-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Fecal Viral Flora of Wild Rodents
The frequent interactions of rodents with humans make them a common source of zoonotic infections. To obtain an initial unbiased measure of the viral diversity in the enteric tract of wild rodents we sequenced partially purified, randomly amplified viral RNA and DNA in the feces of 105 wild rodents (mouse, vole, and rat) collected in California and Virginia. We identified in decreasing frequency sequences related to the mammalian viruses families Circoviridae, Picobirnaviridae, Picornaviridae, Astroviridae, Parvoviridae, Papillomaviridae, Adenoviridae, and Coronaviridae. Seventeen small circular DNA genomes containing one or two replicase genes distantly related to the Circoviridae representing several potentially new viral families were characterized. In the Picornaviridae family two new candidate genera as well as a close genetic relative of the human pathogen Aichi virus were characterized. Fragments of the first mouse sapelovirus and picobirnaviruses were identified and the first murine astrovirus genome was characterized. A mouse papillomavirus genome and fragments of a novel adenovirus and adenovirus-associated virus were also sequenced. The next largest fraction of the rodent fecal virome was related to insect viruses of the Densoviridae, Iridoviridae, Polydnaviridae, Dicistroviriade, Bromoviridae, and Virgaviridae families followed by plant virus-related sequences in the Nanoviridae, Geminiviridae, Phycodnaviridae, Secoviridae, Partitiviridae, Tymoviridae, Alphaflexiviridae, and Tombusviridae families reflecting the largely insect and plant rodent diet. Phylogenetic analyses of full and partial viral genomes therefore revealed many previously unreported viral species, genera, and families. The close genetic similarities noted between some rodent and human viruses might reflect past zoonoses. This study increases our understanding of the viral diversity in wild rodents and highlights the large number of still uncharacterized viruses in mammals.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002218
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002218Summary
The frequent interactions of rodents with humans make them a common source of zoonotic infections. To obtain an initial unbiased measure of the viral diversity in the enteric tract of wild rodents we sequenced partially purified, randomly amplified viral RNA and DNA in the feces of 105 wild rodents (mouse, vole, and rat) collected in California and Virginia. We identified in decreasing frequency sequences related to the mammalian viruses families Circoviridae, Picobirnaviridae, Picornaviridae, Astroviridae, Parvoviridae, Papillomaviridae, Adenoviridae, and Coronaviridae. Seventeen small circular DNA genomes containing one or two replicase genes distantly related to the Circoviridae representing several potentially new viral families were characterized. In the Picornaviridae family two new candidate genera as well as a close genetic relative of the human pathogen Aichi virus were characterized. Fragments of the first mouse sapelovirus and picobirnaviruses were identified and the first murine astrovirus genome was characterized. A mouse papillomavirus genome and fragments of a novel adenovirus and adenovirus-associated virus were also sequenced. The next largest fraction of the rodent fecal virome was related to insect viruses of the Densoviridae, Iridoviridae, Polydnaviridae, Dicistroviriade, Bromoviridae, and Virgaviridae families followed by plant virus-related sequences in the Nanoviridae, Geminiviridae, Phycodnaviridae, Secoviridae, Partitiviridae, Tymoviridae, Alphaflexiviridae, and Tombusviridae families reflecting the largely insect and plant rodent diet. Phylogenetic analyses of full and partial viral genomes therefore revealed many previously unreported viral species, genera, and families. The close genetic similarities noted between some rodent and human viruses might reflect past zoonoses. This study increases our understanding of the viral diversity in wild rodents and highlights the large number of still uncharacterized viruses in mammals.
Introduction
The order Rodentia is the single largest group of mammalian species accounting for 40% of all mammal species [1]. There are ca 2200 living rodent species, including mice, rats, voles, squirrels, prairie dogs, beavers, chipmunks, and guinea pigs. Many rodents have mixed diets but some eat mostly seeds or green vegetation. Rodents are known to vector more than 60 known human infectious diseases [2]. Some rodents live in close association with humans offering numerous opportunities for cross-species viral transmission through their urine, feces, or their arthropod ectoparasites such as ticks, mites, and fleas [2]–[8].
Rodents have been associated with numerous viruses including members of the Arenaviridae, Reoviridae, Togaviridae, Picornaviriade, and Flaviviridae families [2], [9]–[12]. The hantavirus pulmonary syndrome (HPS), an infection with an exceptionally high mortality first identified in the southwestern United States, was recognized as a zoonotic viral infection with Sin Nombre virus (SNV) in the Hantavirus genus in the Bunyaviridae family originating from deer mouse (Peromyscus maniculatus) [13]. Since then, HPS has been identified throughout the United States [14]–[17] with SNV responsible for most cases [18]–[23]. Deer mice captured in Montana showed an SNV antibody prevalence of approximately 11% [24]. Other members of the Hantavirus genus transmitted from rodents include Hantaan, Dobrava-Belgrade, Seoul, and Puumala viruses, causing hemorrahagic fever with renal syndrome (HFRS) worldwide [25]–[31]. HFRS was endemic in 28 of 31 provinces of China and is considered a major public health concern. Over 1,200 HFRS cases occurred in 2007 in China [32], [33]. It was reported that there were approximately 8,300 patients with HFRS in Inner Mongolia and 261 (3.14%) died during 1955–2006 [33]. Recently, Seoul virus was detected in 47 of 649 Norway rats (Rattus norvegicus) [34]. Several vole species (Microtus arvalis, Pitymys subterraneus, and M. subterraneus) have been linked with Tula virus also in the Hantavirus genus [35]–[39]. In Switzerland, acute infection with Tula virus was found in a 12-year-old boy after a rodent's bite [40]. Tick-borne encephalitis virus (TBEV) in the Flavivirus genus of the family Flaviviridae can cause fatal encephalitis in humans [41]–[43]. Several rodent species such as voles (Microtus agrestis and Myodes glareolus), field mice (Apodemus agrarius) are natural hosts of ticks that cause TBE [43], [44]. Lassa fever, an acute viral hemorrhagic fever first described in 1969 in Nigeria, is caused by Lassa virus, a member of the family Arenaviridae [45]. Its primary animal host is the Multimammate mouse (Mastomys [Praomys] natalensis) [3]. Lassa fever, endemic in West Africa, causes 30,000–500,000 cases and 5,000 deaths annually [46].
Because of their health and economic impact there is a growing awareness of emerging (and re-emerging) zoonotic infections [2], [7]. Increased interactions between rodents and humans occur when people build homes in wildlife habitat or conduct more recreational activities there [2], [47], [48]. Irruptions in density of rodent hosts, as occurred prior to the SNV infections in the southwestern US, also increase the risk of human viral exposure [49]. Viral surveys in wild and domesticated animals with extensive contacts with humans can be used to monitor for the presence of known zoonotic viruses or closely related viral species and to provide a baseline of the viruses present to help detect future changes associated with disease outbreaks. The identification of animal viruses closely related to human viruses also provides information regarding past successful zoonoses. To assist in these goals we performed an initial characterization of the fecal viromes of rodents from two locations in the US.
Results
Viral metagenomic overview
Viral particles in fecal samples were purified by filtration, digested with DNase and RNase enzymes to remove unprotected nucleic acids, amplified by random RT-PCR, and subjected to 454 pyrosequencing. A total of 1,441,930 sequence reads with an average of 177-bp were generated from the extracted nucleic acids in the present study and the sequences from each animal were assembled de novo into contigs of variable length. Both singlets and contigs longer than 100-bp were then classified using BLASTx and BLASTn as likely virus, phage, bacteria, or eukaryota based on the taxonomic origin in the annotation of the best-hit sequence (E score <10−5) with the GenBank non-redundant database. Fecal samples of mice, voles, and a woodrat revealed a large degree of microbial diversity. 26,846 sequence reads had best matches with viral protein, RNA or DNA sequences as shown in Figure 1. There were also ∼585,000 sequences for bacteria, 30,400 for eukaryota, and 154,000 for phage. A large proportion of the total reads (45%) did not have any significant hits to nucleotide or amino acid sequences in GenBank in agreement with viral metagenomic studies of feces from bats, turkeys, and humans [50]–[52].
Fig. 1. Taxonomic classification of sequences with similarity to eukaryotic viruses. 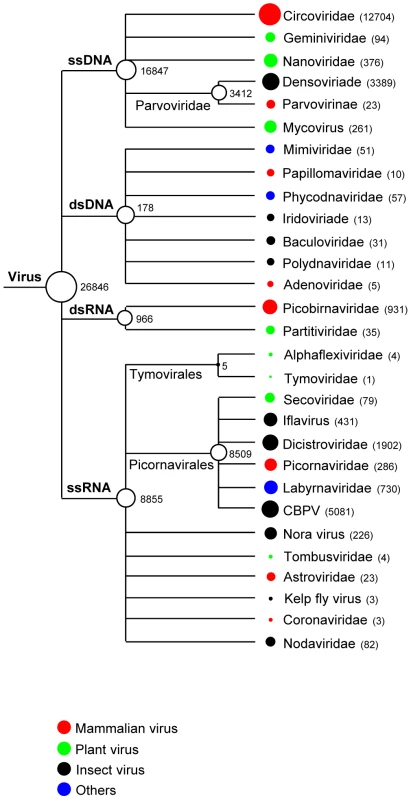
Circles located next to taxa are logarithmically proportional to the total number of sequence reads with BLASTx E<10−5. The numbers of sequence read are also included. Mammalian viruses
The largest proportion of the rodent fecal virus-related sequences (52%) was related to mammalian viruses, with 91% of these being related to DNA viruses. Viral sequences related to single-stranded DNA viruses in the Circoviridae were abundant, comprising 90% of the mammalian DNA virus-like sequence reads. DNA viruses in the Parvovirinae subfamily, Papillomaviridae, and Adenoviridae families were also detected. RNA viral sequences were mostly related to the families Picobirnaviridae and Picornaviridae. A few RNA virus sequences (n = 23) related to the family Astroviridae were also identified. While some sequences showed >90% similarity at the amino acid level with known viruses, the majority exhibited <70% similarity. We further characterized some of these novel mammalian virus-like sequences by full or near full genome sequencing and compared them to their closest relatives by phylogenetic analyses. Partial viral genomes were similarly analyzed.
Mouse papillomavirus
Papillomaviruses (PVs) are a highly diverse family of double-stranded circular DNA genomes ca 8-kb in size. PVs are known to infect a wide variety of mammals, as well as birds and reptiles. Some PV types cause benign or malignant epithelial tumors of the skin and mucous membranes in their natural hosts, while others are commonly present in the healthy skin of healthy humans, as well as a range of different animal species. PVs are highly species-specific and rarely transmitted between species. More than 100 human PV types have been detected, and the genomes of more than 80 have been completely sequenced [53], [54]. Only a few full genomes of PVs have been reported in non-human species.
We characterized the full-length genome of the deer mouse (Peromyscus maniculatus) PV type 1, hereafter referred to as PmPV1 (GenBank JF755418). The complete circular PmPV1 genome was 7,704-bp, with a GC content of 51%. Six distinct ORFs on the same coding strand were identified, including the early genes E6, E7, E1, and E2 and the late genes L2, and L1 (Figure 2A). Analysis of the deduced amino acid sequences revealed two characteristic zinc-binding domains (C-X2-C-X29-C-X2-C) in E6, separated by 36 amino acids. E7 also contained a zinc-binding domain and the conserved retinoblastoma tumor suppressor-binding motif (L-X-C-X-E). The C-terminal region of E1 protein had an ATP-dependent helicase motif (GPPDTGKS) and also contained a cyclin interaction RXL motif required for viral replication. The long control region (LCR) between the end of the L1 gene and the start of the E6 gene, was 469 bp. In the C-terminus of LCR, two consensus E2-binding sites (ACC-X6-GGT) were present. The TATA box (TATAAA) of the E6 promoter was located at position 7661 and a polyadenylation site (AATAAA; nt 7240) for processing of the L1 and L2 capsid mRNA transcripts were found at the N-terminus of the LCR. Taken together, many of the classic PV specific elements were identified in PmPV1.
