-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Sustained Dietary Change Increases Epigenetic Variation in Isogenic
Mice
Epigenetic changes can be induced by adverse environmental exposures, such as
nutritional imbalance, but little is known about the nature or extent of these
changes. Here we have explored the epigenomic effects of a sustained nutritional
change, excess dietary methyl donors, by assessing genomic CpG methylation
patterns in isogenic mice exposed for one or six generations. We find stochastic
variation in methylation levels at many loci; exposure to methyl donors
increases the magnitude of this variation and the number of variable loci.
Several gene ontology categories are significantly overrepresented in genes
proximal to these methylation-variable loci, suggesting that certain pathways
are susceptible to environmental influence on their epigenetic states. Long-term
exposure to the diet (six generations) results in a larger number of loci
exhibiting epigenetic variability, suggesting that some of the induced changes
are heritable. This finding presents the possibility that epigenetic variation
within populations can be induced by environmental change, providing a vehicle
for disease predisposition and possibly a substrate for natural selection.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001380
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001380Summary
Epigenetic changes can be induced by adverse environmental exposures, such as
nutritional imbalance, but little is known about the nature or extent of these
changes. Here we have explored the epigenomic effects of a sustained nutritional
change, excess dietary methyl donors, by assessing genomic CpG methylation
patterns in isogenic mice exposed for one or six generations. We find stochastic
variation in methylation levels at many loci; exposure to methyl donors
increases the magnitude of this variation and the number of variable loci.
Several gene ontology categories are significantly overrepresented in genes
proximal to these methylation-variable loci, suggesting that certain pathways
are susceptible to environmental influence on their epigenetic states. Long-term
exposure to the diet (six generations) results in a larger number of loci
exhibiting epigenetic variability, suggesting that some of the induced changes
are heritable. This finding presents the possibility that epigenetic variation
within populations can be induced by environmental change, providing a vehicle
for disease predisposition and possibly a substrate for natural selection.Introduction
Epigenetic modifications lie at the interface between genes and the environment, and thus have the potential to create functional diversity in response to environmental cues. There is mounting evidence that the establishment of epigenetic states during mammalian development can be influenced by the gestational and neonatal milieu, resulting in lifelong phenotypic changes. Epigenetic changes have been observed after early exposure to a variety of insults including environmental toxins [1], variations in maternal care [2], in vitro culture [3] and nutritional stressors [4]–[12]. In some cases the epigenetic effects are heritable, giving rise to environmentally-induced phenotypes in subsequent, unexposed generations [1], [5].
The epigenetic response to altered nutrition is of great interest because it may explain how nutritional stress during gestation can have health effects beyond the neonatal period. Suboptimal nutrition or exposure to environmental toxins or stress during gestation increases the susceptibility of offspring to a number of adult-onset diseases, a phenomenon known as fetal programming [13]. It has been widely speculated that epigenetic changes underlie the phenotypic response to early nutritional stress [14]–[17], but the genes responsible for the phenotypic changes are not known, and few studies have examined the magnitude and extent of epigenetic changes in response to altered nutrition.
Perhaps the best-studied model of epigenetic response to nutrition is the effect of methyl donor supplementation on the murine Avy allele. Supplementation of pregnant dams with methyl donors influences the epigenetic state of the Avy allele in offspring, resulting in suppression of the obese yellow phenotype characteristic of Avy mice [4]–[5], [9]. We have previously shown that this environmentally-induced epigenetic change can be passed from one generation to the next [5]. However, there is no reason to suppose that the Avy allele is the only locus whose epigenetic state is susceptible to dietary influence. Epigenetic changes have been observed at various individual loci after exposure to general nutritional deprivation or excess [7], [18]–[21] and more recent genome-wide screens in cases of intrauterine growth restriction have suggested that changes may occur at loci throughout the genome [22]–[23].
We have investigated the extent of epigenetic changes induced by methyl donors, by assessing cytosine methylation at CpG island promoters across the genome in mice exposed to methyl donors for one or six generations. We find that methyl donors induce stochastic changes in methylation at thousands of loci throughout the genome, leading to an increase in epigenetic variability among individuals that is more pronounced in mice exposed for multiple generations. While affected genes differed among individual mice, similar functional groups were affected: genes involved in gene expression and transcription, organogenesis, and cellular development were highly overrepresented, suggesting that these genetic programs may be more susceptible to environmental influence.
Results
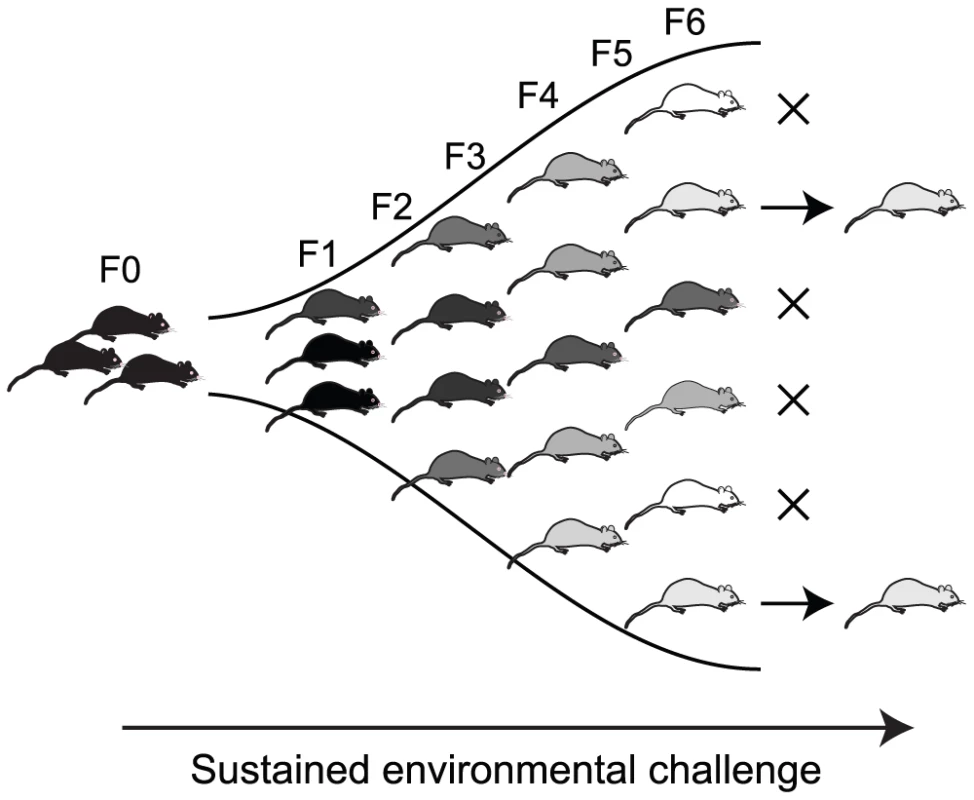
In order to assess the extent of epigenetic changes in response to dietary methyl donors, we examined changes in DNA methylation across the genomes of isogenic C57Bl/6J mice. Dietary supplementation with methyl donors commenced in founder pairs two weeks prior to mating, and was continued throughout pregnancy and lactation. We collected hepatocytes for analysis from mice in the first generation of exposure, and after supplementation for six generations. These mice were compared with C57Bl/6J mice that had never been exposed to methyl donors.
Methyl donors do not alter global 5-methylcytosine levels
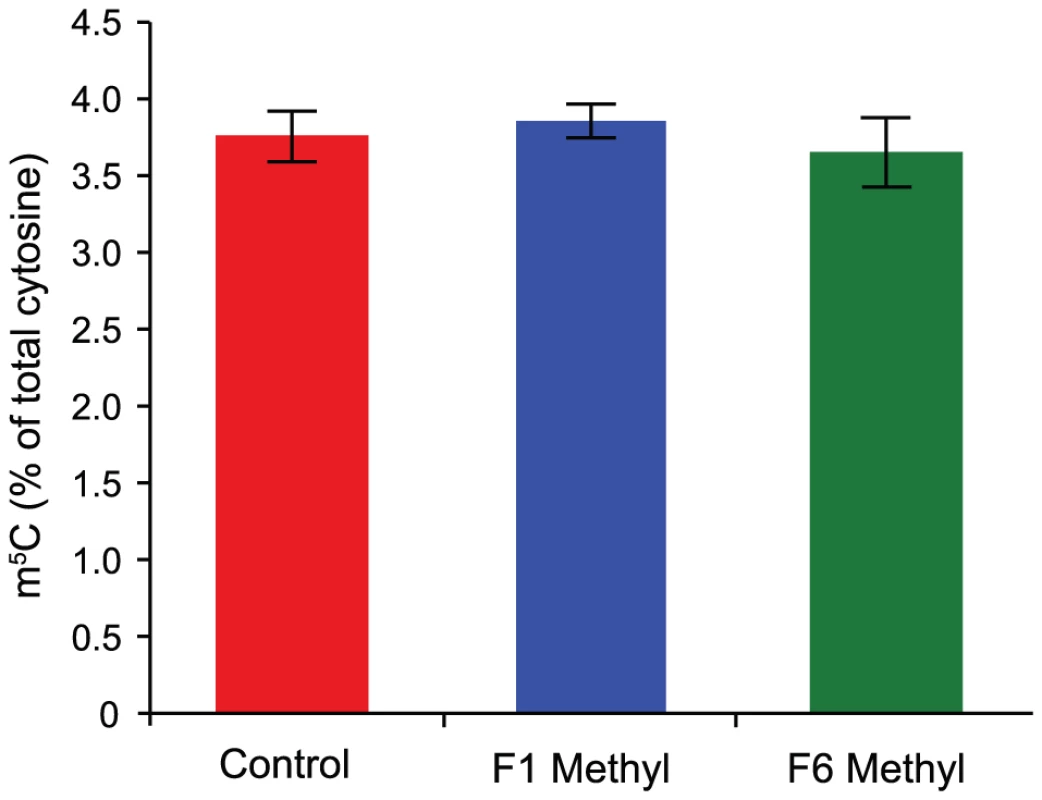
Methyl donors participate in an arm of one-carbon metabolism that creates methyl groups for donation to various molecules, including DNA, via the conversion of S-adenosylmethionine to S-adenosylhomocysteine. The observed effect of methyl donors on the Avy allele – epigenetic silencing of the IAP element that drives ectopic expression of the agouti gene [4]–[5], [9] – has been supposed to result from increased cytosine methylation due to an increase in the availability of methyl groups [9]. To determine if methyl donor supplementation leads to a global increase in the level of cytosine methylation, we assessed 5-methylcytosine (m5C) levels in genomic DNA from the livers of supplemented and unsupplemented mice by high-performance liquid chromatography (HPLC). We find that the m5C content of DNA from supplemented mice is not increased, even after six generations of supplementation (Figure 1).
Epigenetic variability is increased by methyl-donor supplementation
The absence of gross changes in genomic m5C levels does not preclude changes at some loci in supplemented mice. Methyl donors have been reported to induce epigenetic changes in at least two discrete loci (Avy and AxinFu) [5], [12] but it is not known if other genomic loci are also affected. To determine whether methyl donors exert epigenetic changes at other loci, and to resolve the extent of any changes, we compared genomic methylation patterns of supplemented and unsupplemented mice using a recently described method that combines enrichment of the unmethylated fraction of DNA with promoter microarray analysis [24]. Enrichment of the unmethylated fraction gives a better signal-to-noise ratio than other methods based on enrichment of methylated DNA, because removal of most repetitive sequences reduces the size of the DNA pool; moreover, since unmethylated CpG dinucleotides are less abundant in the genome than methylated CpG dinucleotides, this method is considerably more sensitive to DNA methylation changes at CpG islands [25].
We constructed libraries enriched for the unmethylated fraction of genomic DNA from liver using sequential HpaII and McrBC digestion and ligation-mediated PCR [24], and hybridised them to Agilent Mouse CpG Island 105K arrays representing approximately 16,000 CpG islands. We chose to examine CpG islands for two reasons: first, methylation changes at CpG islands are more likely to reflect regulatory changes than methylation changes at low-CpG density loci [26]; second, the enzymatic enrichment method we used preferentially targets CpG islands. We compared libraries from five F1 and five F6 supplemented mice to those from five unsupplemented controls; pooled libraries from 10 unsupplemented controls acted as the reference sample for each array. We analysed normalised array data using Partek Genomics Suite software.
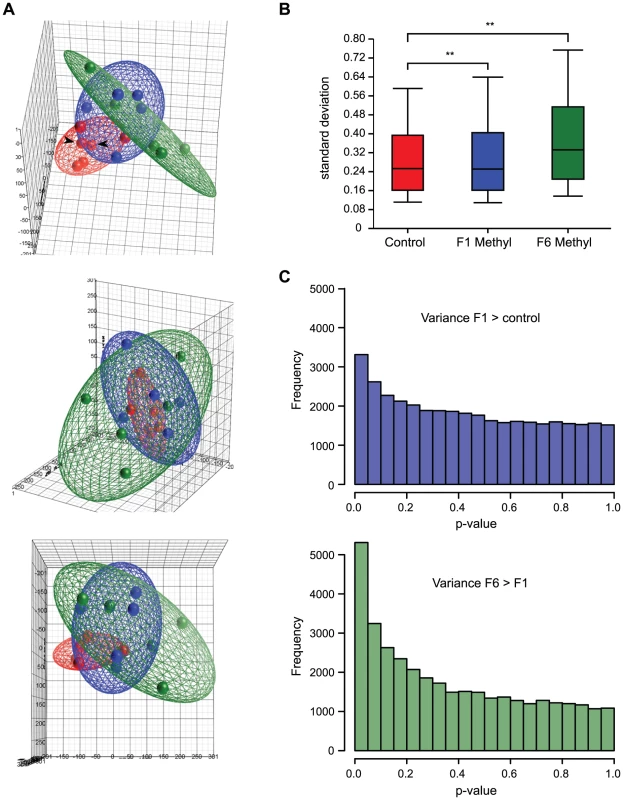
To view the overall distribution of array data from each group of mice, we performed a principal component analysis (PCA). PCA is a variable reduction procedure by which data with many variables is reduced to a few artificial variables, called principal components, which together account for most of the variance in the actual variables. The first three components of our data accounted for 38.7% of the variability and are visualized as a pseudo three-dimensional score plot in Figure 2A. In this visualization, array datasets from control mice cluster more closely than datasets from supplemented mice, suggesting that there is less variability between datasets from control animals than between those from supplemented animals. But control datasets do not overlap each other entirely, showing that there is some variability between controls. This variability cannot be attributed to technical variation between arrays, as principal component scores from array replicates were highly similar, so it is most likely due to methylation differences between control animals. This suggests that isogenic mice exposed to the same environment exhibit intrinsic epigenetic variation.
To confirm that the inter-individual epigenetic variation we observed was indeed biological in origin and not due to some intrinsic variability in probe signal, we measured the intrinsic variability of each probe by calculating the standard deviation of the signals from the reference pool across all 15 arrays. We compared this value with the probe's array signal standard deviation in each group. We found no correlation between reference pool standard deviation and array signal standard deviation (Figure S1). We also find no correlation between array signal standard deviation and probe GC content, which is the primary source of intrinsic variation in probe hybridization behavior [27] (Figure S1). This data indicates that the inter-sample variation we observe is due not to technical variation, but rather to methylation differences between animals.
Array datasets from supplemented mice show a broader range of principal component scores than those from controls (Figure 2A), indicating that array data from supplemented mice are more variable. Datasets from supplemented mice are also spatially distinct from control datasets in the PCA. Together, this suggests that supplemented mice have methylation patterns that are both more variable than, and different from, unsupplemented mice. Principal component scores from F6 supplemented animals show even greater dispersal than those from F1 animals, suggesting that the increased variability in methylation patterns seen in methyl donor supplemented animals is amplified with multigenerational exposure. Datasets from long-term supplemented mice are also more distant from controls than those from short-term supplemented mice. This suggests that in addition to increasing methylation variability, long-term supplementation may cause mice to become progressively more epigenetically distinct from mice that have never been supplemented.
As a second measure of overall variability in the array data, we calculated the range of probe signal standard deviations within each treatment group (Figure 2B). The average standard deviation was significantly higher for both F1 and F6 supplemented mice than for controls (p<0.001, unequal variance t-test), consistent with greater variability in methylation patterns between individual supplemented mice than between individual controls.
Third, we analysed each probe to determine whether it was more variable in one treatment group than another (Bartlett's test): this revealed significantly more variability in short term supplemented mice than control mice, and in long term than short term supplemented mice (Figure 2C). Finally, consistent with the idea that methyl donor supplementation increases epigenetic variability, histogram plots of array signals show an increased frequency of very low and very high signals in exposed mice (Figure 3A). Taken together, these results indicate that supplemented mice harbor many loci that carry more or less methylation relative to control mice.
Methylation changes at individual loci are stochastic among individuals
The measures that we performed indicated variability in methylation at individual CpG island loci in the genomes of both unsupplemented and supplemented mice. To identify candidate changes at individual loci induced by methyl donor supplementation, the conventional approach would be an analysis of variance (ANOVA). But candidate identification by ANOVA relies on within-group variance being lower than between-group variance, and our measures of overall variability indicated high within-group variance (particularly within the supplemented groups). Thus an ANOVA of our datasets yielded very few candidate loci, which when subjected to validation by extensive bisulphite sequencing showed no change in methylation (data not shown). We therefore took a different approach and first attempted to identify where methylation variability occurs, regardless of the treatment group: to do this, we interrogated the array probes that showed the most variable signals between mice of the same group, rather than between groups.
We identified probes with standard deviation values above the 95th percentile of the control group and mapped them to their respective CpG islands; we arbitrarily defined these loci as “methylation-variable”. We find 2110 methylation-variable loci in the control group, 2606 in F1 and 3640 in F6 (Figure 3B; for a list of all methylation-variable loci, see Table S1). There were 1490 methylation-variable loci in common between the short-term and long-term supplemented groups; 800 of these were also methylation-variable in the controls. A considerable proportion of methylation-variable loci were unique to each treatment group: long-term supplemented animals display the most (1752 or 48% of all this group's methylation-variable loci) and control animals the least (601 or 28%). Thus, not all the loci that are methylation-variable in control animals were affected by methyl donors in our sample supplemented population; this may be a reflection of the small sample size.
Representative methylation-variable loci are illustrated in Figure 3C. The variable regions are tightly defined and are flanked by sequence that is methylation-invariant among animals. Consistent with our finding that methyl donors do not alter global levels of m5C, we find that methylation-variable loci in supplemented animals are as likely to lose methylation as to gain it (Figure 3A and 3C). This challenges the assumption that methyl donors exert epigenetic effects via an increase in cytosine methylation [7], [9], and is consistent with our previous finding that methyl donors increase the probability of silencing at Avy without increasing the level of cytosine methylation [28]. At any given methylation-variable region, differences invariably occur in the same direction, although the amplitude differs among mice. Four loci interrogated by bisulphite allelic sequencing are shown in Figure S3. We found that just over half of validated loci (5/9) showed small methylation changes in the direction indicated by the array; the verification rate (FDR ∼0.55), and the small magnitude of changes we observe, are comparable to that of previous studies using this array strategy [29]–[30].
Taken together these results show that methylation variability occurs at many loci across the genomes of isogenic mice, and that the number of loci that exhibit variability increases with exposure to dietary methyl donors. Methylation changes in response to methyl donors are therefore stochastic and act to increase the epigenetic variability extant in an isogenic population.
Genes associated with methylation-variable loci are overrepresented in developmental ontologies
We find significantly more methylation-variable loci that are common to the three groups than expected by chance (800 vs 150; p<0.0001, χ2 test, 6 degrees of freedom); this suggests that methylation variability does not occur randomly, but rather that some genes are more epigenetically “plastic” than others. We performed a gene ontology (GO) analysis of the methylation-variable loci using two independent methods (Ingenuity Pathways Analysis (IPA) and GOstat [31]), to determine whether genes associated with these loci had functions in common. Both methods showed that genes involved in transcription, development and organogenesis are significantly overrepresented in methylation-variable loci, and that this is independent of dietary intervention (Figure 4 and Table S2). This applied to the loci that were common among groups as well as those unique to a group; thus, although genes may be idiosyncratically methylation-variable from one individual to the next, the variations appear to occur in common pathways.
Methylation variability is independent of local sequence characteristics
We considered the possibility that the methylation variability we observed was conditioned by the underlying genetic sequence, and so compared the sequence composition of the promoter regions (−1000 bp to +500 bp relative to the TSS) associated with the 100 most variable probes in the control group to that of the promoters associated with the 1000 least variable probes. We found no difference in GC content between methylation-variable and methylation-invariant promoters (Figure S2). We ran a de novo motif prediction pipeline (GimmeMotifs) to uncover any DNA motifs common to variable promoters, then compared the frequency of these motifs between the methylation-variable and methylation-invariant promoters. We identified nine motifs in the promoters of variable genes, but none of these were enriched relative to the methylation-invariant set (data not shown). Finally, given the known role of repetitive elements in affecting the epigenetic state of nearby genes, we examined the frequency and relative location of genomic repeat elements (LINE, SINE, LTR retrotransposons, simple repeats, low complexity repeats, microsatellites and DNA transposons) in the same promoter regions as above. We found no evidence for a difference in either repeat frequency or distribution between methylation-variable and methylation-invariant promoters (Figure S2). Taken together, these results indicate that local sequence context is unlikely to account for the methylation-variable regions that we have observed.
Discussion
We have conducted a genomewide DNA methylation analysis to investigate the epigenomic consequences of a sustained nutritional change, methyl donor supplementation. The epigenetic effect of dietary methyl donors has been well documented at the retrotransposon-derived murine Avy allele, but the extent to which the genome as a whole is affected by any sustained dietary intervention is largely unexplored. We found that methyl donor supplementation has widespread effects which increase epigenetic variation and are exacerbated by long-term exposure.
The increase in epigenetic variation induced by methyl donors occurred on a background of inter-individual epigenetic variation already extant in C57BL/6J mice. DNA from different control mice did not give identical array signals; these differences cannot be attributed to technical variation or genetic differences, and indicate epigenetic variation between isogenic mice reared in the same environment. The methylation-variable regions we defined usually do not span entire CpG islands, but are restricted to a subset of probes within each affected island, with surrounding probes showing no variability. Since the CpG islands on the array were chosen using computational (rather than functional) criteria, the methylation-variable regions we have identified may represent functional components within CpG islands. Our finding of well-defined methylation-variable loci in a control population of isogenic individuals is consistent with previous observations of variably methylated regions (VMRs) in the genomes of inbred mice by Feinberg and Irizarry [32]. Although the two studies used different methods of analysis, they identified methylation-variable regions that show striking overlap in gene ontology. It would be interesting to examine whether the widespread epigenetic differences that have been observed between human monozygotic twins [33]–[34] occur in genes from the same ontologies.
While several independent studies (including this one) now suggest that epigenetic variation persists in the absence of any genetic or environmental change, this study provides the first indication that additional epigenetic variation can be induced by environmental exposure. Methyl donor supplementation resulted in an increase in the number of methylation-variable loci: the epigenetic changes induced by dietary methyl donors were small in magnitude but widespread throughout the genome. Importantly, changes were stochastic, occurring at different loci in different individuals. Long-term exposure to excess methyl donors further increased the epigenetic variability within the population. That the effect becomes more pronounced with multigenerational exposure suggests that at least some of the induced changes are heritable. If so, phenotypic diversity created by an environmentally-induced increase in epigenetic variability might be acted upon by natural selection independently of genotype (Figure 5). This could enable rapid (within a few generations) adaptation to new environments [35]–[37], and because no genetic change is required, the acquired phenotypes would potentially be reversible if environmental conditions reverted. A sustained environmental change over a longer period might eventually result in a permanent epigenetic change which can in turn facilitate genetic mutation through the increased mutability of 5-methylcytosine [32], [38]–[39].
The idea that nutritional perturbations result in epigenetic changes throughout the genome, as opposed to at a few key regulatory genes, is consistent with the findings of several recent studies investigating the epigenetic contribution to fetal programming. Most candidate-approach studies report small, subtle methylation changes (typically <10%) [7], [19], [21]–[23]; reports of larger changes are less common [40]–[41]. An immediate question that arises is whether such small methylation changes are likely to exert any significant effect on phenotype. The VMRs identified by Feinberg and Irizarry were associated with gene expression variability [32], so small methylation changes may well have the potential to alter phenotype. Small differences in the methylation level of a locus, such as we have detected by array, could be due to a small methylation change in many cells, or a large methylation change in a small subset of cells. A large methylation change would likely be reflected in a change in gene expression within those particular cells; small changes in methylation might be considered less likely to be associated with a change in gene expression. However, the methylation status of critical CpG dinucleotides at some loci (e.g. within transcription factor binding motifs) can be tightly linked to gene expression [2]; changes at these CpGs could alter gene expression without large methylation changes across the locus. It is also possible that small, widespread changes in methylation induced by a poor intrauterine environment may become magnified over a lifetime and hence accelerate age-associated epigenetic decline [15]; this may go some way to explaining why fetal programming effects are observed later in life.
Fetal programming consistently increases the risk of the metabolic syndrome, despite being induced by a variety of environmental insults. This raises the question of whether specific metabolic genes are targeted by altered nutrition. In our model, methylation changes do not always occur at the same loci in different animals, but affected loci cluster in common gene ontologies. Metabolic ontologies are notable by their absence: rather, the most significant enrichment is seen in gene expression, organ development and cellular development. The fact that control animals (both in our study, and that of Feinberg and Irizzary) also show epigenetic variation within these ontologies suggests that genes in these pathways are “normally” epigenetically plastic; their increased epigenetic variability after supplementation implies that this plasticity (or “metastability”) renders the genes more susceptible to environmental influence. If so, even opposing environmental insults such as gestational undernutrition and overnutrition could produce epigenetic changes in these same pathways. The absence of metabolic ontologies does not necessarily preclude the generation of metabolic phenotypes: changes in organ development, for example, could have indirect metabolic consequences [42].
It has been proposed that adaptation though intrinsic epigenetic diversity may rely ultimately on genetic change within a species [32], but there is no reason to suppose that altered epigenetic states might not become stable in a population (or a subset of a population) without leading to a genetic mutation. The Lcyc epimutation of Linaria vulgaris represents one example of a potentially adaptive (and reversible) phenotypic change that is purely epigenetic [43]; the epimutation allows the plant to alter its floral symmetry, perhaps in response to environmental cues, and has remained in this species for centuries without effecting a permanent genetic change. Evaluating the heritability of more subtle epigenetic alterations induced by environmental changes, such as those induced by dietary methyl donors in mice, will be key to understanding the impact of early environment on the epigenetic contribution to complex disease risk.
Methods
Mice, diets, and tissue
All animals were handled in strict accordance with good practice as defined by the NHMRC (Australia) Statement on Animal Experimentation, and the requirements of NSW State Government legislation. All animal work was approved by the St Vincents/Garvan Animal Ethics Committee (animal research authorities #06/12 and #09/12). C57BL/6 mice were fed ad libitum on either (control) NIH-31 diet or (methyl donor supplemented) NIH-31 diet supplemented with (per kg) 15 g of choline, 15 g of betaine, 7.5 g of L-methionine, 150 mg of ZnSO4, 15 mg of folic acid and 1.5 mg of vitamin B12 (Specialty Feeds, Glen Forrest, Western Australia). Supplementation was commenced two weeks prior to mating founder pairs and continued for six generations; mice to be tested were sacrificed at 5 weeks of age for DNA collection. We extracted DNA from liver tissue, chosen because of its relative cellular homogeneity and high DNA yield.
Genomic 5-methylcytosine analysis
Genomic 5-methylcytosine (m5C) levels in supplemented and unsupplemented mice were assessed using high performance liquid chromatography (HPLC). 1 µg liver genomic DNA was denatured, digested into single nucleotides and dephosphorylated as previously described [44]. HPLC was performed using a method modified from Kovacheva et al. [45] with an Atlantis dC18 column (5 µm, 4.6×150 mm) and a 2.5%–16% methanol gradient in 50 mM K3PO4 (pH 4.5).
CpG island microarrays and analysis
For CpG island microarray, genomic DNA from supplemented and unsupplemented mice was enriched for the unmethylated fraction as previously described [25]. Briefly, 250 ng liver genomic DNA was subject to HpaII digestion and adaptor ligation followed by a second digestion with McrBC and adaptor-specific PCR. Library preparation was performed in triplicate and replicate libraries pooled for microarray analysis. Libraries were subject to two quality control steps. First, a fraction of each amplified library was analysed by gel electrophoresis and any libraries showing anomalous amplification (low amplicon quantity or unusual size range) were discarded. Second, in vitro methylated pCMV DNA and unmethylated pIRES DNA were spiked in to each sample before the McrBC digestion step. After library construction, the control plasmids were PCR amplified and amplicons quantified by densitometry; any libraries showing significant amplification of pCMV (>10% of an unmethylated control sample) or poor amplification of pIRES were discarded.
The DNA libraries were hybridized to Agilent 105K Mouse CpG Island microarrays. Before analysis of microarray data, outliers and low signal intensity features (within 2.6 standard deviations of background) were removed. Data was analysed using Partek Genomics Suite with LOESS normalization and median scaling to zero. We chose to use LOESS normalization because both test and reference samples underwent enrichment, and signals would thus be expected to center around 0, as required by LOESS normalization.
A Shapiro Wilks test in R 2.11.1 [46] was used to confirm that normalized probe signals were normally distributed. Differences in the variance of probe signals between groups were assessed using a Bartlett's test in R 2.11.1, with a post hoc analysis comparing the magnitude of probe standard deviation used to identify probes with increased variability.
Bisulphite methylation analysis
Allelic methylation patterns of selected methylation-variable loci were assessed by bisulphite allelic sequencing [47]. For bisulphite PCR, 2 µg liver genomic DNA was treated with sodium bisulphite using the Epitect Bisulphite kit (Qiagen) and 10% of the reaction was used in each PCR. Amplicons were cloned into pGEM-T and transformed into DH5-α E. coli cells, and plasmid DNA from individual colonies was sequenced.
Motif discovery in methylation-variable regions
For each of the 100 most variable probes in the control samples, we defined the genomic location of the closest known gene's promoter region as 1000 bp upstream and 500 bp downstream of the transcription start site using Galaxy [48] and the mm9 build of the UCSC Genome Browser [49]. As a control we used the 1000 least variable promoters in the control samples. We used GimmeMotifs [50] (version 0.61, using default options and medium motif size, with a randomized genomic background) to discover sequence motifs common to methylation-variable loci. The program Clover (version Jun 12 2006, with default options, and 1000 randomizations and a p-value threshold of 0.05) [51] was used to interrogate whether any of the motifs discovered were enriched in the methylation-variable dataset relative to the 1000 least variable.
Repeat element associations of methylation-variable regions
Using the same promoter regions as described above, we obtained the GC content of each promoter using the geecee tool from Galaxy, the genomic location of the microsatellites from the microsat track, and the LINE, SINE, LTR, Simple_repeat, Low_complexity, and DNA repeats from the RepeatMasker track, all at UCSC Genome Browser. We compared the distribution of the distance from the TSS to the midpoint of each element for variable versus control promoters using a two-sample unpaired t-test, and compared the frequency of these elements using a χ2 test, in R 2.11.1 [46].
Gene ontology of methylation-variable regions
To identify genes associated with methylation-variable probes, the list of array probes with intra-group standard deviation above the 95th percentile of control standard deviations was matched to overlapping annotated genes using Ingenuity Pathways Analysis (IPA) software. Functional analysis of the resulting gene list was performed independently in both IPA and GOStat (http://gostat.wehi.edu.au/), using the array genes and all RefSeq genes (mm9) as reference sets for both analyses.
Supporting Information
Zdroje
1. Anway
MD
Cupp
AS
Uzumcu
M
Skinner
MK
2005
Epigenetic transgenerational actions of endocrine disruptors and
male fertility.
Science
308
1466
1469
2. Weaver
ICG
Cervoni
N
Champagne
FA
Alessio
ACD
Sharma
S
2004
Epigenetic programming by maternal behaviour.
Nature Neuroscience
7
847
854
3. Young
LE
Fernandes
K
McEnvoy
TG
Butterwith
SC
Broadbent
PJ
2001
Epigenetic change in IGF2R is associated with fetal overgrowth
after sheep embryo culture.
Nature Genetics
27
153
154
4. Cooney
CA
Dave
AA
Wolff
GL
2002
Maternal methyl supplements in mice affect epigenetic variation
and DNA methylation in offspring.
The Journal of Nutrition
132
2393S
2400S
5. Cropley
JE
Suter
CM
Beckman
KB
Martin
DIK
2006
Germ-line epigenetic modification of the murine Avy allele by
nutritional supplementation.
Proceedings of the National Academy of Sciences of the United States of
America
103
17308
17312
6. Dolinoy
DC
Weidman
JR
Waterland
RA
Jirtle
RL
2006
Maternal genistein alters coat color and protects Avy mouse
offspring from obesity by modifying the fetal epigenome.
Environmental Health Perspectives
114
567
572
7. Lillycrop
KA
Phillips
ES
Jackson
AA
Hanson
MA
Burdge
GC
2005
Dietary protein restriction of pregnant rats induces and folic
acid supplementation prevents epigenetic modification of hepatic gene
expression in the offspring.
Journal of Nutrition
135
1382
1386
8. Sinclair
KD
Allegrucci
C
Singh
R
Gardner
DS
Sebastian
S
2007
DNA methylation, insulin resistance and blood pressure in
offspring determined by maternal periconceptional B vitamin and methionine
status.
Proceedings of the National Academy of Sciences of the United States of
America
104
19351
19356
9. Waterland
RA
Jirtle
RL
2003
Transposable elements: targets for early nutritional effects on
epigenetic gene regulation.
Molecular and Cellular Biology
23
5293
5300
10. Waterland
RA
Lin
JR
Smith
CA
Jirtle
RL
2006
Post-weaning diet affects genomic imprinting at the insulin-like
growth factor 2 (Igf2) locus.
Human Molecular Genetics
15
705
716
11. Zhang
S
Rattanatray
L
Maclaughlin
SM
Cropley
JE
Suter
CM
2010
Periconceptional undernutrition in normal and overweight ewes
leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19
gene in offspring.
FASEB J
12. Waterland
RA
Dolinoy
DC
Lin
JR
Smith
CA
Shi
X
2006
Maternal methyl supplements increase offspring DNA methylation at
Axin Fused.
Genesis
44
401
406
13. McMillen
IC
Robinson
JS
2005
Developmental origins of the metabolic syndrome: prediction,
plasticity and programming.
Physiological Reviews
85
571
633
14. Gallou-Kabani
C
Junien
C
2005
Nutritional epigenomics of metabolic syndrome: new perspective
against the epidemic.
Diabetes
54
1899
1906
15. Thompson
RF
Einstein
FH
2010
Epigenetic basis for fetal origins of age-related
disease.
Journal of Women's Health
19
581
587
16. Cropley
JE
Suter
CM
2008
An epigenetic basis for fetal programming.
Highlights
16
22
25
17. Waterland
RA
Garza
C
1999
Potential mechanisms of metabolic imprinting that lead to chronic
disease.
American Journal of Clinical Nutrition
69
179
197
18. Burdge
GC
Slater-Jefferies
J
Torrens
C
Phillips
ES
Hanson
MA
2007
Dietary protein restriction of pregnant rats in the F0 generation
induces altered methylation of hepatic gene promoters in the adult male
offspring in the F1 and F2 generations.
British Journal of Nutrition
97
435
439
19. Tobi
EW
Lumey
LH
Talens
RP
Kremer
D
Putter
H
2009
DNA methylation differences after exposure to prenatal famine are
common and timing - and sex specific.
Human Molecular Genetics
18
4046
4053
20. Heijmans
BT
Tobi
EW
Stein
AD
Putter
H
Blauw
GJ
2008
Persistent epigenetic differences associated with prenatal
exposure to famine in humans.
Proceedings of the National Academy of Sciences of the United States of
America
105
17046
17049
21. Gemma
C
Sookoian
S
Alvariñas
J
García
SI
Quintana
L
2009
Maternal pregestational BMI is associated with methylation of the
PPARGC1A promoter in newborns.
Obesity
17
1032
1039
22. Einstein
F
Thompson
RF
Bhagat
TD
Fazzari
MJ
Verma
A
2010
Cytosine Methylation Dysregulation in Neonates Following
Intrauterine Growth Restriction.
PLoS ONE
5
e8887
doi:10.1371/journal.pone.0008887
23. Thompson
RF
Fazzari
MJ
Niu
H
Barzilai
N
Simmons
RA
2010
Experimental intrauterine growth restriction induces alterations
in DNA methylation and gene expression in pancreatic islets of
rats.
Journal of Biological Chemistry
285
15111
15118
24. Schumacher
A
Weinhausl
A
Petronis
A
2008
Application of microarrays for DNA methylation
profiling.
Methods in Molecular Biology
439
109
129
25. Schumacher
A
Kapranov
P
Kaminsky
Z
Flanagan
J
Assadzadeh
A
2006
Microarray-based DNA methylation profiling: technology and
applications.
Nucleic Acids Research
34
528
542
26. Weber
M
Hellmann
I
Stadler
MB
Ramos
L
Paabo
S
2007
Distribution, silencing potential and evolutionary impact of
promoter DNA methylation in the human genome.
Nature Genetics
39
457
466
27. Irizarry
RA
Ladd-Acosta
C
Carvalho
B
Wu
H
Brandenburg
SA
2008
Comprehensive high-throughput arrays for relative methylation
(CHARM).
Genome Research
18
780
790
28. Cropley
JE
Suter
CM
Beckman
KB
Martin
DIK
2010
CpG methylation of a silent controlling element in the murine
A(vy) allele is incomplete and unresponsive to methyl donor
supplementation.
PLoS ONE
5
e9055
doi:10.1371/journal.pone.0009055
29. Flanagan
JM
Popendikyte
V
Pozdniakovaite
N
Sobolev
M
Assadzadeh
A
2006
Intra - and interindividual epigenetic variation in human germ
cells.
American Journal of Human Genetics
79
67
84
30. Mill
J
Tang
T
Kaminsky
Z
Khare
T
Yazdanpanah
S
2008
Epigenomic profiling reveals DNA-methylation changes associated
with major psychosis.
American Journal of Human Genetics
82
696
711
31. Beissbarth
T
Speed
TP
2004
GOstat: find statistically overrepresented Gene Ontologies within
a group of genes.
Bioinformatics
20
1464
1465
32. Feinberg
AP
Irizarry
RA
2010
Stochastic epigenetic variation as a driving force of
development, evolutionary adaptation, and disease.
Proceedings of the National Academy of Sciences of the United States of
America
107
1757
1764
33. Kaminsky
Z
Tang
T
Wang
S
Ptak
C
Oh
GHT
2009
DNA methylation profiles in monozygotic and dizygotic
twins.
Nature Genetics
41
240
245
34. Fraga
MF
Ballestar
E
Paz
MF
Ropero
S
Setein
F
2005
Epigenetic differences arise during the lifetime of monozygotic
twins.
Proceedings of the National Academy of Sciences of the United States of
America
102
10604
10609
35. Jablonka
E
Lamb
MJ
1989
The inheritance of acquired epigenetic
variations.
Journal of Theoretical Biology
139
69
83
36. Monk
M
1995
Epigenetic programming of differential gene expression in
development and evolution.
Developmental Genetics
17
188
197
37. Guerrero-Bosagna
C
Sabat
P
Valladares
L
2005
Environmental signaling and evolutionary change: can exposure of
pregnant mammals to environmental estrogens lead to epigenetically induced
evolutionary changes in embryos?
Evolution and Development
7
341
350
38. Skinner
MK
Manikkam
M
Guerrero-Bosagna
C
2010
Epigenetic transgenerational actions of environmental factors in
disease etiology.
Trends in Endocrinology and Metabolism
21
214
222
39. Sved
J
Bird
A
1990
The expected equilibrium of the CpG dinucleotide in vertebrate
genomes under a mutation model.
Proceedings of the National Academy of Sciences of the United States of
America
87
4692
4696
40. Park
JH
Stoffers
DA
Nicholls
RD
Simmons
RA
2008
Development of type 2 diabetes following intrauterine growth
retardation in rats in associated with progressive epigenetic silencing of
Pdx1.
The Journal of Clinical Investigation
118
2316
2324
41. Kovacheva
VP
Mellot
TJ
Davison
JM
Wagner
N
Lopez-Coviella
I
2007
Gestational choline deficiency causes global and Igf2 gene DNA
hypermethylation by upregulation of Dnmt1 expression.
Journal of Biological Chemistry
282
31777
31788
42. Pham
TD
MacLennan
NK
Chiu
CT
Laksana
GS
Hsu
JL
2003
Uteroplacental insufficiency increases apoptosis and alters p53
gene methylation in the full-term IUGR rat kidney.
American Journal of Physiology - Regulatory Integrative &
Comparative Physiology
285
962
970
43. Cubas
P
Vincent
C
Coen
E
1999
An epigenetic mutation responsible for natural variation in
floral symmetry.
Nature
401
157
161
44. Crain
PF
1990
Preparation and enzymatic hydrolysis of DNA and RNA for mass
spectrometry.
Methods in Enzymology
193
782
790
45. Kovacheva
VP
Mellott
TJ
Davison
JM
Wagner
N
Lopez-Coviella
I
2007
Gestational choline deficiency causes global and Igf2 gene DNA
hypermethylation by up-regulation of Dnmt1 expression.
Journal of Biological Chemistry
282
31777
31788
46. Ihaka
R
Gentleman
R
1996
R: A Language for Data Analysis and Graphics.
Journal of Computational and Graphical Statistics
5
299
314
47. Clark
SJ
Harrison
J
Paul
CL
Frommer
M
1994
High sensitivity mapping of methylated cytosines.
Nucleic Acids Research
22
2990
2997
48. Goecks
J
Nekrutenko
A
Taylor
J
Team
G
2010
Galaxy: a comprehensive approach for supporting accessible,
reproducible, and transparent computational research in the life
sciences.
Genome Biology
11
R86
49. Kent
WJ
Sugnet
CW
Furey
TS
Roskin
KM
Pringle
TH
2002
The human genome browser at UCSC.
Genome Research
12
996
1006
50. van Heeringen
SJ
Veenstra
GJ
2011
GimmeMotifs: a de novo motif prediction pipeline for
ChIP-sequencing experiments.
Bioinformatics
27
270
271
51. Frith
MC
Fu
Y
Yu
L
Chen
JF
Hansen
U
2004
Detection of functional DNA motifs via statistical
over-representation.
Nucleic Acids Research
32
1372
1381
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání