-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTri6 Is a Global Transcription Regulator in the Phytopathogen
In F.
graminearum, the transcriptional regulator Tri6 is encoded within the trichothecene gene cluster and regulates genes involved in the biosynthesis of the secondary metabolite deoxynivalenol (DON). The Tri6 protein with its Cys2His2 zinc-finger may also conform to the class of global transcription regulators. This class of global transcriptional regulators mediate various environmental cues and generally responds to the demands of cellular metabolism. To address this issue directly, we sought to find gene targets of Tri6 in F. graminearum grown in optimal nutrient conditions. Chromatin immunoprecipitation followed by Illumina sequencing (ChIP-Seq) revealed that in addition to identifying six genes within the trichothecene gene cluster, Tri1, Tri3, Tri6, Tri7, Tri12 and Tri14, the ChIP-Seq also identified 192 additional targets potentially regulated by Tri6. Functional classification revealed that, among the annotated genes, ∼40% are associated with cellular metabolism and transport and the rest of the target genes fall into the category of signal transduction and gene expression regulation. ChIP-Seq data also revealed Tri6 has the highest affinity toward its own promoter, suggesting that this gene could be subject to self-regulation. Electro mobility shift assays (EMSA) performed on the promoter of Tri6 with purified Tri6 protein identified a minimum binding motif of GTGA repeats as a consensus sequence. Finally, expression profiling of F. graminearum grown under nitrogen-limiting conditions revealed that 49 out of 198 target genes are differentially regulated by Tri6. The identification of potential new targets together with deciphering novel binding sites for Tri6, casts new light into the role of this transcriptional regulator in the overall growth and development of F. graminearum.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002266
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002266Summary
In F.
graminearum, the transcriptional regulator Tri6 is encoded within the trichothecene gene cluster and regulates genes involved in the biosynthesis of the secondary metabolite deoxynivalenol (DON). The Tri6 protein with its Cys2His2 zinc-finger may also conform to the class of global transcription regulators. This class of global transcriptional regulators mediate various environmental cues and generally responds to the demands of cellular metabolism. To address this issue directly, we sought to find gene targets of Tri6 in F. graminearum grown in optimal nutrient conditions. Chromatin immunoprecipitation followed by Illumina sequencing (ChIP-Seq) revealed that in addition to identifying six genes within the trichothecene gene cluster, Tri1, Tri3, Tri6, Tri7, Tri12 and Tri14, the ChIP-Seq also identified 192 additional targets potentially regulated by Tri6. Functional classification revealed that, among the annotated genes, ∼40% are associated with cellular metabolism and transport and the rest of the target genes fall into the category of signal transduction and gene expression regulation. ChIP-Seq data also revealed Tri6 has the highest affinity toward its own promoter, suggesting that this gene could be subject to self-regulation. Electro mobility shift assays (EMSA) performed on the promoter of Tri6 with purified Tri6 protein identified a minimum binding motif of GTGA repeats as a consensus sequence. Finally, expression profiling of F. graminearum grown under nitrogen-limiting conditions revealed that 49 out of 198 target genes are differentially regulated by Tri6. The identification of potential new targets together with deciphering novel binding sites for Tri6, casts new light into the role of this transcriptional regulator in the overall growth and development of F. graminearum.Introduction
Fusarium graminearum Schwabe [telemorph Gibberella zeae (Schwein.) Petch] is the causal agent of Fusarium head blight (FHB), one of the most destructive crop diseases in temperate climes throughout the world. In addition to yield reduction, FHB is often associated with the accumulation of the secondary metabolite DON in grain [1]. DON belongs to the trichothecene family of secondary metabolites; it binds to the peptidyltransferase of ribosomes thereby inhibiting protein synthesis [2]. DON accumulates in infected plant tissues, is phytotoxic, and poses considerable health risk to consumers [3]. Although considerable effort has been expended to both detect and regulate the amount of this mycotoxin in the infected cereals, there is less information with regard to the regulation of DON biosynthesis.
Our knowledge of mechanisms involved in the biosynthesis of DON and other secondary metabolites comes largely from in vitro culture studies. A considerable amount of evidence gathered over many years suggested that the physiological status of the fungus and the availability of nutrients are the main contributors for secondary metabolite production [4], [5]. Other growth conditions such as pH have also been shown to influence the production of secondary metabolite in numerous fungi including F. graminearum [6], [7]. Recent studies have assessed the role of various carbon and nitrogen sources in the induction of DON [5], [8]. For example, while the products of the polyamine biosynthesis pathway such as agmatine and putrescine strongly influenced DON biosynthesis, the study also revealed negative effects of other nitrogenous compounds on the induction of DON [5]. Other studies have also demonstrated the importance of carbon sources in the regulation of DON biosynthesis [8]. Growth conditions modified by the addition of salt solutions, hydrogen peroxide, and various phytochemicals and fungicides have also been shown to influence DON production [9].
In addition to the physiological conditions, factors affecting fungal developmental also impact the synthesis of secondary metabolites [4]. In Aspergillus species, the canonical heterotrimeric G protein/cyclic AMP/protein kinase A signalling pathway involved in diverse cellular responses including cell division, morphogenesis and pathogenic development affect the production of the secondary metabolites penicillin and sterigmatocystin. For example, mutations in fadA, a gene encoding for the Gα subunit of the heterotrimeric G protein, negatively impacts aflatoxin biosynthesis [10], [11]. Conversely, a dominant active fadA mutant inhibited expression of the transcription factor AflR, resulting in the blockage of sterigmatocystin synthesis. Interestingly, introduction of the same dominant active fadA mutant in F. sporotrichioides resulted in elevated levels of T-2 toxin, suggesting conservation of signalling pathways between filamentous fungi in the regulation of secondary metabolite synthesis [12].
This level of complexity, integrating various environmental inputs to fungal development leading to the activation of secondary metabolic gene clusters, has led to the current working model which proposes a multilevel regulation of secondary metabolism by both global and pathway-specific transcription factors [6]. In this scenario, the global transcription factors would respond and integrate disparate environmental cues such as temperature, pH and various carbon and nitrogen sources. Examples include AreA which mediates nitrogen catabolite repression in Aspergillus, and PacC which mediates pH regulation of the penicillin and trichothecene gene clusters in Aspergillus,and F. graminearum, respectively [13], [14]. One of the characteristic features of the global regulators is that they possess Cys2His2 zinc-finger domains, important for DNA binding and regulating gene expression [6]. The pathway-specific regulators on the other hand have a characteristic Zn(II)2Cys6 zinc binuclear cluster and positively regulate expression of a specific gene cluster. This is best exemplified by AflR, which regulates the sterigmatocystin biosynthetic gene cluster in Aspergillus, [15]. In F. graminearum and F. sporotrichioides, the transcriptional regulator Tri6 is encoded within the trichothecene gene cluster and regulates expression of structural genes involved in the synthesis of DON and T-2 toxin, respectively [16]–[18]. Targeted disruption of Tri6 in both Fusarium species established its role as a positive regulator of trichothecene genes [16], [18], [19]. Although this criterion designated Tri6 as a pathway-specific transcriptional regulator, evidence accumulated over the past few years have suggested that Tri6 may be representative of a global transcription factor whose expression is influenced by a variety of environmental factors. In addition to possessing Cys2His2 zinc-finger domains, Tri6 is influenced by the pH of the growth media and by a large range of nitrogen and carbon compounds [5], [8]. For example, polyamines such as agmatine and putrescine induced novel genes regulated by Tri6 [20]. Additionally, expression profiling of FHB-infected tissues identified more than 200 genes that are not part of the trichothecene gene cluster differentially regulated by Tri6 [16]. This included genes in the isoprenoid biosynthesis pathway which produces farnesyl pyrophosphate, an immediate precursor for trichothecenes, and genes involved in transport and virulence [16]. These observations suggested that genes outside of the trichothecene gene cluster are subject to Tri6 regulation.
To investigate the possibility that Tri6 is a global transcriptional regulator responsive to both environmental and developmental cues, a genome wide ChIP-Seq experiment was undertaken to identify potential new targets of Tri6. Therefore, ChIP-Seq was performed with Fusarium grown in nutrient-rich conditions, a condition optimal to detect targets involved in both growth and development. We identified 198 potential new targets of Tri6 and functional categorization associated them with energy, metabolism and other cellular processes. Expression profiling of Fusarium grown in nitrogen-deprived conditions, a condition optimal for the production of trichothecenes showed that 47 of 198 targets were differentially regulated in the tri6Δ strain. The over expression of Tri6 in the tri6Δ strain confirmed that Tri6 can auto-regulate its own expression under nutrient rich conditions. Detailed analysis of the Tri6 promoter revealed a new tandem GTGA DNA binding site, located adjacent to the previously described binding site for Tri6. This finding, together with the identification of new targets, signifies a broader regulatory role for Tri6.
Results
Targets of Tri6 include structural and regulatory genes involved in metabolism
Tri6 has been defined as a pathway-specific transcription factor that regulates genes of the trichothecene gene cluster under nitrogen-deprived conditions. However, to characterize Tri6 as a global transcriptional regulator, we sought to identify targets of Tri6 by performing a genome wide ChIP-Seq in F. graminearum grown in nutrient-rich conditions. The ChIP-Seq was performed in the Tri6-HA complemented strain and was compared to the Tri6Δ strain. It should be noted that the addition of the HA epitope to the C-terminus of Tri6 did not compromise its function [21]. Moreover, the presence of Tri6 protein in the Tri6-HA complemented strain was confirmed by immunoblot blot analysis using HA antibodies (Fig. S1A). ChIP DNA samples from the Tri6-HA and the Tri6Δ strains were sequenced by Illumina Genome Analyzer as 38 base tags. The software Novoalign was used to map the tags/reads to the reference genome (F. graminearum, PH-1; NRRL 31084) and the software Site Identification from Short Sequence Reads (SISSRs) was used to identify potential binding sites [22]. A browser shot of the output from the SISSRs analysis is displayed in Fig. 1. Among the 1491 enriched binding sites in the Tri6-HA complemented strain, we identified a total of 198 protein-coding genes with at least one binding site 1 Kb upstream of the ORF as potential targets of Tri6, distributed in all four chromosomes (Fig. 1). The binding site was defined by a high stringent criterion with a minimum of 120 tags and the number of tags per given target is proportional to the affinity of Tri6 to its target genes [22]. This is highlighted by the substantial enrichment of region in chromosome 2, where Tri6 is located (dotted box, Fig. 1). The 198 target genes with their tags are listed in Table S1.
Fig. 1. Browser shot of Tri6 binding sites in all four chromosome of F. graminearum. 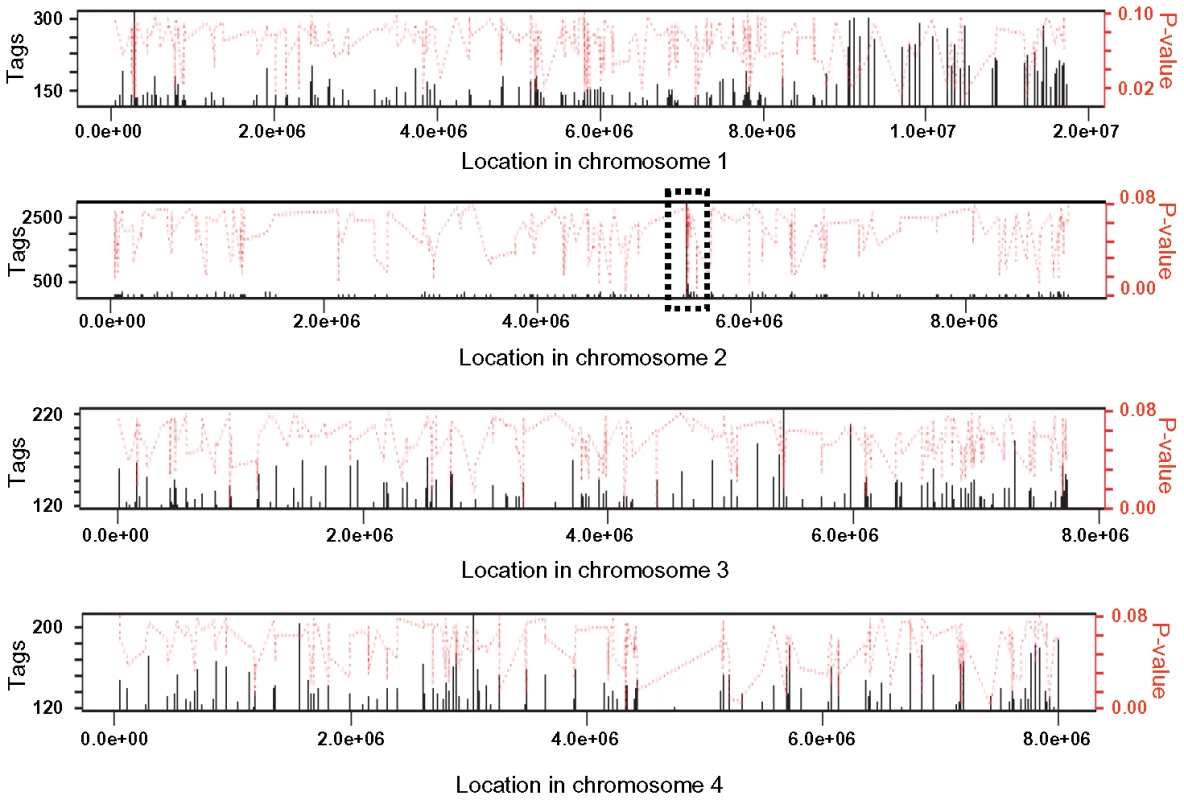
The binding sites were identified by the site identification software SISSR [22]. The black bars in each window refer to the number of tags or reads for a given site. The corresponding P-value is displayed in red. The dotted box in chromosome 2 indicates the site of the Tri6 promoter region. The analysis of the 198 target genes by the MIPS F. graminearum FunCat database (http://mips.helmholtzuenchen.de/genre/proj/DB/Search/Catalogs/searchCatfirstFun.html) and Kyoto encyclopaedia of genes and genomes (http://www.genome.jp/kegg/kegg1.html) categorized them into various aspects of metabolism and cellular processes (Table 1). For example, genes involved in nitrogen metabolism, such as pyridoxal decarboxylase (FGSG_08249) which decarboxylates L-glutamate into GABA and ornithine aminotransferase (FGSG_02304) which transaminates L-ornithine into glutamate - γ -semialdehyde were identified (Table S2) [23], [24]. Genes involved in lipid metabolism were also identified as potential targets of Tri6. For example, triacyl glycerol lipase (FGSG_02082) and acyl-CoA thioesterases (FGSG_03286 and FGSG_02848) yield free fatty acids, which are used in the β-oxidation pathway to produce energy [25]. In addition, acetyl-CoA, the by-product of thioesterase activity, is assimilated into the energy generating TCA cycle (Table S2) [25]. The data also revealed that Tri6 has the highest affinity towards its own promoter and to other genes of the trichothecene biosynthesis pathway, namely Tri1, Tri14, Tri3, Tri12 and Tri7 (Table 2). Since the Tri genes are normally induced in nutrient-limiting conditions, the discovery of these genes as targets of Tri6 in nutrient-rich conditions suggested a new role for Tri6.
Tab. 1. Tri6 targets summarized by functional categories. 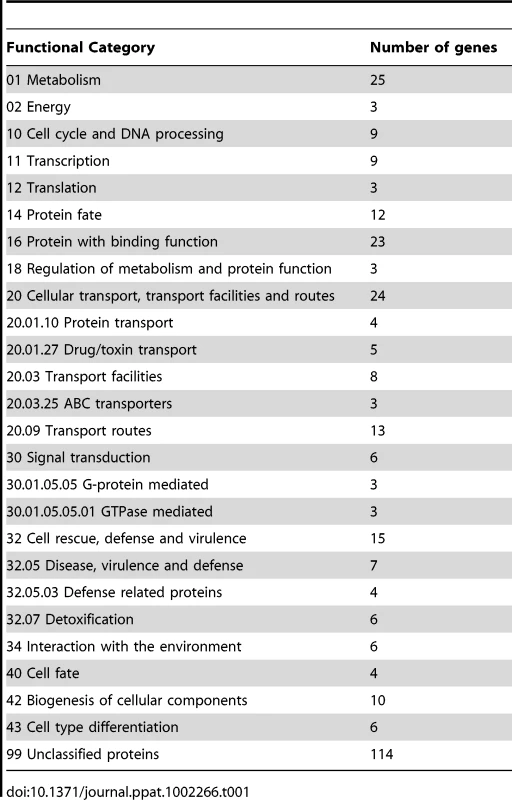
Tab. 2. Enrichment of Tri6 binding sites in the promoters of trichothecene genes in nutrient-rich conditions. 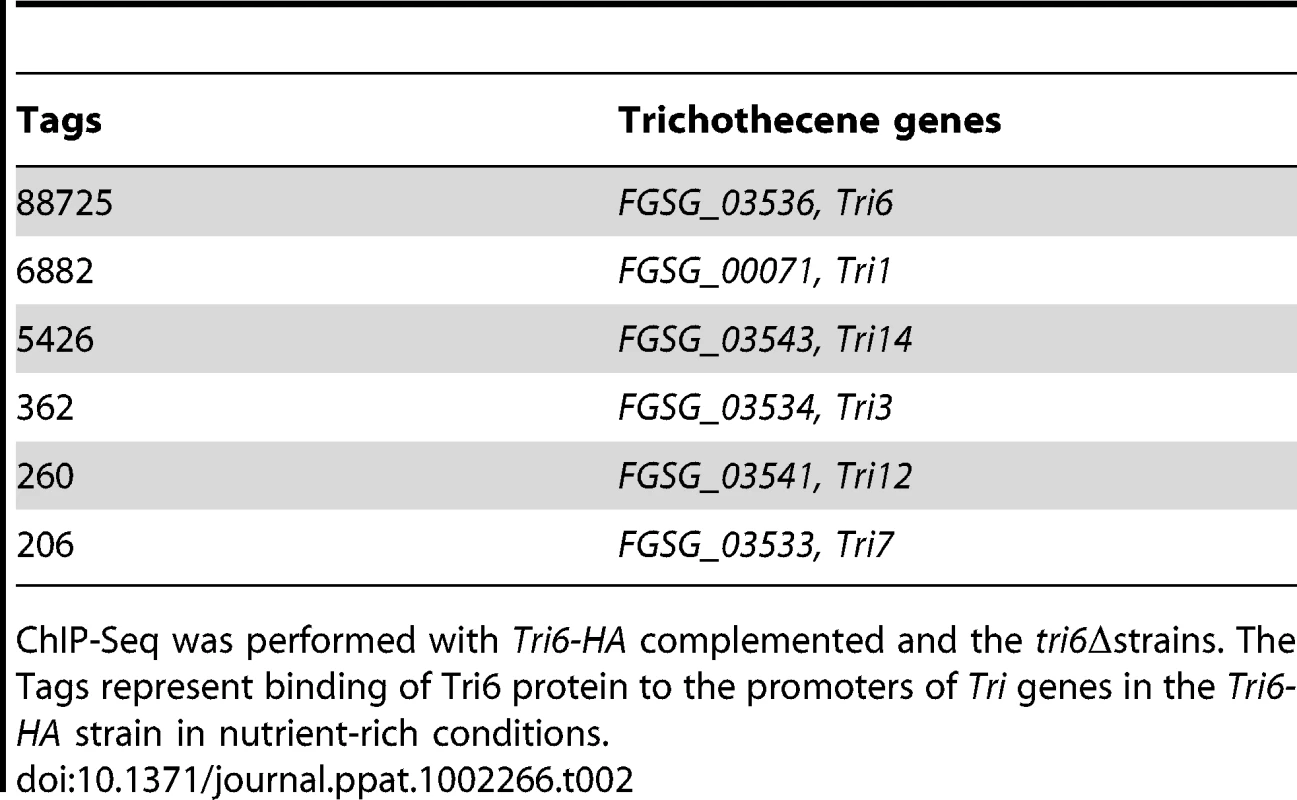
ChIP-Seq was performed with Tri6-HA complemented and the tri6Δstrains. The Tags represent binding of Tri6 protein to the promoters of Tri genes in the Tri6-HA strain in nutrient-rich conditions. In addition to the structural genes involved in metabolism, the targets of Tri6 also included genes involved in regulatory functions and signal transduction processes (Table S2). Many of the transcription factors are classified as zinc-binding proteins and some are known to be involved in nitrogen regulation, including two genes (FGSG_05942 and FGSG_10774) with NmrA domains. Genes with NmrA domains with Rossman fold structures can act as negative regulators of nitrogen catabolite repression [26], [27]. Two members of the RAS family of GTP binding proteins (FGSG_01649 and FGSG_06209) and a homologue of GIT1, a member of the adenyl cyclase associated family of proteins (FGSG_01923), were identified as targets of Tri6. RAS has been shown previously to regulate growth and pathogenesis in Fusarium while GIT1 in S. pombe is an essential component of the cAMP signalling pathway that primarily responds to glucose [28], [29].
In summary, the genome-wide ChIP-Seq performed in nutrient-rich conditions identified new targets involved in various aspects of metabolism. The targets encompassed not only regulatory genes, but also genes involved in primary and secondary metabolism, energy, and transport. The analysis also identified genes of the trichothecene gene cluster. This was particularly interesting given the fact that these genes are activated only in nutrient-deprived conditions. This suggested that that under nutrient-rich conditions, Tri6 could potentially exert transcriptional control over itself and other Tri genes.
Tri6 auto-regulates its own expression in nutrient-rich conditions
The ChIP-Seq data suggested that Tri6 had high affinity to its own promoter so we were interested to know if Tri6 would bind to its own promoter and self-regulate its expression. To demonstrate that Tri6 protein binds to its own promoter, the DNA-Tri6 complex was immunoprecipitated with HA antibodies from both the Tri6Δ and the Tri6-HA complemented strains grown in nutrient-rich conditions and PCR was performed using the primers spanning the upstream region of Tri6 ORF. As shown in Fig. 2, the primer set Tri6-Prom F/R (Table S3) amplified a product of 1.2 kb only from the samples immunoprecipitated from the Tri6-HA complemented strain (Lanes 1-3, Tri6-HA, Fig. 2). We could not amplify a 1.2 kb band in the samples immunoprecipitated from the tri6Δstrain (Lanes 1-3, tri6ΔFig. 2), even from 25 ng of input DNA (Lane 1, tri6ΔFig. 2). Genomic DNA was used as control to monitor the size of the PCR fragment (Lanes 1–3, Genomic, and Fig. 2). These in vivo results validated the Chip-Seq results and indicated that Tri6p can bind to its own promoter.
Fig. 2. Tri6 binds to its cognate promoter in vivo. 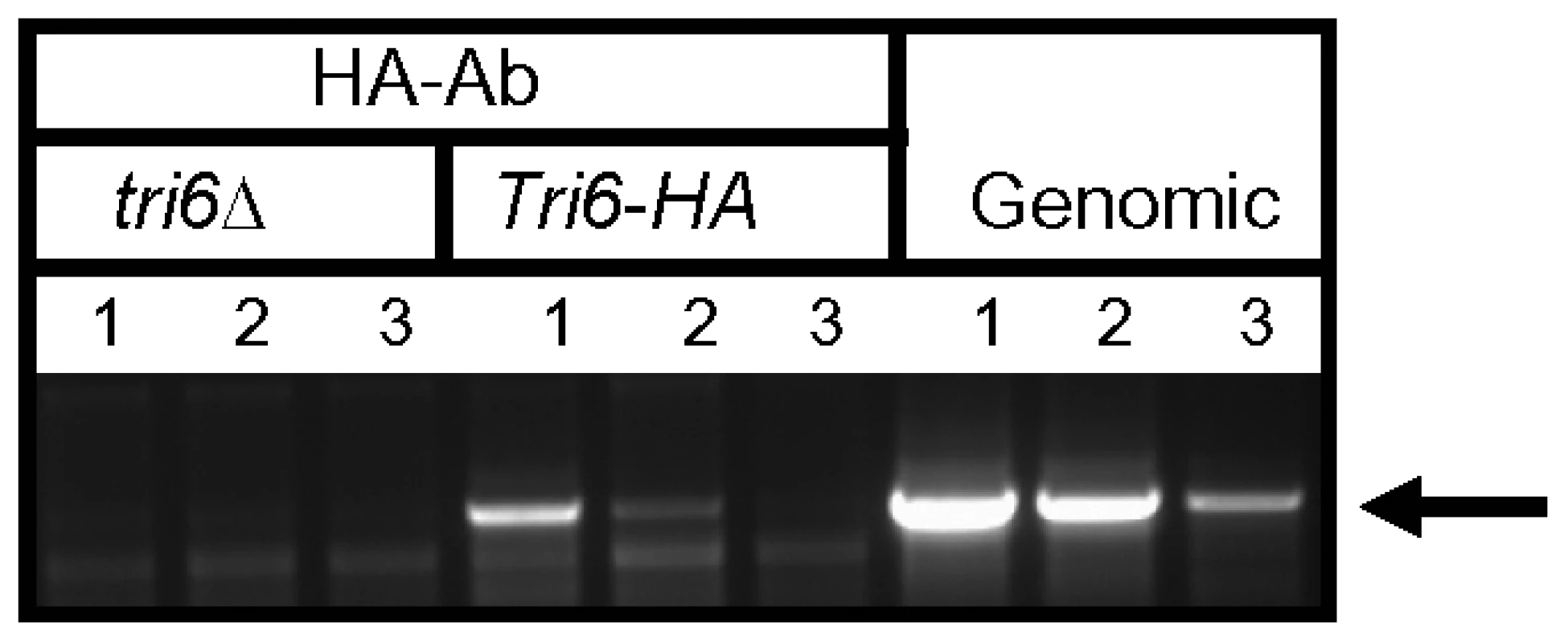
ChIP was performed with HA antibodies (HA-Ab) with the Tri6-HA complemented (Tri6-HA) and Tri6 mutant (tri6Δstrains. PCR was performed on the ChIP samples using the primers FGSG_03536 Promo F and FGSG_03536 Promo R, encompassing the 1.2 Kb upstream of the Tri6 ORF, using different amounts of input DNA in the PCR reaction. Input DNA: Lane 1 = 25 ng, Lane 2 = 2.5 ng and Lane 3 = 0.25 ng of DNA. Fusarium genomic DNA (Genomic) was used as control to monitor the size of the PCR fragment (arrow). This is representative of two independent experiments. To demonstrate that Tri6 can bind to its own promoter and regulate its expression in nutrient-rich conditions, Tri6 expression was monitored in the wildtype strain, the tri6Δstrain, and the strain that over expressed Tri6 in the tri6 mutant background strain (tri6ΔTri6). We designed two distinct primer sets (Table S3) to monitor Tri6 transcripts. The first primer set (Tri6-ORF F/R) was designed in the coding region of Tri6 (vertical open box, Fig. 3A) and as shown, over expression of Tri6 in the tri6Δ strain (tri6ΔTri6) led to a significant increase of Tri6 transcripts compared to the wildtype strain (52±4, Tri6-ORF, Fig. 3B and 77±7; Tri6-ORF, Fig. 3C). As expected, no expression of Tri6 was detected with these primers in the tri6Δstrain (Fig. 3B and 3C). The second primer set was designed to overlap the 5′UTR region and the coding region of Tri6 (Dotted vertical box, Fig. 3A) which allowed us to monitor Tri6 transcripts originating only in the wildtype and the tri6Δstrains. As shown in the Fig. 3B and Fig. 3C, a significant increase of Tri6 expression (Tri6-5′UTR, 4.4±0.4, Fig. 3B and Tri6-5′UTR, 7±0.9, Fig. 3C) was observed in the tri6Δstrain, compared to the wildtype strain. However, over expression of Tri6 in the tri6Δstrain (tri6ΔTri6) resulted in decreased Tri6 expression (Tri6-5′UTR, 0.72±0.1, Fig. 3B and Tri6-5′UTR, 1±0.06, Fig. 3C). This suggested that Tri6 acts as a repressor, regulating its own expression in nutrient-rich conditions. We did not observe any significant change in the expression of other Tri genes in the tri6ΔTri6 strain (tri6ΔTri6; Fig. 3B and 3C), suggesting that additional factors are required for the expression of these genes.
Fig. 3. Tri6 auto-regulates its own expression in nutrient-rich conditions. 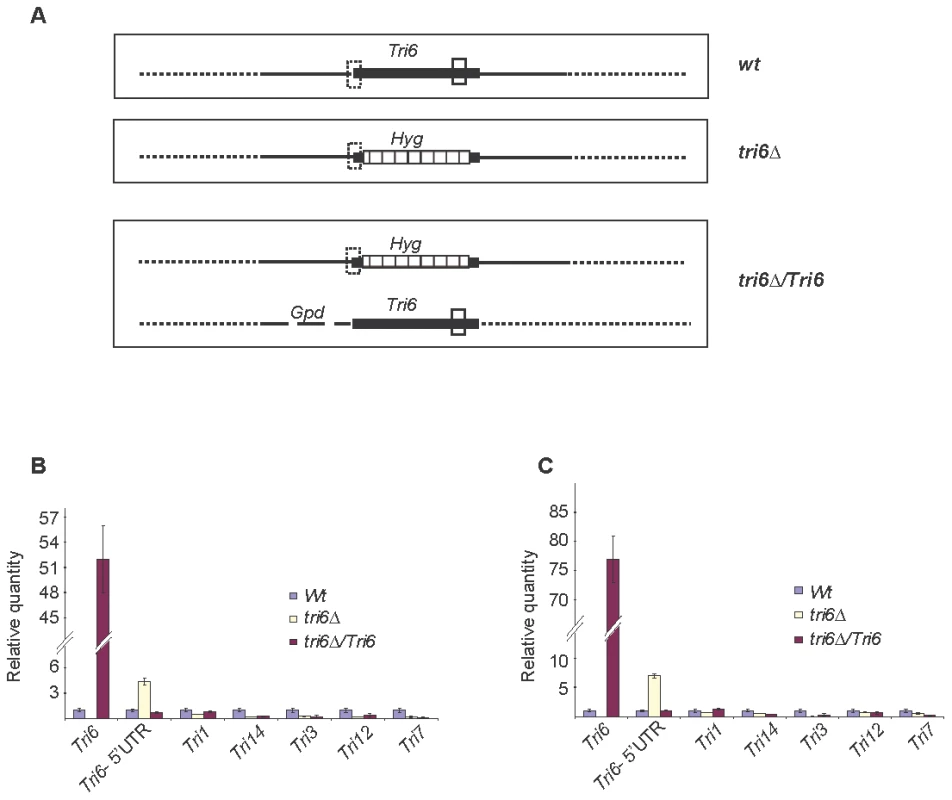
A) Arrangement of the Tri6 gene and location of Tri6 primers used in the RT-qPCR analysis of wildtype (Wt) and transgenic strains. The solid vertical box indicates the location of Tri6-ORF primers in the coding region of Tri6 (Filled horizontal box) of the wildtype and the Tri6 over-expressing transgenic strains (tri6ΔTri6). The dotted vertical box indicates the location of Tri6 5′ UTR primers in the wildtype (Wt), the Tri6 mutant (tri6Δand the Tri6 over-expressing transgenic strains (tri6ΔTri6). The location of the Hygromycin gene within the Tri6 coding region of the tri6Δstrain (tri6Δis indicated by the striped horizontal box. Gpd indicates the promoter used to over-expressTri6 [41]. The solid horizontal lines indicate 5′ and 3′ flanking regions of the Tri6 gene. B) The quantitative real-time PCR (RT-qPCR) analysis of Tri genes in wildtype, tri6Δand the tri6ΔTri6 strains grown in nutrient-rich conditions. RT-qPCR reactions were performed in triplicates using Applied Biosystems Power SYBR Green kit and the Applied Biosystems Step One Plus Real-Time PCR System. A list of qPCR primers for all the Tri genes is listed in the Table S2. The β-tubulin gene (FGSG_09530) was used as the internal control and the data was imported and Relative quantity (RQ) was derived by the Relative standard method included in the StepOne 2.1 software. C) Identical to B), except the internal control used was Gapdh (FGSG_06257). The figures are representative of two independent biological replicates. Identification and characterization of the Tri6 binding site in the Tri6 promoter
A previous study performed in F. sporotrichioides suggested TNAGGCC as a DNA binding site for Tri6 protein [17]. A recent study that examined the promoters of genes differentially regulated by Tri6 during the F. graminearum infection process also suggested a similar DNA binding motif [16]. The results described here confirmed that Tri6 is able to bind its own promoter in vivo (Fig. 2) and regulate its own expression (Fig. 3). Since the promoter of Tri6 (−836 to −768) harbours two RNAGGCC (where R = G or A) binding sites (Fig. 4A), we employed EMSA analyses to further delineate the binding site for Tri6. All the EMSA assays were performed with purified recombinant Tri6 protein (Fig. S1B). First, we tested the probe which contained two of the RNAGGCC motifs and as the results indicated, Tri6 did not bind to this probe (Tri6-1, Fig. 4B). This prompted us to examine the region that surrounds this motif. The probe Tri6-2 which included sequences proximal to the binding sites (Fig. 4A) also did not bind the Tri6p (Fig. 4B). However, a probe (Tri6-3) which included sequences distal to the binding sites was able to bind Tri6 protein. The specificity of the binding to the Tri6-3 probe was confirmed by the addition of 10-fold excess non-labelled Tri6-3 probe in the EMSA assays (Tri6-3, competitor, ‘+’, Fig. 4B). In addition, we also used a probe which did not contain this consensus sequence as a negative control (Tri6-NS, Fig. 4B). These results suggested that RNAGGCC motif is not involved in Tri6 binding in vitro. To further confirm this, nucleotides GGCC within the motif were mutated in the Tri6-3 probe and the results indicated that the mutations did not abolish Tri6 binding (Tri6-3-1, Fig. 2). These results confirmed that the YNAGGCC motif is not required for binding of Tri6, but suggested that nucleotides outside of this motif in the Tri6-3 probe are involved in Tri6 binding. Outside of the RNAGGCC motif, three domains in the Tri6-3 probe were recognized that could potentially bind Tri6 (Fig. 5A). We designated CTGA sequence which partially overlaps the AGGCC site as Domain I and the two GTGA repeats separated by six nucleotides as Domain II and III, respectively (Fig. 5A). As the EMSA results indicated, individual nucleotide mutations within Domain I did not have any noticeable effect on Tri6 binding (Fig. 5B). However, individual mutations in Domain II and Domain III of the GTGA sequences, respectively, dramatically decreased Tri6 binding (Fig. 5C). Furthermore, combined mutations in both Domain II and III completely abolished the Tri6 binding (Fig. 5D). These results suggested that either a single GTGA sequence or GTGA repeats in the promoter of Tri6 are required for Tri6 binding.
Fig. 4. The Tri6 protein does not recognize the YNAGGCC motif by EMSA analyses. 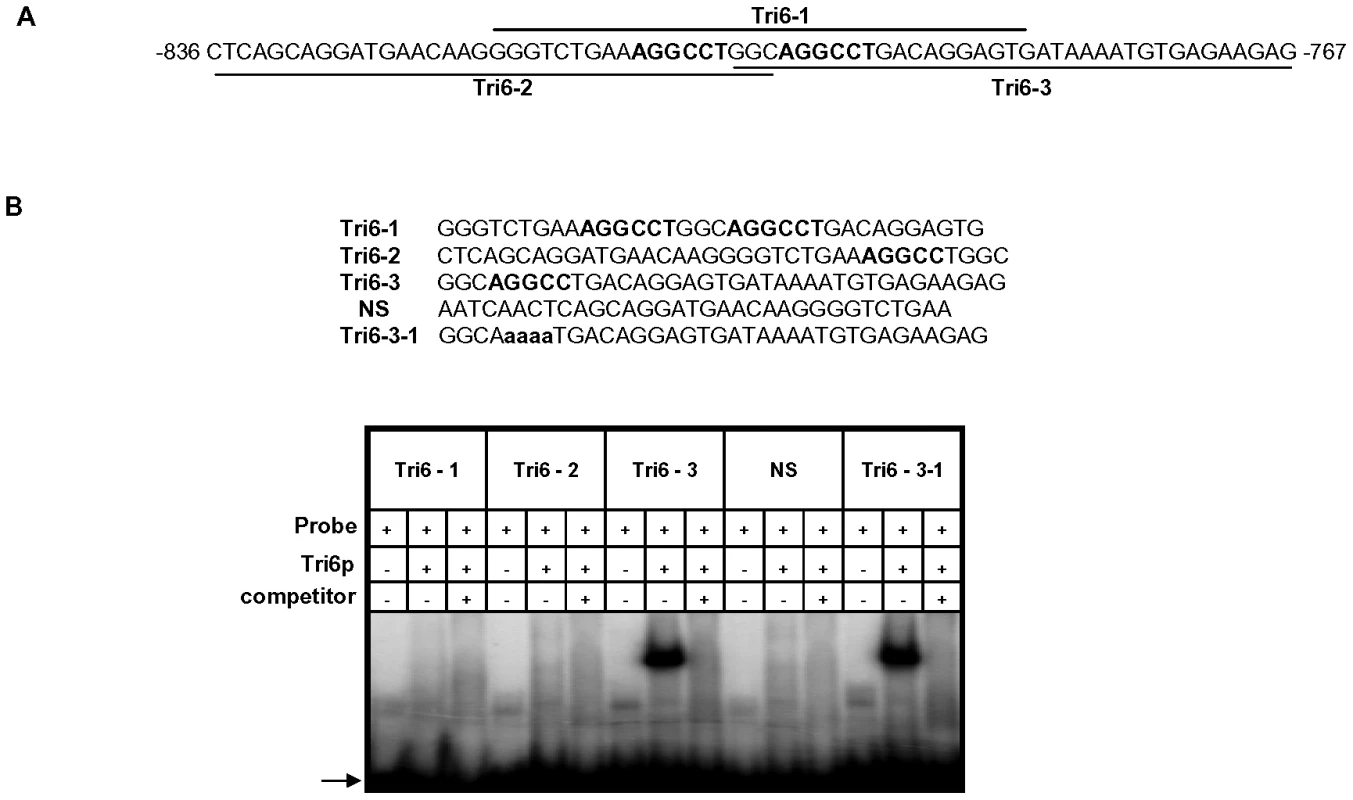
A) Sequence of probes Tri6-1, Tri6-2 and Tri6-3 in the Tri6 promoter (-836 to -767) used in the EMSA analyses. The bold letters indicate the putative consensus binding sequence YNAGGCC for Tri6p. B) EMSA analyses were performed using the probes Tri6 -1, Tri6-2, Tri6-3, a non-specific probe (NS) and a probe with mutations within the binding motif (Tri6-3-1). The addition of Tri6 protein (Tri6p), the probe and the unlabelled probe (competitor) is indicated by ‘+’ sign and the omission is indicated by the ‘-’ sign. The capital bold letters indicate the consensus binding sequence and the bold non-capital letters represent mutations within consensus binding sequence YNAGGCC. Arrow indicates the migration of the free probe. This is representative of at least three independent experiments. Fig. 5. Tri6 binds to GTGA repeats in the Tri6 promoter by EMSA analyses. 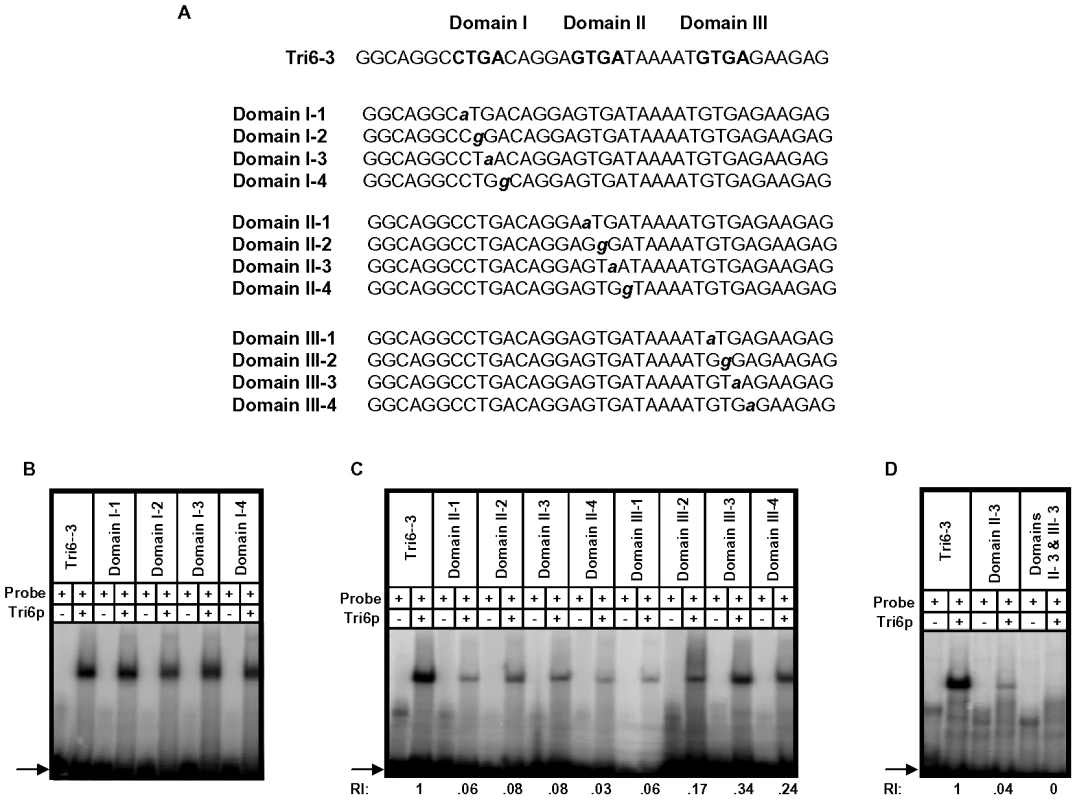
A) Sequence of the probes used in the EMSA analyses. The three domains and the mutation within each Domain I, II, and III in the Tri6-3 probe are indicated. Bold capital letters indicate the three Domains in the Tri6-3 probe and the bold non-capital letters represent mutations in each Domain. B) Domain I with CTGA motif is not involved in Tri6p binding. EMSA analyses with individual mutation in the CTGA sequence was assessed for Tri6p binding. C) Mutations in the two GTGA domains represented by Domain II and Domain III, respectively, reduce Tri6p binding. EMSA analyses with individual mutation in the GTGA sequence in both domains were assessed for Tri6p binding. D) Domain II and III are required for complete Tri6 binding. A combination of mutations in Domain II and III (Domain II-3 and Domain III-3) were assessed for Tri6 binding. Tri6-3 was used as positive control and Domain II-3 was used as reference. The addition of Tri6 protein (Tri6p) and the probe is indicated by ‘+’ sign and the omission is indicated by the ‘-’ sign. RI represents relative intensity and is the densitometer reading corresponding to the binding of Tri6p to each probe with respect to the control probe Tri6-3. The arrow represents the migration of free probe. This is representative of at least three independent experiments. Tri6 targets are differentially regulated under nitrogen limiting conditions
The 198 Tri6 targets identified in nutrient-rich conditions included several genes of the trichothecene gene cluster (Table 2). Since the Tri genes are expressed only under nitrogen-limiting conditions, we were interested to know how many of the non-Tri gene targets are co-regulated with the Tri genes. To address this, we performed a genome-wide expression analysis of wildtype and tri6Δstrains grown in nitrogen-limiting conditions. As the microarray analysis indicated, we identified a total of 1614 genes that were differentially regulated in the tri6Δstrain (2-fold cut off; Table S4). Among the 870 down-regulated genes, 18 of the Tri6 targets were represented and another eight were represented in the 744 up-regulated genes (Fig. 6). Top five genes from each of the up and -down-regulated genes from the microarray analyses were selected for validation by RT-qPCR analyses (Fig. 6). If the expression threshold was set at 1.5, 49 of the 198 target genes (∼25% of the ChIP targets) were shown to be differentially regulated by Tri6 in this nutrient-limiting condition (Table S5). Out of 49 targets, 23 genes or ∼53% were annotated as unclassified by the MIPS functional annotation program and although others were classified into four major groups, only one category associated with cellular transport comprised of eight target genes were enriched to a significant level (enrichment of 19% vs 10% in the genome, p-value 0.06). Thus expression profiling provided a strong evidence to suggest that Tri6 extends its regulatory control beyond the trichothecene cluster and additionally, Tri6 can act both as a positive and negative regulator.
Fig. 6. Tri6 differentially regulates 26 targets under nitrogen-deprived conditions. 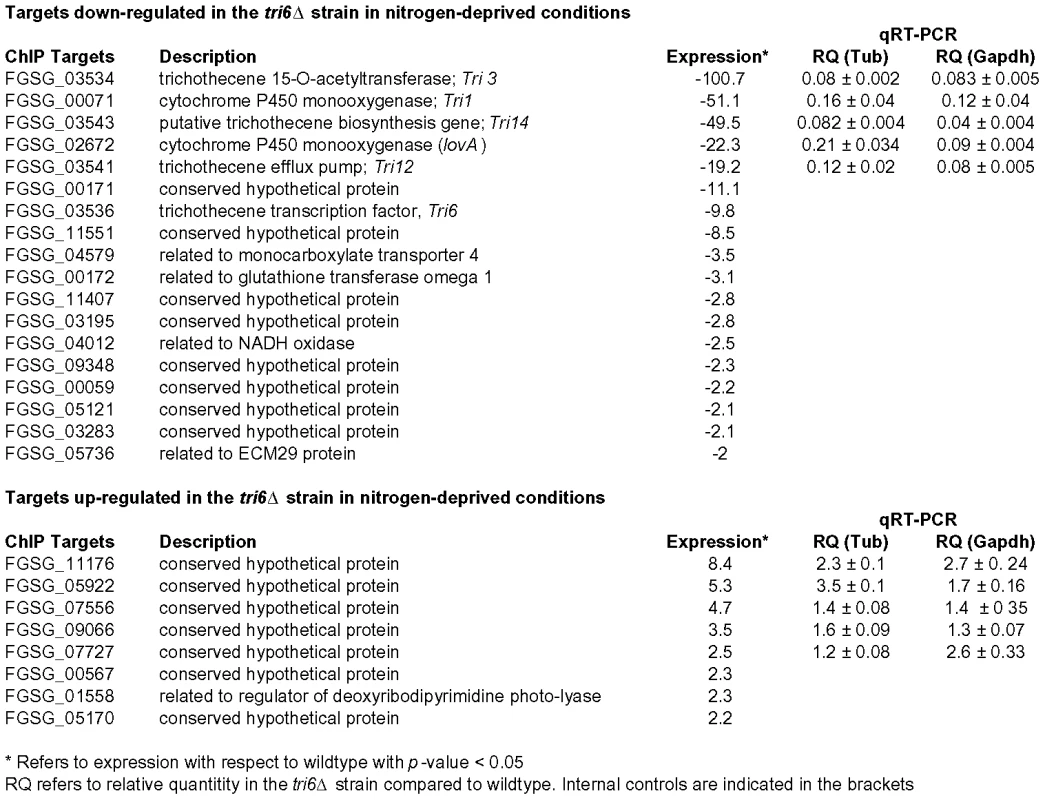
Expression profiling was performed with wildtype and Tri6 mutant (tri6Δstrain grown in nitrogen-limiting conditions. Eighteen targets that are down-regulated and eight targets that are up-regulated in the tri6Δstrain are represented. The top five genes in each category were verified by RT-qPCR analysis represented by relative quantity (RQ). Two internal standards (β- tubulin and Gapdh) were used. This is a representative of three independent experiments. Discussion
Much of our understanding regarding the transcription regulator Tri6 stems from earlier studies that identified its control of the mycotoxin pathway leading to trichothecene biosynthesis and designated it as a pathway-specific transcriptional regulator [17], [18]. Since then, evidence from numerous laboratories have challenged this perception and thus prompted us to hypothesize that Tri6 is a global transcriptional regulator whose influence extends beyond the trichothecene gene cluster [16], [20]. Accordingly, the ChIP-Seq experiments performed in nutrient-rich conditions demonstrated that Tri6 could bind to promoters of both structural and regulatory genes associated with many aspects of nitrogen, carbon and lipid metabolisms (Table S2). One of the intriguing revelations was that even under these conditions Tri6 was bound to the promoters of genes involved in the biosynthesis of the secondary metabolite, trichothecene 15-ADON (Table 2). Since this cluster is only activated in Fusarium grown under nitrogen-deficient conditions, binding of Tri6 to the promoters of Tri genes suggested that Tri6 could potentially act as a negative regulator, suppressing these genes under nutrient-rich conditions. The results from the RT-qPCR clearly demonstrated that with the exception of Tri6, none of the other Tri genes are subject to negative regulation by Tri6 (Fig. 3). Since the switch from nutrient-rich to deprived conditions led to differential regulation of the target genes, we conclude that Tri6 can act both as a positive and a negative regulator. Furthermore, since the regulation by Tri6 extended beyond the trichothecene gene cluster, we propose that Tri6 is a global transcriptional regulator.
Tri6 and other global transcriptional regulators
Nitrogen catabolite repression, or NCR, refers to genes that are repressed when a preferred source of nitrogen such as ammonia or glutamine are present in the environment [24]. NCR is mediated by the action of global nitrogen regulators which regulate the expression of structural genes required to metabolize alternate nitrogen sources. AreA is a prototypic NCR gene regulator and has been identified in many fungi, including A. nidulans, homologues Nut1 from M. grisea and AreA-GF from F. fujikuroi [30]–[33]. Under nutrient-rich conditions, expression of AreA is repressed by the binding of the repressor protein NmrA to the promoter of AreA [31], [32]. However, under nitrogen-starving conditions, AreA repression is relieved and target genes involved in the utilization of non-preferred sources of nitrogen are expressed [31], [32]. Coincidentally, some of the known AreA target genes such as the branched amino acid transferase (FGSG_05696; ∼2.2 –fold, Table S4) and the general amino acid permease (FGSG_05574; ∼4-fold, Table S4) that are normally expressed in the wildtype Fusarium strain under nitrogen-deprived conditions are repressed in the tri6Δstrain [34]. This leads one to speculate that Tri6, similar to AreA, is involved in NCR. Studies in F. fujikuroi have also shown that AreA, in addition to regulating those genes involved in NCR, also regulates expression of genes that synthesize secondary metabolites like gibberellins and the pigment bikaverin [35], [36]. An additional feature that is shared between Tri6 and AreA is that they are both subject to auto-regulation [31]. Taken together, our evidence suggests one possible scenario where Tri6 regulates functions of AreA through NmrA in F. graminearum. In support, we identified two genes (FGSG_05942 and FGSG_10774, Table S2) with Rossman-fold domains as targets of Tri6. Rossman-folds are distinguishing features of NmrA proteins [26]. A further link between NmrA and virulence was recently established in F. oxysporum where the deletion of the transcription factor MeaB, which regulates Nmr1, an orthologue of NmrA resulted in the repression of the virulence gene Six1 [37].
DNA binding properties of Tri6
The EMSA studies demonstrated that mutations in either one of the GTGA elements in the promoter of Tri6 reduced the binding of Tri6. However, combined mutations in both the GTGA elements completely abolished this binding (Fig. 5D). This suggested that the presence of two GTGA elements in close proximity to each other in the Tri6 promoter likely contributes to the affinity of Tri6 to this site. Studies with other global regulators, specifically those responding to nitrogen, such as AreA in A. nidulans or its counterpart Nit2 in N. crassa showed that binding sites located within 30bp of each other in either orientation enhances their DNA binding affinity [38]. When these criteria were applied to the analysis of Tri6 target genes, 109 of the 198 target genes harboured multiple GTGA/TCAC binding sites in their promoters, separated by eight nucleotides or less, suggesting a common mechanism of binding and regulation by Tri6 (Fig. 7). It should be noted that GTGA/TCAC motifs separated by more than eight nucleotides are represented in the promoters of all the Tri6 target genes (Table S1). The GTGA/TCAC motif described in this study is distinct from the previously reported YNAGGCC for Tri6 binding [17]. Unlike this study, Hohn et al. did not use purified Tri6 protein and furthermore, their EMSA analysis with regions of the Tri3 promoter which contained the putative YNAGGCC consensus binding site did not result in a shift [17]. This led them to speculate an alternate binding site for Tri6. It is noteworthy to indicate that Tri3 is one of the genes identified as targets of Tri6 with the GTGA repeats in its promoter.
Fig. 7. GTGA/TCAC repeats are present in the promoters of Tri6 target genes. 
One kilobase region upstream of the open reading frame of each of the Tri6 target genes were analyzed for GTGA/TCAC repeats separated by 0 to 8 nucleotides (N). Studies with global regulators have outlined several mechanisms that influence both binding affinity and specificity [31]. For example, proteins that binds to direct repeats most likely bind as dimers. As an example, Dal80 protein in S. cerevisiae, involved in nitrogen catabolism, binds to two closely spaced GATA elements as a dimer in either a tail-to-tail or head-to-head orientation [39]. Interactions between global and pathway-specific regulators also influence both DNA binding specificity and affinity. For example, some of the genes involved in nitrate assimilation are regulated by the interaction between the global regulators AreA and NirA in A. nidulans, while other genes are regulated by the global regulator Nit2 and the pathway-specific regulator Nit4 in N. crassa [24]. Since we observed closely spaced GTGA repeats in the Tri6 promoter of F. graminearum, we could envisage a scenario, where Tri6 could function as a dimer and repress the expression of Tri6 in nutrient-rich conditions. However, under nutrient-deprived conditions, where the expression of Tri6 increases, Tri6 could interact with other known regulators such as Tri10 or PacC to provide specificity and regulate expression in condition-specific manner [14], [19]. This interaction may also provide a platform for the Tri6-complexes to bind to an alternate site, such as the YNAGGCC proposed in several studies. Such a scenario could account for the differences between the genes regulated by Tri6 in this study from those seen in a recent study of F. graminearum infection in planta [16]. It is noteworthy to mention that the authors in that study also observed stark differences between the genes regulated by Tri6 in culture and in planta, albeit the comparison was made between two different Fusarium strains. One of the intriguing findings from the in planta study was that the promoters of genes, specifically the genes of the isoprenoid pathway essential for trichothecene production in both in planta and culture, showed enrichment of the YNAGGCC motif only in F. graminearum and not in other Fusarium species unable to make trichothecenes or in other related fungi [16]. It was proposed that co-regulation of the isoprenoid genes and the trichothecene genes represent an evolutionary adaptation specific to F. graminearum. A logical extension of this argument is that the source of nutrition, determined by the environment such as culture conditions or the type of host that this fungus infects, is the driving force behind this adaptation.
In conclusion, this study has identified new or additional role for the transcriptional regulator, Tri6. The findings from this study together with earlier studies have led us to conceive a new mode of action for Tri6. In this model, Tri6 recognizes and binds the GTGA/TCAC elements under one condition such as the nutrient-rich condition where it regulates itself and other genes involved in various aspects of metabolism. However, when the environmental conditions change, be it in culture or in planta, activation of other regulators including Tri10 and PacC may change the dynamics of Tri6p-complex and initiate new interactions with the DNA, which could involve recognition of an alternate site such as the YNAGGCC motif. Although, we have no evidence to show that Tri6 directly interacts with either Tri10 or PacC, isolation and identification of Tri6 and Tri10 protein complexes in vivo will shed more light on both the dynamics and the links between these regulators.
Materials and Methods
Strains and growing conditions
Fusarium graminearum wildtype strain GZ3639 (NRRL 38155) was provided by C. Babcock of the Canadian Collection of Fungal Cultures (CCFC), Agriculture and Agri-Food Canada, Ottawa. F. graminearum and transformants were grown on PDA (Sigma Chemical Co. USA) plates.
Construction of Tri6Δdeletion, Tri6-HA complement and tri6Δ/Tri6 over expression strains
Constructs for the tri6Δand Tri6-HA complement strains have been described (Fig. S1; http://apsjournals.apsnet.org/doi/suppl/10.1094/MPMI-09-10-0210). To construct the Tri6 over expression vector, we first PCR amplified Tri6 ORF with the primer set Tri6-GUE-F/ Tri6-GUE-R (Table S2) and F. graminearum genomic DNA as template. The PCR fragment was cloned into the vector pSW-GU. The backbone of pSW-GU vector is identical to pRF-HUE [40] except that the selection marker Hygromycin gene was replaced with Geneticin, encoded by the gene neomycin phosphotransferase [40]. Transformation of the tri6Δstrain with the Tri6 over expression vector was performed according to Rasmus et al [40]. The transgenic Fusarium strains were verified by PCR (Fig. S2). PCR conditions: 200 µM Primers, 200 µM NTP's, 1 unit of Expand long Taq polymerase (Roche), 95°C for 30 s, 52°C for 30 s for annealing, 68°C for extension for 37 cycles. All PCR products were purified using Qiagen's PCR purification kit.
Trichothecene induction and detection
To induce trichothecene production in liquid culture, a two stage media protocol, modified from Miller and Blackwell was employed [41]. 20,000 spores of wildtype, tri6Δand tri6ΔTri6 over expression strains were inoculated into 4 mL of first stage growth media and incubated in Falcon Multiwell 6-well culture trays (Fig. S2). The culture trays were affixed to an orbital shaker and the spores were grown for 24 hr at 28°C in the dark with constant shaking at 170 rpm. Following 24 hr growth, the mycelial solids were washed with water and resuspended in 4 mL of second stage media (pH 4.0) [41] and then transferred to the 6-well culture trays. The mycelium was grown in second stage media under the same conditions as described previously. The supernatant was collected after 24 hr for trichothecene analyses. Trichothecenes were analysed on an AKTA 10 purifier (GE Healthcare, Canada) with direct injection of 100 µL of the culture filtrate into a 150×4.6 mm, 5 µm Hypersil ODS column (Thermo-Electron Corp.), using a methanol: water gradient from 15∶85 to 60∶40 over 25 min at a flow rate of 1 ml/min. Under these conditions, 15-ADON elutes at a retention time ∼10 min, monitored by UV 220nm.
Chromatin Immunoprecipitation
Spores from the tri6Δand the complemented (Tri6-HA) strains were used to inoculate first stage media and grown for 19 hr as described before. The mycelia were washed with water and filtered (sterile 1MM; Whatman). The ChIP-enriched DNA was prepared according to Saleh et al. [42] with modifications. The mycelial pellet was incubated in the cross-linking buffer for 30 min with continuous shaking. After thorough washing, the pellet was ground in liquid N2 and resuspended in the lysis buffer (250 mM, HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton, 0.1% DeoxyCholate, 10 mM DTT and Protease inhibitor (Roche complete Mini) and incubated for 1 hr on ice. To obtain uniform fragments of cross-linked DNA, the suspension was sonicated 6x for 15 sec (60% amplitude, Misonix 4500 Sonicator). A small aliquot was used to confirm a dominant diffused band between 500 and 1000 bp. Approximately 750 µg of protein was incubated with 50 µL HA-magnetic beads (Miltenyi Biotech) and left overnight at 4°C. Immunoprecipitates were washed six times with lysis buffer and eluted with 250 µL of freshly prepared elution buffer (0.5% SDS and 0.1 M NaHCO3) with incubation at room temperature (RT) for 15 min with gentle agitation. The elution step was repeated once with 30 min of incubation at RT. To reverse the cross-linking, 20 µL of 5 M NaCl was added to the 500 µL elutes and incubated overnight at 65°C. The samples were further incubated for 1.5 hr at 45°C with 1 µL of Proteinase K (20 mg/mL), 30 mM Tris-HCl pH 6.5 and 10 mM EDTA to digest the proteins. DNA from these samples was precipitated according to Saleh et al. [42].
Preparation of DGE and ChIP libraries and Illumina sequencing
ChIP - enriched DNA was prepared for sequencing using the Illumina ChIP sample prep protocol with minor modifications. In brief, 2 µg of ChIP-enriched DNA was end repaired, and an adenosine overhang added to the 3′ ends. Standard Illumina adapters were ligated to the DNA and 240 bp fragments size selected from a 1.5% agarose gel. The eluted products were enriched using 18 cycles of amplification with Illumina PCR primers. Libraries were validated on the Bioanalyzer 2100 (Agilent) and Qubit fluorometer (Invitrogen, Carlsbad, CA). Each sample was run on two lanes of the Illumina GAII sequencer at the Centre for the Analysis of Genome Evolution and Function (CAGEF, University of Toronto). For each sample, 6 pmol was loaded in one lane of a standard flow cell, and 8 pmol was loaded in a second lane. The GAII was run for 38 cycles.
Statistical analysis of ChIP data
In total, 24,201,991 out of 29,933,903 tags from Tri6-HA complemented strain and 15,756,703 out of 22,010,411 tags from tri6Δstrain were normalized and uniquely mapped to the genome by Novoalign (http://www.novocraft.com) [43], [44]. The rest of the tags were either of low quality or mapped to multiple locations. Of the mapped tags, ∼50% were mapped to sense strand and another 50% were mapped to anti-sense strand. The mapping data were analyzed by the Site Identification from Short Sequence Reads (SISSRs) software for identification of the binding sites with tags originating from the tri6Δstrain as control [22]. The following parameters were used to run the program: 36,898,000 base pairs for the genome size, 0.8 for the fraction of genome mappable by reads, 1 for the E-value (minimum number of directional tags required on each side of the inferred binding site), 0.1 for the p-value (for fold enrichment of ChIP tags at a binding site location compared to that at the same location in the control data), 2 for the scanning window size, 240 base pairs for the average DNA fragment length, and 350 base pairs for the upper bound DNA fragment length. The switch -u was turned on to allow for identification of the binding sites supported only by reads mapped to one strand. Under these conditions, we were able to identify 1491 potential binding sites with at least 120 tags (Tag#, the sum of the tags mapped to positive strand of the left half of the binding site and the tags mapped to negative strand of the right half of the binding site) and at least 2.15 fold more tags in treatment vs control. We then compiled a list of 198 protein-coding genes with at least one binding site in 1 Kb upstream of the start site. The sum of the Tag# indicates the relative affinity of Tri6 to the binding sites in the promoter of the gene.
RNA isolation and Quantitative RT-PCR Analysis
The two-stage media was employed to grow F. graminearum as described before. Mycelia grown for 5 hr in the second stage (nitrogen-limiting) media were filtered and ground to a powder in liquid nitrogen. The RNA was isolated from 0.25 gm of ground mycelia with 1 mL of Trizol (Invitrogen, USA) according to manufacturer's instructions. The RNA was purified further with the InviTrap Spin Cell RNA mini kit (Invitek, Germany) according to the manufacture's instructions. cDNA for the quantitative RT-PCR Analysis (RT-qPCR) experiments was synthesized from 1 µg total RNA using random hexamers using the high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). RT-qPCR was performed using the Applied Biosystems StepOne Plus Real Time PCR system (ABI, Foster City, USA). Standard curves were created for the house keeping genes (β-tubulin; FGSG_09530 and Gapdh; FGSG_06257) and the genes of interest according to Relative standard curve method outlined in StepOne 2.1 software. Primers used in the RT-qPCR are listed in supplementary Table S2. Using the relative standard curve, the relative quantity (RQ) was determined by comparing target quantity in each sample to the reference sample. P values <0.05 were considered to be statistically significant.
Purification of Tri6 protein and Electromobility shift assays
Tri6 was cloned into pDEST17 vector (Invitrogen, USA) and expressed in BL21 pLys E.coli and the Tri6 protein was purified by the His Trap FF affinity column (GE Health care, Sweden). Double stranded DNA probes (100 ηg/mL) were labelled using T4 Polynucleotide Kinase (Fermentas MBI) and 20 µci of γP32 (Perkin Elmer, USA). The labelled probes were purified using the Quick spin column (Roche, USA) and specific activity varied from 4–7×104 cpm/ηg. Binding reactions for the EMSA were performed in 15 µL volume with 2 µg purified Tri6-HA recombinant protein, labelled probes (20,000 cpm; 2ηg) for 30 minutes at room temperature in a buffer containing 20 mM HEPES pH 7.9, 2 mM DTT, 5% (V/V) Glycerol and 1 µg poly(dIdT). The reaction was run on 6% polyacrylamide gels (acrylamide/bis ratio of 19∶1 w/w) in 50 mM Tris-HCl, pH 8.0, 50 mM sodium borate and 1 mM EDTA. The competition experiments were performed by adding unlabelled oligonucleotides to the reaction at 10X molar excess (20 ηg) of the labelled probe. The shift obtained by the Tri6 was scanned by the Storm 840 densitometer (GE healthcare, USA) and quantified by Image Quant TL 7.0 software (GE healthcare, USA). All the DNA probes used in EMSA were synthesized by Sigma Genosys (SigmaAldrich, Canada).
DNA microarray and statistical analysis
Wildtype and the tri6Δstrains were grown in the second stage media for 5 hrs and the RNA was extracted as described before. The integrity of RNA was confirmed by an Agilent 2100 Bioanalyzer (Agilent, Canada). RNA was then converted into cDNA using the Agilent Quick Amp Labeling Kit and converted back into labeled RNA using T7 RNA polymerase and cyanine 3-labeled CTP or 5-lableled CTP from the Agilent two-color RNA Spike-in kit. The cRNA was then hybridized to a custom F. graminearum 4X44K oligomer microarray (Agilent Technologies, CA, USA), using an Agilent gene expression hybridization kit. Both dye combinations were performed for each biological replicate. Each array consisted of 1,417 spike-in and negative controls and up to three 60-mer oligos designed for each of 13,918 predicted F. graminearum genes (NCBI GEO, Platform Accession #GPL11046). The hybridizations were scanned using the GenePix Professional 4200A scanner, and the signals quantified using GenePix Pro 6. The microarray data was transferred into Acuity 4.0 and the data was normalized using Lowess Normalization. Data points with low intensities were removed, and the dye swapped replicates were combined and expressed in log2 ratio. ANOVA was used to determine the consistency between samples for each hybridization (P<0.05). The data points were then averaged first between three biological replicates, then within the three average hybridizations. The data was then back transformed from the log scale as fold-expression. Separate datasets were then generated that contained genes that were positively identified in the chromatin immunoprecipitation data, as well as the genes that are up and down regulated in the entire genome by either 1.5-fold or greater and 2.0-fold or greater. Raw data can be accessed at NCBI GEO, Accession # GSE30892.
Supporting Information
Zdroje
1. KangZBuchenauerH 2002 Studies on the infection process of Fusarium culmorum in wheat spikes: Degradation of host cell wall components and localization of trichothecene toxins in infected tissue. Eur J Plant Pathol 108 653 660
2. BennettJWKlichM 2003 Mycotoxins. Clinical Micro Reviews 16 497 516
3. PestkaJJSmolinskiAT 2005 Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Critical Review 9 39 69
4. CalvoAMWilsonRABokJWKellerNP 2002 Relationship between secondary metabolism and fungal development. Microbiol and Mol Bio Reviews 66 447 459
5. GardinerDMKazanKMannersJM 2009 Nutrient profiling reveals potent inducers of trichothecene biosynthesis in fusarium graminearum. Fungal Genet Biol 46 604 613
6. YuJ-HKellerN 2005 Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 43 437 58
7. GardinerDMOsborneSKazanKMannersJM 2009 Low pH regulates the production of deoxynivalenol by Fusarium graminearum. Microbiology 155 3149 3156
8. JiaoFKawakamiANakajimaT 2008 Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol Lett 285 212 219
9. OchiaiNTokaiTTakahashi-AndoNFujimuraMKimuraM 2007 Genetically engineered Fusarium as a tool to evaluate the effects of environmental factors on initiation of trichothecene biosynthesis. FEMS Microbiol Lett 275 53 61
10. HicksJYuJ-HKellerNAdamsTH 1997 Aspergillus sporulation and mycotoxin production both require inactivation of FadA G α protein-dependent signaling pathway. EMBO J 16 4916 4923
11. RozeLVBeudryRMKellerNPLinzJE 2004 Regulation of aflatoxin synthesis by FadA/cAMP/protein kinase A signaling in Aspergillus parasiticus. Mycopathologia 158 219 32
12. TagAHicksJGarifullinaGAkeCjrPhillipsTD 2000 G-protein signaling mediates differential production of toxic secondary metabolites. Mol Microbiol 38 658 665
13. PeñalvaMAArstHN 2004 Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu Rev Microbiol 58 425 451
14. MerhejJRichard-ForgetFBarreauC 2011 The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet Biol 48 275 284
15. FernandesMKellerNAdamsTH 1998 Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol Microbiol 28 1355 1365
16. Kye-YSeongPasqualiMHilburnKLMcCormickSPXuJ 2009 Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol Microbiol 72 354 367
17. HohnTMKrishnaRProctorRH 1999 Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet Biol 26 224 235
18. ProctorRHHohnTMMcCormickSPDesjardinsAE 1995 Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl Environ Microbiol 61 1923 1930
19. PeplowAWTagAGGarifullinaGFBeremandMN 2003 Identification of new genes positively regulated by Tri10 and a regulatory network for trichothecene mycotoxin production. Appl Environ Microbiol 69 2731 2736
20. GardinerDMKazanKMannersJM 2009 Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol Plant-Microbe Interact 12 1588 1600
21. SchreiberKNasmithCAllardGSinghJSubramaniamR 2011 Found in translation: High-throughput chemical screening in Arabidopsis thaliana identifies small molecules that reduce Fusarium head blight disease in wheat. Mol Plant-Microbe Interact 24 640 648
22. JothiRCuddapahSBarskiACiuKZhaoK 2008 Genome-wide identification of in vivo protein-DNA binding sites from ChIP-Seq data. Nucleic Acids Res 36 5221 5231
23. SlaughterJC 1988 Nitrogen metabolism. BerryDR Physiology of Industrial Fungi Blackwell Scientific Publications 58 76
24. MarzlufGA 1997 Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol Biol Rev 61 17 32
25. BeopoulosAMrozovaZTheveniauFLe DallM-THapalaI 2008 Mastering lipid accumulation in the yeast Yarrowia lipolytica. Appl Env Microbiol 74 7779 7789
26. StammersDKRenJLeslieKNicholsCELambHK 2001 The structure of the negative transcriptional regulator NmrA reveals a structural superfamily which includes the short-chain dehydrogenase/reductases. The EMBO Journal 20 6619 6626
27. AndrianopoulosAKourambasSSharpJADavisMAHynesMJ 1998 Characterization of the Aspergillus nidulans nmrA gene involved in nitrogen metabolite repression. J Bacteriol 180 1973 1977
28. BluhmBHZhouXFlahertyJEXuJDunkleLD 2007 RAS2 regulates growth and pathogenesis in Fusarium graminearum. Mol Plant Microbe Interact 20 627 636
29. HoffmanCS 2005 Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem Soc Trans 33 257 260
30. FroeligerEHCarpenterBE 1996 NUT1, a major nitrogen regulatory gene in Magnaporthe grisea, is dispensable for pathogenicity. Mol Gen Genet 251 647 56
31. WongKHHynesMJDavisMA 2008 Recent advances in nitrogen regulation: A comparison between Saccharomyces cerevisiae and filamentous fungi. Eukaryotic Cell 7 917 925
32. MihlanMHomannVLiuT-WDTudzynskiB 2003 AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol Microbiol 47 975 991
33. WilsonRAArstrHNJr 1998 Mutational analysis of AREA, a transcriptional activator mediating nitrogen metabolite repression in Aspergillus nidulans and a member of the “streetwise” GATA family. Microbiol Mol Biol Reviews 62 586 596
34. SchönigBBrownDWOeserBTudzynskiB 2008 Cross-species hybridization with Fusarium verticillioides microarrays reveals new insights into Fusarium fujikuroi nitrogen regulation and the role of AreA and NMR. Eukaryotic Cell 7 1831 1846
35. TeichertSWottawaMSchönigBTudzynskiB 2006 Role of the Fusarium fujikuroi TOR kinase in nitrogen regulation and secondary metabolism. Eukaryotic Cell 5 1807 1819
36. TudzynskiB 1999 Biosynthesis of gibberellins in Gibberella fujikuroi: biomolecular aspects. Appl Microbiol Biotechnol 52 298 310
37. López-BergesMSRisapailNPrados-RosalesRCDi PietroA 2010 A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. The Plant Cell 22 2459 2475
38. ChiangTYMarzlufGA 1994 DNA recognition by NIT2 nitrogen regulatory protein: importance of number, spacing, and orientation of GATA core elements and their flanking sequences upon NIT2 binding. Biochemistry 33 576 582
39. CunninghamTSCooperTG 1993 The Saccaromyces cerevisiae DAL80 represssor protein binds to multiple copies of GATAA-containing sequences (URSGATA). J Bacteriol 175 5851 5861
40. FrandsenFJNKristensenMBAnderssonJAGieseH 2008 Efficient four fragment cloning for the construction of vectors for targeted gene replacement in filamentous fungi. BMC Mol Biol 9 70
41. MillerJDBlackwellBA 1986 Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLX 1503 in a stirred jar fermenter. Can J Bot 64 1 5
42. SalehAAlvarez-VenegasRAvramovaZ 2008 An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protocols 3 1018 1025
43. LiHHomerN 2010 A survey of sequence alignment algorithms for next-generation sequencing. Briefings in Bioinformatics 11 473 483
44. KrawitzPRödelspergerCJägerMJostinsLBauerS 2010 Microindel detection in short-read sequence data. Bioinformatics 26 722 729
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



