-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
A key prediction of the prion hypothesis is that autocatalytic, misfolded PrPSc molecules should be highly infectious. Various recombinant PrPSc conformers are able to self-propagate in vitro, yet paradoxically only some of these conformers possess significant levels of specific infectivity in bioassays. Here we use two closely-matched autocatalytic recombinant PrP conformers that share the same origin but differ by >105-fold in specific infectivity to study the molecular basis of prion infectivity. We show that infectious and non-infectious autocatalytic recombinant PrP conformers have subtle structural differences, and that GPI-anchored PrP substrate molecules can only adopt the infectious PrPSc conformation. We conclude that post-translational modifications of host PrPC molecules play a critical role in restricting the range of recombinant PrPSc conformers that are biologically infectious.
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1005017
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005017Summary
A key prediction of the prion hypothesis is that autocatalytic, misfolded PrPSc molecules should be highly infectious. Various recombinant PrPSc conformers are able to self-propagate in vitro, yet paradoxically only some of these conformers possess significant levels of specific infectivity in bioassays. Here we use two closely-matched autocatalytic recombinant PrP conformers that share the same origin but differ by >105-fold in specific infectivity to study the molecular basis of prion infectivity. We show that infectious and non-infectious autocatalytic recombinant PrP conformers have subtle structural differences, and that GPI-anchored PrP substrate molecules can only adopt the infectious PrPSc conformation. We conclude that post-translational modifications of host PrPC molecules play a critical role in restricting the range of recombinant PrPSc conformers that are biologically infectious.
Introduction
The conformational conversion of the host-encoded prion protein (PrP) is a central pathogenic event in the prion diseases [1]. In healthy individuals, PrP adopts a fold that is rich in α-helix, termed PrPC, and is post-translationally modified by the incorporation of N-linked glycans and a C-terminal glycosylphosphatidylinositol (GPI) anchor. In individuals suffering from prion disease, PrPC is misfolded into a β-sheet rich conformation, termed PrPSc, which is capable of acting as a template for the conformational conversion of additional PrPC molecules into PrPSc. This self-propagating activity of PrPSc is referred to as autocatalysis and is thought to underlie the infectious nature of the prion diseases. A critical prediction of the protein-only hypothesis is that autocatalytic PrPSc molecules should be infectious.
A number of in vitro techniques for generating misfolded, autocatalytic PrPSc conformers have been developed and refined, including the cell-free conversion assay [2] and the serial protein misfolding cyclic amplification (sPMCA) technique [3,4]. With few exceptions, PrPSc conformers derived from post-translationally modified, native PrPC substrates have been highly infectious when bioassayed in wild-type animals [4–8]. In contrast, various autocatalytic PrPSc conformers derived from recombinant PrP substrates lacking post-translational modifications have displayed large variations in specific infectivity levels as determined by bioassay in wild-type animals [9–15]. The structural and functional basis of this striking variability in specific infectivity between different autocatalytic recombinant PrPSc molecules remains unknown.
Recently, using only bacterially expressed recombinant PrP and a single endogenous phospholipid cofactor molecule, phosphatidylethanolamine (PE), as substrates, Deleault et al. successfully produced high titer (2.2 x 106 LD50 U/μg PrP), chemically defined mouse prions in vitro [10]. Interestingly, it was observed that the prions produced from these minimal components always formed into a single infectious strain with unique, novel biological properties regardless of the seed originally used to template the in vitro reactions. Importantly, when this novel prion strain was subsequently propagated in the absence of PE cofactor, a new misfolded recombinant PrP conformer was produced, which could also self-propagate in sPMCA reactions, but which surprisingly failed to cause disease upon injection into wild-type mice [10]. This new autocatalytic conformer, which we refer to as protein-only PrPSc, therefore had a >105-fold lower level of specific infectivity as compared to the PrPSc conformer produced in the presence of PE cofactor, which we refer to as cofactor PrPSc.
We saw an opportunity to identify structural and functional properties associated with recombinant PrPSc infectivity by directly comparing these two related PrPSc conformers, which share the same origin and autocatalytic behavior, but differ strikingly in biological infectivity.
Results
Functional differences between cofactor and protein-only PrPSc can be localized to their respective C-terminal, protease-resistant cores
Cofactor and protein-only PrPSc are distinct misfolded recombinant PrP conformers that differ >105-fold in their specific infectivity for wild-type mice [10]. While both of these conformers demonstrate autocatalytic activity when used to seed sPMCA reactions containing recombinant PrP substrate (Fig 1A and [10]), only cofactor PrPSc also demonstrates autocatalysis when used to seed sPMCA reactions containing normal brain homogenate as the substrate (Fig 1B and [10]). The complete failure of protein-only PrPSc to function as a seed for conversion reactions containing native PrPC substrate (Fig 1B, left sample group) provides a logical explanation for this conformer’s lack of infectious activity in vivo, and may apply more generally to other recombinant PrPSc conformers which demonstrate low levels of specific infectivity in bioassays. Using cofactor PrPSc as a well-matched control, we therefore sought to gain structural and mechanistic insight into the substrate-dependence of protein-only PrPSc autocatalytic activity as a means to understand the structural and functional determinants of recombinant PrPSc infectivity.
Fig. 1. Cofactor and protein-only PrPSc are stably propagating recombinant PrP conformers that differ in their ability to template the conversion of native PrPC. 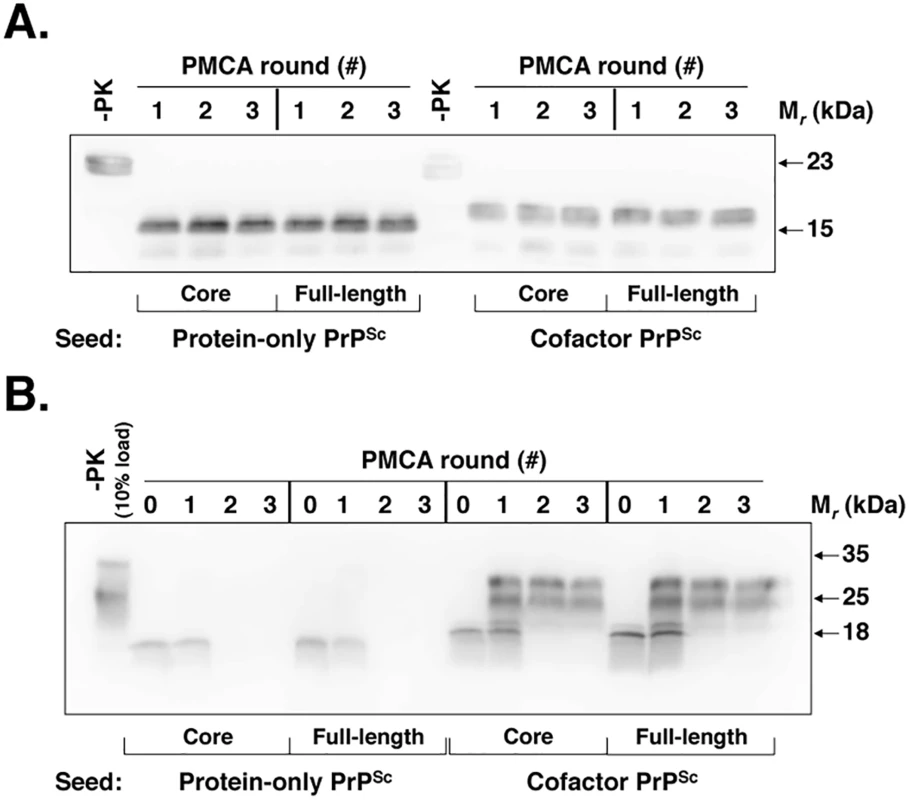
(A) Western blot showing three-round sPMCA reactions using recombinant PrP as the substrate and seeded with full-length or PK-digested cofactor and protein-only PrPSc, as indicated. (B) Western blot showing three-round sPMCA reactions using normal mouse brain homogenate as the substrate and seeded with full-length or PK-digested cofactor and protein-only PrPSc, as indicated. To help focus the structural comparison between cofactor and protein-only PrPSc molecules, we first tested whether the protease-resistant cores of both conformers, which contain approximately two thirds of the residues of mature full-length PrP, have the same substrate-specific activity as their respective parent PrPSc molecules in in vitro propagation experiments (Fig 1). We found that in sPMCA reactions containing recombinant PrP substrate and defined cofactors, both full-length and truncated cofactor and protein-only PrPSc molecules function as competent seeds which faithfully propagate the characteristic PK-resistant bands associated with their parent PrPSc molecules (Fig 1A) [10]. Moreover, in in vitro conversion reactions containing native, brain-derived PrPC substrate we found that full-length and PK-digested cofactor PrPSc drive the conversion of native PrPC (Fig 1B, third and fourth panels), while full-length and truncated protein-only PrPSc do not (Fig 1B, first and second panels), indicating that functional differences in the in vitro activity of cofactor and protein-only PrPSc molecules can be localized to the PK-resistant core. Epitope mapping of the cofactor and protein-only PrPSc PK-resistant cores (S1 Fig), revealed that both are C-terminal PrP fragments that include the 6D11 epitope (residues 93–109, with 97–100 as the major determinants of binding [16]) (S1 Fig, top panel) and the extreme C-terminus (S1 Fig, bottom panel). Based on these results, we focused our subsequent structural analyses on C-terminal residues beginning at glycine 89 (G89), the primary PK-cleavage site for PrPSc 27–30 [17].
Structural comparison of cofactor and protein-only PrPSc by DXMS identifies conformational differences in restricted C-terminal domains
To compare the structures of cofactor PrPSc and protein-only PrPSc molecules, we performed hydrogen/deuterium exchange MS (DXMS) on these two conformers generated in parallel from the same OSU prion strain seed (Fig 2A). The OSU prion strain was originally synthesized by Wang et al. using recombinant PrP, total liver RNA and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) [15], and it has previously been referred to as the OSU strain by Deleault et al. [10]. OSU-seeded cofactor and protein-only PrPSc samples used for structural analysis in this study were generated with high conversion efficiency in sPMCA (S2 Fig) and then purified prior to DXMS analysis by a series of ultracentrifugation steps described in Materials and Methods. Co-sedimentation of significant quantities of non-specifically aggregated, protease-sensitive PrP was ruled out in the OSU-seeded cofactor PrPSc DXMS sample due to its near complete conversion to PK-resistant PrPSc (96% PK-resistant conversion efficiency, S2 Fig). To assess the potential contribution of non-specifically aggregated, protease-sensitive PrP to the OSU-seeded protein-only PrPSc sample analyzed by DXMS (77% PK-resistant conversion efficiency, S2 Fig), we mock-seeded protein-only PMCA reactions and subjected the resulting material to the DXMS purification protocol (S3 Fig, top panel). We did not detect any non-specifically aggregated PrP in these mock-seeded PMCA reactions (S3 Fig, top panel, samples S0 vs P3), indicating that the protein-only PrPSc DXMS sample does not contain appreciable quantities of non-specifically aggregated, protease sensitive PrP.
Fig. 2. Regional solvent accessibility of cofactor and protein-only PrPSc conformers. 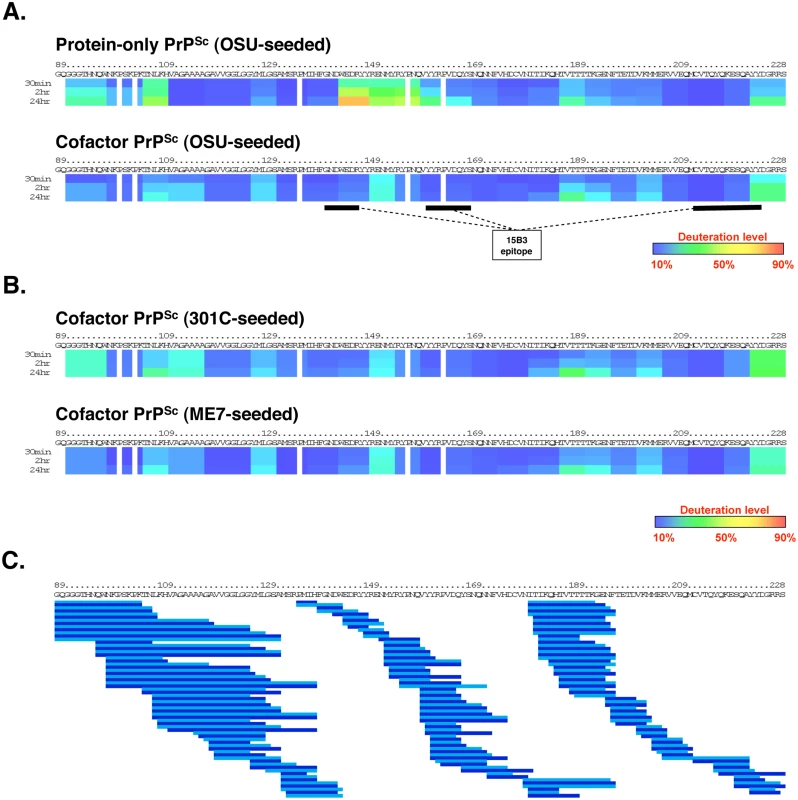
Purified PrPSc conformers were incubated in D2O-containing exchange buffer for 30 min, 2 h, or 24 h and the reaction products were quenched and subjected to LC/MS to measure peptide-specific deuterium incorporation. Data from overlapping peptides was used to quantify localized deuterium exchange and construct ribbon diagrams from a representative experiment. (A) Regional solvent accessibility comparison between cofactor and protein-only PrPSc conformers derived from the same OSU prion strain. The discontinuous epitope of mAb 15B3, which selectively binds infectious PrPSc, is indicated with black bars [18]. (B) Regional solvent accessibility of two additional cofactor PrPSc conformers, derived from the 301C and ME7 prion strains. (C) Map of the 188 high quality deuterated peptides, including different peptide charge states, identified in all four PrPSc experimental samples and used to construct ribbon diagrams in parts (A) and (B). Alternating shades of blue in part (C) are used to highlight neighboring peptides. Despite complete coverage of amino acids 89–230 by overlapping peptides, gaps exist in the ribbon diagrams shown in parts (A) and (B) for two reasons: 1. Deuterium incorporation cannot be quantified for the two N-terminal residues of a peptide as a result of rapid back exchange [19], and 2. Proline residues lack amide hydrogen atoms and therefore do not contribute to deuterium incorporation measurements. Regional solvent accessibility of cofactor and protein-only PrPSc was determined by incorporating deuteration data from 188 overlapping C-terminal peptides (Fig 2C) recovered in a representative experiment from each deuterium-labeled PrPSc sample. This high density of overlapping deuterated peptides provides PrPSc solvent accessibility measurements with resolution down to segments of ~5 amino acids. In examining and discussing the results of this study, specific regions of the PrP primary sequence are referred to either by explicit residue numbering, based on the mouse PrP sequence, or with reference to the location of known secondary structural elements in monomeric, α-helical recombinant PrP (α-PrP). For example, the domain corresponding to residues 178–216 could also be described as α2-α3 because it encompasses the second and third α-helices in α-PrP.
The C-terminal cores of cofactor and protein-only PrPSc are both substantially protected from solvent exchange (Fig 2A) as compared to α-PrP (S4 Fig). This solvent protection is consistent with widespread conversion to β-sheet secondary structure and/or the formation of large solvent-excluding aggregates [20]. Within the misfolded, solvent-protected PrPSc core there are large regions in which cofactor PrPSc and protein-only PrPSc have remarkably similar solvent accessibility profiles—in particular, the regions containing residues 118–143 and 165–230. However, there are also specific domains in which the cofactor and protein-only conformations can be distinguished by solvent accessibility. Most clearly, the domain encompassing residues 144–163, corresponding to α1 and β2 in α-PrP [21], is more solvent-exposed in protein-only PrPSc than in cofactor PrPSc (Fig 2A). The relatively exposed structure of the α1-β2 domain was preserved, and in fact accentuated, in an independently prepared protein-only PrPSc sample (S5 Fig). In addition, residues 91–110 appear to be slightly more exposed in protein-only PrPSc than in cofactor PrPSc while residues in the palindromic region (amino acids 111–120) are more obviously protected in protein-only PrPSc. Differences in these relatively N-terminal regions may contribute to the differing susceptibility to PK cleavage observed for the cofactor and protein-only PrPSc conformations (Fig 1 and S1 and S2 Figs).
Convergence of biological strain properties is correlated with cofactor PrPSc structural convergence
Deleault et al. [10] originally used three prion strains with distinct infectious phenotypes to seed the chemically-defined, recombinant PrP conversion system used to produce cofactor and protein-only PrPSc molecules. Interestingly, the three input strains converged into a single strain with a novel biological phenotype upon propagation in sPMCA reactions containing only recombinant PrP substrate and a single cofactor. We used DXMS to examine the structures of the two additional cofactor PrPSc samples generated by Deleault et al. in sPMCA reactions that were initially seeded with mouse prion strains distinct from the OSU strain (301C - and ME7-seeded cofactor PrPSc) (Fig 2B). The results revealed that the PK-resistant cores of all three cofactor PrPSc molecules have nearly identical solvent accessibility profiles (Fig 2A and 2B), consistent with convergence into a single cofactor PrPSc conformation. As was the case with OSU-seeded cofactor and protein-only PrPSc, we assessed the contribution of non-specifically aggregated, protease-sensitive PrP to the DXMS data for 301C - and ME7-seeded cofactor PrPSc by determining sample conversion efficiency (S2 Fig) and performing a mock-seeding experiment (S3 Fig, bottom panel). 301C-seeded cofactor PrPSc is almost entirely converted to PK-resistant PrPSc (99% PK-resistant conversion efficiency, S2 Fig), ruling out any significant contribution of non-specific PrP aggregation to the presented DXMS data. Mock-seeding of cofactor-supplemented PMCA reactions resulted in the recovery of approximately 8% of the starting material as non-specifically aggregated, protease-sensitive PrP (S3 Fig, bottom panel, samples S0 vs P3). For ME7-seeded cofactor PrPSc (82% PK-resistant conversion efficiency, S2 Fig), we therefore estimate that such non-specifically aggregated PrP accounts for no more than 2% of the sample analyzed by DXMS (ie. 8% of the unconverted, PK-sensitive material yields the estimate for non-specifically aggregated, PK-sensitive PrP, or ~1.4% of the total input PrP, which is then divided by the sum of the PK-resistant and PK-sensitive insoluble PrP, or ~83.4% of the total input PrP).
The data from cofactor PrPSc and protein-only PrPSc sample replicates were aggregated, yielding 63 shared peptides for which individual deuteration curves could be plotted (S6 and S7 Figs). Interestingly, peptides that include residues N-terminal to G89 were less frequently recovered and showed irregular deuteration profiles specifically in cofactor PrPSc samples (S6 and S7 Figs).
Domain-specific conformational differences between cofactor and protein-only PrPSc are supported by immunoprecipitation and Raman spectroscopy studies
Having identified the α1-β2 domain by DXMS as a region of conformational divergence in our cofactor and protein-only PrPSc samples, we sought to confirm this finding using additional biochemical and biophysical approaches. The α1-β2 region contains a portion of the epitope for 15B3, a well-characterized PrPSc-specific conformational antibody [18]. The regions of PrP primary structure that comprise the discontinuous 15B3 epitope are shown schematically in Fig 2A. In a single immunoprecipitation experiment, 15B3 efficiently pulled down all three of our cofactor PrPSc samples (Fig 3, top three panels), but only weakly bound protein-only PrPSc (Fig 3, bottom panel), indicating a disruption of the 15B3 conformational epitope, consistent with our DXMS results.
Fig. 3. Immunoprecipitation with conformation-specific mAb 15B3 distinguishes between cofactor and protein-only PrPSc. 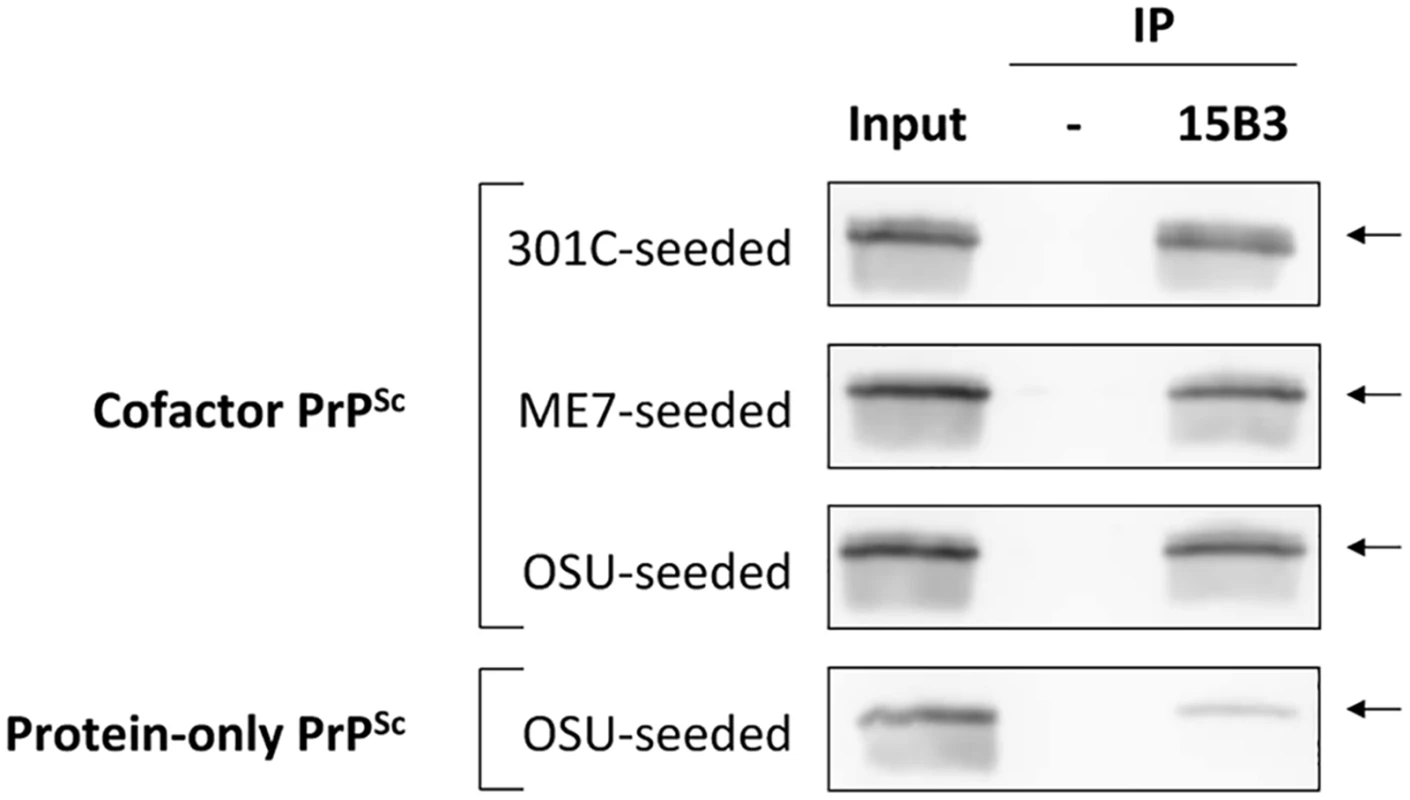
Converted sPMCA products were purified by ultracentrifugation with nOG washes to remove unconverted α-PrP and excess lipid, and immunoprecipation was performed using 15B3-coated or uncoated rat anti-mouse IgM-conjugated magnetic beads, as indicated. The location of the discontinuous, 15B3 conformational epitope is shown in Fig 2A. Arrows indicate an Mr of ~23 kDa, the expected mobility of full-length recombinant PrP. By densitometry, the efficiency of 15B3 immunoprecipitation in this experiment is 79%, 71% and 74% for 301C-seeded, ME7-seeded, and OSU-seeded cofactor PrPSc, respectively, and 15% for OSU-seeded protein-only PrPSc. We further sought to confirm a conformational difference between cofactor and protein-only PrPSc in the α1-β2 domain using Raman spectroscopy (Fig 4). Analysis of the Raman spectra acquired from these two conformers identified multiple Raman shifts that could be assigned to tyrosine residues, which are plentiful in the PrP C-terminus and specifically enriched in the α1-β2 domain (6 of 11 total C-terminal tyrosines). By Raman spectroscopy, protein-only PrPSc appears to contain more exposed tyrosine residues than cofactor PrPSc as evidenced from the increased ring ν(C = C) intensity at ~1620 cm-1 (Fig 4, left panel), the 850 cm-1/830 cm-1 ratio being greater than 1 (Fig 4, right panel) [22], and the increased ring ν(CH) intensity at ~3075 cm-1 (S8 Fig, left panel) [23,24], consistent with our DXMS results. In addition, and also consistent with our DXMS data, protein-only PrPSc appears to contain more exposed CNH groups than cofactor PrPSc as indicated by the increased intensity in the 1530–1580 cm-1 Amide II region, corresponding to ν(CN) and δ(CNH) Raman shifts (S8 Fig, right panel), as well as an increased ν(CN) intensity at ~3300 cm-1 (S8 Fig, left panel). These exposed CNH groups likely originate from the 4 exposed arginine (R) and single exposed glutamine (Q), asparagine (N) and tryptophan (W) residues in the α1-β2 domain, or from CNH-containing side chains in the N-terminal portion of the PK-resistant PrPSc core (residues ~91–115).
Fig. 4. Raman spectroscopy of cofactor and protein-only PrPSc, focusing on spectral regions assigned to tyrosine side chains. 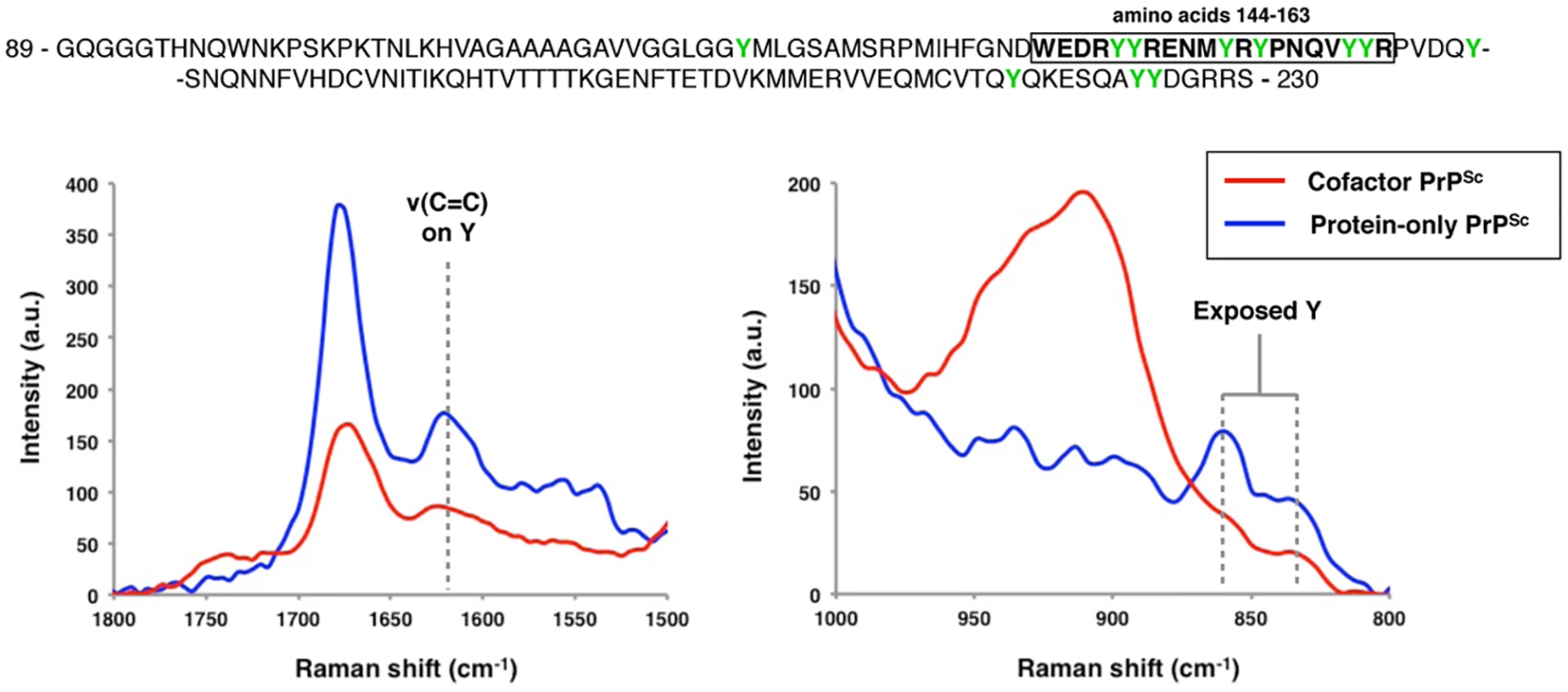
The primary sequence of the region examined by DXMS is shown, with residues 144–163 boxed and tyrosine residues highlighted in green. Raman shifts corresponding to the ν(C = C) ring mode (~1620 cm-1) and the tyrosine Fermi-doublet (~850 and 830 cm-1) are shown. Cofactor and protein-only PrPSc differ functionally in the ability to convert GPI-anchored substrates
Our DXMS data suggests that structural differences between cofactor and protein-only PrPSc are limited to specific domains (Fig 2A) and that these structural differences affect the ability of recombinant PrPSc to convert native PrPC (Fig 1B). As conversion substrates, α-PrP and native PrPC share the same primary sequence, but PrPC also contains bulky N-linked glycans and a GPI anchor as post-translational modifications. Therefore, we hypothesized that limited conformational differences might dramatically alter a recombinant conformer’s infectious activity by impinging on spatial regions that would be occupied by N-linked glycans or a GPI anchor should PrPC adopt the same conformation. To test this hypothesis, we partially purified native PrPC and performed enzymatic deglycosylation with PNGase F. The resulting PrPC molecules, which uniformly contain a GPI anchor as the sole post-translational modification, were used as the substrate in sPMCA experiments seeded with cofactor and protein-only PrPSc (Fig 5 and repeated in S9 Fig). Like brain-derived prions, recombinant cofactor PrPSc was able to template the conversion of unglycosylated PrPC (Fig 5 and S9 Fig, middle and right sample groups), whereas protein-only PrPSc failed to function as an autocatalytic seed in the same substrate (Fig 5 and S9 Fig, left sample group). Note that initial conversion of unglycosylated PrPC substrate molecules to a PK-resistant form was detected after seeding with both cofactor and protein-only PrPSc and 24 h of intermittent sonication, as indicated by the appearance of PK digestion products of slightly higher apparent molecular weight than the respective input seeds (Fig 5 and S9 Fig, left and middle sample groups, round 1 vs 0). In the case of protein-only PrPSc, this higher molecular weight product is diluted out in proportion to the initial seed (Fig 5 and S9 Fig, left sample group, round 2 vs 1), suggesting stoichiometric, as opposed to autocatalytic, PrP conversion. In contrast, the higher molecular weight band generated from infectious PrPSc seed was able to propagate independently of the initial PrPSc seed, suggesting autocatalytic PrP conversion (Fig 5 and S9 Fig, left and middle sample groups, rounds 1–3). This result indicates that the existence of bulky N-linked substrate glycans is not solely responsible for the failure of recombinant protein-only PrPSc to function as a seed for native PrPC. Moreover, by isolating the effect of a single substrate post-translational modification on recombinant PrPSc function, this result provides proof of principle that such modifications may impair the efficient replication of certain recombinant PrPSc conformers in vivo.
Fig. 5. Cofactor and protein-only PrPSc differ in their ability to template the conversion of PrP substrates containing a GPI anchor. 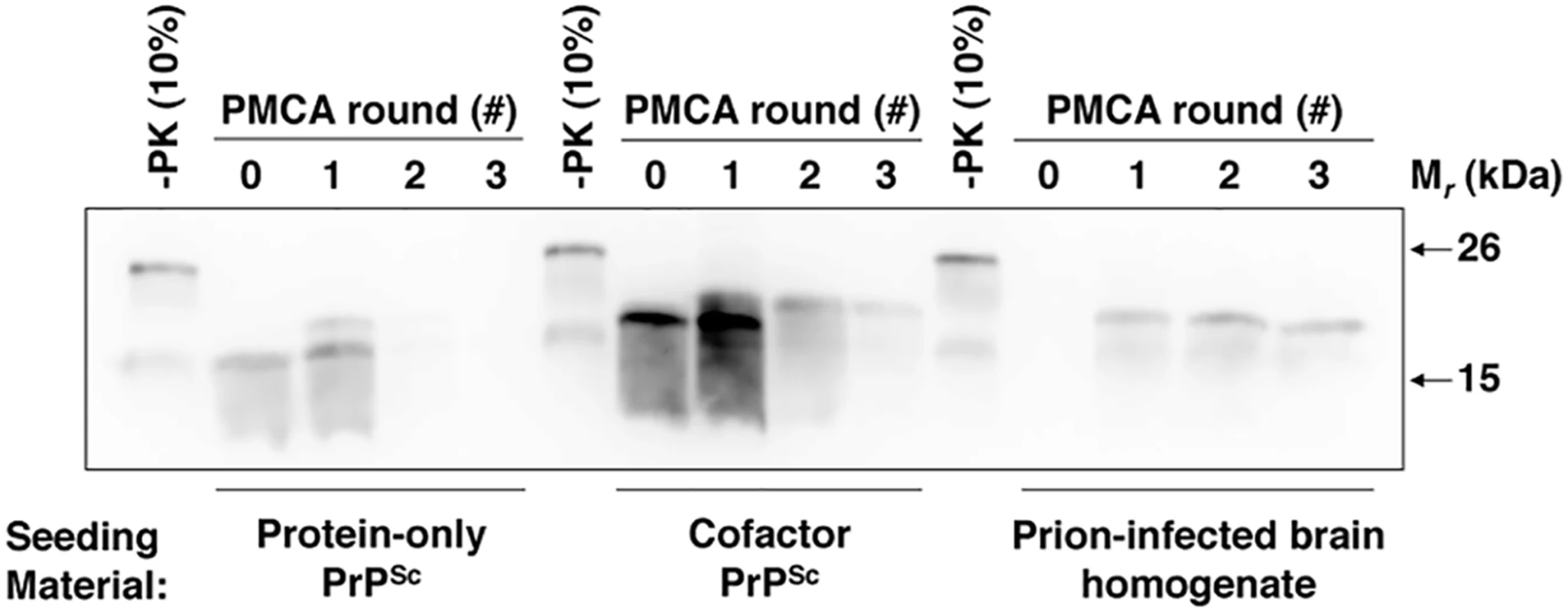
Western blot showing three round sPMCA reactions using partially purified and deglycosylated PrPC as the substrate and seeded with protein-only PrPSc, cofactor PrPSc, or prion-infected brain homogenate, as indicated. A replicate of this experiment is shown in S9 Fig Discussion
A misfolded conformer of the prion protein, PrPSc, is an essential, and possibly the sole, component of infectious prions [1]. Although PrPSc is known to be rich in β-sheet [25–27], a high-resolution structure of this PrP conformer is lacking, and the structural features of PrPSc that determine its infectious activity remain obscure.
Recently, Deleault et al. used a chemically-defined, minimal PrP conversion system and identical seeding material to generate two distinct, autocatalytic recombinant PrPSc conformers that differ >105-fold in their specific infectivity [10]. One recombinant conformer, produced in the presence of PE cofactor molecules and termed cofactor PrPSc, had a titer in normal C57BL mice nearly equivalent to that of brain-derived PrPSc (2.2 x 106 LD50 U/μg PrP), while the other conformer, produced in the absence of cofactor molecules and termed protein-only PrPSc, failed to cause disease in the same host, even at the most concentrated dose tested. In the present study, we have taken advantage of the uniquely controlled opportunity presented by these two PrPSc conformers, which share the same origin and autocatalytic behavior but differ strikingly in their biological activity, in order to investigate the structural and functional determinants of recombinant PrPSc infectivity.
