-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
HoxA genes exhibit central roles during development and causal mutations have been found in several human syndromes including limb malformation. Despite their importance, information on how these genes are regulated is lacking. Here, we report on the first identification of bona fide transcriptional enhancers controlling HoxA genes in developing limbs and show that these enhancers are grouped into distinct topological domains at the sub-megabase scale (sub-TADs). We provide evidence that target genes and regulatory elements physically interact with each other through contacts between sub-TADs rather than by the formation of discreet “DNA loops”. Interestingly, there is no obvious relationship between the functional domains of the enhancers within the limb and how they are partitioned among the topological domains, suggesting that sub-TAD formation does not rely on enhancer activity. Moreover, we show that suppressing the transcriptional activity of enhancers does not abrogate their contacts with HoxA genes. Based on these data, we propose a model whereby chromatin architecture defines the functional landscapes of enhancers. From an evolutionary standpoint, our data points to the convergent evolution of HoxA and HoxD regulation in the fin-to-limb transition, one of the major morphological innovations in vertebrates.
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004018
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004018Summary
HoxA genes exhibit central roles during development and causal mutations have been found in several human syndromes including limb malformation. Despite their importance, information on how these genes are regulated is lacking. Here, we report on the first identification of bona fide transcriptional enhancers controlling HoxA genes in developing limbs and show that these enhancers are grouped into distinct topological domains at the sub-megabase scale (sub-TADs). We provide evidence that target genes and regulatory elements physically interact with each other through contacts between sub-TADs rather than by the formation of discreet “DNA loops”. Interestingly, there is no obvious relationship between the functional domains of the enhancers within the limb and how they are partitioned among the topological domains, suggesting that sub-TAD formation does not rely on enhancer activity. Moreover, we show that suppressing the transcriptional activity of enhancers does not abrogate their contacts with HoxA genes. Based on these data, we propose a model whereby chromatin architecture defines the functional landscapes of enhancers. From an evolutionary standpoint, our data points to the convergent evolution of HoxA and HoxD regulation in the fin-to-limb transition, one of the major morphological innovations in vertebrates.
Introduction
The Hox gene family encodes transcription factors with central roles in patterning of the body plan and organogenesis. Hox genes are grouped into clusters in most animal species, and mammals possess 39 genes divided into four clusters named HoxA to HoxD. In mice, deletion of the HoxA cluster is embryonic lethal [1]–[2] whereas mutants lacking HoxB, HoxC, or HoxD are viable at least until birth [3]–[5]. Inactivation of individual Hox genes identified Hoxa13 as a gene required for proper placenta function and thus embryonic survival [2], [6]–[7]. Mutations in HoxA genes have been found in various human syndromes (e.g. HFGS-OMIM140000, Guttmacher syndrome-OMIM176305, MRKH-OMIM277000) including limb malformations. Studies of gene inactivation in mice demonstrated that genes located at the 5′ end of the HoxA cluster (Hoxa9–13) are required for proper patterning of the three limb segments: the upper arm (humerus; Hoxa9, 10), lower arm (radius and ulna; Hoxa10, 11), and the hand/foot (autopod; Hoxa13) [6], [8]–[11].
Despite their pivotal roles during embryogenesis, little is known about the regulation of HoxA genes. This is in contrast to HoxD, which transcriptional control has been more thoroughly studied, especially in the limb where the HoxD genes play partially overlapping functions with HoxA [12]. Expression at the HoxA and D clusters follows similar dynamics during limb development, and occurs in two phases [12]. In the first phase, which starts at embryonic day 9.5 of development (E9.5), expression at both clusters is comparable suggesting that the control mechanisms are likely similar. During this phase, gene expression generally follows the collinear strategy observed in the trunk, characterized by sequential gene activation from one end of the cluster (Hox1) to the other (Hox13), with early activated genes expressed throughout the limb bud and those activated later (Hox10-13) gradually restricted to posterior cells [13]. In contrast, the expression domains of HoxA and HoxD genes partly differ in the second phase (from E11.5 onwards), suggesting some differences in the regulatory mechanisms controlling the clusters in this later phase.
Previous studies show that transcription at the HoxD cluster is regulated long-distance by enhancers in several tissues (reviewed in [14]). Notably, expression of Hoxd10 to Hoxd13 in the distal part of the limb bud (presumptive hand/foot) is controlled by several remote cis-regulatory sequences located in the gene desert upstream of the cluster [15]. Hands/feet, in particular digits, are evolutionary novel structures and the hallmark of Vertebrate adaptation to terrestrial habitats. The fact that Hoxa10 and Hoxa13 are also expressed in the presumptive hand/foot domain therefore raised the possibility that specific recruitment of HoxA and HoxD gene functions in developing digits stem from the implementation of similar cis-regulatory elements during the fin-to-limb transition. Whereas sequence conservation analysis of the region upstream of these clusters did not identify cognate cis-regulatory elements driving HoxA expression in limbs [16], BAC transgenesis revealed the existence of a “digit” enhancer activity located between 250 and 500 kb upstream of the Hoxa13 gene, in the neighborhood of the 3-hydroxyisobutyrate dehydrogenase (Hibadh) gene [17]. As Hibadh is also expressed in distal limb buds [16], this study could not resolve whether the “digit” enhancer activity detected within that region controls Hibadh, Hoxa10/13, or both. Thus, the enhancer sequence(s) and whether HoxA expression in limbs is regulated by long-range control mechanisms has remained unknown.
It was previously shown that control DNA elements could regulate the expression of remote genes by physically interacting with them [18]. Physical contacts between chromatin segments can be measured using the chromosome conformation capture (3C) methods, a series of assays that use proximity-based ligation to infer the three-dimensional organization of genomes [19]. 3C assays were used to show that regulation of HoxD genes in the presumptive digit domain is mediated by physical contacts with remote enhancers, and led to a model whereby expression of Hoxd10 to d13 associates with the formation of DNA loops between the genes and regulatory islands [15]. This was further supported by Fluorescence In Situ Hybridization data showing the co-localization of HoxD genes and one of its regulatory islands, specifically in digit progenitor cells [20]. Whether the proximity between target genes and regulatory DNA elements requires transcription appears to be loci-dependent and it remains unknown whether a given mechanism prevails over others. Indeed, while such “loops” were sometimes reported in absence of transcription [21]–[22], transcription factors requirement for DNA looping was uncovered at the β-globin locus [23]–[24] and Igh gene [25].
Here, we show that during limb development, expression of HoxA genes is controlled by multiple remote enhancers located upstream of the cluster. In limb cells, these enhancers are grouped into distinct sub-megabase topological domains (sub-TADs) that contact each other and the sub-TADs containing target genes. In head tissues, the topology is drastically different, modifying both gene-enhancer and enhancer-enhancer interactions. Interestingly, enhancers located in the same sub-TAD are active in distinct subset of limb cells suggesting that spatial clustering of enhancers does not simply reflect enhancer co-activity. We also present evidence that enhancer-HoxA contacts are maintained even when enhancer activity is suppressed, suggesting that the HoxA regulatory region acquires a permissive conformation prior to gene activation. We suggest a model whereby sub-TAD formation and/or contacts between sub-TADs define the cis-regulatory network controlling gene expression. From an evolutionary perspective, this first extensive characterization of HoxA regulation in developing limbs provides new insights into the evolution of Hox regulation in the emergence of hand/foot. Our study suggests that while the DNA sequences of the distal limb enhancers for HoxA and HoxD genes are different and have likely emerged independently, chromosome partitioning into topological domains has similarly constrained the evolution of HoxA and HoxD cis-regulatory landscapes underlying the emergence of digits, one of the major morphological innovations in Vertebrates.
Results
Multiple candidate limb enhancers are located upstream of the HoxA cluster
To identify enhancer sequences regulating HoxA expression during limb development, we used a combination of genetic and genomics approaches that probe enhancer features in mouse embryos. We first tested whether HoxA transcription in developing limbs involves cis-regulatory sequences outside of the gene cluster itself. To this end, we used two mutant lines with targeted genomic rearrangements at the HoxA cluster [1]–[2] to monitor activation of reporter transgenes by surrounding enhancer activity (Figure 1A,B). Whole mount in situ hybridization shows that a neomycin reporter transgene located downstream of Hoxa1 is not expressed in limbs at E11.5 (Figure 1A, left). In contrast, we found that a hygromycin transgene inserted at the opposite end of the cluster, 3.5 kb upstream of Hoxa13 is robustly transcribed in distal limbs at this stage (Figure 1A, right). These expression patterns correlate well with the expression profile of the endogenous HoxA genes adjacent to the reporter transgenes. Upon deletion of the entire HoxA cluster, the neomycin transgene becomes activated in distal limbs suggesting that sequences upstream of the cluster are sufficient to trigger distal expression (Figure 1B). These results support the previously proposed hypothesis that a 250 kb region in the neighborhood of Hibadh contains an enhancer activity controlling HoxA expression in developing limbs [17].
Fig. 1. Candidate limb enhancers reside on the telomeric side of the HoxA cluster. 
