-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
article has not abstract
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002178
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002178Summary
article has not abstract
Malaria afflicts 350–500 million people annually, and this debilitating and deadly infectious disease exacts a heavy toll on susceptible populations around the globe. Efforts to find effective, safe, and low-cost drugs for malaria have sharply increased in recent years. Almost all of these efforts have focused on the cyclic blood stage of the disease, partly because the parasites can be easily maintained in culture through addition of human red blood cells to the growth medium, and partly because blood stage infection causes malaria's characteristic symptoms. However, the asymptomatic liver stage, which the parasite goes through only once in its life history, presents the best opportunity for developing drugs that both hit new targets and also could be used in highly desirable eradication campaigns. Recent research, especially on the frequency of differentially expressed genes in blood and liver stage parasites, supports the feasibility of discovering stage-specific drugs. Discovering these drugs will require a high-throughput liver stage phenotypic screen comparable to the existing blood stage screens, and the basic tools for such a screen have recently been created.
Background
Humans have suffered from the burden of malarial infections for thousands of years, and the disease has greatly influenced human evolution and history [1]–[3]. Malaria remains a devastating disease, and in developing countries within Africa, South America, and Asia, the size of its burden has stifled economic growth and development [2]. Despite successful eradication campaigns in North America and Europe, global cases of the disease show little decline, and current improvements rely on pyrethroid treated bed nets and combination therapeutics containing artemisinin derivatives, both of which are susceptible to emerging resistance [4]. Our ability to counter these vulnerabilities with new agents is hampered by the modest number of fully validated drug targets and our limited understanding of many aspects of parasite biology.
Today we understand that malaria is a parasitic disease spread to humans through the bite of an Anopheles mosquito. During their obligatory blood meals, infected female mosquitoes transmit protozoan parasites belonging to the genus Plasmodium, and their proliferation in the human host causes malaria's symptoms (Figure 1). Parasites from infected humans are transmitted back to the mosquito host in subsequent blood meals. The parasite has a complicated life history. In the mosquito gut, the parasites taken up from the human host differentiate into male or female gametes and produce a motile form that migrates through the mosquito gut wall and transforms into an oocyst that in approximately 2 weeks produces and releases thousands of sporozoites that invade the mosquito's salivary glands. Mosquitoes inject sporozoites into the human host during their blood meals, and the sporozoites travel from the skin, through the blood stream, to the liver. Once in the liver, a sporozoite migrates through several liver cells before it settles, propagates, and produces thousands of merozoites (reviewed in [5]) (Figure 1). This exoerythrocytic phase produces no host symptoms. But once the merozoites infect red blood cells, the symptomatic cyclical erythrocytic stage begins. Its waves of bursting red blood cells and the invasion of fresh red blood cells by the newly released parasites produce malaria's characteristic symptoms. In total, the parasite's complicated life history involves two hosts (mosquito and human), three major life-stages (vector stage, liver stage, and blood stage), and multiple forms. The human stages entail vastly different numbers of parasites as an initial infection can begin with as few as 10 parasites from the mosquito, followed by an expansion by a factor of a thousand in the liver, and ultimately an expansion to several trillion in a mature blood stage infection [5].
Fig. 1. Parasite life cycle in the human host and mosquito vector. 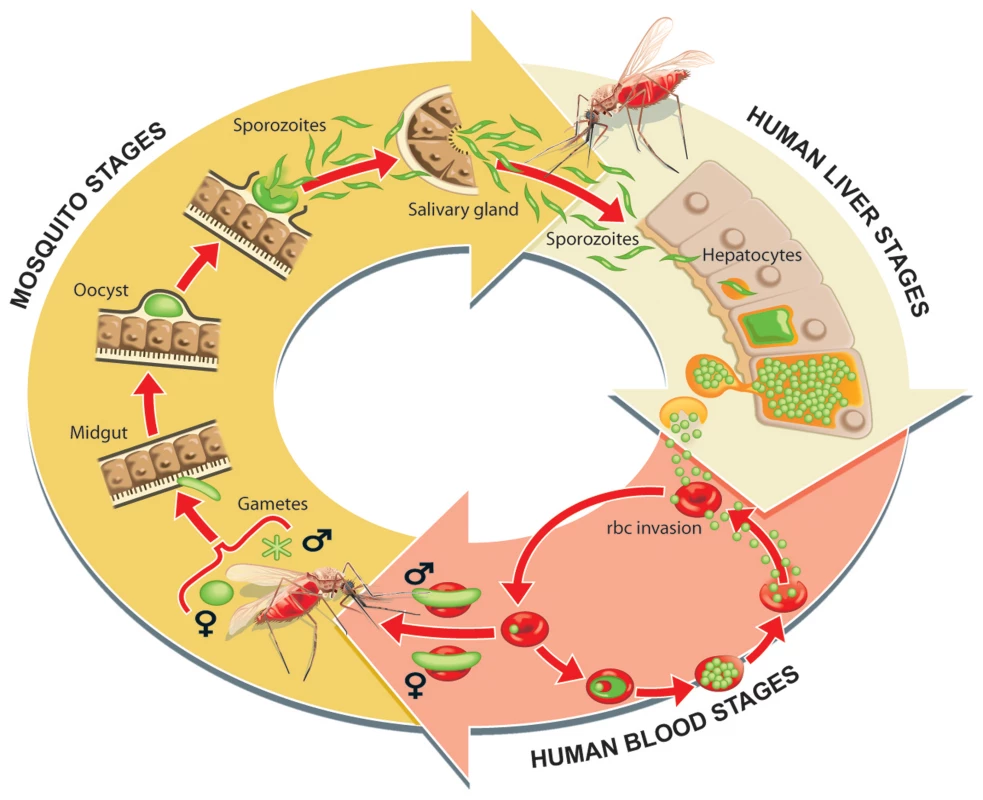
Sporozoites that are found in a mosquito's salivary gland are injected into the skin during the blood meal. The sporozoites that reach a blood vessel travel to the liver and traverse several cells before developing in a hepatocyte. Here the parasite numbers grow significantly and they develop into a form that can invade red blood cells to initiate the cyclic asexual stage. During this time some sexual gametocytes also develop and it is this form of the parasite that is taken up by a mosquito. The parasite invades the mosquito's midgut and develops into sporozoites that can infect a new human host. Graphic prepared by Ian Moores Graphics. P. falciparum is the deadliest of the species of Plasmodium that infect humans, and it accounts for the majority of malaria infections and virtually all of the malaria-related mortality worldwide [6]. P. vivax, P. falciparum's less deadly relative, contributes substantially to malaria's morbidity if not mortality [7], [8]. P. vivax and P. ovale can hide out in the liver for prolonged periods as hypnozoites, the latent hepatic stage, which can cause relapsing malaria months or even years after the initial infection [9]. Only primaquine clears both liver stage parasites and latent stage hypnozoites, while others target only the liver stage parasites (atovaquone). Parasites can rapidly develop drug resistance but at unpredictable and varying rates. There are global concerns that the efficacy of all currently used anti-malarial drugs will erode, thereby creating a pressing need to develop inexpensive yet effective agents that can both treat and eradicate malaria. Ironically, while current drug development focuses on the well-studied blood stage, the vector and liver stages present better options for an eradication campaign, as targeting these stages will prevent infection and disease.
The Past and Present of Anti-Malaria Drug Discovery
Several drugs are used in various combinations to treat malaria, but chloroquine and arteminsinin are arguably the most abundantly used agents (reviewed in [10]). Artemisinin comes from a plant (Artemisia annua) used in traditional Chinese medicine to treat fevers, and the compound and its derivatives are presently used worldwide in anti-malarial combination therapies (reviewed in [11]). Chloroquine was first synthesized early in the last century, although its history can be traced back to quinine from the bark of cinchona trees, the new world's ethnobotanical contribution to the treatment of malaria. Both artemisinin and chloroquine are effective against the parasite's blood stage. Chloroquine targets heme polymerization in the parasite's food vacuole, while artemisinin's target is not known with any certainty [12]. Neither arteminsinin nor chloroquine is effective against the liver stage in vivo. Drug-resistant Plasmodium strains have become prevalent for the currently used blood-stage anti-malarials (reviewed in [13]) and reports of decreased sensitivity towards artensunate in Cambodia (reviewed in [11]) are particularly worrisome.
The high risk of parasite resistance to current therapies highlights the need for replacements, and the most effective replacements are likely to have new targets and new chemotypes to which the parasite has not yet developed resistance. The search for these targets has been frustrated by several factors. Unlike other systems, the sequencing of Plasmodium genomes provides few clues about essential genes and/or processes as ∼50% of the genome contains open reading frames of unknown function [14]. Determining gene function has been hampered by the limited tools available to carry out genetic manipulations on the parasite. And to complete the list of complicating factors, parasite proteins are unusually difficult to express and characterize.
In spite of these obstacles, current approaches to discovering drugs with new targets and chemotypes have had some successes, which have come largely from high-throughput screening of libraries of structurally diverse small molecules. Drug discovery screens can be either target-based or phenotypic. Target-based screens are often operationally simpler as they can use pure proteins in a biochemical assay, but they require selecting the target prior to screening, and they will only discover candidate molecules that modulate the chosen target. Several target-based screens for suitable therapeutic agents have been developed in the past 10 years. Histone deacetylases (HDAC) [15], [16], dihydroorotate dehydrogenase (DHODH) [17], [18], dihydrofolate reductase (DHFR) [19], heat shock protein 90 (Hsp90), and enzymes involved in fatty acid biosynthesis [20], [21] have been among the most promising. While these assays have provided compounds that inhibit malaria growth, the lack of species specificity between the Plasmodium and human enzymes has limited drug development.
Phenotypic screens involving whole cells or even whole organisms have the obvious advantage of being able to find new targets. But they have the drawback of being more, often much more, complicated to run. The only widely used phenotypic malaria screens involve blood stage parasites and a growth/no growth phenotype. There are several published screens that use varying reporters, but they all exploit the ability of the parasite to be grown in red blood cell culture. The first published high-throughput assay utilized a fluorescent DNA dye to quantify the parasite's growth, or lack thereof [22], and most recently GlaxoSmithKline reported the results of a high-throughput screen measuring the parasite's lactate dehydrogenase (LDR) activity [23]. In addition to these methods, luciferase transgenic parasites [24], RNA probes [25], antibody, and PCR-based [26] detection methods have all been developed and have the potential to be optimized toward the goal of anti-malarial drug discovery. Phenotypic screens typically do not reveal an active molecule's mechanism, and linking a molecule to its target can be very difficult [27]. Target identification for phenotypic blood stage screens has repeatedly identified already known targets, like cytochrome bc1 [23]. In this regard, a recent blood stage phenotypic screen by Novartis that identified a gene important for protein synthesis, a P-type cation-transporter ATPase4, is especially noteworthy [28]. In spite of this and hopefully similar successes in the future, we believe that the greatest opportunity for anti-malarial drug discovery lies in the development of non-blood stage parasite screens.
The Need for Anti-Malarial Drugs Targeting the Liver Stage
Liver stage drugs have the potential to hit new targets that are not present or essential during the blood stages of malaria, and these drugs would enjoy a tactical advantage over blood stage drugs because many fewer parasites are involved, which should delay the development of resistance [12]. Liver stage drugs may also have activity against the hypnozoites of P. vivax, like primaquine, and thereby prevent relapsing malaria. Despite their apparent advantages, progress in developing liver stage drugs has been very limited, and this lack of success undoubtedly reflects the way drugs are currently discovered: large numbers of molecules are screened for blood stage activity and only selected active candidates are screened in the liver stage [12]. Primaquine, anti-folates, and atovaquone are currently used drugs with liver stage activity, but among these only primaquine also has anti-hypnozoite activity.
Primaquine, a distant chemical relative of chloroquine whose target has yet to be determined, can inhibit both liver stage infections and blood stage parasites in vitro [29], [30], and also prevent relapsing malaria [9], [31]. Unfortunately, its tendency to cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency, the most common human enzyme deficiency, has severely restricted its use (review in [32]). This deficiency is most common in certain parts of Africa (review in [32]), thus eliminating any hope of using primaquine in an eradication campaign. However, while primaquine itself can never be a widely used anti-malarial agent, its existence argues that other molecules with better therapeutic potential probably exist [12].
Additional evidence for the likelihood of such drugs comes from transcriptional profiling of blood and liver stage parasites. Several recent reports have analyzed both transcriptome and proteome expression levels of malaria parasites in different life stages. These studies uniformly revealed a remarkable number of genes and proteins that are expressed only during the liver stage and thus represent likely stage-specific drug targets (see Figure 2) [33]–[35]. This marked difference between the two stages is unsurprising as the different parasite forms not only find, bind to, and efficiently invade different host cells, but also their replication rates differ by three orders of magnitude. Interestingly, the majority of the differentially expressed liver stage genes encode hypothetical proteins of unknown function [35], a finding that also suggests that they could reveal many new targets. A phenotypic liver stage screen would provide the most efficient path to discovering new drugs that would hit these targets.
Fig. 2. Potential for liver stage specific inhibitors. 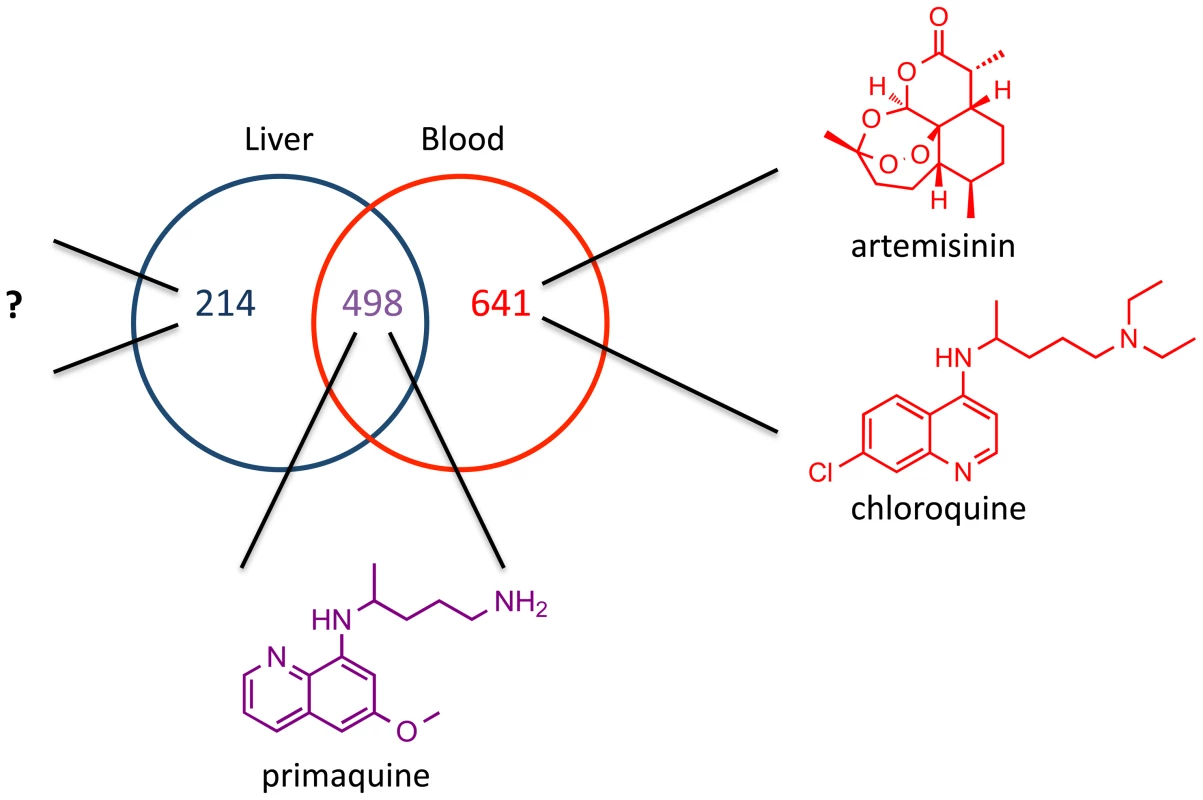
Venn diagram of overlap between P. yoelii liver stage schizont proteins [52] and P. berghei mixed blood stage proteins [53]. Analysis of Plasmodium proteomes was done by Tarun et al. Malaria drugs known to target the various stages are shown. Currently there is no drug on the market that selectively inhibits liver stage parasites. Prospects for a High-Throughput Phenotypic Screen for the Liver Stage
Our understanding of liver stage malaria research has advanced considerably in recent years, and the pieces for a high-throughput screen for liver stage infection appear to be in place. Arguably the most important advance was developing an in vitro culture system for liver stage infection. Now both human hepatoma cell lines and primary hepatocytes can be infected with sporozoites from different Plasmodium species isolated from mosquitoes (review in [36]), although the efficiency is still low. Infection monitoring was initially done with antibody imaging, which was very time consuming [37]–[39]. Still other methods including RT-PCR [40], [41] and fluorescence-activated cell sorting (FACS) using GFP-expressing parasites [42], [43] have emerged, and they allow both overall parasite load and Plasmodium development to be evaluated [44]. Plasmodium sporozoite infection and quantification of liver cells has even been accomplished in 96-well format [45]–[47], suggesting low to medium chemical screening is possible. Luciferase transgenic parasites have been reported in rodent Plasmodium strains (P. yoelii and P. berghei) [48], [49] and these bioluminescent parasites reduce the time of analysis and provide an efficient method to quantify parasite load both in vitro and in vivo.
With both in vitro culturing and reporting methods coming together for a quantitative high-throughput liver stage screen, we anticipate a shift from blood stage to liver stage for high-throughput phenotypic drug screens. It is likely that the rodent malaria strains, including P. yoelii and P. berghei, will be the first to be evaluated as the essential tools are already at hand. Of course, running an assay with today's tools requires the continual infection of liver cells with sporozoites and dissection of infected mosquitoes to isolate these sporozoites. Sporozoite harvesting would currently limit any high-throughput assay. In addition to being tedious, parasite harvesting has an inherent variability as it is difficult to standardize mosquito infections over long periods of time [12]. Still, cryopreservation of large batches of Plasmodium sporozoites may in the future overcome this limitation [50].
Unfortunately, less progress has been made towards reliable in vitro models for hypnozoite assays. To date the only widely used model to screen for anti-hypnozoite activity is via the infection of rhesus monkeys with P. cynomolgi sporozoites (reviewed in [9]). Recently slow growing P. cynomolgi hepatic forms were characterized after sporozoite infection of Macaca fascicularis primary hepatocytes [51]. Similarly, small parasite forms that may be hypnozoites have also been reported after infection of hepatoma cells with purified, cyropreserved P. vivax sporozoites [50]. These reports represent an important first step to establishing in vitro hypnozoite models, but further validation of the system is needed before screening efforts can begin. In particular, re-activation of such putative hypnozoites is required prior to any further development. In that context, in vitro infection of primary hepatocytes or hepatoma cells for longer periods of time must be optimized.
In summary, a high-throughput screen against liver stage parasites should be an attainable goal, although there are still challenges to overcome for truly high throughput. The urgent need for better anti-malaria strategies and the eventual eradication of malaria coupled with the remarkable recent progress from several laboratories make us optimistic about achieving a new generation of liver stage inhibitors in the near future.
Gene IDs for mentioned proteins are 2539 (glucose-6-phosphate dehydrogenase), 812272 (histone deacetylase), 9221804 (dihydrofolate reductase), 811999 (heat shock protein 90), and 3885966 (dihydroorotate dehydrogenase).
Zdroje
1. CarterRMendisKN 2002 Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev 15 564 594
2. SachsJMalaneyP 2002 The economic and social burden of malaria. Nature 415 680 685
3. SnowRWGuerraCANoorAMMyintHYHaySI 2005 The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434 214 217
4. Wolrd Health Organization. 2010 World Malaria Report 2010
5. PrudencioMRodriguezAMotaMM 2006 The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol 4 849 856
6. MendisKSinaBJMarchesiniPCarterR 2001 The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 97 106
7. BassatQAlonsoPL 2011 Defying malaria: fathoming severe Plasmodium vivax disease. Nat Med 17 48 49
8. PriceRNTjitraEGuerraCAYeungSWhiteNJ 2007 Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77 79 87
9. WellsTNBurrowsJNBairdJK 2010 Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol 26 145 151
10. BairdJK 2005 Effectiveness of antimalarial drugs. N Engl J Med 352 1565 1577
11. DondorpAMYeungSWhiteLNguonCDayNP 2010 Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8 272 280
12. MazierDReniaLSnounouG 2009 A pre-emptive strike against malaria's stealthy hepatic forms. Nat Rev Drug Discov 8 854 864
13. WhiteNJ 2004 Antimalarial drug resistance. J Clin Invest 113 1084 1092
14. GardnerMJHallNFungEWhiteOBerrimanM 2002 Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419 498 511
15. PatelVMazitschekRColemanBNguyenCUrgaonkarS 2009 Identification and characterization of small molecule inhibitors of a class I histone deacetylase from Plasmodium falciparum. J Med Chem 52 2185 2187
16. SriwilaijaroenNBoonmaSAttasartPPothikasikornJPanyimS 2009 Inhibition of Plasmodium falciparum proliferation in vitro by double-stranded RNA directed against malaria histone deacetylase. Biochem Biophys Res Commun 381 144 147
17. BaldwinJMichnoffCHMalmquistNAWhiteJRothMG 2005 High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem 280 21847 21853
18. BookerMLBastosCMKramerMLBarkerRHJrSkerljR 2010 Novel inhibitors of plasmodium falciparum dihydroorotate dehydrogenase with anti-malarial activity in the mouse model. J Biol Chem 285 33054 33064
19. BrobeyRKSanoGItohFAsoKKimuraM 1996 Recombinant Plasmodium falciparum dihydrofolate reductase-based in vitro screen for antifolate antimalarials. Mol Biochem Parasitol 81 225 237
20. LeePJBhonsleJBGaonaHWHuddlerDPHeadyTN 2009 Targeting the fatty acid biosynthesis enzyme, beta-ketoacyl-acyl carrier protein synthase III (PfKASIII), in the identification of novel antimalarial agents. J Med Chem 52 952 963
21. SharmaSSharmaSKModakRKarmodiyaKSuroliaN 2007 Mass spectrometry-based systems approach for identification of inhibitors of Plasmodium falciparum fatty acid synthase. Antimicrob Agents Chemother 51 2552 2558
22. BanieckiMLWirthDFClardyJ 2007 High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother 51 716 723
23. GamoFJSanzLMVidalJde CozarCAlvarezE 2010 Thousands of chemical starting points for antimalarial lead identification. Nature 465 305 310
24. GoonewardeneRDailyJKaslowDSullivanTJDuffyP 1993 Transfection of the malaria parasite and expression of firefly luciferase. Proc Natl Acad Sci U S A 90 5234 5236
25. CervantesSPrudhommeJCarterDGopiKGLiQ 2009 High-content live cell imaging with RNA probes: advancements in high-throughput antimalarial drug discovery. BMC Cell Biol 10 45
26. EckerAMoonRSindenREBillkerO 2006 Generation of gene targeting constructs for Plasmodium berghei by a PCR-based method amenable to high throughput applications. Mol Biochem Parasitol 145 265 268
27. GuiguemdeWAShelatAABouckDDuffySCrowtherGJ 2010 Chemical genetics of Plasmodium falciparum. Nature 465 311 315
28. RottmannMMcNamaraCYeungBKLeeMCZouB 2010 Spiroindolones, a potent compound class for the treatment of malaria. Science 329 1175 1180
29. BascoLKBickiiJRingwaldP 1999 In-vitro activity of primaquine against the asexual blood stages of Plasmodium falciparum. Ann Trop Med Parasitol 93 179 182
30. BrayPGDeedSFoxEKalkanidisMMungthinM 2005 Primaquine synergises the activity of chloroquine against chloroquine-resistant P. falciparum. Biochem Pharmacol 70 1158 1166
31. BairdJKHoffmanSL 2004 Primaquine therapy for malaria. Clin Infect Dis 39 1336 1345
32. CappelliniMDFiorelliG 2008 Glucose-6-phosphate dehydrogenase deficiency. Lancet 371 64 74
33. Le RochKGZhouYBlairPLGraingerMMochJK 2003 Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301 1503 1508
34. WestenbergerSJMcCleanCMChattopadhyayRDhariaNVCarltonJM 2010 A systems-based analysis of Plasmodium vivax lifecycle transcription from human to mosquito. PLoS Negl Trop Dis 4 e653 doi:10.1371/journal.pntd.0000653
35. WilliamsCTAzadAF 2010 Transcriptional analysis of the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium yoelii. PLoS ONE 5 e10267 doi:10.1371/journal.pone.0010267
36. KappeSHDuffyPE 2006 Malaria liver stage culture: in vitro veritas? Am J Trop Med Hyg 74 706 707
37. Calvo-CalleJMMorenoAElingWMNardinEH 1994 In vitro development of infectious liver stages of P. yoelii and P. berghei malaria in human cell lines. Exp Parasitol 79 362 373
38. HollingdaleMRLeefJLMcCulloughMBeaudoinRL 1981 In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei from sporozoites. Science 213 1021 1022
39. MazierDBeaudoinRLMelloukSDruilhePTexierB 1985 Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227 440 442
40. Bruna-RomeroOHafallaJCGonzalez-AseguinolazaGSanoGTsujiM 2001 Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31 1499 1502
41. WitneyAADoolanDLAnthonyRMWeissWRHoffmanSL 2001 Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol Biochem Parasitol 118 233 245
42. NatarajanRThathyVMotaMMHafallaJCMenardR 2001 Fluorescent Plasmodium berghei sporozoites and pre-erythrocytic stages: a new tool to study mosquito and mammalian host interactions with malaria parasites. Cell Microbiol 3 371 379
43. TarunASBaerKDumpitRFGraySLejarceguiN 2006 Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int J Parasitol 36 1283 1293
44. PrudencioMRodriguesCDAtaideRMotaMM 2008 Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell Microbiol 10 218 224
45. GegoASilvieOFranetichJFFarhatiKHannounL 2006 New approach for high-throughput screening of drug activity on Plasmodium liver stages. Antimicrob Agents Chemother 50 1586 1589
46. PrudencioMRodriguesCDHannusMMartinCRealE 2008 Kinome-wide RNAi screen implicates at least 5 host hepatocyte kinases in Plasmodium sporozoite infection. PLoS Pathog 4 e1000201 doi:10.1371/journal.ppat.1000201
47. RodriguesCDHannusMPrudencioMMartinCGoncalvesLA 2008 Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe 4 271 282
48. MwakingweATingLMHochmanSChenJSinnisP 2009 Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J Infect Dis 200 1470 1478
49. PloemenIHPrudencioMDouradinhaBGRamesarJFonagerJ 2009 Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS ONE 4 e7881 doi:10.1371/journal.pone.0007881
50. ChattopadhyayRVelmuruganSChakiathCAndrews DonkorLMilhousW 2010 Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS ONE 5 e14275 doi:10.1371/journal.pone.0014275
51. DembeleLGegoAZeemanAMFranetichJFSilvieO 2011 Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS ONE 6 e18162 doi:10.1371/journal.pone.0018162
52. TarunASPengXDumpitRFOgataYSilva-RiveraH 2008 A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A 105 305 310
53. HallNKarrasMRaineJDCarltonJMKooijTW 2005 A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307 82 86
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