By comparing the structures of these two conformers using DXMS, we find that cofactor and protein-only PrPSc molecules have remarkably similar solvent accessibility profiles within the PK-resistant PrPSc core (Fig 2A). Indeed, they are virtually indistinguishable by this measure in the domains that correspond roughly to α2-α3 in α-helical PrP (~ residues 165–230), and to the stretch of residues between the hydrophobic domain and the start of α1 (~118–143) (Fig 2A), both displaying significant protection from solvent exchange, consistent with widespread conversion to β-sheet secondary structure. The degree of solvent protection, especially N-terminal to β2, distinguishes cofactor and protein-only PrPSc from synthetic PrP amyloids [27–31], and is most consistent with previous DXMS studies using brain-derived and recombinant prions [27,32,33]. Like these recent DXMS studies, the data from the present study are consistent only with those models of PrPSc structure that involve a complete refolding of the PrPC C-terminal α-helices to β-sheet—for example, the parallel in-register β-sheet architectures proposed by Cobb et al. [34] and more recently by Groveman et al.[35]. Interestingly, the modest increase in solvent accessibility seen at residues 187–196 in all four PrPSc conformers studied here (Fig 2) corresponds well with the proposed loop of the native disulfide hairpin predicted by both of these models.
In addition to broad similarities between cofactor and protein-only PrPSc in solvent accessibility, we have identified two specific domains in which the cofactor and protein-only PrPSc conformers can be conformationally distinguished: most clearly within the domain that corresponds to α1 through the C-terminus of β2 in PrPC (~ residues 144–163), but also within a domain at the N-terminus of the PK-reistant core, comprising residues ~91–115 (Fig 2A). In both of these domains, protein-only PrPSc appears to be more exposed to solvent exchange than cofactor PrPSc. Given the role PE cofactor molecules play in the formation of cofactor, but not protein-only, PrPSc [10], it is likely that the relatively solvent-protected structural features selectively associated with cofactor PrPSc are cofactor-induced. Moreover, the fact that the PK-resistant cores of all three cofactor PrPSc samples, derived from distinct prion strains but propagated in the same chemically-defined system, are highly similar (Fig 2A and 2B) suggests that the convergence of biological strain properties observed by Deleault et al. [10] is associated with a convergence of PrPSc structure.
The α1-β2 domain, which appears to adopt different conformational states in cofactor and protein-only PrPSc (Figs 2–4), has previously been identified as a region of PrP that has important implications for PrP misfolding. For example, several PrPSc-selective conformational antibodies are known to have epitopes that reside within this domain [18,36], and small deletions towards the C-terminus of this domain produce PrP molecules that do not readily form PrPSc and, in fact, function as dominant-negative inhibitors of PrPSc replication in full-length PrP substrates [37]. Moreover, in the complete absence of the α1-β2 domain, a redacted ‘miniprion’ is capable misfolding to form PrPSc, but does not cause disease when inoculated into animals expressing full-length PrP [38,39]. Interestingly, the α1-β2 domain lies adjacent the β2-α2 loop, a region of PrP known to play an important role in prion formation and interspecies prion transmission [40–43].
Less is known about the second domain (amino acids ~91–115) in which cofactor and protein-only PrPSc appear to adopt different conformational states (Fig 2 and S5 Fig). This domain includes the so-called ‘fifth site’ for Cu2+ binding [44–46].
It is important to acknowledge that any comparisons drawn between DXMS results in the present study and the activities of cofactor and protein-only PrPSc are correlations only, and that it is possible the structural features underlying PrPSc-associated activities such as autocatalysis and infectivity may be subtle and/or beyond the resolution of the DXMS approach. Similarly, it should be acknowledged that DXMS provides a measure of the average deuterium incorporation at a given amide proton position over a population of PrPSc molecules. It has been proposed that PrPSc exists as a heterogeneous conformational mixture of so-called quasi-species [47], but it is not known—for the recombinant PrPSc conformers studied here, or any other PrPSc preparation—what proportion of PrPSc molecules exhibit autocatalytic or infectious activity. Therefore, the solvent accessibility profiles obtained in this study are representative of conformational differences at a PrPSc population level, and may not be representative of rare, and potentially biochemically/biologically active, components within a given PrPSc sample. In addition, it should be made clear that when interpreting ribbon diagrams, as in Fig 2, similar solvent accessibility profiles do not guarantee similar tertiary and/or quaternary structures, although by using a set of matched peptides with dense, overlapping coverage of the region of interest (Fig 2C) to compare the solvent accessibility of the cofactor and protein-only PrPSc conformers, we have increased confidence in such an interpretation of the data. Finally, we do not claim that a relatively solvent inaccessible conformation of either of the two structurally divergent domains identified in the present study is required for PrPSc infectivity generally. The data presented here are specific to the situation in which seed and substrate are both of the mouse PrP sequence, and it is possible that different PrP sequences may have different infectivity-associated conformational spaces. Consistent with a sequence-specific interpretation of our results, it has previously been shown that native mouse prions also appear to have a relatively solvent-protected structure in the α1-β2 domain [27], while a recent study showed that this same domain is relatively solvent exposed in native human prions [33].
The dissociation of in vitro autocatalytic activity and infectivity seen in some, but not all, recombinant PrPSc conformers [10,14,15] presents an interesting functional question: why is it that a PrPSc conformer capable of self-replication in recombinant PrP substrate fails to function as a template for native PrPC conversion in vivo? One obvious possibility relates to substrate complexity: although α-PrP and native PrPC share similar secondary structures [21,48], native PrPC is a more complex conversion substrate due to the post-translational addition of N-linked glycans and a GPI anchor, and due its location within the membrane environment of cells. Using our cofactor and protein-only recombinant PrPSc seeds as a controlled pair, we performed in vitro conversion experiments in which we, in a step-wise manner, modified native PrPC substrate to make it more and more like α-PrP in an effort to identify the factor(s) that prevent protein-only PrPSc from converting PrPC in vivo. Remarkably, the functional difference between cofactor and protein-only PrPSc persisted even after extracting native PrPC from the membrane environment (Fig 1B and [10]) and removing all N-linked glycans (Fig 5 and S9 Fig), suggesting that neither of these factors is responsible for preventing protein-only PrPSc from converting native PrPC in vivo. Unfortunately, the complementary experiment, in which the GPI anchor of PrPC is selectively removed and that modified PrPC substrate used in conversion reactions is not possible for two reasons: 1. Delipidated PrPC is a poor conversion substrate, even for sPMCA experiments seeded with native prions [49], and 2. Detection of delipidated GPI-anchored proteins by Western blotting is technically challenging [50]. Nevertheless, we infer from the available data that the functional difference between cofactor and protein-only PrPSc is mostly likely attributable to differing abilities of these two conformers to template the conversion of wild-type mouse PrP substrates containing a GPI anchor. Interestingly, Kim et al. have previously demonstrated that the GPI anchor plays an important role in the in vitro formation of native PrPSc conformers [49].
To integrate the results of our structural and functional comparison of cofactor and protein-only PrPSc, we propose a model to account for the striking variation in specific infectivity observed for recombinant PrPSc conformers [9–15] (Fig 6). In this model, the PrP polypeptide backbone, represented by recombinant PrP, is capable of adopting a wide variety of autocatalytic conformations. However, post-translationally modified, native PrPC can only adopt a subset of these conformations, and this subset represents the infectious recombinant PrPSc conformers. From previous studies of PrP and other proteins, there is evidence to suggest that post-translational modifications can alter or restrict protein folding pathways. Indeed, N-linked glycans are known broadly to have chaperone-like effects and to contribute to protein stability [51]. In the case of PrP, it has been observed that the presence of glycans can alter the rate of amyloid fibril formation [52,53], and that by restricting available PrP glycoforms it is possible to control prion strain susceptibility [54]. Whether GPI anchors play a role in protein folding or misfolding is less clear. The loss of the GPI anchor of PrP (which is concomitant with a significant reduction in PrPC glycosylation in vivo [55]) appears to have only a modest effect on PrPSc structure [56], but substantially alters the biochemical properties of PrPSc and promotes the formation of fibrillar aggregates [57,58] and interferes with PrPSc replication in vitro [49]. Interestingly, the co-expresssion of anchorless and wild-type PrPC molecules in vivo appears to enhance host susceptibility to recombinant PrP amyloid fibrils [59], while the experimental addition of a GPI anchor to the amyloidogenic yeast protein, Sup35p, prevents the formation of fibrillar structures, leading instead to the formation of PrPSc-like, non-fibrillar aggregates [60]. While the data from the present study specifically point to a role for the GPI anchor of native mouse PrPC in restricting the range of recombinant PrPSc conformers that possess infectious activity, we cannot exclude the possibility that N-linked glycans also influence the infectivity-associated recombinant PrPSc conformational space. In fact, we speculate that the boundaries of this subset of the conformational space are likely to be highly context-dependent, determined by a complex interplay between the polypeptide sequence of a given PrPC substrate molecule and all of its associated post-translational modifications.
Fig. 6. Proposed model, supported by structural and functional data in the present study, to explain variation in specific infectivity between different recombinant PrPSc conformers. 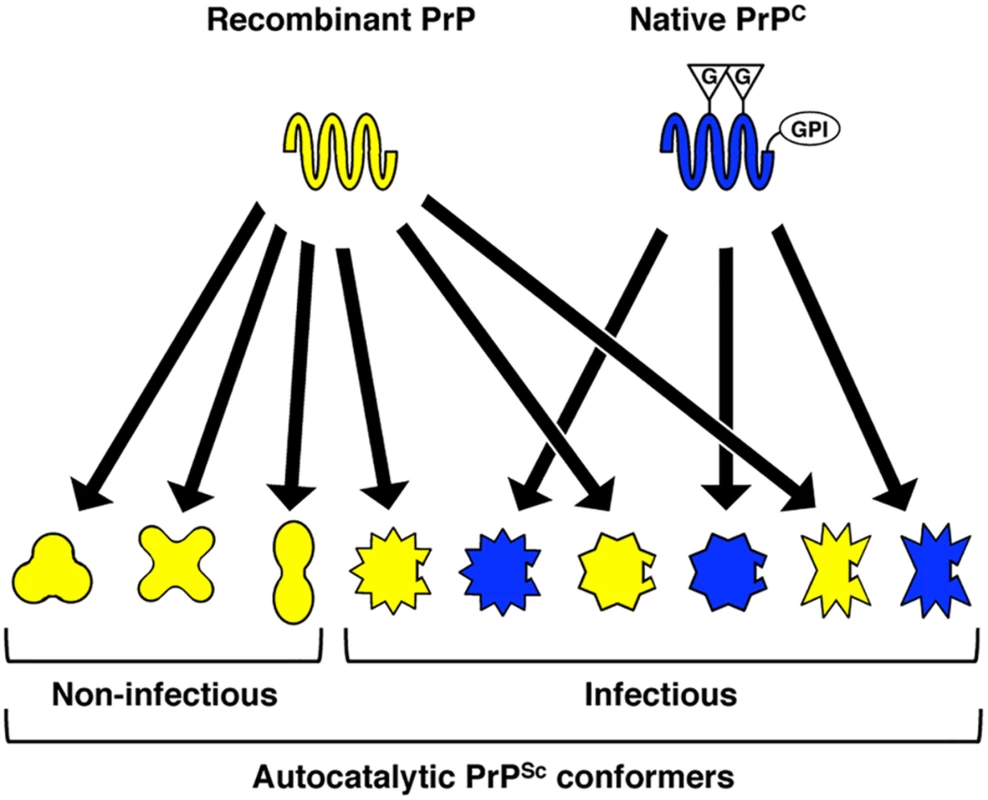
Recombinant PrP, which lacks post-translational modifications, has fewer conformational constraints than native PrPC and can adopt a variety of autocatalytic PrPSc conformations. Only those autocatalytic PrPSc conformers that can also structurally accommodate the post-translational modifications of native PrPC–in the case of the conformers studied presently, a substrate GPI anchor—are capable of biological infectivity. G = N-linked glycan; GPI = GPI anchor. In conclusion, we report for the first time a structural and functional comparison between two autocatalytic recombinant PrPSc conformers that share the same origin and biochemical behavior, but differ >105-fold in infectious titer for wild-type mice. Based on our findings, we suggest that those autocatalytic recombinant PrPSc conformers which are also highly infectious contain specific structural features in a limited number of PrP domains, and that these features may be required in order to accommodate specific substrate post-translational modifications during native PrPC misfolding in vivo.
Materials and Methods
Preparation of cofactor and protein-only PrPSc by sPMCA
Cofactor and protein-only PrPSc [10]were generated by sPMCA as described [10]. Briefly, 100–200 ul reactions containing 6 μg/ml recombinant mouse PrP 23–230 (recombinant PrP) in conversion buffer [20 mM Tris (pH 7.5), 135 mM NaCl, 5 mM EDTA (pH 7.5), 0.15% Triton X-100] were supplemented with either brain-derived cofactor [10] for cofactor PrPSc propagation, or water for protein-only PrPSc propagation. Reactions were seeded with 1/10th volume of converted cofactor or protein-only PrPSc PMCA product and sonicated with 15-s pulses every 30 min for 24 h at 37°C. After 24 h of PMCA, 1/10th volume of the reaction was used to seed fresh substrate cocktail and the 24 h sonication program was repeated. PMCA reactions were sonicated in microplate horns using a Misonix S-4000 power supply (Qsonica) set to amplitude 50–60. Sample tubes were sealed with Parafilm (Bemis Company) and the sonicator horn was soaked in 100% bleach prior to switching to propagation of a different PrPSc species in order to prevent cross-contamination.
Detection of cofactor and protein-only PrPSc
Formation of cofactor and protein-only PrPSc was monitored by digestion of PMCA samples with proteinase K (PK) and Western blotting. Samples were treated with 25 μg/ ml PK (Roche) for 30 min at 37°C, and digestion reactions quenched by the addition of SDS-PAGE loading buffer and heating to 95°C for 10 min. SDS-PAGE and Western blotting were performed as described previously [10] using mAb 27/33, unless otherwise specified.
Purification and epitope mapping of cofactor and protein-only PrPSc PK-resistant cores
All centrifugation was done at 4°C. Cofactor and protein-only PrPSc PMCA products were treated or mock-treated with PK as described above and the reaction quenched by the addition of PMSF to 5 mM final concentration. To remove PK, excess lipids, and any soluble recombinant PrP digestion products, digested PrPSc was washed twice with nOG wash buffer (1% n-octyl-beta-D-glucopyranoside (nOG), 150 mM NaCl, 8.3 mM Tris pH 7.2) by centrifugation at 100,000 rcf for 1 h, with resuspension by sonication (60-s pulse, 70 amplitude) followed by brief vortexing. After the second wash, samples were pelleted by centrifugation at 100,000 rcf for 1 hr and resuspended in conversion buffer by sonication and vortexing to a recombinant PrP concentration of 6 μg/ml for use in epitope mapping and sPMCA experiments.
Epitope mapping of the cofactor and protein-only PrPSc PK-resistant cores was performed using mAbs 6D11 [61] and R2 [62]. Aliquots of identical purified samples were run on SDS-PAGE, transferred to PVDF membrane, and processed independently by Western blotting using trays and containers that had never been in contact with anti-PrP antibodies.
sPMCA with native PrPC and deglycosylated PrPC substrates
For normal brain homogenate sPMCA, brain homogenates were prepared at 10% (w/v) in conversion buffer (PBS containing 1% (v/v) Triton X-100 and cOmplete protease inhibitor cocktail (Roche)) from healthy C57BL/6 mice, as described by Castilla et al. [5]. Homogenates were clarified by brief sonication (F60 Sonic Dismembrator (Fisher Scientific), three 5-s pulses at ~1.8 amplitude), followed by centrifugation at 500 rcf for 15 min. Clarified substrates were then seeded with 1/10th volume of purified PrPSc samples, and sPMCA carried out as described above, with 20-s sonication pulses every 30 min.
For deglycosylated PrPC sPMCA, diglycosylated native PrPC was first purified from normal mouse brain and then fully deglycosylated by treatment with PNGase F (New England Biolabs) as described [63]. Deglycosylated PrPC sPMCA reactions [63] were seeded with 5% (v/v) of recombinant or brain-derived PrPSc and sonicated as described above for normal brain homogenate sPMCA.
Deuterium exchange and quenching of cofactor and protein-only PrPSc
All centrifugation was done at 4°C. Samples were prepared for deuterium exchange as described previously [32] with the following modifications. For each PrPSc species, converted PMCA cocktail was washed twice with nOG wash buffer (1% n-octyl-beta-D-glucopyranoside (Anatrace), 150 mM NaCl, 8.3 mM Tris pH 7.2) by centrifugation at 100,000 rcf for 1 h, with resuspension by 60 s of sonication at 70 amplitude followed by brief vortexing. After the second wash, samples were pelleted by centrifugation at 100,000 rcf for 1 hr and resuspended in a volume of mock labeling buffer (150 mM NaCl, 8.3 mM Tris, pH 7.2) by sonication and vortexing immediately prior to the initiation of deuterium exchange. Deuterium exchange was initiated by the addition an equal volume of labeling buffer (D2O containing 150 mM NaCl, 8.3 mM Tris, pH* 7.2, where pH* is the pH meter reading without taking into account the hydrogen isotope effect) and samples were incubated at room temperature (22°C). During the last 30 min of labeling, samples were pelleted by centrifugation at 100,000 rcf. Labeling buffer was removed and the samples were quenched on ice for 2 min with ice-cold quench buffer [0.8% formic acid, 6.4 M guanidine hydrocholride, 150 mM tris(2-carboxyethyl)phosphine (TCEP) (Pierce)]. Quenched samples were diluted with 3 volumes of ice-cold acid diluent (0.8% formic acid, 16.6% glycerol), transferred to chilled autosampler microvials, frozen on crushed dry ice, sealed and stored at -70°C until analysis. For all PrP samples, including those described below, Western blotting of quenched material was performed and confirmed the presence of 1–2 μg recombinant PrP per sample vial.
Deuterium exchange and quenching of α-helical recombinant PrP and preparation of equilibrium-deuterated controls
Deuterium exchange and quenching of normally folded, α-helical recombinant PrP (α-PrP) was performed as described above for cofactor and protein-only PrPSc, with the following modifications. Recombinant PrP was resuspended to 1.0 mg/ml in water and an equal volume of 2x labeling buffer (D2O containing 300 mM NaCl, 16.6 mM Tris, pH* 7.2) was added to initiate deuterium exchange. Thirty minutes prior to quenching, an aliquot of the labeling reaction was placed at 4°C to replicate the temperature change experienced by PrPSc samples during centrifugation. Deuterium-labeled α-PrP was then quenched on ice for 2 min by the addition of 1.25 volumes of ice-cold quench buffer containing 700 mM TCEP. To the quenched samples was added 1.30 volumes of ice-cold acid diluent prior to aliquoting into autosampler microvials and freezing on dry ice, as described above.
Equilibrium-deuterated samples were prepared by resuspension of recombinant PrP in 2.5 or 6.0 M guanidine hydrochloride solution containing a 1 : 1 molar ratio of protons:deuterons by mixing appropriate quantities of H2O, D2O, guanidine HCl and guanidine (D6) DCl (Cambridge Isotope Laboratories, Andover, MA). Deuterium exchange was allowed to proceed for 72 hours at room temperature (22°C) prior to quenching as described above for α-PrP.
Quantification of co-sedimentation of protease-sensitive, insoluble PrP in DXMS PrPSc samples
To estimate the fraction of non-specifically aggregated PrP that could potentially co-sediment during the purification of PrPSc samples for DXMS, mock-seeded PMCA reactions were performed using PMCA cocktail supplemented with brain-derived cofactor or water. After 24 h of PMCA, the mock-seeded PMCA reactions were purified by ultracentrifugation as described above for DXMS samples and the fraction of the input PrP that was recovered as non-specifically aggregated, insoluble PrP was quantified by Western blot.
Analysis of deuterium incorporation
Measurement of deuterium incorporation by LC-MS was performed as described previously [32], with the following modifications. Samples were loaded onto the in-line immobilized fungal protease XIII column at a rate of 60 μl/min, allowed to digest for 3 min, and then pushed onto the in-line immobilized pepsin column at a rate of 20 μl/min. Peptides were collected during pepsin digestion on a C18 trap column (Michrom MAGIC C18AQ, 0.2x2) preceding the C18 resolving column (Michrom MAGIC C18AQ, 0.2x50). All measurements were made on an Orbitrap Elite mass spectrometer (Thermo Fisher Scientific), and data was analyzed as described previously [32].
Immunoprecipitation of cofactor and protein-only PrPSc with mAb 15B3
Rat anti-mouse IgM-conjugated Dynabeads (Life Technologies) were washed according to the manufacturer’s protocol and coated with mAb 15B3 (Prionics) at 5 μg per 10 μl beads with gentle mixing at room temperature for 2 h. Coated beads were washed three times to remove unbound antibody and stored at 4°C for no more than one week prior to use. Converted cofactor or protein-only PrPSc PMCA cocktail was washed twice with nOG wash buffer as described above for the preparation of samples for DXMS. After the second wash, samples were collected by centrifugation at 100,000 rcf for 1 hr at 4°C and the pellet was gently washed with Prionics homogenization buffer (Prionics). Samples were then centrifuged at 100,000 rcf for 10 min at 4°C and the supernatant discarded. The pellet was resuspended in Prionics homogenization buffer by sonication (30-s pulse, 70 amplitude) and vortexing to a final concentration of ~60 ng/μl PrP. For each PrPSc sample, ~250 ng PrP was added to 0.5 ml Prionics IP buffer (Prionics) and to this was added 10 μl of beads that were either coated with 15B3 or uncoated. Samples were allowed to interact with the beads at room temperature for 4 h with gentle mixing, followed by two washes with Prionics IP buffer and resuspension of the beads in 2x SDS-PAGE loading buffer. Samples were incubated at 95°C for 10 min, briefly centrifuged to concentrate the beads and the supernatant was collected for analysis by Western blot.
Raman spectroscopy of cofactor and protein-only PrPSc
Cofactor and protein-only PrPSc were generated by sPMCA supplemented with synthetic plasmalogen PE (Avanti Polar Lipids) as the sole cofactor, as described previously [64]. Converted PMCA cocktail was digested with PK as described above and quenched by the addition of PMSF (Sigma Aldrich) to 2 mM final concentration. Digested samples were washed twice with nOG wash buffer, as described above, and then twice with water to remove residual buffer components and detergent. Samples were then resuspended in water to a concentration of ~140 ng/μl PrP by vortexing and a 15-s sonication pulse. 10 μl of the resulting sample was spotted onto a glass slide and allowed to dry under a stream of nitrogen. Once dry, another 10 μl was spotted on top of the first and again allowed to dry under nitrogen. Spotted samples were scanned using a WITec CRM200 Raman confocal light microscope, equipped with a 100x lens and a 514 nm argon laser with 45 mW output. An f/4, 300 mm imaging spectrograph was employed with 2 exit ports and a 600 lines/mm grating, with a Peltier-cooled CCD, 1340 x 100 pixel format, and a 16-bit camera controller. The fiber optic connecting the microscope with the spectrograph was 50 μm in diameter. Spectra were acquired using an integration time of 8 s, with two hardware and two software accumulations per shot and a spectral resolution of 4 cm-1. Presented spectra are averages of 20–30 shots. In each figure, the baseline was adjusted to zero and data points were joined with a smoothed line in Microsoft Excel. Although spectral normalization was not possible, data were collected with the same instrument at the same time from highly concentrated films of protein, and it can be expected that intensity differences between samples originate in structural differences between conformers.
Ethics statement
All experiments involving mice in this study were conducted in accordance with protocol supa.su.1 as reviewed and approved by Dartmouth College’s Institutional Animal Care and Use Committee, operating under the regulations/guidelines of the NIH Office of Laboratory Animal Welfare (assurance number A3259-01).
Supporting Information
Zdroje
1. Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95 : 13363–13383. 9811807
2. Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, et al. (1994) Cell-free formation of protease-resistant prion protein. Nature 370 : 471–474. 7913989
3. Saborio GP, Permanne B, Soto C (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411 : 810–813. 11459061
4. Castilla J, Morales R, Saa P, Barria M, Gambetti P, et al. (2008) Cell-free propagation of prion strains. Embo J 27 : 2557–2566. doi: 10.1038/emboj.2008.181 18800058
5. Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121 : 195–206. 15851027
6. Deleault NR, Harris BT, Rees JR, Supattapone S (2007) Formation of native prions from minimal componenets in vitro. Proc Natl Acad Sci U S A 104 : 9741–9746. 17535913
7. Piro JR, Harris BT, Nishina K, Soto C, Morales R, et al. (2009) Prion protein glycosylation is not required for strain-specific neurotropism. J Virol 83 : 5321–5328. doi: 10.1128/JVI.02502-08 19297485
8. Piro JR, Harris BT, Supattapone S (2011) In situ photodegradation of incorporated polyanion does not alter prion infectivity. PLoS Pathog 7: e1002001. doi: 10.1371/journal.ppat.1002001 21304885
9. Colby DW, Wain R, Baskakov IV, Legname G, Palmer CG, et al. (2010) Protease-sensitive synthetic prions. PLoS Pathog 6: e1000736. doi: 10.1371/journal.ppat.1000736 20107515
10. Deleault NR, Walsh DJ, Piro JR, Wang F, Wang X, et al. (2012) Cofactor molecules maintain infectious conformation and restrict strain properties in purified prions. Proc Natl Acad Sci U S A 109: E1938–E1946. doi: 10.1073/pnas.1206999109 22711839
11. Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, et al. (2010) Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285 : 14083–14087. doi: 10.1074/jbc.C110.113464 20304915
12. Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, et al. (2004) Synthetic mammalian prions. Science 305 : 673–676. 15286374
13. Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, et al. (2010) Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119 : 177–187. doi: 10.1007/s00401-009-0633-x 20052481
14. Timmes AG, Moore RA, Fischer ER, Priola SA (2013) Recombinant prion protein refolded with lipid and RNA has the biochemical hallmarks of a prion but lacks in vivo infectivity. PLoS One 8: e71081. doi: 10.1371/journal.pone.0071081 23936256
15. Wang F, Wang X, Yuan CG, Ma J (2010) Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science 327 : 1132–1135. doi: 10.1126/science.1183748 20110469
16. Spinner DS, Kascsak RB, Lafauci G, Meeker HC, Ye X, et al. (2007) CpG oligodeoxynucleotide-enhanced humoral immune response and production of antibodies to prion protein PrPSc in mice immunized with 139A scrapie-associated fibrils. J Leukoc Biol 81 : 1374–1385. 17379700
17. Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE (1984) Purification and structural studies of a major scrapie prion protein. Cell 38 : 127–134. 6432339
18. Korth C, Stierli B, Streit P, Moser M, Schaller O, et al. (1997) Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390 : 74–77. 9363892
19. Bai Y, Milne JS, Mayne L, Englander SW (1993) Primary structure effects on peptide group hydrogen exchange. Proteins 17 : 75–86. 8234246
20. Engen JR (2009) Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Anal Chem 81 : 7870–7875. doi: 10.1021/ac901154s 19788312
21. Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, et al. (1996) NMR structure of the mouse prion protein domain PrP(121–321). Nature 382 : 180–182. 8700211
22. Siamwiza MN, Lord RC, Chen MC, Takamatsu T, Harada I, et al. (1975) Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry 14 : 4870–4876. 241390
23. Edwards HG, Hunt DE, Sibley MG (1998) FT-Raman spectroscopic study of keratotic materials: horn, hoof and tortoiseshell. Spectrochim Acta A Mol Biomol Spectrosc 54A: 745–757. 9679318
24. Günzler H, Gremlich H-U (2002) IR spectroscopy: an introduction. Weinheim: Wiley-VCH. xiii, 361 p. p.
25. Caughey B, Raymond GJ, Bessen RA (1998) Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J Biol Chem 273 : 32230–32235. 9822701
26. Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, et al. (1991) Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30 : 7672–7680. 1678278
27. Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, et al. (2011) Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 18 : 504–506. doi: 10.1038/nsmb.2035 21441913
28. Lu X, Wintrode PL, Surewicz WK (2007) Beta-sheet core of human prion protein amyloid fibrils as determined by hydrogen/deuterium exchange. Proc Natl Acad Sci U S A 104 : 1510–1515. 17242357
29. Nazabal A, Hornemann S, Aguzzi A, Zenobi R (2009) Hydrogen/deuterium exchange mass spectrometry identifies two highly protected regions in recombinant full-length prion protein amyloid fibrils. J Mass Spectrom 44 : 965–977. doi: 10.1002/jms.1572 19283723
30. Smirnovas V, Kim JI, Lu X, Atarashi R, Caughey B, et al. (2009) Distinct structures of scrapie prion protein (PrPSc)-seeded versus spontaneous recombinant prion protein fibrils revealed by hydrogen/deuterium exchange. J Biol Chem 284 : 24233–24241. doi: 10.1074/jbc.M109.036558 19596861
31. Singh J, Udgaonkar JB (2013) Dissection of conformational conversion events during prion amyloid fibril formation using hydrogen exchange and mass spectrometry. J Mol Biol 425 : 3510–3521. doi: 10.1016/j.jmb.2013.06.009 23811055
32. Miller MB, Wang DW, Wang F, Noble GP, Ma J, et al. (2013) Cofactor Molecules Induce Structural Transformation during Infectious Prion Formation. Structure.
33. Kim HJ, Raphael AR, LaDow ES, McGurk L, Weber RA, et al. (2014) Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet 46 : 152–160. doi: 10.1038/ng.2853 24336168
34. Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK (2007) Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proc Natl Acad Sci U S A 104 : 18946–18951. 18025469
35. Groveman BR, Dolan MA, Taubner LM, Kraus A, Wickner RB, et al. (2014) Parallel In-register Intermolecular beta-Sheet Architectures for Prion-seeded Prion Protein (PrP) Amyloids. J Biol Chem 289 : 24129–24142. doi: 10.1074/jbc.M114.578344 25028516
36. Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, et al. (2003) A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 9 : 893–899. 12778138
37. Taguchi Y, Mistica AM, Kitamoto T, Schatzl HM (2013) Critical significance of the region between Helix 1 and 2 for efficient dominant-negative inhibition by conversion-incompetent prion protein. PLoS Pathog 9: e1003466. doi: 10.1371/journal.ppat.1003466 23825952
38. Muramoto T, Scott M, Cohen FE, Prusiner SB (1996) Recombinant scrapie-like prion protein of 106 amino acids is soluble. Proc Natl Acad Sci U S A 93 : 15457–15462. 8986833
39. Supattapone S, Bosque P, Muramoto T, Wille H, Aagaard C, et al. (1999) Prion protein of 106 residues creates an artifical transmission barrier for prion replication in transgenic mice. Cell 96 : 869–878. 10102274
40. Kurt TD, Bett C, Fernandez-Borges N, Joshi-Barr S, Hornemann S, et al. (2014) Prion transmission prevented by modifying the beta2-alpha2 loop structure of host PrPC. J Neurosci 34 : 1022–1027. doi: 10.1523/JNEUROSCI.4636-13.2014 24431459
41. Kurt TD, Jiang L, Bett C, Eisenberg D, Sigurdson CJ (2014) A proposed mechanism for the promotion of prion conversion involving a strictly conserved tyrosine residue in the beta2-alpha2 loop of PrPC. J Biol Chem 289 : 10660–10667. doi: 10.1074/jbc.M114.549030 24596090
42. Sigurdson CJ, Nilsson KP, Hornemann S, Heikenwalder M, Manco G, et al. (2009) De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci U S A 106 : 304–309. doi: 10.1073/pnas.0810680105 19073920
43. Sigurdson CJ, Nilsson KP, Hornemann S, Manco G, Fernandez-Borges N, et al. (2010) A molecular switch controls interspecies prion disease transmission in mice. J Clin Invest 120 : 2590–2599. doi: 10.1172/JCI42051 20551516
44. Hasnain SS, Murphy LM, Strange RW, Grossmann JG, Clarke AR, et al. (2001) XAFS study of the high-affinity copper-binding site of human PrP(91–231) and its low-resolution structure in solution. J Mol Biol 311 : 467–473. 11493001
45. Jackson GS, Murray I, Hosszu LL, Gibbs N, Waltho JP, et al. (2001) Location and properties of metal-binding sites on the human prion protein. Proc Natl Acad Sci U S A 98 : 8531–8535. 11438695
46. Jones CE, Abdelraheim SR, Brown DR, Viles JH (2004) Preferential Cu2+ coordination by His96 and His111 induces beta-sheet formation in the unstructured amyloidogenic region of the prion protein. J Biol Chem 279 : 32018–32027. 15145944
47. Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15 : 233–249. doi: 10.1038/nrn3689 24619348
48. Hornemann S, Schorn C, Wuthrich K (2004) NMR structure of the bovine prion protein isolated from healthy calf brains. EMBO Rep 5 : 1159–1164. 15568016
49. Kim JI, Surewicz K, Gambetti P, Surewicz WK (2009) The role of glycophosphatidylinositol anchor in the amplification of the scrapie isoform of prion protein in vitro. FEBS Lett 583 : 3671–3675. doi: 10.1016/j.febslet.2009.10.049 19854187
50. Nishina KA, Supattapone S (2007) Immunodetection of glycophosphatidylinositol-anchored proteins following treatment with phospholipase C. Anal Biochem 363 : 318–320. 17321480
51. Mitra N, Sinha S, Ramya TN, Surolia A (2006) N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem Sci 31 : 156–163. 16473013
52. Chen PY, Lin CC, Chang YT, Lin SC, Chan SI (2002) One O-linked sugar can affect the coil-to-beta structural transition of the prion peptide. Proc Natl Acad Sci U S A 99 : 12633–12638. 12235358
53. Stohr J, Elfrink K, Weinmann N, Wille H, Willbold D, et al. (2011) In vitro conversion and seeded fibrillization of posttranslationally modified prion protein. Biol Chem 392 : 415–421. doi: 10.1515/BC.2011.048 21476870
54. Cancellotti E, Mahal SP, Somerville R, Diack A, Brown D, et al. (2013) Post-translational changes to PrP alter transmissible spongiform encephalopathy strain properties. EMBO J 32 : 756–769. doi: 10.1038/emboj.2013.6 23395905
55. Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, et al. (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308 : 1435–1439. 15933194
56. Baron GS, Hughson AG, Raymond GJ, Offerdahl DK, Barton KA, et al. (2011) Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry 50 : 4479–4490. doi: 10.1021/bi2003907 21539311
57. Bett C, Kurt TD, Lucero M, Trejo M, Rozemuller AJ, et al. (2013) Defining the conformational features of anchorless, poorly neuroinvasive prions. PLoS Pathog 9: e1003280. doi: 10.1371/journal.ppat.1003280 23637596
58. Chesebro B, Race B, Meade-White K, Lacasse R, Race R, et al. (2010) Fatal transmissible amyloid encephalopathy: a new type of prion disease associated with lack of prion protein membrane anchoring. PLoS Pathog 6: e1000800. doi: 10.1371/journal.ppat.1000800 20221436
59. Raymond GJ, Race B, Hollister JR, Offerdahl DK, Moore RA, et al. (2012) Isolation of novel synthetic prion strains by amplification in transgenic mice coexpressing wild-type and anchorless prion proteins. J Virol 86 : 11763–11778. doi: 10.1128/JVI.01353-12 22915801
60. Marshall KE, Offerdahl DK, Speare JO, Dorward DW, Hasenkrug A, et al. (2014) Glycosylphosphatidylinositol anchoring directs the assembly of Sup35NM protein into non-fibrillar, membrane-bound aggregates. J Biol Chem 289 : 12245–12263. doi: 10.1074/jbc.M114.556639 24627481
61. Pankiewicz J, Prelli F, Sy MS, Kascsak RJ, Kascsak RB, et al. (2006) Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur J Neurosci 23 : 2635–2647. 16817866
62. Williamson RA, Peretz D, Pinilla C, Ball H, Bastidas RB, et al. (1998) Mapping the prion protein using recombinant antibodies. J Virol 72 : 9413–9418. 9765500
63. Nishina KA, Deleault NR, Mahal SP, Baskakov I, Luhrs T, et al. (2006) The stoichiometry of host PrPC glycoforms modulates the efficiency of PrPSc formation in vitro. Biochemistry 45 : 14129–14139. 17115708
64. Deleault NR, Piro JR, Walsh DJ, Wang F, Ma J, et al. (2012) Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc Natl Acad Sci U S A 109 : 8546–8551. doi: 10.1073/pnas.1204498109 22586108
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



