-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
Toll-like receptor 3 (TLR3) and cytosolic RIG-I-like helicases (RIG-I and MDA5) sense viral RNAs and activate innate immune signaling pathways that induce expression of interferon (IFN) through specific adaptor proteins, TIR domain-containing adaptor inducing interferon-β (TRIF), and mitochondrial antiviral signaling protein (MAVS), respectively. Previously, we demonstrated that hepatitis A virus (HAV), a unique hepatotropic human picornavirus, disrupts RIG-I/MDA5 signaling by targeting MAVS for cleavage by 3ABC, a precursor of the sole HAV protease, 3Cpro, that is derived by auto-processing of the P3 (3ABCD) segment of the viral polyprotein. Here, we show that HAV also disrupts TLR3 signaling, inhibiting poly(I:C)-stimulated dimerization of IFN regulatory factor 3 (IRF-3), IRF-3 translocation to the nucleus, and IFN-β promoter activation, by targeting TRIF for degradation by a distinct 3ABCD processing intermediate, the 3CD protease-polymerase precursor. TRIF is proteolytically cleaved by 3CD, but not by the mature 3Cpro protease or the 3ABC precursor that degrades MAVS. 3CD-mediated degradation of TRIF depends on both the cysteine protease activity of 3Cpro and downstream 3Dpol sequence, but not 3Dpol polymerase activity. Cleavage occurs at two non-canonical 3Cpro recognition sequences in TRIF, and involves a hierarchical process in which primary cleavage at Gln-554 is a prerequisite for scission at Gln-190. The results of mutational studies indicate that 3Dpol sequence modulates the substrate specificity of the upstream 3Cpro protease when fused to it in cis in 3CD, allowing 3CD to target cleavage sites not normally recognized by 3Cpro. HAV thus disrupts both RIG-I/MDA5 and TLR3 signaling pathways through cleavage of essential adaptor proteins by two distinct protease precursors derived from the common 3ABCD polyprotein processing intermediate.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002169
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002169Summary
Toll-like receptor 3 (TLR3) and cytosolic RIG-I-like helicases (RIG-I and MDA5) sense viral RNAs and activate innate immune signaling pathways that induce expression of interferon (IFN) through specific adaptor proteins, TIR domain-containing adaptor inducing interferon-β (TRIF), and mitochondrial antiviral signaling protein (MAVS), respectively. Previously, we demonstrated that hepatitis A virus (HAV), a unique hepatotropic human picornavirus, disrupts RIG-I/MDA5 signaling by targeting MAVS for cleavage by 3ABC, a precursor of the sole HAV protease, 3Cpro, that is derived by auto-processing of the P3 (3ABCD) segment of the viral polyprotein. Here, we show that HAV also disrupts TLR3 signaling, inhibiting poly(I:C)-stimulated dimerization of IFN regulatory factor 3 (IRF-3), IRF-3 translocation to the nucleus, and IFN-β promoter activation, by targeting TRIF for degradation by a distinct 3ABCD processing intermediate, the 3CD protease-polymerase precursor. TRIF is proteolytically cleaved by 3CD, but not by the mature 3Cpro protease or the 3ABC precursor that degrades MAVS. 3CD-mediated degradation of TRIF depends on both the cysteine protease activity of 3Cpro and downstream 3Dpol sequence, but not 3Dpol polymerase activity. Cleavage occurs at two non-canonical 3Cpro recognition sequences in TRIF, and involves a hierarchical process in which primary cleavage at Gln-554 is a prerequisite for scission at Gln-190. The results of mutational studies indicate that 3Dpol sequence modulates the substrate specificity of the upstream 3Cpro protease when fused to it in cis in 3CD, allowing 3CD to target cleavage sites not normally recognized by 3Cpro. HAV thus disrupts both RIG-I/MDA5 and TLR3 signaling pathways through cleavage of essential adaptor proteins by two distinct protease precursors derived from the common 3ABCD polyprotein processing intermediate.
Introduction
Hepatitis A virus (HAV) [1] and hepatitis C virus (HCV) [2] are positive-strand RNA viruses that cause hepatitis in humans. Despite important differences in virion structure, they share similar genome structures and many aspects of their replication strategies. Both viruses demonstrate strong tropism for the hepatocyte, and replicate their RNA genomes in replicase complexes contained within cytoplasmic vesicles. Both produce double-stranded RNA (dsRNA), a potent pathogen-associated molecular pattern (PAMP) recognized by innate immune sensors, as replication intermediates. Thus, both HAV and HCV face similar challenges posed by the innate immune system early in the course of hepatic infection. However, HAV and HCV infections have dramatically different outcomes. HAV never causes chronic hepatitis while HCV does so in the majority of those it infects. Prolonged shedding of HAV has been reported in premature infants [3], but long-term persistent infection has never been documented. This contrasts sharply with HCV, which persists for decades in the majority of those infected [2], [4].
Although factors controlling HCV infection outcome are poorly understood, T cell responses are critical [reviewed in 2]. T cells also appear to be important for HAV clearance [5], [6]. In both cases, the vigor and breadth of the virus-specific T response is likely to be profoundly influenced by early interferon (IFN) and other cytokine responses evoked by innate antiviral response pathways. How HCV both induces and disrupts signaling initiated by retinoic acid-inducible gene I (RIG-I) and Toll-like receptor 3 (TLR3) has been studied in depth. Proteolytic cleavage of mitochondrial antiviral signaling protein (MAVS, also known as IPS-1, VISA or Cardif) and TIR domain-containing adaptor inducing IFN-β (TRIF, also known as TICAM-1) by the NS3/4A serine protease of HCV effectively blocks the activation of IFN-regulatory factor 3 (IRF-3) and nuclear factor-κB (NF-κB) induced by RIG-I and TLR3, respectively [7], [8], [9]. Much less is known about how HAV stimulates or antagonizes these innate signaling pathways.
Both clinical and experimental observations indicate that there is extensive replication of HAV within the liver prior to the onset of hepatic inflammation 3–4 weeks after infection [10]. This lengthy, clinically silent incubation period suggests that HAV either blocks or otherwise fails to induce innate immune responses to dsRNA in the early stages of the infection. Consistent with this, HAV, like HCV, disrupts virus-induced signaling initiated by RIG-I-like receptors (RLRs, RIG-I and melanoma differentiation associated gene 5, MDA-5) [11], [12]. Our previous work shows that it does this by targeting MAVS for proteolysis by a precursor of its 3Cpro cysteine protease, 3ABC [12]. Cleavage requires both the protease activity of 3Cpro and a transmembrane domain in 3A that directs 3ABC to the mitochondrial outer membrane where MAVS is localized [12]. The shared capacity of the HAV 3ABC and HCV NS3/4A proteases to cleave MAVS and disrupt signaling from RLRs suggests that MAVS-dependent signaling is critical to antiviral defense in the liver (as it is in other tissues), but also indicates that NS3/4A cleavage of MAVS is not primarily responsible for the unique ability of HCV to establish persistent infections.
In addition to RLRs, TLR3 is functionally expressed in primary human hepatocytes [13]. During HCV infection, signaling initiated by TLR3 recognition of dsRNA is blocked by NS3/4A cleavage of the adaptor protein, TRIF [7], [13]. This led us to ask whether HAV also antagonizes TLR3 signaling. We show here that HAV strongly inhibits TLR3 signaling by also targeting TRIF for degradation. We demonstrate that TRIF is proteolytically cleaved by a distinct intermediate in the polyprotein processing cascade, the viral 3CD protease-polymerase. Cleavage requires expression of the cysteine protease activity of 3Cpro fused in cis to 3Dpol sequence. Mutational studies reveal an unexpected role of the 3Dpol domain in modulating the substrate specificity of 3Cpro such that it is able to achieve scission of non-canonical 3Cpro cleavage sites within TRIF. The role played by the polymerase sequence in innate immune evasion represents a remarkable and unique mechanism of viral adaptation to the intrahepatic environment, and provides a second major evasive strategy by which HAV can escape innate immunity.
Results
HAV inhibits TLR3 signaling by reducing abundance of the adaptor protein TRIF
Although hepatocytes express TLR3, Huh7 hepatoma cells, which are permissive for replication of cell culture-adapted HAV, are defective in TLR3 signaling [14], [15]. We therefore studied the impact of HAV infection on TLR3 signaling in Huh7 cells in which signaling was functionally reconstituted by retroviral transduction of TLR3 expression [13]. Previous studies of these cells include extensive control experiments showing that the activation of IRF-3 by extracellular poly-(I:C) occurs specifically through TLR3 signaling [13]. Control cells used in here included Huh7 cells transduced in parallel with a TIR-domain TLR3 deletion mutant or empty vector. Stimulation of Huh7-TLR3 cells with extracellular poly(I:C), a synthetic dsRNA analog, induced transcriptional activation of the IFN-β promoter and expression of the interferon-stimulated gene (ISG) ISG15, neither of which were observed in Huh7-ΔTIR or Huh7-Vector cells (Fig. 1A, B). However, prior infection of Huh7-TLR3 cells with HM175/18f, a cell culture-adapted HAV variant [16], strongly inhibited both responses (Fig. 1A, B). The expression levels of TLR3 and its ΔTIR mutant were not affected by HAV (Fig. 1B), suggesting that HAV does not disrupt TLR3 signaling by reducing TLR3 abundance. HAV inhibition of TLR3 signaling was also observed in Huh7.5-TLR3 cells, TLR3-reconstituted Huh-7.5 cells that are deficient in RIG-I signaling (Fig. S1A in Text S1) [13].
Fig. 1. HAV inhibits TLR3 signaling prior to IRF-3 phosphorylation and nuclear translocation. 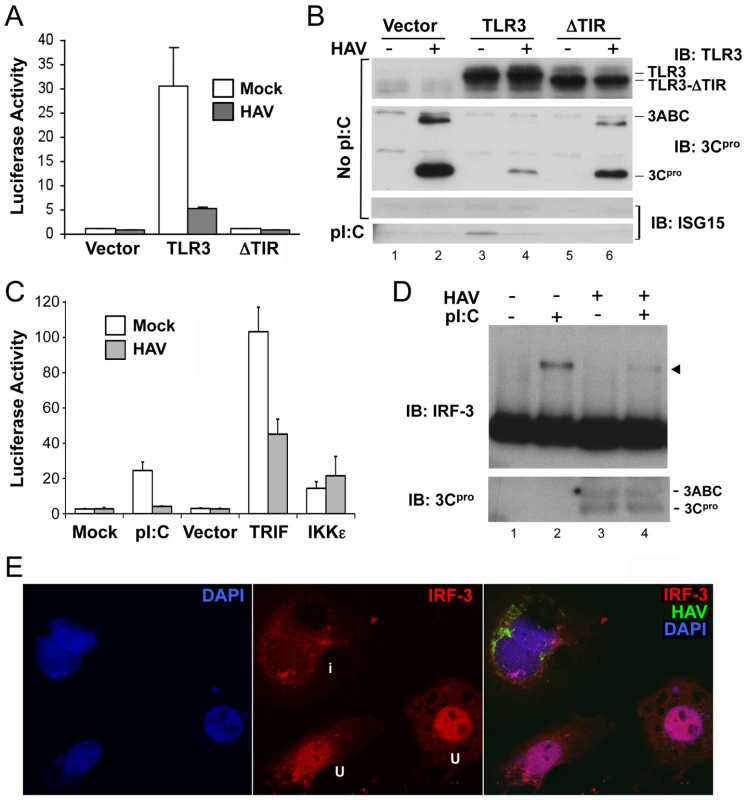
(A) IFN-β-Luc reporter assay of Huh7-TLR3, Huh7-ΔTIR, and Huh7-Vector cells that were mock- or HAV-infected (m.o.i. = 3) for 5 days and stimulated with extracellular poly(I:C) for 6 hours. Luciferase activity was normalized to an internal β-gal transfection control and is presented fold-induction by poly(I:C). (B) Immunoblots of the cells in panel (A) showing expression of TLR3 and TLR3-ΔTIR (top panel), HAV 3ABC and 3Cpro (middle panel), and poly(I:C)-induced ISG15 (bottom panel). (C) IFN-β-Luc reporter assay of poly(I:C), TRIF, or IKKε-induced activation of the IFN-β promoter in mock- or HAV-infected Huh7-TLR3 cells. (D) HAV infection blocks poly-(I:C)-induced dimerization of IRF-3 in Huh7/TLR3 cells. (top panel) Immunoblot of IRF-3 in extracts of HAV- or mock-infected cells resolved by native PAGE. Cells were lysed 2 hrs after addition of poly-(I:C) (50 µg/ml) to media. IRF-3 dimers are indicated by the arrowhead. (bottom panel) Immunoblot of native PAGE with 3Cpro-specific antibody. (E) Laser-scanning confocal microscopy images of Huh7-TLR3 cells infected with HAV at low m.o.i. for 5 days and stimulated with poly-(I:C). Cells were labeled with antibodies to IRF-3 (red) and HAV (green), while nuclei were counterstained with DAPI (blue). The merged image on the right shows that IRF-3 has undergone nuclear translocation in two uninfected (‘u’, see middle panel) cells, but not in an infected cell (‘i’) containing HAV antigen. The IFN-β promoter is activated by overexpression of the TLR3 adaptor protein, TRIF, or downstream kinases, TBK-1 or IKKε, that phosphorylate IRF-3 [17]. In HAV-infected cells, however, its activation by TRIF was reduced by about 50%, while there was no reduction in its stimulation by IKKε (Fig. 1C). This result is similar to that reported previously by Fensterl et al. [11]. We also observed a marked reduction in the abundance of IRF-3 dimers in HAV-infected Huh7-TLR3 cells stimulated with extracellular poly-(I:C) (Fig. 1D). In addition, confocal microscopy revealed that IRF-3 did not undergo nuclear translocation upon poly-(I:C) stimulation of HAV-infected Huh7-TLR3 cells (Fig. 1E), while this occurred uniformly in uninfected cells (Fig. S1B in Text S1, right panel). Importantly, HAV infection itself induced neither IRF-3 dimerization (Fig. 1D) nor nuclear translocation (Fig. S1B in Text S1, left panel), indicating an absence of IRF-3 activation. Collectively, these results indicate that HAV infection disrupts the signal transduction pathway from TLR3 prior to the kinases responsible for IRF-3 activation.
Consistent with a defect in signaling at this level, we found the abundance of endogenous TRIF was substantially reduced in HAV-infected Huh7 or Huh7.5-TLR3 cells (Fig. 2A). In addition, we could not detect TRIF in a stable Huh7 cell line harboring an autonomously replicating subgenomic HAV replicon (HAV-Bla cells) [18], while TRIF expression was restored after eliminating the replicon by IFN treatment (Bla-C cells, Fig. 2B). Thus, HAV infection disrupts TLR3 signaling by substantially decreasing the expression of TRIF.
Fig. 2. TRIF abundance is reduced by HAV infection. 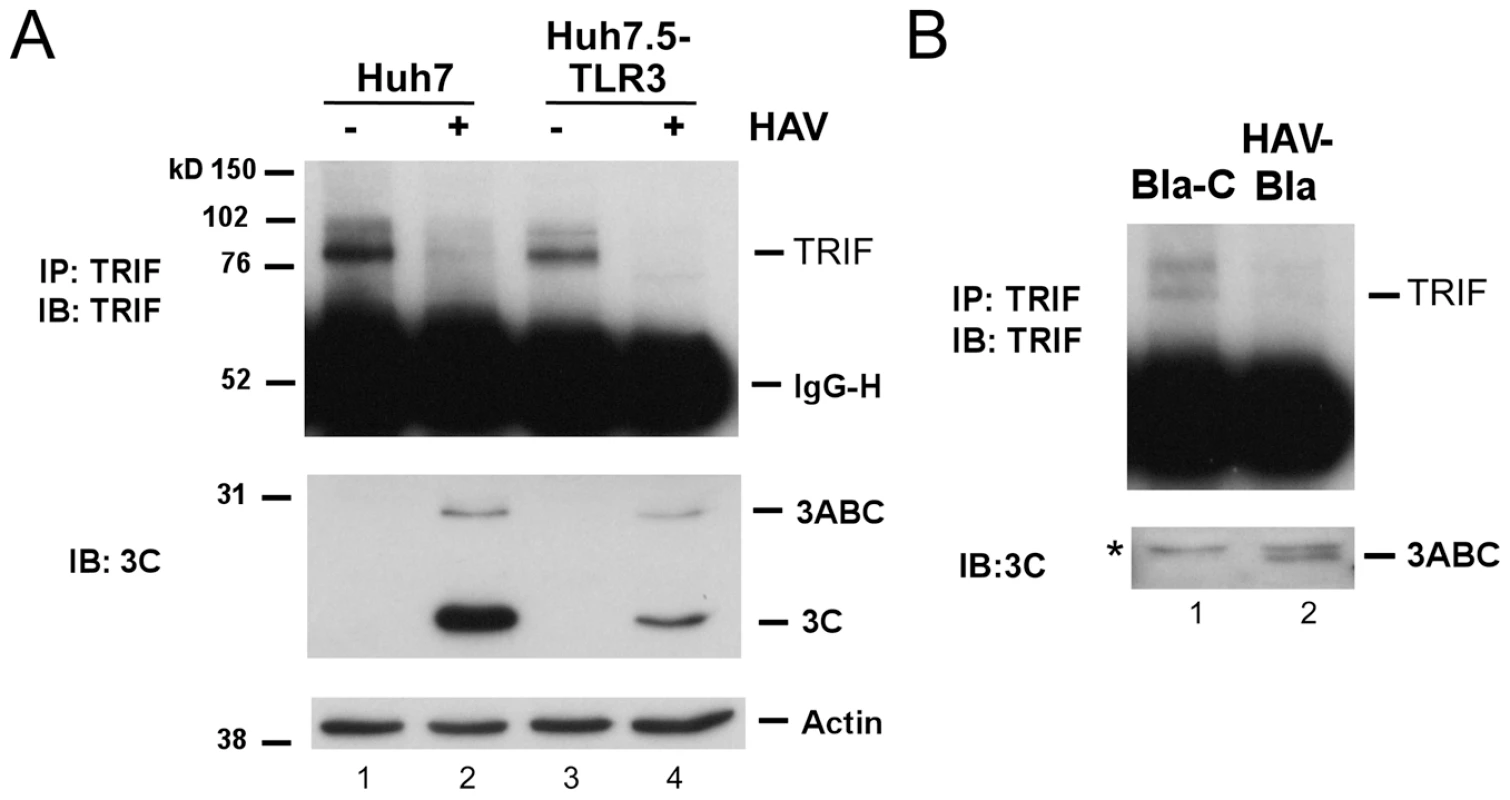
(A) (top panel) Endogenous TRIF in mock- or HAV-infected Huh7 and Huh7.5-TLR3 cells was immunoprecipitated using a rabbit anti-TRIF antibody [7] and detected by immunoblotting. (middle panel) HAV infection was confirmed by immunoblot of viral proteins 3ABC and 3Cpro. (bottom panel) Actin served as loading control. (B) Endogenous TRIF and 3ABC expression in the HAV replicon HAV-Bla cells and IFN-α-cured Bla-C cells [12] (top). TRIF was detected as in panel A. ‘*’ indicates a nonspecific protein band. The HAV 3CD protease-polymerase processing intermediate disrupts TLR3 signaling by cleaving TRIF
To determine whether a specific HAV protein or polyprotein processing intermediate was responsible for the reduction in TRIF abundance and, as a result, the inhibition of TLR3 signaling, we over-expressed individual proteins in Huh7-TLR3 cells, assessing the impact on poly(I:C)-induced, TLR3-dependent activation of the IFN-β promoter. While ectopic expression of the P1-2A structural proteins (P1-2A, VP0, VP3, VP1-2A) and 2B or 2C had little impact on TLR3 signaling, the processing intermediate 3ABCD and its 3CD protease-polymerase derivative strongly blocked promoter activation (Fig. 3A, top panel). An intermediate degree of suppression was observed with 2BC and 3Cpro expression. The effect of 2BC on signaling may be related to its capacity to induce intracellular membrane rearrangement [19], and was not studied further.
Fig. 3. The HAV 3CD protease-polymerase precursor disrupts TLR3 signaling through cleavage of TRIF. 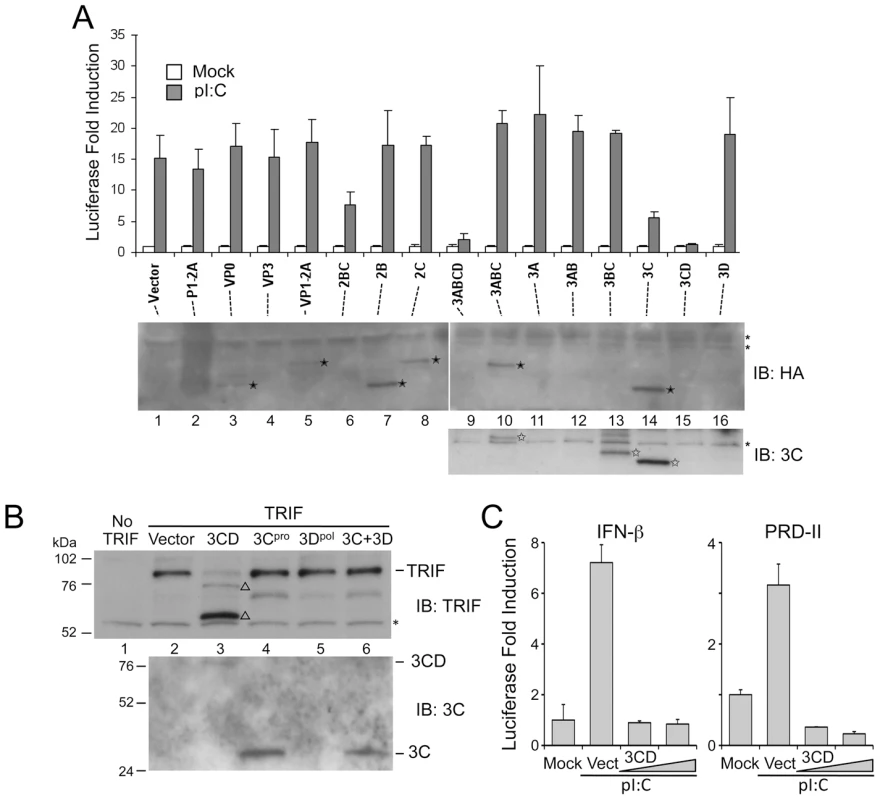
(A) (top panel) IFN-β-Luc reporter assay of Huh7.5-TLR3 cells transfected with expression vectors encoding HA-tagged HAV proteins and stimulated with extracellular poly(I:C). (bottom panel) Extracts of similarly transfected cells were analyzed by immunoblotting with HA antibody to detect HAV proteins (solid star, middle panel), or with specific antibody to 3Cpro (open star, bottom panel). ‘*’ indicates a nonspecific protein band. 3CD and 3Dpol were not visualized with any antibody. (B) HEK 293FT cells were co-transfected with vectors expressing TRIF and HA-tagged HAV proteins. Cell lysates were analyzed by immunoblotting for TRIF (top) or 3Cpro and 3CD (bottom). In addition to the 90-kDa full-length TRIF, two TRIF fragments, 75- and 55-kDa in size (‘Δ’), were detected in cells expressing 3CD. A nonspecific protein band detected by TRIF antibody (‘*’) indicates equal loading. (C) IFN-β-Luc and PRD-II-Luc (NF-κB specific) reporter assays of Hela cells transfected with control or 3CD expression vectors and stimulated with extracellular poly(I:C). Increasing amounts of 3CD expression vector (50 ng and 100 ng) were used. The common presence of the 3Cpro cysteine protease domain in 3ABCD, 3CD and 3Cpro suggested it may play a role in disrupting TLR3 signaling. However, 3Cpro alone was significantly less inhibitory than either 3CD or 3ABCD (Fig. 3A, top panel, p<0.002 by Student's t test), despite being expressed in much greater abundance (Fig. 3A, lower panel). Since 3ABCD is the precursor of 3CD, its inhibitory effect on TLR3 signaling could be due entirely to 3CD. 3ABC, which is also derived from 3ABCD and targets MAVS for cleavage [12], had no effect. Consistent with these results, the co-expression of 3CD, but not 3Cpro or 3Dpol, or a combination of these two proteins, resulted in a marked reduction of ectopically expressed TRIF in HEK 293FT cells (in which endogenous TRIF expression is negligible) (Fig. 3B). The reduced abundance of full-length TRIF in cells expressing 3CD was accompanied by the appearance of two TRIF fragments with apparent molecular masses of 75 and 55 kDa that were detected by an antibody recognizing residues surrounding Ser-219 (Fig. 3B, open triangles). Since full-length TRIF has a mass of ∼90-kDa, these are likely to be overlapping degradation products. An antibody to residues 4–31 of TRIF identified an additional fragment with an apparent mass of 20 kDa, likely derived from the N-terminus of TRIF (not shown). The accumulation of at least three different fragments suggests that 3CD causes multiple scission events within TRIF. 3CD-mediated cleavage of TRIF was not dependent upon the cell culture-adaptive mutations present in the 3CD sequence of the HM175/18f virus [16] used in these studies, as it was also observed with ectopically expressed, wild-type 3CD (Fig. S3 in Text S1). Consistent with these observations, 3CD overexpression resulted in a marked reduction in poly-I:C-stimulated IFN-β and PRD-II (NF-κB-responsive) promoter activity in HeLa cells (Fig. 3C). Thus, 3CD effectively antagonizes an endogenous TLR3 pathway, as well as the reconstituted pathway in Huh7-TLR3 cells.
Since co-expression of 3Cpro and 3Dpol did not result in detectable TRIF cleavage (Fig. 3B), efficient scission appears to require expression of the protease and polymerase domains in cis. 3CD is known to be a catalytically active precursor of 3Cpro [20], thus 3CD could directly cleave TRIF. To test this hypothesis, we expressed a 3CD mutant with an Ala substitution of the active-site nucleophile, Cys-172 (Fig. 4A). This lacked any capacity to cleave ectopically expressed TRIF (Fig. 4B), confirming that the protease activity of 3CD is responsible. In contrast, a mutant in which the conserved GDD motif required for RNA-dependent RNA polymerase activity was ablated (3CD-GAA) remained capable of cleaving TRIF. The 3CD-GAA mutant also inhibited poly(I:C)-induced activation of the IFN-β promoter in Huh7-TLR3 cells, whereas the proteinase-deficient 3CD-C172A mutant did not (Fig. 4C). Collectively, the results shown in Figs. 3 and 4 indicate that TRIF cleavage results from the 3Cpro protease acting in cis with 3Dpol.
Fig. 4. 3CD cleavage of TRIF requires both 3Cpro protease activity and 3Dpol in cis. 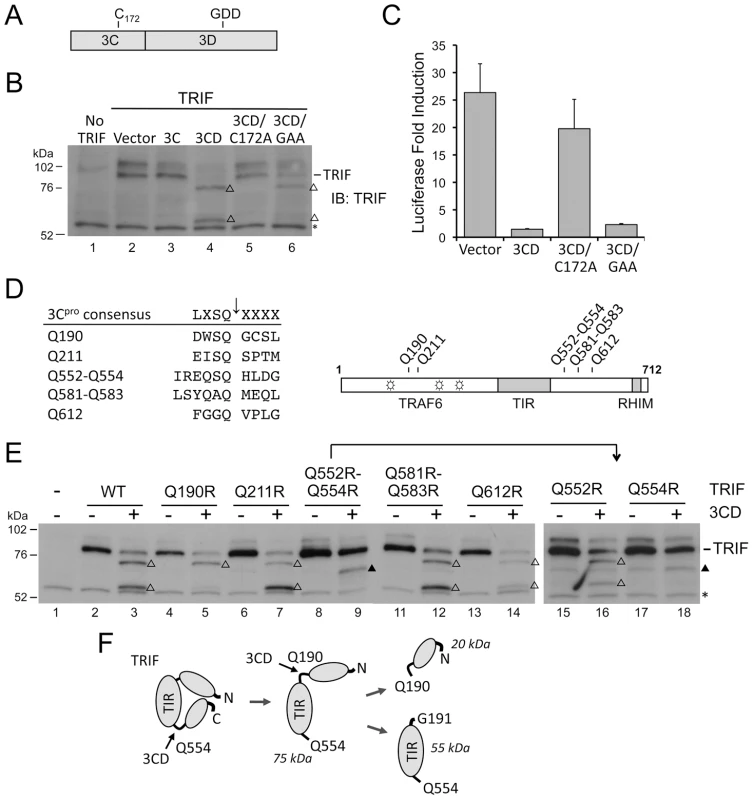
(A) Organization of 3CD, showing the position of Cys-172 at the 3Cpro active site and the GDD polymerase motif in 3Dpol. (B) Huh7 cells were co-transfected with vectors expressing TRIF and 3C, 3D, or 3CD, as indicated. Cell extracts were analyzed by immunoblotting for TRIF. TRIF cleavage fragments are marked (‘Δ’). A nonspecific protein band detected by TRIF antibody (‘*’) indicates equal loading. (C) IFN-β-Luc reporter assay of Huh7-TLR3 cells transfected with vectors expressing 3CD or the related C172A and GAA mutants, and stimulated with extracellular poly-(I:C). (D) Alignment of 3Cpro consensus cleavage sequence and possible cleavage sites in TRIF (left), and locations of these sites relative to the TRAF6-binding motifs (open star) and TIR and RHIM domains (grey boxes) within TRIF (right). (E) Identification of Q190 and Q554 as 3CD cleavage sites in TRIF. HEK 293FT cells were co-transfected with vectors expressing TRIF mutants and 3CD, and cell extracts were analyzed by immunoblotting of TRIF. TRIF cleavage fragments and a nonspecific protein band were marked as in (B). A TRIF degradation band (solid triangle), independent of 3CD cleavage, was occasionally observed. (F) A model for 3CD cleavage of TRIF. Cleavage at the preferred primary Q554 site induces a conformational change that exposes the Q190 site for the second cleavage. To assess whether TRIF is cleaved by a 3Cpro–3Dpol complex forming after auto-processing of 3CD, we constructed 3CD mutants with modifications at the 3C–3D junction that accelerate or retard autoprocessing. The 3C–3D junction is comprised of a primary 3Cpro cleavage site, IESQ↓R, and an alternative cleavage site, EFTQ↓C, separated by 9 residues (Fig. S2A in Text S1). In one mutant, 3CD-QQRR, both cleavage sites were abolished by Gln-to-Arg mutations such that expression resulted only in the 3CD precursor (Fig. S2B in Text S1). In the other, 3CD-LWG, the primary cleavage site was optimized to LWSQ↓G, making it identical to the efficiently cleaved 2C–3A junction (Fig. S2A in Text S1). Expression of 3CD-LWG led to less 3CD precursor, and a greater abundance of mature 3Cpro due to enhanced 3C/3D processing (Fig. S2B in Text S1). While the 3CD-QQRR mutant remained capable of cleaving TRIF, the hyper-processing 3CD-LWG mutant did not (Fig. S2C in Text S1). We conclude that the cleavage of TRIF results from the cysteine protease activity of the unprocessed 3CD sequence.
TRIF is cleaved by cellular caspases at residues D281 and D289 under conditions favoring apoptosis, including over-expression of TRIF [21]. This generates a 38-kDa fragment that is distinct from those generated by 3CD (Fig. S4A in Text S1). Moreover, a D281E-D289E (DDEE) TRIF mutant resistant to caspase cleavage [21] remained subject to cleavage by 3CD (Fig. S4A in Text S1). Thus, TRIF is not degraded indirectly by cellular caspases when 3CD is expressed. While a broadly active caspase inhibitor, z-VAD-fmk, partially inhibited the 3CD-mediated cleavage of TRIF (Fig. S4B in Text S1), z-VAD-fmk is known to inhibit cellular proteases other than caspases that, like 3Cpro, contain cysteine nucleophiles [22].
To show that TRIF is cleaved directly by the viral protease, we attempted to purify GST-3CD and GST-3Cpro fusion proteins produced in E. coli. The GST-3CD fusion product formed an insoluble pellet upon extraction, precluding its purification. This likely reflects the extreme insolubility of 3Dpol, which has hindered previous efforts to purify the HAV polymerase [23]. We were able to produce purified GST-3Cpro. In cell-free cleavage assays, this demonstrated a limited ability to process [35S]-labeled TRIF prepared by in vitro translation (Fig. S5 in Text S1), but did produce the expected ∼75-, ∼55-, ∼27-, and ∼18-kDa cleavage products when incubated with full-length TRIF, or fragments representing amino acids 1–372 or 373–712 of TRIF, in vitro (Fig. S5B in Text S1). Taken collectively with the data shown in Fig. S4 in Text S1, these results confirm that TRIF cleavage is caused by the HAV protease directly, and not by indirect activation of a caspase or other cellular protease. The incomplete proteolysis of TRIF observed in the cell-free cleavage reactions is consistent with the partial inhibition of IFN-β promoter activation by poly-(I:C) we observed following high-level expression of 3Cpro in Fig. 3A. Thus, while 3Cpro is capable of cleaving TRIF, its capacity to do so is much less than 3CD.
3CD cleaves TRIF at Gln-190 and Gln-554 in an ordered process
We next examined the sequence of human TRIF for potential 3Cpro cleavage sites. Previous studies of 3Cpro substrate specificity have documented a preference for Gln at the P1 position and a consensus sequence (L,V,I)X(S,T)Q↓X where X is any amino acid [24]. The 3ABC cleavage site in MAVS, LASQ↓V, fits this consensus perfectly [12]. In contrast, TRIF does not contain any consensus 3Cpro cleavage sites, although several sites are partial fits that could serve as non-canonical cleavage sites with consensus P1 and P2 resides. We focused on two clusters of such sites (Fig. 4D) that could potentially generate cleavage fragments of appropriate size (75, 55, and 20 kDa, see above). We constructed a series of mutants in which the invariant Gln at each potential P1 position was substituted with Arg, and examined their cleavage by 3CD. 3CD cleavage was not affected by Q211R, Q581R-Q583R, or Q612R mutations (Fig. 4E, left), excluding these as 3CD cleavage sites. In contrast, a Q190R mutation blocked the cleavage event that generates the 55 kDa but not the 75 kDa fragment, while Q552R-Q554R mutations completely abolished 3CD cleavage of TRIF (Fig. 4E, left). We then constructed individual Q552R and Q554R mutants, and showed the loss of cleavage in Q552R-Q554R was due to Q554R (Fig. 4E, right). These results establish Q190 and Q554 as 3CD cleavage sites within TRIF, and clarify the identities of the observed TRIF cleavage fragments. The 75 kDa fragment results from cleavage at Q554 and corresponds to aa 1–554 of TRIF. This fragment is further cleaved at Q190, giving rise to the 55-kDa and 20-kDa fragments that correspond to aa 191–554 and 1–190, respectively.
The different effects of the Q190R and Q554R mutations on 3CD cleavage of TRIF indicate that 3CD cleaves TRIF in an ordered process. The fact that cleavage at Q190 cannot proceed when cleavage at Q554 is blocked (Fig. 4E) suggests that the cleavage at Q554 is a prerequisite to cleavage at Q190. We thus propose a “two-step” model for the 3CD cleavage of TRIF, in which primary cleavage at Q554 site induces a conformational change that exposes the Q190 site, allowing the second cleavage to occur (Fig. 4F).
The N-terminal region of TRIF contains three TRAF6-binding motifs that are important for activation of the transcription factors NF-κB and IRF-3 in TLR3 signaling [25], [26]. Q190 is located between the first and second of these TRAF6-binding motifs, while the Q554 cleavage site is located between the TIR domain and RHIM motif (Fig. 4D, right), both important for transcriptional activation of IFN-β [27]. Cleavage at these residues could yield fragments with reduced signaling ability, or potentially dominant negative activity against signal transduction. We thus ectopically expressed the predicted, individual 3CD-generated TRIF fragments: N-190 (aa 1–190), N-554 (aa 1–554), M-364 (aa 191–554) and C-158 (aa 555–712) (Fig. S6A in Text S1), and examined their abilities to activate IFN-β and NF-κB-specific (PRD-II) promoters in luciferase reporter assays. While N-190 and C-158 were incapable of activating either promoter, overexpression of N-554 and M-364 stimulated both the IFN-β and PRD-II promoters (Fig. S6B in Text S1, left and right, respectively). When co-expressed with wild-type TRIF at a 1∶1 ratio, these fragments did not demonstrate any dominant negative effects (Fig. S6C in Text S1). Other evidence suggests they do not transduce signals from TLR3 (data not shown).
Altered substrate specificity of 3CD contributes to TRIF cleavage
We next addressed the question of why TRIF is cleaved by 3CD but very inefficiently or not at all by 3Cpro. The ability of 3ABC to cleave MAVS, while 3Cpro cannot, is related to its unique mitochondrial targeting [12]. To determine if differences in intracellular localization could similarly account for the unique activity of 3CD, we compared the cellular localization of 3Cpro and 3ABCD by confocal microscopy. When expressed ectopically with an N-terminal Flag tag, 3Cpro was diffusely present throughout the cytoplasm, while Flag-3ABC, included as a control, demonstrated prominent mitochondrial localization, as reported previously [12] (Fig. 5A). In contrast, 3ABCD, expressed with a C-terminal V5 tag, was present at much lower abundance and with a perinuclear, ER-like distribution. Both 3CD and 3ABCD are known to be subject to ubiquitin-mediated proteolysis [28], potentially explaining the low abundance of 3ABCD-V5, much of which is likely processed to 3CD or 3Dpol. Confocal microscopy of cells ectopically expressing both TRIF and proteolytically-inactive C172A mutants of 3Cpro and 3CD revealed no evidence for specific co-localization of these proteins (Fig. 5B). Thus, the unique ability of 3CD to cleave TRIF is not due to its localization to a TRIF-rich compartment.
Fig. 5. Altered substrate specificity of 3CD contributes to TRIF cleavage. 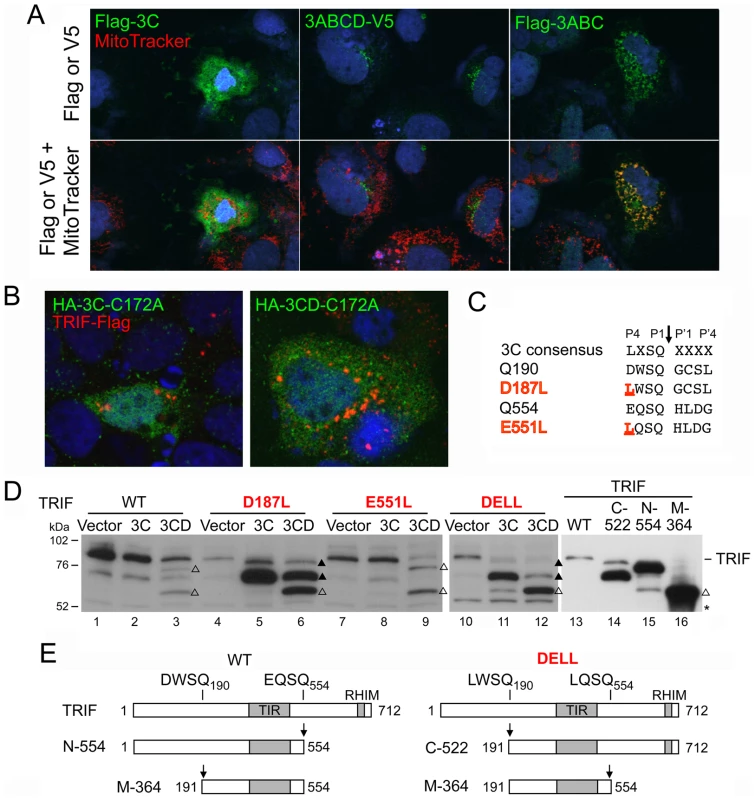
(A) Confocal immunofluorescence microscopic images showing subcellular localization of Flag- or V5-tagged 3Cpro, 3ABCD and 3ABC (green), merged with DAPI (blue) staining of nucleus (top) and additional MitoTracker (red) staining of mitochondria (bottom). (B) Confocal immunofluorescence microscopic images of cells expressing C-terminally Flag-tagged full-length TRIF and N-terminally HA-tagged protease-inactive mutant (C172A) forms of 3Cpro and 3CD. No co-localization is evident between 3Cpro or 3CD proteins and TRIF. (C) Alignment of the 3Cpro consensus cleavage sequence and TRIF 3CD cleavage sites. The P4 positions at the Q190 and Q554 cleavage sites (D187 and E551, respectively) were substituted with the 3Cpro-preferred Leu (underlined) in the D187L, E551L and DELL (D187L-E551L) double mutant. (D) Cleavage of TRIF mutants by 3Cpro and 3CD. HEK 293FT cells were co-transfected with vectors expressing TRIF mutants and 3Cpro or 3CD (lane 1 to 12), or transfected with full-length or truncated TRIF constructs corresponding to the cleavage fragments (lane 13–16). Cell extracts were analyzed by immunoblotting of TRIF. The D187L mutant and DELL double mutant were cleaved by both 3Cpro and 3CD, resulting in intermediate cleavage fragments (solid triangle) different from that of 3CD-cleaved wild-type (WT) TRIF (open triangle, 75-kDa), but the end product was the same (open triangle, 55-kDa). A nonspecific protein band (‘*’) detected by TRIF antibody indicates equal loading. (E) Schematic showing TRIF fragments created by different cleavage order at Q190 vs. Q554, corresponding to cleavage fragments observed in (C) and (D). The noncanonical nature of the 3CD cleavage sites in TRIF, DWSQ190 and EQSQ554, in which the P4 position is occupied by an amino acid residue with an acidic (Asp or Glu) rather than hydrophobic side chain (Leu, Ile or Val) (Fig. 5C), could explain why TRIF is not efficiently cleaved by 3Cpro. The fact that they are nonetheless cleaved by 3CD suggests that the substrate specificity of 3CD may differ from that of 3Cpro in tolerating or possibly preferring an acidic residue at the P4 position. To assess this potential difference in substrate specificity, we altered the P4 positions within the non-canonical cleavage sites in TRIF, substituting the acidic P4 residues in each with Leu, thereby generating consensus 3Cpro sites (TRIF-D187L and TRIF-E551L, respectively, Fig. 5C). When expressed ectopically, the D187L mutant, now carrying a LWSQ cleavage sequence, was readily processed by both 3Cpro and 3CD, yielding a novel fragment with an apparent molecular mass of 70 kDa (Fig. 5D, lane 5, 6). This 70 kDa fragment co-migrated in SDS-PAGE with the TRIF C-522 fragment corresponding to aa 191–712 (Fig. 5D, compare lane 5 vs. 14), confirming that cleavage had occurred at Q190. As expected, this fragment was not further cleaved by 3Cpro, but was further processed by 3CD at Q554, generating the same 55 kDa fragment produced from wild-type TRIF by 3CD (Fig. 5D, lane 3 vs. 6). On the other hand, there was no difference in the processing of the wild-type and the E551L mutant TRIF, the latter of which was only marginally cleaved by 3Cpro despite carrying a canonical LQSQ sequence (Fig. 5D, lane 7 vs. 8). Nonetheless, when both cleavage sites were changed to a 3Cpro consensus, the resulting double mutant (TRIF-DELL) was readily cleaved by both 3Cpro and 3CD (Fig. 5D, lanes 11 and 12). This produced a 70 kDa fragment similar to that observed with the D187L mutant, suggesting that the order of cleavage had been altered to occur first at Q190 (Fig. 5E). Additional processing led to the 55 kDa fragment, although there was less of this product produced by 3Cpro than 3CD. Collectively, these results demonstrate that 3CD differs in its substrate specificity from 3Cpro, tolerating an acidic residue at the P4 position while 3Cpro does not, and that this accounts, at least in part, for its ability to cleave TRIF.
TLR3 signaling and cellular permissiveness to HAV
The ability of HAV to antagonize TLR3 signaling is likely to have evolved because antiviral responses evoked by TLR3 act in someway to restrict infection. To test this hypothesis, we assessed viral replication by a variety of methods over a range of multiplicity of infection. Immunoblots demonstrated that viral protein abundance (3Cpro) was reduced in Huh7.5-TLR3 cells infected at an m.o.i. of 3, compared to cells expressing empty vector or TLR3-ΔTIR (Fig. 1B, lane 4 vs. lanes 2 or 6). Similarly, the fluorescence intensity of HAV antigen was noticeably less in infected Huh7.5-TLR3 cells compared with the control ΔTIR cells (Fig. 6A), although the proportion of cells expressing HAV antigen was not reduced 5 days after infection at an m.o.i. of 1. HAV antigen-specific ELISA assays also showed that Huh7.5-TLR3 cells (infected at an m.o.i. of 0.05) produced less than 50% of the amount of assembled HAV capsid antigen produced by cells expressing empty vector, or a TLR3 mutant incapable of binding dsRNA (TLR3-H539E) [13] (Fig. 6B). Infectious virus yields were also reduced (by 30–60%) in Huh7.5-TLR3 cells infected at low m.o.i. (Fig. 6C).
Fig. 6. TLR signaling and TRIF regulate cellular permissiveness for HAV. 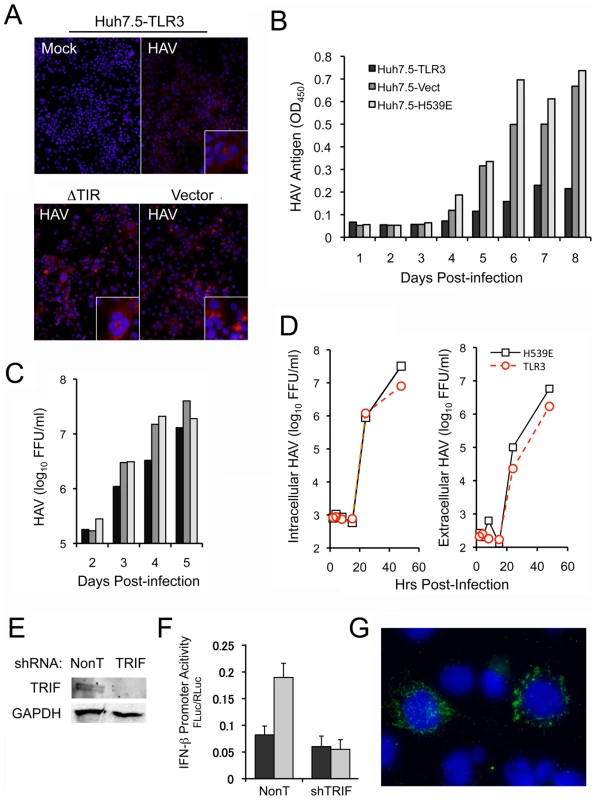
(A) Immunofluorescence microscopy of HAV antigen in Huh7.5-TLR3, -ΔTIR, and -Vector cells 5 days after infection of the cells with HAV at an m.o.i. of 1. (B–C) Cells were infected with virus at an m.o.i. of 0.05. Virus yields were determined in harvests of the entire culture (cells plus supernatant media) after 3 freeze-thaw cycles. Lysates were clarified by brief centrifugation and supernatants were used for (B) HAV antigen ELISA, or (C) quantal infectivity assay. (D) Paired cultures of Huh7.5-TLR3 and -H539E cells were inoculated with HAV at m.o.i. = 5 to determine one-step replication kinetics. After 1 hr adsorption, cells were washed extensively with PBS, and refed with fresh medium. Culture supernatants (extracellular) and cell lysates (intracellular) were harvested at the times indicated and infectious virus titer determined. (E) Immunoblot showing TRIF expression in PH5CH8 cells transduced with lentivirus expressing TRIF-specific shRNA or non-targeting (NonT) shRNA. GAPDH served as a loading control. (F) IFN-β promoter activity in cells from panel (E) with (grey bar) and without (black bar) poly-(I:C) stimulation. (G) HAV-specific immunofluorescence in PH5CH8 cells after depletion of TRIF. Similar antigen expression was observed very rarely in control cells inoculated in parallel with HAV. Somewhat different results were obtained in one-step growth assays done at an m.o.i. of 5.0. HAV replicates very slowly compared to other picornaviruses, with an infectious cycle of approximately 24 hrs evident in such assays (Fig. 6D). Somewhat surprisingly, we observed no differences in the kinetic of intracellular infectious virus accumulation between Huh7.5-TLR3 vs. H539E cells, up to 24 hrs after inoculation of the cells under one-step growth conditions (Fig. 6D, left). Subsequent to this time point, however, less virus was produced in cells expressing functional TLR3. This restriction on virus replication was also reflected in slightly lower (about half log10) yields in extracellular infectious virus released from the cells (Fig. 6D). Collectively, these data suggest that TLR3 signaling imposes a modest restriction on HAV infection, particularly at low m.o.i., and after the first round of viral RNA replication. This is reminiscent of the effects of TLR3 expression on low vs. high m.o.i. HCV infections that we have observed in previous studies [13].
