-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
Angelman syndrome (AS) is a severe neurodevelopmental disorder caused by maternal deficiency of the imprinted gene UBE3A. Individuals with AS suffer from intellectual disability, speech impairment, and motor dysfunction. Currently there is no cure for the disease. Here, we evaluated the phenotypic effect of activating the silenced paternal allele of Ube3a by depleting its antisense RNA Ube3a-ATS in mice. Premature termination of Ube3a-ATS by poly(A) cassette insertion activates expression of Ube3a from the paternal chromosome, and ameliorates many disease-related symptoms in the AS mouse model, including motor coordination defects, cognitive deficit, and impaired long-term potentiation. Studies on the imprinting mechanism of Ube3a revealed a pattern of biallelic transcription initiation with suppressed elongation of paternal Ube3a, implicating transcriptional collision between sense and antisense polymerases. These studies demonstrate the feasibility and utility of unsilencing the paternal copy of Ube3a via targeting Ube3a-ATS as a treatment for Angelman syndrome.
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004039
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004039Summary
Angelman syndrome (AS) is a severe neurodevelopmental disorder caused by maternal deficiency of the imprinted gene UBE3A. Individuals with AS suffer from intellectual disability, speech impairment, and motor dysfunction. Currently there is no cure for the disease. Here, we evaluated the phenotypic effect of activating the silenced paternal allele of Ube3a by depleting its antisense RNA Ube3a-ATS in mice. Premature termination of Ube3a-ATS by poly(A) cassette insertion activates expression of Ube3a from the paternal chromosome, and ameliorates many disease-related symptoms in the AS mouse model, including motor coordination defects, cognitive deficit, and impaired long-term potentiation. Studies on the imprinting mechanism of Ube3a revealed a pattern of biallelic transcription initiation with suppressed elongation of paternal Ube3a, implicating transcriptional collision between sense and antisense polymerases. These studies demonstrate the feasibility and utility of unsilencing the paternal copy of Ube3a via targeting Ube3a-ATS as a treatment for Angelman syndrome.
Introduction
Angelman syndrome (AS) is clinically manifested by features of intellectual and developmental disability, absence of speech, ataxic movement, epilepsy, and unique behaviors such as frequent laughter and fascination with water [1], [2]. Despite absence of effective treatment currently, therapeutic development for Angelman syndrome could be potentially optimistic, since patients with AS have overall normal development and brain architecture early in life.
Genetically, the disease is caused by deficiency of an E3 ubiquitin ligase termed UBE3A, which participates in many important neuronal functions such as synaptic development, signal transduction, and plasticity [3]. The gene encoding UBE3A is among a handful of human genes that are subject to genomic imprinting. In neuronal cells, it is highly expressed from the maternal allele, but silenced on the paternal allele. Disruption of the maternal allele, through genomic deletion, paternal uniparental disomy, imprinting defects, or point mutations, leads to the absence of UBE3A expression in neuronal tissues and hence Angelman syndrome. Indeed, in all cases of the disorder, at least one copy of paternal UBE3A is intact. One could speculate that by correcting the expression level of UBE3A via activating the silenced paternal allele, the disease might be treated.
Imprinted genes usually form clusters in the genome and are controlled by the imprinting center (IC). On human chromosome 15q11–q13, paternally expressed genes, including MAGEL2, NDN, SNRPN, SNORD115 and SNORD116, are critical genes for Prader-Wiili syndrome (PWS) and form an 2-Mb imprinting cluster together with the AS gene UBE3A. Although not fully understood, it is generally believed that the PWS/AS region is regulated by a bipartite imprinting center composed of PWS-IC, which activates genes located in its proximity via looping and direct interacting with them, and AS-IC, which suppresses PWS-IC by transcription-mediated DNA methylation [4], [5]. As a result of combined action of both PWS-IC and AS-IC, the paternal and maternal alleles of NDN and SNRPN show very distinct epigenetic patterns of DNA methylation and histone modifications [6], [7], [8], which define the paternal alleles as transcriptionally active and maternal alleles as transcriptionally silent.
Imprinting of UBE3A, however, is not associated with differential DNA methylation at the promoter region [9], [10]. Instead, it is regulated by its antisense RNA, UBE3A-ATS, which is expressed from the paternally inherited chromosome in the brain [11], [12]. As part of the large non-coding transcript (Shng14) initiated from the Snrpn promoter in mice [13], Ube3a-ATS expression is always negatively associated with Ube3a sense transcript. For example, when the Snrpn promoter was deleted, with or without the Prader-Willi syndrome imprinting center (PWS-IC), the Ube3a-ATS level was found to be reduced, coupling with significant up-regulation of paternal Ube3a [12], [14]. On the other hand, when maternal Ube3a-ATS was activated through replacement of the mouse imprinting center (IC) with the human one, or deletion of the putative AS-IC, maternal Ube3a was found to be repressed to some extent [15], [16]. Recently, by terminating Ube3a-ATS transcription in neuronally differentiated ES cells, we have showed that paternal Ube3a can be activated to a comparable level as maternal Ube3a [12], suggesting a direct role of Ube3a-ATS in suppressing paternal Ube3a.
In the present study, we continue evaluating Ube3a-ATS as a potential therapeutic target for treating Angelman syndrome. By characterizing a novel mouse model expressing the truncated form of Ube3a-ATS, we provide the first in vivo evidence that eliminating Ube3a-ATS is sufficient to restore Ube3a expression and improve the abnormal behaviors in the AS mouse model. Mechanisms underlying paternal Ube3a silencing are also studied, and a hypothesis of transcriptional collision between Ube3a and Ube3a-ATS is proposed.
Results
Truncation of Ube3a-ATS unsilences paternal Ube3a in vivo
In order to test if suppression of Ube3a-ATS alone is sufficient to unsilence the paternal allele of Ube3a, mice with the Ube3aATS-stop allele were generated by inserting the triple SV40 poly(A) cassette [12] in between Snord115 and Ube3a (chr7 : 66573289 NCBI37/mm9) (Figure 1). This design aims to prevent overlap between Ube3a and Ube3a-ATS and to minimize its effect on expression of the snoRNA clusters. The inserted cassette also contains a neomycin selection marker in the opposite transcriptional orientation to Ube3a-ATS to facilitate and enhance transcriptional termination. The mice were backcrossed to C57/BL6 background for six generations before subsequent expression and behavioral analysis.
