-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Genome of and the Basis of Host-Microsporidian Interactions
Microsporidia are obligate intracellular parasites with the smallest known eukaryotic genomes. Although they are increasingly recognized as economically and medically important parasites, the molecular basis of microsporidian pathogenicity is almost completely unknown and no genetic manipulation system is currently available. The fish-infecting microsporidian Spraguea lophii shows one of the most striking host cell manipulations known for these parasites, converting host nervous tissue into swollen spore factories known as xenomas. In order to investigate the basis of these interactions between microsporidian and host, we sequenced and analyzed the S. lophii genome. Although, like other microsporidia, S. lophii has lost many of the protein families typical of model eukaryotes, we identified a number of gene family expansions including a family of leucine-rich repeat proteins that may represent pathogenicity factors. Building on our comparative genomic analyses, we exploited the large numbers of spores that can be obtained from xenomas to identify potential effector proteins experimentally. We used complex-mix proteomics to identify proteins released by the parasite upon germination, resulting in the first experimental isolation of putative secreted effector proteins in a microsporidian. Many of these proteins are not related to characterized pathogenicity factors or indeed any other sequences from outside the Microsporidia. However, two of the secreted proteins are members of a family of RICIN B-lectin-like proteins broadly conserved across the phylum. These proteins form syntenic clusters arising from tandem duplications in several microsporidian genomes and may represent a novel family of conserved effector proteins. These computational and experimental analyses establish S. lophii as an attractive model system for understanding the evolution of host-parasite interactions in microsporidia and suggest an important role for lineage-specific innovations and fast evolving proteins in the evolution of the parasitic microsporidian lifecycle.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003676
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003676Summary
Microsporidia are obligate intracellular parasites with the smallest known eukaryotic genomes. Although they are increasingly recognized as economically and medically important parasites, the molecular basis of microsporidian pathogenicity is almost completely unknown and no genetic manipulation system is currently available. The fish-infecting microsporidian Spraguea lophii shows one of the most striking host cell manipulations known for these parasites, converting host nervous tissue into swollen spore factories known as xenomas. In order to investigate the basis of these interactions between microsporidian and host, we sequenced and analyzed the S. lophii genome. Although, like other microsporidia, S. lophii has lost many of the protein families typical of model eukaryotes, we identified a number of gene family expansions including a family of leucine-rich repeat proteins that may represent pathogenicity factors. Building on our comparative genomic analyses, we exploited the large numbers of spores that can be obtained from xenomas to identify potential effector proteins experimentally. We used complex-mix proteomics to identify proteins released by the parasite upon germination, resulting in the first experimental isolation of putative secreted effector proteins in a microsporidian. Many of these proteins are not related to characterized pathogenicity factors or indeed any other sequences from outside the Microsporidia. However, two of the secreted proteins are members of a family of RICIN B-lectin-like proteins broadly conserved across the phylum. These proteins form syntenic clusters arising from tandem duplications in several microsporidian genomes and may represent a novel family of conserved effector proteins. These computational and experimental analyses establish S. lophii as an attractive model system for understanding the evolution of host-parasite interactions in microsporidia and suggest an important role for lineage-specific innovations and fast evolving proteins in the evolution of the parasitic microsporidian lifecycle.
Introduction
Microsporidia are a diverse phylum of obligate intracellular parasites related to fungi. Over 1300 species have been described in approximately 160 genera and, as is the case for other microbial eukaryotes, a vast undescribed diversity is thought to exist in the environment [1]. Microsporidia are important pathogens of a broad range of animal groups: they can infect immunocompromized humans, such as those with HIV/AIDS, and are major pathogens of fish and invertebrates, representing a significant threat to sericulture [2] and fisheries [3]. Their unusual lifecycle has also attracted attention, particularly the unique mechanism by which microsporidia gain entrance to host cells. Outside the host cell, microsporidia exist as a resistant spore containing a coiled polar tube. Upon coming into contact with a host cell, or appropriate stimulus, the spore rapidly everts this tube, penetrating the host cell membrane and delivering the spore contents to the host cytoplasm, where proliferation and the next round of spore production occurs [4].
In addition to their importance as parasites of animals, Microsporidia have attracted much attention as eukaryotic model systems for reductive genome evolution. The 2.9 Mb genome of the microsporidian Encephalitozoon cuniculi was one of the first eukaryotic genomes to be sequenced [5]. Analysis of the E. cuniculi genome revealed a highly reduced and streamlined genome which had lost or simplified many biochemical pathways, had truncated genes, shortened intergenic spaces and had almost entirely lost introns and repetitive DNA [5]; its close relative Encephalitozoon intestinalis has an even smaller genome, at 2.3 Mb [6]. Interestingly, while microsporidian genomes are consistently smaller than those of their opisthokont relatives, there is a ten-fold difference in genome size within the phylum, with some genomes as large as 24 Mb [7]. To date, it has proven difficult to relate this genomic variation to differences in parasite biology or host preference. This is because all microsporidia sequenced so far share a broadly conserved core proteome, with differences in genome size due largely to changes in gene density, transposon content, and expansions of uncharacterized, lineage-specific or fast evolving protein families [8]–[10]. As the main differences in coding capacity among sequenced microsporidian lineages, it seems reasonable to hypothesize that these lineage-specific or fast evolving proteins play a role in mediating host-parasite interactions. However, because they lack any detectable similarity to genes from model eukaryotes, the functions of these proteins are difficult to predict using bioinformatics. Combined with the current lack of a system for genetic manipulation in these parasites, this makes understanding the basis of host-microsporidian interactions extremely challenging. Beyond identification of proteins of the spore wall and polar tube, very little known about molecular basis of spore germination and eversion of the polar tube, or the exact details of how the microsporidian sporoplasm is transferred into the host cell. Even though microsporidia can have drastic effects on the organization of the host cell, we know little of the virulence factors and effector proteins that bring about these changes or how they are delivered into the host cell environment either directly or via the parasitophorous vacuole in human infective species such as E. cuniculi and E. intestinalis.
Spraguea lophii is a microsporidian that infects the monkfish Lophius piscatorius and Lophius budegassa, inhabiting both the North Atlantic and Mediterranean regions [11]. Compared to those microsporidia that have been sequenced so far, S. lophii is an attractive model for identifying microsporidian effector proteins and investigating host-parasite interactions, despite the current lack of an in vitro culture system [12]. Infection with S. lophii results in the formation of xenomas, large clusters of spore-filled cells in the vagal nerves of the fish that can be several centimeters in diameter and contain spores in various stages of development (Figure 1). In the monkfish, no fitness effects are known to be associated with Spraguea infection, and the prevalence rate can be as high as 83% [13], [14]. However, xenoma formation in salmon infected with related microsporidia commonly localizes to the gills and is a considerable threat in aquaculture [15]. S. lophii spores are easily purified from xenomas in large quantities, providing an opportunity to study germination and to perform experiments that are difficult or impossible in other microsporidia due to the difficulty of obtaining sufficient amounts of parasite material. In addition, S. lophii is the first microsporidian parasite of fish to be sequenced, with the potential to provide new insights into host-parasite interactions in these economically important vertebrates. Our aim here is to provide a genomic resource that will facilitate future work on this promising model microsporidian.
Fig. 1. Microsporidian xenomas in situ. 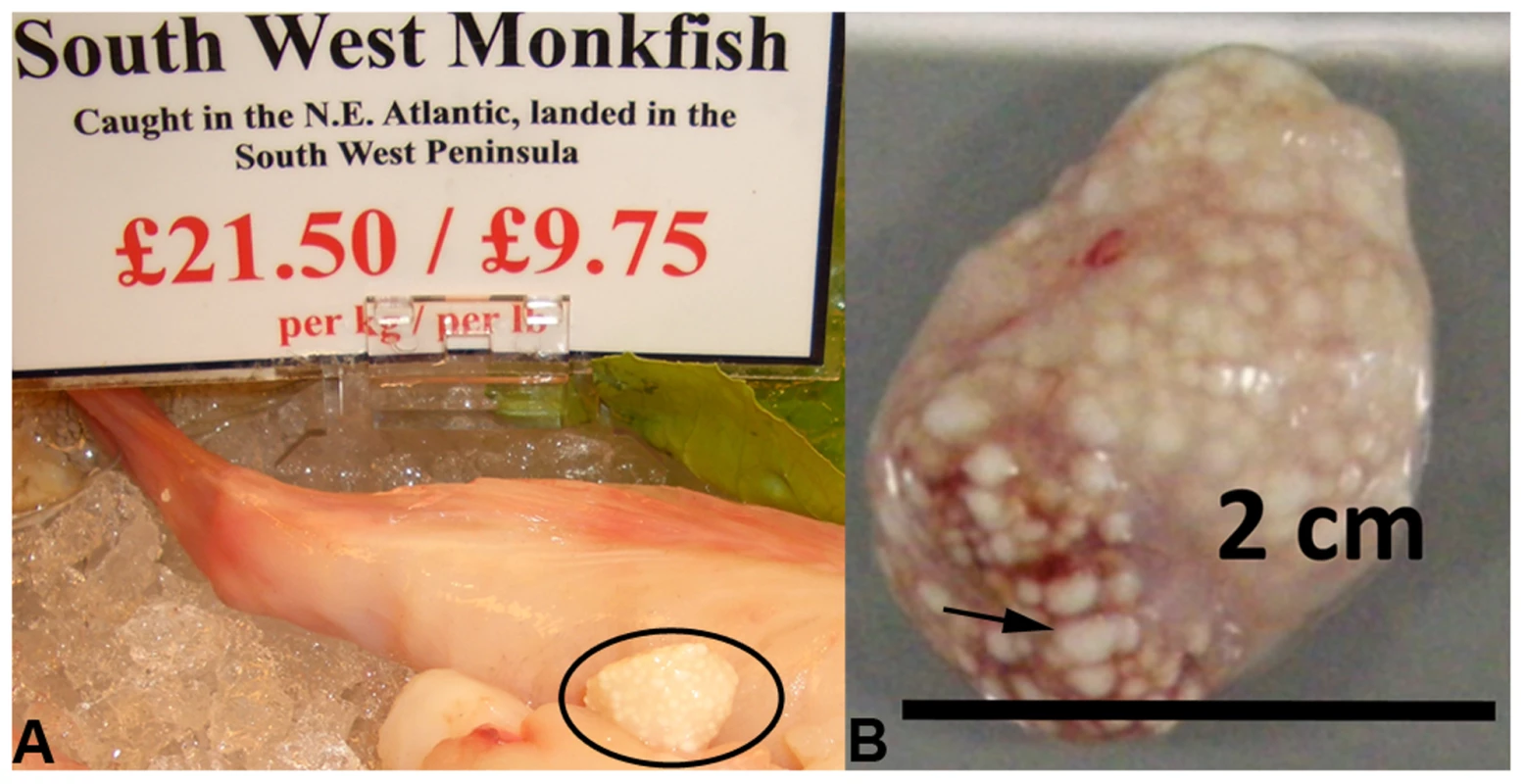
S. lophii grows and replicates inside large cysts visible to the naked eye; these cysts are comprised of swollen, spore-filled fish cells called xenomas. A. Cyst of S. lophii xenomas in situ in commercially available monkfish (circled). B. Close up of a cyst of xenomas. Arrow points to a single enlarged fish cell (xenoma). S. lophii was the first microsporidian to be explored with genome-scale sequencing, and 120 Kb of the genome has previously been published [16]. Here we present 4.98 Mb of unique sequence from the S. lophii genome as determined by Illumina sequencing, representing 70–80% of the complete genome (estimated at 6.2–7.3 Mb [17]–[19]) and, based on our analyses, the great majority of the coding DNA. We investigated the evolution of the S. lophii proteome, using OrthoMCL [20] to identify proteins that are unique to S. lophii and may therefore be associated with the unique xenoma formation seen in microsporidian infections of fish. To explore the utility of S. lophii as a model for understanding core features of microsporidian biology, we combined complex mix proteomics with TruSeq transcriptomics to characterize the proteins expressed and secreted during spore germination, a key time point in the lifecycle of this intracellular parasite. Our results highlighted the importance of microsporidia-specific and fast evolving proteins in germination and host interaction.