Fig. 2. Mouse papillomavirus. 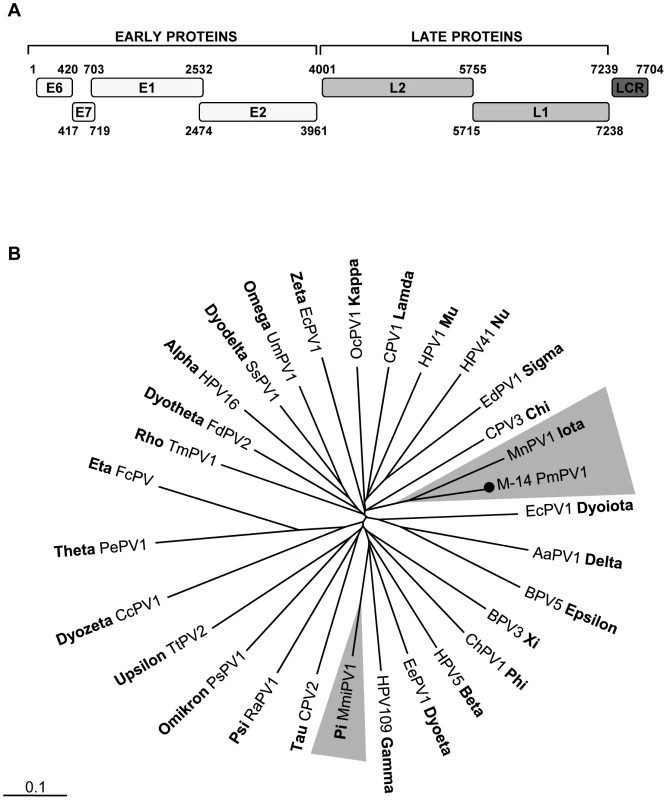
A. Genome organization of a novel mouse papillomavirus. B. Phylogenetic tree generated with concatenated L1 and L2 capsid proteins of PmPV1_M-14 and representatives of all genera in the family Papillomaviridae. The novel mouse papillomavirus in this study is labeled with a black circle. The other murine papillomavirus MmiPV1 is highlighted in grey. The scale in this and every tree indicates amino acid substitutions per position. The family Papillomaviridae currently contains at least 29 genera and mouse PVs have been found in the Pipapillomavirus and Iotapapillomavirus genera [53]. Phylogenetic analysis of the complete L1 protein was performed. PmPV1 shared the same root as the Multimammate mouse (Mastomys natalensis) PV type 1 (MnPV1) in the Iotapapillomavirus genus (Figure 2B). PmPV1 had the highest L1 similarity of 67% to the L1 of MnPV1 (Table S1). In addition, the closest amino acid similarities of other major PmPV1 proteins (E1, E2 and L2) were also to MnPV1. According to the International Committee on Taxonomy of Viruses (ICTV), different PV species share between 60% and 70% of nucleotide sequence similarity in the L1 ORF [53]. PmPV1 is therefore a proposed new PV species within the Iotapapillomavirus genus sharing 63% similarity with MnPV1.
Rodent circo-like viruses
Circular ssDNA viruses known to infect animals have the smallest viral genomes and are classified in the Circoviridae family and the unassigned genus Anellovirus. Circular ssDNA genome infecting plants belong to the Germiniviridae and Nanoviridae families. Despite very distinct host-specificities, these viruses share conserved motifs in their Replication initiator proteins (Rep) including a helicase domain [55]. Several circovirus-like genomes were also recently characterized from reclaimed water and marine environments [56], [57] and directly from a single cell of a marine protist [58]. Rep-like sequences were found in feces from 23% (12/52) of mice, 63% of voles (33/52), and 100% of cotton rat (1/1). Seventeen full circular DNA viral genomes were then sequenced using inverse PCR targeting the initial Rep sequence matches. The GenBank accession numbers are in Table S2. One genome was derived from feces of the Woodrat Neotoma cinerea, four from the mice Peromyscus maniculatus and Mus musculus and twelve from the voles Microtus pennsylvanicus. The smallest genome was 1,124-bp and the largest 3,781-bp.
These replicase-containing circular genomes could be placed into four different types of ORF organization following the classification of Rosario et al [56] (Figure 3). The type I genome had features similar to circoviruses and was characterized by a small circular DNA genome (approximately 2-kb), with the Rep protein and an unknown major ORF in opposite orientation. However, a majority of the type I genome had a stem-loop structure located in the 3′ downstream intergenic region distinct from the 5′ intergenic location seen in circoviruses, nanoviruses, and geminiviruses. The type II genome had the unique feature of encoding two separate Rep ORFs. The type III genomes contained two major ORFs in the same orientation in a manner similar to anelloviruses, a highly diverse but phylogenetically unrelated group of viruses with circular DNA genomes. The type IV genome had the largest genomes of nearly 4-kb, with up to eight ORFs. Due to their diverse genomic architectures, we preliminarily named these circular DNA genomes rodent stool-associated circular viruses (RodSCVs).
Fig. 3. Genomic organization of RodSCVs. 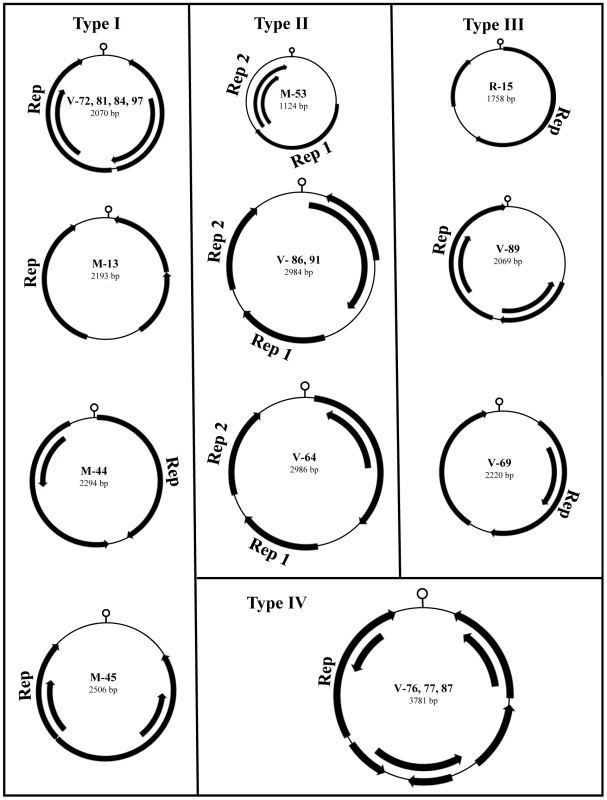
The novel rodent stool-associated circular viruses (RodSCVs) are classified into four different genomic organization categories. The location of putative Rep genes and other ORFs (greater than 100 amino acids long) are indicated by arrows. A DNA stem-loop containing a conserved nonamer is thought to have an important role in initiating rolling-cycle and a binding site for the Rep protein during circovirus replication [59]–[61]. The nonamer sequences in RodSCVs were slightly different from each other, but shared 5–7 nucleotide identity with those in circoviruses, nanoviruses, and geminiviruses [62]. Interestingly, RodSCV-M-13, M-45, and V-89 had the same nonamer sequence GGGTAATAC although they differed in genomic size and organization (Table S2).
The Rep proteins in RodSCVs possessed conserved motifs (DRYP and WWDGY) but not the DDFYGW motif typical of circoviruses. The Rep proteins in RodSCVs contained at least one well-known superfamily domain, either viral Rep, RNA helicase, or Gemini-AL1. In the type I genome, M-45 carried a homologue of the viral Rep superfamily; however, M-13, -44, V-72, -81, -84 and -97 contained both the viral Rep plus the RNA helicase superfamily domains within a single Rep ORF. By BLASTx, the Rep proteins of M-44 and 45 showed the most significant hits (E-value = 1×10−73 and 2×10−19, respectively) with circovirus-like genomes found in the environment [56] (Table S2). The Rep proteins of RodSCV-M-13, -44, V-72, -81, -84 and -97 showed the top BLAST hits (E-value = 4×10−35 and 2×10−82, respectively sharing 20–52% protein similarity) to the Rep protein of Giardia intestinalis, a major diarrhea causing parasite in humans. In the type II genome, M-53 had both the viral Rep and the RNA helicase superfamilies in two separate Rep ORFs. V-64, -86 and -91 did not contain any recognizable viral Rep superfamily protein domain, but their Rep-like proteins showed best BLASTx matches with the Rep of the plant geminiviruses. In the type III and IV genomes, RodSCV-R-15, V-76, -77 and -87 contained the Gemini-AL1 superfamily motif that is commonly found in geminiviruses (Table S2). None of the other ORFs in the all RodSCVs showed detectable similarities to known protein superfamilies or significant matches in GenBank.
In order to phylogenetically classify the RodSCVs their Rep proteins were aligned to those of circoviruses, cycloviruses, geminiviruses, nanoviruses, gyrovirus (Chicken anemia virus), environmental circovirus-like genomes, Bifidobacterium pseudocatenulatum plasmid p4M, and the protozoans Giardia intestinalis and Entamoeba histolytica. The RodSCV Rep were located on separate branches with some clustering with circovirus-like genomes derived from marine and reclaimed waters while other clustered with an integrated Rep in the genome of the parasitic protozoan Giardia intestinalis (Figure 4). Theses findings indicated that the RodSCVs likely represent several novel viral families.
Fig. 4. Phylogenetic analysis of Rep proteins of RodSCV and single stranded circular viral genomes. 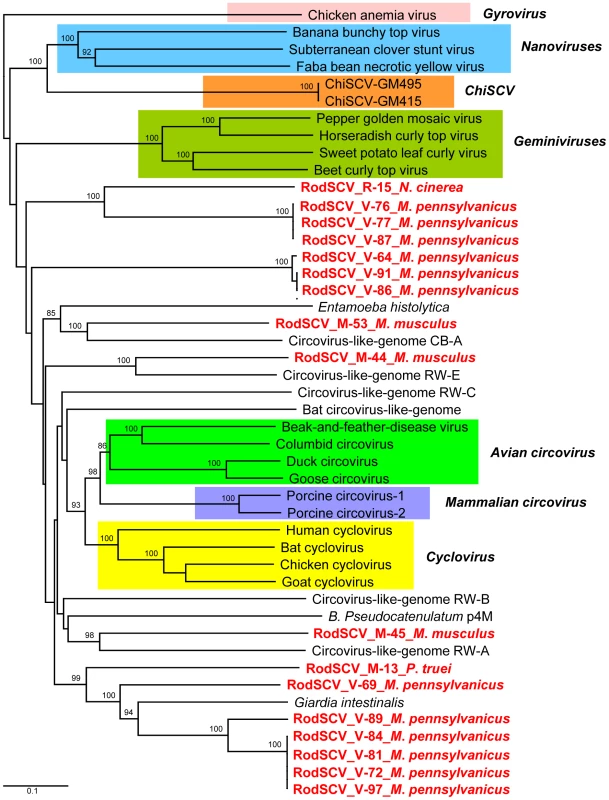
Phylogenetic tree obtained from amino acid sequences of complete Rep genes of members of the families Geminiviridae, Nanoviridae, Circoviridae, Gyrovirus, Canarypox virus, Circovirus-like genomes, Bifidobacterium pseudocatenulatum plasmid p4M, Giardia intestinalis, and Entamoeba histolytica. The novel RodSCVs in this study are highlighted in red. Mouse kobuvirus
The Aichi picornavirus was first identified in human cases of oyster-associated gastroenteritis in 1989 [63]–[65]. Aichi virus, a second species of human kobuvirus named salivirus/klassevirus also associated with diarrhea [66]–[70], bovine kobuvirus, porcine kobuvirus and a partial genome from a bat constitute the Kobuvirus genus in the family Picornaviridae (http://www.picornaviridae.com/kobuvirus/kobuvirus.htm). The reference Aichi virus genome is approximately 8-kb and similar to other picornaviruses contains a single large ORF that encodes a large polyprotein of 2,433 amino acids proteolytically cleaved into structural and non-structural proteins.
We found several sequence reads from two mouse (Peromyscus crinitus and P. maniculatus) fecal samples with similarities to Aichi virus. The sequence reads shared a high nucleotide similarity of 93% to each other, suggesting that the picornaviruses in these mice were from the same species. We successfully acquired the complete genome (8,171-bp, excluding the poly(A) tail) of the kobuvirus M-5 from Peromyscus crinitus mouse (GenBank JF755427), including 5′ UTR (610-bp), polyprotein ORF (2,439-aa) and 3′ UTR (244-bp). An optimal Kozak sequence, RNNAUGG (ATCATGG), was found at the beginning of the translated polyprotein. The leader (L) protein, located in the amino-terminal part of the polyprotein, showed the closest match (68%) to the L of human Aichi virus. The N-terminal zinc finger protein-binding motif C-X-H-X(6)-C-X(2)-C essential for the cytosol-dependent phosphorylation cascade found in L proteins of the Cardiovirus genus, such as Encephalomyocarditis virus and Theiler's murine encephalomyelitis virus could not be found in the mouse kobuvirus [71], [72]. In the 2C protein a highly conserved nucleotide-binding domain of the helicase (GPPGTGKS) was identified. In addition, the RNA-binding domain GLCG and the H-D-C catalytic triad were present at amino acid positions 42, 84 and 143 in the 3Cpro region as were the characteristic RdRp KDELR, YGDD and FLKR motifs in 3Dpol. Phylogenetic analysis of 3Dpol showed that the mouse kobuvirus was the closest relative to the human Aichi virus (Figure 5). This finding was also supported by pair-wise comparison in which mouse Kobuvirus M-5 had 81–84% similarities to human Aichi virus and 53–63% similarities to bovine kobuvirus at the amino acid level for the P1, P2 and P3 regions (Table S3). Genome analysis therefore indicated that the M-5 should be considered a new species and the first murine kobuvirus.
Fig. 5. Phylogenetic analysis of mouse kobuvirus. 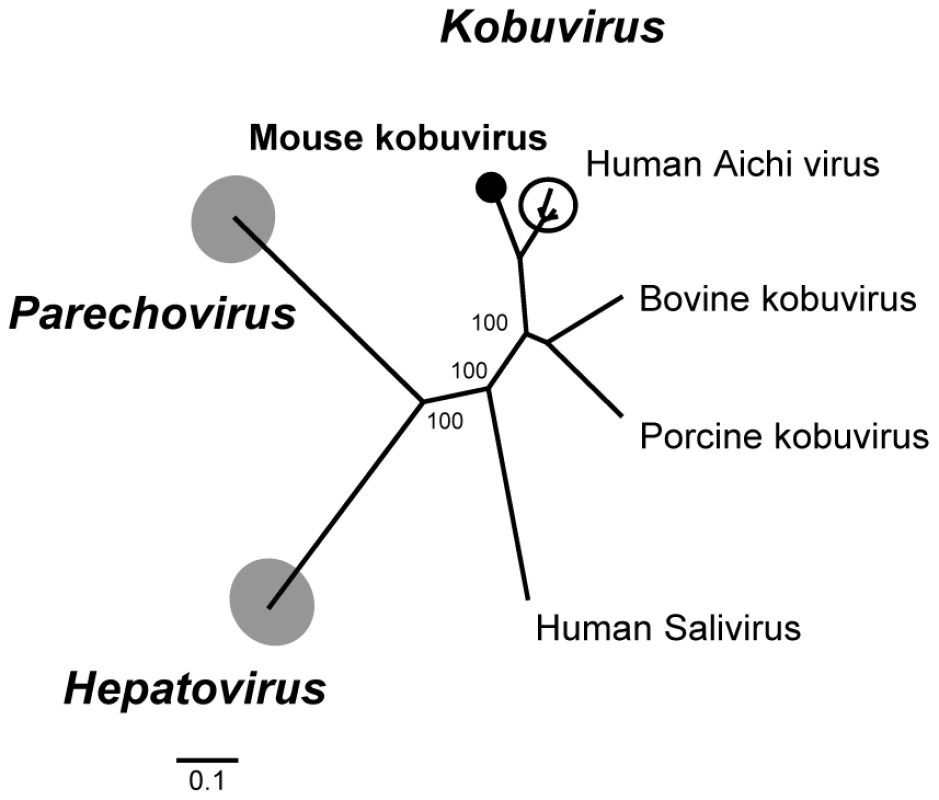
Phylogenetic tree generated with complete 3Dpol protein of members of the genera Kobuvirus, Hepatovirus and Parechovirus in the family Picornaviridae. The human Aichi viruses are encircled. The novel mouse kobuvirus in this study is labeled by a black circle. Molecular epidemiology of human Aichi virus worldwide has been performed using specific primers targeting the 3CD junction [73]–[75]. In order to understand the prevalence of Aichi-like viruses in rodents, we designed consensus primers based on 2C-3B regions conserved between rodent and human Aichi viruses and used RT-nPCR to screen other rodent fecal samples. Two additional mice (Peromyscus crinitus and P. maniculatus) were positive for Aichi virus. Their nucleotide sequences over that region (∼700-bp) were 94–95% similar to the mouse kobuvirus with which they also clustered phylogenetically (data not shown).
Mouse Sapelovirus
We detected a Sapelovirus-related sequence encoding 207 amino acids in house mouse (Mus musculus) feces M-58 (GenBank JF755421). This sequence covered about 25% of the capsid encoding P1 region and showed the best amino acid similarity (53%) to a porcine Sapelovirus. According to the ICTV, members of a picornavirus genus should share at least 40% amino acid similarity in the P1 region. Phylogenetically this sequence was also related to the Sapelovirus genus, indicating that it represents a fraction of the first reported murine Sapelovirus genome (Figure S1).
Proposed new mouse picornavirus genus
The family Picornaviridae currently consists of 12 genera, although recently characterized genomes are expected to nearly double that number (www.picornaviridae.com). In this study, we found a novel picornavirus in the feces of a canyon mouse (Peromyscus crinitus) we temporarily named Mosavirus for mouse stool associated picornavirus. We successfully acquired the nearly complete genome (6934-bp, excluding the poly(A) tail) of Mosavirus (GenBank JF973687) including a partial 5′ UTR (138-bp), complete polyprotein ORF (2235-aa) and complete 3′ UTR (88-bp). The hypothetical cleavage map of the Mosavirus polyprotein was derived from alignments with other known picornaviruses. Similar to Cardioviruses and Senecavirus, Mosavirus had a conserved cleaved site Q↓GN for a putative L protein preceding the capsid region. The P1 polypeptide (758-aa) contained the rhv-like superfamily and best BLASTx match to Theiler's encephalomyelitis virus in Cardiovirus genus, sharing 37% aa similarity. The P1 was hypothesized to be cleaved at VP4/VP2 (M↓D), VP2/VP3 (Q↓G) and VP3/VP1 (Q↓G). The VP4 was found to have the GXXX[T/S] myristylation site. The P2 polypeptide (501-aa) encoded non-structural proteins cleaved at 2A/2B (G↓P) and 2B/2C (Q↓G). BLAST showed that the P2 region had the highest aa similarity of 36% to the Saffold virus belonging to Cardiovirus genus. Similar to cardioviruses the 2A protein in Mosavirus had the conserved NPGP motif for 2A-mediated cleavage at the 2A/2B junction. The 2A protein was predicted to be 36 amino acids shorter than those of any known cardioviruses (∼150-aa). The 2C protein contained some characteristic features of picornaviruses such as the RNA-helicase superfamily, NTPase and helicase motifs. The P3 polypetide encoded proteins 3A, 3B (VPg, small genome-linked protein), 3Cpro (protease) and 3Dpol (RNA-dependent RNA polymerase). P3 with 822 amino acids in length was cleaved at 3A/3B (E↓G), 3B/3C (Q↓G) and 3C/3D (Q↓G). 3Cpro contained the peptidase C3 superfamily and conserved GXCG motifs. Some conserved YGDD, FLKR and GG[LMN]PSG motifs were identified in the 3D protein. The pair-wise amino acid sequence analysis demonstrated that the P3 polyprotein in Mosavirus shared very low aa similarity, less than 29%, to the genetically-closest picornaviruses (Table S4). Based on the 3Dpol phylogenetic tree (Figure 6) and genetic distance calculations, Mosavirus is not significantly linked to any recognized or proposed genera. According to the ICTV, the member of a picornavirus genus should share >40%, >40% and >50% amino acid similarity in P1, P2 and P3 regions respectively. The similarities over these three regions in Mosavirus were less than 40% at the amino acid level to those of any reported piconaviruses. Mosavirus is therefore proposed as a novel genus in the family Picornaviridae.
