-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
Neisseria meningitidis (Nm) and N. gonorrhoeae (Ng) are adapted to different environments within their human host. If the basis of this difference has not yet been fully understood, previous studies (including our own data) have reported that, unlike Ng, Nm tolerates high manganese concentrations. As transition metals are essential regulators of cell growth and host pathogen interactions, we aimed to address mechanisms of Nm Mn2+ tolerance and its pathogenic consequences. Using bioinformatics, gene deletion and heterologous expression we identified a conserved bacterial manganese resistance factor MntX (formerly YebN). The predicted structure suggests that MntX represents a new family of transporters exporting Mn. In the Neisseria genus, this exporter is present and functional in all Nm isolates but it is mutated in a majority of Ng strains and commonly absent in nonpathogenic species. In Nm, Mn2+ export via MntX regulates the intracellular Mn/Fe ratio and protects against manganese toxicity that is exacerbated in low iron conditions. MntX is also important for N. meningitidis to resist killing by human serum and for survival in mice blood during septicemia. The present work thus points to new clues about Mn homeostasis, its interplay with Fe metabolism and the influence on N. meningitidis physiology and pathogenicity.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002261
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002261Summary
Neisseria meningitidis (Nm) and N. gonorrhoeae (Ng) are adapted to different environments within their human host. If the basis of this difference has not yet been fully understood, previous studies (including our own data) have reported that, unlike Ng, Nm tolerates high manganese concentrations. As transition metals are essential regulators of cell growth and host pathogen interactions, we aimed to address mechanisms of Nm Mn2+ tolerance and its pathogenic consequences. Using bioinformatics, gene deletion and heterologous expression we identified a conserved bacterial manganese resistance factor MntX (formerly YebN). The predicted structure suggests that MntX represents a new family of transporters exporting Mn. In the Neisseria genus, this exporter is present and functional in all Nm isolates but it is mutated in a majority of Ng strains and commonly absent in nonpathogenic species. In Nm, Mn2+ export via MntX regulates the intracellular Mn/Fe ratio and protects against manganese toxicity that is exacerbated in low iron conditions. MntX is also important for N. meningitidis to resist killing by human serum and for survival in mice blood during septicemia. The present work thus points to new clues about Mn homeostasis, its interplay with Fe metabolism and the influence on N. meningitidis physiology and pathogenicity.
Introduction
It is largely accepted that access to metals impacts on the equilibrium of host pathogen interface [1], [2]. In fact, bacteria must acquire nutrients for survival from the host environment during the course of the interaction. These nutrients comprise transition metals (such as Fe, Mn, Zn, Ni, Cu, Co and Mo) [3] which have the specific characteristic of an incompletely filled “d” orbital. This permits different state of oxidation (e.g. Fe2+ and Fe3+) and their use for proteins structural stabilization or as enzymes cofactors in a majority of metabolic processes [4]. Accordingly, transition metals are essential for the survival of bacteria. However, their accumulation can be toxic if the quantity, state of oxidation or intracellular localization and regulation are inadequate [5]. In these cases, metals can cause deleterious oxido-reduction reactions with proteins or other compounds (e.g. H202 and iron also known as Fenton reaction), generating toxic compounds (e.g. OH. OH-) that alter macromolecular structures such as proteins, membranes and DNA, leading to cell death [5]. In bacteria, this duality has forced selection of strategies to orchestrate essential transition metals homeostasis by sensing, acquiring, storing or, when needed, exporting them properly. On the host side, the duality of metal functionality is also true and metal homeostasis is also tightly controlled. Furthermore, prokaryote-eukaryote co-evolution has selected immune strategies aimed at controlling metals availability and restricting bacterial growth [3], [6], [7].
If the dominant role of iron in host-pathogen interactions has been extensively documented the impact of other transition metals and the interplay between their metabolisms are just emerging. One can cite the example of Mn for which the importance in bacterial physiology and pathogenesis just became apparent [8], [9], [10]. In addition to its role as cofactor in several bacteria, manganese has been suggested to quench reactive oxygen species (ROS) [11], [12] which could be endogenous (generated during bacterial metabolism) or exogenous (host immune defense mechanism). Bacterial manganese importers have been shown to influence host-pathogen interactions but depending on the infectious model and type of pathogen they were found to either contribute or not to virulence [13], [14], [15], [16]. Their definition in terms of general bacterial pathogenicity determinants is therefore unclear. Besides, the homeostasis of manganese has to be considered in a more complex situation when several metals are acting in concert. Examples of metals interplay have been described with manganese control of bacterial iron homeostasis [17] or more importantly, adequate intracellular Mn/Fe ratio critical to resist to certain stress [18], [19], [20].
The impact of metal availability on pathogenesis is particularly exemplified by Neisseria human pathogens. The Neisseria genus is composed of bacteria which are part of the normal human microbiome and live in harmless symbiosis with humans. Unfortunately, in some cases this relation may evolve to parasitism. This is particularly the case of two species namely N. meningitidis and N. gonorrhoeae. These two closely related bacteria are exclusively found in humans but in different ecological niches consequently causing distinct diseases. N. meningitidis is frequently isolated from the upper respiratory tract of asymptomatic carriers but can also be the causative agent of life threatening invasive infections such as septicemia and meningitis. N. gonorrhoeae is isolated from the genitourinary tract and is the causative agent of gonorrhoeae a sexually transmitted disease generally characterized by a localized inflammation with, in some circumstances, severe consequences. In both cases, the importance of Fe acquisition for Neisseria pathogenesis has been previously established. As a matter of fact, deletion of genes encoding metallo-transporters [21], [22], [23], [24], [25] leads to a decreased of virulence in model of infection. Regarding N. meningitidis, an additional approach has been used by providing a compatible iron source that enhances virulence in a mice model [26], [27]. In contrast, the role of Mn and its potential interplay with Fe metabolism remains unclear in this pathogen.
Some studies have demonstrated that N. gonorrhoeae is more sensitive to manganese than N. meningitidis [28], [29], [30]. The work in this article aims to extend this observation by: 1) identifying the genetic determinant responsible for Mn sensitivity and 2) describing the impact of its alteration on N. meningitidis pathogenesis. Our hypothesis was that Mn homeostasis may be different between Neisseria spp., which evolved to fill distinct host niches, and these differences might give clues about N. meningitidis virulence.
Results
Manganese tolerance is an attribute conserved in N. meningitidis
To confirm that N. gonorrhoeae is more sensitive to manganese than N. meningitidis [28], [29], [30], we tested the sensitivity of a sample (≈20 strains) of each species isolated during the last twenty years by the Centre National de Reference des Meningocoques (Table S1). The Figure 1 presents Mn-dependent growth inhibition of these isolates measured by disk assay. Approximately 60% of strains of N. gonorrhoeae were Mn-sensitive compared to none in the case of N. meningitidis isolates. This result suggests that the capacity to resist to manganese toxicity is globally present in both species but that this capacity is strongly conserved only in N. meningitidis. In both bacteria, genes encoding the manganese/zinc transporter system MntABC are present and conserved [30], implying a similar capacity for manganese import. Thus we postulated the existence of a new actor in the manganese homeostasis.
Fig. 1. Resistance to manganese toxicity is a conserved trait in Meningococcus. 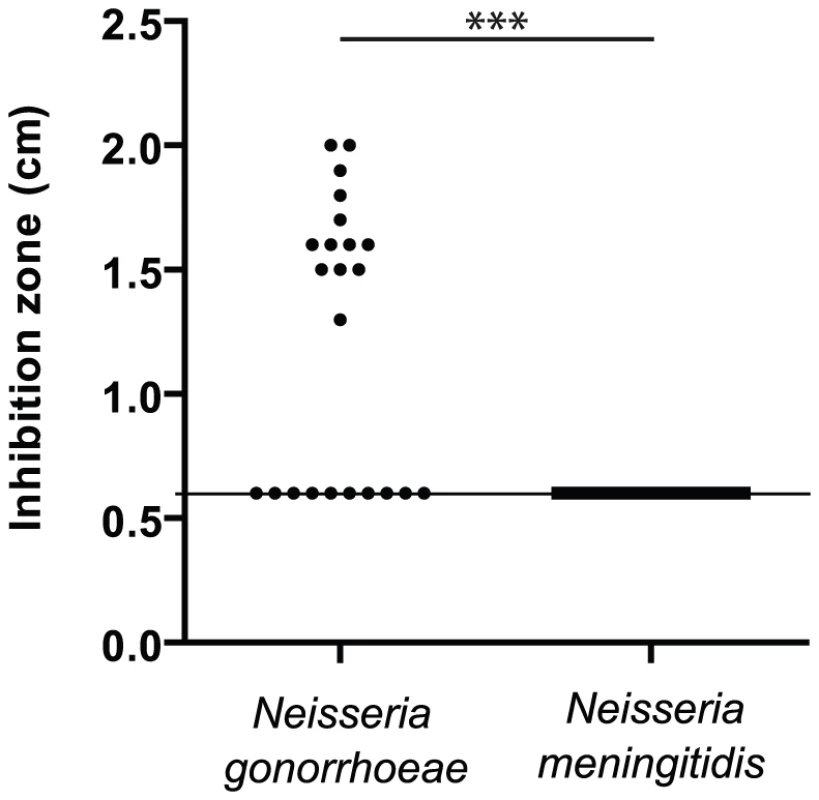
Disk assay of the sensitivity of bacterial growth to 1M MnCl2 in GCB agar media was carried out for several clinical isolates of N. gonorrhoeae and N. meningitidis. (*** p<0.01) Search for a new Mn2+-regulated bacterial factors
Such an actor in manganese homeostasis should be regulated by Mn2+ to act when required. At the transcriptional level, the best known manganese regulators are the DtxR-related factor MntR [31] or Mur, derived from the general ferric uptake regulator (Fur) [17], [32]. Since, the genome of N. meningitidis does not code for these specialized metallo-regulators (our observations), we have preferred an alternative strategy which has consisted to first identify new actors in a model organism and to later verify their presence and evolution in the Neisseria genus. The bold speculation was that this factor may be present and regulated by MntR in other proteobacteria pathogens. We chose the phytopathogen Xanthomonas campestris, which possesses a simple predicted Mn regulon, including an mntRH locus, but lacks other Fur-derived transcription factor such as the oxido-metallo-regulator PerR or additional Mn importers such as the P-type ATPase MntA and the ABC-driven, periplasmic binding protein-based SitABCD transport system (our observation).
