-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
The functionality of sensory neurons is defined by the expression of specific sensory receptor genes. During the development of the Drosophila larval eye, photoreceptor neurons (PRs) make a binary choice to express either the blue-sensitive Rhodopsin 5 (Rh5) or the green-sensitive Rhodopsin 6 (Rh6). Later during metamorphosis, ecdysone signaling induces a cell fate and sensory receptor switch: Rh5-PRs are re-programmed to express Rh6 and become the eyelet, a small group of extraretinal PRs involved in circadian entrainment. However, the genetic and molecular mechanisms of how the binary cell fate decisions are made and switched remain poorly understood. We show that interplay of two transcription factors Senseless (Sens) and Hazy control cell fate decisions, terminal differentiation of the larval eye and its transformation into eyelet. During initial differentiation, a pulse of Sens expression in primary precursors regulates their differentiation into Rh5-PRs and repression of an alternative Rh6-cell fate. Later, during the transformation of the larval eye into the adult eyelet, Sens serves as an anti-apoptotic factor in Rh5-PRs, which helps in promoting survival of Rh5-PRs during metamorphosis and is subsequently required for Rh6 expression. Comparably, during PR differentiation Hazy functions in initiation and maintenance of rhodopsin expression. Hazy represses Sens specifically in the Rh6-PRs, allowing them to die during metamorphosis. Our findings show that the same transcription factors regulate diverse aspects of larval and adult PR development at different stages and in a context-dependent manner.
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004027
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004027Summary
The functionality of sensory neurons is defined by the expression of specific sensory receptor genes. During the development of the Drosophila larval eye, photoreceptor neurons (PRs) make a binary choice to express either the blue-sensitive Rhodopsin 5 (Rh5) or the green-sensitive Rhodopsin 6 (Rh6). Later during metamorphosis, ecdysone signaling induces a cell fate and sensory receptor switch: Rh5-PRs are re-programmed to express Rh6 and become the eyelet, a small group of extraretinal PRs involved in circadian entrainment. However, the genetic and molecular mechanisms of how the binary cell fate decisions are made and switched remain poorly understood. We show that interplay of two transcription factors Senseless (Sens) and Hazy control cell fate decisions, terminal differentiation of the larval eye and its transformation into eyelet. During initial differentiation, a pulse of Sens expression in primary precursors regulates their differentiation into Rh5-PRs and repression of an alternative Rh6-cell fate. Later, during the transformation of the larval eye into the adult eyelet, Sens serves as an anti-apoptotic factor in Rh5-PRs, which helps in promoting survival of Rh5-PRs during metamorphosis and is subsequently required for Rh6 expression. Comparably, during PR differentiation Hazy functions in initiation and maintenance of rhodopsin expression. Hazy represses Sens specifically in the Rh6-PRs, allowing them to die during metamorphosis. Our findings show that the same transcription factors regulate diverse aspects of larval and adult PR development at different stages and in a context-dependent manner.
Introduction
Even though the complexity of eyes varies between animal species, their function remains the same: perception of visual information from the environment. Drosophila employs simple eyes during the larval stage and complex compound eyes during adulthood. The adult compound eye is a widely used model system to study eye development, sensory receptor expression and function [1], [2]. However, only little is known regarding the development of the visual system in the larva. The larval eye (also termed Bolwig Organ, BO) is simple, but plays important roles in navigation, circadian rhythm and even the formation of associative memories [3], [4], [5], [6]. Each larval eye is composed of 12 photoreceptor neurons (PRs) that are divided into two subtypes depending on the rhodopsin gene they express. Four PRs express the blue-sensitive Rhodopsin 5 (Rh5), while the remaining eight PRs express the green-sensitive Rhodopsin 6 (Rh6) [7]. All PRs of the larval eye develop during embryogenesis and are fully functional at larval hatching [8]. The development of larval PRs occurs in a two-step process: first, three or four primary precursors are specified by expressing the proneural gene atonal (ato) [9], [10]. In a second step, primary precursors recruit surrounding cells to develop into secondary precursors through Epidermal Growth Factor Receptor (EGFR) signaling [9]. Subsequently, primary precursors differentiate into Rh5-PRs, while secondary precursors develop into Rh6-PRs. Interestingly approximately the same ratio of Rh5 - to Rh6-expressing PRs (30∶70) exists in the adult retina [7], [11]. However, conversely to adult R8 PRs, where mutually exclusive expression of Rh5 and Rh6 is based on a stochastic mechanism [11], [12], [13], [14], Rh5 and Rh6 expression is initiated through a deterministic cell-fate specification mechanism in the larval eye.
Terminal differentiation and PR subtype specification in the larval eye requires the action of the transcription factors Spalt (Sal), Seven-up (Svp) and Orthodenticle (Otd) [7], three genes that are also involved in PR fate decision in the compound eye [15], [16], [17], [18]. During metamorphosis, the larval eye undergoes a transformation to become a group of extraretinal PRs (termed “eyelet”) involved in entrainment of the circadian clock [19], [20]. During this transformation, larval Rh5-PRs switch expression from Rh5 to Rh6, while Rh6-PRs undergo apoptotic cell death. Both processes are controlled by ecdysone signaling: interfering with Ecdysone Receptor (EcR) function in Rh6-PRs inhibits apoptosis, while inhibiting EcR signaling in Rh5-PRs blocks the switch of rhodopsins [21]. The genetic network acting downstream of EcR to control the rhodopsin switch or to induce apoptosis is currently unknown.
Here, we investigate the function of two key transcription factors controlling the development of the larval PRs and transformation of the larval eye to the adult eyelet. We show that the zinc finger transcription factor Senseless (Sens) acts in three steps in larval PR and eyelet development. First, a short pulse of Sens expression in primary precursors initiates a genetic feedforward loop to maintain the Rh5-cell fate, thereby acting as a binary switch between Rh5 - versus Rh6-PR cell fates. Moreover, Sens provides a second function during metamorphosis to suppress EcR-induced apoptosis in the Rh5-PR subtype. Finally, Sens is also necessary to promote Rh6 expression in the adult eyelet.
We further show that the homeodomain transcription factor Hazy (Flybase: Pph13 for PvuII-PstI homology 13) has two distinct roles during larval eye development and a third one during metamorphosis. Hazy is necessary for the initiation and maintenance of Rh5 and Rh6 expression in the larval eye, while initial subtype specification occurs normally. Hazy acts through a conserved motif present in the rhodopsin promoters, called Rhodopsin Core Sequence I (RCSI) [22], [23], [24]. The analysis of RCSI function led to two new findings that differ from the situation in the adult retina: First, Hazy acts through the RCSI of both Rh5 and Rh6 in the larva, whereas it affects only Rh6 in the adult. Second, neither the RCSI nor Hazy are required for activation of Rh6 in the eyelet, demonstrating that the regulation of the rh6 promoter is distinct in the larval and adult eyes compared to the adult eyelet. During metamorphosis, Hazy represses Sens in Rh6-PRs, thus allowing them to undergo apoptosis. Our findings show that a small set of transcription factors are used to regulate diverse aspects of larval and adult PR development at different stages and in a context-dependent manner.
Results
An early pulse of Sens initiates a feedforward loop to maintain primary precursor differentiation into Rh5-PRs
Specification of larval Rh5-PRs depends on the combinatorial action of Sal and Otd [7]. Sal is exclusively expressed in Rh5-PRs and promotes their differentiation. In sal mutants, primary precursors fail to fully differentiate and remain “empty” PRs lacking rhodopsin expression. Otd promotes Rh5 and represses Rh6 expression in Rh5-PRs. Conversely, Svp is exclusively expressed in Rh6-PRs, where it represses Sal and promotes Rh6 expression [7]. However, the mechanisms of how the cell fate choice of primary and secondary precursors is initially controlled remain unknown.