Fig. 6. Phylogenetic analysis of Mosavirus and Rosavirus. 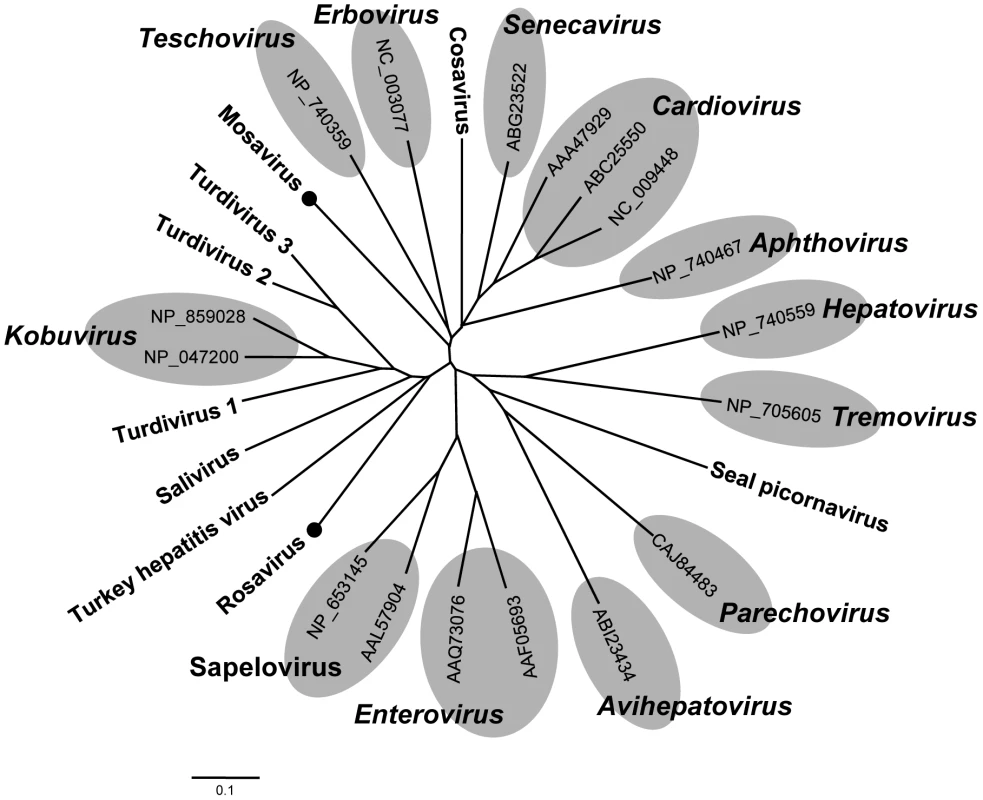
Phylogenetic tree obtained from amino acid sequences of complete 3D proteins of all taxonomic genera in the family Picornaviridae. Mosavirus and Rosavirus are labeled with black circles. A second proposed new picornavirus genus
In the same house mouse feces where Mosavirus was found, we also found another picornavirus temporarily named Rosavirus for rodent stool associated picornavirus. A genome fragment of 3,956-bp was sequenced, including a partial 2C gene, the complete P3 region and a complete 3′ UTR (GenBank JF973686). The 2C segment contained the conserved NTPase motif GXXGXGKS (GGPGCGKS) and helicase motif DDLGQ typical of picornaviruses. The P3 region of Rosavirus was hypothesized to be cleaved at 3A/3B (E↓G), 3B/3C (Q↓I) and 3C/3D (Q↓G). The 3A (106-aa) and 3B (31-aa) proteins had typical lengths but did not show any detectable sequence similarity to other picornaviruses. 3B did have a conserved tyrosine at position 3 and another conserved glycine at position 5, consistent with its function as the genome-linked protein, VPg.
The 3C, with 206 amino acids, had the H-D-C catalytic triad at positions 47, 90, and 158, followed by 15 amino acids downstream of the GIH motif, similar to the substrate-binding site of chymotrypsin-like proteases. By BLAST, 3C contained the peptidase C3 superfamily and was genetically closest to turkey hepatitis virus, sharing 30% amino acid similarity. Rosavirus 3D contained conserved RdRp motifs KDELR, YGDD and FLKR. Interestingly, the 3D protein in Rosavirus has a mutated motif GAMPSG compared with conserved GG[LMN]PSG in other picornaviruses. The 3D protein in Rosavirus was most closely related to the 3D of avian turdivirus 2, sharing 44% amino acid similarity. In addition, Rosavirus had the longest reported 3′ UTR of 795-bp in length.
The P3 region in Rosavirus showed very low amino acid similarity (<31%) to the closest picornavirus (Table S4). The P3 regions of Rosavirus and Mosavirus shared only 23% similarity at the amino acid level. The 3Dpol-based phylogenetic grouping demonstrated that Rosavirus shared a monophyletic root with members of the genus Kobuvirus, turdiviruses and turkey hepatitis virus (Figure 6). A similar topology was found in a 3Cpro-based phylogenetic analysis (data not shown). Based on genetic distance criteria Rosavirus is therefore a candidate prototype for a novel genus in the family Picornaviridae.
Rodent picobirnaviruses
Picobirnavirus (PBV), the only genus in the new Picobirnaviridae viral family, has a bi-segmented dsRNA genome. The large RNA segment encodes the capsid protein while the small segment encodes the viral RdRp. Originally found in the intestines of rat [76] PBV has since been found in numerous mammals [77]. In humans, ca 20% of fecal diarrheal samples in the Netherlands were positive for PBV [78]. This virus has also been reported as a causative agent of gastroenteritis in HIV-positive patients [79], [80]. Based on the small segment sequence, PBV has been classified into two genogroups, I and II [81]. However, there are few full-length sequences of this segment.
23% of feces from mice (12/52) and 19% from voles (10/52) contained PBV related sequences, the second highest prevalence and number of mammalian virus-like sequences after the Circoviridae-like reads (Figure 1). Previous reports indicated that the genogroup I was predominant in humans [78], [82]. Two fecal specimens had large number of PBV-like reads that assembled into nearly full-length RdRp for two strains, M-58 (house mouse Mus musculus) (412-aa at GenBank JF755419) and V-111 (vole Microtus pennsylvanicus) (414-aa at GenBank JF755420). Amino acid sequence alignment of nearly full-length RdRp allowed the construction of a phylogenetic tree using all other available sequences of similar length (Figure 7). M-58 and V-111 clustered with strains in genogroup I. M58 and V-111 were 63% similar at the amino acid level to each other and only 54% to 63% similar to human and bovine PBVs in genogroup I. These two picobirnaviruses therefore represent new PBV species, the first reported in mice and voles.
Fig. 7. Phylogenetic analysis of rodent picobirnaviruses. 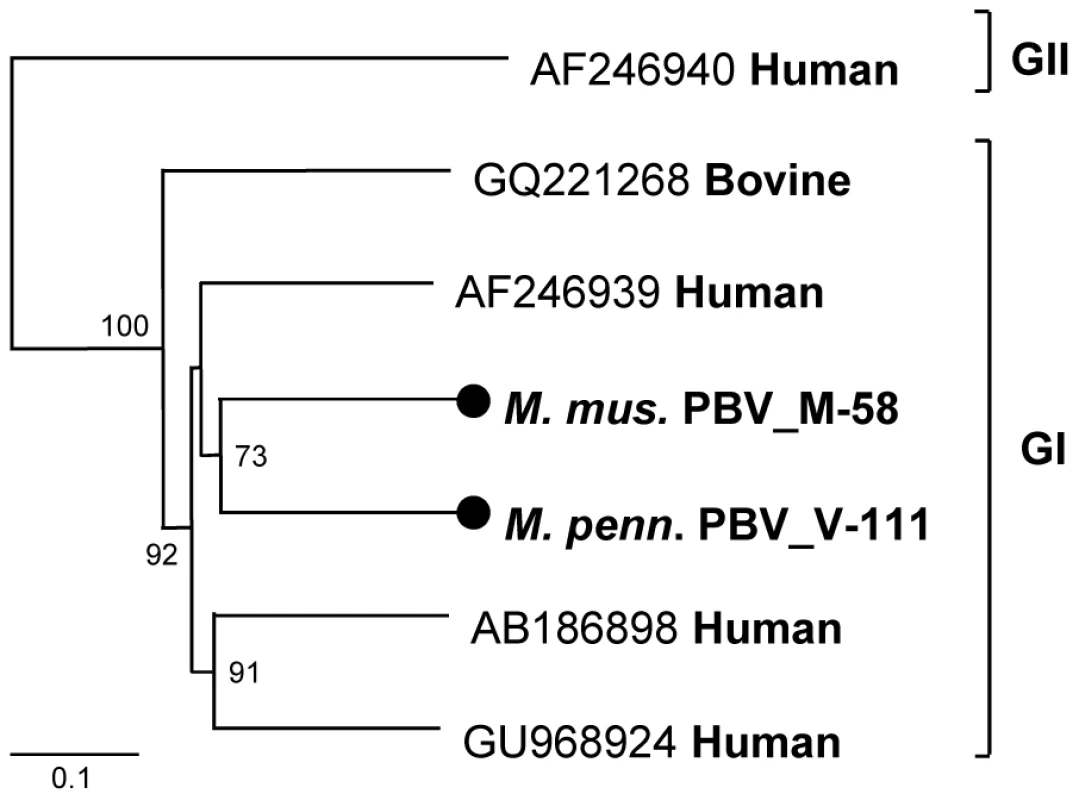
Phylogenetic tree obtained from nearly full-length RdRp protein of genogroup I and II picobirnaviruses in the family Picobirnaviridae. The novel picobirnaviruses are labeled by black circles. Mouse astrovirus
The family Astroviridae consists of two genera, namely, Mamastrovirus and Avastrovirus, that infect mammalian and avian species, respectively. Astrovirus (AstV), a member of the Mamastrovirus genus, is a small, non-enveloped, single-strand RNA virus that has been associated with human gastroenteritis and detected in association with other enteric pathogens [83]. The genome of astroviruses range from 6.1 to 7.3-kb and contains ORF1a, 1b, and 2, coding for serine protease, RdRp, and capsid protein, respectively. Astroviruses have been reported in fecal specimens from humans, bats, rats, pigs, cattle, and other animals [51], [84]-[88]. Astroviruses recently detected in human specimens were genetically related to animal astroviruses, indicating their possible zoonotic origins [89]–[91].
We sequenced the complete genome of an astrovirus from house mouse (Mus musculus) feces (M-52) (GenBank JF755422). The 6,519-bp genome length included a 14 bases 5′ UTR, ORF1a (848-aa), ORF1b (545-aa), ORF2 (707-aa) and 3′ UTR (332-nt, excluding the poly(A) tail). ORF1a contained a trypsin-like serine protease domain and ORF1b encoded RdRp following a -1 ribosomal frameshift induced by the presence of a heptameric “slippery sequence” AAAAAAC. The consensus promoter initiating ORF2 subgenomic RNA synthesis in the mouse astrovirus was identified as CUUUGGAGGGGUGGACCAAGAGGAGACAAUGGC (start codon in boldface). The 3′ UTR (332-nt) was longer than that of previously described astroviruses. This region contained the highly conserved 35-nt motif also found in other astroviruses from human, bat, porcine, and duck, in the avian picornavirus turdivirus as well as in a dog norovirus [92], [93]. Phylogenetic analysis based on the capsid protein showed that M-52 was linked to an astrovirus clade including bat, human, mink, and sheep astroviruses (Figure 8). BLASTx searches demonstrated that complete ORF1b and ORF2 of M-52 were most closely related to those of a bat astrovirus, sharing 65% and 38% amino acid similarities, respectively (Table S5). M-52 and rat astrovirus shared only 43% and 19% similarities in ORF1b and ORF2, respectively. M-52 is therefore the first characterized mouse astrovirus species.