Thus, we deleted mntH (Mn-importer), mntR (Mn-regulator) or both genes and measured Mn sensitivity by disk diffusion assay. The results are illustrated in Figure 2A. The mntH deletion did not alter growth in the presence of Mn. In contrast, a drastic Mn-dependent growth inhibition was observed when mntR was deleted. First, we attributed this effect to an over-expression of MntH in absence of MntR but the double mutant ΔmntH-R was as affected as the single ΔmntR mutant. These results argued for the presence of at least one other MntR-regulated actor in manganese homeostasis in X. campestris.
Fig. 2. Search for Mn2+-regulated bacterial factor(s) in a simple model organism. 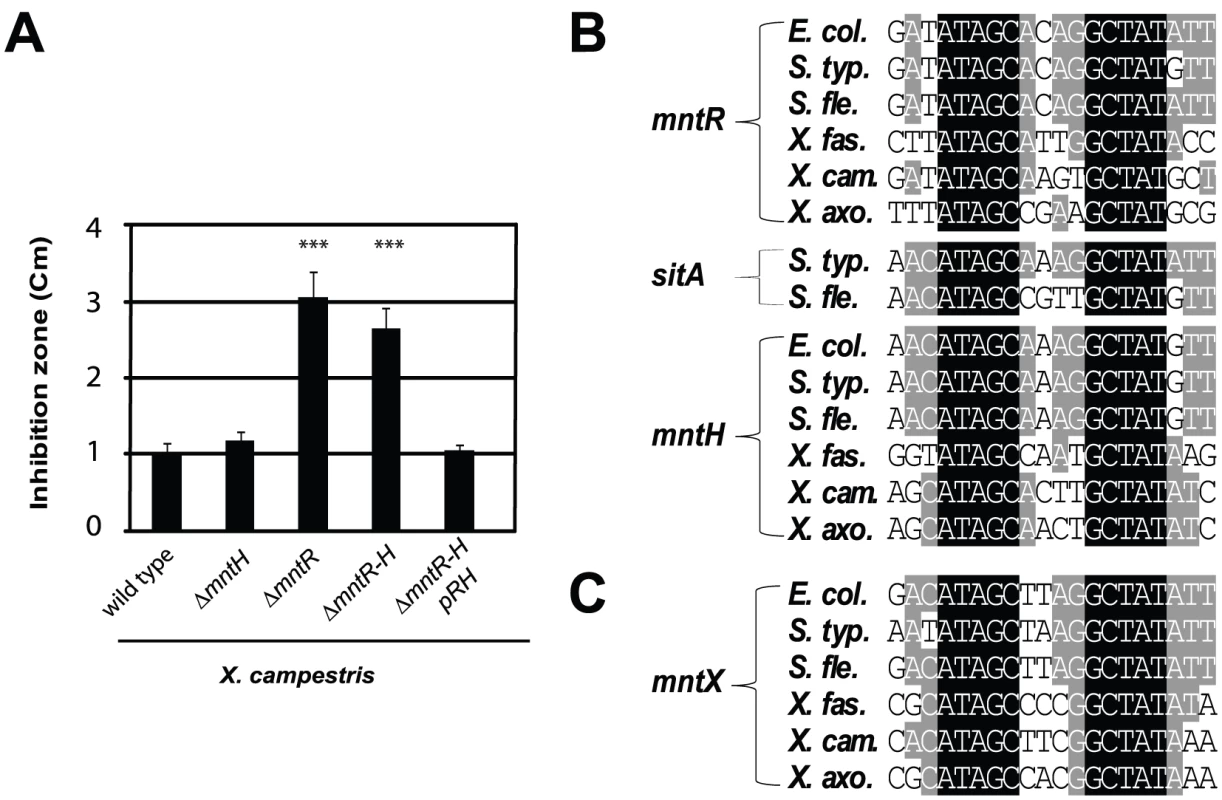
A) Xanthomonas campestris MntR controls Mn transport: genetic studies of bacterial sensitivity to 1M MnCl2, measured by disk assay, revealed a novel Mn resistance factor, as mntR disruption increased Mn sensitivity independent of MntH import (*** p<0.01 compare to the wild type sensitivity). This experiment done in triplicates is representative of several experiments. B) Multiple sequence alignments of MntR binding sites found in the promoters of genes which contribute to Mn homeostasis (mntH, mntR, sitA) in γ-proteobacterial plant or animal pathogens (respectively Escherichia coli, Salmonella typhimurium, Shigella flexneri and Xylella fastidiosa, X. campestris, X. axonopodis). C) Sequence alignment of the MntR binding site detected upstream of one gene (yebN or XCC4075 herein referred to as mntX), using the PredictRegulon web site, and that is conserved γ-proteobacteria. To identify candidates, we used MntR-regulated promoters [33], [34], [35] to derive a matrix for MntR binding site (Figure 2B), which allowed consistent detection of one DNA motif (Figure 2C) in the 5′ region of a Xanthomonas gene termed XCC4075 (formerly yebN; in this article designated mntX).
MntX is a conserved proteobacterial Mn-resistance factor not functional in a majority N. gonorrhoeae strains
In X. campestris
We constructed a mntX deletion mutant and tested its manganese tolerance. The Figure 3A presents the results of this analysis and revealed that the mntX deletion leads to an increased manganese sensitivity as compared to the wild type bacteria. From this result, it can be concluded that mntX encodes a Mn-resistance factor in X. campestris (MntXXc). In support of this interpretation, the comparison between ΔmntX, ΔmntR, ΔmntR-mntX and ΔmntR-mntH-mntX showed the dominant role of MntXXc in conferring Mn sensitivity and its regulation by MntR (Figure S1).
Fig. 3. MntX which confers Mn resistance, is often not functional in N. gonorrhoeae. 
Disk assay of bacterial sensitivity to 1M MnCl2 was carried out for A) X. campestris wild type and mutants lacking mntH and mntR or mntX. B) N. meningitidis MC58 wild type, lacking mntX or back complemented (insertion of p5Y3 by double recombination). C) N. gonorrhoeae 16626 wild type or transformed with mntX from N. meningitidis. These experiments done in triplicates are representative of several experiments (*** p<0.01 compare to the wild type sensitivity). In Neisseria
We next searched for possible homologs of MntXXc, in the Neisseria genomes and whether genetic variation could explain the difference in manganese sensitivity between N. meningitidis and N. gonorrhoeae. A gene encoding a similar protein (48% overall identity, 64% similarity) was found in N. meningitidis (8 out of the 8 sequenced strains). Additionally, mntX was present in the genome of the closely related species N. cinerea (1/1), N. polysaccharea (1/1), N. lactamica (2/2) and N. gonorrhoeae (16/16). But we noticed in this latter organism that the gene was frameshift mutated in 66% of sequenced strains (once 7/16 or twice 4/16). Also, the gene was absent from the more distantly related species N. elongata (1/1), N. flavescens (2/2), N. mucosa (1/1), N. sicca (1/1), N. subflava (1/1) and N. sp. oral taxon 014 str. F0314 (1/1).
To test that N. meningitidis mntX (MntXNm) encodes also a Mn resistance factor we constructed a deletion mutant, ΔmntX, which showed increased sensitivity toward manganese in disk diffusion assay (Figure 3B). Notably, inactivation of mntX did not increase sensitivity toward other metal salts such as FeSO4, NiCl2 and ZnCl2 for both X. campestris and N. meningitidis homologs (data not shown). Lastly, to determine whether N. gonorrhoeae mntX mutation (mntXNg) was the cause of the difference in manganese sensitivity observed between both species (Figure 1), we replaced mntXNg (frameshift mutated) by mntXNm (via p5Y3::Km) in one of the sensitive strain of N. gonorrhoeae (16626). As shown in the Figure 3C, complementation by mntXNm restored manganese tolerance in the N. gonorrhoeae sensitive strain 16626. Overall these results strongly suggest that a manganese resistance factor is conserved in distant species and that specific evolutionary events explain the difference in sensitivity of two closely related Neisseria.
MntX represents a novel family of prokaryotic metal transporters
To our knowledge MntXNm and MntXXc are the first members of a large family of highly hydrophobic putative membrane proteins (Figure 4A) of previously unknown function. As a first clue toward the mechanism of MntX-mediated manganese tolerance, we examined the phylogenetic distribution of 202 MntX homologues by Neighbor Joining (NJ) analysis [36]. The result, presented as a tree topology in the Figure S2A, shows significant discrepancy between the phylogenetic and taxonomic distributions of the sequences studied. The simplest explanation can be that mntX were frequently transmitted by horizontal gene transfer. Nevertheless, MntXXc and MntXNm are found in separate subgroups among most of the other proteobacterial sequences from α-, β - and γ-divisions.
Fig. 4. MntX identifies a family of predicted bacterial metal transporters. 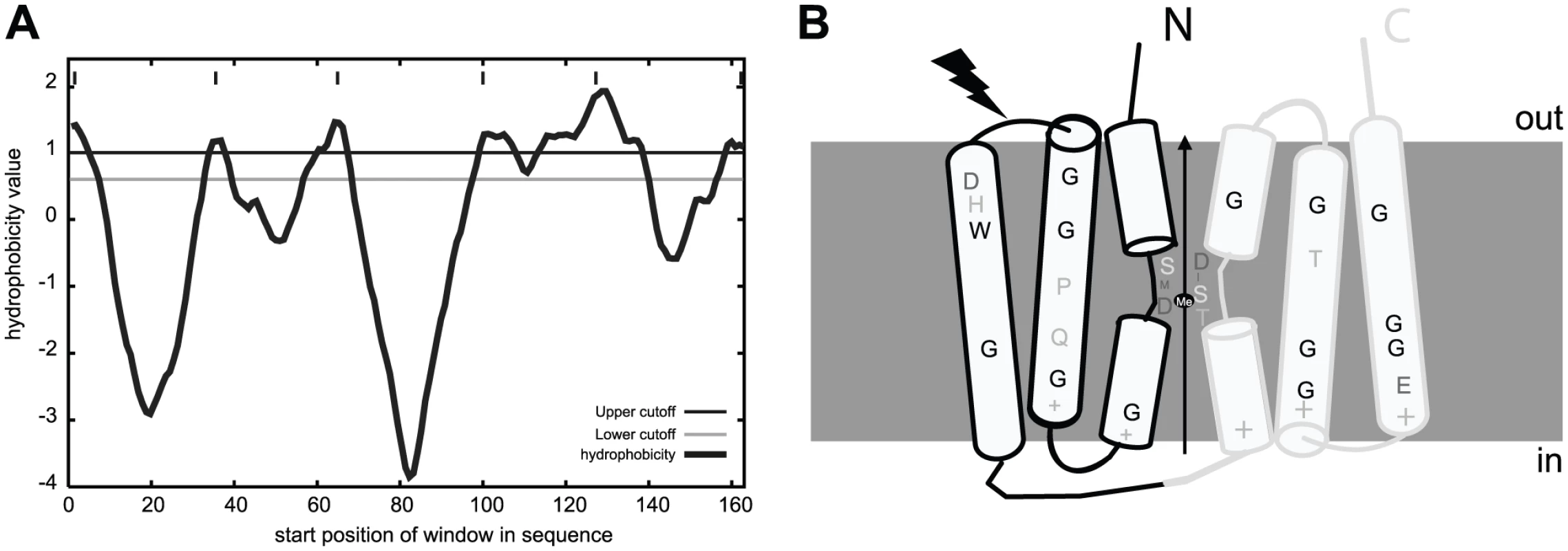
A) MntXXc hydrophobicity plot based on the Goldman, Engelman, Steitz hydropathy scale, using core and wedge windows of 14 and 4 a.a. residues, respectively. B) Proposed model of structure-function relationships in MntX deduced from transmembrane topology prediction and evolutionary sequence analyses. MntX inverted structural symmetry and pattern of sequence conservation suggest a functional interface that may act as a conformational switch for metal transport. The black lightening form represents the localization of the truncation of MntXNg. To gain functional information on MntX, we generated logos of aligned protein sequences presented in Figure S2B. These graphical representations of amino acid frequencies by position allow simultaneous visualization of patterns and variations among MntX homologues that correspond to selected phylogenetic groups [37]. To identify features typical of the MntX family, we also included a phylogenetic outgroup of divergent sequences (in dark blue) [38]. Transmembrane segments (TMS) are the most conserved parts of the molecule and logos were obtained for each (Figure S2B, TMS1-3, top; TMS4-6, bottom). Examining these logos provided important structural information that is summarized in the predicted topology presented in Figure 4B.
First, similar patterns of conservation between TMS1-3 and TMS4-6 could be observed (underlined in the Figure S2B). This corresponds to the two directly repeated Domains of Unknown Function 204 (DUF204) [39] that form the MntX structure. In addition, conserved accumulations of positive charges found between TMS1/2, 3/4 and 5/6 (Figure S2B) were detected. Based on the ‘positive-inside’ rule, it was predicted that the two halves may adopt an inverted transmembrane configuration forming an overall pseudo-symmetric structure (Figure 4B). We also noticed periodicity of residue conservation in TSM2/3 and TSM5/6 compatible with α-helical structure and also the abundance of conserved glycine implying tight inter-helix packing interactions [40]. In comparison, the sequence conservation pattern in TMS1&4 is more local and central and involves adjacent residues which could form short extended segments. As reviewed in [41], such a configuration of repeated structures with a central extended segment is a characteristic of several membrane transporters. In these cases, the extended segments may provide contact points for substrate binding. In accordance with this hypothesis, MntX putative extended peptides (TMS1&4 as schematized in Figure 4B) contain invariant D residues which are know to be preferred ligands for divalent metals and polar moieties (S, T) also known to interact with inorganic cations [42]. In conclusion, this structural prediction suggests that MntX family is composed of metallo-transporters with conserved features putatively implicated in metal binding and transport
MntX is an active manganese exporter
MntX can protect bacteria against manganese toxicity and sequences analyses suggest transport function. To test whether MntX encodes a metallo-exporter, we first used heterologous expression in E. coli using a model strain that reports on gene expression controlled by the intracellular pools of metals. In this strain (schematized in the top of Figure 5A), the mntH Orf was replaced by the firefly luciferase Orf. Furthermore, it is in a Δfur background. Hence, luminescence is repressed exclusively by MntR (Figure 5A bar 1), with Mn2+ as co-repressor, and less efficiently with other metals as Fe2+ [43] (Bergevin and Cellier, unpublished). In this system, incubation of bacteria with a membrane permeant Me2+ chelator (2,2′-dipyridyl: DP) [16], [44] rapidly de-repressed significantly PmntH-luc expression (Figure 5A bar 9), and this could be prevented by co-incubation with some Me2+ (Figure 5A bar 13). With this assay, it is expected that a difference in luciferase expression may be observed if metals are pumped out of the cell via an exporter (i.e. the metal will not reverse the effect of DP).
Fig. 5. MntX is a manganese exporter. 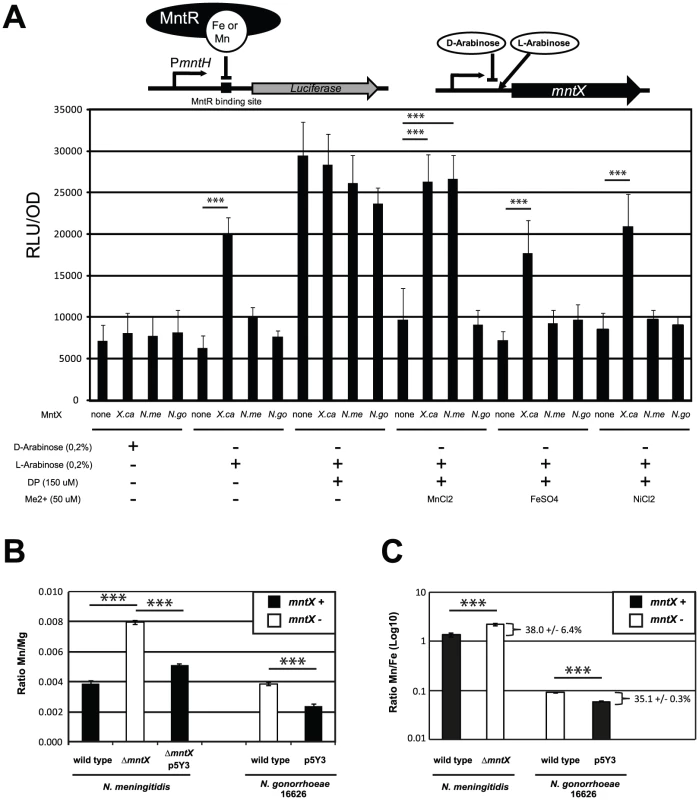
A) MntX depletes E. coli intracellular metal pools. The E. coli K-12 strain Δfur PmntH::luc emits light upon intracellular metal depletion with the divalent metal chelator 2,2′-Dipyridyl (DP). This process is suppressed by co-incubation of DP with divalent metals, therefore allowing monitoring potential metal export activity. Plasmid-driven expression of MntX was induced with L-arabinose. Each bar represents the average of four independent measurements and is representative of several experiments B and C) ICP-MS quantification of divalent metals contents (Mg, Mn, and Fe), for strains of N. meningitidis and N. gonorrhoeae harboring mntXNm (black bars) or not (white bars) and grown in rich medium. The data are expressed in ratio Mn/Mg in B and Mn/Fe in C. Each bar represents the average of three independent measurements for N. meningitidis and two for N. gonorrhoeae (*** p<0.01). To measure metal export in this E. coli strain, we expressed MntXXc, MntXNm and MntXNg (strain 16626; frameshift mutated) via a L-arabinose inducible promoter present in the pBad plasmid (depicted in the top of Figure 5A). As a negative control we used bacteria transformed with an empty plasmid.
In rich medium, (LB broth) metals are in sufficient amount to control the expression of luciferase. The basal expression (D-arabinose) without production of MntX proteins was similar in all strains (bar 1 to 4). In contrast, when the production of MntX was induced (bar 5 to 8) the expression of luciferase was significantly increased only in the case of MntXXc (bar 6). This suggests that MntXXc is strongly active and able to deplete enough intracellular metals for MntR to be free from all possible metal cofactors (including Fe2+) in this rich medium. Other strains, in particular the one harboring mntXNm did not differently express the luciferase when the production of MntX was induced (bar 3 compared to 7). This implies that MntXNm is not active or it is not able to deplete, in rich medium, all possible MntR metallo-cofactors (including Fe2+).
In metallo-depleted medium (using DP bars 9 to 12), a maximum expression of the luciferase, independent of the presence of MntX, was observed. To test export of specific metal, bacteria were co-incubated with a Me2+ concentration allowing to significantly reverse DP intracellular action (Mn2+ Fe2+ or Ni2+). This reversion of the DP effect with metals could be observed for strains carrying the empty plasmid (bars 13, 17 and 21) or the plasmid encoding MntXNg (bars 16, 20 and 24). In contrast, this reversion of the DP effect by Mn2+, Fe2+ and Ni2+ was not observed for the strain expressing MntXXc (bars 14, 18 and 22). On the other hand, the luciferase activity was de-repressed in presence of MntXNm only when DP was co-incubated with Mn2+ (bar 15) but not with Fe2+ (Bar 19) or Ni2+ (bar 23). As a note, similar results were observed for MntXXc and MntXNm using up to 300 µM of Mn2+ or Fe2+ (Figure S3). Overall, these results strongly suggest that MntXXc exports a broad variety of metals whereas MntXNm exports only Mn2+ when expressed in E. coli.
To confirm these results and examine MntXNm endogenous function we performed ICP-MS analyses of metal content in N. meningitidis and N. gonorrhoeae harboring a functional MntX or not. We quantified intracellular atoms of manganese using bacteria grown in rich media with added Mn and using Mg or Fe content for normalizing the metal measurements (Figure 5B and 5C). For both strains, the lack of MntX activity (white) correlated with ∼two fold increase in Mn intracellular content compared to their parental or complemented strains (black). Similar results were obtained when Mn intracellular content was normalized with Fe (Figure 5C) suggesting that MntXNm did not interfere with iron homeostasis and support E. coli data showing lack of MntXNm specificity for Fe. Thus N. meningitidis MntX native activity supports the prediction that it is a membrane transporter that exports Mn and was therefore renamed MntX.
Of note, the intracellular amount of Mn measured in WT N. gonorrhoeae and N. meningitidis were close (respectively 3.0×105 and 6.5×105 Mn atoms by bacteria). However, about 10-fold more Fe was associated with WT N. gonorrhoeae compared to WT N. meningitidis (respectively 3.9×106 and 4.9×105 Fe atoms by bacteria), suggesting unsuspected differences in metal homeostasis between these close relatives, including an unusually high Mn/Fe ratio for N. meningitidis.
MntX regulates the intracellular Mn/Fe ratio in N. meningitidis
Manganese is typically considered as less toxic transition metal than iron (less Fenton's reaction) and beneficial in the context of an oxidative stress [45]. Thus, the existence of a manganese exporter in the genome of a bacterium which is only found in human, in metallo-restricted condition seemed rather surprising. Consequently, we tested if MntX could protect from oxidative stress. In X. campestris and E. coli, we were able to correlate the presence of MntX (expected to decrease intracellular manganese) with reduced resistance to organic peroxide toxicity (Figure S4). However, this was not true for N. meningitidis (Figure S4) as previously reported by others [30]. More importantly this trait was specific to N. meningitidis since N. gonorrhoeae was shown to accumulate manganese in defense against oxidative species [30], [46]. As N. meningitidis does not seems to rely on Mn to detoxify oxidative compounds, we reasoned that Mn over-accumulation may be deleterious, for example by competing with other essential metals when available in limited quantity (such as iron or zinc) for the binding to the active site of N. meningitidis proteins. Using a N. meningitidis strain harboring, in its native locus, the mntX promoter fused to the luciferase orf we observed that regulation of mntX was consistent with this hypothesis. The Figure 6A presents the key experiment which has consisted in growing N. meningitidis PmntX::luc in media with increased concentrations of manganese and Desferal (iron chelator). The results show that luciferase activity increased in presence of manganese but more importantly this expression was potentiated in low iron condition (i.e. manganese 10 µM and Desferal 5 µM) (Figure 6A).
Fig. 6. MntX protects N. meningitidis from Mn cytotoxicity exacerbated in low iron condition. 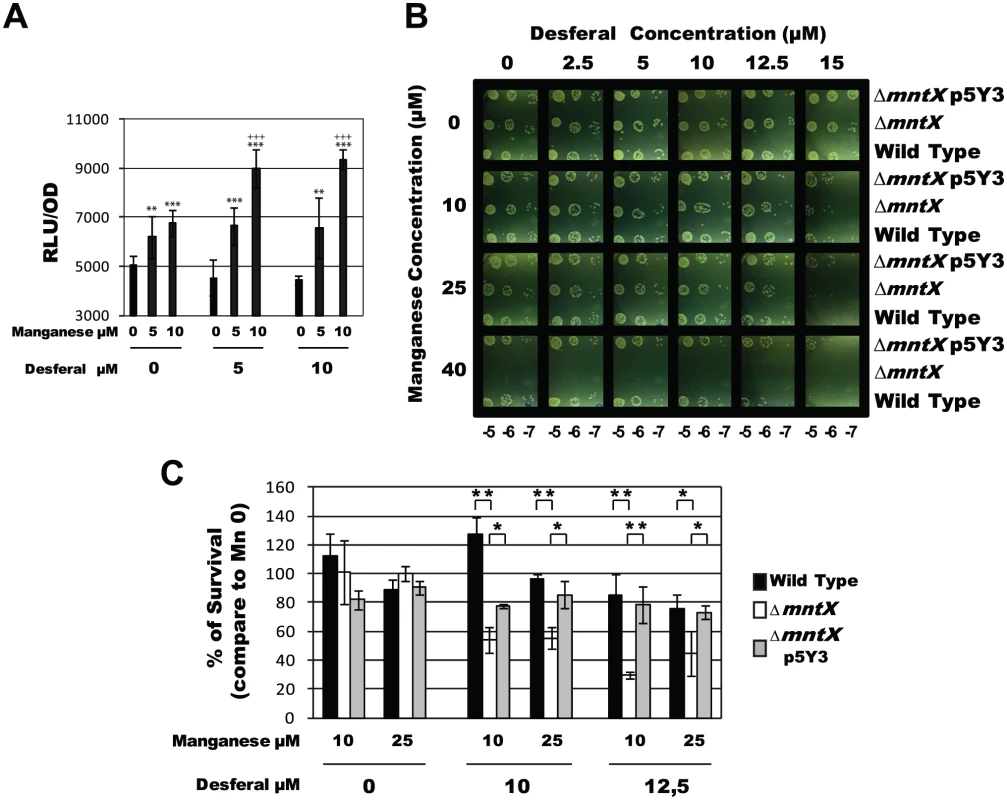
A) Light emission normalized with OD, and done in triplicates, of N. meningitidis harboring PmntX::luc and grown in GCB agar for 4 hours containing indicated concentration of Desferal and manganese. (*** p<0.01, ** p<0.05 in comparison to the same Desferal concentration without Mn; +++ p<0.01 in comparison to the same Mn concentration without Desferal). B) Serial dilution plate assays were performed to compare the tolerance to iron chelation (Desferal) of N. meningitidis wild type strain, ΔmntX mutant and the complemented strain, in the presence of increased concentrations of MnCl2. C) Percentage of CFU growing on Desferal and manganese compared to those growing in the same concentration of Desferal but without manganese. Each bar represents the mean of three measurements. (*** p<0.01, ** p<0.05) To test a possible role for MntX under low iron/high manganese condition, we performed a serial dilution plate assay under increased concentrations of Desferal in combination with increased amount of manganese. The results presented in Figure 6B revealed that the growth of N. meningitidis harboring mntX (wild type or complemented) was neither affected by manganese alone (up to 40 µM) nor by Desferal alone (up to 15 µM). Most importantly, a drastic inhibitory effect of Desferal (15 µM) on the growth of N. meningitidis was strictly dependent on the presence of manganese (Figure 6, bottom right of panel B). This strongly suggests that with low iron availability manganese excess becomes lethal for N. meningitidis. Indeed, ΔmntX could not grow in manganese concentrations above 40μM, independently of iron concentration. To determine whether the ΔmntX mutation could increase sensitivity toward Desferal in the presence of sublethal Mn concentration (<40μM) we performed CFU counting experiments. The results presented in Figure 6C showed reduced survival in strains lacking MntX only in the presence of both Desferal and manganese but not in the presence of either compound alone.
Measurements of intracellular metal content showed that an active MntX decreases the Mn/Fe ratio by 38.0 +/- 6.4% in the case of N. meningitidis and 35.1 +/- 0.3% for N. gonorrhoeae (Figure 5C). All together, these data illustrate the importance of MntX to maintain an optimal Mn/Fe ratio in N. meningitidis via Mn export and in proportion with intracellular iron pool depletion. To test whether reduced fitness of bacteria lacking MntX under low iron/high manganese condition could be due to the abnormal replacement of Fe cofactors by Mn, we monitored the expression of Fur regulated genes. Accordingly, in absence of MntX, a miss-regulation of Fur regulated genes was observed in low iron/high manganese, illustrating these Mn interferences (Figure S5).
MntX is important for virulence in sepsis models
Next we addressed the possibility that, since iron availability is restricted in the human body, N. meningitidis may require manganese export to survive in the host. First, we used the murine model of sepsis to determine if the gene was expressed in blood. To avoid residual expression due to culture media, we performed an in vivo passage (infecting mice with blood from a previously infected mouse) and extracted RNA. We employed two types of mice: wild type BALB/c mice and BALB/c mice expressing the human transferrin (hTf) [26]. N. meningitidis iron acquisition from transferrin is host-specific [47]. Therefore we used mice expressing the hTf (which is a compatible iron source) as an alternate model which sustains a better bacterial survival [26]. The Figure 7A presents the Δ (Δ Cycle threshold or Ct) of bacterial genes expressed in blood, normalized with gyrA and relative to the expression level upon growth on culture media. As a note, the Ct is defined as the number of cycles required for the fluorescent signal to cross the threshold. In this experiment, we measured the expression of mntX and genes encoding the pillin pilE, the global iron regulator fur, the transferrin binding protein A tbpA and the porin porA. The Δ (Δ Ct) of mntX was positive and similar between Balb/C and hTf mice. Thus, mntX was expressed in mouse blood suggesting that export of manganese is required in these conditions.
Fig. 7. MntX is expressed during infection and is important to survive in serum. 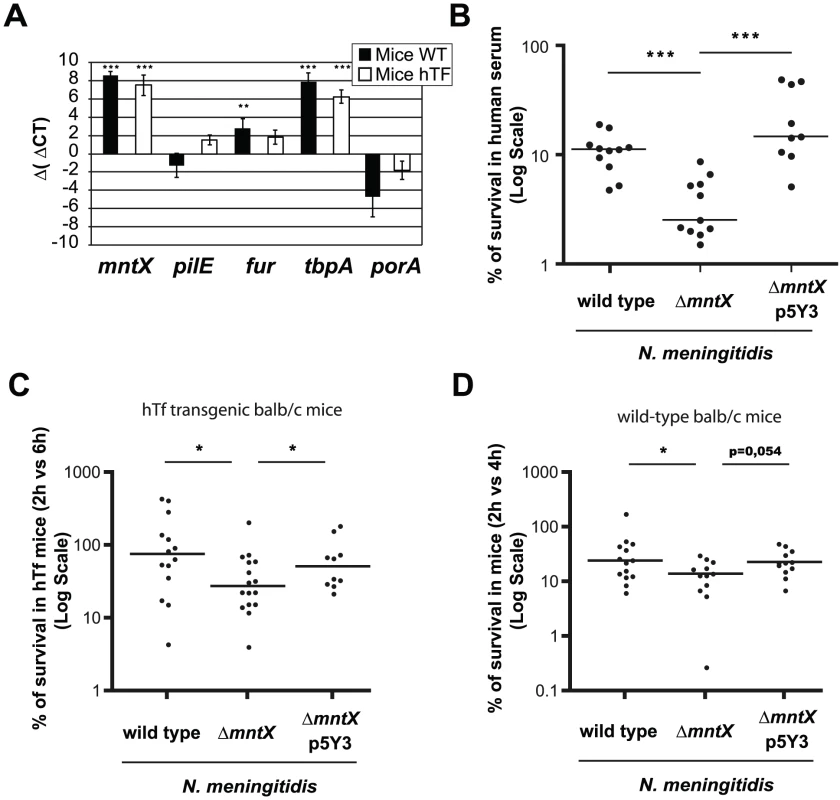
A) Expression of selected genes in bacteria from blood 6h post IP infection of hTf mice (black) or wild type mice (white), quantified by qRT-PCR. gyrA was used as endogenous house keeping gene whereas the reference is MC58 growing on GCB. Each bar represents the mean of three infections and representative of several experiments (*** p<0.001, ** p<0.005). B) Standard human serum bactericidal assays (50%) were performed for N. meningitidis wild type, ΔmntX or complemented. Each point represents a measure done in three independent experiments. (*** p<0.01) C) BALB/c mice expressing the human transferrin were infected intraperitoneally with 106 bacteria from GCB agar. The graph represents the number of CFUs recovered from blood 6h post-infection compared to 2h p.i. and expressed in %. D) Similarly, BALB/c mice were infected intraperitoneally with 106 bacteria from GCB agar containing 12.5 µM Desferal. The number of CFU recovered from blood 4h after infection was compared to 2h. For C and D, the graph presents the median % of CFU from two pooled independent experiments (* p<0.05). Secondly, we infected mice (BALB/c wild type or hTf) i.p. with N. meningitidis harboring or not mntX and determined the amount of bacteria in the blood at early (2h after infection) and later time point (4 h or 6h as indicated). The results of these experiments are expressed as % bacteria that survived in blood compared to those found at earlier time point (2 h) and are depicted in Figure 7C for hTf mice and Figure 7D for wild type mice. Median survival of N. meningitidis mntX mutant was significantly reduced compared to the wild type or complemented strains in both models of murine infection. Interestingly, the number of bacteria at 2h post-infection was similar independent of mntX indicating that the ΔmntX strain could still cross the epithelial barrier from the peritoneal cavity to the circulation. Nevertheless, to support a reduced survival of N. meningitidis ΔmntX in host blood, we assayed a human serum bactericidal activity with N. meningitidis wild type, ΔmntX or the complemented strain. As shown in Figure 7B, there was 3 to 4 times less mntX mutant surviving in the presence of this specific serum compared to the wild type or complemented bacteria, therefore supporting a role for manganese export in the survival of N. meningitidis during septicemia.
Discussion
The present work establishes the manganese export function of MntX (formerly YebN), the first characterized members of a new family of metal exporter. Members of the MntX family have been found so far only in prokaryotes and phylogeny suggests pervasive mntX horizontal transfer. While these observations require functional validation, it is of interest that several known major pathogenic bacteria were found to harbor a homologue of MntX such as Actinobacillus, Bacillus, Corynebacterium, Clostridum, Campylobacter, Fusobacteria, Legionella, Proteus, Pseudomonas and enterobacteria (such as Escherichia, Klebsiella, Yersinia, Salmonella, Shigella) species (data not shown). In addition, members from this novel family display sequence characteristics that could be related to the evolution of their functional properties. MntX protein sequences are composed of direct repeats with inverted topology. A common evolutionary scenario reminiscent of the MFS, EmrE and LeuT superfamilies [48], [49], [50], [51] would propose that initially, an ancestral 3 TMS repeat (i.e., DUF204) could adopt inverted topologies and dimerize pseudo-symmetrically to catalyze a substrate translocation process. Subsequent steps of genetic fusion and evolution would have yielded a fixed 6TMS topology, and allowed substrate-driven divergence. MntX xenologs display appreciable sequence conservation despite inverted symmetrical organization; it is tempting to suggest that this family may have relatively recent origins, following the genetic fusion of two DUF204 domains. Future studies will establish whether N. meningitidis MntX Mn2+ selectivity can be related to site-specific sequence divergence within TMS and if other members could have evolved toward another specific metal export.
Beyond physico-chemistry, the novel family of manganese exporters MntX sheds new light on manganese impact on bacterial physiology. In this work, we studied the importance of Mn export via MntX in N. meningitidis. Unlike N. gonorrhoeae, N. meningitidis is not known to use Mn as quenching compounds during oxidative stress [30]. The selection of different strategies may reflect conditions encountered within their specific ecological niche. In fact, N. meningitidis (but also N. lactamica) are frequently isolated from the human nasopharynx, which is aerobic. The strong conservation of manganese export attribute in N. meningitidis suggests a damaging effect of manganese and a need for export in some conditions. It is generally recognized that the homeostasis of manganese and iron are linked; manganese importers expression is influenced by iron (e.g. via fur) [34] and, inversely, iron importer could be regulated by manganese [17]. In addition, several studies have recently correlated the ratio of Mn/Fe with the resistance toward specific stresses [18]. The results presented in this study, strongly suggest that in order to survive in iron restricted environment, N. meningitidis needs to regulate the Mn/Fe intracellular ratio and that MntX is contributing by exporting manganese. Consistent with this, inactivation of mntX reduces N. meningitidis virulence by limiting survival during septicemia in mouse model of infection. Similar attenuation has been observed using another human pathogen, Streptococcus pneumoniae and another family of manganese exporter. Lack of the CDF manganese exporter MntE reduced S. pneumoniae virulence by diminishing nasal colonization, blood invasion and mice mortality [10]. In the absence of MntX (or other manganese exporters), the replacement of iron by manganese in proteins may lead to suboptimal enzymatic activities or inadequate regulation. In stringent environments where bacteria need to be high-performers, a lack of competitiveness may reduce survival of the microorganism due to absence of vigorous responses.
However, a different strategy has been preferred for N. gonorrhoeae in order to survive in the genitourinary tract. This environment is anaerobiotic and rich in peroxides produced by host cells (e.g., neutrophils) and other host-adapted bacteria such as Lactobacillus spp. which compete for the same ecological niche [52]. Lactobacilli are well-known for their high intracellular manganese content and peroxide stress resistance [11]. It is therefore not surprising that N. gonorrhoeae uses also Mn-dependent peroxide detoxification strategies (reviewed in [53]). However, though Lactobacilli strategy relies on redundant Mn uptake systems [54], N. gonorrhoeae approach seems to exploit mntX gene mutation. Importantly, the mntX gene is not deleted from the genome and a reversion of the mutation(s) by phase variation may be possible if a modification of the local environment occurred (e.g., less H202). During our study we have also noticed that N. gonorrhoeae contains more intracellular iron than N. meningitidis. This result is similar to previous N. gonorrhoeae measurement [18] and may suggest that because N. gonorrhoeae accumulates more Fe than N. meningitidis, the Mn quantities tolerated in the cytoplasm are higher and the need for MntX is not as crucial as it is in N. meningitidis. In addition, this situation may have favored the use of a different strategy for peroxide stress protection aiming to increase manganese concentration during oxidative stress [46]. As our results reveal, this is not possible in N. meningitidis possibly due to lower intracellular iron content not permissive for this Mn-increase.
Bacterial homeostasis of iron and manganese or modulations of the Mn/Fe ratio are complex phenomena as they have to integrate information from both the extracellular environment and bacterial intrinsic physiology. In addition, the metabolism of other divalent metals such as Zn may also be coordinately regulated. In this sense, MntX and other manganese exporters have a crucial role to play in the interplay of metal metabolisms. In the future, it will be also important to precisely determine how the innate immune system is interfering with this ratio (versus iron alone) to understand how host defense could affect pathogenicity and if perturbation of this equilibrium could inspire new therapeutic strategies.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the European Union Directive 2010/63/EU (and its revision 86/609/EEC) on the protection of animals used for scientific purposes. Our laboratory has the administrative authorization for animal experimentation (Permit Number 75–1554) and the protocol was approved by the Institut Pasteur Review Board that is part of in the Regional Committee of Ethics of Animal Experiments of Paris region (Permit Number: 99–174). All surgery was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimize suffering.
Bacterial strains and culture conditions
Xanthomonas campestris pv campestris str. ATCC 33913 was purchased at the ATCC and was grown in SB (Silva Buddenhagen) medium at 30°C. Neisseria meningitis MC58 and clinical isolates of N. meningitidis and N. gonorrhoeae were obtained from the Centre National de Reference des Meningocoques (CNRM, Institut Pasteur, Paris). The description of these clinical isolates is provided in Table S1. All Neisseria were grown in GCB medium with Kellogg supplements. For cloning experiments, E. coli DH5α was grown at 37°C in Luria-Bertani Media (Difco). As required, antibiotics were added as follows: chloramphenicol (10 µg.ml−1), Spectinomycin (100 µg.ml−1), streptomycin (50 µg.ml−1), tetracyclin (10 µg.ml−1), gentamycin (30 µg.ml−1), kanamycin (50 µg.ml−1 for E. coli; 100 µg.ml−1 for Neisseria sp.) and Erythromycin (300 µg.ml−1 for E. coli; 3 µg.ml−1 for Neisseria sp.).
Construction of Xanthomonas campestris mutants and complemented strain
The mntH (XCC2171) and mntR (XCC2170) genes are in the same locus and in an opposite direction in the genome of X. campestris. Therefore, to construct the plasmid used for double recombination, the 3′ part of mntH (amplified using the primers XcaSmaIF and XcampR2) was first inserted into pK18mobsacB [55] (gift of CVector) using the enzymes SmaI and EcoRI. Secondly, the plasmid generated, called pK18clon1, has served to generate all the other constructs by adding a specific 5′ part: as a part of mntR (to construct ΔmntH-R), or mntR and the rest of mntH (complemented) or the full mntR and a 5′ part of mntH (ΔmntH). These specifics 5′ parts were generated by specific enzymatic digestion of the same PCR product called PCR2 (obtained using XcaHindIIIF and XcaNoMutR primers). In more details, the construction of plasmids was as follow: 1) pK18mntH::Tet used to make the mntH deletion: PCR2 was inserted into pK18clon1 using SmaI and BamHI. Following this, the tetracycline resistance cassette from p34S-tet [56] was inserted between the 5′ and 3′ fragments using SmaI. 2) pK18mntH-R::Gm used to make the mntH-R deletion: The chloramphenicol resistance cassette from p34s-Cm [56] was inserted into pK18clon1 using SmaI. The resulting plasmid was ligated with PCR2, after a digestion with SalI and HindIII, to generate pK18mntH-R::Cm. Due to spontaneous resistance, the chloramphenicol resistance gene was changed by the gentamycin cassette. This one was extracted from p34S-Gm [56] using SmaI and inserted into pK18mntH-R::Cm digested with SmaI to generate the plasmid pK18mntH-R::Gm. 3) pRH used to complement the mntR-H deletion: PCR2 was inserted into pK18clon1 using PstI and XbaI to generate pK18comp this has led to the reconstruction of the complete locus. The mntH-R genes were extracted to pK18comp using NheI and XbaI and were transferred into a X. campestris compatible plasmid, pBBR1-Tet digested by XbaI. Of note, pBBR1-Tet was generated by the ligation of pBBR1-Tp [57] digested with NheI and NcoI and treated with T4 DNA polymerase with the SmaI-digested tetracyclin cassette from p34S-Tet [56]. 4) pK18mntR::Sm used to make the mntR deletion: The plasmid pK18comp was digested with AgeI and SalI and, after treatment with T4 DNA polymerase, was ligated to a SmaI-digested spectinomycin resistance cassette from p34S-Sm3 [56]. 5) pK18yebN::Tet used to make the yebN deletion: A PCR product containing yebN and obtained with XCC4075F and XCC4075R was digested by NheI and XbaI and cloned in pK18mobsacB digested with the same enzyme. This new plasmid was digested by BglI (two sites in yebN), treated with T4 DNA polymerase and ligated with the tetracycline resistance cassette obtained by SmaI digestion of p34S-Tet, to finally generate pK18yebN::Tet [56]. All these plasmids were introduced in X. campestris using E. coli S17λpir as previously described [58]. The allelic exchange events were selected in SB medium containing 10% sucrose, ampicillin 100 µg.ml-1 (in order to inhibit E. coli) and the specific antibiotic for the deletion.
Construction of Neisseria mutants and complemented strain
As a first step, the kanamycin and erythromycin resistance genes were amplified using Km6-KmUp and ERAM3-ERAMUp and subcloned into pGEM-T-easy (Promega) to give pGEM::Km and pGEM::Ery respectively. Secondly, the 5′ and 3′ DNA fragments were amplified from genomic DNA of the N. meningitidis MC58, using respectively KOYebN5′F-KOYebN5′R or KOYebN3′F-KOYebN3′R couple of primers. Lastly, the 5′ and 3′ DNA fragments were inserted sequentially into this pGEM::Km using respectively, NcoI and SphI or NdeI and PstI to finally generate p5′KOYebN3′::Km.
