-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
Dyskeratosis congenita (DC) is a heterogeneous inherited bone marrow failure and cancer predisposition syndrome in which germline mutations in telomere biology genes account for approximately one-half of known families. Hoyeraal Hreidarsson syndrome (HH) is a clinically severe variant of DC in which patients also have cerebellar hypoplasia and may present with severe immunodeficiency and enteropathy. We discovered a germline autosomal recessive mutation in RTEL1, a helicase with critical telomeric functions, in two unrelated families of Ashkenazi Jewish (AJ) ancestry. The affected individuals in these families are homozygous for the same mutation, R1264H, which affects three isoforms of RTEL1. Each parent was a heterozygous carrier of one mutant allele. Patient-derived cell lines revealed evidence of telomere dysfunction, including significantly decreased telomere length, telomere length heterogeneity, and the presence of extra-chromosomal circular telomeric DNA. In addition, RTEL1 mutant cells exhibited enhanced sensitivity to the interstrand cross-linking agent mitomycin C. The molecular data and the patterns of inheritance are consistent with a hypomorphic mutation in RTEL1 as the underlying basis of the clinical and cellular phenotypes. This study further implicates RTEL1 in the etiology of DC/HH and immunodeficiency, and identifies the first known homozygous autosomal recessive disease-associated mutation in RTEL1.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003695
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003695Summary
Dyskeratosis congenita (DC) is a heterogeneous inherited bone marrow failure and cancer predisposition syndrome in which germline mutations in telomere biology genes account for approximately one-half of known families. Hoyeraal Hreidarsson syndrome (HH) is a clinically severe variant of DC in which patients also have cerebellar hypoplasia and may present with severe immunodeficiency and enteropathy. We discovered a germline autosomal recessive mutation in RTEL1, a helicase with critical telomeric functions, in two unrelated families of Ashkenazi Jewish (AJ) ancestry. The affected individuals in these families are homozygous for the same mutation, R1264H, which affects three isoforms of RTEL1. Each parent was a heterozygous carrier of one mutant allele. Patient-derived cell lines revealed evidence of telomere dysfunction, including significantly decreased telomere length, telomere length heterogeneity, and the presence of extra-chromosomal circular telomeric DNA. In addition, RTEL1 mutant cells exhibited enhanced sensitivity to the interstrand cross-linking agent mitomycin C. The molecular data and the patterns of inheritance are consistent with a hypomorphic mutation in RTEL1 as the underlying basis of the clinical and cellular phenotypes. This study further implicates RTEL1 in the etiology of DC/HH and immunodeficiency, and identifies the first known homozygous autosomal recessive disease-associated mutation in RTEL1.
Introduction
Hoyeraal Hreidarsson syndrome (HH) is a clinically severe variant of the telomere biology disorder dyskeratosis congenita (DC) [1]. DC is a heterogeneous inherited bone marrow failure syndrome (IBMFS) diagnosed by the presence of the classic triad of dysplastic nails, abnormal skin pigmentation, and oral leukoplakia. However, substantial clinical heterogeneity has been observed and the phenotype may include pulmonary fibrosis, liver disease, esophageal, urethral, or lacrimal duct stenosis, developmental delay, and/or other complications. Individuals with DC are at very high risk of bone marrow failure (BMF), myelodysplastic syndrome, and cancer [2]. The clinical consequences of DC manifest at variable ages and in different patterns, even within the same family. Independent of the classic triad, lymphocyte telomere lengths less than the first percentile for age are diagnostic of DC [3]. Depending on the affected gene, DC can be inherited in X-linked recessive (XLR), autosomal dominant (AD), or autosomal recessive (AR) patterns. Germline mutations in DKC1 result in XLR inheritance, mutations in TERC, TERT, RTEL1, or TINF2 result in AD inheritance, and mutations in TERT, RTEL1, CTC1, NOP10, NHP2, or WRAP53 result in AR inheritance [4]–[7] [8]; mutations in these genes account for approximately one-half of classic DC cases.
Patients with HH have many of the DC features listed above; however, severe immunodeficiency [9], non-specific enteropathy, intrauterine growth retardation (IUGR), and developmental delay may be the presenting features. In addition to features of DC, the presence of cerebellar hypoplasia is often the basis for a diagnosis of HH [1]. Patients with HH have extremely short telomeres, even when compared with other DC patients [3]. Germline mutations in DKC1 (XLR), TINF2 (AD), or TERT (AR) have been shown to cause HH. The causative mutation in HH is known in less than one-half of cases.
We clinically characterized individuals with HH from two different families. The affected individuals had IUGR, immunodeficiency, enteropathy, and extremely short telomeres. In both families, we discovered homozygous recessive germline mutations in Regulator of Telomere Elongation Helicase 1 (RTEL1) and characterized the telomere defect that resulted from these mutations. While RTEL1 mutations have been previously implicated in AD and AR compound heterozygous cases of DC, HH, and DC-like cases [6], [7], this report is the first instance of a homozygous DC-causative mutation in this gene.
Results
Clinical Characterization
Family NCI-318
The female proband, NCI-318-1 (family NCI-318) was born prematurely at 32 weeks gestation due to placental clots (Table 1, Figure 1A). Her parents were unrelated and of AJ ancestry. She was small for age and had poor postnatal growth. At 6 months of age she developed recurrent, chronic diarrhea and rectal prolapse. An extensive evaluation for allergic and infectious etiologies was negative. At 11 months of age, a colonoscopy showed severe colitis with evidence of apoptosis in the colonic epithelium. A concurrent immunologic evaluation showed low total B cells (CD 20+) at 14 cells/mm3, NK cells at 65 cells/mm3, and CD8+ T cells were 487 cells/mm3 (normal tenth percentiles are 1,310 cells/mm3, 360 cells/mm3, and 2,100 cells/mm3, respectively [10]), and her mitogen studies were abnormal. Her IgG was low at 26 mg/dL, IgA<5 mg/dL, IgM 29 mg/dL (lower limits of normal for age are 453 mg/dL, 20 mg/dL, and 19 mg/dL, respectively). Chromosome breakage studies were not consistent with Fanconi anemia. Subsequent testing identified peripheral blood telomere length as very short for her age (Figure 2A). An MRI of her brain showed cerebellar hypoplasia. Based on her clinical history and very short telomeres, she was diagnosed with the HH variant of DC. Genetic testing for TERT, TERC, TINF2, NOP10, NHP2, and WRAP53 was negative. She died due to complications following bone marrow transplant at two years of age. The mother and father are both clinically healthy, and their telomeres are normal (30 percentile and 70 percentile for age, respectively) (Figure 2A).