Fig. 8. Phylogenetic analysis of mouse astrovirus. 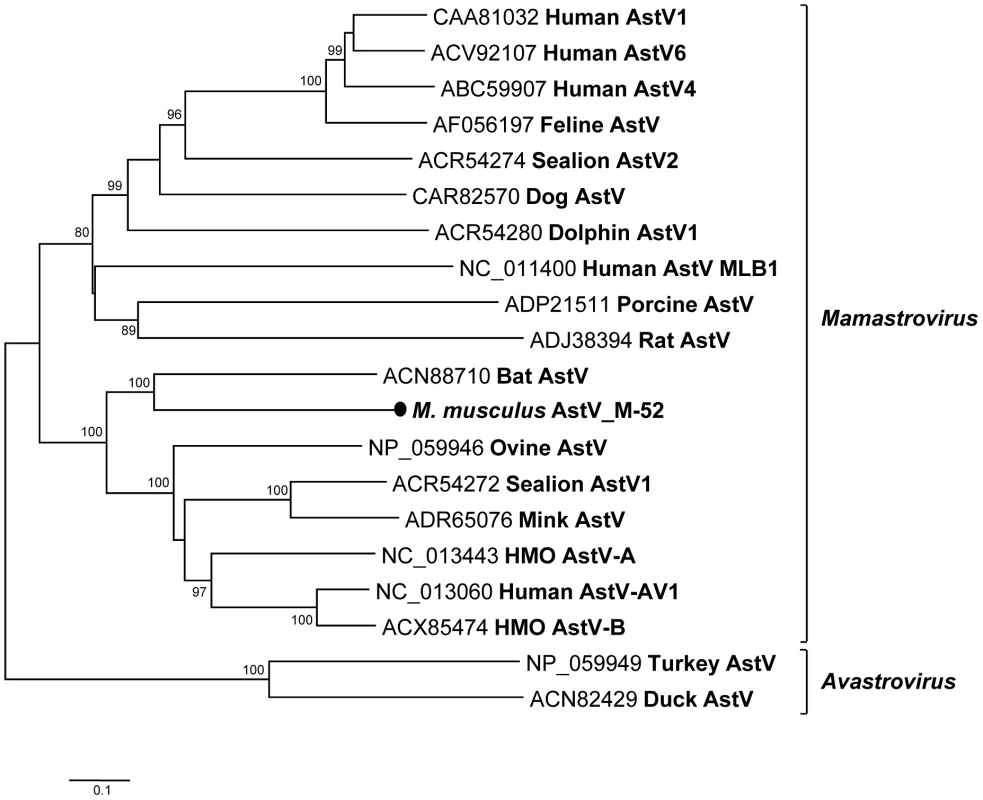
Phylogenetic tree obtained from complete ORF2 protein of astroviruses in the family Astroviridae. The novel astrovirus is labeled with a black circle. Rodent adeno-associated virus and adenovirus
Adeno-associated viruses (AAV) are small, ssDNA viruses with icosahedral capsid symmetry belonging to the Dependovirus genus of the family Parvoviridae. A characteristic of AAVs is their dependence on co-infection with adenovirus as a helper virus for their replication. AAV genomes are ca 4.7-kb in length and contain two major ORFs, and 145-bp inverted terminal repeats, which serve as the origins of replication [94]. AAV contains two major ORFs encoding the nonstructural Rep proteins and structural Cap proteins. Adenoviruses (AdV) are non-enveloped viruses composed of an icosahedral capsid and a double-stranded linear DNA genome ranging from 26 to 45-kb in length.
In our study, we found both AAV and AdV sequences in one deer mouse (Peromyscus maniculatus) fecal sample (M-6). The AAV sequence reads resulted in three separate contigs (GenBank JF755424-JF755426), covering 40% (304 amino acids) of the AAV VP7 protein showing the highest similarity to porcine AAV (61%) and AAV-5 (60%), followed by rat AAV (55%). It shared a lower similarity with mouse AAV-1 of only 43%. The phylogenic tree confirmed that AAV M-6 clustered with porcine AAV, AAV-5 and rat AAV (Figure S2A). AdV sequence reads, consisted of two separate contigs encoding 10% (154 amino acids) of the AdV hexon protein. M-6 (GenBank JF755423) exhibited the closet match of 80% to murine AdV2 with which it clustered phylogenetically (Figure 2B).
Insect viruses
Thirty-nine percent of the rodent virome sequences were related to insect viruses (Figure 1). Detection of these viral sequences may be due to insect consumption. Insect RNA virus matches were more abundant than insect DNA viruses, making up 67% of insect viral sequences. Of note, 72% of RNA sequences were related to chronic bee paralysis virus (CBPV, an unassigned member of the Dicistroviridae family or of a distinct family in the picornavirales) known to infect honeybees [95]. Feces of infected honeybees are positive for CBPV (91). Other sequences also belonged to the family Dicistroviriade and had closest matches to newly identified viruses, including kelp fly virus and Nora virus [96], [97]. Insect DNA viruses from the viral families Iridoviridae and Polydnaviridae and the subfamily Densovirinae were found. The majority of insect virus-like sequences shared protein similarity of less <70% with annotated insect viral proteins.
Plant viruses
About 3.4% of the rodent virome (911 sequence reads) were related to plant viruses. DNA viruses were predominant with 86% of the reads and the largest proportion was single-stranded DNA viruses from the family Nanoviridae (48%), followed by the families Geminiviridae (12%) and Phycodnaviridae (7.2%). Interestingly, 33% of the DNA viral sequences were related to fungi infecting Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1), the first reported mycovirus with a DNA genome [98]. RNA viruses accounted for only 14% of plant viral sequences and the majority was related to single-stranded RNA viruses in the family Secoviridae (64%), followed by the families Tymoviridae, Alphaflexiviridae and Tombusviridae. Double-stranded RNA viruses were responsible for 28% of the plant viral RNA sequences, belonging to the family Partitiviridae.
Discussion
Animal and human viral discovery has long been focused on pathogenic infections and viruses that could be readily grown in cell cultures and cause visible cytopathic effects. Viral metagenomics is a recent approach to analyzing mixtures of viral nucleic acids enriched directly from a variety of sources including animals, plants, protozoa, bacteria, archaea, and diverse environments without a prerequisite in vitro or in vivo amplification [20], [50]–[52], [99]–[110]. Viral metagenomics has been successfully employed to identify both commensal viruses and viral pathogens and has the potential to detect all viruses recognizable through sequence similarity searches [111]. We describe here the fecal viral flora in several species of wild rodents. Sequences closely and distantly related to known viral sequences were identified as well as many sequences of unknown taxonomic origin [51], [99], [101], [108], [112]–[114]. A fraction of these currently unclassifiable sequences may be derived from still genetically uncharacterized viral families refractory to nucleotide or protein sequence similarity based searches. Multiple known DNA and RNA viral families infecting plants, insects, and mammals were also detected. Plant and insect viral sequences likely reflect the omnivorous diet of these rodents.
The number of Rep-containing circular DNA genomes recently found in various animal and environmental viral surveys has greatly expanded the genetic diversity in this group of viruses [56], [62], [115]–[119]. Some of the Rep proteins of the small circular DNA genomes characterized here clustered with viruses in marine environment or in protozoan genomes. Type II RodSCVs contained two separate Rep ORFs in their genomes, separately encoding the viral Rep and RNA helicase with each gene individually smaller than those in single Rep containing genomes. The many novel circular DNA genomes seen here indicate the likely existence of several previously unknown viral families. The closest relatives of some Rep sequences found in rodent feces are those integrated in the Giardia intestinalis genome, possibly reflecting their replication in protozoans in rodent guts.
We report on the first identification of a kobuvirus in mice (Peromyscus crinitus and P. maniculatus). Since its genetic characterization in 1998 in Japan [65], Aichi virus, the archetypical kobuvirus, has been associated with acute gastroenteritis worldwide [74], [120], [121]. Other kobuvirus species have been reported in pigs and cows as well as in humans and associated with diarrhea [68]–[70], [121]. Our phylogenetic analysis revealed that the mouse kobuvirus clustered with human Aichi virus. A recent study describes a similar close relationship between a canine kobuvirus and Aichi virus [122]. This close relationship between human, mouse, and canine kobuviruses suggests past zoonotic transmission of kobuviruses between these hosts or their recent ancestors followed by independent evolution leading to the close but distinct species seen today.
Sapelovirus is another genus in the family Picornaviridae and currently includes 3 species (www.picornaviridae.com). While phylogenetically related to the enteroviruses, these viruses have a different type of internal ribosome entry site and a leader protein (www.picornaviridae.com). A Sapelovirus-like genome fragment was identified in house mouse feces. Phylogenetically, this fragment clustered with the other Sapeloviruses. Mice therefore also appear to harbor Sapeloviruses, expanding the known host range of this group of viruses.
Two potential new picornaviridae genera we provisionally labeled Mosavirus and Rosavirus were genetically characterized. Only distantly related to the kobuvirus genus, the Rosavirus is also part of a larger clade containing several avian picornaviruses, including the recently described avian turdiviruses and turkey hepatitis virus [93], [123]. The recent characterization of numerous deep-branched members of the picornaviridae family (likely new genera) from mammals, birds, reptiles, and fish indicates this viral family is still greatly under sampled and likely to rapidly expand as more potential host species are analyzed (www.picornaviridae.com). A second potential new genus distantly related to the cardioviruses was also characterized. Until recently cardioviruses consisted of the Theiloviruses and Encephalomyocarditis viruses infecting largely rodents and the Saffold virus group infecting humans [124]–[126]. The Mosavirus also exhibited the large genetic distance relative to other picornaviruses required to tentatively qualify Mosavirus as a founding member of a new Picornaviridae genus.
Using consensus PCR primers PBV has been detected in the feces of mammals, birds, and snakes with or without diarrhea [127]–[132]. Infection was common in wild rodents with 22/105 animals shedding PBV-like nucleic acids, the second highest group of mammalian virus-like sequences after the highly diverse small circular DNA genomes (Figure 1). PBV therefore represent the largest quantity of viral nucleic acids released by these rodents into the environment. Two nearly complete PBV RdRp sequences clustered together with a larger clade of human PBV cluster I sequences possibly reflecting past rodent-human transmission. Complete PBV RdRp sequences from more animal species will be required to further test this hypothesis.
The number of mammalian astroviruses species has recently undergone a rapid expansion [85], [88]–[91], [133], [134]. The first astrovirus genome from a mouse clustered with a clade containing mink, sheep, and recently identified human astroviruses [89], [91]. The detection of multiple unrelated astrovirus species within some host species (human, pigs, and sea lions) may reflect frequent cross-species transmission rather than diversification from a common source.
AAV infects humans and other primate species [16], [135], [136]. Although over 80% of humans are seropositive, AAV has not been associated with any diseases in humans [137]. Members of the family Adenoviridae infect various species of vertebrates, including humans [51], [138]–[140]. The majority of AdV infection cause upper respiratory diseases; however, AdV also causes other symptoms, such as acute gastroenteritis [141]. The small AAV and AdV fragments identified here are too limited for definitive classification but provisionally appear to represent a fourth species of murine adenovirus [142].
Rodents are known reservoirs of numerous viruses capable of causing human diseases [2]. The characterization of the viromes of animal species with frequent contacts with humans can provide baseline viral content to more quickly identify the possible sources of future zoonotic infections and therefore assist in their control. Changes in the baseline virome of various animal may also assist in identifying changes associated with future population crashes. The addition of annotated viral genomes to public databases will also facilitate their inclusion on microarrays as well as assist in their precise identification after high-throughput sequencing [143], [144]. Monitoring for rodent viruses in humans will require extensive geographical sampling in persons with high exposure such as hunters, hikers, and farmers, and in regions of high biodiversity. The large increase in rodent viral species and higher level taxa revealed here by a limited species, geographic, and individual sampling reflects the large extent of mammalian virus diversity awaiting characterization.
Materials and Methods
Rodents and samples
Fecal specimens were collected from 52 mice, 52 voles and one Woodrat and stored at −80°C. Rodents were humanely captured and released according to the international guidelines of the American Society of Mammalogists (www.mammalsociety.org). Four mouse species Peromyscus crinitus (Canyon mouse), P. maniculatus (Deer mouse), P. truei (Pinyon mouse), and P. boylii (Brush mouse) were trapped in three different California counties, Siskiyou, El Dorado and Lassen, in May and June 2010 (Table 1). Two species of voles were analyzed: one Microtus longicaudus (Long-tailed vole) was caught in El Dorado Co., CA in June 2010 and 51 Microtus pennsylvanicus (Meadow vole) plus 20 Mus musculus mice were caught in an old field habitat in southeastern Virginia during April-November 2008. A Neotoma cinerea (Woodrat) was trapped in Siskiyou Co., CA in May 2010. The feces collected from the traps were used in this study. Sample collection was exempted from review by the IACUC committees of the CDPH and of the Old Dominion University.