To construct the plasmid for the complementation, the full length yebN gene was amplified using YebNNsiIF and YebNPstIR and was inserted into p5′KOYebN3′::Km after a digestion with NsiI and PstI to generate p5Y3::Km. Subsequently, the kanamycine resistance gene has been removed and replaced by the erythromycin gene from pGEM::Ery using EcoRI to generate p5Y3::Ery. The luciferase was amplified using LucF and LucR, digested with NsiI-PvuII and ligated to p5Y3::Km digested with NsiI-BsaBI to generate p5L3::Km (luciferase under the control of the yebN promoter).
The p5′KOYebN3′::Km and p5L3::Km were then used to transform N. meningitidis MC58, and transformants were selected on GCB medium in the presence of 100 µg/ml Km. After verification of the deletion of yebN, the plasmid p5Y3::Ery was used to transform N. meningitidis MC58 ΔyebN and transformants were selected on GCB medium in the presence of 3 µg/ml Ery. Therefore, the functional yebN gene was introduced back in its original locus in the genome of N. meningitidis ΔyebN. Additionally, the plasmid p5Y3::Km was used to transform and complement N. gonorrhoeae 16626.
Sensitivity to metal
First, to evaluate the metal sensitivity of X. campestris, Neisseria sp. and respective mutants, a disk assay of metal sensitivity was used. X. campestris and mutants were grown during a 16-h period at 30°C and 250 rpm. The cultures were then diluted in GTA broth to obtain a final OD580 of 0.1 and were further incubated at 30°C until OD580 0.4. A volume of 1 ml was then taken, mixed with 9 ml of Top-SB medium and poured in 15 cm Petri plates containing 40 ml of solidified SB medium. After 15 min, a disk containing 10 µl of solution was placed on the center of the plates. For Neisseria, an aliquot of a 16h old culture (on plate) was taken and diluted on GCB media plus supplements to an OD600 of 1. Following this, 100 µl of this suspension were spread on a Petri dish containing 20 ml of GCB agar medium plus supplements. After 15 min, a disk containing 10 µl of solutions was deposed on the center of the plates. A standard t-test was done in order to assess statistically significant differences.
Secondly, growth sensitivity to the ratio manganese/iron was evaluated for the different strains of N. meningitidis. Serial dilutions of bacteria were spotted on plate containing increasing amount of Desferal and manganese. These plates were incubated overnight at 37°C after what, pictures were taken. To measure more precisely the effect of the ratio on bacterial growth, ≈250 bacteria were plated on GCB agar plate containing the indicated amount of manganese and Desferal, and the percentage of growth was calculated compared to the GCB agar with no manganese. A standard t-test was done in order to assess statistically significant differences.
Measurement of intracellular metals depletion using E. coli Δfur; pmntH-Luc over-expressing YebN
Construction of the plasmids
the yebN ORF from X. campestris was amplified using YNcoIF and YXbaIR whereas the ORF from N. meningitidis MC58 and N. gonorrhoeae 16691 was amplified using YebNF and YebNR. All these ORFs were cloned into pBAD24 using NcoI and XbaI.
Luciferase assay
Overnight cultures of each strain were diluted 1/40 into 1.8 ml of LB media and incubated during 2 h at 37°C with agitation. Following this, 200 µl of 10X cocktail (arabinose L or D; DP; Metals) was added at concentration indicated in Figure 5A. In parallel to the OD600 measurements, the bacterial lysis was done according to manufacturer recommendation (Luciferase assay system; promega) and directly after the addition of substrate, RLU were measured using LB96V MicroLumat Plus (Berthold) during 20 seconds and a standard t-test was applied to assess statistical significance.
yebN gene expression in vitro and in vivo
For in vitro expression, we used the firefly luciferase fused to the yebN promoter and inserted in the genome as described above. The strain was grown on GCB agar plates overnight at 37°C and subculture in GCB agar plates containing the indicated concentration of MnCl2 and Desferal during 5 h. The bacterial layer was harvested in physiologic water to obtain an OD600. The luciferase activity was measured as described in the “Luciferase assay system” protocol (Promega) and a standard t-test was applied to assess statistical significance.
For in vivo expression, we performed qRT-PCR. Briefly, RNA was extracted using bacterial RNA protect and RNeasy Mini Kit (Qiagen) from blood of mice infected i.p. with 500 ml of blood from a mice previously infected i.p. with 10° bacteria. A passage from one mouse to another was done in order to attenuate the GCB media interference. qRT-PCR was performed using Power SYBR Green PCR master mix and StrepOne plus (Applied Biosystems) using the primers listed in table 1. In addition, a standard t-test was applied to assess statistically significant difference in comparison to the gyrA ΔCt.
Tab. 1. Oligonucleotide primers used in this study. 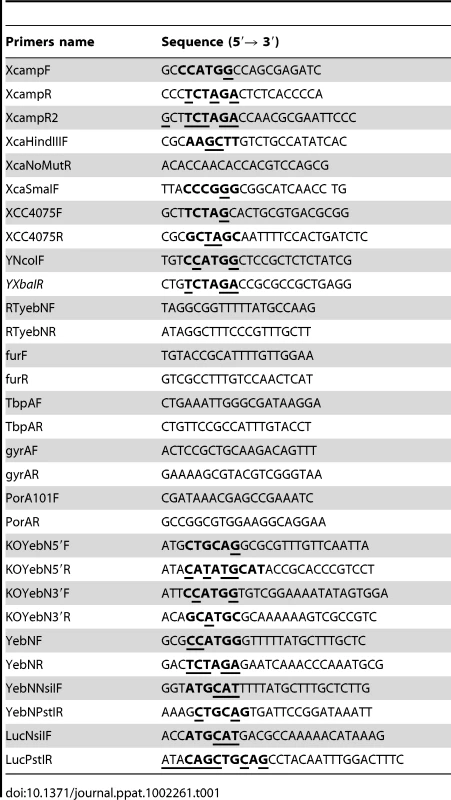
Determination of metal content using ICP-MS
The amount of metals accumulated in Neisseria sp. cells was determined by growing cells overnight on complete GCB and sub-culturing them in GCB agar plate containing 10 µM of Mn. After an incubation of 6h for N. meningitidis and 16h for N. gonorrhoeae (slow grower) the cells were resuspended in PBS and centrifuged. The pellets were subjected to acidic digestion using 500 µL of 65% acid nitric during one hour at 80°C. The preparation has been diluted with H2O (HPLC grade, Fisher) to obtain a concentration of 2% acid nitric. The samples were sent to Intertek Analytical Services (Chalon sur Saone; France) and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) with Agilent 7500 cx (Agilent Technologies, USA). Results were expressed as the calculated ratio of number of atoms of the specified metal (MW used for Mn: 54,938) normalized with the number of Mg atoms (MW: 24,305) or Fe (MW: 55,845). Each strain has been cultured in triplicates for N. meningitidis and in duplicates for N. gonorrhoeae and a standard t-test was applied to assess significance.
Sensitivity to human serum
To measure the percentage of survival, a specific human serum was selected due to its capacity to induce death in 30 to 50% of bacteria without addition of external source of complement. The assay was done as previously described [59] by incubating ≈1000 bacteria in HSSB2+ containing 50% of human serum during 30 min at 37°C. The results of several experiments (at least three) were pooled and one tailed Mann–Whitney test was used to determine statistical significance of observed differences (GraphPad Prism v5.0; GraphPad Software, CA).
Survival in a mouse model of septicemia
The day of infection, inocula of N. meningitidis strains were prepared in PBS to obtain a final concentration of 2×107 CFU/ml (OD600 0,1). Six week-old BALB/c (Janvier; France) or six to nine week-old human transferrin (hTf) transgenic mice [26] were infected by intraperitoneal challenge with 500 µl of inocula (1×107 CFU). Bacterial counts in the blood were determined at 2h and 4h or 6h (for wild type or hTf mice respectively) after meningococcal challenge by plating serial dilutions of blood samples on GCB medium. Results are expressed in percentage of bacteria counted at 4 h or 6 h (for wild type or hTf mice respectively) compare to those counted at 2 h post infection. The results of several experiments (at least two) were pooled and one tailed Mann–Whitney test was used to determine statistical significance of observed differences (GraphPad Prism v5.0; GraphPad Software, CA).
Bioinformatic analyses
To search for MntR regulated genes in bacterial genomes, we first sought the presence of putative conserved MntR binding sites using several predictive approaches. For this purpose, the sequence of the promoters of mntH, mntR, sitA from different proteobacteria were aligned using the AlignX program from VectorNTI (Invitrogen), and the resulting multiple alignment of similar motifs was used as a matrix to detect conserved binding sites in different genomes using the programs PredictRegulon [60] and PREDetector [61].
The protein hydropathy profile was calculated using the server TOPPRED [62] using a 20-residue long sliding window (core window: 14 residues, wedge windows: 4 residues). The default parameters suggested for predicting the presence of transmembrane segments in prokaryotic proteins were used, such as the Goldman, Engelman, Steitz hydropathy scale cut-off values (Lower, 0.6; putative; Upper, 1.0; certain). Protein transmembrane topology prediction was calculated using the MEMSAT-SVM server [63].
Molecular evolutionary sequence analyses were performed as previously described [38]. Sequences were classified by similarity clustering using CLANS [64] and multiple sequence alignment and phylogenetic analyses were conducted using the package Mega [36]. Briefly, BlastP searches using MntX revealed numerous similar bacterial sequences showing unexpected taxonomic distribution. To perform tractable sequence analyses we selected from databases of reduced complexity (entries <70% identity) candidate homologs co-linear with and displaying more than 30% identity to MntX. Clustering and tree-making approaches validated our sequence set as representative of the putative MntX family and including a possible phylogenetic outgroup [38]. To visualize MntX sequence conservation patterns we used a multiple-logo alignment tool [37] to compare the variability/homogeneity of aligned sequences representing hierarchically defined subclusters, such as the outgroup and the MntX family, as well as MntX family subgroups.
Supporting Information
Zdroje
1. FinkelsteinRASciortinoCVMcIntoshMA 1983 Role of iron in microbe-host interactions. Rev Infect Dis 5 Suppl 4 S759 777
2. WeinbergED 1971 Role of iron in host-parasite interactions. J Infect Dis 124 401 410
3. SchaibleUEKaufmannSH 2005 A nutritive view on the host-pathogen interplay. Trends Microbiol 13 373 380
4. BarondeauDPGetzoffED 2004 Structural insights into protein-metal ion partnerships. Curr Opin Struct Biol 14 765 774
5. StadtmanER 1990 Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9 315 325
6. CellierMFCourvillePCampionC 2007 Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect 9 1662 1670
7. CorbinBDSeeleyEHRaabAFeldmannJMillerMR 2008 Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319 962 965
8. JakubovicsNSValentineRA 2009 A new direction for manganese homeostasis in bacteria: identification of a novel efflux system in Streptococcus pneumoniae. Mol Microbiol 72 1 4
9. Kehl-FieTESkaarEP 2010 Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14 218 224
10. RoschJWGaoGRidoutGWangYDTuomanenEI 2009 Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol 72 12 25
11. ArchibaldFSFridovichI 1981 Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol 145 442 451
12. SeibKLWuHJKiddSPApicellaMAJenningsMP 2006 Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev 70 344 361
13. ChampionOLKarlyshevAVCooperIAFordDCWrenBW 2011 Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation that is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157 Pt 4 1115 22
14. AndersonESPaulleyJTGainesJMValderasMWMartinDW 2009 The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect Immun 77 3466 3474
15. ZaharikMLCullenVLFungAMLibbySJKujat ChoySL 2004 The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun 72 5522 5525
16. BoyerEBergevinIMaloDGrosPCellierMF 2002 Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun 70 6032 6042
17. PuriSHohleTHO'BrianMR 2010 Control of bacterial iron homeostasis by manganese. Proc Natl Acad Sci U S A 107 23 10691 10695
18. DalyMJGaidamakovaEKMatrosovaVYVasilenkoAZhaiM 2004 Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306 1025 1028
19. AnjemAVargheseSImlayJA 2009 Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72 844 858
20. McEwanAG 2009 New insights into the protective effect of manganese against oxidative stress. Mol Microbiol 72 812 814
21. Renauld-MongenieGPoncetDMignonMFraysseSChabanelC 2004 Role of transferrin receptor from a Neisseria meningitidis tbpB isotype II strain in human transferrin binding and virulence. Infect Immun 72 3461 3470
22. HagenTACornelissenCN 2006 Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol Microbiol 62 1144 1157
23. LarsonJAHigashiDLStojiljkovicISoM 2002 Replication of Neisseria meningitidis within epithelial cells requires TonB-dependent acquisition of host cell iron. Infect Immun 70 1461 1467
24. GencoCADesaiPJ 1996 Iron acquisition in the pathogenic Neisseria. Trends Microbiol 4 179 184
25. GencoCAChenCYArkoRJKapczynskiDRMorseSA 1991 Isolation and characterization of a mutant of Neisseria gonorrhoeae that is defective in the uptake of iron from transferrin and haemoglobin and is avirulent in mouse subcutaneous chambers. J Gen Microbiol 137 1313 1321
26. ZarantonelliMLSzatanikMGiorginiDHongEHuerreM 2007 Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 75 5609 5614
27. OftungFLovikMAndersenSRFroholmLOBjuneG 1999 A mouse model utilising human transferrin to study protection against Neisseria meningitidis serogroup B induced by outer membrane vesicle vaccination. FEMS Immunol Med Microbiol 26 75 82
28. OdugbemiTMcEntegartMHafizS 1978 Effects of various divalent cations on the survival of Neisseria gonorrhoeae in liquid media. Br J Vener Dis 54 239 242
29. OdugbemiTOMcEntegartMGHafizS 1978 A simple manganous chloride and Congo red disc method for differentiating Neisseria gonorrhoeae from Neisseria meningitidis. J Clin Pathol 31 936 938
30. SeibKLTsengHJMcEwanAGApicellaMAJenningsMP 2004 Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis 190 136 147
31. QueQHelmannJD 2000 Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35 1454 1468
32. RodionovDAGelfandMSToddJDCursonARJohnstonAW 2006 Computational reconstruction of iron - and manganese-responsive transcriptional networks in alpha-proteobacteria. PLoS Comput Biol 2 e163
33. PatzerSIHantkeK 2001 Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol 183 4806 4813
34. KehresDGJanakiramanASlauchJMMaguireME 2002 Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+). J Bacteriol 184 3151 3158
35. IkedaJSJanakiramanAKehresDGMaguireMESlauchJM 2005 Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J Bacteriol 187 912 922
36. TamuraKDudleyJNeiMKumarS 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
37. ShihACLeeDTPengCLWuYW 2007 Phylo-mLogo: an interactive and hierarchical multiple-logo visualization tool for alignment of many sequences. BMC Bioinformatics 8 63
38. CellierM 2011 Nutritional Immunity: Homology Modeling of Nramp Metal Import. Current Topics in Innate Immunity II. LambrisJDHajishengallisGN Advances in Experimental Medicine and Biology 946
39. Marchler-BauerALuSAndersonJBChitsazFDerbyshireMK CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 39 D225 229
40. NamHJJeonJKimS 2009 Bioinformatic approaches for the structure and function of membrane proteins. BMB Rep 42 697 704
41. BoudkerOVerdonG 2010 Structural perspectives on secondary active transporters. Trends Pharmacol Sci 31 418 426
42. KuntalBKAparoyPReddannaP 2010 Development of tools and database for analysis of metal binding sites in protein. Protein Pept Lett 17 765 773
43. MakuiHRoigEColeSTHelmannJDGrosP 2000 Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35 1065 1078
44. BreuerWEpsztejnSCabantchikZI 1995 Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J Biol Chem 270 24209 24215
45. KehresDGMaguireME 2003 Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27 263 290
46. TsengHJSrikhantaYMcEwanAGJenningsMP 2001 Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol 40 1175 1186
47. SchryversABGonzalezGC 1989 Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect Immun 57 2425 2429
48. KorkhovVMTateCG 2009 An emerging consensus for the structure of EmrE. Acta Crystallogr D Biol Crystallogr 65 186 192
49. KhafizovKStaritzbichlerRStammMForrestLR 2010 A study of the evolution of inverted-topology repeats from LeuT-fold transporters using AlignMe. Biochemistry 49 10702 10713
50. ForrestLRRudnickG 2009 The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 24 377 386
51. RadestockSForrestLR 2011 The alternating-access mechanism of MFS transporters arises from inverted-topology repeats. J Mol Biol 407 5 698 715
52. ZhengHYAlcornTMCohenMS 1994 Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis 170 1209 1215
53. FaulknerMJHelmannJD 2011 Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal 15 1 175 189
54. GrootMNKlaassensEde VosWMDelcourJHolsP 2005 Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology 151 1229 1238
55. SchaferATauchAJagerWKalinowskiJThierbachG 1994 Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145 69 73
56. DennisJJZylstraGJ 1998 Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl Environ Microbiol 64 2710 2715
57. DeShazerDWoodsDE 1996 Broad-host-range cloning and cassette vectors based on the R388 trimethoprim resistance gene. Biotechniques 20 762 764
58. SlaterHAlvarez-MoralesABarberCEDanielsMJDowJM 2000 A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38 986 1003
59. McQuillenDPGulatiSRicePA 1994 Complement-mediated bacterial killing assays. Methods Enzymol 236 137 147
60. YellaboinaSSeshadriJKumarMSRanjanA 2004 PredictRegulon: a web server for the prediction of the regulatory protein binding sites and operons in prokaryote genomes. Nucleic Acids Res 32 W318 320
61. HiardSMareeRColsonSHoskissonPATitgemeyerF 2007 PREDetector: a new tool to identify regulatory elements in bacterial genomes. Biochem Biophys Res Commun 357 861 864
62. ClarosMGvon HeijneG 1994 TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci 10 685 686
63. NugentTJonesDT 2009 Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics 10 159
64. FrickeyTLupasA 2004 CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20 3702 3704
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