A, B. Distal limb enhancer activity lies upstream of the HoxA cluster and does not require sequences within it. Expression of the Neomycin and Hygromycin reporter genes flanking the cluster were analyzed by whole mount in situ hybridization on E11.5 embryos. In embryos where the HoxA cluster is intact (A), expression of the upstream Hygromycin reporter was detected in the distal part of the limb while downstream neomycin transcripts were not. TKNeo: minimal thymidine kinase promoter upstream of Neomycin reporter gene. PGKHygro: minimal phosphoglycerate kinase-1 promoter upstream of Hygromycin reporter gene. Arrow above the HoxA cluster diagram shows transcription direction. B. Neomycin expression after deletion of the cluster and PGKHygro by recombination of loxP sites flanking the reporter genes shows that sequences within the cluster are not required for distal limb enhancer activity. C. Distal limb cells analyzed in this study express 5′ HoxA genes (Hoxa9 to a13). HoxA gene expression in developing limbs is illustrated on the left. The dotted line indicates the area micro-dissected to collect distal limb cells for analysis. Stylopod: upper arm, zeugopod: lower arm, mesopod: wrist, autopod: hand. D. The position of candidate enhancer sequences was identified by ChIP-seq. Proteins known as being enriched at active enhancers (RNAP2, Med12, p300) and the H3K27Ac histone mark was examined as described in Materials and Methods. The y-axis corresponds to “reads per million” except for the p300 data where the number of sequence reads is shown. Colored rectangles below each track indicate the position of significant peaks. The position of candidate enhancers (e1 to e19) is highlighted in green below the genomic region characterized, where transcriptionally active genes are in red and arrows indicate transcription direction. Sequence conservation in the chicken is shown on the bottom. Given the results described above, we focused our analysis on the genomic region upstream of the cluster. To identify active enhancers in distal limbs, we used dissected distal forelimbs (Figure 1C), which are composed of cells expressing mainly Hoxa10 and a13, but also a small amount of Hoxa9 and a11 from the presumptive wrist domain (mesopod). Active enhancers are characterized by the binding of several proteins including RNA polymerase II (RNAP2), and subunits of Mediator like Med12 [26]. We therefore mapped candidate enhancer sequences by identifying genomic sites enriched in these proteins using chromatin immunoprecipitation combined with deep sequencing (ChIP-seq) in cells isolated from E12.5 distal limb buds (Figure 1D). This data was considered together with previously published datasets derived from whole limb buds for the transcriptional co-activator p300 [27] and acetylated histone H3 lysine 27 (H3K27Ac), which also mark active enhancers [28]. Sequences distinct from proximal promoters (RefSeq) that were bound by RNAP2 and at least one other mark, or by both p300 and H3K27Ac were retained as candidate enhancers. Using these criteria, 19 putative enhancers were identified within 850 kb upstream of Hoxa13 (Figure 1D, top).
HoxA expression in developing limbs relies on several enhancer elements
The number of candidate sequences identified upstream of HoxA was rather large, particularly compared to HoxD for which seven enhancers have been identified [15]. Also, in contrast to the gene desert surrounding HoxD, the region upstream of HoxA encompasses several genes (Figure 1D). Candidate HoxA enhancers therefore reside amidst other genes including Hibadh, Tax1bp1, and Jazf1, for which expression has been reported in the limb [16]. As ChIP-seq datasets cannot resolve the targets of enhancers, we used a structural approach to assess the potential interactions of the candidate enhancers with HoxA genes. We profiled the interaction pattern of the HoxA cluster with the upstream 850 Kb region in distal limb buds and head tissues using 5C technology combined with deep sequencing [29]–[30], which provides insights into chromatin architecture at high resolution (down to 4–6 kbs on average). We found that the 5′ part of HoxA, containing Hoxa9 to Hoxa13, frequently interacts with several regions upstream of the cluster (Figure 2, top, Figure S1), and that most of these regions contain the candidate limb enhancers (Figure 2, bottom). In contrast, none of the enhancers interact with the 3′ part of the cluster containing Hoxa1 to Hoxa7 (Figure 2), which have no detectable expression in limb buds at this developmental stage. This result is reminiscent of the distal enhancers controlling the 5′ HoxD genes, which are also located upstream of the cluster and specifically interact with genes located in the 5′ part [15]. Interestingly, previous studies based on Hi-C analysis revealed that the HoxA and HoxD clusters each span a junction between two so-called “topologically associated domains” (TADs), with 3′ genes residing into one TAD and the 5′ part extending into the other [31]. TADs are thought to represent a basic unit of chromatin organization at the megabase-scale that is largely conserved between cell types [32]. Our data therefore points to a common relationship between chromatin topology and the limb regulatory landscapes of the HoxA and HoxD clusters.
Fig. 2. Several candidate enhancers interact specifically with 5′ HoxA genes in the limb. 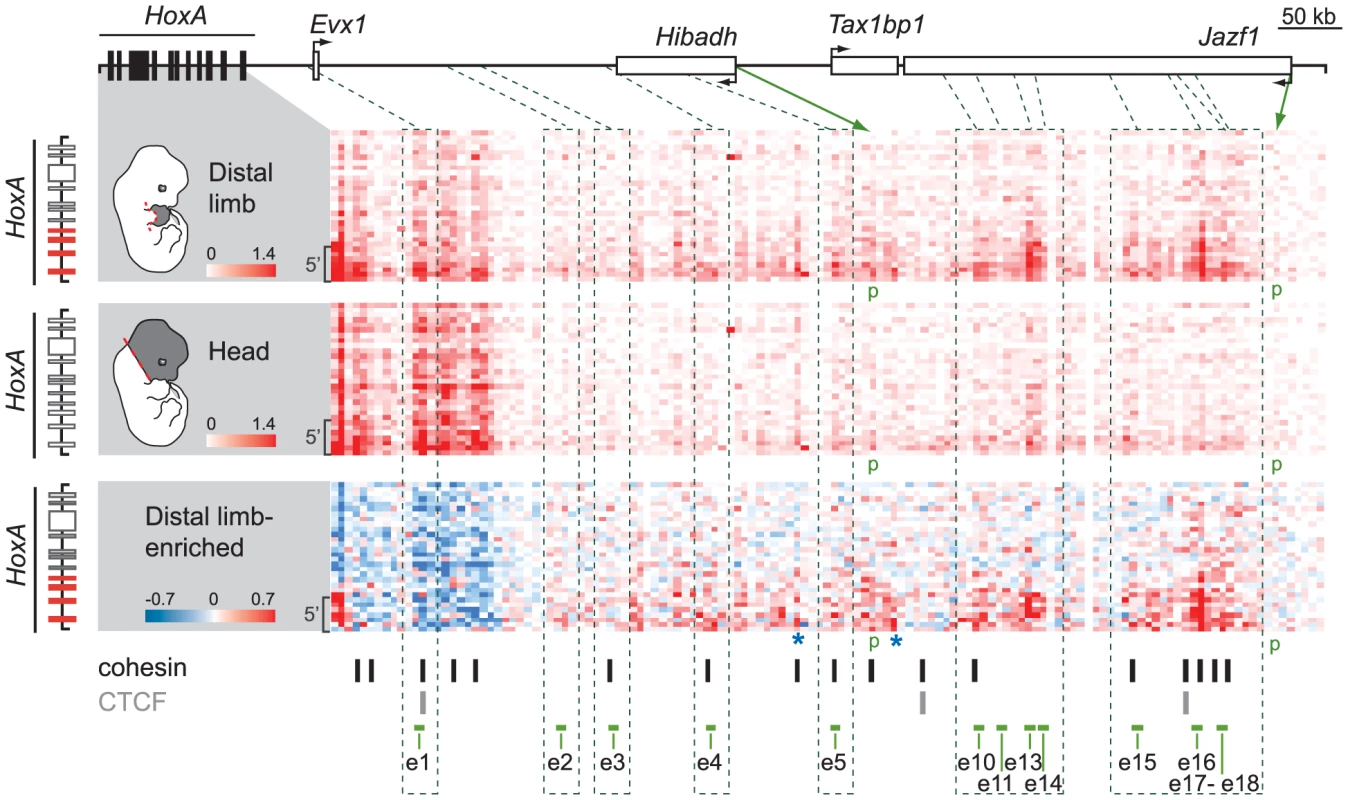
Physical contacts between the HoxA cluster and the upstream genomic region containing candidate enhancers were measured by 5C-seq in distal limb (top) and the head (middle) of E12.5 embryos. 5C data is represented in heatmap form with the color intensity of each pixel reflecting the frequency of interaction between two genomic regions. Contact frequency is according to the respective color scales and corresponds to the number of sequence reads. Most predicted enhancers (4;5;10;11;13;14;15;16;17;18) interacted long-distance specifically with 5′ HoxA genes and these interactions were enriched in the limb (bottom panel). Color scale in the bottom panel contrasts interactions enriched in the limb (red) and in the head (blue). Green dotted lines link the position of enhancers along the genomic region to the corresponding 5C fragments. Brackets on the left hand side of each heatmap show the area containing Hoxa9, a10, a11, and a13. Green arrows point to the chromatin fragments containing the Hibadh and Jazf1 promoters (p). Blue stars highlight other limb-enriched interactions with HoxA genes that do not correspond to candidate enhancers. Restriction fragments corresponding to enhancer e6–8, 12, and 19 could not be included in the 5C design as they fell into regions that were not amenable to 5C (see Materials and Methods). Loci bound by cohesin (black bars) and/or CTCF (grey bars) in limb bud cells at E11.5 [35] are indicated below the 5C heatmaps. Note that most interactions identified by 5C correspond to loci bound by cohesin. Interestingly, sequences with the highest interaction frequencies with 5′HoxA genes (e10, 13, 14 and e15, 16, 18) locate farther from the cluster, within the Jazf1 gene, and correspond to those loci most enriched in marks typical of active enhancers (Figure 1D). High interaction frequencies being associated either with stronger, more abundant and/or stable spatial contacts, these data likely reflect a prevalent activity of these enhancers in distal limbs. In contrast to the other enhancers, e1 and e3 do not show enriched interactions with the 5′ part of the HoxA cluster in distal limbs compared to head tissue (Figure 2, bottom). e1 is located close to Evx1, within a region of high interaction frequencies with HoxA both in limb and head tissues. This is not the case for e3 so we further tested interaction frequencies between Hoxa13 and e2 to e5 using 3C (Figure S2). This analysis shows higher frequency of interactions between these enhancers and Hoxa13 specifically in the limb. Yet, based on our 5C data, these interactions are modest compared to those observed for the other enhancers (Figure 2). Interestingly, contacts such as those with e5, e13 and e15 were also observed in the head at low frequencies (Figure 2). As there is no evidence of HoxA expression in the head at the stage analyzed, the contacts observed might be evidence of default chromatin architecture in this tissue. Alternatively, these enhancers may drive HoxA expression in head tissues at levels below detection by whole-mount in situ hybridization. Finally, our 5C data also reveals high interaction frequencies with at least two loci that have no apparent characteristics of transcriptional enhancers (Figure 2 bottom, blue stars). These may reflect additional structural anchors that stabilize the chromatin architecture, such as those mediated by CTCF and Cohesin [33]–[34]. Interestingly, loci bound either by CTCF or cohesin in limb buds have been recently identified [35] and comparison with our data shows that almost all loci interacting with 5′ HoxA genes overlap with either CTCF or cohesin binding (Figure 2, bottom).
Having confirmed the spatial proximity between 5′ HoxA genes and most of our candidate enhancers, we proceeded to test their in vivo activity in the mouse by transgenesis. Putative enhancer sequences were subcloned into vectors carrying the β-globin minimal promoter and lacZ reporter. Except for e1 and e2, X-Gal staining in transgenic embryos shows that all candidate enhancers tested activates transcription in developing limbs (Figure 3, Table S1). Interestingly, the confirmed enhancers exhibit distinct but overlapping activity domains in limb buds, and all trigger expression in the presumptive hand/foot (Figure 3). While some are active mostly in the distal part of the limb (e3, 4, 5, 10, 12, 13), others are functional also in the proximal domain (e5, 16, 18). The only candidate enhancers that fail to trigger reporter expression in our transgenic assays are e1 and e2 (Table S1). The absence of activity for these two candidates indicates either that these sequences are not limb enhancers or that the transgenes did not include all the necessary sequences to reflect their transcriptional activities. For e1, our 5C data (Figure 2) neither supports nor disagrees with it being a HoxA enhancer since it lies within a large region of high interaction frequency. Interestingly, e1 is located within a 50 kb DNA fragment that was previously shown to trigger gene expression in distal limbs [2], suggesting that it is possibly a bona fide limb enhancer but that some sequences required for its activity are absent from the 2.9 kb fragment tested in our transgenic assay. Similarly, absence of X-Gal staining in e2 transgenic embryos does not prove that e2 is not an enhancer. Yet, the fact that it does not strongly interact with 5′ HoxA genes in our 3C and 5C assays suggest that e2 may not be tightly linked to the regulation of HoxA genes. Nonetheless, analysis of the other identified enhancers shows that multiple enhancers with overlapping domain-specific activities regulate transcription at the HoxA cluster in the limb.
Fig. 3. Overlapping domain-specific enhancer activity regulates 5′ HoxA genes in distal limbs. 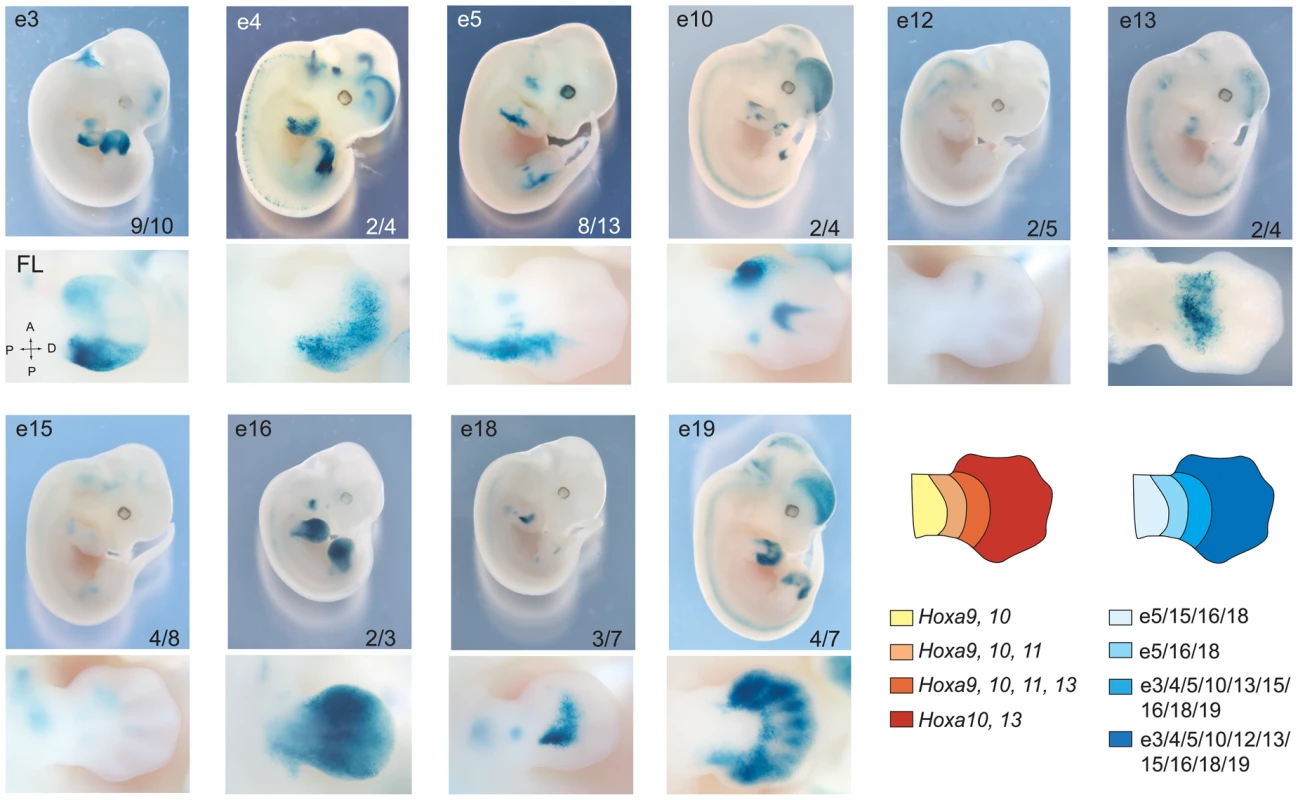
Transgenic analysis of enhancer candidates. In each row, top panels show LacZ staining in whole embryos and higher magnification of the limb bud is shown below. Tested enhancers are indicated at the top of each panel, and the number at the bottom represents embryos positive for the pattern reported over the total number of transgenic specimens analyzed. Lower panels present a dorsal view of corresponding forelimbs (FL) except for e13, which is shown ventrally. Diagrams on the right summarize the expression patterns of HoxA genes (left), and the activity of each enhancer (right) in the developing limb at E12.5, respectively. HoxA-enhancer contacts do not require enhancer activity
While “DNA looping” is associated with long-range transcriptional control, the extent to which spatial structure exists prior to or as a consequence of enhancer activation remains elusive. This issue partly originates from the fact that most studies have compared the spatial distance of enhancers and target gene(s) in tissues expressing the genes with others where they are never expressed. To gain insight into the causative relationship between spatial proximity and long-distance enhancer regulation, we examined the outcome of enhancer silencing on long-range enhancer-gene interactions in developing limbs. During limb development, the transcriptional repressor Gli3R negatively regulates the expression of HoxA genes [36]–[37]. While Gli3 is expressed almost throughout the limb in wild type (wt) embryos, the Gli3R domain is restricted anteriorly as a consequence of posterior Sonic hedgehog (Shh) signaling emanating from the Zone of Polarizing Activity (ZPA), which blocks processing of the full length Gli3 protein into its truncated repressor form [38]. In Shh−/ − limbs, the Gli3R functional domain extends posteriorly leading to the down-regulation of HoxA as well as HoxD genes [36]–[37].
Amongst the HoxA-associated limb enhancers identified, we found several that overlap with loci bound by Gli3R in the limb (e3, e5, e9 and e16; [39]). The activity of these enhancers should thus be suppressed in Shh−/− mutant. Of these, e5 is of particular interest because it triggers robust gene expression (Figure 3), and there is no other limb enhancer in its genomic neighborhood allowing us to assess its interaction frequency with HoxA without potential interference from surrounding enhancers. We first verified the activity of e5 in Shh−/ − by generating mutant embryos homozygous for Shh inactivation and carrying the e5 transgene. X-Gal staining shows that e5 activity is suppressed in limbs upon inactivation of Shh (Figure 4Aa–d, compare a to b) while still functional in the developing genitalia (Figure 4A, panel d). In contrast, a transgene containing the e1 enhancer, which does not overlap with a Gli-bound locus, remained expressed in a Shh−/ − background (Figure 4Af–h) although in a smaller domain consistent with Shh−/−embryos having reduced limb size ([40]; Figure 4A, compare e to f).
