-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Coordination of fetal maturation with birth timing is essential for mammalian reproduction. In humans, preterm birth is a disorder of profound global health significance. The signals initiating parturition in humans have remained elusive, due to divergence in physiological mechanisms between humans and model organisms typically studied. Because of relatively large human head size and narrow birth canal cross-sectional area compared to other primates, we hypothesized that genes involved in parturition would display accelerated evolution along the human and/or higher primate phylogenetic lineages to decrease the length of gestation and promote delivery of a smaller fetus that transits the birth canal more readily. Further, we tested whether current variation in such accelerated genes contributes to preterm birth risk. Evidence from allometric scaling of gestational age suggests human gestation has been shortened relative to other primates. Consistent with our hypothesis, many genes involved in reproduction show human acceleration in their coding or adjacent noncoding regions. We screened >8,400 SNPs in 150 human accelerated genes in 165 Finnish preterm and 163 control mothers for association with preterm birth. In this cohort, the most significant association was in FSHR, and 8 of the 10 most significant SNPs were in this gene. Further evidence for association of a linkage disequilibrium block of SNPs in FSHR, rs11686474, rs11680730, rs12473870, and rs1247381 was found in African Americans. By considering human acceleration, we identified a novel gene that may be associated with preterm birth, FSHR. We anticipate other human accelerated genes will similarly be associated with preterm birth risk and elucidate essential pathways for human parturition.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001365
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001365Summary
Coordination of fetal maturation with birth timing is essential for mammalian reproduction. In humans, preterm birth is a disorder of profound global health significance. The signals initiating parturition in humans have remained elusive, due to divergence in physiological mechanisms between humans and model organisms typically studied. Because of relatively large human head size and narrow birth canal cross-sectional area compared to other primates, we hypothesized that genes involved in parturition would display accelerated evolution along the human and/or higher primate phylogenetic lineages to decrease the length of gestation and promote delivery of a smaller fetus that transits the birth canal more readily. Further, we tested whether current variation in such accelerated genes contributes to preterm birth risk. Evidence from allometric scaling of gestational age suggests human gestation has been shortened relative to other primates. Consistent with our hypothesis, many genes involved in reproduction show human acceleration in their coding or adjacent noncoding regions. We screened >8,400 SNPs in 150 human accelerated genes in 165 Finnish preterm and 163 control mothers for association with preterm birth. In this cohort, the most significant association was in FSHR, and 8 of the 10 most significant SNPs were in this gene. Further evidence for association of a linkage disequilibrium block of SNPs in FSHR, rs11686474, rs11680730, rs12473870, and rs1247381 was found in African Americans. By considering human acceleration, we identified a novel gene that may be associated with preterm birth, FSHR. We anticipate other human accelerated genes will similarly be associated with preterm birth risk and elucidate essential pathways for human parturition.
Introduction
Despite the important public health consequences of preterm birth [1], [2], determinants of human parturition remain largely uncharacterized. While some important physiological antecedents of labor have been identified in model organisms, such as progesterone withdrawal in rodents, such signals do not seem to precede human labor. Because humans are born developmentally less mature than other mammals [3], [4], birth timing mechanisms may differ between humans and model organisms that have been typically studied [5].
Evidence suggests that parturition has changed along the human lineage in response to other uniquely human adaptations. The dramatic increase in brain size, along with the human pelvis becoming narrower to facilitate bipedalism, places unique constraints on birth in humans compared even with evolutionarily close relatives such as Neanderthals and chimpanzees [6], [7]. Given the historically high mortality rate associated with pregnancy, these human traits may generate selective pressure to initiate parturition at a relatively earlier time in gestation compared to non-human primates to avoid cephalopelvic disproportion and arrested labor by delivery of a relatively smaller, less mature fetus. High rates of human versus non-human primate divergence in human pregnancy-related genes, such as genes in the reproduction Gene Ontology (GO) category [8], [9] as well as GO categories related to fetal development, including transcription factors [10], nuclear hormone receptors [10], transcriptional regulation [11] and development [9], support the notion that human gestation length has been altered to accommodate features unique to human pregnancy.
Genetic influences on birth timing in humans appear to be substantial, based on family and twin studies [12], [13], [14]. However, association studies using candidates selected from suspected pathways have not detected robust susceptibility variants for preterm birth. Genome-wide association studies (GWAS) are promising but will require large numbers of well-characterized subjects in order to overcome the challenge of multiple statistical comparisons. Here, we test the hypothesis that the set of genes accelerated on the human lineage will include genes that play important roles in regulating parturition and harbor variants that influence preterm birth risk. We identified and analyzed genes showing marked divergence between humans and other mammals, defined by relative nucleotide substitution rates in coding and highly conserved noncoding regions, for association with preterm birth. We find that genes with evidence of rate acceleration in humans may provide an informative group of candidates, and demonstrate that the human accelerated gene, follicle-stimulating hormone receptor (FSHR), may alter risk for preterm birth.
Results/Discussion
Life history traits
Because of large human head size and narrow birth canal cross-section compared to other primates [6], we hypothesized that genes involved in parturition have evolved rapidly along the human phylogenetic lineage to decrease the length of gestation and alleviate the complications arising from these constraints. We performed a comparative analysis of life history traits in mammals to further evaluate whether the relative gestational period in humans has decreased compared to other primates and mammals. Data acquired by Sacher and Staffeldt [15] and reanalyzed by us show that both adult and neonatal higher primates (simians) have higher brain to body weight ratios compared to other mammals (Figure 1A, 1B and Table S1 for list of species). The difference in brain/body size ratios in higher primates relative to other mammals makes it possible to ask whether gestation in higher primates is linked to brain size or body size. Higher primates and other mammals have equivalent gestational periods with respect to brain weight (Figure 1C). In contrast, the gestational period in higher primates is longer relative to the length of gestation in mammals with equivalent neonatal body weights (Figure 1D). This suggests that the length of gestation is expected to change with brain size but not body size.
Fig. 1. Allometric analysis of brain size, body size, and gestational length by linear regression. 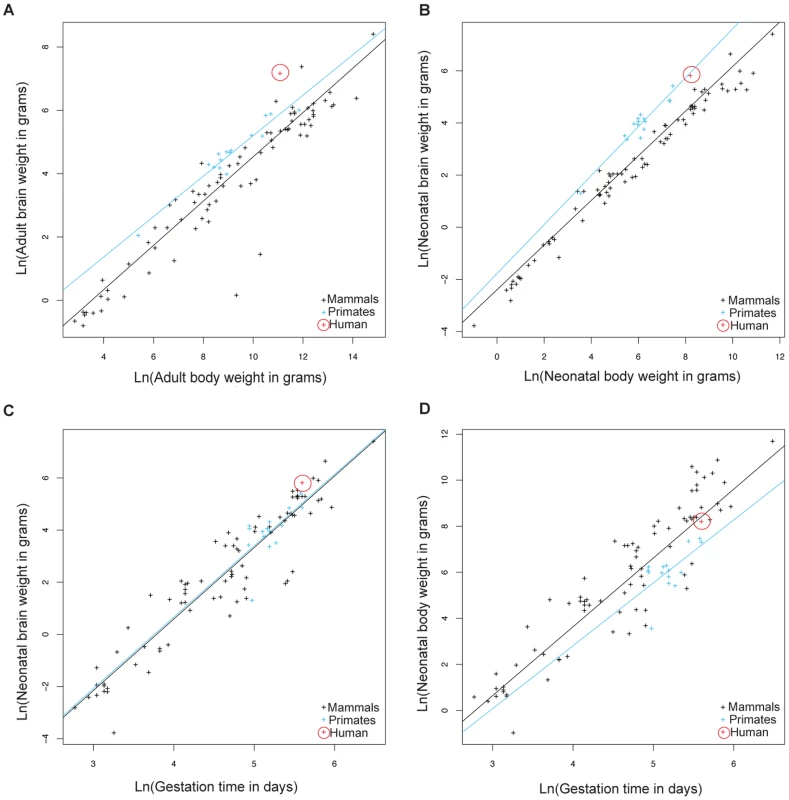
Brain to body weight ratios for adults (A) and neonates (B) are shown for humans (red), other higher primates (blue), and other mammals (black). The black line shows least squares fits to the 91 mammalian species. Neonatal brain (C) and body size (D) to gestational time ratios are displayed for the same species. The blue line shows least squares fits to 15 higher primate species. Allometric data was acquired by Sacher and Staffeldt (1974) [15]. Humans have evolved the highest adult brain to body weight ratio of any mammal [16]. In contrast to the evolution of brain/body ratios in higher primates, where both adult and neonatal ratios are increased relative to other mammals, the increase in the brain/body ratio in humans relative to other primates is present in adults but not neonates (Figure 1B). The simplest explanation is that human adult brain/body ratios have changed independently of neonatal ratios. However, the ratio of brain/body weight is highest at birth and declines until adulthood. Thus, an alternative explanation is that both adult and neonatal brain/body ratios have increased in humans, as in other higher primates, but that a concurrent decrease in the length of gestation lowered the neonatal brain/body ratio. This second possibility is supported by the relative immaturity of human neonates compared to other primates [3], [4] and that the length of human gestation, relative to either neonatal brain or body weight, is shorter than most other higher primates (Figure 1C, 1D).
To examine the evolution of gestation length relative to neonatal brain and body weight in primates we inferred the evolution of these characters across a phylogenetic tree. For both gestation-neonatal body ratio (Figure 2A) and gestation-neonatal brain ratio (Figure 2B) there is a consistent trend of a relatively shorter length of gestation on branches leading to humans. Of note, humans have the lowest gestation-neonatal body ratio (Figure 2A) or gestation-neonatal brain ratio (Figure 2B) of all the 20 primates evaluated. The gestation-neonatal brain ratio for humans is 69% that of gorilla and 45% that of chimpanzee. The gestation-neonatal body ratio of human is 49% that of gorilla and 50% that of chimpanzee.
Fig. 2. Phylogenetic analysis of brain size, body size, and gestational length in primates. 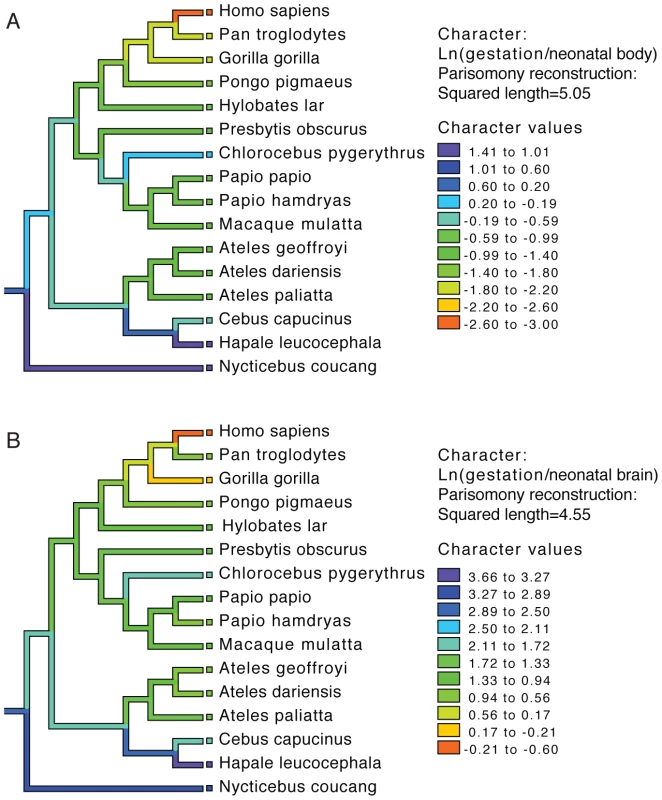
Gestational time to neonatal brain (A) and neonatal body size (B) natural logarithm-transformed ratios are shown for each species and color coded along each lineage as inferred by parsimony. Allometric data was acquired by Sacher and Staffeldt (1974) [15] and phylogeny by Purvis [41]. Accelerated gene evolution in the human lineage
In light of this evidence for human adaptation for birth timing, we examined whether genes involved in parturition would display accelerated protein evolution along the human lineage measured by an increased rate of amino acid altering to synonymous nucleotide substitutions (dN/dS; Figure S1). We found that, of 120 suggested candidate genes for preterm birth that were included in the ENSEMBL database, 7 showed statistically significant increased rate acceleration (i.e. increased dN/dS; p<0.05) along the human lineage in comparison to the other lineages. Table 1 shows these 7 genes plus 2 other genes significantly accelerated along the human-chimpanzee ancestor lineage (complete analysis of dN/dS provided in Dataset S1). Of these, common variants of PGR [17] and MMP8 [18] have previously been found to contribute to preterm birth risk. Using criterion agnostic to possible involvement with preterm birth, and measuring genome-wide changes, we identified 175 genes either accelerated along the human (40 genes) or on the human and human-chimpanzee ancestor lineages combined (135 genes) at a 5% false discovery rate (FDR) [19] from this analysis of protein-coding sequences.
Tab. 1. Sample of candidate genes showing coding region rate acceleration in humans. 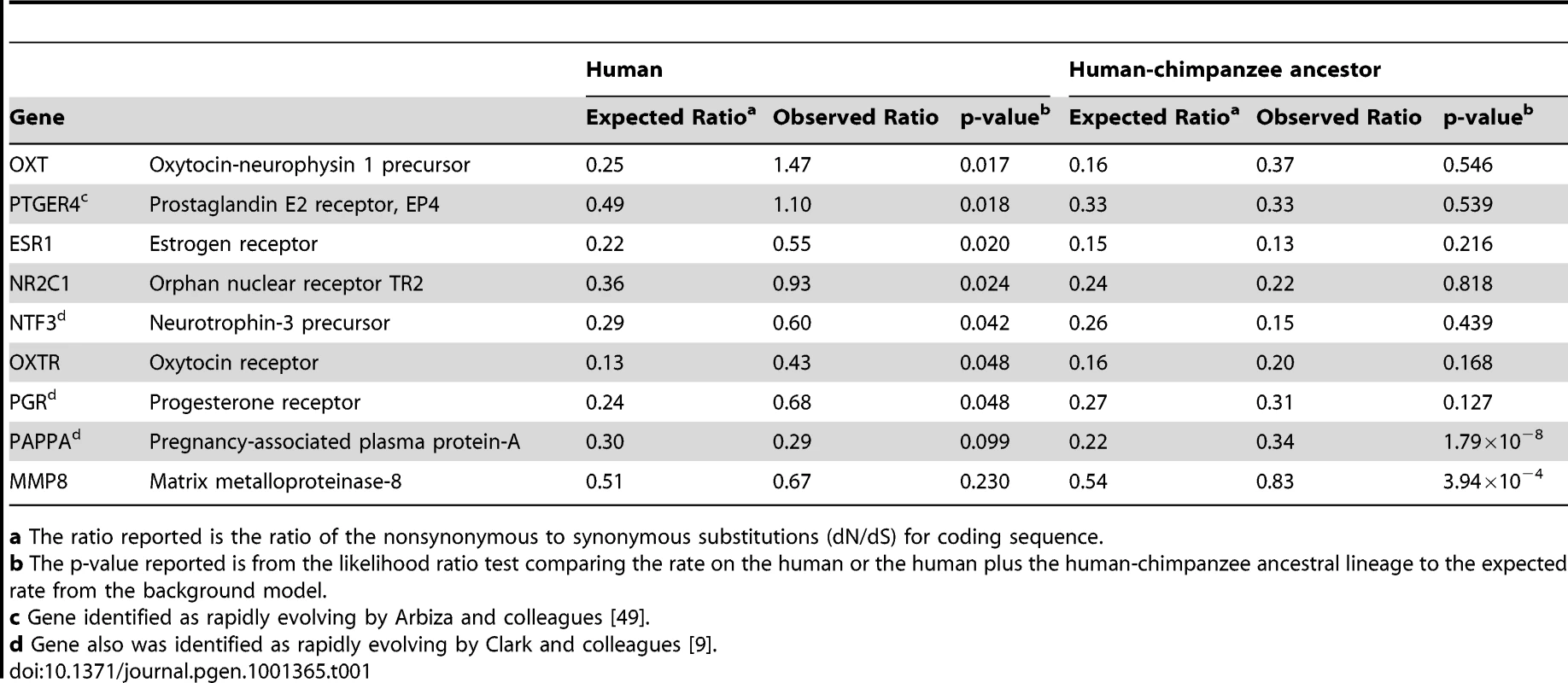
a The ratio reported is the ratio of the nonsynonymous to synonymous substitutions (dN/dS) for coding sequence. Motivated by this evidence of protein coding region evolution for genes involved in parturition and that acceleration has also been found to act on noncoding regions, we developed a method to identify human accelerated noncoding sequences [11], [20]. We identified a total of 401 elements significant along the human lineage and 2,103 elements significant along the human and human-chimpanzee ancestor lineages at a 5% FDR. To choose candidate genes, we calculated gene-wise p-values for each gene locus by assigning each conserved element to its nearest RefSeq gene [21] and a Fisher's combined p-value across the locus. This resulted in identification of a total of 279 candidate genes (complete analysis of human accelerated non-coding regions provided in Dataset S2). 150 of the genes identified as human accelerated in the protein-coding sequence and highly conserved noncoding elements screens, selected based on expression and functional information suggesting potential roles in parturition, were analyzed for association with preterm birth (Table S2).
Association analysis of human accelerated genes
Because recent data suggests that heritability of risk of preterm birth acts largely through the maternal genome [14], and the Finnish have low environmental risk and high genetic homogeneity compared to other populations, we genotyped Finnish (165 case, 163 control) mothers for 8,490 SNPs in the gene regions of our prioritized list of 150 human accelerated genes. The most significant finding was rs6741370 (p = 8.1×10−5) in the follicle-stimulating hormone (FSH) receptor gene (FSHR). 91 SNPs were significant at the p<0.01 level by allelic tests (Table S3). However, no SNPs were significant after correcting for 5,377 independent tests, considering relationships among markers, by the Bonferroni method (p<9.3×10−6). Of note, 8 of the 10 most statistically significant SNPs were located in FSHR. We identified FSHR as human accelerated in the noncoding analysis, with 40 changes in 4,218 bp of 17 conserved elements (human lineage p = 5.4×10−5, Dataset S2). Moreover, FSHR was revealed as rapidly evolving in a study of noncoding conserved elements by Prabhakar and colleagues [20], which otherwise had limited overlap with our gene list (see Methods). FSHR also harbors SNPs with extreme iHS values in the Yoruban population, reflecting extended haplotype homozygosity and suggesting a recent selective sweep [23]. Bird and colleagues [24] identified a region less than 1 megabase downstream of the FSHR gene boundaries as rapidly evolving in their study, further supporting human acceleration of the locus. Finally, because of being paralogous with other G-protein coupled receptors, such as the luteinizing hormone receptor, FSHR was excluded from our genome-wide coding region analysis. Therefore, we separately analyzed FSHR coding region acceleration along the human lineage. We found that the human-specific dN/dS was 1.41 which was significantly accelerated (p = 0.0045) in comparison to a constrained model for other primates and mammals using a 5 way multi-Z alignment in HYPHY where dN/dS was 0.174 over the entire tree (human, chimpanzee, rhesus, dog, mouse). The human-specific dN/dS for FSHR greater than 1 provides evidence for recent positive selection in addition to rate acceleration in humans. This information, together with the known importance of variation in human FSHR in subfertility [25], [26], a risk factor for preterm delivery independent of the use of assisted reproductive technologies [27], [28], and evidence suggesting its expression in uterus and cervix [29], [30], [31], motivated its specific study.
11 SNPs in FSHR showing potential association in the screening analysis (p<0.1) were genotyped in European American (147 preterm, 157 control), African American (79 cases, 171 controls) and Hispanic (Mexican) American (73 preterm, 292 control) mothers (Table 2 and Table S4). Several SNPs exhibited suggestive association (p<0.01) with preterm birth risk. Three SNPs in the African American mothers, rs11686474, rs11680730 and rs12473815, were significant after correcting for multiple testing (OR 1.63–1.82 (95% CI 1.11–1.21), 10 independent tests; p≤0.005). The allele frequency for this high linkage disequilibrium block differs considerably between HapMap CEU and YRI populations. To determine whether this association reflects a functional effect of local variation and not an artifact of population stratification with greater African ancestry in the case population relative to controls, we analyzed a limited set of ancestry informative markers using STRUCTURE. We found a small number of individuals (10, 3 cases and 7 controls) in our African American cohort that grouped more closely with the HapMap CEU cluster than the HapMap YRI cluster, though the relative distribution of these between cases and controls did not statistically differ from the relative sizes of the group. We performed a logistic analysis including the quantitative measure of CEU clustering as a covariate. The CEU cluster value was not significant in the model (p = 0.77), and adjusting for this in the regression model had little effect on statistical significance (e.g., unadjusted allelic p-value for rs12473815 = 0.0032, adjusted p = 0.0047). While we do not find evidence that population substructure confounds the association study in our African American cohort, we acknowledge that further study exploiting a larger number of subjects along with more dense ancestry markers will be needed for definitive conclusions to be drawn regarding association in this population. We did not find a statistically significant association in our European American or Hispanic cohorts for this LD block in FSHR, though risk trends for the minor allele (OR 1.08–1.38) were in the same direction as the Finnish and African American populations. This finding may reflect the limited sample size analyzed, or a specific role for variants in this LD block in the genetically isolated, homogeneous Finnish population and ancestrally distinct African American population.
Tab. 2. Demographic profile of study populations. 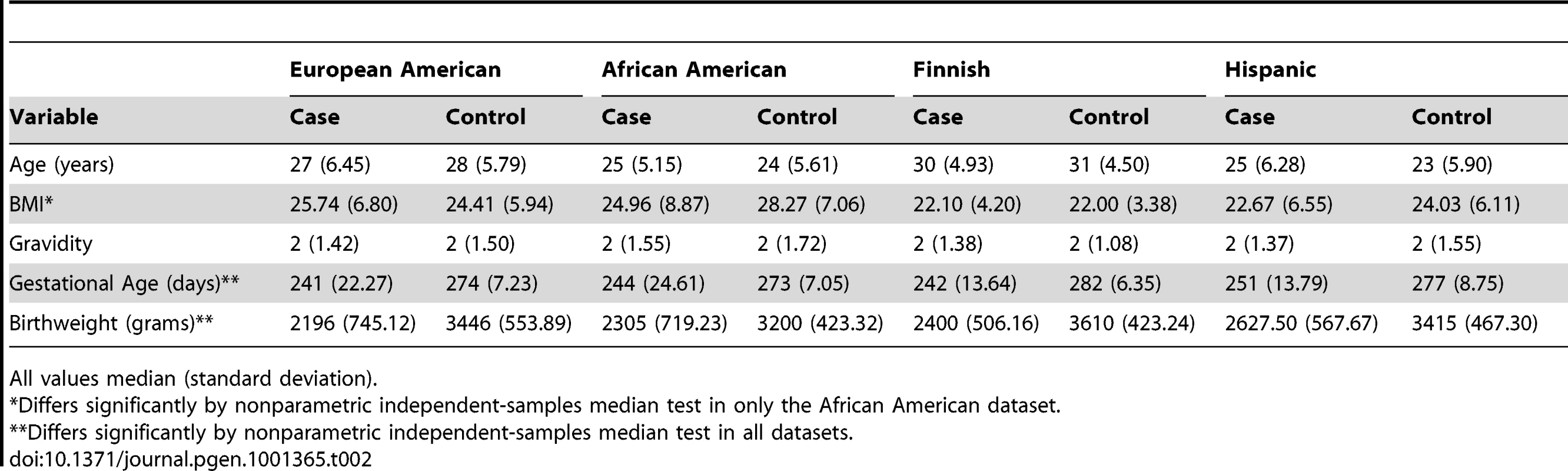
All values median (standard deviation). In FSHR, these 4 SNPs in high LD lie within intron 2 of FSHR (Figure 3) and show little LD with variants outside of this intron, based on available information from the International HapMap Project database [32]. Variants in this intron may tag yet uncharacterized variants in coding regions or nearby regulatory sequences. Alternatively, an intronic variant in FSHR may affect risk directly by altering functional sequences contained within the intron, such as microRNA binding sites, splice regulatory sites or transcription regulation sites. For instance, a variant in a splice enhancer site may change splicing patterns in favor of transcripts that promote preterm birth risk, as several alternatively spliced FSHR isoforms have been observed with altered function [33]. Further suggesting functional importance of this LD block, rs12473870 is significantly associated (p<0.0001) with altered expression of CCNJ, FURIN, DDR1, TBCD10A, and NAGA in quantitative trait databases for YRI populations (http://scan.bsd.uchicago.edu/newinterface/about.html). Risk-promoting variation in this gene may contribute to birth timing, rather than size at birth, based on additional tests examining gestational age or birth-weight Z-score as a quantitative trait, rather than preterm birth affection status (Table S5). Hence, FSHR may represent a novel gene involved in birth timing and preterm birth risk.
Fig. 3. Overview of the SNPs tested in the FSHR gene region. 
The gene structure for FSHR is represented by an arrow in which black rectangles designate 3′ and 5′ untranslated regions and dark grey rectangles designate coding exons. Diamonds represent SNPs on the Affymetrix SNP 6.0 array examined in the Finnish cohort. Triangles represent SNPs tested in the replication cohorts. A star indicates rs12473815, and the LD block that includes rs11686474 and rs11680730, which is significant after multiple testing correction in African Americans (p≤0.005). Circles represent conserved elements examined in the region. FSHR encodes the follicle-stimulating hormone (FSH) receptor. FSH is secreted from the pituitary and, in females, acts primarily on receptors in the ovaries to stimulate follicle development and synthesis of estrogens. Investigators also have observed FSHR protein and mRNA expression in nongonadal tissues, including uterus and cervix [29], [30], [31]. In these tissues, FSHR may mediate uterine relaxation, as suggested by FSH's ability to modify electrical signaling in the myometrium, independent of estrogen and progesterone [29]. Padmanabhan and colleagues [34] noted a progressive rise in bioactive serum FSH levels during pregnancy. Because high levels of FSH are known to downregulate FSHR expression [35], increasing levels of FSH may lead to gradual desensitization to the hormone and resultant increase in contractility as term approaches. Additionally, evidence from the FSHR haploinsufficient mouse [36] suggests that FSHR levels affect the relative abundance of progesterone receptor isoforms A (PR-A) and B (PR-B). Increased PR-A: PR-B ratios, occurring in human pregnancy normally near term and observed in FSHR haploinsufficient mice in non-pregnant states, are correlated with increased myometrium contractility. Hence, dysregulation of FSHR may contribute to early uterine contractility and promote preterm birth.
Aspects of our approach pose limitations on interpretation of this work. First, we assigned conserved elements to the nearest RefSeq gene to calculate gene-wise p-values; however, conserved elements may not be associated with the nearest gene per se, potentially affecting the accuracy of the estimate gene-wise p-values. Additionally, because we use adjacent genes to estimate expected synonymous and nonsynonymous rates for a given locus, human accelerated genes that are located physically nearby other genes undergoing human acceleration, such as gene families with multiple members in the same region, may miss detection. The variability in number of probes represented on the Affymetrix Genome-Wide Human SNP Array 6.0 within the gene regions of the 150 human accelerated genes tested poses another limitation. Although the coverage is adequate for most human accelerated genes, there are some genes with too few probes tested to support or refute their potential association with preterm birth; as a result, this study may have failed to detect association between preterm birth and human accelerated genes underrepresented on this genotyping array. Lastly, while precedence exists for intronic variants affecting protein structure and function [37], [38], additional study is needed to prove whether any of the SNPs associated with preterm birth in this work have a functional effect.
We find that human gestational length has been altered relative to other non-human primates and mammals. Using allometric scaling, we demonstrate that human gestation is shorter than predicted based upon gestational length in other mammalian species. By using comparative genomics to identify genes with an accelerated rate of change in humans, we identified a gene that shows evidence of association with preterm birth that otherwise would not have been revealed by current models of parturition physiology [39]. Moreover, our approach exploits a filter for relevant genes based upon rate of evolution in humans to more efficiently utilize currently available datasets for preterm birth, which are probably underpowered to detect variants of effect sizes reported in GWAS of other complex traits. Our approach represents an alternative method for a priori gene discovery in which fewer comparisons are made than in GWAS, thus potentially retaining more power to detect effect sizes typical for common variants. We provide evidence that FSHR, identified by these means, may alter risk for preterm birth. We anticipate that other human accelerated genes will similarly be associated with preterm birth risk and elucidate the essential pathways for human parturition.
Materials and Methods
Allometric analysis
Data acquired by Sacher and Staffeldt [15] was used to examine the relationships among brain size, body size and gestation length among mammalian species. Specifically, we compared logarithm-transformed values for these traits between human, primate and non-primate mammals, using linear regression implemented in R [40]. Additionally, we used allometric data from this paper and the primate phylogeny delineated by Purvis [41] to trace the evolution of gestation-neonatal body size ratio, and gestation-neonatal brain size ratio, using Mesquite [42]. Given a phylogenic tree, the Mesquite method uses parsimony to reconstruct the ancestral states by assuming a squared change for a continuous character from state x to state y is (x–y)2.
Coding sequence multiple sequence alignments
We obtained a set of 10,639 human gene predictions from the ENSEMBL database with one-to-one orthologs in the chimpanzee, macaque, mouse, rat, dog, and cow genomes (Release 46) [43]. We limited our analysis to only those proteins where the human, chimpanzee, macaque, and at least 75% of the mammalian genomes were present (Text S1). The list of 120 possible candidate genes for preterm birth assessed for dN/dS included those in the Institute of Medicine report [39], SPEED (pregnancy), GeneCards (parturition), and progesterone/prostaglandin metabolic pathways.
Noncoding sequence multiple sequence alignments
We obtained a set of highly conserved elements from UCSC Genome Browser [44] and tested 443,061 noncoding sequences with a conservation score > = 400. From the 17-way MultiZ alignments that are publicly available (downloaded March, 2007) [45], we extracted the human, chimpanzee, macaque, mouse, rat, dog and cow sequences (Text S1).
Likelihood ratio tests
We used the phylogeny ((Human, Chimpanzee), Macaque), ((Mouse, Rat), (Dog, Cow))). The evolutionary models were implemented in the HYPHY package [46] and we used the Q-value software [19] to establish statistical thresholds to achieve 5% false discovery rates (p-value distributions and pi_0 values in Figure S2).
Previous studies of both coding [9], [46] and noncoding [11], [21] sequences identify regions evolving under positive selection by a rate of evolution faster than a neutral rate. However, we felt that this criterion is too restrictive since some genes may have an increased rate of evolution along the human lineage relative to other mammals, but not increased above the neutral rate. To include genes with a significantly increased rate in humans compared to other mammals for testing in a population association study, we identify genes as human accelerated by testing whether omega along the human (or human+human-chimpanzee ancestor) lineage is significantly higher than omega along the non-human lineages (or non-human+non-human-chimpanzee ancestor). Here, omega is dN/dS-adj or dNC/dNC-adj, where dNC is the noncoding rate and dS-adj and dNC-adj are the adjacent synonymous rates from the 10 upstream and 10 downstream genes and the adjacent noncoding rates from 25 kb of conserved noncoding sequences, respectively. Thus, we test whether the data is more likely under a model with 1 omega value or 2 omega values (Figure S1). The coding sequence model used the MG94×HKY85 [47] model of codon evolution. The noncoding sequences model used an HKY85 model. For both tests, the alternative model has one additional degree of freedom and the significance of the change in likelihood was determined using chi-squared statistics. Both models use adjacent coding or conserved noncoding sequences to estimate the expectation for a given sequence that accounts for variable mutation rates across the genome and lineage-specific differences in effective population size, by allowing for branch-specific differences in selective constraint. Our list of human accelerated coding region gene list showed low overlap with previous studies that required for dN/dS>1 in their analyses (6% with Clark et al. [9], 0% Nielson et al. [48]) and more overlap with Arbiza et al. [49] (26%) which considered rate acceleration on the human lineage by methods more similar to ours than those used by [9], [48] (Figure S3). For human accelerated conserved noncoding elements in humans, 22% of the elements we identified were in common with Prabhakar et al. [20]. Considering unique genes associated with human accelerated conserved noncoding elements in humans, 11% of our genes also were identified by Prabhakar et al. [20], and 4% identified by Pollard et al. [11]. Similar to our study, 4% of unique genes in the Prabhakar study overlapped with those identified by Pollard et al. (Figure S4).
We calculated gene-wise p-values for each gene locus by assigning each conserved element to its nearest RefSeq gene [21] and a Fisher's combined p-value across the locus. Chi-squared analysis was used to determine the statistical significance of observed and expected genes with p<0.05 in suggested preterm birth candidate and overall human gene lists.
Candidate human accelerated gene list
To minimize the number of tests we would perform and thereby retain more power to detect small effects, we selected a subset of genes likely to be involved in parturition, based on expression and functional information, to use as candidate genes. Duplicated genes from a list developed by Bailey and colleagues [50] identified as pregnancy, fetal, placental or hormone-related genes were also included as candidates. A total of 150 of genes were used as candidate genes in subsequent analysis (Table S2).
Human subjects
Mothers of preterm or term infants were enrolled for genetic analysis by methods approved by Institutional Review Boards/Ethics Committees at each participating institution. Informed consent was obtained for all participants. Mothers with preterm birth were included if the birth was spontaneous (non-iatrogenic), singleton, had no obvious precipitating stimulus (trauma, infection, drug use), and was less the 37 weeks (Yale University; New York University) or 36 weeks (University of Helsinki; University of Oulu; Centennial Hospital, Nashville, TN) of completed gestation. DNA from blood or saliva was prepared by standard methods. Race/ethnicity was assigned by self-report. For the African American cohort, no differences in allele frequency were found in the distribution of 24 ancestry informative markers selected across the genome comparing cases and controls (all p>0.05 performing Chi square analysis between cases and controls; data not shown). All specimens were linked with demographic and medical data abstracted from maternal/neonatal records.
Genotyping
Initial genotyping of the Finnish cohort was performed using the Affymetrix Genome-Wide Human SNP Array 6.0. Genotypes were called from cell intensity data by the birdseed v2 algorithm, implemented in Affymetrix Genotyping Console 3.0. We selected SNPs represented on the array within the gene regions of candidate genes for analysis. SNPs examined in replication cohorts were genotyped using the Sequenom iPLEX massARRAY technology (Sequenom, San Diego, CA).
Data analysis
Data cleaning and analysis was performed with Whole-genome Association Study Pipeline (WASP) [51] and PLINK [52]. We excluded individuals in the Affymetrix Genome-Wide Human SNP Array 6.0 analysis based on genotyping quality (<95% call rate) and possible cryptic relatedness, and SNPs based on the following criteria: not in Hardy-Weinberg Equilibrium in controls (p<0.001 chi-squared test), <95% genotype call rate, minor allele frequency (MAF) <0.05, duplicate probes. Our primary analysis considered preterm birth affection status (i.e. delivery <36 weeks) as a binary trait, comparing allele and genotype frequencies between case and control groups by chi-squared test. We also examined gestational age and birth-weight Z-score as quantitative traits, standardized to normal distributions (μ = 0, σ = 1) using a Wald test to compare the mean phenotype between different allele or genotype classes. We corrected for multiple testing using the simpleM method [53], which estimates the number of independent tests, given the LD relationships among SNPs, used to adjust the significance level. Genetic ancestry in the African American population was inferred using STRUCTURE 2.3.1 [54] and the available ancestry informative markers that had been genotyped. Assuming K = 4 with the admixture function on and allowing 10,000 iterations and 10,000 burn-in cycles, genetic ancestry was determined for study samples using unrelated individuals from Hapmap Phase 3 (112 CEU, 113 YRI, and 48 ASW) as learning populations for STRUCTURE.
Supporting Information
Zdroje
1. EsplinMS
2006 Preterm birth: a review of genetic factors and future directions for genetic study. Obstet Gynecol Surv 61 800 806
2. GreenNS
DamusK
SimpsonJL
IamsJ
ReeceEA
2005 Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 193 626 635
3. BootheRG
DobsonV
TellerDY
1985 Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci 8 495 545
4. SmithBH
1989 Dental Development as a Measure of Life-History in Primates. Evolution 43 683 688
5. SmithR
2007 Parturition. N Engl J Med 356 271 283
6. RosenbergK
TrevathanW
2002 Birth, obstetrics and human evolution. BJOG 109 1199 1206
7. WeaverTD
HublinJJ
2009 Neandertal birth canal shape and the evolution of human childbirth. Proc Natl Acad Sci U S A 106 8151 8156
8. Chimpanzee Sequencing and Analysis Consortium 2005 Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437 69 87
9. ClarkAG
GlanowskiS
NielsenR
ThomasPD
KejariwalA
2003 Inferring nonneutral evolution from human-chimp-mouse orthologous gene trios. Science 302 1960 1963
10. BustamanteCD
Fledel-AlonA
WilliamsonS
NielsenR
HubiszMT
2005 Natural selection on protein-coding genes in the human genome. Nature 437 1153 1157
11. PollardKS
SalamaSR
LambertN
LambotMA
CoppensS
2006 An RNA gene expressed during cortical development evolved rapidly in humans. Nature 443 167 172
12. ChaudhariBP
PlunkettJ
RatajczakCK
ShenTT
DeFrancoEA
2008 The genetics of birth timing: insights into a fundamental component of human development. Clin Genet 74 493 501
13. PlunkettJ
BoreckiI
MorganT
StamilioD
MugliaLJ
2008 Population-based estimate of sibling risk for preterm birth, preterm premature rupture of membranes, placental abruption and pre-eclampsia. BMC Genet 9 44
14. PlunkettJ
FeitosaMF
TrusgnichM
WanglerMF
PalomarL
2009 Mother's genome or maternally-inherited genes acting in the fetus influence gestational age in familial preterm birth. Hum Hered 68 209 219
15. SacherGA
StaffeldtEF
1974 Relation of gestation time to brain weight for placental mammals: implications for the theory of vertebrate growth. The American Naturalist 108 593 615
16. WilcoxAJ
SkaervenR
LieRT
2008 Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol 167 474 479
17. EhnNL
CooperME
OrrK
ShiM
JohnsonMK
2007 Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res 62 630 635
18. WangH
ParryS
MaconesG
SammelMD
FerrandPE
2004 Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet 13 2659 2669
19. StoreyJD
TibshiraniR
2003 Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100 9440 9445
20. PrabhakarS
NoonanJP
PaaboS
RubinEM
2006 Accelerated evolution of conserved noncoding sequences in humans. Science 314 786
21. WheelerDL
BarrettT
BensonDA
BryantSH
CaneseK
2007 Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 35 D5 12
22. KistkaZA
DeFrancoEA
LigthartL
WillemsenG
PlunkettJ
2008 Heritability of parturition timing: an extended twin design analysis. Am J Obstet Gynecol 199 43 e41 45
23. VoightBF
KudaravalliS
WenX
PritchardJK
2006 A map of recent positive selection in the human genome. PLoS Biol 4 e72 doi:10.1371/journal.pbio.0040072
24. BirdCP
StrangerBE
LiuM
ThomasDJ
IngleCE
2007 Fast-evolving noncoding sequences in the human genome. Genome Biol 8 R118
25. LussianaC
GuaniB
MariC
RestagnoG
MassobrioM
2008 Mutations and polymorphisms of the FSH receptor (FSHR) gene: clinical implications in female fecundity and molecular biology of FSHR protein and gene. Obstet Gynecol Surv 63 785 795
26. MeduriG
BachelotA
CoccaMP
VasseurC
RodienP
2008 Molecular pathology of the FSH receptor: new insights into FSH physiology. Mol Cell Endocrinol 282 130 142
27. LudwigM
2009 Are adverse outcomes associated with assisted reproduction related to the technology or couples' subfertility? Nat Clin Pract Urol 6 8 9
28. RomundstadLB
RomundstadPR
SundeA
von DuringV
SkjaervenR
2008 Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet 372 737 743
29. HascalikS
CelikO
TaglukME
YildirimA
AydinNE
2009 Effects of highly purified urinary FSH and human menopausal FSH on Uterine Myoelectrical Dynamics. Mol Hum Reprod
30. MizrachiD
ShemeshM
1999 Follicle-stimulating hormone receptor and its messenger ribonucleic acid are present in the bovine cervix and can regulate cervical prostanoid synthesis. Biol Reprod 61 776 784
31. ShemeshM
MizrachiD
GurevichM
StramY
ShoreLS
2001 Functional importance of bovine myometrial and vascular LH receptors and cervical FSH receptors. Semin Reprod Med 19 87 96
32. FrazerKA
BallingerDG
CoxDR
HindsDA
StuveLL
2007 A second generation human haplotype map of over 3.1 million SNPs. Nature 449 851 861
33. LiY
GantaS
ChengC
CraigR
GantaRR
2007 FSH stimulates ovarian cancer cell growth by action on growth factor variant receptor. Mol Cell Endocrinol 267 26 37
34. PadmanabhanV
SonsteinJ
OltonPL
NippoldtT
MenonKM
1989 Serum bioactive follicle-stimulating hormone-like activity increases during pregnancy. J Clin Endocrinol Metab 69 968 977
35. SimoniM
GromollJ
NieschlagE
1997 The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18 739 773
36. DanilovichN
RoyI
SairamMR
2002 Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology 143 3618 3627
37. FaustinoNA
CooperTA
2003 Pre-mRNA splicing and human disease. Genes Dev 17 419 437
38. PaganiF
StuaniC
TzetisM
KanavakisE
EfthymiadouA
2003 New type of disease causing mutations: the example of the composite exonic regulatory elements of splicing in CFTR exon 12. Hum Mol Genet 12 1111 1120
39. Committee on Understanding Premature Birth and Assuring Healthy Outcomes 2006 Preterm Birth: Causes, Consequences, and Prevention;
BehrmanRE
ButlerAS
Washington D.C. The National Academies Press
40. R Development Core Team 2009 R: A language and environment for statistical computing: R Foundation for Stastical Computing
41. PurvisA
1995 A composite estimate of primate phylogeny. Philos Trans R Soc Lond B Biol Sci 348 405 421
42. MaddisonW
MaddisonD
2009 Mesquite: a modular system for evolutionary analysis: http://mesquiteproject.org. Accessed September 2010.
43. HubbardTJ
AkenBL
BealK
BallesterB
CaccamoM
2007 Ensembl 2007. Nucleic Acids Res 35 D610 617
44. KuhnRM
KarolchikD
ZweigAS
TrumbowerH
ThomasDJ
2007 The UCSC genome browser database: update 2007. Nucleic Acids Res 35 D668 673
45. BlanchetteM
KentWJ
RiemerC
ElnitskiL
SmitAF
2004 Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res 14 708 715
46. PondSL
FrostSD
MuseSV
2005 HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 676 679
47. MuseSV
GautBS
1994 A likelihood approach for comparing synonymous and nonsynonymous nucleotide substitution rates, with application to the chloroplast genome. Mol Biol Evol 11 715 724
48. NielsenR
BustamanteC
ClarkAG
GlanowskiS
SacktonTB
2005 A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol 3 e170 doi:10.1371/journal.pbio.0030170
49. ArbizaL
DopazoJ
DopazoH
2006 Positive selection, relaxation, and acceleration in the evolution of the human and chimp genome. PLoS Comput Biol 2 e38 doi:10.1371/journal.pcbi.0020038
50. BaileyJA
GuZ
ClarkRA
ReinertK
SamonteRV
2002 Recent segmental duplications in the human genome. Science 297 1003 1007
51. HaflerDA
CompstonA
SawcerS
LanderES
DalyMJ
2007 Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357 851 862
52. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
53. GaoX
BeckerLC
BeckerDM
StarmerJD
ProvinceMA
2009 Avoiding the high Bonferroni penalty in genome-wide association studies. Genet Epidemiol
54. PritchardJK
StephensM
DonnellyP
2000 Inference of population structure using multilocus genotype data. Genetics 155 945 959
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