Fig. 1. The transcriptional stop cassette was inserted downstream of Ube3a via homologous recombination. 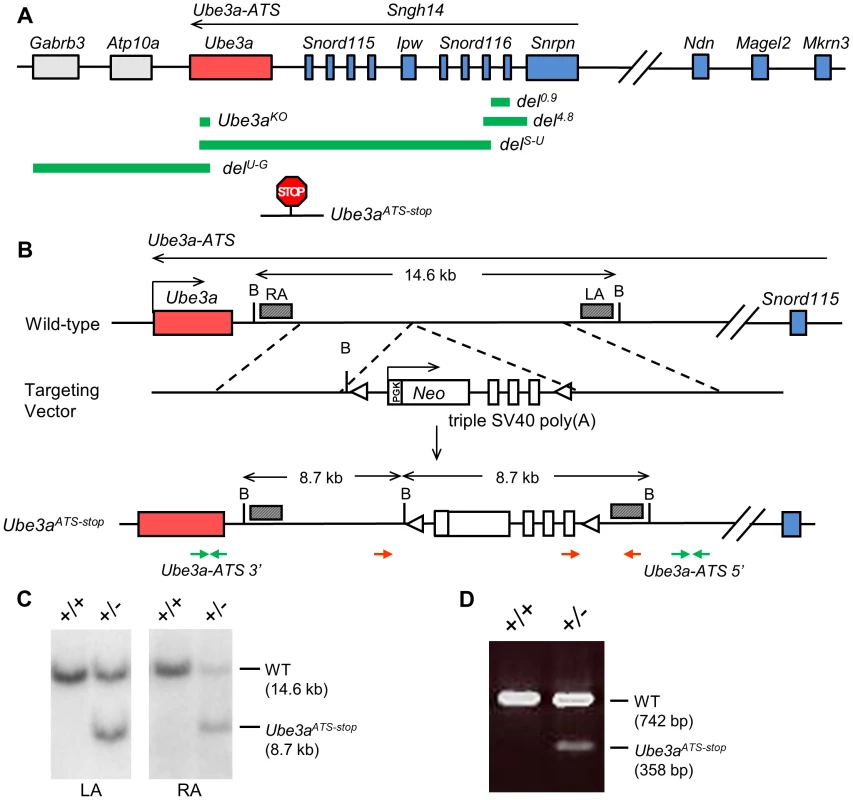
(A) The murine genomic structure of the PWS/AS region is shown (not to scale), with paternally expressed genes in blue, maternally expressed genes in red, and biallelically expressed genes in grey. Genomic regions deleted in the knock-out alleles of del0.9, del4.8, delS-U, delU-G, and Ube3aKO are marked by the green bars. Insertion site of the poly(A) cassette in Ube3aATS-stop allele is shown by the “STOP” sign. (B) The mouse wild-type locus is shown with enlargement of the region between Snord115 and Ube3a. The targeting vector contains the poly(A) cassette and the neomycin selection marker. The slanted boxes are left and right probes used in the Southern blot analysis. LA, left arm; RA, right arm; B, BglII; red arrows, genotyping primers; green arrows, q-PCR primers of Ube3a-ATS 5′ and 3′; triangles, loxP sites. (C) Recombinant ES cell clones were identified by Southern blot with BglII digesion. The WT allele gives a band of 14.6 kb and the knock-in allele giving a band of 8.7 kb, hybridizing to either LA or RA probe. (D) Chimeric mice were confirmed by multiplex PCR, with WT allele of 742 bp and mutant allele of 358 bp. We first determined the effect of the termination cassette on the expression level of Ube3a-ATS and other genes located in the imprinting cluster. The Ube3a-ATS level downstream of the insertion site (Ube3a-ATS 3′, green arrows in Figure 1B) was found to be significantly down-regulated by qPCR analysis when the stop allele was inherited paternally, while maternal inheritance of the allele has no effect (Figure 2A). To exclude the possibility that the PCR amplification site is spliced out instead of terminated, a custom designed strand-specific microarray was further performed as previously reported [12]. A significantly lower level of Ube3a-ATS was detected beyond the stop cassette insertion site (Figure S1). Expression of most other imprinted genes located nearby, including Mkrn3, Magel2, Snrpn, Snord116, and Ipw remained unchanged in both Ube3aATS-stop/+ and Ube3a+/ATS-stop mice (maternal genotype precedes the paternal genotype), indicating that the imprinting status of the PWS/AS region is not disrupted by the insertion. The level of Ndn was found to be approximately doubled in Ube3a+/ATS-stop mice compared to the other two genotypes. It is interesting that similar observation has been found in delS-U/0.9 mice previously [12], which expresses Ube3a-ATS at a lower level due to Snrpn promoter deletion. The reason for the observed up-regulation is unclear.
Fig. 2. Insertion of the poly(A) cassette into Ube3a-ATS leads to its transcriptional termination and unsilencing of paternal Ube3a. 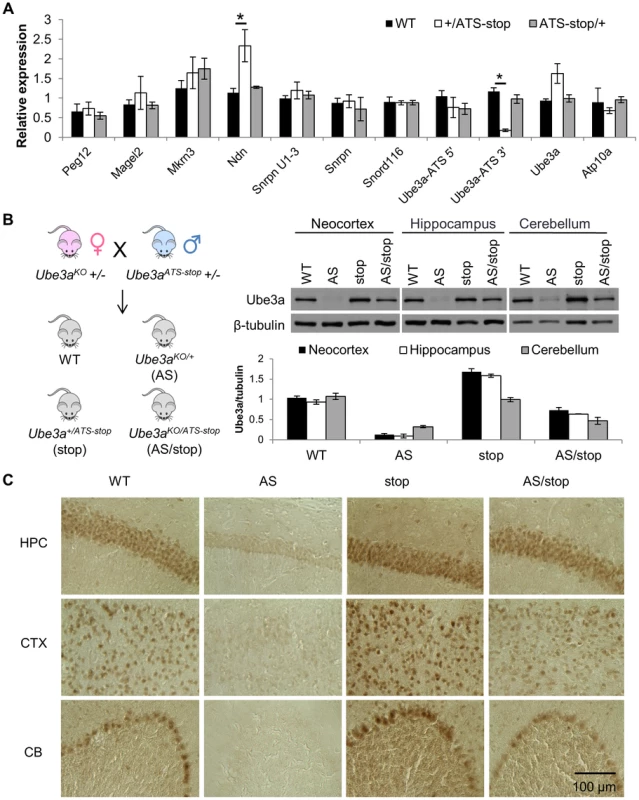
(A) The expression profile of genes in the PWS/AS region was assessed by qRT-PCR in cortices from WT mice and mice inheriting Ube3aATS-stop maternally (ATS-stop/+) or paternally (+/ATS-stop). One set of primers were designed to target Ube3a-ATS at loci 5′ and another 3′ to the insertion site. Gapdh is used as the internal control. N = 3 per genotype group. Data are averages ± standard error of means (SEM). *p<0.05. (B) Male Ube3aATS-stop heterozygous mice were bred with female Ube3aKO heterozygous mice to generate progeny of four different genotypes, WT, Ube3a+/ATS-stop (stop), Ube3aKO/+ (AS), and Ube3aKO/ATS-stop (AS/stop). Western blot with anti-Ube3a was performed in neocortex, hippocampus, and cerebellum from these mice. The protein level of Ube3a was normalized to β-tubulin. N = 3 mice per genotype group. Data are averages ± SEM. (C) Brain sections from WT, AS, stop, and AS/stop mice were stained with anti-Ube3a. Representative images from CA2 region of hippocampus (HPC), layer II/III of cerebral cortex (CTX), and cerebellum (CB) are shown. Ube3a mRNA is doubled in the Ube3a+/ATS-stop mice, suggesting that paternal Ube3a may be unsilenced. To confirm this, male mice heterozygous for Ube3a-ATSstop were crossed with female mice heterozygous for Ube3aKO [17] (C57/BL6 background), which is a constitutive Ube3a knock-out allele (Figure 1 and 2B). In the progeny, littermates of wild-type (WT), Ube3aKO/+ (AS), Ube3a+/ATS-stop (stop), Ube3aKO/ATS-stop (AS/stop) were compared. In the AS/stop mice, Ube3a protein was found to be activated to ∼70% of the WT level in neocortex, ∼60% in hippocampus, and ∼50% in cerebellum. The incomplete activation may be due to leaky termination of Ube3a-ATS, as about 20% of Ube3a-ATS can still be detected in Ube3a+/ATS-stop mice (Figure 2A). Immunostaining with anti-Ube3a showed that in AS/stop mice, paternal Ube3a is expressed in most brain regions, including all layers of neocortex, CA1-3 and dentate gyrus of hippocampus, and Purkinje neurons of cerebellum (Figure 2C and S2). Its expression pattern is very similar to that of maternal Ube3a in the WT mice. The incomplete unsilencing of paternal Ube3a may be due to a smaller number of Ube3a positive neurons, or a lower expression level in each single neuron, or more likely a combination of both. Male mice heterozygous for Ube3aATS-stop were also crossed with female mice heterozygous for Ube3aYFP [18], which carries the C-terminal YFP tag (Figure S3). Since Ube3a-YFP is expressed as a fusion protein with a higher molecular weight, it can be easily distinguished from wild-type Ube3a protein by western blot. Inheritance of Ube3aATS-stop from the paternal side leads to biallelic expression of Ube3a, while in contrast, maternal inheritance of the allele had no effect.
