-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
While in Northern hemisphere countries, the pandemic H1N1 virus (H1N1pdm) was introduced outside of the typical influenza season, Southern hemisphere countries experienced a single wave of transmission during their 2009 winter season. This provides a unique opportunity to compare the spread of a single virus in different countries and study the factors influencing its transmission. Here, we estimate and compare transmission characteristics of H1N1pdm for eight Southern hemisphere countries/states: Argentina, Australia, Bolivia, Brazil, Chile, New Zealand, South Africa and Victoria (Australia). Weekly incidence of cases and age-distribution of cumulative cases were extracted from public reports of countries' surveillance systems. Estimates of the reproduction numbers, R0, empirically derived from the country-epidemics' early exponential phase, were positively associated with the proportion of children in the populations (p = 0.004). To explore the role of demography in explaining differences in transmission intensity, we then fitted a dynamic age-structured model of influenza transmission to available incidence data for each country independently, and for all the countries simultaneously. Posterior median estimates of R0 ranged 1.2–1.8 for the country-specific fits, and 1.29–1.47 for the global fits. Corresponding estimates for overall attack-rate were in the range 20–50%. All model fits indicated a significant decrease in susceptibility to infection with age. These results confirm the transmissibility of the 2009 H1N1 pandemic virus was relatively low compared with past pandemics. The pattern of age-dependent susceptibility found confirms that older populations had substantial – though partial - pre-existing immunity, presumably due to exposure to heterologous influenza strains. Our analysis indicates that between-country-differences in transmission were at least partly due to differences in population demography.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002225
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002225Summary
While in Northern hemisphere countries, the pandemic H1N1 virus (H1N1pdm) was introduced outside of the typical influenza season, Southern hemisphere countries experienced a single wave of transmission during their 2009 winter season. This provides a unique opportunity to compare the spread of a single virus in different countries and study the factors influencing its transmission. Here, we estimate and compare transmission characteristics of H1N1pdm for eight Southern hemisphere countries/states: Argentina, Australia, Bolivia, Brazil, Chile, New Zealand, South Africa and Victoria (Australia). Weekly incidence of cases and age-distribution of cumulative cases were extracted from public reports of countries' surveillance systems. Estimates of the reproduction numbers, R0, empirically derived from the country-epidemics' early exponential phase, were positively associated with the proportion of children in the populations (p = 0.004). To explore the role of demography in explaining differences in transmission intensity, we then fitted a dynamic age-structured model of influenza transmission to available incidence data for each country independently, and for all the countries simultaneously. Posterior median estimates of R0 ranged 1.2–1.8 for the country-specific fits, and 1.29–1.47 for the global fits. Corresponding estimates for overall attack-rate were in the range 20–50%. All model fits indicated a significant decrease in susceptibility to infection with age. These results confirm the transmissibility of the 2009 H1N1 pandemic virus was relatively low compared with past pandemics. The pattern of age-dependent susceptibility found confirms that older populations had substantial – though partial - pre-existing immunity, presumably due to exposure to heterologous influenza strains. Our analysis indicates that between-country-differences in transmission were at least partly due to differences in population demography.
Introduction
In late April 2009, the first cases of the novel swine-derived H1N1pdm influenza A virus were detected in Mexico and the United States, prompting the World Health Organization (WHO) to raise the level of influenza pandemic alert to phase 5 [1]. By the end of 2009, the H1N1pdm virus had spread to more than 208 countries, resulting in hundreds of thousands of cases and at least 18000 deaths [2], [3]. Following WHO and Centers for Disease Control and Prevention (CDC) recommendations, generalized media coverage and international mobilization, many countries initiated mitigation measures and enhanced surveillance of H1N1pdm virus infection in humans, providing an abundance of epidemiological data for this epidemic [3], [4]. As a result the H1N1pdm is one of the most documented pandemics with enhanced surveillance established in many regions of the globe, with the exception of Africa [5], [6].
The H1N1pdm virus was introduced into most northern and southern hemisphere countries during the spring and summer of 2009. This period is outside the typical influenza season in temperate countries in the Northern hemisphere, but in the typical winter season for influenza transmission for countries from temperate regions of the Southern Hemisphere. In most Southern hemisphere temperate countries, a full epidemic of H1N1pdm influenza was observed and the pandemic strain quickly became the predominant circulating influenza virus, replacing seasonal strains in many countries [7].
Influenza transmission in a given community may depend on several factors: e.g. climatic characteristics as temperature and humidity [4], [8], [9], virus intrinsic transmissibility, acquired immunity in affected populations, contact patterns in the community, collective and individual measures limiting virus spread [10]. The 2009 H1N1 pandemic was a unique opportunity for comparing the spread of a novel influenza virus in a community setting in different countries with different population structures and contact patterns. In this context, countries from temperate regions of the Southern Hemisphere, which present different demographic patterns and experienced the virus during their usual winter season, present an opportunity to evaluate the impact of these characteristics on transmission.
Here we use mathematical modelling to assess the transmission characteristics of H1N1pdm virus using epidemiological data from Southern hemisphere countries in temperate regions. We address the question of the origins of the observed differences between countries by investigating the role of seasonality (with latitude used as a proxy), population density and population demography (with proportion of children used as a proxy). We then explore more precisely the contributions of demography in the spread of the disease by fitting different transmission models to the set of countries.
Material and Methods
H1N1 influenza data
The epidemiological data analysed here were weekly case incidence of laboratory-confirmed H1N1pdm or influenza-like-illness (ILI) and the distribution of cumulative incidence by age-group over the study period for seven Southern hemisphere countries and one state (Argentina, Australia -whole country and Victoria-, Bolivia, Brazil, Chile, New Zealand and South Africa). The data were extracted from websites or public reports issued by the countries surveillance systems. Country datasets and corresponding sources are described and listed in Table 1. Neither daily case incidence nor age-stratified weekly case incidence data were available. Depending on the country, weekly incidence data were either laboratory confirmed H1N1pdm cases (H1N1CC) (Argentina, Australia, Bolivia, Brazil, Chile, New Zealand, South Africa) or influenza-like-illness (ILI) (Australia, Chile, New Zealand, Victoria). All available datasets were used in the analysis, even when multiple datasets were available for a given country.
