-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
In the search for new drug targets, we evaluated the biotin synthetic pathway of Mycobacterium tuberculosis (Mtb) and constructed an Mtb mutant lacking the biotin biosynthetic enzyme 7,8-diaminopelargonic acid synthase, BioA. In biotin-free synthetic media, ΔbioA did not produce wild-type levels of biotinylated proteins, and therefore did not grow and lost viability. ΔbioA was also unable to establish infection in mice. Conditionally-regulated knockdown strains of Mtb similarly exhibited impaired bacterial growth and viability in vitro and in mice, irrespective of the timing of transcriptional silencing. Biochemical studies further showed that BioA activity has to be reduced by approximately 99% to prevent growth. These studies thus establish that de novo biotin synthesis is essential for Mtb to establish and maintain a chronic infection in a murine model of TB. Moreover, these studies provide an experimental strategy to systematically rank the in vivo value of potential drug targets in Mtb and other pathogens.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002264
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002264Summary
In the search for new drug targets, we evaluated the biotin synthetic pathway of Mycobacterium tuberculosis (Mtb) and constructed an Mtb mutant lacking the biotin biosynthetic enzyme 7,8-diaminopelargonic acid synthase, BioA. In biotin-free synthetic media, ΔbioA did not produce wild-type levels of biotinylated proteins, and therefore did not grow and lost viability. ΔbioA was also unable to establish infection in mice. Conditionally-regulated knockdown strains of Mtb similarly exhibited impaired bacterial growth and viability in vitro and in mice, irrespective of the timing of transcriptional silencing. Biochemical studies further showed that BioA activity has to be reduced by approximately 99% to prevent growth. These studies thus establish that de novo biotin synthesis is essential for Mtb to establish and maintain a chronic infection in a murine model of TB. Moreover, these studies provide an experimental strategy to systematically rank the in vivo value of potential drug targets in Mtb and other pathogens.
Introduction
Mtb causes approximately 8 million new cases of active tuberculosis (TB) and 2 million deaths each year [1]. Efforts to combat the TB pandemic have been hampered by the emergence of drug resistant strains of Mtb [2]. TB drug development thus represents a major area of unmet medical need. Mtb is shielded from the environment by a complex envelope that consists of an inner membrane, a periplasmic space, an outer membrane and a loosely attached capsule [3]–[5]. Isoniazid (INH), ethionamide (ETH) and ethambutol (EMB) constitute three anti-tuberculosis drugs that specifically inhibit synthesis of this envelope and validate cell envelope biosynthesis as a target pathway in Mtb.
Biotin is an essential cofactor required for synthesis of the fatty acid component of Mtb's cell envelope [6]–[10] and is synthesized from pimeloyl-CoA via a pathway consisting of four enzymes, BioF, BioA, BioD and BioB (Figure 1A) [11]. Bioactivity of biotin further requires covalent attachment to an enzyme, via a biotin ligase [12]. While the source of pimeloyl-CoA in Mtb is unknown [13], mammalian cells lack the enzymes to synthesize biotin de novo and must acquire it from external sources. Based on this presumed essentiality and intrinsic bacterial specificity, we sought to validate biotin biosynthesis as a potential target for the development of new antibiotics.
Fig. 1. Biotin synthesis pathway and construction of Mtb ΔbioA. 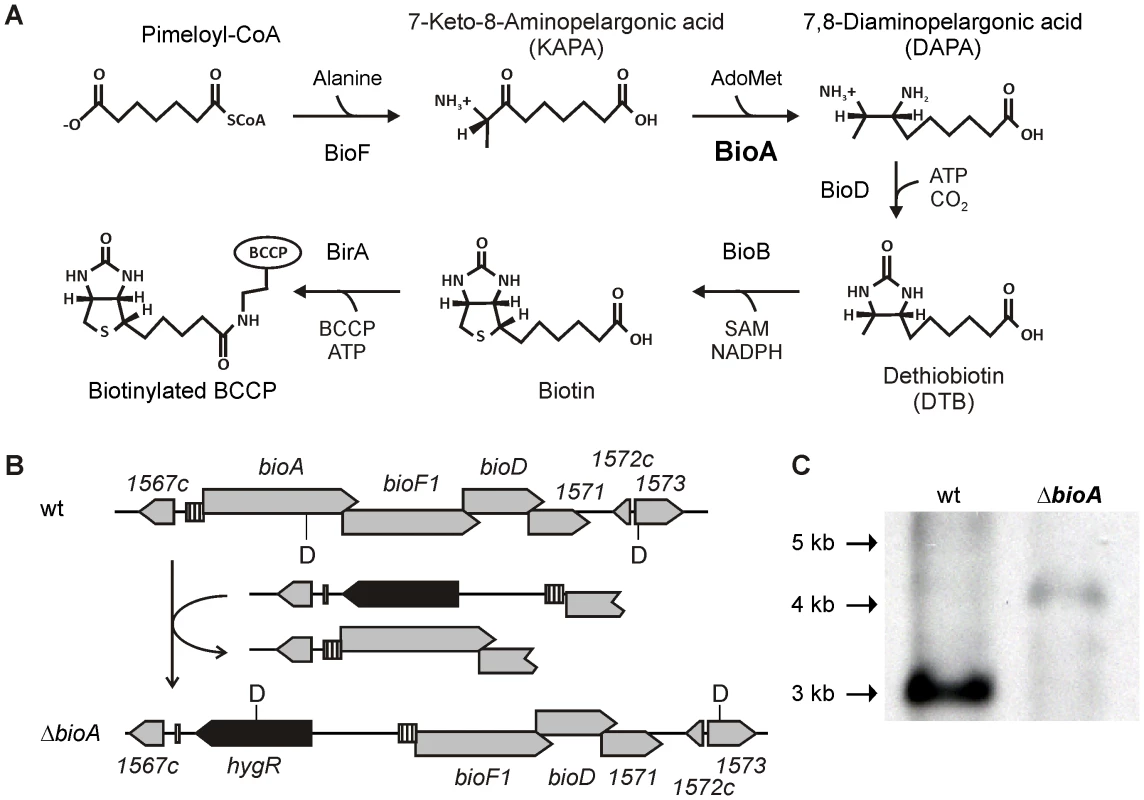
(A) Shown are the reactions catalyzed by KAPA synthase (BioF), DAPA synthase (BioA), DTB synthase (BioD) and biotin synthase (BioB), which convert pimeloyl-CoA to biotin, and biotin ligase, which attaches the cofactor to biotin-dependent enzymes. (B) The upper panel displays the genetic organization of the bioA region in wt Mtb; the lower panel displays that of ΔbioA. Gray boxes represent open reading frames of the genes specified above or below, the striped boxes mark the putative bioA promoter, and the black boxes represent hygR. The localization of recognition sites for the restriction endonuclease DraIII are labeled “D”. (C) Southern blot of DraIII-digested genomic DNA from wt Mtb and ΔbioA. The expected sizes for the DraIII fragments hybridizing to the bioF1-derived probe that was used in this blot are 3068 bp for wt and 4021 bp for ΔbioA. That efficient inhibition of biotin synthesis by a small molecule can be achieved was demonstrated by the ability of amiclenomycin, a natural product inhibitor of BioA isolated from Streptomyces lavendulae [14], to prevent in vitro growth of Mtb in media lacking exogenous biotin [15]. Treatment of mice with amiclenomycin had no impact on infection with Mtb, which was interpreted as evidence that Mtb does not depend on biotin synthesis during infection and is instead able to scavenge this cofactor from the host [16]. However, the lack of pharmacokinetic data showing that the organism was, in fact, exposed to the drug in these experiments temper this conclusion. In contrast, genome wide mutagenesis studies identified transposon insertion mutants of biotin synthesis genes as among the most highly attenuated mutants of Mtb observed in mice [17], suggesting that Mtb cannot access exogenous biotin in mice. Here, we constructed genetically defined Mtb bioA mutants to determine the consequences of inhibiting biotin synthesis on growth and survival of Mtb in vitro and during acute and chronic mouse infections.