Finally, the effect of Ube3aATS-stop on paternal Ube3a was compared with the other two alleles of del4.8 and del0.9. The allele of del4.8 removes 4.8 kb of Snrpn promoter and functions as a PWS-IC deletion, while the allele of del0.9 removes 0.9 kb of Snrpn promoter and is equivalent to a Snrpn promoter deletion (Figure 1) [19]. After crossing with female mice carrying genomic deletion over the Snrpn-Ube3a region (delS-U/+, Figure 1) [20], the mRNA and protein levels of paternal Ube3a were found to be the highest in delS-U/4.8 mice, intermediate in delS-U/Ube3aATS-stop mice and the lowest in delS-U/0.9 mice (Figure S4A, B). Interestingly, such order is in accordance with the suppression level of Ube3a-ATS (Figure S4C). Plotting of paternal Ube3a against Ube3a-ATS fits into the curve of exponential decay (R2 = 0.997, Figure S4D), suggesting that suppression of paternal Ube3a by Ube3a-ATS is “dose-dependent”.
Unsilenced paternal Ube3a ameliorates phenotypic defects in the Angelman syndrome mouse model
We next tested whether inheritance of Ube3aATS-stop paternally can correct the phenotypic defects in the Angelman syndrome (AS) mouse model. To address this, male Ube3aATS-stop heterozygous mice were crossed with female Ube3aKO heterozygous mice [17] (C57/BL6 background) and the littermates of WT, Ube3aKO/+ (AS), Ube3a+/ATS-stop (stop), Ube3aKO/ATS-stop (AS/stop) were studied for various AS-related phenotypes.
Obesity is associated with a small portion of AS patients [2], [9] and constantly observed in many Angelman syndrome mouse models [15], [21], [22]. Ube3aKO/+ mice become overweight starting from three month of age, in both males and females (Figure 3A, p(WT vs. AS)<0.01 for 4, 5, 6 months of age, two-way ANOVA of repeated measures). Activation of paternal Ube3a in the AS/stop mice completely reversed the obese phenotype (p(AS vs. AS/stop)<0.05 for 4, 5, 6 months of age).
Fig. 3. Expression of paternal Ube3a improves phenotypic defects in the AS mouse model. 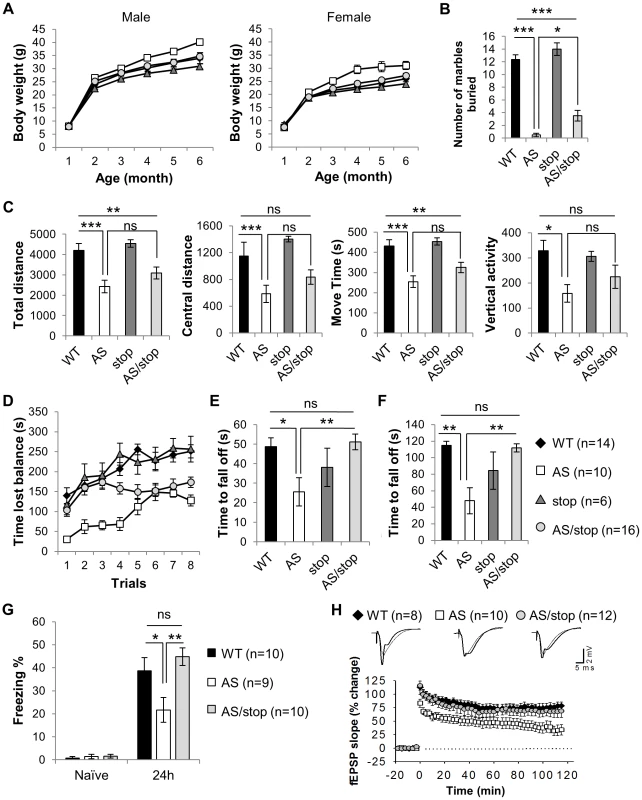
Littermates of WT (black diamonds), AS (white squares), stop (dark gray triangles), and AS/stop (light grey circles) were studied for AS-related phenotypes, including development, behaviors, and electrophysiology. (A) Growth curve of male and female mice. (B) Marble burying test. (C) Open field assay. (D) Accelerating rotarod. (E) Wire hanging test. (F) Dowel test. (G) Contextual fear conditioning. (H) Long-term potentiation. For the growth curve, the number of animals measured are WT(male) = 9, WT(female) = 10, AS(male) = 9, AS(female) = 5, stop(male) = 7, stop(female) = 5, AS/stop(male) = 9, AS/stop(female) = 11. For the rest experiments, both males and females were tested. Data are averages ± SEM. Error bars in (A) are very tight and are obscured by the symbols. Labels of Black diamonds are overlaid by other symbols. *p<0.05; **p<0.01; ***p<0.001. The marble burying test measures repetitive behavior as potentially analogous to an autistic phenotype. Interestingly, AS mice were found to be dramatically impaired in performing this task (Figure 3B, WT: 12.36±0.75, AS: 0.50±0.27, p(WT vs. AS)<0.001, one-way ANOVA with Newman-Keuls post-hoc test). AS/stop mice showed a slight but significant improvement over AS mice (AS/stop: 3.50±0.84, p(AS vs. AS/stop)<0.05).
Hyperactivity with short attention span is a pronounced problem in young children with AS. Different from humans, AS mice have been reported to display hypoactivity [22], [23]. When placed in an open field and allowed for exploration, AS mice showed significantly lower activity level as measured by total distance and central distance traveled, movement time, and vertical activity (Figure 3C). A slight trend of improvement was consistently observed in the AS/stop mice for these parameters. However, the difference between AS mice and AS/stop mice does not reach statistical significance (one-way ANOVA with Newman-Keuls post-hoc test).
Ataxia and movement difficulty is one of the most severe defects in human AS patients and AS mouse models [17]. AS mice display severe motor coordination defects during the accelerating rotarod test (Figure 3D, p(WT vs. AS)<0.05 for all eight trials, two-way ANOVA of repeated measures). AS/stop mice show restoration in the first few trials of accelerating rotarod, although they fail to improve in later trials (p(AS vs. AS/stop)<0.05 for trial 1–4). They also show full restoration of other motor defects during wire hanging test and dowel test, indicating a significant improvement of their motor coordination skills (Figure 3E, F, and Figure S5, p(AS vs. AS/stop)<0.01 for wire-hanging test and <0.001 for dowel test, one-way ANOVA with Newman-Keuls post-hoc test). It is noted that maternal inheritance of the Ube3aATS-stop allele does not affect the performance of the mice in all three motor tests (Figure S6), suggesting that the presence of neomycin cassette has minimal or no effect on motor coordination in mice.
Individuals with AS are frequently affected with specific cognitive deficits [1], [2] and Ube3aKO/+ mice are known to have learning and memory problems [17]. During a fear conditioning test, AS mice exhibited significantly less freezing behavior than did WT littermates (Figure 3G, WT: 38.66±5.78%, AS: 21.68±5.35%, p(WT vs. AS)<0.05, one-way ANOVA with Newman-Keuls post-hoc test). Remarkably, the freezing behavior displayed in the AS/stop mice is comparable to the WT mice, suggesting that long-term memory is fully restored (AS/stop: 44.82±3.85%, p(AS vs. AS/stop)<0.01).