Tab. 1. Summary of epidemiological data. 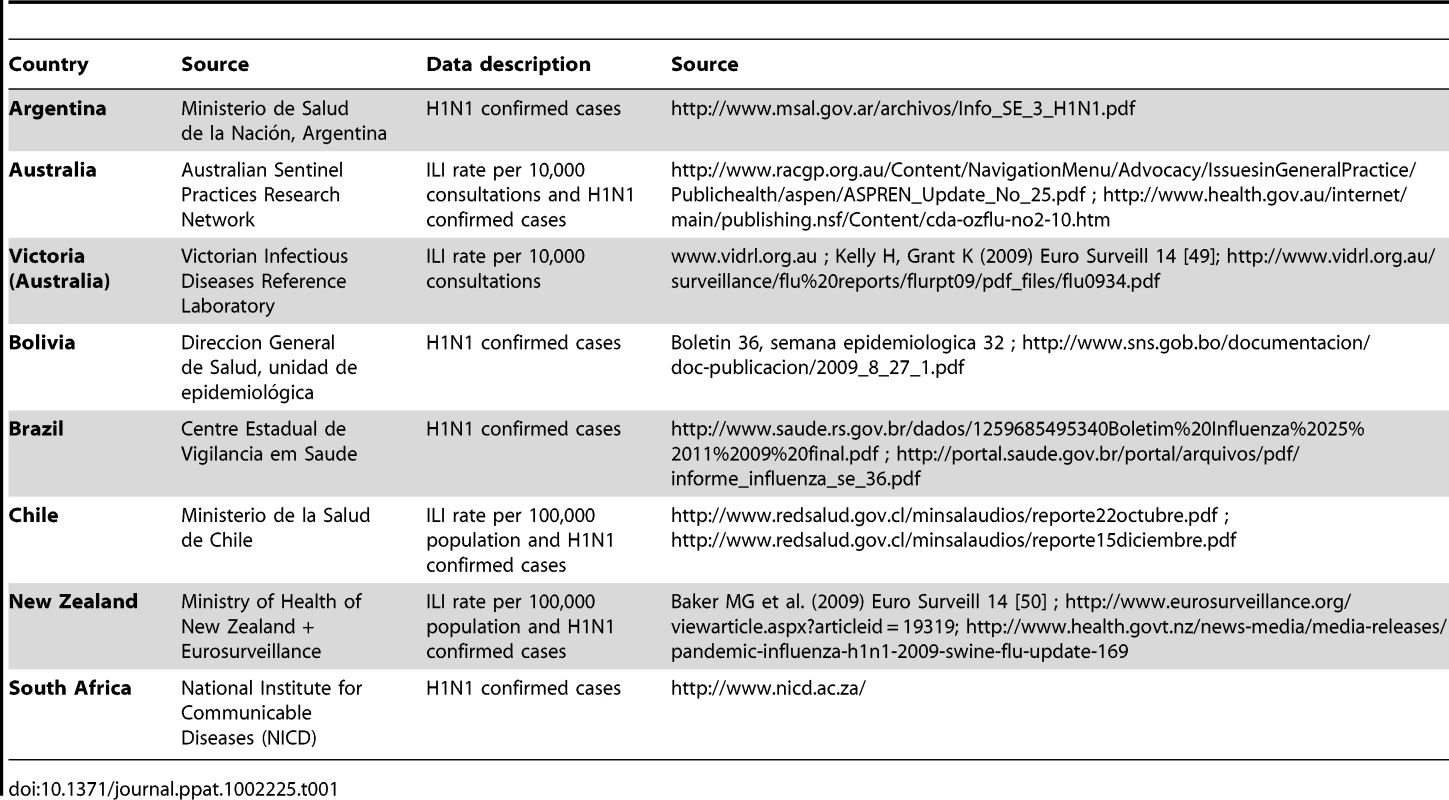
Cumulative distributions of cases by age were extracted from the same data sources (Table 1). These were generally of H1N1pdm confirmed cases, except for Australia and New Zealand, where we used the age distribution of ILI cases.
Due to differences between countries in the age stratification of available H1N1pdm data, country-associated age-groups were broken down into the following: Argentina (0–5, 5–19, 20–49, 50–59, ≥60 years old); Australia (0–5, 5–19, 20–49, 50–64, ≥65 years old); Victoria (0–5, 5–19, 20–49, 50–64, ≥65 years old); Bolivia (0–5, 5–19, 20–44, 45–49, ≥50 years old); Brazil (0–5, 5–14, 15–49, 50–59, ≥60 years old); Chile (0–5, 5–14, 15–54, 55–64, ≥65 years old); New Zealand (0–5, 5–19, 20–49, 50–59, ≥60 years old); South Africa (0–5, 5–19, 20–49, 50–64, ≥65 years old).
Demographic data
Demographic information was extracted from census data of the national statistics institute of the corresponding countries (data are presented in details and electronic URL for sources are listed in Table S1 in Text S1).
Model
A deterministic model was constructed to describe the spread of the virus in a population structured by age-groups. Model parameters and their values are summarized in Table 2.
Tab. 2. List of model parameters and their values. 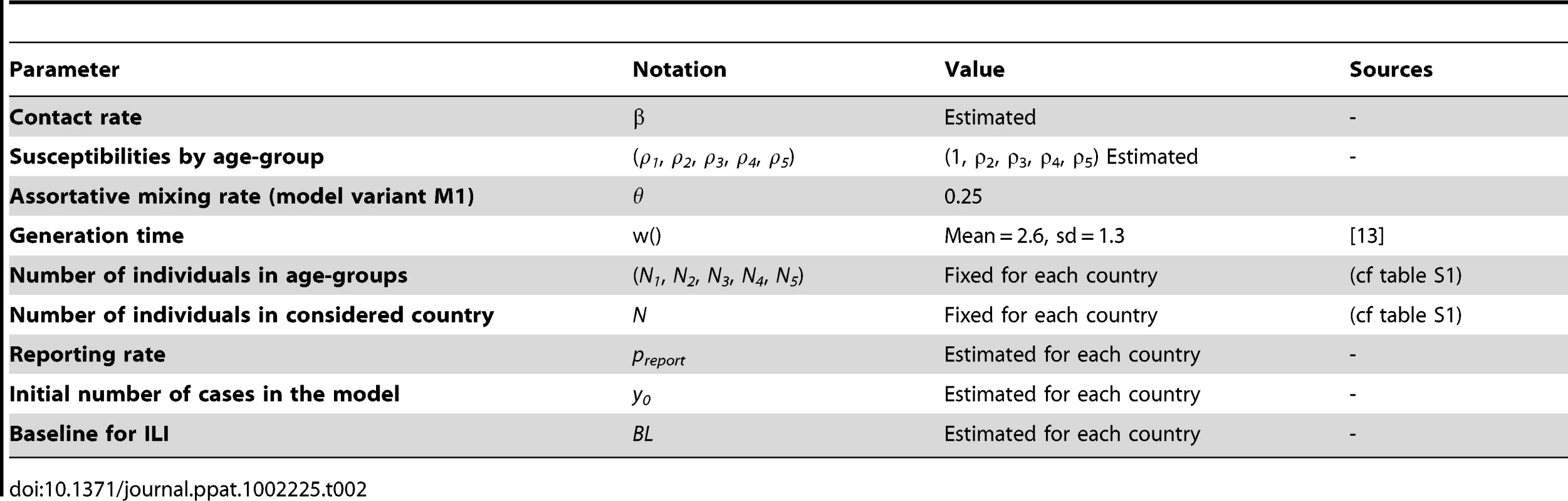
Five age-groups were defined in the model (NA = 5): young children, children, young adults, adults, older adults (with breakdowns as defined above). Population structure was described by the vector Ni, with Ni representing the number of individuals in age-group i. Total population size was noted NP.
Individuals in the population were assumed to be either susceptible, infected or recovered (classical SIR model). Each age group of the population was initialized with y0 (a fitted parameter) infections at the beginning of the simulation (ten weeks before the first week of observation). The model incorporated heterogeneous mixing by age, with a variety of mixing patterns being explored (more details are presented below and in section 1 of Text S1). The parameter β defined the transmission coefficient. Susceptibility to infection was hypothesized to vary with age and given by the vector ρi . To avoid confounding with the parameter β, the susceptibility of young children was fixed at 1 (ρ1 = 1) and the susceptibility of other groups was estimated. Therefore, for a given individual of age i, the risk of infection per contact with an infected individual is given by βρi.
The generation time was assumed to be Gamma distributed [11], [12] with mean µ = 2.6 days and standard deviation σ = 1.3 days [13]. Although some previous studies have suggested that children infected with influenza may be more infectious than adults, there was no evidence of any significant age-specific transmission risk of H1N1pdm [13], [14]. Consequently, no age-specific infectiousness was considered in the model.
We also assumed that only a proportion of infected individuals were effectively reported to the surveillance system, represented in the model by a reporting rate preport (underreporting included here both unreported symptomatic cases and asymptomatic cases). No incubation period or reporting delay was considered, since so long as the generation time distribution is captured accurately, ignoring these factors does not affect transmission parameter estimates.
We finally assumed that ILI surveillance data included a constant incidence of non-influenza related cases (baseline), defined as BL.
Technical details of the model can be found in section 1 of Text S1.
Basic reproduction number and infection attack rate
The basic reproduction number of the virus spread, R0, was computed as the largest eigenvalue of the next generation matrix K of the model. The next generation matrix defines the next generation of new infected from a previous generation of infected [15] with element Ki,j representing the expected number of new cases from age-group i generated by one infected individual of age-group j. K was defined as:with β being the contact rate, ρ the susceptibilities and M the mixing matrix among age-groups, defined as the proportion of contacts an infected individual in age class j makes with individuals in age class i.
The infection attack rate pI was defined as the proportion of individuals in the population having been infected after the epidemic ends.
Parameter estimation
Parameters of the dynamic model were estimated in a likelihood-based Bayesian setting using Markov Chain Monte Carlo (MCMC) methods with a Metropolis Hastings sampler to explore the space of parameters. The posterior median and 95% credible interval were reported for each parameter. See Text S1 for more details.
Initially, parameters were estimated for each country independently (country-specific fits). In order to better understand the role of demography on H1N1pdm spread, estimation was also run for all the countries together (global fits).
Model variants
We defined three model variants which differed in the assumption made on mixing patterns between age-groups. In the first two models, assortative mixing between age groups was assumed [16]. For a given age group, individuals had a proportion of their contacts θ occurring in their own age-group, with the remaining 1-θ fraction of contacts occurring at random in the whole population. Model variant one (M1) involved a simple assortative mixing in which individuals mixed preferentially in their own age-group (with fixed probability θ = 0.25) and randomly with all age-groups with probability (1-θ). Although higher values for assortative parameter were proposed in previous studies [16], θ = 0.25 was chosen as it was consistent with mixing patterns measured in the UK via diary studies [17].
Model variant two (M2) involved a more elaborate description of mixing. Three different assortativity parameters were defined: θ1 = 0.15 for young children; θ2 = 0.4 for older children; and θ3 = 0.14 for adults. The numerical values were estimated by fitting the mixing matrix to the mixing patterns measured in the UK [17].
For M1 and M2, the contact rate parameter (β) was assumed to be common to all age-groups. Given that contact rates vary among age-groups [17], this means that the estimates of age-dependent susceptibility obtained for these model variants also implicitly incorporate variation in contact rates as well as actual variation in susceptibility arising from pre-existing immunity.
Model variant three (M3) differed from M1 and M2 as it used an empirical contact matrix. The matrix was derived from the POLYMOD study data published for casual contacts in United Kingdom [17]. In order to derive appropriate matrices for each of the studied countries, two assumptions were made. First, we assumed that in a country in which a given age-group is more prevalent than in the UK, any individual will have a higher proportion of his contacts appearing in this age-group than individuals from the same age-group in the UK. Second, we assumed that contact rates varied between age-groups but were constant across countries (see supplementary material).
Model parameters and their values (if these were not fitted) are listed in table 2.
Model fitting
Firstly, we fitted model variant M1 to weekly case incidence data and to the cumulative age distribution of cases for each country independently, using a negative binomial likelihood with fitted variance parameter (to allow for over-dispersion in the case data). For each country, nine parameters were estimated: reporting rate (preport), four age-related susceptibilities (ρi)i = 2..5, dispersion parameter for the negative binomial likelihood, baseline for ILI incidence in the sample population (BL), initial number of cases at the beginning of the simulation (y0) and reproduction number (R0).
Secondly, to assess the extent to which a single model could explain the patterns seen in different countries' epidemics, we fitted model variants M1 to M3 to all the countries simultaneously, keeping most parameters common to all countries. For these global fits, susceptibilities by age and contact rate were assumed to be common to all the locations (five global parameters) whereas reporting rate (preport), ILI incidence baseline (BL), and the initial number of cases (y0) were fitted on a country-specific basis (four country-specific parameters).
Further details of the models and fitting procedures are given in the supplementary material. MCMC methods were used to obtain parameter estimates. For the country-specific fits, MCMC samples of 3×106 were generated for each country with the first 100000 iterations discarded to allow the chain to converge. For the global fits equilibration of the MCMC chains was slower, so we generated samples of 6×106 and discarded the first 2×106 of these.
Descriptive statistical analysis of factors influencing R0
In order to assess which factors could influence the spread of the virus in the different countries, the R0 estimates were regressed on countries demographic age-distribution, latitude of the capital city (except for South Africa where the biggest city was considered) and densities of populations (see supplementary material). This analysis was conducted for two different set of R0 estimates: the R0 values estimated from the exponential growth of confirmed cases in the early weeks of the epidemic in each country, using the renewal equation [11], [12] (supplementary material) and the median posterior estimates from the country-specific fits. H1N1 confirmed cases were used for those countries where such data was available and ILI data was used for the one area (Victoria) where such data were not available.
Results
The 2009 H1N1pdm influenza in the Southern hemisphere
With the exception of South Africa, the H1N1pdm epidemic started at the end of May (epidemiological weeks [EW] 20–22) and finished by the end of September (around EW 40). South Africa experienced a first wave of seasonal H3N2 influenza followed by H1N1pdm influenza peaking in early August 2009 [6] (Table 1)(Figures 1 and 2).
Fig. 1. Surveillance data and model estimates for weekly incidence of cases. 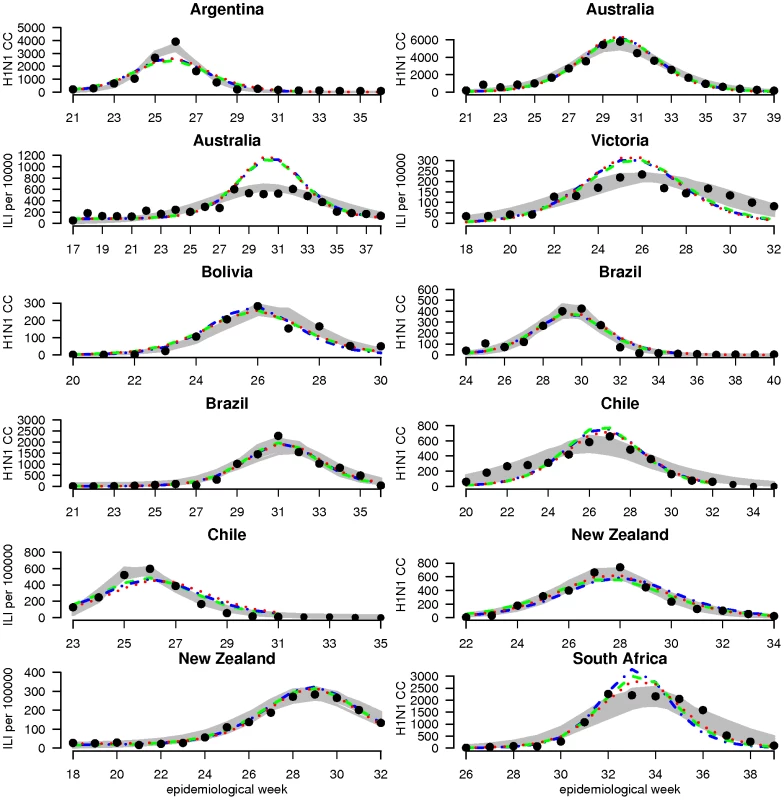
For each country, graphs show observed case incidence from surveillance data (black points), the 95% credibility region on incidence from the country-specific fits (grey region) and predicted incidence for the posterior median set of parameters obtained from the global fits (dashed lines) for model variants M1 (blue), M2 (green) and M3 (red). Weekly incidence from the models is plotted in all cases, with lines being drawn between weeks for visual clarity. Depending on the country, observed case incidence are either confirmed H1N1pdm cases (H1N1CC) or influenza like illness rate (ILI) - showing ILI rate per 100,000 population for Chile and New Zealand and ILI rate per 10,000 consultations for Australia and Victoria. Fig. 2. Surveillance data and model estimates for the age-distribution of cases. 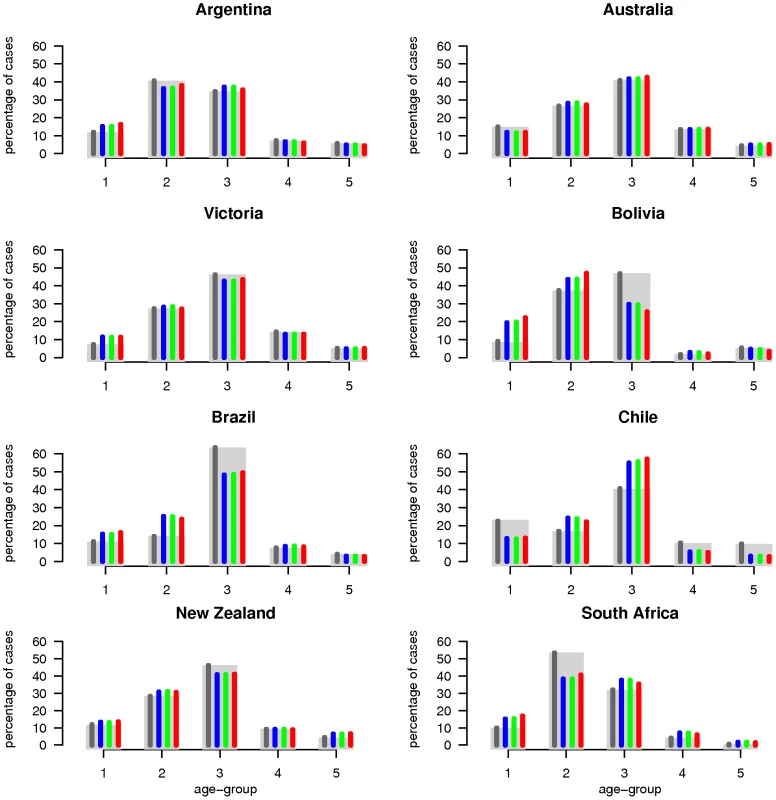
Observed cumulative cases distribution among age-groups (grey rectangles) and model median posterior estimates (coloured thin bars). The dark grey bars correspond to country-specific fits, whereas blue, green and red bars represent the results for M1, M2 and M3 model variants of the global model, respectively. Cumulative age-specific incidence is summarized in Table S1 of Text S1, as well as demographic data and sources.
Estimated empirical R0-values derived from the early exponential growth rate of the epidemic were positively correlated with the proportion of children in the population (p = 0.004) as illustrated in figure 3a. No significant association was found with latitude and density (supplementary material).