Results
BioA is required for protein biotinylation, and therefore the growth and survival of Mtb without exogenous biotin
The Mtb bioA gene is located upstream of bioF1, bioD, and a gene of unknown function, rv1571 (Figure 1B). The start codons of bioF, bioD, and rv1571 are each positioned directly upstream of the stop codons of their respective upstream genes. This overlapping genetic organization suggests that both transcription and translation of bioA, bioF1, bioD and rv1571 mRNAs are coupled. We therefore generated a knockout cassette consisting of a hygromycin resistance gene flanked by 451 bp of rv1567c and its putative promoter on one end, and the putative bioA promoter followed by the first 723 bp of bioF1 on the other (Figure 1B). Integration of this cassette into the genome of Mtb H37Rv deleted bioA and placed the DNA fragment predicted to contain the promoter and the translational initiation site of bioA upstream of bioF1. Creation of this deletion mutant, ΔbioA, was confirmed by Southern Blot (Figure 1C).
As expected, ΔbioA did not grow in liquid media without added biotin but could be rescued with exogenous biotin or des-thiobiotin (DTB), the substrate of the biotin synthase, BioB (Figure 2A). No rescue occurred with 7-keto-8-aminopelargonic acid (KAPA), the substrate of BioA. ΔbioA growth was dependent on the concentration of biotin in the media (Figure 2B); with little to no growth with exogenous biotin concentrations below 25 nM and wild-type (wt) levels of growth above 250 nM biotin. Anti-biotin immunoblotting similarly revealed a selective loss of immunoreactivity corresponding to a single protein in ΔbioA, but not wt Mtb, following 6 days of biotin starvation (Figure 2C). The electrophoretic mobility and anti-biotin immunoreactivity of this protein suggests that it corresponds to AccA3, one of three proteins predicted to be biotinylated in Mtb [6], [18].
Fig. 2. In vitro characterization of Mtb ΔbioA. 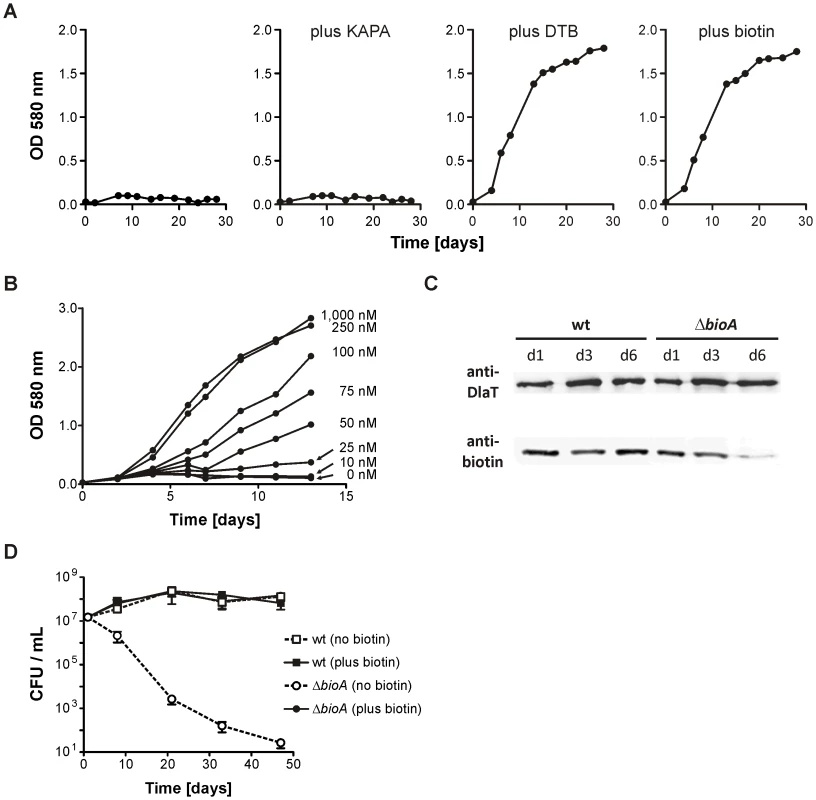
(A) Growth of ΔbioA in Sauton's medium (left) or Sauton's medium supplemented with KAPA, DTB or biotin. Data are from individual cultures and representative of at least two independent experiments. (B)Growth of ΔbioA in Sauton's medium with varying concentrations of biotin. Data are from individual cultures and representative of at least two independent experiments. (C) Immunoblots performed with both an anti-biotin antiserum and rabbit serum recognizing Mtb's dihydrolipoamide acyltransferase (DlaT). The lower panel shows the signal from the anti-biotin antibody; the upper panel shows the DlaT signal, which served as the loading control. Protein extracts were prepared after the indicated days of cultivation in biotin-free Sauton's medium. Data are from individual cultures and representative of at least two independent experiments. (D) Survival of wt and ΔbioA in Sauton's media with and without biotin. Data are averages from triplicate cultures and representative of at least two independent experiments. Error bars indicate standard deviations. We also assessed the impact of biotin starvation on ΔbioA viability by enumerating the colony forming units (CFUs) recovered after incubation for various time in Sauton's medium without biotin. ΔbioA CFUs decreased from 107 CFUs per ml to less than 102 CFUs per ml over a period of 50 days (Figure 2D). In the same medium, the CFUs of wt Mtb increased during the first two weeks and remained stable thereafter. CFUs recovered for wt and ΔbioA from biotin-containing Sauton's medium were almost identical. These experiments thus demonstrate that selective deletion of bioA caused a specific defect in Mtb's biotin biosynthesis pathway as reported by biotin-dependent changes in protein biotinylation, growth and survival of ΔbioA but not wt Mtb.
BioA is required for Mtb to establish acute infection in mice
Mtb mutants harboring transposon insertions in biotin synthesis genes were among the most strongly attenuated during infection of mouse spleens by pooled mutants [17]. We sought to extend these studies by measuring the extent to which Mtb depends on de novo biotin biosynthesis for growth in mouse lungs during acute, single-strain infection and for persistence in the chronic phase of infection. Whereas ΔbioA could address the first question, a mutant that enabled conditional silencing of bioA during the chronic phase of infection was required to answer the second. We therefore constructed a tetracycline repressor (TetR)-regulated mutant in which transcription of bioA can be induced with either anhydrotetracycline (atc) or doxycycline (doxy), but was inhibited in their absence. To achieve this, we cloned the bioA gene downstream of the TetR-controlled promoter Pmyc1tetO [19] into a plasmid, which also contained a tetR whose codon usage was adapted to improve expression in mycobacteria [20]. Integration of the resulting plasmid, pGMCK-T2M1-bioA, into the attachment site of the phage L5 (attL5) yielded Mtb bioA TetON-1.
Growth of bioA TetON-1 in biotin-free medium was indistinguishable from that of wt Mtb in the presence of atc, but reduced, though not abolished as observed for ΔbioA, in its absence (Figure 3A). Growth of wt, ΔbioA, and bioA TetON-1 in biotin-containing media, by contrast, was indistinguishable. Following aerosol infection, ΔbioA did not grow in mouse lungs. The bioA TetON-1 mutant grew slightly more slowly in mice not receiving doxy than in mice that were fed doxy but it reached a similar final bacterial load in lungs with and without doxy (Figure 3B). In vitro growth analyses confirmed that bioA TetON-1 bacteria recovered from mouse lungs at day 56 post-infection were still TetR regulated and did not represent suppressor mutants (not shown). In contrast to the muted phenotype of bioA TetON-1, ΔbioA was strongly attenuated and no or few CFUs were recovered 56 days post infections (Figure 3B). BioA is thus required for Mtb to establish an acute infection in mouse lungs.