Since Sens is involved in initial specification steps in PRs of the adult compound eye [25], [26], we tested whether it has a similar role in the larval eye. First, we analyzed the expression of Sens. During embryonic stage 11, when primary and secondary precursors have been formed, cells of the larval eye detach from the optic lobe placode and start to express differentiation markers such as, Elav, Fasciclin II (FasII), Krüppel (Kr) and Hazy [27] (see below). Sens is specifically expressed in all primary precursors in a short highly dynamic pulse during embryonic stages 11 and 12 (Figure 1A–1E). Sens expression initiated first in two primary precursors at mid stage 11, and is subsequently upregulated in the remaining two primary precursors (Figure 1A, 1B and 1C). Expression of Sens ceases during mid-late stage 12, when primary precursors start to express Sal (Figure 1C, 1D and 1E), which is then maintained until their maturation into fully differentiated Rh5-PRs (Figure 1C, 1D, 1E and 1F). Since Sens is exclusively and transiently expressed in the precursors of the Rh5-PR subtype, we analyzed the expression of Rh5 and Rh6 in sens mutants at the end of embryogenesis when these mutants die. Even though the correct number of PRs is produced, no Rh5 expression can be found, while all 12 PRs express Rh6 (Figure 2B).
Fig. 1. Pulsed Sens expression during precursor development. 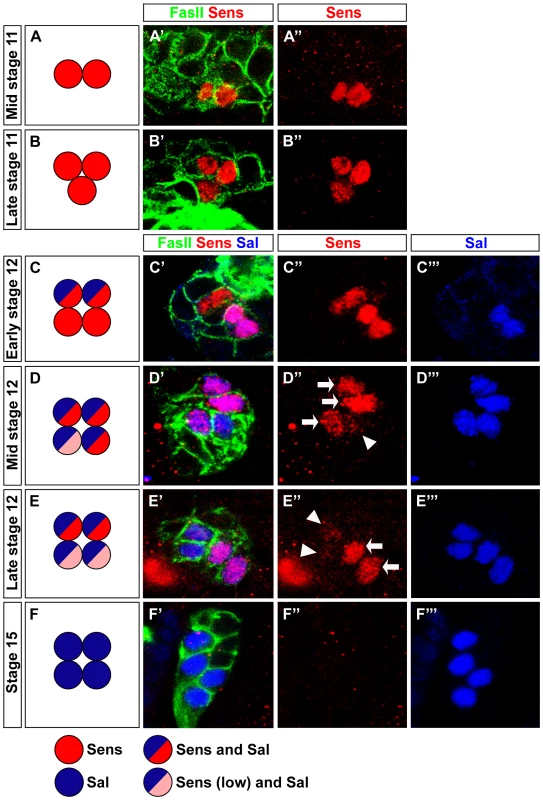
(A–F) Schematic representation of Sens and Sal expression during stage 11 and stage 12 in wild-type embryonic PRs. (A′–F′) Sens expression (red) from stage 11 to 15 in wild-type embryonic PRs stained with anti-FasII (green) and anti-Sal (blue); single confocal sections are shown. (A′, A″) Sens staining in mid stage 11 is detected in two cells. (B′, B″) In late stage 11, Sens staining is detected in three cells. (C′, C″, C‴) Sens staining in early stage 12 in four cells, co-expressed with Sal in two cells. (D′, D″, D‴) At mid stage 12, all cells express Sal, co-expression with Sens is found in three cells (high, arrow) and one cell (low, arrowhead). (E′, E″, E‴) Sens staining in late stage 12, all cells express Sal, co-expression with Sens is restricted to two cells (high) and very low residual expression is detected in the remaining two cells. (F′, F″, F‴) No Sens expression is seen at stage 15, while Sal expression is observed in all four cells. Fig. 2. Sens is required for Rh5-PR identity and acts in parallel with Otd. 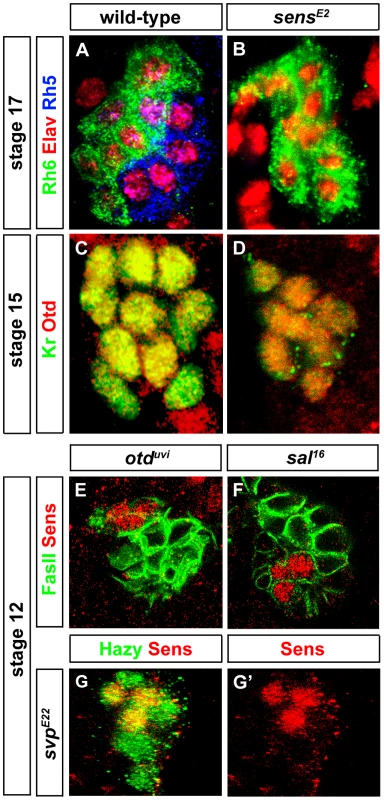
(A, B) Rh5 and Rh6 expression in wild-type and sensE2 mutant PRs during embryonic stage 17, stained with anti-Rh6 (green), anti-Rh5 (blue) and anti-Elav (red); z-projection of confocal sections. No Rh5 expression was seen in sensE2 mutants and all the cells were marked by Rh6. (C, D) Otd expression in wild-type and sensE2 mutant PRs during embryonic stage 15 stained with anti-Kr (green) and anti-Otd (red). Both in wild-type and sensE2 mutants, all the PR nuclei expresses Otd, showing that Otd was not affected in sensE2 mutant; z-projection of confocal sections. (E, F, G) Sens expression (red) in otduvi, sal16 and svpE22 mutant PRs during embryonic stage 12. Staining against FasII or Hazy (green), shows that Sens expression was not affected in these mutants; z-projection of confocal sections. Since sens mutants display the same rhodopsin expression phenotype as otd mutants [7], i.e., loss of Rh5 and gain of Rh6, we investigated the interactions between otd and sens. In sens mutants, all PRs express Otd comparable to wild-type (Figure 2C and 2D) and therefore, Otd does not act downstream of Sens. Conversely, we tested if sens expression depends on Otd. No change of Sens expression is observed in otd mutants (Figure 2E), suggesting that Sens and Otd act in parallel.
Since both Sal and Svp are key factors orchestrating differentiation of Rh5 - and Rh6-PRs, we analyzed the interaction between sens and sal or svp. In primary precursors, the pulse of Sens precedes Sal expression, suggesting that Sens regulates Sal. Indeed, in sens mutants, Sal expression is abolished (Figure 3B). Conversely, no change of Sens expression is observed in sal mutants (Figure 2F), suggesting that Sens acts genetically upstream of sal and its transient expression may function as a trigger that initiates Sal expression. Moreover, in sens mutants, all precursors express Svp, the repressor of Rh5-PR fate (Figure 3E), but Sens expression is normal in svp mutants (Figure 2G and 2G′). Thus, Sens acts upstream of both Sal and Svp: it has a dual role in primary precursor cell fate specification, as it a) genetically promotes the expression of Sal (an activator of Rh5 fate) and b) genetically represses Svp (a repressor of sal and Rh5 fate) (Figure 4E).
Fig. 3. Role of Sens in regulation of precursor differentiation and rhodopsin expression. 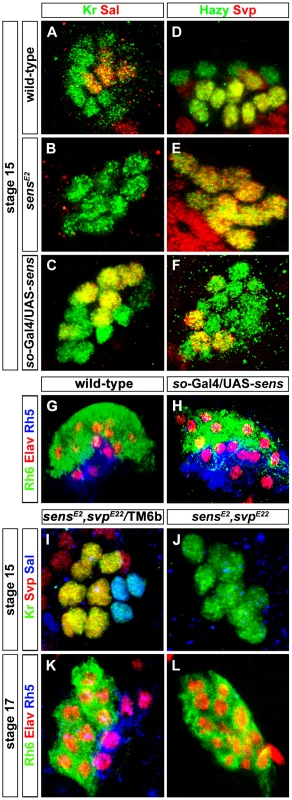
(A, B, C) Sal expression (red) in wild-type, sensE2 mutant and Sens over-expression (so-Gal4/UAS-sens) PRs during embryonic stage 15 stained with anti-Kr (green); z-projection of confocal sections. Sal expression was not detected in the sensE2 mutant, while an increase of Sal expressing cells was found in so-Gal4/UAS-sens over-expression, showing that Sens genetically interacts with Sal and promotes its activation (D, E, F) Svp expression (red) in wild-type, sensE2 mutant and Sens over-expression (so-Gal4/UAS-sens) PRs during embryonic stage 15, stained with anti-Hazy (green); z-projection of confocal sections. Svp was de-repressed in sensE2 mutant in all PRs, while a reduced number of Svp expressing cells was found in so-Gal4/UAS-sens over-expression, suggesting that Sens genetically interacts with Svp and promotes its repression. (G, H) Rh5 (blue) and Rh6 (green) expression in wild-type and so-Gal4/UAS-sens over-expression in third instar larval eye, stained with anti-Elav (red); z-projection of confocal sections. An increased number of Rh5 expressing cells were found in so-Gal4/UAS-sens over-expression. (I, J) Sal expression (Blue) in the heterozygous (control) and homozygous (sensE2, svpE22) double mutant PRs during stage 15, stained with anti-Kr (green) and anti-Svp (red); z-projection of confocal sections. Sal expression was not found in the homozygous double mutant PRs. (K, L) Rh5 and Rh6 expression in heterozygous (control) and homozygous (sensE2, svpE22) double mutant PRs during stage 17, stained with anti-Rh6 (green), anti-Elav (red) and anti-Rh5 (blue); z-projection of confocal sections. Rh5 expression was absent in the homozygous double mutant PRs, whereas all PRs expressed Rh6. Fig. 4. Role of Sens in the transformation of larval eye into the adult eyelet. 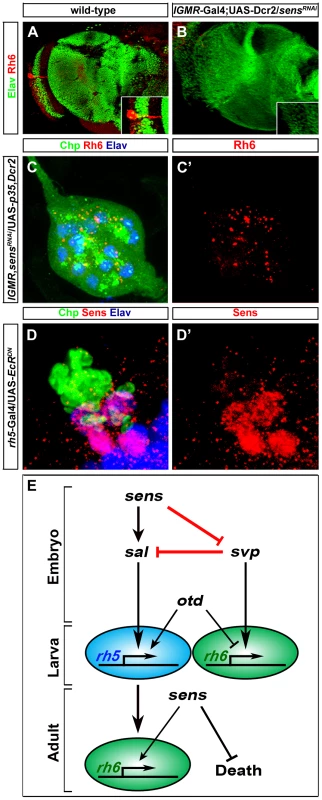
(A, B) Rh6 expression in wild-type and sensRNAi (lGMR-Gal4; UAS-Dcr2/UAS-sensRNAi) adult eyelets, stained with anti-Rh6 (red) and anti-Elav (green). In all lGMR-Gal4; UAS-Dcr2/UAS-sensRNAi animals, the eyelet was absent (inset: high magnification of eyelet position). (C, C′) Rh6 expression (red) in sensRNAi when p35 was ectopically expressed in the eyelet to keep the cells alive (UAS-sensRNAi/lGMR-Gal4; UAS-p35/UAS-Dcr2), stained with anti-Chp (green), and anti-elav (blue); z-projection of confocal sections. No Rh6 expression was found in the eyelet. (D, D′) Sens expression (red) in the eyelet when a dominant-negative form of EcR was ectopically expressed in Rh5-PRs (rh5-Gal4/UAS-EcRDN) and stained with anti-Chp (green) and anti-Elav (blue); z-projection of confocal sections. Sens was expressed in all four eyelet cells. (E) A model describing the role of Sens during different developmental stages. Since Sens regulates Svp and Sal in primary precursors, we next addressed whether Sens is sufficient for genetically activating sal and repressing svp in precursors of the larval eye. We ectopically expressed sens under the control of sine oculis-Gal4 (so-Gal4), which starts to be expressed early in precursors of the optic epithelium. Ectopic expression of Sens results in an increased number of Sal expressing cells and a reduction of Svp expressing cells (Figure 3C and 3F). This is in line with the data above that Sens acts to promote sal and to inhibit svp expression. The switch of Svp-expressing precursors to Sal-expressing precursors suggests that some of the secondary precursors have changed their identity to primary precursors, and thus, might have switched their Rhodopsin expression. Indeed, we found an increase of Rh5-PRs and a decrease of Rh6-PRs, while the overall number of PRs remained unaltered in so-Gal4/UAS-sens larvae (Figure 3H). Our findings support a model in which a pulsed expression of Sens acts to allow primary precursors to adopt Rh5-cell fate by genetically repressing the default Rh6-cell fate (Figure 4E).
svp is not only necessary for Rh6 expression, but also for the repression of sal in secondary precursors [7]. We therefore next investigated whether Sens-dependent Sal expression in primary precursors occur in a Svp-dependent or independent manner. In other words, Sal expression could be either due to direct activation by Sens or it could be an indirect result of Sens repressing Svp, which in turn represses Sal. To address this, we generated a sens, svp double mutant. If the activation of Sal is an indirect consequence of Svp repression by Sens, we would expect to observe a de-repression of Sal in all the PRs. Conversely if it is Svp-independent, we would expect to see the same phenotype as in sens mutant alone. We found that in sens, svp double mutants, Sal expression was still absent (Figure 3J), further suggesting that Sal expression is most likely due to activation by sens and not indirect due to relief or repression by Svp.
We next investigated whether Sens also functions genetically downstream of the Sal/Svp fate decision to regulate Rh5 expression or whether ectopic sal expression or loss of svp in sens mutants can rescue Rh5 expression. To address this, we investigated Rh5 and Rh6 expression in sens, svp double mutants at the end of embryogenesis, when these mutants die. Although the same number of PRs was produced compared to the wild-type, no Rh5 expression was found and all of them expressed Rh6 (Figure 3K and 3L). This finding suggests that Sens does not function downstream of Svp to regulate Rh5 expression.
We have previously shown that EGFR signaling is required to inhibit apoptosis in secondary precursors during the formation of the larval eye. Genetically inhibiting the EGFR pathway results in a larval eye comprising only 3–4 Rh5-expressing PRs [7], [9]. We next tested if EGFR signaling is also required for Sens expression in the developing primary precursors. We found that Sens expression was normal compared to the wild-type in the remaining primary precursors when inhibiting EGFR signaling by ectopic expression of a dominant negative form of EGFR (UAS-EGFRDN) under the control of so-Gal4 (Figure S1A and S1B). Thus, expression of Sens in developing PRs during embryogenesis occurs independently of EGFR signaling. ato has been shown to promote Sens expression in the eye antennal disc [25]. Since in ato mutant embryos primary precursors fail to develop, we were unable to assess if ato is necessary to promote Sens expression. Instead, we tested if ectopic expression of ato in all precursors is sufficient to induce and maintain Sens expression in the larval eye. However, ectopic expression of ato was not sufficient to induce Sens expression during embryogenesis (Figure S1E and S1F).
Reinitiated Sens expression in Rh5-PRs is required for cell survival during metamorphosis and Rh6 expression in the adult eyelet
During late third instar larval stage, Sens expression is reinitiated in Rh5-PRs and is maintained during metamorphosis, when these cells transform to become the adult eyelet [21]. To address the role of Sens in Rh5-PRs at this stage, we knocked-down Sens in all larval PRs by expressing sensRNAi together with Dicer-2 (Dcr-2), which has been shown to enhance RNAi potency [28] with the pan-PR lGMR-Gal4 driver. sens knockdown leads to a complete loss of eyelet PRs (Figure 4B). Rh6-PRs, which do not express Sens, undergo normal EcR mediated apoptotic cell death [21]. Misexpression of Sens in these cells is sufficient to inhibit apoptosis [21]. Thus, Sens acts as a PR-subtype specific survival factor for Rh5-PRs that become the adult eyelet (Figure 4E).
We next addressed whether besides blocking apoptotic cell death Sens might also be required for rhodopsin expression in the eyelet. We therefore knocked down sens in all larval PRs, but at the same time we kept the PRs alive by concomitantly expressing the apoptosis inhibitor p35 with the lGMR-Gal4 driver. This resulted in eyelets consisting of 12 PRs that failed to express Rh6 expression (Figure 4C and 4C′), suggesting that Sens is also required for Rh6 expression in the eyelet (Figure 4E).
Ecdysone signaling is required for the transformation of the larval eye into the adult eyelet. Genetically inhibiting EcR signaling in Rh6-PRs blocks apoptosis, while inhibiting EcR signaling in Rh5-PRs blocks the switch of rhodopsins [21]. We next asked if EcR signaling is required for Sens expression in the adult eyelet by ectopically expressing a dominant negative form of EcR (UAS-EcRDN) in Rh5-PRs using rh5-Gal4. Sens expression was unaltered (Figure 4D and D′), indicating that Sens expression in the eyelet is independent of EcR signaling.
In summary, Sens fulfills three temporally and functionally separable roles in the same cells at different developmental stages: First, it initiates precursor specification in early embryonic stages; second, it suppresses apoptosis and thus enables survival during metamorphosis in fully differentiated Rh5-PRs; and third, it is required in the eyelet for Rh6 expression (Figure 4E).
Hazy is not required for larval PR subtype specification
Hazy is expressed in developing precursors of the larval eye [27] and we therefore investigated its function in larval PRs. Hazy is expressed in all larval PRs starting in precursors at embryonic stage 12 (Figure 2G) and continues to be expressed throughout embryogenesis (Figure 5A). Hazy expression is further maintained in fully differentiated larval PRs (Figure 5B) and PRs of the adult eyelet (Figure 5C). Since Hazy is already expressed early in both precursor types, we investigated initial specification of primary and secondary precursors in hazy mutants. Specification of precursors appears to occur normally since Otd, Sal, Svp and Sens expression was not affected (Figure 5D, 5E, 5F and 5G). In hazy mutants, PRs differentiate normally as indicated by the expression of canonical PR differentiation markers such as Kr, Elav or FasII (Figure 5D, 5E and 5G). We next addressed if EGFR signaling is required for Hazy expression in PR precursors. We found that Hazy expression was not altered when blocking EGFR signaling (Figure S1C and S1D).
Fig. 5. Hazy functions in larval eye development by acting through the promoters of rh5 and rh6. 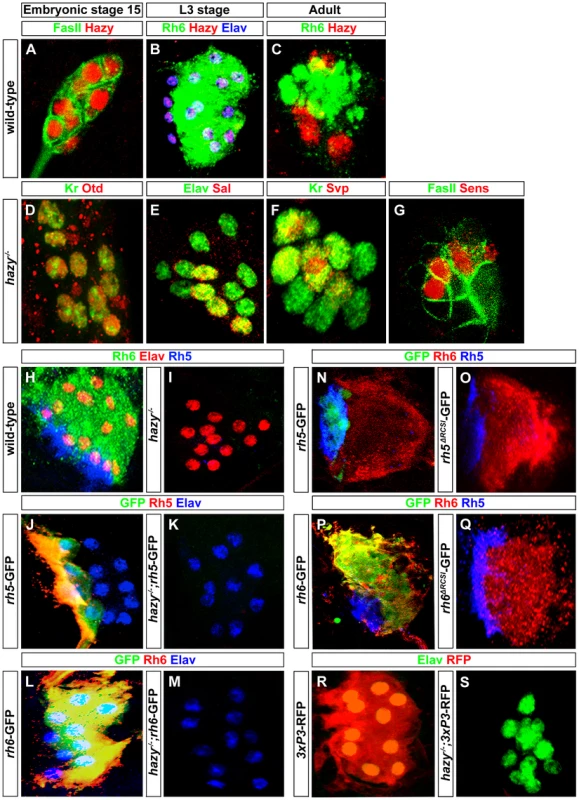
(A) Wild-type embryonic larval eye precursors of stage 15 stained with anti-Hazy (red) and anti-FasII (green); single confocal section. (B) Wild-type third instar larval eye stained with anti-Hazy (red), anti-Rh6 (green) and anti-Elav (blue). (C) Wild-type adult eyelet stained with anti-Hazy (red) and anti-Rh6 (green); z-projection of confocal sections. Hazy was expressed in all the PRs of embryonic larval eye precursors, third instar larval eye and in adult eyelet. hazy−/− mutant larval eyes stained with (D) anti-Otd (red) and anti-Kr (green), (E) anti-Elav (green) and anti-Sal (red). Embryonic larval eye precursors were stained with (F) anti-Kr (green) and anti-Svp (red), (G) anti-FasII (green) and anti-Sens (red). No change of Otd, Kr, Sal, Svp, and Sens expression was observed in hazy−/− mutants. (H, I) Wild-type and hazy−/− mutant third instar larval eyes stained with anti-Elav (red), anti-Rh6 (green) and anti-Rh5 (blue). Rh5 and Rh6 expression was lost in hazy−/− mutants. (J, K) Third instar larval eyes of wild-type reporter line of rh5 (rh5-GFP) and rh5-GFP in hazy−/− null background (hazy−/−; rh5-GFP), stained with anti-Rh5 (red), anti-GFP (green) and anti-Elav (blue). (L, M) Third instar larvae of wild-type reporter line of rh6 (rh6-GFP) and rh6-GFP in hazy−/− null background (hazy−/−; rh6-GFP) stained with anti-Rh6 (red), anti-GFP (green) and anti-Elav (blue); z-projection of confocal sections. No GFP expression was observed in the hazy−/− mutant background. (N, O, P, Q) rh5-GFP, rh5ΔRCSI-GFP, rh6-GFP and rh6ΔRCSI-GFP larval eyes stained with anti-Rh6 (red), anti-GFP (green) and anti-Rh5 (blue); z-projection of confocal sections. No GFP expression was observed in the larval eye of rh5ΔRCSI-GFP (O) and rh6ΔRCSI-GFP animals (Q). (R, S) Third instar larval eyes of 3XP3-RFP and 3XP3-RFP in hazy−/− null background (hazy−/−; 3XP3-RFP), stained with anti-RFP (red) and anti-Elav (green). No RFP expression was observed in the larval eye in hazy−/−; 3xP3-RFP animals. Hazy controls rhodopsin expression in the larval eye
Since Hazy controls rh6 expression, but not rh5 expression in the adult retina [29], we next investigated expression of Rh5 and Rh6 in hazy null mutant larvae. Neither Rh5 nor Rh6 expression was detected (Figure 5I). To address whether the lack of rh5 and rh6 expression occurs at the transcriptional level, we used rh5-GFP and rh6-GFP reporter lines: GFP expression was completely abolished in both hazy; rh5-GFP and hazy; rh6-GFP mutants (Figure 5J, 5K, 5L and 5M). Thus, Hazy is necessary for expression of both Rhodopsins in the larval eye, whereas it is only required for Rh6 in the adult eye.
In the adult retina, it has been suggested that Hazy acts through the rhodopsin core sequence I (RCSI) that is found in all proximal rhodopsin promoters [23], [30], [31]. We used rh5ΔRCSI -GFP and rh6ΔRCSI -GFP reporter lines in which the RCSI is deleted to study the requirement of the RCSI in the larval eye. In both cases, we observed a complete loss of GFP expression (Figure 5N, 5O, 5P and 5Q), which demonstrates that the RCSI is necessary for rh5 and rh6 expression in larval PRs. Moreover, Hazy is required for the activation of a generic version of the RCSI called P3 that is sufficient when multimerized to drive reporter expression in all PRs [30]. After introducing the 3×P3-RFP in a hazy mutant background, we observed a complete lack of RFP expression in the larval eye (Figure 5R and 5S). This result further supports that Hazy acts directly through the RCSI sites of rh5 and rh6 to activate their expression in larval PRs.
Temporal rescue unveils a dual role of hazy in Rhodopsin expression
In order to address at which time point during PR development Hazy functions, we rescued the hazy mutant phenotype by expressing hazy under the control of a heat shock inducible promoter (hs-hazy) at distinct developmental stages. A heat-shock was given at 37°C for 30 minutes and rhodopsin expression was assessed after larval hatching. Heat shocks at embryonic stage 12 resulted in a rescue of both Rh5 and Rh6 expression in the first larval instar (Figure 6B). However, the expression of rhodopsins was not maintained: We neither detect Rh5 nor Rh6 in the second larval instar (Figure 6C). This suggests that Hazy expression is continuously required to maintain Rhodopsin expression. To further test this, we took animals that had received a heat shock at embryonic stage 12 and applied a second heat shock during the second larval instar. Indeed, Rh5 and Rh6 expression was restored in the third larval instar (Figure 6G), supporting that continuous Hazy expression is essential for maintained Rh5 and Rh6 expression.
Fig. 6. Rescue of the hazy−/− mutant phenotype in the larval eye. 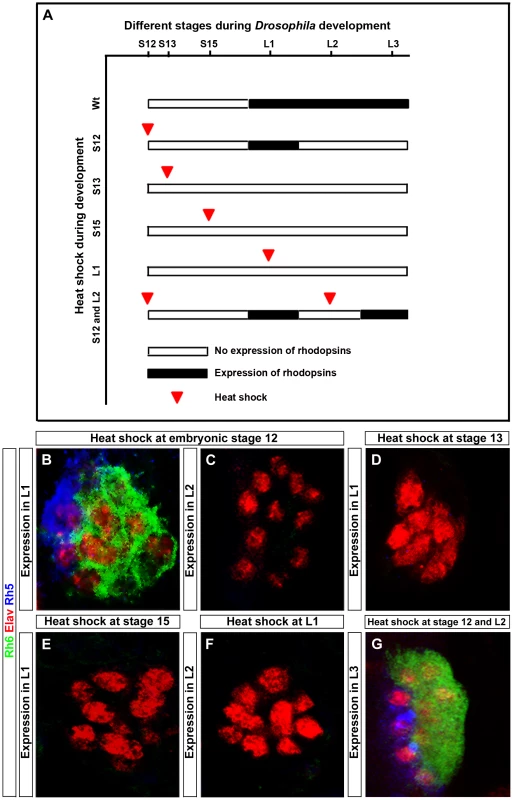
(A) Heat shock mediated rescue of hazy−/− mutant at different stages during development and consequence for Rhodopsin expression. White bar indicates no Rhodopsin expression, while black bar indicates Rhodopsin expression. Red arrowhead marks the stage at which heat shock was given. (B–G) All panels show larval eyes stained with anti-Elav (red), anti-Rh6 (green) and anti-Rh5 (blue); z-projection of confocal sections. (B, C) Heat shocks were performed three times at embryonic stage 12. At the L1 stage, Rh5 and Rh6 expression was detected (B), while at L2 stage, Rh5 and Rh6 expression was lost (C). Heat shocks performed at stage 13 (D), stage 15 (E) and at L1 stage (F) did not result in a rescue of Rh5 and Rh6 expression in the larval eyes at L1 in (D, E) and L2 in (F). Heat shocks at embryonic stage 12 and at L2 stage restores Rh5 and Rh6 expression in L3 larvae (G). Surprisingly, heat shocks after stage 12 (during embryonic stage 13, 15 or even in the first instar) did not rescue the lack of rhodopsin expression (Figure 6D, 6E and 6F). Since rhodopsin expression starts at embryonic stage 16/17, Hazy appears to provide an important function at embryonic stage 12 during the specification process from precursors to PRs prior to its role in rhodopsin regulation.
Thus, Hazy is playing two distinct roles in the larval eye: first, it is required during embryogenesis for proper PRs differentiation, and second, expression of Hazy throughout development is essential for larval PRs to express Rh5 and Rh6.
Hazy is necessary for apoptosis of larval Rh6-PRs
Hazy expression is maintained in larval PRs throughout metamorphosis to the adult eyelet (Figure 5C). We therefore analyzed rhodopsin expression in hazy mutant eyelets. Surprisingly, we found that originally “empty” Rh5-PRs correctly turn on rh6 during metamorphosis (Figure 7B). Thus, in the adult eyelet, Rh6 expression depends on Sens (see above), but occurs independently of Hazy, while in the larval eye as well as in the adult retina, Hazy is essential for Rh6 expression. As Hazy is not required for Rh6 expression in the eyelet, we reasoned that the RCSI should be dispensable for activation of the rh6-GFP reporter. Indeed, GFP expression was still observed in the eyelet when the RCSI was deleted from rh6-GFP (Figure 7D), further supporting that rh6 regulation is distinct in the larval eye and the adult eye from the eyelet.
Fig. 7. Role of Hazy in the adult eyelet. 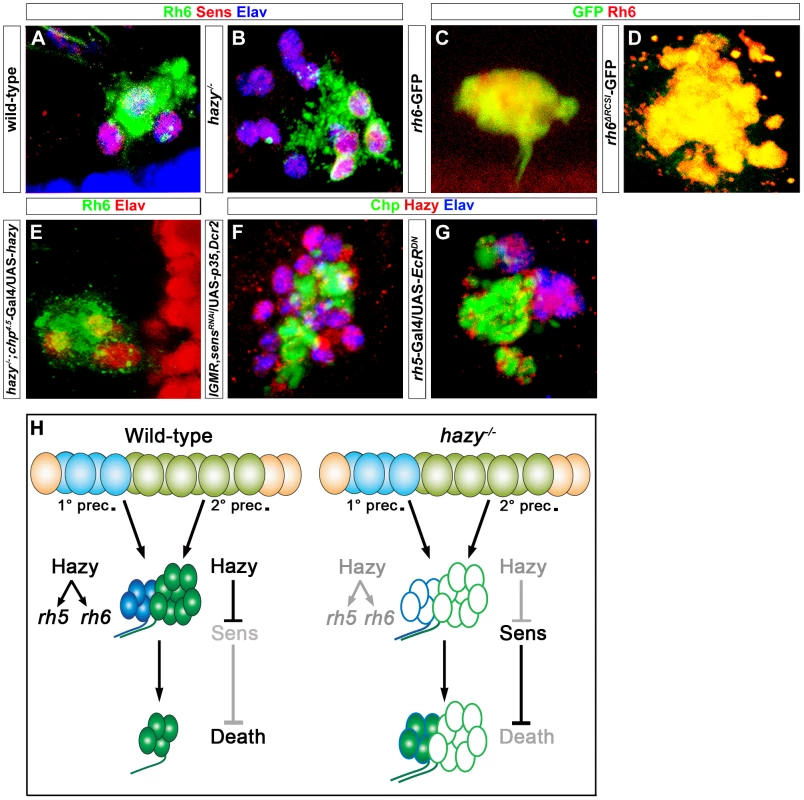
(A, B) Wild-type and hazy−/− mutant eyelets were stained against Sens (red), Rh6 (green) and Elav (blue); z-projection of confocal sections. In hazy−/− mutant, the eyelet consisted of 12 cells and all of them expressed Sens, while Rh6 expression was restricted to four cells. (C, D) rh6-GFP and rh6ΔRCSI-GFP eyelets, stained with anti-Rh6 (red) and anti-GFP (green); z-projection of confocal sections. GFP expression was still observed in the eyelets of rh6ΔRCSI-GFP. (E) UAS-hazy was expressed under the control of chp4.5-Gal4 in a hazy−/− null background (hazy−/−; chp4.5-Gal4/UAS-hazy) and the adult eyelet was analyzed for Elav (red) and Rh6 (green); z-projection of confocal sections. Normal number of Rh6 expressing PRs was found in hazy−/−; chp4.5-Gal4/UAS-hazy animals. (F) Hazy expression was assessed in sensRNAi when p35 was ectopically expressed in the eyelet to keep the cells alive (UAS-sensRNAi/lGMR-Gal4; UAS-p35/UAS-Dcr2) and stained with anti-Hazy (red), anti-Chp (green) and anti-Elav (blue). Eyelet consists of 12 cells and Hazy was expressed in all the cells in UAS-sensRNAi/lGMR-Gal4;UAS-p35/UAS-Dcr2 animals; z-projection of confocal sections. (G) Hazy expression (red) when a dominant-negative form of EcR was ectopically expressed in Rh5-PRs (rh5-Gal4/UAS-EcRDN) in the eyelet and stained with anti-Chp (green) and anti-Elav (blue); z-projection of confocal sections. Eyelet consists of four cells and Hazy was expressed in all PR cells. (H) A Model describing the role of Hazy in the larval eye and the adult eyelet. Interestingly, in hazy mutants, larval Rh6-PRs do not undergo apoptosis during pupation and are maintained into the adult, leading to a bigger eyelet that consists of about 12 PRs (Figure 7B). The larval Rh5-PRs switch on Rh6 expression during metamorphosis, while the former larval Rh6-PRs are empty. Since we have identified Sens as a survival factor counteracting EcR-induced apoptosis (see above), we investigated if Sens is expressed in the adult eyelet in hazy mutants and we found that this is indeed the case (Figure 7B). Therefore, Hazy is necessary to repress Sens in Rh6-PRs to allow them to die during metamorphosis. In line with this conclusion, the mutant phenotypes could be rescued with a PR-specific chaoptin-Gal4 driver (chp4.5-Gal4), driving hazy (Figure 7E; Figure S2).
We next assessed the genetic interaction between Hazy and Sens in the eyelet. Genetic knock-down of sens using RNAi, while inhibiting apoptosis by expressing p35 results in an eyelet consisting of 12 Hazy expressing PRs (Figure 7F), supporting that Hazy expression does not depend on Sens.
We next investigated if Hazy expression in the eyelet depends on EcR signaling. We expressed EcRDN in Rh5-PRs using rh5-Gal4 and did not observe a change in Hazy expression (Figure 7G), suggesting that Hazy expression in the eyelet is independent of EcR signaling.
Taken together, these results provide evidence for distinct functions of Hazy for larval PR development and their transformation into the adult eyelet: First, Hazy is necessary for the differentiation of PRs during embryogenesis; second, maintained Hazy expression promotes Rh5 and Rh6 expression in larval PRs; third, in Rh6-PRs Hazy is necessary to repress sens during metamorphosis, allowing these cells to undergo apoptotic cell death (Figure 7H).
Discussion
Sens initiates a binary cell fate decision in larval PRs
In the larval eye, determination of primary or secondary precursors to acquire either Rh5-PR or Rh6-PR identity depends on the transcription factors Sal, Svp and Otd [7]. Primary as well as secondary precursors have the developmental potential to express Rh5 or Rh6. During differentiation, a pulsed expression of Sens acts as a trigger to initiate a distinct developmental program: Sens acts genetically in a feedforward loop to inhibit the Rh6-PR cell-fate determinant Svp and to promote the Rh5-PR cell-fate determinant Sal. Similarly, in the adult retina, differentiation of ‘inner’ PRs R7 and R8 requires sens and sal [32], [33]. Sal is necessary for Sens expression in R8-PRs and misexpression of Sal is sufficient to induce Sens expression in the ‘outer’ PRs R1-R6 [15].
Svp is exclusively expressed in R3/R4 and R1/R6 pairs of the outer PRs in early retina development. Initially, Sal is expressed in the R3/R4 PRs in order to promote Svp expression. Later, Svp represses Sal in R3/R4 PRs in order to prevent the transformation of R3/R4 into R7 [16]. Similarly in larval PRs Svp is repressing Sal in secondary precursors [7].
Intriguingly, in R8 development in the adult retina Sens also provides two temporally separable functions: First, during R8 specification, lack of Sens in precursors results in a transformation of the cell into R2/R5 fate [26]; second, during differentiation, Sens counteracts Pros to inhibit R7 cell fate and promotes R8 cell fate [25], [33], [34]. Thus, Sens is an early genetic switch in R8-PRs and larval Rh5-PRs that represses an alternate cell fate.
The lack of Sens results in a larval eye composed of only Rh6-PRs. Thus, the default state for both primary and secondary precursors is to differentiate into Rh6-expressing PRs. Rh6 is also the default state in adult R8 PRs: In the absence of R7 PRs (e.g. sevenless mutants) that send a signal to a subset of underlying R8 PRs, the majority of R8 PRs express Rh6 [12], [35]. Thus, the genetic pathway initiated by the Sens pulse ensures that primary precursors choose a distinct developmental pathway by repressing the Rh6 ground state. The mechanisms that initiate and control this pulse of Sens remain to be discovered.
In larval PRs as well as in the formation of sensory organ precursors (SOP) in the wing, Sens functions as a binary switch between two alternative cell fates. In the larval eye, this switch occurs when Sens is expressed in one cell type and not in the other. However, during wing disc development the cell fate choice in SOP formation is controlled by the levels, and not the presence or absence of Sens expression: high levels of Sens act synergistically with proneural genes to promote a neuronal fate, while in neighboring cells, low levels of Sens repress proneural gene expression, thereby promoting a non-SOP fate [36]. Thus, Sens uses distinct molecular mechanisms to act as a switch between Rh5 versus Rh6-PR cell fate and SOP versus non-SOP cell fate.
Sens mediates a survival signal in many developmental contexts
Transcription factors regulate developmental programs in a context - dependent fashion [34]. An example is Sens, which has distinct functions in BO and eyelet development. First, during embryonic development, Sens acts as a key cell fate determinant by regulating transcription factors controlling PR-subtype specification. Second, during metamorphosis Sens inhibits ecdysone-induced apoptotic cell death. Third, in the adult eyelet Sens promotes Rh6 expression. Interestingly, the pro-survival function of Sens appears to be a conserved feature of Sens in other tissues and also in other animal species. In the salivary gland of Drosophila, Sens acts also as a survival factor of the salivary gland cells under the control of the bHLH transcription factor Sage [37]. pag-3, a C.elegans homolog of Sens is involved in touch neuron gene expression and coordinated movement [38], [39]. Pag-3 was shown to act as a cell-survival factor in the ventral nerve cord and involved in the neuroblast cell fate and may affect neuronal differentiation of certain interneurons and motorneurons [40]. In mice, Gfi1 is expressed in many neuronal precursors and differentiating neurons during embryonic development and is required for proper differentiation and maintenance of inner ear hair cells. Gfi1 mutant mice lose all cochlear hair cells through apoptosis, suggesting that its loss causes programmed cell death [41]. Taken together, these findings support that Sens and its orthologs function in cell fate determination and cell differentiation both in nervous system formation, but also play an essential role in the suppression of apoptosis.
Hazy is critical for larval, adult and eyelet PR development
Hazy plays distinct roles in larval PRs and during metamorphosis. First, Hazy is essential during embryogenesis for proper PR differentiation. This early function of Hazy is essential for PRs to differentiate properly during embryogenesis, to express Rhodopsins and to subsequently maintain Rhodopsin expression during larval stages. This function of Hazy is similar to its role in rhabdomere formation in adult PRs and subsequent promotion of Rh6 expression, although it is not required for Rh5 in the adult retina [29]. It is likely that Hazy exerts this function by binding to the RCSI site of the rhodopsin promoters, as has been suggested for the adult retina [31]. Second, during metamorphosis Hazy is required in Rh6-PRs to repress sens, thus allowing these cells to undergo apoptosis. This highlights the reuse of a small number of TFs for distinct functions in the same cell type at distinct time points of PR development. How these temporally distinct developmental programs are controlled on a molecular level remains unresolved. It seems likely that the competence of the cell to respond to a specific transcription factor changes during development.
Comparison between gene regulatory networks specifying the same Rhodopsin fate in larval and adult PRs
rh5 and rh6 are expressed in different PRs at different developmental stages: rh5 is expressed in the larval eye and in the adult retina, whereas rh6 is expressed in the larval eye, the adult eyelet and the adult retina. However, the gene regulatory networks controlling rhodopsin expression are distinct in these organs. In the adult retina, a bistable feedback loop of the growth regulator melted and the tumor suppressor warts acts to specify Rh5 versus Rh6 cell fate, respectively [11], while in the larva, Sens, Sal, Svp and Otd control Rh5 versus Rh6 identity [7] whereas Hazy has been shown to maintain Rhodopsin expression. A third genetic program acts downstream of EcR during metamorphosis in Rh5-PRs to switch to Rh6, which requires Sens.
An intriguing question is how the developmental pathways to specify Rh5 - or Rh6-cell fates converge on the regulatory sequences of these two genes. It seems likely that parts of the regulatory machinery acting on the rh5 and rh6 promoters are shared between the larval eye, adult retina and eyelet, especially as short minimal promoters are functional in all three different contexts (Rister, Tsachaki and Sprecher, unpublished). Future experiments will show how the activity of the identified trans-acting factors is integrated on these promoters to yield context-specific outcomes.
Materials and Methods
Drosophila strains and genetics
Wild-type Canton S or the Sp/CyO; TM2/TM6b strains were used as controls in all cases. The following fly strains used were sal16 [42], svpE22 [43], otduvi [18], sensE2 and UAS-sens [44], Pph13hazy (here termed hazy), heat shock-Hazy [27], UAS-EGFRDN [45], UAS-H2B::YFP (anti-GFP antibody/biogenesis recognizes the YFP antigen), UAS-ato, UAS-p35, UAS-EcRDN (isoform B2), UAS-mCD8::GFP, GMR-Gal4 and rh5-Gal4 (Bloomington Stock Center), UAS-sensRNAi (VDRC Stock Center), and so-Gal4 [46]. sensE2, svpE22 double mutants were generated by recombination.. All the crosses were grown at 25°C except RNAi experiments, which were performed at 29°C. For analysis of 3×P3-RFP [30] expression, we used the 3×P3-RFP marked attB integration site at 86Fb [47].
Generation of transgenic flies
Chp4.5-Gal4 flies were made by amplification of 4.5 kb of sequence upstream of the chaoptin gene from genomic DNA using primer pairs AC25/AC27 and the GeneXL PCR amplification system and introducing a NotI restriction site to the 5′ end and a BglII site to the 3′ end. Amplified fragments were cloned into the NotI/BamHI site of a Gal4 vector containing a hs43 promoter (hs43-Gal4) [48]. The primers were:
AC25 TGACGCGGCCGCGTCGACGAGTCTTTATGC NotI
AC27 TGACAGATCTCGATCGAACATGGAGGCGCGA BglII
The cDNA of hazy was subcloned into the pUAST/attB vector [47] between the BglII and NotI sites. The pCDNA3 plasmid containing the cDNA of hazy was kindly provided by A. Zelhof (Indiana University, Bloomington).
The rh6 (−227/+121) and rh5 (−256/+50) minimal promoters were generated using the following primers flanked with 5′BglII and 3′ NotI sites for directional cloning into a transformation plasmid containing eGFP, a miniwhite marker and an attB site:
rh5 fw: AGATCTAACATGTAAAGCTTGTAAAA
rh5 rev: GCGGCCGCTAGTTTCCTTTGCAGGTCGAC
rh6 fw: AGATCTGGGTGGGTGGTACCTCAAAC
rh6 rev: GCGGCCGCGGTGGCGCTTCGGTGGTGGCTTC
RCSI deletions were generated using the Stratagene QuikChange site-directed mutagenesis kit with the following primers:
DRCSRh6A: TGGATTGGCCAAGTGCCGGCGGGCAATTAGTCTAAGACG
DRCSRh6B: CGTCTTAGACTAATTGCCCGCCGGCACTTGGCCAATCCA
DRCSRh5A: AATGGTCACCACTTAATCCGTCTTTTGGCGGGCTATAAAAGCAT
DRCSRh5B: TTACCAGTGGTGAATTAGGCAGAAAACCGCCCGATATTTTCGTA
The UAS-hazy construct, as well as rh6-GFP, rh5-GFP, rh5-GFPΔRCSI and rh6-GFPΔRCSI reporters were all inserted into the 86Fb site on the third chromosome using the φC31 site-specific integration system [47].
Immunohistochemistry
Embryos were dechorionated, fixed and immunostained according to a previously described protocol [49]. Dissection and immunostaining of the larval eye and the eyelet have been described previously [7], [21]. The samples were mounted in Vectashield H-1000 (Vector laboratories). Primary antibodies and dilutions were as follows: rat anti-Elav 1∶30 and mouse anti-FasII 1∶30 (Developmental studies Hybridoma bank), rabbit anti-Sal 1∶300 [42], mouse anti-Svp 1∶100 [50], rabbit anti-Hazy 1∶500 [27], sheep anti-GFP 1∶1000 (Invitrogen), guinea pig anti-Sens 1∶800 [44], rat anti-Kr 1∶200 [51], rabbit anti-Rh6 1∶10000 [52], mouse anti-Rh5 1∶20 [53] and rabbit anti-Otd 1∶200 [54]. The secondary antibodies were anti-rabbit, anti-mouse, anti-rat conjugated with Alexa-488, Alexa-555 or Alexa-647, anti-guinea pig DyLight 549 and anti-Sheep DyLight 488 (Jackson Immunoresearch). All secondary antibodies were developed in donkey and/or goat and used in 1∶200 dilution.
Laser confocal microscopy and image processing
The confocal microscope for the analysis of the samples was a Leica TCS. The picture size was 512×512 or 1024×1024 pixels and the optical sections ranged from 0.8–1.5 µm depending on the sample. The images acquired were post-processed using the Fiji software and Adobe Photoshop CS3.
Supporting Information
Zdroje
1. RisterJ, DesplanC (2011) The Retinal Mosaics of Opsin Expression in Invertebrates and Vertebrates. Developmental Neurobiology 71 : 1212–1226.
2. RisterJ, DesplanC, VasiliauskasD (2013) Establishing and maintaining gene expression patterns: insights from sensory receptor patterning. Development 140 : 493–503.
3. KeeneAC, MazzoniEO, ZhenJ, YoungerMA, YamaguchiS, et al. (2011) Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J Neurosci 31 : 6527–6534.
4. KeeneAC, SprecherSG (2012) Seeing the light: photobehavior in fruit fly larvae. Trends Neurosci 35 : 104–110.
5. MazzoniEO, DesplanC, BlauJ (2005) Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron 45 : 293–300.
6. von EssenAMHJ, PaulsD, ThumAS, SprecherSG (2011) Capacity of Visual Classical Conditioning in Drosophila Larvae. Behavioral Neuroscience 125 : 921–929.
7. SprecherSG, PichaudF, DesplanC (2007) Adult and larval photoreceptors use different mechanisms to specify the same rhodopsin fates. Genes & Development 21 : 2182–2195.
8. GreenP, HartensteinAY, HartensteinV (1993) The embryonic development of the Drosophila visual system. Cell Tissue Res 273 : 583–598.
9. DanielA, DumstreiK, LengyelJA, HartensteinV (1999) The control of cell fate in the embryonic visual system by atonal, tailless and EGFR signaling. Development 126 : 2945–2954.
10. SuzukiT, SaigoK (2000) Transcriptional regulation of atonal required for Drosophila larval eye development by concerted action of eyes absent, sine oculis and hedgehog signaling independent of fused kinase and cubitus interruptus. Development 127 : 1531–1540.
11. Mikeladze-DvaliT, WernetMF, PistilloD, MazzoniEO, TelemanAA, et al. (2005) The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 122 : 775–787.
12. ChouWH, HuberA, BentropJ, SchulzS, SchwabK, et al. (1999) Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development 126 : 607–616.
13. ThanawalaSU, RisterJ, GoldbergGW, ZuskovA, OlesnickyEC, et al. (2013) Regional modulation of a stochastically expressed factor determines photoreceptor subtypes in the Drosophila retina. Dev Cell 25 : 93–105.
14. WernetMF, MazzoniEO, CelikA, DuncanDM, DuncanI, et al. (2006) Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 440 : 174–180.
15. DomingosPM, BrownS, BarrioR, RatnakumarK, FrankfortBJ, et al. (2004) Regulation of R7 and R8 differentiation by the spalt genes. Dev Biol 273 : 121–133.
16. DomingosPM, MlodzikM, MendesCS, BrownS, StellerH, et al. (2004) Spalt transcription factors are required for R3/R4 specification and establishment of planar cell polarity in the Drosophila eye. Development 131 : 5695–5702.
17. HiromiY, MlodzikM, WestSR, RubinGM, GoodmanCS (1993) Ectopic expression of seven-up causes cell fate changes during ommatidial assembly. Development 118 : 1123–1135.
18. VandendriesER, JohnsonD, ReinkeR (1996) orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev Biol 173 : 243–255.
19. Helfrich-ForsterC, EdwardsT, YasuyamaK, WisotzkiB, SchneuwlyS, et al. (2002) The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci 22 : 9255–9266.
20. HofbauerA, BuchnerE (1989) Does Drosophila Have 7 Eyes. Naturwissenschaften 76 : 335–336.
21. SprecherSG, DesplanC (2008) Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature 454 : 533–537.
22. FortiniME, RubinGM (1990) Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev 4 : 444–463.
23. PapatsenkoD, NazinaA, DesplanC (2001) A conserved regulatory element present in all Drosophila rhodopsin genes mediates Pax6 functions and participates in the fine-tuning of cell-specific expression. Mech Dev 101 : 143–153.
24. MismerD, RubinGM (1987) Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics 116 : 565–578.
25. FrankfortBJ, NoloR, ZhangZ, BellenH, MardonG (2001) senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron 32 : 403–414.
26. PeppleKL, AtkinsM, VenkenK, WellnitzK, HardingM, et al. (2008) Two-step selection of a single R8 photoreceptor: a bistable loop between senseless and rough locks in R8 fate. Development 135 : 4071–4079.
27. ZelhofAC, KoundakjianE, ScullyAL, HardyRW, PoundsL (2003) Mutation of the photoreceptor specific homeodomain gene Pph13 results in defects in phototransduction and rhabdomere morphogenesis. Development 130 : 4383–4392.
28. DietzlG, ChenD, SchnorrerF, SuKC, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156.
29. JukamD, XieB, RisterJ, TerrellD, Charlton-PerkinsM, et al. (2013) Opposite Feedbacks in the Hippo Pathway for Growth Control and Neural Fate. Science 342 : 1238016.
30. ShengG, ThouvenotE, SchmuckerD, WilsonDS, DesplanC (1997) Direct regulation of rhodopsin 1 by Pax-6/eyeless in Drosophila: evidence for a conserved function in photoreceptors. Genes Dev 11 : 1122–1131.
31. MishraM, OkeA, LebelC, McDonaldEC, PlummerZ, et al. (2010) Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137 : 2895–2904.
32. MollereauB, DominguezM, WebelR, ColleyNJ, KeungB, et al. (2001) Two-step process for photoreceptor formation in Drosophila. Nature 412 : 911–913.
33. XieB, Charlton-PerkinsM, McDonaldE, GebeleinB, CookT (2007) Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development 134 : 4243–4253.
34. FrankfortBJ, MardonG (2004) Senseless represses nuclear transduction of Egfr pathway activation. Development 131 : 563–570.
35. PapatsenkoD, ShengG, DesplanC (1997) A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development 124 : 1665–1673.
36. Jafar-NejadH, AcarM, NoloR, LacinH, PanH, et al. (2003) Senseless acts as a binary switch during sensory organ precursor selection. Genes Dev 17 : 2966–2978.
37. ChandrasekaranV, BeckendorfSK (2003) senseless is necessary for the survival of embryonic salivary glands in Drosophila. Development 130 : 4719–4728.
38. JiaY, XieG, AamodtE (1996) pag-3, a Caenorhabditis elegans gene involved in touch neuron gene expression and coordinated movement. Genetics 142 : 141–147.
39. JiaY, XieG, McDermottJB, AamodtE (1997) The C. elegans gene pag-3 is homologous to the zinc finger proto-oncogene gfi-1. Development 124 : 2063–2073.
40. CameronS, ClarkSG, McDermottJB, AamodtE, HorvitzHR (2002) PAG-3, a Zn-finger transcription factor, determines neuroblast fate in C. elegans. Development 129 : 1763–1774.
41. WallisD, HamblenM, ZhouY, VenkenKJ, SchumacherA, et al. (2003) The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development 130 : 221–232.
42. KuhnleinRP, FrommerG, FriedrichM, Gonzalez-GaitanM, WeberA, et al. (1994) spalt encodes an evolutionarily conserved zinc finger protein of novel structure which provides homeotic gene function in the head and tail region of the Drosophila embryo. EMBO J 13 : 168–179.
43. MlodzikM, HiromiY, WeberU, GoodmanCS, RubinGM (1990) The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell 60 : 211–224.
44. NoloR, AbbottLA, BellenHJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102 : 349–362.
45. O'KeefeL, DouganST, GabayL, RazE, ShiloBZ, et al. (1997) Spitz and Wingless, emanating from distinct borders, cooperate to establish cell fate across the Engrailed domain in the Drosophila epidermis. Development 124 : 4837–4845.
46. ChangT, Younossi-HartensteinA, HartensteinV (2003) Development of neural lineages derived from the sine oculis positive eye field of Drosophila. Arthropod Struct Dev 32 : 303–317.
47. BischofJ, MaedaRK, HedigerM, KarchF, BaslerK (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104 : 3312–3317.
48. CookT, PichaudF, SonnevilleR, PapatsenkoD, DesplanC (2003) Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell 4 : 853–864.
49. TherianosS, LeuzingerS, HirthF, GoodmanCS, ReichertH (1995) Embryonic development of the Drosophila brain: formation of commissural and descending pathways. Development 121 : 3849–3860.
50. KanaiMI, OkabeM, HiromiY (2005) seven-up Controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell 8 : 203–213.
51. KosmanD, SmallS, ReinitzJ (1998) Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol 208 : 290–294.
52. TahayatoA, SonnevilleR, PichaudF, WernetMF, PapatsenkoD, et al. (2003) Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Dev Cell 5 : 391–402.
53. ChouWH, HallKJ, WilsonDB, WidemanCL, TownsonSM, et al. (1996) Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron 17 : 1101–1115.
54. HirthF, KammermeierL, FreiE, WalldorfU, NollM, et al. (2003) An urbilaterian origin of the tripartite brain: developmental genetic insights from Drosophila. Development 130 : 2365–2373.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



