-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
The insulin/IGF-1 signaling (IIS) pathway is a conserved regulator of longevity, development, and metabolism. In Caenorhabditis elegans IIS involves activation of DAF-2 (insulin/IGF-1 receptor tyrosine kinase), AGE-1 (PI 3-kinase), and additional downstream serine/threonine kinases that ultimately phosphorylate and negatively regulate the single FOXO transcription factor homolog DAF-16. Phosphatases help to maintain cellular signaling homeostasis by counterbalancing kinase activity. However, few phosphatases have been identified that negatively regulate the IIS pathway. Here we identify and characterize pdp-1 as a novel negative modulator of the IIS pathway. We show that PDP-1 regulates multiple outputs of IIS such as longevity, fat storage, and dauer diapause. In addition, PDP-1 promotes DAF-16 nuclear localization and transcriptional activity. Interestingly, genetic epistasis analyses place PDP-1 in the DAF-7/TGF-β signaling pathway, at the level of the R-SMAD proteins DAF-14 and DAF-8. Further investigation into how a component of TGF-β signaling affects multiple outputs of IIS/DAF-16, revealed extensive crosstalk between these two well-conserved signaling pathways. We find that PDP-1 modulates the expression of several insulin genes that are likely to feed into the IIS pathway to regulate DAF-16 activity. Importantly, dysregulation of IIS and TGF-β signaling has been implicated in diseases such as Type 2 Diabetes, obesity, and cancer. Our results may provide a new perspective in understanding of the regulation of these pathways under normal conditions and in the context of disease.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001377
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001377Summary
The insulin/IGF-1 signaling (IIS) pathway is a conserved regulator of longevity, development, and metabolism. In Caenorhabditis elegans IIS involves activation of DAF-2 (insulin/IGF-1 receptor tyrosine kinase), AGE-1 (PI 3-kinase), and additional downstream serine/threonine kinases that ultimately phosphorylate and negatively regulate the single FOXO transcription factor homolog DAF-16. Phosphatases help to maintain cellular signaling homeostasis by counterbalancing kinase activity. However, few phosphatases have been identified that negatively regulate the IIS pathway. Here we identify and characterize pdp-1 as a novel negative modulator of the IIS pathway. We show that PDP-1 regulates multiple outputs of IIS such as longevity, fat storage, and dauer diapause. In addition, PDP-1 promotes DAF-16 nuclear localization and transcriptional activity. Interestingly, genetic epistasis analyses place PDP-1 in the DAF-7/TGF-β signaling pathway, at the level of the R-SMAD proteins DAF-14 and DAF-8. Further investigation into how a component of TGF-β signaling affects multiple outputs of IIS/DAF-16, revealed extensive crosstalk between these two well-conserved signaling pathways. We find that PDP-1 modulates the expression of several insulin genes that are likely to feed into the IIS pathway to regulate DAF-16 activity. Importantly, dysregulation of IIS and TGF-β signaling has been implicated in diseases such as Type 2 Diabetes, obesity, and cancer. Our results may provide a new perspective in understanding of the regulation of these pathways under normal conditions and in the context of disease.
Introduction
Insulin/IGF-1 signaling (IIS) is a conserved neuroendocrine pathway that regulates longevity, development and energy metabolism across phylogeny [1], [2]. In the roundworm Caenorhabditis elegans (C. elegans), activation of the DAF-2 insulin/IGF-1 receptor tyrosine kinase intiates an AAP-1/AGE-1 PI 3-kinase signaling cascade involving the downstream serine/threonine kinases PDK-1, AKT-1, and AKT-2 [3]–[7]. Activated AKT-1 and AKT-2 phosphorylate DAF-16, the single Forkhead Box O (FOXO) family transcription factor homolog in C. elegans [8]. Phosphorylation of DAF-16 results in its inactivation and sequestration in the cytosol [9], [10]. Under low signaling conditions, DAF-16 translocates into the nucleus, where it can transactivate/repress hundreds of target genes [9]–[13].
The dauer is an alternative survival stage that worms can enter upon poor environmental conditions such as crowding [14]. Mutations in the kinases upstream of DAF-16 such as daf-2, age-1, pdk-1, akt-1 and akt-2 result in an increase in lifespan, dauer formation, fat storage and/or stress resistance, and loss-of-function mutations in daf-16 completely suppress these phenotypes [15]–[18]. In addition to the IIS pathway, dauer formation in C. elegans is also regulated by the DAF-7/TGF-β-like signaling pathway [19]–[21]. Activation of TGF-β signaling is achieved through binding of the DAF-7 BMP-like ligand to the DAF-1/DAF-4, the Type I/II receptors, which phosphorylate and activate the downstream receptor-associated SMAD (R-SMAD) proteins DAF-8 and DAF-14, presumably through a conserved SSXS phosphorylation motif that has been shown to be important for R-SMAD activation in mammals [22]–[24]. Upon activation, R-SMADs can associate with a Co-SMAD to regulate the transcription of hundreds of genes [23], [25]. In C. elegans, DAF-8 and DAF-14 act to antagonize the transcriptional activity of the DAF-3 Co-SMAD and the DAF-5 SNO-SKI repressor [22], [24], [26]–[29]. Reduction of function mutations in daf-7, daf-1, daf-4, daf-8 and daf-14 show temperature-sensitive constitutive dauer formation and mutations in daf-3 and/or daf-5 completely suppress this phenotype [19], [21], [30]. Genetic epistasis studies have suggested that the TGF-β pathway acts in a parallel manner with IIS to modulate dauer formation [31]–[33].
The PTEN lipid phosphatase homolog DAF-18, which antagonizes signaling at the level of AGE-1/PI 3-kinase, is a major negative regulator of IIS. In contrast to the kinases in this pathway, loss-of-function mutations in daf-18 reduces lifespan, fat storage, dauer formation and stress resistance [32], [34]–[39]. Besides DAF-18, few negative modulators of the pathway have been identified. In particular, less is known about serine/threonine phosphatases that counterbalance kinase activity in the IIS pathway. We recently performed a directed RNA interference (RNAi) screen for serine/threonine phosphatases that regulate C. elegans IIS using dauer formation as an output [39]. We identified the PP2A regulatory subunit PPTR-1 as an important regulator of AKT-1 dephosphorylation as well as DAF-16-dependent phenotypes [39]. Here we characterize another candidate from this screen, pdp-1, as a positive regulator of dauer formation. PDP-1 is homologous to pyruvate dehydrogenase phosphatase (PDP) in higher organisms, an enzyme that positively regulates the pyruvate dehydrogenase enzyme complex (PDHc). RNAi of the other components of PDHc do not result in changes in dauer formation. Interestingly, we report that although PDP-1 is a robust modulator of multiple IIS-regulated processes as well as DAF-16 activity, genetic epistasis studies place pdp-1 in the DAF-7/TGF-β pathway. Through this study, we find that IIS and TGF-β signaling are more tightly connected than previously suggested, with distinct roles for the Co-SMAD DAF-3 in modulating the IIS pathway. Our data suggests that PDP-1 modulates the gene expression of several insulins, and that insulins may be a potential mediator of the crosstalk between these two pathways.
Results
C. elegans PDP-1 regulates daf-2 dauer formation independent of PDH
Our RNAi screen was designed to identify serine/threonine phosphatases that modulated dauer formation of daf-2(e1370), a non-null, temperature-sensitive mutant of the C. elegans insulin/IGF-1 receptor gene, daf-2 [39]. We were particularly interested in phosphatases that would negatively regulate IIS similar to DAF-18/PTEN, and for all RNAi based assays described below, daf-18 RNAi was used as a positive control [39]. From this screen, we identified pdp-1 as a modulator of daf-2(e1370) dauer formation (Figure 1A and Figure S2). BLAST analyses using amino acid sequence revealed that PDP-1 is homologous to fly and mammalian PDP (∼52% positive and ∼38% identical). pdp-1 RNAi significantly reduces dauer formation of daf-2(e1370) worms, similar to daf-18 RNAi (Figure 1A and Figure S2). This phenotype is not allele-specific, as pdp-1 RNAi results in suppression of dauer formation in a second allele of daf-2, daf-2(e1368) (Figure 1B and Figure S2). Similar to the results with the RNAi, a mutation in pdp-1 also affects dauer formation - pdp-1(tm3734); daf-2(e1370) double mutants form significantly fewer dauers when compared to the daf-2(e1370) parental strain (Figure S2).
Fig. 1. PDP-1 regulates daf-2 dauer formation independent of the PDHc. 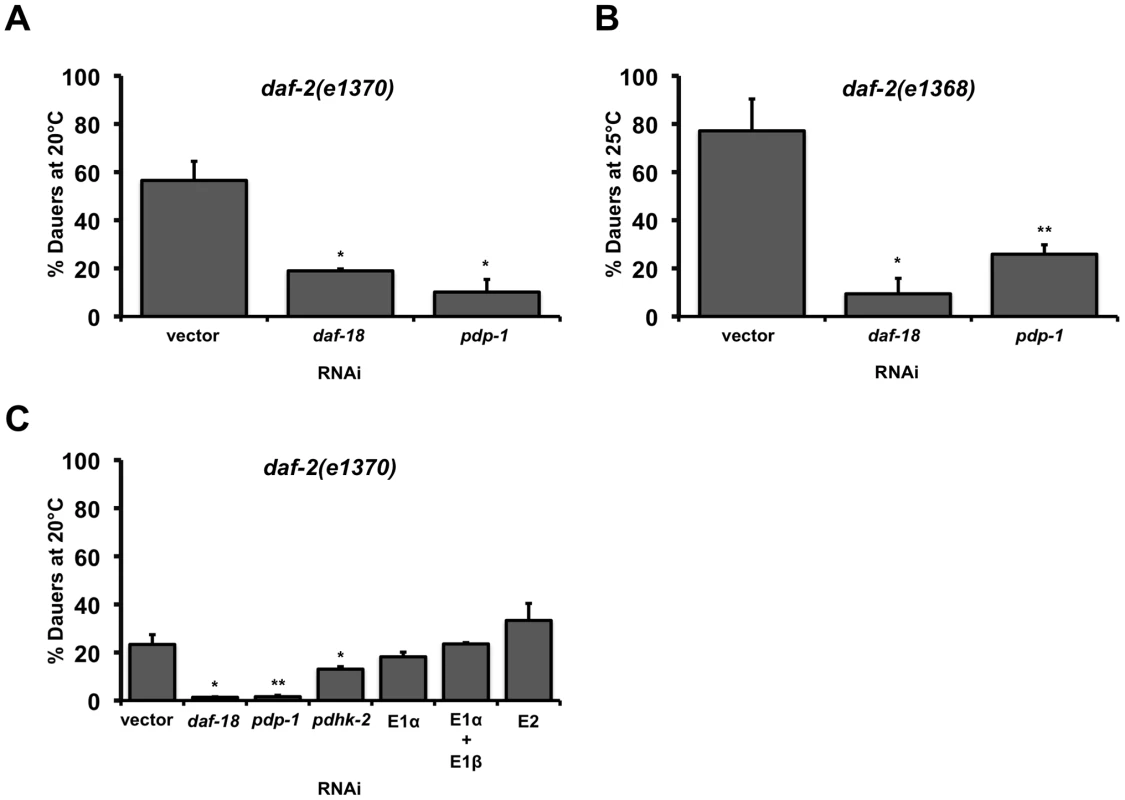
Error bars indicate the standard deviation among the different RNAi plates within one experiment. Data shown are from one representative experiment. (A) pdp-1 RNAi suppresses daf-2(e1370) dauer formation similar to daf-18 RNAi. Dauer formation of daf-2(e1370) is 56.5±8.0% (n = 278) on vector RNAi, 18.9±0.8% (n = 79) on daf-18 RNAi (p<0.05) and 10.5±5.3% (n = 293) on pdp-1 RNAi (p<0.05). (B) pdp-1 RNAi suppresses dauer formation of daf-2(e1368) worms similar to daf-18 RNAi. Dauer formation of daf-2(e1368) is 77.1±13.2% dauers (n = 297) on vector RNAi, daf-2(e1368) worms form only 9.4±6.4% (n = 258) dauers on daf-18 RNAi (p<0.06) and 25.9±3.9% (n = 636) dauers on pdp-1 RNAi (p<0.05). (C) RNAi of other components of the PDHc including the E1α subunit does not affect daf-2(e1370) dauer formation. Dauer formation of daf-2(e1370) on PDHc RNAi is 23.3±4.1% (n = 282) on vector RNAi, 1.3±0.2% (n = 219) on daf-18 RNAi (p<00.04), 1.6±0.6% (n = 185) on pdp-1 RNAi (p<0.03), 13.1±1.0% (n = 233) on pdhk-2 on RNAi (p<0.05), 18.2±2.0% (n = 193) on E1α RNAi, 23.5±0.5% (172) on a combination of E1α and E1β RNAi and 33.3±7.1% (n = 25) on E2 RNAi. Given its homology to PDP in higher organisms, we wondered whether the effect of pdp-1 knockdown on daf-2 dauer formation was a consequence of modulating the activity of the PDHc. The PDHc is a multi-subunit enzyme complex consisting of three major enzymes: E1 pyruvate dehydrogenase, E2 dihydrolipoyl acetyltransferase and E3 dihydrolipoyl dehydrogenase that regulate energy metabolism [40]. PDHc converts pyruvate to acetyl-coA, which can either enter the TCA cycle or be used for fatty acid synthesis. In mammals, regulation of PDHc activity is primarily achieved through reversible phosphorylation/dephosphorylation of the E1α subunit by pyruvate dehydrogenase kinase (PDHK) and PDP, with phosphorylation inactivating the enzyme complex [40]. All of the components of the PDH complex have conserved C. elegans homologs, encoded by the genes T05H10.6 (E1α), C04C3.3 (E1β), F23B12.5 (E2), LLC1.3 (E3), pdhk-2 (PDHK) and pdp-1 (PDP).
