-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
As a para-retrovirus, hepatitis B virus (HBV) is an enveloped virus with a double-stranded (DS) DNA genome that is replicated by reverse transcription of an RNA intermediate, the pregenomic RNA or pgRNA. HBV assembly begins with the formation of an “immature” nucleocapsid (NC) incorporating pgRNA, which is converted via reverse transcription within the maturing NC to the DS DNA genome. Only the mature, DS DNA-containing NCs are enveloped and secreted as virions whereas immature NCs containing RNA or single-stranded (SS) DNA are not enveloped. The current model for selective virion morphogenesis postulates that accumulation of DS DNA within the NC induces a “maturation signal” that, in turn, triggers its envelopment and secretion. However, we have found, by careful quantification of viral DNA and NCs in HBV virions secreted in vitro and in vivo, that the vast majority of HBV virions (over 90%) contained no DNA at all, indicating that NCs with no genome were enveloped and secreted as empty virions (i.e., enveloped NCs with no DNA). Furthermore, viral mutants bearing mutations precluding any DNA synthesis secreted exclusively empty virions. Thus, viral DNA synthesis is not required for HBV virion morphogenesis. On the other hand, NCs containing RNA or SS DNA were excluded from virion formation. The secretion of DS DNA-containing as well as empty virions on one hand, and the lack of secretion of virions containing single-stranded (SS) DNA or RNA on the other, prompted us to propose an alternative, “Single Strand Blocking” model to explain selective HBV morphogenesis whereby SS nucleic acid within the NC negatively regulates NC envelopment, which is relieved upon second strand DNA synthesis.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002255
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002255Summary
As a para-retrovirus, hepatitis B virus (HBV) is an enveloped virus with a double-stranded (DS) DNA genome that is replicated by reverse transcription of an RNA intermediate, the pregenomic RNA or pgRNA. HBV assembly begins with the formation of an “immature” nucleocapsid (NC) incorporating pgRNA, which is converted via reverse transcription within the maturing NC to the DS DNA genome. Only the mature, DS DNA-containing NCs are enveloped and secreted as virions whereas immature NCs containing RNA or single-stranded (SS) DNA are not enveloped. The current model for selective virion morphogenesis postulates that accumulation of DS DNA within the NC induces a “maturation signal” that, in turn, triggers its envelopment and secretion. However, we have found, by careful quantification of viral DNA and NCs in HBV virions secreted in vitro and in vivo, that the vast majority of HBV virions (over 90%) contained no DNA at all, indicating that NCs with no genome were enveloped and secreted as empty virions (i.e., enveloped NCs with no DNA). Furthermore, viral mutants bearing mutations precluding any DNA synthesis secreted exclusively empty virions. Thus, viral DNA synthesis is not required for HBV virion morphogenesis. On the other hand, NCs containing RNA or SS DNA were excluded from virion formation. The secretion of DS DNA-containing as well as empty virions on one hand, and the lack of secretion of virions containing single-stranded (SS) DNA or RNA on the other, prompted us to propose an alternative, “Single Strand Blocking” model to explain selective HBV morphogenesis whereby SS nucleic acid within the NC negatively regulates NC envelopment, which is relieved upon second strand DNA synthesis.
Introduction
The hepatitis B virus (HBV) is a global human pathogen that chronically infects hundreds of millions and causes a million fatalities yearly. It belongs to the Hepadnaviridae family, which also includes several related animal viruses such as the duck hepatitis B virus (DHBV) [1]. Hepadnaviruses contain a small (ca 3 kb), partially double-stranded (DS) DNA genome enclosed within an icosahedral capsid that is formed by 240 (or 180 in a minority population) copies of the same viral protein, the core or capsid protein (HBc), and is, in turn, coated with an outer envelope. As pararetroviruses, hepadnaviruses assemble initially as immature nucleocapsids (NCs), packaging an RNA pregenome (pgRNA). These immature NCs undergo a process of maturation first to NCs containing a single-stranded (SS) DNA (still considered immature) and subsequently to mature NCs containing the DS DNA genome, via reverse transcription of pgRNA inside the maturing NCs. Only the mature NCs are then enveloped by the viral envelope or surface (HBs) proteins and secreted extracellularly [2], [3].
How genome maturation, within NCs, is coupled to envelopment, from without, remains poorly understood. In particular, the exact nature of the viral genome that is ultimately responsible for regulating virion secretion is not yet clear. As SS RNA or DNA is not secreted in virions but DS DNA, in either the major, relaxed circular (RC) or minor, double-stranded linear (DSL) form, or RNA-DNA hybrid, is [3]–[10], the prevailing model posits that the accumulation of DS DNA as a result of second strand elongation during reverse transcription triggers a structural change in the maturing NC that, in turn, signals envelopment and secretion [2], [3], [11], [12]. Thus, this so-called maturation signal would emerge on the mature NC only as reverse transcription approaches completion and positively regulate virion secretion.
On the other hand, it has been suggested that HBV may secrete virions containing no DNA at all. Two populations of HBV virion particles were found to circulate in the blood of infected patients decades ago, with one having a lighter buoyant density than the other [13]–[15]. These so-called “light” virion particles contained HBV envelope and core proteins but in contrast to the “heavy” particles, displayed no endogenous polymerase activity, which reflects DNA synthesis by the virion reverse transcriptase (RT) using the endogenous DNA template. These light particles also appeared empty under electron microscopy (EM) and were assumed to be devoid of viral DNA. However, these early reports did not directly determine the levels of viral DNA in the light virions or whether they contained viral RNA or host nucleic acid. A more recent study suggested that the light virion particles might actually contain, instead of the normal capsid protein, an aberrantly processed precore protein [16]. Another recent report found that small amounts of enveloped HBV capsids devoid of viral genome were secreted in transfected cell cultures but those were deemed aberrant [9], [16]. Thus, it has remained unclear if HBV does secrete DNA-free virions and if so, whether it is part of the normal virion morphogenesis process.
In our efforts to further define the nature of the viral genome that underlies selective NC envelopment and virion secretion, we have found that genome packaging or DNA synthesis, per se, was actually not required at all for virion secretion as HBV mutants able to form normal capsids but incapable of viral RNA packaging or DNA synthesis, due to defects in RT, were secreted readily as enveloped, empty (nucleic acid-free) virions. Furthermore, quantitative analyses of virion-associated DNA and capsids revealed that the vast majority of virions secreted by the wild type (WT) HBV in cell culture as well as in infected chimpanzees contained no viral DNA. We propose a new model to explain the selective HBV virion morphogenesis that can reconcile the seemingly contradictory observations that virion formation selects stringently for DS, and against SS, nucleic acid genome and yet empty HBV virions devoid of any nucleic acid are secreted.
Results
Dramatic reduction of HBV DNA synthesis did not decrease virion secretion
We obtained the first hint that HBV virion secretion may not necessarily require viral DNA synthesis during our recent studies on the anti-HBV effect of the cellular antiviral protein, Apobec3G (A3G) [17]. As shown in Figure 1 and reported earlier [18], over-expression of A3G led to a dramatic reduction of HBV DNA associated with non-enveloped (naked) NCs as well as virions secreted into the culture medium of transfected human hepatoma (HepG-2) cells, which were resolved by native agarose gel electrophoresis [19]. However, we found, unexpectedly, that the amount of enveloped capsids secreted into the culture medium was not decreased at all by A3G. The identity of the virion signal was further verified by its association with, and dependence on, the viral envelope proteins (Figures 1 & 2) and its authentic buoyant density (see below). Naked capsids, which are routinely released into cell culture medium via an unknown mechanism unrelated to virion secretion and are of uncertain significance [20], were not affected by A3G though their DNA content was reduced as reported before [18]. HBsAg particles, which contain only the viral envelope proteins but no capsids nor genome [1], were not affected by A3G either (Figure 1). As HBsAg particles are normally released in large excess (100-fold or more) over virions [1], the surface protein signals detected in Figures 1 and 2 were virtually all from the HBsAg but the virion and HBsAg comigrated on the gel. This was also evidenced by the surface protein signal migrating at the virion position from a mutant defective in core and polymerase protein expression (C-P-) and thus could only secrete HBsAg particles but no virions (neither empty nor DNA-filled) at all (Figure 2B). These results indicated that viral DNA synthesis might not be required for NC envelopment and virion formation, in apparent contradiction to the prevailing maturation signal hypothesis.
Fig. 1. Inhibition of HBV DNA synthesis by Apobec3G did not block virion secretion. 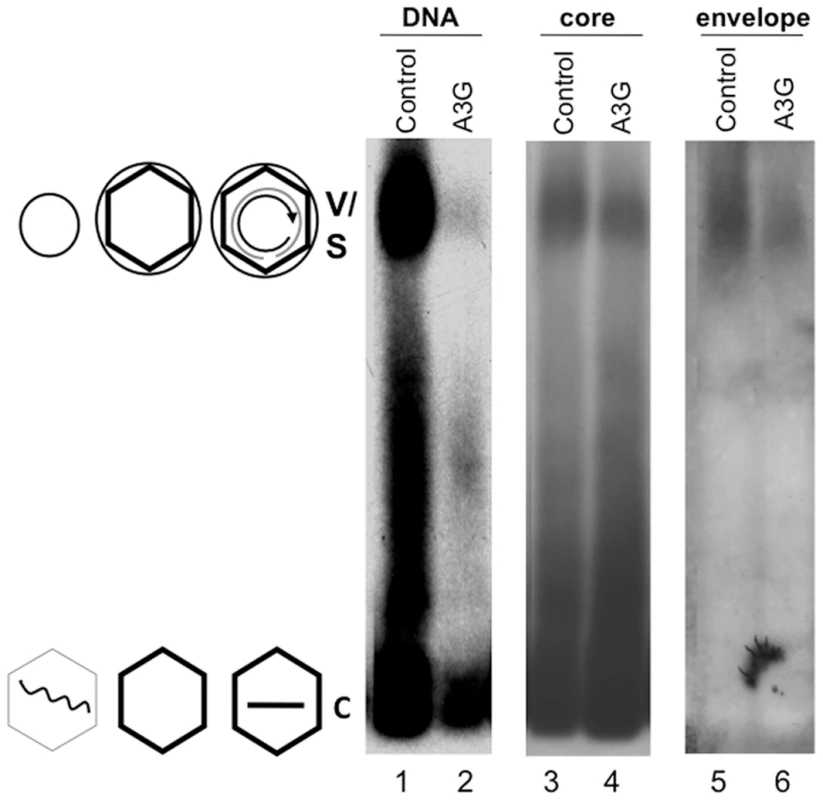
Culture media from HepG-2 cells transfected with pCMV-HBV plus pA3G-Flag (lanes 2, 4, 6; A3G or Apobec3G), or plus vector control (lanes 1, 3, 5; control) were collected and viral particles concentrated from the medium were resolved by agarose gel electrophoresis and transferred to nitrocellulose membrane. Viral DNA (lanes 1–2), and the core (lanes 3–4) and envelope (lanes 5–6) proteins were detected sequentially using a 32P-labeled HBV DNA probe, the anti-core antibody, and the anti-S antibody respectively, all on the same piece of membrane. V, virions, containing either RC DNA or empty; C, naked capsids, containing DNA (mostly SS), RNA, or empty. The diagrams on the side depict the structures of the virions and capsids. Wavy line, viral RNA; straight single line, SS DNA; concentric partial circle, RC DNA; hexagon, capsid; large circle, virion envelope; small circle, HBsAg particles (S, containing no capsid nor genome) comigrating with the virions. Fig. 2. Analyses of HBV virion secretion by native agarose gel electrophoresis. 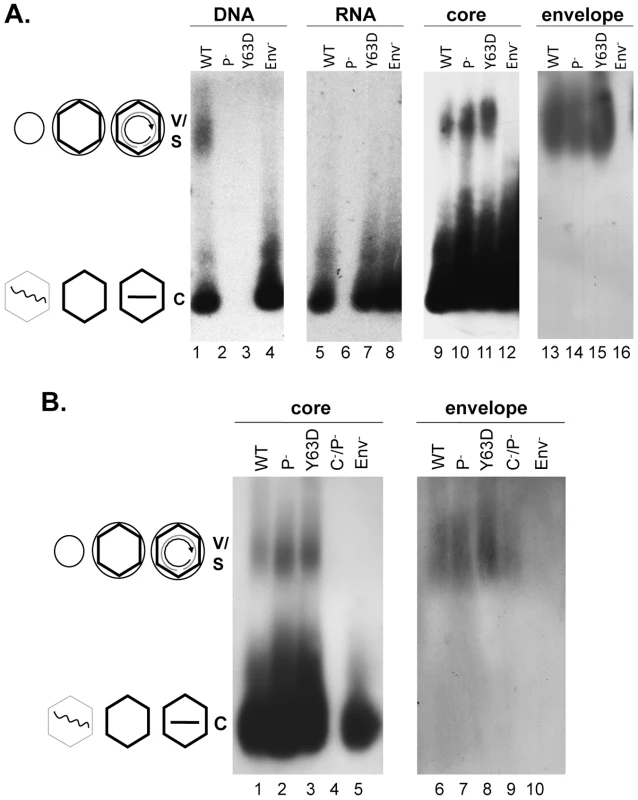
WT or mutant HBV genomes (Pol-, defective in pgRNA packaging; Y63D, competent in pgRNA packaging but defective in DNA synthesis; Env-, envelope deletion; C-P-, deficient for both core and polymerase expression) were transfected into HepG-2 cells. Viral particles concentrated from the culture medium were resolved by agarose gel electrophoresis and transferred to nitrocellulose membrane. Viral DNA (A, lanes 1–4) or core (A, lanes 9–12; B, lanes 1–5) and envelope proteins (A, lanes 13–16; B, lanes 6–10) were detected as in Figure 1. Viral RNA (A, lanes 5–8) was detected as for viral DNA, except that the samples were resolved on a separate gel that was not treated with NaOH prior to transfer and the membrane probed with a plus-strand specific riboprobe. V, virions, containing either RC DNA or empty; C, naked capsids, containing DNA, RNA, or empty. The diagrams on the side depict the structures of the virions and capsids as described in Figure 1. HBV mutants defective in RNA packaging or DNA synthesis secreted enveloped capsids containing no viral RNA or DNA
To further examine the relationship between genome content and virion secretion in HBV, we determined the virion secretion capacity of two mutants, Pol- and Y63D; Pol- is defective in polymerase expression and unable to package viral RNA [21] whereas Y63D is defective in DNA synthesis but fully functional in RNA packaging [22] (Figure 2). Native agarose gel analyses of HBV particles concentrated from the culture medium of transiently transfected HepG-2 cells showed that as reported before [5], pgRNA was not found in enveloped virions though readily detectable in naked capsids of either WT or the Y63D mutant (Figure 2). As anticipated, no viral RNA was detected in either NC or virion particles from the Pol- mutant, and only the WT, but not the Y63D or Pol- mutant, contained viral DNA in virions or naked capsids. However, capsid protein signal was clearly detected not only with the naked capsids but also with the virion particles from the Y63D and Pol- mutants, at levels at least as high as those of the WT. As a negative control, the Env- mutant did not show any capsid or DNA signal migrating at the virion position.
The co-migration of the putative Y63D and Pol- virions with the WT virions as well as with the envelope signals supported their identification as authentic virions, which was further verified by density gradient centrifugation (Figure 3; Figure S1). Native agarose gel electrophoresis analyses of viral particles fractionated on the CsCl gradient confirmed that enveloped capsids, i.e., virions, judged both by their density profile and mobility on the agarose gel, were indeed secreted by the Y63D and Pol- mutants even though they contained no viral DNA. Consistent with the notion that these enveloped capsids contained no nucleic acid (viral or cellular; also see below) at all, the empty virions (marked as peak #2) produced by the Y63D or Pol- mutant had a slightly lower density (as predicted by their lack of nucleic acid) than the authentic, DNA-containing virions secreted by the WT HBV (peak #1). Furthermore, the capsid signal peak (marked as #2) from the WT virion fractions also had a lower density than the DNA peak. This later result suggested that most of the WT HBV virions might also be empty such that the bulk virions (empty) had a density lighter than the minor amounts of DNA-containing virions.
Fig. 3. Analyses of HBV virion secretion by CsCl density gradient analyses. 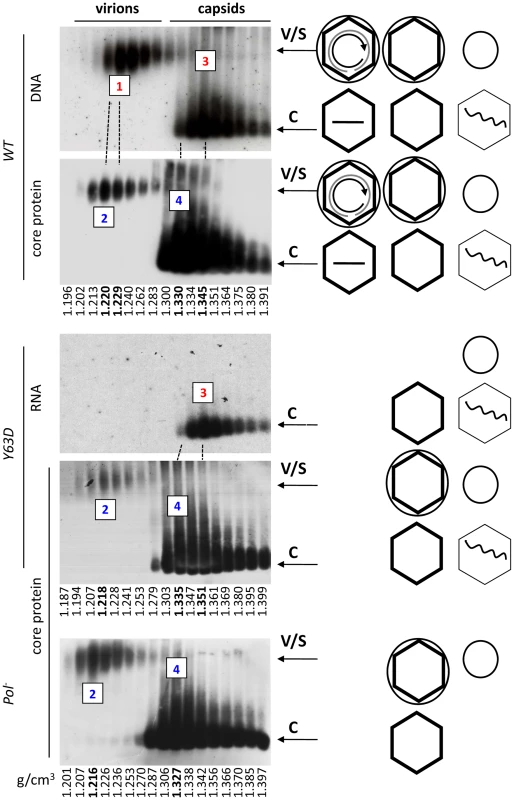
WT or mutant HBV genomes were transfected into HepG-2 cells and viral particles concentrated from the culture media as in Figure 1. The concentrated media were then fractionated by CsCl gradient centrifugation. Gradient fractions were analyzed by resolving viral particles on native agarose gels. HBV DNA (panel 1), RNA (panel 3), and core protein (panels 2, 4, 5) were detected as described in Figures 1 & 2. WT, panels 1 & 2; Y63D, panels 3 & 4; Pol-, panel 5. The numbered fractions mark the DNA-containing (#1) or DNA-free (#2) virion peaks, and the naked capsid peaks containing viral DNA or RNA (#3) or no nucleic acid (empty, #4), with their respective buoyant densities indicated in bold at the bottom. Note that the first two panels from the top were derived from the same membrane sequentially probed for viral DNA and the core protein, and the 3rd and 4th panels also from the same membrane sequentially probed for viral RNA and core protein. The dashed lines are used to align the identical lanes from the same membrane. The diagrams on the side depict the structures of the virions and capsids as described in Figure 1. Although small amounts of naked NCs migrated near the position of the virions after the CsCl gradient fractionation step, presumably in aggregated forms as a result of exposure to the high salt concentration of the gradient as suggested earlier [9], the naked NCs were clearly separated from the virions by the CsCl density gradient fractionation such that there was no naked NC contamination in the virion fractions (Figure 3). In addition, consistent with numerous previous reports (see Introduction), immature SS DNA was found in naked NCs but not in the virion fractions (Figure S2; and also Figure 4 below), again indicating clear separation of virions from naked NCs. On the other hand, no naked capsid signal was detected near the virion position on the agarose gel without the CsCl gradient centrifugation step as indicated by the absence of any capsid protein or viral DNA signal at the virion position from an HBV mutant defective in envelope protein expression (Figure 2A, lanes 4, 12; Figure 2B, lane 5). Furthermore, abolishing viral envelope protein expression eliminated any capsid or viral DNA signal in the virion fractions on the CsCl gradient (Figure S3), again indicating the secretion of the empty virions, just like the DNA-containing virions, was absolutely dependent on the viral envelope proteins and no naked capsids contaminated the virion fractions following the density gradient fractionation.
Fig. 4. Quantitative analyses of DNA-filled and empty virions secreted by WT HBV in vitro and in vivo. 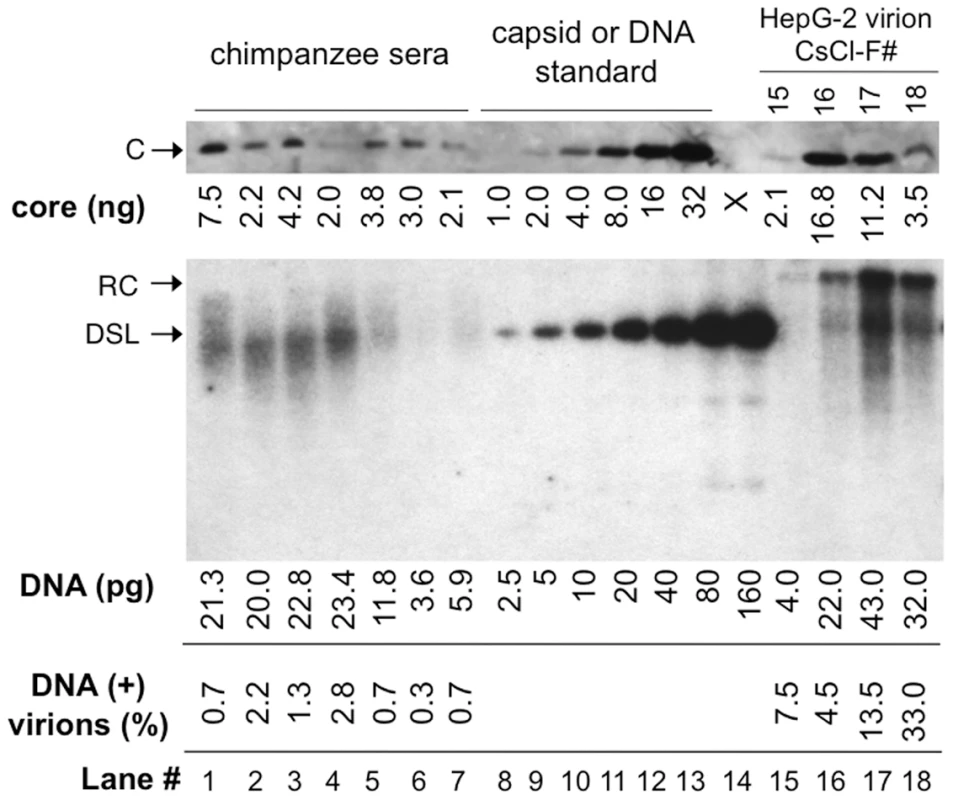
HBV virions in the sera of experimentally infected chimpanzees (lanes 1–7; 2 µl each serum) and purified from WT HBV transfected HepG-2 cells by CsCl density gradient centrifugation (lanes 15–18; 30 µl each fraction), along with known amounts of HBV capsid standard (1–32 ng; lanes 8–13), were resolved by SDS-PAGE; Virion-associated core protein was detected by western blot analysis using the anti-HBV core monoclonal antibody (top image). The virion DNA released by SDS-protease digestion from the same chimpanzee sera (lanes 1–7; 2 µl each serum) and HepG-2 virion fractions (lanes 15–18; 2 µl each fraction) were also resolved on an agarose gel, along with known amounts of HBV DNA (3 kb, 2.5–160 pg, lanes 8–14), and detected by Southern blot analysis (bottom image). Lane 1 was from chimpanzee A0A006 at week 7 post-infection (PI); lanes 2 & 3, chimpanzee 1603 at week 15 and 16 PI, respectively; lanes 4 & 5, chimpanzee 1618 at week 14 and 17; lanes 6 & 7, chimpanzee 1616 at week 20 and 23 PI. The amounts of HBV core protein (top) and virion DNA (bottom) are indicated at the bottom of the lanes. The percentages of DNA-containing virions, calculated as the molar amounts of HBV DNA divided by the molar amounts of HBV capsid (240 copies of core subunits per capsid) and multiplied by 100, are also indicated. Please note that 15-fold more HepG-2 virions were loaded on the SDS-PAGE gel than on the agarose gel, which was taken into account in the calculation of DNA-containing virions. F#, virion fraction number from the CsCl gradient. C, core protein; RC and DSL, RC and DSL DNA; X, blank lane (lane 14) on the SDS-PAGE gel. WT HBV secreted mostly empty virions in vitro and in vivo
To follow up on the above suggestion, we decided to quantify the amount of HBV DNA and the capsid protein signals within the virion fractions secreted by WT HBV transfected HepG-2 cells. This revealed that most secreted HBV virions (from 92.5% in the lighter fraction to 67% in the heavier fraction) from transfected cells (Figure 4, lanes 15–18) were indeed devoid of any viral DNA. These estimates were based on quantifications of the levels of virion-associated capsids (based on 240 copies of core protein per capsid) vs. the virion DNA. Although it is theoretically possible that the DNA-free virion capsids may have reacted differently than the DNA-containing virion capsids with the anti-HBc antibody used for the western blotting, this was made unlikely by the fact that the relative signals of the capsid proteins remained constant across the lighter (virtually DNA-free) and heavier (with more DNA-containing virions) fractions (Figure S4), whether the capsid protein levels were estimated as native particles resolved on an agarose gel or as denatured subunits resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by either a polyclonal antibody or a monoclonal directed at a linear epitope at the N-terminus of the capsid protein [23].
As reported earlier [13], [14] using HBV positive human sera, two kinds of WT HBV virions were observed by EM after negative staining, with either a filled or empty looking inner capsid under EM (Figure 5A). However, for most virions, the stain did not penetrate the envelope thus making it difficult to discern if the capsids within the virions were filled or empty. To visualize better the capsids contained in the virions, the virion envelope was removed by detergent lysis [12], [14] and the released capsids were observed under EM. Upon removal of the virion envelope by the detergent treatment, we found that the majority of the released capsids (ca 70%) had an empty appearance consistent with their containing no nucleic acid while the remaining showed a filled appearance presumably containing the DS DNA (Figure 5B). We also released the capsids from the Pol- mutant virions using the same method and found that all released Pol- capsids showed the empty appearance, consistent with their lower density and containing no nucleic acid (Figure 5C). As higher concentrations of capsids were obtainable from intracellular sources, more detailed analyses of the empty and filled capsids, including 3-D image reconstruction, were carried out on those capsids and are described below (Figures 6 & 7; Figures S8 & S9).
Fig. 5. EM of HBV virions and virion-derived capsids. 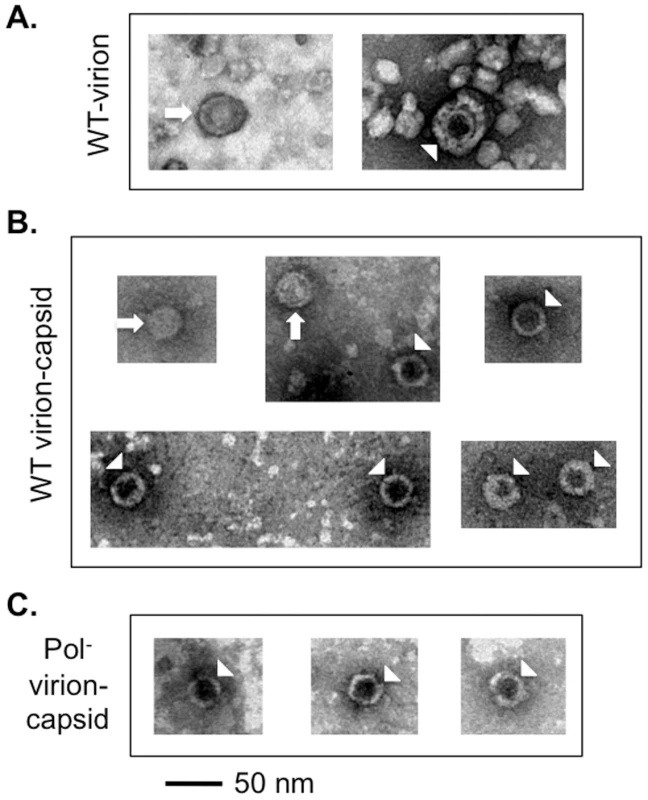
HBV WT or Pol- virions released from transfected HepG-2 cells were fractionated by CsCl density gradient centrifugation. The purified WT virions (A), or capsids released from the WT (B) or Pol- (C) virions by NP40 treatment were visualized by EM after negative staining. The arrows indicate the filled virion or virion-derived capsids and the arrowheads denote the empty virion or virion-derived capsids. Note that the smaller particles in A represent the HBsAg spheres, which were much more numerous than the virions and were removed by the detergent treatment in B and C. Fig. 6. Nucleic acid and protein staining of intracellular capsids. 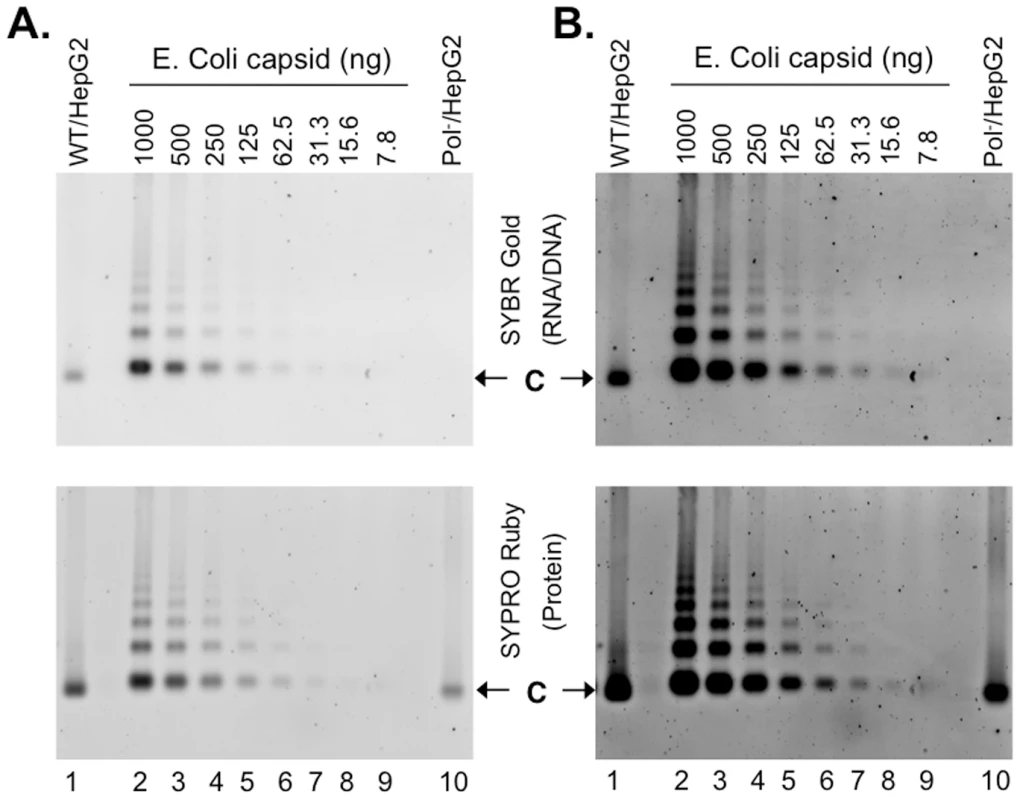
WT HBV NCs (lane 1) and HBV capsids devoid of the polymerase (Pol-) (lane 10) were purified from HepG-2 cells transiently transfected with pCMV-HBV and pCMV-HBV-Pol-, respectively. WT HBV capsids purified from E. Coli (lanes 2–9) were purchased from Virogen. The capsids were resolved by native agarose gel electrophoresis. Capsid-associated nucleic acid was detected by SYBR Gold staining (top) and the capsid proteins were detected by destaining of the SYBR Gold signal and subsequent restaining of the same gel with SYPRO Ruby (bottom). Note that A & B are from the same gel and the images in B are contrast-enhanced to display the weak signals. C, capsids. Fig. 7. EM of intracellular capsids. 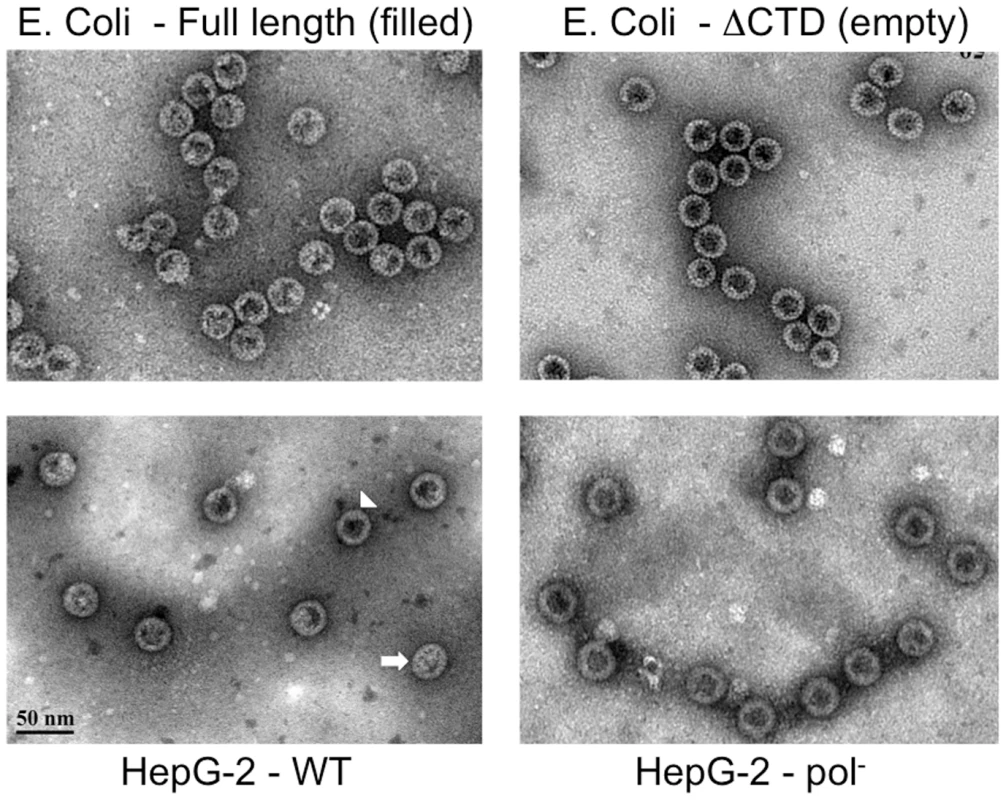
Native HBV NCs purified by sucrose gradient centrifugation from transfected HepG-2 cells (WT, bottom left; Pol-, bottom right) were visualized by EM after negative staining with phosphotungstic acid. E. Coli-derived HBV WT (top left) or C-terminally truncated (at position 144, C144 or ΔCTD; top right) capsids were examined in parallel as (RNA) filled and empty capsid controls, respectively. Two WT capsids with distinct appearances, empty (arrowhead) and filled (arrow), are indicated. Intrigued by these results, we decided to quantify the levels of viral DNA and capsid protein in the sera of HBV infected chimpanzees [24]. As shown in Figure 4 (lanes 1–7) and Figure S5, the vast majority (97.8–99.7%) of HBV virions found in the sera from all four infected chimpanzees harvested at two different times post-infection contained no viral DNA. HBV virions in one of the chimpanzee sera were also fractionated by CsCl density gradient centrifugation. As with the virions released by transfected cells in culture, the core protein peak (Figure S5B, lane 2) in the serum virions had a lighter density than the DNA peak (Figure S5B, lane 4), indicating again the majority of the WT virions in the serum were DNA-free and had a lighter density. The core protein signal in the DNA peak fraction (lane 4) was below the limit of detection since our core protein Western blotting had a sensitivity of ca 1 ng/lane while the Southern blotting had a sensitivity of less than 2.5 pg/lane (Figure 4). The virion DNA secreted by the infected chimpanzees in vivo migrated mostly just below the 3 kb DS DNA standard (Figure 4, bottom image, lanes 1–7), typical for the incompletely DS HBV virion RC DNA isolated from infected patients [10], [25]. For reasons yet to be understood, HBV virion DNA secreted by the transfected hepatoma cells in vitro appeared more fully DS [26], migrating above the 3 kb linear DNA (Figure 4, bottom, lanes 15–18).
As the HBsAg particles are present in large excess (100–1,000 fold or more) over virions [1] and we detected also a large excess of empty virions over DNA-containing ones (with total virion core proteins reaching 1–3.75 µg/ml or 1.6–4.4×1011 virions/ml), it was of interest to estimate the levels of HBsAg in the sera from the infected chimpanzees. To this end, purified recombinant HBsAg (eEnzyme) was used a quantitative standard for western blotting. Due to the undefined oligomeric states (a mixture of monomers, dimers and higher order oligomers; eEnzyme) of the HBsAg standard, it was resolved by SDS-PAGE along with different dilutions of two serum samples from the same chimpanzee, one before and one after HBV infection. The surface proteins were detected using a rabbit polyclonal antibody that can recognize the denatured HBsAg (Figure S6A) and the HBsAg concentration in the HBV-positive serum was estimated based on comparison with the standard. The HBsAg concentrations in the other chimpanzees were then estimated by comparison to this serum after they were resolved together on an agarose gel (Figure S6B). Our estimates of the serum HBsAg levels in all four infected chimpanzees ranged from 124–1,899 µg/ml, or ca 3×1013–4.4×1014/ml assuming 100 copies of the small surface protein per HBsAg sphere [1], [27]. Thus, the HBsAg levels were ca 167 - to 1,688-fold above the virions (both DNA-containing and empty). These estimates were in good agreement with earlier estimates of HBsAg concentrations in the serum of HBV infected human patients of 1013/ml or 40 µg/ml [28] to more recent quantifications of up to 1015 or 4 mg/ml [29]–[31].
Empty HBV capsids were assembled in human cells that did not package non-specific RNA
The secretion of HBV virions containing no nucleic acid at all implied that empty HBV capsids, containing no viral or cellular nucleic acid, were assembled in host cells. In support of this, the naked capsids harvested from the WT and Y63D transfections peaked at a lower density (Figure 3 & Figure S1, peak #4;) than the viral RNA or DNA signals (i.e., filled NCs) that peaked at a higher density (peak #3). Although this capsid protein peak contained some viral RNA or DNA, it likely represented the overlap from the heavier, nucleic acid-containing NCs; earlier (still lighter) capsid fractions contained little to no viral RNA or DNA. The naked capsids of the polymerase-null mutant, being devoid of any viral RNA or DNA (Figure 2) or cellular nucleic acid (see Figure 6 below), peaked at the same light density as the capsid protein peak (#4) from the WT and the Y63D mutant, lighter than the RNA or DNA-containing NCs peak (#3).
To further verify that empty HBV capsids, containing no viral or cellular nucleic acid, were indeed made in cells, we purified intracellular capsids from transfected HepG-2 cells by sucrose gradient centrifugation, resolved them on agarose gels along with HBV capsids purified from E. Coli that are known to be filled with nonspecific RNA [32]–[34], and detected the capsids (the protein signal) or their associated nucleic acid with the highly sensitive general protein (SYPRO Ruby) or nucleic acid (SYBR Gold) stain. The detection sensitivity of the capsid-associated RNA (approximately 8 ng capsids, or 1.6 ng RNA assuming each capsid packages 3 kb RNA; Figure 6) was about 5-fold less than that of purified RNA (approximately 0.3 ng RNA; Figure S7), presumably due to the sequestration of RNA inside the capsids. Therefore, instead of attempting to quantify the absolute amounts of nucleic acid associated with the HepG-2 cell-derived capsids, we used the E. Coli-derived capsids, not purified RNA or DNA, for relative comparison.
The HBV Pol- capsids did not show any detectable nucleic acid signal (Figure 6, top, lane 10), as anticipated. Given that 244 ng of Pol- capsids (Figure 6, bottom, lane 10), estimated based on comparison to the E. Coli-derived capsid standard (lanes 2–9), was loaded and the RNA associated with 7.8 ng bacterially derived capsids was detectable, the amount of RNA in the pol- capsids, if any, was thus less than 3% of that in the E. Coli-derived capsids. The capsids harvested from WT HBV transfected HepG-2 cells (Figure 6, lane 1) were found to contain detectable but much less (ca 35%) nucleic acid when compared to the E. Coli-derived capsids. Thus, the amount of WT HBV capsids purified from HepG-2 cells loaded was 707 ng (Figure 6, bottom, lane 1) but its nucleic acid content was equivalent only to 269 ng E. Coli-derived capsid (Figure 6, top, lane 1). Titration of the WT HBV capsids harvested from HepG-2 cells showed that the nucleic acid content could be detected by the staining method when ca 100 ng of capsid was loaded per lane (Figure S8). As the nucleic acid signal detected in the WT HBV NCs was a mixture of capsid-associated viral DNA as well as RNA, and the SYBR Gold stain had a higher (by ca 7 fold) detection sensitivity for DNA than RNA (Figure S7), the amount of nucleic acid associated with the WT capsids from HepG-2 cells, relative to that associated with the bacterially-derived capsids that contain only RNA, were likely overestimated. Thus, these results clearly indicated that the majority of WT HBV capsids assembled in hepatoma cells were also empty.
The purified capsids were further subjected to negative staining and EM (Figure 7), which showed that most (ca 80–90%) (Table S1) HBV capsids harvested from the WT HBV transfected HepG-2 cells, and virtually all capsids from the HBV Pol- transfected cells, displayed the thin-walled or empty appearance characteristic of nucleic acid-free capsids such as the empty, C-terminally truncated HBV capsids purified from E. Coli [15], [35], [36]. The rest (ca 10–20%) of the WT HBV capsids from HepG-2 cells showed the thick-walled or filled appearance consistent with their containing RNA or DNA, similar to the full-length capsids derived from E. Coli that are known to contain non-specific RNA.
To obtain further information about the interior contents of the various HBV capsid populations, 3-D capsid maps were reconstructed from selected negatively stained particles from the EM images (Table S1). To check how the 3-D reconstruction program translated the EM images into a 3-D model, the WT HBV capsids from the HepG-2 cells were separated into two groups, those that appeared to be empty and those that appeared to be filled, and reconstructions were performed separately for each group. The empty group resulted in a map with a hollow core, and the filled group depicted electron densities inside of the capsid (Figure S9). In comparison, the 3-D map of the HBV Pol- capsids also had a hollow core, in agreement with the results above showing that these capsids contained no detectable nucleic acid (Figures 2 & 6; Figure S8). Therefore, the results indicated that a 3-D reconstruction from negatively stained EM images could help distinguish between empty and filled viral particles. Capsids assembled from the full-length and C-terminally truncated capsid proteins expressed in E. Coli were also examined. The 3-D map of the full-length capsids from bacteria showed electron densities protruding inwards from the shell again consistent with the fact they contained non-specific RNA. In contrast, the C-terminally truncated capsids that appeared empty in the EM image also showed a hollow core in the corresponding 3-D model. These results agreed well with previously published cryo-EM structures that revealed that the amount of the RNA content in the E. Coli-derived HBV capsids depends on the length of the C-terminal nucleic acid binding domain of the core proteins [37].
A previous report suggested that an aberrant precore protein, lacking the C-terminal nucleic acid binding region of the core protein but retains the precore region preceding the core protein including the signal peptide, could assemble into abnormal capsids that were enveloped and secreted [16]. The constructs we used here to express the HBV core protein lacks the coding sequence for part of the precore region including the precore initiation codon and thus is not expected to produce any precore protein [38]. Also, the core protein in the purified capsids or virions migrated on SDS-PAGE as the full-length core protein standard (Figure 4; Figures S4 & S5), suggesting that no truncation or degradation occurred. To confirm directly that the HBV core protein made in transfected cells indeed contained the C-terminal region, we employed an antibody specific for the last 14 residues of the core protein [39]. As anticipated, this antibody failed to detect a C-terminally truncated core protein but could detect the core protein in both the WT and Pol- capsids; an antibody specific for the N-terminal sequence of the core protein detected the full-length as well as the truncated core proteins (Figure S10). These results thus verified that the HBV core protein expressed in our system was full-length and no aberrantly processed core or precore proteins were made.
Discussion
Hepadnaviruses select only mature, DS DNA-containing, but not immature, RNA - or SS DNA-containing NCs for envelopment and extracellular secretion as virions. The molecular mechanism underlying this selective virion morphogenesis, which is a defining characteristic of all hepadnaviruses as pararetroviruses, remains to be elucidated. We have provided here multiple, complementary lines of evidence to demonstrate the secretion of empty HBV virions with no viral or cellular nucleic acid, i.e., enveloped capsids with no RNA or DNA. First, the secretion of empty virions, like the DNA-containing ones, depended on the expression of the viral envelope proteins as viral mutants defective in envelope expression did not secrete any virions, empty or DNA-filled. Second, the co-migration of the capsid and envelope proteins on the agarose gel and their co-fractionation on the density gradient indicated the capsids detected at the virion position were enveloped, as confirmed by EM observation. Third, the buoyant density of the empty virions were very close to the DNA-containing virions and much lower than the naked NCs, as expected for enveloped capsids, Fourth, the absence of viral DNA or RNA in the empty virions was shown by the secretion of virions by the Pol- mutant that is incapable of packaging viral RNA or synthesizing DNA and by the Y63D mutant that can package pgRNA but can't synthesize any DNA, and by the lack of detection of any viral RNA or DNA by Southern blotting in these virions. Fifth, the absence of any nucleic acid, viral or cellular, in the empty virions was indicated by their slightly but reproducibly lower density than the DNA-containing virions and the EM observation of virions containing empty capsids as well as empty (mostly for the WT and entirely for the Pol- mutant) capsids released from the virions. Sixth, the naked (non-enveloped) capsids from the WT were shown to be mostly, and those from the Pol- HBV entirely, empty, with no viral or cellular nucleic acid. This was demonstrated by the absence of viral RNA or DNA by Southern blot analyses, by EM observation and 3-D image reconstruction, by their expected lighter density on the CsCl gradient, and by direct measurement of total nucleic acid in the capsids using sensitive nucleic acid staining methods. Thus, in the case of the Pol- virions, it would have been very difficult to imagine that the secreted virions would have contained any RNA or DNA since the intracellular Pol- capsids, which were the substrates for envelopment and virion formation, contained no detectable nucleic acid. Also, given that (a) most of the WT intracellular capsids contained no viral or cellular nucleic acid and the remaining WT capsids contained either viral RNA or DNA, (b) the intracellular capsids were the precursors to the enveloped virions, and (c) viral RNA-containing capsids were excluded from envelopment, it would have been difficult too to explain how the DNA-negative WT virions would have contained any nucleic acid.
The secretion of enveloped capsids, without any nucleic acid inside, is incompatible with the prevailing model of hepadnavirus morphogenesis, whereby virion formation requires DS DNA synthesis, i.e., NC maturation. Instead, we propose here that the paradox, i.e., the seemingly stringent selection of DS DNA over RNA or SS DNA in virion formation on one hand and yet secretion of empty HBV virions devoid of any nucleic acid on the other, can be resolved by invoking a new model, which we call “single strand (SS) blocking.” In this, negative signal model, the presence of SS DNA or pgRNA in the immature NCs actively prevents their envelopment by triggering a signal that negatively regulates NC envelopment (Figure 8A). The requirement of DS DNA for efficient virion secretion would NOT be due to a need to accumulate a threshold amount of total nucleic acid with the resulting increase in internal pressure, negative charge, genome rigidity, or some other unknown effect, which in turn would trigger a structural change (i.e., the maturation signal) on the maturing NC leading to envelopment and virion secretion, as envisioned by the classical maturation signal model (Figure 8B) [2], [3], [11], [12]. Rather, the apparent requirement for DS DNA synthesis in virion secretion reflects the need to remove the pgRNA or SS DNA, the trigger of the blocking signal, so as to relieve its inhibition on NC envelopment. Those capsids that do not package pgRNA (nor any other RNA, see below) would not display this negative signal and are thus competent for envelopment and secretion (as empty virions) (Figure 8A). Although the secretion of empty virions could be explained, in theory, by invoking a second envelopment signal for the empty capsids, independent from that emerging on the DS DNA-containing capsids, the simplest explanation is that the empty capsids and the DS DNA-containing capsids share the same envelopment signal.
Fig. 8. The single strand blocking hypothesis for selective hepadnavirus virion morphogenesis. 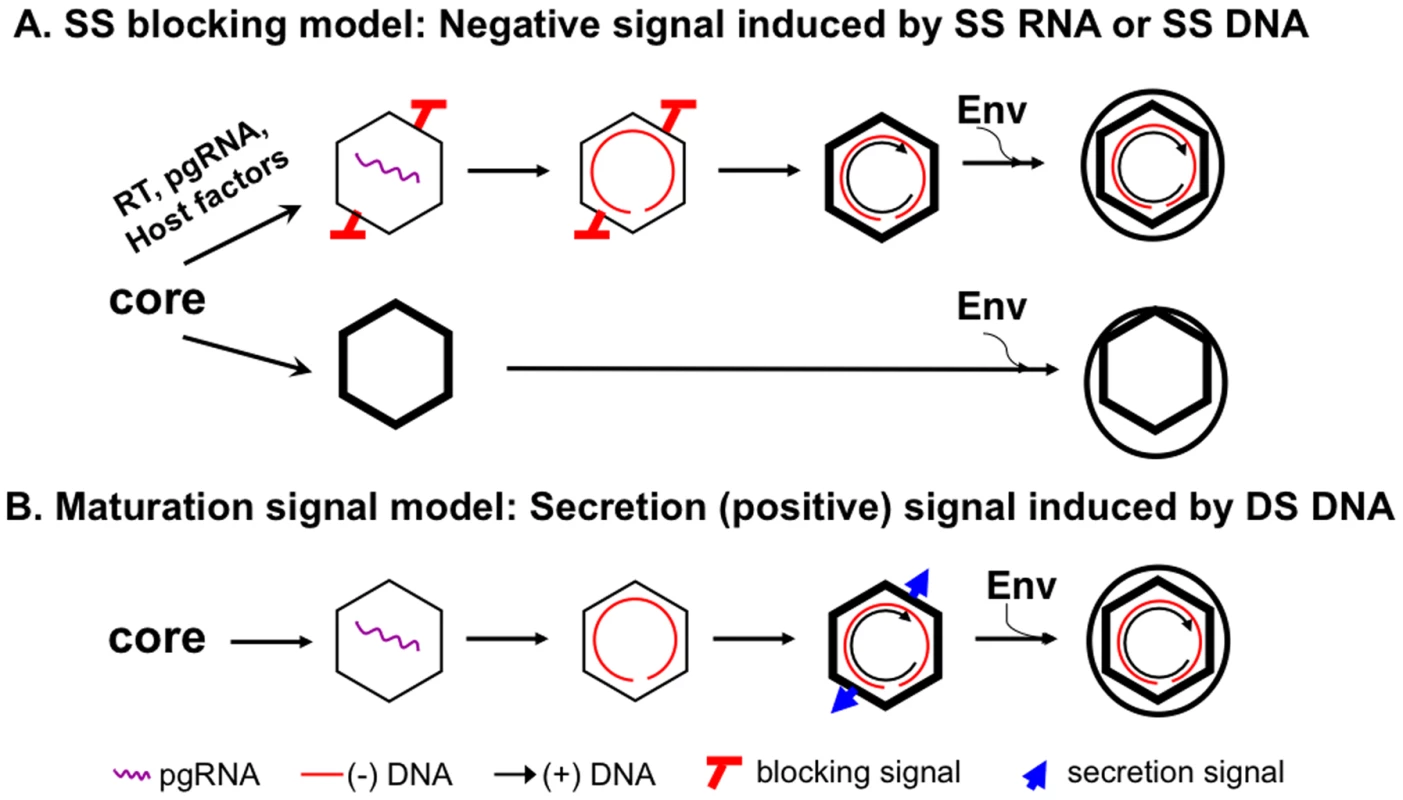
The new model is presented in panel A, in comparison with the classical maturation signal model depicted in panel B. See Discussion for details. The SS blocking model also predicts that decreasing the DS DNA (thus the total amount of nucleic acid inside the NC) may not block virion secretion. In support of this model, internally deleted short (ca 2 kb or shorter) DNAs, derived from packaging and reverse transcription of spliced HBV pgRNA have been detected commonly in the blood of HBV-infected patients [40], [41] and in transfected cell culture medium [42]–[44]. In contrast, the secretion of these shortened genomes in virions would be more difficult to explain with the classical maturation signal model.
The secretion of empty HBV virions requires assembly of empty capsids inside cells (Figure 8A). Three lines of evidence support the existence of these empty capsids: (1) their lower buoyant density than the nucleic acid-containing NCs, (2) lack of detection of nucleic acid with highly sensitive, general nucleic acid stain, and (3) their empty appearance under EM observation and 3-D capsid image reconstruction. HBV capsids assembled in insect cells did not package any cellular RNA either [45], [46] and presumably empty HBV capsids (so-called light cores) were also detected in the liver of HBV infected patients [14], [15], [47]. Thus, in contrast to capsids assembled in bacteria which package non-specific RNA [32]–[34], HBV capsids assembled in insect and mammalian cells do not package non-specific RNAs and are empty if they fail to package the viral pgRNA. The underlying mechanism for this difference remains to be elucidated but may be at least partly due to capsid phosphorylation in mammalian and insect cells that decreases its RNA binding activity [46], [48], [49].
We previously reported that the capsid protein associated with the DHBV virions, as well as intracellular mature NCs containing DS DNA, is unphosphorylated, in contrast to immature NCs that consist of heterogeneously phosphorylated core protein [11]. If DHBV, like HBV, secretes empty virions, it would suggest that not all unphosphorylated DHBV capsids are mature and some unphosphorylated DHBV capsids are in fact empty. If all unphosphorylated DHBV capsids do contain DS DNA, it would suggest that DHBV does not secrete empty virions, in contrast to HBV. For the single strand blocking model to accommodate the absence of empty DHBV virions, we suggest that DHBV capsids may package viral pgRNA more efficiently or could package non-specific RNA in host cells, unlike HBV. Consequently, empty DHBV capsids may not be assembled in host cells and thus unavailable for virion formation. Studies are currently underway to determine the relationship between genome content and virion secretion in DHBV.
How SS DNA or RNA triggers the proposed blocking signal is not yet understood. One NC maturation model suggests that the mature NC, by containing DS DNA and thus twice the amount of negative charges as the immature NC containing either the SS DNA or pgRNA, is destabilized as a result of the charge imbalance, triggering an NC conformational change that may be part of the maturation/envelopment signal [50], [51]. In the context of the single strand blocking model, it can be suggested that NC destabilization due to the charge imbalance can not only result from DS DNA accumulation within the maturing NC but also from the lack of any nucleic acid within the empty capsids. Indeed, empty HBV capsids were shown to be unstable especially under low salt conditions [51]. This structural instability may preclude the generation of the blocking signal, thus allowing the secretion of both empty and DS DNA-containing virions. The secretion of SS DNA-containing virions by certain HBV core mutants (the so-called immature secretion mutants) [9], [52], [53] and an avian hepadnavirus (the snowgoose HBV or SGHBV) [54] indicates that the blocking signal may be absent from, or sequestered by, these immature NCs. In addition, low level secretion of SS DNA-containing virions by the WT HBV has also been observed [52], [53] although we saw little, if any, SS DNA in WT HBV virions in our experiments here. It is possible that the stringency with which the single strand blocking signal controls virion morphogenesis may vary, to some extent, with the viral strains, the host cells, and the exact experimental conditions.
In conclusion, we propose that SS DNA or RNA within immature hepadnavirus NCs triggers a blocking signal that negatively regulates their envelopment and secretion, thus ensuring the secretion of only DS DNA (or RNA-DNA hybrid) in virions. However, empty capsids assembled in host cells that are devoid of any viral or cellular nucleic acid, thus lacking the negative signal, can be enveloped and secreted as “empty” virions containing capsids but no nucleic acid. Therefore, the long-sought-for maturation signal, a positive signal for envelopment, may in fact represent the removal of this negative signal, as the maturing NC retains ever-shorter SS DNA due to second (plus-) strand DNA elongation. In common with the maturation signal hypothesis, the SS blocking hypothesis also entails an as yet ill-defined structural change accompanying plus-strand DNA elongation (i.e., loss of the blocking signal). However, the blocking hypothesis predicts that mature, DS DNA-containing NCs share a structural characteristic with empty capsids (both lacking the blocking signal) and furthermore, this characteristic is absent from the pgRNA or SS DNA-containing NCs. The potential pathophysiological significance, if any, of the empty HBV virions remains to be determined. Among other possibilities, these empty virions may function as immune modulators, defective interfering particles, and markers of viral production or tissue damage [13], [55]. As the decision to secrete DNA-filled vs. empty virions is actually made at an earlier step during HBV assembly, i.e., during NC formation and pgRNA packaging (Figure 8A), the relative abundance of these two different virion populations reflects the efficiency of pgRNA packaging and may be a convenient marker for monitoring this complex step in the HBV life cycle that requires not only the viral RT and core proteins and pgRNA but also cellular factors [56], [57].
Methods
Plasmids
pCMV-HBV contains the HBV (ayw) 1.1-mer over-length genomic sequence driven by the cytomegalovirus (CMV) immediate-early promoter [38]. pCMV-HBV-Pol- and pCMV-HBV-Env- were derived from pCMV-HBV and are defective in polymerase and envelope protein expression, respectively [26]. pCMV-HBV-Core-Pol- was derived from pCMV-HBV-Pol- by introducing a 4 nt (GATC) insertion at position 1986 creating a frameshift mutation after codon 30 in the core gene. pCMV-HBV-Y63D bears a Y63D substitution in the RT gene eliminating DNA synthesis [22]. All site-specific mutations were confirmed by automated DNA sequencing. pA3G-Flag expresses the Flag-tagged human Apobec3G protein [18].
Transient transfection and stably transfected cell line
The human hepatoma cell line HepG-2 were transfected by FuGene 6 (Roche) [18], [58]. The HepAD38 cell line was derived from HepG-2 cell and replicates HBV in a tetracycline (Tet)-repressible manner [59].
Analyses of viral core protein expression and DNA synthesis
Core protein expression levels in the cytoplasmic lysate were analyzed by SDS-PAGE and western blotting using the anti-HBV core antibody (Dako, a rabbit polyclonal antibody; or a mouse monoclonal specific for the N-terminal end of the core protein [23]) as described previously [18], [58]. Briefly, primary antibodies were diluted at 1∶1,000 and incubated with proteins bound to Immobilon-P membrane (Millipore) overnight. Secondary anti-mouse or anti-rabbit peroxidase labeled antibodies were used at 1∶20,000 dilution. Chemilluminescence was used for detection of the bound antibody. A rabbit polyclonal antibody specific for the last 14 residues of the HBV core protein [39] was used where indicated to specifically detect the C-terminal sequence of the core protein. Virion - or naked capsid-associated DNA was isolated and analyzed by Southern blotting as described previously [18], [58].
Native agarose gel analysis of capsids and capsid-associated nucleic acid
Native HBV NCs were purified from transfected HepG-2 cells by sucrose gradient centrifugation [11], [60]. Recombinant HBV capsids purified from bacteria were obtained from Virogen. Purified NCs and capsids were resolved by native agarose gel electrophoresis [18], [58], [61], [62] and the gel was stained by SYBR Gold (Invitrogen) to detect RNA and DNA; after destaining with 10% methanol and 7% glacial acetic acid for 2 hr, the same gel was restained with SYPRO Ruby (Sigma) to detect proteins. The protein and nucleic acid signals were detected and quantified by using a Molecular Imager (BioRad FX-PRO Plus).
Analysis of virion secretion and CsCl density gradient centrifugation
Culture medium containing HBV virions and naked NCs was concentrated by polyethylene glycol precipitation and digested with DNase I (1 mg/ml at 37°C for 1 h) to eliminate residual plasmid DNA and fractionated by isopycnic CsCl gradient ultracentrifugation [7], [11], [61] to remove naked (non-enveloped) NCs. Purified virion fractions or DNase digested concentrated medium samples were analyzed by native agarose gel electrophoresis as described [63]. Encapsidated RNA or DNA in viral particles was detected using 32P-labeled RNA or DNA probe as indicated, followed by detection of core proteins associated with virions or naked NCs on the same membrane using the anti-core antibody. Goat polyclonal anti-HBV surface protein (Dako) was then used to detect the viral envelope proteins after stripping the membrane. The nature of virion DNA was determined by Southern blotting after extraction from purified virions using SDS/proteinase K digestion [7], [11]. The core protein associated with virions was also analyzed by SDS-PAGE and western blot analysis as described above. Sera from HBV infected chimpanzees have been described before [24]. DNA levels were quantified using phosphoimaging following Southern blot analysis and protein levels using densitometry following western blot analysis. Viral DNA and core protein standards were used to generate standard curves from which sample virion DNA and core protein levels were quantified. To estimate the levels of HBsAg, purified recombinant HBsAg (eEnzyme) was used as a quantitative standard. Following SDS-PAGE and western transfer, the HBV envelope proteins were detected by a polyclonal rabbit anti-HBs antibody (Virostat). To prepare virion-derived capsids for EM, the virion fractions were treated with 1% NP40-10 mM dithiothreitol (DTT) for 30 minutes on ice to remove the viral envelope and release the capsids [12], [14]. The released virion capsids, along with complete virions were observed under EM after neagative staining as described below.
EM and 3-D image reconstruction
For each sample, an aliquot of 3 µl was placed on a freshly glow-discharged continuous carbon coated copper grid. Phosphotungstic acid negative stain was applied by standard drop method and the sample was examined in JEOL 1400 TEM at 120 kV. For the 3-D reconstruction, 10–20 micrographs were collected with a Gatan Orius SC1000 CCD camera with Digital Micrograph at a calibrated magnification of 22,510X and 27,845X. Viral particles were selected and processed using Robem [64]. The reconstruction processes were performed without CTF correction using icosahedral averaging with the program Auto3dem, which generated a random model directly from the raw data as the initial starting structure [64]. The final resolution was determined where the Fourier shell correlation fell below 0.5 as reported in the summary output file of Auto3dem. The number of viral particles selected, the final resolution and diameters of the 3-D reconstructions are listed in Table S1. The final pixel sizes were 1.26 for the WT HBV capsids and 2.52 for all other constructs. The final reconstructions were colored radially using the program Chimera [65].
Supporting Information
Zdroje
1. SeegerCZoulimFMasonWS 2007 Hepadnaviruses. KnipeDMHowleyPM Fields Virology Philadelphia Lippincott, Williams & Wilkins 2977 3030
2. SeegerCHuJ 1997 Why are hepadnaviruses DNA and not RNA viruses? Trends Microbiol 5 447 450
3. SummersJMasonWS 1982 Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell 29 403 415
4. MillerRHTranCTRobinsonWS 1984 Hepatitis B virus particles of plasma and liver contain viral DNA-RNA hybrid molecules. Virology 139 53 63
5. GerelsaikhanTTavisJBrussV 1996 Hepatitis B virus nucleocapsid envelopment does not occur without genomic DNA synthesis. J Virol 70 4269 4274
6. LienJMPetcuDJAldrichCEMasonWS 1987 Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J Virol 61 3832 3840
7. PerlmanDHuJ 2003 Duck hepatitis B virus virion secretion requires a double-stranded DNA genome. J Virol 77 2287 2294
8. WeiYTavisJEGanemD 1996 Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J Virol 70 6455 6458
9. SchormannWKraftAPonselDBrussV 2006 Hepatitis B virus particle formation in the absence of pregenomic RNA and reverse transcriptase. J Virol 80 4187 4190
10. SummersJO'ConnellAMillmanI 1975 Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A 72 4597 4601
11. PerlmanDHBergEAO'Connor PBCostelloCEHuJ 2005 Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc Natl Acad Sci U S A 102 9020 9025
12. RosemanAMBerrimanJAWynneSAButlerPJCrowtherRA 2005 A structural model for maturation of the hepatitis B virus core. Proc Natl Acad Sci U S A 102 15821 15826
13. GerinJLFordECPurcellRH 1975 Biochemical characterization of Australia antigen. Evidence for defective particles of hepatitis B virus. Am J Pathol 81 651 668
14. KaplanPMFordECPurcellRHGerinJL 1976 Demonstration of subpopulations of Dane particles. J Virol 17 885 893
15. SakamotoYYamadaGMizunoMNishiharaTKinoyamaS 1983 Full and empty particles of hepatitis B virus in hepatocytes from patients with HBsAg-positive chronic active hepatitis. Lab Invest 48 678 682
16. KimuraTOhnoNTeradaNRokuharaAMatsumotoA 2005 Hepatitis B virus DNA-negative dane particles lack core protein but contain a 22-kDa precore protein without C-terminal arginine-rich domain. J Biol Chem 280 21713 21719
17. ChiuYLGreeneWC 2008 The APOBEC3 Cytidine Deaminases: An Innate Defensive Network Opposing Exogenous Retroviruses and Endogenous Retroelements. Annu Rev Immunol 26 317 353
18. NguyenDHGummuluruSHuJ 2007 Deamination-Independent Inhibition of Hepatitis B Virus Reverse Transcription by APOBEC3G. J Virol 81 4465 4472
19. GuoHJiangDZhouTCuconatiABlockTM 2007 Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol 81 12472 12484
20. WatanabeTSorensenEMNaitoASchottMKimS 2007 Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci U S A 104 10205 10210
21. NguyenDHHuJ 2008 Reverse transcriptase - and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids. J Virol 82 6852 6861
22. LanfordRENotvallLLeeHBeamesB 1997 Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J Virol 71 2996 3004
23. ZhangZTianYLiLFiedlerMSchmidE 2006 A conserved linear B-cell epitope at the N-terminal region of woodchuck hepatitis virus core protein (WHcAg). J Virol Methods 135 17 25
24. AsabeSWielandSFChattopadhyayPKRoedererMEngleRE 2009 The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 83 9652 9662
25. DeliusHGoughNMCameronCHMurrayK 1983 Structure of the hepatitis B virus genome. J Virol 47 337 343
26. GaoWHuJ 2007 Formation of Hepatitis B Virus Covalently Closed Circular DNA: Removal of Genome-Linked Protein. J Virol 81 6164 6174
27. HeermannKHGoldmannUSchwartzWSeyffarthTBaumgartenH 1984 Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol 52 396 402
28. KannMGerlichW 1998 Hepadnaviridae - Structure and Molecular Virology. ZuckermanAJThomaHC Viral Hepatitis 2nd ed London Edinburgh Philadelphia Sydney Toronto Churchill Livingstone 77 105
29. VolzTLutgehetmannMWachtlerPJacobAQuaasA 2007 Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 133 843 852
30. DeguchiMYamashitaNKagitaMAsariSIwataniY 2004 Quantitation of hepatitis B surface antigen by an automated chemiluminescent microparticle immunoassay. J Virol Methods 115 217 222
31. ThompsonAJNguyenTIserDAyresAJacksonK 2010 Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 51 1933 1944
32. GallinaABonelliFZentilinLRindiGMuttiniM 1989 A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self assembles into capsid particles but fails to bind nucleic acids. J Virol 63 4645 4652
33. BirnbaumFNassalM 1990 Hepatitis B virus nucleocapsid assembly: Primary structure requirements in the core protein. J Virol 64 3319 3330
34. WingfieldPStahlSWilliamsRStevenA 1995 Hepatitis core antigen produced in Escherichia coli: subunit composition, conformational analysis, and in vitro capsid assembly. Biochemistry 34 4919 4932
35. NewmanMSukFMCajimatMChuaPKShihC 2003 Stability and morphology comparisons of self-assembled virus-like particles from wild-type and mutant human hepatitis B virus capsid proteins. J Virol 77 12950 12960
36. PorterfieldJZDhasonMSLoebDDNassalMStraySJ 2010 Full-length HBV Core Protein Packages Viral and Heterologous RNA With Similar High Cooperativity. J Virol 84 7174 7184
37. LiuSHeJShihCLiKDaiA 2010 Structural comparisons of hepatitis B core antigen particles with different C-terminal lengths. Virus Res 149 241 244
38. FallowsDAGoffSP 1995 Mutations in the epsilon sequences of human hepatitis B virus affect both RNA encapsidation and reverse transcription. J Virol 69 3067 3073
39. GuoHMaoRBlockTMGuoJT 2010 Production and function of the cytoplasmic deproteinized relaxed circular DNA of hepadnaviruses. J Virol 84 387 396
40. TerreSPetitMABrechotC 1991 Defective hepatitis B virus particles are generated by packaging and reverse transcription of spliced viral RNAs in vivo. J Virol 65 5539 5543
41. RosmorducOPetitMAPolSCapelFBortolottiF 1995 In vivo and in vitro expression of defective hepatitis B virus particles generated by spliced hepatitis B virus RNA. Hepatology 22 10 19
42. Le PogamSChuaPKNewmanMShihC 2005 Exposure of RNA templates and encapsidation of spliced viral RNA are influenced by the arginine-rich domain of human hepatitis B virus core antigen (HBcAg 165-173). J Virol 79 1871 1887
43. NassalM 1992 The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol 66 4107 4116
44. SommerGvan BommelFWillH 2000 Genotype-specific synthesis and secretion of spliced hepatitis B virus genomes in hepatoma cells. Virology 271 371 381
45. HilditchCMRogersLJBishopDH 1990 Physicochemical analysis of the hepatitis B virus core antigen produced by a baculovirus expression vector. J Gen Virol 71 (Pt 11) 2755 2759
46. LanfordRENotvallL 1990 Expression of hepatitis B virus core and precore antigens in insect cells and characterization of a core-associated kinase activity. Virology 176 222 233
47. PetitMAPillotJ 1985 HBc and HBe antigenicity and DNA-binding activity of major core protein P22 in hepatitis B virus core particles isolated from the cytoplasm of human liver cells. J Virol 53 543 551
48. HattonTZhouSStandringD 1992 RNA - and DNA-binding activities in hepatitis B virus capsid protein: a model for their role in viral replication. J Virol 66 5232 5241
49. MachidaATsudaOYoshikawaAHoshiYTanakaT 1991 Phosphorylation in the carboxyl-terminal domain of the capsid protein of hepatitis B virus: evaluation with a monoclonal antibody. J Virol 65 6024 6030
50. ChuaPKTangFMHuangJYSuenCSShihC 2010 Testing the balanced electrostatic interaction hypothesis of hepatitis B virus DNA synthesis by using an in vivo charge rebalance approach. J Virol 84 2340 2351
51. NewmanMChuaPKTangFMSuPYShihC 2009 Testing an electrostatic interaction hypothesis of hepatitis B virus capsid stability by using an in vitro capsid disassembly/reassembly system. J Virol 83 10616 10626
52. YuanTTSahuGKWhiteheadWEGreenbergRShihC 1999 The mechanism of an immature secretion phenotype of a highly frequent naturally occurring missense mutation at codon 97 of human hepatitis B virus core antigen. J Virol 73 5731 5740
53. YuanTTTaiPCShihC 1999 Subtype-independent immature secretion and subtype-dependent replication deficiency of a highly frequent, naturally occurring mutation of human hepatitis B virus core antigen. J Virol 73 10122 10128
54. ChangSFNetterHJBrunsMSchneiderRFrolichK 1999 A new avian hepadnavirus infecting snow geese (Anser caerulescens) produces a significant fraction of virions containing single-stranded DNA. Virology 262 39 54
55. AlbertiADianaSScullardGHEddlestonWFWilliamsR 1978 Full and empty Dane particles in chronic hepatitis B virus infection: relation to hepatitis B e antigen and presence of liver damage. Gastroenterology 75 869 874
56. HuJLinL 2009 RNA-protein interactions in hepadnavirus reverse transcription. Front Biosci 14 1606
57. HuJSeegerC 1996 Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci U S A 93 1060 1064
58. HuJFloresDToftDWangXNguyenD 2004 Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J Virol 78 13122 13131
59. LadnerSKOttoMJBarkerCSZaifertKWangGH 1997 Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother 41 1715 1720
60. HuJToftDOSeegerC 1997 Hepadnavirus assembly and reverse transcription require a multi - component chaperone complex which is incorporated into nucleocapsids. EMBO J 16 59 68
61. YuMSummersJ 1994 Multiple functions of capsid protein phosphorylation in duck hepatitis B virus replication. J Virol 68 4341 4348
62. BasagoudanavarSHPerlmanDHHuJ 2007 Regulation of hepadnavirus reverse transcription by dynamic nucleocapsid phosphorylation. J Virol 81 1641 1649
63. LenhoffRJSummersJ 1994 Coordinate regulation of replication and virus assembly by the large envelope protein of an avian hepadnavirus. J Virol 68 4565 4571
64. YanXSinkovitsRSBakerTS 2007 AUTO3DEM--an automated and high throughput program for image reconstruction of icosahedral particles. J Struct Biol 157 73 82
65. PettersenEFGoddardTDHuangCCCouchGSGreenblattDM 2004 UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25 1605 1612
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