Tab. 1. Summary of rodent sample information. 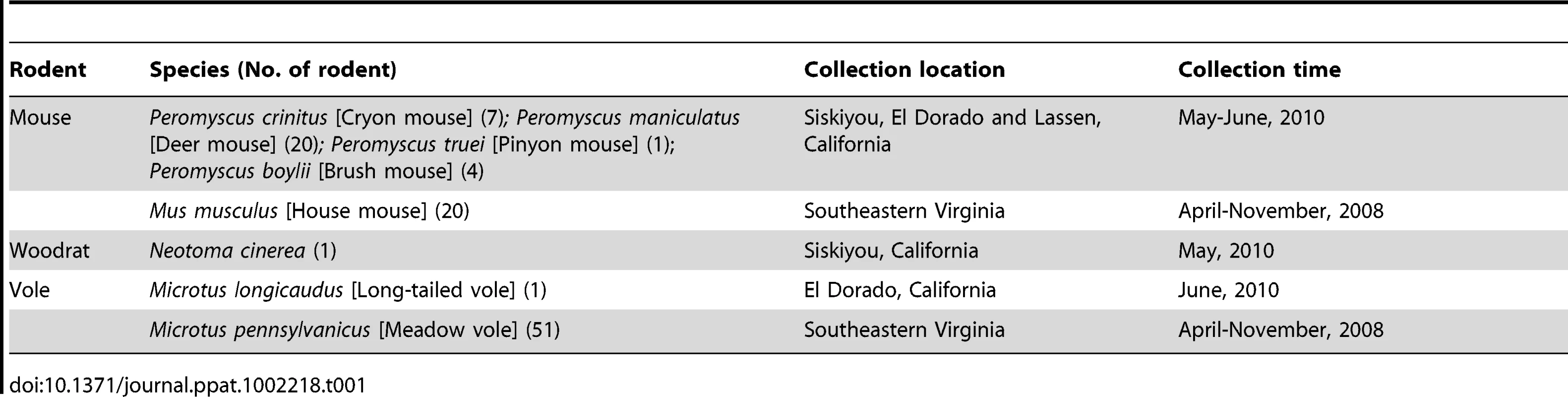
Viral particle purification and nucleic acid extraction
Fecal samples were resuspended in Hanks buffered saline solution (Gibco BRL) and vortexed. The samples were clarified by 15,000 x g centrifugation for 10 min. A total of 200 µl of supernatants was filtered through a 0.45-µm filter (Millipore) to remove bacterium-sized particles. The filtrate was then treated with a cocktail of DNases (Turbo DNase from Ambion, Baseline-ZERO from Epicentre, and Benzonase from Novagen) and with RNase (Fermentas) to digest unprotected nucleic acids [52]. Nucleic acids protected from nuclease digestion within viral capsids were then extracted using QIAamp spin-columns according to the manufacturer's instructions (Qiagen).
Viral metagenome library construction for 454 pyrosequencing
cDNA synthesis was performed as described previously [52]. Briefly, RNA only and DNA plus RNA virus sequence-independent amplifications were separately performed and then combined before sequencing. For RNA virus-only amplification, 10 µl of the extracted nucleic acid was treated with DNase (Ambion) and was used as a template to synthesize cDNA, using SuperScript III reverse transcriptase (Invitrogen) and a primer containing an arbitrary set sequence followed by a randomized eight nucleotides at the primer's 3′ end. For the RNA plus DNA virus amplification, the DNase step prior to RT was excluded. Following reverse transcription followed by heat denaturation and re-annealing of the primer a single round of primer extension DNA synthesis was performed using Klenow fragment polymerase (New England Biolabs). PCR amplification was then performed using primers consisting of only the set portion of the random primer. To increase sampling of the viral nucleic acids, the random PCR amplifications were performed in duplicate resulting in four PCR products (2 from viral RNA-only and 2 from viral RNA plus DNA). The four PCR products were pooled and purified using the QIAquick Purification Kit (Qiagen). The purified DNA level was determined by Nanodrop (Thermo Scientific). Equal amounts of amplified DNA from up to 40 different fecal samples (using different primers subsequently recognizable by their set sequences) were combined into larger pools to generate 3 libraries. A total of 120 µg of DNA from each library was run on a 2% agarose gel, yielding a DNA smear. DNA ranging in apparent size from 500 to 1,000-bp was cut from the gel and purified using the QIAquick Gel Extraction Kit (Qiagen). The extremities of the PCR products DNA were then polished using T4 polynucleotide kinase. The Roche/454 adaptors were then ligated, and small DNA fragments removed, according to the manufacturer's protocol (GS FLX Titanium General Library Preparation Kit, Roche).
Sequence read classification
The set sequences on the different random PCR primers were used to assign sequence reads to the corresponding fecal samples. Sequence reads were trimmed of their set primer sequences and adjacent eight nucleotides corresponding to the randomized part of the primers. Trimmed sequences from each bin were then de novo assembled into contigs using Sequencher (Gene Codes), with a criterion of at least 95% identity over 35-bp to merge two fragments. The assembled sequence contigs and singlet sequences greater than 100-bp were compared to the GenBank nonredundant nucleotide and protein databases using BLASTn and BLASTx, respectively. Based on BLAST output, sequences were classified as viruses, phage, bacteria, and eukaryota based on the taxonomic origin of the best-hit (lowest E score) sequence match. An E value of 10−5 was the cutoff value for significant hits. Sequences whose best alignment E value was >10−5 were deemed unclassifiable.
Complete genome sequencing of circular DNA viruses
Complete circular DNA viral genomes were amplified using inverse PCR (iPCR) with specific primers designed from 454 derived short-sequence fragments. iPCR amplicons were then directly sequenced by primer walking. PCR reactions contained 2.5 U of LA Taq polymerase (Takara) in 2.5 µl of 10X LA PCR buffer (Takara), 4 µl of 2.5 mM dNTP (Takara), 2.5 µl of forward and reverse primers (10 pmol/µl), and 2.5 µl of nucleic acids (for first round) or 1 µl of the first-round PCR product (for second PCR round) as a template in a 25 µl total volume. PCR was performed at 94°C for 1 min, followed by 30 cycles of 98°C for 10 s, 68°C for 4–10 min depending on the sizes of the expected amplicon, and a final extension at 72°C for 10 in, and then held at 4°C.
Genome acquisition and detection of linear RNA viruses
All primers are described in Table S6. Sequence reads showing significant BLASTn or BLASTx hits to Aichi virus were linked together using RT-PCR. The 5′ and 3′ rapid amplification of cDNA end (5′ and 3′ RACE) was used to acquire the 5′ and 3′ extremities of the Aichi-like virus genome [89], [145]–[147]. For the complete genome of astrovirus, pairs of specific reverse (Ast-R1 and Ast-R2) and forward primers (Ast-F1 and Ast-F2) designed from an initial astrovirus-like-sequence of 414-bp were used in 5′ and 3′ RACE [89], [145]–[147] to amplify ∼1.5-kb and 5-kb PCR products, respectively. In a mouse (Peromyscus crinitus) fecal specimen two small picornavirus-like-genome fragments (300-bp and 251-bp, respectively) were detected. PCR failed to link these two fragments together, suggesting that they belonged to two different viruses. For the complete genome of the Mosavirus, specific reverse (Mosa-R1 and Mosa-R2) and forward primers (Mosa-F1 and Mosa-F2) were used in 5′ and 3′ RACE [89], [145]-[147] to amplify ∼1.5-kb and 6-kb PCR products, respectively. For the Rosavirus, a pair of specific forward primers (Rosa-F1 and Rosa-F2) were used in 3′ RACE [89], [146], [147] to amplify ∼4-kb amplicon. 5′ RACE was not successful. To investigate the prevalence of Aichi-like virus, consensus primers were used for PCR screening designed on a nucleotide alignment of the 2C-3B region of all human Aichi virus genotypes available in GenBank and the mouse Aichi-like virus strain characterized here. For the RT reaction, 10 µl of extracted RNA was added to 10 µl of RT mixture containing 4 µl of 5X First-Strand buffer (Invitrogen), 1 µl of 10 mM dNTP (Fermentas), 1 µl of random primer, 1 µl of SuperScript III Reverse Transriptase (Invitrogen), 1 µl of RNase inhibitor (Fermentas), and 1 µl of DEPC-treated water. The RT reaction mixture was incubated at 25°C for 5 min, 50°C for 60 min, 70°C for 15 min to inactivate the enzyme, and then held at 4°C. For the first round PCR, 2.5 µl of cDNA template was mixed with 2.5 µl of 10X ThermoPol Reaction buffer (New England Biolabs), 0.5 µl of 10 mM dNTP (Fermentas), 2.5 µl of each primer (10 pmol/µl) (Ai-Deg-F1 and Ai-Deg-R1), targeting the Achi-like virus, 0.4 µl of Taq DNA Polymerase (New England Biolabs). DEPC-treated water was added up to a 25 µl total volume. The PCR condition was as follows: denaturation at 95°C for 5 min, 35 cycles of 95°C for 30 s, 63°C for 30 s and 72°C for 1 min, a final extension at 72°C for 10 min, and then held at 4°C. The second round of amplification was performed using the same conditions except that the annealing temperature was 60°C, and inner primers Ai-(Deg-F2 and Ai-Deg-R2). The second round PCR amplification resulted in the amplicon size of 735-bp.
Phylogenetic analysis and genomic structure prediction
Reference viral sequences from different viral families were obtained from GenBank. Sequence analysis was performed using CLUSTAL X with the default settings. Sequences were trimmed to match the genomic regions of the viral sequences obtained in the study. A phylogenetic tree with 100 bootstrap resamples of the alignment data sets was generated using the neighbor-joining method [148]. The genetic distance was calculated using Kimura's two-parameter method (PHYLIP) [149]. Sequence identity matrix was measured using BioEdit. GenBank accession numbers of the viral sequences used in the phylogenetic analyses were shown in the trees. Putative ORFs in the genome were predicted by NCBI ORF finder. The circular genome architectures were predicted using Vector NTI 11.5 Advance (Invitrogen) with the following conditions: minimum ORF size of 100 codons, start codons ATG and GTG, stop codons TAA, TGA and TAG. To identify stem-loop structures, nucleotide sequences were analyzed with Mfold. Full and partial genome sequences are at GenBank accession numbers JF755401-JF755427, and JF973686-JF973687. The 454 pyrosequencing data is in the short read archive at SRA030869.