To confirm these findings, we sought evidence of a gain in permissiveness for HAV infection in PH5CH8 cells in which TLR3 signaling was impaired by RNAi-mediated depletion of TRIF. PH5CH8 cells are T-antigen transformed adult human hepatocytes that possess robust TLR3 and RLR signaling [14] and are generally nonpermissive for HAV. TRIF was depleted by lentiviral transduction of a TRIF-specific short-hairpin RNA (shRNA) (Fig. 6E), eliminating IFN-β promoter activation by extracellular poly-I:C (Fig. 6F). The cells were infected with HAV at an m.o.i. of 1, and examined 8 days later by immunofluorescence microscopy for viral antigen expression. This was rarely observed in PH5CH8 cells transduced with a non-targeting control shRNA, but detected in ∼2% of the TRIF depleted cells (Fig. 6G). Similar results were obtained in cells transduced with a TLR3-specific shRNA (data not shown).
Thus, reconstitution of TLR3 signaling in Huh7.5 cells results in a modest inhibition of HAV infection, while the ablation of TLR3 signaling in PH5CH8 cells provides a significant replication advantage to HAV. In both cases, these effects are of relatively small magnitude, likely reflecting the presence of redundant innate antiviral defense mechanisms, including responses generated by RLRs or protein kinase R. The abrogation of pro-inflammatory signals, the effects of which cannot be deduced from in vitro experiments, may represent a more substantial advantage to the virus in vivo in HAV-infected persons.
Discussion
Here, we show that HAV disrupts TLR3 signaling by targeting the essential adaptor protein TRIF for degradation by the 3CD protease-polymerase processing intermediate. The ability of poly-I:C to stimulate the IFN-β promoter or induce the expression of ISGs when added to media was markedly attenuated in HAV-infected Huh7 hepatoma cells in which TLR3 expression had been reconstituted by retroviral gene transduction (Fig. 1A and B). This disruption of TLR3 signaling was associated with a loss of detectable TRIF (Fig. 2), and could be recapitulated by ectopic expression of 3ABCD or 3CD in both Huh7-TLR3 cells and HeLa cells which possess an endogenous TLR3 signaling pathway (Fig. 3). The loss of TRIF expression was linked to the cysteine protease activity residing within the 3C sequence of 3CD, which we demonstrate cleaves TRIF sequentially at two noncanonical 3Cpro cleavage sites (Fig. 4B and E). Additional studies suggested that this is due to the ability of the 3D sequence in 3CD to alter the substrate specificity of the protease such that it better accommodates the acidic residues present at the P4 position of cleavage sites in TRIF (Fig. 5D). These observations add to our understanding of the pathogenesis of HAV, a significant human pathogen that has received scant attention in recent years.
In previous work, we demonstrated that HAV also antagonizes the induction of IFN responses by the cytosolic RLR pattern recognition receptors, RIG-I and MDA-5, by inducing proteolysis of the adaptor protein MAVS [12] (Fig. 7). As we report here with poly-I:C-induced TLR3 signaling, we found that ectopically expressed 3ABCD was capable of disrupting Sendai virus-induced RIG-I signaling. 3ABCD results from secondary processing of the HAV polyprotein at the P2-P3 junction, and is itself subject to further processing via two distinct pathways, one leading to production of 3ABC and the other to 3CD (Fig. 7). Both intermediates contain the catalytically active 3Cpro cysteine protease domain, but they have distinct cellular localization and substrate specificities (Fig. 5). 3ABC, due to the presence of a mitochondrial targeting transmembrane domain in 3A, localizes to the mitochondrial membrane where it cleaves MAVS (Fig. 5A). In contrast, 3CD appears to be localized primarily to the perinuclear ER, and its ability to cleave TRIF is dependent upon its unique substrate specificity rather than its intracellular localization (Figs. 5B and 5D).
Fig. 7. Interferon-activating pathways disrupted during HAV infection by 3Cpro precursor-mediated proteolysis of signaling adaptor proteins. 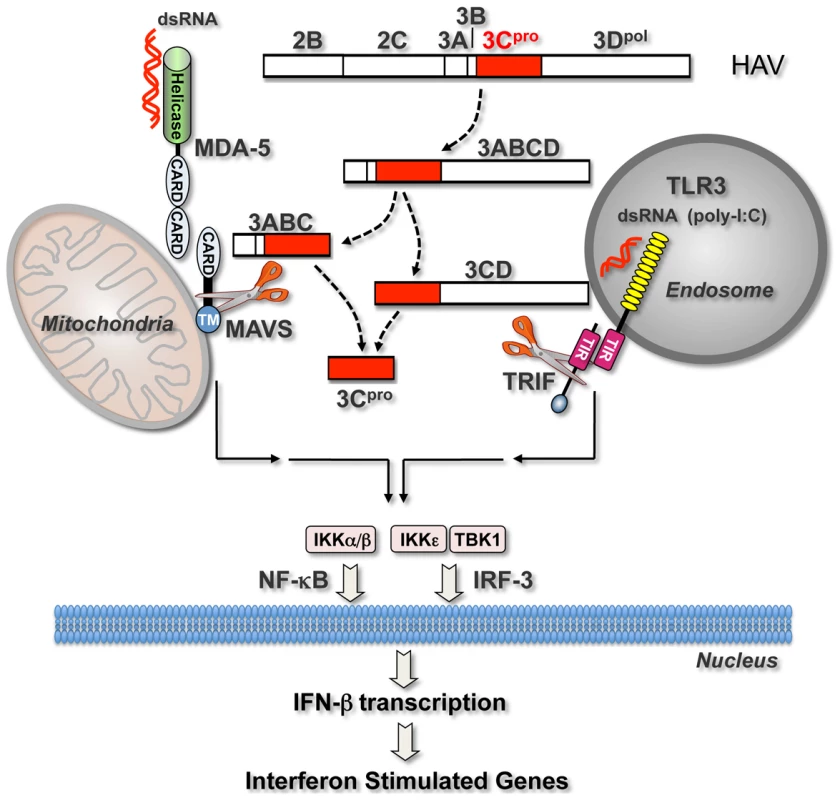
Cytosolic HAV RNA is sensed by RNA helicases (most likely MDA-5) which interact through shared caspase-recruitment domains (CARDS) with the adaptor protein, MAVS, localized on the mitochondrial outer membrane. This induces formation of a macromolecular signaling complex that leads to activation of non-canonical (IKKε and TBK-1) and canonical (IKKα/β) kinases of the IκB complex, and subsequent activation of latent cytoplasmic transcription factors, IRF-3 and NF-κB. These activated transcription factors translocate to the nucleus where they induce the transcription of IFN-β mRNA, thereby initiating the production of IFNs and ISGs. TLR3 activates these same transcription factors via a parallel signaling pathway that is initiated upon the sensing of viral dsRNA (or poly-I:C) by TLR3 within an endosomal compartment. Binding of its dsRNA ligand induces the dimerization of TLR3 and subsequent recruitment of the adaptor protein, TRIF, to its cytoplasmic domain through shared Toll/Interleukin-1 receptor (TIR) domains. Additional details of these pathways are available elsewhere [45]. Precursors of the HAV 3Cpro cysteine protease block both signaling pathways by directing cleavage of the critical adaptor proteins, MAVS [12] and, as shown in this communication, TRIF. These processing intermediates, 3ABC and 3CD, represent products of alternative processing pathways by which 3Cpro is derived from the P2P3 polyprotein fragment (2B to 3Dpol shown at the top), a product of the primary HAV polyprotein cleavage between 2A/2B [1]. MAVS cleavage by 3ABC is dependent upon 3ABC localization to the mitochondria, while 3CD cleavage of TRIF, as shown here, is dependent upon altered substrate specificity of 3Cpro induced by its fusion to 3Dpol. Our results reveal an unexpected role of the 3D sequence in modulating the substrate specificity of 3CD. 3Cpro cleavage sites within the HAV polyprotein, as well as MAVS, contain a hydrophobic amino acid (Leu, Ile, or Val) at the P4 position [12], [24] that fits into the hydrophobic S4 binding pocket within the crystal structure of 3Cpro [29],[30]. In contrast, both 3CD cleavage sites within TRIF contain an acidic amino acid residue (Asp-190 and Glu-551) at the P4 position (Fig. 5C), and therefore do not conform to the canonical cleavage sequence. A previous study showed that a peptide substrate with a Glu substitution (underlined) at the P4 position, Ac-EERTQ↓SFS-NH2, which is similar to the TRIF cleavage site EQSQ554, was not cleaved by 3Cpro [31]. Our data suggest that 3CD possesses a unique substrate specificity that allows it to recognize and hydrolyze cleavage sites within TRIF that are otherwise relatively resistant to 3Cpro. In support of this notion, we showed that changing the non-canonical cleavage site at Gln-190 of TRIF to a canonical 3Cpro cleavage sequence resulted in efficient 3Cpro proteolysis and a reversal of the order of cleavage at the two sites in TRIF (.5D). A similar change at the Gln-554 site did not make it fully permissive for 3Cpro cleavage, however, suggesting that there are other differences in the substrate specificities of 3Cpro and 3CD. Our data indicate that the change in substrate specificity of 3CD is conferred in cis by the 3D sequence (Fig. 3B), although the structural basis for this remains to be determined.
In addition to their differentiated roles in evading innate immune responses, 3ABC and 3CD are likely to have specialized roles in the viral life cycle. 3ABC is a stable intermediate that is important in processing of the P1-2A segment of the polyprotein required for assembly of the viral capsid [32]. 3CD, based on studies with other picornaviruses, may play a role in the uridylyation of the protein primer of RNA synthesis, 3B (VPg) [33]. The multiple functions of these viral proteins reflect a strategy used by picornaviruses to create processing intermediates that are functionally distinct from their mature products [34], [35], [36].
The dual targeting of RLR and TLR3 signaling by HAV 3ABCD processing intermediates is reminiscent of the HCV NS3/4A protease, which disrupts both RIG-I and TLR3 signaling pathways by proteolytically cleaving the same signaling adaptor proteins, MAVS and TRIF, respectively [7], [8], [9]. 3Cpro and NS3/4A are both chymotrypsin-like proteases with double β-barrel folds [30], [37], but they are not closely related phylogenetically. The HAV 3Cpro protease has a cysteine nucleophile in its active site, while NS3/4A has a serine. These viral proteases have very different substrate specificities, and they cleave MAVS and TRIF at distinctly different sites [7,8,9,12, and Fig. 3]. The fact that both of these hepatotropic viruses express proteases targeting these two critical adaptor molecules is thus a remarkable example of convergent evolution. It also speaks strongly to the importance of these signaling pathways in the control of RNA viruses in the liver. However, since HAV infection is always successfully controlled by the host (except in rare cases of fulminant disease), these data indicate that the disruption of RLR and TLR3-mediated antiviral defenses is not sufficient for a virus to establish the longterm persistence that typifies most HCV infections. HCV must possess additional immune evasion strategies to account for its unique capacity to establish chronic infections.
We demonstrated a minimal gain of permissiveness for HAV replication in hepatocyte-derived cells in which TLR3 or TRIF expression was depleted (Fig. 6G), and a reduction in viral antigen expression in hepatoma cells with active TLR3 signaling (Fig. 6B). However, these effects were modest, potentially reflecting very efficient control of TLR3 signaling by 3CD in infected cells such that TLR3 has little impact on viral replication. Alternatively, it may be that the primary advantage gained by HAV in antagonizing TLR3 signaling is impaired production of proinflammatory cytokines and reduced inflammation associated with the infection. TLR3 signaling is critically important to murine host defense against coxsackievirus B, another picornavirus [38], and it is plausible that the disruption of TLR3 signaling has significance beyond impairing the type I IFN response.
The subversion of both RLR and TLR3 signaling likely contributes to the relatively lengthy, clinically silent incubation period that precedes acute liver injury in hepatitis A. This period is characterized by robust viral replication within the liver and shedding of virus in feces, which reaches a maximum at the onset of hepatic inflammation [10], [39]. The absence of a type I IFN response in acute infectious hepatitis was hinted at in clinical studies done almost 40 years ago [40]. Consistent with this, we recently documented a paucity of type I IFN-dependent ISG expression (e.g., IFIT-1, ISG15) within the liver of HAV-infected chimpanzees during the first weeks of infection despite high viral RNA copy numbers [41]. The cleavage of MAVS and TRIF by 3ABC and 3CD, respectively, provides a partial mechanistic explanation for this. By dealing a double blow to two major cellular antiviral response pathways, HAV appears able to block somatic cell expression of IFN-α/β, thus facilitating its replication. Yet to be explained is how it evades recognition by plasmacytoid dendritic cells (pDCs), which may play a significant role in sensing HCV infection in the liver and generating the strong intrahepatic ISG responses that are often observed in acute and chronic hepatitis C [42], [43].
Methods
Cells and viruses
HEK 293FT cells, Huh7, Huh-7.5 and Bla-C cells [12] were cultured in DMEM with 8% FBS. Huh7-TLR3, Huh-7.5-TLR3 and related control cells [13], and HAV-Bla subgenomic replicon cells were cultured in the same medium supplemented with blasticidin [12]. The cell culture-adapted HAV strain HM175/18f [16] was amplified in Huh7 cells; on fetal rhesus kidney FRhK4 cells.
Plasmids and antibodies
pCDNA6-TRIF [7] and pCMV-HA vectors expressing N-terminally HA-tagged HAV proteins derived from HM-175/18f virus [12] have been described previously. Similar pCMV-HA vectors expressing the wild-type HM175 3Cpro and 3CD proteins were constructed by amplification of the corresponding sequences from pHAV/8y (Suzanne Emerson, NIAID). Truncations of TRIF were generated by PCR mutagenesis, and mutations in TRIF and 3CD constructed by site-directed mutagenesis (Strategene). Other plasmids were obtained from the following sources: pIFN-β-Luc (Rongtuan Lin, McGill University), pPRD-II-Luc (Michael Gale, University of Washington), pCMV-β-gal (Clontech), pRL-CMV (Promega), pEF-Bos-TRIF (Kate Fitzgerald, University of Massachusetts) and pCDNA3-Flag-IKKε (Tom Maniatis, Harvard University). Antibodies used in these studies included: anti-TLR3 (Ilkka Julkunen, National Institute for Health and Welfare, Finland), anti-TRIF S219 (Cell Signaling Technology), anti-TRIF aa 4–31 (Alexia), anti-IRF-3 sc-9082 (Santa Cruz), monoclonal anti-HAV K2-4F2, K3-4C8 (Commonwealth Serum Laboratories, Victoria, Australia), and 6A5 (John Hughes, Merck, Sharp & Dohme), anti-HAV 2A (David Sangar, Wellcome Biotech), anti-3Cpro (Verena Gauss-Muller, University of Lübeck), anti-ISG15 (Santa Cruz), and anti-HA and anti-Actin (Sigma). Rabbit anti-TRIF antibody S537-2 was obtained by immunization of rabbits with recombinant TRIF protein [7].
Transfection and luciferase reporter assays
For protein expression, cells were transfected with plasmid DNA using Lipofectamine 2000 (Invitrogen) and lysates prepared 20 hrs later using 1% NP-40 lysis buffer. HAV-infected cells (m.o.i. = 3) were cultured for 4 days prior to transfection. For luciferase reporter assays, expression and/or Luc reporter plasmids were transfected into cells (seeded in triplicate in 96-well format) with an internal β-galactosidase (pCMV-β-gal) or Renilla luciferase (pRL-CMV, Promega) transfection control. At 20 hours posttransfection, when indicated, poly(I:C) (Sigma) was added to the medium and cells incubated for additional 6 hours. Cells were lysed in Reporter Lysis Buffer (Promega) and equal quantity of lysate used for luciferase and β-galactosidase assays (Promega). In experiments using the Renilla luciferase control, cells were lysed in Passive Lysis Buffer (Promega) and tested by Dual-Luciferase assay (Promega). For each sample, the luciferase activity was normalized to the β-galactosidase or renilla luciferase activity. In the case of poly(I:C) stimulation, results were presented as fold induction compared to unstimulated cells. Statistical analysis was performed using two-tailed Student's t test.
HAV assays
HAV antigen-specific ELISA was carried out using a post-convalescent human antibody for capture [44], and a murine monoclonal antibody (K24F2) for detection. Absorption at 450 nm was determined on a Synergy (Biotek, Inc) plate reader. The infrared fluorescent immunofocus assay (IR-FIFA) for infectious HAV was done using FRhK-4 cells as previously described [12]. For detection of HAV antigen by immunofluorescence microscopy, cells were fixed with 4% PFA for 25 min, labeled with murine mAb 6A5 and after extensive washing incubated with goat anti-mouse IgG Alexa-594 conjugate (Invitrogen).
Expression and purification of GST-3C from E. coli
The 3Cpro coding sequence from HAV strain HM175/18f was cloned into bacterial expression vector pGEX-4T3 (GE Life Sciences), fused in-frame with an N-terminal GST tag. For protein expression, an overnight culture of E. coli strain BL21(DE) (Novagen) containing the expression construct was diluted 10-fold and cultured at 37°C for 2 hrs. Expression was induced by addition of 0.1 mM IPTG and continued culture at 25°C for 3 hrs. Bacterial cells were harvested and lysed in BugBuster solution (EMD Biosciences) containing 37.5 U/ml Benzonase, 15 KU/ml recombinant lysozyme and 2 mM DTT. GST-3Cpro fusion protein was purified from the bacterial lysate by affinity chromatography using the GST MicroSpin Purification Module (GE Life Sciences) according to the manufacturer's instructions.
In vitro cleavage assay
Myc-TRIF and its truncated forms were synthesized in vitro and labeled with [35S]-Met/Cys using T7 Coupled Transcription/Translation System (TNT, Promega) according to the manufacturer's instructions. Cleavage assays were performed in a 10-µl mixture containing 1 µl TNT product and 0.5 µM purified GST-3Cpro in a buffer containing 50 mM Tris-HCl pH 8.0, 2.5 mM EDTA and 2 mM DTT. Similarly purified GST was used as a negative control at the same concentration. Reactions were carried out overnight at 3°C and stopped by addition of an equal volume of 2X SDS sample buffer. Cleavage products were analyzed by SDS-PAGE followed by autoradiography.
Immunoprecipitation and immunoblotting
For detection of endogenous TRIF, 1 mg of cell lysate was immunoprecipitated with 1 µg of rabbit anti-TRIF antibody S537-2 [7], followed by immunoblotting with anti-TRIF S219. HA-3CD was detected with a similar method using anti-HA for immunoprecipitation and anti-3Cpro for immunoblotting.
IRF-3 dimerization
Huh7-TLR3 cells were mock-infected or infected with HAV at m.o.i. = 5 and cultured for 5 days, then stimulated by the addition of poly-(I:C) (50 µg/ml) to the medium for 2 hours and lysed with 1% NP-40 lysis buffer. Cell lysates (10 µg) were mixed with deoxycholate (DOC) sample buffer (final concentration 1% DOC) and separated by Tris-Glycine/1% DOC native PAGE. IRF-3 monomer and dimer were detected by immunoblotting with rabbit anti-IRF-3 sc-9082.
Laser-scanning confocal microscopy
Ectopically expressed Flag-tagged TRIF, 3Cpro and 3ABC, HA-tagged 3Cpro and 3CD, and V5-tagged 3ABCD were imaged as described previously [12]. For visualization of poly(I:C)-induced changes in IRF-3 localization, Huh7-TLR3 cells were grown on chamber slides, infected at low m.o.i. and cultured for 5 days. Cells were then mock-stimulated or stimulated with poly-(I:C) (50 µg/ml) added to the medium for 2 hours, and fixed with 4% paraformaldehyde. After permeabilization with 0.25% Triton X-100, the cell monolayer was incubated with rabbit anti-IRF-3 sc-9082 and murine anti-HAV K3-4C8, followed by secondary antibodies goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594 (Invitrogen). Nuclei were counterstained stained with DAPI. Images were collected using a Leica DMIRB Inverted Microscope in the Michael Hooker Microscopy Facility.
GenBank accession numbers
HAV strain HM175/18f (including individual HAV proteins), M59808; wild-type HAV strain HM175, M14707.1; TLR3, NP_003256; ISG15, NP_005092; TRIF (TICAM-1), NP_891549; IKKe, NP_054721; IRF-3, NP_001562; MDA5, NP_071451; RIG-I, O95786; MAVS (IPS-1, Cardif, VISA), Q7Z434; GAPDH, P04406; Actin, P60709.
Supporting Information
Zdroje
1. MartinALemonSM 2002 The molecular biology of hepatitis A virus. OuJ Hepatitis Viruses Norwell, MA Kluwer Academic Publishers 23 50
2. LemonSMWalkerCAlterMJYiMKnipeD 2007 Hepatitis C viruses. Fields Virology. Philadelphia Lippincott Williams & Wilkins 1253 304
3. RosenblumLSVillarinoMENainanOVMelishMEHadlerSC 1991 Hepatitis A outbreak in a neonatal intensive care unit:Risk factors for transmission and evidence of prolonged viral excretion among preterm infants. J Infect Dis 164 476 82
4. ChisariFV 2005 Unscrambling hepatitis C virus-host interactions. Nature 436 930 2
5. SchulteIHitzigerTGiuglianoSTimmJGoldH 2010 Characterization of CD8+ T-cell response in acute and resolved hepatitis A virus infection. J Hepatol 54 201 8
6. FleischerBFleischerSMaierKWiedmannKHSacherM 1990 Clonal analysis of infiltrating T lymphocytes in liver tissue in viral hepatitis A. Immunology 69 14 9
7. LiKFoyEFerreonJCNakamuraMFerreonAC 2005 Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA 102 2992 7
8. MeylanECurranJHofmannKMoradpourDBinderM 2005 Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437 1167 72
9. LiXDSunLSethRBPinedaGChenZJ 2005 Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA 102 17717 22
10. LemonSMBinnLNMarchwickiRMurphyPCPingLH 1990 In vivo replication and reversion to wild type of a neutralization-resistant antigenic variant of hepatitis A virus. J Infect Dis 161 7 13
11. FensterlVGrotheerDBerkISchlemmingerSVallbrachtA 2005 Hepatitis A virus suppresses RIG-I-mediated IRF-3 activation to block induction of beta interferon. J Virol 79 10968 77
12. YangYLiangYQuLChenZYiM 2007 Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci USA 104 7253 8
13. WangNLiangYDevarajSWangJLemonSM 2009 Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol 83 9824 34
14. LiKChenZKatoNGaleMJrLemonSM 2005 Distinct poly-I:C and virus-activated interferon signaling pathways in hepatocytes. J Biol Chem 280 16739 47
15. KonduruKKaplanGG 2006 Stable growth of wild-type hepatitis A virus in cell culture. J Virol 80 1352 60
16. LemonSMMurphyPCShieldsPAPingLHFeinstoneSM 1991 Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection:evidence for genetic recombination. J Virol 65 2056 65
17. FitzgeraldKAMcWhirterSMFaiaKLRoweDCLatzE 2003 IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4 491 6
18. YiMLemonSM 2002 Replication of subgenomic hepatitis A virus RNAs expressing firefly luciferase is enhanced by mutations associated with adaptation of virus to growth in cultured cells. J Virol 76 1171 80
19. TeterinaNLBienzKEggerDGorbalenyaAEEhrenfeldE 1997 Induction of intracellular membrane rearrangements by HAV proteins 2C and 2BC. Virology 237 66 77
20. ProbstCJechtMGauss-MullerV 1998 Processing of proteinase precursors and their effect on hepatitis A virus particle formation. J Virol 72 8013 20
21. RebsamenMMeylanECurranJTschoppJ 2008 The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ 15 1804 11
22. MisaghiSKorbelGAKesslerBSpoonerEPloeghHL 2006 z-VAD-fmk inhibits peptide: N-glycanase and may result in ER stress. Cell Death Differ 13 163 5
23. TesarMPakIJiaXYRichardsOCSummersDF 1994 Expression of hepatitis A virus precursor protein P3 in vivo and in vitro: Polyprotein processing of the 3CD cleavage site. Virology 198 524 33
24. SeipeltJGuarneABergmannEJamesMSommergruberW 1999 The structures of picornaviral proteinases. Virus Res 62 159 68
25. SasaiMTatematsuMOshiumiHFunamiKMatsumotoM 2010 Direct binding of TRAF2 and TRAF6 to TICAM-1/TRIF adaptor participates in activation of the Toll-like receptor 3/4 pathway. Mol Immunol 47 1283 91
26. SatoSSugiyamaMYamamotoMWatanabeYKawaiT 2003 Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 171 4304 10
27. KaiserWJOffermannMK 2005 Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 174 4942 52
28. LosickVPSchlaxPEEmmonsRALawsonTG 2003 Signals in hepatitis A virus P3 region proteins recognized by the ubiquitin-mediated proteolytic system. Virology 309 306 19
29. BergmannEMMosimannSCChernaiaMMMalcolmBAJamesMN 1997 The refined crystal structure of the 3C gene product from hepatitis A virus:specific proteinase activity and RNA recognition. J Virol 71 2436 48
30. AllaireMChernalaMMMalcolmBAJamesMNG 1994 Picornaviral 3C cysteine proteinases have a fold similar to chymotrypsin-like serine proteinases. Nature 369 72 6
31. JewellDASwietnickiWDunnBMMalcolmBA 1992 Hepatitis A virus 3C proteinase substrate specificity. Biochemistry 31 7862 9
32. KusovYGauss-MullerV 1999 Improving proteolytic cleavage at the 3A/3B site of the hepatitis A virus polyprotein impairs processing and particle formation, and the impairment can be complemented in trans by 3AB and 3ABC. J Virol 73 9867 78
33. PaulAVYinJMugaveroJRiederELiuY 2003 A “slide-back” mechanism for the initiation of protein-primed RNA synthesis by the RNA polymerase of poliovirus. J Biol Chem 278 43951 60
34. ParsleyTBCornellCTSemlerBL 1999 Modulation of the RNA binding and protein processing activities of poliovirus polypeptide 3CD by the viral RNA polymerase domain. J Biol Chem 274 12867 76
35. Ypma-WongMFDewaltPGJohnsonVHLambJGSemlerBL 1988 Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology 166 265 70
36. PathakHBOhHSGoodfellowIGArnoldJJCameronCE 2008 Picornavirus genome replication:roles of precursor proteins and rate-limiting steps in oriI-dependent VPg uridylylation. J Biol Chem 283 30677 88
37. LoveRAPargeHEWickershamJAHostomskyZHabukaN 1996 The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 87 331 42
38. NegishiHOsawaTOgamiKOuyangXSakaguchiS 2008 A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc Natl Acad Sci USA 105 20446 51
39. MartinALemonSM 2006 Hepatitis A virus:From discovery to vaccines. Hepatology 43 S164 72
40. WheelockEFSchenkerSCombesB 1968 Absence of circulating interferon in patients with infectious and serum hepatitis. Proc Soc Exp Biol Med 128 251 3
41. LanfordREFengZChavezDGuerraBBraskyKM 2011 Acute hepatitis A virus infection is associated with a limited type I interferon response and persistence of intrahepatic viral RNA. Proc Natl Acad Sci USA 108 11223 8
42. TakahashiKAsabeSWielandSGaraigortaUGastaminzaP 2010 Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA 107 7431 6
43. LauDTFishPMSinhaMOwenDMLemonSM 2008 Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology 47 799 809
44. BinnLNLemonSMMarchwickiRHRedfieldRRGatesNL 1984 Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J Clin Micro 20 28 33
45. LemonSM 2010 Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem 285 22741 7
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



