-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
Transient Receptor Potential (TRP) channels serve as temperature receptors in a wide variety of animals and must have played crucial roles in thermal adaptation. The TRP vanilloid (TRPV) subfamily contains several temperature receptors with different temperature sensitivities. The TRPV3 channel is known to be highly expressed in skin, where it is activated by warm temperatures and serves as a sensor to detect ambient temperatures near the body temperature of homeothermic animals such as mammals. Here we performed comprehensive comparative analyses of the TRPV subfamily in order to understand the evolutionary process; we identified novel TRPV genes and also characterized the evolutionary flexibility of TRPV3 during vertebrate evolution. We cloned the TRPV3 channel from the western clawed frog Xenopus tropicalis to understand the functional evolution of the TRPV3 channel. The amino acid sequences of the N - and C-terminal regions of the TRPV3 channel were highly diversified from those of other terrestrial vertebrate TRPV3 channels, although central portions were well conserved. In a heterologous expression system, several mammalian TRPV3 agonists did not activate the TRPV3 channel of the western clawed frog. Moreover, the frog TRPV3 channel did not respond to heat stimuli, instead it was activated by cold temperatures. Temperature thresholds for activation were about 16 °C, slightly below the lower temperature limit for the western clawed frog. Given that the TRPV3 channel is expressed in skin, its likely role is to detect noxious cold temperatures. Thus, the western clawed frog and mammals acquired opposite temperature sensitivity of the TRPV3 channel in order to detect environmental temperatures suitable for their respective species, indicating that temperature receptors can dynamically change properties to adapt to different thermal environments during evolution.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1002041
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002041Summary
Transient Receptor Potential (TRP) channels serve as temperature receptors in a wide variety of animals and must have played crucial roles in thermal adaptation. The TRP vanilloid (TRPV) subfamily contains several temperature receptors with different temperature sensitivities. The TRPV3 channel is known to be highly expressed in skin, where it is activated by warm temperatures and serves as a sensor to detect ambient temperatures near the body temperature of homeothermic animals such as mammals. Here we performed comprehensive comparative analyses of the TRPV subfamily in order to understand the evolutionary process; we identified novel TRPV genes and also characterized the evolutionary flexibility of TRPV3 during vertebrate evolution. We cloned the TRPV3 channel from the western clawed frog Xenopus tropicalis to understand the functional evolution of the TRPV3 channel. The amino acid sequences of the N - and C-terminal regions of the TRPV3 channel were highly diversified from those of other terrestrial vertebrate TRPV3 channels, although central portions were well conserved. In a heterologous expression system, several mammalian TRPV3 agonists did not activate the TRPV3 channel of the western clawed frog. Moreover, the frog TRPV3 channel did not respond to heat stimuli, instead it was activated by cold temperatures. Temperature thresholds for activation were about 16 °C, slightly below the lower temperature limit for the western clawed frog. Given that the TRPV3 channel is expressed in skin, its likely role is to detect noxious cold temperatures. Thus, the western clawed frog and mammals acquired opposite temperature sensitivity of the TRPV3 channel in order to detect environmental temperatures suitable for their respective species, indicating that temperature receptors can dynamically change properties to adapt to different thermal environments during evolution.
Introduction
Animals adapt to environmental temperature changes by sensing both their body and ambient temperatures. Thermal stimuli are detected by temperature receptors and transmitted by the peripheral nerves in which they reside [1]–[4]. Thus, temperature receptors must have served crucial roles in adaptation to thermal environments during the course of evolution. In mammals, temperature receptors are ion channels that are activated by thermal stimuli [1]–[4]. In humans and rodents, nine temperature receptors have currently been identified and all of them belong to the transient receptor potential (TRP) cation channel superfamily and are called “thermoTRPs”. These nine thermoTRPs are further classified into three subfamilies: four belong to the TRP vanilloid subfamily (TRPV1-TRPV4), four to the TRP melastatin subfamily (TRPM2, TMPM4, TRPM5, and TRPM8) and one to the TRP ankyrin subfamily (TRPA1) [1], [3]. Phylogenetic analysis of vertebrate thermoTRP homologs revealed that the genes encoding TRPV1-TRPV4, TRPM2, TRPM4, TRPM5, and TRPM8 are unique to vertebrates [5]. Most of these genes emerged in the common ancestor of teleost fishes and terrestrial vertebrates through repeated gene duplications; subsequent sequence divergence resulted in a thermoTRP repertoire with different physiological properties. In humans and rodents, TRPV1 and TRPV2 are activated by noxious high temperatures, TRPM8 and TRPA1 by cold temperatures, and TRPV3, TRPV4, TRPM2, TRPM4, and TRPM5 by warm temperatures [1]–[4]. In addition to thermal stimuli, thermoTRPs are also activated by various physical and chemical stimuli [1]–[4]. Thus, thermoTRPs are involved in various sensory transductions and required for the adaptation to ambient environments.
TRPV3 and TRPV4 perceive warm temperatures in homeothermic animals such as mammals [6]–[8] and thus must play important roles in body temperature regulation. Consistent with this idea, TRPV3 knockout mice showed abnormalities in sensing ambient temperatures near their body temperature [9]. However, whether these warm-temperature receptors are also physiologically important for the ectothermic vertebrates remains unknown. With respect to the TRPV4 gene, all of the vertebrate species thus far examined possess TRPV4 orthologs with highly conserved amino acid sequences among these different vertebrate species [5]. Regarding the TRPV3 gene, although all terrestrial vertebrate species thus far examined possess one copy of a TRPV3 orthologous gene, it has been lost in two teleost fish species [5]. Additionally, in the genome sequence database of the western clawed frog Xenopus tropicalis (belonging to the amphibian class) [10], the predicted gene for TRPV3 is much shorter than mammalian orthologs due to the lack of the N - and C-terminal portions. Sequences homologous to the mammalian terminal regions were searched, but such regions have not been found in the genome sequence of the western clawed frog [5]. This implies that the N - and C-terminal regions of western clawed frog TRPV3 have diverged from those of the mammalian orthologs, thus the complete coding sequence of the TRPV3 gene could not be annotated bioinformatically utilizing mammalian TRPV3s. Since amino acid sequences of the terminal regions of TRPV3s are well conserved among several mammalian species, divergence of TRPV3 may reflect a functional shift of the TRPV3 channel between mammals and the western clawed frog. Thus TRPV3 is a suitable model for understanding how thermoTRPs have changed their amino acid sequences as well as function during the course of evolution. Moreover, comparison of TRPV3 channel properties between homeothermic and ectothermic vertebrates may supply new insights into the functional evolution of thermoTRPs related to body temperature differences among species.
In contrast to the well characterized TRPV3 channels in homeothermic animals such as mammals, information on the TRPV3 channel in ectothermic animals is quite limited. Grandl et al. (2008) cloned western clawed frog TRPV3 (which lacked the N - and C-terminal regions) into a mammalian expression vector [11], but this truncated TRPV3 was nonfunctional. They subsequently fused the N - and C-terminal regions of mouse TRPV3 to western clawed frog TRPV3 and found that this chimeric TRPV3 responded to heat, camphor, and 2-aminoethoxydiphenyl borate (2-APB) [11], [12], known activators of mammalian TRPV3 in cultured mammalian cells [6]–[9], [13], [14]. However, since these observations were obtained using chimeric TRPV3, they did not show the native channel properties of western clawed frog TRPV3. In order to characterize the amino acid sequence as well as the channel properties of native TRPV3 from the western clawed frog, determination of the entire cDNA sequence is necessary.
The aim of the present study is to understand evolutionary changes in the TRPV3 channels. In this study, we sequenced cDNA of western clawed frog TRPV3 including the 5′ - and 3′-untranslated regions (UTR) and compared the amino acid sequences among various terrestrial vertebrate species. To characterize its channel properties, we cloned TRPV3 into an expression vector and used a heterologous expression system to compare properties between mammals and amphibians. Additionally, we conducted comprehensive comparative and phylogenetic analyses of the vertebrate TRPV subfamily utilizing various genome sequence databases to elucidate the evolutionary processes that occurred within the vertebrate lineages. Here we report the evolutionary changes of the TRPV3 channels, and highlight the differences in the temperature sensitivities between mammals and anurans.
Results
Reconstruction of the vertebrate TRPV subfamily phylogenetic tree
In order to understand the evolutionary changes within the TRPV subfamily, a comprehensive phylogenetic tree containing a broad range of vertebrate species including mammals, chicken, green anole, western clawed frog, and teleost fishes was reconstructed (Figure 1) [15]–[17]. The TRPV5 and TRPV6 genes that code for non-temperature-sensitive channels first diverged from the TRPV1-TRPV4 genes. Among the TRPV1-4 genes, each member was monophyletic to each other with high bootstrap value (>96%). The TRPV4 cluster was first to diverge, followed by a split of the TRPV3 cluster from the vertebrate TRPV1/2 cluster. However, the order of divergence between the TRPV3 and TRPV4 clusters was not clearly resolved since the connection between the vertebrate TRPV1/2 and TRPV3 clusters was supported only by a moderate bootstrap value (61%). Within the TRPV1/2 cluster, the terrestrial vertebrate TRPV2 cluster first split from the clusters containing the teleost fish TRPV1/2 and terrestrial vertebrate TRPV1 genes, although this branching order was supported by a moderate bootstrap value (61%). In dog, cow, and horse, one copy each of the TRPV1-TRPV6 genes were found. In the green anole, one copy each of the TRPV1-4 genes, and two copies of the TRPV6 gene were found. Unfortunately, due to low coverage in the green anole genome sequence database, only short sequenced fragments existed for the TRPV1, TRPV2, and TRPV4 genes. Thus, only the TRPV3 gene and two copies of the TRPV6 genes were included in the phylogenetic tree (Figure 1). One copy of the TRPV6 gene (TRPV6a) clustered with the chicken TRPV6 gene; the other copy of TRPV6 (TRPV6b) clustered with the former two genes with high bootstrap value (82%). Thus the gene duplication event which created the two copies of the TRPV6 gene in the green anole occurred within the reptile/bird lineages independent from the gene duplication event that produced the mammalian TRPV5 and TRPV6 genes. This latter duplication event likely occurred within the common ancestor of mammals since opossum also possesses TRPV5 and TRPV6 genes.
Fig. 1. Phylogenetic relationship of the TRPV gene subfamily of vertebrates. 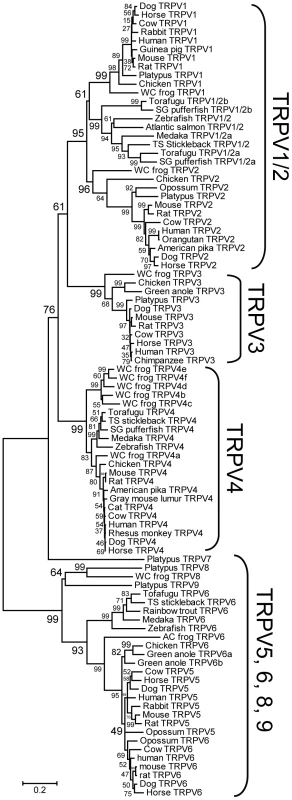
Statistical confidence (bootstrap value) is indicated beside the respective branch [15]. The TRPV5/6 genes were used as outgroups. WC frog, AC frog, SG pufferfish, and TS stickleback indicate western clawed frog, African clawed frog, spotted green pufferfish, and three-spined stickleback, respectively. Teleost fish TRPV1/2 genes showed copy number variation among the different species. Zebrafish and three-spined stickleback possessed only one copy, while torafugu, spotted green pufferfish, and medaka possessed two copies (note that the medaka TRPV1/2b gene was excluded from the phylogenetic tree in Figure 1 since it has large deletions in the central region which reduces the resolution of the phylogenetic tree; Figure 1 and Figure 2A). The TRPV1/2a and TRPV1/2b genes in medaka and torafugu were located in different genomic regions in which syntenic relationships were preserved around TRPV1/2s (Figure 2A).
Fig. 2. Conserved gene arrangements in the genomic regions encompassing vertebrate TRPV1-TRPV3 genes. 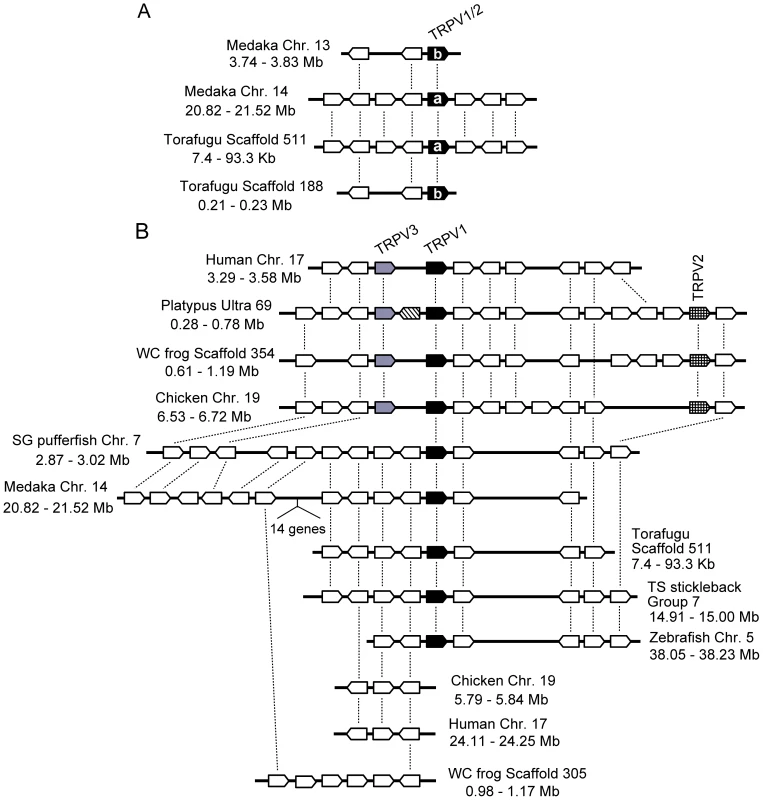
The gene orders around TRPV1/2 of medaka and torafugu (A) and TRPV1-TRPV3 of vertebrates (B) are shown. The genes are shown as boxes with their directions indicated. Filled, hatched, gray, and striped boxes represent TRPV1, TRPV2, TRPV3, and TRPV7 (only found in platypus genome), respectively. The open boxes indicate non-TRP genes. The orthologous genes among different species are connected by lines. The physical distances of the genomic regions are indicated. Chr., chromosome. The syntenic relationship also existed in the genomic regions around the TRPV1-TRPV3 genes among vertebrate species (Figure 2B). In platypus, western clawed frog, and chicken, the TRPV1 and TRPV3 genes were located adjacently, and TRPV2 was positioned several genes away from TRPV1 and TRPV3. In humans, the TRPV1 and TRPV3 genes were also located adjacently although the TRPV2 gene was distantly located in the same chromosome [5]. The teleost fish TRPV1/2 gene was located in a position corresponding to the terrestrial vertebrate TRPV1 gene (Figure 2B). Although a syntenic relationship can be observed around the TRPV1-TRPV3 genes among vertebrate species, the genes corresponding to the terrestrial vertebrate TRPV3 and TRPV2 were not found in the teleost fish genome sequences (Figure 2B).
In the course of phylogenetic analysis, we found several novel TRPV genes that have not previously been described. We found one novel gene from platypus that formed a sister group to a cluster of vertebrate TRPV1-TRPV4 genes, but was located outside of them (tentatively named TRPV7) (Figure 1). The TRPV7 gene was flanked by the TRPV1 and TRPV3 genes in the platypus genome sequence (Figure 2B). We could not find a corresponding gene in the other vertebrate species examined, including human, mouse, dog, cow, opossum, chicken, western clawed frog, medaka, and zebrafish. The predicted amino acid sequence of platypus TRPV7 possessed the putative ankyrin repeat and six transmembrane domains that are highly conserved among TRP channels. TRPV7 showed 44.2%, 42.7%, and 44.0% amino acid sequence similarity to platypus TRPV1, TRPV2, and TRPV3, respectively, in the central conserved regions (from ankyrin repeat domain 1 to the TRP domain; Figure 3A). In addition to platypus TRPV7, we also found three genes that are closely related to the vertebrate TRPV5 and TRPV6 genes (Figure 1). Two of them, from platypus and western clawed frog, formed a monophyletic cluster (tentatively named TRPV8). Platypus possessed one additional gene that clustered together with the TRPV8 genes (tentatively named TRPV9). The western clawed frog also possessed a TRPV6 gene that formed a sister group with the African clawed frog TRPV6, although the western clawed frog TRPV6 gene has a large portion that has not been sequenced yet, thus it was excluded from the phylogenetic tree shown in Figure 1.
Fig. 3. Comparison of TRPV3 among representative terrestrial vertebrate species. 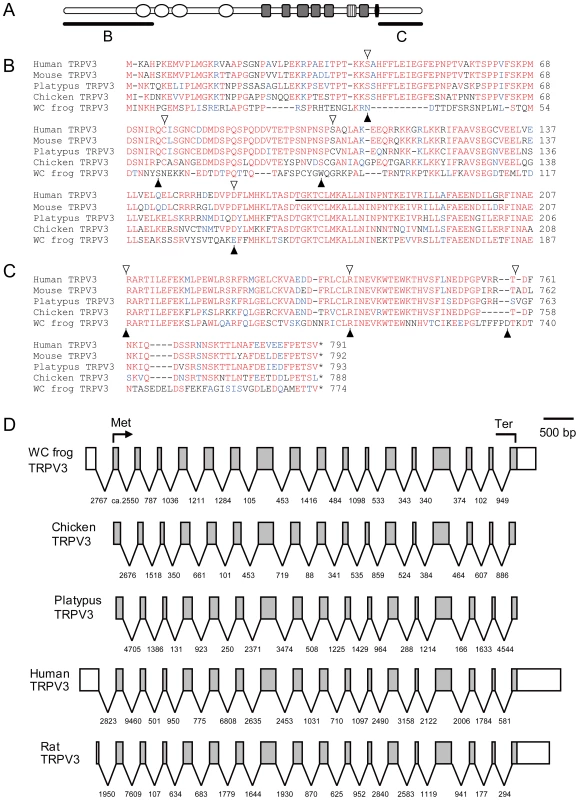
The schematic structure of the TRPV3 channel (A) is shown and the N- (B) and C- (C) terminal regions of amino acid alignments are indicated. The open circles, gray boxes, striped boxes, and black box represent the putative ankyrin repeat, transmembrane, pore loop, and TRP domains, respectively. The amino acids identical to, similar to, and different from the consensus residues are indicated in red, blue, and black letters, respectively. Exon boundaries for human, mouse, platypus, and chicken are indicated by open triangles and those for western clawed frog by filled triangles. (B) The first ankyrin repeat domain is underlined. Gene structures of TRPV3s in several vertebrate species (D). Exons are indicated by open boxes according to their scale. Introns are indicated by solid lines with their lengths (bp). The open reading frame is indicated by a shaded area with its initiation (Met) and termination (Ter) codons. Gene structures of chicken and platypus were predicted in the genome sequence databases (Ensembl) but not supported by cDNA data thus exons for the 5′- and 3′-UTR regions are not known. Gene structures of human and rat TRPV3s are based on full length cDNA nucleotide sequences and that of the western clawed frog TRPV3 is base on the cDNA nucleotide sequence determined in the present study. Determination of the cDNA sequences of the western clawed frog TRPV3 gene
Detailed comparative analyses from this and previous studies [5] raised the possibility that TRPV3 of the western clawed frog is diversified from that of mammals. However, as mentioned above, the predicted TRPV3 gene lacks the N - and C-terminal portions as they could not be annotated bioinformatically from the genome sequence database of the western clawed frog. We performed RT-PCR, 3′ - and 5′-RACE using total RNA extracted from the toe of the western clawed frog to sequence the cDNA of western clawed frog TRPV3 from the 5′ - to 3′-UTRs to obtain the full length coding sequence. We obtained a 2819-bp cDNA fragment (AB588024) which had a 2319-bp open reading frame (773 amino acid residues) starting near the 5′ end in the 2nd exon and ending in the last exon (Figure S1). Comparison of this cDNA sequence with the genome sequence database of the western clawed frog revealed that the nucleotide sequence corresponding to the second exon (114 bp) did not exist in the database. To confirm the result obtained by 5′-RACE, the cDNA fragment spanning exons 1 to 6 was amplified by RT-PCR. We obtained an approximately 650-bp DNA fragment that contained the second exon (Text S1). We further amplified and sequenced the genomic regions containing the second exon of the TRPV3 gene (AB588025) and confirmed that the second exon was located within the genomic portions that had yet to be sequenced by the genome sequence project (Text S1 and Figure S2). Therefore, the existence of the second exon was not an artifact.
Comparison of the amino acid sequences of western clawed frog TRPV3 with those of other terrestrial vertebrate orthologs revealed that it possesses conserved motifs such as four ankyrin repeat domains and six transmembrane domains (Figure S3). The amino acid sequences in the central portion of TRPV3 were relatively conserved among the tetrapod species examined. In contrast, amino acid sequences in the N - and C-terminal regions of western clawed frog TRPV3 were highly divergent from those of amniote TRPV3s although the corresponding regions of TRPV3 among amniotes were relatively well conserved (Figure 3B, 3C and Figure S3). In both terminal regions, a large number of amino acid substitutions as well as many gaps existed between western clawed frog and amniotes TRPV3s. The exon-intron structure of the TRPV3 gene of the western clawed frog was highly similar to those of other vertebrate TRPV3 genes (Figure 3D). Highly divergent regions in western clawed frog TRPV3 spanned across several exons although exon boundaries were conserved among the vertebrate species compared (Figure 3). This suggests that the divergence of the terminal regions of TRPV3 was not the result of modification of gene structure; rather, the divergence can be attributed to the accumulation of considerable amino acid substitutions.
Characterization of ion channel properties of TRPV3 in the western clawed frog
We next examined the ion channel properties of TRPV3 in the western clawed frog by expressing it in oocytes of the African clawed frog (Xenopus laevis) from which we recorded ionic currents using a two-electrode voltage-clamp method. As the mammalian TRPV3 channel is activated by temperatures >31-39°C [6]-[8], we first asked whether the TRPV3 channel of the western clawed frog is also activated by heat. Heat stimuli, however, did not induce any response in the oocytes injected with western clawed frog complementary RNA (cRNA; Figure 4A). Instead, surprisingly, cold stimulations induced large currents in the oocytes injected with western clawed frog TRPV3 cRNA (Figure 4A and 4B), but not in water-injected oocytes (Figure 4C). The currents induced by cold temperatures were also observed without prior heat stimulation (Figure 4B). The cold-induced currents were desensitized rapidly during the first cold stimulation and were considerably smaller during the second cold stimulation (Figure 4A). This property is different from that of mammalian TRPV3 which becomes sensitized with repeated heat stimulations [6], [8]. The average temperature threshold for activation was 16.35±0.51°C (n = 14) when analyzed with Arrhenius plots (Figure 4D).
Fig. 4. Cold temperature activation of the TRPV3 channel of the western clawed frog. 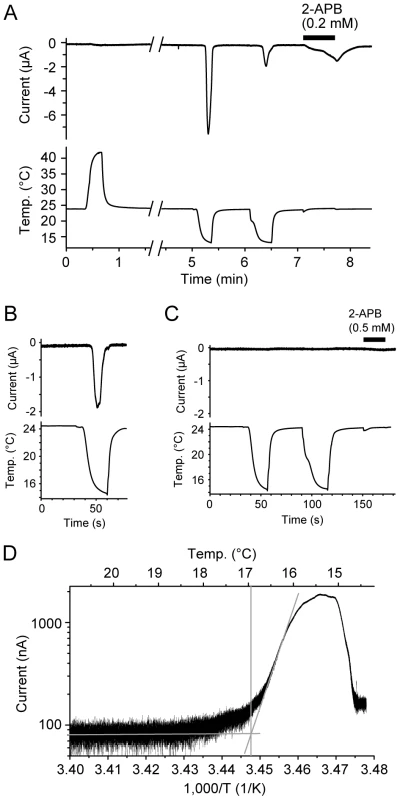
(A and B) Representative current traces (Upper) from oocytes injected with TRPV3 cRNA of the western clawed frog and corresponding temperatures (Lower). The bar indicates the application of 2-ABP (0.2 mM). (C) A representative trace of an oocyte injected with water alone. (D) An Arrhenius plot of the current in panel B. Average temperature threshold for activation was 16.35±0.51°C (n = 14). We next examined the pharmacological properties of western clawed frog TRPV3 currents. The oocytes expressing western clawed frog TRPV3 also responded to 2-APB, a known agonist of mammalian TRPV3 [13], [14], in a dose-dependent manner (Figure 4A, Figure 5A and 5B). In human, dog, and chicken, histidine residues at position 426 in TRPV3 are reported to be involved in 2-APB sensitivity [12]. The corresponding residue of western clawed frog TRPV3 was also histidine as reported previously [12] (Figure S3). We also confirmed that western clawed frog TRPV3 responded to 2-APB (Figure 5A and 5B). The 2-APB current tended to be sensitized when short-period stimulations (20 seconds) were repeatedly applied to the oocytes expressing western clawed frog TRPV3 (Figure S4A). This observation is similar to that of mammalian TRPV3, which showed sensitization upon heat, camphor, and 2-APB [6], [8], [9], [13]. In mouse TRPV3, a synergistic effect has been reported for temperature and 2-APB stimuli [13], [14]. Thus, temperature effects on 2-APB stimulation in TRPV3 of the western clawed frog were examined. Unexpectedly, cold stimulations suppressed 2-APB currents (Figure S4B), while heat stimulations showed potentiation effects (Figure S4C), implying that the activation mechanisms may be different between the cold and 2-APB responses for western clawed frog TRPV3.
Fig. 5. The activation and inhibition properties of the TRPV3 channel of the western clawed frog. 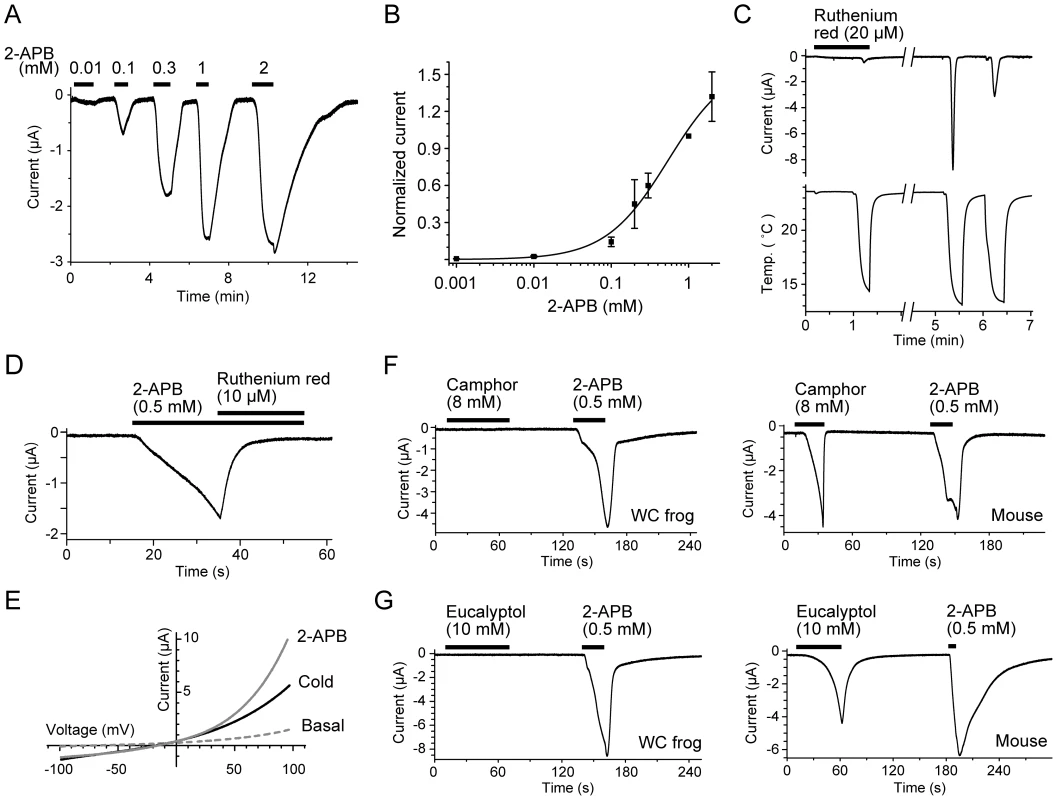
The data in panels A-E were obtained from oocytes injected with TRPV3 cRNA of the western clawed frog. (A and B) Oocytes responded to 2-APB in a dose-dependent manner. A representative current trace evoked by 2-APB (A) and its dose-response curve (B). Currents were normalized to the values at 1 mM. The EC50 was 0.54±0.24 mM, and the Hill coefficient was 1.1±0.3. Error bar indicate SEMs. (C) Inhibition of cold-induced currents in the oocytes. Ruthenium red (20 µM) was applied prior to and during the first cold stimulation. The second and third cold stimuli were applied four minutes after washing out the ruthenium red. (D) A 2-APB (0.5 mM)-induced current in the oocyte was inhibited by ruthenium red (10 µM) even in the presence of 2-APB. (E) Current-voltage relationships of cold- and 2-APB-evoked responses in the oocyte. (F and G) Representative current traces in response to initial applications of camphor (8 mM) (F) or eucalyptol (10 mM) (G) with secondary applications of 2-APB (0.5 mM) in oocytes injected with TRPV3 cRNA of the western clawed frog (Left) or mouse (Right). Ruthenium red, a broad TRP channel antagonist [4], [6]–[8], inhibited cold-induced currents in a reversible manner (Figure 5C) and also inhibited 2-APB-induced currents (Figure 5D) in oocytes expressing western clawed frog TRPV3. Moreover, the currents induced by both 2-APB and cold temperatures showed an outwardly-rectifying current-voltage relationship with slightly negative reversal potentials (–12.35±3.24 mV, n = 4; and –9. 33±2.03 mV, n = 4 for cold - and 2-APB-induced currents, respectively; Figure 5E). These results indicate that TRPV3 of the western clawed frog is a nonselective cation channel with a property similar to that of mammalian TRPV3 [6]–[8], [14]. On the other hand, western clawed frog TRPV3 did not respond to camphor (8 mM), eucalyptol (10 mM; Figure 5F and 5G, Left), menthol (2 mM), vanillin (10 mM), and eugenol (2 mM) (Figure S4D–S4F), well known activators of mammalian TRPV3 (Figure 5F and 5G, Right) [9], [18], [19]. These observations suggest that while western clawed frog TRPV3 shares some electrophysiological properties with mammalian TRPV3, it also possesses distinct properties, which may be related to its opposite temperature sensitivity from mammalian TRPV3.
Expression profile of TRPV3 mRNA in the western clawed frog
To compare the expression profiles of TRPV3 between mammals and western clawed frog, the tissue distribution of TRPV3 mRNAs in the western clawed frog was examined by semi-quantitative RT-PCR. TRPV3 mRNAs of the western clawed frog were expressed in skin from various parts of its body, toes of both fore and hind limbs, as well as testis (Figure 6). TRPV3 mRNAs were not detected in the gastrointestinal tract, peripheral nerve or brain where expression has been reported in mammals [6]–[8].
Fig. 6. Expression of TRPV3 in the skin of the western clawed frog. 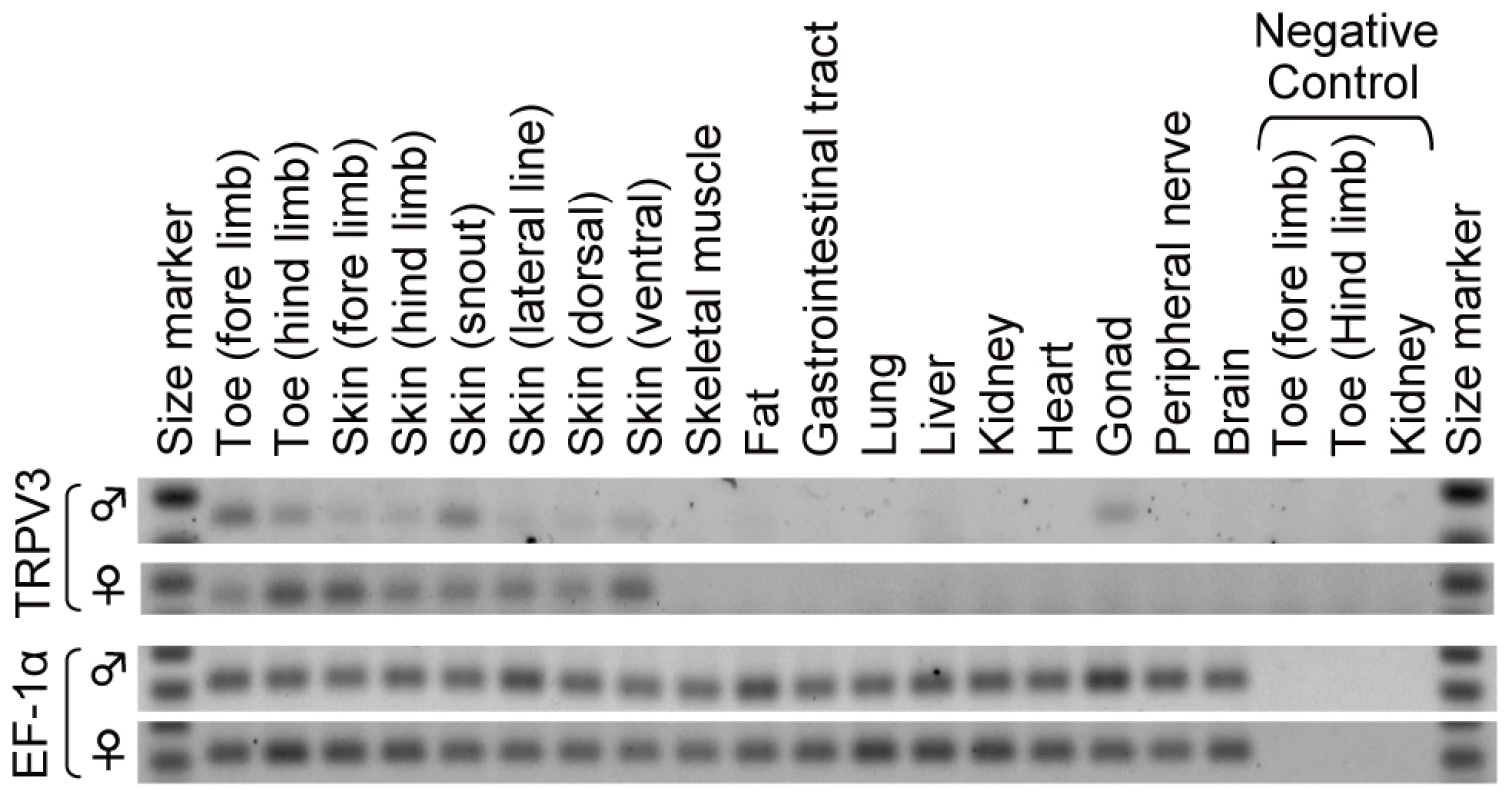
Transcription profiles of TRPV3 in the western clawed frogs were examined by semi-quantitative RT-PCR. The gene for elongation factor 1α (EF-1α) was used as an internal control. For negative control experiments, RNA samples from toes of both fore- and hind-limbs as well as kidney were used. The upper and lower bands of the size markers were 200-bp and 100-bp, respectively for TRPV3, and 300-bp and 200-bp, respectively for EF-1α. Discussion
Evolution of TRPV gene subfamily in vertebrates
In the present study, we performed comprehensive phylogenetic analysis on genes belonging to the TRPV subfamily from various kinds of vertebrate species (Figure 1). As previously reported [5], the TRPV3 and TRPV4 genes diverged earlier than the timing of the gene duplication between the TRPV1 and TRPV2 genes. Given that the teleost fish genes were included in the TRPV1/2 cluster, TRPV3 and TRPV4 genes emerged, at the latest, in the common ancestor of teleost fishes and terrestrial vertebrates. Whether the gene duplication of TRPV1 and TRPV2 occurred before or after the divergence of teleost fishes and terrestrial vertebrates is unclear since statistical support for the branch connecting the terrestrial vertebrate TRPV1 and teleost fish TRPV1/2 genes had a moderate value. In the teleost fishes, the TRPV1/2 genes showed copy number variation. Only one copy of the TRPV1/2 genes was found for stickleback and zebrafish, while two copies were found for medaka, torafugu, and spotted green pufferfish (Figure 1 and Figure 2A). Since teleost fish TRPV1/2 genes were clustered together with 99% bootstrap value, the gene duplication events producing teleost fish TRPV1/2s and vertebrate TRPV1 and TRPV2 must have independently occurred in each lineage. Around the genomic region encompassing the two paralogous TRPV1/2 genes, syntenic relationships are preserved (Figure 2A), suggesting that the two copies of the TRPV1/2 genes were produced by the whole genome duplication that occurred in the common ancestor of teleost fishes [20]. One copy has subsequently been lost in the stickleback and zebrafish lineages. In contrast, given that the TRPV1-TRPV3 genes are closely located in the terrestrial vertebrate genome, they were produced by tandem gene duplications.
In the course of phylogenetic analysis, we found four novel TRPV genes that had yet to be reported (Figure 1). Three of these novel genes clustered with the TRPV5 and TRPV6 genes - two genes were from platypus (TRPV8 and TRPV9) and one from the western clawed frog (TRPV8). That these three genes split from the TRPV5/6 cluster suggests that they emerged in the common ancestor of teleost fish and terrestrial vertebrates. We also found another novel TRPV gene (TRPV7) in platypus that formed a monophyletic cluster with the TRPV1-TRPV4 clusters (Figure 1). Since TRPV7 was located outside of the vertebrate TRPV1-TRPV4 genes in the phylogenetic tree, we expected that other vertebrate species also possess orthologous genes. Our search for the orthologs to platypus TRPV7 in several vertebrate genome sequences, however, failed to find any orthologous genes. One of the explanations for this observation is that the TRPV7 gene may have emerged in the common ancestor of teleost fish and terrestrial vertebrates, and has been lost in most of the lineages. However, this scenario is unlikely because we have to assume independent gene loss events in the lineages leading to the different vertebrate classes. Another explanation is that TRPV7 was specifically produced in the lineage leading to platypus and a large amount of amino acid substitutions have subsequently been accumulated. The fact that TRPV7 was located between the TRPV1 and TRPV3 genes in the genome sequence of platypus suggests that TRPV7 was produced from either TRPV1 or TRPV3, or from both genes (Figure 2B). At present, it is unclear if platypus TRPV7 is a functional gene; further characterization of TRPV7 will prove interesting for future study since phylogenetically it is closely related to the TRPV1-TRPV4 genes that code for temperature sensitive channels.
In dog, cow, and horse, we found one copy each of the TRPV1-TRPV6 genes, as has been found for human and rodents (Figure 1). Chicken and green anole possessed one copy each of the TRPV1-4 genes. Western clawed frog possessed one copy each of the TRPV1-3 genes and possessed six copies of TRPV4 genes as reported previously [5]. Copy numbers also varied for TRPV1/2 genes among teleost fishes and for TRPV5-9 genes among vertebrate species. In addition, TRPV3 has been lost in the teleost fishes (Figure 1 and Figure 2B). In conclusion, the repertoires of the TRPV gene subfamily in vertebrates are essentially conserved, but gene duplication and loss events that occurred in specific lineages resulted in copy number variation; which potentially contributed to adaptation in the respective species.
Functional evolution of the TRPV3 channel in vertebrates
In the present study, we attempted to identify thermoTRPs that have changed their functional properties within specific evolutionary lineages as these thermoTRPs must have been involved in adaptation to thermal environments. In some cases, the functional shift of thermoTRPs was accompanied by diversifications of amino acid sequences. To identify these changes, we performed detailed comparative analyses of mammalian thermoTRP homologs utilizing the genome sequence database of various vertebrate species. In the first phase of our study, we comprehensively collected thermoTRP homologs from various vertebrate species and conducted comparative analyses (Figure 1 and Figure 2) [5]. These analyses showed that the N - and C-terminal regions of the western clawed frog were missing from the predicted gene in the genome sequence database. A search for the homologous sequences to mammalian TRPV3 terminal regions in the genome sequence database of the western clawed frog failed to detect such regions. Thus we predicted that both terminal regions of western clawed frog TRPV3 are different those of from mammalian orthologs. To elucidate the amino acid sequences of western clawed frog TRPV3, the cDNA sequence was determined and the deduced amino acid sequence was compared to those of other vertebrate TRPV3s. As expected, the N - and C-terminal regions of TRPV3 in the western clawed frog were highly diversified from those regions of TRPV3 in other terrestrial vertebrate species, although the central portions were relatively conserved among all terrestrial vertebrates examined (Figure 3B, 3C, and Figure S3). Characterization of western clawed frog TRPV3 channel properties revealed striking differences from those of mammalian TRPV3 channels. The TRPV3 channel of the western clawed frog was not activated by chemical compounds that are known to activate the mammalian TRPV3 channel (Figure 5F and 5G) [9], [18], [19]. Furthermore, the TRPV3 channel of the western clawed frog was activated by cold temperatures whereas the mammalian TRPV3 channels has been reported to be activated by warm temperatures (Figure 4) [6]–[8]. Thus through a combination of interdisciplinary approaches including bioinformatics, molecular evolution, molecular biology, and electrophysiology, we were able to successfully identify a thermoTRP that has undergone a functional shift during the course of vertebrate evolution.
Opposite temperature sensitivities among orthologs have been reported in other thermoTRPs. For instance, the TRPA1 channels are activated by warm temperatures in several snake and insect species [21], [22], while it is activated by cold temperatures in mouse, although cold activation of mouse TRPA1 is the subject of some debate [23]–[27]. In the present study, we clearly demonstrate that the TRPV3 gene of the western clawed frog and mammals are orthologous genes by showing a monophyletic relationship among them (Figure 1) as well as conserved syntenic relationships of the genes flanking the TRPV3 genes among terrestrial vertebrate species (Figure 2B). These results indicate that TRPV3 channels have acquired opposite temperature sensitivities during the course of terrestrial vertebrate evolution. This, in turn, indicates that the temperature sensitivity of thermoTRPs is not always stable but can dynamically change, even reveres in some cases, during the course of evolution.
The molecular determinants for the difference in temperature sensitivities are not clear at present, but several lines of evidence suggest the N - and C-terminal regions as candidate domains. First, amino acid sequences of TRPV3 channels in these regions are highly divergent between mammals and the western clawed frog (Figure 3B, 3C, and Figure S3). Second, the C-terminal regions of thermoTRP channels have been reported to be involved in the modulations of temperature sensitivities. It has been shown that the swapping of the C-terminal regions between TRPV1 and TRPM8 channels of rat results in an exchange of temperature sensitivities [28]. Furthermore, it has also been reported that gradual truncations of the C-terminal regions of rat TRPV1 channels gradually shift temperature thresholds for activation [29]. Additionally, in the case of TRPV2, the N - and C-terminal regions are reported to play crucial roles in heat sensitivity in rodents [30]. Lastly, a chimeric mutant of the TRPV3 channel (in which the N - and C-terminal regions of mouse TRPV3 were fused to the central portion of TRPV3 from the western clawed frog) exhibited warm temperature activation when expressed in cultured mammalian cells (HEK293T) [11]. This chimeric mutant was also reported to be activated by camphor, thus the N - and C-terminal regions are likely to be involved in both chemical and temperature sensitivity of the TRPV3 channel. Future detailed study using chimeric mutants of the TRPV3 channel will be necessary to understand the molecular basis for the differences in temperature as well as chemical sensitivities of TRPV3 channels between mammals and the western clawed frog.
What is the physiological role of TRPV3 in the western clawed frog? TRPV3 was mainly expressed in the skin in the western clawed frog similar to the expression pattern of mammalian TRPV3 (Figure 6). In mammals, it has been proposed that thermal stimuli perceived by the skin are transmitted to peripheral nerves [3], [4], [31], [32]. Therefore, TRPV3 of the western clawed frog is likely to be involved in sensing temperatures at the body surface. The western clawed frog is a fully aquatic anuran that inhabits tropical areas, and its optimal ambient temperature range is 22–28°C [33], [34]. Temperatures below 18–20°C have detrimental effects [35], [36], and here we show that the temperature threshold for activation of the TRPV3 channel is slightly below its temperature limit (about 16°C) (Figure 4D). Thus, the physiological role of the TRPV3 channel is likely to detect noxious cold temperatures in the western clawed frog.
It is expected that the physiological importance of warm temperature perception is higher in homeothermic vertebrates than for ectothermic vertebrates. TRPV3 channels serve crucial roles in homeothermic mammals by detecting innocuous temperatures near the body temperatures such as in mouse [9], while it serves as a sensor to detect noxious cold temperatures in ectothermic vertebrates such as the western clawed frog (Figure 4). Similar observations have been reported for TRPM8 channels that act as cold temperature receptors. Activation temperatures of TRPM8 channels of the western and African clawed frog are much lower than those of rat and chicken TRPM8 channels [37]. Since body temperatures of frogs are lower than mammals and birds, it has been interpreted that the shift in temperature sensitivity of TRPM8 channels reflects the differences in the physiological requirements of body temperatures between homeothermic and ectothermic vertebrates. Characterization of TRPV3 channels from more diverse amniote species including both homeothermic and ectothermic animals will provide new insights into the functional evolution of temperature receptors related to homeothermy in vertebrates.
In conclusion, detailed comparative analyses on the TRPV subfamily performed in the present study identified novel TRPV genes that have not been reported previously (Figure 1) and also elucidated the flexible nature of TRPV3 in vertebrate evolution (Figure 7). The TRPV3 gene emerged in the common ancestor of teleost fishes and terrestrial vertebrates but has subsequently been lost in teleost fish lineages. Terrestrial vertebrates retained the TRPV3 channel, however, the western clawed frog and mammals acquired opposite temperature sensitivity to detect environmental temperatures suitable for their respective species. Thus the results of the present study reveal that thermoTRPs can dynamically change channel properties to adapt to different thermal environments during the course of evolution.
Fig. 7. The evolutionary changes of TRPV3 channels in the vertebrate lineages. 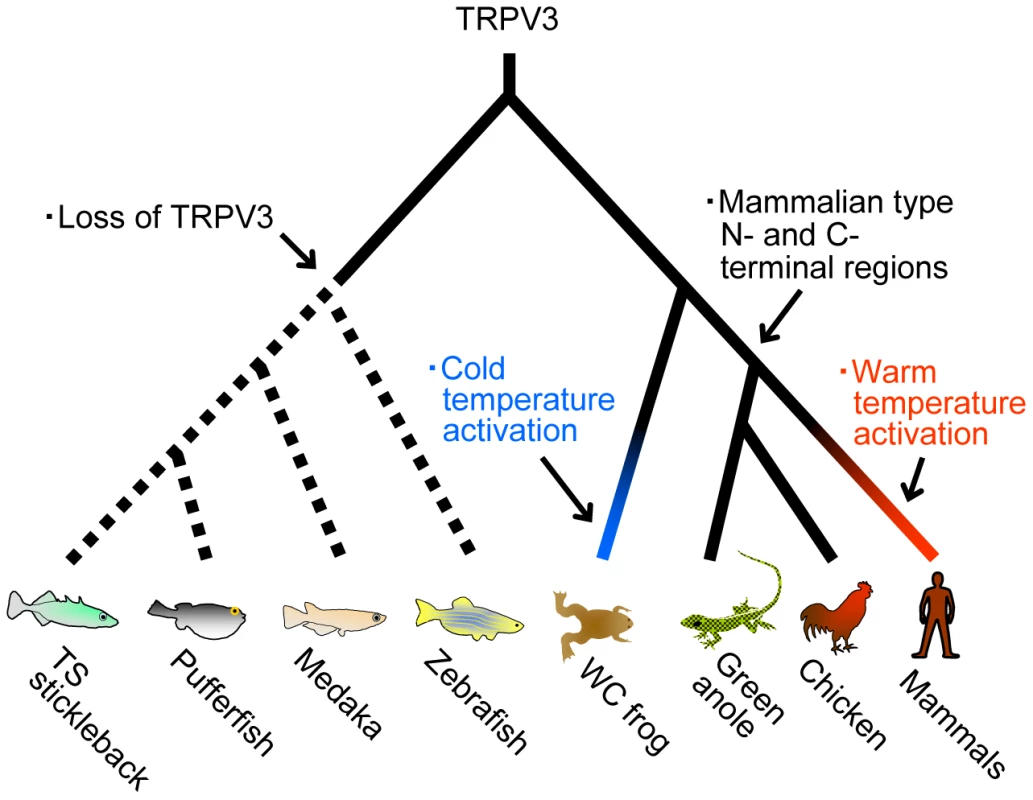
The major evolutionary events are indicated on the respective branches. The amino acid sequences of the N- and C-terminal regions of the TRPV3 channels were conserved among amniote species, while the N- and C-terminal regions of TRPV3 in the western clawed frog were highly diversified from those regions of TRPV3 in other terrestrial vertebrate species. The ancestral states of the terminal regions are ambiguous since teleost fishes have lost the TRPV3 gene. Western clawed frog and mammals acquired opposite temperature sensitivities of TRPV3 channels; however, the timing of the shift is not clearly determined since the temperature sensitivities of TRPV3 channels of birds and reptiles have not been reported. Materials and Methods
Retrieving nucleotide sequences of the TRPV genes
The TRPV1-5 genes from human, mouse, rat, chicken, western clawed frog, zebrafish, and torafugu were previously collected [5]. For the present study we collected TRPV homologous genes from several mammalian species, green anole, western clawed frog, and teleost fishes utilizing the orthologue prediction based on the draft genome sequence database published by Ensembl (http://www.ensembl.org/index.html).
Molecular phylogenetic analysis
Multiple sequence alignments were performed using the CLUSTAL W algorithm [38], with minor manual adjustments. Evolutionary distances between the amino acid sequences were calculated using the central conserved portions containing the ankyrin repeat and transmembrane domains (381 residues) by applying the JTT model [16] after all alignment gap sites were eliminated. The phylogenetic tree was then reconstructed using the minimum-evolution method [17]. The statistical confidence of each branch in the phylogenetic tree was estimated by the bootstrap method with 1,000 replications [15]. All of the above analyses were performed using MEGA4 software [39]. The TRPV genes and species used for phylogenetic reconstruction are listed in Table S1.
Western clawed frog
All procedures involving the care and use of animals were approved by the National Institute for Physiological Sciences. Western clawed frogs (Xenopus tropicalis) were kindly provided by the National Bio-resource Project (NBRP) of the Ministry of Education, Science, Sports and Culture of Japan. The western clawed frog strain used was the Yasuda line [34].
Sequencing and cloning of the TRPV3 gene of the western clawed frog
Using total RNA extracted from the toe of a fore-limb of an adult female western clawed frog as the template, a cDNA fragment spanning the 5′ - to 3′-UTR of the TRPV3 gene was amplified by RT-PCR and 5′ - to 3′-RACE. A DNA fragment containing the western clawed frog TRPV3 gene was amplified by RT-PCR and cloned into the pGEMHE vector. The PCR primers used are listed in Table S2. The mouse TRPV3 gene that was cloned into the pcDNA3 vector (Invitrogen) was the kind gift of Mike Caterina (Johns Hopkins, Baltimore, USA) and was subcloned into pOX+ vector.
Oocyte electrophysiology
The TRPV3 channel of the western clawed frog was heterologously expressed in oocytes of the African clawed frog Xenopus laevis, and ionic currents were recorded using the two-electrode voltage-clamp method. Western clawed frog TRPV3 cRNA was injected into defolliculated oocytes and ionic currents were recorded 1–4 days post-injection. The oocytes were voltage-clamped at −60 mV. All chemical compounds were diluted into ND96 bath solution and applied to the oocytes by perfusion. For thermal stimulations, heated or cold ND96 bath solutions were applied by perfusion. The current-voltage relationship was obtained using 200 ms voltage-ramp pluses from −100 to +100 mV applied every 1.5 seconds. The data values are expressed as mean ± SEM.
Semi-quantitative RT-PCR
Total RNA was extracted from skin from various parts of the body, toes of the fore - and hind-limbs, thigh skeletal muscle, fat body, gastrointestinal tract, lung, liver, kidney, heart, testis, ovary, peripheral nerve, and brain of male and female adult western clawed frogs. A 149-bp and a 206-bp cDNA fragment containing the TRPV3 and EF-1α (as internal control) genes, respectively, were amplified by RT-PCR.
Details of the Materials and Methods are described in Text S1.
Supporting Information
Zdroje
1. BandellMMacphersonLJPatapoutianA 2007 From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol 17 490 497
2. BenhamCDGunthorpeMJDavisJB 2003 TRPV channels as temperature sensors. Cell Calcium 33 479 487
3. DhakaAViswanathVPatapoutianA 2006 TRP ion channels and temperature sensation. Annu Rev Neurosci 29 135 161
4. PatapoutianAPeierAMStoryGMViswanathV 2003 ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4 529 539
5. SaitoSShingaiR 2006 Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 27 219 230
6. PeierAMReeveAJAnderssonDAMoqrichAEarleyTJ 2002 A heat-sensitive TRP channel expressed in keratinocytes. Science 296 2046 2049
7. SmithGDGunthorpeMJKelsellREHayesPDReillyP 2002 TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418 186 190
8. XuHRamseyISKotechaSAMoranMMChongJA 2002 TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418 181 186
9. MoqrichAHwangSWEarleyTJPetrusMJMurrayAN 2005 Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307 1468 1472
10. HellstenUHarlandRMGilchristMJHendrixDJurkaJ 2010 The genome of the Western clawed frog Xenopus tropicalis. Science 328 633 636
11. GrandlJHuHBandellMBursulayaBSchmidtM 2008 Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci 11 1007 1013
12. HuHGrandlJBandellMPetrusMPatapoutianA 2009 Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc Natl Acad Sci U S A 106 1626 1631
13. ChungMKLeeHMizunoASuzukiMCaterinaMJ 2004 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci 24 5177 5182
14. HuHZGuQWangCColtonCKTangJ 2004 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem 279 35741 35748
15. FelsensteinJ 1985 Confidence-Limits on Phylogenies - an Approach Using the Bootstrap. Evolution 39 783 791
16. JonesDTTaylorWRThorntonJM 1992 The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8 275 282
17. RzhetskyANeiM 1992 A Simple Method for Estimating and Testing Minimum-Evolution Trees. Molecular Biology and Evolution 9 945 967
18. Vogt-EiseleAKWeberKSherkheliMAVielhaberGPantenJ 2007 Monoterpenoid agonists of TRPV3. Br J Pharmacol 151 530 540
19. MacphersonLJHwangSWMiyamotoTDubinAEPatapoutianA 2006 More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 32 335 343
20. TaylorJSBraaschIFrickeyTMeyerAVan de PeerY 2003 Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 13 382 390
21. GrachevaEOIngoliaNTKellyYMCordero-MoralesJFHollopeterG 2010 Molecular basis of infrared detection by snakes. Nature 464 1006 1011
22. ViswanathVStoryGMPeierAMPetrusMJLeeVM 2003 Opposite thermosensor in fruitfly and mouse. Nature 423 822 823
23. BautistaDMJordtSENikaiTTsurudaPRReadAJ 2006 TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124 1269 1282
24. JordtSEBautistaDMChuangHHMcKemyDDZygmuntPM 2004 Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427 260 265
25. KarashimaYTalaveraKEveraertsWJanssensAKwanKY 2009 TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 106 1273 1278
26. KwanKYAllchorneAJVollrathMAChristensenAPZhangDS 2006 TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50 277 289
27. StoryGMPeierAMReeveAJEidSRMosbacherJ 2003 ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112 819 829
28. BrauchiSOrtaGSalazarMRosenmannELatorreR 2006 A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 26 4835 4840
29. VlachovaVTeisingerJSusankovaKLyfenkoAEttrichR 2003 Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci 23 1340 1350
30. NeeperMPLiuYHutchinsonTLWangYFloresCM 2007 Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem 282 15894 15902
31. MandadiSSokabeTShibasakiKKatanosakaKMizunoA 2009 TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch 458 1093 1102
32. LeeHCaterinaMJ 2005 TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch 451 160 167
33. HirschNZimmermanLBGraingerRM 2002 Xenopus, the next generation: X. tropicalis genetics and genomics. Dev Dyn 225 422 433
34. KashiwagiKKashiwagiAKurabayashiAHanadaHNakajimaK 2010 Xenopus tropicalis: an ideal experimental animal in amphibia. Exp Anim 59 395 405
35. KhokhaMKChungCBustamanteELGawLWTrottKA 2002 Techniques and probes for the study of Xenopus tropicalis development. Dev Dyn 225 499 510
36. RosenblumEBPoortenTJSettlesMMurdochGKRobertJ 2009 Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus. PLoS ONE 4 e6494 doi:10.1371/journal.pone.0006494
37. MyersBRSigalYMJuliusD 2009 Evolution of thermal response properties in a cold-activated TRP channel. PLoS ONE 4 e5741 doi:10.1371/journal.pone.0005741
38. ThompsonJDHigginsDGGibsonTJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
39. TamuraKDudleyJNeiMKumarS 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



