Is Development of a Vaccine against Feasible?
article has not abstract
Published in the journal:
. PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004843
Category:
Pearls
doi:
https://doi.org/10.1371/journal.ppat.1004843
Summary
article has not abstract
Introduction
Cryptococcus neoformans, the predominant etiological agent of cryptococcosis, is an encapsulated fungal pathogen that can cause fungal pneumonia and life-threatening infections of the central nervous system (CNS) [1]. C. neoformans can be found ubiquitously throughout the environment [1]. Inhalation of airborne yeast or desiccated basidiospores typically results in asymptomatic disease or dormant infections; however, progression towards clinical disease commonly occurs in persons with severely compromised immune responses. Global estimates suggest that 1 million cases of cryptococcal meningitis occur each year, resulting in approximately 625,000 deaths [2]. Morbidity and mortality rates due to cryptococcosis are significantly higher in resource-limited settings and in individuals with impaired CD4+ T cell-mediated immune responses (reviewed in [3–5]). Current therapies are often rendered ineffective because of the development of drug resistance by C. neoformans, drug toxicity, and treatment cost. Thus, a need remains for a cost-effective approach to prevent cryptococcosis.
Is Developing a Vaccine against C. neoformans a Wise Thing to Do?
Simply restoring immune function in immune-compromised individuals has resulted in a decline in cryptococcosis; however, Cryptococcus-related immune reconstitution inflammatory syndrome (IRIS), which is also life threatening, is observed in a significant percentage of HIV+ individuals receiving highly active antiretroviral therapy (HAART) and in solid organ transplant recipients following administration of antifungal drugs and a reduction in immune-suppressive therapy [6]. The need for prophylactic measures to prevent cryptococcosis among immune-compromised persons is clearly evident. However, considering that the very arm of the immune response tasked with defending against C. neoformans is absent in the majority of individuals at the highest risk for developing cryptococcosis leads to questions regarding the feasibility of developing a vaccine that is effective in immunocompromised patients. Also, will the protective immune response generated by an anticryptococcal vaccine have the unintended consequence of predisposing the vaccinated population to Cryptococcus-related IRIS, thus augmenting host damage?
Can Protection Be Achieved in Immune-Compromised Patients?
Clinical and experimental evidence suggests that protective immunity against cryptococcosis is dependent upon Th1-type CD4+ T cell-mediated immune responses (reviewed in [5]). Th1-type CD4+ T cells orchestrate protective immune responses against C. neoformans through the generation of a Th1-type cytokine profile characterized by the production of interleukin (IL)-2, IL-12, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, which induce lymphocyte and phagocyte recruitment to the site of infection and increase phagocyte uptake and killing of C. neoformans. Consequently, it may seem counterintuitive to suggest that development of an effective anticryptococcal vaccine that (1) confers protection in persons with low CD4+ T cell counts (i.e., HIV+ patients) and (2) induces protection that endures during the subsequent development of immune suppression is feasible.
Antibody-mediated immunity (AMI) has long been considered an obvious target mechanism for inducing vaccine-mediated protection against cryptococcosis in patients with suppressed cell-mediated immunity. HIV is associated with B cell defects that have been linked to increased susceptibility to cryptococcosis [7]. Studies focusing on AMI to cryptococcosis have provided promising results. Monoclonal antibodies (mAb) against the C. neoformans capsular polysaccharide glucuronoxylomannan (GXM) are capable of reducing organ cryptococcal burden and prolonging survival in mice [8]. Furthermore, protective antibodies against C. neoformans are able to aid in phagocytosis, modulate the inflammatory response, and alter gene expression of the yeast, rendering it more susceptible to antifungal drugs [7]. This demonstrates potential for antibodies as effective treatment options against cryptococcosis; however, more study is needed to evaluate the efficacy of antibodies to control cryptococcosis in immunocompromised hosts.
Previous studies by Huffnagle et al. have shown that CD8+ T cells may compensate for the loss of CD4+ T cells to facilitate protection against cryptococcosis in an IFN-γ-dependent manner [9]. Similarly, immunization with a C. neoformans strain genetically engineered to produce IFN-γ, designated H99γ [10], can induce protective immunity against cryptococcosis in mice depleted of CD4+ T cells [11]. Immune-competent mice immunized with C. neoformans H99γ and subsequently rendered both CD4+ and CD8+ T cell deficient (>98% depletion of each population) prior to and during challenge with wild-type (WT) C. neoformans were completely protected, as evidenced by 100% survival and sterilizing immunity [10,11]. Protective immunity has been observed up to 100 days postimmunization with the IFN-γ-producing strain [12]. These studies provided proof of concept that vaccines designed to combat C. neoformans infections are capable of inducing potent, long-lasting anticryptococcal immunity in immune-compromised patients.
Is the Phagocyte’s (Macrophage or Dendritic Cell) Activation Status Critical for Protection against C. neoformans?
Since inhalation is the principle route for entry of C. neoformans propagules, resident pulmonary macrophages and dendritic cells (DCs) are well situated to contain the pathogen and prevent dissemination. Macrophages, and perhaps DCs, are capable of polarizing toward a fungicidal, classically (M1) activated phenotype or a cryptococcal growth-permissive, alternatively (M2) activated phenotype, depending on the cytokine milieu (Fig 1) (reviewed in [5,13]). Pulmonary infection with C. neoformans in mice typically induces Th2-type cytokine responses and M2 macrophage activation, resulting in uncontrolled fungal growth, dissemination, and disease exacerbation [14,15]. In stark contrast, pulmonary inoculation with C. neoformans H99γ results in Th1-type and IL-17A cytokine responses, M1 macrophage activation, and resolution of disease [16,17]. Additionally, mice protectively immunized with C. neoformans strain H99γ and subsequently challenged with WT C. neoformans develop an M1 macrophage activation phenotype with enhanced fungistasis and nitric oxide (NO) production associated with enhanced signal transducer and activator of transcription 1 (STAT1) signaling [18].
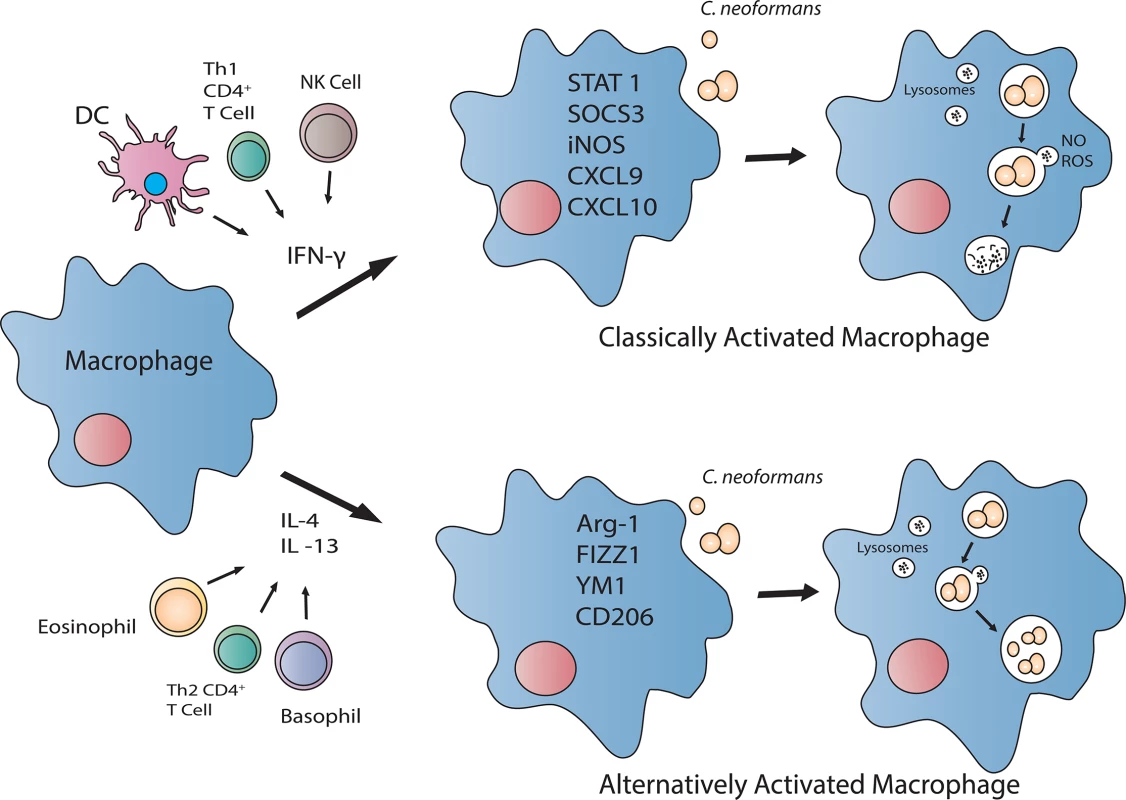
During inoculation with C. neoformans strain H99γ, STAT1-/- mice show a decrease in M1 and increase in M2 macrophage activation markers and uncontrolled intramacrophage proliferation of the yeast, correlating with increased pulmonary and central nervous system (CNS) fungal burden and 90% mortality [19]. Furthermore, the increased intramacrophage cryptococcal growth in STAT1-/- mice coincided with decreased NO production [19]. These studies indicate that NO production by M1 macrophages is critical for their anticryptococcal activity.
DCs phagocytose and kill C. neoformans by oxidative and nonoxidative methods and subsequently present antigen to T cells to help guide the adaptive response [20–23] via the production of cytokines and chemokines that induce cellular infiltration and the production of antimicrobial peptides and inflammatory mediators (reviewed in [24,25]). Recognition of cryptococcal CpG DNA by toll-like receptor (TLR) 9, for example, results in the production of IL-12p40 and co-stimulatory molecule CD40 in murine DCs [26]. Similar to the activation phenotype observed with macrophages, DCs exhibit characteristics akin to an inflammatory (DC1) or alternative (DC2) activation phenotype [27,28]. Specifically, stimulation of DCs with IL-4 induces expression of multiple alternative activation markers both in vivo and in vitro, albeit with a different expression pattern compared to that of macrophages [28]. Concurrent stimulation of DCs with IL-4 and TLR ligands including lipopolysaccharide (LPS) and CpG DNA results in boosted IL-12p70 and inhibited IL-10, resistin-like molecule α (RELMα), and chitinase3-like 3 (Chil3 or YM1) production [28]. The ability of DCs to respond to antigen by producing cytokines and chemokines that drive protective immune responses makes the targeting of these innate cells an attractive option for antifungal vaccine design. We postulate that the efficacy for a vaccine to enhance anticryptococcal activity of innate cell populations, particularly when T cell numbers are diminished, will depend upon the cytokines/chemokines produced by DCs and their subsequent influence on effector cell responses.
Could Innate Cells “Trained” to Provide Protection against Cryptococcosis Be a Potential Vaccine Strategy?
Studies by Netea et al. showed that an initial exposure of monocytes/macrophages to a microbe or antigen enhances their innate immune responses against restimulation, a concept termed “trained immunity” [29,30]. Altogether, the studies indicate that monocytes and macrophages exhibit memory-like responses following exposure to the fungal cell wall component β-glucan and the enhanced responses are associated with changes in the epigenetic programming of the monocytes [31]. These findings suggest that therapies targeting monocytes and/or macrophages could potentially provide protective immunity, even in immune-compromised patients; however, the duration of these “trained” effects beyond 4–8 weeks remains to be assessed. Immune-based therapies and/or vaccines designed to augment macrophage responses against C. neoformans are plausible strategies considering the critical role of STAT1-mediated M1 macrophage activation in mediating protection. Recent studies demonstrate that macrophages polarized with IL-4 to an M2 phenotype can be repolarized to a functional M1 phenotype upon exposure to IFN-γ [32], demonstrating the plasticity of macrophage activation and their susceptibility to therapeutic manipulation. In theory, DCs “trained” to polarize towards an inflammatory phenotype are prime candidates to sustain a Th1-type cytokine environment that is critical for maintenance of an M1 macrophage activation phenotype in T cell-deficient hosts. However, will the induction of vaccine-mediated immune responses in immune-deficient hosts predispose these individuals to Cryptococcus-related IRIS? This may be best tested in an experimental model system by looking for signs of IRIS following immunization of a T cell-depleted host and subsequently allowing the host to reconstitute their T cell populations during the recall response to C. neoformans. In human patients, sensitive tests designed to detect cryptococcal antigen could be used to screen asymptomatic individuals in order to reduce the risk of potentially provoking cryptococcal-induced IRIS following reconstitution of the immune system. The absence of IRIS will support the efficacy and safety of vaccine strategies to mediate protection in immune-compromised hosts. In contrast, any observation of IRIS may provide a model system by which to test therapies designed to ameliorate complications due to IRIS in patients.
Is a Vaccine against Cryptococcosis Feasible?
The discussion and evidence provided above suggest that a vaccine is possible; however, specific considerations must be made when designing immunotherapeutics. Patients lacking intact T cell responses will undoubtedly suffer from reduced memory T cell responses, rendering common vaccine strategies ineffectual. Thus, novel therapies that target innate immune cells such as macrophages and DCs have the potential to mediate protective immunity against C. neoformans. Recent studies indicate that these phagocytes are capable of trained immunity exhibiting enhanced protective responses upon secondary exposure. However, it is not yet known how long-lasting these “trained” effects are and if this approach to vaccine design is plausible. Nonetheless, protective immunity has been observed in the absence of traditional adaptive immunity, thus providing proof of concept that vaccines targeting innate immune responses may prove effectual in prevention or treatment of cryptococcosis.
Zdroje
1. Kwon-Chung KJ, Fraser JA, Doering TL, Wang Z, Janbon G, Idnurm A, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harbor perspectives in medicine. 2014;4(7):a019760. doi: 10.1101/cshperspect.a019760 24985132
2. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525–30. Epub 2009/02/03. doi: 10.1097/QAD.0b013e328322ffac 19182676
3. Hole CR, Wormley FL Jr. Vaccine and immunotherapeutic approaches for the prevention of cryptococcosis: lessons learned from animal models. Front Microbiol. 2012;3:291. doi: 10.3389/fmicb.2012.00291 22973262
4. Chaturvedi AK, Wormley FL Jr. Cryptococcus antigens and immune responses: implications for a vaccine. Expert review of vaccines. 2013;12(11):1261–72. doi: 10.1586/14760584.2013.840094 24156284
5. Olszewski MA, Zhang Y, Huffnagle GB. Mechanisms of cryptococcal virulence and persistence. Future microbiology. 2010;5(8):1269–88. doi: 10.2217/fmb.10.93 20722603
6. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7(6):395–401. 17521592
7. Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell host & microbe. 2012;11(5):447–56.
8. Datta K, Subramaniam K. Host Defense Against Cryptococcal Disease: Is there a Role for B cells and Antibody-Mediated Immunity. Current fungal infection reports. 2014;8:287–95.
9. Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. Journal of immunology. 2005;174(12):7920–8. 15944298
10. Wormley FL Jr., Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75(3):1453–62. 17210668
11. Wozniak KL, Young ML, Wormley FL Jr. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clin Vaccine Immunol. 2011;18(5):717–23. doi: 10.1128/CVI.00036-11 21450975
12. Wozniak KL, Levitz SM. Isolation and purification of antigenic components of Cryptococcus. Methods Mol Biol. 2009;470:71–83. doi: 10.1007/978-1-59745-204-5_7 19089377
13. Leopold Wager CM, Wormley FL. Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014;7(5):1023–35. doi: 10.1038/mi.2014.65 25073676
14. Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. Journal of immunology. 2005;174(10):6346–56. 15879135
15. Muller U, Stenzel W, Kohler G, Werner C, Polte T, Hansen G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. Journal of immunology. 2007;179(8):5367–77. 17911623
16. Hardison SE, Ravi S, Wozniak KL, Young ML, Olszewski MA, Wormley FL Jr. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am J Pathol. 2010;176(2):774–85. doi: 10.2353/ajpath.2010.090634 20056835
17. Hardison SE, Wozniak KL, Kolls JK, Wormley FL Jr. Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun. 2010;78(12):5341–51. doi: 10.1128/IAI.00845-10 20921149
18. Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL Jr. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. Journal of immunology. 2012;189(8):4060–8. doi: 10.4049/jimmunol.1103455 22984078
19. Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Wormley FL Jr. STAT1 Signaling Is Essential for Protection against Cryptococcus neoformans Infection in Mice. Journal of immunology. 2014;193(8):4060–71. doi: 10.4049/jimmunol.1400318 25200956
20. Hole CR, Bui H, Wormley FL Jr., Wozniak KL. Mechanisms of dendritic cell lysosomal killing of Cryptococcus. Scientific reports. 2012;2:739. doi: 10.1038/srep00739 23074646
21. Kelly RM, Chen J, Yauch LE, Levitz SM. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect Immun. 2005;73(1):592–8. 15618199
22. Wozniak KL, Vyas JM, Levitz SM. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect Immun. 2006;74(7):3817–24. 16790753
23. Wozniak KL, Levitz SM. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun. 2008;76(10):4764–71. doi: 10.1128/IAI.00660-08 18678670
24. Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harbor perspectives in biology. 2012;4(8):a007401. doi: 10.1101/cshperspect.a007401 22855722
25. Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host-pathogen interface. Nature immunology. 2006;7(2):117–20. 16424884
26. Nakamura K, Miyazato A, Xiao G, Hatta M, Inden K, Aoyagi T, et al. Deoxynucleic acids from Cryptococcus neoformans activate myeloid dendritic cells via a TLR9-dependent pathway. Journal of immunology. 2008;180(6):4067–74. 18322216
27. Weitnauer M, Schmidt L, Ng Kuet Leong N, Muenchau S, Lasitschka F, Eckstein V, et al. Bronchial epithelial cells induce alternatively activated dendritic cells dependent on glucocorticoid receptor signaling. Journal of immunology. 2014;193(3):1475–84. doi: 10.4049/jimmunol.1400446 24965772
28. Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):9977–82. doi: 10.1073/pnas.1121231109 22660926
29. Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell host & microbe. 2011;9(5):355–61.
30. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17537–42. doi: 10.1073/pnas.1202870109 22988082
31. Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell host & microbe. 2012;12(2):223–32.
32. Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4(3):e00264–13. doi: 10.1128/mBio.00264-13 23781069
Štítky
Hygiena a epidemiologie Infekční lékařství LaboratořČlánek vyšel v časopise
PLOS Pathogens
2015 Číslo 6
- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
Nejčtenější v tomto čísle
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- A 21st Century Perspective of Poliovirus Replication
- Battling Phages: How Bacteria Defend against Viral Attack
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
