-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32
and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell
Lymphoma
Non-Hodgkin lymphoma (NHL) represents a diverse group of hematological
malignancies, of which follicular lymphoma (FL) is a prevalent subtype. A
previous genome-wide association study has established a marker, rs10484561 in
the human leukocyte antigen (HLA) class II region on 6p21.32 associated with
increased FL risk. Here, in a three-stage genome-wide association study,
starting with a genome-wide scan of 379 FL cases and 791 controls followed by
validation in 1,049 cases and 5,790 controls, we identified a second independent
FL–associated locus on 6p21.32, rs2647012
(ORcombined = 0.64,
Pcombined = 2×10−21)
located 962 bp away from rs10484561 (r2<0.1 in controls). After
mutual adjustment, the associations at the two SNPs remained genome-widesignificant (rs2647012:
ORadjusted = 0.70,
Padjusted = 4×10−12;rs10484561:
ORadjusted = 1.64,
Padjusted = 5×10−15).
Haplotype and coalescence analyses indicated that rs2647012 arose on an
evolutionarily distinct haplotype from that of rs10484561 and tags a novel
allele with an opposite (protective) effect on FL risk. Moreover, in a follow-up
analysis of the top 6 FL–associated SNPs in 4,449 cases of other NHL
subtypes, rs10484561 was associated with risk of diffuse large B-cell lymphoma
(ORcombined = 1.36,
Pcombined = 1.4×10−7).
Our results reveal the presence of allelic heterogeneity within the HLA class II
region influencing FL susceptibility and indicate a possible shared genetic
etiology with diffuse large B-cell lymphoma. These findings suggest that the HLA
class II region plays a complex yet important role in NHL.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001378
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001378Summary
Non-Hodgkin lymphoma (NHL) represents a diverse group of hematological
malignancies, of which follicular lymphoma (FL) is a prevalent subtype. A
previous genome-wide association study has established a marker, rs10484561 in
the human leukocyte antigen (HLA) class II region on 6p21.32 associated with
increased FL risk. Here, in a three-stage genome-wide association study,
starting with a genome-wide scan of 379 FL cases and 791 controls followed by
validation in 1,049 cases and 5,790 controls, we identified a second independent
FL–associated locus on 6p21.32, rs2647012
(ORcombined = 0.64,
Pcombined = 2×10−21)
located 962 bp away from rs10484561 (r2<0.1 in controls). After
mutual adjustment, the associations at the two SNPs remained genome-widesignificant (rs2647012:
ORadjusted = 0.70,
Padjusted = 4×10−12;rs10484561:
ORadjusted = 1.64,
Padjusted = 5×10−15).
Haplotype and coalescence analyses indicated that rs2647012 arose on an
evolutionarily distinct haplotype from that of rs10484561 and tags a novel
allele with an opposite (protective) effect on FL risk. Moreover, in a follow-up
analysis of the top 6 FL–associated SNPs in 4,449 cases of other NHL
subtypes, rs10484561 was associated with risk of diffuse large B-cell lymphoma
(ORcombined = 1.36,
Pcombined = 1.4×10−7).
Our results reveal the presence of allelic heterogeneity within the HLA class II
region influencing FL susceptibility and indicate a possible shared genetic
etiology with diffuse large B-cell lymphoma. These findings suggest that the HLA
class II region plays a complex yet important role in NHL.Introduction
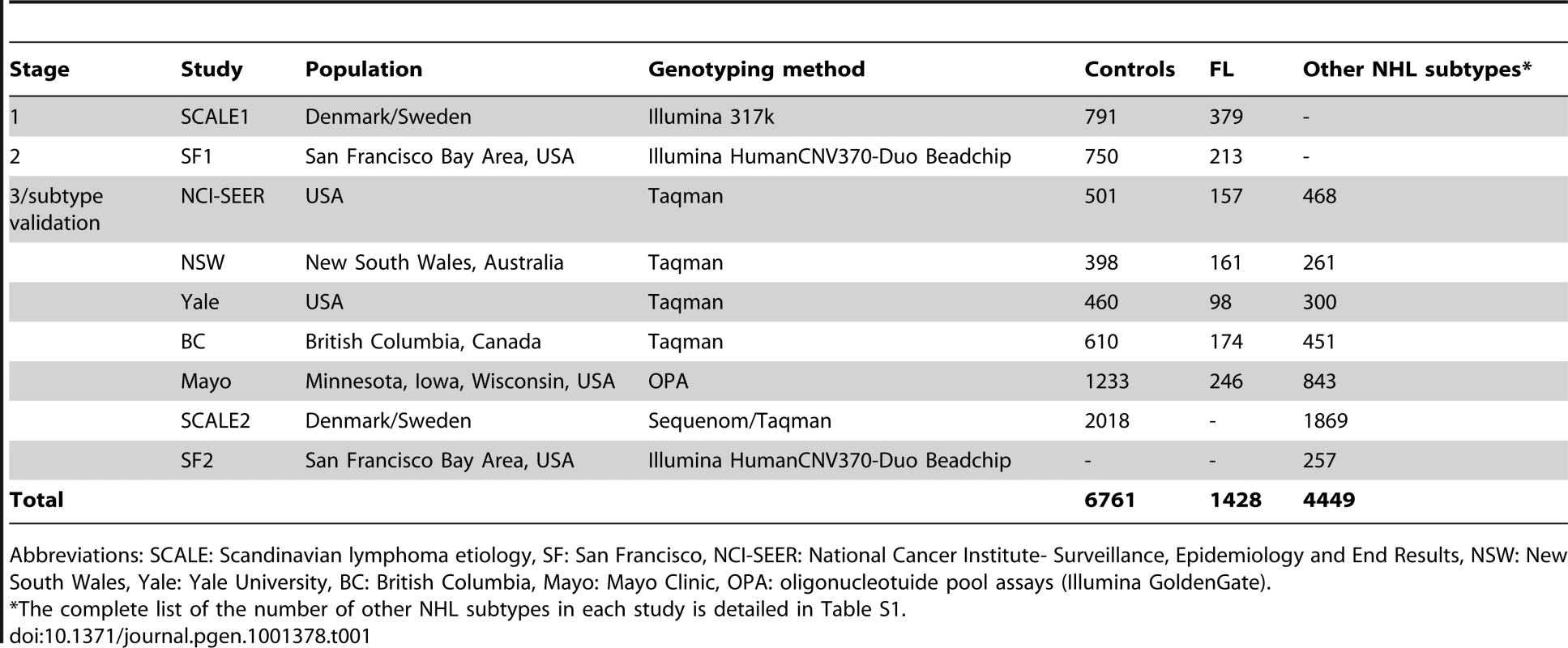
Non-Hodgkin lymphoma (NHL) represents a diverse group of B - and T-cell malignancies of lymphatic origin. The most common subtypes are of B-cell origin and are further classified on the basis of their resemblance to normal stages of B-cell differentiation [1]. Epidemiological studies indicate that these may have different environmental and genetic risk factors, although some etiological factors may also be shared [2]. Familial studies provide substantial evidence for a genetic influence on susceptibility to the major mature B-cell neoplasms, including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) [3], [4]. Recent genome-wide association studies (GWAS) of the FL subtype of NHL identified associations with two variants within the human leukocyte antigen (HLA) region, one at 6p21.33 (rs6457327) [5] and the other at 6p21.32 (rs10484561) [6]. Additional true associations, particularly in the HLA region, may have been missed because a limited number of samples were used in the initial genome-wide screens, and the selection of a few top single nucleotide polymorphisms (SNPs) for validation is further subject to chance. In this study, we conducted a larger independent genome-wide scan of FL using 379 cases and 791 controls from the Scandinavian Lymphoma Etiology (SCALE) study of Sweden and Denmark, which was used in the validation of the previous GWAS [6]. This scan was followed by two stages of validation in European-ancestry cases of FL and other common B-cell NHL subtypes and controls from the US, Canada and Australia (Table 1, Table S1, Table S2, Figure 1).
Fig. 1. Schematic representation of the three-stage study design. 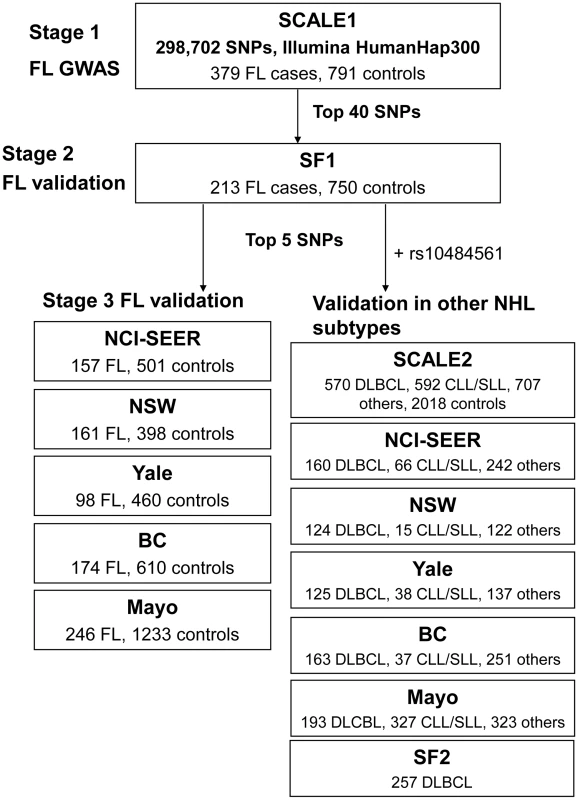
Results
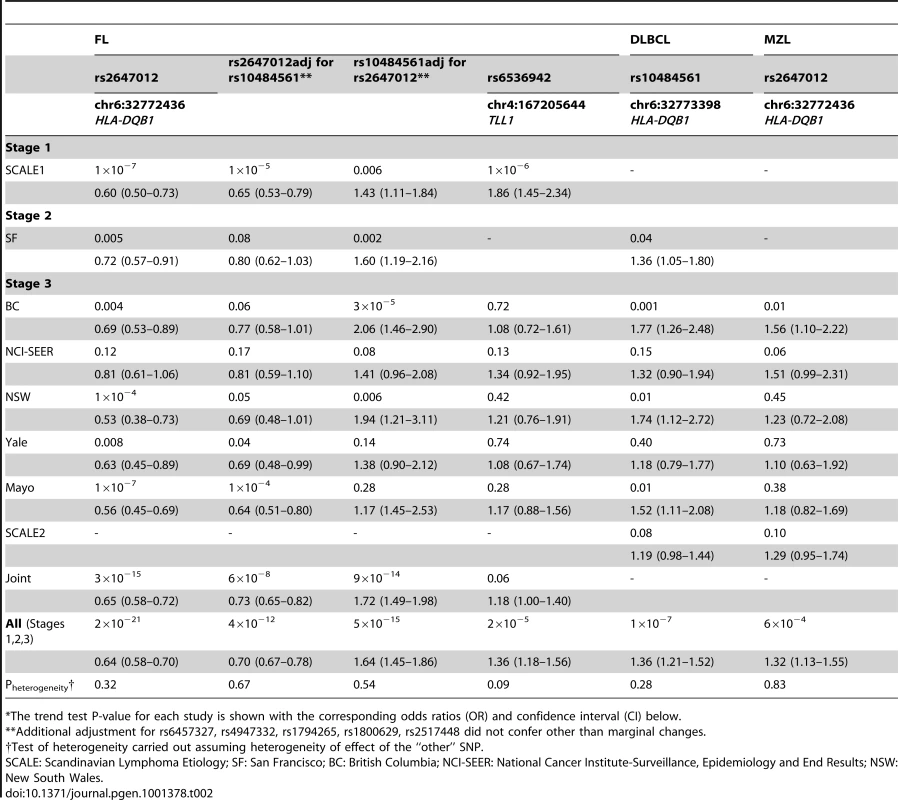
In total, 298,168 SNPs were analyzed in Stage 1 (λ = 1.028; λ1000 = 1.055 [7]), in which we observed suggestive associations (adjusted trend P-value<10−5) at 4q32.3, 6p21.32 and 10q25.3 (Table S3) with the strongest at rs2647012 (odds ratio (OR) = 0.58, PPCAadjusted = 1.59x10−7) within the HLA class II region on 6p21.32. Sixteen SNPs in close proximity to the HLA-DQ genes showed association with adjusted P-values<10−4, including the previously reported rs10484561 (Figure 2, Table S4) [6]. The previously reported HLA class I associated SNP rs6457327 [5] was modestly associated with FL risk (OR = 0.82, P = 0.03) in Stage 1, and was not in linkage disequilibrium (LD; r2 = 0) with any of the top 100 SNPs.