Results
Genome architecture of Spraguea lophii
The sequence data are summarized in Table 1. Our sequencing resulted in 1392 contigs over 500 bp in length to give a total of 4982 Kb (This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession ATCN00000000. The version described in this paper is version ATCN01000000). These contigs had a maximum length of 46788 bp and an N50 length of 5923. The average coverage across these contigs is 70× (Mode = 29×, Median = 59×). The overall GC content is low at 23.4%, rising slightly to 25.7% in protein coding regions. The karyotype has been investigated both by pulse field electrophoresis and by using an ultrathin multiwire proportional chamber-based detector [17]–[19]. This predicts a variable karyotype between isolates from different geographic regions, but the consensus is that there are 15 chromosomes, of which 10–13 are unique, and the overall genome size is estimated at 6.2 to 7.3 Mb. In our assembly we identified 2,573 predicted open reading frames or fragments of, which made up 52% of our assembly. This gives it an intermediate coding density amongst microsporidia, which is consistent with an emerging pattern for microsporidian genomes where coding density decreases with increasing genome size. For example, Trachipleistophora hominis has 34% coding DNA and an estimated genome size of 8.5–11.6 Mb, while 86% of the 2.9 Mb E. cuniculi genome is made up of coding DNA [5], [8]. Our assembly covers 70–80% of the S. lophii genome based on size estimates, but is likely enriched for coding regions. To evaluate the completeness of our assembly, we searched the S. lophii genome for the presence of genes involved in a range of core metabolic pathways as described previously [8], [21] (Table S1). Our assembly encodes most major metabolic pathways in full, including glycolysis and trehalose metabolism as well as a full complement of transfer RNAs (tRNAs) and protein kinases (Table S1). We identified only a few absences, many of which are also absent in related microsporidia such as T. hominis, lending support to our coverage of the protein-coding component of the genome. As an independent check on the completeness of our assembly, we compared the transcripts from our de novo transcriptome assembly (see below) to the genes predicted on the genome. Based on BLAST similarity to genes from other microsporidia, there were only 20 additional S. lophii genes in the transcriptome that did not map to the genome assembly, 5 of which represent transcripts from LTR retrotransposons. Taken together, these analyses suggest that our assembly represents a largely complete sampling of the coding component of the S. lophii genome.
Tab. 1. Summary of sequence data. 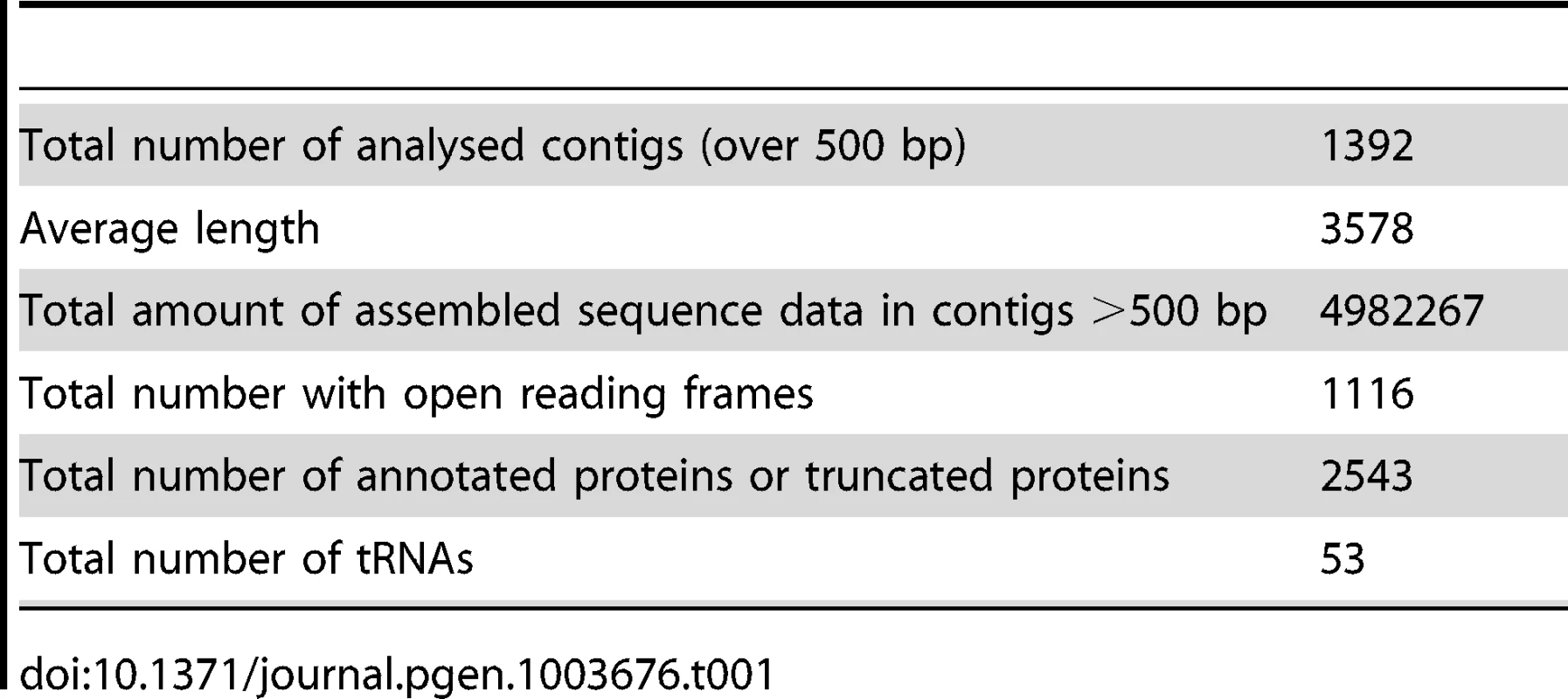
Loss and retention of metabolic pathways in Spraguea lophii
To investigate the evolution of gene content in Spraguea, we mapped the taxonomic distribution of microsporidian gene families onto a cladogram (Figure 2A) (derived from a multiprotein phylogeny - see below). We built gene families using OrthoMCL [20] on a broad sampling of microsporidian genomes, with Homo sapiens and Saccharomyces cerevisiae as opisthokont outgroups. Our analysis included genomes covering a broad taxonomic spectrum of sequenced microsporidia, including Nematocida parisii Ertm1 [22], T. hominis [8], Nosema ceranae [23], E. cuniculi [5], and Enterocytozoon bieneusi [24]. The results indicated that 19% of the predicted proteins are shared with all sampled opisthokonts, 1% are specific to sampled fungi, 4% are specific to microsporidia and conserved across the group, 3% are found in clusters of proteins only present in T. hominis and S. lophii. 42% the 2499 analysed S. lophii proteins, do not cluster with proteins from any of the other organisms in our analysis and of these 30% cluster with other S. lophii proteins, indicating that they are part of multiprotein families within the S. lophii genome. Table S2 gives a complete classification of the S. lophii proteome by OrthoMCL analysis.