Fig. 3. Reproduction numbers. 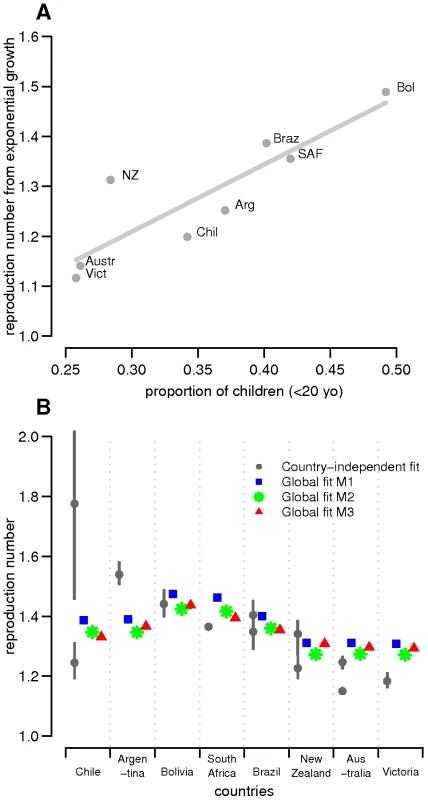
(A) Estimated empirical R0-values derived from the early exponential growth rate of the epidemic versus proportion of children in the eight studied countries/states. R0-values estimated from data on H1N1 confirmed cases were used in the regression analysis except for Victoria for which only ILI data was available. (B) Distribution of estimated reproduction numbers by country obtained in country-specific and global fits. For each country, the posterior median estimates of R0 for country-specific and global fits are plotted with 95% credible intervals. The grey circles correspond to country-specific estimates, whereas blue squares, green stars and red triangles represent estimates for M1, M2 and M3 model variants of the global fits, respectively. For those countries where two datasets were available, the two estimates are plotted. For the global fits, because R0 differences among countries derived from population demography only, fitting resulted in one estimate only even when both ILI and confirmed case data were available. Country-specific estimates
Estimates of R0, attack rate and reporting rate are summarized in Table 3. For each country and dataset, Figure 1 compares the fits of the model (grey lines) with the H1N1pdm incidence data. The match to the age distribution of cases is shown in Figure 2, and estimates of R0 for the 8 countries are plotted in Figure 3B. Estimated posterior median values of R0 ranged from 1.2 and 1.8, with the highest values (1.5 and 1.8 respectively) being obtained from for Argentina and Chile (though for Chile, only the ILI data gave a high estimate). We found estimated age-related susceptibilities to vary markedly by country. With the exception of Bolivia and Brazil, a consistent pattern of decreasing susceptibility with age and higher susceptibility for children under 20 was found (Figure 4).
Fig. 4. Estimated age-dependent susceptibilities. 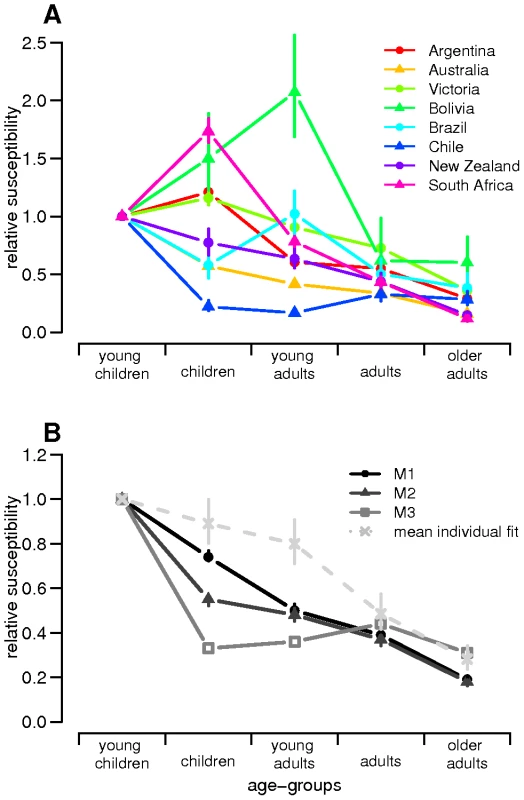
Estimated susceptibilities (posterior median with 95% credible intervals) are plotted according to age in the 8 countries/states for (A) country-specific fits and (B) global fits (M1, M2 and M3). Tab. 3. Estimated parameters for country-specific model (median posterior with 95% credible interval indicated in parenthesis). 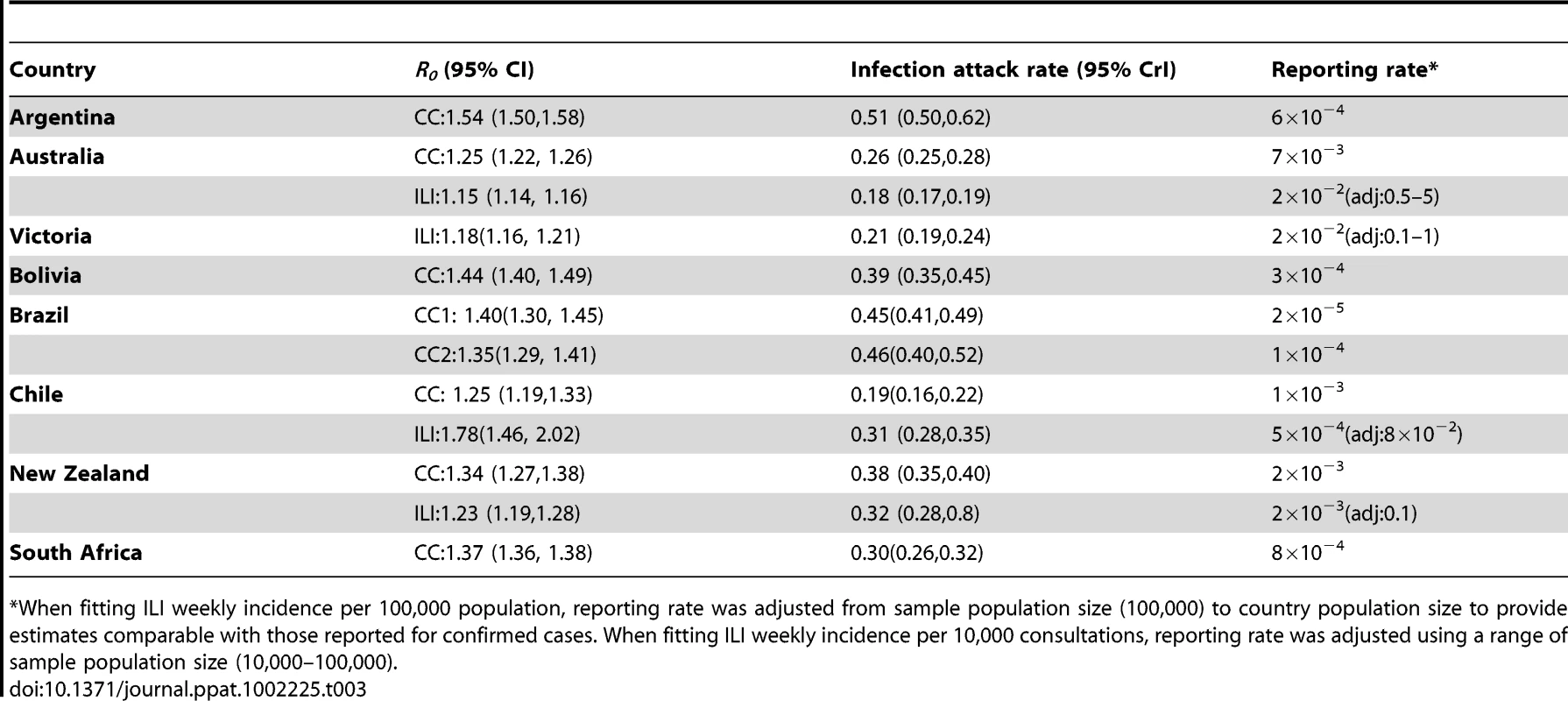
*When fitting ILI weekly incidence per 100,000 population, reporting rate was adjusted from sample population size (100,000) to country population size to provide estimates comparable with those reported for confirmed cases. When fitting ILI weekly incidence per 10,000 consultations, reporting rate was adjusted using a range of sample population size (10,000–100,000). We obtained estimated posterior median infection attack rates of between 20% and 50% of the population (Table 3). These values also varied markedly from one country to another: from 20% for Australia to 40% for Argentina and Brazil.
Global estimates
Common and country specific parameter estimates from the fits of the global model are summarized for model variants M1-M3 in Table 4, while fit quality to the incidence time series is illustrated in Figures 1 and 2. Overall, the global fits reproduce temporal and age trends in the surveillance data well, albeit not as precisely as the fits of the country-specific model (see section 6 of Text S1 for evaluation of model fitting). Peak incidences were slightly underestimated for Argentina, Chile-ILI and New Zealand-H1N1CC and overestimated for Australia-ILI, Victoria, Chile-H1N1CC and South Africa. Likelihood comparison did not allow one of the 3 model variants examined to be identified as superior (section 6 of Text S1). The global fits well reproduced the age distribution of cases for Argentina, Australia, Victoria and New Zealand, although the contribution of adult cases were underestimated for Bolivia and Brazil, and overestimated for South Africa and Chile (Figure 2). Resulting R0 estimates were similar for the three model variants, with still significant (albeit much reduced compared with the country-specific model) variation between countries: the highest values were obtained for South Africa and Bolivia and the lowest ones for New Zealand, Australia and Victoria (Figure 3B).
Tab. 4. Estimated parameters for global model variants. 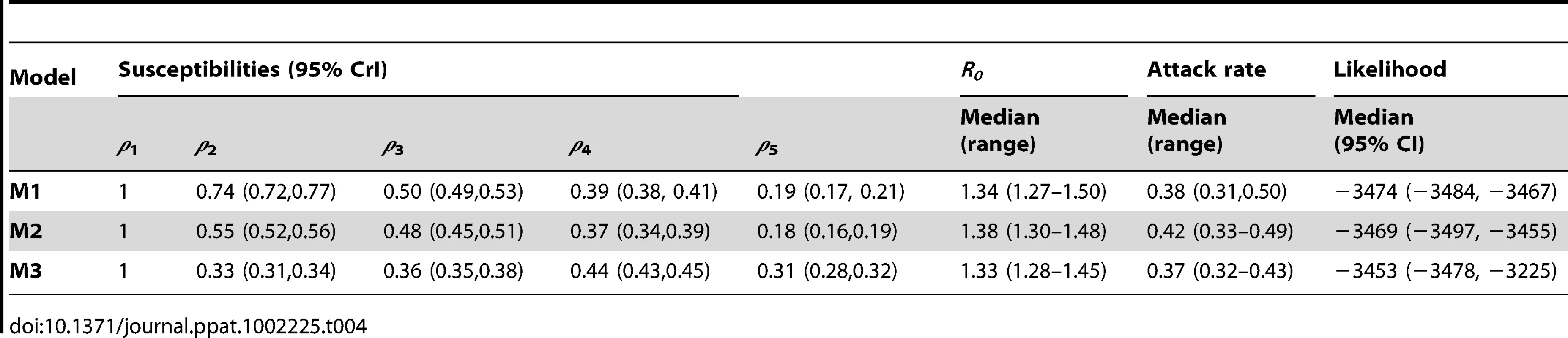
Lastly, age-dependent susceptibilities to H1N1pdm were still found to decrease with age (Figure 4B). This effect was higher in model M1 and M2 suggesting that children had both higher susceptibility to the virus and higher numbers of contacts. Estimates from model M3 also suggested that resulting differences in relative susceptibilities among adult age-groups might largely be due to variation in contacts rates between these age-groups.
Only two country-specific parameters were fitted for the global fits: the initial number of cases (y0) and the reporting rate (preport). As y0, and preport mainly influence epidemic timing and the scaling required to match surveillance incidence data, the variation in R0 seen between countries and the qualitatively good fits obtained support the idea that demographic differences between countries may have had a substantial impact on H1N1pdm transmission.
Discussion
Our results suggest transmission of H1N1pdm in 2009 varied significantly between the eight countries/states included in our analysis. Differences were found in transmissibility (R0 median estimates ranged between 1.2 and 1.8) and in the size of the epidemic (estimated median infection attack rates ranging 20–50%).
Estimates of R0 are relatively low compared with previous estimates from past pandemics, for which values in the range 1.7–2.2 have been more typical [18]–[24], though it should be noted that some of the higher values of R0 obtained for previous pandemics assumed a longer mean generation time than we do here. Our estimates are comparable to typical flu seasons (R0∼1.3) [25] and consistent with other studies for H1N1pdm in 2009 obtained from other countries [26]–[30].
Our results further reinforce existing evidence that children (<20 years old) were substantially more susceptible to infection with H1N1pdm than adults [31]–[33], with adults having 30–80% the susceptibility of children, depending on the model variant examined. The country-specific fits led to differences in susceptibility estimates among countries, maybe indicating that some over-specification exists in the country-specific model. However, this might also suggest that levels of prior existing immunity differ among the studied populations, which has been documented in some countries [31], [34], [35], playing a role in the variation in patterns of H1N1pdm spread observed. If real, such differences in pre-existing population immunity may have contributed to the unexplained variance of the global fits relative to the country-specific fits. It should be noted that models M1 and M2 assumed simple assortative mixing by age with no age-dependent variation in contact rates, so that estimates of age-dependent susceptibility may be confounded with variation in contact rates with age. Model M3 used data from a diary survey of contact patterns [17] and thus incorporated higher contact rates in children, and the resulting estimated differences in susceptibility between adults and children were therefore lower for that model variant. In addition, in a context of high media coverage and public concern, it is possible that cases in children might have been more likely to lead to health-care seeking behaviour, affecting estimates.
Nevertheless, our finding that susceptibility decreased with age is consistent with recent serological study results which demonstrated a significant proportion of immune adults prior to the start of the 2009 H1N1 epidemics [31], [34], [35]. Age-dependent susceptibility might arise from the effect of immune system maturation or cross-reactive immunity due to prior infections with other (non H1N1pdm) influenza subtypes/strains. In a completely naive population, the reproduction number would therefore be expected to be substantially larger. The lack of serological data during the pandemic prevented explicit incorporation of pre-existing immunity in the model [36], though age-dependent susceptibility implicitly represents its effects. Sensitivity analyses in which we assumed pre-existing immunity at the beginning of the pandemic suggested including immunity would substantially affect our estimates of R0 (given the estimates provided here are implicitly in the presence of substantial pre-existing immunity) , but also of attack rate.
Although H1N1pdm was a new virus, our results further reinforce the evidence base that there was substantial pre-existing partial cross-immunity to the virus prior to the 2009 epidemic, particularly in adults. Cross-immunity, an important feature of seasonal influenza epidemiology, was not expected to play such a key role in a pandemic situation. Clearly the experience of H1N1 in 2009 has highlighted the need for more research – both experimental and theoretical - on heterosubtypic immunity (and perhaps non-HA mediated immunity).
Pre-existing immunity impeded the estimation of the classic basic reproduction number (R0) from the data examined here. Our R0 estimates are really estimates for R[0], the reproduction number at the beginning of the epidemic (at time 0), rather than for the reproduction number in the absence of prior immunity. However, for ease of notation (and because one might argue that transmission may never occur in a truly immunologically naïve population), we have chosen still to refer to the reproduction number of the 2009 virus at the start of each country's epidemic as R0.
Each of the three tested mixing matrices was clearly a simplification of the true mixing patterns that might be observed in the studied countries. M1 and M2 assumed a simple assortativity model (moderate preference for mixing preferentially within one's own age group). The value of 0.25 assumed for the assortativity parameter is broadly consistent with the levels of assortativity seen in the mixing matrices provided by the UK POLYMOD survey [17]. However, in order to test whether this choice influenced the estimates, we undertook a sensitivity analysis and looked at values in the range 0–0.5. This indicated that neither reproduction numbers nor susceptibility estimates were strongly affected by varyingθ.
The models presented here were intentionally parsimonious. Our aim was to compare in the simplest way possible the initial epidemic of a novel influenza in different countries. The models developed here cannot generate multiple waves of transmission, and do not capture potentially important behavioural changes that may have affected transmission and disease surveillance during the pandemic [37]–[39], such as early risk avoidance and higher rates of health-care seeking behaviour early in the pandemic. In addition we did not allow for the potential impact of school holidays and seasonal climate variation on transmission [40]–[42], which may have improved the models fits. Lastly, only local transmission was considered here. Imported cases were not considered in the model as one would expect importations to be a substantial proportion of cases only in the first weeks before the epidemic starts and that the transmission would thereafter be predominantly local. However, by exploring multiple model variants we have demonstrated that estimates of R0 and attack rates are largely robust to uncertainty in the parameterisation of age-specific mixing patterns in the population.
The differences in pandemic surveillance [43] in the countries considered may be the most influential factor affecting the reliability of our estimates and the variation found between countries. Surveillance to detect virologically confirmed cases of influenza was likely to have been highly non-systematic in several countries and variable throughout the pandemic, meaning the relationship between measured incidence and true incidence of infection may have been highly non-linear. In particular, many countries which initially undertook highly intensive case finding in 2009 moved to less intensive surveillance once case numbers grew too large for routine virological testing to be undertaken. Syndromic surveillance of ILI, by comparison, is typically more systematic but suffers from ILI being non-specific for influenza. All surveillance systems were subject to the effects of changes in health-care seeking behaviour over time. While we estimate the proportion of infections appearing in surveillance incidence data (the reporting rate), we did not have the statistical power to do anything other than assume that reporting rates were constant over time.
Perhaps the most interesting aspect of our results is that demographic differences between countries may have contributed strongly to the differences in observed H1N1pdm spread. In particular, we found countries with higher proportions of children (under 20) had higher estimated R0 values and attack rates. Fits for the global models with shared parameters between countries are clearly poorer than the country-specific fits, but nevertheless capture much of the country to country variation. That said, fit quality for Argentina and for South Africa may indicate other factors playing a role in determining the observed patterns of transmission (or alternatively may result from imperfections in surveillance). Several other factors have been demonstrated to impact the Influenza virus transmission, notably seasonal climatic variations, such as absolute humidity and temperature [8], [44]. Although the countries examined here have substantial geographical differences between them (e.g. capital city latitudes between 15°S and 41°S and mean population densities between 3 and 24/km2), no significant association between estimated R0 and latitude or densities of populations were found (Section 8 and Figure S8 in Supplementary material). More generally, our estimates of reproduction numbers did not differ strongly from those obtained from analyses of the spring/summer wave in countries from the Northern Hemisphere (US, Mexico and UK) [16], [27], [45], suggesting a limited impact of seasonal variation in H1N1pdm transmissibility. Prior immunity could also explain differences between countries as pointed out by recent serological surveys showing that immunity to H1N1pdm varied by country of tested individuals [31], [34], [35], [46]–[48] .
Results presented here suggest there may be country-to-country differences in epidemiology (driven in part by demographic variation, but not entirely so), suggesting some need to allow for appropriate modification of control policies on a country by country basis. In particular, targeting vaccination at children may be more optimal for countries with populations with a high proportion of school-age children. They also support the importance of developing accurate age-structured models for the analysis of influenza epidemics and the potential benefit of extending real time data collection by age-group, on serology and/or reporting rate.
To conclude, this study is one of the first attempts to gain insight into the dynamics of disease transmission via inter-country comparison. Our analysis has shown that, although differences in spread of H1N1pdm were observed during the Southern hemisphere winter wave, many features of transmission were shared between countries and could be explained with largely common parameters for all countries. We showed that differences between countries could be partially explained by differences in population demography. Our results confirm that susceptibility to the virus decreased with age but also that higher contact rates in children may have partly shaped the way H1N1pdm influenza spread in 2009.
Supporting Information
Zdroje
1. World Health Organization 2009 Statement by WHO Director-General, Dr Margaret Chan. Available: http://www.who.int/mediacentre/news/statements/2009/h1n1_20090429/en/index.html
2. World Health Organization 2009 Pandemic (H1N1) 2009 - weekly update 81. Available: http://www.who.int/csr/don/2009_12_30/en/index.html
3. Center for Disease Control and Prevention 2010 Updated CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009 – April 10, 2010. Available: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm
4. World Health Organization 2009 Human infection with pandemic (H1N1) 2009 virus: updated interim WHO guidance on global surveillance. 10 July 2009. Available: http://www.who.int/csr/resources/publications/swineflu/interim_guidance/en/index.html
5. YazdanbakhshMKremsnerPG 2009 Influenza in Africa. PLoS Med 6 e1000182
6. Van KerkhoveMDMountsAMallSVandemaeleKChamberlandM 2011 Epidemiologic and Virologic Assessment of the 2009 Influenza A (H1N1) Pandemic on Selected Temperate Countries in the Southern Hemisphere: Argentina, Australia, Chile, New Zealand and South Africa. Influenza Other Respi Viruses 5 10.1111/j.1750-2659.2011.00249.x
7. World Health Organization 2009 Pandemic (H1N1) 2009 - update 61 - Epidemiological Update on the Global Situation and Preliminary Overview of the Southern Hemisphere Winter Influenza Season (as of 6 August 2009). Available: http://www.who.int/csr/don/2009_08_12/en/index.html
8. ShamanJKohnM 2009 Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A 106 3243 3248
9. du PrelJBPuppeWGrondahlBKnufMWeiglJA 2009 Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis 49 861 868
10. LipsitchMViboudC 2009 Influenza seasonality: lifting the fog. Proc Natl Acad Sci U S A 106 3645 3646
11. WallingaJLipsitchM 2007 How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci 274 599 604
12. GrasslyNCFraserC 2008 Mathematical models of infectious disease transmission. Nat Rev Microbiol 6 477 487
13. CauchemezSDonnellyCAReedCGhaniACFraserC 2009 Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med 361 2619 2627
14. CauchemezSBhattaraiAMarchbanksTLFaganRPOstroffS 2011 Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci U S A 108 2825 2830
15. DiekmannOHeesterbeekJARobertsMG 2010 The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 7 873 885
16. FraserCDonnellyCACauchemezSHanageWPVan KerkhoveMD 2009 Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324 1557 1561
17. MossongJHensNJitMBeutelsPAuranenK 2008 Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 5 e74
18. SertsouGWilsonNBakerMNelsonPRobertsMG 2006 Key transmission parameters of an institutional outbreak during the 1918 influenza pandemic estimated by mathematical modelling. Theor Biol Med Model 3 38
19. MillsCERobinsJMLipsitchM 2004 Transmissibility of 1918 pandemic influenza. Nature 432 904 906
20. ChowellGAmmonCEHengartnerNWHymanJM 2006 Estimation of the reproductive number of the Spanish flu epidemic in Geneva, Switzerland. Vaccine 24 6747 6750
21. CooperBSPitmanRJEdmundsWJGayNJ 2006 Delaying the international spread of pandemic influenza. PLoS Med 3 e212
22. GaniRHughesHFlemingDGriffinTMedlockJ 2005 Potential impact of antiviral drug use during influenza pandemic. Emerg Infect Dis 11 1355 1362
23. FergusonNMCummingsDACauchemezSFraserCRileyS 2005 Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 437 209 214
24. FergusonNMCummingsDAFraserCCajkaJCCooleyPC 2006 Strategies for mitigating an influenza pandemic. Nature 442 448 452
25. ChowellGMillerMAViboudC 2008 Seasonal influenza in the United States, France, and Australia: transmission and prospects for control. Epidemiol Infect 136 852 864
26. HsiehYH 2010 Pandemic influenza A (H1N1) during winter influenza season in the southern hemisphere. Influenza Other Respi Viruses 4 187 197
27. LesslerJSantosTDAguileraXBrookmeyerRCummingsDA 2010 H1N1pdm in the Americas. Epidemics 2 132 138
28. PaineSMercerGNKellyPMBandaranayakeDBakerMG 2010 Transmissibility of 2009 pandemic influenza A(H1N1) in New Zealand: effective reproduction number and influence of age, ethnicity and importations. Euro Surveill 15 19591
29. KellyHAMercerGNFieldingJEDowseGKGlassK 2010 Pandemic (H1N1) 2009 influenza community transmission was established in one Australian state when the virus was first identified in North America. PLoS One 5 e11341
30. PedroniEGarciaMEspinolaVGuerreroAGonzalezC 2010 Outbreak of 2009 pandemic influenza A(H1N1), Los Lagos, Chile, April-June 2009. Euro Surveill 15 19456
31. MillerEHoschlerKHardelidPStanfordEAndrewsN 2010 Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 375 1100 1108
32. DelangueJSalezNNinoveLKiefferAZandottiC Serological study of the 2009 pandemic due to influenza A H1N1 in the metropolitan French population. Clin Microbiol Infect 10.1111/j.1469-0691.2011.03545.x
33. XuCBaiTIulianoADWangMYangL 2011 The Seroprevalence of Pandemic Influenza H1N1 (2009) Virus in China. PLoS One 6 e17919
34. IkonenNStrengellMKinnunenLOsterlundPPirhonenJ 2010 High frequency of cross-reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill 15 19478
35. HancockKVeguillaVLuXZhongWButlerEN 2009 Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med DOI:10.1056/NEJMoa0906453
36. Van KerkhoveMDAsikainenTBeckerNGBjorgeSDesenclosJC 2010 Studies needed to address public health challenges of the 2009 H1N1 influenza pandemic: insights from modeling. PLoS Med 7 e1000275
37. CowlingBJNgDMIpDKLiaoQLamWW 2010 Community psychological and behavioral responses through the first wave of the 2009 influenza A(H1N1) pandemic in Hong Kong. J Infect Dis 202 867 876
38. LauJTGriffithsSChoiKCTsuiHY 2010 Avoidance behaviors and negative psychological responses in the general population in the initial stage of the H1N1 pandemic in Hong Kong. BMC Infect Dis 10 139
39. IbukaYChapmanGBMeyersLALiMGalvaniAP 2010 The dynamics of risk perceptions and precautionary behavior in response to 2009 (H1N1) pandemic influenza. BMC Infect Dis 10 296
40. SloanCMooreMLHartertT 2011 Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci 4 48 54
41. ShamanJGoldsteinELipsitchM 2011 Absolute humidity and pandemic versus epidemic influenza. Am J Epidemiol 173 127 135
42. CauchemezSValleronAJBoellePYFlahaultAFergusonNM 2008 Estimating the impact of school closure on influenza transmission from Sentinel data. Nature 452 750 754
43. BriandSMountsAChamberlandM 2011 Challenges of global surveillance during an influenza pandemic. Public Health 125 247 256
44. ShamanJPitzerVEViboudCGrenfellBTLipsitchM 2010 Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol 8 e1000316
45. GhaniACBaguelinMGriffinJFlascheSPebodyR 2009 The Early Transmission Dynamics of H1N1pdm Influenza in the United Kingdom. PLoS Curr 1 RRN1130
46. TsaiTFPedottiPHilbertALindertKHohenbokenM 2010 Regional and age-specific patterns of pandemic H1N1 influenza virus seroprevalence inferred from vaccine clinical trials, August-October 2009. Euro Surveill 15 19624
47. ZimmerSMCrevarCJCarterDMStarkJHGilesBM 2010 Seroprevalence following the second wave of Pandemic 2009 H1N1 influenza in Pittsburgh, PA, USA. PLoS One 5 e11601
48. GilbertGLCretikosMAHuestonLDoukasGO'TooleB 2010 Influenza A (H1N1) 2009 antibodies in residents of New South Wales, Australia, after the first pandemic wave in the 2009 southern hemisphere winter. PLoS One 5 e12562
49. KellyHGrantK 2009 Interim analysis of pandemic influenza (H1N1) 2009 in Australia: surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill 14 19288
50. BakerMGWilsonNHuangQSPaineSLopezL 2009 Pandemic influenza A(H1N1)v in New Zealand: the experience from April to August 2009. Euro Surveill 14 19319
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