Fig. 4. Regulatory HoxA contacts are independent of enhancer activity. 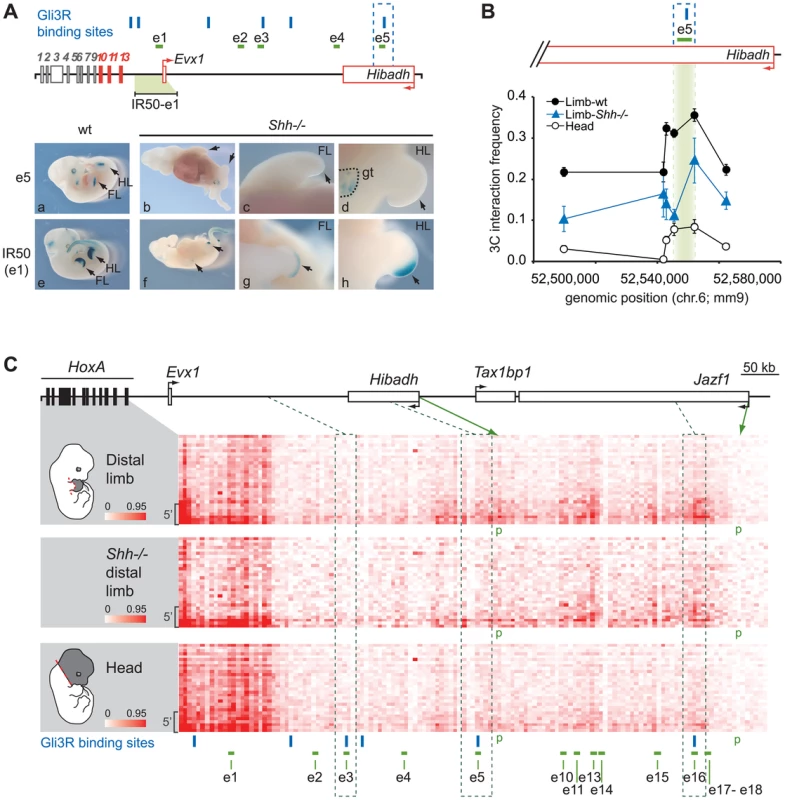
A. Analysis of e1 and e5 activity in Shh−/− limbs. Upper panel: Scheme representing loci bound by Gli3R (blue bars) along the HoxA regulatory region. The IR50 transgene used as control contains the 50 kb intergenic region to Hoxa13 and Evx1, which includes e1. Active genes are shown in red, and arrows indicate the position of promoters and transcription direction. Lower panel: LacZ staining showing e5 (a–d) and e1 (e–h) transcriptional activity, in wt (a; e) and Shh−/− embryos (b–d; f–h). B. Long-range e5 interaction with Hoxa13 is independent of its activity. The physical proximity between the Hoxa13 gene and e5 was measured by 3C. The position of e5 is highlighted in green. Interaction frequency were measured compared to a BAC 3C library as described in the Materials and Methods. Error bars correspond to the standard error of the mean. C. Physical contacts between the HoxA cluster and the upstream genomic region measured by 5C-seq in wild-type distal limb (top), Shh−/− mutant distal limb (middle), and the head (bottom) of mouse embryos. 5C data is presented in the form of heatmaps according to color scales as described in Figure 2. The limb-specific interaction pattern between enhancers and the 5′ HoxA genes are similar in Shh−/− (middle panel) and wt distal limb buds (top panel) albeit with some interaction frequencies slightly reduced. Dotted lines delineate the regions containing the enhancers bound by Gli3R (e3, e5 and e16). Gli3R sites are represented with blue bars. Brackets on the left hand side of each heatmap show the area containing Hoxa9, a10, a11, and a13. Green arrows indicate the chromatin fragments containing the Hibadh and Jazf1 promoters (p). Restriction fragments corresponding to enhancer e6–8, 12, and 19 are not shown in the heatmaps as they fell into regions that were not amenable to 5C (see Materials and Methods). To assess whether HoxA-enhancer proximity requires enhancer activity, we measured contacts between Hoxa13 and e5 in wt and Shh−/ − distal limb buds from E11.5 embryos. As e5 activity is suppressed in the absence of Shh, the enhancer should no longer interact with Hoxa13 if enhancer activity is required for the contact. 3C analysis shows that e5 interacts with Hoxa13 even in the absence of Shh (Figure 4B). Although interaction frequencies are lower than in wt limbs, the interaction pattern is similar and contacts are much stronger than in the head, which was used as control. These data show that even though e5 silencing may affect the robustness of the interactions, the spatial proximity between e5 and Hoxa13 does not require the transcriptional activity of the enhancer. As Hoxa13 expression is severely reduced in the absence of Shh [37], [41], we next wondered whether the contact pattern of HoxA genes with the distal limb enhancers was similarly preserved in Shh−/− limbs. To address this question, we compared the interaction profile of the HoxA cluster with its upstream regulatory region in wt and Shh−/− limbs, and in the head. For these 5C experiments, we used a modified 3C library protocol optimized for the production of libraries from a small number of cells (see Materials and Methods). This protocol largely recapitulated the contact pattern detected in wt limbs and the head with our standard approach (compare corresponding panels in Figures 2 and 4C). Consistent with our 3C data, this 5C analysis revealed a similar contact pattern between the 5′ HoxA genes and upstream regulatory region in the Shh−/− mutant and wt limbs (Figure 4C, Figure S3). These include contacts with e5 and e16 enhancers, which overlap with Gli3R sites and others like e10 and e13 that are not regulated by Shh. As observed in our preliminary 3C analysis, the contacts were weaker in the Shh−/− suggesting that strengthening a given enhancer-promoter contact upon enhancer activation may impact on the stabilization/strength of other interactions. Together, these data indicate that enhancer activity strengthens, but is not mandatory for spatial proximity between enhancers and their target genes.
An extensive physical chromatin network regulates multiple genes in distal limb cells
The observation that different enhancers drive transcription in the same areas of the limb suggests a possible physical link between some of them. To test this possibility, we extended our 5C analysis to the whole regulatory region. The HoxA cluster was previously found to span the junction between two adjacent TADs in human IMR90 and mouse embryonic stem cells (ES) analyzed with Hi-C at the mega-base scale [31]. We observed a similar megabase scale organization in our samples, where 5′ HoxA genes and distal limb enhancers are located in the same TAD (Figure 5A, B, and Figure S4). We found that this TAD is subdivided into domains of interactions that differ between the limb and head tissues at E12.5 (Figure 5A, B). In addition, contacts between sub-TADs are different in the two tissues. For example, the HoxA sub-TAD containing Hoxa9 to Hoxa13 preferentially forms long-range contacts with the enhancers in the limb (e.g. e10–14, e15–18; Figure 5A, Figure S5), while it interacts strongly with the 3′ HoxA genes in the head (Figure 5C, bottom, Figure S4). Similarly, Evx1, which spatially localizes within its own domain, interacts long-distance with a subset of the identified enhancers in limbs, consistent with its expression pattern being similar to Hoxa13. This is different in the head where Evx1 and HoxA are mostly inactive and the genes form a large interacting domain (Figure 5B,C), which likely reflects chromatin compaction at transcriptionally silent loci. This result raises the possibility that chromatin conformation within TADs could vary in a tissue-specific manner. In support of this, a recent 5C analysis in mouse ES and neural progenitor cells identified tissue-specific topological domains at the sub-megabase scale, termed “sub-TADs” [33]. Our 5C analysis therefore revealed the existence of tissue-specific sub-TAD interactions underlying the regulation of HoxA genes in developing limbs.
Fig. 5. Extensive clustering of genes and enhancers highlights a complex regulation network in distal limbs. 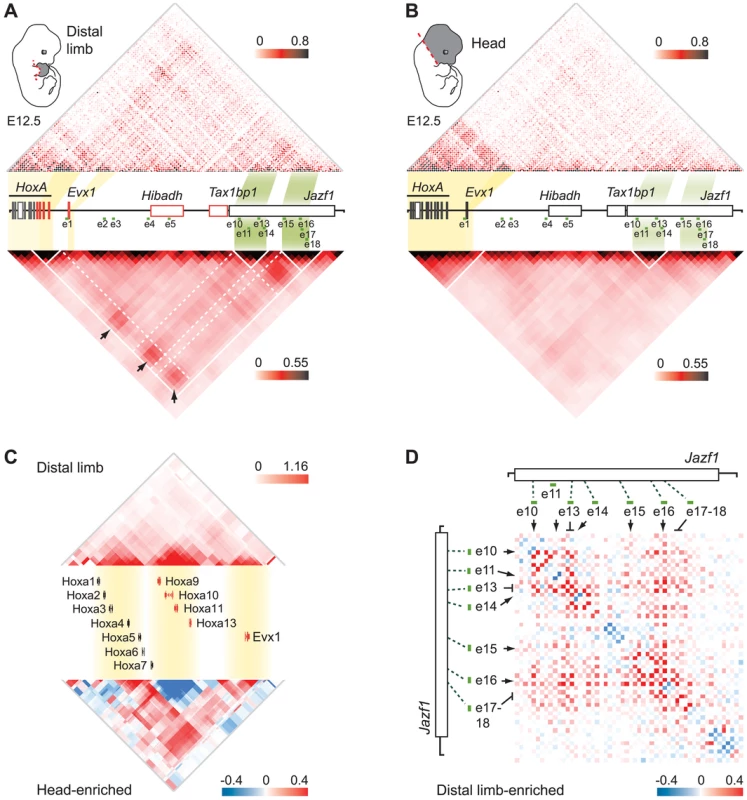
A,B. 5C interaction matrix of the HoxA cluster and its upstream regulatory region in distal limb (A) and head (B). The 5C data was generated by 5C-seq using tissues from E12.5 embryos, and is presented in the form of heatmaps according to color scales as described in Figure 2. Heatmaps above the linear diagram of the genomic region show interaction frequencies for each restriction fragment, irrespective of their size. Heatmaps at the bottom show the mean interaction frequencies per 20 kb DNA fragment and were obtained from binning and smoothing of the 5C raw data. Expressed genes within the region are colored in red. The yellow and green shading links the genomic position of HoxA and Evx1 genes, and the enhancer clusters to the corresponding areas in heatmaps. Black arrows point to interactions between the gene sub-TADs and enhancer sub-TADs. White lines delineate the TAD and sub-TADs therein. Dashed white lines are drawn to highlight the sub-TAD interactions. C. Topological organization of the HoxA cluster and Evx1. Genes are organized in three sub-TADs in the limb (top). Interaction enrichment in head tissues compared to the limb (bottom) shows significant increase in interaction between the gene sub-TADs in the head. Smoothing was performed based on distance (8 kb) and heatmap intensities represent the mean of interaction frequency for each 8 kb window. D. Extensive limb-enriched interactions between distal HoxA enhancers suggest that a physical network regulates 5′ HoxA genes in the limb. The interaction matrix of the region containing enhancer e10 to e18 is shown in the form of a heatmap. Limb-enriched contacts are shown in red according to the color scale as described in Figure 2. The chromatin architecture resulting in the spatial proximity between 5′ HoxA genes, the enhancers, and the promoter of Hibadh suggests that Hibadh itself interacts with HoxA-associated limb enhancers. Indeed, Hibadh shows enriched interaction with e5, e13 and e16 in limb compared to head tissue (Figure S5). Interactions between Evx1 and Hibadh are also enriched in limb compared to head tissue. Our experimental design unfortunately did not retain the promoter region of Tax1bp1 and thus we could not profile its connectivity with the region. As for the promoter of Jazf1, it contacts neither the genes nor the enhancers consistent with the absence of RNAP2 and Med12 at its promoter (Figure 1D), and in agreement with previous work showing that Jazf1 is expressed in distal limbs only at later developmental stages [16]. Together, these results show that a subset of HoxA-associated enhancers likely regulate also Evx1 and Hibadh. Interestingly, there is an extensive connectivity between the enhancers themselves in the limb but not in head tissues (Figure 5A, B). Similarly to the genes, the enhancers partition among different sub-TADs that interact together. This is particularly visible for the most distal ones where e10–14 localized within one sub-TAD, and e15–18 into another (Figure 5A, D). This organization suggests that enhancers are spatially grouped into regulatory modules, which can interact with each other, eventually triggering specific expression patterns. Such interaction between genomic domains is reminiscent of the contacts identified in Drosophila [42]. It is thus likely that long-range gene regulation relies on sub-TAD interactions rather than discrete looping between specific DNA elements. Moreover, interactions between gene and enhancer sub-TADs in the limb strengthened and better defined the position of the corresponding TAD as compared to head tissues (Figure 5, compare A and B). This result suggests that although largely invariant, the partitioning of chromosomes into TADs can be affected by the tissue-specific folding of the chromatin at the sub-megabase level.
Discussion
Enhancer and gene sub-TADs interact long-range to control transcription in developing limbs
In this study, we identified the very first set of bona fide limb enhancers controlling 5′ HoxA gene expression. We show that these enhancers, like the HoxA genes, are grouped into distinct topological domains at the sub-megabase scale, and that long-range contacts between the sub-TADs underlie the expression of 5′ HoxA genes in the developing limb. This result suggests that long-distance regulation of HoxA genes is based on sub-TAD interactions rather than discrete looping between enhancers and target genes. In the head, sub-TAD interactions are barely detectable thus indicating that the chromatin architecture of the region upstream HoxA varies in a tissue-specific manner at the sub-megabase scale. The apparent lack of sub-TAD interactions in the head could also be the consequence of the greater cellular complexity of this tissue, which would equally imply that sub-TAD interactions are cell type/tissue-specific (Figure 5B). A similar conclusion was recently reached based on the comparison between 5C data in mouse ES cells and neural progenitor cells {Nora, 2012 #165} [32]–[33]. The cell-type/tissue specificity of sub-TADs contrasts with the mostly invariant nature of TADs, which partition the genome into topological domains at the megabase scale [31]–[32]. While it was proposed that TADs could represent the structural basis of regulatory landscapes [43], the actual chromatin folding associated with transcriptional activity likely relies mostly on sub-TAD interactions (Figure 6). The diametrically opposed invariant nature of TADs and the tissue-specificity of sub-TADs also imply that distinct structural parameters define them. Accordingly, while arrays of CTCF sites characterize TAD boundaries [31], there is no obvious correlation between CTCF binding and the sub-TAD boundaries observed in limb buds (Figure S5).
Fig. 6. Model illustrating how genome topology underlies the tissue-specific regulation of HoxA genes. 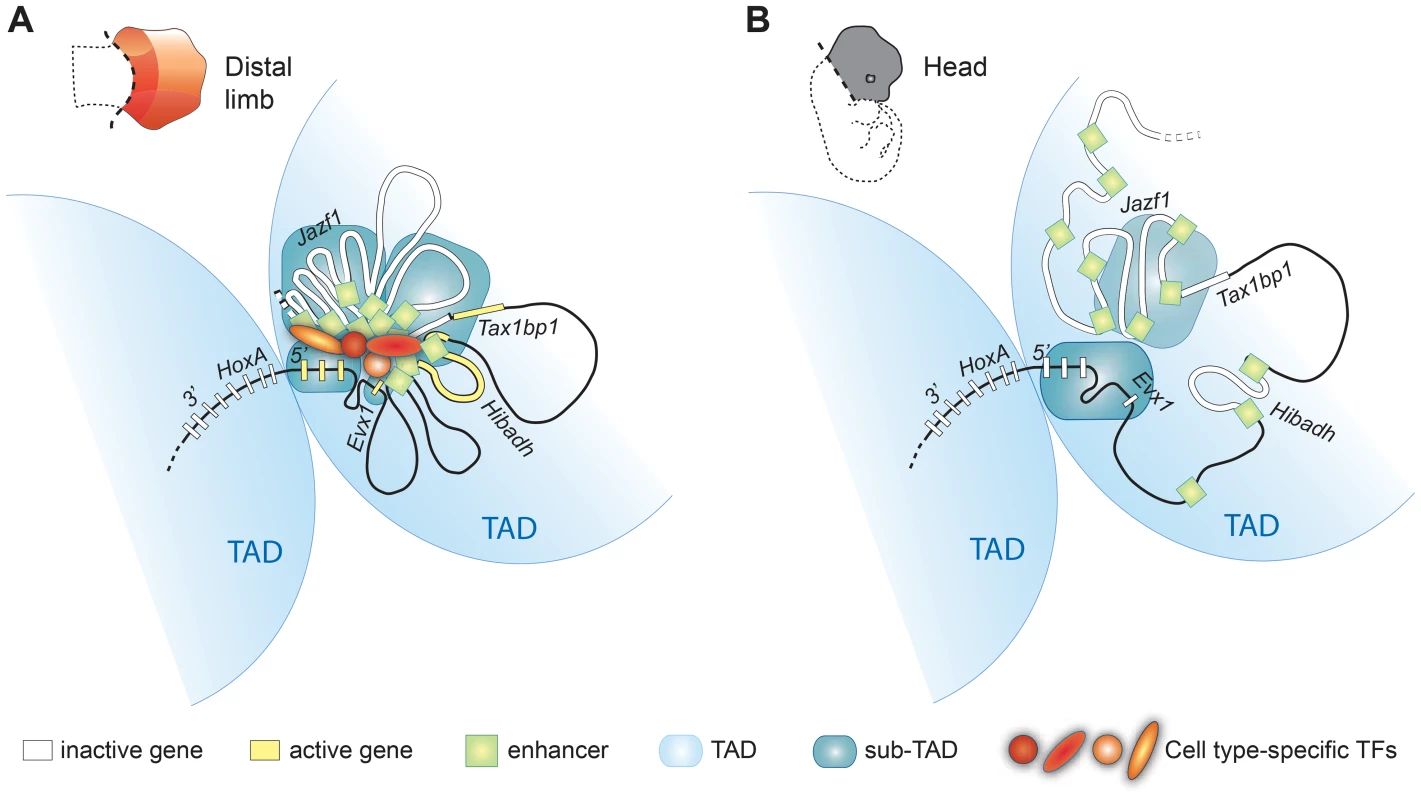
The HoxA cluster is partitioned between two TADs (light blue), physically segregating 3′HoxA from 5′HoxA genes in a mostly cell-type independent manner. In contrast, the sub-TAD interaction pattern is drastically different in the limb (A) compared to the head (B). Limb enhancer sub-TADs (dark blue) interact with each other and with gene-sub-TADs in distal limb but not head tissue. Enhancer and gene interactions occur between sub-TADs from the same TAD (5′HoxA containing TAD) but not with 3′HoxA genes that are located in the adjacent TAD. The limb-specific sub-TAD interactions create a platform architecture controlling HoxA expression by the remote distal limb enhancers upon enhancer activation by transcription factors. The schemes of the chromatin conformation were designed assuming cellular homogeneity within each tissue. Our results also indicate that at least some of the gene-enhancer contacts form independently of enhancer transcriptional activity (Figure 4), and we suggest that this structure largely exists before the gene transcription begins. This view is supported by the existence of interactions with loci for which there is no evidence of transcriptional activity (Blue stars in Figure 2). Moreover, our data shows that enhancers triggering distinct expression patterns in the limb (i.e. active in different cells) actually belong to the same sub-TAD, which further supports the notion that organization of the chromatin into sub-TADs does not simply reflect physical clustering of active enhancers. Chromatin interactions nonetheless appear strengthened by enhancer activity consistent with the recent concept of self-enforcing structure-function feedbacks, considered as a mechanism propagating cell-fate memory [44]. Our data also reveal better-defined boundaries of the 5′ HoxA-containing TAD in limbs, where sub-TADs robustly interact (Figure 5). This result raises the possibility that upon enhancer activation, the robustness of sub-TAD interactions within two adjacent TADs may change and consequently re-define the position of the TAD boundary. This potential TAD/sub-TAD interplay may actually provide a mechanistic explanation for Hoxd9 to Hoxd11 switching from one TAD to the neighboring one in proximal limb compared to distal limb cells [45].
The identification of multiple enhancers controlling 5′HoxA genes in distal limbs raises questions about the potential role and benefits for this apparently complex control mechanism. The evidence that the various enhancers identified have distinct spatial specificities, together with the eventual morphological diversity of the hand/foot, points to the existence of an early molecular heterogeneity among the mesenchymal progenitors of the hand/foot. Accordingly, Shh signaling regulates a subset of enhancers identified here while others are not (Figure 4). Nonetheless, most enhancers also appear to share overlapping functional domains. Interactions between some enhancers may reflect their co-function in some cells, which could correspond to specific cell populations in which a higher HoxA dosage is required. Alternatively, enhancer interactions could be the consequence of a “pre-set” chromatin architecture whereby a series of enhancers is brought in the vicinity of the same target genes, without having necessarily a combined transcriptional input in the same cell. Finally, it should also be mentioned that multiple enhancers with overlapping function can be beneficial, as exemplified with the discovery of shadow enhancers, which compensate for each another in sub-optimal conditions [46]–[47].
Emergence of the hand and foot in Tetrapods is associated with the convergent evolution of HoxA and HoxD regulation
The hand/foot (autopod) is one of the major morphological novelties that accompanied Vertebrates adaptation to terrestrial habitats. As autopod development requires the function of HoxA and HoxD genes, the mechanism that led to the emergence of this new Hox function appears as a key molecular event associated with the fin-to-limb transition. By mapping active enhancers in the presumptive hand/foot and testing their interaction with HoxA genes, we provide evidence that HoxA expression in this tissue relies on long-range regulation by multiple enhancers. Previous studies on the regulation of HoxD genes led to the same conclusion [15], [48]–[49] suggesting that HoxA and HoxD genes have been recruited in this evolutionary novel structure through the implementation of a similar regulatory strategy. Yet, sequence comparison between HoxA and HoxD specific enhancers failed to identify obvious conservation thereby favoring a model whereby the recruitment of HoxA and HoxD genes in the presumptive hand/foot was likely achieved through independent implementations of novel cis-regulatory elements. Since these enhancers were identified with a resolution varying between 0.5 and 2 kb, it is possible that they are bound by the same transcription factors but with a distinct layout of their binding sites, as it would be expected from the independent evolution of the HoxA and HoxD regulatory landscapes. It is also possible that some ‘HoxA’ and ‘HoxD’ enhancers are bound by distinct combinations of transcription factors, in agreement with a subset of ‘HoxA’ enhancers having domains of activity within the developing limb distinct from the ‘HoxD’ enhancers (Figure 3; [15]). Notably, the differences in enhancer functional domains are consistent with the specificities of HoxA expression as illustrated in the presumptive digit one domain: while HoxD expression in digit one is restricted to Hoxd13 as a result of the quantitative collinearity [50]–[52], no such phenomenon is observed for HoxA genes, the regulation of which involves a digit one-specific enhancer not identified for HoxD genes [15].