Fig. 2. Comparison of the gene content of S. lophii to published microsporidian genomes. 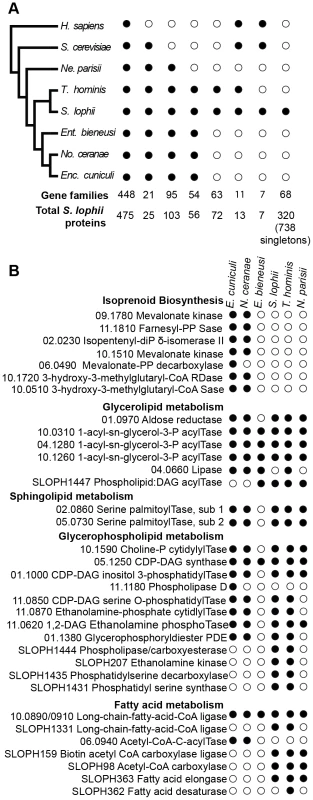
A. Phylogenetic distribution of annotated S. lophii proteins. Gene families (OrthoMCL clusters) were mapped on to a multigene phylogeny of microsporidia and their opisthokont relatives (see also Figure 7A.). B. Graphical illustration of the variation in fatty acid synthesis enzymes encoded by six different microsporidian genomes. Although the S. lophii genome is larger than that of E. cuniculi, it has lost some components of the pathway that are conserved in the smaller genome, such as isoprenoid biosynthesis. Reference numbers are given for E. cuniculi or S. lophii locus ID's. E. cuniculi and other microsporidia have a reduced lipid metabolism repertoire [5], [24]. A key missing step is the initial reaction of fatty acid synthesis, the carboxylation of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase. Homologues of both acetyl-CoA carboxylase and the biotin-[acetyl-CoA-carboxylase] ligase that it depends upon are present in the S. lophii genome as well as in the T. hominis and N. parisii genomes, suggesting that they are capable of performing this reaction. However, as in E. cuniculi, no fatty acid synthase is evident, though both a fatty acid elongase and desaturase are present (Figure 2B). Interestingly, the presence of these additional components in S. lophii was predicted on the basis of comparative liquid chromatography of the lipid composition of E. cuniculi and S. lophii spores which showed a higher level of docosahexaenoic acid, an unsaturated fatty acid, in S. lophii than E. cuniculi [25]. In comparison to E. cuniculi, the S. lophii genome also encodes more enzymes for glycerophospholipid synthesis, allowing for a greater variety of interconversions between different types of phospholipid for membrane integration. In contrast, and despite more complexity in some aspects of fatty acid metabolism, no components of the isoprenoid biosynthesis pathway were present in the S. lophii genome, though these are encoded in the E. cuniculi and N. ceranae genomes [5], [23] (Figure 2B). These are also absent from our transcriptome data, and from the genomes of T. hominis, N. parisii and E. bieneusi. Taken together, these results suggest that several lineages of microsporidia have independently lost the ability to biosynthesize isoprenoids, a capability that is otherwise conserved across the tree of life [24]. This pathway has been shown to be essential in prokaryotes, and whilst some parasitic Apicomplexa have replaced the classical mevalonate biosynthesis pathway with the alternative MEP pathway [26], it too is absent from these microsporidian genomes. These data suggest that some microsporidia scavenge sterols from the environment. This pathway has been lost in microsporidia with diverse hosts: E. bieneusi (mammals, insects), T. hominis (mammals, insects), N. parisii (worms) and S. lophii (fish), suggesting that there is nothing specific about the host biochemical environment that is driving the loss of this pathway.
Key components of the RNAi system are encoded by the S. lophii genome, including a dicer protein, an argonaute protein and fragments of an RNA dependent RNA polymerase. This is consistent with emerging genomic data for microsporidia with larger genomes such as N. ceranae and T. hominis that possess transposable elements [8], [23]. This suggests that RNAi was present in the common ancestor of microsporidia but has been secondarily lost in highly reduced genomes such as E. cuniculi and E. bieneusi [5], [8], [24]. A comparable situation exists in ascomycete fungi, where RNAi has been lost from the compact S. cerevisiae genome but is conserved in other budding yeasts [27].
Relatively few proteins with homology to characterized domains are found in S. lophii but no other microsporidia. There are proteins annotated on the basis of similarity to PFAM domains such as kinases, phosphatases and acetyltransferases, which are difficult to relate to specific functions within the cell. One notable exception is a glutamate-ammonia ligase domain containing protein, which can catalyze the generation of glutamine from glutamate and ammonia [28]. This protein is nested between genes with homologs in other microsporidian genomes (a DNA binding protein and acetyl-CoA carboxylase) (Figure S1) and in phylogenetic analysis does not fall into a clade with other fungi, but rather with prokaryotes meaning that it is not clear whether this gene was acquired by lateral transfer or by vertical inheritance. Fish excrete their nitrogenous waste products as ammonia across gills, but glutamate-ammonia ligases are expressed in the brains of fish and other vertebrates to protect from fluctuations in ammonia levels [29]. This protein may have a similar role in protection against ammonia stress in the microsporidian: Whilst the fish is alive the microsporidia may be protected by host ammonia defense mechanisms, however, once the fish dies, microbial degradation of the fish can increase ammonia levels [30]. As the spores of S. lophii are embedded deeply within the nervous tissue of the monkfish, they may have to be liberated after the death of the fish, and this glutamate-ammonia ligase may allow the spores to survive fluctuations in ammonia levels in the decaying fish tissue.
Despite the relatively close relationship of T. hominis to S. lophii (Figure 2A), these two species share few genes that are not found in other microsporidian genomes in our comparison, and most of these are uncharacterized, lineage-specific or fast evolving genes with no similarity to genes in other lineages. A handful of genes shared between T. hominis and S. lophii have a function that can be predicted on the basis of homology to characterized proteins from model organisms. These include an arsenite transporting ATPase, which might act as an efflux pump in the cell membrane and a cold shock domain protein, which could allow the cell to survive at lower than optimal temperatures [31].
Leucine rich repeat (LRR) proteins
The largest protein family expansion present in S. lophii is a family of proteins containing LRRs (Figure 3). Fungal genomes generally encode fewer LRR proteins than their animal relatives [32] though expansion of LRR protein families as pathogenicity factors is known in pathogenic fungi [33]. The genome of E. cuniculi encodes just 9 LRR genes in total [5], yet in stark contrast, we have found 97 ORFs encoding fragments of LRR proteins in the S. lophii genome, 52 of which appear complete (that is, they have a predicted start and stop codon), and 35 of which appear in our transcriptome data. We used PFAM and MEME [34], [35] to identify common conserved motifs, SignalP 4.1 and TargetP 1.1 to look for presence or absence of a secretion signal, and TMHMM 2.0 [36], [37] to look for evidence of transmembrane domains that could anchor the proteins in the membrane of the parasite as a potential LRR receptor protein. MEME analysis shows the presence of three different leucine-enriched motifs in the proteins (Figure 3). We also found that the majority of the proteins (37/52) have a predicted signal peptide but no transmembrane domain, meaning that they are potentially a family of secreted parasite effector proteins (Figure 3). Leucine rich repeat proteins often mediate protein-protein interactions, particularly through the formation of dimers [38]. One possibility is that, if these microsporidian LRR proteins are secreted into the host, they could potentially interfere with the formation of dimers of host proteins, disturbing the functional dimer-monomer cycle by sequestering them into inactive dimers, a mechanism seen in mammalian cells [39]. Perhaps surprisingly, similar sequences are also found in the distantly related microsporidian parasite of humans, Vittaforma corneae, but in no other microsporidian species for which there is an available genome sequence (Table S3). A large family of leucine-rich proteins was recently reported in another microsporidian, T. hominis, although the two families are not related and no predicted secretion signals were reported for the T. hominis family [8].
Fig. 3. An expanded family of 52 complete leucine-rich repeat proteins in the S. lophii genome. 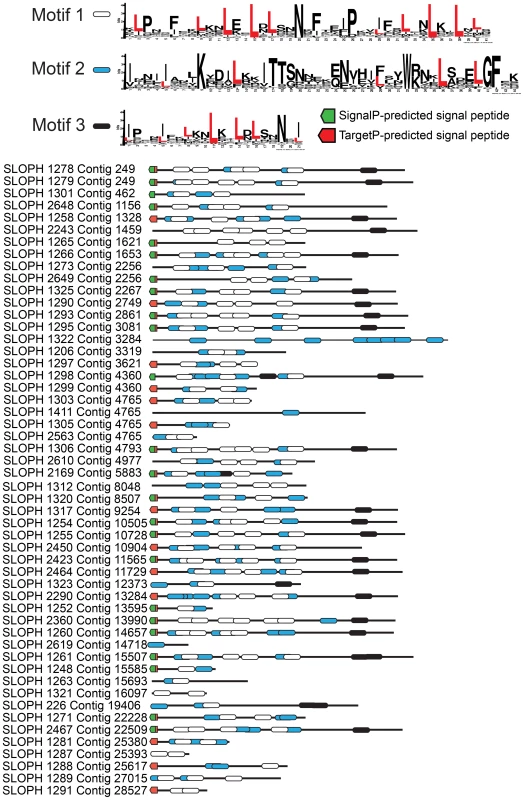
This family represents the largest S. lophii-specific protein family expansion, and interestingly, many of its members have N-terminal signal peptides, raising the possibility that some may be secreted or targeted to the parasite cell surface for host interactions. Leucine-rich motifs at non-overlapping sites with a p-value lower than 0.0001 were identified using MEME [34] and signal peptides identified using SignalP 4.1 (green) and TargetP (red) [37]. Although the S. lophii genome encodes 97 LRR-containing ORFs, we have evidence of expression for only 35. Therefore, we cannot exclude the possibility that some family members are never expressed and are pseudogenes. In other eukaryotic parasites such as Trypanosoma and Giardia, large protein families contain many pseudogenes which provide the genetic variation for the ongoing process of antigenic switching [40], [41]. If microsporidian multigene families interact with the host, switching of expression from gene to gene may allow escape from the fish adaptive immune response over the course of infection [42].
The transcriptome of germinated S. lophii spores
The cDNAs of artificially germinated spores were sequenced by the non strand-specific TruSeq approach using Illumina sequencing. This Transcriptome Shotgun Assembly project has been deposited at DDBJ/EMBL/GenBank under the accession GALE00000000. The version described in this paper is the first version, GALE01000000. We used Trinity RNA-Seq [43] to assemble the spore transcriptome, and RSEM [44] to quantify the relative abundance of transcripts. Our de novo transcriptome assembly contained 12,932 unique transcripts, of which 2,896 mapped to the S. lophii genome. Although relatively small in number, these transcripts made up 67.7% of the transcriptome by abundance, and likely represent the majority of the S. lophii genes in the dataset. 2,514 of these transcripts mapped to existing S. lophii genes, so that 1,598 of the predicted 2,539 open reading frames had at least one matching transcript. A small proportion of transcripts (398) mapped to regions of the genome assembly without gene predictions; manual inspection of these cases provided evidence for 30 additional genes that had been missed by the initial annotation process.
The remaining third of transcripts that did not map to the S. lophii genome assembly might represent contaminants, unmapped S. lophii genes, or artifacts of the assembly process. To distinguish between these possibilities, we searched these transcripts against the NCBI nr database using BLASTX [45]. Many showed high sequence identity to Pseudomonas and Flavobacterium genes and likely represent contaminants. We did, however, identify 20 additional genes with best BLAST hits among the Microsporidia, particularly T. hominis and Vavraia culicis; these are most likely S. lophii genes from the unassembled portion of the genome (Table S4). Interestingly, this set of 20 genes included one LTR and four non-LTR retrotransposons with similarity to those found on the T. hominis genome [8]. While the T. hominis retrotransposons are largely fragmented and pseudogenized, these results demonstrate that at least some of their homologues in S. lophii remain active.
Overall, our analyses of the S. lophii transcriptome provided support for the completeness of our genome assembly and for our gene calling approach, and also provided some new insights into microsporidian biology. Of the 1,986 genes in our genomic data that have complete open reading frames, that is, they have a start and a stop codon, 265 have complete coverage and for these, the RNA transcript was on average 132 base pairs longer than the gene. Five of these show more than one gene in the transcripts. However, given the short read-length of Illumina sequences and the possibility that transcripts of adjacent divergent genes could erroneously assemble, it is not possible to say whether these transcripts are overrunning into downstream genes as seen in other microsporidian species [46], [47].
Interestingly, although the most abundant transcripts corresponded to 18S ribosomal RNA (Table 2), the most highly expressed protein in S. lophii spores is an uncharacterized ORF, with homologues found only in a limited number of other microsporidia. Indeed, while the list of highly transcribed proteins contained many of the expected candidates (ribosomal proteins, ATP-binding proteins, transcription factors, and proteins involved in energy metabolism), these were intermingled with a number of uncharacterized proteins annotated as hypotheticals (see Table 2), strongly suggesting an important role for novel, lineage-specific or very fast evolving proteins in S. lophii and microsporidian biology. An interesting observation is that microsporidia-specific proteins conserved in multiple species make up a larger proportion of the transcriptome than Spraguea-specific proteins (Figure 4), and a larger proportion of the transcriptomic data than they do of the genomic data. These potentially novel proteins originating in the common ancestor of microsporidia may be good targets for future experimental work on the maintenance of the parasitic lifecycle both in S. lophii and other microsporidian species.
Fig. 4. Taxonomic distribution of predicted ORFs in the genome and transcriptome of Spraguea. 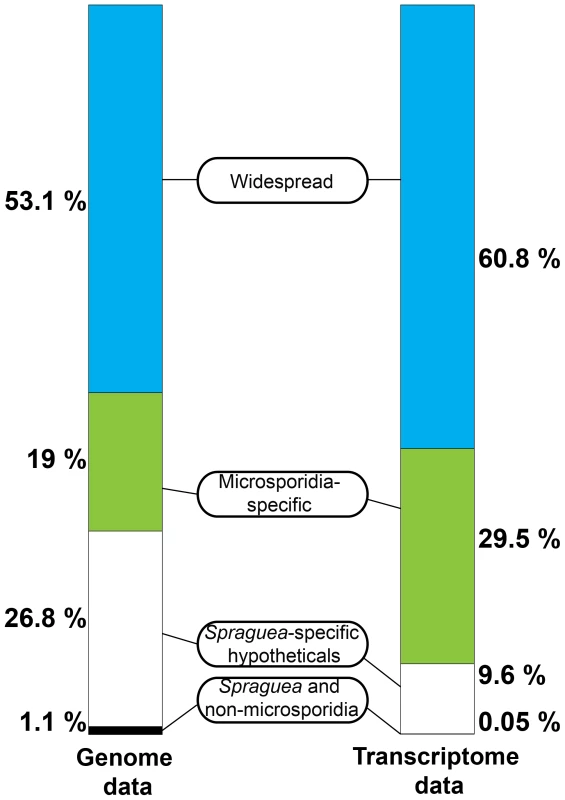
For taxonomic profiling, BLASTP was used to search against the NCBI nr database in January 2013, with a cutoff value of e<1×10−5. ORFs were classified into the four illustrated categories according to the results. Microsporidia- and S. lophii-specific ORFs and fast-evolving proteins with no similarity to proteins from other lineages make up a substantial proportion of the transcriptome, suggesting an important role for lineage-specific (or fast evolving) genes in the evolution of microsporidia. Tab. 2. The twenty most abundant protein-coding transcripts in the S. lophii transcriptome. 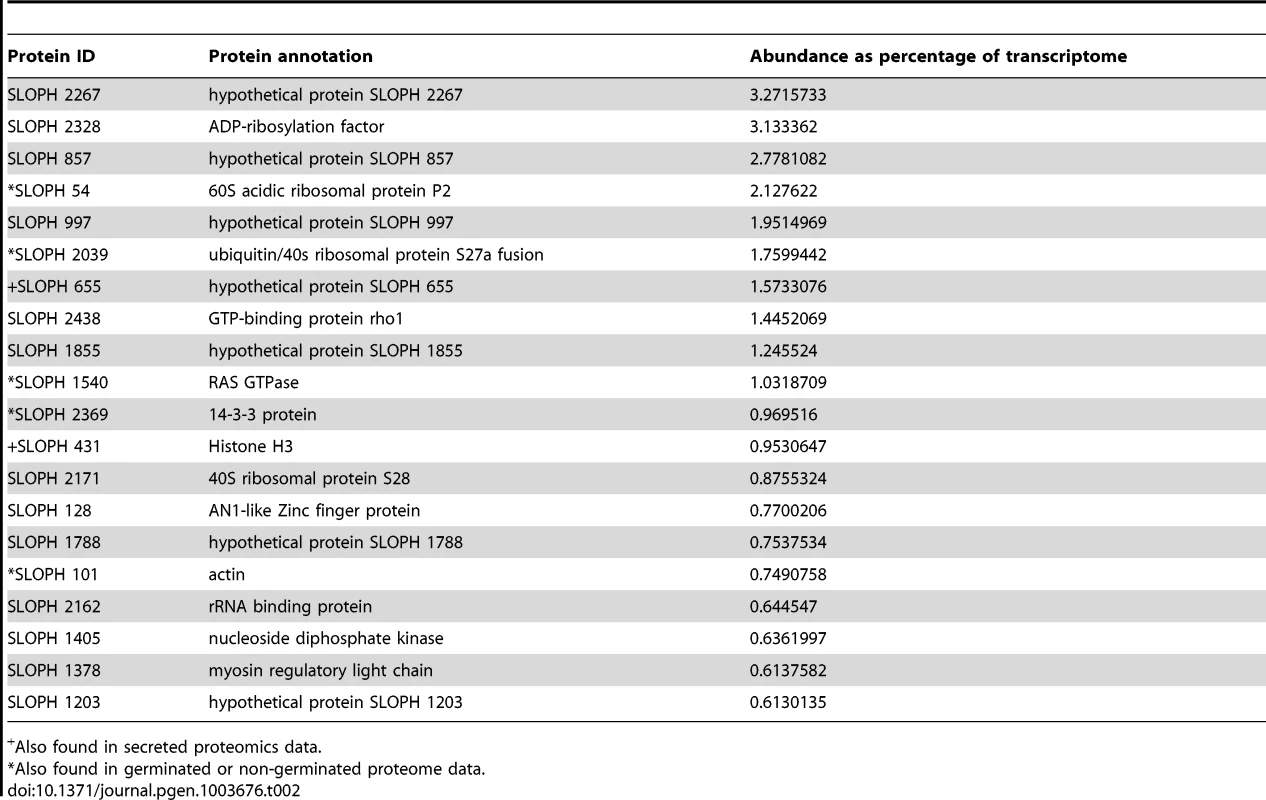
Also found in secreted proteomics data. Splicing and introns in S. lophii
Our de novo transcriptome assembly also enabled us to investigate the splicing of putative introns in S. lophii protein-coding genes. The number of introns in microsporidian genomes is greatly reduced compared to their opisthokont relatives [48]. E. cuniculi, N. ceranae, and T. hominis encode a relatively small (6–78) number of spliceosomal introns, which are largely confined to ribosomal proteins, while the Nematocida genus appears to have lost both introns and the splicing machinery entirely [22]. S. lophii does encode conserved components of the splicing machinery, so we searched its coding sequences for introns using a two-step approach. First, we scanned the genome with a consensus microsporidian intron motif built from comparisons of the introns in E. cuniculi and T. hominis [5], [8], [49]. This search returned hits to 8 genes, 6 of which encode ribosomal proteins (Figure 5); the two non-ribosomal proteins included genes encoding the DNA replication licensing factor Mcm1 and a poly(A) binding protein. We then searched the S. lophii transcriptome for transcripts containing deletions relative to the genome assembly, which might also indicate the presence of introns. Surprisingly, this analysis identified only two transcripts from which the predicted intron sequence had been spliced, corresponding to two of the eight genes identified by our motif scan (ribosomal protein S23 and poly(A) binding protein, Figure 5), transcripts for the other six genes still contained the putative intron motif. A comparison of the sequences of the two actively spliced introns with those of the other intron-like sequences revealed three striking differences (Figure 5): the spliced introns are much longer, are out of frame with the coding sequence, and are located further downstream from the 5′ end of the gene (89 and 152 nucleotides 3′ of the start codon, as opposed to directly adjacent to that codon in all other cases). Of the eight putative intron-containing genes we identified in Spraguea, five have orthologues in E. cuniculi that also contain an intron (S17, L27a, S24, L5 and poly(A) binding protein), and the efficiency with which those introns are spliced parallels our results with the S. lophii transcriptome. The introns in E. cuniculi S17, L27a, S24 and L5, for which we did not detect splicing in Spraguea, are also short [49] and are among the least efficiently spliced genes in E. cuniculi, with less than 15% of transcripts experiencing splicing (a figure which drops to 5% for L5) [50]. In contrast, the E. cuniculi orthologue of the actively-spliced poly(A) binding protein contains the longest and most frequently spliced intron in E. cuniculi, with over 80% of transcripts spliced. Thus, it appears that the properties determining intron splicing efficiency are conserved between these two distantly related microsporidia; it will be interesting to see if they hold more generally for other intron-containing microsporidian genomes.
Fig. 5. Putative introns in S. lophii. 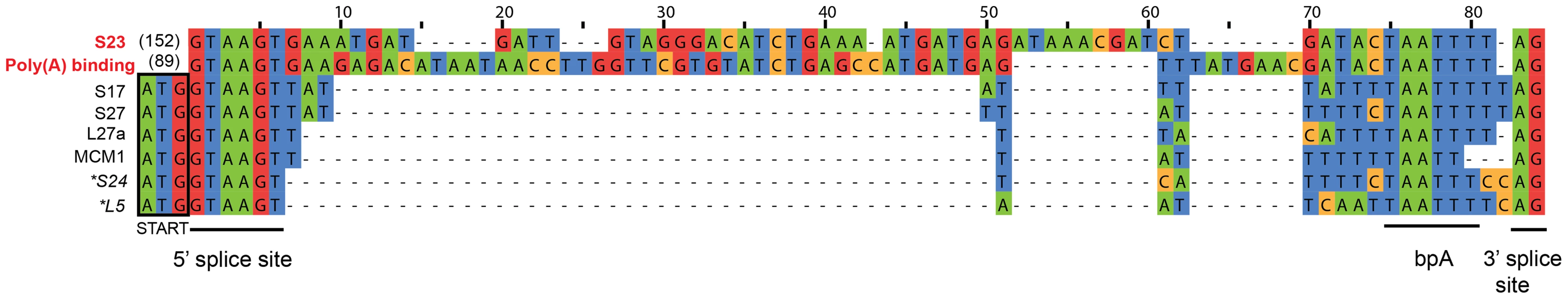
The regions corresponding to the conserved microsporidian motifs that interact with the splicing machinery (5′ and 3′ splice sites, branch point bpA) are indicated [49]. The splicing of introns highlighted in red (S23 and poly(A) binding protein) was confirmed at the transcriptome level; transcripts from the other genes retained the intron sequence, suggesting that they are rarely, if ever, spliced in S. lophii. The actively spliced introns are located 152 and 89 nucleotides downstream of the translation start site, while all others are immediately adjacent to the start codon (outlined in black). Genes marked with an asterisk could, in principle, be read through to produce a short insertion in the protein sequence, but the others cannot be read through because they contain an in-frame stop codon. These observations raise the question of how genes containing intron-like sequences that are rarely, if ever, spliced can be adequately expressed in Spraguea. The genes containing these motifs encode some of the most widely conserved and functionally important proteins in cellular life forms, including components of the ribosome and a DNA replication factor. Several of these intron-containing proteins were identified in our whole cell protein analysis of germinated and non-germinated spores. Ribosomal proteins S27, S24 and L5 were present in our germinated sample and S27 and L5 were also present in the proteome of dormant spores (Table S5). For two of the six genes (S24 and L5), inspection of the intron-like sequence suggests a simple explanation: indels in these sequences have caused a frameshift such that the intron can be read through from the upstream ATG without encountering an in-frame stop codon. Thus, a full-length protein containing a short N-terminal insertion could be expressed from these transcripts in the absence of splicing. The other four introns contain in-frame stop codons such that translation from the upstream ATG is not possible unless the intron is spliced. We considered the possibility that translation could instead begin at an alternative start codon downstream of the intron. However, initiation at the next available, in-frame ATG would result in substantial N-terminal deletions (covering 25–37% of the coding sequence) for three of these genes, and in the case of ribosomal protein S17 no suitable alternative start codon is available. Thus, it remains unclear whether these genes can be expressed without splicing, or whether translation depends on a rate of splicing too low to be detected in our assay; it was recently suggested that low rates of transcript degradation might partially ameliorate this problem in E. cuniculi [50].
Proteomics of germination and secretion
Dissecting the interactions between microsporidia and their host requires an understanding of the process of spore germination, in which the spore leaves dormancy and rapidly expels a long polar tube, through which the spore's cellular contents exit the spore and enter the host cell. The specific host cell stimuli inducing microsporidian spore germination are unidentified and likely complex, however several successful methods for artificial spore germination in-vitro have been described in a range of species [51]–[53]. At present, both the changes within the spore that trigger germination, and the identity of the secreted effector proteins used by the parasite gain entry into the host cell and control its biology are unknown. Genetic manipulation is a powerful tool for identifying these factors in many pathogens, but is not yet available for any microsporidian. However, S. lophii xenomas are densely packed with spores, providing an abundant source of parasite material for proteomic comparisons between the dormant and germinated spore stages.
To identify any proteins present in artificially germinated but not dormant spores, we analyzed whole protein extractions of both lifecycle stages with mass spectrometry of complex protein mixtures. After pooling and filtering 3 biological replicates our analysis showed no consistent variation at the proteomic level between the two lifecycle stages (Table S5). We did find components of many core pathways in germinated and non-germinated spores, such as histones, heat shock protein and ribosomal proteins. We also find glycolytic enzymes in both germinated and non-germinated spores, which is consistent with recent work that glycolytic pathways are specifically active in the spore stage [8], [54]. Two components of the secretory pathway (Sec23 and Sec24) were identified only in germinated spores, which may indicate its activation specifically upon germination, however these were not consistently found in all three replicates and overall we found a surprisingly conserved repertoire of proteins between the two samples. It may be that germination happens too rapidly to allow for translation, with microsporidia pre-packaging the proteins needed for immediate use upon recognition of the germination stimulus and therefore that obvious changes in protein complement may come later in development in meront and sporogonial stages. Alternatively it may be that the samples are dominated by highly expressed housekeeping proteins, and the proteins that vary between the two samples may be present at low levels not easily detectable by complex mix proteomics.
Next, we investigated the complement of proteins present in the extracellular medium after in-vitro germination. Here, we consistently retrieved a small subset of proteins that were visualized by SDS-PAGE (Figure S2). Importantly, no proteins were identified from the supernatant of non-germinated S. lophii spores, suggesting that the identified proteins are released specifically by the parasite upon germination. These could be proteins released by the sporoplasms on early infection or by the spore during germination. We retrieved 37 proteins from three quality-filtered replicates (Figure 6). Of these, 11/37 proteins are predicted by SignalP 4.1 to have a secretion signal and 17 are predicted by TargetP 1.1 to be directed to the secretory pathway; in some cases these predictions overlap (Figure 6). Proteins secreted to the extracellular medium range in size from 101 amino acids to 935 amino acids however there is no obvious correlation between size and the presence or absence of a predicted secretion signal (Figure 6). Five proteins are consistently present in all three replicates. Three of these have no similarity to any other proteins in the NCBI nr database (e<1×10−5) (SLOPH 477, 723, 762), while two of the proteins are found in other microsporidia (SLOPH 1766, 1854). One of these has features which make it particularly interesting in the context of potential effector proteins: it is part of a multigene family whose members have predicted Ricin-B-lectin domains and is found in several copies in S. lophii, as well as in several other microsporidian genomes (SLOPH 1766-see below). The other secreted protein shared with other microsporidia has significant sequence similarity to a spore wall protein (SWP7) in Nosema bombycis (SLOPH 1854).
Fig. 6. Proteins found in mass spectrometry analysis of medium from germinated S. lophii spores. 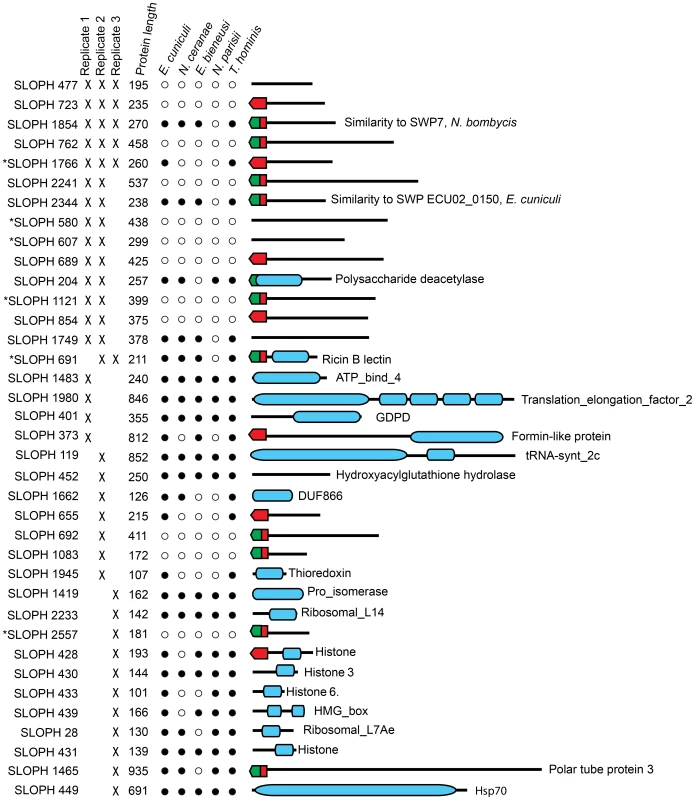
Targeting signals predicted by SignalP (green) or TargetP (red) are indicated. Presence in each replicate of the experiment is indicated with a cross. Stars next to proteins indicate the presence of multiple orthologues of the protein in the S. lophii genome. Presence or absence of the protein in other microsporidian genomes is indicated with a full or empty circle respectively. Recognized PFAM domains are indicated by blue bars. Ten proteins are found in two of three replicates. Six of these proteins have no similarity to any proteins in the NCBI database (e<1×10−5) and are potentially unique to S. lophii, demonstrating that species-specific innovations may play a crucial role in S. lophii invasion of the host cell. Four proteins are found in S. lophii and other microsporidia, including SLOPH 1749, which contains an exonuclease/endonuclease/phosphatase domain, SLOPH 2344, a spore wall protein and another RICIN-B lectin domain protein (SLOPH 691 - see below). A chitin deacetylase, which is conserved across the Microsporidia, was also identified in 2/3 biological replicates. The orthologue of this protein has been studied in E. cuniculi. In this species it is expressed at high levels during sporogonial lifecycle stages and localizes to the endospore, where it accumulates in paramural bodies [55], [56]. However, later functional analysis demonstrated that the protein was unable deacetylate chitooligosaccharides or bind chitin or any glycans, suggesting a non-canonical function [57]. Urch et al speculate that this chitin deacetylase may have evolved from a carbohydrate active enzyme to a lectin and may bind carbohydrates in the E. cuniculi cell wall [57]. A total of five microsporidia-specific uncharacterized proteins were observed in at least 2/3 biological replicates (SLOPH 1854, 1766, 2344, 1749, 691) (Figure 6). Orthologues of these proteins display a varied taxonomic distribution and secretion prediction profile across microsporidia, although two of them - SLOPH 2344 and SLOPH 1749 - are present in all microsporidia examined (with the exception of N. parisii) and are predicted to be secreted in all species expect E. intestinalis and E. bieneusi (SLOPH 2344), or just E. bieneusi (SLOPH 1749) (Table S6). Some proteins lacking predicted secretion signals are potentially highly expressed proteins that are released during the germination protocol and are therefore potentially false positives, for example histone proteins and translation elongation factor which were found in single replicate. Others, that are present in 2 or 3 replicates, may be secreted via a non-canonical secretion signal not detected by bioinformatics prediction programs.
The S. lophii-specific proteins may be host-driven innovations that mediate interactions specifically with the fish host, representing cases of lineage-specific adaptation or microsporidian-specific proteins that are fast evolving and not easily recognized between species. Some of the identified proteins are part of multigene families with other homologs in the S. lophii genome (Starred in Figure 6, Table S7) though proteomic data correspond to one member of the family. In agreement with our transcriptome analysis, these results demonstrate the importance of uncharacterized, hypothetical proteins in microsporidian germination, host cell invasion and early infection. This approach also represents a powerful method for identifying the presumably small proportion of novel parasite effectors or virulence factors from among the hundreds, or thousands, of hypothetical proteins found in sequenced microsporidian genomes. The need for such streamlining approaches is obvious, as a genetic manipulation protocol for microsporidia is currently lacking and hence protein characterization relies on heterologous expression systems or indirect assays which are time consuming and not easily applied to a large number of proteins.
A family of lectin-like proteins conserved in the Microsporidia
Two of the identified secreted proteins (SLOPH 1766 and SLOPH 691) are conserved in other microsporidia and show similarity to RICIN B-lectin proteins, with weakly conserved motifs involved in carbohydrate binding. Lectins have diverse roles in parasites, and can mediate adhesion of the parasite to the host cell during infection, but they can also play roles in immune evasion and their binding to host proteins can trigger different developmental pathways in the parasite [58], [59]. The members of this family form clusters in the genomes of E. cuniculi and N. ceranae (Figure 7A), reminiscent of clusters of effector proteins in fungal pathogens such as Ustilago maydis [60] and the RXLR and Crinkler effector families in oomycetes of the genus Phytophthora [61]. In E. cuniculi, four proteins are found in a single syntenic block, whereas in N. ceranae, a block of six proteins sit together in the genome with two others elsewhere. Interrogating the E. cuniculi genome, which has well-assembled chromosomes, these genes are not found at the end of chromosomes in the subtelomeric regions as is the case for effector proteins in other parasite species [62]. It is difficult to draw conclusions about the relative genomic location of the corresponding genes from our S. lophii data as several are found on short contigs, but we did identify two clusters of two genes (Figure 7A). A phylogeny of this protein family suggests that most of the paralogs within each species arose from species-specific duplications (Figure S3), again mirroring the pattern seen for effector families in other eukaryotic pathogens such as Ustilago maydis [60], Phytophthora [61] and Trichomonas [63]. The distribution of lectin-like proteins and expanded gene families were plotted onto a multigene phylogeny of microsporidia to give an overview of their distribution across the phylum (Figure 7B); the patchy distribution of these families suggests a process of differential loss and expansion during the radiation of microsporidia. In particular, we did not detect homologues of the lectin-like proteins in the N. parisii genome, suggesting that this family may have evolved after the divergence of N. parisii from the other sequenced microsporidia.
Fig. 7. Genomic context of lectin-like proteins and phylogenetic distribution of expanded gene families in microsporidia. 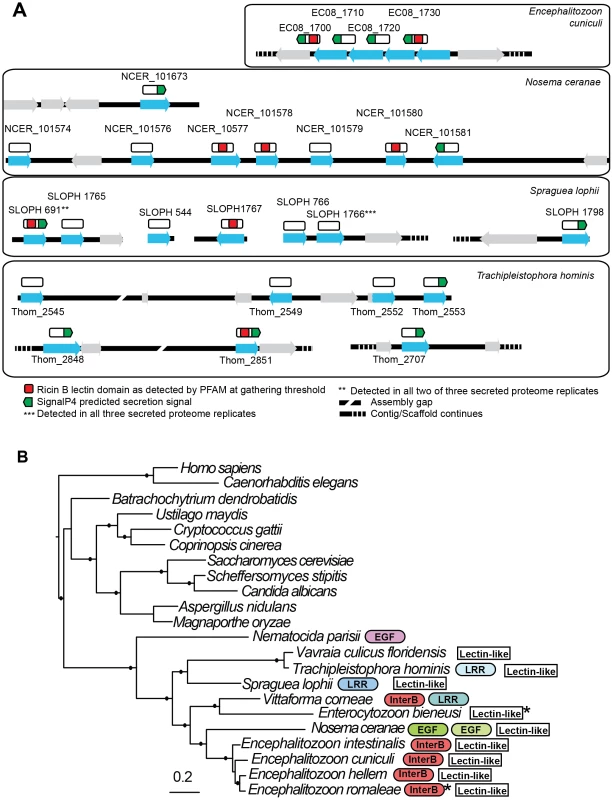
A. Putative lectin-like proteins are shown in their genomic context as blue arrows and unrelated flanking genes are shown as grey arrows. Above these (white rectangles) are shown hypothetical translated proteins (not to scale) to illustrate the positions of predicted motifs. The proteins SLOPH 691 and 1766 that were identified in our secretion proteomics were BLASTed against the Spraguea lophii genome at a cutoff of e<1×10−5 to identify other members of the lectin-like family. These proteins also show BLASTP similarity to lectin-like proteins in E. cuniculi and N. ceranae, as indicated. B. RAxML 8 protein phylogeny of microsporidia showing the phylogenetic context of S. lophii. Published expanded gene families were mapped onto this phylogeny, including InterB proteins [66], leucine rich-repeat proteins (LRR) [8], and other expanded gene families (EGF) as published [22], [23]. Stars indicate the detection of a single homolog of the protein in the genome. Shading is used to denote different families of apparently unrelated LRR proteins. Solid circles on branches indicate a posterior probability of 1 in our Bayesian phylogenetic analysis. Discussion
Although the S. lophii genome is larger than that of E. cuniculi, there is no clear trend towards a greater retention of broader ancestral metabolism. Like T. hominis, S. lophii has apparently lost the ability to biosynthesize isoprenoids, although it has retained more enzymes involved in other aspects of lipid metabolism. These results demonstrate that the loss of major biosynthetic pathways did not occur only in the common ancestor of all microsporidia, but has been an ongoing process throughout the evolution of the group, giving rise to important lineage-specific differences in metabolism.
Beyond reduction in genome size, a relative scarcity of introns is a conserved and striking feature of microsporidian genomes. The remaining introns tend to be short and, in many cases, located at the 5′ ends of genes encoding ribosomal proteins [5]. Our comparisons of splicing in S. lophii and E. cuniculi suggest that the molecular determinants of splicing efficiency are conserved in these distantly-related species, with longer, out-of-frame introns experiencing the highest levels of splicing. The observation that some inefficiently spliced, intron-like sequences can be read through without producing a truncated or frameshifted protein suggests a plausible, though speculative, mechanism by which introns could be lost from microsporidian genomes: once read-through is possible, subsequent deletions could reduce or remove the newly expressed insertion, resulting in complete loss of the former intronic sequence. We note, however, that the introns we compared in our assay are conserved between S. lophii and E. cuniculi, and so have not been lost in the period of time since the divergence of these species.
One of the most striking features of the S. lophii genome is the presence of a leucine rich repeat protein family, which may represent an expanded gene family of pathogenicity factors. Such expanded gene families are characteristic of other fungal pathogens such as Batrachochytrium dendrobatidis and Blumeria graminis, have also been reported in the microsporidia T. hominis, E. cuniculi, Anncaliia algerae and Vittaforma corneae [8], [64]–[66], and are also considered of importance in host-parasite interactions more generally [67]. Whilst the S. lophii members of this family have predicted N-terminal secretion signals, there is no evidence of an obvious conserved peptide motif involved in directing protein secretion into the host such as the oomycete crinkler motifs or the conserved tripeptide motif found in B. graminis which could help to define the microsporidian secretome [65], [68].
In addition to the conserved microsporidian proteome and an expanded complement of LRR proteins, S. lophii encodes a large number of predicted hypothetical proteins. Some of these were only expressed at low levels in our transcriptome analysis, potentially representing false positive ORF calls, but there were also a significant number of highly expressed microsporidia-specific transcripts and together with S. lophii-specific transcripts, these made up 39.1% of the total expression in S. lophii spores (Figure 4). Thus, lineage-specific or fast evolving proteins likely play an important role in S. lophii biology, and the same may well be true for all microsporidia. Although we now have genome sequences for a number of microsporidia, our understanding of their basic biology, pathogenicity and host interactions is limited by the current lack of a genetic manipulation system for these intracellular parasites. Recent work has shown that identification of proteins bearing secretion signals through bioinformatics can reveal interesting strategies by which microsporidia may alter the host environment in their favor. Cuomo et al. have identified functional secretion signals in a family of microsporidian hexokinases; when secreted, these effectors may stimulate host metabolism, providing more metabolites for the parasite [22]. These results demonstrate the utility of bioinformatics in predicting signal peptides and identifying microsporidian proteins that may be targeted to the cell surface or secreted. However, signal peptide prediction tools are trained on model eukaryotes, and their accuracy in predicting the full secretome from the highly divergent sequences of microsporidia is unclear. Even when these signals are accurately identified, heterologous characterization is laborious and expensive. One way forward may be through alternatives to genetic transformation, such as those we have explored in this article. Here we have used secretion proteomics to detect candidate virulence factors that are likely to be secreted from the parasite as it germinates, leaves the polar tube and enters the host cell. Two of these identified secreted proteins are a part of a family that is broadly conserved across the Microsporidia, with local duplications giving rise to syntenic blocks of family members in several microsporidian genomes. Based on their sequence similarity to lectin domains, they may be involved in binding to carbohydrates on host proteins, and their conservation suggests they play an important general role in microsporidian parasitism. We combined our secretion proteomics with an analysis of the S. lophii transcriptome, which identified a further set of highly expressed, microsporidia-specific hypotheticals. These proteins are ideal candidates for further characterization to better understand the molecular basis of parasitism in the Microsporidia, both within the parasite cell and in its host interactions.
Materials and Methods
Spore purification
DNA was extracted from two separate clusters of cysts both collected from local Monkfish (Lophius piscatorius) landed at either Plymouth or Brixham caught in the North Atlantic. Spores were separated from fish material in a three-step process. Xenomas filled with microsporidia were removed from the fish tissue manually. Samples were then soaked overnight at 4°C in EDTA (10 mM) containing Triton X 100 at 0.05% and trypsin 0.025% (w/v). The samples were then homogenized in a glass homogenizer until they formed a fine suspension, and washed three times in sterile PBS. The spore suspension was then cleaned by centrifugation through 100% Percoll (Sigma) at 4°C at 1600×g for 15 minutes. Samples were further washed three times in sterile 1× PBS before resuspension and storage at 4°C in 1 ml of sterile 1× PBS with addition of an antibiotic cocktail of 10 µg/ml Ampicillin, Penicillin/Streptomycin and Kanamycin.
DNA extraction and library preparation
Aliquots of 200 µl of purified spores were resuspended in 400 µl TE 10/1 pH 7.5 and ground in a pestle and mortar in liquid nitrogen for 15 minutes. Powdered frozen material was transferred to 800 µl phenol (pH 7.9) and mixed by inversion then centrifuged at 10,000×g for 10 minutes. 400 µl chloroform was added to the aqueous supernatant and this was centrifuged at 10,000×g for 5 minutes. DNA was precipitated from the aqueous layer using a standard ethanol precipitation protocol [69].
Preparation of DNA library for Illumina sequencing
For sample one, the DNA was sheared using a Covalis Nebuliser. Sheared fragments of approximately 400 bp in size were selected using a polyacrylamide gel and used to create a library using the Illumina paired-end sample preparation protocol (revision A June 2008). A single lane of paired-end 36 bp sequence reads was sequenced. For sample two, DNA was sheared by Biorupter sonication and fragments of approximately 600 bp were selected by polyacrylamide electrophoresis for library preparation as above. A single lane of paired-end reads of 76 bp was sequenced giving a total of 325 Mb of data.
Data assembly and annotation
The data from the two runs were pooled and assembled together using Velvet 0.7.50 using a kmer length of 31 and coverage cutoff of 5. Estimated expected kmer coverage using automatic calibration was 6. Contigs of 500 bp or larger were retained and analysed further. This resulted in a set of 3080 contigs, which showed a bimodal distribution of GC content with peaks at approximately 23% GC and 58% GC, indicative of DNA from a second organism (based on sequence identity, a close relative or strain of Pseudomonas fluorescens). A histogram of GC content revealed two distinct distributions with little overlap (Figure S4); we therefore discarded all contigs with GC content greater than 40% as potential Pseudomonas contaminants. All retained contigs were used to BlastN search gi229587578 Pseudomonas fluorescens SBW25 chromosome and gi146343893 Pseudomonas fluorescens SBW25 plasmid pQBR103, to verify that there was no P. fluorescens DNA in the remaining contigs. Removed high GC contigs were also used to BLAST search the same genomes and any contigs with a BLASTN hit value higher than 1×10−30 were used to BLASTX search the same microsporidia used in our comparative analysis to verify that there was no obvious microsporidian content. Remaining contigs were parsed to find unknown stretches of DNA left by paired-end assembly of 5 base pairs or more and split at these points. This left a set of 1392 contigs that were further analyzed. These contigs were annotated using Artemis 13.2.0. All ORFs of 100 amino acids or more were analyzed by BLASTP and PFAM search and an annotation given on the basis of these searches [35]. Each contig was searched for the presence of tRNAs using tRNAscan-SE v.1.23 [70], which were annotated onto the contigs using Artemis.
OrthoMCL and BLAST analysis
56274 proteins sequences from eight species (predicted proteomes of H. sapiens, S. cerevisiae, N. ceranae, E. bieneusi, E. cuniculi, N. parisii, T. hominis and those 2499 S. lophii proteins without predicted frameshifts) were clustered using OrthoMCL: BLAST e-value cutoff = 1e-5, Inflation value = 1.5. Output files were parsed and sorted into categories shown in Figure 2A.
In vitro germination
300 µl of purified spores were washed 3 times in 1× PBS. A non-germinated control sample was then treated with 100 µl of 0.25 mM EGTA per 100 µg of spores to inhibit germination. The sample to be germinated was treated with 100 µl of 0.5 M Gly-Gly buffer (pH 7.0) per 100 µg of spores and incubated at room temperature for 30 minutes. 40 µl of Calcium Ionophore A23187 (1 µg/µl) (Sigma-Aldrich) dissolved in DMSO was added to the sample followed immediately by 40 µl of 0.5 M Gly-Gly buffer (pH 9.0) per 100 µg of spores. Germination was verified instantly by light microscopy and efficiency was estimated to reach a maximum of 80%.
RNA preparation for sequencing
200 µl of germinated S. lophii spores were frozen with liquid nitrogen and disrupted manually using a pestle and mortar. RNA was then extracted following the standard TRIzol (Invitrogen) protocol. RNA was resuspended in 50 µl Milli-Q water (Millipore) and quantified as 2260 ng/ul (absorbance 260/280 = 2.12; 260/230 = 2.41). RNA integrity was verified on 1.5% TAE agarose gel prior to sequencing. PolyA RNA was isolated from 4 µg total RNA and the TruSeq library was prepared according to the Illumina TruSeq RNA sample preparation guide (Part # 15008136 Rev. A November 2010). 15 cycles of PCR were used to amplify the library and 5 µl of the 6 nM library was loaded onto a flow cell with two other TruSeq libraries and run on the HiSeq 2000 with a paired-end 100 bp run.
Transcriptome assembly and analysis
The raw Illumina reads obtained from RNA sequencing were filtered with fastq-mcf [71] to remove adapter sequences and low quality reads (with a quality score <28). The filtered reads were assembled using the Trinity package [43]. Reads were mapped back onto the transcriptome assembly using Bowtie [72], and the abundance of each transcript was estimated using RSEM [44].
Identification of proteins secreted into the extracellular medium during spore germination
500 µl of purified S. lophii spores were germinated using the protocol described previously. Germinated S. lophii spores along with non-germinated control were then spun at 12,000×g for 15 minutes at 4°C. The resultant supernatant was then collected and concentrated using Millipore Amicon 3 kDa centrifugal concentration column at 2,000×g for 30 minutes at 4°C. The concentrated extracellular protein was then quantified and checked on 12% SDS gels. Complex mixtures of extracellular secreted proteins from germinated and non-germinated samples were sent for analysis on an 6520 accurate mass quadrupole time of flight (Q-TOF) mass spectrometer (Agilent Technologies) and resultant masses were searched against our translated S. lophii genome database. The experiment was conducted in triplicate and any protein without two distinct peptide hits and a percent score peak intensity (% SPI) of ≥60% was removed from the analysis.
Supporting Information
Zdroje
1. KeelingP (2009) Five Questions about Microsporidia. PLoS Pathog 5: e1000489.
2. SinghT, BhatMM, KhanMA (2012) Microsporidiosis in the Silkworm, Bombyx mori L. (Lepidoptera: Bombycidae). Pertanika Journal of Tropical Agricultural Science 35 : 387–406.
3. Speare DJ, Lovy J (2011) Loma salmonae related species. In: Woo PTK, Buchmann K, editors. Fish Parasites: Pathobiology and Protection. UK: CABI Publishing.
4. SchotteliusJ, SchmetzC, KockNP, SchulerT, SobottkaI, et al. (2000) Presentation by scanning electron microscopy of the life cycle of microsporidia of the genus Encephalitozoon. Microbes Infect 2 : 1401–1406.
5. KatinkaMD, DupratS, CornillotE, MetenierG, ThomaratF, et al. (2001) Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414 : 450–453.
6. CorradiN, PombertJF, FarinelliL, DidierES, KeelingPJ (2010) The complete sequence of the smallest known nuclear genome from the microsporidian Encephalitozoon intestinalis. Nat Commun 1 : 77.
7. CorradiN, HaagKL, PombertJF, EbertD, KeelingPJ (2009) Draft genome sequence of the Daphnia pathogen Octosporea bayeri: insights into the gene content of a large microsporidian genome and a model for host-parasite interactions. Genome Biol 10: R106.
8. HeinzE, WilliamsTA, NakjangS, NoëlCJ, SwanDC, et al. (2012) The Genome of the Obligate Intracellular Parasite Trachipleistophora hominis: New Insights into Microsporidian Genome Dynamics and Reductive Evolution. PLoS Pathog 8: e1002979.
9. WilliamsBA, LeeRC, BecnelJJ, WeissLM, FastNM, et al. (2008) Genome sequence surveys of Brachiola algerae and Edhazardia aedis reveal microsporidia with low gene densities. BMC Genomics 9 : 200.
10. KrylovDM, WolfYI, RogozinIB, KooninEV (2003) Gene loss, protein sequence divergence, gene dispensability, expression level, and interactivity are correlated in eukaryotic evolution. Genome Res 13 : 2229–2235.
11. DöfleinF (1898) Studien zur Naturgeschichte der Protozoen. III. Über die Myxosporidien. Zool Jahrb Abt Anat 11 : 281–350.
12. WeidnerE, ByrdW (1982) The microsporidian spore invasion tube. II. Role of calcium in the activation of invasion tube discharge. J Cell Biol 93 : 970–975.
13. FreemanMA, YokoyamaH, OsadaA, YoshidaT, YamanobeA, et al. (2011) Spraguea (Microsporida: Spraguidae) infections in the nervous system of the Japanese anglerfish, Lophius litulon (Jordan), with comments on transmission routes and host pathology. J Fish Dis 34 : 445–452.
14. CañásL, SampedroMP, FariñaAC (2010) Influence of host biological features on macroparasites of the two European anglerfish species, Lophius piscatorius and Lophius budegassa, off north and northwest Spain. J Parasitol 96 : 191–193.
15. PutzRE, HoffmanGL, DunbarCE (1965) 2 New Species of Plistophora (Microsporides) from North American Fish with a Synopsis of Microsporidea of Freshwater and Euryhaline Fishes. Journal of Protozoology 12 : 228–236.
16. HinkleG, MorrisonHG, SoginML (1997) Genes coding for reverse transcriptase, DNA-directed RNA polymerase, and chitin synthase from the microsporidian Spraguea lophii. Biol Bull 193 : 250–251.
17. MansourL, CheikaliC, DesaunaisP, CoulonJP, DaubinJ, et al. (2004) Description of an ultrathin multiwire proportional chamber-based detector and application to the characterization of the Spraguea lophii (Microspora) two-dimensional genome fingerprint. Electrophoresis 25 : 3365–3377.
18. BiderreC, PagesM, MetenierG, DavidD, BataJ, et al. (1994) On small genomes in eukaryotic organisms: molecular karyotypes of two microsporidian species (Protozoa) parasites of vertebrates. C R Acad Sci III 317 : 399–404.
19. MansourL, Ben HassineOK, VivaresCP, CornillotE (2013) Spraguea lophii (Microsporidia) parasite of the teleost fish, Lophius piscatorius from Tunisian coasts: Evidence for an extensive chromosome length polymorphism. Parasitol Int 62 : 66–74.
20. LiL, StoeckertCJJr, RoosDS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189.
21. KeelingPJ, CorradiN, MorrisonHG, HaagKL, EbertD, et al. (2010) The Reduced Genome of the Parasitic Microsporidian Enterocytozoon bieneusi Lacks Genes for Core Carbon Metabolism. Genome Biology and Evolution 2 : 304–309.
22. CuomoCA, DesjardinsCA, BakowskiMA, GoldbergJ, MaAT, et al. (2012) Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res 12 : 2478–2488.
23. CornmanRS, ChenYP, SchatzMC, StreetC, ZhaoY, et al. (2009) Genomic analyses of the microsporidian Nosema ceranae, an emergent pathogen of honey bees. PLoS Pathog 5: e1000466.
24. AkiyoshiDE, MorrisonHG, LeiS, FengX, ZhangQ, et al. (2009) Genomic survey of the non-cultivatable opportunistic human pathogen, Enterocytozoon bieneusi. PLoS Pathog 5: e1000261.
25. El AlaouiH, BataJ, BauchartD, DoreJC, VivaresCP (2001) Lipids of three microsporidian species and multivariate analysis of the host-parasite relationship. J Parasitol 87 : 554–559.
26. LangeBM, RujanT, MartinW, CroteauR (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci U S A 97 : 13172–13177.
27. DrinnenbergIA, WeinbergDE, XieKT, MowerJP, WolfeKH, et al. (2009) RNAi in budding yeast. Science 326 : 544–550.
28. EisenbergD, GillHS, PflueglGM, RotsteinSH (2000) Structure-function relationships of glutamine synthetases. Biochim Biophys Acta 1477 : 122–145.
29. SuarezI, BodegaG, FernandezB (2002) Glutamine synthetase in brain: effect of ammonia. Neurochem Int 41 : 123–142.
30. Huss HH (1995) Quality and quality changes in fresh fish. Food and agriculture organization of the united nations.
31. PhadtareS, AlsinaJ, InouyeM (1999) Cold-shock response and cold-shock proteins. Curr Opin Microbiol 2 : 175–180.
32. SoanesDM, TalbotNJ (2010) Comparative genome analysis reveals an absence of leucine-rich repeat pattern-recognition receptor proteins in the kingdom Fungi. PLoS One 5: e12725.
33. ButlerG, RasmussenMD, LinMF, SantosMA, SakthikumarS, et al. (2009) Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459 : 657–662.
34. BaileyTL, ElkanC (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2 : 28–36.
35. PuntaM, CoggillPC, EberhardtRY, MistryJ, TateJ, et al. (2012) The Pfam protein families database. Nucleic Acids Res 40: D290–301.
36. KroghA, LarssonB, von HeijneG, SonnhammerEL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305 : 567–580.
37. EmanuelssonO, BrunakS, von HeijneG, NielsenH (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2 : 953–71.
38. KobeB, DeisenhoferJ (1995) A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 374 : 183–186.
39. BertholdJ, SchenkovaK, RamosS, MiuraY, FurukawaM, et al. (2008) Characterization of RhoBTB-dependent Cul3 ubiquitin ligase complexes–evidence for an autoregulatory mechanism. Exp Cell Res 314 : 3453–3465.
40. DeitschKW, LukehartSA, StringerJR (2009) Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol 7 : 493–503.
41. MarcelloL, BarryJD (2007) Analysis of the VSG gene silent archive in Trypanosoma brucei reveals that mosaic gene expression is prominent in antigenic variation and is favored by archive substructure. Genome Res 17 : 1344–1352.
42. RautaPR, NayakB, DasS (2012) Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms. Immunol Lett 148 : 23–33.
43. GrabherrMG, HaasBJ, YassourM, LevinJZ, ThompsonDA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29 : 644–652.
44. LiB, DeweyCN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12 : 323.
45. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410.
46. CorradiN, GangaevaA, KeelingPJ (2008) Comparative profiling of overlapping transcription in the compacted genomes of microsporidia Antonospora locustae and Encephalitozoon cuniculi. Genomics 91 : 388–93.
47. WilliamsBA, SlamovitsCH, PatronNJ, FastNM, KeelingPJ (2005) A high frequency of overlapping gene expression in compacted eukaryotic genomes. Proc Natl Acad Sci U S A 102 : 10936–10941.
48. KeelingPJ, SlamovitsCH (2004) Simplicity and complexity of microsporidian genomes. Eukaryot Cell 3 : 1363–1369.
49. LeeRCH, GillEE, RoySW, FastNM (2010) Constrained Intron Structures in a Microsporidian. Molecular Biology and Evolution 27 : 1979–1982.
50. GrisdaleC, BowersL, DidierE, FastN (2013) Transcriptome analysis of the parasite Encephalitozoon cuniculi: an in-depth examination of pre-mRNA splicing in a reduced eukaryote. BMC Genomics 14 : 207.
51. PleshingerJ, WeidnerE (1985) The microsporidian spore invasion tube. IV. Discharge activation begins with pH-triggered Ca2+ influx. J Cell Biol 100 : 1834–1838.
52. FrixioneE, RuizL, UndeenAH (1994) Monovalent cations induce microsporidian spore germination in vitro. J Eukaryot Microbiol 41 : 464–468.
53. FrixioneE, RuizL, CerbonJ, UndeenAH (1997) Germination of Nosema algerae (Microspora) spores: conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J Eukaryot Microbiol 44 : 109–116.
54. DolgikhVV, SenderskiyIV, PavlovaOA, NaumovAM, BeznoussenkoGV (2011) Immunolocalization of an alternative respiratory chain in Antonospora (Paranosema) locustae spores: mitosomes retain their role in microsporidial energy metabolism. Eukaryot Cell 10 : 588–593.
55. BrossonD, KuhnL, PrensierG, VivaresCP, TexierC (2005) The putative chitin deacetylase of Encephalitozoon cuniculi: a surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol Lett 247 : 81–90.
56. BrossonD, KuhnL, DelbacF, GarinJ, P. VivarèsC, et al. (2006) Proteomic analysis of the eukaryotic parasite Encephalitozoon cuniculi (microsporidia): a reference map for proteins expressed in late sporogonial stages. PROTEOMICS 6 : 3625–3635.
57. UrchJE, Hurtado-GuerreroR, BrossonD, LiuZ, EijsinkVG, et al. (2009) Structural and functional characterization of a putative polysaccharide deacetylase of the human parasite Encephalitozoon cuniculi. Protein Sci 18 : 1197–1209.
58. LoukasA, MaizelsRM (2000) Helminth C-type lectins and host-parasite interactions. Parasitol Today 16 : 333–339.
59. PetriWAJr, HaqueR, MannBJ (2002) The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu Rev Microbiol 56 : 39–64.
60. KamperJ, KahmannR, BolkerM, MaLJ, BrefortT, et al. (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 : 97–101.
61. SchornackS, HuitemaE, CanoLM, BozkurtTO, OlivaR, et al. (2009) Ten things to know about oomycete effectors. Mol Plant Pathol 10 : 795–803.
62. SargeantTJ, MartiM, CalerE, CarltonJM, SimpsonK, et al. (2006) Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol 7: R12.
63. NoelCJ, DiazN, Sicheritz-PontenT, SafarikovaL, TachezyJ, et al. (2010) Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC Genomics 11 : 99.
64. AbramyanJ, StajichJE (2012) Species-specific chitin-binding module 18 expansion in the amphibian pathogen Batrachochytrium dendrobatidis. MBio 3: e00150–00112.
65. SpanuPD, AbbottJC, AmselemJ, BurgisTA, SoanesDM, et al. (2010) Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 330 : 1543–1546.
66. DiaN, LavieL, MetenierG, ToguebayeBS, VivaresCP, et al. (2007) InterB multigenic family, a gene repertoire associated with subterminal chromosome regions of Encephalitozoon cuniculi and conserved in several human-infecting microsporidian species. Curr Genet 51 : 171–186.
67. FankhauserN, Nguyen-HaTM, AdlerJ, MaserP (2007) Surface antigens and potential virulence factors from parasites detected by comparative genomics of perfect amino acid repeats. Proteome Sci 5 : 20.
68. SchornackS, van DammeM, BozkurtTO, CanoLM, SmokerM, et al. (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc Natl Acad Sci U S A 107 : 17421–17426.
69. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning : a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory.
70. SchattnerP, BrooksAN, LoweTM (2005) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33: W686–689.
71. Aronesty E (2011) Command-line tools for processing biological sequencing data. ea-utils http://code.google.com/p/ea-utils: Expression Analysis, Durham, NC
72. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