To test whether modulation of PDHc activity affects daf-2 dauer formation, we grew daf-2(e1370) worms on PDHc RNAi. Quantification the RNAi efficiency of the PDHc components revealed that we achieved 60–90% knockdown (Figure S1). To our surprise, RNAi of the E1α subunit had no effect on daf-2 dauer formation, while pdp-1 RNAi resulted in dauer suppression (Figure 1C and Figure S2). In addition, RNAi of either the other E1 subunit E1β, or the E2 subunit, did not affect daf-2 dauer formation (Figure 1C and Figure S2). Knockdown of the E3 subunit resulted in lethality (data not shown). Interestingly, pdhk-2 RNAi resulted in slight suppression daf-2(e1370) dauer formation but had no effect on dauer formation of daf-2(e1368) mutants (Figure 1C and Figure S2). Therefore pdhk-2 modulates the IIS pathway in an allele-specific manner and we did not perform further characterization of this gene.
To further evaluate the components of the PDH complex, we examined their expression patterns. The expression pattern of PDP-1 does not completely overlap with that of the E1 and E2 subunits of PDHc (Figure S3). PDP-1 expression was enriched in the head and tail neurons, head muscle and the intestine. We did not observe any expression in the pharynx. In contrast, the expression of the E1 and E2 subunits, was observed throughout the body of the worm and was significantly enriched in the pharynx. Taken together, PDP-1 modulates daf-2 dauer formation and this function is likely to be independent of its role in regulating the PDHc.
PDP-1 regulates multiple outputs of the IIS pathway
In addition to dauer formation, the IIS pathway also regulates longevity, stress resistance and fat storage [17], [18]. Mutations in daf-2 and age-1 result in a significant extension in lifespan, enhanced resistance to various stresses and increased fat storage [7], [35], [41]–[44]. These phenotypes are suppressed by loss-of-function mutations in daf-18 and daf-16 [32], [34], [35], [39]. We therefore investigated whether dosage modulation of pdp-1 would affect additional outputs of the pathway. We first tested the role of PDP-1 in regulating lifespan (Figure 2 and Figure S4). The lifespan of wild-type worms was not affected by pdp-1 RNAi and slightly reduced by a mutation in pdp-1 (Figure 2A and 2D). In contrast, the mean and maximal lifespan of long-lived daf-2(e1370) and age-1(hx546) mutants was significantly reduced by pdp-1 RNAi (Figure 2B and 2C). Similarly, pdp-1(tm3734); daf-2(e1370) double mutants lived significantly shorter than the parental daf-2(e1370) strain (Figure S4).
Fig. 2. PDP-1 regulates multiple outputs of the IIS pathway. 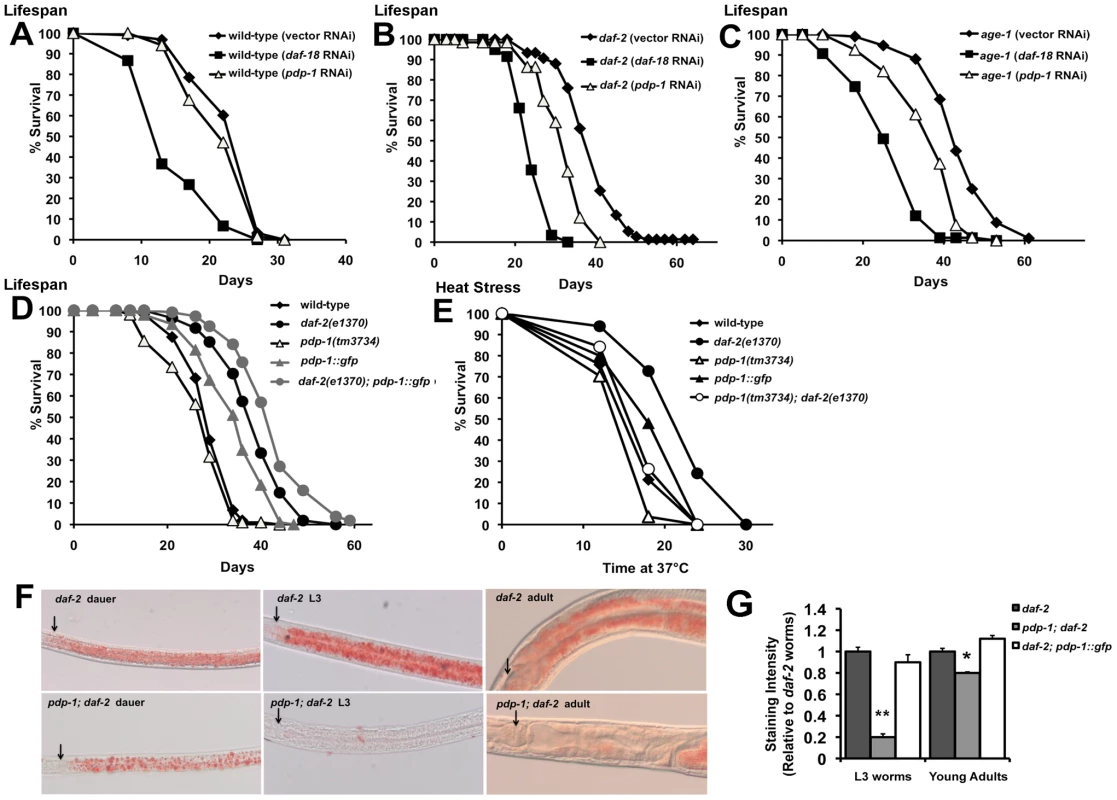
Data shown are from one representative experiment. (A) pdp-1 RNAi does not significantly reduce the lifespan of wild-type worms. Mean lifespan of wild-type worms is 23.8±0.5 days (n = 93) on vector RNAi, 14.5±0.9 days (n = 34) on daf-18 RNAi (p<0.0001) and 22.6±0.6 days (n = 68) on pdp-1 RNAi (p<0.08). (B) The increased lifespan of daf-2(e1370) worms is reduced by pdp-1 RNAi. Mean lifespan of daf-2(e1370) worms is 38.9±0.9 days (n = 75) on vector RNAi, 24.5±0.5 days (n = 59) on daf-18 RNAi (p<0.0001) and 31.7±0.8 days (n = 66) on pdp-1 RNAi (p<0.0001). (C) pdp-1 RNAi reduces the increased lifespan of age-1(hx546) mutants. Mean lifespan of daf-2(e1370) worms is 42.8±0.8 days (n = 84) on vector RNAi, 28.0±0.9 days (n = 81) on daf-18 RNAi (p<0.0001) and 36.5±1.0 days (n = 67) on pdp-1 RNAi (p<0.0001). (D) pdp-1 overexpression increases the lifespan of wild-type and daf-2(e1370) worms while pdp-1 mutants live slightly shorter than wild-type animals. Mean lifespan of wild-type worms is 29.4±0.5 days (n = 104), pdp-1(tm3734) mutants was 27.1±0.7 days (n = 98), p<0.05, pdp-1::gfp mutants is 34.5±0.8 days (n = 92) p<0.0001, daf-2(e1370) is 38.7±0.7 days (n = 108) and daf-2(e1370); pdp-1::gfp is 42.8±0.7 days (n = 105) days p<0.0001. (E) PDP-1 regulates thermotolerance. Mean survival of wild-type worms is 18.3±0.7 hours (n = 37), pdp-1(tm3734) mutants is 17.1±0.8 hours (n = 27) p<0.2, pdp-1::gfp worms is 19.7±0.9 days (n = 25) p<0.09, daf-2(e1370) worms is 21.6±0.6 hours (n = 30) and pdp-1(tm3734); daf-2(e1370) worms is 18.6±0.9 hours (n = 19), p<0.0007). (F) Oil Red O staining reveals that pdp-1(tm3734); daf-2(e1370) worms store less fat than daf-2 worms across different stages in the worm life cycle: dauers (left), L3 worms (middle) and adults (right). Arrows indicate the lower bulb of the pharynx. (G) Quantification of Oil Red O staining in L3 and young adults of daf-2(e1370), pdp-1(tm3734); daf-2(e1370) and daf-2(e1370); pdp-1::gfp worms. A mutation in pdp-1 significantly reduces daf-2(e1370) fat storage in both, L3s (p<0.0001) and young adults (p<0.01). In adult worms, daf-2(e1370); pdp-1::gfp worms store slightly more fat than daf-2(e1370) not in younger L3 animals (p<0.02). To examine the effect of increased dosage of pdp-1, we generated transgenic worms bearing a translational fusion containing pdp-1 fused to gfp and driven by its own promoter (pdp-1::gfp). In addition, we also crossed the pdp-1::gfp worms to daf-2(e1370) mutants to generate the daf-2(e1370); pdp-1::gfp strain. Overexpression of pdp-1 results in a significant extension in lifespan compared to wild-type worms (Figure 2D and Figure S4). Interestingly, pdp-1 overexpression further extends the lifespan of daf-2(e1370) mutants (Figure 2B and Figure S4). In both of these cases, the increased lifespan was suppressed by daf-16 RNAi (Figure S5). Therefore, dosage modulation of pdp-1 regulates lifespan in a DAF-16 dependent manner.