Fig. 1. NCI-318 and MSK-41 pedigrees with RTEL1 mutation and shared risk haplotype. 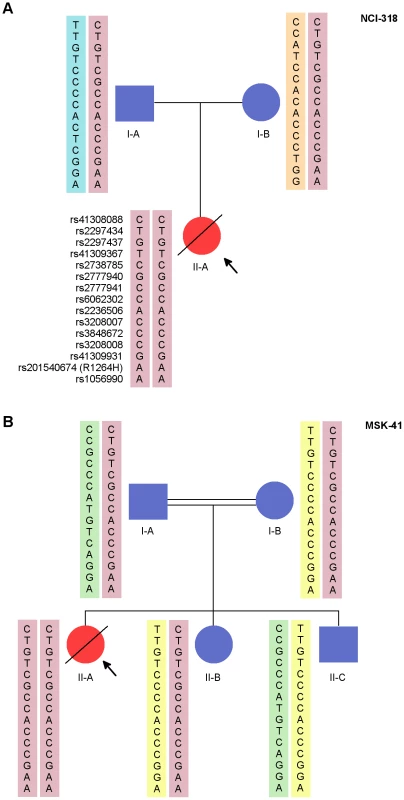
NCI-318 (A) and MSK-41 (B) pedigrees are shown. Red symbols indicate affected individuals. The pink rectangles indicate the shared haplotype between the pedigrees. Each other colored rectangle indicates a unique haplotype. Fig. 2. Telomere length is altered in individuals with RTEL1R1264H. 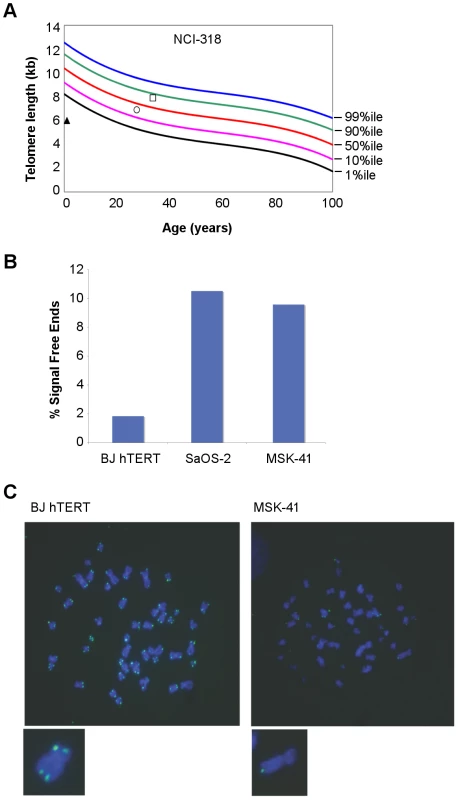
(A) Primary lymphocyte telomeres in family NCI-318 were measured by flow cytometry with fluorescent in situ hybridization (FISH) [3]. The proband is indicated by a triangle, the mother by a circle, and the father by a square. (B) Telomere FISH analysis of MSK-41 hTERT-immortalized fibroblasts revealed extreme telomere length heterogeneity. Quantitation of chromatids lacking detectable telomeric signal is shown. BJ hTERT, a normal hTERT-immortalized fibroblast line, and SaOS-2, an osteosarcoma cell line that relies on recombination-based telomere maintenance (ALT), are presented for comparison. (C) Representative metaphase spreads of MSK-41 and BJ hTERT are shown. Tab. 1. Clinical characteristics of families with RTEL1 mutations. 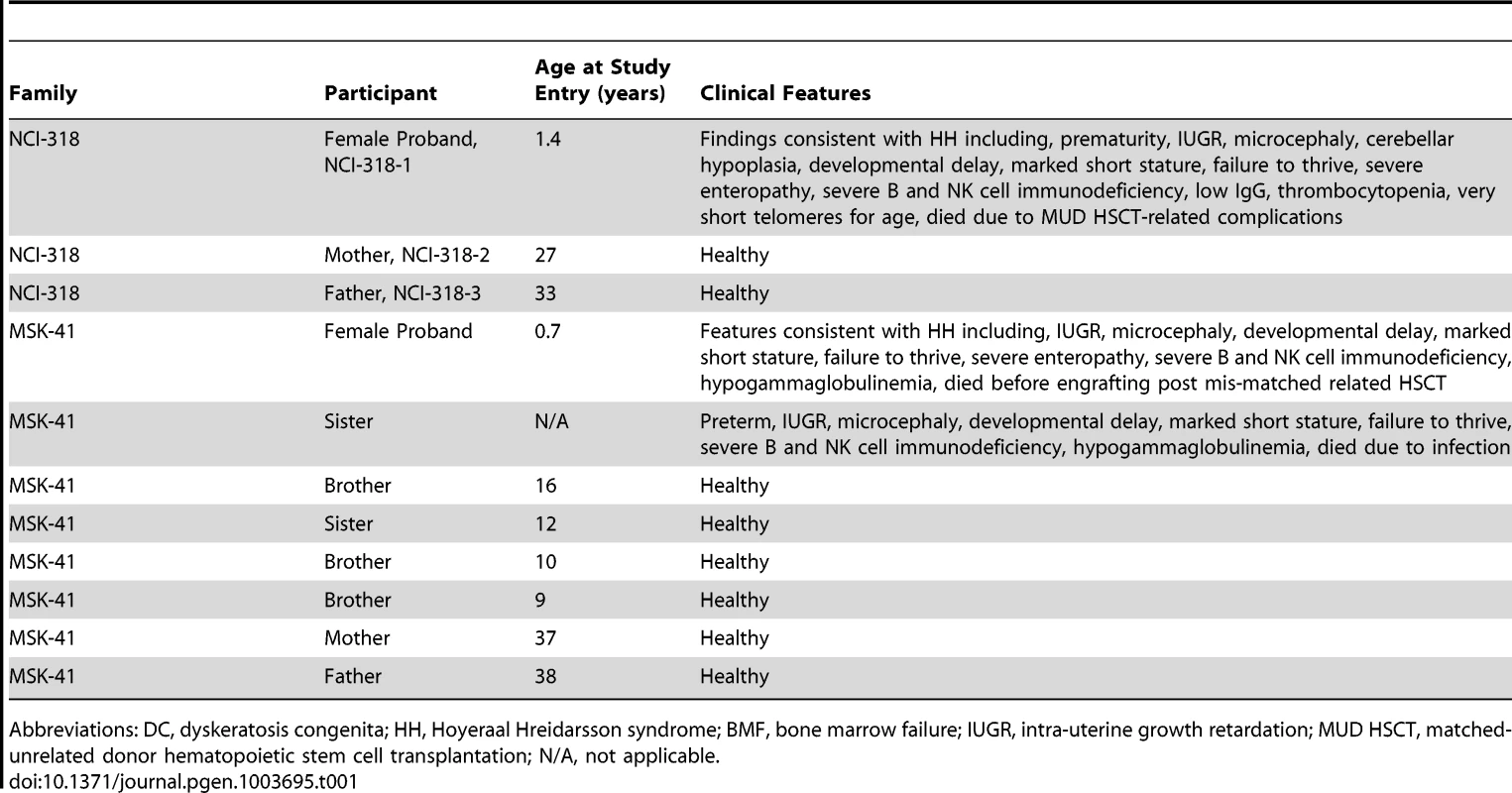
Abbreviations: DC, dyskeratosis congenita; HH, Hoyeraal Hreidarsson syndrome; BMF, bone marrow failure; IUGR, intra-uterine growth retardation; MUD HSCT, matched-unrelated donor hematopoietic stem cell transplantation; N/A, not applicable. MSK-41 Patient
The female proband, MSK-41, was born prematurely at 29 weeks gestation with IUGR, weight 615 grams (Table 1). Her parents, both of whom are healthy, are consanguineous and of AJ ancestry (Figure 1B). She had poor postnatal growth, gastroesophageal reflux, and vesicouretal reflux. She was evaluated for a potential immunodeficiency at the referring institution, as an older sister also born prematurely with IUGR had died at 15 months of age of systemic adenovirus prior to the family's enrollment in the study. The sister had microcephaly, developmental delay, failure to thrive, severe B and NK cell immunodeficiency, and hypogammaglobulinemia. At 6 months of age, MSK-41 developed an upper respiratory tract infection due to influenza and at 7.1 months of age, she was hospitalized for fever, but had negative cultures. At 7.2 months of age, she was readmitted for fever and diarrhea, and was found to have high-grade cytomegalovirus (CMV) viremia. She was placed on anti-viral therapy and referred to Memorial Sloan-Kettering Cancer Center for evaluation for transplant. Although her total white blood cell (WBC), hemoglobin, and platelet counts were normal prior to the development of CMV viremia, she developed count suppression secondary to the virus and anti-viral therapy. Her initial immunologic evaluation showed mildly decreased numbers of circulating CD4+ and CD8+ T-cells, low NK-cell numbers, and low B-cell numbers for age. She subsequently developed progressive T-, B-, and NK-cell lymphopenia and hypogammaglobulinemia, and she lacked specific B-cell responses to vaccines administered at 2 and 4 months of age. Her T-cell function waxed and waned but at 8.5 months of age, she had a normal T-cell response to phytohemagglutinin and allogeneic cells, but lacked response to Candida or CMV. CT scan and subsequent MRI of the head showed normal sized ventricles and sulci, and the gray-white matter differentiation was considered normal for her gestational age. She was neurologically normal for her gestational age until she developed the CMV infection. Laboratory work-up revealed normal levels of adenosine deaminase (ADA) and purine nucleoside phosphorylase (PNP), and absence of mutations in genes associated with immunodeficiency including RAG1, RAG2, CD3D, CD3E, and DCLRE1C. Although lymphocyte defects and impaired growth can be caused by inherited defects in DNA repair genes, DNA sequencing did not reveal evidence of DNA ligase IV deficiency, Cernunnos defects, ataxia telangiectasia, Nijmegen breakage syndrome, Bloom syndrome, or Fanconi anemia. She died 41 days following a T-cell depleted HLA-mis-matched related stem cell transplant without evidence of engraftment. There are four additional healthy siblings in the family: three brothers and one sister.
Sequence Analyses
DNA from the family trio NCI-318 was analyzed by whole exome sequencing (WES). Variants identified by WES were evaluated in AD, AR, and XLR inheritance models (Tables S1 and S2). We also ensured that there was adequate coverage of known DC genes, including the recently-discovered DC-associated gene CTC1 [11] and the non-protein-coding TERC locus. After filtering out common variants (Table S1), the top candidate variants that fit the most likely inheritance model were validated by an orthogonal sequencing technology (Materials and Methods). While we found variants in several telomere maintenance and DNA damage repair genes (Table S3), most were heterozygous in the proband and her father. Given that the father had longer-than-average telomeres for his age and was clinically healthy, we proposed that an autosomal recessive model was more likely than a paternal autosomal dominant one. An analysis of rare AR variants revealed three candidate single nucleotide variants (SNVs) (Table S2), of which RTEL1, an evolutionarily conserved helicase involved in telomere replication and stability, was the most biologically plausible. The proband was homozygous for a mutation (g.20 : 62326972G>A (hg19), hereafter referred to as RTEL1R1264H), and each parent was a heterozygous carrier of this mutation (Figure 1A). We did not observe any compound heterozygous variants in this family that met our filtering criteria.
Fibroblast DNA from MSK-41 underwent targeted sequencing of approximately 300 genes involved in the DNA damage response or implicated in maintaining genome stability. Amongst those candidate genes, the only variant found was a homozygous RTEL1R1264H mutation (Figure 1B). Importantly, except for RTEL1, most other candidate variants found in NCI-318 by exome sequencing were not recapitulated in MSK-41 (Table S2). Follow-up sequencing indicated that both the mother and father of MSK-41 were heterozygous carriers of RTEL1R1264H.
The RTEL1R1264H mutation affects three RTEL1 protein-coding isoforms (UniProt identifiers Q9NZ71-6, Q9NZ71-2 and Q9NZ71-5, in which the affected amino acid is R509; Ensembl IDs ENST00000360203462/ENSP00000353332, ENST00000318100/ENSP00000322287, and ENST00000370003/ENSP00000359020) and encodes a previously undefined C4C4 RING finger domain (Figure 3). This domain is characterized by a specific pattern of cysteine residues conforming to the consensus sequence Cx2C x9 Cx2C x4 Cx2C x10 Cx2C. Despite the somewhat conservative amino acid change, R1264 is highly conserved (Figure 3), and is centrally located within the putative C4C4 Zn2+ coordination domain; therefore, the R1264H change is likely to exert a substantial impact on RTEL1 function. In silico prediction algorithms (SIFT, PolyPhen-2, and Condel) indicate that this amino acid substitution is likely to be damaging to the protein. The TNFRSF6B gene is adjacent to the RTEL1 locus, and RTEL1 exon 34 sequences are present in non-coding exons of the TNFRSF6B transcript as well as in a non-coding RTEL1-TNFRSF6B read-through transcript, raising the possibility that the mutation may also affect TNFRSF6B expression. However, western blotting of MSK-41 whole cell extracts indicated no change in the TNFRSF6B levels (Figure S1), arguing that the effects of the mutation are confined to RTEL1.