The independent evolution of HoxA and HoxD regulatory landscapes suggested by the absence of obvious sequence conservation of their respective enhancers is further supported by several findings. First, the recent evidence that HoxA and HoxB clusters most likely stem from the duplication of an ancestral HoxA/B cluster [53] implies that putative ancestral regulatory modules common only to HoxA and HoxD should have been lost at HoxB and HoxC. This scenario however appears unlikely to account for the specific HoxA and HoxD regulation associated with hand/feet development as the tandem duplications of the ancestral Hox cluster that led to the four Vertebrate Hox clusters occurred prior to the fin-to-limb transition. Second, there is a major difference in the layout of the HoxA and HoxD regulatory landscapes controlling their expression in the developing hand/feet. While HoxD-associated enhancers are part of a gene desert [15], [48]–[49], a large number of HoxA-associated enhancers are embedded in genes. Notably, HoxA enhancers with the highest enrichment of RNAP2, Med12 and p300, which also show the highest frequencies of interaction with HoxA genes, are located within Hibadh and Jazf1. Moreover, the genomic domain between HoxA and Jazf1, has undergone significant expansion from fish to mice (about 50 kb in fish and 800 kb in mice), indicating that an extensive genomic reshuffling at the HoxA regulatory landscape occurred during the fin-to-limb transition, which further support an independent evolution of the HoxA and HoxD regulatory landscapes. Interestingly, this genome expansion affected both the size of Hibadh, Jazf1 and the intergenic regions. The absence of preferential localization of HoxA-associated enhancers in gene-free regions thus suggests that introns are equally amenable to sequence evolution and emergence of new regulatory elements.
Although enhancers controlling HoxA and HoxD expression in distal limb most likely emerged independently, in both cases the distal limb regulatory landscape is located within the TAD containing the 5′ genes ([45] and this work). As long-range physical contacts between DNA sequences preferentially occur within TADs, it is conceivable that topological constraints have influenced the evolution of HoxA and HoxD regulatory landscapes associated with their distal limb expression. Interestingly, the early/proximal limb regulatory landscape of HoxD was identified on the opposite side of the gene cluster, within a TAD containing the 3′ HoxD genes, and not contacting Hoxd13 [45]. Whether the existence of a TAD boundary within the HoxA and HoxD clusters has favored the differential expression of Hox genes in proximal and distal limb bud or spatially constrained the emergence of proximal and distal limb enhancers remains unclear. Nonetheless, the deleterious modification of proximal limb development upon expression of Hoxa13 or Hoxd13 in early/proximal limb bud [54] raises the possibility that the TAD boundary embedded in both the HoxA and HoxD clusters has influenced the evolution of the tetrapod limb morphology. Although, chromosome partitioning into TADs remains to be characterized in most animal species, the presence of a TAD boundary embedded in each Hox cluster both in mice and humans [31] suggests a possible widespread impact of genome topology on the evolution of Hox regulation, and perhaps more generally on the evolution of regulatory landscapes.
In summary, our study reveals that extensive three-dimensional chromatin interactions control the expression of HoxA genes in developing limbs by forming distinct topological domains containing limb enhancers, which interact with each other and with the topological domains containing their target genes. Although this chromatin architecture is tissue-specific, our data suggests that it forms independently of enhancer activity, and is strengthened upon enhancer activation. Importantly, our data provide evidence that target genes and regulatory elements physically interact with each other through contacts between sub-TADs rather than by the formation of discreet “DNA loops”. From an evolutionary standpoint, the identification of HoxA-associated enhancers in limbs reveals major differences with the HoxD regulatory landscape suggesting that the changes in HoxA and HoxD regulation associated with the emergence of the hand/foot likely occurred through the independent emergence of regulatory sequences but common topological constraints.
Materials and Methods
Mice lines and transgenics
The HoxAFlox, HoxADelNeo, IR50, and Shh−/− mice lines were described previously [1]–[2], [40]. Candidate enhancer sequences identified from ChIP-seq data were amplified by PCR using the primer sequences reported in Table S1 and PCR products were verified by sequencing. Enhancer sequences were cloned upstream of the chicken β-globin minimal promoter and the LacZΔCpG NLS reporter. Transgenic embryos were generated by pronuclear injections, and at least three transgenic embryos per construct were analyzed. The stable mouse line for e5 was generated using the same protocol.
X-Gal staining and whole-mount in situ hybridization
X-Gal staining was performed on E12.5 embryos following standard procedures. In situ hybridization was conducted using a standard procedure [55]. Hygromycin and Neomycin probes were generated as described previously [2].
Isolation and fixation of cells for ChIP-seq, 3C, and 5C analysis
Distal limb and head tissues were dissected at E12.5 for wt and at E11.5 for Shh−/ − mice. Tissues were collected in 1×PBS containing 10% FBS (100 µl per embryo), and the samples were incubated 20 min at 37°C with collagenase (0.025% final concentration) to obtain single-cell suspensions. The number of cells in suspension was then counted under the microscope, and each sample was diluted in 9 ml of 1×PBS containing 10% FBS (5 ml for Shh−/ − embryos). The cells were then fixed with 1% formaldehyde for 10 min at room temperature. Crosslinking was stopped with glycine (125 mM final concentration), and incubated 5 min at room temperature followed by 15 min on ice. Cells were centrifuged at 400 g for 10 min at 4°C. Supernatants were removed and the cell pellets were flash frozen on dry ice.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as previously described with some modifications [56]–[57]. Briefly, chromatin from 5 million cells was sonicated using a Branson Sonicator 450D to obtain fragments with average sizes ranging between 100–600 bp. Cell debris was removed by centrifugation at 20,000 g for 15 min at 4°C and aliquots of the supernatant were taken for quantification and to confirm proper sonication. Remaining samples were stored at −80°C until use. Chromatin from 5 million cells was used for each immunoprecipitation. Protein G Dynal Beads (Invitrogen) were incubated 8 hours at 4°C with either 5 or 10 µg of antibodies. The chromatin was then incubated with the beads overnight. Immunoprecipitated complexes were sequentially washed in low salt (150 mM NaCl, 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.0)), medium salt (250 mM NaCl, 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.0)), LiCl (0.25 M LiCl, 0.5% NP40, 0.5% Na-Deoxycholate, 1 mM EDTA, 10 mM Tris-HCl (pH 8.0)), and 1×TE buffers. The protein/DNA complexes were eluted in an SDS buffer (1% SDS, 50 mM Tris (pH 8.0), 10 mM EDTA) by incubation at 65°C for 15 min on a rotating platform. Crosslinks were reversed by incubating the complexes at 65°C overnight. Samples were treated one hour at 55°C with RNAseA (0.2 µg/ml final concentration) and then with Proteinase K for two hours. Finally, the DNA was purified on QIAquick columns (Qiagen). Specific antibodies for Med12 and RNAP2 were purchased from Bethyl (A300-774A) and Abcam (ab5131), respectively.
ChIP sequencing and analysis
The ChIPed material was sequenced on a Hi-Seq 2000 high-throughput DNA sequencer. Sequencing libraries were prepared from 31 ng (RNAP2), 5 ng (Med12), and 345 ng (input) of ChIPed DNA. The libraries and flow cells were prepared by the IRCM Molecular Biology and Functional Genomics platform. The libraries were multiplexed and sequenced on one lane. The sequencing was performed by the McGill University and Génome Québec Innovation Centre following recommendations by the manufacturer (Illumina, San Diego, CA).
For RNAP2, Med12, and the input, we obtained a total of 151,045,509, 110,507,927, and 98,043,425 sequence reads, respectively. The first base of each sequence read was trimmed to ensure high base calling quality. The trimmed reads were mapped to the mouse mm9 genome assembly with Bowtie using the –best parameters [58]. To identify the highly significant RNAP2 and Med12 peaks, we used the MACS 1.4.1 peak finder with the following parameters: --format SAM --wig --bw 250 --mfold 7,30 -pvalue 1e–5 -g mm [59].
Redundant reads were filtered out for peak finding and wiggle file generation. Thus the wiggle files enclose the total number of uniquely mapped and non-redundant reads. After processing the data, the number of sequence reads we obtained was 129,222,085 for RNAP2, 91,816,355 for Med12, and 88,141,136 for the input. The position of RNAP2 and Med12 peaks genome-wide identified in our study is provided in Tables S21 and S22, respectively. We also provide the wig files for the data on chromosome 6 (Dataset S1, S2 and S3).
3C library preparation for a large number of cells (2×106–107 cells)
Limb and head cell pellets were treated as previously described [29], [60]. Briefly, 10 million fixed cells (2.87 million for Shh−/ − library used for the 3C experiments) were incubated for 15 min on ice in 200 µl of lysis buffer (10 mM Tris (pH 8.0), 10 mM NaCl, 0.2% NP40, supplemented with fresh protease inhibitor cocktail). Cells were then disrupted on ice with a dounce homogenizer (pestle B; 2×20 strokes). Cell suspensions were transferred to eppendorf tubes and centrifuged 5 min at 2000 g. Supernatants were removed and the cell pellets were washed twice with 100 µl of 1×EcoRI buffer (NEB). After the second wash, the cell pellet was resuspended in 100 µl of 1×EcoRI buffer, and divided into two eppendorf tubes containing 50 µl of cell suspension. 1×EcoRI buffer (337 µl) was added to each tube, and the mixture was incubated 10 min at 65°C with 0.1% SDS final (38 µl). Triton X-100 (44 µl of 10% Triton X-100) was added before overnight digestion with EcoRI (400 Units). The restriction enzyme was then inactivated by adding 86 µl of 10% SDS, and incubating 30 min at 65°C. Samples were then individually diluted into 7.62 ml of ligation mix (750 µl of 10% Triton X-100, 750 µl of 10×ligation buffer, 80 µl of 10 mg/ml of BSA, 80 µl of 100 mM ATP and 3000 Cohesive end Units of T4 DNA ligase). Ligation was carried out at 16°C for 2 hours.
3C libraries were then incubated overnight at 65°C with 50 µl Proteinase K (10 mg/ml), and with an additional 50 µl Proteinase K the following day for 2 hours. The DNA was purified by one phenol and one phenol-chloroform extraction, and precipitated with 0.1 volume of 3M NaOAc pH 5.2 (800 µl) and 2.5 volumes of cold EtOH (20 ml). After at least 1 h at −80°C, the DNA was centrifuged 25 min at 20,000 g at 4°C, and the pellets were washed with cold 70% EtOH. The DNA was resuspended in 400 µl of 1×TE pH 8.0, and transferred to eppendorf tubes for another phenol-chloroform extraction and precipitation with 0.1 volume of 3M NaOAc pH 5.2 (40 µl) and 2.5 volumes of cold EtOH (1.1 ml). DNA was recovered by centrifugation (25 min at maximum speed at 4°C), and washed eight times with cold 70%EtOH. The pellets were then dissolved in 100 µl of 1×TE pH 8.0, and incubated with RNAse A (1 µl at 10 mg/ml) for 15 min at 37°C.
3C library preparation for a small number of cells (106 cells)
This protocol was used to produce the 5C data for the distal limb, Shh−/− distal limb, and head shown in Figure 4. The protocol is essentially the same as the one described for samples containing 2 to 10 million cells, with some modifications. Briefly, one million fixed cells were incubated for 15 min on ice in 200 µl of lysis buffer (10 mM Tris (pH 8.0), 10 mM NaCl, 0.2% NP40 supplemented with fresh protease inhibitor cocktail). Cells were then disrupted on ice with a dounce homogenizer (pestle B; 2×20 strokes). Cell suspensions were transferred to eppendorf tubes and centrifuged 5 min at 2000 g. Supernatants were removed and the cell pellets were washed twice with 100 µl of 1×EcoRI buffer (NEB).
After the second wash, the cell pellet was resuspended in 50 µl of 1×EcoRI buffer. 1×EcoRI buffer (337 µl) was added to each tube, and the mixture was incubated 10 min at 65°C with 0.1% SDS final (38 µl). Triton X-100 (44 µl of 10% Triton X-100) was added before overnight digestion with EcoRI (400 Units). The restriction enzyme was then inactivated by incubating 30 min at 65°C. Ligation was performed in 600 µl (450 µl of digestion product, 15 µl of 10% Triton-X-100, 60 µl of ligase buffer, 6 µl of 10 mg/ml of BSA, 6 µl of 10 mM ATP, and 300 Cohesive end Units of T4 DNA ligase). Ligation was carried out at 16°C for 4 hours.
3C libraries were then incubated overnight at 65°C with 15 µl Proteinase K (10 mg/ml), and with an additional 15 µl Proteinase K the following day for 2 hours. The DNA was purified by one phenol and two phenol-chloroform extractions, and precipitated with 0.1 volume of 3M NaOAc pH 5.2 (64 µl) and 2.5 volumes of cold EtOH (1740 µl). After at least 1 h at −80°C, the DNA was centrifuged 25 min at maximum speed at 4°C, and the pellets were washed once with cold 70% EtOH. The DNA was resuspended in 50 µl of 1×TE pH 8.0, and incubated with RNAse A (1 µl at 10 mg/ml) for 15 min at 37°C.
Design and preparation of control 3C libraries
As 3C products are quantified by PCR amplification of expected ligation junctions with different primer pairs, differences in PCR primer pair efficiencies must be corrected using control 3C libraries. Control libraries were generated from bacterial artificial chromosomes (BACs) as previously described [29] and contain equimolar ratios of all possible 3C contacts. Briefly, BAC clones covering the HoxA region (mm9, chr6 : 51,946,668–52,656,241), and one USP22 control region (mm9, chr11 : 60,890,403–61,093,236)) were mixed in equimolar ratio. Mixed BACs were digested with EcoRI and randomly ligated with T4 DNA ligase (5700 Cohesive end Units) overnight at 16°C. BAC libraries were then purified by phenol-chloroform extraction. The libraries were generated with the following BACs: RP23-420L19, RP24-359H1, RP24-242G11, RP-347D14, RP23-305I5 (Invitrogen, CHORI). These libraries were used only to correct primer pair efficiencies during 3C analysis and not in the 5C experiments.
3C analysis
3C primers were designed using the ‘3CPrimer’ program (http://dostielab.biochem.mcgill.ca), and sequences are listed in Table S2. Three reactions using the control BAC library and three reactions using each 3C library were generated for each primer pair. The PCR conditions were described elsewhere [29]. 3C PCR products were resolved on agarose gel containing ethidium bromide and quantified using a ChemiDoc XRS system featuring a 12-bit digital camera and the Quantity One computer software (version 4.6.3; BioRad). Interaction frequencies (IF) were measured by dividing the value of each template PCR reactions by the value of each of the three control PCR reactions. The nine values were then average to determine the normalized interaction frequency. Three biological replicates were averaged after normalization for the wt limb and head. Normalization between different libraries was done using the compaction profiles for the USP22 region and an intergenic region within HoxA region as a reference.
5C primer and library design
5C primers covering the USP22 region (mm9, chr11 : 60,917,307–61,017,307) and the HoxA region (mm9, chr6 : 52,099,908–53,050,000) were designed using ‘my5C.primer’ [61] and the following parameters: optimal primer length of 30 nt, optimal TM of 65°C, default primer quality parameters (mer:800, U-blast:3, S-blasr:50). The sequences of these primers are listed in Table S3 and S4. Primers were not designed for large (>20 kb) and small (<100 bp) restriction fragments. Low complexity and repetitive sequences were excluded from our experimental designs such that not all fragments could be probed in our assays. Primers with several genomic targets were also removed.
The universal A-key (CCATCTCATCCCTGCGTGTCTCCGACTCAG-(5C-specific)) and the P1-key tails ((5C-specific)-ATCACCGACTGCCCATAGAGAGG) were added to the Forward and Reverse 5C primers, respectively. Reverse 5C primers were phosphorylated at their 5′ ends. Two experimental designs were used in our study. In the “cluster R” design (anchored 5C scheme, Figure 2, Figure S1, Figure 4C, Figure S3), Reverse 5C primers covered the HoxA cluster while Forward 5C primers tiled the surrounding upstream region. In this design, we used 142 Forward and 39 Reverse 5C primers (133 Forward/30 Reverse for the HoxA region, 9 Forward/9 Reverse USP22 region). In the “FR” design (alternating 5C scheme, Figure 5, Figure S3), alternating Forward and Reverse 5C primers covering the entire HoxA region were used to generate the 5C libraries. This design used 194 primers (86 Forward/90 Reverse for the HoxA region, 9 Forward/9 Reverse USP22 region). Primer sequences are listed in Table S3 (anchored “R” design) and S4 (alternating “FR” design).
5C library preparation
5C libraries were prepared and amplified with the A-key and P1-key primers following a procedure described previously [30]. Briefly, 3C libraries were first titrated by PCR for quality control (single band, absence of primer dimers, etc.), and to verify that contacts were amplified at frequencies similar to what is usually obtained from comparable libraries (same DNA amount from the same species and karyotype) [29], [62]–[63]. We also verified the quality of the 3C libraries by generating a compaction profile in the USP22 region. In general, we used approximately 1.5 µg of 3C library per 5C ligation reaction when the libraries were generated from a large number of cells (2×106 to 107 cells). When 3C libraries were generated from a small cell number (106 cells), we used approximately 1 µg of DNA.
Before adding the 3C libraries to the reaction tubes, 5C primer stocks (20 µM) were diluted individually in water on ice, and mixed to a final concentration of 0.002 µM. Mixed diluted primers (1.7 µl) were combined with 1 µl of annealing buffer (10×NEBuffer 4, New England Biolabs Inc.) on ice in reaction tubes. Salmon testis DNA (1.5 µg) was added to each 5C reaction, followed by the 3C libraries and water for a final volume of 10 µl. Samples were denatured at 95°C for 5 min, and annealed at 55°C for 16 hours. Ligation with Taq DNA ligase (10 U) was performed at 55°C for one hour. One tenth (3 µl) of each ligation was then PCR-amplified individually with primers against the A-key and P1-key primer tails. We used 28 cycles based on dilution series showing linear PCR amplification within that cycle range. The products from 2 (for the 3C libraries prepared from a large number of cells) to 8 (for the 3C libraries prepared from 106 cells) PCR reactions were pooled before purifying the DNA on MinElute columns (Qiagen).
5C libraries were quantified on agarose gel and diluted to 0.0534 ng/µl (for Xpress Template Kit v2.0) or 0.0216 ng/µl (for Ion PGM Template OT2 200 kit). One microliter of diluted 5C library was used for sequencing with an Ion PGM Sequencer. Samples were sequenced onto Ion 316 Chips following either the Ion Xpress Template Kit v2.0, and Ion Sequencing Kit v2.0 protocols, or the Ion PGM Template OT2 200 Kit, and Ion PGM Sequencing 200 Kit v2.0 protocols as recommended by the manufacturer's instructions (Life Technologies).
5C analysis
Analysis of the 5C sequencing data was performed as described earlier [30]. The sequencing data was processed through a Torrent 5C data transformation pipeline on Galaxy (https://main.g2.bx.psu.edu/). Briefly, the data was mapped against a customized reference file with TMAP. The reference file contained a list of all possible contacts between Forward and Reverse 5C primers covering our regions. The data was then filtered to remove low-quality reads (MAQ quality score of lower than 30), reads aligning more than two nucleotides away from the reference sequence start site, and reads which do not contain EcoRI restriction sites. This analysis generates an excel sheet containing interaction frequency lists (IFL) as well as a text file, which was used to visualize results using ‘my5C-heatmap’ [61]. Limb-enriched 5C interactions were obtained by subtracting limb and head 5C-seq data. Data was normalized by dividing the number of reads of each 5C contact by the total number of reads from the corresponding sequence run. All scales correspond to this ratio multiplied by 103. The number of total reads and of used reads is provided for each experiment in Table S5. 5C data are provided in Tables S6 to S20 and can be downloaded from our website: http://dostielab.biochem.mcgill.ca/
Databases and URLs
The limb p300 and H3K27Ac datasets (Acc. No. GSE13845 and GSE30641) are from E11.5 embryos, and were downloaded from the Gene Expression Omnibus (GEO) website http://www.ncbi.nlm.nih.gov/geo/. The my5C-primer and my5C-heatmap bioinformatics tools can be found at http://3dg.umassmed.edu/my5Cheatmap/heatmap.php
Supporting Information
Zdroje
1. KmitaM, TarchiniB, ZakanyJ, LoganM, TabinCJ, et al. (2005) Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature 435 : 1113–1116.
2. ScottiM, KmitaM (2012) Recruitment of 5′ Hoxa genes in the allantois is essential for proper extra-embryonic function in placental mammals. Development 139(4): 731–9.
3. Medina-MartinezO, BradleyA, Ramirez-SolisR (2000) A large targeted deletion of Hoxb1-Hoxb9 produces a series of single-segment anterior homeotic transformations. Dev Biol 222 : 71–83.
4. SpitzF, GonzalezF, PeichelC, VogtTF, DubouleD, et al. (2001) Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations. Genes Dev 15 : 2209–2214.
5. SuemoriH, NoguchiS (2000) Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Dev Biol 220 : 333–342.
6. Fromental-RamainC, WarotX, MessadecqN, LeMeurM, DolleP, et al. (1996) Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development 122 : 2997–3011.
7. ShautCA, KeeneDR, SorensenLK, LiDY, StadlerHS (2008) HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification. PLoS Genet 4: e1000073.
8. Fromental-RamainC, WarotX, LakkarajuS, FavierB, HaackH, et al. (1996) Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 122 : 461–472.
9. WellikDM, CapecchiMR (2003) Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science 301 : 363–367.
10. DavisAP, WitteDP, Hsieh-LiHM, PotterSS, CapecchiMR (1995) Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375 : 791–795.
11. BouletAM, CapecchiMR (2004) Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development 131 : 299–309.
12. ZakanyJ, DubouleD (2007) The role of Hox genes during vertebrate limb development. Current opinion in genetics & development 17 : 359–366.
13. TarchiniB, DubouleD (2006) Control of Hoxd genes' collinearity during early limb development. Dev Cell 10 : 93–103.
14. MontavonT, DubouleD (2012) Landscapes and archipelagos: spatial organization of gene regulation in vertebrates. Trends Cell Biol 22 : 347–354.
15. MontavonT, SoshnikovaN, MascrezB, JoyeE, ThevenetL, et al. (2011) A regulatory archipelago controls Hox genes transcription in digits. Cell 147 : 1132–1145.
16. LehoczkyJA, WilliamsME, InnisJW (2004) Conserved expression domains for genes upstream and within the HoxA and HoxD clusters suggests a long-range enhancer existed before cluster duplication. Evolution & development 6 : 423–430.
17. LehoczkyJA, InnisJW (2008) BAC transgenic analysis reveals enhancers sufficient for Hoxa13 and neighborhood gene expression in mouse embryonic distal limbs and genital bud. Evolution & development 10 : 421–432.
18. DostieJ, BickmoreWA (2012) Chromosome organization in the nucleus - charting new territory across the Hi-Cs. Curr Opin Genet Dev 22 : 125–131.
19. EthierSD, MiuraH, DostieJ (2012) Discovering genome regulation with 3C and 3C-related technologies. Biochimica et biophysica acta 1819 : 401–410.
20. WilliamsonI, EskelandR, LetticeLA, HillAE, BoyleS, et al. (2012) Anterior-posterior differences in HoxD chromatin topology in limb development. Development 139 : 3157–3167.
21. AmanoT, SagaiT, TanabeH, MizushinaY, NakazawaH, et al. (2009) Chromosomal dynamics at the Shh locus: limb bud-specific differential regulation of competence and active transcription. Dev Cell 16 : 47–57.
22. MeloCA, DrostJ, WijchersPJ, van de WerkenH, de WitE, et al. (2013) eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 49 : 524–535.
23. VakocCR, LettingDL, GheldofN, SawadoT, BenderMA, et al. (2005) Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell 17 : 453–462.
24. DengW, LeeJ, WangH, MillerJ, ReikA, et al. (2012) Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell 149 : 1233–1244.
25. RenX, SiegelR, KimU, RoederRG (2011) Direct interactions of OCA-B and TFII-I regulate immunoglobulin heavy-chain gene transcription by facilitating enhancer-promoter communication. Mol Cell 42 : 342–355.
26. KageyMH, NewmanJJ, BilodeauS, ZhanY, OrlandoDA, et al. (2010) Mediator and cohesin connect gene expression and chromatin architecture. Nature 467 : 430–435.
27. ViselA, BlowMJ, LiZ, ZhangT, AkiyamaJA, et al. (2009) ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457 : 854–858.
28. CotneyJ, LengJ, OhS, DeMareLE, ReillySK, et al. (2012) Chromatin state signatures associated with tissue-specific gene expression and enhancer activity in the embryonic limb. Genome research 22 : 1069–1080.
29. DostieJ, DekkerJ (2007) Mapping networks of physical interactions between genomic elements using 5C technology. Nature protocols 2 : 988–1002.
30. FraserJ, EthierSD, MiuraH, DostieJ (2012) A Torrent of Data: Mapping Chromatin Organization Using 5C and High-Throughput Sequencing. Methods in enzymology 513 : 113–141.
31. DixonJR, SelvarajS, YueF, KimA, LiY, et al. (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485 : 376–380.
32. NoraEP, LajoieBR, SchulzEG, GiorgettiL, OkamotoI, et al. (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485 : 381–385.
33. Phillips-CreminsJE, SauriaME, SanyalA, GerasimovaTI, LajoieBR, et al. (2013) Architectural Protein Subclasses Shape 3D Organization of Genomes during Lineage Commitment. Cell 153 : 1281–1295.
34. MerkenschlagerM, OdomDT (2013) CTCF and cohesin: linking gene regulatory elements with their targets. Cell 152 : 1285–1297.
35. DeMareLE, LengJ, CotneyJ, ReillySK, YinJ, et al. (2013) The genomic landscape of cohesin-associated chromatin interactions. Genome Res 23 : 1224–1234.
36. LitingtungY, DahnRD, LiY, FallonJF, ChiangC (2002) Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418 : 979–983.
37. te WelscherP, ZunigaA, KuijperS, DrenthT, GoedemansHJ, et al. (2002) Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298 : 827–830.
38. MethotN, BaslerK (1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96 : 819–831.
39. VokesSA, JiH, WongWH, McMahonAP (2008) A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes & development 22 : 2651–2663.
40. ChiangC, LitingtungY, LeeE, YoungKE, CordenJL, et al. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383 : 407–413.
41. ShethR, GregoireD, DumouchelA, ScottiM, PhamJM, et al. (2013) Decoupling the function of Hox and Shh in developing limb reveals multiple inputs of Hox genes on limb growth. Development 140 : 2130–2138.
42. SextonT, YaffeE, KenigsbergE, BantigniesF, LeblancB, et al. (2012) Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148 : 458–472.
43. NoraEP, DekkerJ, HeardE (2013) Segmental folding of chromosomes: A basis for structural and regulatory chromosomal neighborhoods? Bioessays 35(9): 818–28.
44. CavalliG, MisteliT (2013) Functional implications of genome topology. Nat Struct Mol Biol 20 : 290–299.
45. AndreyG, MontavonT, MascrezB, GonzalezF, NoordermeerD, et al. (2013) A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science 340 : 1234167.
46. BoettigerAN, LevineM (2009) Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science 325 : 471–473.
47. PerryMW, BoettigerAN, BothmaJP, LevineM (2010) Shadow enhancers foster robustness of Drosophila gastrulation. Curr Biol 20 : 1562–1567.
48. SpitzF, GonzalezF, DubouleD (2003) A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 113 : 405–417.
49. GonzalezF, DubouleD, SpitzF (2007) Transgenic analysis of Hoxd gene regulation during digit development. Dev Biol 306 : 847–859.
50. DolleP, DierichA, LeMeurM, SchimmangT, SchuhbaurB, et al. (1993) Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell 75 : 431–441.
51. KmitaM, FraudeauN, HeraultY, DubouleD (2002) Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature 420 : 145–150.
52. MontavonT, Le GarrecJF, KerszbergM, DubouleD (2008) Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev 22 : 346–359.
53. SoshnikovaN, DewaeleR, JanvierP, KrumlaufR, DubouleD (2013) Duplications of hox gene clusters and the emergence of vertebrates. Dev Biol 378 : 194–199.
54. WilliamsME, LehoczkyJA, InnisJW (2006) A group 13 homeodomain is neither necessary nor sufficient for posterior prevalence in the mouse limb. Dev Biol 297 : 493–507.
55. KondoT, ZakanyJ, DubouleD (1998) Control of colinearity in AbdB genes of the mouse HoxD complex. Molecular cell 1 : 289–300.
56. LeeTI, JohnstoneSE, YoungRA (2006) Chromatin immunoprecipitation and microarray-based analysis of protein location. Nature protocols 1 : 729–748.
57. MiuraH, TomaruY, NakanishiM, KondoS, HayashizakiY, et al. (2009) Identification of DNA regions and a set of transcriptional regulatory factors involved in transcriptional regulation of several human liver-enriched transcription factor genes. Nucleic acids research 37 : 778–792.
58. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25.
59. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome biology 9: R137.
60. FerraiuoloMA, RousseauM, MiyamotoC, ShenkerS, WangXQ, et al. (2010) The three-dimensional architecture of Hox cluster silencing. Nucleic acids research 38 : 7472–7484.
61. LajoieBR, van BerkumNL, SanyalA, DekkerJ (2009) My5C: web tools for chromosome conformation capture studies. Nature methods 6 : 690–691.
62. FraserJ, RousseauM, BlanchetteM, DostieJ (2010) Computing chromosome conformation. Methods in molecular biology 674 : 251–268.
63. DostieJ, ZhanY, DekkerJ (2007) Chromosome conformation capture carbon copy technology. Curr Protoc Mol Biol Chapter 21: Unit 21 14.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