Lastly, we studied long-term potentiation (LTP) at Schaffer collateral–CA1 synapses, using high-frequency stimulation as the LTP-inducing protocol [17], [24]. As expected, this protocol induced a stable LTP in WT slices but caused a decaying LTP in AS slices (Figure 3H, LTP at 120 min, WT: 78±6.8%, AS: 33±8.6%, p(WT vs. AS)<0.01, one-way ANOVA). Notably, the expression of paternal Ube3a reverses the LTP deficits (AS/stop: 63±9.1%, p(AS vs. AS/stop)<0.05). The LTP rescue in AS/stop slices cannot be attributed to abnormal basal synaptic transmission, since the relation of fiber volley versus stimulation intensity, initial slope of field EPSPs versus afferent volley size, and paired pulse facilitation were unaltered in these slices (Figure S7).
Paternal Ube3a is transcriptionally active
In developing therapies for treating AS via activating paternal UBE3A, it is important to understand the molecular mechanism underlying genomic imprinting of Ube3a. Promoters of both the paternal and maternal UBE3A remain unmethylated in human brains [9], [10], [25], therefore DNA methylation at the promoter cannot account for silencing of paternal UBE3A. In order to look for parent-of-origin epigenetic markers that may account for UBE3A imprinting, we first analyzed histone modifications of H3K4 trimethylation (H3K4me3) in human cerebellum tissues by ChIP-on-chip experiment (Figure 4A). In contrast to healthy controls, a PWS patient with a paternal class II deletion (common 4 Mb deletion from break point 2 to 3) lacked H3K4me3 at the SNRPN promoter, suggesting that this modification is paternal specific, as previously reported [8]. However, in AS patients with maternal class II deletion, the peak of H3K4me3 was still present at the UBE3A promoter, and was indistinguishable from control and PWS samples. Therefore H3K4me3 is equally distributed between the paternal and maternal promoters of UBE3A in human cerebellum, regardless of the mono-allelic expression pattern. This conclusion from human was later supported by a recent ChIP-seq study in mice [26], in which equal enrichment of H3K4me3 and H3K27 trimethylation at both parental promoters of Ube3a was observed.
Fig. 4. Both promoters of Ube3a are enriched with histone H3 lysine 4 trimethylation (H3K4me3), bound by RNA polymerase II, and actively transcribed. 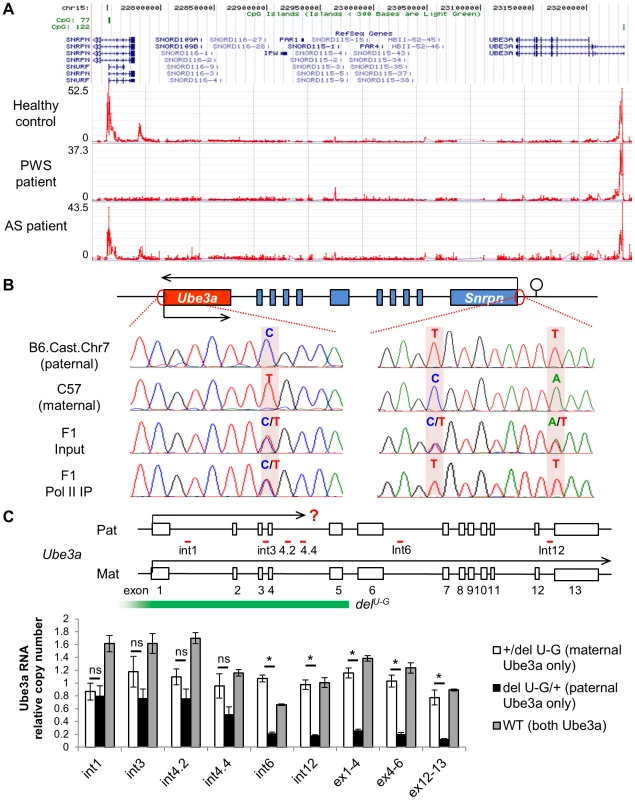
(A) H3K4me3 of the PWS/AS region was measured in human postmortem cerebellum from healthy control, PWS, and AS patients by ChIP-on-chip. The relative enrichment (signal intensity of IP vs. input) was plotted over the genomic coordinates. (B) Binding of RNA polymerase II, a key component of the PIC complex, to the Ube3a and Snrpn promoters was analyzed by ChIP followed by PCR and sequencing. Cerebral cortices from F1 hybrid of C57 and Cast.Chr7 mice were used for the analysis. SNPs between the two lines were used for allelic identification. (C) Relative expression level of paternal versus maternal Ube3a was analyzed in +/delU-G, delU-G/+, and WT mice. The copy number of maternal Ube3a RNA in +/delU-G mice was set equal to 1 and the relative expression level of paternal Ube3a in delU-G/+ mice or total Ube3a in WT mice was calculated. Pre-mRNA of Ube3a was measured by strand-specific qRT-PCR using primers located in intronic regions (int1, int3, int4.2, int4.4, int6, and int12), while mature mRNA was measured using primers spanning exon junctions (ex1-4, ex4-6, and ex12-13). Exons of mouse Ube3a are displayed as white boxes and deletion region of delU-G is marked by the green bar. The locations of primers directed to introns are shown with red bars. Pat: paternal; Mat: maternal. N = 3 mice per genotype group. Data are averages ± SEM. *p<0.05. We next measured binding of the transcription preinitiation complex (PIC) at the Ube3a promoter by chromatin immunoprecipitation (ChIP). The PIC is a large protein complex composed of RNA polymerase II, TATA binding protein (TBP), TFIIB, and many other proteins assembled at the promoter of active genes. ChIP with antibody against RNA polymerase II was performed in brain samples of F1 hybrid of C57 (female) crossed with B6.Cast.Chr7 (male), which carries Mus. musculus castaneus chromosome 7 on the Mus. musculus domesticus C57BL/6 background. Single nucleotide polymorphisms (SNPs) between the two lines allow detection of parental specific alleles. In contrast to the Snrpn promoter, from which only the transcriptionally active paternal allele was precipitated, both parental alleles of the Ube3a promoter can be detected in the same IP fraction (Figure 4B). ChIP in F1 hybrids of the reciprocal cross and ChIP with anti-TFIIB and anti-TBP revealed the same result (Figure S8). Altogether, the results suggest that the PIC is able to be properly assembled at the promoter of both paternal and maternal Ube3a alleles, regardless of theirs different expression status.
Since paternal Ube3a shows multiple features of an active gene as we demonstrated above, we considered the hypothesis that it is actually transcriptionally active despite the absence of mature mRNA. To test this, a mouse model carrying a deletion from Ube3a to Gabrb3 (delU-G, Figure 1A and 4C) was used [27]. Since the deletion covers the promoter of Ube3a, only maternal Ube3a RNA is present in the paternal deletion +/delU-G mice and only paternal Ube3a RNA is present in the maternal deletion delU-G/+ mice. We set the RNA copy number of maternal Ube3a in +/delU-G mice equal to 1 across different portions of the gene and used it as the reference to calculate the relative RNA copy number of paternal Ube3a in delU-G/+ mice or total Ube3a in WT mice. Consistent with the known mono-allelic expression pattern, the mature mRNA of paternal Ube3a (quantified by qPCR using primers spanning exon-exon junction) is about 0.2 copy at both the 5′ - and 3′-portions in delU-G/+ mice (Figure 4C, ex1-4, ex4-6, and ex12-13). However, when pre-mRNA of paternal Ube3a was quantified (by strand-specific qRT-PCR [28] using tagged primers directed to introns), it is around one copy at the 5′ portion of Ube3a (black bars of int1, int3, and int4.2 in Fig. 4C) and drops to about 0.2 copy as the primers are moved to the 3′ portion of Ube3a (black bars of int4.4, int6, and int12 in Fig. 4C). Altogether, our data supports a model that paternal Ube3a is transcribed at a comparable level as maternal Ube3a from the promoter, but later becomes suppressed during the process of transcription elongation.
Ube3a-ATS was localized to its transcription site
Airn and Kcnq1ot1, two antisense RNAs playing a regulatory role in their respective imprinting cluster, are known based on FISH analysis to be localized around the transcribed regions [29], [30], consistent with their functional roles. Ube3a-ATS has been shown to be localized exclusively to the nucleus [12], [31], but the subnuclear detail was unknown. To address this question, a combined RNA/DNA FISH was performed in mouse brain sections. Signals of Ube3a-ATS form a single bright dot inside the nucleus (Figure 5A) and can be observed in multiple regions throughout the brain including olfactory bulb, neocortex, hippocampus, cerebellum, and hindbrain. Interestingly, the signal co-localizes with only one of the two foci formed by Ube3a DNA signal (Figure 5B). Such co-localization is not random overlapping between the DNA and RNA probes because Ube3a-ATS does not overlap with the control DNA probe (targeting an irrelevant gene on mouse chromosome 4). Therefore, similar to Airn and Kcnq1ot1, Ube3a-ATS remains located proximate to its transcription site after it being synthesized.
Fig. 5. Ube3a-ATS localizes to its transcription site in the nucleus. 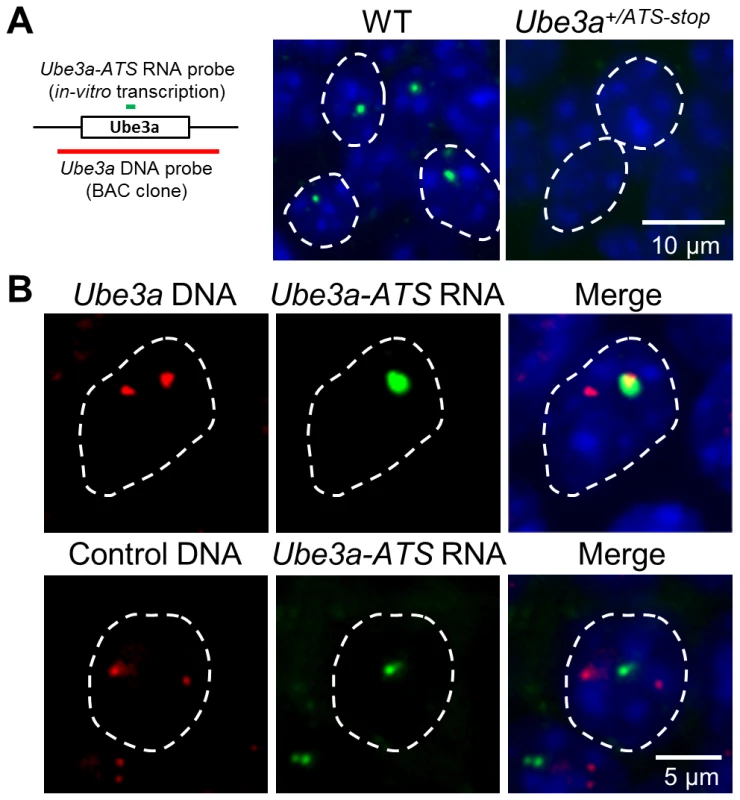
(A) Localization of Ube3a-ATS RNA was detected by FISH analysis (probe position indicated by the green bar). The signal forms a single dot in the nucleus and is absent in the Ube3a+/ATS-stop mice, indicating the specificity of the analysis. (B) With combined RNA/DNA FISH analysis, signal of Ube3a-ATS RNA was found to be co-localized with one of the two foci formed by Ube3a DNA (probe position indicated by the red bar, from chromosome 7qC), but not by control DNA targeting irrelevant gene on chromosome 4qD. White dashed lines indicate the boundaries of the nuclei. Discussion
Patients with Angelman syndrome suffer from developmental delay, speech impairment, and epilepsy. Therapies for AS are limited and focus mainly on symptomatic management [2]. Recently, topoisomerase inhibitors have been identified as the first compounds to successfully unsilence paternal Ube3a in mice [32], [33]. In the current research, we investigated a potential therapeutic strategy by activation of the silenced paternal allele of UBE3A via suppressing its antisense RNA. Previous studies have defined Ube3a-ATS as the negative regulator of Ube3a imprinting [12], [14]. However, it was unknown if depletion of Ube3a-ATS without modulating other epigenetic factors is sufficient to activate paternal Ube3a. This question is crucial in determining whether knock-down of Ube3a-ATS is a suitable strategy for treating AS. By generating a mouse model with Ube3a-ATS being prematurely terminated, we observed unsilencing of Ube3a in multiple brain regions, implying that the antisense RNA plays a regulatory role in modulating Ube3a imprinting. We then compared mice which express paternal Ube3a on the maternal Ube3a knock-out background (AS/stop) with AS and WT mice. The AS/stop mice exhibit complete reversal of obesity, motor tests of wire-hanging and dowel walking, fear conditioning defect, and plasticity-related electrophysiology. They also display slight but significant improvement in the tests of accelerating rotarod and marble burying. Therefore, our research confirmed the clinical benefit of activating paternal Ube3a in treating Angelman syndrome and provided a mouse model as the positive control for future drug testing. Given the conservation of the PWS/AS region between mouse and human, activation of paternal UBE3A through inhibiting UBE3A-ATS expression/transcription should be a promising strategy for developing AS therapy.
One important question in activating paternal UBE3A is how much UBE3A protein is needed to achieve phenotypic improvement in AS patients. In the mouse model of AS/stop, we observed some phenotypic reversal, such as obesity, and cognitive deficits. However, their performance during accelerating rotarod and marble bury test is only partially or moderately improved and their decreased locomotive activity is not restored. This may be due to the incomplete activation of paternal Ube3a, which is quantified to be 50–70% of the WT level in different parts of the brain by western blot. Some of the behavioral phenotypes might be more sensitive to the protein level of Ube3a and therefore are more difficult to reverse. Another possibility is the interference from the remaining neomycin cassette. However, paternal inheritance of the cassette on the WT background does not affect mouse behaviors, and maternal inheritance of the cassette does not change Ube3a expression and rotarod performance in mice, suggesting that the presence of the selection marker has no or minimal effect on Ube3a function. Among the human UBE3A mutations that have been reported so far, there is a striking preponderance of frameshift and nonsense mutations [34]. It is possible that individuals with less pathogenic missense mutation in UBE3A display some, but not all, clinical features associated with AS and thus are excluded from AS diagnosis and research. A patient with C21Y missense mutation located outside the HECT domain of UBE3A has been reported to have a less classical phenotype [35], suggesting that partial activity of UBE3A may be beneficial.
Another relevant issue is to understand the molecular mechanism underlying UBE3A imprinting. Interestingly, several pieces of evidence have suggested that the paternal allele of UBE3A/Ube3a is transcriptionally active. For example, the promoter of paternal UBE3A is unmethylated [9], [10], [36], modified with active histone markers (Figure 4A), and bound with transcription pre-initiation factors (Figure 4B). Indeed, Ube3a pre-mRNA can be detected equally from the 5′-portion of both paternal and maternal alleles in mice (Figure 4C). Therefore, paternal Ube3a is transcriptionally active and its suppression may occur during the process of transcription elongation. The previous observation of “biallelic” expression pattern at the 5′-portion of mouse Ube3a by SNP analysis is consistent with this conclusion [37].
As demonstrated in this and many other studies, Ube3a-ATS has a direct role in silencing paternal Ube3a. However the detailed mechanism is unclear. Research on the other two imprinted ncRNA Airn and Kcnq1ot1 has raised two different working models, promoter occlusion and RNA-directed targeting. When silencing the overlapping gene in embryonic tissues, Airn transcribes through the Igf2r promoter and precludes binding of RNA polymerase II to the Igf2r promoter [38]. In contrast, when silencing the respective non-overlapping genes in extraembryonic tissues, the RNA product of Kcnq1ot1 or Airn will bind to trans-acting protein factors and induce repressive higher-order chromatin changes [29], [39], [40], [41]. Can either of the two models be applied to Ube3a-ATS? Promoter occlusion is unlikely to be the cause of Ube3a imprinting since paternal Ube3a promoter is transcriptionally active. Components of PIC such as RNA polymerase II, TBP, and TFIIB are found to bind paternal and maternal Ube3a equally. Currently, it is unknown whether the RNA product of Ube3a-ATS is essential in mediating Ube3a imprinting. However, Ube3a-ATS has very low homology between mouse and human, and is quickly degraded [12], implying a low functional importance of the RNA product.
Here we proposed an alternative hypothesis of transcriptional collision as the mechanism for Ube3a-ATS mediated Ube3a imprinting (Figure 6). Our previous strand-specific microarray data revealed a significant decrease of Ube3a-ATS RNA signal around intron 4 of Ube3a, although the transcript remains detectable until ∼40 kb upstream of Ube3a promoter [12]. Interestingly, this is around the same region where the pre-mRNA level of paternal Ube3a becomes suppressed. Therefore, on the paternal chromosome, Ube3a sense and antisense RNAs are transcribed head-to-head at a relatively high level until the polymerases reach intron 4, where both drop to a lower level. These findings are similar to what has been described for transcriptional collision occurring during convergent transcription [42]. Such collision will result in stalling, dissociation of both polymerases, and abortive transcription of both. Research in budding yeast has demonstrated both in vitro and in vivo that convergent transcription will result in collision of the two opposing polymerases [42], [43]. The collision event can also be detected by atomic force microscopy in vitro when two promoters are aligned convergently on a linear DNA template [44]. Currently, we still lack direct evidence to support the Ube3a transcriptional collision hypothesis. It will be necessary to test it in the future by mapping RNA polymerase II stalling sites along Ube3a using GRO-seq or NET-seq technology [45], [46].
Fig. 6. Transcriptional collision hypothesis of Ube3a imprinting. 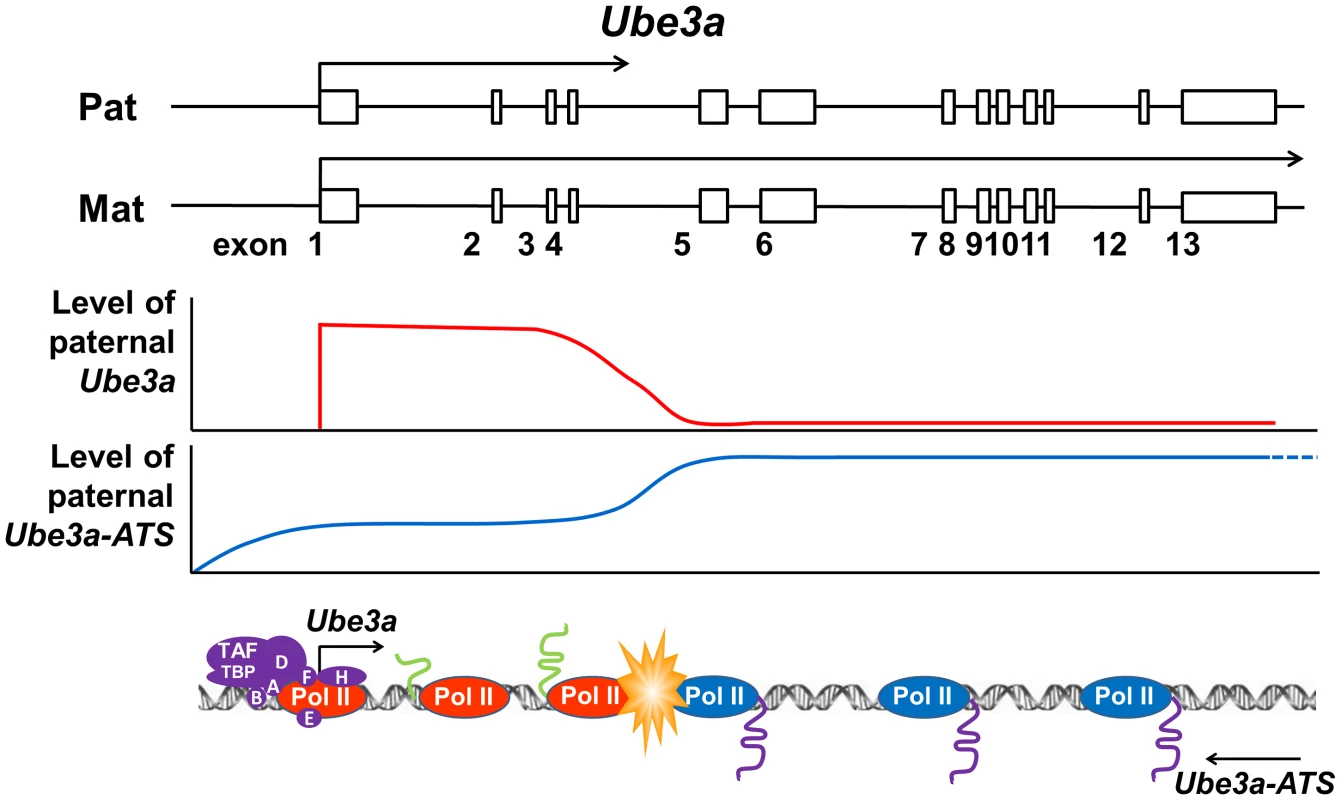
Paternal Ube3a was found to be transcribed at a similar level as maternal Ube3a at the 5′ portion of the gene. The levels of both paternal Ube3a sense and antisense transcripts were found to be decreased around intron 4 of Ube3a. Based on these observations, a hypothesis of transcriptional collision is proposed in which the two opposing polymerases of Ube3a and Ube3a-ATS on the paternal chromosome collide into each other around intron 4 of Ube3a. The collision causes the polymerases to stall or fall off from the templates, thereby completely or partially terminating the transcription of Ube3a and Ube3a-ATS. The incomplete sense transcript of Ube3a is then degraded and unable to be processed into full-length mature mRNA. Materials and Methods
Ethics statement
All animal procedures were performed in accordance with NIH guidelines and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). All human studies were performed in accordance with NIH guidelines and approved by the Baylor College of Medicine Institutional Review Board (IRB).
Generation of Ube3aATS-stop mice
The insertion cassette composed of SV40 triple poly(A) signal and neomycin selection marker was inserted downstream of Ube3a by gene targeting in wild-type AB2.2 ES cells. After microinjecting into blastocysts of C57/BL6 mice, high percentage male agouti chimeras were obtained and germline transmission was established. The lines were then backcrossed to C57/BL6 mice for more than six generations. PCR genotyping was developed with TS-F (TTCCCAGTGCTGAGACTAAAG), TS-R (CCACAATCTGAA-CCCTAAAAC) and SV40-R (AAAAGGGACAGGATAAGTATG).
RNA extraction and qRT-PCR
Total RNA was prepared with miRNeasy Mini Kit (Qiagen, Valencia, CA). On-column DNase treatment was performed for all the samples. The cDNA was generated using 0.2–1 µg of total RNA with SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA), and qRT-PCR was performed using Applied Biosystems StepOnePlus Real-Time PCR System and SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA). Primers used are listed in Table S1.
Western blot
Western blot against Ube3a and β-tubulin was performed as previously described [12]. Quantification was performed based on densitometry with ImageJ.
Histology and immunohistochemistry
Tissue preparation and immunohistochemistry were performed by Neuropathology Core of Baylor College of Medicine, as previously described [47]. Immunostaining was carried out with Rabbit polyclonal anti-Ube3a (1∶500, A300-352A, Bethyl Laboratories, Montgomery, TX) and horseradish peroxidase conjugated goat anti-rabbit (1∶200, Dako Inc., Carpinteria, CA). The localization of the antibody was visualized using diaminobenzidine (DAB, 0.5 mg/ml, Vector Laboratories Inc., Burlingame, CA) as a chromogen.
Behavioral tests
A battery of behavioral tests was performed using a protocol previously described and used in Behavioral Core facilities at Baylor College of Medicine [27], [48]. A detailed protocol for each test is described in Text S1. Tests start when the mice are 2 month-old in both males and females and the order of tests are kept the same as listed in the supplementary material. The interval between two tests is one week, except wire hanging, dowel tests and rotarod were performed in two consecutive days.
Electrophysiology
Horizontal hippocampal slices (350 µm) were cut with a Leica (VT 1000S) vibratome (Buffalo Grove, IL) from brains of WT, AS and AS/stop mice in 4°C artificial cerebrospinal fluid (ACSF) and kept in ACSF at room temperature for at least one hour before recording, as previously described [49], [50]. Slices were maintained in an interface-type chamber perfused (2–3 ml/min) with oxygenated ACSF (95% O2 and 5% CO2) containing in mM: 124 NaCl, 2.0 KCl, 1.3 MgSO4, 2.5 CaCl2, 1.2 KH2PO4, 25 NaHCO3, and 10 glucose. Bipolar stimulating electrodes were placed in the CA1 stratum radiatum to excite Schaffer collateral and commissural fibers. Field EPSPs were recorded at 30–31°C, with ACSF-filled micropipettes. The recording electrodes were placed in the stratum radiatum and the intensity of the 0.1 ms pulses was adjusted to evoke 40–50% of maximal response. A stable baseline of responses at 0.033 Hz was established for at least 20 min. Tetanic LTP was induced by using two 1 s, 100 Hz tetani, 20 s apart at baseline stimulus intensity, as previously described [17].
Human postmortem brain samples
Postmortem brain tissues from control, PWS, and AS individuals were obtained from NICHD Brain and Tissue Bank for Developmental Disorders from University of Maryland School of Medicine.
Chromatin immunoprecipitation and microarray analysis of human tissues
ChIP-on-chip analysis was performed as previously described [51]. Immunoprecipitation was performed with Protein A Dynabeads (Invitrogen) coated with normal rabbit IgG or anti-H3K4me3 antibodies (17-614, Millipore) according to manufacturer's instructions. Precipitated and input DNA was amplified with GenomePlex Complete Whole Genome Amplification (WGA) kit (Sigma, St. Louis, MO) and labeled with Cy3 (input DNA) or Cy5 (ChIP DNA) using BioPrime Array CGH Genomic Labeling System (Invitrogen). The DNA was then applied to a custom designed human chromosome 15q11.2–q12 focused array (Agilent, Santa Clara, CA), with genomic tilling probes covering regions from MAGEL2 to GABRB3 (chr15 : 21,361,151–25,487,147, genome build NCBI36/hg18) in the 4X44k format. Hybridization, wash and scanning were performed according to manufacturer's instructions. The image files were processed with Agilent Feature Extraction software using protocol CGH-v4_95_Feb07 and further analyzed with Agilent G4477AA ChIP Analytics 1.3 software.
ChIP-PCR and SNP identification
Brain tissues of 50 mg from newborn mice was chopped into fine pieces, crosslinked with 1% formaldehyde in DMEM and lysed in SDS lysis buffer (50 mM Tris, 10 mM EDTA, 1% SDS). The lysate was then sonicated (Fisher Scientific 500 Sonic Dismembrator) and centrifuged. The supernatant was collected and combined with IP buffer (2 mM Tris, 15 mM NaCl, 0.2 mM EDTA, 0.1% Triton X-100, 1× proteinase inhibitor). Immunoprecipitation was then performed with Protein G Dynabeads (Invitrogen) coated with anti-pol II (05-623, Millipore), anti-TFIIB (sc-225, Santa Cruz Biotechnology), or anti-TBP (MAB3658, Millipore) overnight.
Immunoprecipitated DNA was PCR amplified with Snrpn or Ube3a promoter primers, purified with MinElute PCR purification kit (Qiagen) and analyzed by Sanger sequencing to identify allelic SNPs. Alternatively, unpurified PCR products were digested with BsaI for the Snrpn promoter or BbsI for the Ube3a promoter, and analyzed by eletrophoresis on a 1.5% agarose gel.
Strand-specific qRT-PCR
Total RNA of 200 ng from cortices of newborn mice was used as the input in the analysis. The cDNA synthesis was performed using tagged gene-specific primers in the RT reaction to detect Ube3a pre-mRNA in a strand-specific manner [28], and then amplified in the SYBR Green q-PCR system using the tag as the reverse primer and locus specific forward primer. All primers used are listed in Table S2.
DNA/RNA combined fluorescent in-situ hybridization (FISH)
Tissue preparation and RNA FISH were carried out by RNA In-Situ Hybridization Core at Baylor College of Medicine as previously described [52]. Briefly, brains of adult mice were embedded in O.C.T., fresh frozen, and sectioned sagittally at 25 µm thickness. After paraformaldehyde fixation, acetylation, and dehydration, the slides were assembled into flow-through hybridization chambers and placed into a Tecan (Mannedorf, Switzerland) Genesis 200 liquid-handling robot. The DIG labeled RNA probes were prepared with in-vitro transcription and corresponded to chr7 : 66,530,657–66,531,391(NCBI37/mm9). Primers for DNA template synthesis are SP6-Ube3a-int5.1F ATTTAGGTGACACTATAGAAGCGAAGATGAGTCAG-TTTGGTTTT and T7-Ube3a-ex6.1R TAATACGACTCACTATAGGGAGATTCTGAGTCTTCTTCCATA-GC). The T7 promoter was used to generate Ube3a-ATS probe. Hybridized probes were detected by a dual amplification strategy and visualized by Alexa488 conjugated streptavidin [52].
After RNA FISH, the slides were washed in 2XSSC at 37°C for 15 min, dehydrated in 70%, 85%, 95% ethanol at −20C for 2 min each and denatured in 70% formamide/2XSSC at 70°C for 2 min. After washing with 70%, 85% and 100% ethanol, the slides were air-dried before hybridization with the DNA FISH probe. The probe was prepared from Ube3a BAC clone bMQ311i10 (Source BioScience, UK) or Lepre1 (chr 4) with FISH Tag DNA Red Kit (Invitrogen) and hybridized to the sections at 37°C overnight. The slides were washed in 50% formamide/2X SSC solution twice for 8 min at 42°C, once in 2XSSC for 8 min at 37°C and mounted with SlowFade Gold antifade reagent (Invitrogen).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA). One-way ANOVA with Newman–Keuls post-hoc test and two-way ANOVA with repeated measures were used.
Supporting Information
Zdroje
1. DagliA, BuitingK, WilliamsCA (2012) Molecular and Clinical Aspects of Angelman Syndrome. Mol Syndromol 2 : 100–112.
2. WilliamsCA, DriscollDJ, DagliAI (2010) Clinical and genetic aspects of Angelman syndrome. Genet Med 12 : 385–395.
3. MabbAM, JudsonMC, ZylkaMJ, PhilpotBD (2011) Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci 34 : 293–303.
4. RabinovitzS, KaufmanY, LudwigG, RazinA, ShemerR (2012) Mechanisms of activation of the paternally expressed genes by the Prader-Willi imprinting center in the Prader-Willi/Angelman syndromes domains. Proc Natl Acad Sci U S A 109 : 7403–7408.
5. SmithEY, FuttnerCR, ChamberlainSJ, JohnstoneKA, ResnickJL (2011) Transcription is required to establish maternal imprinting at the Prader-Willi syndrome and Angelman syndrome locus. PLoS Genet 7: e1002422.
6. JayP, RougeulleC, MassacrierA, MonclaA, MatteiMG, et al. (1997) The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nat Genet 17 : 357–361.
7. GlennCC, PorterKA, JongMT, NichollsRD, DriscollDJ (1993) Functional imprinting and epigenetic modification of the human SNRPN gene. Hum Mol Genet 2 : 2001–2005.
8. XinZ, AllisCD, WagstaffJ (2001) Parent-specific complementary patterns of histone H3 lysine 9 and H3 lysine 4 methylation at the Prader-Willi syndrome imprinting center. Am J Hum Genet 69 : 1389–1394.
9. LossieAC, WhitneyMM, AmidonD, DongHJ, ChenP, et al. (2001) Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J Med Genet 38 : 834–845.
10. MakedonskiK, AbuhatziraL, KaufmanY, RazinA, ShemerR (2005) MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression. Hum Mol Genet 14 : 1049–1058.
11. RougeulleC, CardosoC, FontesM, ColleauxL, LalandeM (1998) An imprinted antisense RNA overlaps UBE3A and a second maternally expressed transcript. Nat Genet 19 : 15–16.
12. MengL, PersonRE, BeaudetAL (2012) Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a. Hum Mol Genet 21 : 3001–3012.
13. LandersM, BancescuDL, Le MeurE, RougeulleC, Glatt-DeeleyH, et al. (2004) Regulation of the large (approximately 1000 kb) imprinted murine Ube3a antisense transcript by alternative exons upstream of Snurf/Snrpn. Nucleic Acids Res 32 : 3480–3492.
14. ChamberlainSJ, BrannanCI (2001) The Prader-Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics 73 : 316–322.
15. JohnstoneKA, DuBoseAJ, FuttnerCR, ElmoreMD, BrannanCI, et al. (2006) A human imprinting centre demonstrates conserved acquisition but diverged maintenance of imprinting in a mouse model for Angelman syndrome imprinting defects. Hum Mol Genet 15 : 393–404.
16. WuMY, ChenKS, BresslerJ, HouA, TsaiTF, et al. (2006) Mouse imprinting defect mutations that model Angelman syndrome. Genesis 44 : 12–22.
17. JiangYH, ArmstrongD, AlbrechtU, AtkinsCM, NoebelsJL, et al. (1998) Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21 : 799–811.
18. DindotSV, AntalffyBA, BhattacharjeeMB, BeaudetAL (2008) The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet 17 : 111–118.
19. BresslerJ, TsaiTF, WuMY, TsaiSF, RamirezMA, et al. (2001) The SNRPN promoter is not required for genomic imprinting of the Prader-Willi/Angelman domain in mice. Nat Genet 28 : 232–240.
20. TsaiTF, JiangYH, BresslerJ, ArmstrongD, BeaudetAL (1999) Paternal deletion from Snrpn to Ube3a in the mouse causes hypotonia, growth retardation and partial lethality and provides evidence for a gene contributing to Prader-Willi syndrome. Hum Mol Genet 8 : 1357–1364.
21. CattanachBM, BarrJA, BeecheyCV, MartinJ, NoebelsJ, et al. (1997) A candidate model for Angelman syndrome in the mouse. Mamm Genome 8 : 472–478.
22. HuangHS, BurnsAJ, NonnemanRJ, BakerLK, RiddickNV, et al. (2013) Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res 243 : 79–90.
23. AllensworthM, SahaA, ReiterLT, HeckDH (2011) Normal social seeking behavior, hypoactivity and reduced exploratory range in a mouse model of Angelman syndrome. BMC Genet 12 : 7.
24. WeeberEJ, JiangYH, ElgersmaY, VargaAW, CarrasquilloY, et al. (2003) Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci 23 : 2634–2644.
25. JiangYH, SahooT, MichaelisRC, BercovichD, BresslerJ, et al. (2004) A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet A 131 : 1–10.
26. XieW, BarrCL, KimA, YueF, LeeAY, et al. (2012) Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome. Cell 148 : 816–831.
27. JiangYH, PanY, ZhuL, LandaL, YooJ, et al. (2010) Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PLoS One 5: e12278.
28. McBeathA, BainN, FourrierM, ColletB, SnowM (2013) A strand specific real-time RT-PCR method for the targeted detection of the three species (vRNA, cRNA and mRNA) of infectious salmon anaemia virus (ISAV) replicative RNA. J Virol Methods 187 : 65–71.
29. NaganoT, MitchellJA, SanzLA, PaulerFM, Ferguson-SmithAC, et al. (2008) The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322 : 1717–1720.
30. RedrupL, BrancoMR, PerdeauxER, KruegerC, LewisA, et al. (2009) The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development 136 : 525–530.
31. PowellWT, CoulsonRL, GonzalesML, CraryFK, WongSS, et al. (2013) R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1305426110
32. HuangHS, AllenJA, MabbAM, KingIF, MiriyalaJ, et al. (2012) Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 481 : 185–189.
33. KingIF, YandavaCN, MabbAM, HsiaoJS, HuangHS, et al. (2013) Topoisomerases facilitate transcription of long genes linked to autism. Nature 501 : 58–62.
34. FangP, Lev-LehmanE, TsaiTF, MatsuuraT, BentonCS, et al. (1999) The spectrum of mutations in UBE3A causing Angelman syndrome. Hum Mol Genet 8 : 129–135.
35. MatsuuraT, SutcliffeJS, FangP, GaljaardRJ, JiangYH, et al. (1997) De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet 15 : 74–77.
36. Mancini-DinardoD, SteeleSJ, LevorseJM, IngramRS, TilghmanSM (2006) Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev 20 : 1268–1282.
37. NumataK, KohamaC, AbeK, KiyosawaH (2011) Highly parallel SNP genotyping reveals high-resolution landscape of mono-allelic Ube3a expression associated with locus-wide antisense transcription. Nucleic Acids Res 39 : 2649–2657.
38. LatosPA, PaulerFM, KoernerMV, SenerginHB, HudsonQJ, et al. (2012) Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 338 : 1469–1472.
39. PandeyRR, MondalT, MohammadF, EnrothS, RedrupL, et al. (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32 : 232–246.
40. MohammadF, MondalT, KanduriC (2009) Epigenetics of imprinted long noncoding RNAs. Epigenetics 4 : 277–286.
41. TerranovaR, YokobayashiS, StadlerMB, OtteAP, van LohuizenM, et al. (2008) Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev Cell 15 : 668–679.
42. PrescottEM, ProudfootNJ (2002) Transcriptional collision between convergent genes in budding yeast. Proc Natl Acad Sci U S A 99 : 8796–8801.
43. HobsonDJ, WeiW, SteinmetzLM, SvejstrupJQ (2012) RNA polymerase II collision interrupts convergent transcription. Mol Cell 48 : 365–374.
44. CramptonN, BonassWA, KirkhamJ, RivettiC, ThomsonNH (2006) Collision events between RNA polymerases in convergent transcription studied by atomic force microscopy. Nucleic Acids Res 34 : 5416–5425.
45. CoreLJ, WaterfallJJ, LisJT (2008) Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322 : 1845–1848.
46. ChurchmanLS, WeissmanJS (2011) Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469 : 368–373.
47. ReeberSL, SillitoeRV (2011) Patterned expression of a cocaine - and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. J Comp Neurol 519 : 1781–1796.
48. NelsonED, KavalaliET, MonteggiaLM (2008) Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci 28 : 395–406.
49. ZhuPJ, HuangW, KalikulovD, YooJW, PlaczekAN, et al. (2011) Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell 147 : 1384–1396.
50. HuangW, ZhuPJ, ZhangS, ZhouH, StoicaL, et al. (2013) mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16 : 441–8.
51. DindotSV, PersonR, StrivensM, GarciaR, BeaudetAL (2009) Epigenetic profiling at mouse imprinted gene clusters reveals novel epigenetic and genetic features at differentially methylated regions. Genome Res 19 : 1374–1383.
52. YaylaogluMB, TitmusA, ViselA, Alvarez-BoladoG, ThallerC, et al. (2005) Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn 234 : 371–386.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



