-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
Pathogenic bacteria have to resist host immune response and MgtC is used by several intracellular pathogens to promote bacterial multiplication inside macrophages. Here we investigated MgtC’s role in the virulence of an extracellular pathogen, Pseudomonas aeruginosa. A P. aeruginosa mgtC mutant is attenuated in zebrafish embryos, but only in the presence of macrophages. Moreover, this mutant is more rapidly killed by macrophages than the wild-type strain. Both phenotypes can be mimicked upon production of a MgtC antagonistic peptide in wild-type Pseudomonas strain. MgtC thus provides a singular example of a virulence determinant that promotes strategies to subvert the antimicrobial behavior of macrophages, in both intracellular and extracellular pathogens and our results support an intramacrophage stage during in P. aeruginosa acute infection, as well as an interplay between MgtC role and phagosome acidification. In addition, P. aeruginosa MgtC is required for growth in Mg2+ deprived medium, a property shared by MgtC factors from intracellular pathogens, and limits biofilm formation. MgtC may share a similar function in intracellular and extracellular pathogens, with an outcome adapted to the different bacterial lifestyles
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004969
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004969Summary
Pathogenic bacteria have to resist host immune response and MgtC is used by several intracellular pathogens to promote bacterial multiplication inside macrophages. Here we investigated MgtC’s role in the virulence of an extracellular pathogen, Pseudomonas aeruginosa. A P. aeruginosa mgtC mutant is attenuated in zebrafish embryos, but only in the presence of macrophages. Moreover, this mutant is more rapidly killed by macrophages than the wild-type strain. Both phenotypes can be mimicked upon production of a MgtC antagonistic peptide in wild-type Pseudomonas strain. MgtC thus provides a singular example of a virulence determinant that promotes strategies to subvert the antimicrobial behavior of macrophages, in both intracellular and extracellular pathogens and our results support an intramacrophage stage during in P. aeruginosa acute infection, as well as an interplay between MgtC role and phagosome acidification. In addition, P. aeruginosa MgtC is required for growth in Mg2+ deprived medium, a property shared by MgtC factors from intracellular pathogens, and limits biofilm formation. MgtC may share a similar function in intracellular and extracellular pathogens, with an outcome adapted to the different bacterial lifestyles
Introduction
Pathogenic bacteria have developed numerous strategies to adapt to host environment and common strategies can be used by pathogens that share similar lifestyle or similar environmental niches. MgtC is a virulence factor common to several intracellular pathogens [1]. It was first described in Salmonella enterica serovar Typhimurium (S. Typhimurium) as required for intramacrophage multiplication and systemic infection in mice [2–4]. MgtC appears as a singular factor that promotes pathogenicity by inhibiting the Salmonella's own F1Fo ATP synthase [5]. MgtC has been shown to directly interact with F1Fo ATP synthase, thereby altering its ability to translocate protons and to couple translocation to ATP synthesis. Salmonella mgtC is highly regulated both at the transcriptional and post-transcriptional levels. This regulation includes a positive regulation by Mg2+ deprivation [6] and by an increase in cytosolic ATP [7] as well as a negative regulation by the MgtR peptide [8]. MgtC was also described as a critical factor for the intramacrophage growth of Mycobacterium tuberculosis, Brucella suis, Yersinia pestis, Burkholderia cenocepacia and Salmonella enterica serovar Typhi, being a virulence factor in a mouse model for M. tuberculosis and B. cenocepacia [9–13]. More recently, a contribution for MgtC in phagocytosis has been uncovered in Mycobacterium marinum [14]. In addition, MgtC has been involved in adaptation to low Mg2+ environments in these many pathogens [1]. However, the role of MgtC in low Mg2+ environments can be dissociated from its role in macrophages, suggesting that MgtC has a dual function [15].
Genes encoding MgtC-like proteins are found in a limited number of eubacterial genomes and phylogenetic analysis suggested that mgtC has been acquired by horizontal gene transfer repeatedly throughout bacterial evolution [16]. Alignment of MgtC-like proteins as well as hydrophobicity pattern clearly define two domains: an hydrophobic N-terminal part, highly conserved in all MgtC-like proteins, and a soluble C-terminal part that is much more variable, but specifically conserved in the subgroup of MgtC proteins from intracellular pathogens [16]. Noticeably, this phylogenetic subgroup also contains two MgtC-like proteins, PA4635 and PA2558, from Pseudomonas aeruginosa [16], suggesting a potential common function with proteins from intracellular pathogens. Because of this phylogenetic clustering, we have previously investigated whether the P. aeruginosa mgtC-like genes can complement a S. Typhimurium ΔmgtC strain [15]. PA4635 fully complemented the Salmonella mgtC mutant for growth in low Mg2+ medium but not in macrophages. On the other hand, PA2558 failed to complement the Salmonella ΔmgtC mutant for growth both in low Mg2+ medium and in macrophages. In addition, amino-acid sequence alignment of MgtC-like proteins from Salmonella phylogenetic subgroup indicated that some conserved residues important for Salmonella MgtC function are not conserved in PA2558 [15]. Taken together, these results suggested that only PA4635 shares functional properties with the Salmonella MgtC and is herein referred as P. aeruginosa MgtC.
In the present study, we investigated for the first time the role of MgtC in an extracellular pathogen, P. aeruginosa. The environmental bacterium and opportunistic human pathogen P. aeruginosa is a major cause of mortality in cystic fibrosis (CF) patients. Interestingly, MgtC has been highlighted as a horizontally-acquired gene shared by several opportunistic bacteria infecting CF patients [17]. P. aeruginosa virulence and resistance to treatment is largely due to its ability to form biofilms, whose stability can be affected by extracellular cations [18,19]. P. aeruginosa is known to impair host phagocytic functions in chronic lung infections of CF patients. However, macrophages can play a protective role against P. aeruginosa infection in a systemic model of infection in zebrafish embryos, where P. aeruginosa has been shown to be phagocytosed by macrophages [20]. In addition, despite the fact that P. aeruginosa is an extracellular pathogen, an intracellular step in airway epithelial cells might occur before the formation of biofilm during the acute phase of infection [21,22]. In the present study, we have constructed a P. aeruginosa mgtC mutant and have analysed its infection phenotype in animal and cellular models, as well as biofilm formation. We also investigated mgtC gene expression in vitro and in cellulo. In addition, MgtR, a peptide that has been proposed as a MgtC antagonist in Salmonella [8] has been heterologously produced in a wild-type P. aeruginosa strain (P. aeruginosa does not encode a MgtR homologue). We establish that MgtC contributes to Pseudomonas acute infection and resistance to macrophage killing, thus being a factor that subverts the antimicrobial behavior of macrophages both in intracellular and extracellular pathogens.
Results
Phylogenetic analysis of MgtC-like proteins from Pseudomonas species
The genome of P. aeruginosa strain PAO1 encodes two MgtC-like proteins, PA4635 and PA2558, which both belong to the same phylogenetic subgroup as Salmonella MgtC [16]. In Fig 1A, we provided a phylogenetic analysis of this subgroup that focused on proteins from bacterial pathogens able to replicate in macrophages for which MgtC role in virulence has been studied, as well as proteins from opportunistic bacteria infecting CF patients.
Fig. 1. Phylogenetic analysis of Pseudomonas MgtC-like proteins. 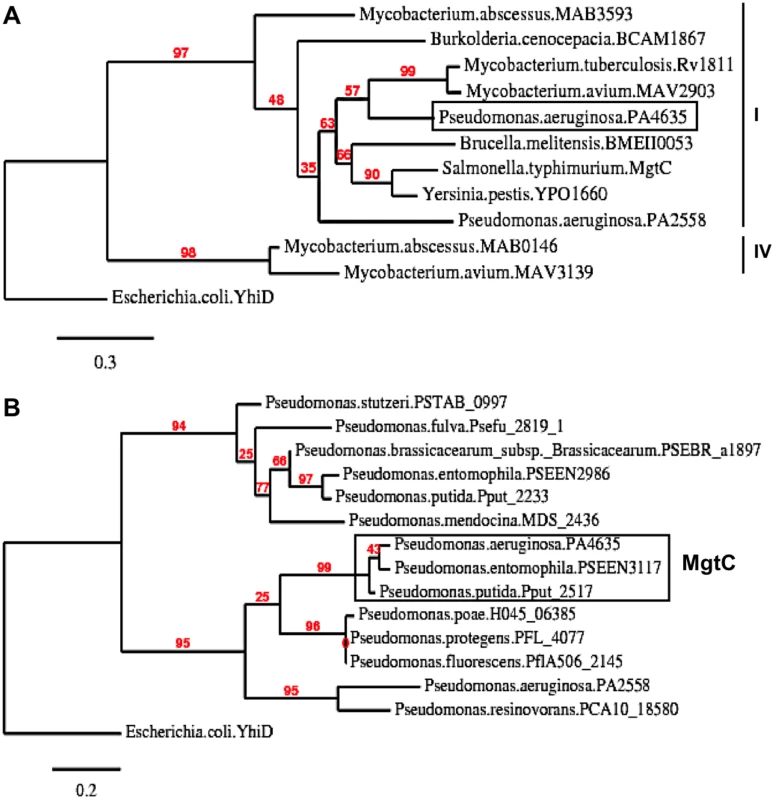
(A) Phylogenetic tree of MgtC-like proteins from bacterial pathogens that survive in macrophages for which MgtC role in virulence has been studied (S. Typhimurium, M. tuberculosis, B. suis, B. cenocepacia, Y. pestis) and opportunist bacterial pathogens found in CF patients (P. aeruginosa, B. cenocepacia, M. avium, M. abscessus). Several bacteria, including P. aeruginosa, encode two MgtC-like proteins. Phylogenetic analyses were carried out with the “Phylogeny.fr” web server (http://www.phylogeny.fr) [57] using MUSCLE, PhyML and TreeDyn softwares. Numeration of phylogenetic groups follows a previous analysis [58]. Escherichia coli YhiD is used as a distantly related MgtC-like protein [16] to root the tree. (B) Phylogenetic tree of MgtC-like sequences recovered from Pseudomonas genomes. MgtC-like protein sequences were recovered from BlastP analysis using PA4635 sequence on completed genomes from the Pseudomonas genome database (http://v2.pseudomonas.com/) [23]. PA4635 is conserved (>99% identity) in the 10 P. aeruginosa genomes other than PAO1 found in the Pseudomonas database, including the divergent strain PA7. Besides P. aeruginosa genomes, MgtC is found in P. entomophila and P. putida (>85% identity with PA4635). Other genomes harbors MgtC-like proteins that differ from PA4635 (<40% identity with PA4635). As mgtC sequences may have been acquired by horizontal gene transfer [16], we investigated the distribution of mgtC coding sequence in Pseudomonas genomes and performed phylogenetic analysis. MgtC appeared to be conserved in all P. aeruginosa strains present in the Pseudomonas genome database [23,24], including the atypical PA7 strain [24]. In addition, MgtC was found in the insect pathogen Pseudomonas entomophila as well as the closely related Pseudomonas putida (> 85% identity with PA4635, Fig 1B). In contrast, other Pseudomonas species harbor MgtC-like proteins that largely differ from PA4635 (below 40% identity, Fig 1B) or do not harbor any MgtC-like protein (Pseudomonas syringae). This analysis indicates a strong association of MgtC with Pseudomonas species that are pathogenic for humans and insects, suggesting a putative role during animal/human host infection.
To address the role of MgtC in P. aeruginosa, we constructed an in-frame deletion in mgtC (PA4635) to avoid any polar effect on the expression of the downstream PA4636 gene (S1 Fig). The resulting ΔmgtC mutation was complemented by a single copy of the mgtC gene with its own promoter inserted into the unique attB site in strain PAO1 (S1 text).
MgtC is important for P. aeruginosa virulence in the Danio rerio infection model in a macrophage-dependent manner
To evaluate the role of MgtC in P. aeruginosa virulence, we used the zebrafish (Danio rerio) embryo model. This model, which has been used for various intracellular and extracellular bacterial pathogens, is a model of choice to investigate the contribution of cells from the innate immune system during infection [25]. The Danio has been successfully used to monitor the role of P. aeruginosa determinants in virulence based on survival curves of larvae infected with mutants deficient in type III secretion system (T3SS) or Quorum Sensing [20,26]. Bacteria producing a fluorescent protein were injected intravenously in the caudal vein of embryos (Fig 2A) at 30 hours post-fertilization, a time where macrophages, but not neutrophils, are fully functional and capable of engulfing invading bacteria [27]. The survival curves of infected embryos indicated that MgtC is a critical virulence determinant in this model since the mgtC mutant is significantly attenuated as compared to the wild-type PAO1 strain (Fig 2B). In agreement, fluorescence microscopy of 18 hpi infected embryos showed a lower bacterial burden with PAO1 strain than mgtC mutant (Fig 2C).
Fig. 2. Infection of zebrafish embryos with the P. aeruginosa mgtC mutant. 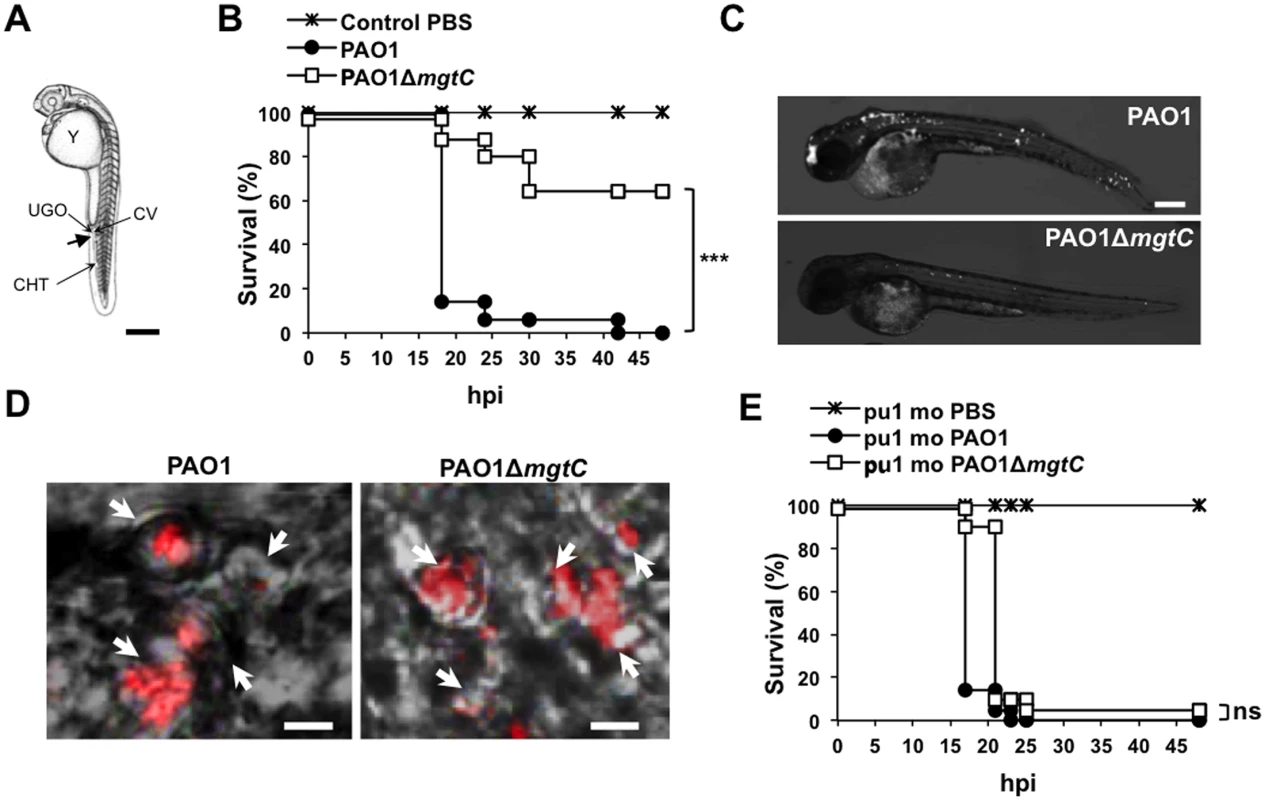
(A) Diagram of 30 hours post-fertilization (hpf) zebrafish embryo showing the injection site used in this study (arrow). All injections were done in the caudal vein (CV) just behind to the urogenital opening (UGO). Y: Yolk, CHT: Caudal Hematopoietic Tissue. Scale bar, 100 μm. (B) Survival curves of embryos infected with PAO1 wild-type stain or PAO1 ΔmgtC mutant and PBS-injected (control). Approximately 1200–1400 CFU P. aeruginosa were microinjected into the caudal vein (n = 20 per group). Results are expressed as the percentage of surviving on each hour post-infection. Representative results of three biologically independent replicates are shown. Embryos are significantly more susceptible to infection with PAO1 wild-type than ΔmgtC mutant (P<0.001). (C) 30 hpf embryons are intravenously infected with PAO1 or ΔmgtC mutant expressing mCherry. Representative fluorescence microscopy images of 18 hpi embryos infected with PAO1 (top panel) or PAO1 ΔmgtC mutant (bottow panel) are shown (1200–1400 CFU). Scale bar, 200 μm. (D) 30 hpf zebrafish embryos are intravenously infected with mCherry-expressing PAO1 (left panel) or PAO1 ΔmgtC mutant (right panel) and imaged by confocal microscopy. Arrows indicate maximum intensity projection of macrophages that phagocytose bacteria close to the site of injection at 1 hpi. Scale bar, 10 μm. (E) Survival curves of pu.1 morphant (pu.1 mo) embryos (n = 20 each) infected with 900 CFU of PAO1 wild type stain or PAO1 ΔmgtC mutant compared to the PBS- injected control. Representative results of three biologically independent replicates are shown. No statistically significant difference is obtained between wild type-infected embryos and ΔmgtC mutant-infected embryos (ns: non significant). Confocal microscopy at early time after injection allowed us to visualize bacteria phagocytosed by macrophages close to the site of injection (Fig 2D). Macrophages can be depleted from zebrafish embryos with a validated method that uses antisens oligonucleotides (morpholinos) against the myeloid transcription factor gene pu.1 [28,29]. Macrophage-depleted zebrafish embryos have been shown to be hypersusceptible to P. aeruginosa infection [20]. In addition, it has been shown that macrophage depletion restored the virulence of the attenuated T3SS mutant in zebrafish embryos [20]. We carried out experiments with Tg(mpeg1::mCherry) embryos in which macrophages are visualized as red cells [29]. Therefore, macrophage depletion can be checked upon pu.1 morpholinos injection (S2 Fig). Interestingly, the survival curve of macrophage-depleted embryos was similar upon infection with wild-type and mutant strains (Fig 2E and S3 Fig), indicating that macrophage depletion suppresses the difference between wild-type and mutant strain. Taken together, these results suggest that MgtC acts by protecting P. aeruginosa against macrophages.
MgtC contributes to P. aeruginosa resistance to macrophage killing in J774 macrophages
The virulence of P. aeruginosa is associated with its ability to resist the innate immune system notably because of a cytotoxic action towards macrophages and the T3SS plays a major role in Pseudomonas cytotoxicity [30]. We thus tested the cytotoxicity of the mgtC mutant on J774 macrophages and showed that the mutant is slightly but significantly less cytotoxic than the wild-type strain or the complemented strain (Fig 3A). However, the reduction of cytotoxicity was much lower than the one observed with a T3SS mutant (Fig 3A). Given our results in zebrafish embryos, we hypothesized that MgtC may play a role in the ability of P. aeruginosa strains to resist killing by phagocytic cells. We therefore tested the sensitivity of P. aeruginosa strains to the bactericidic activity of J774 macrophages. Whereas the T3SS mutant behaves similarly than the wild-type strain towards the killing by macrophages after phagocytosis, our results indicated that the mgtC mutant is more sensitive to macrophage killing than the wild-type or complemented strain, with a striking difference at time 1.5 hr (Fig 3B). The survival decrease of the wild-type strain at the second time point may account for a slower killing, but may also be due in part to the damage of infected macrophages.
Fig. 3. Behaviour of mgtC mutant towards J774 macrophages. 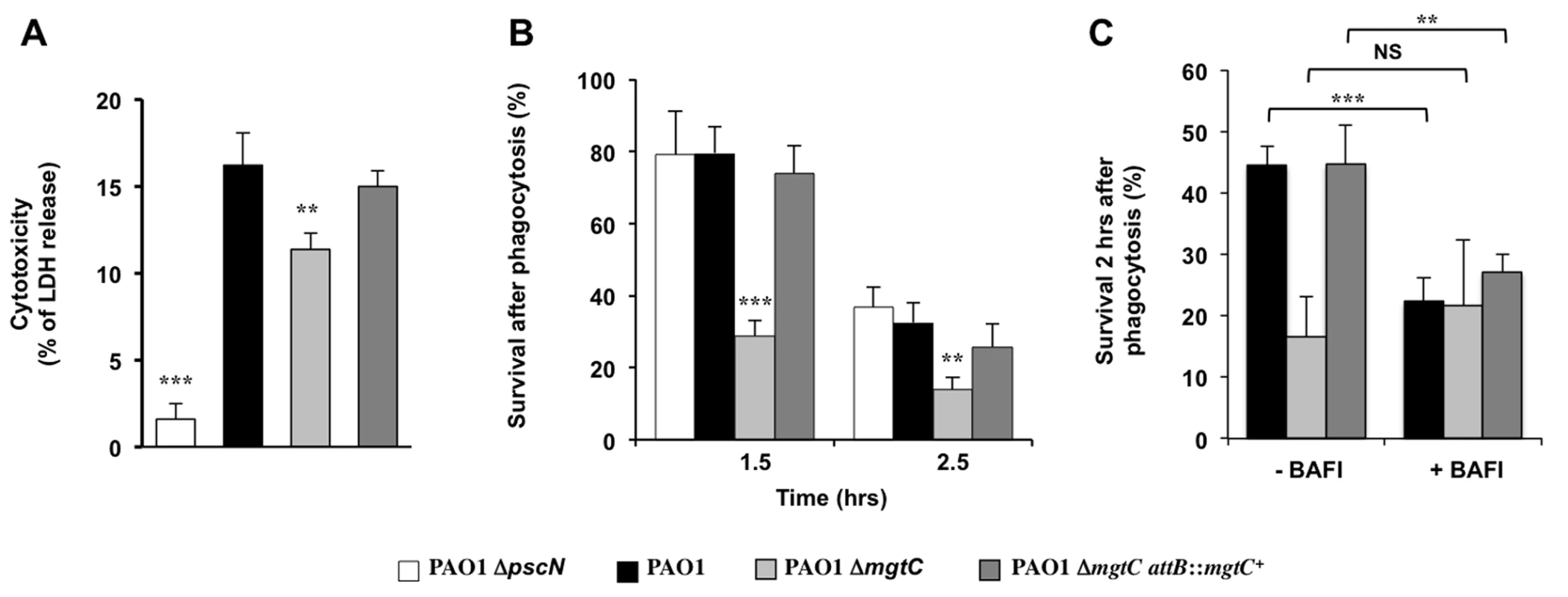
(A) Strain cytotoxicity was evaluated using a LDH assay. A T3SS mutant (ΔpscN) is included as negative control. Error bars correspond to standard deviations from three independent experiments. (B) Survival of bacteria upon phagocytosis. Results are expressed as percentage of surviving bacteria at the indicated time comparatively to the number of bacteria internalized after 30 min of phagocytosis. Error bars correspond to standard errors (SE) from five independent experiments. (C) Effect of inhibition of vacuolar proton ATPase on bacterial survival. The survival of bacteria was measured from macrophages treated or not with bafilomycin A1. Error bars correspond to standard errors (SE) from three independent experiments. In all panels the asterisks indicate P values (Student’s t test, **P <0.01, ***P <0.001). Salmonella MgtC can inhibit the bacterial F-ATP synthase and modulate physiological ATP levels and cytosolic pH, which has been proposed to play a role in the ability of Salmonella strain to replicate in macrophages [5]. Moreover, acidification of the phagosome has been proposed to promote Salmonella MgtC production by increasing bacterial ATP level [7]. The acidification of the phagosome is dependent on the activity of the host vacuolar ATPase, which can be specifically inhibited by the inhibitor bafilomycin A1 [31]. To decipher the contribution of phagosome acidification in the intramacrophage role of Pseudomonas MgtC protein, macrophage infection was performed in presence of bafilomycin A1 (Fig 3C). The addition of bafilomycin A1 did not affect significantly the mutant strain whereas the survival rate of the PAO1 strain or the complemented strain was significantly reduced. As a consequence, the survival rate of wild-type and mutant strains did not differ when cells are treated with vacuolar ATPase inhibitor.
To visualize intracellular bacteria upon infection of J774 cells, we imaged slides of fixed macrophages infected with fluorescent bacteria (Fig 4A). Moreover, bacterial counts on images were made at time 0 and 2 hrs after phagocytosis (Fig 4B). Our results indicated that the number of bacteria per cell after phagocytosis is slightly higher for the mutant strain than for the wild-type strain. We further quantified the phagocytosis rate with CFUs counts and showed that the mgtC mutant is phagocytosed at a slightly but significantly higher rate than the wild-type strain (S4 Fig). The evolution of the patterns between time 0 and time 2 hrs (Fig 4B) indicated that the bacterial number per cell rather increased with the wild-type but not with the mutant. These experiments, which do not discriminate between live and dead/dying bacteria, suggested a survival of wild-type bacteria with limited bacterial multiplication.
Fig. 4. Visualisation and quantification of intracellular bacteria in fixed macrophages. 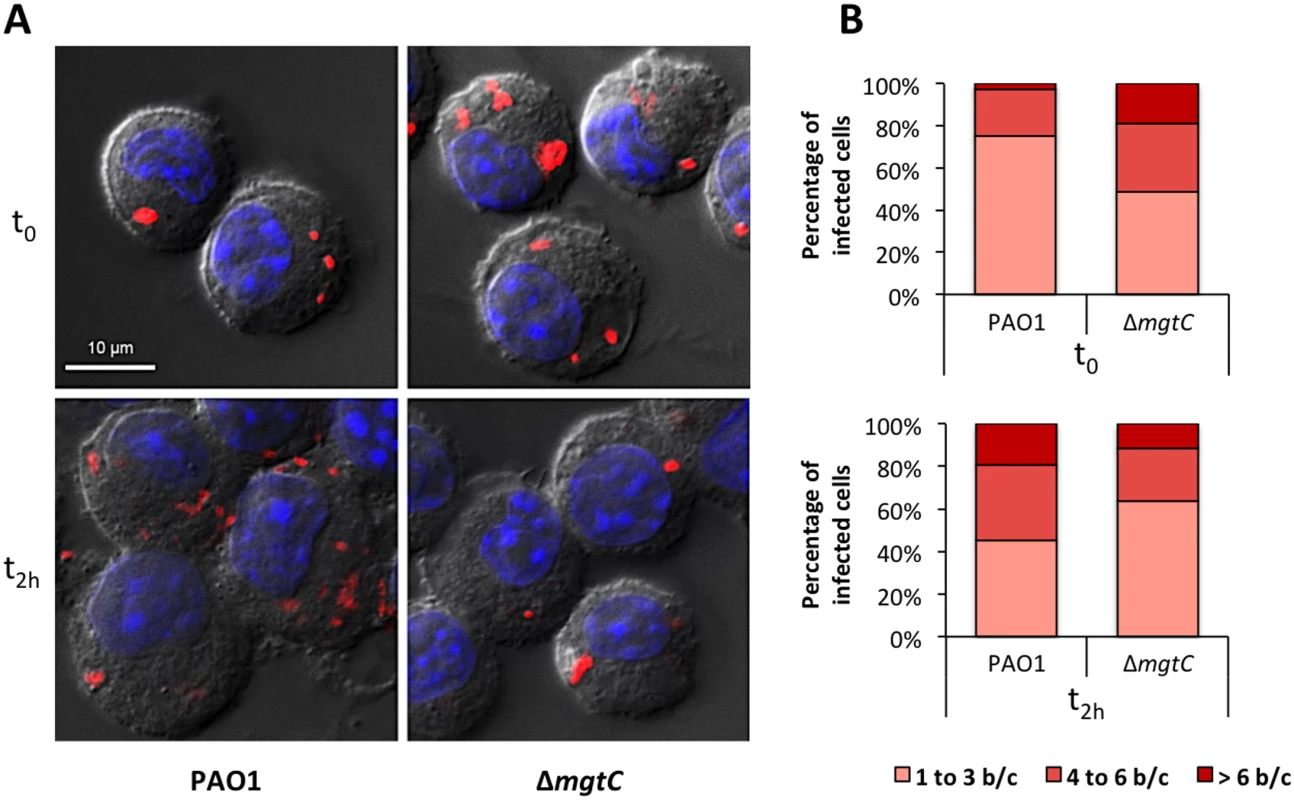
Macrophages infected with PAO1 or ΔmgtC mutant expressing mCherry were fixed after phagocytosis and 20 min (t0) or 2 hours (t2h) treatment with amikacin. (A) Visualization of intracellular bacteria. The images shown were obtained by making a maximum projection of 12 central planes from a Z-stack. The merged image shows Differential Interference Contrast (DIC), nucleus staining (blue) and bacteria expressing mCherry (red). (B) Count of the number of bacteria in infected macrophages from images obtained with the maximum projection. The numbers of bacteria per cell (b/c) were classified in three groups and percentage of each class is shown. Count is done from at least 20 cells and results are expressed as means from three independent experiments. We also investigated the behavior of the mgtC mutant towards non-phagocytic cells that can be targeted by the pathogen during the infection [22]. Gentamycin protection assays performed to measure internalisation in HeLa cells did not show significant difference between wild-type and mgtC mutant strains (S4 Fig).
Cumulatively, ex vivo experiments indicate that, similarly to intracellular pathogens, the MgtC virulence determinant of P. aeruginosa plays a role towards macrophages. Wild-type bacteria resisted better to phagocytosis and to bacterial killing by the phagosome than the mgtC mutant. Moreover, our results indicate an interplay between Pseudomonas MgtC role in macrophages and phagosome acidification.
Expression of P. aeruginosa mgtC is induced within macrophages
We first investigated the regulation of mgtC expression in liquid media depending on Mg2+ concentration and pH. Quantitative RT-PCR experiment showed that mgtC gene (PA4635) is strongly induced by low Mg2+ environment (Fig 5A). Moreover, in low Mg2+ condition, the level of mgtC gene expression can be further increased by lowering the pH (Fig 5A). On the other hand, the other mgtC-like gene (PA2558) was not regulated by Mg2+ nor pH (Fig 5B). We then quantified the mgtC mRNA level from infected macrophages. Importantly, we showed that mgtC is highly induced in macrophages, which agrees with a specific role in this step. On the other hand PA2558, as well as the T3SS gene pcrV or the house-keeping gene gyrA (S5 Fig), were similarly expressed in liquid medium and macrophages, and are therefore not induced in macrophages. Bafilomycin A1 treatment of macrophages, which decreases phagosome acidification, lowered mgtC expression intracellularly, which is consistent with the pH regulation observed in vitro. Taken together, these results indicate that mgtC is specifically expressed in macrophages and that phagosome acidification contributes to an optimal expression of the gene.
Fig. 5. Expression of P. aeruginosa mgtC gene in liquid medium and intracellularly. 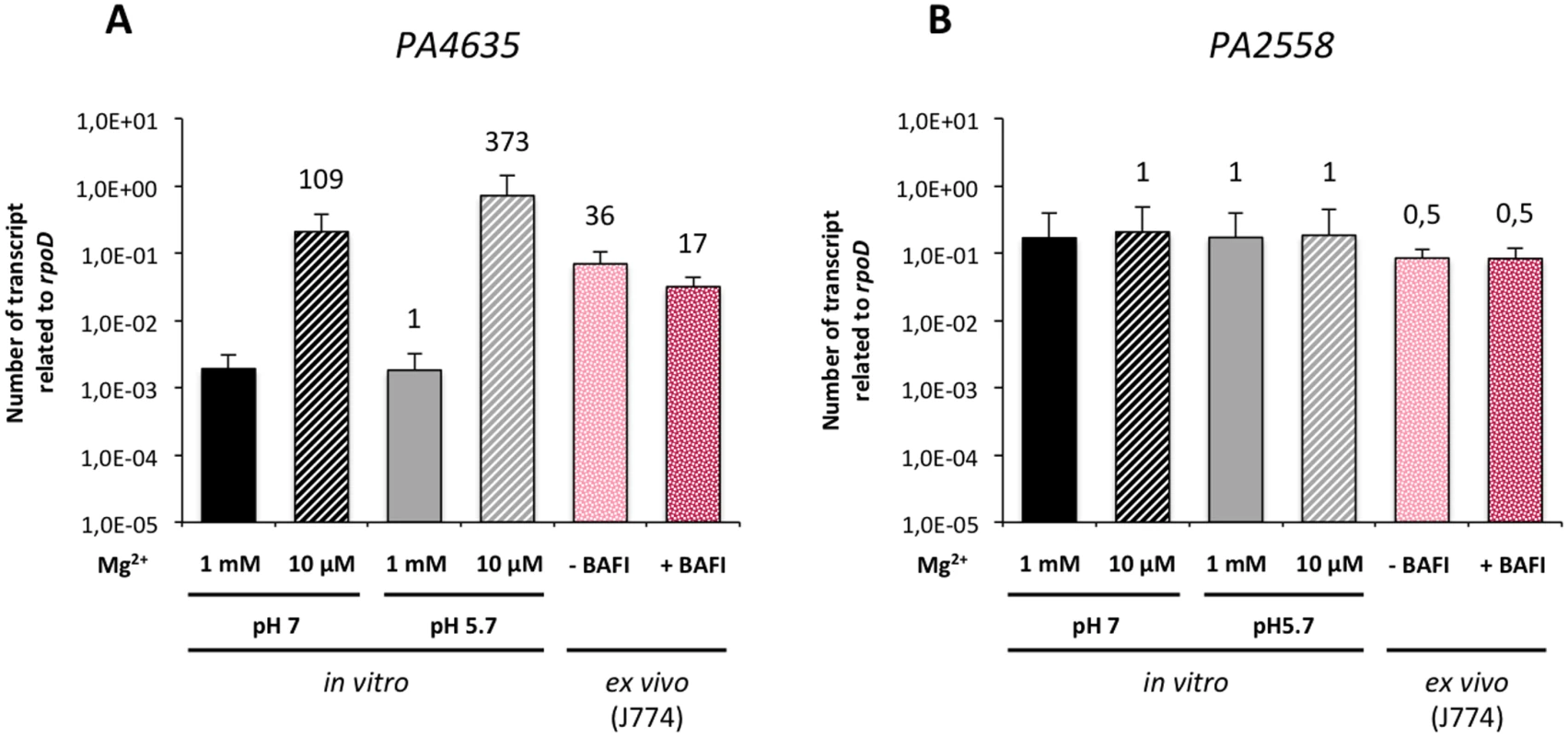
The levels of PA4635 (A) and PA2558 (B) transcripts relative to those of the rpoD gene were measured by qRT-PCR. RNA was extracted from bacteria grown in liquid medium (in vitro) containing a high (1 mM) or low (10 μM) concentration of MgSO4 and a pH of 7 or at 5.7. A pH of 5.7 is expected to mimic the pH faced by bacteria in the phagosome. Bacterial RNA was also extracted from infected J774 macrophages (ex vivo) that were treated (+ BAFI) or not (- BAFI) with bafilomycin A1. For all conditions, RNA were prepared two times independently. Results are expressed as means ± SD from at least three independent measurements (each performed in triplicate). The numbers indicate the induction fold relatively to the condition 1 mM MgSO4 pH 7. Role of MgtC for P. aeruginosa growth in low magnesium medium and biofilm formation on abiotic surface
Bacterial growth of P. aeruginosa strains was measured in minimal medium deprived for magnesium (without added Mg2+ or with 10 μM Mg2+) or containing 1 mM Mg2+ (corresponding to physiological concentration). Growth of the mgtC mutant was significantly impaired in low Mg2+ media (Fig 6A and Fig 6B), while it did grow similarly to the PAO1 wild-type strain in high Mg2+ medium (Fig 6C). The complemented strain displayed a wild-type growth in these conditions. Hence, MgtC contributes to the adaptation of P. aeruginosa to low Mg2+ environment, similarly to MgtC homologues from intracellular pathogens [1]. This result is consistent with the induction of mgtC gene expression in low Mg2+ environment (Fig 5A). To test whether the phenotype observed with the mgtC mutant in macrophages may be related to the growth defect upon Mg2+ limitation, the killing assay by J774 macrophages was repeated upon addition of 25 mM Mg2+ in the DMEM medium. However, addition of Mg2+ did not rescue the enhanced sensitivity of the mgtC mutant to macrophages (S6 Fig), suggesting that the increased killing of the mutant is not related to intracellular Mg2+ limiting environment.
Fig. 6. MgtC is required for growth of P. aeruginosa in Mg2+ deprived medium. 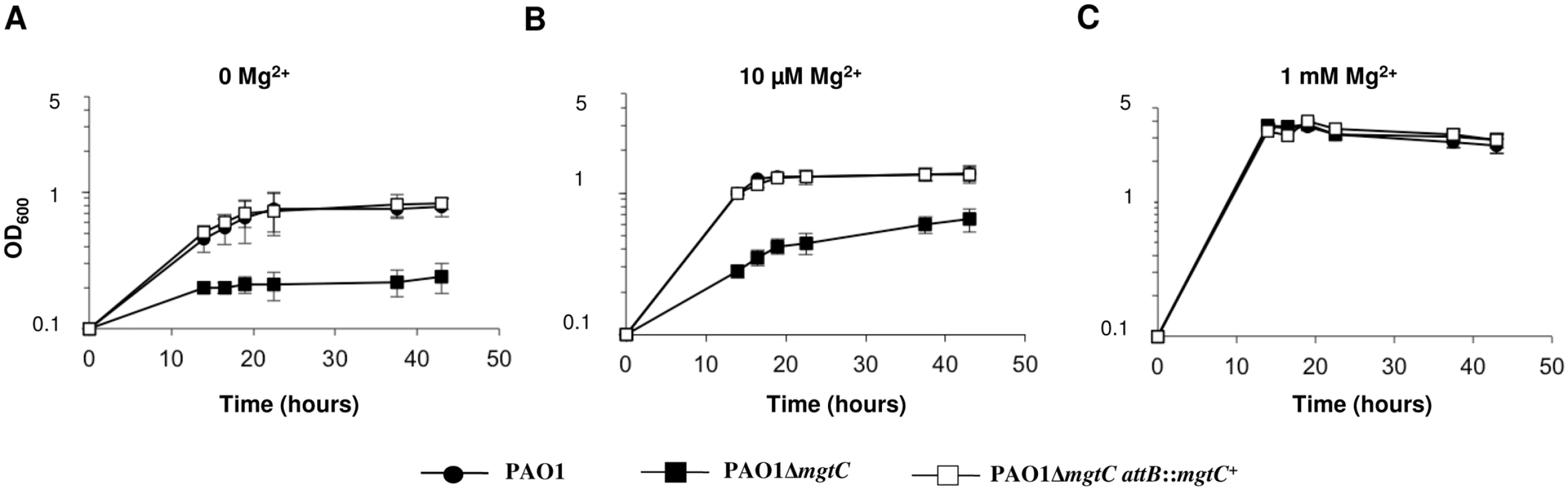
(A) P. aeruginosa strains PAO1, PAO1ΔmgtC, and PAO1ΔmgtC attB::mgtC+ were grown at 30°C in minimal medium without MgSO4. OD600 is indicated over the growth period. The experiment was independently repeated three times and results are expressed as means ± SD. Statistical analyses indicate significant difference between growth of the mgtC mutant and the wild-type or the complemented strain (Student’s t test, P <0.001 at times 20 hrs and 40 hrs). (B) The same strains were grown in medium with 10 μM MgSO4. Statistical analyses indicate significant difference between growth of the mgtC mutant and the wild-type or the complemented strain (Student’s t test, P <0.001 at times 20 hrs and 40 hrs). (C) The same strains were grown in high magnesium medium (1 mM MgSO4). No significant difference is found between the three strains. P. aeruginosa ability to form biofilm is a crucial virulence determinant, more particularly in chronic infection. Interestingly, biofilm formation has been shown to be sensitive to EDTA through chelation of divalent cations including Mg2+ [18]. To check the role of both MgtC and Mg2+ ions during biofilm formation, wild-type, mutant and complemented strains were grown in different Mg2+ concentrations in glass tubes for 24 h and bacterial adherence to the glass was visualized (Fig 7A) and quantified using crystal violet staining (Fig 7B) to infer the ability of strains to form biofilm. Profiles of the three strains were the same in both assays if bacteria are grown in high Mg2+, indicating similar biofilm formation at physiological Mg2+ concentration. However, in the absence of Mg2+, wild-type and complemented strains poorly attached to the glass, indicating that Mg2+ is required for biofilm initiation by P. aeruginosa. In contrast, the ΔmgtC mutant was still able to form biofilm. The increased biofilm formation for the ΔmgtC mutant comparatively to the wild-type strain was also observed in the presence of 10 μM Mg2+. Cumulatively, these results indicate that when expressed, i.e. under Mg2+ limitation, MgtC limits biofilm formation. Since exopolysaccharides (EPS) are essential biofilm matrix components, important for initial adherence and biofilm formation [32,33], EPS production was measured by Congo red assay [34] in low and high Mg2+ (S7 Fig). Whereas, all strains produced similar EPS level at 1 mM Mg2+, the mutant strain produced significantly more EPS than wild-type and complemented strain in low Mg2+ medium.
Fig. 7. Quantification of bacterial adherence to glass tubes to infer the ability of strains to form biofilm. 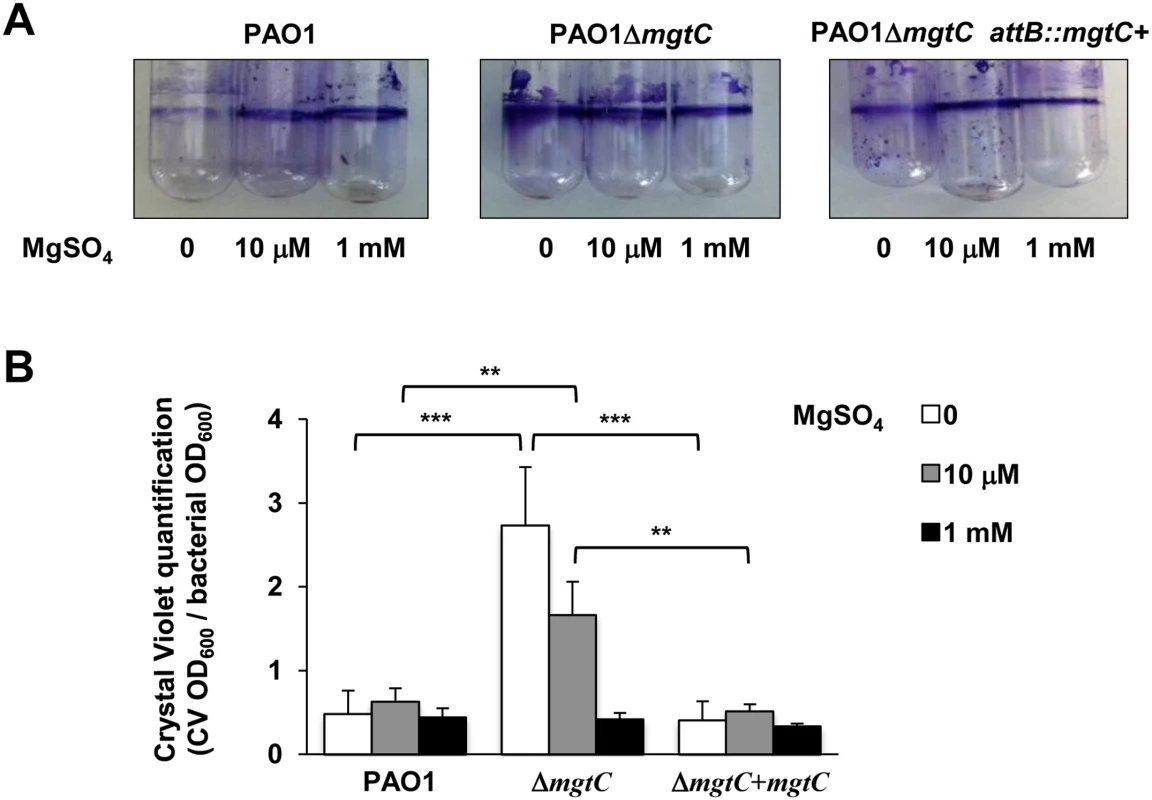
(A) Adherence assay with P. aeruginosa strains PAO1, PAO1ΔmgtC, and PAO1ΔmgtC attB::mgtC+ grown at 30°C for 24 hrs in minimal medium without MgSO4 or with MgSO4 (10 μM and 1 mM). The biofilm quantification is visualized by crystal violet ring on the glass tube. (B) Crystal violet quantification (CV OD600) is divided by the bacterial density (bacterial OD600). Error bars correspond to standard errors (+ SE) from three independent experiments and the asterisks indicate P values (Student’s t test, **P <0.01, ***P <0.001). We also investigated whether the increased biofilm formation of the mgtC mutant strain under Mg2+ limitation may be related to altered properties of the flagellum or the type IV pili, which are major adhesins required for initial attachment [35]. Our results indicated that the twitching and swimming motilities, which depend on type IV pili and flagellum, respectively, were similar in wild-type, mutant and complemented strain (S8 Fig). On the other hand, we observed that P. aeruginosa ΔmgtC bacteria grown under Mg2+starvation were more elongated than wild-type bacteria (S9 Fig). Hence the increased biofilm formation of the mgtC mutant strain under Mg2+ limitation may be due to increased EPS production and/or bacterial cell elongation.
Heterologous production of the Salmonella MgtR peptide in PAO1 mimics the mgtC mutant virulence phenotypes
MgtR is a membrane peptide (30 amino-acid long) encoded downstream of mgtC in S. Typhimurium that regulates negatively MgtC expression [8]. MgtR interacts directly with Salmonella MgtC in a bacterial two-hybrid system and over-expression of mgtR in a wild-type Salmonella strain reduced significantly the ability of the strain to grow within macrophages, thus displaying an anti-infective effect [8]. Site-directed mutagenesis and structural analysis have suggested that MgtR interacts with the 4th transmembrane domain of MgtC [8,36]. Examination of the DNA region adjacent to PA4635 as well as Blast analyses did not reveal any peptide similar to MgtR in P. aeruginosa. Because the 4th transmembrane domain of MgtC is well conserved between S. Typhimurium and P. aeruginosa (S10 Fig), we have tested whether the Salmonella MgtR peptide could interact with P. aeruginosa MgtC (PaMgtC) using the bacterial two-hybrid system BACTH [37]. The interaction between T18-PaMgtC and T25-MgtR fusion proteins was evaluated by measuring β-galactosidase activity (Fig 8A). Negative controls consisted in T18-PaMgtC with empty pKT25 (Fig 8A) and empty pUT18 with T25-MgtR (S11 Fig). The high level of β-galactosidase activity, which was 60 fold the value of the negative control, was indicative of an interaction between the MgtR peptide and PaMgtC in this system. On the other hand, PaMgtC did not interact with another membrane peptide, the Salmonella KdpF peptide (S11 Fig), indicating that the interaction with MgtR is not due to sticky properties of PaMgtC.
Fig. 8. Virulence phenotypes of a PAO1 strain expressing mgtR. 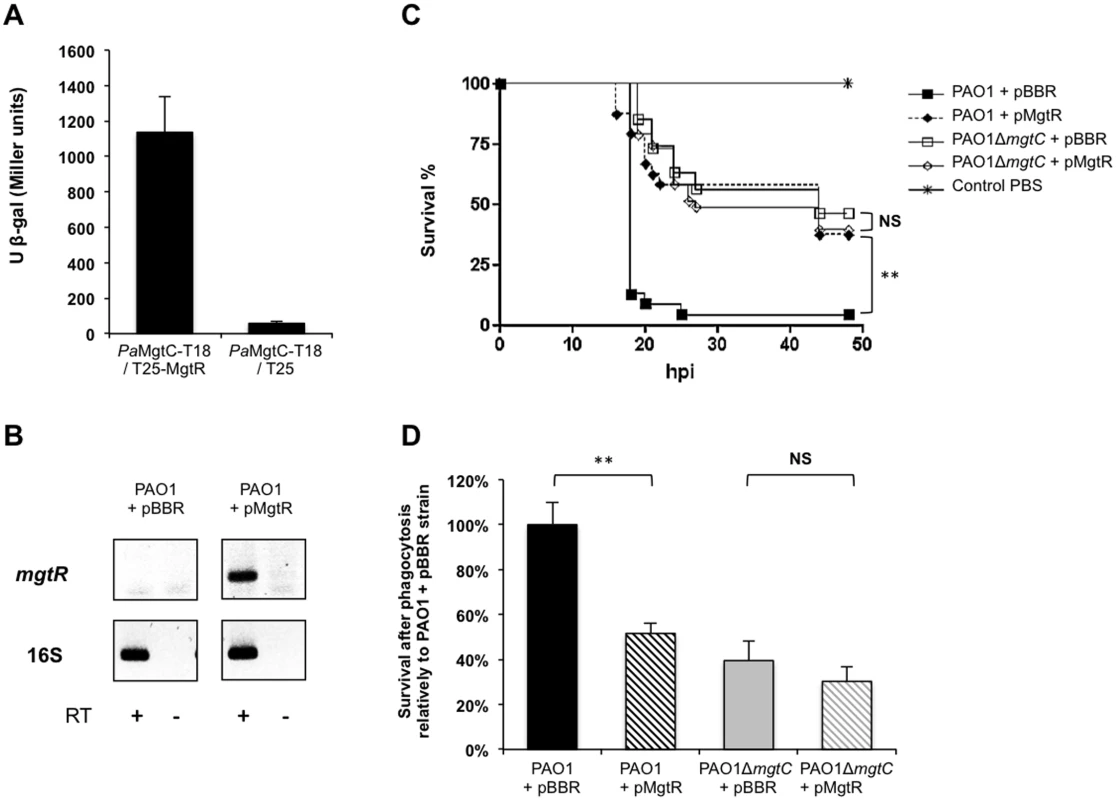
(A) In vivo interaction of Pa MgtC with MgtR peptide. The interaction was assayed using the BACTH system by transforming E. coli BTH101 cells with plasmids producing PaMgtC-T18 and T25-MgtR. Liquid β-galactosidase assays were performed from six independent experiments. As negative control, BTH101 bacteria were cotransformed with a plasmid expressing PaMgtC-T18 and the pKT25 vector. Error bars represent SD. (B) Expression of Salmonella mgtR in PAO1 strain. RT-PCR experiment was performed with primers specific for mgtR gene from RNA isolated from PAO1 strain carrying the pBBR1MCS vector (pBBR) or a pBBR1MCS derivative that encodes mgtR (pMgtR). Primers specific for 16S gene were used as positive control. Controls where reverse transcriptase was omitted are indicated (RT-). (C) Survival curve of embryos infected with PAO1 strain expressing or not mgtR. PAO1ΔmgtC strain expressing or not mgtR is also included in the experiment and non-injected embryos were used as control. Approximately 600–1000 CFU of P. aeruginosa were microinjected into the caudal vein (n = 24 per group). Results are expressed as the percentage of surviving on hour post-infection (hpi). Representative results of at least three biologically independent replicates are shown. Embryos are significantly more resistant to infection with PAO1 strain expressing mgtR than PAO1 strain with empty vector (P<0.01). (D) Behaviour of the PAO1 strain expressing mgtR in J774 macrophages. PAO1ΔmgtC strain expressing or not mgtR is included in the experiment. Results are normalized to 100% for the PAO1-pBBR strain and are expressed as means +SE from four independent experiments. Asterisks indicate statistical significance ** P <0.01). NS, non significant. We then transformed the wild-type P. aeruginosa strain with pMgtR, a plasmid that harbors the Salmonella mgtR gene [8]. Expression of mgtR in PAO1 was verified by RT-PCR (Fig 8B). The virulence of the strain was evaluated using the zebrafish embryo model. As shown in Fig 8C, a wild-type PAO1 strain that expressed mgtR was attenuated comparatively to a control PAO1 strain that harbored an empty vector. On the other hand, no effect of the pMgtR plasmid was detected in the context of the PAO1 mgtC mutant strain. Expression of mgtR also increased significantly the killing of wild-type PAO1 strain, whereas no significant effect was found in the presence of the mgtC mutation (Fig 8D). In addition, a mild but significant increase in phagocytosis was observed (S12 Fig). Taken together, these results suggest that heterologous production of the Salmonella MgtR peptide can lower PAO1 virulence and resistance to macrophage killing by targeting the P. aeruginosa MgtC. Regarding other phenotypes related to MgtC defect in P. aeruginosa, production of the Salmonella MgtR peptide did not modulate bacterial growth in magnesium deprived medium (S13 Fig). This correlates with results obtained in Salmonella and is consistent with the finding of a dual role for MgtC [15].
Discussion
Phylogenetic analysis indicated that the MgtC protein from the extracellular pathogen P. aeruginosa clustered in the same subgroup as MgtC proteins from intracellular pathogens, which are known to subvert macrophages and promote intracellular bacterial multiplication [1,16]. MgtC exhibits a sporadic distribution in Pseudomonas species, being mostly associated with strains that are pathogenic for humans and insects. In the present study, we show that MgtC plays a role in P. aeruginosa virulence in a systemic model of infection in a macrophage-dependent manner and in P. aeruginosa ability to escape the killing action of the macrophage. MgtC thus provides a singular example of a virulence determinant that subverts the antimicrobial behavior of macrophages both in intracellular and extracellular pathogens (Fig 9).
Fig. 9. Role of MgtC in intracellular and extracellular pathogens. 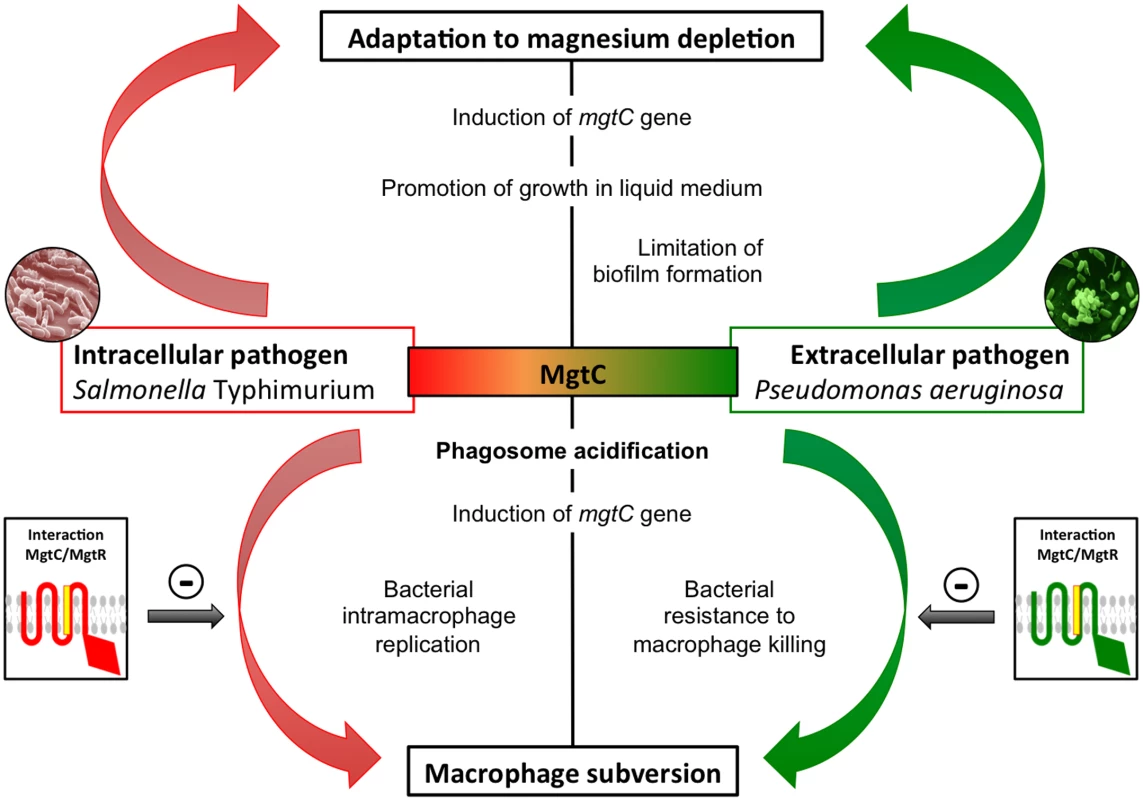
MgtC is involved in adaptation to magnesium deprivation and macrophage subversion both in intracellular and extracellular pathogens. We propose that MgtC share a similar function in intracellular and extracellular pathogens and that the bacterial lifestyle determines whether MgtC will promote intramacrophage bacterial multiplication or resistance to killing by macrophages. Expression of mgtC gene is induced in macrophages and phagosome acidification contributes to its optimal expression. The MgtR peptide can interfere with MgtC function, thus acting as a natural antagonist, and its production limits the macrophage subversion. There is growing evidence that some cytotoxic extracellular pathogens can actually survive inside macrophages after phagocytosis to hide from the immune system [38], but little is known about the intracellular behavior of these pathogens. The zebrafish embryo model has contributed significantly to the study of an intracellular phase in phagocytic cells in the life cycle of Staphylococcus aureus, a pathogen that has long been considered as an extracellular pathogen [39]. Interestingly, MgtC has been shown to promote intramacrophage survival of Yersinia. pestis [12], which is a facultative intracellular pathogen that replicates mainly extracellularly and produces antiphagocytic factors. Our present results identify MgtC as a novel actor in P. aeruginosa virulence, playing a role in an intramacrophage phase of this extracellular pathogen. The ability of P. aeruginosa to inhibit the microbicidal mechanisms of phagocytic cells is much less documented than its cytotoxicity towards mammalian cells, which is linked to the production of exotoxins and injected effector proteins by the T3SS. We have shown that the P. aeruginosa mgtC mutant is attenuated for acute infection in zebrafish embryos, which possess professional phagocytes that can engulf and kill P. aeruginosa upon infection [20]. MgtC acts by protecting P. aeruginosa against phagocytes since macrophage depletion suppressed the difference between mgtC mutant and PAO1 strain. Interestingly, a T3SS mutant has been reported to have a similar behavior in zebrafish embryos [20]. However, the phenotypes of the two mutants differ in ex vivo experiments because, contrary to a T3SS mutant, the mgtC mutant is more sensitive than the wild-type strain to the bactericidal effect of J774 macrophages. In contrast, the effect of the mgtC mutant on cytotoxicity towards macrophages is minor relatively to the one of T3SS mutant.
Consistent with the role of P. aeruginosa mgtC within macrophages, expression of the gene is highly induced when the bacteria reside inside macrophages, which is not the case for the mgtC-like PA2558 and for a T3SS gene. Strong intramacrophage induction has also been reported for the Salmonella mgtC gene [40] and to our knowledge, this is the first report of a P. aeruginosa gene induced within phagocytic cells. It has been proposed that phagosome acidification may contribute to Salmonella MgtC protein production via an increased level of cytoplasmic ATP [5,7]. Our results further support an interplay between MgtC role in macrophages and the function of the V-ATPase because inhibition of the V-ATPase suppressed the difference between wild-type strain and mgtC mutant strain. Noticeably, Pseudomonas wild-type strain, but not mgtC mutant strain, had a reduced macrophage survival in the presence of V-ATPase inhibitor. A reduced resistance of wild-type bacteria to macrophage killing upon bafilomycin treatment has been also described for intracellular bacteria, for which acidification is important for proper expression of genes required intracellularly [41,42]. We demonstrated that phagosome acidification contributes to an optimal production of Pseudomonas mgtC, which agrees with the maximization of mgtC induction in vitro at low pH. Cumulatively, our results identify mgtC as a key gene for P. aeruginosa intramacrophage stage. Moreover, uptake by macrophages, but not epithelial cells, was enhanced with the P. aeruginosa mgtC mutant compared to the wild-type strain. A similar phenotype has been described for a M. marinum mgtC mutant, with MgtC playing a role in later steps of the internalization process rather than initial attachment events of bacteria to macrophages [14]. Further experiments will be required to investigate the fate of intracellular bacteria but we propose that P. aeruginosa has acquired a macrophage subversion factor to resist to killing in case of phagocytosis by macrophages. Moreover, our findings highlight the importance of macrophage subversion by P. aeruginosa in a model of acute infection. In agreement, mgtC has been recovered in a recent screen as a Pseudomonas gene involved in bacterial fitness in a systemic model of infection in mice [43]. It would be of interest to further explore the role of Pseudomonas MgtC in the mice model, both during acute and chronic infection.
Growth kinetic analyses showed that MgtC is required for optimal growth in magnesium deprived medium in P. aeruginosa, which is a common feature with intracellular pathogens. This result agrees with previous complementation studies showing that heterologous expression of P. aeruginosa mgtC (PA4635) in a S. Typhimurium mgtC mutant restored the growth defect in Mg2+ deprived medium [15]. Moreover, we have shown that mgtC expression in P. aeruginosa is induced in conditions of Mg2+ starvation, which is consistent with a previous proteomic analysis [44], and implies a role of MgtC in such stressing condition. Hence, our results indicate a mechanism of convergent evolution between pathogens that have different niches in their ability to use MgtC to adapt to Mg2+ limitation (Fig 9). We believe that this ability does not account for P. aeruginosa MgtC role in macrophages because Mg2+ measurement in a phagosome indicated a concentration in the millimolar range [45] and because addition of extracellular Mg2+ did not rescue the increased sensitivity of mgtC mutant to macrophages.
Magnesium has been shown to influence attachment and subsequent Pseudomonas biofilm formation [46], which is a hallmark of Pseudomonas chronic infection, and biofilms are disrupted by cation chelators [18]. Moreover, Mg2+ is required for biofilm formation in fungal pathogens [47]. In Mg2+ limiting condition, the P. aeruginosa mgtC mutant retains the ability to form biofilm, while the wild-type strain is severely impaired for biofilm formation, indicating that MgtC limits biofilm formation. The biofilm phenotype of the mgtC mutant is associated with increased production of EPS, which are essential biofilm matrix components. In addition, biofilm formation could be favored by the elongated shape of the mgtC mutant grown under Mg2+ limitation, as bacterial elongation can promote biofilm formation [48]. Very interestingly, a recent report provides an unsuspected link between MgtC, cellulose (a polysaccharide associated with the formation of biofilm) and intramacrophage survival in Salmonella [49]. MgtC repressed cellulose biosynthesis during growth in low Mg2+ medium and cellulose production was shown to hinder Salmonella replication inside macrophages. Despite the fact that cellulose is not produced by P. aeruginosa strains (as PAO1), this new finding, combined to our finding of an increased EPS production by the P. aeruginosa mgtC mutant in low Mg2+ medium, suggests a potential link between EPS production and P. aeruginosa MgtC role in macrophages. Further studies will be required to determine the nature of the EPS involved, to investigate their production intracellularly and the putative link with intramacrophage phenotypes.
MgtC proteins consist of two structural domains, a highly conserved membrane N-terminal domain and a more divergent soluble C-terminal domain. Structural data as well as bioinformatic modeling indicate that the P. aeruginosa MgtC C-terminal domain adopts the same fold as the one of MgtC proteins from intracellular pathogens, and this despite a low level of sequence identity ([50]; G. Labesse, personal communication). This suggests that MgtC may share a similar function in P. aeruginosa and intracellular pathogens, which is supported by the common role in adaptation to Mg2+ deprivation. Furthermore, whereas expression of P. aeruginosa mgtC does not complement the macrophage replication defect of a S. Typhimurium mgtC mutant, a significant complementation is observed when a P. aeruginosa MgtC variant carrying a single amino-acid change is produced [15]. Hence, MgtC proteins from P. aeruginosa and S. Typhimurium may have evolved in a very subtle way, possibly to optimally interact with partner protein(s).
The MgtR membrane peptide has been proposed as a natural MgtC antagonist because in S. Typhimurium, the intramacrophage replication of a wild-type strain can be reduced upon over-production of MgtR, which plays a negative regulatory role on mgtC expression [8]. In the present study, we have found that the phenotypes observed with Pseudomonas mgtC mutant in animal and cellular infection models can be mimicked upon production of the Salmonella MgtR peptide in a wild-type P. aeruginosa strain. Experiments in mgtC mutant strain indicated that the peptide requires a functional MgtC protein to play a role. Hence, these results support the action of MgtR natural peptide as an antagonist of MgtC and highlight MgtC as a promising new target for anti-virulence strategies.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids are described in S1 Table. P. aeruginosa was grown at 37°C in Luria broth (LB) or at 30°C in MM63 medium supplemented with 0.5% casamino acids, 0.2% glucose, and MgSO4 (10 μM or 1mM) or without MgSO4. Plasmids were introduced in P. aeruginosa by conjugation, using an E. coli strain containing pRK2013. Recombinant bacteria were selected on Pseudomonas isolation agar (PIA). P. aeruginosa mutants obtained by using the pKNG101 suicide vector were selected on plates containing 6% sucrose. Antibiotic concentrations (μg/ml) were: for E. coli: ampicillin, kanamycin, streptomycin, gentamicin, 50; tetracycline, 40; for P. aeruginosa: gentamicin, 50 or 125 (plates); tetracycline, 200; streptomycin, 2000.
Construction of a P. aeruginosa mgtC mutant and complemented strain
To generate a non-polar deletion of the mgtC (PA4635) gene, 526 bp upstream and 580 bp downstream of the mgtC gene were amplified, cloned in pCR2.1 and subcloned in pKNG101 suicide vector giving the mutator pSBC44 which was mobilized in the wild-type P. aeruginosa strain PAO1 to select for double recombination event (S1 Text).
Complementation by a single copy of the mgtC+ gene under its own promoter was carried out with the miniCTX integration method at attB region [51]. The entire mgtC gene with the 525 bp-upstream region, in which a σ70 dependent promoter was predicted (S1 Fig), was amplified, cloned in the pCR2.1 giving pSBC46, which was subcloned in mini-CTX1 plasmid vector giving pSBC47. Chromosomal insertion of pSBC47 carrying mgtC+ and excision of plasmid DNA sequences resulted in an unmarked integrant (S1 Text).
Infection of Danio rerio embryos
Experiments were performed using the golden zebrafish mutant [52] or the Tg(mpeg1::mCherry) [29] zebrafish line and maintained under standard conditions (supplemental material). Bacterial strains, which were freshly streaked out from glycerol stocks, were grown in LB medium to mid-log phase (DO = 0.6), recovered by centrifugation and washed twice in Phosphate-Buffered Saline (PBS). Suspensions were homogenized through a 26-gauge needle and resuspended in PBS at about 109 bacteria/ml added with 10% phenol red to aid visualization of the injection process. Infections were carried out by microinjection of 1–2 nl of bacterial suspensions into the caudal vein of 30 hpf embryos, which were previously dechorionated and anesthetized with 0.02% tricaine. For survival kinetics after infection, the number of dead embryos was determined visually based on the absence of heartbeat. Depletion of macrophages was carried out upon microinjection of pu.1 morpholinos into zebrafish eggs and visualized by fluorescence microscopy (S1 Text).
Microscopic analysis of zebrafish embryos
For live imaging, anesthetized infected embryos were mounted in 35 mm dishes and immobilized with 1% low-melting point agarose. Direct visualization is performed using an Olympus MVX10 epifluorescent microscope equipped with a digital color camera (Olympus XC50). Fluorescence and bright-field images are acquired and processed with CellSens (Olympus) and assembled using GIMP 2.6 freeware to adjust levels and brightness and to remove out-of-focus background fluorescence.
For fixed samples observations, anesthetized embryos are fixed overnight at 4°C with 4% paraformaldehyde in PBS, then washed twice in PBS and transferred gradually from PBS to 50% glycerol-PBS. Fixed fishes were positioned flat onto depression transparent slides covered with a cover slip for observation with 40x Leica Apo oil 1.15 NA or 63x Leica Apo oil 1.33 NA objectives and using a Leica DM2500CSQ upright microscope equiped with a Leica TCS SPE confocal scan head and fluorescence and differential interference contrast (DIC) optics. Widefield fluorescence and DIC images were captured and assembled by LAS-AF software (Leica Microsystems).
Ethics statement
All animal experiments described in the present study were conducted at the University Montpellier 2 according to European Union guidelines for handling of laboratory animals (http://ec.europa.eu/environment/chemicals/lab_animals/home_en.htm) and were approved by the Direction Sanitaire et Vétérinaire de l'Hérault and Comité d'Ethique pour l'Expérimentation Animale under reference CEEA-LR-13007. The breeding of adult fish adhered to the international guidelines specified by the EU Animal Protection Directive 2010/63/EU and adult zebrafish were not sacrificed for this study. All experiments were performed before the embryos free-feeding stage and did not fall under animal experimentation law according to the EU Animal Protection Directive 2010/63/EU. For survival curves, cardiac rhythm was used as a clinical criterion to fix the endpoint at which embryos are euthanized using the anaesthetic Tricaine up to a lethal dose (500 mg/ml). Embryos that survive infection were anaesthetized with Tricaine up to a lethal dose before bleach treatment.
Cytotoxicity towards macrophages
The cytotoxicity of P. aeruginosa strains grown to mid-log phase in LB broth was assayed by using J774 macrophages as described in [53], except that macrophages were infected for 1.5 hr at a multiplicity of infection (MOI) of 20. The percentage of LDH release was calculated relatively to that of the uninfected control, which was set at 0% LDH release, and that of uninfected cells lysed with Triton X-100, which was set at 100% LDH release.
Macrophage-mediated bactericidal assay
The killing of P. aeruginosa strains by J774 macrophages was carried out essentially as described previously [54]. Mid-log phase P. aeruginosa grown in LB broth was centrifuged and resuspended in PBS to infect J774 macrophages grown in DMEM medium supplemented with 10% FBS at a MOI of 10. After centrifugation of 24-well culture plates, bacterial phagocytosis was allowed to proceed for 30 min before washing three times with sterile PBS and adding fresh DMEM media supplemented with 400 μg ml-1 gentamicin. Macrophages were lysed after 1 h 30 min and 2 h 30 by using 1% Triton X-100 and the number of viable bacteria was determined by subsequent plating onto LB agar plates. The percentage of survival represents the ratio between the number of bacteria at time 1.5 hr or 2.5–3 hrs and the number of bacteria internalized after phagocytosis. In case of treatment with bafilomycin A1, the inhibitor was added at a concentration of 100 nM 30 min before phagocytosis and was also added after phagocytosis. Bafilomycin does not exhibit any bacterial toxicity at the working concentration. In experiments with strains expressing mgtR, macrophages were lysed after 20 min or 2 hrs of gentamicin treatment and the ratio between bacteria enumerated at the two time points was calculated.
Analysis of intracellular bacteria by fluorescence microscopy
For analysis of bacteria in fixed cells, infection of J774 macrophages was carried out as described above for the CFU assay, except that macrophages were seeded on glass coverslips and infected at a MOI of 5 to 10 with bacteria that were previously passed four times through a 26G needle. Because fluorescent bacterial strains carry a plasmid-encoded gentamycin resistant gene, amikacin (300 μg ml-1) was used to kill extracellular bacteria. For time 0, cells have been in contact with amikacin for 20 min. At the end of the incubation period, the cells were fixed with 4% paraformaldehyde in PBS for at least 1 hr. Cells were washed three times with PBS and mounted on glass slides in Vectashield with DAPI (Vector Laboratories, Inc). The slides were examined using an upright fluorescence microscope (Axioimager Z2, Zeiss) equipped with an Apotome 1 for optical sectioning. A 63X Apochromat Objective (NA 1.4) was used, transmitted light was acquired using differential interference contrast (DIC), and m-Cherry fluorescence was acquired using a texas red filter set. Z-stacks were systematically acquired using a Z-step of 240 nm. Counting was performed taking into account a maximum projection of the Z-stack. Images were processed using ZEN blue software (Zeiss).
RNA extraction and quantitative RT-PCR (qRT-PCR)
For the study of expression of PA4635 and PA2558 genes, RNA was prepared from mid-logarithmic bacterial cultures grown for 1 hour in NCE-minimal medium supplemented with 0.1% casamino acids, 38 mM glycerol and 10 μM MgSO4 or 1 mM MgSO4 [8] at pH 7 or pH 5.7. For mgtR expression study, RNA was prepared from mid-logarithmic bacterial cultures grown in LB. For bacterial RNA extraction from infected J774, 4.106 macrophages were seeded into a 100 cm2 tissue culture dish and infected at an MOI of 10 as described above. One hour after phagocytosis, cells were washed three times with PBS, lysed with 0.1% Triton X100 and pelleted by centrifugation at 13 000 rpm for 10 min at 15°C. Bacteria were resuspended by adding 500 μl PBS and transferred to a new tube for RNA preparation (thus discarding non resuspended cellular debris).
RNA was prepared and reverse transcribed as previously described [55] except that bacteria were incubated only 5 min with RNA protect reagent and were disrupted with lyzozyme. Controls without reverse transcriptase were done on each RNA sample to rule out possible DNA contamination. Quantitative real-time PCR was performed using a Light Cycler 480 SYBR Green I Master mix and a 480 Light Cycler instrument (Roche). PCR conditions were as follows: 3 min denaturation at 98°C, 45 cycles of 98°C for 5 sec, 60°C for 10 sec and 72°C for 10 sec. PCR conditions for mgtR and 16S rRNA control gene were as follows: 25 cycles of 95°C for 30 sec, 46°C (59°C for 16S rRNA) for 20 sec and 72°C for 30 sec. The sequences of primers used for RT-PCR are listed in S1 Table.
Internalization in non-phagocytic cells
Invasion assay of HeLa cells were done as described [22] with mid-log phase P. aeruginosa grown in LB broth.
Adherence assay on inert surfaces and analysis of exopolysaccharide (EPS) production
The adherence assay was performed as previously described [56] except that the biofilm visualisation was done in glass tubes. O/N cultures were done in 2 ml of MM63 medium supplemented with glucose, Casamino acids and 1 mM MgSO4, cells were washed with PBS before resuspension in the same medium with various concentrations of MgSO4. The absorbance of the culture was measured at 600 nm (A600) before attached bacteria were stained with 0.1% crystal violet for a period of 15 min and washed twice with water. The stain was then dissolved in ethanol and absorbance was measured at 600 nm.
For liquid Congo red (CR) assays, bacteria were grown as previously in a minimal medium containing 40 μg/ml CR, under vigorous agitation. At 24 h, the A600 (absorbance at 600 nm) was determined and the bacterial cells were pelleted by centrifugation at 15493 g. For quantification of CR binding, the A490 of the supernatant of each sample was determined. The ratio of A490/A600 indicates the EPS production.
Bacterial two-hybrid analysis
The P. aeruginosa mgtC gene, amplified using PAO1 chromosomal DNA and primers PA4635-pUT18-F/PA4635-pUT18-R, was cloned at the HindIII and EcoRI sites of the pUT18 vector, to produce a fusion protein PaMgtC-T18. This recombinant plasmid was cotransformed with a plasmid producing a fusion protein T25-MgtR [8] into BTH101 bacteria. Quantification of interaction between fusion proteins was carried out at 30°C as described previously [8]. Values are average from at least six independent cultures. A level of β-galactosidase activity at least fivefold higher than that measured for vectors indicates an interaction.
Statistical analysis
Statistical analyses of comparisons between survival curves were performed using the log rank test with Prism 4.0 (Graphpad, Inc). Statistical significance was assumed at P values <0.05. For ex vivo experiments (J774 or HeLa cells), paired Student’s t test was performed using Excel software (Microsoft). In the figures, * means P values <0.05, ** <0.01, and *** <0.001.
Supporting Information
Zdroje
1. Alix E, Blanc-Potard AB MgtC: a key player in intramacrophage survival. Trends Microbiol. 2007; 15 : 252–256. 17416526
2. Blanc-Potard AB, Groisman EA The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997; 16 : 5376–5385. 9311997
3. Lawley TD, Chan K, Thompson LJ, Kim CC, Govoni GR, et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006; 2: e11. 16518469
4. Thompson JA, Liu M, Helaine S, Holden DW Contribution of the PhoP/Q regulon to survival and replication of Salmonella enterica serovar Typhimurium in macrophages. Microbiology. 2011; 157 : 2084–2093. doi: 10.1099/mic.0.048926-0 21511762
5. Lee EJ, Pontes MH, Groisman EA A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium's own F1Fo ATP synthase. Cell. 2013; 154 : 146–156. doi: 10.1016/j.cell.2013.06.004 23827679
6. Garcia Vescovi E, Soncini FC, Groisman EA Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996; 84 : 165–174. 8548821
7. Lee EJ, Groisman EA Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature. 2012; 486 : 271–275. doi: 10.1038/nature11090 22699622
8. Alix E, Blanc-Potard AB Peptide-assisted degradation of the Salmonella MgtC virulence factor. EMBO J. 2008; 27 : 546–557. doi: 10.1038/sj.emboj.7601983 18200043
9. Buchmeier N, Blanc-Potard A, Ehrt S, Piddington D, Riley L, et al. A parallel intraphagosomal survival strategy shared by mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000; 35 : 1375–1382. 10760138
10. Lavigne JP, O'Callaghan D, Blanc-Potard AB Requirement of MgtC for Brucella suis intramacrophage growth: a potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect Immun. 2005; 73 : 3160–3163. 15845525
11. Maloney KE, Valvano MA The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect Immun. 2006; 74 : 5477–5486. 16988222
12. Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006; 74 : 3727–3741. 16790745
13. Retamal P, Castillo-Ruiz M, Mora GC Characterization of MgtC, a virulence factor of Salmonella enterica Serovar Typhi. PLoS One. 2009; 4: e5551. doi: 10.1371/journal.pone.0005551 19436747
14. Belon C, Gannoun-Zaki L, Lutfalla G, Kremer L, Blanc-Potard AB Mycobacterium marinum MgtC plays a role in phagocytosis but is dispensable for intracellular multiplication. PLoS One. 2014; 9: e116052. doi: 10.1371/journal.pone.0116052 25545682
15. Rang C, Alix E, Felix C, Heitz A, Tasse L, et al. Dual role of the MgtC virulence factor in host and non-host environments. Mol Microbiol. 2007; 63 : 605–622. 17176255
16. Blanc-Potard AB, Lafay B MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens: evidence from molecular phylogeny and comparative genomics. J Mol Evol. 2003; 57 : 479–486. 14708580
17. Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One. 2009; 4: e5660. doi: 10.1371/journal.pone.0005660 19543527
18. Banin E, Brady KM, Greenberg EP Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006; 72 : 2064–2069. 16517655
19. Mulcahy H, Lewenza S Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One. 2011; 6: e23307. doi: 10.1371/journal.pone.0023307 21858064
20. Brannon MK, Davis JM, Mathias JR, Hall CJ, Emerson JC, et al. Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell Microbiol. 2009; 11 : 755–768. doi: 10.1111/j.1462-5822.2009.01288.x 19207728
21. Garcia-Medina R, Dunne WM, Singh PK, Brody SL Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infect Immun. 2005; 73 : 8298–8305. 16299327
22. Sana TG, Hachani A, Bucior I, Soscia C, Garvis S, et al. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J Biol Chem. 2012; 287 : 27095–27105. doi: 10.1074/jbc.M112.376368 22665491
23. Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, et al. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011; 39: D596–600. doi: 10.1093/nar/gkq869 20929876
24. Roy PH, Tetu SG, Larouche A, Elbourne L, Tremblay S, et al. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS One. 2010; 5: e8842. doi: 10.1371/journal.pone.0008842 20107499
25. Torraca V, Masud S, Spaink HP, Meijer AH Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Dis Model Mech. 2014; 7 : 785–797. doi: 10.1242/dmm.015594 24973749
26. Clatworthy AE, Lee JS, Leibman M, Kostun Z, Davidson AJ, et al. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect Immun. 2009; 77 : 1293–1303. doi: 10.1128/IAI.01181-08 19168742
27. Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001; 98 : 3087–3096. 11698295
28. Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, et al. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007; 2 : 29–39. 18005715
29. Bernut A, Herrmann JL, Kissa K, Dubremetz JF, Gaillard JL, et al. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc Natl Acad Sci U S A. 2014; 111: E943–952. doi: 10.1073/pnas.1321390111 24567393
30. Engel J, Balachandran P Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009; 12 : 61–66. doi: 10.1016/j.mib.2008.12.007 19168385
31. Bowman EJ, Siebers A, Altendorf K Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988; 85 : 7972–7976. 2973058
32. Friedman L, Kolter R Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol. 2004; 51 : 675–690. 14731271
33. Vasseur P, Vallet-Gely I, Soscia C, Genin S, Filloux A The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology. 2005; 151 : 985–997. 15758243
34. Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol. 2006; 188 : 8213–8221. 16980452
35. O'Toole GA, Kolter R Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998; 30 : 295–304. 9791175
36. Jean-Francois FL, Dai J, Yu L, Myrick A, Rubin E, et al. Binding of MgtR, a Salmonella transmembrane regulatory peptide, to MgtC, a Mycobacterium tuberculosis virulence factor: a structural study. J Mol Biol. 2014; 426 : 436–446. doi: 10.1016/j.jmb.2013.10.014 24140750
37. Karimova G, Pidoux J, Ullmann A, Ladant D A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A. 1998; 95 : 5752–5756. 9576956
38. Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, et al. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 2012; 8: e1003016. doi: 10.1371/journal.ppat.1003016 23209405
39. Prajsnar TK, Hamilton R, Garcia-Lara J, McVicker G, Williams A, et al. A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model. Cell Microbiol. 2012; 14 : 1600–1619. doi: 10.1111/j.1462-5822.2012.01826.x 22694745
40. Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003; 47 : 103–118. 12492857
41. Rathman M, Sjaastad MD, Falkow S Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996; 64 : 2765–2773. 8698506
42. Porte F, Liautard JP, Kohler S Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect Immun. 1999; 67 : 4041–4047. 10417172
43. Skurnik D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, et al. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 2013; 9: e1003582. doi: 10.1371/journal.ppat.1003582 24039572
44. Guina T, Wu M, Miller SI, Purvine SO, Yi EC, et al. Proteomic analysis of Pseudomonas aeruginosa grown under magnesium limitation. J Am Soc Mass Spectrom. 2003; 14 : 742–751. 12837596
45. Martin-Orozco N, Touret N, Zaharik ML, Park E, Kopelman R, et al. Visualization of vacuolar acidification-induced transcription of genes of pathogens inside macrophages. Mol Biol Cell. 2006; 17 : 498–510. 16251362
46. Song B, Leff LG Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol Res. 2006; 161 : 355–361. 16517137
47. Robertson EJ, Wolf JM, Casadevall A EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl Environ Microbiol. 2012; 78 : 7977–7984. doi: 10.1128/AEM.01953-12 22941091
48. Yoon MY, Lee KM, Park Y, Yoon SS Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PLoS One. 2011; 6: e16105. doi: 10.1371/journal.pone.0016105 21267455
49. Pontes MH, Lee EJ, Choi J, Groisman EA Salmonella promotes virulence by repressing cellulose production. Proc Natl Acad Sci U S A. 2015; 112 : 5183–5188. doi: 10.1073/pnas.1500989112 25848006
50. Yang Y, Labesse G, Carrere-Kremer S, Esteves K, Kremer L, et al. (2012) The C-terminal domain of the virulence factor MgtC is a divergent ACT domain. J Bacteriol 194 : 6255–6263. doi: 10.1128/JB.01424-12 22984256
51. Hoang TT, Kutchma AJ, Becher A, Schweizer HP Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000; 43 : 59–72. 10610820
52. Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005; 310 : 1782–1786. 16357253
53. Soscia C, Hachani A, Bernadac A, Filloux A, Bleves S Cross talk between type III secretion and flagellar assembly systems in Pseudomonas aeruginosa. J Bacteriol. 2007; 189 : 3124–3132. 17307856
54. Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggio MA, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011; 6: e19970. doi: 10.1371/journal.pone.0019970 21625641
55. Gannoun-Zaki L, Belon C, Dupont C, Hilbert F, Kremer L, et al. Overexpression of the Salmonella KdpF membrane peptide modulates expression of kdp genes and intramacrophage growth. FEMS Microbiol Lett. 2014; 359 : 34–41. doi: 10.1111/1574-6968.12559 25197761
56. Vallet I, Olson JW, Lory S, Lazdunski A, Filloux A The chaperone/usher pathways of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc Natl Acad Sci U S A. 2001; 98 : 6911–6916. 11381121
57. Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008; 36: W465–469. doi: 10.1093/nar/gkn180 18424797
58. Gastebois A, Blanc Potard AB, Gribaldo S, Beau R, Latge JP, et al. Phylogenetic and functional analysis of Aspergillus fumigatus MGTC, a fungal protein homologous to a bacterial virulence factor. Appl Environ Microbiol. 2011; 77 : 4700–4703. doi: 10.1128/AEM.00243-11 21602378
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