Fig. 3. Growth of wt Mtb, ΔbioA, and bioA TetON-1 in liquid media and mice. 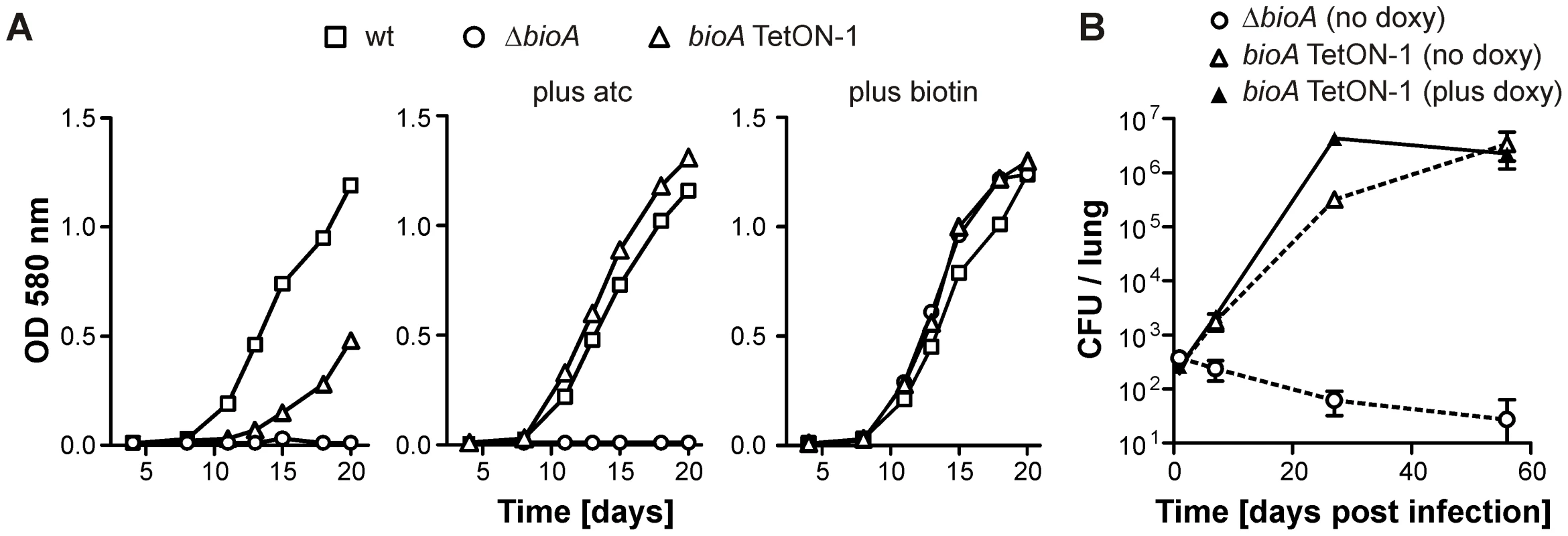
(A) Growth of wt, ΔbioA, and bioA TetON-1 in Sauton's media, Sauton's media with atc or Sauton's media with biotin. Squares, circles, and triangles represent data for wt, ΔbioA, and bioA TetON-1, respectively. Data are from individual cultures and representative of several independent experiments. (B)Growth of ΔbioA and bioA TetON-1 in mouse lungs. Circles represent data for ΔbioA, triangles represent data for bioA TetON-1. Doxy was either given from day 1 of the infection or not at all. Data displayed by open symbols and dotted lines are from doxy-free mice, closed symbols represent data from doxy-fed mice. Data are averages from four mice per group; error bars represent the standard error of the mean. Construction of tightly controlled Mtb bioA-TetON mutants
To compare BioA protein levels of bioA TetON-1 with those of wt and ΔbioA, we grew them (with biotin) in the presence and absence of atc and analyzed total protein extracts with BioA-specific polyclonal antiserum. As expected, no BioA was detected in ΔbioA and BioA expression was not changed by atc in wt Mtb (Figure 4A). In bioA TetON-1 extracts prepared from atc-containing cultures BioA protein exceeded the wt levels, but BioA protein was not detected in extracts of cultures grown without atc. Because bioA TetON-1 grew, albeit more slowly, in the absence of both atc and biotin (Figure 3A), these results suggested that the amount of BioA expressed by wt is actually significantly higher than required for growth in biotin-free media.
Fig. 4. BioA protein levels of wt Mtb, ΔbioA, and bioA TetON mutants. 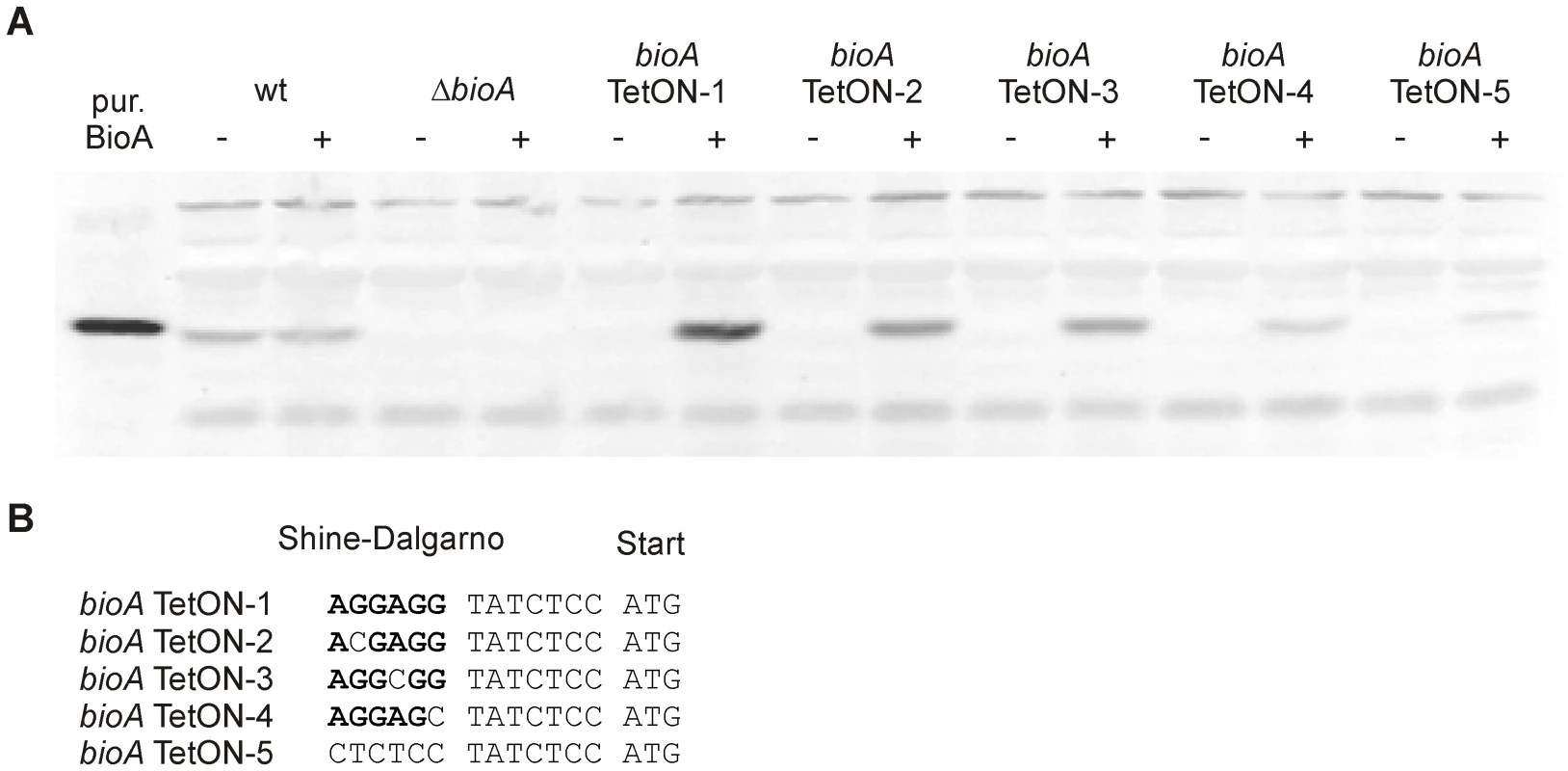
(A) Immunoblots performed with anti-BioA antiserum using total protein extracts prepared from cultures grown with biotin. The plus and minus signs indicate presence or absence of atc in the culture medium. The first lane on the left contained purified BioA. (B) The putative Shine-Dalgarno sequence of bioA TetON-1 is shown in bold. The mutations that were introduced and distinguish the different TetON mutants are shown below. “ATG” indicates the start of the bioA. The immunoblots also indicated that induction of BioA by atc in bioA TetON-1 was stronger than necessary to achieve full complementation. Seven nucleotides upstream of the bioA start codon, the bioA mRNA of bioA TetON-1 contains a hexamer that is perfectly complementary to the region of Mtb's 16S rRNA thought to bind mRNAs during the initiation of translation. We sought to improve regulation of BioA expression by mutating this putative ribosome binding site (Shine-Dalgarno site, SD) and constructed three mutants, bioA TetON-2/-3/-4, in which individual nucleotides were mutated as shown in Figure 4B. In the fifth mutant, bioA TetON-5, all nucleotides of the SD were changed to decrease binding of the bioA TetON-5 mRNA to the ribosome. With atc, bioA TetON-2/-3/-4/-5 all expressed less BioA than bioA TetON-1 and the lowest amount of BioA was detected in extracts of bioA TetON-5 (Figure 4A). The experiments shown in Figure 4A did not allow us to measure BioA expression in the different TetON mutants without atc, but we expected that mutations in the SD also decreased the amount of BioA expressed by each mutant in the absence of atc and tested this by measuring growth without biotin.
In contrast to bioA TetON-1 (Figure 3A), bioA-TetON-2/-3/-4/-5 all failed to grow in biotin-free media lacking atc. With atc, growth of the new mutants was indistinguishable from growth of wt and from growth with biotin (Figure 5A and Figure S1). Moreover, growth of the mutants was strictly atc-dose dependent (Figure S2 and not shown). Next, we infected mice and monitored growth of ΔbioA and all five bioA TetON mutants without doxy (Figure 5B). Similar CFU counts were recovered from mouse lungs for all mutants 1 day after the aerosol infection. 21 days post infection, the CFUs recovered from the mice infected with bioA TetON-1 had increased from 161 (±11) to 136,800 (±31,704) whereas the CFUs of ΔbioA and bioA TetON-2/-3/-4/-5 were close to or below the limit of detection.
Fig. 5. Growth of Mtb bioA TetON-5 in biotin free liquid media and of all mutants in mice without doxy. 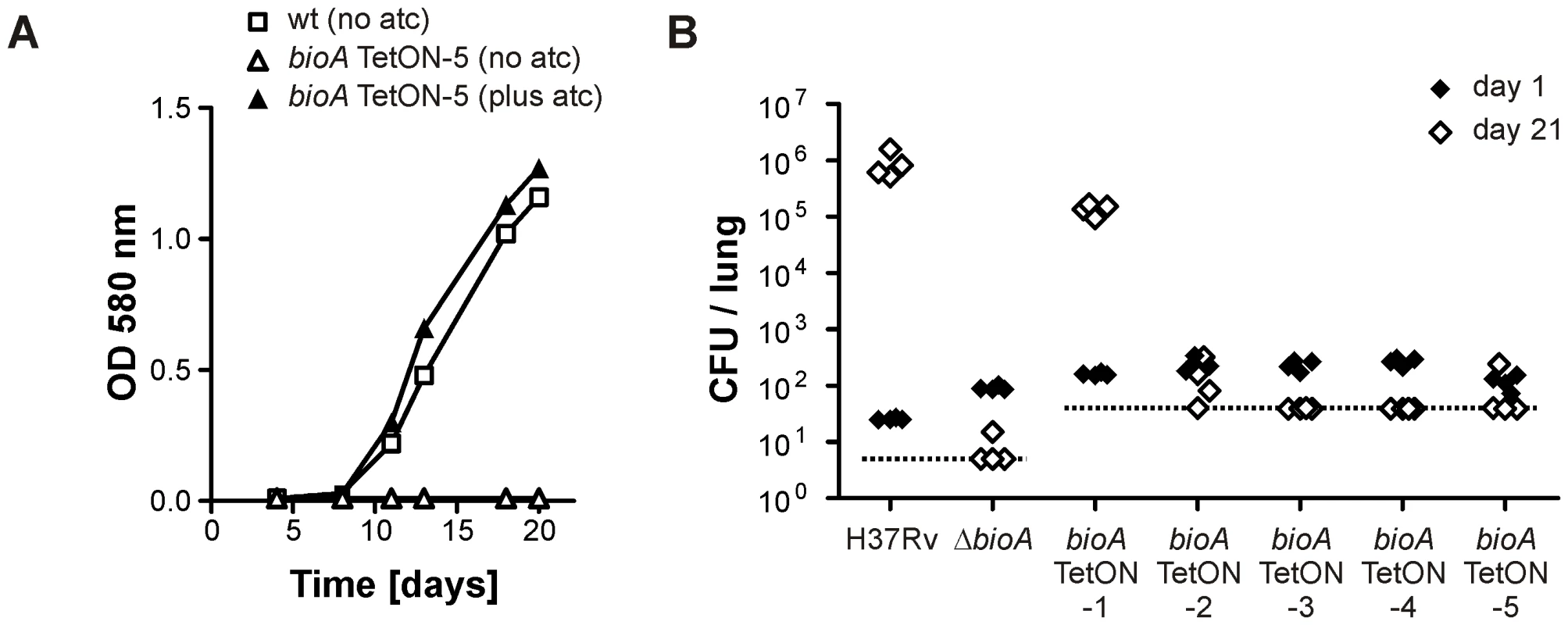
(A) Growth of wt and bioA TetON-5 in biotin-free Sauton's medium with and without atc. Squares represent data for wt, triangles represent data for bioA TetON-5. Open symbols indicate data from medium without atc, closed symbols represent data from medium containing atc. Data are from individual cultures and representative of at least two independent experiments. (B) Growth of wt, ΔbioA and the different bioA TetON mutants in mouse lungs. Closed symbols represent CFUs from day 1 post infection, open symbols represent data from day 21. The dotted line indicates the limit of detection (which was lower for wt and ΔbioA than for the TetON mutants due to the different amounts of lung homogenates that were plated). Each symbol represents CFUs obtained from one mouse. Together, these experiments demonstrated that (i) BioA levels in wt Mtb are significantly above those required for growth without exogenous biotin, (ii) efficient regulation of BioA expression by TetR and Pmyc1tetO required mutation of the ribosome binding sequence, and (iii) conditionally silenced Mtb bioA strains TetON-2/3/4/5 could not replicate in mouse lungs in the absence of doxy.
BioA is essential for persistence of Mtb during chronic infections
Expression of BioA was lowest in bioA TetON-5 (Figure 4) suggesting that this mutant might also allow most efficient silencing of BioA without atc. We therefore selected it to determine the extent to which Mtb requires BioA to persist during the chronic phase of infection in a murine model of TB and silenced bioA transcription on days 1, 10, 28 or 56 post infection. In accordance with the infection described above (Figure 5B), bioA TetON-5 failed to grow in mice that never received doxy (Figure 6), with no more than 4 CFUs isolated from lungs 56 days post infection and none recovered after 112 days. In the absence of doxy, bioA TetON-5 thus reproduced the phenotype of ΔbioA (Figure 3B).
Fig. 6. Growth and survival of Mtb bioA TetON-5 in mouse lungs and spleens. 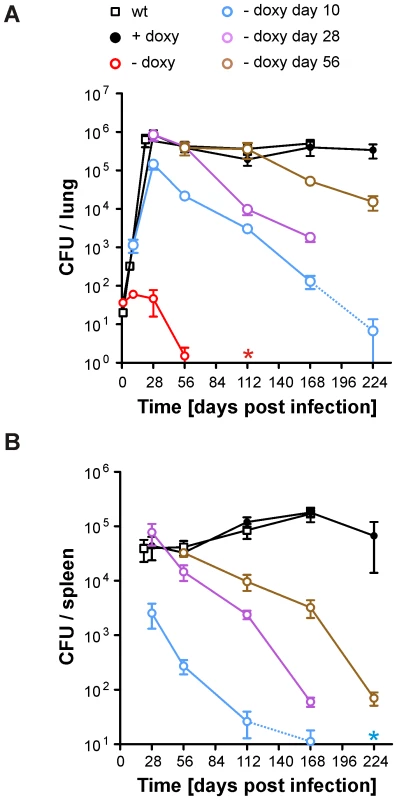
(A) Growth and persistence in lungs. Squares represent data for wt, circles represent data for bioA TetON-5. Mtb bioA TetON-5 was analyzed in mice that did not receive doxy (red circles), received doxy from day 1 to day 10 (blue circles), from day 1 to day 28 (purple circles), from day 1 to 56 (brown circles) or received doxy throughout the infection (black circles). Data are averages from at least four mice and two independent infections; error bars represent the standard error of the mean. The limit of detection was 4 CFUs per lung. Dotted lines end in data points for which most of the lungs contained 4 or fewer CFUs. The red asterisk indicates that no CFUs were recovered from any of the no-doxy mice 112 days post infection. (B) Persistence in spleens. The blue asterisk indicates that on day 224 no CFUs were recovered from any of the mice that received doxy up to day 10. Otherwise as described for (A). In mice receiving doxy continuously, CFUs recovered from lungs increased during approximately four weeks, were almost constant in lungs and spleens thereafter, and were very similar to CFUs of wt Mtb H37Rv. Deprivation of biotin after the first 10 days resulted in growth impairment of bioA TetON-5 in lungs and spleens and perhaps more importantly caused a subsequent decline of CFUs to less than 0.02% of the day 28 CFUs from lungs by 224 days post infection. By that time no CFUs could be recovered from the spleens of these bioA TetON-5 infected mice. When biotin deprivation was initiated 28 days post-infection, the CFUs in lungs decreased by more than 99% at day 168. Due to a technical problem we did not obtain data for this group of mice 224 days post infection (or the wt control). However, silencing bioA even later in the chronic phase of the infection, at day 56, resulted in a ∼96% reduction in bacterial numbers recovered from lungs at day 224. Histological analyses demonstrated that silencing of BioA expression 10 days post infection prevented the severe pathology caused by bioA TetON-5 with doxy and that inactivation of BioA at day 56 reduced lung pathology at later time points (Figure S3). In summary, these experiments demonstrated that bioA expression is required for Mtb to both establish and maintain infection in a murine model of TB.
Quantitative analyses of the BioA levels and activities of different mutants
Our final goal in this study was to determine the minimal level of BioA expression required for in vitro growth and survival of Mtb in mice. To do so, we first analyzed immunoblots that contained serial dilutions of total protein extract for all TetON mutants. The intensity of the BioA band in these blots was measured using an Infrared Imaging System, normalized to the total protein amount loaded, and compared to wt protein extracts. Two representative immunoblots of this kind are shown in Figure 7 and the relative BioA levels measured by this approach are shown in Table 1.
Fig. 7. Quantitative BioA immunoblotting of bioA TetON-1 and bioA TetON-5. 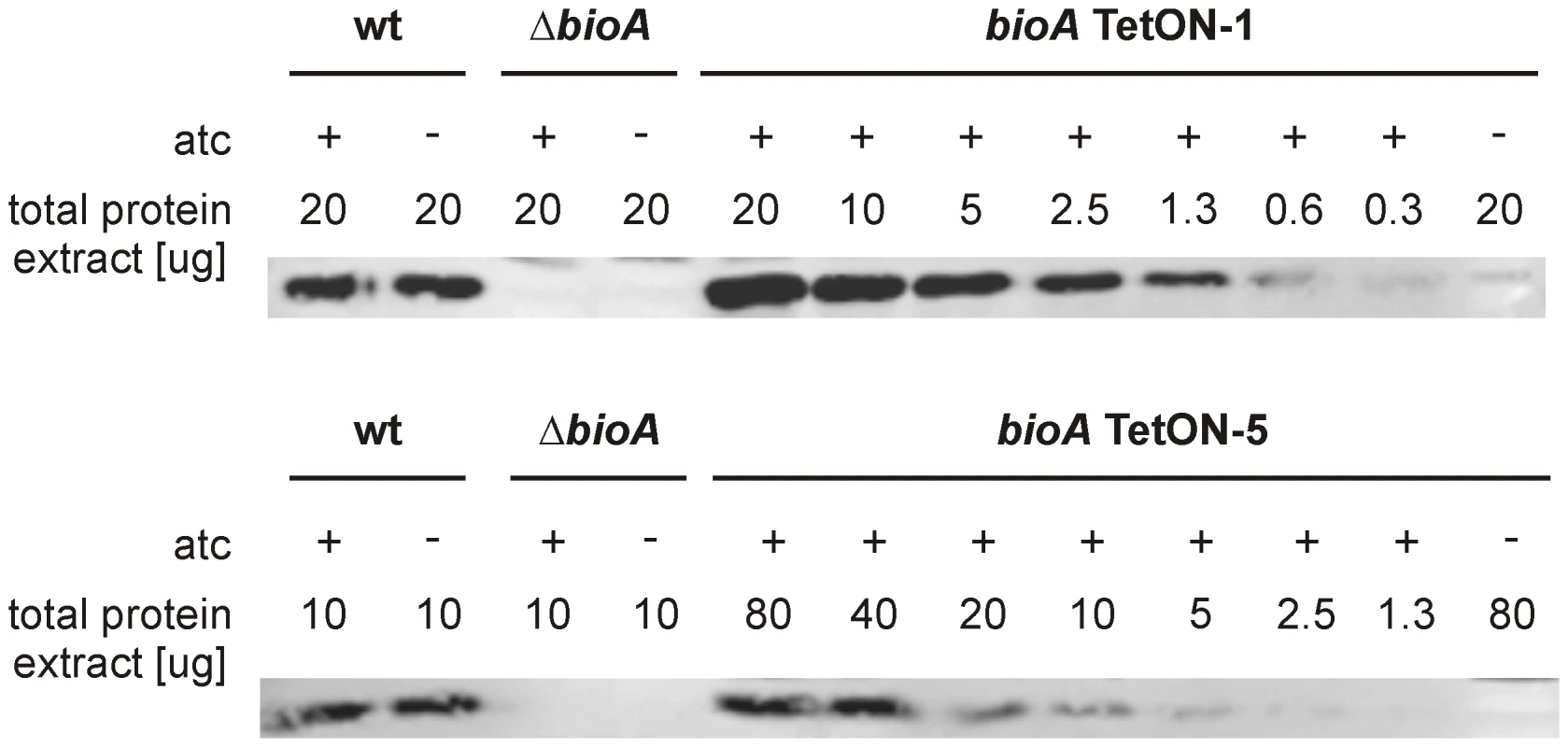
Immunoblots were performed as described in the main text and in the material and methods. Tab. 1. Relative BioA protein levels and activities. 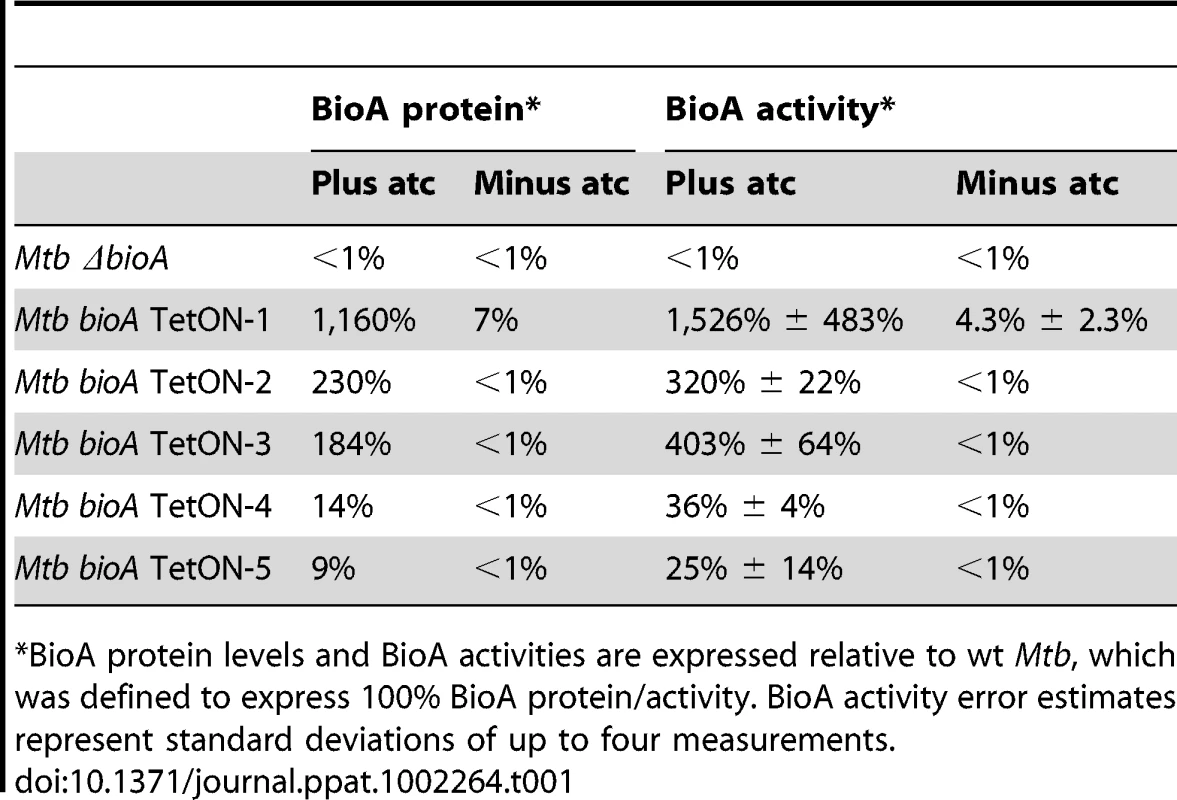
*BioA protein levels and BioA activities are expressed relative to wt Mtb, which was defined to express 100% BioA protein/activity. BioA activity error estimates represent standard deviations of up to four measurements. We next developed a coupled assay to measure BioA activities in cell lysates using BioD catalyzed formation of DTB. In this assay, saturating concentrations of substrates and cofactors were added to cell lysates along with recombinant BioD to catalyze conversion of DAPA into DTB. DTB was then purified from the reaction mixture using streptavidin agarose resin and eluted into buffer by thermal treatment of the resin (Figure S4). DTB was subsequently detected by displacement of the fluorescent DTB probe N1-[3-[2-(2-{3-[(Fluorescein-5-yl)carbonyl]aminopropoxy}ethoxy)ethoxy]propyl}dethiobiotinamide (Fl-DTB) from streptavidin resulting in an increase in the fluorescent signal. This assay proved to be extremely sensitive and enabled detection of as little as 30 femtomoles of BioA in the cell lysates. The amount of DTB produced was directly proportional to the concentration of BioA (Figure S5). For each assay a standard curve was generated by adding known amounts of DTB to ΔbioA lysates (Figure S6). The relative BioA activity measured by this approach is also shown in Table 1.
BioA expression levels estimated by immunoblotting were somewhat lower than those obtained by the coupled enzyme assay but generally in good agreement with the results of this BioA activity assay. Both assays demonstrated that regulation of expression of bioA in bioA TetON-1 was efficient and without atc the BioA activity of this mutant was reduced by ∼96% compared to wt Mtb (Table 1). For bioA TetON-2/3/4/5 BioA protein levels and activities were reduced by at least 99%.
Discussion
Here, we report an Mtb mutant, ΔbioA, in which the entire open reading frame encoding DAPA synthase was deleted, to evaluate the in vitro and in vivo characteristics of Mtb's BioA as a potential drug target. Deletion of bioA was confirmed genotypically (Figure 1C) and phenotypically in experiments showing that ΔbioA expressed no BioA protein (Figure 4A), had no BioA activity (Table 1), and produced decreasing amounts of biotinylated protein after transfer into biotin-free media (Figure 2C). In biotin-free medium, ΔbioA lost viability (Figure 2D) with kinetics similar to those observed for an Mtb bioF mutant [11]. The in vitro growth defect of ΔbioA could be complemented with biotin at concentrations as low as 50 nM (Figure 2B), which is at least 25-times higher than the biotin concentration in human serum [21], [22]. Growth of ΔbioA was also rescued with DTB, but not using KAPA as a substrate since conversion of KAPA to biotin is BioA-dependent (Figures 1A and 2A). Following aerosol infection, ΔbioA failed to replicate in mice and was cleared from the lungs of several mice during the first 8 weeks of the infection (Figures 3B and 5B).
Survival of an M. smegmatis mutant, which contains a transposon insertion in bioA (referred to as 272A), was impaired during stationary phase even in the presence of exogenous biotin [23]. That we did not observe such a defect for Mtb ΔbioA (Figure 2D) suggests that M. smegmatis and Mtb differ with respect to the conditions in which they are able to utilize exogenous biotin. However, what exactly caused the survival defect of M. smegmatis 272A is not entirely clear because this mutant required several vitamins to grow and it is unknown if a wt copy of bioA would complement its stationary phase survival defect [23]. What is clear, however, is that the growth and survival defects that Mtb ΔbioA displayed in vitro and during infections can be complemented by expression of an intact copy of bioA (Figures 3, 5A, 6, S1, S2, and S3). Our experiments thus demonstrate that Mtb cannot acquire biotin from the host and requires de novo biotin biosynthesis to both establish and maintain a chronic infection in a murine model of TB. This dependence on de novo biotin biosynthesis is likely conserved in other mycobacterial pathogens, as a biotin auxotroph of M. marinum was also attenuated [13].
To determine Mtb's dependency on biotin synthesis over the course of infection, we constructed TetON mutants, in which transcription of bioA was induced with atc or doxy and repressed in the absence of both tetracycline derivatives. This allowed us to deprive Mtb of its ability to synthesize biotin after it had grown to more than 1,000 CFUs in both lungs and spleens or had already established a chronic infection. Irrespective of the stage of the infection at which shutdown of bioA was initiated, it always had a severe impact on growth and survival of Mtb (Figure 6). In mice that received doxycycline from day 1 to day 10, CFUs increased for approximately 18 more days. That the switch from doxy-containing to doxy-free food did not result in a more immediate impact is likely because BioA, biotin, and the biotinylated enzymes that were synthesized prior to removal of doxy have to be depleted or inactivated before survival of Mtb is affected. Beginning on day 28 post infection, CFUs in the lungs declined with a rate of 14% (±5%) per week (Figure 6A), which caused most mice to have less than 4 CFUs per lungs 224 days post infection. If silencing was initiated at 28 or 56 days post infection lung CFUs decreased with rates of 12% (±1%) and 10% (±1%) per week, respectively, at later time points. Silencing of bioA also had a drastic impact on survival of Mtb in mouse spleens (Figure 6B) and histological studies demonstrated that silencing bioA during chronic infections decreased lung pathology at later timer points (Figure S3). These data do not prove that silencing bioA at the later time points can actually sterilize a chronic infection in the lungs. However, once the CFUs started to decrease they continued to do so with similar kinetics irrespectively of the time point at which silencing was initiated, which suggests that sterilization would have occurred eventually. Taken together, this demonstrates that Mtb relies on biotin synthesis for growth and survival throughout the course of mouse infections. It furthermore indicates that the reported inactivity of amiclenomycin in Mtb infected mice [16], may have been due to poor pharmacokinetic behavior of this compound, a conjecture supported by our observation that amiclenomycin spontaneously decomposes through aromatization (Orisadipe, A., Boshoff, H. I., Barry, C. E., unpublished results).
We previously pointed out that genetic drug target validation studies often fall short of assessing the vulnerability of a target to partial inhibition [24]. This is relevant because chemical inactivation of a target during an infection is likely incomplete and enzymes that need to be inactivated by, for example, 80% will, on average, make better targets than those that need to be inactivated by 99% before a pathogen stops replicating. We employed two approaches, protein level and protein activity measurements, to determine the extent to which BioA needs to be inactivated before Mtb stops growing. In general, these two approaches gave similar results but minor discrepancies were apparent for mutants that expressed low levels of BioA in the presence of atc. This was likely due to very low signal intensities in the respective immunoblots. Both analysis methods indicated that inactivation of BioA has to reach more than 90% before growth of Mtb is severely affected in vitro or during infections. This is because Mtb bioA TetON-1 still grew without atc and without biotin in vitro and also grew without doxy in mice even though its BioA protein level and activity were reduced by more than 90% without atc/doxy. Mutants in which the expression and activity of BioA was reduced by at least 99% showed strong growth and survival defects in vitro and during infection.
It is impossible to compare vulnerability of BioA to incomplete inhibition to that of other potential Mtb drug targets because BioA is the first Mtb protein for which vulnerability has been measured. But studies in which vulnerability of different essential enzymes to incomplete depletion was measured have recently been published for M. smegmatis [25], [26]. These studies suggest that BioA might not be a highly vulnerable target because reducing expression of the RNA polymerase subunit B, RpoB, by as little as 80% was sufficient to prevent growth in M. smegmatis [26] and similar levels of inactivation might suffice to stop replication of Mtb as well. It thus seems prudent to determine if other enzymes in the biotin synthesis pathway might be more susceptible to partial inactivation than BioA. However, the reported activity of amiclenomycin against whole Mtb cells suggests that sufficiently potent inhibitors of BioA can be obtained and the structure of amiclenomycin provides a starting point for their design. We expect the mutants we described here to help with the development of such amiclenomycin derivatives and the identification, evaluation and development of other inhibitors of Mtb's biotin metabolism, because they should be more susceptible than wt to such inhibitors.
Amiclenomycin is known to covalently inactivate BioA in the process of aromatization [27] thus leading to an unusual situation analogous to 100% enzyme inhibition. A recently appreciated variable in determining target vulnerability is the ability to identify inhibitors with long residence times such as those that form covalent linkages or that induce conformational changes upon binding their targets. In TB residence time may be correlated with in vivo effect [28]. Such factors would seem likely to be more important with relatively invulnerable targets such as BioA than with more vulnerable targets such as RpoB. Percent inhibition required for an effect on growth is, of course, only one factor important in determining the overall target vulnerability. Nonetheless such information offers an important new way to prioritize potential targets within a biochemical pathway and may explain some of the failure in translating highly potent inhibitors of essential enzymes into compound series with high cellular potency [29].
That so little is known about vulnerability of essential proteins to incomplete depletion is in part due to the difficulties we and others have experienced in constructing phenotypically well-regulated conditional Mtb knockdown mutants. Here, we overcame a key technical hurdle associated with evaluating a potential drug target in vitro and in mice: the leakiness of transcriptional regulatory systems, which can often prevent efficient silencing of proteins required only in small amounts, for which we developed a novel translational regulatory strategy. This may provide a generally applicable approach that will enable vulnerability to be added as a parameter of target validation. Such information should help to further focus target-based drug discovery efforts on pathways and enzymes that are essential under a variety of conditions and are susceptible to incomplete inhibition.
Material and Methods
Ethics statement
All procedures including animal studies were conducted following the National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after protocol review and approval by the Institutional Animal Care and Use Committee of Weill Cornell Medical College (protocol # 0802-713A, TetR-controlled gene silencing in mycobacteria during mouse infections).
Strains, media and culture conditions
Mtb H37Rv was obtained from Dr. Robert North (Trudeau Institute) and grown in Sauton's liquid medium containing 0.02% tyloxapol, or in Middlebrook 7H9 liquid medium (Difco) containing 0.2% glycerol, 0.5% BSA, 0.2% dextrose, 0.085% NaCl, and 0.05% Tween 80, or on Middlebrook 7H11 agar plates containing 10% OADC supplement (Becton Dickinson) and 0.5% glycerol. When required, hygromycin B was used at a concentration of 50 or 100 µg/ml and kanamycin at a concentration of 15 µg/ml. Preparation of competent cells, electroporations, and preparation of genomic DNA were performed as described [19]. Where indicated, 7-keto-8-aminopelargonic acid (KAPA; Toronto Research Chemicals), DTB (Sigma), and biotin (Sigma) were added at a concentration of 1 µM.
Mutant construction and southern blotting
BioA was deleted by homologous recombination following transduction with a derivative of the temperature sensitive mycobacteriophage phAE87 [30]. To generate this phage the 3′-portion of the rv1567c was amplified by PCR, digested with BglII and NcoI and cloned into likewise digested pJSC284 (gift of J. S. Cox) resulting in pJSC284-Rv1567c. We then amplified the bioA promoter region and the 5′-portion of bioF using primers that allowed us to fuse these two amplicons in a third PCR. The resulting PCR product (in which the bioA promoter region was located upstream of the 5′-portion of bioF) was cloned downstream of the hygromycin resistance cassette of pJSC284 using AflII and AgeI. This resulted in pJSC284-Rv1567c-bioF, which contained the complete bioA knockout cassette. The integrity of pJSC284-Rv1567c-bioF was confirmed by restrictions and DNA sequencing. pJSC284-Rv1567c-bioF was used to construct a bioA knockout phage as described [31]. Hygromycin-resistant transductants of Mtb were selected on 7H11 agar and obtained after 3 weeks of incubation at 37°C. Mutants were analyzed by PCR and Southern blotting. For Southern blotting genomic DNA was digested with DraIII and analyzed with a probe specific for bioF. Hybridization and detection were carried out with an ECL chemoluminescent detection system (GE Healthcare) as instructed by the manufacturer. BioA-TetON mutants were obtained by transformation of ΔbioA with expression plasmids that integrate into the attachment site of the mycobacteriophage L5, contain bioA downstream of Pmyc1tetO [19] and also contained tetR#2 [20]. These plasmids were constructed using gateway recombination as described [32].
Immunoblots
Cell lysates were prepared by bead-beating cell pellets in lysis buffer (25 mM Tris HCl [pH 7.6], 1 mM EDTA, and 1 mM PMSF) and sterilized by passage through a 0.22 µm Spin-X filter (Costar). For the detection of biotinylated proteins, 15 µg cell lysates were subjected to SDS-PAGE, followed by transfer to a nitrocellulose membrane. Biotin-specific mouse serum (Sigma) and DlaT-specific rabbit serum [33] were used at 1∶1,000 dilutions in blocking buffer. For the detection of BioA proteins, cell lysates were subjected to SDS-PAGE and blots were probed with BioA-specific rabbit serum (1∶1,000) generated against purified BioA protein. Goat anti-mouse and goat anti-rabbit (LI-COR Biosciences) were diluted 1∶10,000 in Odyssey blocking buffer. Blots were developed using the Odyssey Infrared Imaging System (LI-COR Biosciences).
BioA activity assay
Bacteria were grown in at least 500 ml Sauton's media containing 1 µM biotin in the presence or absence of atc. Once the culture reached an OD580 of ∼ 1.0, bacteria were harvested by centrifugation, washed, and resuspended in lysis buffer (100 mM bicine pH 8.6, 50 mM NaHCO3, 1 mM MgCl2, 0.0025% igepal CA-630, and protease inhibitor cocktail tablet [Roche]). Cell free lysates were prepared by beating the solution with 0.1 mm Zirconia/Silica beads (BioSpec Products, Inc) three times at 6000 rpm for 50 sec at 4°C, followed by 10 min centrifugation at 13000 rpm, 4°C and sterilized by passage through a 0.22 µm Spin-X filter (Costar). BioA activity in the lysates was quantified using a coupled assay employing BioD to generate DTB, which was subsequently detected in a fluorescence displacement assay. BioA and BioD were expressed and purified as described (Wilson, D. J. et al., submitted) Biotin and biotinylated proteins were removed from cell free lysates by the addition of 100 µL streptavidin agarose resin (Thermo Scientific 20349) that had been pre-washed with PBS to remove storage buffer.
Samples were incubated for 30 min at 4°C, spun at 1000 × g for 1 min, and the biotin free supernatant was retrieved. Reactions were then prepared in duplicate in 500 µL total volume and contained 200 µL biotin-free Mtb lysate (1–20 mg protein/mL), 12.5 µM KAPA, 5 mM SAM, 1 mM TCEP, 5 mM ATP, 200 µM PLP in reaction buffer (100 mM bicine pH 8.6, 50 mM NaHCO3, 1 mM MgCl2, 0.0025% igepal CA-630). Reactions were initiated by the addition of 320 nM BioD and incubated at 37°C for 16 hours. Control reactions were made by omitting BioD or freezing control reactions at −80°C. Both controls yielded identical results. DTB formed was removed from reactions by the addition of 30 µL of streptavidin agarose resin followed by incubation at 4°C for 30 min. Resin was pelleted at 1000 × g for 1 min and the resin was washed with 300 µL reaction buffer plus 10 mM MgCl2. DTB was removed from the resin by incubating the beads with 47 µL of water for 5 min at 110°C. The free DTB was detected by transferring 45 µL of the reaction mixture into a black 384 well plate (corning 3575) and adding N1-[3-[2-(2-{3-[(Fluorescein-5-yl)carbonyl]aminopropoxy}ethoxy)ethoxy]propyl}dethiobiotinamide (Fl-DTB) [34] and streptavidin (Sigma S4762) providing a final concentration of 20 nM and 185 nM, respectively in a total of 50 µL. Plates were incubated for 10 min and read on a molecular devices M5e multi mode plate reader using excitation 485, emission 530, and cutoff 530 nm. To quantitate the amount of DTB produced a standard curve was produced by adding known quantities of DTB to reactions containing lysate from the ΔbioA mutant (see Figure S6 for a representative standard curve). The curve was fit to equation (1) using GraphPad Prism (version 4):(1)where F is the fluorescence intensity at DTB concentration [S], Fmax and Fmin are the maximum and minimum fluorescence signals respectively and IC50 is the concentration of DTB that produces 50% Fmax. Fluorescence was then converted back into DTB concentration using equation (2).(2)
Experiments to determine that the amount of DTB produced was directly proportional to the concentration of BioA were performed by adding 0, 0.5, 1,0, and 2.0 nM recombinant BioA to the standard reaction above using lysate from the ΔbioA mutant (2 mg/mL). The amount of DTB produced per concentration of BioA was fit with linear regression analysis using Prism (Figure S5).
Mouse infections
8–10 week old C57BL/6 mice were purchased from Jackson Laboratory and infected with the indicated Mtb strains by aerosol as described [35]. When indicated, mice received doxycycline containing mouse chow (2,000 ppm; Research Diets). Serial dilutions of organ homogenates from four mice per data point were plated onto 7H11 agar plates to quantify CFUs. When histopathology analyses were performed, the upper left lobes of infected lungs were fixed in 10% buffered formalin, sectioned and stained with hematoxylin and eosin (Laboratory of Comparative Pathology at Memorial Sloan-Kettering Cancer Center).
Supporting Information
Zdroje
1. DyeCWilliamsBG 2010 The population dynamics and control of tuberculosis. Science 328 856 861
2. LoBueP 2009 Extensively drug-resistant tuberculosis. Curr Opin Infect Dis 22 167 173
3. HoffmannCLeisANiederweisMPlitzkoJMEngelhardtH 2008 Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A 105 3963 3967
4. ZuberBChamiMHoussinCDubochetJGriffithsG 2008 Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol 190 5672 5680
5. SaniMHoubenENGeurtsenJPiersonJde PunderK 2010 Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6 e1000794
6. DanielJOhTJLeeCMKolattukudyPE 2007 AccD6, a member of the Fas II locus, is a functional carboxyltransferase subunit of the acyl-coenzyme A carboxylase in Mycobacterium tuberculosis. J Bacteriol 189 911 917
7. GagoGKurthDDiacovichLTsaiSCGramajoH 2006 Biochemical and structural characterization of an essential acyl coenzyme A carboxylase from Mycobacterium tuberculosis. J Bacteriol 188 477 486
8. KurthDGGagoGMde la IglesiaABazet LyonnetBLinTW 2009 ACCase 6 is the essential acetyl-CoA carboxylase involved in fatty acid and mycolic acid biosynthesis in mycobacteria. Microbiology 155 2664 2675
9. OhTJDanielJKimHJSirakovaTDKolattukudyPE 2006 Identification and characterization of Rv3281 as a novel subunit of a biotin-dependent acyl-CoA Carboxylase in Mycobacterium tuberculosis H37Rv. J Biol Chem 281 3899 3908
10. Massengo-TiasséRCronanJ 2009 Diversity in enoyl-acyl carrier protein reductases. Cell Mol Life Sci 66 1507 1517
11. DeySLaneJMLeeRERubinEJSacchettiniJC 2010 Structural characterization of the Mycobacterium tuberculosis biotin biosynthesis enzymes 7,8-diaminopelargonic acid synthase and dethiobiotin synthetase. Biochemistry 49 6746 6760
12. PurushothamanSGuptaGSrivastavaRRamuVGSuroliaA 2008 Ligand specificity of group I biotin protein ligase of Mycobacterium tuberculosis. PLoS One 3 e2320
13. YuJNiuCWangDLiMTeoW 2011 MMAR_2770, a new enzyme involved in biotin biosynthesis, is essential for the growth of Mycobacterium marinum in macrophages and zebrafish. Microbes Infect 13 33 41
14. OkamiYKitaharaTHamadaMNaganawaHKondoS 1974 Studies on a new amino acid antibiotic, amiclenomycin. J Antibiot (Tokyo) 27 656 664
15. MannSPlouxO 2006 7,8-Diaminoperlargonic acid aminotransferase from Mycobacterium tuberculosis, a potential therapeutic target. Characterization and inhibition studies. FEBS J 273 4778 4789
16. KitaharaTHottaKYoshidaMOkamiY 1975 Biological studies of amiclenomycin. J Antibiot (Tokyo) 28 215 221
17. SassettiCMRubinEJ 2003 Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A 100 12989 12994
18. RheeKYErdjument-BromageHTempstPNathanCF 2005 S-nitroso proteome of Mycobacterium tuberculosis: Enzymes of intermediary metabolism and antioxidant defense. Proc Natl Acad Sci U S A 102 467 472
19. EhrtSGuoXVHickeyCMRyouMMonteleoneM 2005 Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33 e21
20. KlotzscheMEhrtSSchnappingerD 2009 Improved tetracycline repressors for gene silencing in mycobacteria. Nucleic Acids Res 37 1778 1788
21. HayakawaKOizumiJ 1987 Determination of free biotin in plasma by liquid chromatography with fluorimetric detection. J Chromatogr 413 247 250
22. HartheCClaustratB 2003 A sensitive and practical competitive radioassay for plasma biotin. Ann Clin Biochem 40 259 263
23. KeerJSmeuldersMJGrayKMWilliamsHD 2000 Mutants of Mycobacterium smegmatis impaired in stationary-phase survival. Microbiology 146 Pt 9 2209 2217
24. BoshoffHIDartoisVDickTEhrtS 2009 The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7 845 855
25. KimJHWeiJRWallachJBRobbinsRSRubinEJ 2010 Protein inactivation in mycobacteria by controlled proteolysis and its application to deplete the beta subunit of RNA polymerase. Nucleic Acids Res 39 2210 2220
26. WeiJRKrishnamoorthyVMurphyKKimJHSchnappingerD 2011 Depletion of antibiotic targets has widely varying effects on growth. Proc Natl Acad Sci U S A 108 4176 4181
27. MannSLesageDTabetJ-CMarquetA 2003 Identification of the products of reaction between pyridoxal phosphate and amiclenomycin and other related 1-amino-cyclohexa-2,5-dienes. Tetrahedron 59 5209 5214
28. LuHTongePJ 2010 Drug-target residence time: critical information for lead optimization. Curr Opin Chem Biol 14 467 474
29. PayneDJGwynnMNHolmesDJPomplianoDL 2007 Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6 29 40
30. BardarovSKriakovJCarriereCYuSVaamondeC 1997 Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 94 10961 10966
31. BardarovSBardarovJrSJrPavelkaJrMSJrSambandamurthyVLarsenM 2002 Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148 3007 3017
32. BlumenthalATrujilloCEhrtSSchnappingerD 2010 Simultaneous analysis of multiple Mycobacterium tuberculosis knockdown mutants in vitro and in vivo. PLoS One 5 e15667
33. ShiSEhrtS 2006 Dihydrolipoamide acyltransferase is critical for Mycobacterium tuberculosis pathogenesis. Infect Immun 74 56 63
34. WilsonDJShiCDuckworthBPMurettaJMManjunathaU 2011 A continuous fluorescence displacement assay for BioA: An enzyme involved in biotin biosynthesis. Anal Biochem 416 27 38
35. GandotraSSchnappingerDMonteleoneMHillenWEhrtS 2007 In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med 13 1515 1520
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