Supporting Information
Zdroje
1. HuchonDMadsenOSibbaldMJAmentKStanhopeMJ 2002 Rodent phylogeny and a timescale for the evolution of Glires: evidence from an extensive taxon sampling using three nuclear genes. Mol Biol Evol 19 1053 1065
2. MeerburgBGSingletonGRKijlstraA 2009 Rodent-borne diseases and their risks for public health. Crit Rev Microbiol 35 221 270
3. CharrelRNde LamballerieX 2010 Zoonotic aspects of arenavirus infections. Vet Microbiol 140 213 220
4. CutlerSJFooksARvan der PoelWH 2010 Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg Infect Dis 16 1 7
5. KleinSLCalisherCH 2007 Emergence and persistence of hantaviruses. Curr Top Microbiol Immunol 315 217 252
6. LeDucJW 1987 Epidemiology of Hantaan and related viruses. Lab Anim Sci 37 413 418
7. MillsJNChildsJE 1998 Ecologic studies of rodent reservoirs: their relevance for human health. Emerg Infect Dis 4 529 537
8. ZeierMHandermannMBahrURenschBMullerS 2005 New ecological aspects of hantavirus infection: a change of a paradigm and a challenge of prevention--a review. Virus Genes 30 157 180
9. BengisRGLeightonFAFischerJRArtoisMMornerT 2004 The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech 23 497 511
10. ChomelBBBelottoAMeslinFX 2007 Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis 13 6 11
11. EnriaDAPinheiroF 2000 Rodent-borne emerging viral zoonosis. Hemorrhagic fevers and hantavirus infections in South America. Infect Dis Clin North Am 14 : 167-184, x
12. MackenzieRB 1972 Public health importance of rodents in South America. Bull World Health Organ 47 161 169
13. NicholSTSpiropoulouCFMorzunovSRollinPEKsiazekTG 1993 Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 262 914 917
14. KhanASSpiropoulouCFMorzunovSZakiSRKohnMA 1995 Fatal illness associated with a new hantavirus in Louisiana. J Med Virol 46 281 286
15. KhanASKhabbazRFArmstrongLRHolmanRCBauerSP 1996 Hantavirus pulmonary syndrome: the first 100 US cases. J Infect Dis 173 1297 1303
16. MonroeMCMorzunovSPJohnsonAMBowenMDArtsobH 1999 Genetic diversity and distribution of Peromyscus-borne hantaviruses in North America. Emerg Infect Dis 5 75 86
17. TurellMJKorchGWRossiCASeslineDEngeBA 1995 Short report: prevalence of hantavirus infection in rodents associated with two fatal human infections in California. Am J Trop Med Hyg 52 180 182
18. GoldsmithCSElliottLHPetersCJZakiSR 1995 Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch Virol 140 2107 2122
19. RiversMNAlexanderJLRohdeREPierceJRJr 2009 Hantavirus pulmonary syndrome in Texas: 1993-2006. South Med J 102 36 41
20. RoweJESt JeorSCRioloJOttesonEWMonroeMC 1995 Coexistence of several novel hantaviruses in rodents indigenous to North America. Virology 213 122 130
21. SchwarzTFZakiSRMorzunovSPetersCJNicholST 1995 Detection and sequence confirmation of Sin Nombre virus RNA in paraffin-embedded human tissues using one-step RT-PCR. J Virol Methods 51 349 356
22. Torres-Perez F, Wilson L, Collinge SK, Harmon H, Ray C, et al. Sin Nombre virus infection in field workers, Colorado, USA. Emerg Infect Dis 16 308 310
23. WhiteDJMeansRGBirkheadGSBoslerEMGradyLJ 1996 Human and rodent hantavirus infection in New York State: public health significance of an emerging infectious disease. Arch Intern Med 156 722 726
24. LonnerBNDouglassRJKuenziAJHughesK 2008 Seroprevalence against Sin Nombre virus in resident and dispersing deer mice. Vector Borne Zoonotic Dis 8 433 441
25. PetterssonLBomanJJutoPEvanderMAhlmC 2008 Outbreak of Puumala virus infection, Sweden. Emerg Infect Dis 14 808 810
26. HjelleBJenisonSAGoadeDEGreenWBFeddersenRM 1995 Hantaviruses: clinical, microbiologic, and epidemiologic aspects. Crit Rev Clin Lab Sci 32 469 508
27. HjertqvistMKleinSLAhlmCKlingstromJ 2010 Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 16 1584 1586
28. MakaryPKanervaMOllgrenJVirtanenMJVapalahtiO 2010 Disease burden of Puumala virus infections, 1995-2008. Epidemiol Infect 138 1484 1492
29. OnculOAtalayYOnemYTurhanVAcarA 2011 Hantavirus infection in Istanbul, Turkey. Emerg Infect Dis 17 303 304
30. PapaAChristovaI 2011 Genetic detection of dobrava/belgrade virus, bulgaria. Emerg Infect Dis 17 308 309
31. VapalahtiOMustonenJLundkvistAHenttonenHPlyusninA 2003 Hantavirus infections in Europe. Lancet Infect Dis 3 653 661
32. ZhangYZZouYFuZFPlyusninA Hantavirus infections in humans and animals, China Emerg Infect Dis 16 1195 1203
33. ZhangYZZhangFXWangJBZhaoZWLiMH 2009 Hantaviruses in rodents and humans, Inner Mongolia Autonomous Region, China. Emerg Infect Dis 15 885 891
34. ZuoSQZhangPHJiangJFZhanLWuXM 2008 Seoul virus in patients and rodents from Beijing, China. Am J Trop Med Hyg 78 833 837
35. ZouYWangJBGaowaHSYaoLSHuGW 2008 Isolation and genetic characterization of hantaviruses carried by Microtus voles in China. J Med Virol 80 680 688
36. KorvaMDuhDPuterleATrilarTZupancTA 2009 First molecular evidence of Tula hantavirus in Microtus voles in Slovenia. Virus Res 144 318 322
37. PlyusninAVapalahtiOLankinenHLehvaslaihoHApekinaN 1994 Tula virus: a newly detected hantavirus carried by European common voles. J Virol 68 7833 7839
38. SongJWBaekLJSongKJSkrokAMarkowskiJ 2004 Characterization of Tula virus from common voles (microtus arvalis) in Poland: evidence for geographic-specific phylogenetic clustering. Virus Genes 29 239 247
39. SongJWGligicAYanagiharaR 2002 Identification of Tula hantavirus in Pitymys subterraneus captured in the Cacak region of Serbia-Yugoslavia. Int J Infect Dis 6 31 36
40. SchultzeDLundkvistABlauensteinUHeymanP 2002 Tula virus infection associated with fever and exanthema after a wild rodent bite. Eur J Clin Microbiol Infect Dis 21 304 306
41. LindquistLVapalahtiO 2008 Tick-borne encephalitis. Lancet 371 1861 1871
42. MansfieldKLJohnsonNPhippsLPStephensonJRFooksAR 2009 Tick-borne encephalitis virus - a review of an emerging zoonosis. J Gen Virol 90 1781 1794
43. BakhvalovaVNDobrotvorskyAKPanovVVMatveevaVATkachevSE 2006 Natural tick-borne encephalitis virus infection among wild small mammals in the southeastern part of western Siberia, Russia. Vector Borne Zoonotic Dis 6 32 41
44. TonteriEJaaskelainenAETikkakoskiTVoutilainenLNiemimaaJ 2011 Tick-borne encephalitis virus in wild rodents in winter, Finland, 2008–2009. Emerg Infect Dis 17 72 75
45. FrameJDBaldwinJMJrGockeDJTroupJM 1970 Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg 19 670 676
46. KhanSHGobaAChuMRothCHealingT 2008 New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res 78 103 115
47. KlempaB 2009 Hantaviruses and climate change. Clin Microbiol Infect 15 518 523
48. TersagoKSchreursALinardCVerhagenRVan DongenS 2008 Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector Borne Zoonotic Dis 8 235 244
49. DavisSCalvetELeirsH 2005 Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector Borne Zoonotic Dis 5 305 314
50. DayJMBallardLLDukeMVSchefflerBEZsakL 2010 Metagenomic analysis of the turkey gut RNA virus community. Virol J 7 313
51. LiLVictoriaJGWangCJonesMFellersGM 2010 Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol 84 6955 6965
52. VictoriaJGKapoorALiLBlinkovaOSlikasB 2009 Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol 83 4642 4651
53. BernardHUBurkRDChenZvan DoorslaerKHausenH 2010 Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401 70 79
54. de VilliersEMFauquetCBrokerTRBernardHUzur HausenH 2004 Classification of papillomaviruses. Virology 324 17 27
55. GibbsMJSmeianovVVSteeleJLUpcroftPEfimovBA 2006 Two families of rep-like genes that probably originated by interspecies recombination are represented in viral, plasmid, bacterial, and parasitic protozoan genomes. Mol Biol Evol 23 1097 1100
56. RosarioKDuffySBreitbartM 2009 Diverse circovirus-like genome architectures revealed by environmental metagenomics. J Gen Virol 90 2418 2424
57. RosarioKNilssonCLimYWRuanYBreitbartM 2009 Metagenomic analysis of viruses in reclaimed water. Environ Microbiol 11 2806 2820
58. YoonHSPriceDCStepanauskasRRajahVDSierackiME Single-cell genomics reveals organismal interactions in uncultivated marine protists. Science 332 714 717
59. FaurezFDoryDGraslandBJestinA 2009 Replication of porcine circoviruses. Virol J 6 60
60. SteinfeldtTFinsterbuschTMankertzA 2001 Rep and Rep' protein of porcine circovirus type 1 bind to the origin of replication in vitro. Virology 291 152 160
61. SteinfeldtTFinsterbuschTMankertzA 2007 Functional analysis of cis - and trans-acting replication factors of porcine circovirus type 1. J Virol 81 5696 5704
62. BlinkovaOVictoriaJLiYKeeleBFSanzC 2010 Novel circular DNA viruses in stool samples of wild-living chimpanzees. J Gen Virol 91 74 86
63. YamashitaTKobayashiSSakaeKNakataSChibaS 1991 Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis 164 954 957
64. YamashitaTSakaeKIshiharaYIsomuraSUtagawaE 1993 Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J Clin Microbiol 31 2938 2943
65. YamashitaTSakaeKTsuzukiHSuzukiYIshikawaN 1998 Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J Virol 72 8408 8412
66. GreningerALHoltzLKangGGanemDWangD 2010 Serological evidence of human klassevirus infection. Clin Vaccine Immunol 17 1584 1588
67. ShanTWangCCuiLYuYDelwartE 2010 Picornavirus salivirus/klassevirus in children with diarrhea, China. Emerg Infect Dis 16 1303 1305
68. LiLVictoriaJKapoorABlinkovaOWangC 2009 A novel picornavirus associated with gastroenteritis. J Virol 83 12002 12006
69. HoltzLRFinkbeinerSRZhaoGKirkwoodCDGironesR 2009 Klassevirus 1, a previously undescribed member of the family Picornaviridae, is globally widespread. Virol J 6 86
70. GreningerALRunckelCChiuCYHaggertyTParsonnetJ 2009 The complete genome of klassevirus - a novel picornavirus in pediatric stool. Virol J 6 82
71. DvorakCMHallDJHillMRiddleMPranterA 2001 Leader protein of encephalomyocarditis virus binds zinc, is phosphorylated during viral infection, and affects the efficiency of genome translation. Virology 290 261 271
72. PaulSMichielsT 2006 Cardiovirus leader proteins are functionally interchangeable and have evolved to adapt to virus replication fitness. J Gen Virol 87 1237 1246
73. KaikkonenSRasanenSRametMVesikariT 2010 Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol Infect 138 1166 1171
74. PhamNTKhamrinPNguyenTAKantiDSPhanTG 2007 Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J Clin Microbiol 45 2287 2288
75. Sdiri-LouliziKHassineMGharbi-KhelifiHSaklyNChouchaneS 2009 Detection and genomic characterization of Aichi viruses in stool samples from children in Monastir, Tunisia. J Clin Microbiol 47 2275 2278
76. PereiraHGFlewettTHCandeiasJABarthOM 1988 A virus with a bisegmented double-stranded RNA genome in rat (Oryzomys nigripes) intestines. J Gen Virol 69 (Pt11) 2749 2754
77. ChandraR 1997 Picobirnavirus, a novel group of undescribed viruses of mammals and birds: a minireview. Acta Virol 41 59 62
78. van LeeuwenMWilliamsMMKorakaPSimonJHSmitsSL 2010 Human picobirnaviruses identified by molecular screening of diarrhea samples. J Clin Microbiol 48 1787 1794
79. GiordanoMOMartinezLCRinaldiDEspulCMartinezN 1999 Diarrhea and enteric emerging viruses in HIV-infected patients. AIDS Res Hum Retroviruses 15 1427 1432
80. GiordanoMOMartinezLCRinaldiDGuinardSNarettoE 1998 Detection of picobirnavirus in HIV-infected patients with diarrhea in Argentina. J Acquir Immune Defic Syndr Hum Retrovirol 18 380 383
81. FregolenteMCGattiMS 2009 Nomenclature proposal for picobirnavirus. Arch Virol 154 1953 1954
82. GaneshBNatarajuSMRajendranKRamamurthyTKanungoS 2010 Detection of closely related Picobirnaviruses among diarrhoeic children in Kolkata: evidence of zoonoses? Infect Genet Evol 10 511 516
83. WalterJEMitchellDK 2003 Astrovirus infection in children. Curr Opin Infect Dis 16 247 253
84. VilarinoMLLe GuyaderFSPoloDSchaefferJKrolJ 2009 Assessment of human enteric viruses in cultured and wild bivalve molluscs. Int Microbiol 12 145 151
85. ChuDKChinAWSmithGJChanKHGuanY 2010 Detection of novel astroviruses in urban brown rats and previously known astroviruses in humans. J Gen Virol 91 2457 2462
86. JeongAYJeongHSJoMYJungSYLeeMS 2011 Molecular epidemiology and genetic diversity of human astrovirus in South Korea from 2002 to 2007. Clin Microbiol Infect 17 404 408
87. ReuterGPankovicsPBorosA 2011 Identification of a novel astrovirus in a domestic pig in Hungary. Arch Virol 156 125 128
88. RiveraRNollensHHVenn-WatsonSGullandFMWellehanJFJr 2010 Characterization of phylogenetically diverse astroviruses of marine mammals. J Gen Virol 91 166 173
89. KapoorALiLVictoriaJOderindeBMasonC 2009 Multiple novel astrovirus species in human stool. J Gen Virol 90 2965 2972
90. FinkbeinerSRHoltzLRJiangYRajendranPFranzCJ 2009 Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 6 161
91. FinkbeinerSRLiYRuoneSConrardyCGregoricusN 2009 Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J Virol 83 10836 10839
92. MartellaVLorussoEDecaroNEliaGRadognaA 2008 Detection and molecular characterization of a canine norovirus. Emerg Infect Dis 14 1306 1308
93. WooPCLauSKHuangYLamCSPoonRW 2010 Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds, and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in the family Picornaviridae. J Gen Virol 91 2433 2448
94. SrivastavaALusbyEWBernsKI 1983 Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol 45 555 564
95. OlivierVBlanchardPChaouchSLallemandPSchurrF 2008 Molecular characterisation and phylogenetic analysis of Chronic bee paralysis virus, a honey bee virus. Virus Res 132 59 68
96. HabayebMSEkengrenSKHultmarkD 2006 Nora virus, a persistent virus in Drosophila, defines a new picorna-like virus family. J Gen Virol 87 3045 3051
97. HartleyCJGreenwoodDRGilbertRJMasoumiAGordonKH 2005 Kelp fly virus: a novel group of insect picorna-like viruses as defined by genome sequence analysis and a distinctive virion structure. J Virol 79 13385 13398
98. YuXLiBFuYJiangDGhabrialSA 2010 A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci U S A 107 8387 8392
99. BreitbartMHewsonIFeltsBMahaffyJMNultonJ 2003 Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol 185 6220 6223
100. BreitbartMSalamonPAndresenBMahaffyJMSegallAM 2002 Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci U S A 99 14250 14255
101. BrieseTPaweskaJTMcMullanLKHutchisonSKStreetC 2009 Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog 5 e1000455
102. LipkinWIPalaciosGBrieseT 2009 Diagnostics and discovery in viral hemorrhagic fevers. Ann N Y Acad Sci 1171 Suppl 1 E6 11
103. CulleyAILangASSuttleCA 2003 High diversity of unknown picorna-like viruses in the sea. Nature 424 1054 1057
104. CulleyAILangASSuttleCA 2006 Metagenomic analysis of coastal RNA virus communities. Science 312 1795 1798
105. KennedyJMarchesiJRDobsonAD 2008 Marine metagenomics: strategies for the discovery of novel enzymes with biotechnological applications from marine environments. Microb Cell Fact 7 27
106. QuanPLWagnerTABrieseTTorgersonTRHornigM 2010 Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis 16 918 925
107. SimonCDanielR 2011 Metagenomic analyses: past and future trends. Appl Environ Microbiol 77 1153 1161
108. SvrakaSRosarioKDuizerEvan der AvoortHBreitbartM 2010 Metagenomic sequencing for virus identification in a public-health setting. J Gen Virol 91 2846 2856
109. TangPChiuC 2010 Metagenomics for the discovery of novel human viruses. Future Microbiol 5 177 189
110. TownerJSSealyTKKhristovaMLAlbarinoCGConlanS 2008 Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 4 e1000212
111. DelwartEL 2007 Viral metagenomics. Rev Med Virol 17 115 131
112. CannAJFandrichSEHeaphyS 2005 Analysis of the virus population present in equine faeces indicates the presence of hundreds of uncharacterized virus genomes. Virus Genes 30 151 156
113. DonaldsonEFHaskewANGatesJEHuynhJMooreCJ 2010 Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J Virol 84 13004 13018
114. FinkbeinerSRAllredAFTarrPIKleinEJKirkwoodCD 2008 Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog 4 e1000011
115. BlinkovaORosarioKLiLKapoorASlikasB 2009 Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol 47 3507 3513
116. LiLKapoorASlikasBBamideleOSWangC 2010 Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J Virol 84 1674 1682
117. LiLShanTOderindeSBMasroorAMKunzT 2011 Possible cross-species transmission of circoviruses and cycloviruses in farm animals. J Gen Virol 92 768 772
118. NgTFManireCBorrowmanKLangerTEhrhartL 2009 Discovery of a novel single-stranded DNA virus from a sea turtle fibropapilloma by using viral metagenomics. J Virol 83 2500 2509
119. RosarioKMarinovMStaintonDKrabergerSWiltshireEJ 2011 Dragonfly cyclovirus, a novel single-stranded DNA virus discovered in dragonflies (Odonata: Anisoptera). J Gen Virol 92 1302 1308
120. Le GuyaderFSLe SauxJCAmbert-BalayKKrolJSeraisO 2008 Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J Clin Microbiol 46 4011 4017
121. ReuterGBorosAPankovicsP 2011 Kobuviruses - a comprehensive review. Rev Med Virol 21 32 41
122. LiLPesaventoPShanTLeuteneggerCMWangC 2011 Viruses in diarrhetic dogs include novel kobuviruses and sapoviruses. J Gen Virol [Epub ahead of print]
123. HonkavuoriKSShivaprasadHLBrieseTStreetCHirschbergDL 2011 Novel picornavirus in Turkey poults with hepatitis, california, USA. Emerg Infect Dis 17 480 487
124. JonesMSLukashovVVGanacRDSchnurrDP 2007 Discovery of a novel human picornavirus in a stool sample from a pediatric patient presenting with fever of unknown origin. J Clin Microbiol 45 2144 2150
125. BlinkovaOKapoorAVictoriaJJonesMWolfeN 2009 Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J Virol 83 4631 4641
126. ChiuCYGreningerALKanadaKKwokTFischerKF 2008 Identification of cardioviruses related to Theiler's murine encephalomyelitis virus in human infections. Proc Natl Acad Sci U S A 105 14124 14129
127. ArdenKEMackayIM 2010 Newly identified human rhinoviruses: molecular methods heat up the cold viruses. Rev Med Virol 20 156 176
128. BhattacharyaRSahooGCNayakMKSahaDRSurD 2006 Molecular epidemiology of human picobirnaviruses among children of a slum community in Kolkata, India. Infect Genet Evol 6 453 458
129. BuzinaroMGFreitasPPKisielliusJJUedaMJerezJA 2003 Identification of a bisegmented double-stranded RNA virus (picobirnavirus) in calf faeces. Vet J 166 185 187
130. GiordanoMOMartinezLCMasachessiGBarrilPAFerreyraLJ 2011 Evidence of closely related picobirnavirus strains circulating in humans and pigs in Argentina. J Infect 62 45 51
131. MasachessiGMartinezLCGiordanoMOBarrilPAIsaBM 2007 Picobirnavirus (PBV) natural hosts in captivity and virus excretion pattern in infected animals. Arch Virol 152 989 998
132. FregolenteMCde Castro-DiasEMartinsSSSpilkiFRAllegrettiSM 2009 Molecular characterization of picobirnaviruses from new hosts. Virus Res 143 134 136
133. ZhuHCChuDKLiuWDongBQZhangSY 2009 Detection of diverse astroviruses from bats in China. J Gen Virol 90 883 887
134. ChuDKPoonLLGuanYPeirisJS 2008 Novel astroviruses in insectivorous bats. J Virol 82 9107 9114
135. BrownKE 2010 The expanding range of parvoviruses which infect humans. Rev Med Virol 20 231 244
136. OlsonEJHaskellSRFrankRKLehmkuhlHDHobbsLA 2004 Isolation of an adenovirus and an adeno-associated virus from goat kids with enteritis. J Vet Diagn Invest 16 461 464
137. HenckaertsELindenRM 2010 Adeno-associated virus: a key to the human genome? Future Virol 5 555 574
138. BanyaiKEsonaMDLiuAWangYTuX 2010 Molecular detection of novel adenoviruses in fecal specimens of captive monkeys with diarrhea in China. Vet Microbiol 142 416 419
139. KunzANOttoliniM 2010 The role of adenovirus in respiratory tract infections. Curr Infect Dis Rep 12 81 87
140. TongSSinghJRuoneSHumphreyCYipCC 2010 Identification of adenoviruses in fecal specimens from wild chimpanzees (Pan trogylodytes schweinfurthii) in western Tanzania. Am J Trop Med Hyg 82 967 970
141. SmithJGWiethoffCMStewartPLNemerowGR 2010 Adenovirus. Curr Top Microbiol Immunol 343 195 224
142. KlempaBKrugerDHAusteBStankoMKrawczykA 2009 A novel cardiotropic murine adenovirus representing a distinct species of mastadenoviruses. J Virol 83 5749 5759
143. WangDCoscoyLZylberbergMAvilaPCBousheyHA 2002 Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A 99 15687 15692
144. PalaciosGQuanPLJabadoOJConlanSHirschbergDL 2007 Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg Infect Dis 13 73 81
145. YamashitaYOotsukaYKondoROsetoMDoiM 2010 Molecular characterization of Sapovirus detected in a gastroenteritis outbreak at a wedding hall. J Med Virol 82 720 726
146. KatayamaKShirato-HorikoshiHKojimaSKageyamaTOkaT 2002 Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299 225 239
147. IshidaSYoshizumiSMiyoshiMIkedaTOkuiT 2008 Characterization of sapoviruses detected in Hokkaido, Japan. Jpn J Infect Dis 61 504 506
148. SaitouNNeiM 1987 The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4 406 425
149. KimuraM 1980 A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16 111 120
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