Next, we asked if PDP-1 modulated additional outputs of the IIS signaling pathway. We first tested whether PDP-1 regulates stress resistance by assaying the survival of pdp-1 mutants and transgenic animals when exposed to heat stress at 37°C (Figure 2E and Figure S7). Dosage modulation of pdp-1 affects the response to heat stress, with a pdp-1 mutation decreasing and pdp-1 overexpression slightly enhancing thermotolerance (Figure 2E). Importantly a pdp-1 mutation drastically reduced the thermotolerance of daf-2 mutants (Figure 2E).
To examine the role of pdp-1 in regulating fat storage, we used both Oil Red O [45] and Sudan Black [7] staining (Figure 2F and 2G and Figure S7). pdp-1 mutants had similar levels of fat compared to wild-type worms, while overexpression of pdp-1 slightly enhanced fat storage (Figure S7). In contrast, a pdp-1 mutation drastically reduced the increased fat of daf-2(e1370) mutants (Figure 2F and 2G and Figure S7). This was observed in dauers, larval stage 3 (L3) animals and adults, suggesting that PDP-1 is an important regulator of fat storage in daf-2 mutants. We did observe any further enhancement of the increased fat storage in the daf-2(e1370); pdp-1::gfp worms (Figure S7). Importantly, the increased fat storage of pdp-1::gfp and daf-2(e1370); pdp-1::gfp worms was suppressed by daf-16 RNAi, similar to daf-2 mutants (Figure S7). Thus, PDP-1 modulates all four well-characterized outputs of the IIS pathway.
In addition to these phenotypes, pdp-1(tm3734) mutants exhibit a slow movement phenotype, which we quantified using locomotion assays (Figure S6). This slow movement was rescued by the pdp-1::gfp transgene. In addition, we performed brood size analysis of wild-type, pdp-1(tm3734) mutants, daf-2(e1370) mutants, and pdp-1(tm3734); daf-2(e1370) double mutants (Figure S6). pdp-1(tm3734) worms showed a slight decrease in the number of progeny compared to wild-type worms. However, when compared to daf-2 mutants, only 5% of the pdp-1(tm3734); daf-2(e1370) eggs yielded progeny (Figure S6). daf-2 mutants have a slightly reduced brood size [46], [47], and a mutation in pdp-1 severely enhances this phenotype . Taken together, PDP-1 regulates multiple outputs of IIS and acts as a negative regulator the pathway, similar to DAF-18/PTEN.
PDP-1 positively regulates DAF-16
The FOXO transcription factor DAF-16 is the major target of the C. elegans IIS pathway [2], [48]. Under conditions of reduced IIS, DAF-16 is able to translocate into the nucleus, where it regulates the expression of hundreds target genes [12], [13], [49], [50]. We therefore asked whether PDP-1 modulates DAF-16 subcellular localization as well as activity (Figure 3A and Figure S8). daf-2(e1370); daf-16::gfp worms were grown on vector, daf-18 and pdp-1 RNAi, and DAF-16 nuclear/cytosolic localization was visualized using fluorescence microscopy and quantified. Throughout the body of the worm, while DAF-16::GFP was mostly nuclear on vector RNAi, its localization was enriched in the cytosol on pdp-1 RNAi, similar to daf-18 RNAi (Figure 3A and Figure S8).
Fig. 3. PDP-1 regulates DAF-16 nuclear localization and transcriptional activity. 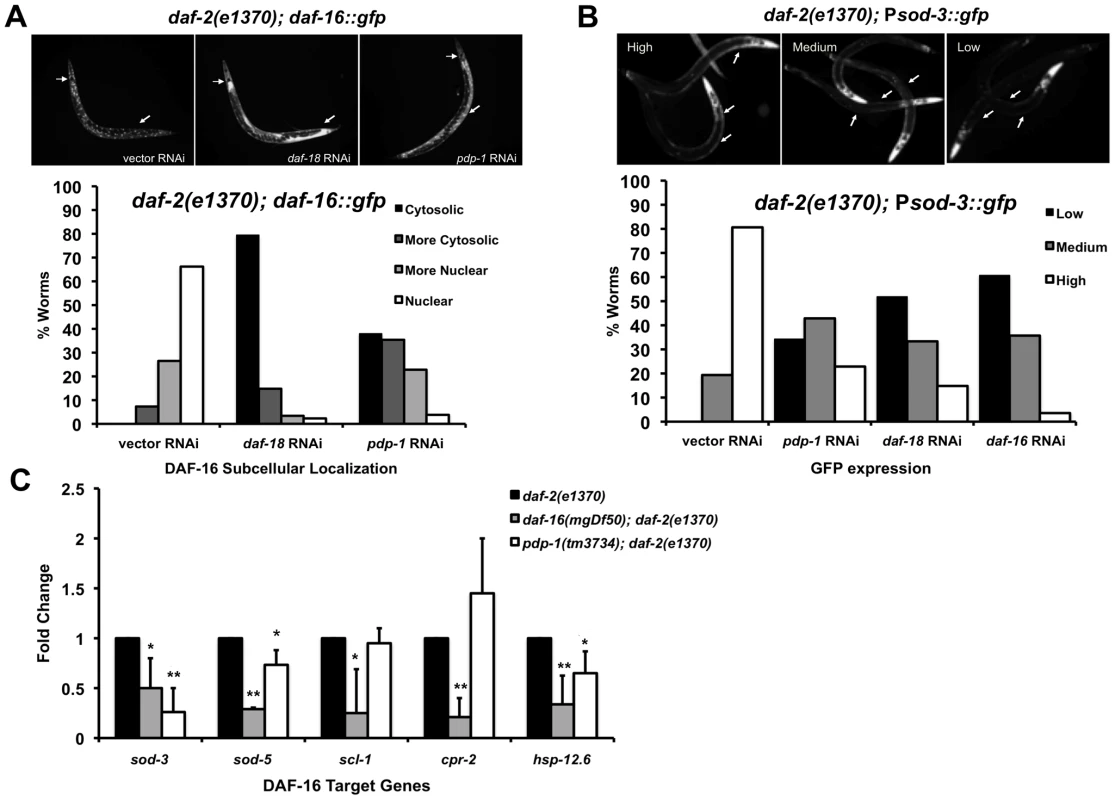
(A) DAF-16::GFP localization visualized in daf-2(e1370); daf-16::gfp worms on vector, daf-18 and pdp-1 RNAi (top panel, 100× magnification) and quantification of DAF-16::GFP nuclear-cytosolic localization (lower panel). Data shown are from one representative experiment. (n = 68 on vector RNAi, n = 88 on daf-18 RNAi and n = 79 on pdp-1 RNAi). (B) Representative images of high, medium and low GFP expression in daf-2(e1370); Psod-3::gfp worms (top panel, 100× magnification). Quantification of GFP expression in daf-2(e1370);Psod-3::gfp worms on vector, daf-18, pdp-1 and daf-16 RNAi (Lower panel). Data shown are from one representative experiment (n = 31 on vector RNAi, n = 35 on pdp-1 RNAi, n = 27 on daf-18 RNAi and n = 28 on daf-16 RNAi). (C) Levels of known DAF-16 targets are reduced in pdp-1(tm3734); daf-2(e1370) worms when compared to daf-2(e1370) worms. Data shown is an average of three independent repeats. * p<0.05, **p<0.01. The gene superoxide dismutase 3 (sod-3) is a direct DAF-16 target [11]. To test whether PDP-1 modulates transcriptional activity of DAF-16, we used a Psod-3::gfp reporter strain in a daf-2(e1370) background [51]. daf-2(e1370); Psod-3::gfp worms were grown on vector, pdp-1, daf-18 and daf-16 RNAi and GFP expression was visualized using fluorescence microscopy and scored as low, medium or high (Figure 3B and Figure S8). GFP expression was markedly lower on pdp-1 RNAi compared to vector RNAi, similar to daf-18 and daf-16 RNAi, suggesting that PDP-1 positively modulates DAF-16 transcriptional activity. To further validate these results, we used quantitative real-time PCR (Q-PCR) to look at the expression levels of well-known DAF-16 target genes [52] in daf-2(e1370), pdp-1(tm3734); daf-2(e1370) and daf-16(mgDf50); daf-2(e1370) worms (Figure 3C). Notably, the expression of sod-3, sod-5 and hsp-12.6 was significantly reduced in pdp-1(tm3734); daf-2(e1370) mutants relative to daf-2(e1370). Therefore PDP-1 positively regulates a subset of DAF-16 targets.
PDP-1 acts in the DAF-7/TGF-β signaling pathway
Thus far our data indicates that PDP-1 regulates multiple outputs of IIS as well as DAF-16 activity. Using dauer formation as the readout, we performed genetic epistasis experiments to identify the substrate of PDP-1. We first tested whether pdp-1 acted directly through the IIS pathway by focusing on kinase mutants downstream of daf-2 (Table 1 and Figure S9). pdk-1(sa680), daf-2(e1370); akt-1(ok525) and daf-2(e1370); akt-2(ok393) mutants were maintained on vector, daf-18 and pdp-1 RNAi and dauer formation of these strains was assayed at the appropriate temperatures. Interestingly, pdp-1 RNAi resulted in suppression of dauer formation of pdk-1(sa680) mutants, daf-2(e1370); akt-1(ok525) and daf-2(e1370); akt-2(ok393) worms (Table 1 and Figure S9). DAF-16 is downstream of the AKT kinases in the pathway, but we were unable to detect a physical interaction between PDP-1 and DAF-16 (data not shown).
Tab. 1. Genetic epistasis analysis using IIS mutants. 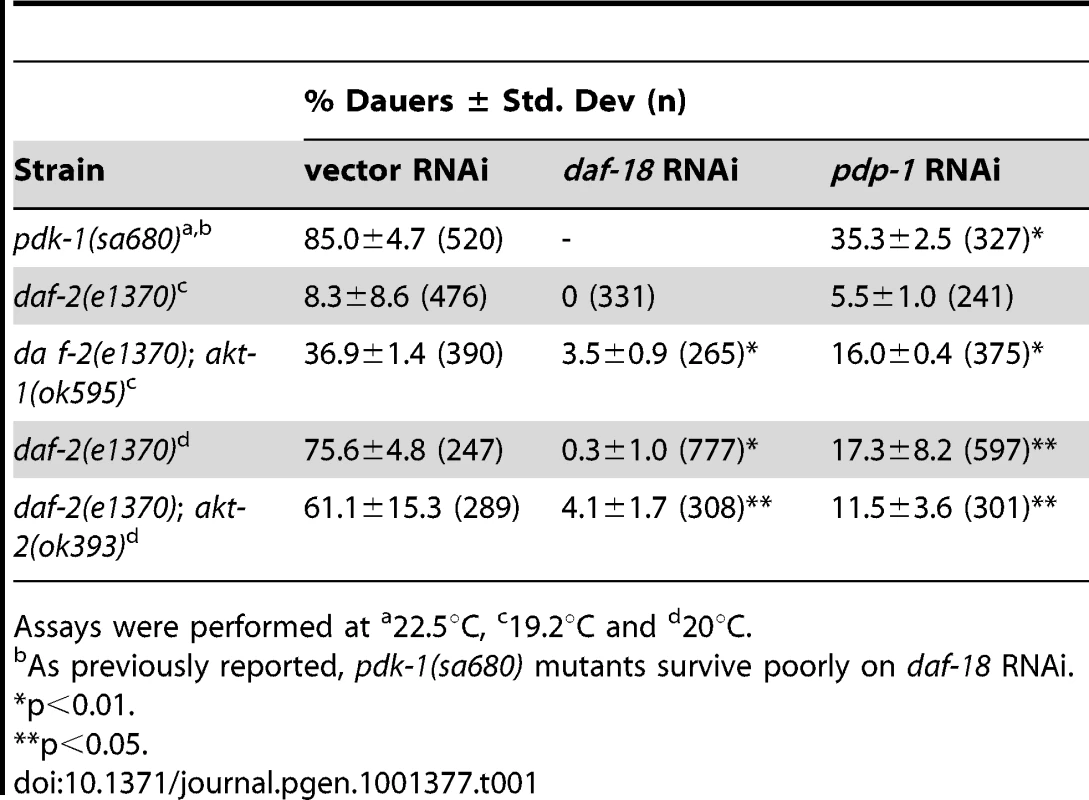
Assays were performed at We next examined a TGF-β pathway that also regulates dauer formation [19]–[21] using genetic epistasis analyses with mutants of this pathway. In these assays, TGF-β pathway mutants were maintained on vector RNAi, pdp-1 RNAi and daf-3 RNAi (as a positive control; Table 2 and Figure S10). We first tested daf-7 mutants, which contain a mutation in the gene encoding the TGF-β ligand [53]. Dauer formation of daf-7(e1372) mutants was suppressed on pdp-1 RNAi similar to daf-3 RNAi, suggesting that pdp-1 does not function at the level of daf-7 (Table 2 and Figure S10). Next, we tested dauer formation with mutants of the SMADS daf-8 and daf-14 [22]. We grew daf-14(m77) mutants on vector, pdp-1 and daf-3 RNAi. Interestingly, pdp-1 RNAi had no effect on daf-14 dauer formation, while daf-3 RNAi still resulted in suppression (Table 2 and Figure S10). We next looked at dauer formation of daf-8(m85) mutants and again observed that pdp-1 RNAi had no effect, while daf-3 RNAi suppressed dauer formation (Table 2 and Figure S10). Therefore, our genetic epistasis results indicate a genetic interaction between pdp-1 and daf-14/daf-8.
Tab. 2. Genetic epistasis analysis using TGF-β signaling mutants. 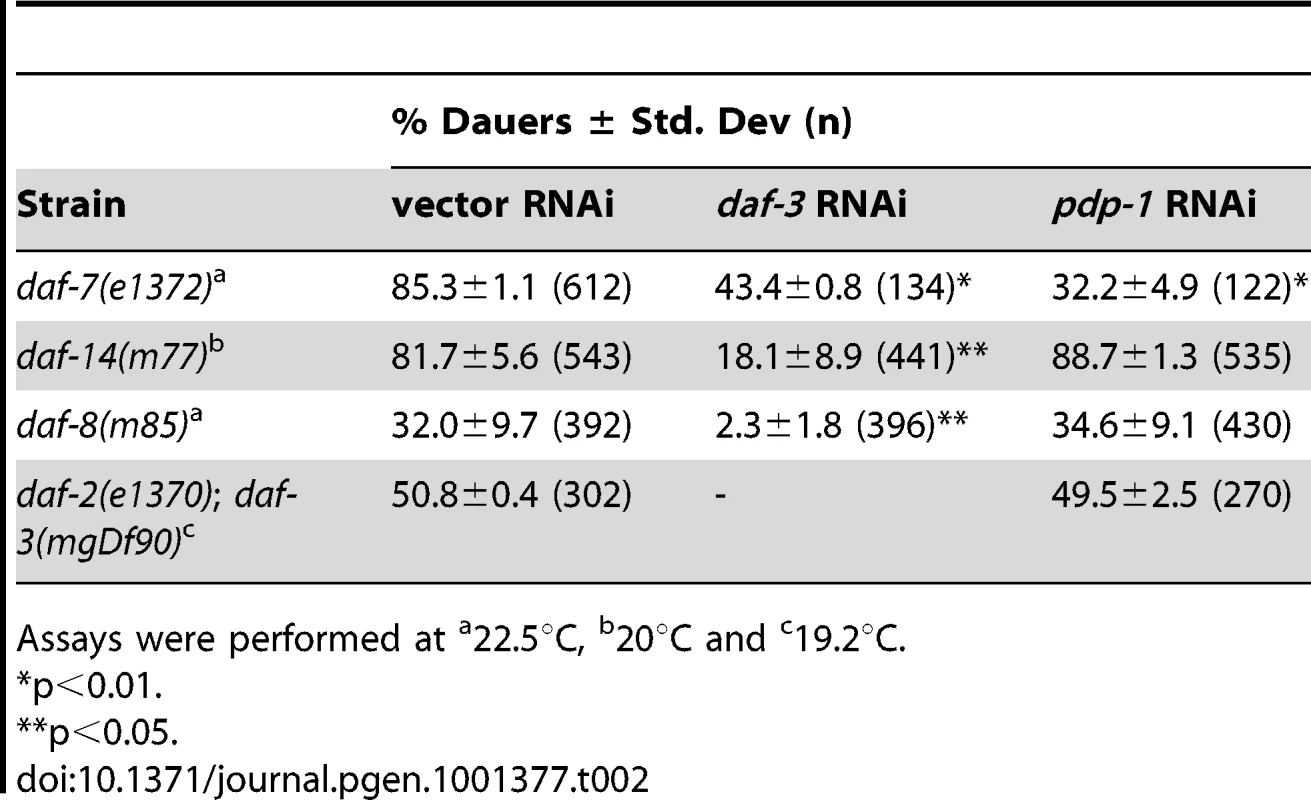
Assays were performed at To confirm these results, we investigated whether pdp-1 RNAi could suppress dauer formation of daf-2(e1370); daf-3(mgDf90) double mutants (Table 2 and Figure S10). In this strain, input from the TGF-β pathway is removed due to the daf-3 null mutation, and dauer formation is presumably mediated through activated DAF-16 [39]. Therefore, if pdp-1 was indeed acting in the TGF-β pathway, we would not see any effect of pdp-1 RNAi on daf-2(e1370); daf-3(mgDf90) double mutants. Expectedly, pdp-1 RNAi had no effect on daf-2(e1370); daf-3(mgDf90) double mutants (Table 2 and Figure S10). DAF-3 itself is unlikely to be a substrate for PDP-1, as similar to mammalian Co-SMADs, it lacks the SMAD phosphorylation motif [28]. Therefore, our genetic epistasis analysis supports a model whereby pdp-1 acts in the DAF-7 TGF-β pathway at the level of daf-8 and daf-14.
TGF-β signaling can modulate the IIS pathway
How does a phosphatase in the TGF-β signaling pathway have such robust effects on the outputs of the IIS pathway and DAF-16? A number of studies have previously identified roles for the TGF-β pathway in lifespan and fat storage [7], [54], [55]. However, genetic epistasis analysis on dauer formation placed DAF-7 TGF-β signaling and IIS as two parallel pathways where components of one pathway did not affect the other [14], [56], [57]. Yet in our studies, PDP-1 was able to regulate multiple outputs of IIS. Therefore, we decided to further investigate the potential crosstalk between the IIS and TGF-β signaling pathways. First, we focused on DAF-3 and DAF-5, which are positive regulators of dauer formation similar to PDP-1, and asked whether mutations in daf-3 or daf-5 could also affect phenotypes of the IIS pathway [14], [28], [29].
We tested lifespan, fat storage, dauer formation and stress resistance of TGF-β pathway mutants in a wild-type as well as daf-2(e1370) background. (Figure 4A–4C, Figure S11, 12, S13 and Table S1). As previously reported, the lifespan of daf-3 and daf-5 single mutants is slightly shorter than wild-type worms (Table S1) [55]. In our hands, mutations in the upstream components of the TGF-β pathway such as daf-7 and daf-14 enhance dauer formation but do not significantly extend lifespan (Table S1 and Figure S4). Intriguingly, mutations in daf-3 and daf-5 have opposite effects on daf-2(e1370) phenotypes. When compared to the daf-2(e1370) parental strain, daf-2(e1370); daf-3(mgDf90) mutants lived significantly longer. This was also observed in daf-2(e1370); daf-3(e1376) worms, which is a weaker allele of daf-3. In contrast, daf-5(e1386); daf-2(e1370) double mutants live much shorter than daf-2(e1370) worms (Figure 4A, Figure S13 and Table S1). A mutation in daf-5 also decreased the increased lifespan of age-1(hx546) worms, with age-1(hx546); daf-5(e1385) double mutants living significantly shorter than the parental strain (Figure S13). Importantly, for daf-2 worms, the effect of a daf-3 null mutation on lifespan was more pronounced at 20°C where signaling through the IIS pathway is further reduced. Therefore, under low IIS conditions, DAF-3 as well as DAF-5 can modulate longevity.
Fig. 4. Crosstalk between IIS and TGF-β signaling pathways. 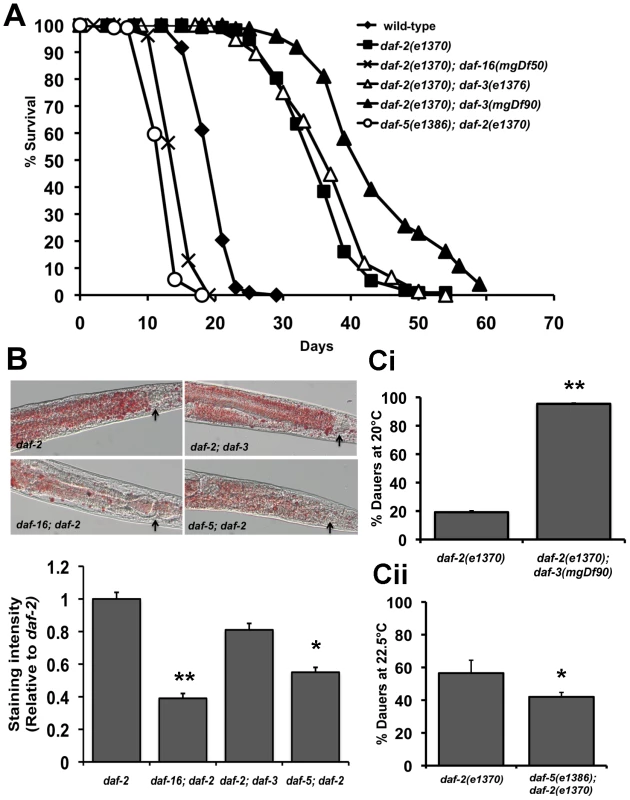
Data shown are from one representative experiment. For the dauer assays, error bars indicate the standard deviation among the different plates within one experiment. (A) Lifespan graph showing the opposite effects of daf-3 and daf-5 mutations in a daf-2(e1370) background. Mean survival of wild-type worms is 20.1±0.2 days (n = 108), daf-2(e1370) worms is 35.6±0.5 days (n = 111), daf-16(e1370); daf-2(e1370) worms is 14.9±0.6 days (n = 74) p<0.0001, daf-2(e1370); daf-3(e1376) worms is 37.2±0.8 days (n = 76) p<0.02 , daf-2(e1370); daf-3(mgDf90) is 40.5±0.7 days (n = 57) p<0.0001 and daf-5(e1386); daf-2(e1370) worms is 13.0±0.2 days (n = 104), p<0.0001). (B) Top panel: Oil Red O staining showing the modulation of fat stores in daf-2(e1370) by mutations in daf-16, daf-3 and daf-5. Arrows indicate the lower bulb of the pharynx. Lower panel: Quantification of Oil Red O staining shows a significant reduction in daf-2(e1370) fat storage by a mutation in daf-16 (p<0.0001) and daf-5 (p<0.0001). (C) daf-2(e1370) dauer formation is enhanced or decreased by mutations in daf-3 and daf-5. i) Dauer formation of daf-2(e1370) is 19.2±0.7% (n = 1062) and daf-2(e1370); daf-3(mgDf90) is 95.3±0.3% (n = 393), p<0.0001. (ii) Dauer formation of daf-2(e1370) is 57.1±9.8% (n = 176) and daf-5(e1386); daf-2(e1370) is 43.8±5.4% (n = 396), p<0.02. We next tested the role of DAF-3 and DAF-5 on fat storage, dauer formation and stress resistance. Oil Red O staining for fat storage showed comparable levels between daf-2(e1370) and daf-2(e1370); daf-3(mgDf90) worms, but markedly lesser amounts of fat in daf-5(e1386); daf-2(e1370) worms (Figure 4B top and bottom panel and Figure S12). Similarly, age-1(hx546); daf-5(e1385) had less fat than age-1(hx546) worms (Figure S12). Both daf-3 and daf-5 single mutants have slightly reduced levels of fat when compared to wild-type worms (Figure S12).
A similar trend was seen with our data for dauer formation. daf-2(e1370); daf-3(mgDf90) worms show significant enhancement of daf-2(e1370) dauer formation across several temperatures tested, whereas a daf-5 mutation or daf-5 RNAi results in reduced daf-2(e1370) dauer formation (Figure 4Ci, Figure 4Cii and Figure S11). In addition, daf-5(e1386); daf-2(e1370) worms fail to completely arrest at the restrictive temperature of 25°C (data not shown). A mutation in daf-5 also significantly reduces thermotolerance of daf-2(e1370) worms at 37°C (Figure S13). Taken together, similar to PDP-1, DAF-3 and DAF-5 modulate multiple outputs of the IIS pathway. Unexpectedly, we find that while DAF-3 promotes dauer formation under conditions of reduced TGF-β signaling, it negatively regulates dauer formation and longevity under conditions of reduced IIS.
To further explore the crosstalk between both pathways, we next asked whether DAF-18 and DAF-16, which are components of the IIS pathway, affect TGF-β pathway signaling. For this, we assayed dauer formation of TGF-β pathway mutants on daf-18 and daf-16 RNAi (Table 3 and Figure S10). Interestingly, dauer formation of daf-7(e1372), daf-14(m77) and daf-8(m8 5) worms was robustly suppressed by daf-16 RNAi. We observed similar results for dauer formation daf-7(e1372) and daf-14(m77) mutants on daf-18 RNAi. However, in the case of daf-8(m85) mutants, daf-18 RNAi had no effect on dauer formation of (Figure S10), suggesting a complex crosstalk between both pathways. The enhanced dauer formation of daf-2(e1370); daf-3(mgDf90) is suppressed by both daf-18 and daf-16 RNAi but not pdp-1 RNAi (Table 3 and Figure S10). Therefore, we not only observe DAF-3 and DAF-5 affecting various phenotypes of the IIS pathway, but also the converse, where DAF-16 and DAF-18 robustly regulates TGF-β dauer formation. These results unravel a more complex interaction between the two pathways, where DAF-16 is likely to be the major downstream effector regulating longevity, dauer formation and other physiological outputs.
Tab. 3. Dauer formation of TGF-β signaling mutants is regulated by DAF-18 and DAF-16. 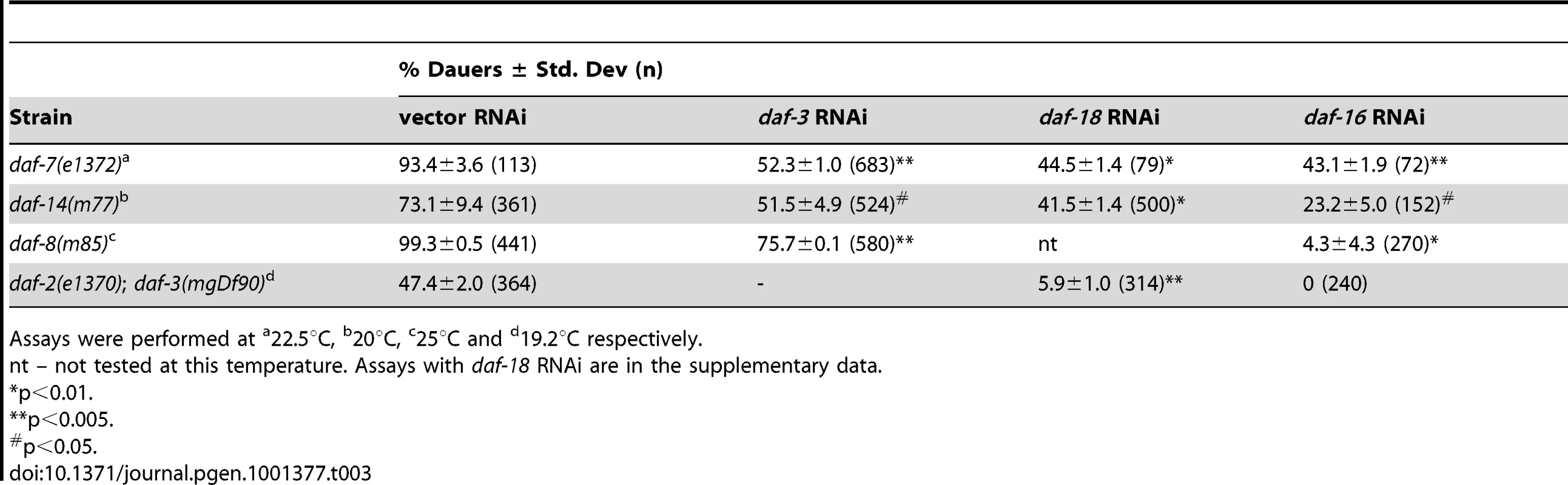
Assays were performed at Insulins are a possible connection between TGF-β signaling and IIS
How can these two pathways, once considered to be parallel to each other, be mechanistically linked? Thus far our data suggests that PDP-1, a component of the TGF-β pathway can modulate multiple phenotypes of IIS by positively regulating DAF-16. In addition, we observe extensive crosstalk between the two pathways at multiple levels. A feed-forward model that has been proposed to connect TGF-β signaling to the IIS pathway suggests insulins as a possible link [55], [58]. The C. elegans genome encodes 40 insulin genes [59], [60] (WormBase 215: www.wormbase.org). Studies using mutants and RNAi have characterized some of the insulins as agonists or antagonists of the IIS pathway [13], [59]–[61]. Importantly, microarray studies have identified several insulin genes that are regulated by TGF-β signaling, including ins-1, ins-4, ins-5, ins-6, ins-7, ins-17, ins-18, ins-30, ins-33, ins-35 and daf-28 [55], [57]. We tested changes in the levels of these insulins using Q-PCR in TGF-β pathway mutants such as daf-3(mgDf90), daf-14(m77) as well as pdp-1(tm3734) and compared them to wild-type worms (Figure 5A–5C, Figure S14, Tables S2 and S3). Interestingly, both pdp-1(tm3734) and daf-3(mgDf90) showed elevated levels of several insulins as compared to wild-type worms (Figure 5A and Figure S14). In contrast, expression of these insulins was markedly reduced in daf-14(m77) mutants (Figure 5B and Figure S14). We next looked at the effects of overexpressing DAF-3 and PDP-1 on insulin gene expression (Figure 5C and Figure S14). The levels of several insulins are markedly reduced in daf-3::gfp and pdp-1::gfp animals when compared to wild-type worms. Therefore, dosage modulation of DAF-3 and PDP-1 modulates insulin gene expression. INS-4, for example, has been reported as a positive regulator TGF-β pathway and a suppressor of dauer formation of daf-7 and daf-8 mutants [62]. ins-4 transcript levels were elevated in pdp-1 and daf-3 mutants but reduced in daf-14.
Fig. 5. PDP-1 modulates the expression of insulin genes that possibly feed into the IIS pathway. 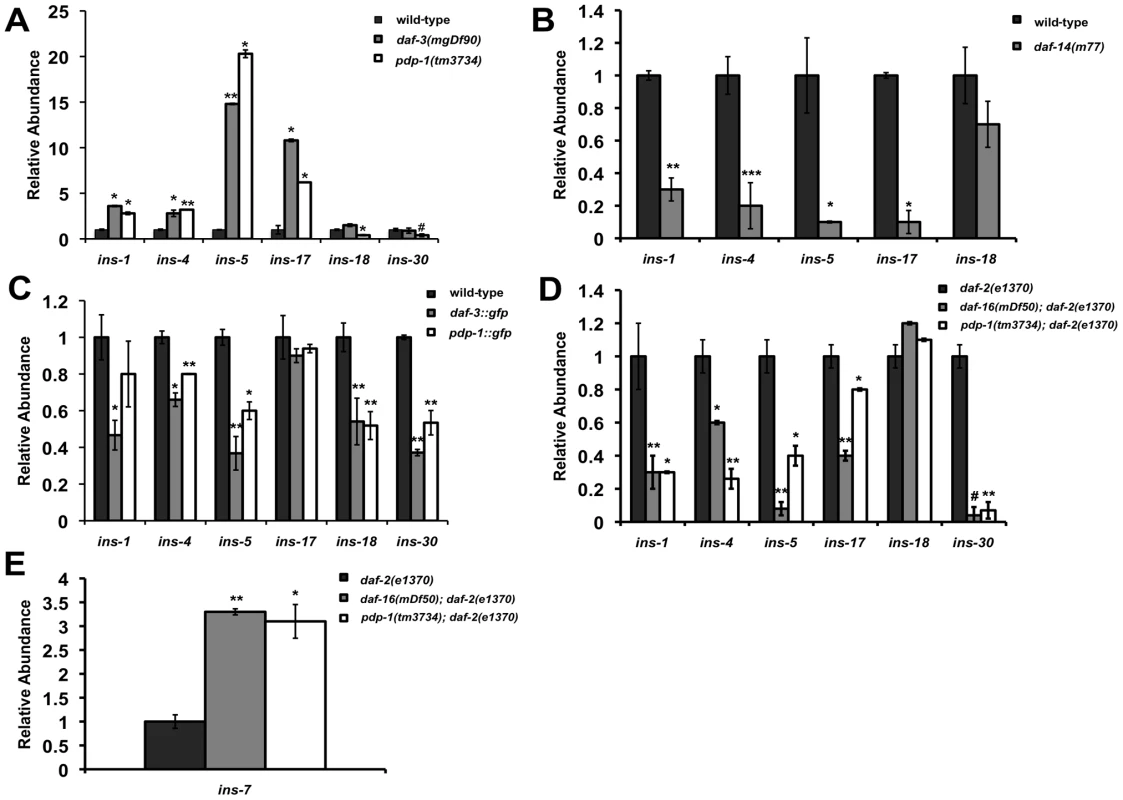
Data are representative of one experiment. Error bars represent standard error of the mean within triplicates. All experiments were performed at least twice. (A) The expression of several insulins is elevated in both pdp-1(tm3734) and daf-3(mgDf90) mutants. * p<0.05, ** p<0.007, # a significant reduction in ins-30 levels is observed in pdp-1(tm3734) worms this set but not in others, p<0.03. (B) The same insulins show decreased expression on daf-14(m77) mutants. *p<0.008, **p<0.005, ***p<0.0001. (C) In contrast to the mutants, daf-3::gfp and pdp-1::gfp worms show reduced levels of the insulins tested. *p<0.05, **p<0.005. (D) The trend in Insulin levels are similar between pdp-1(tm3734); daf-2(e1370) and daf-16(mgDf50); daf-2(e1370) double mutants compared to the daf-2(e1370) parental strain. *p<0.03, **p<0.01, # a significant reduction in ins-18 levels is observed in daf-16(mgDf50; daf-2(e1370) mutants this set but not in others, p<0.001. (E) ins-7 levels are drastically elevated in daf-16(mgDf50); daf-2(e1370) and pdp-1(tm3734); daf-2(e1370) worms as compared daf-2(e1370) worms. *p<0.01, **p<0.004. To investigate insulin gene expression regulated by DAF-16, we tested daf-2(e1370), pdp-1(tm3734); daf-2(e1370) and daf-16(mgDf50); daf-2(e1370) mutants. Several insulins were changed relative to daf-2(e1370) worms, with the trend between pdp-1(tm3734); daf-2(e1370) and daf-16(mgDf50); daf-2(e1370) being quite similar (Figure 5D and Figure S14). Interestingly, ins-7 levels were elevated both double mutants (Figure 5E and Figure S14). Previous studies have shown ins-7 to be an agonist of the IIS pathway as well as a DAF-16 target gene [13], [63]. In contrast, ins-1 levels were drastically reduced, and INS-1 has been characterized as a potential antagonist of IIS [59]. We did not observe a significant change in ins-18, another potential DAF-16 target [13]. We also did not detect any appreciable differences in insulin gene expression in daf-16(mgDf50) single mutants (Figure S14). In addition, we were unable to detect ins-33 and ins-35 transcripts in all the strains tested, and the trend observed with daf-28 was inconclusive (Table S2 and S3). Taken together, our results suggest the possibility that insulins downstream of TGF-β signaling mediate at least part of the cross talk between the two pathways. Therefore, PDP-1 would modulate to regulate expression of several insulins that can potentially feed into or antagonize the IIS pathway to regulate DAF-16 and its associated phenotypes.
Discussion
We identified pdp-1 from a RNAi screen for serine/threonine phosphatases that modulate daf-2 dauer formation. C. elegans PDP-1 is homologous to mammalian pyruvate dehydrogenase phophatase (PDP), a metabolic enzyme that is a positive regulator of the pyruvate dehydrogenase enzyme complex (PDHc). Remarkably, other components of the PDHc in C. elegans do not affect daf-2 dauer formation. Microarray and SAGE studies on dauers have indicated that genes involved in anaerobic metabolism are upregulated while genes involved in the TCA cycle and mitochondrial oxidative phosphorylation are downregulated, suggesting that PDHc activity may not be critical for dauer diapause [64]–[66]. Further, annotations indicate that the C. elegans genome encodes approximately 60 serine/threonine phosphatases, in contrast to the 400 plus protein kinases, suggesting that phosphatases are likely to have a number of cellular substrates [39], [67]. We find that PDP-1 also regulates longevity, fat storage and stress resistance in addition to dauer formation. Interestingly, these phenotypes are more severe in mutants such as daf-2 and age-1, where IIS is reduced. Further, PDP-1 positively regulates DAF-16 activity. We reason that PDP-1 function is critical under conditions of stress or low food availability, when DAF-16 activation is required [39].
Intriguingly, genetic epistasis analyses place PDP-1 in the DAF-7/TGF-β pathway, at the level of the R-SMAD proteins DAF-14 and DAF-8. A recent functional RNAi screen for serine/threonine phosphatases that modulate BMP signaling identified PDP as a SMAD1 phosphatase in Drosophila S2 cells and mammalian 293T cells [68]. Our study complements these findings and reveals a molecular conservation in the role of PDP-1 in regulating TGF-β signaling. Early genetic epistasis studies had suggested that TGF-β signaling and IIS pathways are parallel signaling pathways that modulate dauer diapause [31]. Importantly, in these studies, the conclusion was that both these pathways acted independently, and it was the IIS pathway that regulated longevity and stress resistance [31], [32].
However, the effect of PDP-1 on DAF-16 activity led us to re-investigate the interaction between the IIS and TGF-β signaling. Previous studies have shown that DAF-3 and DAF-5 are negatively regulated by TGF-β signaling, and function similarly as repressors of gene expression to ultimately promote dauer formation [28],[29],[69],[70]. We find that under conditions of reduced IIS, DAF-3 and DAF-5 affect various outputs of the IIS pathway in opposite ways. DAF-3 in particular regulates IIS depending upon the level of signaling through the pathway (Figure 6). In our hands, mutants of the TGF-β signaling pathway do not exhibit a pronounced increase in lifespan. However, components of this pathway are important for the long lifespan of mutants in the IIS pathway, as well as other phenotypes such as dauer formation, fat storage and stress resistance. Our epistasis studies reveal that daf-18 and daf-16 RNAi can strongly suppress dauer and fat storage of TGF-β pathway mutants. Together, these results point to a feed-forward model where signals through the TGF-β pathway are relayed to modulate activity of the IIS pathway as well as DAF-16. Indeed, recent studies have suggested that TGF-β pathway regulates the expression of insulins, leading to a feed-forward model, where signals from the TGF-β pathway are relayed to modulate activity of the IIS pathway as well as DAF-16 [55], [58].
Fig. 6. PDP-1 links TGF-β signaling to the IIS pathway and DAF-16. 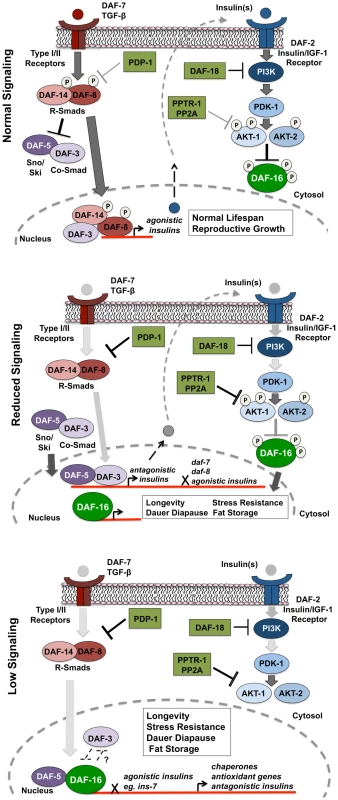
Top panel: Under favorable environmental conditions, signaling through the TGF-β pathway activates the R-SMAD proteins DAF-8 and DAF-14, which regulate insulin gene expression while antagonizing DAF-3 and DAF-5 function. These insulins may act as agonists and activate IIS, thereby promoting phosphorylation and suppression of DAF-16 activity. In this feed-forward model, the worm undergoes reproductive growth and has a normal life span. Middle panel: PDP-1 negatively regulates TGF-β signaling through dephosphorylation of DAF-8 and DAF-14. Under these conditions, DAF-3 and DAF-5 repress the transcription of agonistic insulins as well as expression of the daf-7 TGF-β ligand and daf-8, leading to further downregulation of the TGF-β pathway. Alternatively, DAF-3 and DAF-5 may promote transcription of potential antagonistic insulins. This results in reduced signaling through the IIS pathway, enhancing DAF-16 nuclear localization. Lower panel: Under low IIS conditions, DAF-16 localization is predominantly nuclear, where it regulates the transcription of hundreds of target genes that act in combination to regulate longevity, stress resistance, dauer formation and the response to stress. Paradoxically, under low IIS conditions, DAF-3 and DAF-5 play opposite roles. DAF-5 is likely to synergize with DAF-16 and modulate the activity of its target genes. DAF-3 acts to antagonize DAF-16, either directly or through suppression of DAF-16 target genes. Therefore, the role of DAF-3 in modulating IIS depends upon the level of signaling through the pathway. In support of this model, we find TGF-β signaling regulates the expression of several insulin genes with DAF-3 and PDP-1 negatively modulating insulin gene expression. This is in agreement with previous studies that identify DAF-3 as a repressor of gene expression [69], [70]. The expression of several insulins is also modulated by DAF-16, with pdp-1(tm3734); daf-2(e1370) and daf-16(mgDf50); daf-2(e1370) worms showing similar trends in insulin levels. Therefore, in the absence of PDP-1, increased levels of agonists or reduced levels of antagonists hyperactivate the DAF-2 pathway to negatively regulate DAF-16, thereby affecting the enhanced lifespan, stress resistance, dauer formation and fat storage of daf-2 mutants.
Our results suggest a model where under favorable growth conditions, signals through the TGF-β pathway activate the SMAD transcriptional complex to regulate the expression of insulins that activate the IIS pathway to phosphorylate and inhibit DAF-16 activity, thereby promoting growth, reproduction and normal lifespan (Figure 6, top panel). However, when food is limiting or under harsh survival conditions, TGF-β signaling is downregulated by PDP-1 to activate DAF-3 and DAF-5, to regulate the repression of insulin genes that may feed into the IIS pathway (Figure 6, middle panel). DAF-3 has also been reported to negatively regulate daf-7 and daf-8 gene expression in a feedback loop [24]. We find that pdp-1 expression is elevated in daf-3(mgDf90) mutants, suggesting a similar feedback regulation (Figure S15). Repression of TGF-β and insulin gene expression by DAF-3 results in a reduction in signaling through the IIS pathway, and promotes DAF-16 nuclear localization. DAF-16 then regulates the transcription of hundreds of target genes that ultimately modulate longevity, stress resistance, dauer formation and fat storage. Under low TGF-β signaling and IIS conditions, DAF-3 and DAF-5 regulate these outputs in an opposite manner, with DAF-5 synergizing and DAF-3 antagonizing DAF-16 function (Figure 6 lower panel). With our Q-PCR data, we found that PDP-1 affected only a subset of the DAF-16 target genes tested. These could represent genes that are regulated by DAF-16 and SMAD proteins. SMAD proteins have low affinity for binding DNA, and the orchestration of cellular signals into defined outputs requires their association with additional co-factors [71]. Mammalian SMAD proteins can bind several co-activators and co-repressor proteins to modulate gene transcription [23]. Specifically, a synergy between mammalian FOXO (FOXO1, FOXO3a and FOXO4) and SMAD2/3 was identified for the regulation of several genes involved in cell cycle regulation and the response to stress [72]. Importantly, these interactions required the function of the co-SMAD protein SMAD-4, which is homologous to DAF-3 [72]. Therefore, DAF-3 and DAF-5 could also directly modulate the IIS pathway at the transcriptional level.
A clear interpretation of our results is complicated by three main factors. First, the sheer number of insulins in the worm makes it difficult to assess whether they are functionally distinct. Secondly, the role of temperature in modulating the readouts of the pathway has not been closely explored. For example, we observe the effects of pdp-1 RNAi on daf-2 lifespan at 15°C but the effect decreases at a higher temperature, as the pathway gets more inactive. It is therefore likely that a certain level of signaling through the pathway is required to activate and target PDP-1 to its substrate(s). At higher temperatures such as 20°C or 25°C, there may be extremely low levels of phosphorylated substrate available for PDP-1. Similarly, the effect of a daf-3 null mutation on daf-2 phenotypes is more pronounced at higher temperatures but not at 15°C. Third, the lack of null alleles may provide an incomplete picture of the phenotypes observed. For example, previous studies using non-null alleles of daf-16 only partially suppressed dauer formation of TGF-β pathway mutants and therefore DAF-16 was thought to only affect the IIS pathway [31]. Therefore, temperature, level of signaling and the kind of mutants used (null versus weak) are important additional inputs that need to be considered to better understand the crosstalk between the IIS and the TGF-β pathways.
In conclusion, our studies show that PDP-1 acts through the TGF-β pathway to negatively regulate IIS and promote DAF-16 activity. PDP-1 may mediate this function in part by negatively regulating TGF-β signaling to repress expression of several insulins that feed into the IIS pathway. In humans, dysregulation of TGF-β signaling and the insulin/IGF-1 signaling axis have been implicated in the onset of age-associated diseases such as Type 2 Diabetes and cancer [73]–[77]. Future studies exploring the interactions between these two pathways as well as the factors that modulate these interactions may ultimately provide a better understanding of the pathophysiology of these diseases.
Materials and Methods
Strains
All strains were maintained at 15°C using standard C. elegans techniques [78]. For all RNAi assays, worms were maintained on the RNAi bacteria for two generations except for the assays on the PDHc RNAi. Strains used in this manuscript are listed in Table S4.
RNAi–based assays
RNAi plates were prepared as previously described [39]. All RNAi clones were sequenced and verified before any assays were carried out. L4 worms were picked onto fresh RNAi plates and maintained for two generations prior to the assay, with the exception PDHc RNAi plates. Worms exhibit lethality when maintained on the following RNAi clones: T05H10.6 (E1α), C04C3.3 (E1β), F23B12.5 (E2), or LLC1.3 (E3) [79]. To circumvent this problem, strains were maintained on vector RNAi for two generations and transferred to E1α, E1β, E2 or E3 plates prior to the assay.
Strain construction
For the pdp-1(tm3734);daf-2(e1370) double mutant, daf-2(e1370) males were mated to pdp-1(tm3734) hermaphrodites at 15°C. A total of 30 F1 progeny were picked onto individual plates and allowed to have progeny at 25°C. From the F2 progeny on each plate, dauers were selected and transferred to fresh plates and incubated for an additional 24 hours at 25°C. The next day, the dauers were allowed to recover at 15°C until they reached adulthood. Subsequently, adult worms were picked onto individual plates and transferred to 25°C and allowed to have progeny. Among the F3 progeny, we observed that some plates had 100% dauers at 25°C, while worms in some of the plates exhibited a developmental delay and could not form complete dauers even after 5–6 days at 25°C. Worms from both sets of plates were recovered, picked to individual plates and allowed to self at 15°C. Parents were then tested for pdp-1(tm3734) deletion by PCR. As anticipated, the pdp-1(tm3734);daf-2(e1370) double mutants are unable to form 100% dauers at 25°C.
The daf-2(e1370);pdp-1::gfp strain was made by crossing daf-2(e1370) males to pdp-1::gfp hermaphrodites at 15°C. About 30 F1 animals were transferred to individual plates and allowed to have progeny at 25°C. From the progeny, F2 dauers were selected from each plate and allowed to recover at 15°C. The recovered adult worms were then checked for the presence of GFP, and GFP-positive worms were transferred to individual plates and incubated at 25°C. Plates where 100% of the progeny were dauers and GFP positive were selected and established as the strain for the assays.
Dauer assays
Strains were maintained on RNAi plates for two generations or regular OP50 plates at 15°C. Dauer assays were performed by picking approximately 100 eggs onto 2 fresh plates and incubated at the appropriate temperature. The pdk-1(sa680), daf-7(e1372) and daf-14(m77) worms have a strong Egl phenotype. For dauer assays on these strains, gravid adult worms growing on the RNAi plates were washed off the plate with sterile PBS onto a 1.5 mL eppendorf tube. After 2 washes at 2000 g for 30 seconds, the adults were vortexed for 5 mins in 5 mL of 1 N sodium hydroxide and 3% sodium hypochlorite (final concentration). The samples were then washed twice with sterile PBS and eggs were aspirated with a glass pipette onto fresh RNAi plates. For all dauer assays, plates were scored for the presence of dauers or non-dauers after 3.5–5.5 days, depending upon the strain. Dauer assays were performed at the temperature indicated. Significance was determined by Student's t-test.
Lifespan assays
Strains were maintained at 15°C and synchronized by picking eggs onto fresh RNAi or OP50 plates. Approximately 60 young adult worms were transferred per plate to a total of three fresh RNAi or regular OP-50 plates containing 5-fluorodeoxyuridine (FUDR) at final concentration of 0.1 mg/mL [80]. All RNAi-based lifespan assays were carried out at 15°C. Lifespans on OP50 plates were performed at the temperature indicated. Survival was scored by tapping with a platinum wire every 2–3 days. Worms that died from vulval bursting were censored from the analysis. Statistical analyses for survival were conducted using the standard chi-squared-based log rank test.
Heat stress assay
Strains were maintained on RNAi or regular OP50 bacteria at 15°C, as described above. From these plates, approximately 30 young adult worms were picked onto fresh RNAi or regular plates and upshifted to 20°C for 6 hrs. The plates were then transferred to 37°C and heat stress-induced mortality was determined every few hours till all the animals died. Statistical analyses for survival were conducted using the standard chi-squared-based log rank test.
Fat staining
Strains maintained RNAi or on regular OP50 plates were synchronized by picking eggs on to fresh plates and grown synchronously at 15°C. The plates were then upshifted to 20°C for 8 hours, at the L2 stage to get L3 worms and at the L4 stage to get young adult worms. Worms were then washed off the plates into microcentrifuge tubes and incubated in 1× PBS buffer for 20 minutes on a shaker at RT. After 2 washes at 3000 rpm for 30 seconds with 1× PBS, the strains were fixed according to the type of staining performed. Oil Red O and Sudan black staining was performed as previously described [39], [45], [81], [82]. After incubation overnight at RT, worms were mounted on slides and visualized using the Zeiss Axioscope 2+ microscope.
Quantification of fat staining
For Sudan Black Staining, we used Image J software to measure the average pixel intensity for a 84-pixel radius below the pharynx of each animal in the anterior intestine area. Next, an 84-pixel radius of the background was measured, and subtracted from the values obtained for the staining. At least 10 animals were measured for each RNAi clone. Significance was determined by Student's t-test.
For Oil Red O Staining, Image J was used to separate out each color image into its RGB channel components. As previously described [45], Oil Red O absorbs light at 510 nm and therefore, the green channel was used for further analysis. We measured the average pixel intensity for a 84-pixel radius below the pharynx of each animal in the anterior pharynx area. We next measured a 84-pixel radius of the background, which was later subtracted from the values obtained from the staining. At least 10 animals was measured for each RNAi clone. Significance was determined by Student's t-test.
DAF-16::GFP localization assay
DAF-16 localization assays were performed as previously described [39], [52]. daf-2(e1370); daf-16::gfp worms were maintained on RNAi plates at 15°C similar to the dauer assays. Approximately 30 L4 worms were transferred to fresh RNAi bacteria and the plates were shifted to 20°C for 1 hr. The worms were visualized under a fluorescence microscope (Zeiss Axioscope 2+ microscope). Worms were classified into four categories based on the extent of DAF-16::GFP nuclear-cytoplasmic distribution: completely cytosolic, more cytosolic than nuclear in most tissues, more nuclear than cytosolic in most tissues and completely nuclear.
Psod-3::gfp expression
Quantification of Psod-3::gfp was performed as previously described [39]. daf-2(e1370);sod-3::gfp worms were grown at 15°C on RNAi as described above. Approximately 30 L4 animals were transferred to fresh RNAi bacteria and shifted to 25°C for 1 hr. The expression of sod-3::gfp was visualized using Zeiss Axioscope 2+ microscope. GFP expression was categorized as follows:
High: GFP expression seen throughout the worm
Medium: Weak expression detected in the body of the worm along with the head and the tail
Low: Low GFP expression only detected in the head and tail
Transgenic worms
Promoter and ORF entry clones of pdp-1 obtained from the promoterome and ORFeome were combined using multisite Gateway cloning (Invitrogen) into the pDEST-DD03 or the R4-R2 GFP destination vectors to create the Ppdp-1::gfp or Ppdp-1::pdp-1ORF::gfp constructs [83], [84]. All constructs contain the unc-119 minigene. The vectors were verified by sequencing as well as restriction digestion. Transgenic worms were generated by ballistic transformation into unc-119(ed3) mutant worms as previously reported (Biorad, USA) [83]. Integrated lines that were obtained were used for further analyses. For the pdp-1::gfp translational fusion strain, additional lines were generated by integration of extrachromosomal array lines by UV irradiation as previously described [85]. All translational fusion lines were backcrossed 4× to wild-type prior to analysis.
RT-PCR experiments
For all RT-PCR experiments, strains were maintained at 15°C. Eggs were obtained from gravid adult worms by hypochlorite treatment described earlier. The eggs were seeded onto large plates maintained at 15°C until the worms entered the L4 stage. The plates were then upshifted to 20°C for 8 hours until they became young adults. Worms were then collected with sterile 1×PBS and washed twice at 2000 g for 30 seconds. The supernatant was removed, and 0.5 mL of AE buffer (50 mM acetic acid, 10 mM EDTA), 0.1 mL of 10% SDS, and 0.5 mL of phenol was added to the worm pellet and the mixture was vortexed vigorously for 1 min, followed by incubation at 65°C for 4 min. Total RNA was purified by phenol:chloroform extraction and ethanol precipitation. The quality of the RNA isolated was determined by checking the 28 S and 18 S RNA on an agarose gel. 2 ug of total RNA was used for making cDNA using the SuperScript cDNA synthesis kit (Invitrogen, USA). The expression of the DAF-16 target and insulin genes was checked by RT-PCR using the SYBR Green PCR Master Mix and 7000 Real-Time PCR System (Applied Biosystems, USA). The relative expression of the genes tested was compared to actin as an internal loading control. Significance was determined by Student's t-test. Primers used for the RT-PCR experiments are listed in Table S5.
Locomotion assay
Young adult wild-type and pdp-1(tm3734) worms were picked onto 6 individual plates each. After 5 minutes, the worms were picked off the plate. The average distance covered was calculated by measuring the traces on the bacterial lawn using ImageJ. Significance was determined by Student's t-test.
Brood size measurements
Wild type, daf-2(e1370), pdp-1(tm3734) and pdp-1(tm3734); daf-2(e1370) worms were maintained at 15°C. 5 L4 worms were picked onto individual plates and allowed to lay eggs at 22.5°C. Worms were transferred to a new plate every 12 hours. After 22.5 hours, the parental worms were picked off the plates, and the total number of eggs laid was scored. The number of progeny from these eggs was scored again after 38 hours. The % hatched eggs was calculated as a percentage of the average number of progeny over the average number of eggs laid. Significance was determined by Student's t-test.
Software used in this study
Statistical analyses were performed using JMP and Microsoft Excel. NIH Image J was used for quantification of locomotion and fat storage.
Supporting Information
Zdroje
1. BarbieriM
BonafeM
FranceschiC
PaolissoG
2003
Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans.
Am J Physiol Endocrinol Metab
285
E1064
1071
2. NarasimhanSD
YenK
TissenbaumHA
2009
Converging pathways in lifespan regulation.
Curr Biol
19
R657
666
3. ParadisS
RuvkunG
1998
Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor.
Genes Dev
12
2488
2498
4. ParadisS
AilionM
TokerA
ThomasJH
RuvkunG
1999
A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans.
Genes Dev
13
1438
1452
5. WolkowCA
MunozMJ
RiddleDL
RuvkunG
2002
Insulin receptor substrate and p55 orthologous adaptor proteins function in the Caenorhabditis elegans daf-2/insulin-like signaling pathway.
J Biol Chem
277
49591
49597
6. MorrisJZ
TissenbaumHA
RuvkunG
1996
A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans.
Nature
382
536
539
7. KimuraKD
TissenbaumHA
LiuY
RuvkunG
1997
daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans.
Science
277
942
946
8. HertweckM
GobelC
BaumeisterR
2004
C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span.
Dev Cell
6
577
588
9. LeeRY
HenchJ
RuvkunG
2001
Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway.
Curr Biol
11
1950
1957
10. LinK
DormanJB
RodanA
KenyonC
1997
daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans.
Science
278
1319
1322
11. OhSW
MukhopadhyayA
DixitBL
RahaT
GreenMR
2006
Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation.
Nat Genet
38
251
257
12. McElweeJ
BubbK
ThomasJH
2003
Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16.
Aging Cell
2
111
121
13. MurphyCT
McCarrollSA
BargmannCI
FraserA
KamathRS
2003
Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans.
Nature
424
277
283
14. RiddleDBT
MeyerB
PriessJ
1997
C. elegans II
Cold Spring Harbor
Cold Spring Harbor Press
1222
15. KenyonCJ
2010
The genetics of ageing.
Nature
464
504
512
16. MukhopadhyayA
OhSW
TissenbaumHA
2006
Worming pathways to and from DAF-16/FOXO.
Exp Gerontol
41
928
934
17. AntebiA
2007
Genetics of aging in Caenorhabditis elegans.
PLoS Genet
3
e129
doi:10.1371/journal.pgen.0030129
18. WolffS
DillinA
2006
The trifecta of aging in Caenorhabditis elegans.
Exp Gerontol
41
894
903
19. Savage-DunnC
2005
TGF-beta signaling.
WormBook
1
12
20. PattersonGI
PadgettRW
2000
TGF beta-related pathways. Roles in Caenorhabditis elegans development.
Trends Genet
16
27
33
21. FielenbachN
AntebiA
2008
C. elegans dauer formation and the molecular basis of plasticity.
Genes Dev
22
2149
2165
22. InoueT
ThomasJH
2000
Targets of TGF-beta signaling in Caenorhabditis elegans dauer formation.
Developmental Biology
217
192
204
23. MassagueJ
2000
How cells read TGF-beta signals.
Nat Rev Mol Cell Biol
1
169
178
24. ParkD
EstevezA
RiddleDL
2010
Antagonistic Smad transcription factors control the dauer/non-dauer switch in C. elegans.
Development
137
477
485
25. MassagueJ
GomisRR
2006
The logic of TGFbeta signaling.
FEBS Lett
580
2811
2820
26. RenP
LimC
JohnsenR
AlbertPS
PilgrimD
1996
Control of C. elegans Larval Development by Neuronal Expression of a TGF - β homologue.
Science
274
1389
1391
27. GuntherCV
GeorgiLL
RiddleDL
2000
A Caenorhabditis elegans type I TGF beta receptor can function in the absence of type II kinase to promote larval development.
Development
127
3337
3347
28. PattersonGI
KoweekA
WongA
LiuY
RuvkunG
1997
The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway.
Genes Dev
11
2679
2690
29. da GracaLS
ZimmermanKK
MitchellMC
Kozhan-GorodetskaM
SekiewiczK
2004
DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF beta pathway to regulate C. elegans dauer development.
Development
131
435
446
30. Savage-DunnC
2001
Targets of TGF beta-related signaling in Caenorhabditis elegans.
Cytokine Growth Factor Rev
12
305
312
31. VowelsJJ
ThomasJH
1992
Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans.
Genetics
130
105
123
32. LarsenPL
AlbertPS
RiddleDL
1995
Genes that regulate both development and longevity in Caenorhabditis elegans.
Genetics
139
1567
1583
33. HuPJ
2007
Dauer.
WormBook
1
19
34. DormanJB
AlbinderB
ShroyerT
KenyonC
1995
The age-1 and daf-2 Genes Function in a Common Pathway to Control the Lifespan of Caenorhabditis elegans.
Genetics
141
1399
1406
35. OggS
RuvkunG
1998
The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway.
Molecular Cell
2
887
893
36. RouaultJP
KuwabaraPE
SinilnikovaOM
DuretL
Thierry-MiegD
1999
Regulation of dauer larva development in Caenorhabditis elegans by daf-18, a homologue of the tumour suppressor PTEN.
Current Biology
9
329
332
37. MihaylovaVT
BorlandCZ
ManjarrezL
SternMJ
SunH
1999
The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway.
Proc Natl Acad Sci U S A
96
7427
7432
38. GilEB
Malone LinkE
LiuLX
JohnsonCD
LeesJA
1999
Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene.
Proc Natl Acad Sci U S A
96
2925
2930
39. PadmanabhanS
MukhopadhyayA
NarasimhanSD
TeszG
CzechMP
2009
A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation.
Cell
136
939
951
40. VassylyevDG
SymerskyJ
2007
Crystal structure of pyruvate dehydrogenase phosphatase 1 and its functional implications.
J Mol Biol
370
417
426
41. KenyonC
ChangJ
GenschE
RudnerA
TabtiangR
1993
A C. elegans mutant that lives twice as long as wild type.
Nature
366
461
464
42. FriedmanDB
JohnsonTE
1988
A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility.
Genetics
118
75
86
43. LithgowGJ
WalkerGA
2002
Stress resistance as a determinate of C. elegans lifespan.
Mech Ageing Dev
123
765
771
44. GarsinDA
VillanuevaJM
BegunJ
KimDH
SifriCD
2003
Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens.
Science
300
1921
45. SoukasAA
KaneEA
CarrCE
MeloJA
RuvkunG
2009
Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans.
Genes Dev
23
496
511
46. GemsD
SuttonAJ
SundermeyerML
AlbertPS
KingKV
1998
Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans.
Genetics
150
129
155
47. TissenbaumHA
RuvkunG
1998
An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans.
Genetics
148
703
717
48. KenyonC
2005
The plasticity of aging: insights from long-lived mutants.
Cell
120
449
460
49. HendersonST
JohnsonTE
2001
daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans.
Curr Biol
11
1975
1980
50. LinK
HsinH
LibinaN
KenyonC
2001
Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling.
Nat Genet
28
139
145
51. LibinaN
BermanJR
KenyonC
2003
Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan.
Cell
115
489
502
52. KwonES
NarasimhanSD
YenK
TissenbaumHA
2010
A new DAF-16 isoform regulates longevity.
Nature
466
498
502
53. RenP
LimCS
JohnsenR
AlbertPS
PilgrimD
1996
Control of C. elegans larval development by neuronal expression of a TGF-beta homolog.
Science
274
1389
1391
54. OggS
ParadisS
GottliebS
PattersonGI
LeeL
1997
The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans.
Nature
389
994
999
55. ShawWM
LuoS
LandisJ
AshrafJ
MurphyCT
2007
The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling.
Curr Biol
17
1635
1645
56. ThomasJH
BirnbyDA
VowelsJJ
1993
Evidence for parallel processing of sensory information controlling dauer formation in C. elegans.
Genetics
134
1105
1117
57. LiuT
ZimmermanKK
PattersonGI
2004
Regulation of signaling genes by TGFbeta during entry into dauer diapause in C. elegans.
BMC Dev Biol
4
11
58. Liu TZK
PattersonGI
2004
Regulation of signaling genes by TGFβ during entry into dauer diapause in C. elegans.
BMC Dev Biol
4
59. PierceSB
CostaM
WisotzkeyR
DevadharS
HomburgerSA
2001
Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family.
Genes and Development
15
672
686
60. LiW
KennedySG
RuvkunG
2003
daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway.
Genes Dev
17
844
858
61. KawanoT
ItoY
IshiguroM
TakuwaK
NakajimaT
2000
Molecular cloning and characterization of a new insulin/IGF-like peptide of the nematode Caenorhabditis elegans.
Biochem Biophys Res Commun
273
431
436
62. KaoG
NordensonC
StillM
RonnlundA
TuckS
2007
ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells.
Cell
128
577
587
63. MurphyCT
LeeSJ
KenyonC
2007
Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans.
Proc Natl Acad Sci U S A
104
19046
19050
64. WangJ
KimSK
2003
Global analysis of dauer gene expression in Caenorhabditis elegans.
Development
130
1621
1634
65. McElweeJJ
SchusterE
BlancE
ThorntonJ
GemsD
2006
Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans.
Mech Ageing Dev
127
458
472
66. HoltSJ
RiddleDL
2003
SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva.
Mech Ageing Dev
124
779
800
67. ManningG
2005
Genomic overview of protein kinases.
WormBook
1
19
68. ChenHB
ShenJ
IpYT
XuL
2006
Identification of phosphatases for Smad in the BMP/DPP pathway.
Genes Dev
20
648
653
69. ThatcherJD
HaunC
OkkemaPG
1999
The DAF-3 Smad binds DNA and represses gene expression in the Caenorhabditis elegans pharynx.
Development
126
97
107
70. DeplanckeB
MukhopadhyayA
AoW
ElewaAM
GroveCA
2006
A gene-centered C. elegans protein-DNA interaction network.
Cell
125
1193
1205
71. WranaJL
2000
Crossing Smads.
Sci STKE
2000
re1
72. GomisRR
AlarconC
HeW
WangQ
SeoaneJ
2006
A FoxO-Smad synexpression group in human keratinocytes.
Proc Natl Acad Sci U S A
103
12747
12752
73. RaneSG
LeeJH
LinHM
2006
Transforming growth factor-beta pathway: role in pancreas development and pancreatic disease.
Cytokine Growth Factor Rev
17
107
119
74. GordonKJ
BlobeGC
2008
Role of transforming growth factor-beta superfamily signaling pathways in human disease.
Biochim Biophys Acta
1782
197
228
75. VirkamakiA
UekiK
KahnCR
1999
Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance.
J Clin Invest
103
931
943
76. VivancoI
SawyersCL
2002
The phosphatidylinositol 3-Kinase AKT pathway in human cancer.
Nat Rev Cancer
2
489
501
77. AkhurstRJ
DerynckR
2001
TGF-beta signaling in cancer–a double-edged sword.
Trends Cell Biol
11
S44
51
78. StiernagleT
2006
Maintenance of C. elegans.
WormBook
1
11
79. KamathRS
FraserAG
DongY
PoulinG
DurbinR
2003
Systematic functional analysis of the Caenorhabditis elegans genome using RNAi.
Nature
421
231
237
80. HosonoR
MitsuiY
SatoY
AizawaS
MiwaJ
1982
Life span of the wild and mutant nematode Caenorhabditis elegans. Effects of sex, sterilization, and temperature.
Exp Gerontol
17
163
172
81. YenK
LeTT
BansalA
NarasimhanSD
ChengJX
2010
A comparative study of fat storage quantitation in nematode Caenorhabditis elegans using label and label-free methods.
PLoS ONE
5
e12810
doi:10.1371/journal.pone.0012810
82. ArdaHE
TaubertS
MacNeilLT
ConineCC
TsudaB
2010
Functional modularity of nuclear hormone receptors in a Caenorhabditis elegans metabolic gene regulatory network.
Mol Syst Biol
6
367
83. DupuyD
LiQR
DeplanckeB
BoxemM
HaoT
2004
A first version of the Caenorhabditis elegans Promoterome.
Genome Res
14
2169
2175
84. ReboulJ
VaglioP
RualJF
LameschP
MartinezM
2003
C. elegans ORFeome version 1.1: experimental verification of the genome annotation and resource for proteome-scale protein expression.
Nat Genet
34
35
41
85. TissenbaumHA
GuarenteL
2001
Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans.
Nature
410
227
230
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



