. Among them, Pentagon (Pent) regulates BMP morphogen gradient via the glypican Dally in the larval wing imaginal discs [40]. Although loss of pent did not induce evident PCV defects, Dally and Dally-like are required for BMP signal at the PCV region non-cell autonomously [35]. Pentagon may be involved in PCV formation together with HSPGs.
Our results also provide insights into the positional information for the PCV formation. Since sog mutant could not induce evident ectopic CVs [11], [13] and PCV formation could be rescued in some cases despite ubiquitous sog expression [26], it has been argued that sog repression in the PCV region may not be sufficient to provide the positional information for the PCV development [10], [13]. We showed that the initial PCV morphogenesis provides prepattern information independently of sog transcription (Figure 2). Thus the initial sog transcription and ß-integrin localization may cooperate to establish Sog gradient to instruct BMP transport toward the PCV region. BMP transport is then maintained by a positive feedback mechanism through Cv-C (Figure 8). Although the factors that regulate the initial PCV morphogenesis remain to be elucidated, they may regulate Rho1 activity in the initial PCV region (Figure S3C, Figure 8).
Instructive role of morphogenesis for the cell specification was also recently reported in pancreatic tubulogenesis, where Cdc42 mediated tubulogenesis controls cell specification [42]. Our data further suggest the coupling of extracellular cues and dynamic morphogenesis via instructive role of morphogenesis. The coupling mechanism provides two general implications. First, the coupling mechanism ensures spatial distribution of secreted factors at the tissues undergoing dynamic morphogenesis without restricting the competence to respond to signaling. Indeed, the intervein regions can respond to BMP signaling [13]. Second, a positive feedback mechanism allows for continuous signaling to the target tissues. This would be especially important when continuous signaling is required for further differentiation. In fact, adult wing vein morphogenesis requires continuous BMP signaling (Figure 1O, Figure 6D and 6F).
In conclusion, we identified a Cv-C-mediated feed-forward mechanism that couples BMP transport and PCV morphogenesis. Given that BMP signaling and a human homolog of Cv-C, Deleted in liver cancer (DLC1), act as tumour suppressors in a variety of contexts [43], [44], a similar coupling mechanism may be operated in tissue homeostasis as well as tissue morphogenesis. As illustrated in our study, investigating simple in vivo models would provide further insights into the coordination between extracellular cues and dynamic morphogenesis.
Materials and Methods
Fly strains
The cv70, sogP129D, cv2KO1, shv3Kpn-Gal4, and UAS-GFP-Dpp flies were described previously [13]. The cv-c1and cv-cc524 flies were obtained from H. Skaer [19]. The UAS-PNKG58AeGFP flies were kindly provided by J.C. Hombria [23]. The mysnj42 flies were obtained from F. Schoeck. The dppshv-lacZ, dpps11, dpps4, cdc422, Rho172O, Rac2Δ, Rac1J11, UAS-GFP, sogP11885, UAS-Rho1RNAi, BS1348-Gal4, and mys1 flies were obtained from Bloomington Drosophila Stock Center. Vkg-GFP flies were obtained from Fly Trap stock collection. cv-c MARCM clones were generated using y w hs-FLP; tubP-GAL4 UAS-mCD8::GFP; FRT82B cv-cc524/FRT82B tubP-GAL80.
Immunostaining and in situ hybridization
Drosophila pupal wings were fixed at 4°C overnight and then dissected from the pupae. All immunohistochemistry and in situ hybridizations were performed, as previously described [11]. The primary antibodies were as follows: rabbit anti-pMad at 1∶1000 (a gift from P. ten Dijke), mouse anti-LacZ at 1∶1000 (Promega), mouse anti-ß-integrin (CF.6G11) at 1∶100 (Developmental Studies Hybridoma Bank (DSHB)), rabbit anti-aPKC (C-20) at 1∶200 (Santa Cruz Biotechnology, Inc.), and mouse anti-Dlg (4F3) at 1∶100 (DSHB). The secondary antibodies were as follows: anti-rabbit IgG-Alexa 568 or 647 and anti-mouse IgG-Alexa 488 were used at 1∶1000, respectively (Invitrogen). Can Get Signal Solution B (TOYOBO) was used for staining with anti-pMad and anti-ß-integrin. The fluorescent images were obtained with a Leica TCS SP5 confocal microscope.
Quantification of the images
To analyze the number of GFP-Dpp dots, approximately 10 confocal sections were taken at app. 1-µm intervals to cover a single cell layer and the images were processed by maximum intensity profile and quantified using analyze particle command in ImageJ software (National Institutes of Health, Bethesda, MD, USA). To analyze GFP-Dpp distribution along apical-basal axis, relative position of GFP-Dpp dots from basal surface of the PCV region (the basal surface is set to 0% and the apical surface is set to 100%) was measured individually in a single confocal image using Image J. The heatmap of the intensity of pMad signal was produced using the “HeatMap Histogram” plugin of ImageJ.
Supporting Information
Zdroje
1. AffolterM, BaslerK (2007) The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet 8 : 663–674.
2. WuMY, HillCS (2009) Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell 16 : 329–343.
3. ParkerL, StathakisDG, AroraK (2004) Regulation of BMP and activin signaling in Drosophila. Prog Mol Subcell Biol 34 : 73–101.
4. JaffeAB, HallA (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21 : 247–269.
5. SchwartzMA, ShattilSJ (2000) Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci 25 : 388–391.
6. RossmanKL, DerCJ, SondekJ (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6 : 167–180.
7. MoonSY, ZhengY (2003) Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 13 : 13–22.
8. CorderoJB, LarsonDE, CraigCR, HaysR, CaganR (2007) Dynamic decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina. Development 134 : 1861–1871.
9. WidmannTJ, DahmannC (2009) Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J Cell Sci 122 : 1362–1373.
10. RalstonA, BlairSS (2005) Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev Biol 280 : 187–200.
11. ShimmiO, RalstonA, BlairSS, O'ConnorMB (2005) The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol 282 : 70–83.
12. SerpeM, RalstonA, BlairSS, O'ConnorMB (2005) Matching catalytic activity to developmental function: tolloid-related processes Sog in order to help specify the posterior crossvein in the Drosophila wing. Development 132 : 2645–2656.
13. MatsudaS, ShimmiO (2012) Directional transport and active retention of Dpp/BMP create wing vein patterns in Drosophila. Dev Biol 366 : 153–162.
14. ShimmiO, UmulisD, OthmerH, O'ConnorMB (2005) Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120 : 873–886.
15. WangYC, FergusonEL (2005) Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature 434 : 229–234.
16. O'ConnorMB, UmulisD, OthmerHG, BlairSS (2006) Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133 : 183–193.
17. HoganBL, KolodziejPA (2002) Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet 3 : 513–523.
18. SternC (1934) On the Occurrence of Translocations and Autosomal Non-Disjunction in Drosophila melanogaster. Proc Natl Acad Sci U S A 20 : 36–39.
19. DenholmB, BrownS, RayRP, Ruiz-GomezM, SkaerH, et al. (2005) crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development 132 : 2389–2400.
20. FristromD, WilcoxM, FristromJ (1993) The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development 117 : 509–523.
21. St JohnstonRD, HoffmannFM, BlackmanRK, SegalD, GrimailaR, et al. (1990) Molecular organization of the decapentaplegic gene in Drosophila melanogaster. Genes Dev 4 : 1114–1127.
22. BroduV, CasanovaJ (2006) The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev 20 : 1817–1828.
23. SimoesS, DenholmB, AzevedoD, SotillosS, MartinP, et al. (2006) Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 133 : 4257–4267.
24. FehonRG, OrenT, LaJeunesseDR, MelbyTE, McCartneyBM (1997) Isolation of mutations in the Drosophila homologues of the human Neurofibromatosis 2 and yeast CDC42 genes using a simple and efficient reverse-genetic method. Genetics 146 : 245–252.
25. LeeT, LuoL (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 : 451–461.
26. SerpeM, UmulisD, RalstonA, ChenJ, OlsonDJ, et al. (2008) The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14 : 940–953.
27. TelemanAA, CohenSM (2000) Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103 : 971–980.
28. BaronM, O'LearyV, EvansDA, HicksM, HudsonK (2000) Multiple roles of the Dcdc42 GTPase during wing development in Drosophila melanogaster. Mol Gen Genet 264 : 98–104.
29. GenovaJL, JongS, CampJT, FehonRG (2000) Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev Biol 221 : 181–194.
30. AraujoH, NegreirosE, BierE (2003) Integrins modulate Sog activity in the Drosophila wing. Development 130 : 3851–3864.
31. WilcoxM, DiAntonioA, LeptinM (1989) The function of PS integrins in Drosophila wing morphogenesis. Development 107 : 891–897.
32. BementWM, MillerAL, von DassowG (2006) Rho GTPase activity zones and transient contractile arrays. Bioessays 28 : 983–993.
33. YanJ, LuQ, FangX, AdlerPN (2009) Rho1 has multiple functions in Drosophila wing planar polarity. Dev Biol 333 : 186–199.
34. Dominguez-GimenezP, BrownNH, Martin-BermudoMD (2007) Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J Cell Sci 120 : 1061–1071.
35. ChenJ, HoneyagerSM, SchleedeJ, AvanesovA, LaughonA, et al. (2012) Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development 139 : 2170–2176.
36. BuntS, HooleyC, HuN, ScahillC, WeaversH, et al. (2010) Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell 19 : 296–306.
37. WangX, HarrisRE, BaystonLJ, AsheHL (2008) Type IV collagens regulate BMP signalling in Drosophila. Nature 455 : 72–77.
38. Pastor-ParejaJC, XuT (2011) Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev Cell 21 : 245–256.
39. MurrayMA, FesslerLI, PalkaJ (1995) Changing distributions of extracellular matrix components during early wing morphogenesis in Drosophila. Dev Biol 168 : 150–165.
40. VuilleumierR, SpringhornA, PattersonL, KoidlS, HammerschmidtM, et al. (2010) Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol 12 : 611–617.
41. SzuperakM, SalahS, MeyerEJ, NagarajanU, IkmiA, et al. (2011) Feedback regulation of Drosophila BMP signaling by the novel extracellular protein larval translucida. Development 138 : 715–724.
42. KesavanG, SandFW, GreinerTU, JohanssonJK, KobberupS, et al. (2009) Cdc42-mediated tubulogenesis controls cell specification. Cell 139 : 791–801.
43. LahozA, HallA (2008) DLC1: a significant GAP in the cancer genome. Genes Dev 22 : 1724–1730.
44. MassagueJ (2008) TGFbeta in Cancer. Cell 134 : 215–230.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