Fig. 3. RTEL1R1264H affects a putative conserved C4C4 domain. 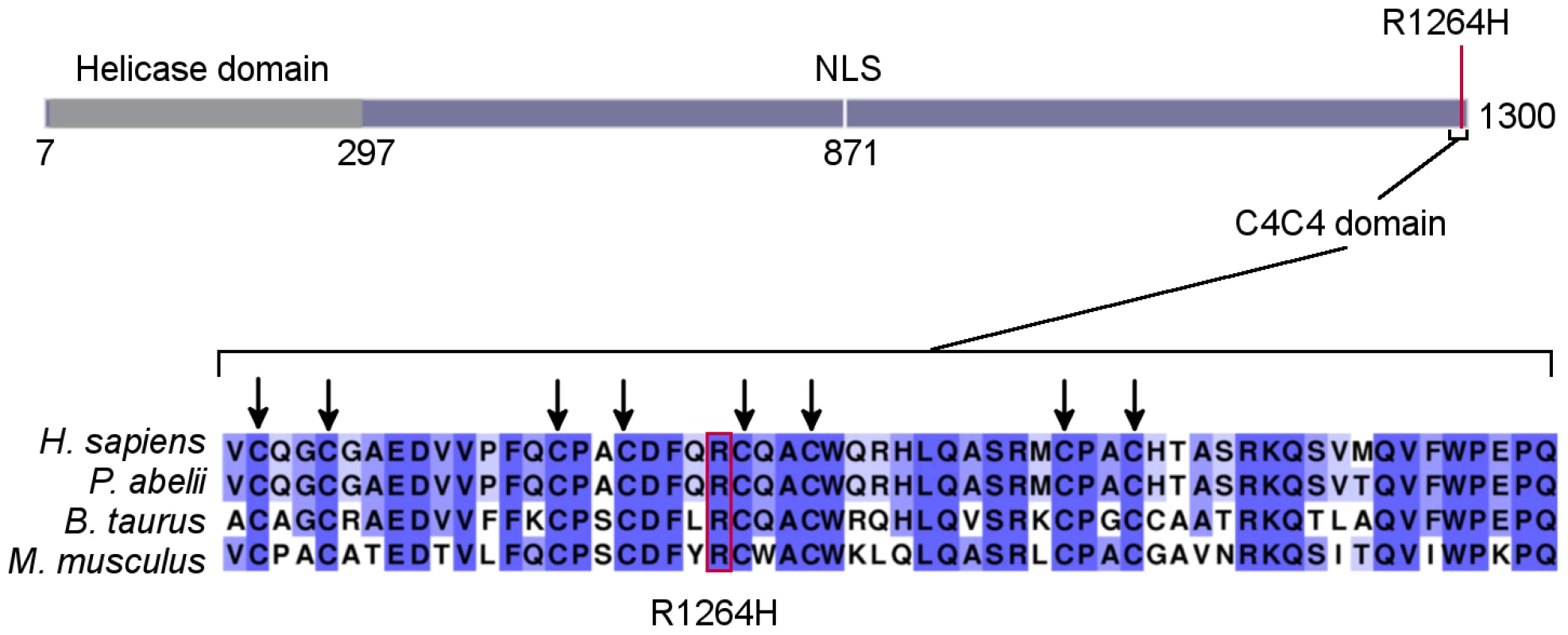
As displayed on the schematic (representing ENSP00000353332), the RTEL1 mutation is at the C-terminus of the protein, distal to the helicase domain. The affected amino acid is in a putative C4C4 domain. All eight key cysteines and R1264 are conserved in human, orangutan, cattle, and mouse sequences. Higher percent identity at a given amino acid position is indicated by a deeper purple color. Haplotype Analysis
An analysis of 15 common SNPs in the 1000 Genomes European populations distributed over the RTEL1 locus indicated low linkage disequilibrium in the ∼34,000 bases surrounding the g.20 : 62326972G>A mutation that encodes RTEL1R1264H. This results in several haplotypes in healthy populations within the 1000 Genomes Project [12]. The carrier parents and affected individuals in our families were the only individuals we found to have haplotypes containing the G>A mutation (compared with 378 of 1000 Genomes samples of European ancestry). Sanger sequencing was performed to determine the genotypes of 12 common single nucleotide polymorphisms in all the available family members of both families. These included the trio from NCI-318 and five individuals from MSK-41 (see pedigree, Figure 1A and 1B). Three SNPs that were in strong linkage disequilibrium (r2 = 1) with the genotyped SNPs were also included in the analysis. These polymorphisms were chosen to be within the region chr20 : 62,292,868–62,327,449 (hg19) that encompasses RTEL1 exons 4 through 35, a region that also includes the RTEL1R1264H mutation. The probands in both families were homozygous for the mutation and all genotyped SNPs (Figure 1A and 1B). Haplotypes were reconstructed based on allele sharing in the unaffected siblings and parents. No recombinants were seen in either family and the segregating risk haplotype was identical in NCI-318 and MSK-41. In MSK-41, the unaffected individuals II-B and II-C inherited one copy and no copies of the risk haplotype containing the mutant allele, respectively. Thus we show that the R1264H variant is carried on a common haplotype, likely from a common AJ founder. Notably, the variant is not seen in the publically available data on approximately 9,000 individuals (ESP 6500 or the 1000 Genomes); however, dbSNP 137 shows the entry rs201540674 with a minor allele frequency (MAF) of 0.002 in a population of approximately 600 individuals of European descent. The combined data from these three sampled populations suggests a very low carrier frequency of approximately 1 in 9,600 individuals (MAF ∼0.0001). Because this is a recessive allele, the disease-associated genotype frequency would then be approximately 1 in 100 million in the general population, which is consistent with the low prevalence of this disorder.
Cellular Phenotype
As expected for DC patients, primary lymphocytes from the NCI-318 proband (Figure 2A) and the MSK-41 hTERT-immortalized fibroblast line exhibited clear indications of defects in telomere maintenance (Figure 2B and 2C). Notably, extreme heterogeneity in telomere length was evident in MSK-41 cells despite immortalization with hTERT. The frequency of chromatid ends lacking telomeric FISH signal in MSK-41 cells was approximately 10%, approaching that seen in SaOS2, a cell line with the alternative lengthening of telomeres (ALT) phenotype [13]. A similar outcome was observed upon inactivation of the RTEL1 gene in murine embryonic fibroblasts (MEFs) [14], indicating that the telomere defects observed are likely attributable to a decrement in RTEL1 function due to the RTEL1R1264H mutation.
Loss of telomeric sequence upon conditional deletion of RTEL1 in MEFs is accompanied by the formation of extrachromosomal T-circles [14]. T-circles are proposed to arise in RTEL1-deficient cells when the DNA replication machinery collides with the T-loop structure that would otherwise be dismantled by RTEL1 to permit replication of the chromosome end. Therefore, we examined the MSK-41 hTERT-immortalized cell line for the presence of T-circles to determine whether the RTEL1R1264H mutant phenocopied RTEL1 deficiency in this regard. T-circles are detected by annealing a telomere-specific primer to denatured genomic DNA, followed by treatment with Phi29 DNA polymerase. In this setting, circular DNA is amplified by a rolling circle mechanism, whereas linear telomeric DNA is not [14], [15]. When subjected to the amplification assay, genomic DNA from MSK-41 cells gave rise to levels of T-circles approximating those seen upon conditional activation of RTEL1 in mouse embryonic fibroblasts (Figure 4A and 4B). This suggests that in cells bearing the RTEL1R1264H mutation, telomeres are compromised due to an inability to appropriately resolve the T-loop structure.
Fig. 4. Inhibiting DNA replication blocks T-circle formation in MSK-41 RTEL1R1264H cells. 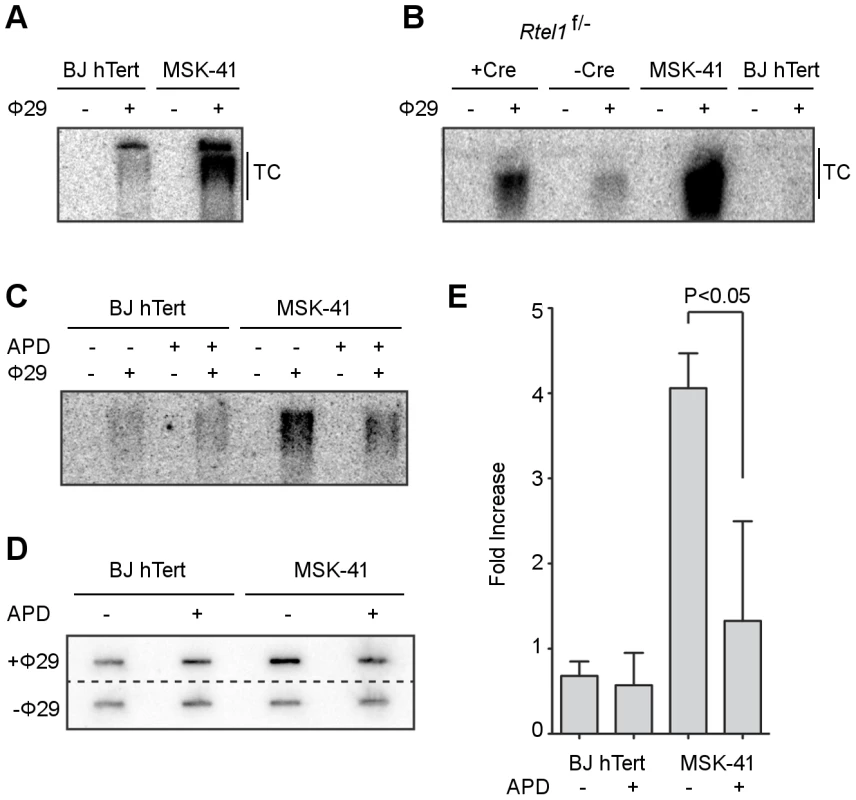
(A) Phi29-dependent T-circles in BJ hTERT and MSK-41. (B) Phi29-dependent T-circles in RTEL1 floxed/- MEFs ± Cre, BJ hTERT and MSK-41. (C) Phi29-dependent T-circles in BJ hTERT and MSK-41 ± aphidicolin (APD; 5 µM). (D) Dot blot of the Phi29-dependent T-circles in BJ hTERT and MSK-41 ± aphidicolin (APD; 5 µM). (E) Quantification of the fold increase in intensity of Phi29-dependent T-circles in the different cell lines subjected to the indicated treatments. Intensity mean and standard deviation were calculated over two independent experiments; statistical analysis (one-way ANOVA) was calculated with Prism (GraphPad). In further support of this model, the formation of T-circles depends on an intact DNA replication process. MSK-41 hTERT cells exhibited four-fold higher levels of T-circles compared with BJ hTERT control cells (Figure 4C, 4D, 4E); however, when DNA replication was inhibited by the addition of 5 µM aphidicolin, the T-circle-derived signal in MSK-41 cells was significantly reduced, as inferred from electrophoretic analysis and slot blotting of Phi29-treated genomic DNA. Collectively, these data strongly support the interpretation that the RTEL1R1264H mutation impairs the functions of RTEL1 at the telomere.
As reported previously, T-circle formation in RTEL1-deficient cells is dependent on the nuclease SLX4, and knockdown of SLX4 in an RTEL1-deficient background results in a rescue of the telomere loss phenotype [14]. To determine whether the RTEL1R1264H mutation impeded appropriate resolution of T-loops, we reduced the expression of SLX4 in MSK-41 cells. We performed transient knockdown experiments using two different short hairpin RNAs (shRNAs) targeting SLX4 in the MSK-41 hTERT cell line (Figure 5A). Both shRNAs result in efficient knockdown of SLX4 (Figure 5A) and suppression of T-circle formation (Figure 5B); the extent of suppression correlates with the degree of knockdown of SLX4. This confirms that the RTEL1R1264H mutation has a deleterious effect on RTEL1 function. Stable expression of the SLX4 shRNAs in MSK-41 cells did not achieve sufficient knockdown of SLX4 (data not shown), and therefore we were unable to assess the effect on telomere loss in this cell line.
Fig. 5. T-circle formation in MSK-41 cells is dependent on SLX4. 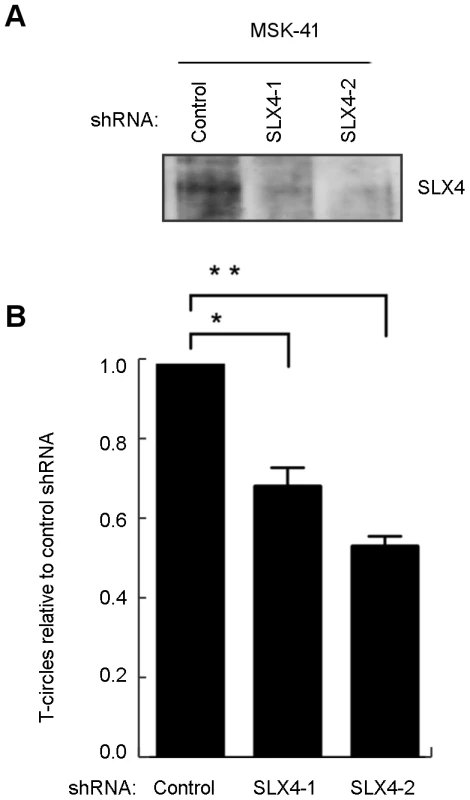
(A) Two shRNAs (SLX4-1 and SLX4-2) were used to knockdown SLX4 expression. (B) T-circle formation was measured in the MSK-41 SLX4 knockdown strains relative to MSK-41 with a control shRNA. Mean and standard deviation were calculated from two independent experiments. *P<0.05, **P<0.01 by unpaired two-tailed t-test. Similar to its proposed role at T-loops, RTEL1 mediates dismantling of displacement loops, or D-loops, which are formed as intermediates in homology-directed DNA double strand break (DSB) repair at telomeres and throughout the genome [16]. This function prevents the execution of inappropriate recombination events, and is proposed to thereby suppress deleterious genome rearrangements and enforce the orderly repair of DSBs [17]. To determine whether non-telomeric functions of RTEL1 were affected by the RTEL1R1264H mutation, we assessed the sensitivity of MSK-41 hTERT cells to the DNA crosslinking agent mitomycin C (MMC). Cells were subjected to MMC for 24 hours (20–80 nM), and plated for colony formation, with BJ hTERT serving as the wild-type control. We observed a modest (8–10 fold) increase in sensitivity to MMC at all doses, indicating that the repair of DNA crosslinks was impaired in the RTEL1R1264H mutant (Figure 6A).
Fig. 6. MSK-41 cells are hypersensitive to DNA damage and experience elevated levels of sister chromatid exchange. 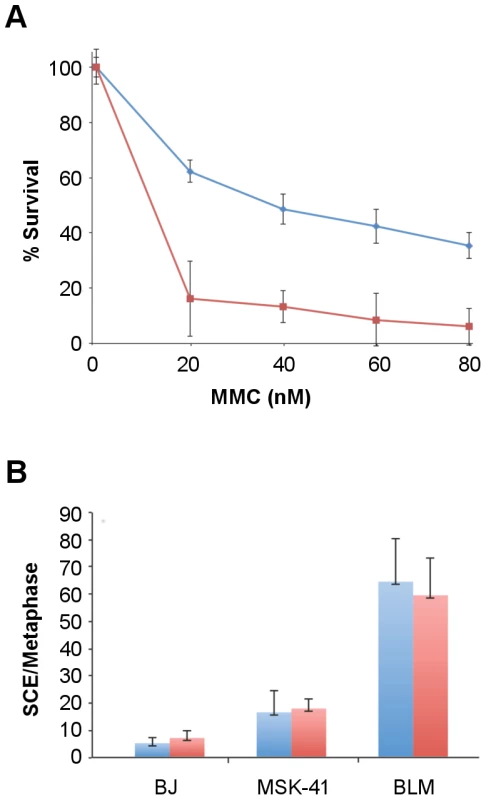
(A) BJ hTERT (blue line) and MSK-41 cells (red line) were treated at the indicated doses of mitomycin C (MMC) for 24 hours, and colony formation was scored 14 days post-treatment. Formation of at least 50 colonies was required at each dose for the experiment to be considered valid. (B) Spontaneous (blue) and MMC-induced (red) sister chromatid exchanges were visualized by Giemsa staining; the number of exchanges per metaphase is shown. Cells were cultured in 20 µM BrdU for 40 hours, with treatment with 25 ng/mL MMC for the final 24 hours. In addition to MMC sensitivity, we observed an increase in the spontaneous levels of sister chromatid exchanges (SCE) in MSK-41 hTERT cells, indicating an increase in genomic instability in the presence of the RTEL1R1264H mutation. SCEs were observed in 18% of MSK-41 metaphase spreads, approximately a two-fold increase over the levels seen in BJ hTERT control cells, but 3-fold less frequently than observed in a Bloom Syndrome fibroblast line (Figure 6B). MMC treatment had no effect on SCE levels in any of the genotypes observed. Although the SCE phenotype in MSK-41 cells is less severe than observed in Bloom Syndrome cells, the increased levels are likely to reflect a reduction in the anti-recombination functions of the RTEL1R1264H gene product. Hence, both the telomeric and non-telomeric functions of RTEL1 are affected by the RTEL1R1264H mutation. However, the general DNA damage repair phenotype in MSK-41 cells is not as severe as that of cells derived from a patient with Bloom Syndrome, a disorder marked by primary dysfunction in the DNA damage repair machinery.
Discussion
This study demonstrates the clinical and molecular consequences of homozygous autosomal recessive mutations in RTEL1. We identified two families with children who had HH, were of AJ ancestry, and had the same homozygous RTEL1R1264H mutations. These data provide further evidence that defects in RTEL1 function can lead to clinical phenotypes consistent with the HH variant of DC [6]. Our molecular analyses indicate that the homozygous RTEL1R1264H mutation results in short, heterogeneous telomeres. Additionally, cell lines bearing this mutation produce excess extrachromosomal T-circles, but only in the presence of functioning DNA replication machinery. RTEL1 is proposed to resolve T-circles to enable proper telomeric replication; in the absence of this activity, T-loops are inappropriately resolved as a circle when encountered by the replication machinery, resulting in a shortened telomere [18]. T-circle formation in the presence of RTEL1R1264H is SLX4-dependent, similar to T-circle formation in RTEL1-deficient cells [14].
RTEL1 also aids in suppressing inappropriate recombination throughout the genome. We have shown that the RTEL1R1264H mutation results in a modest enhancement in sensitivity to DNA damage, as well as an increase in SCE, indicating that the RTEL1R1264H mutation impairs both telomeric and non-telomeric aspects of RTEL1 function.
The fact that both the probands were homozygous for the identical risk haplotype suggests that there is an ancestral haplotype that is shared by parents in both families (Figure 1A and 1B). We were able to reconstruct the haplotype based on the genotypes obtained using Sanger sequencing. This haplotype was also seen without the mutation in 14/378 (TSI/GBR/FIN) samples of EUR ethnicity in the 1000 Genomes data. Together with the occurrence of the risk haplotype in the two families with AJ ethnicity, the evidence supports the interpretation that this mutation is confined to EUR populations and is most likely an AJ founder mutation. We have not extended the 34 kb haplotype further since the number of individuals with this rare recessive disorder in our study is too small to investigate the age of the mutation based on haplotypes and population history.
We and others recently reported that AD nonsense RTEL1 mutations are present in HH and that an additional missense mutation in the helicase domain further exacerbates the clinical and telomere length phenotype, while the presence of only a single missense mutation in the helicase domain resulted in a less clinically severe phenotype [6], [7].[8] The current study provides important insight into the function of the C-terminal end of the human RTEL1 protein. RTEL1 deficiency confers embryonic lethality in mice [19], suggesting that the R1264H allele is hypomorphic. As is the case for the two families described here, hypomorphs are usually recessive; for example, AR partial loss-of-function mutations in FANCD2 cause Fanconi anemia and AR LIG4 mutations result in Ligase IV syndrome [20], [21]. Furthermore, this mutation is distal to the RTEL1 helicase domain, and is thus unlikely to directly affect enzymatic activity. Nevertheless, the phenotypic impact of RTEL1R1264H at the cellular level was pronounced.
The RTEL1R1264H mutation falls within exon 34, which encodes a predicted C4C4 RING domain of RTEL1, lying downstream of a putative PIP box. Many RING domain-containing proteins are E3 ubiquitin ligases that interact with E2 ubiquitin-conjugating enzymes through their RING domains. BRCA1, MDM2, and Parkin are all examples of RING domain-containing proteins that are involved in human disease [22]. The putative RTEL1 RING domain is distant from the helicase domain, suggesting that the RTEL1R1264H mutation may affect the RING domain while leaving the helicase activity intact. Given the severity of the clinical and cellular phenotypes of this mutation, the data suggest that this domain exerts a significant influence on the biological function of RTEL1. Further analysis of this domain to define the mechanism(s) of its influence is ongoing.
These findings, together with the recent report that non-coding SNPs in RTEL1 have been found to be associated with susceptibility to high-grade glioma [23]–[25], broadly implicate the RTEL1 locus in human cancer susceptibility. Given the cellular phenotypes of DC/HH and those reported here, the clinical features of DC are likely sequelae of defects in maintenance and functions of the telomere.
We have demonstrated that the RTEL1R1264H mutation affects both the telomeric and non-telomeric functions of RTEL1. Individually, proteins involved in either telomere maintenance or DNA repair can result in immunodeficiency when perturbed: DC is an example of the former, and Bloom syndrome of the latter. The patients described here exhibit severe immunodeficiency, which may be the result of a mutation affecting both of these pathways. However, future studies are required to better understand this observation.
Materials and Methods
Ethics Statement
This research was approved by the Institutional Review Boards (IRB) of the National Cancer Institute and Memorial Sloan Kettering Cancer Center. All participants or their parents signed IRB-approved informed consent forms.
Patients
Patient NCI-318 and her family were participants in an IRB-approved longitudinal cohort study at the National Cancer Institute (NCI) entitled “Etiologic Investigation of Cancer Susceptibility in Inherited Bone Marrow Failure Syndromes” (NCI 02-C-0052, ClinicalTrials.gov Identifier: NCT00027274). In this study, patients and their family members complete questionnaires and undergo thorough clinical evaluations at the NIH Clinical Center [2]. Telomere length was measured by flow cytometry with fluorescent in situ hybridization (flow FISH) in leukocytes [26].
THE MSKCC proband was ascertained on IRB-approved protocol 95-091 entitled “Collection of Hematopoietic Progenitor Cell and/or Blood Samples From Patients For Research Studies.” Other family members consented to germline testing in the Clinical genetics Service, as well as MSKCC 93-102 “Ascertainment of Peripheral Blood or Saliva Samples for Genetic Epidemiology Studies of Familial Cancers,” as well as a specific consent for the novel homologous recombination gene described in this report.
Exome Sequencing, Analysis, and Variant Prioritization
Whole exome sequencing for family NCI-318 was performed at the NCI's Cancer Genomics Research Laboratory as previously described [6].
Reads were aligned to the hg19 reference genome using Novoalign software version 2.07.14 (http://www.novocraft.com), Picard software version 1.67 (http://picard.sourceforge.net/) and the Genome Analysis Toolkit (GATK, http://www.broadinstitute.org/gatk/) [27]. Variant discovery, genotype calling, and annotation were performed as described [6] using data from the UCSC GoldenPath database (http://hgdownload.cse.ucsc.edu/goldenPath/hg19/database/), the ESP6500 dataset from the Exome Variant Server, NHLBI Exome Sequencing Project (ESP), Seattle, WA (http://evs.gs.washington.edu/EVS/) (accessed August 2012), the Institute of Systems Biology KAVIAR (Known VARiants) database (http://db.systemsbiology.net/kaviar/) [28], the National Center for Biotechnology Information dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) [29] build 137, and the 1000 Genomes (http://www.1000genomes.org/) [12]. Variants were also annotated for their presence in an in-house database consisting of over 700 whole exomes that were sequenced in parallel with our DC families. Variants within each family were filtered and categorized as indicated in Table S1.
RTEL1 Targeted Sequencing
Validation of exome sequencing findings in the NCI-318 trio was performed by sequencing coding exons of RTEL1. Primer sequences are shown in Table S4. All samples were amplified using KAPA2 RobustHotstart Readymix (2×) (Kapa Biosystems, Johannesburg, South Africa) and the following cycling conditions: 3 min at 95°, followed by 30 cycles of 15 sec at 95°, 15 sec at 60°, 15 sec at 72°, followed by 10 min at 72°. Amplicons were purified using Agencourt's Ampure XP beads, then libraries were constructed and barcoded using the Ion Xpress Plus Fragment Library Kit (Life Technologies, Carlsbad, CA, USA). DNA tagged beads were generated for sequencing using Life Technologies' OneTouch and run on an Ion 316 chip on the Ion PGM Sequencer (Life Technologies). The default TMAP aligner and variant caller was used to generate a variant list per sample.
MSK-41 Sequencing
Targeted resequencing of DNA damage response genes was instrumental in the discovery of the RTEL1 mutation at MSKCC. Genomic enrichment via microfluidic PCR was conducted using the primer pool from Raindance Technologies [30]. Resulting libraries were prepared for sequencing using the SOLiD 4 sequencer (Life Technologies, Carlsbad). Read alignment and base-calling was done using the ABI Bioscope software with parameters optimal for targeted resequencing. Reads were filtered for mapping quality. RTEL1 contained the most biologically relevant non-synonymous exonic variant. MSK-41 was included in a panel of 24 cell lines in which targeted DNA sequencing of approximately 300 DNA damage response genes (including RTEL1) was carried out (see methods [13]).
In silico Analysis
PolyPhen-2 [31] (http://genetics.bwh.harvard.edu/pph2), SIFT [32] (http://sift.jcvi.org), and Condel [33] (http://bg.upf.edu/condel/home) were used to predict the severity of RTEL1 amino acid substitutions. Multiple sequence alignments were generated for homologous RTEL1 protein sequences using T-Coffee [34] (www.tcoffee.org) to evaluate conservation. Alignments were generated with NCBI Reference Sequence, GenBank or Ensembl proteins ENSP00000353332 (Homo sapiens), NP_001124929.1 (Pongo abelii), NP_001091044.1 (Bos taurus), and EDL07405.1 (Mus musculus).
Telomere FISH Analysis
Telomere FISH was performed as described [35]. Images were captured at 100× magnification, with precisely the same exposure time for each genotype (MSK-41 hTERT and BJ hTERT). Sensitivity (gain) is increased to saturation, and chromosome ends for which there still appears no signal are scored as signal-free ends. The heterogeneity observed in Figure 2C was reproducible over several experiments, and with different probes (data not shown).
Genomic DNA Extraction and T-Circle Amplification
Cells were collected from 2 to 3 10 cm plates at 70% confluence for each condition. Genomic DNA extraction was performed as described [36]. DNA was double digested by AluI/HinfI restriction enzymes overnight before starting TCA assay and then Southern Blot as described [37] with minor modifications to Phi29 DNA polymerization (MBI Fermentas) with a mammalian telomeric primer and a mammalian telomeric probe for hybridization. Blot images were captured and quantified with Storm 840 scanner and ImageQuant TL software (Amersham Biosciences).
Sister chromatid exchange analysis and telomere FISH were carried out as described previously [35]. Mitomycin C sensitivity assays were as described [38].
SLX4 Knockdown
To detect SLX4 levels in the various knockdown conditions, we immunoprecipitated SLX4 (1.5 mg protein lysate, 10 µg of antibody) with rabbit polyclonal antibody (A302-269A) followed by western blotting with polyclonal rabbit antibody A302-270A. Both antibodies were from Bethyl. T-circles were detected and quantified as previously described [14].
Cell Culture
Immortalized conditional RTEL1F/ - MEFs were as previously described [14] and were cultured in DMEM containing 10% fetal bovine serum. Cre recombination was carried out with Ad5-CMV-Cre adenovirus (Vector Biolabs) for 96 hr as described [39]. Cells were either not treated or treated with aphidicolin (5 µM) for 24 hrs.
Supporting Information
Zdroje
1. SavageSA, BertuchAA (2010) The genetics and clinical manifestations of telomere biology disorders. Genet Med 12 : 753–764.
2. AlterBP, GiriN, SavageSA, PetersJA, LoudJT, et al. (2010) Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol 150 : 179–188.
3. AlterBP, RosenbergPS, GiriN, BaerlocherGM, LansdorpPM, et al. (2012) Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 97 : 353–359.
4. NelsonND, BertuchAA (2012) Dyskeratosis congenita as a disorder of telomere maintenance. Mutat Res 730 : 43–51.
5. WalneA, BhagatT, KirwanM, GitauxC, DesguerreI, et al. (2012) Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica 98 (3) 334–8.
6. BallewBJ, YeagerM, JacobsK, GiriN, BolandJ, et al. (2013) Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet 132 : 473–480.
7. WalneAJ, VulliamyT, KirwanM, PlagnolV, DokalI (2013) Constitutional Mutations in RTEL1 Cause Severe Dyskeratosis Congenita. Am J Hum Genet 92 (3) 448–53.
8. Le GuenT, JullienL, TouzotF, SchertzerM, GaillardL, et al. (2013) Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet 22 (16) 3239–49.
9. JyonouchiS, ForbesL, RuchelliE, SullivanKE (2011) Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum–a single-center pediatric experience. Pediatr Allergy Immunol 22 : 313–319.
10. ShearerWT, RosenblattHM, GelmanRS, OyomopitoR, PlaegerS, et al. (2003) Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 112 : 973–980.
11. KellerRB, GagneKE, UsmaniGN, AsdourianGK, WilliamsDA, et al. (2012) CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr Blood Cancer 59 : 311–314.
12. ConsortiumGP (2010) A map of human genome variation from population-scale sequencing. Nature 467 : 1061–1073.
13. LovejoyCA, LiW, ReisenweberS, ThongthipS, BrunoJ, et al. (2012) Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS genetics 8: e1002772.
14. VannierJB, Pavicic-KaltenbrunnerV, PetalcorinMI, DingH, BoultonSJ (2012) RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 149 : 795–806.
15. ZellingerB, AkimchevaS, PuizinaJ, SchiratoM, RihaK (2007) Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Molecular Cell 27 : 163–169.
16. BarberLJ, YoudsJL, WardJD, McIlwraithMJ, O'NeilNJ, et al. (2008) RTEL1 maintains genomic stability by suppressing homologous recombination. Cell 135 : 261–271.
17. VilleneuveAM (2008) Ensuring an exit strategy: RTEL1 restricts rogue recombination. Cell 135 : 213–215.
18. CesareAJ, GriffithJD (2004) Telomeric DNA in ALT cells is characterized by free telomeric circles and heterogeneous t-loops. Mol Cell Biol 24 : 9948–9957.
19. DingH, SchertzerM, WuX, GertsensteinM, SeligS, et al. (2004) Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell 117 : 873–886.
20. ChistiakovDA (2010) Ligase IV syndrome. Adv Exp Med Biol 685 : 175–185.
21. ShimamuraA, AlterBP (2010) Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 24 : 101–122.
22. DeshaiesRJ, JoazeiroCA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78 : 399–434.
23. WrenschM, JenkinsRB, ChangJS, YehRF, XiaoY, et al. (2009) Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet 41 : 905–908.
24. SheteS, HoskingFJ, RobertsonLB, DobbinsSE, SansonM, et al. (2009) Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet 41 : 899–904.
25. EganKM, ThompsonRC, NaborsLB, OlsonJJ, BratDJ, et al. (2011) Cancer susceptibility variants and the risk of adult glioma in a US case-control study. J Neurooncol 104 : 535–542.
26. BaerlocherGM, VultoI, de JongG, LansdorpPM (2006) Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 1 : 2365–2376.
27. DePristoMA, BanksE, PoplinR, GarimellaKV, MaguireJR, et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43 : 491–498.
28. GlusmanG, CaballeroJ, MauldinDE, HoodL, RoachJC (2011) Kaviar: an accessible system for testing SNV novelty. Bioinformatics 27 : 3216–3217.
29. SherryST, WardMH, KholodovM, BakerJ, PhanL, et al. (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 29 : 308–311.
30. TewheyR, WarnerJB, NakanoM, LibbyB, MedkovaM, et al. (2009) Microdroplet-based PCR enrichment for large-scale targeted sequencing. Nat Biotechnol 27 : 1025–1031.
31. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249.
32. KumarP, HenikoffS, NgPC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4 : 1073–1081.
33. Gonzalez-PerezA, Lopez-BigasN (2011) Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet 88 : 440–449.
34. NotredameC, HigginsDG, HeringaJ (2000) T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302 : 205–217.
35. AttwoollCL, AkpinarM, PetriniJH (2009) The mre11 complex and the response to dysfunctional telomeres. Mol Cell Biol 29 : 5540–5551.
36. Munoz-JordanJL, CrossGA, de LangeT, GriffithJD (2001) t-loops at trypanosome telomeres. EMBO J 20 : 579–588.
37. ZellingerB, AkimchevaS, PuizinaJ, SchiratoM, RihaK (2007) Ku suppresses formation of telomeric circles and alternative telomere lengthening in Arabidopsis. Mol Cell 27 : 163–169.
38. StrackerTH, WilliamsBR, DerianoL, TheunissenJW, AdelmanCA, et al. (2009) Artemis and nonhomologous end joining-independent influence of DNA-dependent protein kinase catalytic subunit on chromosome stability. Molecular and Cellular Biology 29 : 503–514.
39. CelliGB, de LangeT (2005) DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 7 : 712–718.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