In Stage 2, we carried out an in silico validation of the top 40 SNPs from Stage 1 (Table S5) in 213 FL cases and 750 controls from the San Francisco Bay Area, USA (Table 1), the study that reported an association at 6p21.32 [6]. Among 38 out of 40 SNPs, seven showed association (P<0.05) in Stage 2 (Table S5), six of which were located within the 6p21.32 region. We tested the independence of multiple association signals in 6p21.32 using a stepwise logistic regression analysis (entering SNPs based on a criterion of likelihood ratio test p-value<0.05) and found that with rs2647012 (the top SNP within the region) forced in the model, only the addition of rs10484561 contributed significantly to the association with increased risk of FL. The OR for this SNP, adjusted for rs2647012, was 1.43, P = 0.006 (Table S6).
Fig. 2. Recombination plot showing associations in 6p21.32 in Stage 1. 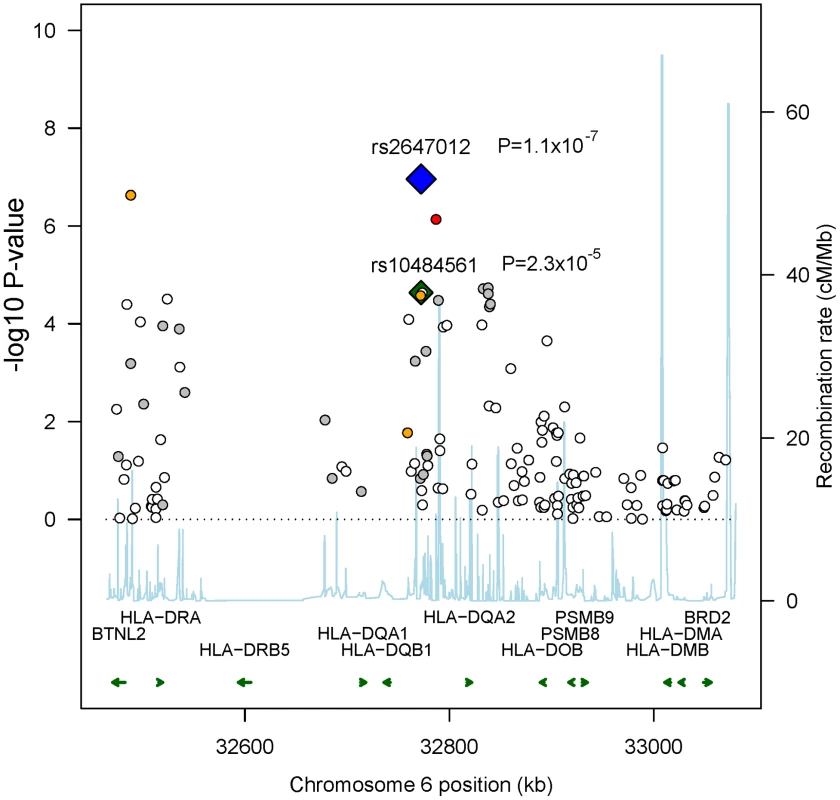
After excluding previously identified and non-independent association signals, we selected rs2647012, and an additional four top SNPs to be taken forward to a third stage (Table S7, S8), wherein these were genotyped in 836 FL cases and 3202 controls from the Mayo Clinic (US) [8], National Cancer Institute-Surveillance, Epidemiology and End Results (NCI-SEER, US) [9], Yale University (US) [10], New South Wales (NSW, Australia) [11] and British Columbia (BC, Canada) [12] studies. The association of rs2647012 with FL was validated, showing consistent associations with similar ORs (no heterogeneity, P = 0.32) across all independent studies and reaching genome-wide significance in both the combined analysis of the validation samples (P = 3×10−15) and the combined analysis of all three stages (1428 FL cases, 4743 controls; OR = 0.64, P = 2×10−21) (Table.2, Figure 3). After adjustment for rs10484561, the association at rs2647012 remained genome-wide significant with minimal change in magnitude (ORadjusted = 0.70, Padjusted = 4×10−12). The LD between the two SNPs is low (r2<0.1 in the SCALE controls and HapMap CEU [Utah residents with northern and western European ancestry] samples release27). Taken together, our results suggest that the association at rs2647012 is independent from rs10484561, and tags a different disease-predisposing variant. We also found suggestive evidence for an association at rs6536942 on 4q32.3 (OR = 1.36, P = 2×10−5) (Table 2, Figure S1A).
Fig. 3. Forest plots of main associations with risk of FL. 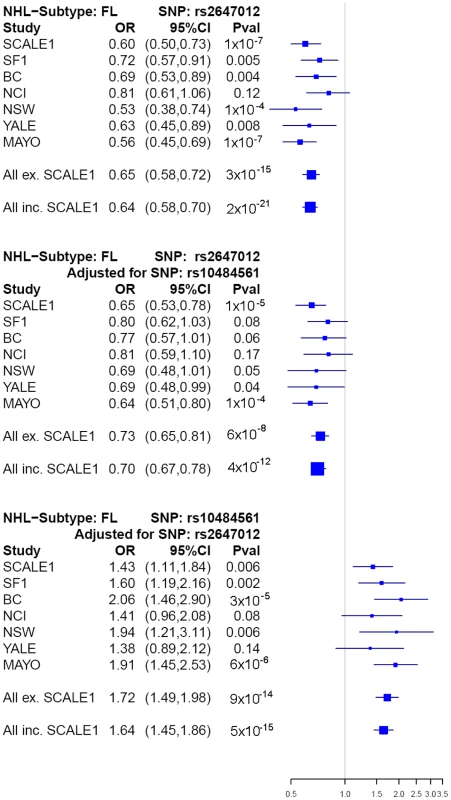
To fine-map the association signals in the HLA class II region, we imputed 10,639 SNPs within 600 kb surrounding the top SNP rs2647012 using data from the 1000 Genomes (1000G, 60 CEU subjects, August 2009) and HapMap projects (HapMapII release 22, CEU) in Stage 1. Among the imputed SNPs, 258 SNPs located in a strong LD block of 236 kb (r2>0.8) showed stronger evidence of association than all the genotyped SNPs within the region (Figure S2). Since a moderate discordance of reference genotypes was observed between 1000 G and HapMapII, we analyzed only SNPs showing a concordance of >95% in the two datasets and identified the strongest association at rs9378212 (OR = 1.66, P = 3.21×10−8), located 219 kb upstream of rs2647012 (r2 = 0.56 in controls). We subsequently confirmed the imputed genotypes by Taqman genotyping in 345 of the FL case subjects used in Stage 1 and found a 99.4% concordance with the imputed genotypes, demonstrating high confidence in the results of the imputation.
Next, we performed a haplotype analysis using rs2647012, rs10484561 and an additional 12 adjacent genotyped SNPs located within a block of minimal recombination. Out of the eight haplotypes identified, three were neutral (OR = 0.9–1.1), three increased risk (ORs>1.2; strongest risk haplotype tagged by rs10484561) and two were protective (OR≤0.8; both tagged by rs2647012) (Table S9), suggesting the presence of at least two susceptibility alleles within the region. Coalescence analysis of the eight haplotypes indicated that rs2647012 and rs10484561 arose on two distal branches of the ancestral recombination graph [13] (Figure S3), which was also supported by the analysis of median-joining network [14] using seven SNPs without any recombination (Figure 4). Further haplotype analysis of the seven genotyped SNPs (Table S9) and the imputed SNP rs9378212 indicated that the two alleles of rs9378212 tag the two different evolutionary lineages (Figure 4), each harboring either rs2647012 or rs10484561. Thus, the associations at the two SNPs are likely due to two distinct susceptibility variants, instead of a single risk allele, that arose independently on different haplotype backgrounds.
Fig. 4. Coalescence analysis of rs2647012 and rs10484561. 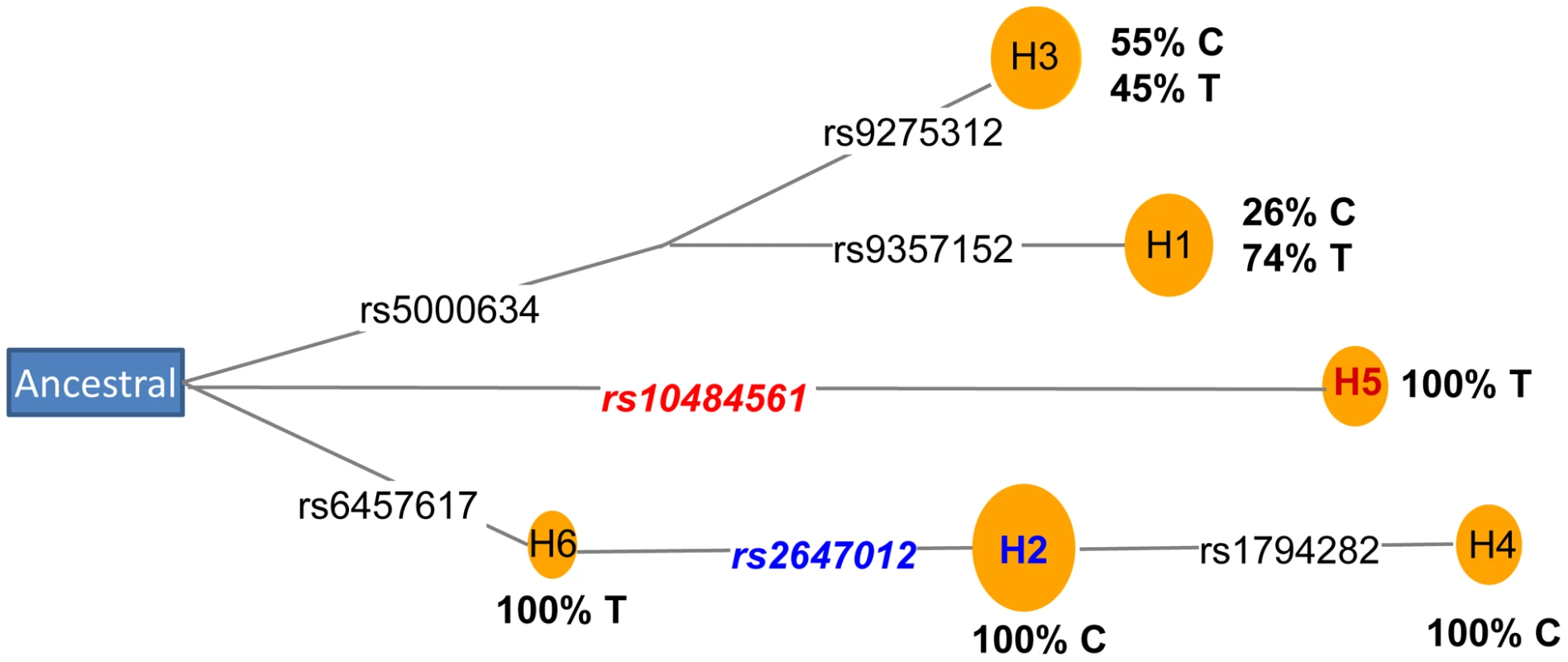
The FL-associated SNP, rs10484561, was previously found to tag the extended haplotype HLA-DQA1*0101-HLA-DQB1*0501-HLA-DRB1*0101 [6]. Here, to test whether any HLA class II alleles may also be responsible for the observed association at rs2647012, we imputed known HLA tag SNPs [15], [16] using data from the 1000G and HapMapII European datasets. We confirmed the association of the HLA-DRB1*0101-HLA-DQA1*0101-HLA-DQB1*0501 extended haplotype, tagged by rs10484561. The association at rs2647012 remained significant after adjustment for these three HLA alleles (OR = 0.64, P = 8.11×10−6), suggesting that these are not driving the association at rs2647012. Furthermore, rs2647012 was not in strong LD (r2<0.8 in HapMap CEU or SCALE controls) with any other known HLA tags [15], including those tagging FL-associated alleles previously reported [17], [18] (r2<0.39 with the six HLA-DRB1*13 tag SNPs [rs2395173, rs2157051, rs4434496, rs6901541, rs424232, rs2050191] [17] and r2<0.25 with the three HLA-B*0801 and HLA-DRB*0301 tag SNPs [rs6457374, rs2844535, rs2040410] [15]). Of the other 17 HLA class II alleles (∼39% of all the class II alleles) that could be imputed, none showed significant association or were found to be responsible for the association at rs2647012 (Table S10). Detailed HLA allelotyping on large numbers of cases and controls is needed to determine if particular HLA class II alleles are responsible for the observed association at rs2647012.
To assess whether the FL-associated SNPs may be involved in the development of other NHL subtypes, we genotyped the five SNPs selected for Stage 3 together with rs10484561 in a total of 1592 DLBCL, 1075 CLL/SLL, 336 marginal zone lymphoma (MZL), 262 mantle cell lymphoma, 306 T-cell lymphoma and 878 rare or unspecified NHL cases and 5220 controls from the SCALE2, SF2, BC, Mayo, NCI-SEER, Yale and NSW studies (Table 1, Table S1, Figure 1). Among these SNPs, rs10484561 showed evidence of association with DLBCL (OR = 1.36, P = 1.41×10−7) (Figure S1B) and all NHL (OR = 1.23, P = 6.81×10−7). ORs were consistent across the seven studies. There was also a suggestive association for rs2647012 with MZL (OR = 1.32, P = 6.34×10−4) (Table.3), consistent across six studies.
Finally, we investigated the possibility of additional susceptibility loci for FL outside of the HLA region by performing a joint analysis of the top 41 to 1000 variants of our scan and the previously published GWAS of follicular lymphoma [6]. From this combined analysis, we did not find any additional markers with a strong association (P<10−6) with FL that were not in LD with our top 5 markers taken forward to stage 3 (data not shown).
Tab. 3. 
*Excluding SCALE1 for FL. Discussion
Through the identification of a second variant, rs2647012, that is independent of the previously identified risk variant rs10484561 [6] within the 6p21.32 region, our findings substantiate a major link between HLA class II loci and genetic susceptibility to FL. In addition, our study revealed evidence that rs10484561 is associated with DLBCL risk suggesting some shared biological mechanisms of susceptibility between these two common NHL subtypes. The association of rs2647012 with FL risk was not detected in earlier GWAS studies [5], [6], and that of rs10484561 with DLBCL risk previously reported was only marginal [6], perhaps because of the smaller sample sizes in Stage 1. The number of FL cases scanned in this study was almost double compared to the previous individual GWAS [6].
HLA class II molecules are expressed in antigen presenting cells such as B-lymphocytes, and act to present exogenous antigens to CD4+ helper T-cells. Efficiency of antigen presentation may influence lymphomagenesis through effects on anti-tumor immunity or on immune response to infections that are directly or indirectly oncogenic (e.g., through viral genome insertion or nonspecific chronic antigenic stimulation) [19]. Allelic variants in coding regions may affect the structure of the peptide binding groove of the class II molecules, leading to differences in the efficiency of oncogenic peptide binding or T-cell recognition. Coding sequence variation in the molecules encoded by the extended HLA-DRB1*0101-HLA-DQA1*0101-HLA-DQB1*0501 haplotype may be responsible for the association at rs10484561 [6].
Alternatively, variants in the regulatory sequences may influence the expression level of the HLA molecules and consequently the efficiency of antigen presentation. We note that rs2647012 is strongly associated with the average expression levels of HLA-DRB4 (β = 0.78, P = 3.4×10-22) and HLA-DQA1 (β = -0.58, P = 5.1×10−13) probes in Epstein-Barr virus-transfected lymphoblastoid cell lines (mRNA by SNP browser) [20], and rs10484561 is also associated with the expression levels of HLA-DQA1 probes (β = -0.884, P = 1.6×10−10). We speculate that this may be an alternative mechanism underlying the observed associations, especially at rs2647012.
Interestingly, SNPs within the same LD block harboring rs2647012 (r2>0.7 in HapMap CEU) have previously been associated with rheumatoid arthritis with the same direction of effect [21]. Since autoimmune disorders such as rheumatoid arthritis and Sjögren syndrome are associated with increased risk of NHL, in particular with DLBCL but also with FL [22], our finding may suggest a molecular link between these diseases, although their associations within this region of high LD could also be due to different causal variants.
Previously, large-scale candidate gene studies have pointed to susceptibility loci in the HLA class III region mainly between the TNF variant –308G->A (rs1800629) and risk of DLBCL [23], [24]. We provide novel evidence of association of DLBCL with an independent HLA marker in the class II region (rs10484561; r2 = 0), 1.1Mb away from rs1800629, strongly suggesting that alleles in the HLA class II region may play an important role in the pathogenesis of this subtype as well. The weaker association of rs10484561 with DLBCL (OR 1.36) than with FL (OR 1.95) [6] could imply that the DLBCL-association is confined to a subset of DLBCL tumors with specific morphological or molecular features more closely related to FL, such as the germinal center-like B-cell phenotype [25]. However, the observed effects could also be due to modification of other concurrent DLBCL-specific susceptibility variants, or rs10484561 could tag a more strongly associated marker in this region of high LD.
Moreover, we found suggestive evidence of association at rs6536942 on 4q32.3, located within an intron of the tolloid-like 1 (TLL1) gene, with FL risk. However, larger studies are needed to validate this finding. Although the strongest associations so far have been observed in the HLA region, and extended pooling of available scan data failed to identify additional loci outside of HLA, we expect that future larger meta-GWAS efforts will more robustly identify additional loci in other regions.
In conclusion, our results strongly suggest that future genetic and functional work focused on the HLA class II region will provide important insight into the disease pathology of FL, DLBCL and other subtypes of NHL. In addition, further studies of this region and potential interaction with environmental factors in NHL risk, and of NHL prognosis are warranted.
Methods
Ethics statement
The studies described in this manuscript have been approved by the ethics committee of the respective institutions: Karolinska Institutet (Sweden), Scientific Ethics Committee system (Denmark), University of California, Berkeley (US), National Cancer Institute, National Institutes of Health (US), Mayo Clinic (US), University of British Columbia (Canada), Yale University (US), University of Sydney (Australia).
Study subjects
The SCALE study is a population-based study of the etiology of NHL carried out in all of Denmark and Sweden during 1999 to 2002 [26]. NHL subtype diagnoses were reviewed and reclassified according to the World Health Organization (WHO) classification [1] as previously described [26]. For this GWAS (SCALE1) we used DNA from 400 cases with follicular lymphoma (FL; 150 from Denmark and 250 from Sweden) and from 150 Danish controls, individually matched to the Danish FL cases by sex and age at study inclusion. We also used material collected from 673 control subjects in a separate Swedish population-based case-control study of rheumatoid arthritis (the Eira study) [21], [27]. The latter was conducted during 1996 to 2005 among residents 18 to 70 years of age in the southern and central parts of Sweden (including 90% of Swedish residents). Hence, the population controls recruited in this study were considered to represent the same study population as the Swedish component of the SCALE study with regard to genetic variation. Genotyping completion rates were similar between cases and controls; out of 400 cases and 823 controls genotyped, 379 cases (95%) and 791 controls (96%) were included in the final analysis. Study subjects used in Stages 2, 3 and validation in other NHL subtypes (Table 1, Table S1, S2) have been previously described [6], [8]–[12], and details are available as supporting text (Text S1). For the SCALE2 NHL subtype validation study, we used the rest of the lymphoma cases with blood samples originally recruited in SCALE (n = 1869), Danish control subjects not included in the GWAS (n = 556), a second set of control subjects from the Eira study (n = 742) and a third group of controls recruited in a national population-based case-control study of breast cancer, the Cancer and Hormones Replacement in Sweden (CAHRES) study [28] (n = 720). The control subjects from this study were randomly selected from the Swedish general population to match the expected age distribution of the participating breast cancer cases (50 to 74 years).
Genotyping
Stage I genotyping of 317,503 single nucleotide polymorphisms (SNPs) was done on the HumanHap300 (version 1.0) array. Validation genotyping was done using Sequenom iPlex; SNPs in the human leukocyte antigen (HLA) region that failed primer design for Sequenom assays were genotyped using Taqman (Applied Biosystems).
Genome-wide association study
The scan included 317,503 SNPs from the HumanHap300 (version 1.0) array. The datasets were filtered on the basis of SNP genotyping call rates (≥>95% completeness), sample completion rate (≥90%), minor allele frequency (MAF; all subjects as well as cases and controls separately ≥0.03) and non-deviation from Hardy-Weinberg equilibrium (HWE; p<10−6). We also excluded SNPs with cluster plot problems, and those on the X and Y chromosomes. Study subjects with gender discrepancies and/or labelling errors were removed. We also removed individual samples with evidence of cryptic family relationships (identified using the–genome command in PLINK). To detect outliers in terms of population stratification, we performed principal component (PC) analysis using the EIGENSTRAT software (Figure S4). A subset of linkage disequilibrium (LD) thinned SNPs was selected such that all pair-wise associations had r2<0.2, and long-range regions of high LD, reported to potentially confound genome scans, were removed [29]. Twenty-five samples were removed as population outliers on the basis of their values on the first three PCs. To adjust for possible stratification in our association analyses we adjusted the regression analyses using the first three PCs; the number of PCs used for adjustment was determined by plotting the eigenvalues and locating the position of the “elbow” on the scree plot (Figure S5). Wald tests, treating minor allele counts as continuous covariates were used to test for association. The genomic inflation factor (λ) was calculated to be 1.0283 after adjusting for the first three PCs, suggesting the presence of minimal stratification. Quantile-quantile plots for the associations before and after adjustment are shown in Figure S6. Finally, we assessed associations of age and sex with main genotypes among the control subjects to address the possibility of confounding by these factors (Table S11). As there was no evidence of associations of age or sex with genotypes among the controls, we did not adjust for them in the final main effects analyses of genotypes.
Validation and meta-analysis
In Stage 2, similar quality control measures were applied as in Stage 1, including genotyping call rate ≥95%, sample completion rate ≥90%, and MAF ≥0.05. We tested each validation study for association using trend tests. For meta-analyses across studies and NHL subtypes, we used the Cochran-Mantel-Haenszel method to calculate the combined odds ratio and P-value, and χ2 tests for heterogeneity. Multivariate logistic regression was used to test for independence of SNP effects. For validation among other NHL subtypes, the control subjects were the same as those in Stages 2 and 3 for validation in FL for all studies except SCALE2. Only European-ancestry subjects were included, and the possibility of population stratification affecting the results has been thoroughly explored and found to be low in earlier investigations in the same populations [6], [8].
Imputation
We used IMPUTEv1 for the imputation of SNPs from the 1000 Genomes pilot1 CEU data (August 2009 release); and the HapMap Phase II release 22 CEU data. We set a strict threshold for imputation, using only SNPs with confidence scores of ≥0.9, call rates ≥90%, non-deviation from Hardy-Weinberg equilibrium P >0.001 and MAF >0.01. The imputation was done on the Stage 1 samples separately for each of the two reference datasets and SNPs showing a discordance of >5% between the genotypes imputed with the two datasets were excluded from further analysis. The data were then merged using HapMap II as the master dataset to which additional imputed SNPs from the 1000 Genomes dataset were added. HLA alleles were imputed by identifying tag SNPs [15] from the genotyped and imputed SNP dataset. We used PLINK for haplotype imputation with the tag SNPs and downstream association analyses. Only haplotypes with call rates >90%, MAF>1% and probability thresholds >0.8 were analyzed.
Haplotype and coalescence analyses
For coalescence analysis all 12 SNPs (genotyped in this study and within a region of ∼177 Kb) adjacent to the two SNPs associated with the FL risk were used to construct haplotypes. These were phased using the PHASE program [30] and tested for association using PLINK. The ancestral haplotype was constructed from the chimpanzee (PanTro2) allele whenever possible, and otherwise from the macaque alleles. An ancestral recombination graph was constructed using the program Beagle [13], [31] which allows recombination assuming an infinite site mutation model. After identifying the first recombination event the haplotype segment before the recombination spot was used to construct a median –joining network using the Network program [14]. The alleles of the imputed SNP rs9378212 were then phased on each haplotype segment using the PHASE program.
The URLs for the data and analytic approaches presented herein are as follows:
1000 Genomes http://1000genomes.org
HapMapII http://www.hapmap.org
IMPUTEv1 https://mathgen.stats.ox.ac.uk/impute/impute_v1.html
mRNA by SNP browser http://www.sph.umich.edu/csg/liang/asthma/
R script for recombination plot http://www.broadinstitute.org/science/projects/diabetes-genetics-initiative/plotting-genome-wide-association-results
Supporting Information
Zdroje
1. Jaffe
ES
Harris
NL
Stein
H
Vardiman
J
2001
World Health Organization classification of tumours pathology and
genetics, tumours of hematopoietic and lymphoid tissues.
Lyon
IARC Press
2. Morton
LM
Wang
SS
Cozen
W
Linet
MS
Chatterjee
N
2008
Etiologic heterogeneity among non-Hodgkin lymphoma
subtypes.
Blood
112
5150
5160
3. Altieri
A
Bermejo
JL
Hemminki
K
2005
Familial risk for non-Hodgkin lymphoma and other
lymphoproliferative malignancies by histopathologic subtype: the Swedish
Family-Cancer Database.
Blood
106
668
672
4. Chang
ET
Smedby
KE
Hjalgrim
H
Glimelius
B
Adami
HO
2006
Reliability of self-reported family history of cancer in a large
case-control study of lymphoma.
J Natl Cancer Inst
98
61
68
5. Skibola
CF
Bracci
PM
Halperin
E
Conde
L
Craig
DW
2009
Genetic variants at 6p21.33 are associated with susceptibility to
follicular lymphoma.
Nat Genet
41
873
875
6. Conde
L
Halperin
E
Brown
KM
Smedby
KE
Rothman
N
2010
Genome-wide association study of follicular lymphoma identifies a
risk locus at 6p21.32.
Nat Genet
42
661
664
7. de Bakker
PI
Ferreira
MA
Jia
X
Neale
BM
Raychaudhuri
S
2008
Practical aspects of imputation-driven meta-analysis of
genome-wide association studies.
Hum Mol Genet
17
R122
128
8. Cerhan
JR
Ansell
SM
Fredericksen
ZS
Kay
NE
Liebow
M
2007
Genetic variation in 1253 immune and inflammation genes and risk
of non-Hodgkin lymphoma.
Blood
110
4455
4463
9. Wang
SS
Cerhan
JR
Hartge
P
Davis
S
Cozen
W
2006
Common genetic variants in proinflammatory and other
immunoregulatory genes and risk for non-Hodgkin lymphoma.
Cancer Res
66
9771
9780
10. Zhang
Y
Holford
TR
Leaderer
B
Boyle
P
Zahm
SH
2004
Hair-coloring product use and risk of non-Hodgkin's
lymphoma: a population-based case-control study in
Connecticut.
Am J Epidemiol
159
148
154
11. Hughes
AM
Armstrong
BK
Vajdic
CM
Turner
J
Grulich
A
2004
Pigmentary characteristics, sun sensitivity and non-Hodgkin
lymphoma.
Int J Cancer
110
429
434
12. Spinelli
JJ
Ng
CH
Weber
JP
Connors
JM
Gascoyne
RD
2007
Organochlorines and risk of non-Hodgkin lymphoma.
Int J Cancer
121
2767
2775
13. Song
YS
Hein
J
2005
Constructing minimal ancestral recombination
graphs.
J Comput Biol
12
147
169
14. Bandelt
HJ
Forster
P
Rohl
A
1999
Median-joining networks for inferring intraspecific
phylogenies.
Mol Biol Evol
16
37
48
15. de Bakker
PI
McVean
G
Sabeti
PC
Miretti
MM
Green
T
2006
A high-resolution HLA and SNP haplotype map for disease
association studies in the extended human MHC.
Nat Genet
38
1166
1172
16. Leslie
S
Donnelly
P
McVean
G
2008
A statistical method for predicting classical HLA alleles from
SNP data.
Am J Hum Genet
82
48
56
17. Wang
SS
Abdou
AM
Morton
LM
Thomas
R
Cerhan
JR
2010
Human leukocyte antigen class I and II alleles in non-Hodgkin
lymphoma etiology.
Blood
115
4820
4823
18. Abdou
AM
Gao
X
Cozen
W
Cerhan
JR
Rothman
N
2010
Human leukocyte antigen (HLA) A1-B8-DR3 (8.1) haplotype, tumor
necrosis factor (TNF) G-308A, and risk of non-Hodgkin
lymphoma.
Leukemia
24
1055
1058
19. Bateman
AC
Howell
WM
1999
Human leukocyte antigens and cancer: is it in our
genes?
J Pathol
188
231
236
20. Dixon
AL
Liang
L
Moffatt
MF
Chen
W
Heath
S
2007
A genome-wide association study of global gene
expression.
Nat Genet
39
1202
1207
21. Plenge
RM
Seielstad
M
Padyukov
L
Lee
AT
Remmers
EF
2007
TRAF1-C5 as a risk locus for rheumatoid arthritis–a
genomewide study.
N Engl J Med
357
1199
1209
22. Baecklund
E
Backlin
C
Iliadou
A
Granath
F
Ekbom
A
2006
Characteristics of diffuse large B cell lymphomas in rheumatoid
arthritis.
Arthritis Rheum
54
3774
3781
23. Rothman
N
Skibola
CF
Wang
SS
Morgan
G
Lan
Q
2006
Genetic variation in TNF and IL10 and risk of non-Hodgkin
lymphoma: a report from the InterLymph Consortium.
Lancet Oncol
7
27
38
24. Skibola
CF
Bracci
PM
Nieters
A
Brooks-Wilson
A
de Sanjose
S
2010
Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA)
polymorphisms and risk of non-Hodgkin lymphoma in the InterLymph
Consortium.
Am J Epidemiol
171
267
276
25. Alizadeh
AA
Eisen
MB
Davis
RE
Ma
C
Lossos
IS
2000
Distinct types of diffuse large B-cell lymphoma identified by
gene expression profiling.
Nature
403
503
511
26. Smedby
KE
Hjalgrim
H
Melbye
M
Torrang
A
Rostgaard
K
2005
Ultraviolet radiation exposure and risk of malignant
lymphomas.
J Natl Cancer Inst
97
199
209
27. Plenge
RM
Padyukov
L
Remmers
EF
Purcell
S
Lee
AT
2005
Replication of putative candidate-gene associations with
rheumatoid arthritis in >4,000 samples from North America and Sweden:
association of susceptibility with PTPN22, CTLA4, and PADI4.
Am J Hum Genet
77
1044
1060
28. Magnusson
C
Baron
J
Persson
I
Wolk
A
Bergstrom
R
1998
Body size in different periods of life and breast cancer risk in
post-menopausal women.
Int J Cancer
76
29
34
29. Price
AL
Weale
ME
Patterson
N
Myers
SR
Need
AC
2008
Long-range LD can confound genome scans in admixed
populations.
Am J Hum Genet
83
132
135; author reply 135-139
30. Stephens
M
Smith
NJ
Donnelly
P
2001
A new statistical method for haplotype reconstruction from
population data.
Am J Hum Genet
68
978
989
31. Lyngsø
R
Song
Y
Hein
J
2005
Minimum recombination histories by branch and bound, proceedings
of workshop on algorithms in bioinformatics.
Lect Notes Comput Sci
3692
239
250
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání