-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDoes Positive Selection Drive Transcription Factor Binding Site
Turnover? A Test with Drosophila Cis-Regulatory Modules
Transcription factor binding site(s) (TFBS) gain and loss (i.e., turnover) is a
well-documented feature of cis-regulatory module (CRM) evolution, yet little
attention has been paid to the evolutionary force(s) driving this turnover
process. The predominant view, motivated by its widespread occurrence,
emphasizes the importance of compensatory mutation and genetic drift. Positive
selection, in contrast, although it has been invoked in specific instances of
adaptive gene expression evolution, has not been considered as a general
alternative to neutral compensatory evolution. In this study we evaluate the two
hypotheses by analyzing patterns of single nucleotide polymorphism in the TFBS
of well-characterized CRM in two closely related Drosophila species,
Drosophila melanogaster and Drosophila
simulans. An important feature of the analysis is classification of
TFBS mutations according to the direction of their predicted effect on binding
affinity, which allows gains and losses to be evaluated independently along the
two phylogenetic lineages. The observed patterns of polymorphism and divergence
are not compatible with neutral evolution for either class of mutations.
Instead, multiple lines of evidence are consistent with contributions of
positive selection to TFBS gain and loss as well as purifying selection in its
maintenance. In discussion, we propose a model to reconcile the finding of
selection driving TFBS turnover with constrained CRM function over long
evolutionary time.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1002053
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002053Summary
Transcription factor binding site(s) (TFBS) gain and loss (i.e., turnover) is a
well-documented feature of cis-regulatory module (CRM) evolution, yet little
attention has been paid to the evolutionary force(s) driving this turnover
process. The predominant view, motivated by its widespread occurrence,
emphasizes the importance of compensatory mutation and genetic drift. Positive
selection, in contrast, although it has been invoked in specific instances of
adaptive gene expression evolution, has not been considered as a general
alternative to neutral compensatory evolution. In this study we evaluate the two
hypotheses by analyzing patterns of single nucleotide polymorphism in the TFBS
of well-characterized CRM in two closely related Drosophila species,
Drosophila melanogaster and Drosophila
simulans. An important feature of the analysis is classification of
TFBS mutations according to the direction of their predicted effect on binding
affinity, which allows gains and losses to be evaluated independently along the
two phylogenetic lineages. The observed patterns of polymorphism and divergence
are not compatible with neutral evolution for either class of mutations.
Instead, multiple lines of evidence are consistent with contributions of
positive selection to TFBS gain and loss as well as purifying selection in its
maintenance. In discussion, we propose a model to reconcile the finding of
selection driving TFBS turnover with constrained CRM function over long
evolutionary time.Introduction
Gene expression in eukaryotes is generally controlled by transcriptional enhancers, also called cis-regulatory modules (CRM), which are short regions in the genome consisting of a cluster of transcription factor binding sites (TFBS) spaced by intervening sequences (spacers). Individual TFBS have been shown repeatedly to be required for CRM function, yet surprisingly they evolve rapidly and are frequently gained and lost in evolution, attributes that have been demonstrated for a large number of CRM and transcription factors [1]–[5]. These observations pose a challenge to understanding the forces driving the process, especially in cases where CRM function has been preserved despite sequence and structural divergence [6]–[8].
The gain or loss of a TFBS is unlikely to be functionally irrelevant, as repeatedly shown in TFBS knockout experiments [9]–[11], and also demonstrated for the evolved differences between two species by a chimeric enhancer study [12]. One possibility for reconciling conservation of CRM function with rapid TFBS turnover is to assume that each loss of a TFBS is precisely balanced by the simultaneous gain of a cognate TFBS elsewhere in the CRM, a process we will call compensatory evolution [13]. The idea draws on a model first proposed by Kimura [14], where he considers a pair of tightly linked mutant genes that are individually deleterious but in combination restore wildtype function. As applied to TFBS, the gain of a novel site on an allele carrying a mutation that decreases the quality of an existing binding site can offset the mutants fitness cost, creating a selectively neutral double-mutant allele. Binding site turnover - fixation of the double mutant allele - is achieved entirely by genetic drift, thus preserving both CRM function and population fitness. Recently, a theoretical model of this compensatory turnover process was developed to ask about the feasibility of compensatory evolution for TFBS [15]. With plausible assumptions about the mutation rate, population size and selection coefficient on the individual mutations, a completely neutral model cannot achieve a high enough level of turnover to explain Drosophila CRM evolution (as exemplified by eve stripe 2 enhancer), whereas a model that assumes the double mutant to be more fit than the wildtype does.
This theoretical finding raises the prospects for positive selection being an important driving force of TFBS gain and loss. Instances of directional selection have been documented in cases where a novel regulatory regime is favored [16]. Functional evolution of a transcription factor (TF) can also drive adaptive co-evolution of its TFBS [17]–[19]. Broad-scale studies in noncoding regions and promoters of genes have identified signatures of both selective constraint and positive selection in fruitfly and human [20]–[24]. However, only a small number of population genetics studies have been carried out to specifically test this hypothesis with TFBS or CRM, and because they focus on a single TF or CRM, they have low statistical power to distinguish between neutrality and selection [13]. The generality of the conclusions reached in these studies is also not established [25], [26].
Several different approaches have been designed to detect and quantify selection in the system. One of them has been to consider the genome-wide ensemble of TFBS as evolving at mutation-selection balance, with the fitness of each instance of TFBS being strictly determined by its binding energy [4], [27], [28]. This approach proves useful in studying the strength of selective constraints on functional TFBS. However, the assumption of a unidirectional fitness function, i.e. selection always favors affinity-increasing mutations and against affinity-decreasing ones, could be violated if the loss of a TFBS were favored or gain (or strengthening) of a TFBS is deleterious. Another approach calculates the sum of mutational effects in TFBS on binding affinity and compares it to the expectation under a no-selection model [29]. A higher than expected sum could imply selective removal of affinity-decreasing mutations and therefore the action of purifying selection. Applying this approach to two of the CRM also included in this study, the author provided evidence for purifying selection acting to preserve the functional TFBS in the anterior Bicoid-dependent hunchback enhancer and the even-skipped stripe 2 enhancer. This test can also be used to detect positive selection, although its power is limited due to the mixed signal with purifying selection, which is expected to be dominant in most cases.
In this study, patterns of polymorphism and divergence are investigated in a pair of closely related Drosophila species, D. melanogaster (mel) and D. simulans (sim). The short evolutionary distance between the two species ensures unambiguous alignment for noncoding sequences and also allows one to capture the potentially rapid dynamics of TFBS gain and loss. A notable challenge in studying TFBS turnover is assembling a high quality set of TFBS that are precisely defined and contain few false positives. Large numbers of potential TFBS can be identified by methods involving genome-wide scans, such as computational prediction or ChIP, but these approaches generally include a large fraction of false positives, thus reducing their attractiveness for investigating the mechanisms of binding site turnover (see Discussion). Instead, we chose to investigate a curated set of high-confidence TFBS identified by DNaseI footprint in well-studied D. melanogaster CRM. Short footprint regions usually contain only a single TFBS motif, which, in most cases, could be perfectly aligned with the other species to allow identification of single nucleotide differences within and between the species. Each of these differences, in turn, was evaluated for the predicted magnitude and direction of effect on TF binding energy. The neutral and selection models generate distinguishable predictions in both divergence to polymorphism ratios and in the site frequency spectra. Analysis of these patterns reveal evidence for purifying selection against affinity-decreasing mutations segregating in the population, while multiple lines of evidence indicate positive selection for both gains and losses of TFBS. These empirical findings challenge the prevailing view of neutral compensatory turnover, and have important implications for understanding CRM functional evolution. In the course of the analysis, we also identified and modeled a potential ascertainment that can impact population genetics studies of genomic features that have been identified only in a reference sequence such as TFBS.
Results
Our analysis focuses on single nucleotide polymorphism (SNP) and divergence in 645 experimentally identified TFBS for 30 transcription factors in 118 autosomal CRM (Table S1), all annotated in REDfly [30]. These 645 TFBS represent the complete set for which we could obtain unambiguous alignment of both within - and between-species sequences without insertion or deletion. We used position weight matrices (PWM) both to identify TFBS within footprints and to predict the magnitude of binding energy differences among variant alleles. Our bioinformatic and experimental validations showed that the PWM used in this study provide reliable and unbiased estimates for the direction of binding affinity change in both mel and sim (Materials and Methods).
Single nucleotide changes within or between mel and sim were polarized with outgroup sequences from D. sechellia, D. yakuba and D. erecta using PAML (Materials and Methods). Each derived mutation, therefore, could be categorized with respect to species lineage and to direction of binding affinity change.
Lineage-specific gain and loss of TFBS as a general pattern across different CRM and TF
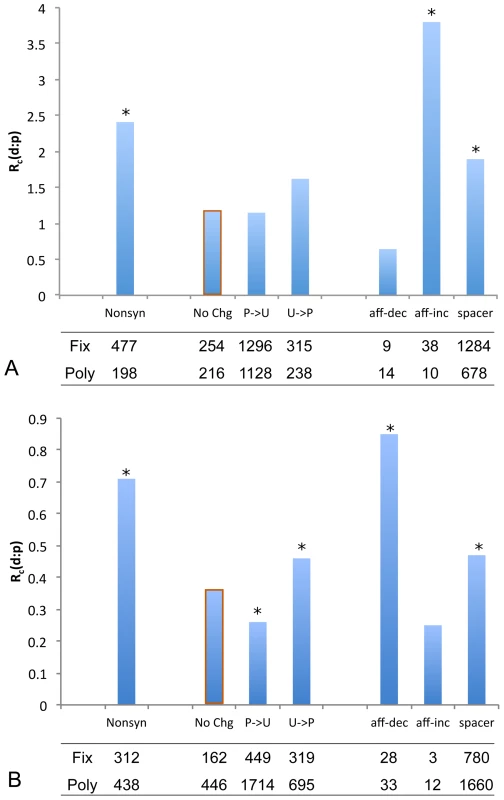
Binding sites for an individual TF or a single CRM usually had too few counts of single nucleotide polymorphism or fixed differences to allow informative statistical analysis. Furthermore, the breadth of the turnover phenomenon across almost all investigated TF and CRM suggests a common underlying evolutionary mechanism [5], [7], [8], [18], [31]. We therefore considered pooling observations from across TFs and CRM. To see if the evolutionary rates in different TFs binding sites are sufficiently uniform, we measured sequence divergence between mel and sim for the 30 TF. After accounting for sample sizes, no significant departure from the average rate is detected by a binomial test (Figure 1). Moreover, the pooling approach should be conservative in deriving a general pattern with respect to among TF variations.
Fig. 1. TFBS divergence for 30 TF. 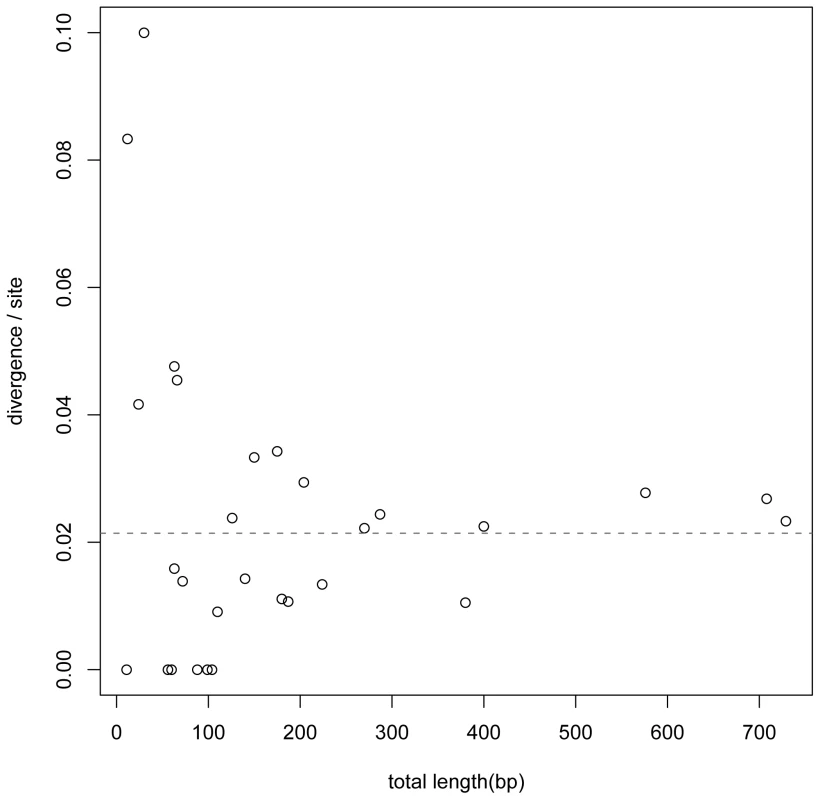
We then estimated percent loss and gain of TFBS on the mel and sim lineages. For each of the 645 footprint TFBS, a PWM score was calculated for each occurrence () in the alignment of mel, sim and the inferred mel-sim ancestor, by taking the log2 ratio of the probability of a sequence under the functional motif distribution versus that under the genomic background distribution (Material and Methods). Using as a cutoff, approximately 2% of all footprint sites were found to be present in mel only and may represent mel specific gains; and about 2.5% were present in the inferred ancestor (and mel) but lost in sim. A set of empirical cutoffs were determined for each TF based on the range of PWM scores among its footprint sites, which produced similar results (Table S2). Consistent with the sequence divergence patterns, gain and loss of TFBS appear to be a general pattern across TF and CRM. A total turnover rate of 4.5% between mel and sim is similar to a previous finding of 5% for a single TF Zeste [5].
We observed approximately equal numbers of gains versus losses in our dataset, although the distribution of these events is asymmetric on the two lineages (16 losses, 0 gain along the sim lineage versus 12 gains, 0 losses along the mel lineage). This is not unexpected, given that all footprint TFBS were identified as being present in mel and the dataset doesn't include sim-specific TFBS. We predicted that identification of TFBS by computational methods would produce a more even pattern of gains and losses in both lineages. We tested this prediction for three TF (Hb,Bcd,Kr) using a stringent cutoff procedure and for each TF we found a similar total number of predicted binding sites in the two lineages (Text S1; Figure S1). We thus rejected the (unlikely) possibility that there has been a large-scale evolutionary gain of TFBS in mel and loss in sim.
Investigating evolutionary forces for TFBS gain, loss, and maintenance
Gain and loss of TFBS may be subject to distinct evolutionary forces. To investigate them separately, we assigned each mutation within a footprint TFBS in mel or sim to either affinity-increasing or affinity-decreasing group based on PWM score difference between the ancestral and the derived mutation (Materials and Methods). Bioinformatic and experimental investigation showed that this PWM-based procedure for inferring the direction of binding affinity change is reliable when PWM predicted magnitude of change is not too small (Materials and Methods, Figure S2 and Figure S3). We established a threshold corresponding to a PWM score difference of one, i.e. at least two-fold change in the likelihood ratio between a motif or background distribution, in order to minimize the chance for mis-assignment. Varying this threshold between zero and two do not affect the results qualitatively.
We employed two approaches to investigate evolutionary forces acting on affinity increasing and decreasing changes. One approach is based on contrasting polymorphism and divergence patterns in a McDonald-Kreitman (MK) test framework [32]. Positive selection is expected to inflate substitution relative to polymorphism while negative selection will have the reverse but weaker effect [33]. We used synonymous changes in the target genes for the CRM as a proxy for a neutrally evolving class. Following established practices, we further classified each synonymous change as according to its expected impact on codon bias – No-Change, Preferred-to-Unpreferred, or Unpreferred-to-Preferred – and used the No-Change class as the neutral reference. The second approach investigates the site frequency spectrum of TFBS polymorphism to make inferences about selective pressures acting more recently on binding sites.
TFBS ascertainment
The fact that all footprints were identified in mel impacts the analysis in two ways. First, gains of TFBS can be observed in mel but not losses, while the reverse is true in sim. Therefore, even though similar processes are most likely operating in both species, our evolutionary analysis of binding site gain will focus on changes in the mel lineage, whereas losses will be restricted to changes in the sim lineage.
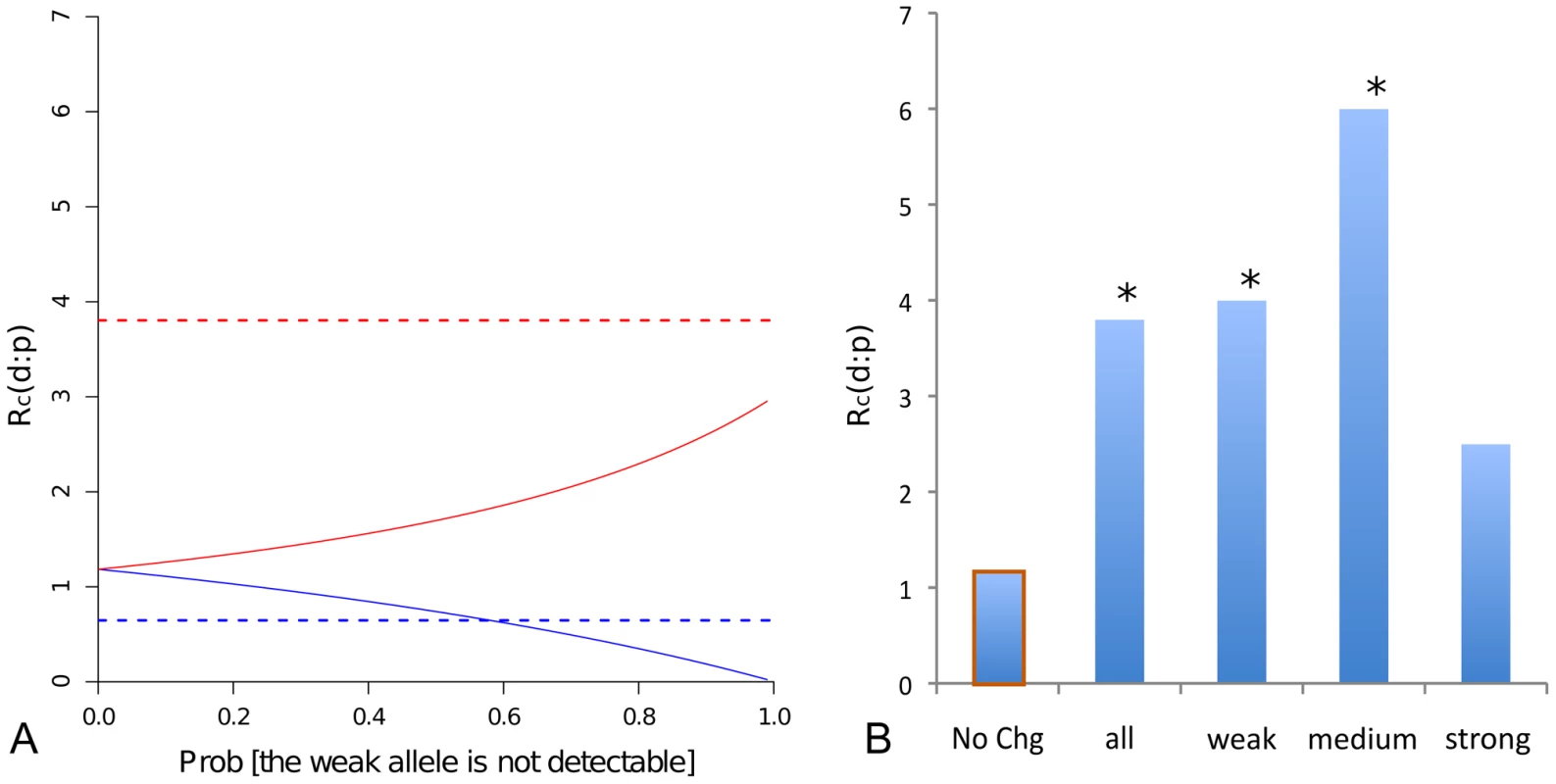
Second, affinity-decreasing and affinity-increasing mutations have the potential to differ in detectability as a footprint site in mel. This arises because mutations in TFBS were sampled conditioned on the TFBS being detected in mel and affinity-changing mutations in mel, in turn, have the potential to affect the detectability of the TFBS. Depending on whether the derived mutation is affinity-increasing or affinity-decreasing, two distinct biases are introduced in the expected neutral frequency spectrum (Figure S4). Given that the dataset consists only of TFBS that are detectable by footprinting, we assume that the high-affinity allele will always be detectable. Consider the possible situation in which the low-affinity allele is not detectable as a footprint: if the derived mutation is affinity-decreasing, the probability of detecting the TFBS will change inversely with the mutant allele frequency; conversely, if the derived mutation is affinity-increasing, the probability of detection will increase with the mutant allele frequency. Substitutions may be viewed as a special instance of a segregating mutation and treated similarly.
This effect of ascertainment on neutral expectations for the MK test and the site frequency spectrum can be modeled analytically (Text S2); there is no ascertainment if both alleles are equally detectable as footprints. To incorporate uncertainty in the detectability of the low-affinity allele, the model incorporates a parameter, f, which specifies the probability that the weaker affinity allele will not be detected in the footprint assay. While f is likely to be greater than 0, it is unlikely to be close to 1 because footprint sites are degenerate and span a range of affinities. Under the conservative assumption that the lowest affinity among the footprint sites is the detection limit, we estimate for the 30 TF (Text S2), indicating that the majority of TFBS changes will be detectable.
In the following sections, we first present our analysis of polymorphism and divergence in mel, focusing on the forces acting to either maintain functional TFBS or to create new ones. We then turn to sim, focusing on TFBS loss. Finally, we analyze the spacer sequences between TFBS in both species.
Analysis in mel suggests potentially positive selection for TFBS gain and purifying selection in maintaining existing TFBS
For each class of change we summarized the data in the MK table by calculating the ratio, . The presence of weakly deleterious mutations can mask signatures of positive selection, and if removed can improve the power of the test [34]. Since most deleterious mutations will be at low frequencies, using 15% as a frequency cutoff has been shown to achieve most of the benefits of a more sophisticated model incorporating the distribution of deleterious effects [35]. We applied this cutoff and denote the ratio of substitutions to common polymorphism by . Under this procedure, is significantly higher for nonsynonymous changes than for the synonymous No-Change class (Figure 2A), consistent with previous findings of positive selection driving amino acid substitutions in Drosophila [36].
To delineate the effect of ascertainment from that of selection for the affinity-increasing and affinity-decreasing mutations, we compared the observed to the expected neutral ratios under the ascertainment with different values (Text S2). For affinity-decreasing mutations in mel, the difference from the synonymous No-Change class is not statistically significant, even in the absence of ascertainment bias (Figure 3A green, Figure 2A). This seems to suggest only neutral or deleterious mutations are present for this class and therefore no positive selection is involved. The validity of this conclusion can be questioned, however, because any affinity decreasing substitutions in mel that led to the loss of a site will not be included in the data while our correction for the ascertainment only accounts for neutral changes but not a potential adaptive excess. Thus, rejection of the neutral model in favor of positive selection is not possible for affinity-decreasing mutations in the mel lineage. However, this test is possible for the sim lineage (reported in the next section), where the loss of a TFBS is observable.
For affinity-increasing mutations no amount of ascertainment under our model can account for the observed relative excess of substitutions (Figure 3A red). We further reasoned that the ascertainment effect should be weaker or non-existent for TFBS with an ancestrally strong binding affinity, which would be identified with or without the affinity-increasing mutations. We therefore investigated whether the excess of affinity-increasing substitutions differed if TFBS changes were grouped according to the strength of the inferred ancestral binding affinity. We found a consistently larger ratio, i.e. an excess of substitutions, across the entire range of inferred ancestral binding affinity classes compared to the No-Change class, including binding sites with the strongest ancestral binding affinity (Figure 3B). These results collectively suggested that positive selection has contributed to the fixation of affinity-increasing changes.
To further investigate evolutionary forces acting on the segregating mutations in TFBS in the population, we utilized the site frequency spectrum, for which we generated the neutral expectations for affinity-increasing and affinity-decreasing mutations separately under ascertainment, with or (corresponding to no bias or complete bias, respectively). For affinity-decreasing mutations, with the ascertainment expected to shift the frequency spectrum to lower frequency classes (Figure 4A, blue versus grey bar), the observed spectrum is shifted in that direction but is even more extremely so than the complete bias expectation (Figure 4A, orange versus blue). Since is clearly an overestimate (compared to our estimate of ), this strongly suggests that forces other than ascertainment must have shaped this pattern. Both a recent selective sweep and population growth can produce an excess of rare variants and one or both mechanisms may be acting in this system, as is suggested by our finding that synonymous changes also show a relative excess of low frequency mutations (Figure S5B). However, as we compared the site frequency spectrum of the affinity-decreasing mutations to that of synonymous sites (corrected for ascertainment), we found the former is again more significantly shifted than the latter (Figure S6). Thus we suggest that the observed frequency spectrum is consistent with on-going purifying selection against affinity decrease in functional TFBS. The observed frequency spectrum for affinity-increasing mutations lies between the two expectations and the differences are not significant from either one, a possible consequence of the small sample size (15 observed affinity-increasing polymorphisms) (Figure 4B). Thus, while positive selection is indicated on the basis of the MK test, inference cannot be made about on-going selection for affinity-increasing mutations.
Fig. 4. Site frequency spectra. 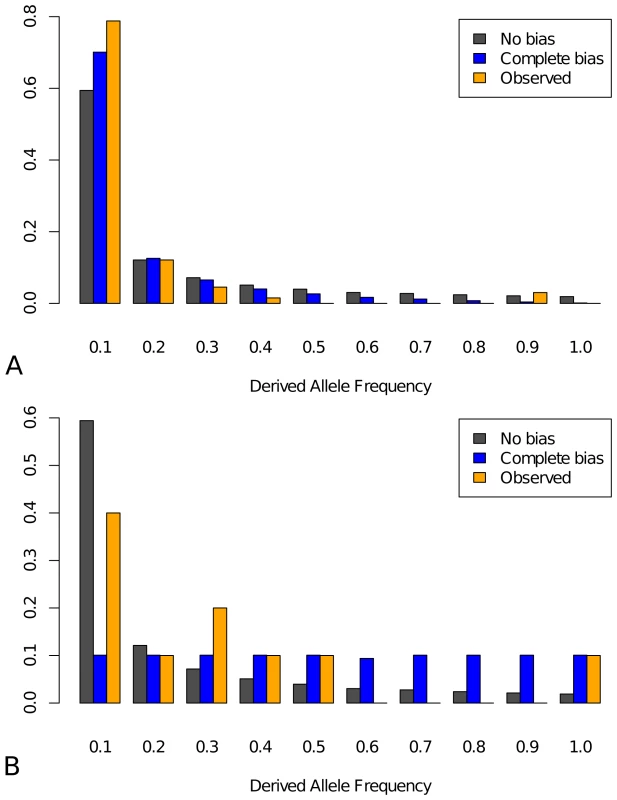
Analysis in sim suggests loss of TFBS may be adaptive
Patterns of polymorphism and divergence in sim are not influenced by the ascertainment because the identification of TFBS in mel is independent of the effect of mutations fixed or segregating in sim. However, the inclusion of binding sites gained in mel may confound the analysis as their orthologous sequences in sim may have evolved under less or different kinds of selective constraints. We thus restricted the analysis to footprint TFBS predicted to be present in the mel-sim common ancestor, where we found a significant excess of substitutions for the affinity-decreasing mutations compared to the synonymous No-Change class (Figure 2B, Fisher's Exact Test ). Statistical significance of this pattern is robust to the cutoff for excluding binding sites gained in mel (Table S3). A relative excess of substitutions might also be a consequence of factors other than selection, such as systematic differences in the genealogical histories of CRM versus synonymous sites. However, these factors seem unlikely to be the cause of this type of departure from neutrality in these two species (Kohn and Wu 2004). Therefore we consider positive selection a more plausible explanation.
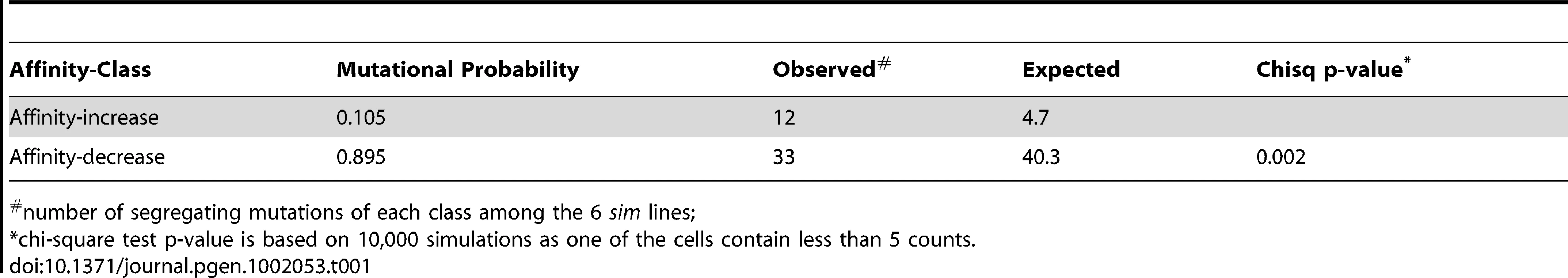
We also compared the ratio between affinity-decreasing and affinity-increasing mutations in polymorphism to the expected ratio of the two classes in the mutational input, i.e. the probability for a new mutation to be one of the two classes (Materials and Methods). Briefly, the expected ratio was obtained by considering all possible mutations in each of the 645 footprint TFBS and their predicted effects on binding affinity the same way as we did before. Assuming polymorphism for both classes were neutral, we expected similar ratios, whereas the observed results showed a significant deficit of affinity-decreasing polymorphism relative to affinity-increasing polymorphism (Table 1), which may suggest that among new mutations, affinity-decreasing ones are more likely to be deleterious, a result consistent with our finding based on frequency spectrum in mel. A similar approach has been applied before, using the sum of (individual mutation's effect on binding affinity predicted by PWM) within a CRM instead of counts of mutations in binary classes [29]. There the author also found evidence for purifying selection against affinity-decreasing mutations. The finding of both on-going purifying selection and potentially positive selection acting is not dissimilar to patterns found in nonsynonymous changes [36]. We reserve for the Discussion section the attempt to reconcile the adaptive loss of TFBS, as observed between the two species, with on-going purifying selection against affinity-decreasing new mutations.
Spacer sequences might contain large numbers of unidentified functional elements
In both mel and sim we found a significant excess of substitutions in spacer sequences, indicative of positive selection in these intervals (Figure 2). Also, the frequency spectrum for this class is strongly shifted towards lower frequencies (Figure S5E, Tajima's D = −1.09), indicative of on-going purifying selection. The implication of these results is that spacer sequences might contain many unidentified functional elements, for example, TFBS for known or uncharacterized transcription factors, or perhaps other structural features not yet understood.
To summarize, analysis of TFBS changes in mel indicates on-going purifying selection against affinity-decreasing polymorphism in the population, and positive selection for affinity-increasing substitutions. In sim, the analysis of affinity-decreasing changes indicates a significant, and potentially adaptive excess of substitutions that contributes to binding site loss. Spacer sequences between footprint TFBS in these well-characterized CRM also exhibit patterns of polymorphism and divergence consistent with both functional constraint and adaptive evolution.
Discussion
Natural selection, both positive and negative, has been shown to act throughout noncoding regions of the Drosophila genome [21], [22], albeit with varying intensities [23]. Against this backdrop of ubiquitous selection in noncoding DNA, should it be surprising to find signatures of positive selection in Drosophila TFBS? We think not. More surprising perhaps is the incompatibility of this finding with the model of neutral compensatory binding site turnover, a simple and appealing mechanism that allows for both rapid binding site turnover and functional stasis of CRM activity. But as explained below, there are good reasons to doubt whether a strictly neutral compensatory process can actually generate rapid TFBS turnover in Drosophila, even with its favorably large population size. Positive selection, in contrast, can drive arbitrarily fast rates of binding site turnover; the question is whether it can also allow for functional stasis of CRM activity. Below, we first discuss the strengths and limits of our analysis and then we describe properties of gene regulatory networks that can promote adaptive binding site turnover and yet also constrain the function of CRM.
One challenge in investigating cis-evolution is the proper alignment of noncoding sequences. To minimize this potential problem, we specifically selected a pair of closely related sibling species, D. melanogaster and D. simulans for investigation. Sequence divergence between the two species in noncoding regions ranges only between 5% and 8% [37], which allowed us to accurately identify single nucleotide differences from unambiguous alignments of binding sites (those with alignment gaps were excluded from the analysis). Working with closely related sequences also provided accurate inference of ancestral states, and thus the direction of mutational change along the phylogeny, as well as minimized trans-cis co-evolution. Independently, Bradley et al also recommended mel and sim for measuring binding site divergence based on these same issues arising in their analysis of divergence between two more distantly related species [31].
Another challenge in studying TFBS turnover is the establishment of a TFBS dataset consisting of biologically functional sites, a difficult task due to both the high false positive rate in binding site prediction (even in ChIP bound regions) and the difficulty in validating the biological functionality of individual binding sites. While many genome-wide datasets for TFBS are becoming available, several properties of the Drosophila DNase I footprint dataset made it the one of choice for use in this study. First, the in vitro footprint experiments were applied not to anonymous noncoding regions but rather to specific sequences that had been identified with in vivo reporter assays as containing a CRM. Furthermore, the transcription factors assayed for each CRM were also chosen based on prior genetic evidence for their involvement in the regulation of the CRM. For both of these reasons, subsequent experimental analysis of Drosophila footprint sites has invariably validated their functionality [38]–[43]. This experimental sampling of footprint site functionality is unique among available TFBS datasets, and provides evidence for a low false positive rate. In contrast, a recent attempt to combine known CRM, ChIP bound regions, and PWM prediction to obtain a genome-wide TFBS dataset estimated false positive rate [4]. Although the footprint sites were identified in lab strains particular to each individual experiment, we provided reasonings and evidence why the annotation is applicable to natural populations (Text S3). In particular, we constructed phylogenetic trees based on the genomic sequences containing the CRM we studied for natural population lines as well as a representative lab strain (the genome sequence reference strain), which shows that the later is indistinguishable from the rest (Figure S8). This also suggests the lab strains were not genetically divergent from the natural population.
Genome-wide studies have identified signals of both positive and negative selection in noncoding sequences in Drosophila, but not the biological or functional basis for this selection. In this study, we distinguished mutations in the footprint sites by their functional impact – either increase or decrease the binding affinity of the corresponding TF – and observed different patterns of polymorphism and divergence between the two classes. For example, we found that affinity-decreasing mutations are on average more deleterious among new mutations than affinity-increasing ones, as revealed by a comparison of the ratio between the two classes in polymorphism with the expectation from mutational input. Such distinctions were not observed when mutations were grouped in other ways irrelevant to the function of TFBS (for example, mutations in the first half of the motif versus the second half). For these reasons we think the evidence supports our specific model of selection acting on binding site gain and loss as opposed to an unidentified functionality in noncoding sequences in general. The mechanism of selection we described here for well-annotated TFBS could in principle be acting more broadly across noncoding regions inasmuch as noncoding DNA is often associated with proteins binding.
Our ability to correctly categorize mutations into affinity-increasing or affinity-decreasing categories hinges on the accuracy of PWM predicted affinity differences. To investigate this issue, we employed a state-of-the-art microfluidics technique, MITOMI [44], to experimentally measure the binding affinity differences for naturally occurring mutations in hunchback and bcd binding sites. To our knowledge, this is the first time that accurate measurements have been made on population-level variants in TFBS. We found that PWM scores correctly predicted the measured direction of affinity change for 21/25 mutations investigated. Of the four mutations that PWM predicted the wrong direction, three have effect sizes predicted to be close to zero. The PWM-based procedure, therefore, may not be accurate for small predicted differences in binding affinity. Taking these results into consideration, we employed a binary classification of mutations with PWM differences exceeding a threshold requirement rather than using quantitative predictions of all PWM score differences as a basis for our analysis.
Another potential issue concerns applying mel derived PWM to score sim TFBS binding affinity. Transcription factor protein evolution between the two species, if it occurred, could lead to underestimation of binding affinity in sim, although the effect should be similarly applied to both substitutions and polymorphism and thus is not expected to cause a relative excess of the former as observed in the sim data. Nevertheless, we show two lines of arguments that suggest this is not the case in our study: first, for the 30 TF whose binding sites we investigated, the DNA bindings domains and other functionally annotated domains are completely conserved except for one biochemically conservative amino acid difference (Asp/Glu) in Dorsals RHD domain (Table S4). Although differences exist in other parts of the proteins, it has been shown that DNA binding domain may singly determine the sequence specificity of the protein [44], [45]. Second, if what we identified as affinity-decreasing mutations in sim reflected on-going adaptations to a slightly different motif, we would expect, but did not find, a consistent pattern in the position and kind of nucleotide changes for a TF (data not shown). To further support this argument, we derived PWM using MEME from the mel footprint sites as well as their aligned sequences in sim. As shown in Figure S7, our classification of binding site differences did not differ between using either the mel PWM or the sim PWM, contrary to what would be expected if TF sequence specificity had evolved between the two species. Therefore we consider it very unlikely for the 30 TF included in this study to have undergone significant evolution in their sequence specificity. In addition, because the SELEX derived PWM produce consistent results with the footprint derived ones (Figure S3), we can also rule out the possibility of over-optimization of the PWM inducing a sequence preference for mel over sim.
Finally, in the course of the analysis, we identified and modeled an ascertainment bias caused by the identification of footprint sites exclusively in a single strain of mel, and the possibility that sequence changes in the same species can lead to creation or destruction of the footprint feature (as described in the Results section). Many other genomic features such as miRNA binding sites and recombination hotspots can also satisfy these two criteria. As new studies attempt comparative evolutionary studies of genomic features often identified in a single reference sequence, we expect this problem to become more common and, therefore, to require greater attention. If not properly accounted for, this form of ascertainment can lead to false rejection of the neutral hypothesis. The analytical model of ascertainment under neutrality we developed here should be applicable to population genetic and evolutionary analysis of many different structural features of genomes.
Our population genetics analysis identified three major forces in TFBS evolution. First, we found functional TFBS were selectively maintained in the population by purifying selection, as revealed by a frequency spectrum skewed towards rare variants for affinity-decreasing polymorphism in mel and a significantly reduced proportion of affinity-decreasing polymorphism compared to mutational input in sim. These results are consistent with previous findings of selective constraints on functional TFBS. Mustonen and Lässig estimated that the average selection coefficient to maintain TFBS in bacteria and yeast genomes are on the order of [28], [46], and a similar estimate has been obtained for Drosophila [4]. The substitution rate with is expected to be less than 0.05% of the neutral rate in a population with a size as large as Drosophila (Equation B6.4.1, [47]). This means TFBS loss is unlikely to happen through fixation of deleterious mutations (0.2 losses expected for 645 footprint TFBS versus 16 inferred in sim). We can think of only three mechanisms by which TFBS loss can occur at an appreciable rate: (1) there is loss of constraint; (2) a pair of tightly linked compensatory mutations creates an effectively neutral allele; or 3) positive selection drives the loss of TFBS. Our second finding – a significant excess of substitutions compared to the neutral class for affinity-decreasing mutations in sim – is consistent only with positive selection for TFBS loss. Occasional adaptive loss of a TFBS is not inconsistent with more ubiquitous selection to maintain binding sites [28], and has been suggested to account for the evolution of fermentation pathways in yeast [16].
Our third finding is positive selection contributing to the gain of TFBS, as revealed by a significant excess of substitutions for affinity-increasing mutations in mel. Collectively, the three findings indicate that natural selection is extensively involved in the maintenance, gain, and loss of TFBS. This conclusion challenges the prevailing view of a neutral TFBS turnover process [4], [13].
We think that a selectionist interpretation of the turnover process is plausible for several reasons. First, the assumption of CRM functional stasis, which is the main argument for the neutral (i.e., compensatory) view, is not well supported experimentally. Reporter transgene assays, in particular, are limited in their quantitative resolution, and yet even in these studies, repeatable differences were found between orthologous CRM [7]. A functional rescue experiment is potentially more sensitive than a reporter transgene assay. As applied to the Drosophila even-skipped stripe 2 enhancer, it demonstrated clear functional differences between CRM that were previously believed to have the same spatial pattern of expression [48].
Second, compensatory neutral evolution cannot account for the patterns of variation observed in this study. According to this model, affinity-decreasing mutations should in general be deleterious but occasionally become “effectively” neutral when a second compensatory mutation occurs in the CRM of the mutant allele. A mixture of deleterious and compensatory mutations, even if the latter is common, may bring patterns of polymorphism and divergence close to a neutral scenario, but cannot produce a signature of positive selection as observed for both classes of mutations in our analysis. In addition, analytical modeling of the compensatory evolution of TFBS finds that the waiting time for a turnover event is long if complete neutrality of the compensating mutations is assumed [15]. To shorten the waiting time to be compatible with the Drosophila TFBS turnover rate, the parameterization of the model requires that the double mutant allele have higher fitness than the non-mutant allele, making it a directional selection model. This supercompensatory scenario could produce signatures of positive selection both for binding site gain and loss, the latter occurring because the fixation of a deleterious mutation in an existing TFBS will have the appearance of being positively selected as it hitchhikes to fixation on the selectively favored allele. However, this scenario is biologically unrealistic, as it requires the second mutation (the gain of a TFBS) to be positively selected only on the background of the first mutation.
As an alternative, consider the following model of positive selection on CRM structure/function. We propose that for CRM with large numbers of interacting partners, the network of cis - and trans-factors will inevitably be constantly evolving – due to both direct selective pressures imposed on the CRM or indirect effects caused by adaptations in other components of the network. For example, egg length variations between and within Drosophila species have been studied as potentially adaptive traits; if egg length evolves, genes such as eve whose expression pattern need to scale with the embryo may need to change its CRM to adapt to the new context [49]. This constant flux of change, we propose, imposes continual selection pressure for CRM function within the network to co-evolve and change. This “moving target” hypothesis finds support in an analytical study, which shows that fluctuating selection may be common in Drosophila, with changes in the sign of selection coefficient occurring at nearly the rate of neutral evolution [50]. Adaptive substitutions could therefore occur before selection switches its sign again, since positively selected mutations fix at rates much higher than the neutral mutation rate.
At the same time, the high connectivity in the regulatory network implies pleiotropic effects while the essentiality of genes controlled by the network may call for accurate regulation, both suggesting that the net change in CRM function will be highly constrained (Figure 5A). Under this conceptual model, functionally significant change will be possible on short evolutionary timescales, but will remain within constrained bounds over longer timescales. This feature of the model would account for adaptive gain and loss of TFBS in CRM, and could explain the strongly non-linear relationship between function and sequence evolution as exemplified by the Drosophila eve stripe 2 enhancer [7], [8]. Moreover, it provides an explanation for the finding of a non-clocklike evolutionary pattern: sequences from D. pseudoobscura rescues a mel eve stripe 2 enhancer deficiency almost as well as the native mel enhancer and substantially better than ones from much more closely related species ([48], Figure 5B).
Fig. 5. Models of CRM evolution with changes in fitness optimum. 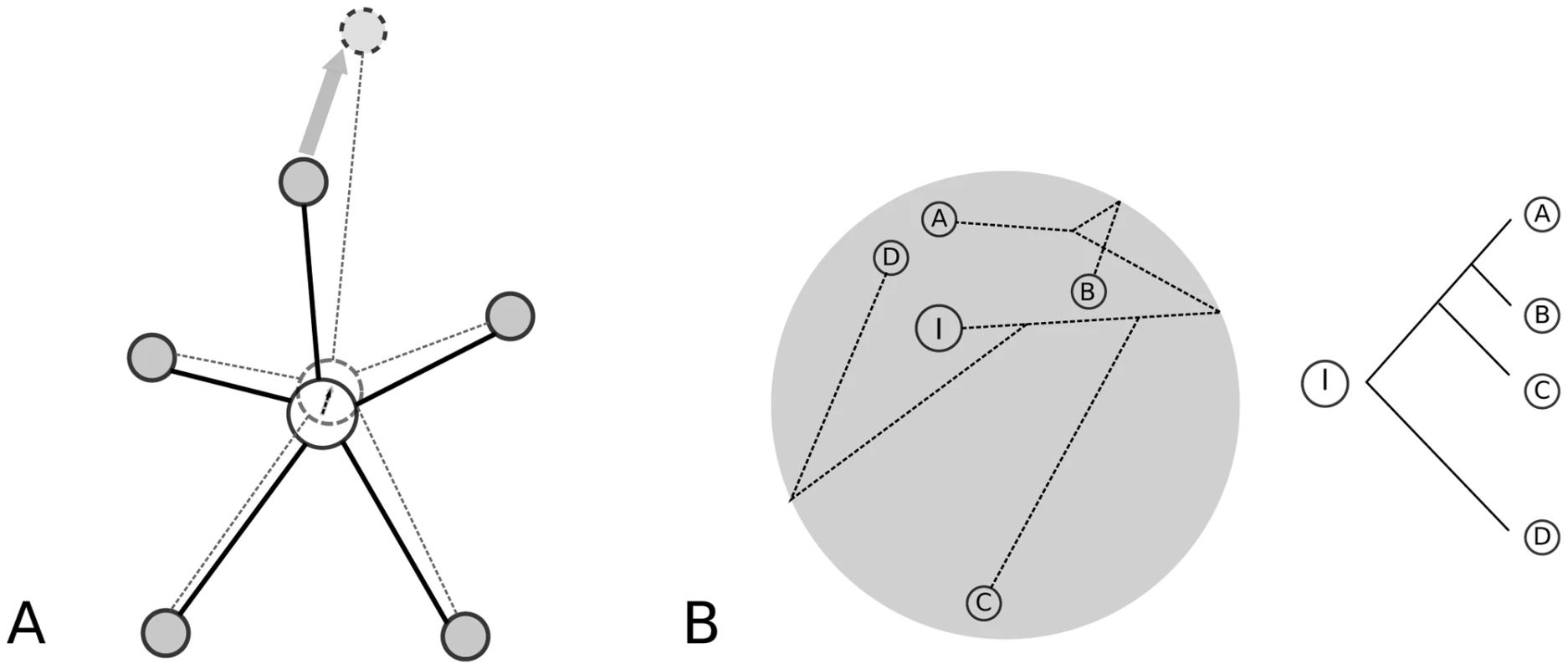
In conclusion, our findings provide empirical evidence for positive natural selection acting in CRM and TFBS evolution. We suggest that CRM are not as functionally static as commonly believed, but rather may experience frequent adaptation through binding site turnover, even though there may be constraints on net change over longer evolutionary time.
Materials and Methods
CRM annotation and sequence alignments
REDfly [30] is a database of manually curated CRM and TFBS obtained from the literature from which we chose 118 non-overlapping autosomal CRM for investigation (Table S1). They regulate 81 target genes and contain binding sites for 82 TF. The 118 CRM range in size from 65 bp to 4.3 kb (median = 515 bp) and contain between 1 to 64 DNase I footprint sites (median = 4). From the set of 82 TF, we identified a subset of 30 with more than 10 footprint sites represented in the dataset and with carefully constructed Position Weight Matrices [51]. In each footprint region plus five flanking bases on each end, we applied the appropriate position weight matrix to identify the highest scoring match as the core motif for the TFBS (referred to as TFBS in the text). We only included those TFBS for which the alignment between mel and sim sequences contain no insertions or deletions (including both fixed or polymorphic sites). As a result, a total of 645 TFBS for these 30 TF were included for analysis.
For each of the 118 CRM (coordinates in dm3 of D. melanogaster reference genome listed in Table S1), we downloaded pre-aligned MAF blocks from UCSC genome browser for D. melanogaster (mel), D. simulans (sim), D. sechellia (sec), and two outgroup species, D. yakuba (yak) and D. erecta (ere). D. sechellia is a sister species to D. simulans and is included to compensate for the low sequence completeness in the reference sim genome. We then used the baseml module in PAML 4.4c [52] to reconstruct the ancestral sequences from the alignments. Following analysis involving polarized changes were done either using a single ancestral sequence for mel and sim determined by the most probable ancestral state (A,C,G or T) at each position, or summing over the posterior probabilities of all four possible states (full Bayesian approach). The two methods produced essentially the same results and therefore we only presented results using the most probable ancestral state. A maximum parsimony method was also investigated and was found to produce consistent results.
For polymorphism analysis, alignments for the same 118 CRM regions were obtained of a population sample of 162 D. melanogaster lines (http://www.hgsc.bcm.tmc.edu/projects/dgrp/) and six D. simulans lines (http://www.dpgp.org/). We also compiled the genome sequences of 150 coding regions corresponding to the target genes of the CRM listed in REDfly, for the purpose of compiling synonymous and nonsynonymous changes. For these data, we used codeml module in PAML 4.4c to reconstruct the ancestral sequence states following otherwise the same procedure as described above for CRM regions.
Position Weight Matrix (PWM)
PWM for 30 TF (Antp, Deaf1, Dfd, Kr, Mad, Trl, Ubx, Abd-A, Ap, Bcd, Br-Z1, Br-Z2, Br-Z3, Brk, Cad, Dl, En, Eve, Hb, Kni, Ovo, Pan, Prd, Slbo, Tin, Tll, Twi, Vvl, Z, Zen) were obtained from [51]. This set represents all the TF for which Down et al. identified a single best motif for the REDfly footprint sites. For comparison, we also constructed five PWM (Hb, Bcd, Kr, Prd, Twi) from SELEX (Systematic Evolution of Ligands by EXponential enrichment) data (kindly provided by Mark Biggin). We ran MEME [53] with parameters “-evt 0.01 -dna -nmotifs 3 -minw A -maxw B -nostatus -mod zoops -revcomp text” on different selection rounds of the SELEX data. The best PWM was chosen based on the MEME score, percentage of footprint sites recovered and a penalty for the number of additional matches predicted in addition to the footprint sites (Table S5).
Use PWM to predict mutation effect on binding affinity
Consider a mutation at the position in a binding site motif involving a change from nucleotide to ( take values 1–4, corresponding to the nucleotides ACGT). We calculated , where is the PWM matrix of size . According to previous theories, the PWM score is proportional to the physical discrimination energy of the protein to the sequence and therefore the above calculation may be used to infer the direction and magnitude of binding energy change due to a mutation [54].
To evaluate the accuracy of the PWM-based inference, we experimentally measured the binding energy change of observed mutations in Hb binding sites, using a state-of-the-art microfluidics device that has high sensitivity for relatively weak molecular interactions (MITOMI, [44]). The experiments were performed as described in Maerkl et al. [44]. Sixty-four oligonucleotides were synthesized to test 25 SNP in Hb footprint sites and their combination in cases of multiple SNPs in a single TFBS between mel and sim. Data were analyzed in GenePix 6.0, R, and Prism 5.0. We found that the PWM we used correctly predicted the direction of change in 21/25 cases (Figure S2). Three of the four disagreements had a predicted PWM score change close to or smaller than one, which indicates that PWM may not be accurate when its predicted binding energy differences are small. To minimize the chance of misassigning the direction of binding energy change to a mutation, we set a threshold corresponding to a PWM score difference of one, and classified mutations within (smaller in absolute value) that bound as uncertain. The conclusions are robust to the setpoint of the threshold (for example, Table S3). We also compared the PWM derived by Down et al. to the five PWM derived from SELEX data: 97% (33/34) of mutations in the TFBS were consistently classified after excluding nine mutations with small predicted effects by either PWM (Figure S3).
Rate of gain and loss of TFBS in mel and sim
To examine the extent of binding sites gain and loss between the two species, we calculated PWM scores for each of the 645 footprint TFBS ( from 1 to 645) in orthologous sequences in mel, sim or the inferred mel-sim ancestor (j from 1 to 3), using patser v3e (by Gerald Z. Hertz, 2002). To determine whether a sequence is a binding site or not, we established two sets of cutoffs for PWM scores. First, we used PWM score , corresponding to the sequence being more likely from a binding site distribution than from a background distribution. For the second we used a set of TF-specific cutoff values chosen by first ranking all footprint sites of a TF by their PWM scores in descending order and then taking the 80% quantile value. The two cutoff set produced similar results (Table S2).
Construct sim-PWM from orthologous sequences to the mel footprint sites
To test whether the mel-derived PWM might be over-optimized so that they would favor mel over sim sequences independent of the binding affinity differences, we ran MEME on both mel footprint sites for three TF (Hb, Bcd, Trl) and their sim orthologous sequences with the same parameters. The two set of ÒorthologousÓ PWM were then applied to score the observed variations in the TFBS of the three TF for comparison (Figure S7).
Mutational probability for affinity-increasing and affinity-decreasing mutations
We attempted to estimate the probability for a random new mutation to be affinity-increasing () or affinity-decreasing () by examining all possible mutations that can occur on the inferred ancestral sequence of mel and sim for the 645 footprint TFBS. At the site in a TFBS for TF x, the probabilities are calculated as:(1)(2)(3)where is the original nucleotide and varies among the three possible mutations. is the position weight matrix for TF x of size . These values were then summed across all 645 TFBS and divided by the total number of nucleotides involved. Mutation matrix is derived from polymorphism of the 4-fold degenerate sites of 9,628 genes in D. simulans [55].
Generalized McDonald Kreitman (MK) test and site frequency spectrum analysis
For the generalized MK test, we counted the number of fixed and segregating sites for different functional categories in both mel and sim lineages. In sim, we required at least two of the six alleles to be non-missing for a site to be included in the analysis. For coding regions, synonymous sites were further classified into No-Change, Preferred-to-Unpreferred and Unpreferred-to-Preferred, following [22]. Polymorphism and divergence sites in both coding and CRM regions were counted using perl scripts adapted from Polymorphorama (Peter Andolfatto, Doris Bachtrog, 2009).
Following the suggestion of [34], we considered only common polymorphism (derived allele frequency 15%) in the generalized MK test to alleviate the problem caused by negatively selected mutations in detecting positive selection. For each mutation category, we compared the substitution-to-polymorphism ratio to the synonymous No-Change class using Fisher's Exact Test. Two-sided p-values are reported.
Site frequency spectrum (mel only): Next-generation sequencing data produce variable coverage. To estimate the site frequency spectrum, for each variable site (TFBS, coding and spacers) with a coverage greater than or equal to 150 (maximum is 162) we randomly chose 150 and combined the counts for each frequency class (from 1/150 to 149/150).
Supporting Information
Zdroje
1. SchmidtDWilsonMDBallesterBSchwaliePCBrownGD
2010
Five-Vertebrate ChIP-seq Reveals the Evolutionary Dynamics of
Transcription Factor Binding.
Science
328
1036
1040
2. BalhoffJPWrayGA
2005
Evolutionary analysis of the well characterized endo16 promoter
reveals substantial variation within functional sites.
Proc Natl Acad Sci U S A
102
8591
8596
3. DermitzakisETClarkAG
2002
Evolution of Transcription Factor Binding Sites in Mammalian Gene
Regulatory Regions: Conservation and Turnover.
Mol Biol Evol
19
1114
1121
4. KimJHeXSinhaS
2009
Evolution of Regulatory Sequences in 12 Drosophila
Species.
PLoS Genet
5
e1000330
doi:10.1371/journal.pgen.1000330
5. MosesAMPollardDANixDAIyerVNLiXY
2006
Large-scale turnover of functional transcription factor binding
sites in Drosophila.
PLoS Comput Biol
2
e130
doi:10.1371/journal.pcbi.0020130
6. GregorTMcgregorAPPWieschausEFF
2008
Shape and function of the Bicoid morphogen gradient in dipteran
species with different sized embryos.
Dev Biol
7. HareEEPetersonBKIyerVNMeierREisenMB
2008
Sepsid even-skipped Enhancers Are Functionally Conserved in
Drosophila Despite Lack of Sequence Conservation.
PLoS Genet
4
e1000106
doi:10.1371/journal.pgen.1000106
8. LudwigMZPatelNHKreitmanM
1998
Functional analysis of eve stripe 2 enhancer evolution in
Drosophila: rules governing conservation and change.
Development (Cambridge, England)
125
949
958
9. ArnostiDNBaroloSLevineMSmallS
1996
The eve stripe 2 enhancer employs multiple modes of
transcriptional synergy.
Development (Cambridge, England)
122
205
214
10. ShimellMJPetersonAJBurrJSimonJAO'ConnorMB
2000
Functional analysis of repressor binding sites in the iab-2
regulatory region of the abdominal-A homeotic gene.
Developmental biology
218
38
52
11. SwansonCIEvansNCBaroloS
2010
Structural rules and complex regulatory circuitry constrain
expression of a Notch - and EGFR-regulated eye enhancer.
Developmental cell
18
359
370
12. LudwigMZBergmanCPatelNHKreitmanM
2000
Evidence for stabilizing selection in a eukaryotic enhancer
element.
Nature
403
564
567
13. LudwigMZKreitmanM
1995
Evolutionary dynamics of the enhancer region of even-skipped in
Drosophila.
Molecular biology and evolution
12
1002
1011
14. KimuraM
1985
The role of compensatory neutral mutations in molecular
evolution.
Journal of Genetics
64
7
19
15. DurrettRSchmidtD
2008
Waiting for Two Mutations: With Applications to Regulatory
Sequence Evolution and the Limits of Darwinian Evolution.
Genetics
180
1501
1509
16. IhmelsJBergmannSGerami-NejadMYanaiIMcClellanM
2005
Rewiring of the Yeast Transcriptional Network Through the
Evolution of Motif Usage.
Science
309
938
940
17. KuoDLiconKBandyopadhyaySChuangRLuoC
2010
Coevolution within a transcriptional network by compensatory
trans and cis mutations.
Genome research
18. McGregorAPShawPJHancockJMBoppDHedigerM
2001
Rapid restructuring of bicoid-dependent hunchback promoters
within and between Dipteran species: implications for molecular
coevolution.
Evol Dev
3
397
407
19. ShawPJWrattenNSMcGregorAPDoverGA
2002
Coevolution in bicoid-dependent promoters and the inception of
regulatory incompatibilities among species of higher
Diptera.
Evolution & development
4
265
277
20. AndolfattoP
2008
Controlling type-I error of the McDonald-Kreitman test in
genomewide scans for selection on noncoding DNA.
Genetics
180
1767
1771
21. AndolfattoP
2005
Adaptive evolution of non-coding DNA in
Drosophila.
Nature
437
1149
1152
22. HaddrillPRBachtrogDAndolfattoP
2008
Positive and negative selection on noncoding DNA in Drosophila
simulans.
Molecular biology and evolution
25
1825
1834
23. KohnMHFangSWuCI
2004
Inference of Positive and Negative Selection on the 5 â
Regulatory Regions of Drosophila Genes.
Molecular Biology and Evolution
21
374
383
24. TorgersonDGBoykoARHernandezRDIndapAHuX
2009
Evolutionary Processes Acting on Candidate cis-Regulatory Regions
in Humans Inferred from Patterns of Polymorphism and
Divergence.
PLoS Genet
5
e1000592
doi:10.1371/journal.pgen.1000592
25. BachtrogD
2008
Positive Selection at the Binding Sites of the Male-Specific
Lethal Complex Involved in Dosage Compensation in
Drosophila.
Genetics
180
1123
1129
26. MacdonaldSJLongAD
2005
Identifying signatures of selection at the enhancer of split
neurogenic gene complex in Drosophila.
Molecular biology and evolution
22
607
619
27. DonigerSWFayJC
2007
Frequent Gain and Loss of Functional Transcription Factor Binding
Sites.
PLoS Comput Biol
3
e99
doi:10.1371/journal.pcbi.0030099
28. MustonenVLässigM
2005
Evolutionary population genetics of promoters: predicting binding
sites and functional phylogenies.
Proceedings of the National Academy of Sciences of the United States of
America
102
15936
15941
29. MosesAM
2009
Statistical tests for natural selection on regulatory regions
based on the strength of transcription factor binding sites.
BMC evolutionary biology
9
286+
30. GalloSMGerrardDTMinerDSimichMDes SoyeB
2010
REDy v3.0: toward a comprehensive database of transcriptional
regulatory elements in Drosophila.
Nucleic Acids Research
31. BradleyRKLiXYTrapnellCDavidsonSPachterL
2010
Binding Site Turnover Produces Pervasive Quantitative Changes in
Transcription Factor Binding between Closely Related Drosophila
Species.
PLoS Biol
8
e1000343
doi:10.1371/journal.pbio.1000343
32. McDonaldJHKreitmanM
1991
Adaptive protein evolution at the Adh locus in
Drosophila.
Nature
351
652
654
33. SawyerSAHartlDL
1992
Population Genetics of Polymorphism and
Divergence.
Genetics
132
1161
1176
34. FayJCWyckoffGJWuCI
2001
Positive and negative selection on the human
genome.
Genetics
158
1227
1234
35. CharlesworthJEyre-WalkerA
2008
The McDonald-Kreitman Test and Slightly Deleterious
Mutations.
Mol Biol Evol
25
1007
1015
36. SmithNGCEyre-WalkerA
2002
Adaptive protein evolution in Drosophila.
Nature
415
1022
1024
37. HalliganDLKeightleyPD
2006
Ubiquitous selective constraints in the Drosophila genome
revealed by a genome-wide interspecies comparison.
Genome research
16
875
884
38. ArnostiDNBaroloSLevineMSmallS
1996
The eve stripe 2 enhancer employs multiple modes of
transcriptional synergy.
Development (Cambridge, England)
122
205
214
39. BeallELManakJRZhouSBellMLipsickJS
2002
Role for a drosophila myb-containing protein complex in
site-specific dna replication.
Nature
420
833
837
40. ChenLO'KeefeSLHodgettsRB
2002
Control of dopa decarboxylase gene expression by the
broad-complex during metamorphosis in drosophila.
Mechanisms of Development
119
145
156
41. LundeKTrimbleJLGuichardAGussKANauberU
2003
Activation of the knirps locus links patterning to morphogenesis
of the second wing vein in drosophila.
Development
130
235
248
42. YanHCanonJBanerjeeU
2003
A transcriptional chain linking eye specification to terminal
determination of cone cells in the drosophila eye.
Developmental Biology
263
323
329
43. YanSJGuYLiWXFlemingRJ
2004
Multiple signaling pathways and a selector protein sequentially
regulate drosophila wing development.
Development
131
285
298
44. MaerklSJQuakeSR
2007
A systems approach to measuring the binding energy landscapes of
transcription factors.
Science (New York, NY)
315
233
237
45. BadisGBergerMFPhilippakisAATalukderSGehrkeAR
2009
Diversity and complexity in DNA recognition by transcription
factors.
Science (New York, NY)
324
1720
1723
46. MustonenVKinneyJCallanCGLässigM
2008
Energy-dependent fitness: A quantitative model for the evolution
of yeast transcription factor binding sites.
Proceedings of the National Academy of Sciences
105
12376
12381
47. CharlesworthBCharlesworthD
2010
Elements of Evolutionary Genetics
Roberts & Company Publishers, 1 edition
48. LudwigMZPalssonAAlekseevaEBergmanCMNathanJ
2005
Functional Evolution of a cis-Regulatory Module.
PLoS Biol
3
e93
doi:10.1371/journal.pbio.0030093
49. LottSEEKreitmanMPalssonAAlekseevaELudwigMZZ
2007
Canalization of segmentation and its evolution in
drosophila.
Proc Natl Acad Sci U S A
50. MustonenVLässigM
2007
Adaptations to uctuating selection in Drosophila.
Proceedings of the National Academy of Sciences of the United States of
America
104
2277
2282
51. DownTABergmanCMSuJHubbardTJP
2007
Large-Scale Discovery of Promoter Motifs in Drosophila
melanogaster.
PLoS Comput Biol
3
e7
doi:10.1371/journal.pcbi.0030007
52. YangZ
2007
PAML 4: Phylogenetic Analysis by Maximum
Likelihood.
Molecular Biology and Evolution
24
1586
1591
53. BaileyTLWilliamsNMislehCLiWW
2006
MEME: discovering and analyzing DNA and protein sequence
motifs.
Nucleic acids research
34
W369
373
54. BergOGvon HippelPH
1987
Selection of DNA binding sites by regulatory proteins.
Statisticalmechanical theory and application to operators and
promoters.
Journal of molecular biology
193
723
750
55. LuJShenYWuQKumarSHeB
2008
The birth and death of microRNA genes in
Drosophila.
Nature Genetics
40
351
355
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání