-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
One of the fundamental questions in biology is how cooperative and altruistic behaviors evolved. The majority of studies seeking to identify the genes regulating these behaviors have been performed in systems where behavioral and physiological differences are relatively fixed, such as in the honey bee. During colony founding in the monogyne (one queen per colony) social form of the fire ant Solenopsis invicta, newly-mated queens may start new colonies either individually (haplometrosis) or in groups (pleometrosis). However, only one queen (the “winner”) in pleometrotic associations survives and takes the lead of the young colony while the others (the “losers”) are executed. Thus, colony founding in fire ants provides an excellent system in which to examine the genes underpinning cooperative behavior and how the social environment shapes the expression of these genes. We developed a new whole genome microarray platform for S. invicta to characterize the gene expression patterns associated with colony founding behavior. First, we compared haplometrotic queens, pleometrotic winners and pleometrotic losers. Second, we manipulated pleometrotic couples in order to switch or maintain the social ranks of the two cofoundresses. Haplometrotic and pleometrotic queens differed in the expression of genes involved in stress response, aging, immunity, reproduction and lipid biosynthesis. Smaller sets of genes were differentially expressed between winners and losers. In the second experiment, switching social rank had a much greater impact on gene expression patterns than the initial/final rank. Expression differences for several candidate genes involved in key biological processes were confirmed using qRT-PCR. Our findings indicate that, in S. invicta, social environment plays a major role in the determination of the patterns of gene expression, while the queen's physiological state is secondary. These results highlight the powerful influence of social environment on regulation of the genomic state, physiology and ultimately, social behavior of animals.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003633
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003633Summary
One of the fundamental questions in biology is how cooperative and altruistic behaviors evolved. The majority of studies seeking to identify the genes regulating these behaviors have been performed in systems where behavioral and physiological differences are relatively fixed, such as in the honey bee. During colony founding in the monogyne (one queen per colony) social form of the fire ant Solenopsis invicta, newly-mated queens may start new colonies either individually (haplometrosis) or in groups (pleometrosis). However, only one queen (the “winner”) in pleometrotic associations survives and takes the lead of the young colony while the others (the “losers”) are executed. Thus, colony founding in fire ants provides an excellent system in which to examine the genes underpinning cooperative behavior and how the social environment shapes the expression of these genes. We developed a new whole genome microarray platform for S. invicta to characterize the gene expression patterns associated with colony founding behavior. First, we compared haplometrotic queens, pleometrotic winners and pleometrotic losers. Second, we manipulated pleometrotic couples in order to switch or maintain the social ranks of the two cofoundresses. Haplometrotic and pleometrotic queens differed in the expression of genes involved in stress response, aging, immunity, reproduction and lipid biosynthesis. Smaller sets of genes were differentially expressed between winners and losers. In the second experiment, switching social rank had a much greater impact on gene expression patterns than the initial/final rank. Expression differences for several candidate genes involved in key biological processes were confirmed using qRT-PCR. Our findings indicate that, in S. invicta, social environment plays a major role in the determination of the patterns of gene expression, while the queen's physiological state is secondary. These results highlight the powerful influence of social environment on regulation of the genomic state, physiology and ultimately, social behavior of animals.
Introduction
Behavior is a complex phenotypic trait, which results from the interactions of multiple intrinsic and extrinsic factors that associate in a nonlinear, often unpredictable fashion [1]. Intrinsic factors include the genetics, the physiology or the phenotype of an organism, while the most typical extrinsic factor is the external environment. In social systems like insect societies, environmental cues primarily are the result of the social environment, i.e. the nature and patterns of interactions among individuals within the colony [2]. The “nature-versus-nurture” debate has long been the major driver of the discussion as to whether internal state of an animal or the external environment (e.g., the social environment) regulates gene expression more [3]. Regardless, extrinsic and intrinsic factors clearly are reciprocally interconnected: the social environment influences the neurogenomic state of the animal, which is responsible for the social behavior performed [4], [5]. A hallmark of advanced social behavior is altruistic behavior, which is achieved through a reproductive division of labor in which few individuals develop into the reproductive caste while most of the colony members become non-reproductive workers and perform all tasks related to colony maintenance and growth. Both fixed (developmental pathways) and plastic (behavioral strategies) factors contribute to this division of labor (reviewed in [6]). Consequently, there has been great interest in studying genes and biological processes that regulate the reproductive and worker divisions of labor [7]. In the advanced eusocial systems examined thus far, differences between queens and workers are largely the result of developmental factors, while differences among workers are often triggered by social signals [8]. However, primitively social systems display reproductive division of labor between females that are anatomically, physiologically and genetically very similar and this reproductive division of labor seems to be primarily established and maintained by social environment. The genes underlying this process have not yet been examined, and potentially may function as core genes associated with sociality.
Variation in colony founding among ant queens is an ideal model to examine the interplay between genes and social environment that has shaped the evolution of cooperative behavior in primitively social systems. Colony founding can occur in two modalities: haplometrosis, where a single queen independently starts a new colony, and pleometrosis, where multiple queens associate and cooperate to start a new colony [9]. Pleometrosis is a fascinating example of cooperative behavior that is not fostered by kin selection, because these groups often comprise unrelated individuals (reviewed [10]). Among social insects, pleometrosis exists in halictine bees [11], termites [12], paper wasps [13], [14], [15] and in several species of ants [8]. In ants, pleometrosis is known to be associated with division of labor in the leaf-cutter ant Acromyrmex versicolor [16] and in the harvester ant Pogonomyrmex californicus [17]. Pleometrotic associations produce a complex social environment, where individuals simultaneously are in cooperation and conflict, and social and reproductive dominance hierarchies are established. These associations represent relatively primitive social systems in which individuals with equivalent anatomical and physiological features develop a division of labor through their behavioral interactions. Thus, identification of the genes underlying establishment of these hierarchies will not only provide insight into the effects of social environment on an individual, but also into the evolution of social behavior.
The red imported fire ant Solenopsis invicta is an excellent system for studying the genes associated with haplometrotic and pleometrotic behaviors, because queens from the monogyne social form (characterized by a single egg-laying queen per nest once established) can adopt either approach for colony founding depending on multiple factors, e.g. the density of newly mated queens in nesting sites [18]. However, monogyne fire ants ultimately only tolerate a single reproductive queen such that the initial cooperation among unrelated pleometrotic cofoundresses slowly transitions to competition and rivalry, which will inevitably produce only one winner and one or multiple losers [19], [20], [21]. Once the first workers emerge, pleometrotic queens engage in open fights where they injure or kill rival cofoundresses and workers actively participate in this process until all the queens are executed but one (see Movie S1). In both haplometrosis and pleometrosis, founding queens initially face a critical period (claustral period) where they are sealed in their nest and must defend it from enemies and competitors, e.g. other fire ant colonies that populate the same area [22], [23]. During the claustral period, fire ant queens rely exclusively on their body mass reserves to produce the first generation of workers. There are physiological and behavioral differences between haplometrotic queens, pleometrotic “winners” and pleometrotic “losers”. Haplometrotic queens lose more weight during the claustral period, and produce more brood per individual than queens in pleometrotic associations [24], [25]. In pleometrotic associations, winners tend to have larger head size, lose less weight [20], [21], [26] and occupy the top of the brood pile while losers are usually found outside the nest chamber [19], attempting to avoid any interaction with the winner or with workers. However, nothing is known about the genes and molecular pathways that underlie these processes.
We performed two separate experiments to characterize the genomic basis for haplometrotic and pleometrotic founding behavior in fire ants. We developed a microarray platform using the official gene set of the fire ant genome [27] plus a set of ESTs obtained from assemblies of the fire ant transcriptome to examine genome-wide expression patterns across founding queens. In the first experiment, we compared whole body gene expression patterns among haplometrotic queens and paired pleometrotic winners and losers that were collected shortly after emergence of the first workers (but prior to execution of the loser). We predicted that haplometrotic queens would be more similar to pleometrotic winners than to pleometrotic losers, because they both will serve as the single queen for the mature colony. For the comparison between winners and losers alone, our expectations were less well-defined: on one hand, we expected to find substantial differences given that their physiology, behavior and fate differ significantly, but on the other hand, winners and losers are not anatomically distinct and there is only weak correlation between morphological measures and outcome of the conflict [20]. In the second experiment, we manipulated queen rank in pleometrotic pairs to determine how changing social rank and social environment would affect an individual's gene expression patterns. This was accomplished by pairing pleometrotic queens with a new partner at the end of the claustral period in order to switch putative winners to losers and vice-versa. Controls in which partners were altered (and social environment was changed) but social rank remained the same were also included. We hypothesized that final social rank would be the primary regulator of gene expression patterns. However, for both experiments our results indicated that social environment (pleiometrosis vs. haplometrosis, switched rank vs. maintained rank) was a much greater driver of gene expression changes than social rank itself, suggesting that social environment, and not reproductive state, is a key regulator of gene expression, physiology and ultimately, behavior.
Results
Experiment 1: Effect of social environment and social rank on global gene expression patterns
Haplometrotic queens (haplo) and paired pleometrotic winners (win) and losers (los) were collected shortly after emergence of the first workers (see methods, N = 8 haplo, 8 win and 8 los). Microarray analysis of gene expression patterns (see Methods for design and validation of microarrays) in whole bodies of these queens revealed that 4080 of the 9388 transcripts included in the analysis were differentially regulated at FDR<0.001 (Table S1). A principal components analysis (PCA) of the differentially regulated transcripts revealed that the social environment is more important than social rank in driving the patterns of gene expression in founding queens (Figure 1A). Differences between haplometrotic and pleometrotic queens accounted for 91.8% of the variation in gene expression while differences between win and los accounted for only 8.2%. Pairwise comparisons of transcripts differentially regulated (FDR<0.001) among the three groups of fire ant queens demonstrated that expression patterns in haplo are more similar to win than to los, since there are fewer genes differentially regulated uniquely between haplo and win (404) than haplo and los (477; Nominal Logistic Fit: df = 1, ChiSquare = 6.78, P = 0.0092; Figure 1B).
Fig. 1. Analyses of global gene expression between haplometrotic and pleometrotic queens. 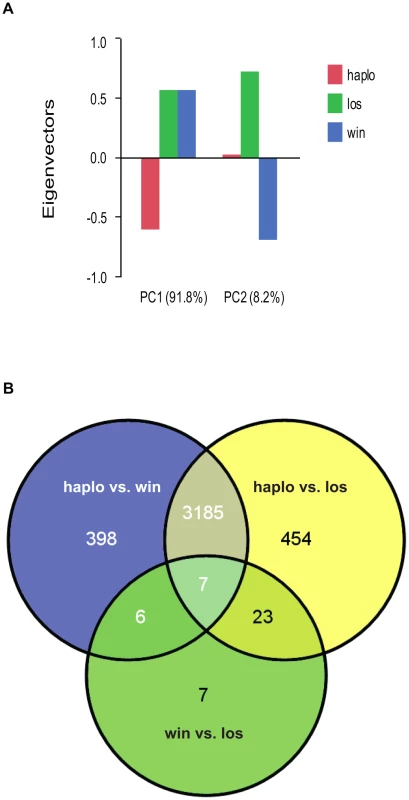
A) Principal Component Analysis of 4080 significantly differentially regulated transcripts. Two PCAs were identified corresponding to the effect of social environment (single queen vs. paired queens, 91.8%) and social rank (winner vs. loser, 8.2%). B) Pairwise comparisons of 4080 significantly differentially regulated transcripts. A total of 3192 transcripts were different between haplometrotic and pleometrotic queens, while only 43 transcripts were different between winner and losers. Haplometrotic queens were more similar to pleometrotic winners than to pleometrotic losers: more transcripts were differentially regulated in haplo vs. los than in haplo vs. win (477 and 404, respectively): this difference was statistically significant (Nominal Logistic Fit: df = 1, ChiSquare = 6.78, P = 0.0092). haplo = haplometrotic queens; los = pleometrotic losers; win = pleometrotic winners. We performed Gene Ontology analysis on the 3003 differentially regulated transcripts (out of the initial pool of 4080) that have Drosophila orthologs with FlyBase annotations using DAVID [28], [29]. 517 GO terms were significantly enriched at a p-value<0.05 (Functional Annotation Chart, see Table S2 for the complete list of GO terms). Additionally, 6 KEGG molecular pathways (Kyoto Encyclopedia of Genes and Genomes, [30]) were significantly enriched (P<0.05): aminoacyl-tRNA biosynthesis, basal transcription factors, dorso-ventral axis formation, endocytosis, RNA degradation and ubiquitin mediated proteolysis (Table S2). To cluster the GO categories into larger functional groups, the 517 significantly enriched GO terms were mapped to the GO_slim2 file in CateGOrizer [31]: 440 GO terms were assigned to one of the ancestor terms by single count (Figure 2). The functional groups containing the greatest number of GO terms were metabolism (19% of the significantly enriched GO terms), cell organization and biogenesis (11%) and development (10%; for a complete list of all ancestor terms represented in this analysis see Table S3).
Fig. 2. Gene Ontology analysis of genes differentially regulated between haplometrotic and pleometrotic queens. 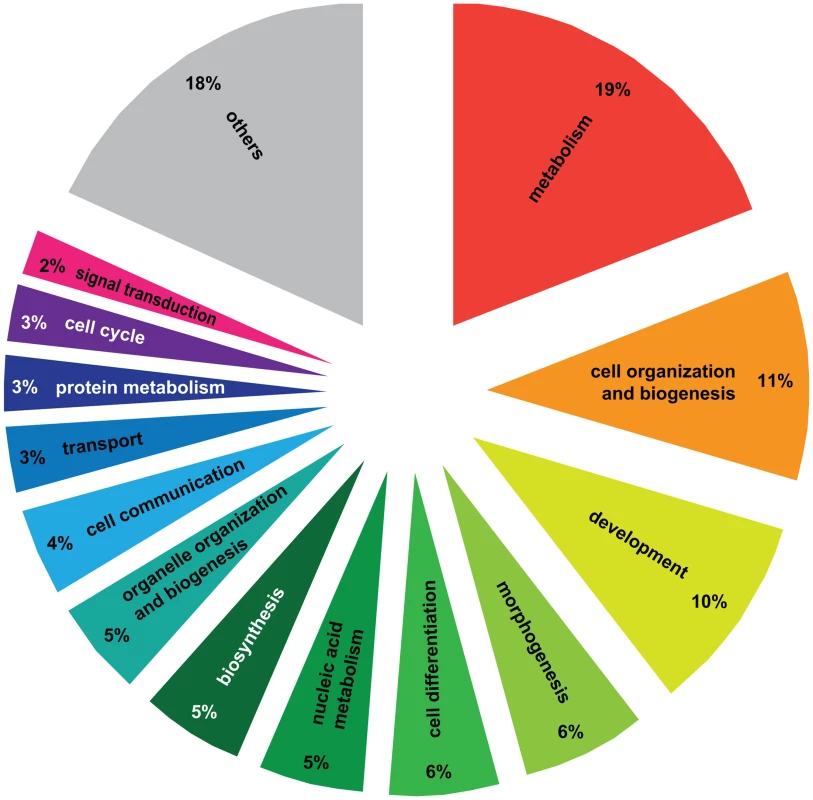
The figure shows the larger functional groups that encompass significantly enriched GO terms resulting from the GO analysis. To obtain this result we overlapped the list of significantly enriched GO terms from experiment 1 (Functional Annotation Chart, P<0.05) with the GO_slim2 list of the cateGOrizer. Others = larger functional groups encompassing less than 2% of the total number of significantly enriched GO terms (see Table S3 for the complete list of ancestor GO terms). To further characterize the genes that were differentially regulated between haplo and pleometrotic queens (pleo), we examined the overlapping set of 3192 transcripts (of which 2541 had Drosophila orthologs with FlyBase annotations) that were differentially regulated between both haplo vs. win and haplo vs. los (Figure 1B). For clearer graphical presentation, we used k-means Clustering in GENESIS [32] to separate these transcripts into two large clusters according to expression patterns: 2280 transcripts that were upregulated in haplo and downregulated in pleo (cluster 1, Figure S1) and 912 transcripts downregulated in haplo and upregulated in pleo (cluster 2, Figure S2). We performed GO analysis on both groups, using Functional Annotation Clustering with medium stringency. For cluster 1 (1925 FlyBase matches), we obtained 88 significantly enriched GO terms (see Table S4; P<0.05) and 1 KEGG pathway (basal transcription factors, P = 0.01). Several of the GO terms were related to aging (determination of adult life span, death), immunity (immune system development, JNK cascade, hemopoiesis), reproduction (reproductive developmental process, oocyte development, eggshell formation, morphogenesis of follicular epithelium, regulation of oocyte development), response to stimuli (response to stress, regulation of response to stimulus, negative regulation of response to stimulus, response to ecdysone), lipid biosynthetic process, locomotion and neurological system processes (neurotransmitter secretion, neurogenesis, central nervous system development, regulation of nervous system development). In cluster 2 (616 FlyBase matches), 34 GO terms (Table S5, P<0.05) and 1 KEGG pathway (glycerophospholipid metabolism, P = 0.01) were significantly enriched. Many GO terms were similar to those in cluster 1, and included determination of adult life span, olfactory behavior, lipid metabolic process, detection of light stimulus, as well as several related to morphogenesis or development of organs and apparatuses like sensory organ, muscle, limb, wing disc, gut and respiratory system.
Interestingly, only 43 transcripts were differentially regulated between win and los queens. A GO analysis performed on this small set of transcripts revealed that fatty acid and hormone metabolic processes were significantly enriched GO terms (Functional Annotation Clustering, P<0.001 and P<0.01, respectively, Table S6). Several transcripts in this group have interesting functions (Table 1). Transcripts upregulated in win included: G protein-coupled receptor kinase 2 (Gprk2), which is involved in the Toll signaling pathway during the response against Gram positive bacteria [33]; endosulfine (endos), which functions in the insulin-signaling pathway during oogenesis [34]; Pheromone-binding protein-related protein 3 (Pbprp3), a member of the odorant binding proteins responsible for chemoreception [35]; and bubblegum (bgm), which is involved in the metabolism of very long-chain fatty acids and prevents neurodegeneration [36]. Transcripts upregulated in los relative to win included: I'm not dead yet (Indy), associated with aging [37]; pale (ple), which plays a role in the response to wounding [38] and in the metabolism of dopamine [39]; desat1, a major regulator of cuticular hydrocarbon biosynthesis involved in pheromone emission and detection [40]; and juvenile hormone acid methyltransferase (jhamt), a key enzyme in the biosynthesis of JH, the major endocrine regulator in insects [41].
Tab. 1. Patterns of expression of the most relevant transcripts that were differentially regulated between winners and losers in experiment 1 (P<0.001). 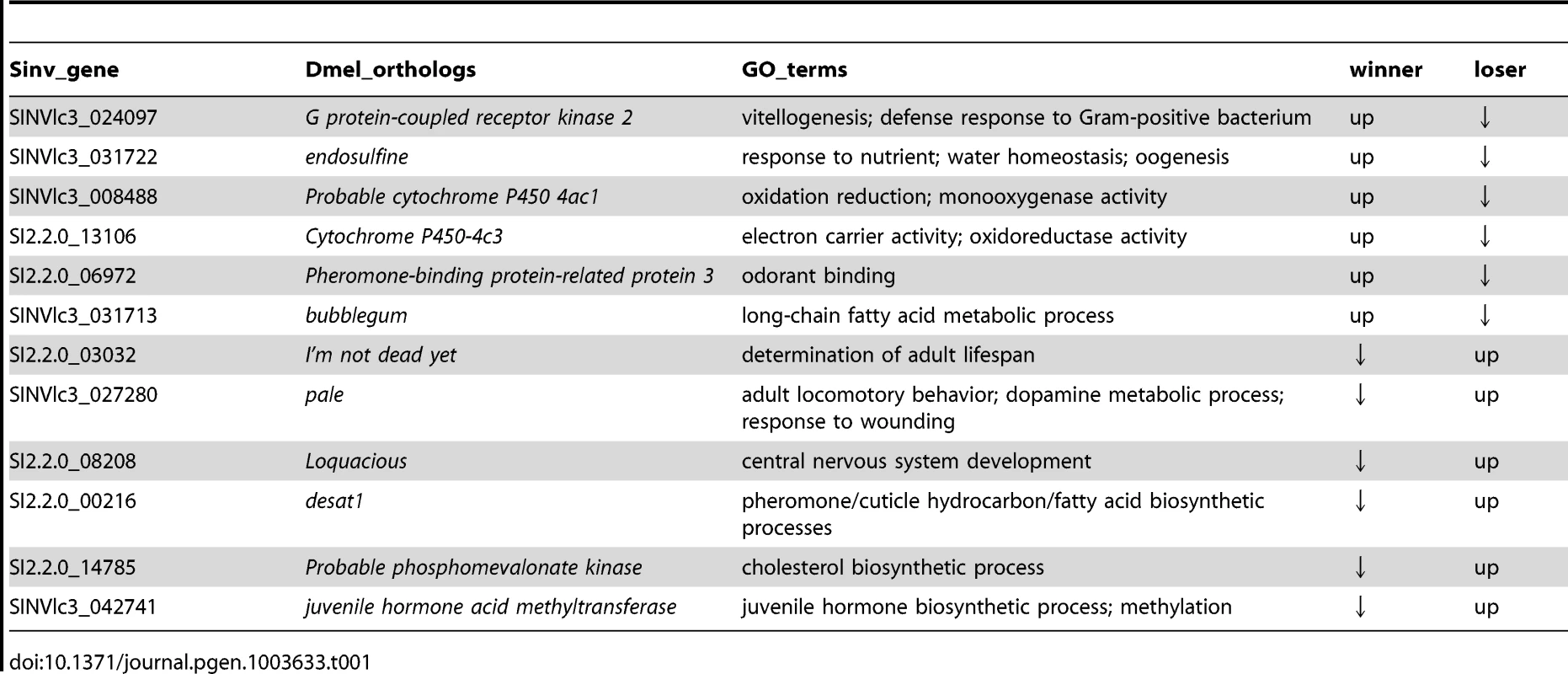
Experiment 1: Candidate gene pathways
GO categories related to aging and longevity were significantly enriched in sets of transcripts that were differentially regulated in haplo vs. pleo (clusters 1 and 2; see Tables S4 and S5). Out of the 129 genes included in the Drosophila aging GO term, oligos representing 93 putative orthologs were present on the fire ant microarray. Of these, 90 were expressed at high enough levels to be included in the microarray analysis and 46 were significantly differentially regulated across the three groups of queens (Figure 3A). The majority of these genes (34) were upregulated in haplo: this number was significantly higher than expected by chance (Nominal Logistic Fit: df = 2, ChiSquare = 29.58, P<0.0001). In addition to their role in regulating aging pathways in Drosophila, several genes in this group have been linked to queen vs. worker caste differentiation and behavioral maturation in honey bees [42]. These include: forkhead box (foxo) and target of rapamycin (TOR), two major players in the insulin-signaling pathway which is associated to caste determination in honey bees [43] and to the workers' transition from nursing to foraging behavior [44], [45], and Peroxiredoxin 5037 (Prx3), which is associated with enhanced learning ability when expressed at higher levels in honey bee workers [46].
Fig. 3. Expression patterns of aging- and immune-associated genes. 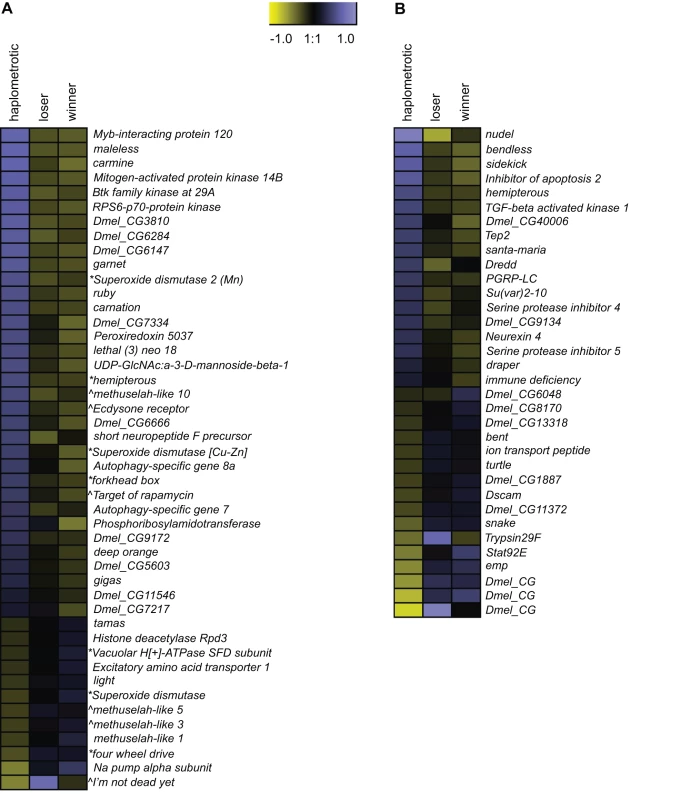
A) Heatmap of log2 transformed and normalized expression values for the fire ant transcripts that are putatively involved in aging processes. This gene list was obtained by overlapping the list differentially regulated transcripts with FlyBase matches in experiment 1 with the list of genes included in the Drosophila GO term “aging”. The directional expression of these transcripts was also compared to the expression patterns of aging genes as found in the review by Paaby and Schmidt [65]. * = gene that extends lifespan when its activity is increased; ∧ = gene that extends lifespan when its activity is decreased. B) Heatmap of log2 transformed and normalized expression values for the fire ant transcripts that are putatively involved in immune processes. This gene list was obtained by overlapping the list differentially regulated transcripts with FlyBase matches in experiment 1 with the list of canonical immune-related genes annotated in honey bees [48]. To further investigate the patterns of expression of aging genes in haplo and pleo queens, we compared our study to a study on aging in Drosophila [47]. In this study, which was aimed at investigating the temporal and spatial (tissue-specific) transcriptional profiles in Drosophila, the authors listed all the age-related GO terms that were significantly enriched and classified them based on the tissue where they were expressed and on their directional expression. We found that 106 GO terms were upregulated in fire ant haplo queens and old Drosophila, while only 36 were shared between old flies and pleo: however, the difference was not statistically significant (Fisher's Exact Test: P = 0.67). When we compared downregulated GO terms, we found that 11 were shared between haplo and old flies while 67 were shared between old flies and pleo: the difference was statistically significant (Fisher's Exact Test: P = 0.0029). Most of these 67 overlapping GO terms (see Table S7 for details) encompassed genes that were regulated in the gut (32) and fat bodies (23), followed by brain (13), muscles (11), malpighian tubules (7) and accessory glands (6).
Immune-related GO terms were significantly enriched in cluster 1 (genes upregulated in haplo vs. pleo, see Table S4). To better examine the overall expression profiles of genes involved in immune pathways, we obtained a list of significantly enriched GO terms for both cluster 1 and cluster 2 (Functional Annotation Chart in DAVID, P<0.05, see Tables S8 and S9) and we mapped these lists to the list of “Immune system gene classes” available on the CateGOrizer website (http://www.animalgenome.org/bioinfo/tools/catego/slims.html). Thereafter, we compared the relative proportions of the parent/ancestor immune categories between the two groups (Figure 4). This analysis confirmed a significant overrepresentation of immune-related classes in cluster 1 relative to cluster 2 (Nominal Logistic Fit: df = 1, ChiSquare = 61.16, P<0.0001), clearly visible in terms of total number of immune categories present and number of GO terms within common categories. These results suggest that haplometrotic queens overall have higher expression levels of immune-related genes and therefore may be better equipped in terms of immune response.
Fig. 4. Directional expression of immune-associated GO terms. 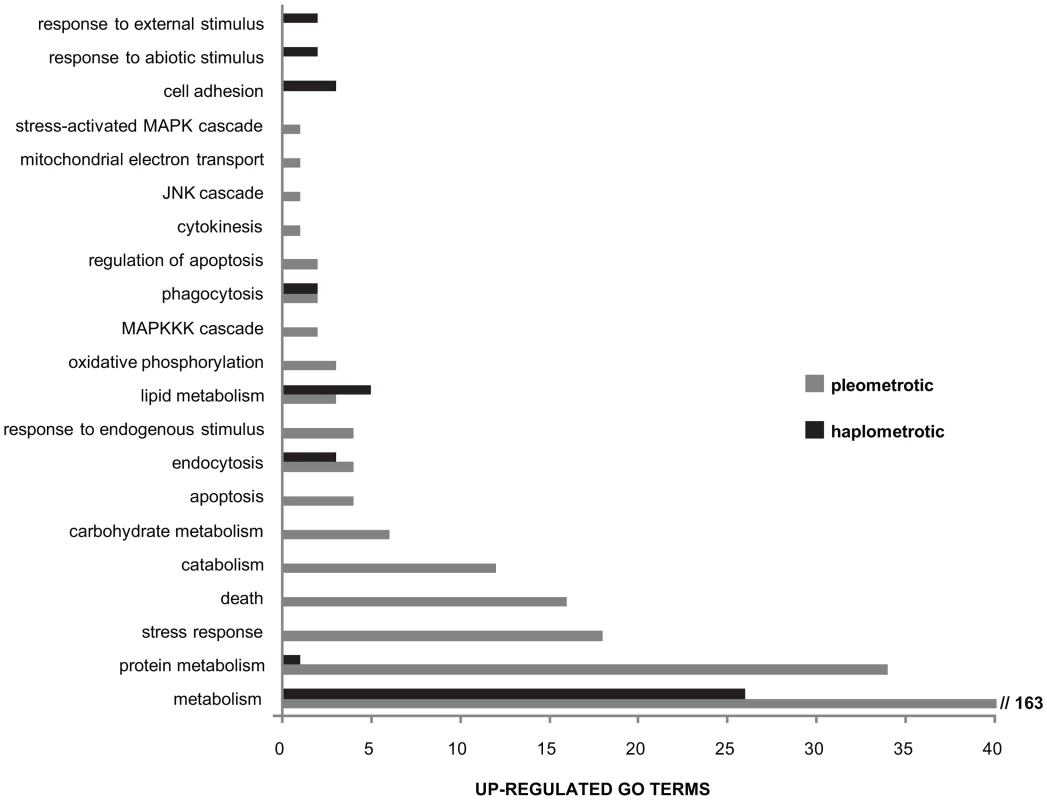
Overview of larger functional categories encompassing the immune-associated GO terms that were overrepresented among genes upregulated in haplometrotic (grey bars) and pleometrotic (black bars) queens. This result was obtained by overlapping the list of immune system gene classes available in the cateGOrizer with the two lists of significantly enriched GO terms in experiment 1 (Functional Annotation Chart, P<0.05), i.e. cluster 1 for transcripts upregulated in haplometrotic queens and cluster 2 for transcripts upregulated in pleometrotic queens (see Tables S8 and S9, respectively). Next, we examined the expression of the fire ant orthologs of the 177 canonical immune-related genes annotated in honey bees [48]. Orthologs for 83 of these genes were included in our array; 82 were expressed at high enough levels to be included in the analysis, and 34 were within our list of 3003 significantly differentially regulated transcripts (Figure 3B). Expression levels of these genes are not strongly coordinated, with similar numbers of up - vs. downregulated genes in haplo vs. pleo queens. Several genes in the Immune-deficiency (IMD) pathway were differentially regulated, including Inhibitor of apoptosis 2 (Iap2), TGF-beta activated kinase 1 (Tak1), immune deficiency (imd), bendless (ben) and Death related ced-3/Nedd2-like protein (Dredd). Furthermore, several members of the Immunoglobulin (IG) Superfamily were differentially regulated, including bent (bt), turtle (tutl), sidekick (sdk) and Down syndrome cell adhesion molecule (Dscam).
Experiment 1: Validation of candidate gene expression levels using quantitative real-time PCR
We examined gene expression levels of candidate genes that were included in one or more GO terms that were significantly enriched in our GO analyses (Figure S3 and Table S10). Expression patterns of all 13 candidate genes were consistent with what observed for haplo and los in the arrays, and these expression differences were significant for 11 genes. We validated Indy and Sod2 for determination of adult life span. In the arrays, Indy was downregulated in haplo and Sod2 was upregulated in haplo: qRT-PCR analysis confirmed these trends and the difference between groups was statistically significant for both genes (P<0.001). For immune response, we validated Dredd and kay, which were both upregulated in haplo in the arrays: qRT-PCR analysis confirmed this trend and the difference between the two groups of queens was statistically significant for kay (P<0.05) but not for Dredd (P = 0.29). Desat1, ifc and putative fatty acyl-CoA reductase CG5065 were analyzed for their involvement in the synthesis and metabolism of fatty acids. In the arrays, desat1 and putative fatty acyl-CoA reductase CG5065 were downregulated in haplo while ifc was upregulated in haplo: these trends were confirmed by qRT-PCR analysis and the difference in the expression levels was statistically significant for putative fatty acyl-CoA reductase CG5065 (P<0.001) and ifc (P<0.05) but not for desat1 (P = 0.11). Reproductive genes included br and Btk29A, both upregulated in haplo in the arrays: this was confirmed by qRT-PCR (P<0.001). We validated Sema-5c and Mer because they play a role in olfactory behavior: these genes were downregulated in haplo in the arrays and in the qRT-PCR analysis (P<0.001 and P<0.05, respectively). Finally, we analyzed fru for aggressive behavior and woc for neurogenesis: these genes were upregulated in haplo in the arrays and in the qRT-PCR analysis (P<0.05 and P<0.001, respectively).
Experiment 2: Effect of the manipulation of social rank on gene expression patterns
We further examined the role of social rank on gene expression patterns by manipulating social rank of individuals in pleometrotic pairs. We swapped winners and losers between groups to generate four groups of queens: winners switched to losers (win/los), losers switched to winners (los/win), continuing winners (win/win) and continuing losers (los/los). Very few transcripts were differentially regulated among these groups, with a total of 616 transcripts at a relatively low significant threshold (FDR<0.1, see Table S11 for a listing of these transcripts). Principal components analysis demonstrated that 48% of the variation in gene expression was associated with switching social rank (win/los and los/win were clustered relative to win/win and los/los), 37% of the variation was associated with the final rank (i.e., win/los and los/los were clustered), while 15% was associated with the initial rank (i.e. win/win and win/los were clustered; Figure 5).
Fig. 5. Analysis of global gene expression in pleometrotic queens after manipulation of social rank. 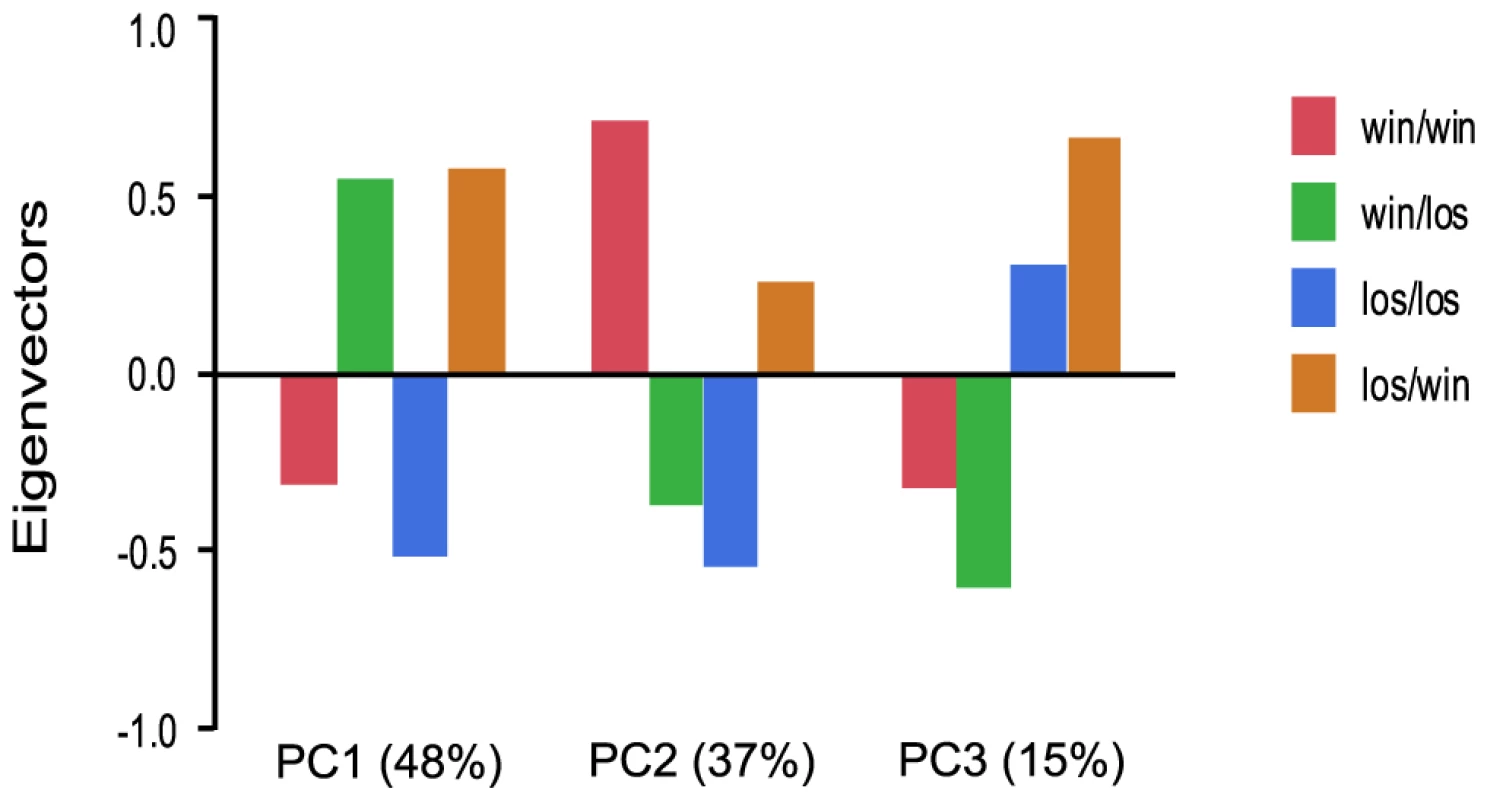
Principal Component Analysis of 606 significantly differentially regulated transcripts revealed three principal effects corresponding to switch of the social rank (48%), final rank (37%) and initial rank (15%). win/los = winners switched to losers; win/win = continuing winners; los/win = losers switched to winners; los/los = continuing losers. We performed GO analysis with Functional Annotation Clustering on the 527 differentially regulated transcripts that have Drosophila orthologs with FlyBase annotations. 21 GO terms were significantly enriched at p-value<0.05 and three survived Benjamini correction: cellular metabolic process, cellular ketone metabolic process and maintenance of protein location (see Table S12). Among the other GO terms, of particular interest was lipid metabolic process, which includes several genes involved in the metabolism of fatty acids such as Helix loop helix protein 106 (HLH106) [49], scully (scu) [50] and two putative fatty acyl-CoA reductases. Additional genes with significant differences in expression included Coenzyme Q biosynthesis protein 2 (Coq2), which plays a role in the response to pathogens, aging and in the insulin-signaling pathway [51], [52], juvenile hormone acid methyltransferase (jhamt), which was also significantly differentially regulated between win and los in experiment 1 (see above), and radish (rad), which is involved in learning and memory [53]. Finally, the GO term “response to stress” was significantly overrepresented, which includes key immune response genes such as immune response deficient 5 (ird5) [54], Ras-related protein Rac1 (Rac1) [55], Hemolectin (Hml) [56], Argonaute 2 (AGO2) [57] and caspar (casp) [58].
Ninety-three transcripts were differentially regulated both between win and los in experiments 1 (548 transcripts, FDR<0.1) and in experiment 2 (616 transcripts, FDR<0.1): this is significantly more than expected by chance (Hypergeometric Test: Representation factor: 2.2, p<1.009−13). These transcripts corresponded to 80 Drosophila orthologs, which were used to perform a GO analysis using Functional Annotation Clustering: 6 GO terms appeared to be significantly enriched, including lipid metabolic process (P<0.001) and regulation of hormone levels (P<0.05; Table S13). The expression patterns of the 9 differentially regulated transcripts involved in lipid metabolic process across the two experiments are shown in Table 2.
Tab. 2. Patterns of expression of transcripts associated with lipid metabolic process that were significantly differentially regulated in both experiment 1 and experiment 2 at FDR<0.1. 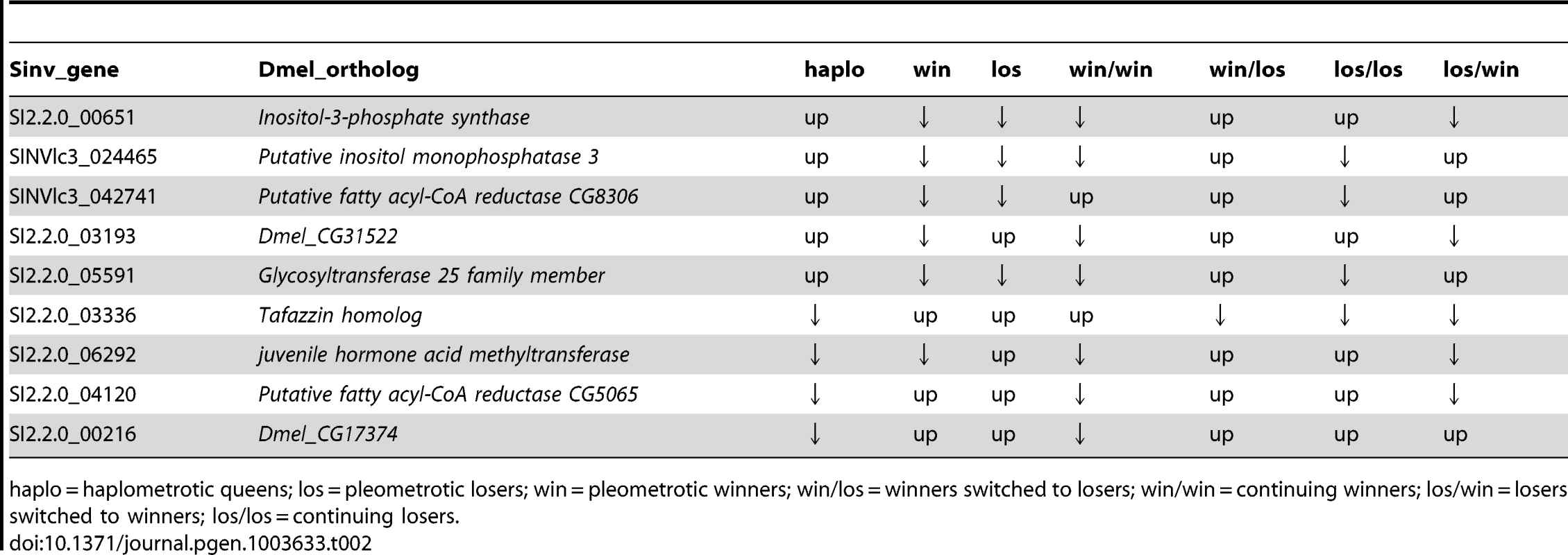
haplo = haplometrotic queens; los = pleometrotic losers; win = pleometrotic winners; win/los = winners switched to losers; win/win = continuing winners; los/win = losers switched to winners; los/los = continuing losers. Discussion
This study demonstrates that social environment greatly influences gene expression in founding queens of the fire ant Solenopsis invicta, and that the effects of social rank are secondary. Social environment in the first experiment (haplometrosis vs. pleometrosis) strongly influenced expression of thousands of genes, and the difference between pleometrotic winners and losers was much smaller. However, in terms of gene expression differences, pleometrotic winners were more similar to haplometrotic queens, suggesting that reproductive and social rank still does impact, albeit more subtly, gene expression patterns. In the second experiment, we manipulated both the social environment and social rank of queens in pleometrotic pairs. Switching social rank significantly affected gene expression patterns more than the initial or final social rank of the individual. Several categories of genes were differentially regulated among these groups of queens, including genes involved in core processes such as metabolism, aging and immunity.
In a recent study Ferreira et al. [59] explored the genetic basis of the early phases of social evolution in a primitively eusocial Polistes wasp. These authors found that 75% of the 2,442 genes differentially expressed between queen and worker phenotypes were novel, having no significant similarity with described sequences. This result supports the hypothesis that novel genes are likely important for eusocial evolution, as previously suggested by other studies [60], [61]. Interestingly, within our pool of 9,388 genes initially analyzed for experiment 1 (haplometrotic vs. pleometrotic queens), 41% were novel but this percentage decreased to 26% (which is a significantly smaller percentage, Fisher's Exact Test, P<0.0001) when we consider only the genes that were differentially regulated between the two phenotypes of founding queens. Thus, while caste differences in Polistes may be associated with novel genes, plasticity in founding behavior in fire ants seems to rely predominantly on more conserved genes.
Haplometrotic vs. pleometrotic queens
Differences in gene expression between haplometrotic and pleometrotic queens were likely due to differences in the physiological demands placed on singly - vs. multiply-founding queens and differences in the costs associated with social environment, where pleometrotic queens are more likely to incur in higher levels of stress due to the establishment of social ranks. We found that genes involved in core physiological processes, including metabolism, cellular processes, development, morphogenesis and biosynthesis were significantly differentially regulated between these groups of queens. Haplometrotic queens produce more eggs and lose more weight than pleometrotic queens during the claustral period of colony founding [20]: this seems to be due to queen-queen reciprocal reproductive inhibition and oophagy/cannibalism of larvae in pleometrotic associations [62]. Genes associated with reproductive functions (including development of reproductive tissues and production of oocytes and eggs) were upregulated in haplometrotic queens. Furthermore, in order to produce eggs, newly mated queens degrade wing muscle tissues and metabolize fat body storage proteins to produce free amino acids [63]. We found 58 protein-related GO terms and 10 amino acid-related that were upregulated in haplometrotic queens versus 5 and 0, respectively that were upregulated in pleometrotic queens (Functional Annotation Chart, see Tables S8 and S9).
Genes associated with stress response were differentially regulated between haplo and pleo queens. Stress tolerance may be achieved by reducing the production of reactive oxidant species (via improved regulation of mitochondrial processes) and/or by increasing the antioxidant activity [64], [65]. In our study, we found that two mitochondria-related GO terms, namely mitochondrial electron transport, NADH to ubiquinone (15 genes) and mitochondrion organization (18 genes) were upregulated in haplo and none in pleo. Moreover, 9 antioxidant genes were upregulated in haplo, including two superoxide dismutases (Sod2 and CCS), two Peroxiredoxins (6005 and 5037), Glutathione S transferase S1 and PTEN-induced putative kinase 1 (Pink1), which plays an essential role in maintaining neuronal survival by preventing neurons from undergoing oxidative stress [66]. These results suggest that haplo queens may experience lower levels of oxidative stress either by producing less ROS or by keeping the levels of antioxidants high. Higher stress levels in pleo queens could be correlated to their social environment, dominated by queen-queen aggressive interactions and competition. Stress tolerance is positively correlated with lifespan [67] and this trait has been used as a proxy for long-lived phenotypes in studies that examine the genetic basis of lifespan [68]. Only SOD was upregulated in pleo. Interestingly, overexpression of SOD has been correlated to increased organismal longevity in Drosophila [69], but this was not confirmed in Lasius niger, where long-lived queens expressed lower levels of this gene than short-lived males and workers [70]. It is evident that the effect of SOD on longevity is highly dependent upon the sex and genetic background [71] and also the social environment [72].
The overrepresentation of GO terms associated to biosynthesis and metabolism (in particular those related to lipids) prompted us to look closer at the nutritional state of founding queens. Nutrition is closely linked to fertility and longevity [73]. In insects, the insulin-signaling pathway regulates nutrient-sensing [74] while juvenile hormone and ecdysone mediate reproductive processes [75]. In honey bees, long-lived queens have low levels of insulin and juvenile hormone, while they have high levels of FOXO, vitellogenin and ecdysone; opposite patterns are found in sterile short-lived workers [76]. Our results show that haplo had higher levels of FOXO and of the ecdysone receptor. Haplo queens also presumably had lower levels of JH, since levels of juvenile hormone acid methyltransferase (jhamt), an enzyme that converts inactive precursors of JHs to active JHs [41], were downregulated, and levels of juvenile hormone epoxide hydrolase 2 (Jheh2), involved in juvenile hormone catabolic process, were upregulated.
There is no clear prediction about which group of queens should have a longer life-span. Our analyses show that a large set of aging-related GO terms was upregulated in haplometrotic queens, while a smaller set was upregulated in pleometrotic queens. This result is not sufficient to establish which group of queens is expected to have longer life-span, since ageing is a quantitative trait determined by both environmental and genetic components. Previous studies of the genetics of longevity in Drosophila melanogaster, identified sets of genes in which upregulated expression either accelerates or decelerates the aging process [65]. However, in our study, genes from both categories were equally up - and downregulated across haplo and pleo queens (see Figure 3A). Therefore, the knowledge of the genetics of longevity in the insect model D. melanogaster cannot be transferred directly to our study system.
Immune-related genes were overexpressed in haplometrotic vs. pleometrotic queens. Most of the overrepresented immune-related GO terms were associated to cellular immunity: endocytosis, phagocytosis, cell adhesion, apoptosis, cytokinesis, the cascade regulating mitogen-activated protein kinase (MAPKKK) and the c-Jun amino-terminal protein kinase (JNK) cascade. In particular, the JNK pathway controls the rapid up-regulation of cytoskeletal genes in response to infection and plays a major role in wound healing [77]. Key genes in the JNK pathways [78] were upregulated in haplo, namely kayak, hemipterous, misshapen, anterior open and Cdc42. Hemopoiesis is the process that is responsible for production and differentiation of immune cells [79]: two key genes involved in this process, serrate and serpent, were upregulated in haplo. Haplo queens may have better constitutive immune responses perhaps because they experience less social stress than pleo queens do: in fact, once initial cooperation transitions into open competition, pleo queens frequently engage in reciprocal aggressions which can lead to serious injuries or death. It is hypothesized that there is a trade-off between reproduction, nutrition and immunity [80], suggesting that highly reproductive haplo queens should have overall reduced immune responses during colony foundation period when food sources are limited. However, previous studies in honey bees demonstrated that reproductive queens have higher expression of immune genes than non-reproductive workers [81], [82], and thus this trade-off may not exist in social insect queens, perhaps because queens have more energy resources than workers.
Pleometrotic queens: Winner vs. loser
Only 43 transcripts were significantly differentially regulated between winners and losers in couples of pleometrotic queens from experiment 1. Although surprising, this result might be explained by the small phenotypic differences between the two types of queens. Previous studies showed that some phenotypic traits such as head width are weakly correlated with the reproductive investment and survival (hence the rank) of pleometrotic cofoundresses [20]. It has been suggested that the relatively weak association between these parameters stems from selection to maintain cooperation [20]. If phenotypic differences strongly correlate with the chances of surviving, smaller queens with lower fighting abilities would be selected not to cooperate and feed the brood in the colony. Thus, the small differences at the genomic level between winners and losers may reflect selection for a system where differences between cofoundresses is sufficiently small so that all of them have a chance of surviving, and thus an interest to cooperate with unrelated individuals.
The two GO terms that were differentially regulated between winners and losers were related to metabolism of lipids and metabolism of hormones. Four transcripts, bubblegum, desat1, Dmel_CG17374 and Dmel_CG31522, which function in fatty acid metabolism, were differentially regulated. Long-chain fatty acids are the precursors of cuticular hydrocarbons in insects, which can function as nestmate recognition cues and social pheromones in many insect species (reviewed [83]). Interestingly, bgm, which encodes a very long-chain fatty acid CoA ligase [36], was downregulated in losers relative to winners and haplo: thus, this gene may be involved in regulating chemical cues related to dominance. Similarly, desat1, which is expressed at higher levels in losers than in winners or haplo functions in pheromonal communication [40]. Altered bgm expression has also been associated with infection (and correlated with changes in cuticular hydrocarbon profiles) in honey bees [84], while desat1 appears to play a role in autophagic responses [85]. Thus, these genes may also be involved in signaling infection, nutrient deprivation or other stress responses.
Behavioral manipulation of the social rank in pairs of pleometrotic queens demonstrated that manipulation of social environment (i.e., conditions in which the social rank of the individual changes) had a much larger effect on gene expression than the initial or final social rank of the individual. Note, however, that all individuals in the study switched social partners, which may have elicited additional (undetected) changes in gene expression. Studies in vertebrates have demonstrated that social interactions and changes in the social environment can be one of the most potent stressors [86]. Indeed, genes associate with ‘response to stress’ were significantly enriched, with a set of 30 transcripts differentially regulated among the four groups of manipulated queens (see Results and Table S12). The effects of restructuring social ranks have not been considered broadly in other species [87], but decreased social rank in dark-eyed junco birds is associated with increased metabolic rates, while increased social rank results in a much lower physiological change [88]. Similarly, for dominant, but not for subordinate, birds there is a measurable metabolic cost to joining a new social group [88].
In both experiments, genes involved in lipid biosynthesis and metabolism were differentially regulated, suggesting that these processes play a key role in mediating fire ant founding behavior and foundress associations. Lipids such as cuticular hydrocarbons play a role in advertising the fertility state in many ant species: these compounds are usually more abundant in reproductive queens and egg-laying workers (reviewed in [89]). Indeed, ‘lipid biosynthetic/metabolic process’ was differentially regulated in haplo vs. pleo and in win vs. los in experiment 1 (Tables S4, S5 and S6) and in experiment 2 (Table S12). These results support the hypothesis that lipids (and in particular fatty acids) are of great importance in regulating social interactions between queens and among nestmates in general. In fire ant pleometrotic associations, the pheromones and nestmate recognition chemicals derived from these fatty acids are most likely an important component of the individual's chemical profile, which is used by nestmate queens to decipher the physiological condition and thus the social rank of the partner.
Conclusions
We used newly developed genomic tools to examine the gene expression patterns associated with complex social behaviors involved in colony founding by fire ant queens. Our results suggest that social environment (haplometrotic vs. pleometrotic, switched vs. maintained social rank) is more important than the social rank or internal condition of the individual in regulating gene expression patterns, and presumably downstream physiological and behavioral traits. Furthermore, because the process of pleometrotic colony founding in fire ants has all the features of a primitively social system in which morphologically, physiologically, and genetically similar individuals perform cooperative behavior to form social groups of unrelated individuals, this is an excellent model to examine the genes that underlie these social behaviors. We found that several core processes were significantly differentially regulated, including metabolism, stress response, aging, reproductive processes, and immunity. Interestingly, lipid metabolic processes were regulated across experiments; these may play a role in both nutrient storage/mobilization and chemical communication. In the future, it will be interesting to investigate whether the molecular pathways characterized in this study also are operating at earlier stages of the co-founding process (e.g., before the emergence of workers). Such studies will help elucidate the mechanisms responsible for the transition from cooperation to conflict in pleometrotic founding queens. Finally, fire ants also display genetically distinct monogyne (colonies headed by a single queen) and polygyne (colonies headed by multiple queens) social forms. It will be of great interest to determine if the same genes that regulate haplometrosis and pleometrosis also are involved in regulating queen number in mature colonies.
Materials and Methods
Insect collection and rearing
A total of 787 fire ant queens were collected immediately after a nuptial mating flight on May 5th, 2010 in a large parking lot (Target, 3970 SW Archer Rd, Gainesville, FL) and weighed. Since the area of collection has a high prevalence of monogyne colonies, we expected these queens to belong to the monogyne social form; this was subsequently confirmed by screening 108 queens for social form using Gp-9 as a marker following the protocol as described in [90]. The remaining 679 queens were split into two groups: 308 queens were set up in pairs (pleometrosis) based on having similar weights (range ±0.2 mg) and paint-marked with different colors, while 371 queens were set up individually (haplometrosis) and paint-marked as well. All the queens were provided with a nesting chamber consisting of a glass tube half-filled with water, which was covered by a cotton ball and a layer of dental plaster: this keeps the chamber moist but avoids an excess of water which is deleterious for the young brood. Tubes were sealed with a loose cap to provide air flow. Specimens were reared in the dark at 28°C, 70% relative humidity under claustral conditions (no food and no water) for 1 month.
Experiment 1: Effect of social environment and social rank on gene expression patterns
After eclosion of the first batch of workers (minims), incipient colonies were provided with water, sugar water and frozen crickets. Glass tubes were set open in pencil boxes coated with Fluon to prevent escape. Queens were subsequently monitored daily until it was possible to identify the social rank of the two cofoundresses in pleometrotic couples. Previous studies have found that the initial cooperation between the two cofoundresses turns into conflict after the emergence of minims, resulting in the execution of one queen [19]. Queens that survive the competition (winners) are usually located at the top of the brood pile within the nest chamber and they are generally tended by workers; conversely, queens that will be executed (losers) are normally seen outside the nest chamber, hiding from workers in order to avoid being attacked (Figure S4). We used these observations to establish the social rank of the two pleometrotic queens, i.e. winner and loser. We collected 25 pleometrotic couples and 25 haplometrotic queens in dry ice and stored them at −80°C to be later processed.
Experiment 2: Effect of the manipulation of social rank on gene expression patterns
This assay was performed with 34 couples of pleometrotic queens from the same pool of newly mated queens as experiment 1. The queens were paired and placed in nesting chambers as before. After emergence of minims, queens' behavior was monitored as before. Once the behavioral observation revealed the social rank of the two cofoundresses, queens were weighed again and re-paired with a different partner. We created the following three groups of queens: a) winner+winner (similar weight), b) loser+loser (similar weight), and c) winner+loser (different weights). Again, we monitored the behavior until the social rank of the newly coupled specimens was evident and we collected 4 new behavioral phenotypes in the same way as above: a) winners switched into losers (win/los, N = 7), b) losers switched into winners (los/win, N = 11), c) continuing winners (win/win, N = 12) and d) continuing losers (los/los, N = 5).
Sample preparation for molecular analyses
Individual fire ant queens were thawed and dissected under cold RNAlater (Qiagen, Valencia, CA) to confirm the mating status: unmated queens were not included in the analysis. Total RNA was extracted using the RNeasy Plus kit (Qiagen) combined with a RNase-Free DNase step (Qiagen) to remove any possible contamination by genomic DNA. Subsequent steps in the microarray analysis were performed at the Penn State Genomic Core Facility. RNA concentration and purity were assessed using NanoDrop and Qubit and RNA quality was assessed using RNA Nano Chips on the Agilent Bioanalyzer. 1 µg of each sample was amplified using the Ambion (Life Technologies) Amino Allyl MessageAmp II aRNA Amplification Kit (AM1753). 15 µg of aRNA were dyed with Cy3 or Cy5 (GE Health Care #RPN5661) and subsequently purified according to the Ambion Kit instructions. 1.5 µg of a Cy3 labeled sample were combined with 1.5 µg of a Cy5 labeled sample and fragmented using RNA Fragmentation Reagents (Ambion AM8740) according to the manufacturer's instructions. Samples were hybridized with mixing in a MAUI hybridization instrument overnight at 42°C. Arrays were scanned using Axon GenePix 4000B.
For the first microarray developed to validate the efficiency of probe sequences, we pooled RNA samples (2 µg total) from different castes, developmental instars and social forms as follows: 3 female alates, 15 workers, 5 larvae and 5 pupae from both monogyne and polygyne social forms and 5 males from monogyne colonies only.
Microarray design and validation
The fire ant genome includes an official gene set of 16,569 protein-coding genes that were generated by a combination of ab initio, EST-based, and sequence similarity-based methods [27]. For our microarray studies, we combined the official gene set with a set of ESTs obtained from assemblies of the fire ant transcriptome for a total set of 63,436 sequences (“transcripts”). We successfully designed 60-mer probes for 51,531 of these transcripts (Roche NimbleGen, Inc., Madison WI). These sequences/probes were grouped into four categories: ESTs with gene models (EWGM, 7433 transcripts), ESTs without gene models (EWOGM, 40,613), gene models (GM, 3246) and gene models redundant with other models (GMRWOM, 239).
We developed and used a first microarray (1-plex 385,000 probe capacity, Roche NimbleGen, Inc., Madison WI) to validate the probe design and test multiple probes per transcript. On average, we designed 7 probes per transcript for a total of 355,930 probes. Each probe was tested for both the red (Cy5) and the green (Cy3) dyes. For transcripts with only one probe (N = 296), we verified that the probe had acceptable intensities for both dyes. For the other transcripts we examined the performance of the probes with the green dye only, because these showed consistently higher intensity compared to the red dye. Probes were ranked in the follow manner: a) if there were only 2 probes per transcript (N = 230), we selected the one with higher intensity; b) if there were 3 to 6 probes (N = 744), we calculated the ratio “probe intensity/median intensity of all probes for that transcript” and selected the probe with highest ratio if the value was <3, otherwise we selected the probe with the second highest ratio; c) for transcripts with 7 probes (N = 50,261), we followed the procedure as in “b” but, in case the probe with the highest ratio was >3, we removed that probe, calculated new ratios and selected a new probe with highest ratio. This procedure allowed us to select the probes with highest intensity that were not outliers.
Selected probes were printed in pairs on two 12-plex microarrays (each array had a 135,000 probe capacity, Roche NimbleGen, Inc., Madison WI). We used a loop design with dye swaps incorporated, allowing us to hybridize 24 RNA samples to each array. For experiment 1 we hybridized 8 haplometrotic queens, 8 pleometrotic winners and 8 pleometrotic losers (Figure S5) and for experiment 2 we compared 6 win/los, 6 los/win, 6 win/win and 5 los/los (Figure S6).
Analysis of gene expression
Any spots with an intensity of less than 300 (the background level on the arrays) were removed from the analyses, as were spots present on less than 20 out of 24 arrays. Expression data were log-transformed and normalized using mixed-model normalization (proc MIXED, SAS, Cary, NC) with the following model:
where Y is expression, dye and block are fixed effects, and array, array*dye and array*block are random effects. Transcripts with significant expression differences between groups were detected by using a mixed-model ANOVA with the model: where Y represents the residual from the previous model. Treatment, spot and dye are fixed effects and array is a random effect. P-values were corrected for multiple testing using a false discovery rate of <0.001 for experiment 1 and <0.1 for experiment 2 (proc MULTTEST, SAS). Because the number of differentially regulated transcripts for experiment 1 was very high (∼13,000 out of ∼50,000), and to avoid an excess of redundancy among the different groups of transcripts, we included only probes corresponding to GM and EWGM (see above).Hierarchical clustering, using the Ward method, and principal component analysis (PCA) for global patterns of gene expression were performed in JMP 9.0.2 (SAS, Cary, NC). We used Genesis 1.7.6 (Graz, Austria) to cluster differentially regulated genes based on average linkage and to perform k-means clustering in experiment 1. Gene Ontology analysis was performed using functional annotation chart/clustering in DAVID version 6 [28], [29] using DAVID default population background and a cutoff of p<0.05. For all Gene Ontology (GO) analyses, fire ant genes were matched to their Drosophila orthologs in FlyBase (http://flybase.org/). CateGOrizer [31] was used to count the occurrences of significantly enriched GO terms within each of the pre-defined set of parent/ancestor GO terms. The array data were deposited on the ArrayExpress website according to MIAME standards (ArrayExpress accession: E-MEXP-3886 for experiment 1, E-MEXP-3898 for experiment 2).
Comparative studies
We compared the results from experiment 1 to the following studies:
-
experiment 2 from this study: we overlapped 548 transcripts that were significantly differentially regulated at FDR<0.1 between win and los in experiment 1 to all the significantly differentially regulated transcripts from experiment 2 (606, FDR<0.1).
-
aging in Drosophila: we compared directional expressions for all the significantly enriched GO terms obtained from Functional Annotation Chart for experiment 1 and the significantly enriched GO terms that were associated to aging in the fruit fly based on Zhan et al. [47].
We performed overlaps between list of transcripts and GO terms with Venny [91]. In the first comparative study we overlapped fire ant transcripts directly, while in the second study we used Drosophila orthologues (FlyBase numbers) to compare fire ant transcripts to the genes of the fruit fly. Statistical significance of the overlap was calculated using a hypergeometric test (http://nemates.org/MA/progs/overlap_stats.html). Selected GO analyses based on study overlap were performed in DAVID as above. In the second study, to test for the significant agreement in the patterns of expression between two studies we performed Fisher's Exact Tests in JMP.
Validation of candidate gene expression using Quantitative Real-Time PCR
We examined gene expression levels of the following candidate genes (Table S10): Indy and Sod2 (determination of adult life span); Dredd and kay (immune response); desat1, ifc and Putative fatty acyl-CoA reductase CG5065 (synthesis and metabolism of fatty acids); br and Btk29A (reproductive functions); Sema-5c and Mer (olfactory behavior); fru (aggressive behavior) and woc (neurogenesis). We used the total RNA extracted from fire ant queens for the microarray analysis and compared gene expression between haplo and los on an ABI Prism 7900 sequence detector (Applied Biosystems, Foster City, CA, USA). cDNA was made using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen-Life Technologies, Carlsbad, CA, USA) and Random Hexamers according to the manufacturer's protocol. The cDNA was then diluted 2(x) with ultra-pure water. Amplification was performed in a 10 µl reaction mixture containing 5 µl of 2× SYBR Green Master Mix (Applied Biosystems-Life Technologies, Carlsbad, CA, USA), 1 µl of each primer (10 µM) and 2 µl of cDNA at the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C for 1 min, a dissociation step of 95°C for 15 sec and 60°C for 15 sec. We used 8 queens per group: triplicate reactions were performed for each of the samples and averaged for use in statistical analysis. Expression levels of candidate genes were normalized to the geometric mean of two housekeeping genes, Rp-9 and Rp-37 [27]. Negative control (cDNA reaction without RT enzyme) was also used. Primer sequences were developed in Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) and primer efficiency was first validated using standard curves. Statistical analysis was performed with nonparametric Kruskall-Wallis rank sums in JMP 10 (SAS, Cary, NC). The data were shown normalized to the haplo group.
Supporting Information
Zdroje
1. HofmannHA (2003) Functional genomics of neural and behavioral plasticity. Journal of Neurobiology 54 : 272–282.
2. RossKG, KellerL (1995) Ecology and evolution of social organization: insights from fire ants and other highly eusocial insects. Annual Review of Ecology and Systematics 631–656.
3. RobinsonGE (2004) Beyond Nature and Nurture. Science 304 : 397–399.
4. RobinsonGE, FernaldRD, ClaytonDF (2008) Genes and Social Behavior. Science 322 : 896–900.
5. ZayedA, RobinsonGE (2012) Understanding the Relationship Between Brain Gene Expression and Social Behavior: Lessons from the Honey Bee. Annual Review of Genetics 46 : 591–615.
6. Duarte A, Weissing FJ, Pen I, Keller L (2011) An Evolutionary Perspective on Self-Organized Division of Labor in Social Insects. In: Futuyma DJ, Shaffer HB, Simberloff D, editors. Annual Review of Ecology, Evolution, and Systematics. Volume 42. Palo Alto: Annual Reviews. pp. 91–110.
7. DolezalAG, BrentCS, HolldoblerB, AmdamGV (2012) Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. Journal of Experimental Biology 215 : 454–460.
8. SmithCR, TothAL, SuarezAV, RobinsonGE (2008) Genetic and genomic analyses of the division of labour in insect societies. Nature Reviews Genetics 9 : 735–748.
9. Hölldobler B, Wilson EO (1990) The ants. Cambridge, MA: Belknap Press. 732 pp.
10. BernasconiG, StrassmannJE (1999) Cooperation among unrelated individuals: the ant foundress case. Trends in Ecology & Evolution 14 : 477–482.
11. KukukP, SageG (1994) Reproductivity and relatedness in a communal halictine bee Lasioglossum (Chilalictus) hemichalceum. Insectes Sociaux 41 : 443–455.
12. RoisinY (1993) Selective pressures on pleometrosis and secondary polygyny: a comparison of termites and ants. Queen number and sociality in insects 402–421.
13. ZanetteLRS, FieldJ (2011) Founders versus joiners: group formation in the paper wasp Polistes dominulus. Animal Behaviour 82 : 699–705.
14. QuellerDC, ZacchiF, CervoR, TurillazziS, HenshawMT, et al. (2000) Unrelated helpers in a social insect. Nature 405 : 784–787.
15. LiebertA, HuiJ, NonacsP, StarksP (2008) Extreme Polygyny: Multi-seasonal “Hypergynous” Nesting in the Introduced Paper Wasp Polistes dominulus. Journal of Insect Behavior 21 : 72–81.
16. RissingSW, PollockGB, HigginsMR, HagenRH, SmithDR (1989) Foraging specialization without relatedness or dominance among co-founding ant queens. Nature 338 : 420–422.
17. JeansonR, FewellJH (2008) Influence of the social context on division of labor in ant foundress associations. Behavioral Ecology 19 : 567–574.
18. TschinkelW, HowardD (1983) Colony founding by pleometrosis in the fire ant, Solenopsis invicta. Behavioral Ecology and Sociobiology 12 : 103–113.
19. BalasMT, AdamsES (1996) The dissolution of cooperative groups: mechanisms of queen mortality in incipient fire ant colonies. Behavioral Ecology and Sociobiology 38 : 391–399.
20. BernasconiG, KellerL (1998) Phenotype and individual investment in cooperative foundress associations of the fire ant, Solenopsis invicta. Behavioral Ecology 9 : 478–485.
21. BernasconiG, KriegerMJB, KellerL (1997) Unequal partitioning of reproduction and investment between cooperating queens in the fire ant, Solenopsis invicta, as revealed by microsatellites. Proceedings of the Royal Society B-Biological Sciences 264 : 1331–1336.
22. AdamsES, TschinkelWR (1995) Effects of foundress number on brood raids and queen survival in the fire ant Solenopsis invicta. Behavioral Ecology and Sociobiology 37 : 233–242.
23. BalasMT, AdamsES (1996) Nestmate discrimination and competition in incipient colonies of fire ants. Animal Behaviour 51 : 49–59.
24. MarkinGP, DillierJH, CollinsHL (1972) Colony founding by queens of red imported fire ant, Solenopsis invicta - Hymenoptera - Formicidae. Annals of the Entomological Society of America 65 : 1053–1058.
25. TschinkelWR (1995) Stimulation of fire ant queen fecundity by a highly specific brood stage. Annals of the Entomological Society of America 88 : 876–882.
26. BernasconiG, KellerL (1996) Reproductive conflicts in cooperative associations of fire ant queens (Solenopsis invicta). Proceedings of the Royal Society B-Biological Sciences 263 : 509–513.
27. WurmY, WangJ, Riba-GrognuzO, CoronaM, NygaardS, et al. (2011) The genome of the fire ant Solenopsis invicta. Proceedings of the National Academy of Sciences of the United States of America 108 : 5679–5684.
28. HuangDW, ShermanBT, LempickiRA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research 37 : 1–13.
29. HuangDW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4 : 44–57.
30. KanehisaM, GotoS, SatoY, FurumichiM, TanabeM (2012) KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Research 40: D109–D114.
31. HuZL, BaoJ, ReecyJM (2008) CateGOrizer: A Web-Based Program to Batch Analyze Gene Ontology Classification Categories. Online Journal of Bioinformatics 9 : 108–112.
32. SturnA, QuackenbushJ, TrajanoskiZ (2002) Genesis: cluster analysis of microarray data. Bioinformatics 18 : 207–208.
33. ValanneS, MyllymäkiH, KallioJ, SchmidMR, KleinoA, et al. (2010) Genome-Wide RNA Interference in Drosophila Cells Identifies G Protein-Coupled Receptor Kinase 2 as a Conserved Regulator of NF-kB Signaling. The Journal of Immunology 184 : 6188–6198.
34. Drummond-BarbosaD, SpradlingAC (2004) alpha-Endosulfine, a potential regulator of insulin secretion, is required for adult tissue growth control in Drosophila. Developmental Biology 266 : 310–321.
35. VieiraFG, RozasJ (2011) Comparative Genomics of the Odorant-Binding and Chemosensory Protein Gene Families across the Arthropoda: Origin and Evolutionary History of the Chemosensory System. Genome Biology and Evolution 3 : 476–490.
36. MinK-T, BenzerS (1999) Preventing Neurodegeneration in the Drosophila Mutant bubblegum. Science 284 : 1985–1988.
37. WangPY, NerettiN, WhitakerR, HosierS, ChangCY, et al. (2009) Long-lived Indy and calorie restriction interact to extend life span. Proceedings of the National Academy of Sciences of the United States of America 106 : 9262–9267.
38. PearsonJC, JuarezMT, KimM, DrivenesØ, McGinnisW (2009) Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proceedings of the National Academy of Sciences 106 : 2224–2229.
39. RiemenspergerT, IsabelG, CoulomH, NeuserK, SeugnetL, et al. (2011) Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proceedings of the National Academy of Sciences of the United States of America 108 : 834–839.
40. HouotB, BousquetFo, FerveurJ-Fo (2010) The Consequences of Regulation of desat1 Expression for Pheromone Emission and Detection in Drosophila melanogaster. Genetics 185 : 1297–1309.
41. ShinodaT, ItoyamaK (2003) Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proceedings of the National Academy of Sciences of the United States of America 100 : 11986–11991.
42. AmdamGV (2011) Social context, stress, and plasticity of aging. Aging Cell 10 : 18–27.
43. WheelerDE, BuckN, EvansJD (2006) Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Molecular Biology 15 : 597–602.
44. AmentSA, ChanQW, WheelerMM, NixonSE, JohnsonSP, et al. (2011) Mechanisms of stable lipid loss in a social insect. J Exp Biol 214 : 3808–3821.
45. AmentSA, CoronaM, PollockHS, RobinsonGE (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. PNAS 105 : 4226–4231.
46. BakerN, WolschinF, AmdamGV (2012) Age-related learning deficits can be reversible in honeybees Apis mellifera. Experimental Gerontology 47 : 764–772.
47. ZhanM, YamazaH, SunY, SinclairJ, LiH, et al. (2007) Temporal and spatial transcriptional profiles of aging in Drosophila melanogaster. Genome Research 17 : 1236–1243.
48. EvansJD, AronsteinK, ChenYP, HetruC, ImlerJL, et al. (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Molecular Biology 15 : 645–656.
49. KunteAS, MatthewsKA, RawsonRB (2006) Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metabolism 3 : 439–448.
50. ShafqatN, MarschallHU, FillingC, NordlingE, WuXQ, et al. (2003) Expanded substrate screenings of human and Drosophila type 10 17-hydroxysteroid dehydrogenases (HSDs) reveal multiple specificities in bile acid and steroid hormone metabolism: characterization of multifunctional 3/7/7/17/20/21-HSD. The Biochemical Journal 376 : 49–60.
51. ChengW, SongC, AnjumKM, ChenM, LiD, et al. (2011) Coenzyme Q plays opposing roles on bacteria/fungi and viruses in Drosophila innate immunity. International journal of immunogenetics 38 : 331–337.
52. LiuJ, WuQ, HeD, MaT, DuL, et al. (2011) Drosophila sbo regulates lifespan through its function in the synthesis of coenzyme Q in vivo. Journal of Genetics and Genomics [Yi chuan xue bao] 38 : 225–234.
53. LeePT, LinHW, ChangYH, FuTF, DubnauJ, et al. (2011) Serotonin-mushroom body circuit modulating the formation of anesthesia-resistant memory in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 108 : 13794–13799.
54. CostaA, JanE, SarnowP, SchneiderD (2009) The Imd pathway is involved in antiviral immune responses in Drosophila. Plos One 4: e7436.
55. HowellL, SampsonCJ, XavierMJ, BolukbasiE, HeckMM, et al. (2012) A directed miniscreen for genes involved in the Drosophila anti-parasitoid immune response. Immunogenetics 64 : 155–161.
56. ScherferC, KarlssonC, LosevaO, BidlaG, GotoA, et al. (2004) Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Current Biology 14 : 625–629.
57. WangXH, AliyariR, LiWX, LiHW, KimK, et al. (2006) RNA interference directs innate immunity against viruses in adult Drosophila. Science 312 : 452–454.
58. KimM, LeeJH, LeeSY, KimE, ChungJ (2006) Caspar, a suppressor of antibacterial immunity in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 103 : 16358–16363.
59. FerreiraPG, PatalanoS, ChauhanR, Ffrench-ConstantR, GabaldonT, et al. (2013) Transcriptome analyses of primitively eusocial wasps reveal novel insights into the evolution of sociality and the origin of alternative phenotypes. Genome Biology 14: R20.
60. JohnsonBR, TsutsuiND (2011) Taxonomically restricted genes are associated with the evolution of sociality in the honey bee. Bmc Genomics 12 : 164.
61. JohnsonBR, LinksvayerTA (2010) Deconstructing the superorganism: social physiology, groundplans, and sociogenomics. The Quarterly Review of Biology 85 : 57–79.
62. TschinkelWR (1993) Resource allocation, brood production and cannibalism during colony founding in the fire ant, Solenopsis invicta. Behavioral Ecology and Sociobiology 33 : 209–223.
63. WurmY, WangJ, KellerL (2010) Changes in reproductive roles are associated with changes in gene expression in fire ant queens. Molecular Ecology 19 : 1200–1211.
64. CoronaM, HughesKA, WeaverDB, RobinsonGE (2005) Gene expression patterns associated with queen honey bee longevity. Mechanisms of ageing and development 126 : 1230–1238.
65. PaabyAB, SchmidtPS (2009) Dissecting the genetics of longevity in Drosophila melanogaster. Fly 3 : 29–38.
66. WangD, QianL, XiongH, LiuJ, NeckameyerWS, et al. (2006) Antioxidants protect PINK1-dependent dopaminergic neurons in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 103 : 13520–13525.
67. VermeulenCJ, LoeschckeV (2007) Longevity and the stress response in Drosophila. Experimental Gerontology 42 : 153–159.
68. WangH-D, Kazemi-EsfarjaniP, BenzerS (2004) Multiple-stress analysis for isolation of Drosophila longevity genes. Proceedings of the National Academy of Sciences of the United States of America 101 : 12610–12615.
69. OrrW, SohalR (1993) Effects of Cu-Zn Superoxide Dismutase Overexpression on Life Span and Resistance to Oxidative Stress in Transgenic Drosophila melanogaster. Archives of biochemistry and biophysics 301 : 34–40.
70. ParkerJD, ParkerKM, SohalBH, SohalRS, KellerL (2004) Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme lifespan. Proceedings of the National Academy of Sciences of the United States of America 101 : 3486–3489.
71. SpencerCC, HowellCE, WrightAR, PromislowDEL (2003) Testing an “aging gene” in long-lived Drosophila strains: increased longevity depends on sex and genetic background. Aging Cell 2 : 123–130.
72. RuanHY, WuCF (2008) Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proceedings of the National Academy of Sciences of the United States of America 105 : 7506–7510.
73. CareyJR, HarshmanLG, LiedoP, MüllerH-G, WangJ-L, et al. (2008) Longevity–fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell 7 : 470–477.
74. WuQ, BrownMR (2006) Signaling and function of insulin-like peptides in insects. Annu Rev Entomol 51 : 1–24.
75. HartfelderK (2000) Insect juvenile hormone: from “status quo” to high society. Brazilian Journal of Medical and Biological Research 33 : 157–177.
76. CoronaM, VelardeRA, RemolinaS, Moran-LauterA, WangY, et al. (2007) Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proceedings of the National Academy of Sciences 104 : 7128–7133.
77. BrennanCA, AndersonKV (2004) Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol 22 : 457–483.
78. RämetM, LanotR, ZacharyD, ManfruelliP (2002) JNK Signaling Pathway Is Required for Efficient Wound Healing in Drosophila. Developmental Biology 241 : 145–156.
79. WilliamsMJ (2007) Drosophila hemopoiesis and cellular immunity. The Journal of Immunology 178 : 4711–4716.
80. Schmid-HempelP (2003) Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society of London Series B: Biological Sciences 270 : 357–366.
81. GrozingerCM, FanYL, HooverSER, WinstonML (2007) Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honey bees (Apis mellifera). Molecular Ecology 16 : 4837–4848.
82. NiñoEL, TarpyDR, GrozingerC (2013) Differential effects of insemination volume and substance on reproductive changes in honey bee queens (Apis mellifera L.). Insect Molecular Biology 22(3): 233–44.
83. Blomquist GJ, Bagnères AG (2010) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press. 504 p.
84. RichardFJ, HoltH, GrozingerC (2012) Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera). Bmc Genomics 13 : 558.
85. KöhlerK, BrunnerE, GuanXL, BouckeK, GreberUF, et al. (2009) A combined proteomic and genetic analysis identifies a role for the lipid desaturase Desat1 in starvation-induced autophagy in Drosophila. Autophagy 5 : 980–990.
86. SelvaN, Cortés-AvizandaA, LemusJA, BlancoG, MuellerT, et al. (2011) Stress associated with group living in a long-lived bird. Biology Letters 7 : 608–610.
87. TannerCJ, SalaliGD, JacksonAL (2011) The ghost of social environments past: dominance relationships include current interactions and experience carried over from previous groups. Biology Letters 7 : 818–821.
88. CristollDA (1995) Costs of switching social groups for dominant and subordinate dark-eyed juncos (Junco hyemalis). Behavioral Ecology and Sociobiology 37 : 93–101.
89. MonninT (2006) Chemical recognition of reproductive status in social insects. Helsinki: Suomen Biologian Seura Vanamo, 1964-. Ann Zool Fennici 43 : 515–530.
90. VallesSM, PorterSD (2003) Identification of polygyne and monogyne fire ant colonies (Solenopsis invicta) by multiplex PCR of Gp-9 alleles. Insectes Sociaux 50 : 199–200.
91. OliverosJ (2009) VENNY. An interactive tool for comparing lists with Venn Diagrams 2007.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





